- 1College of Plant Science and Technology, Innovation Academy of International Traditional Chinese Medicinal Materials, National-Regional Joint Engineering Research Center in Hubei for Medicinal Plant Breeding and Cultivation, Medicinal Plant Engineering Research Center of Hubei Province, Institute for Medicinal Plants, Huazhong Agricultural University, Wuhan, China
- 2Department of Pharmacy, Renmin Hospital, Hubei University of Medicine, Shiyan, China
- 3College of Chemistry and Environmental Science, Laboratory of Xinjiang Native Medicinal and Edible Plant Resources Chemistry. Kashi University, Kashgar, China
- 4Research Institute of Forests and Rangelands, Tehran, Iran
Traditional Chinese medicine (TCM) includes over ten thousand herbal medicines, some of which were introduced from outside countries and territories. The Silk Road enabled the exchange of merchandise such as teas, silks, carpets, and medicines between the East and West of the Eurasia continent. During this time, the ‘Compendium of Materia Medica’ (CMM) was composed by a traditional medicine practitioner, Shizhen Li (1,518–1,593) of the Ming Dynasty. This epoch-making masterpiece collected knowledge of traditional medical materials and treatments in China from the 16th century and before in utmost detail, including the origin where a material was obtained. Of 1892 medical materials from the CMM, 46 came from Persia (now Iran). In this study, the basic information of these 46 materials, including the time of introduction, the medicinal value in TCM theory, together with the current status of these medicines in China and Iran, are summarized. It is found that 20 herbs and four stones out of the 46 materials are registered as medicinal materials in the latest China Pharmacopoeia. Now most of these herbs and stones are distributed in China or replacements are available but saffron, ferula, myrrh, and olibanum are still highly dependent on imports. This study may contribute to the further development, exchange, and internationalization of traditional medicine of various backgrounds in the world, given the barriers of transportation and language are largely eased in nowadays.
1 Introduction
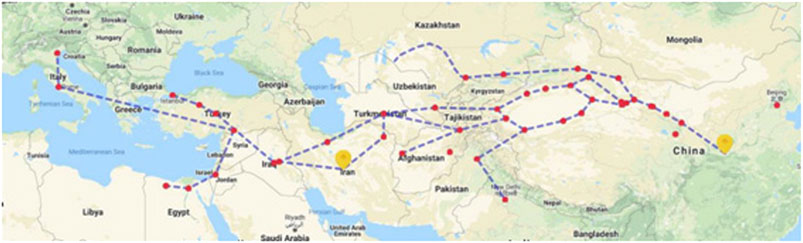
Persia (present Iran) was an important territory along the ancient Silk Road. The Silk Road was first officially proposed by a German geographer Ferdinand von Richthofen in 1877 (Wu, 2019). The Silk Road started from Chang’an (now Xi’an city of China) in the Western Han Dynasty and crossed the Longshan Mountains and Hexi Corridor, and reached to Xinjiang through the Yumenguan and Yangguan passes. Then, passing through modern-day Tajikistan, Turkmenistan, Iran, Iraq, Turkey, it finally reached to Africa and Europe. The Silk Road is a historical route that traverses Eurasia and promotes friendly exchanges between Europe and Asia (Figure 1). In the trade along the Silk Road, China’s export of silk is the most representative; hence, it was named “Silk Road.” Businessmen and messengers from all over the world have continued to carry out economic and cultural exchanges along this road.
The record of Iran in China can be dated back to before Christ (BC), corresponding to the Arsa Dynasty of the Parthian Empire (247 BC–226 AD) in Iran (Lei, 2011). In 138 BC, Qian Zhang was sent as an envoy to greet the Western territories. Qian Zhang opened up a new era of goods and culture exchanges between old China and foreign countries and territories. The route was historically visualized as the “Qian Zhang short-cut.” With the gradual prosperity of the Silk Road, China’s relations with Persia became increasingly close, and many medicinal materials from Persia have also been introduced to China.
The Compendium of Materia Medica (CMM; “本草纲目” in Chinese characters or “Bencao Gangmu” in the Chinese phonetic alphabet) provides a body of medicinal products and agents that was compiled by Shizhen Li (1,518–1,593) in the Ming Dynasty. He devoted his entire life and spent 30 years to complete the masterpiece. The book is divided into 16 sections (waters, fires, earth, metals, stones and minerals, herbs, cereals, vegetables, fruits, wood, fabrics and utensils, insects, worms and amphibians, animals with scales, shells, fowls, animals and humans). With a total of 1.9 million Chinese characters, the book collected 1892 medicines, 11,096 medical prescriptions, and 1,160 illustrations. The book systematically describes the knowledge of each CMM medicine in detail and provides an explanation of the names and background of each medicinal material and it discusses quality, taste, indications, explication, prescriptions, etc. The number of medicinal herbs recorded and the details of the content in the CMM are vast and during the preparation of CMM, Shizhen Li visited a wide range of regions and consulted many people in order to ensure authenticity and accuracy in the recording of ethnobotanical information. He summarized and criticized herbal books written by previous scholars, corrected errors in these books, and then invented a comparative system of drug classification methods. In classifying medicinal materials, he established a system from low level to advanced, from simple to complex, which was a very advanced classification method at that time. The first edition CCM was published over 400 years ago. Since then, it has been translated into other languages, making it accessible to the rest of the world. As a world’s documentary heritage, the CMM was successfully selected in the Memory of the World Register in 2011 (Zhang and Zheng, 2016). Being a treasure of Chinese medicine for thousands of years in China, the CMM is broad and profound. Its scientific achievements encompass many aspects of ancient science and technology but it still has great relevance in modern times as it covers botany, geography, meteorology, mineralogy, biology, physics, etc. Although CMM is primarily a medical book, it is worth studying in many subject fields. The CMM has a far-reaching impact on the development of Chinese medicine and other disciplines.
It has a long history of cultivating medicinal plants in China. These plants account for a major part of traditional Chinese medicine (TCM), however, not all of these herbs are native to China. As exotic plants, they were incorporated and developed into the TCM system despite being introduced to China from overseas. These exotic plants have become naturalized, gradually adapting to local environments and are now widely recognized by Chinese people. According to Tan and Peng (2014), the CMM contained 96 foreign medicines imported from different countries, of which, 46 were imported from Persia. It accounts for nearly half of the number of foreign drugs, including nine herbs, 12 types of wood, seven types of fruit, 15 metals and minerals, two types of vegetables, and one fowl. The exchange of medicinal materials along the ancient Silk Road is a great contribution to the development of traditional Chinese medicine, moreover, it also links the civilizations between Chinese and abroad. In this review study, with these 46 Persian imported medicines as a focus, we endeavored to trace the nomenclature, distribution, pharmacology, and corresponding disease treatments, and most importantly, the changes and evolution of these characteristics in 2 thousand years for each of the materials. The historical, medical, economic, and cultural significance, wherever necessary, were also discussed.
2 Materials and methods
All original information on the medicinal materials was based on a Chinese version of the Compendium of Materia Medica (Li, 2005), of which an English version of the book is also available (Li, 2003). Any TCM documented in the CMM with an origin of Chinese character “波斯” (“Bosi” in Chinese phonics) was thought to be imported from Persia. All TCMs from the CMM were compared with the 2020 edition of the Pharmacopoeia of People’s Republic of China (PPRC) [Chinese version] (Commission, 2020) or 2015 edition of the Pharmacopoeia of People’s Republic of China (ePPRC)[English version] (Commission, 2017). More literature on pharmacology, history, and botany of the plants, were obtained by searching against the following databases: Scifinder, PubMed, and Google Scholar. The Royal Botanic Gardens, Kew (www.mpns.science.kew.org) was used to check the correctness of the nomenclature of the reported plant species.
3 Results
A total of 46 TCMs documented in the CMM were clearly mentioned to have an origin linked to Persia. The name and current distribution of these TCMs are included in Table 1. The CMM was published 500 years ago, but most of the TCMs noted in the book were introduced to China much earlier. In a search of the possible origin, 11 TCMs were introduced to China before Qin and Han dynasties (before 220 AD), 12 were introduced to China in the Wei, Jin, and Nanbei dynasties (220–589 AD), 20 are associated with Sui, Tang, and Five dynasties (618–907 AD). Only three were introduced from the Song to Ming dynasties (960–1644 AD), indicating that the TCM commerce in the Silk Road was established over a thousand of years (Table 2).
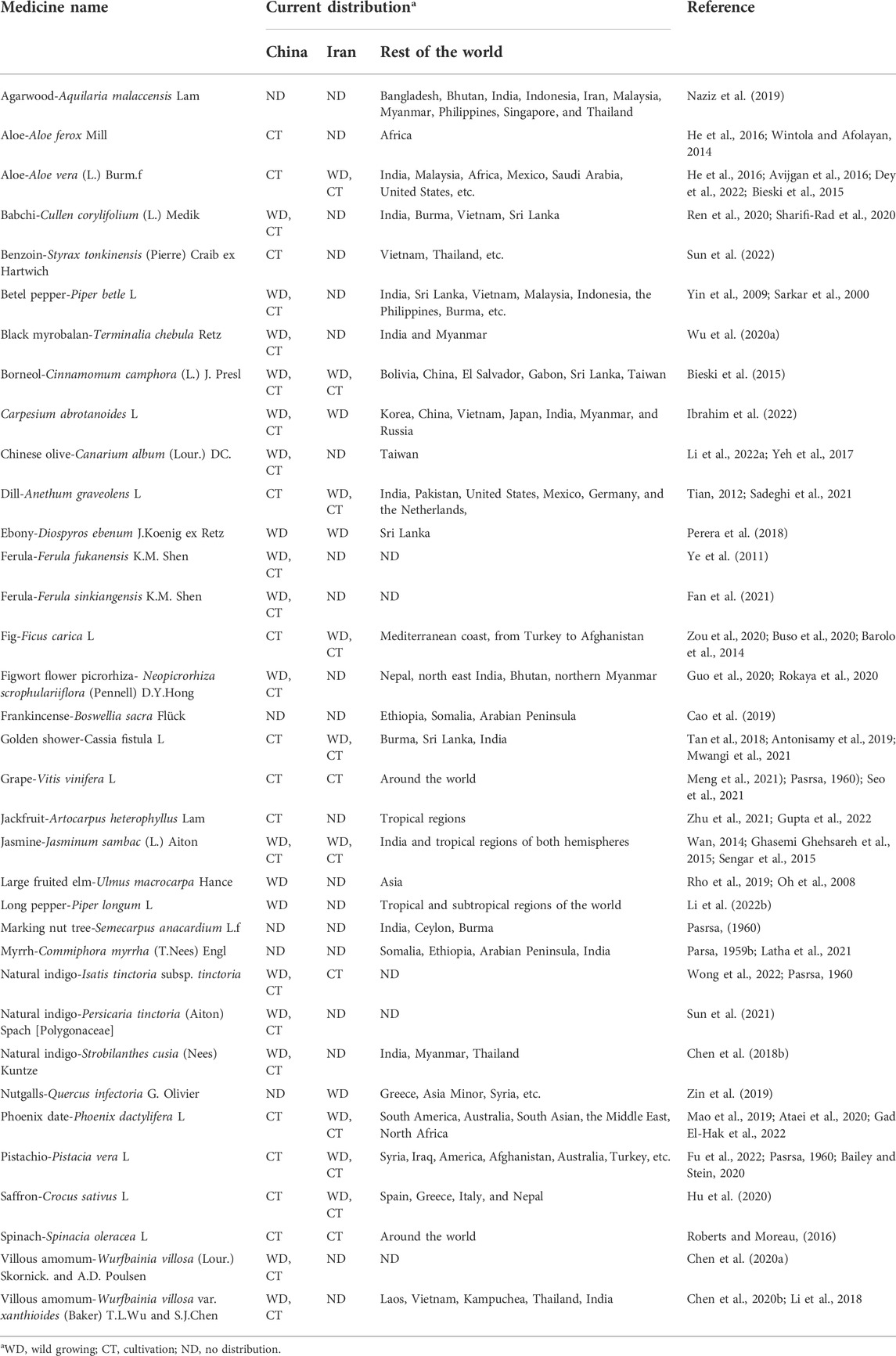
TABLE 1. Medicinal plants exported from Persia to old China as recorded in the Compendium of Materia Medica. The current distribution of these plants is noted.

TABLE 2. Chinese medicinal materials introduced from Persia to China in the Compendium of Materia Medica.
A detail of the basic information of the TCM with a registry of China Pharmacopoeia, is organized in Table 3. Because of the tradition of TCM, one material might be included in two registries with different materials, such as Strobilanthes cusia (Nees) Kuntze and this is used as a single medicine or in combination with Persicaria tinctoria (Aiton) Spach and Isatis tinctoria subsp. tinctoria. On the other hand, two different parts of one herb might be separately registered in the PPRC. The leaf and root of Isatis tinctoria subsp. tinctoria are separately registered in the PPRC as two medicines and their formulations are different. It is worth noting that one stone is not grouped in a formula but most of these materials are still in use with as many as 75 formulae being utilized. According to the CMM method, the TCM from Persia was divided into categories of herbs, vegetables, fruits, woods, metal/stone/mineral, and fowls. The following sections provide details in terms of these medicinal products.
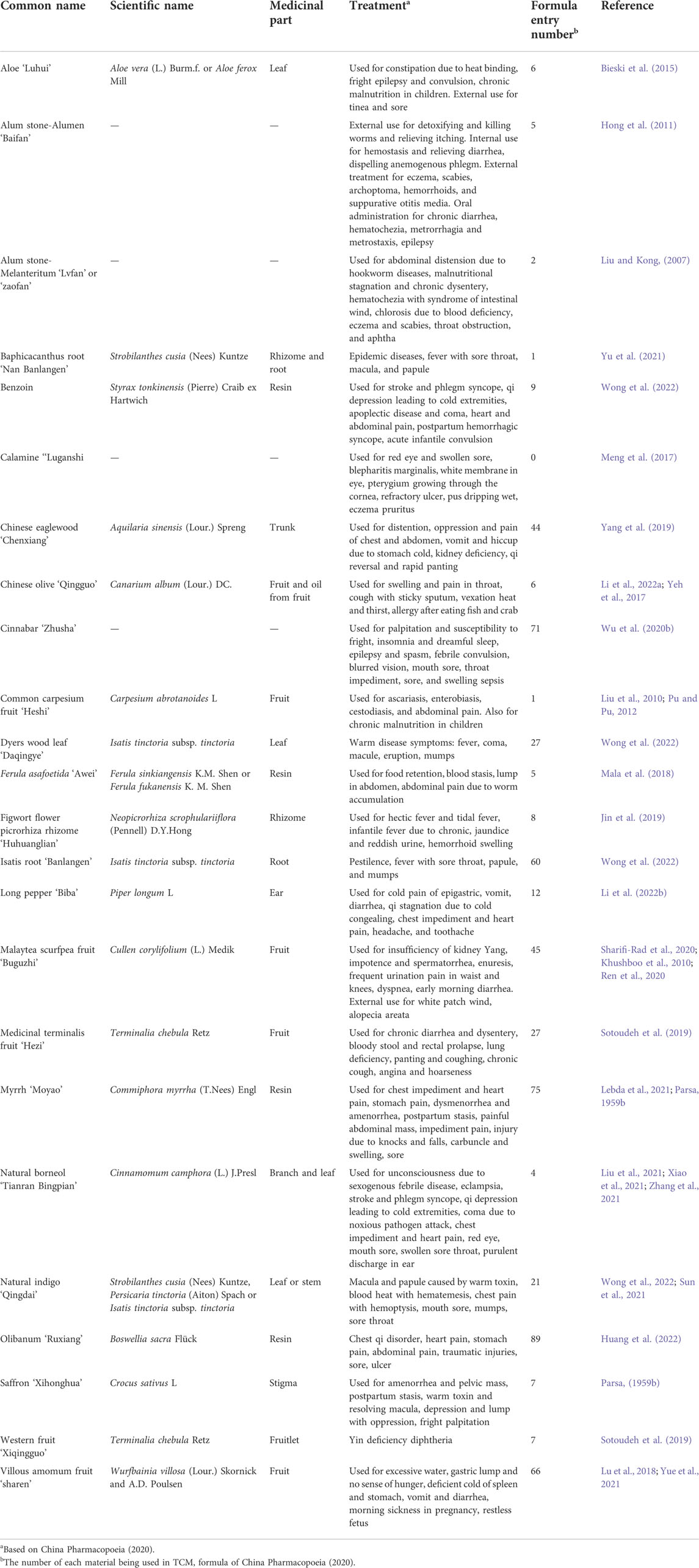
TABLE 3. Basic information in China Pharmacopoeia on medicinal materials recorded in the Compendium of Materia Medica.
3.1 The category of herbs
Natural indigo/Strobilanthes cusia (Nees) Kuntze, Persicaria tinctoria (Aiton) Spach, or Isatis tinctoria subsp. tinctoria/“Qing Dai,”“青黛”1
Natural indigo is used as a dry powder extracted from the leaves or stems of Strobilanthes cusia (Nees) Kuntze, Persicaria tinctoria (Aiton) Spach, or Isatis tinctoria subsp. tinctoria. Natural indigo was commonly used as a dye for cloth and paint in old times but it has been adopted as an important TCM for a long time. It is now included in the 2020 edition of the Pharmacopoeia of People’s Republic of China (PPRC), which is used for reducing body heat and detoxification of the body. I. tinctoria subsp. tinctoria produces a large variety of chemicals including terpenoids, alkaloids, organic acids, and others, which are the chemical foundation of multiple pharmacological effects including anti-inflammation, anti-tumor, anti-allergy, and anti-microbes (Wong et al., 2022). I. tinctoria subsp. tinctoria became a popular TCM in recent years to treat viruses (Mandal et al., 2021). The phenols, polysaccharides, lignans, indole alkaloids, and glycosidic bisindole alkaloids from the plant, were reported to exhibit efficacy against SARS coronavirus (Lin et al., 2005), hepatitis B virus (Wang et al., 2020), Japanese encephalitis virus (Chang et al., 2012), avian influenza A/B virus (Yang et al., 2013), and influenza virus A (Liu et al., 2015), respectively. S. cusia and P. tinctoria are not as commonly used in comparison to I. tinctoria subsp. tinctoria in TCM even though they are regarded as being important in PPRC. There are fewer studies that report on the pharmacological properties of these species. For example, a peptide derivative, aurantiamide acetate from S. cusia was shown to be anti-inflammatory and anti-viral (Zhou et al., 2017). The I. tinctoria subsp. tinctoria was also shown with a strong activity against the protease of SARS-CoV-2, the culprit of the ongoing COVID-19 pandemic (Mandal et al., 2021). A famous, ready-to-use Chinese formula Lianghuaqingwen capsule, which consists of the I. tinctoria, was in a clinical trial against COVID-19 (Lee et al., 2021). In addition to activity against viruses, P. tinctoria was shown to be effective against bacteria (Kataoka et al., 2001) and this was tested against Helicobacter pylori–infected Mongolian gerbils. This species also has been indicated to hold anti-melanogenesis (Chung et al., 2018) and anti-neuroinflammation actions (Lee et al., 2018).
Interestingly, the three medicinal plants are now all cultivated in China and I. tinctoria subsp. tinctoria has a wide application in TCM. I. tinctoria subsp. tinctoria is currently distributed in Iran; however, there are no reports of the distribution of S. cusia and P. tinctoria in Iran. Prepared in coarse or fine powder, the leaves of I. tinctoria subsp. tinctoria are sold as henna in Iran, which is mainly used as a cosmetic for coloring the skin (Pasrsa, 1960).
Long pepper/Piper longum L./“Biba,”“荜茇”
Long pepper is a climbing vine tree that is known to be distributed in southern China but has not been recorded in Iran. In TCM, long pepper is the dried, nearly mature or mature ears of the Piper longum L. (Piperaceae). Shizhen Li in the CMM said that a soup made from boiling long pepper together with milk was excellent in treating headaches, rhinorrhea, and toothache. Long pepper is now included in the PPRC, with functions of warming the interior body, dissipating cold, and for stopping pain. Modern studies have shown that the main components of long pepper are amide alkaloids and volatile oils that have caryophyllene and pentadecane (both about 17.8%) and bisaboline (11%), as well as amino acids and inorganic elements (Li, 2017). Pharmacological studies have shown that long pepper has anti-bacterial and anti-viral properties. Also, it has the capacity for improving lipid and glucose metabolism while showing anti-inflammatory, anti-tumor, anti-gastric ulcer, anti-diarrhea, and anti-oxidant effects (Li D. et al., 2022).
Figwort flower picrorhiza rhizome/Neopicrorhiza scrophulariiflora (Pennell) D. Y. Hong/“Hu Huanglian,” “胡黄连”
The medicinal figwort flower is the dry rhizome of Neopicrorhiza scrophulariiflora (Pennell) D.Y. Hong, a plant of the Scrophulariaceae. It is now predominantly distributed in Tibet and is thus an important Tibetan medicine. There are no records of the plant being grown in Iran. It was listed as a national tertiary protection plant and recorded in the PPRC. In TCM theory, N. scrophulariiflora is used as a tonic for the liver and gallbladder and is said to improve the eyesight. It can also be utilized for treating fevers and it has a reputation as being used for diabetes. Apart from this, it may be used for treating convulsions in pregnant women. It stops diarrhea and dysentery and treats five types of hemorrhoids. Studies showed that its main chemical components are iridoids, cucurbitol, phenylethanol glycosides, and phenol glycosides (Guo et al., 2020). The pharmacological effects of figwort flower include lowering fat of the liver and gallbladder, antibacterial and anti-inflammatory effects, protection of damaged nerve cells, and apoptosis of cardiomyocytes (Jin et al., 2019).
Villous amomum fruit/Wurfbainia villosa (Lour.) Skornick. and A.D. Poulsen or Wurfbainia villosa var. xanthioides (Baker) T.L. Wu and S.J. Chen/“Sushami,”“缩砂蔤”
Villous amomum fruit is the dried, mature fruit of W. villosa or W. villosa var. xanthioides. It is included in the PPRC with the name of Sharen. Both wild and cultivated W. villosa are distributed in China but there are currently no records that indicate it being grown in Iran. The main components of W. villosa are volatile essential oils, such as borneol acetate, camphor, borneol, β-bisabolene, nerolidol, and cubeno (Chen et al., 2020a). There are also nonvolatile components including polysaccharides, flavonoid glycosides, organic acids, and inorganic components (Li et al., 2018). It has been disclosed that W. villosa has various pharmacological effects including gastrointestinal protection of anti-ulcer effects, promoting gastric emptying and gastric peristalsis (Lu et al., 2018; Yue et al., 2021). Other studies have shown this species possesses functions of anti-inflammation (Yin et al., 2019), anti-diarrhea, and anti-obesity (Kim et al., 2022), as well as bacteriostatic effects (Tang et al., 2021). W. villosa has also been extensively utilized for a range of bowel diseases (Chen et al., 2018a).
Betel/Piper betle L./“Jujiang,”“蒟酱”
Betel is a plant that has a long history as a TCM but it is not included in PPRC. Shizhen Li confirmed that Jujiang was made from Piper betle L. but not Biba (Piperis longum L.). Many studies have indicated that betel has a potential for treatment of Leishmania donovani– induced parasitic disease (Misra et al., 2009). It has also been indicated with a profile of anti-fertility (Sarkar et al., 2000). Other medicinal uses include its importance as an anti-malarial(Tamura et al., 2020), anti-gout (Vikrama Chakravarthi et al., 2022), anti-oxidant (Vikrama Chakravarthi et al., 2022), anti-platelet, and anti-inflammation remedy (Saeed et al., 1993). Yin et al. (2009) isolated and identified amide alkaloids, triterpenoids, lignin, flavonoids, and sterols from the stems of the plant. Appreciated for the function of improving the memory, the betel, which was imported from India, is sold under the name of Tanbool or Tanflool in Iran (Pasrsa, 1960).
Babchi/Cullen corylifolium (L.) Medik./“Buguzhi,” “补骨脂”
Babchi is produced from the dry ripening fruits of Cullen corylifolium (L.) Medik plants. The fruit is harvested and dried after it matures in autumn, and the fruit is used as a medicine. It is included in PPRC for treating skin diseases such as psoriasis. C. corylifolia also has a long history in Ayurvedic medicine. It is widely distributed in India. Babchi contains various chemical components including coumarins, monoterpenoids, flavonoids, and benzofurans, and has a wide range of pharmacological effects, including anti-tumor, anti-oxidant, anti-inflammatory, anti-fungal, anti-bacterial, and immunomodulatory properties (Khushboo et al., 2010). In a recent research, Babchi was indicated as a potential inhibitor of the viral cysteine protease of the COVID-19 (Mandal et al., 2021).
Jasmine/Jasminum sambac (L.) Aiton/“Moli,” “茉莉”
The leaves and roots of jasmine, Jasminum sambac (L.) Aiton, are an important component of TCM with the effect of clearing heat and removing excessive water. Jasmine has strong historical and cultural implications in China. Traditionally, flowers of jasmine can be ground into a powder that is used as a mask for detoxification, acne, and facial nourishment. Perfumes made of essential oils from jasmine are popular. Petals of jasmine can be used to make tea with a fragrant smell. Studies have indicated that jasmine exhibits activities of gastroprotective (Alrashdi et al., 2012), anti-inflammation, analgesia, and pyretic (Sengar et al., 2015). Presently, jasmine is not included in PPRC. In Iran, jasmine is widely grown as a valuable plant used for home ornament, landscaping, as well as for essential oils and medicine (Ghasemi Ghehsareh et al., 2015).
Carpesium abrotanoides L./“Tianmingjing,” “天名精”
Carpesium abrotanoides L. has been used as a TCM for a long time in China. It is mainly used for sore throat, tonsillitis, bronchitis, external treatment of traumatic bleeding, furunculosis and pyogenic infections, and insect and snake bites (Qian et al., 2019). It has been shown that C. abrotanoides mainly contains sesquiterpene lactone components, with anti-bacterial and anti-inflammatory, anti-viral, insecticidal, immunosuppressive, and other pharmacological effects (Liu et al., 2010; Pu and Pu, 2012). C. abrotanoides is not included in the PPRC.
Saffron/Crocus sativus L./“Fanhonghua,” “番红花”
Saffron is a famous spice in the Middle East and Europe. In China, saffron is the most representative medicine inherited from Persia to China along the Silk Road. The dried stigma of Crocus sativus L. is now included in PPRC. It has the efficacy of treating depression and tranquilizing the mind, activating blood and resolving stasis, and cooling blood and removing toxins. Saffron contains more than 150 compounds, the most important of which are water-soluble carotenoids including crocetin and crocin. With a limitation of extremely low yield and labor extensive handling, saffron production usually comes with high costs. However, it continuously attracts numerous studies for various health-related diseases in human subjects, such as inflammatory status of elderly hypertensive men (Mojtahedi et al., 2022), obese men with type 2 diabetes mellitus (Hooshmand Moghadam et al., 2022), chemotherapy-induced peripheral neuropathy (Bozorgi et al., 2021), and it has been shown to increase happiness as it reduces depression (Moghadam et al., 2021). In Iran, the dried, red stigmas of the C. sativus are thought to be a stimulant with anti-spasmodic action and are also a favorite coloring spice in food (Parsa, 1959b).
3.2 The category of vegetables
Dill/Anethum graveolens L./Shiluo, “莳萝”
Dill is mainly used in clinical treatment of digestive system diseases, such as stomach pain, indigestion, halitosis, and flatulence. It can also be used to treat child hiccups and promote lactation in lactating women (Tian, 2012). Essential oils made out of the dill contain volatile compounds such as carvone, limonene, and α-phellandrene. It also produces a great amount of flavonoids, phenolic compounds, cardiac glycosides, and terpenoids (Goodarzi et al., 2016), which have anti-bacterial, anti-oxidant, anti-gastric ulcer, and cholesterol-lowering effects (He and Huang, 2011). The essential oil of dill has good bacteriostatic activity and can be used as a powerful natural insecticide (Osanloo et al., 2017). Dill tinctures have the function of increasing aroma and enriching tobacco fragrance, and therefore, can be used in the tobacco industry (Zhan et al., 2007). Dill is a popular spice in Iran for condiment and carminative and is sold in the bazaars. When distilled, dill essential oil and dill water can be made at the same time for medicinal uses and drinking. In Iran, the leaves of dill can be a condiment to cook with rice to restore lost appetite (Parsa, 1959a). The Anethum tablet in Iran is a market medicine for hypolipidemic treatment, which is composed of four herbs, A. Graveolens (68%), Cichorium intybus L. (5%), Fumaria parviflora Lam (5%), and Citrus × aurantiifolia (Christm.) Swingle (4%) (Oshaghi et al., 2016).
Spinach/Spinacia oleracea L./“Boleng,” “菠薐”
Spinach is a common vegetable rich in carotene, vitamin C, protein, calcium, iron, and other minerals. In TCM, it has the functions of heat clearing, dryness moistening, and invigorating of the blood circulation. Although spinach is widely used as a vegetable worldwide, some studies have shown its pharmacological actions as it may help with some diseases, such as metabolic syndrome (Panda et al., 2017), cartilage degeneration and subchondral bone deterioration (Kothari et al., 2020), and also the postmenopausal osteoporosis (Adhikary et al., 2017). In Iran, spinach is mainly cultivated and consumed as a vegetable like in other countries; however, the fruits of spinach have demulcent and diuretic functions, and are sometimes prepared in order to reduce the bowel inflammation (Pasrsa, 1960).
3.3 The category of fruits
Phoenix date/Phoenix dactylifera L./“Wulouzi,” “无漏子”
Phoenix date is the fruit of the Phoenix dactylifera L, which grows in the high temperature and arid desert areas, such as Iraq and Iran. It tonifies the interior and reinforces the qi, eliminates phlegm, and stops coughing. Phoenix date has a high nutritional and medicinal value, and it is rich in vitamin A and vitamins B1 and B2. It has the function of protecting visual acuity, strengthening nerves, and softening blood vessels. In addition, other studies have shown that date also has a certain sedative effect, which has a certain effect on psychological panic and fear, as well as neurological disorders caused by hyperthyroidism (Zhou, 1986). The dates are also shown to relieve the kidney calculi (Mohammadparast Tabas et al., 2021), hypolipidemic and atherosclerosis (Bouhlali et al., 2020), or labor pain during delivery (Bagherzadeh Karimi et al., 2020).
Phoenix dates are widely planted in Iran. It is now cultivated in tropical areas of Fujian, Guangxi, Hainan, Guangdong provinces, and other places in China. The dates are usually used as food in Iran and China. They are not included in the PPRC. In Ahvaz city of Khuzestan province of Iran, a drink made out of phoenix dates is used for colic and sunstroke (Pasrsa, 1960).
Fig/Ficus carica L./“Wuhuaguo,” “无花果”
The medicinal part of fig is the fruit of Ficus carica L. In addition, the roots and leaves can also be used as medicine. The CMM describes the efficacy of the fruits as moistening lungs to stop coughing. Its roots and leaves are good for enteritis and diarrhea. Currently, figs are mainly consumed as fruit and are not included in the PPRC. Figs are rich in amino acids, vitamins, proteins, inorganic salts, and other beneficial ingredients. The fig is good in relieving hyperglycemia and hyperlipidemia (Ramadan et al., 2021), anti-stress of the skin (Dini et al., 2021), ulcerative colitis (Zou et al., 2020), anti-inflammation, and anti-proliferation (Liu et al., 2019). The fig fruit was used as medicine for anti-hemorrhoids, laxation, and tonic in Iran (Buso et al., 2020).
Golden shower/Cassia fistula L./“Alebo,” “阿勒勃”
Golden shower is a tree that grows up to 9–12 m high with 3–5 m wide upon maturity. Based on the TCM theory, C. fistula reduces invading pathogenic heat and wind resting in the heart and diaphragm. In southern China, C. fistula is mostly used in gardens and parks for ornamental purposes. C. fistula is not included in PPRC, however, it is recorded in Tibetan and the Dai nationality medical books in China. In Tibetan medicine, it is used for clearing heat, treating liver disease, and constipation, and reducing swelling of the limbs. The villagers of Dai nationality use it for anti-bacterial effects and for relieving constipation (Xie 1986). Modern pharmacological studies have disclosed that C. fistula is effective against Leishmania donovani (Tabrez et al., 2021) and type 1 diabetes (Indu et al., 2021). It has anti-oxidant actions and prevents neurodegeneration (Thabit et al., 2018). The chemicals in C. fistula are mainly flavonoids, anthraquinones, and disaccharides (Tan et al., 2018).
Pistachio/Pistacia vera L./“Ayuehunzi,” “阿月浑子”
Pistachios are one of the four major nut species in the world. Its dried fruits are rich in fat and various nutrients. Pistachio can moisturize intestines to loosen stools and help body detoxification. The nuts are also known to be a highly nutritious medicine and can treat microbial infection (Ozcelik et al., 2005), inflammation (Paterniti et al., 2017), oxidation (Shi 2014), anemia (Bailey and Stein, 2020), malnutrition (Bailey and Stein, 2020), chronic diarrhea (Sarkhail et al., 2020), wounds (Sarkhail et al., 2020), and obesity (Xia et al., 2020). Pistachios are currently consumed as food in China and are not included in the PPRC or other local pharmacopoeias. In Iran, Pistacia spp. contains a few species and all their fruits are consumed as food. The fruits of P. vera are considered as a tonic in Iran and the outer husk of the fruit is used to treat dysentery (Pasrsa, 1960).
Chinese olive/Canarium album (Lour.) DC./“Ganlan”, “橄榄”
Chinese olive is different from the European olive or common olive, Olea europaea L, which is a common tree in the Mediterranean Basin for olive oil or food. The dried ripe fruit of Canarium album (Lour.) DC. was included in PPRC. In TCM, olive helps produce body fluid, relieves restlessness, and quenches thirst and it is good for relieving throat pain. Chewing the fruit and swallowing the juice also helps to detoxify toxins from all kinds of fishes and turtles. Studies have proven that the chemicals of the fruits exert efficacy against some diseases, such as benzofuran neolignans for anti-inflammation (Li et al., 2022b), methyl brevifolincarboxylate (Chen et al., 2020b) and a polyphenolic compound isocorilagin (Chen et al., 2020c) both for influenza. The ethyl acetate fraction of Chinese olive was shown to regulate metabolic dysfunction in diabetes (Yeh et al., 2017).
Jackfruit/Artocarpus heterophyllus Lam./“Boluomi,” “波罗蜜”
Jackfruit is a famous tropical fruit with delicious flesh and a pleasant aroma. It is rich in sugar, proteins, vitamins, minerals, carbohydrates, and fatty oils (Gupta et al., 2022). In China, jackfruit is mainly consumed as fruit and is not included in the PPRC. In TCM theory, jackfruit is said to act as a tonic and reinforces the qi. It makes people feel happy and vigorous and can alleviate hunger. Recently, studies have discovered many active components with various pharmacological benefits from the jackfruit. Polysaccharides from jackfruit modulated the gut microbiota and they improved the production of the beneficial short-chain fatty acids (Zhu et al., 2021). Coumarin analogs from the jackfruit showed potential activities against inflammation and HIV (Tao et al., 2021). A fraction mostly containing artocarpin produced from jackfruit was effective in inhibiting the colorectal cancer cells (Morrison et al., 2021). Oxyresveratrol from jackfruit was shown to inhibit the production of melanin that is directly related to pigmented age spots and blemishes (Li et al., 2020).
Grape/Vitis vinifera L./“Putao,” “葡萄”
Grapes are now a fruit widely cultivated all over the world with high nutritional value. Grapes are also used as raw materials for making wine (not included in PPRC). In TCM theory, it has the effect of supplementing qi and blood, benefiting the kidney and liver, generating liquid, strengthening muscles and bones, stopping cough and eliminating vexation, and promoting urination. In addition to being used as a fruit, there are huge varieties of grapes for wine and beverage. The rich phytochemical composition of grape makes it an attractive object for pharmacological exploration. Through a systematic review, grape seed extract, which contains resveratrol and other anti-oxidant chemicals, may be beneficial for people with cardiovascular diseases (Foshati et al., 2022). The seed extract was effective to prevent cancer metastasis (You et al., 2021). The proanthocyanidin-rich fraction of the grape seeds attenuated memory impairment in rats (Osuntokun et al., 2021). The grape leaf extract exhibited strong anti-inflammatory and anti-vascular leakage during vein oxidative damage (Seo et al., 2021). The leaf extract can also reduce obesity in a mice model (Meng et al., 2021). Ampelopsin A, a major compound of grape, was found to ameliorate the cognitive and memory capability in neurodegenerative diseases (Hong et al., 2021). In Iran, although mostly used for fruits, dried grapes were also served as medicine with properties of demulcent, laxative, and cooling functions (Pasrsa, 1960).
3.4 The category of woods
Agarwood/Aquilaria malaccensis Lam./“Mixiang,” “蜜香”
Agarwood is regarded as being precious as it is favored by many fragrance enthusiasts because of its special fragrance. Shizhen Li in the CMM concluded that Mixiang is one species of Chenxiang (Chinese Eaglewood). It has similar therapeutic functions to Chenxiang. The name of Chenxiang is derived from its characteristics of sinking in water. When the resin content in Chinese eaglewood is higher than 25%, regardless of its form (tablet, block, or powder), it will sink into water. The agarwood mainly contains volatile chemicals such as sesquiterpenes, 2-(2-phenylethyl) chromone, aromatic, and flavonoids. Although it is believed that the agarwood has analgesic, anti-diarrhea, neuroprotective, anti-inflammatory, and anti-bacterial activities (Huo et al., 2018), thorough studies still fall short.
Marking nut tree/Semecarpus anacardium L. f [Anacardiaceae]/“Poluode,” “婆罗得”
The marking nut tree looks like a Chinese willow tree, and the seeds look like castor beans (Ricinus communis L.). It is also an important Ayurvedic medicine. In TCM theory, it warms the interior, tonifies the waist and kidney. It can dye hair and beard in black. It is not included in PPRC. Pharmacological studies have disclosed the medicinal values of the marking nut tree. The stem barks, or the nuts of the tree have been shown to possess potential against diabetes and inflammation in a rat study (Khan et al., 2013; Ali et al., 2015). The nut milk extract demonstrated remarkable hypolipidemic activity in hypercholesterolemic rats (Vinayagam et al., 2012). The methanol extract of the stem bark promotes wound healing and this was shown using a rat model (Lingaraju et al., 2012). The pericarp can be prepared like tea to relieve flatulence following severe piles in Iran (Pasrsa, 1960).
Myrrh/Commiphora myrrha (T.Nees) Engl./“Moyao,”“没药”
Myrrh is the dry resin from Commiphora myrrha (T.Nees) Engl. As an important TCM with a long history, however, myrrh is not produced in China. According to our research, myrrh was used as a component in 75 formulas out of 1605 TCM formulas registered in the current PPRC, proving its importance in TCM (data not published). In TCM theory, myrrh removes blood stasis, eliminates swelling, relieves pain, and promotes muscle growth. It falls within two categories in PPRC: natural myrrh and colloidal myrrh. Natural myrrh is of irregular granular clump with different sizes, the larger diameter can up to 6 cm or more. Natural myrrh has a special aroma with a bitter taste and slightly pungent smell. The colloidal myrrh is made of irregular lumps and granules with a brown surface, which stick into lumps of different sizes. Colloidal myrrh is opaque, solid or loose in texture with a specific aroma. The main chemical constituents of myrrh are monoterpene, ploidy, triterpenoids, steroids, and lignin (Ge and Zhang, 2019).
Studies have disclosed the modern pharmacology of myrrh. Water extracts and polysaccharides of C. myrrha are effective to treat the osteoporosis in postmenopausal women (Hwang et al., 2021). A 10 min sitz-bath of myrrh extract greatly relieved the wound healing of the episiotomy in a clinical trial (Faraji et al., 2021). C. myrrha can treat the diabetes mellitus and the resin solution stimulates insulin secretion (AlRomaiyan et al., 2021), and anti-microbial activity (AlMadi et al., 2019), human heterophyiasis with the gastro-intestinal troubles with parasitosis (Massoud et al., 2007). In Iran, myrrh is used as a stomachic and for lumbago (Parsa, 1959b).
Benzoin/Styrax tonkinensis (Pierre) Craib ex Hartwich/“Anxixiang,” “安息香”
Benzoin or benjamin is the dried resin of Styrax tonkinensis (Pierre) Craib ex Hartwich, a semi-deciduous tropical tree that can grow up to 20 m high. The outgoing resin is collected and dried when the tree stem is naturally damaged or cut apart in the summer or autumn. Benzoin is included in the PPRC. It is divided into two types: Thai benzoin and Sumatra benzoin. The resin content of benzoin is as high as 70–80%, and the main component of the resin is volatile vanillic acid. In addition, lignins, terpenoids, and steroids were obtained from the studies on the chemical compositions of the genus Bemberine (Zhang et al., 2014). Studies on pharmacological activities showed that benzoin mainly has anti-matrix metalloprotease-1, anti-ulcer, antioxidant, anti-complement, anti-bacterial, and other activities (Lei et al., 2012).
Ferula/Ferula sinkiangensis K.M. Shen or Ferula fukanensis K.M. Shen/“Awei,” “阿魏”
The PPRC stipulates that the medicinal material of this product is the resin of Ferula sinkiangensis K.M. Shen or Ferula fukanensis K.M. Shen. Both plants are native to Xinjiang, China and they are not present in Iran. Most likely the species in old times was classified as Ferula asafoetida (Falc.) H. Karst, which is native to Iran. In Iran, Ferula is used to treat spasmodism, helminth, carmination, and constipation (Iranshahy and Iranshahi, 2011). In TCM theory, Ferula has the effects of regulating qi and eliminating swelling, fatigue, and phlegm. Ferula promotes blood circulation and excites nerves. A pharmacological study in India showed the safety and efficacy of Ferula on functional dyspepsia (Mala et al., 2018). In China, studies on F. sinkiangensis and F. fukanensis provide more pharmacological advances. Kellerin is specific compound from F. sinkiangensis that was verified to improve the neuroprotective effects in a rat model (Mi et al., 2021). Fractions of F. sinkiangensis exerted anti-oxidant and anti-tumor effects in a cell study (Zhang et al., 2015). Sesquiterpenoids from F. fukanensis inhibited nitric oxide production (Motai and Kitanaka, 2005).
Aloe/Aloe vera (L.) Burm. f. or Aloe ferox Mill./“Luhui,” “卢会”
Aloe, originally growing in hot, dry climates, is a popular, succulent plant that is of horticultural importance. Aloe in the PPRC is a dried product of concentrated juice from Aloe vera (L.) Burm. f., Aloe ferox Mill., or other close species. Aloe is used to treat skin diseases, constipation, indigestion, and worms in TCM. The thick juice from A. barbadensis leaves is a good moisturizer, especially in cosmetics. A study has disclosed that a compound, aloin A, might be the bioactive agent that prevents infection of skin wounds when aloe is applied as a topical treatment (Donkor et al., 2020). The dry aerial parts of A. barbadensis can significantly restore the integrity of the hepatocytes toxified by carbon tetrachloride (Chandan et al., 2007). Components from A. barbadensis restore the metabolic and reproductive comorbidities of polycystic ovary syndrome (Dey et al., 2022). A. ferox is also called Cape aloe or bitter alone in South Africa, which is rich in chemicals of flavones, anthraquinones, anthrone-glycosides, and phenolic compounds (Parsa, 1959a). The laxative effect, skin and wound healing, anti-oxidant, anti-inflammation, anti-microbes, anti-cancer, anti-malaria, and permeation-enhancing function, as well as some adverse effects including vomiting were elaborately reviewed (Chen et al., 2012).
In Iranian medicine, aloe is applied as an anti-fever, anti-infection, and wound healing reagent (Dey et al., 2017). Nowadays, this medicinal plant is frequently used in the field of cosmetology (Haghani et al., 2022).
Nutgalls/Quercus infectoria G. Olivier/“Wushizi,” “无食子”
Nutgalls are the cecidum of a special larva (Cynips tinctoria Olivier) parasitized on the young branches of Quercus infectoria G. Olivier. Unpierced cecidum is mainly used for medicinal purposes. Nutgalls are rich in gallotannin (50–70%), followed by gallic acid (2–4%), propionic acid, and resin. It has the effect of strengthening teeth and fixing gums, clearing blood and relieving pain, and inhibiting bacteria. It is a common medicine used in Uyghur as an enema in the treatment of chronic ulcerative colitis (Huang and Fan, 2003). However, it is currently not included in the PPRC.
Frankincense/Boswellia sacra Flück/“Xunluxiang,” “薰陆香”
Frankincense is a resin that is obtained from bark exudates of Boswellia sacra Flück. Its shape and aroma are similar to turpentine. In TCM, frankincense is used for chest, heart, stomach, and other body pains, and it is also used for wounds, carbuncles, and skin ulcers. It has been demonstrated that Boswellia sacra Flück contains a wide range of chemicals that are components of its volatile oils, mainly diterpenoids and triterpenoids. These chemicals have pharmacological properties of anti-inflammation, anti-diabetes, and anti-cancer and are useful for cardiovascular and neurodegenerative diseases (Huang et al., 2022). Frankincense is included in the PPRC. Boswellia sacra Flück is not grown in China, so these products must be imported from other countries but the species is not grown in Iran where it is still being used as a medicine as well.
Borneol/Cinnamomum camphora (L.) J. Presl/“Longnaoxiang,” “龙脑香”
Borneol is the product of crystals obtained by steam distillation of the shoots and leaves of Cinnamomum camphora (L.) J. Presl. Borneol is a common Chinese medicinal material, and there are four kinds of borneol contained in the National Drug Standards of China (Li et al., 2013). Among them, natural borneolum and synthetic borneolum are included in the PPRC. According to our analysis, a total of 168 TCM formulas registered in the PPRC use borneolum as a component. The borneolum is dispensable in the TCM formula for anti-inflammation, anti-microbes and it is included in many TCM formulas for increasing the permeability of other components. The seed kernel extract and the essential oil of C. camphora were demonstrated to hold anti-inflammatory potential (Xiao et al., 2021; Zhang et al., 2021). The seed kernel oil of C. camphora improved lipid metabolism by reducing the body fat mass of obese rats (Fu et al., 2016). The borneol, as a pure compound, can be used to enhance the radiosensitivity of glioma tumor (Li et al., 2021), for neuroprotection in cerebral ischemia (Xie et al., 2022), and for the cardio-cerebrovascular diseases (Liu et al., 2021).
The application of borneol in TCM can be dated back a thousand years ago. All the natural borenolum was imported from overseas until the wildly grown tree of C. camphora was discovered in 1990s in south China. Currently, C. camphora is a medicinal tree in Iran.
Black Myrobalan, Chebule/Terminalia chebula Retz./“Helile,” “诃黎勒”
Chebule is the dry ripe fruit of Terminalia chebula Retz., and now it is included in the PPRC. T. chebula is distributed in Iran. Chebule is used for chronic diarrhea and dysentery, bloody stool and rectal prolapse, lung deficiency, panting and coughing, chronic cough, angina, and hoarseness. Modern pharmacological studies have confirmed that its main chemical components include tannins, phenolic acids, triterpenes, and flavonoids. These components have many effects, such as anti-oxidant, neuroprotective, anti-tumor, anti-viral, and anti-bacterial activity. A combination formula containing Commiphora mukul Engl., Commiphora myrrha (T.Nees) Engl., and T. chebula was comparable to metformin in treating diabetic rats, suggesting its potential remedy in diabetes (Sotoudeh et al., 2019). The fruit of T. chebula, chebule, is frequently used for skin hyperpigmentation in Iran (Khazaeli et al., 2009). The young, unripe nuts which turn black on drying, called Halilah-i-siah or black myrobalans, are mainly used in medicine as a strong purgative for treating stomach pains (Pasrsa, 1960).
Large fruited elm/Ulmus macrocarpa Hance/“Wuyi,” “芜荑”
The product is made by fermenting and drying the fruit pods of Ulmus macrocarpa Hance, which are added with auxiliary materials such as elm bark flour. The CMM recorded that it disperses invading pathogenic factors resting with five Viscera. It also disperses toxins of febrile diseases circulating in the skin and joints. It kills worms and helps digestion. The large-fruited elm is not included in the PPRC.
U. macrocarpa is widely distributed in Korea and north China. However, only the same genus plant but not the same species is present in Iran. Studies have shown that extracts of U. macrocarpa increase the host immunity by modulating the gut microbiota in human subjects (Kim et al., 2021). A taxifolin flavonoid glycoside extracted from U. macrocarpa ameliorates the osteopenic disease (Wang et al., 2022). The root bark of U. macrocarpa exerts anti-hypertensive, vasorelaxant, and anti-oxidant properties with chronic treatments in rats. (Oh et al., 2008).
Ebony/Diospyros ebenum J. Koenig ex Retz./“Wumu,” “乌木”
Ebony is a slow-growing and evergreen tree with a potential to grow as high as 30 m. It is distributed in south China and Iran. In TCM, the product is the wood of D. ebenum. It is used for detoxifying toxins and treating cholera with vomiting and diarrhea (Perera et al., 2018). In addition to being used as medicine, it is also a very precious wood with high collection value. It is not included in the PPRC. Little modern pharmacology on ebony is conducted.
3.5 The category of metals, stones, and minerals
Silver ore/“Xilinzhi,” “锡吝脂”
Xilinzhi, also known as Persian silver, is the silver ore produced in Persia. In old times, it was mainly used to treat pterygium and it is currently not included in the PPRC. No report of using silver ore in the current TCM.
Atacamite/“Lvyan,” “绿盐”
Atacamite is the copper ore and the main component is copper chloride. It is often mixed with aluminum, iron, calcium, and magnesium. The crystal is columnar or plate-shaped with vertical stripes. In old times, it was used for treating inflamed eyes with shedding of tears, and dim vision with profuse secretion and slight pterygium. Atacamite is highly toxic, and when mistakenly swallowed it will stimulate gastric mucosa that causes vomiting and abdominal pain. It is not included in the PPRC and no report has been found on its usage in the current TCM.
Alum stone/“Fanshi,” “矾石”
There are five kinds of alum stones listed in the CMM and these are differentiated by the color: Baifan (white, Alumen), Huangfan (yellow, Fibroferritum), Lvfan (green, Melanteritum), Heifan (black alum), and Jiangfan (red, Alumen perparata). Presently, the PPRC contains Alumen and Melanteritum. In TCM, the alumen treats itching and worms for topical treatment and it stops bleeding by oral administration.
Sulfur/“Shiliuhuang,” “石硫磺”
The massive sulfur is a light yellow crystal that has an unpleasant stench. In TCM, sulfur for external use can kill worms to stop itching, treat scabies, eczema, and skin pruritus. For internal use, it is indicated to nourish fire and invigorate Yang, in addition to strengthening sinews and bones, and to relieving constipation. Sulfur benefits qi and stops bleeding, dispels phlegm and reduces panting. However, sulfur is toxic so it is suggested not to be taken in large quantities or for a long period. Sulfur is not contained in the PPRC. Limited cases of sulfur are used in the TCM formula. Sulfur is a very efficient fumigation reagent and is widely adopted in the TCM material treatment. However, the excessive residue of sulfur is forbidden and it is a monitored substance in the quality control of TCM material.
Salt/“Guangmingyan,” “光明盐”
Salt is a natural crystal that mainly contains sodium chloride. In TCM, it is primarily used for dispelling pathogenic wind for improving eyesight, digestion and accumulation, and detoxification. It is now only a food additive and not a medicine in China. It is not often to be used in TCM formulas but is used in some applications of moxibustion or massage. Although currently sodium chloride is commonly used as an essential condiment for the maintenance of concentration and osmotic pressure crossing cell membrane, treatment with additional sodium chloride is necessary for diseases characterized by lack of salt including cystic fibrosis (Keivanfar et al., 2020), congenital chloride diarrhea (Wedenoja et al., 2008), and small bowel ostomies (Trautmann et al., 2020). No clue is found to connect the traditional and current remedies with the salt.
Litharge/“Mituoseng,” “密陀僧”
Litharge is a red oxide mineral of lead, belonging to the tetrahedral system. Litharge mainly contains lead oxide, which has certain toxicity. Internal use causes lead poisoning. In TCM, litharge treats regurgitation, diabetes, malaria, and dysentery. It also stops bleeding, kills parasites, and removes food retention. It is good for treating sores, eliminating swelling and attacks of noxious agents, dispersing bromhidrosis, and dyeing the hair black. Litharge was externally used to treat eczema, sore poison, burns, scalds, injuries from falls and other surgical diseases. Internal use includes treating diarrhea, malaria, epilepsy, and other diseases. Litharge is currently not included in the PPRC. No report of using it in the current TCM.
Iron/Ferrum/“Tie”, “铁”
Iron is a grayish-black metal, mainly produced from hematite, limonite, and magnetite. The CMM claimed that iron could dissipate extravasated blood and eliminate erysipelas. Iron is used as the main medicinal treatment for incised (metal-inflicted) wounds, chest and diaphragm pain due to being blocked by gas, and retention of food. However, the CMM also stated that it could damage the lung and injure the liver and kidney. Iron is not included in the current PPRC, but in the previous version of PPRC; red, yellow, brown, purple, and black ferric oxides were included. No reports of any use are included in the current TCM.
Gold/“Jin,” “金”
Gold was recorded in many Chinese ancient books as a medicine. However, the gold needs to be made into gold foil before it can be used as medicine. The internal use of gold foil has the effect of leading to tranquility and protecting the liver. The external use is said to detoxify and treat furuncle. Clinically, it is mostly used for treating convulsive epilepsy and insane and disquieted heart spirit. Gold is not included in the PPRC. No report of its current use is now indicated in TCM.
Silver/“Yin,” “银”
Silver has been a medicine in China for thousands of years. The needles for acupuncture and moxibustion were made of silver. In terms of medicinal materials, silver flakes were mainly used for epilepsy and mania, palpitation and trance, treating pox sores, and sudden loss of consciousness in children. Nowadays, silver is mostly used for anti-infection, anti-bacterial, and anti-inflammation purposes. Sliver is not included in the PPRC.
Copper/“Chitong,” “赤铜”
In the CMM, it is noted that the debris of red copper falling off during forging was used for medicinal purposes, and it has the effect of connecting bones and tendons, dispersing blood stasis and relieving pain. Internal treatment can cure blood stasis obstructing the collaterals, and external treatment is implicated in curing bleeding wounds and for eyelid ulcerations. Copper has also been used as a dye to make hair black and remove odors. However, internal use is not necessarily advised as this can lead to stomach and kidney damage and so, copper is suggested not to be taken in large quantities or for a long time. Natural copper (pyrite, mainly containing iron disulfide) is recorded in the PPRC for treating injuries that may be associated with falls, fractures, contusions and strains, and pain due to stasis swelling.
Lead/“Qian,” “铅”
In TCM, lead is good for eliminating scrofula, carbuncles, and swelling. It improves eyesight, stabilizes the teeth, and blackens the hair. It is effective for female suffering absence or atresia of the vagina (a birth defect). It kills parasites and eliminates phlegm. It is also good for treating dysphagia, diabetes, and acute infantile convulsions. Consequently, drugs containing lead should be used carefully in clinical practice to ensure the safety. It is not included in the PPRC. Lead is a poisonous element and a high level of lead in the blood affects brain function and development. Currently lead is monitored in TCM material due to its poisonous nature.
Coral/Corallium/“Shanhu,” “珊瑚”
In TCM theory, coral has a similar medicinal efficacy to that of gold. Due to the specificity of the marine environment, corals have a variety of secondary metabolites and a wide range of biological activities. There are thus opportunities for the development of new pharmaceutical coral–derived ingredients in marine natural drug research. It is not included in the PPRC. At present, it is not common to use coral in TCM treatments.
Cinnabar/“Dansha,” “丹砂”
Cinnabar is a mercuric sulfide mineral, which played a major role in old Chinese alchemy to increase longevity. In TCM it clears the heart, calms the mind, brightens the eyes, and detoxifies the body. When used externally, it can inhibit skin fungi. Mercury is a heavy metal element that is highly toxic. Its presence in common TCM material is thus rigorously monitored and concentrations have to be exceedingly low and limited to ensure quality control aspects linked to safety and toxicity. Cinnabar is included in the PPRC; however, no formula is registered with it.
Calamine/“Luganshi,” “炉甘石”
According to the CCM, calamine is produced in Persia and is similar to gold in appearance. Calamine is a carbonate mineral calcite rhombozite, mainly containing zinc carbonate. It has a sweet and neutral taste and is non-toxic. Calamine is frequently used in ophthalmology and surgery. It has the effect of detoxifying, improving eyesight and removing nebula, dispelling excessive water, relieving itching, and healing sores. It is included in the PPRC. Many modern proprietary medicines contain calamine components, such as medicament for the eyes (Babao, Guangming). Calamine is not suitable for internal use and is frequently made into liniment or lotion with other drugs.
Fibroferrite/“Huangfan,” “黄矾”
The CMM mentioned that fibroferrite ore produced in Persia has a golden thread when cut open. Fibroferrite was used as medicine before the Song Dynasty but was rarely used after that, and it is not included in the PPRC.
3.6 The category of fowls
Ostrich/Struthio camelus/“Tuoniao,” “鸵鸟”
Ostrich originally lived in the semi-arid desert areas of Africa. According to historical records, in 101 AD, the Sabbath Dynasty presented lions, ostriches, and other animals as gifts to Chinese emperors. It is not included in the PPRC. It is a zoo animal in China and there is no report of using it in the current TCM.
4 Discussion
The earliest footprints of Persian businessmen appeared in the Chinese cities of Changan, Luoyang, Guangzhou, and Quanzhou. The book, Taiping Guangji (first edition in 978 of Song Dynasty), recorded a large number of Persian merchants who settled down in Chang’an to trade the jewelry and spice. At the same time, China’s ceramics, paper, silk, tea, porcelain, sericulture, and other expertise were also introduced to Persia, which greatly impact the empire. Yunsheng Hu (Hu and Zhang, 1996) in the study of foreign drugs in the Tang Dynasty indicated that the aromatic drugs are a bulk trade commodity, and a large number of them come from Persia and the Seljuks. The import and export of Persian drugs mainly rely on merchants all over the country. In addition to importing its own drugs, the Persians also collect drugs from foreign countries to trade in China.
Foreign medicines enrich the varieties of traditional Chinese medicines and meet people’s needs to treat diseases and maintain health. There are a few fates of these medicines in China. 1) Some foreign drug resources spread to China, over time, they have gradually adapted to the cultivation environment in China and have been included in the “Chinese herbal medicines,” becoming China’s localized medicine. Saffron, myrrh, and olibanum are as such medicines that are all included in the PPRC for treating diseases even though these medicines are still largely dependent on imports from Iran or other countries. 2) Some medicinal materials are no longer included as TCM materials, but are treated as common food through long-term of experiencing, recognition, and understanding. As mentioned earlier, dill and spinach became common vegetables not only in China but also in the rest of the world. Fig, grape, jackfruit, phoenix date, and pistachio are popular fruits with good taste and nutrition. Jasmine, olives, and other fragrant medicines are primarily being used as raw materials for processing into many products including perfume, essential oil, and skin care products, which can not only make full use of the value of these plants, but also meet the diverse needs of people. Based on the theory of TCM, the aforementioned fruit and vegetables can be classified in a group of food and also medicine due to their tonic factors. 3) In addition, some medicinal materials, due to excessive toxicity, are listed outside the range of commonly used medicinal materials. This part of the medicinal materials is mostly concentrated in the category of metals, stones, and minerals. For example, lead and litharge are no longer used as medicinal materials. Cinnabar, copper, and calamine are also extremely restricted in clinical use. Lead and mercury are closely monitored in the standardization of quality control of all TCM materials for contamination of heavy metals.
Of interest, 25 of the 38 TCM medicinal plants now are cultivated or wild growing in China but they are not found in Iran. There are some possible explanations for this. First, the authentification of material used in TCM is changing with time. In old days without scientific names, it is possible that different plant species could easily be confused, especially if they share similar medicinal efficacy. For example, natural indigo, the three plants, namely, S. cusia, P. tinctoria, and I. tinctoria subsp. tinctoria show similar treatments and people treat them the same but it might be I. tinctoria subsp. tinctoria which was the only one introduced from Persia to China. Second, some of the medicinal plants from Persia might have been replaced by the ones that were naturally found in China. When plants have close pharmacological effects, harvesting from nearby areas is more economical than obtaining plant materials from faraway places that are ten thousand of kilometers away. If this is the case, some of species that are naturally distributed in Iran might be valuable for drug discovery as they have found use in China, such as close species of Cullen corylifolia (L.) Medik., and Terminalia chebula Retz., are all found in Iran. Another example is of the Ferula species. F. sinkiangensis or F. fukanensis are the two species listed in the PPRC but these two plants were discovered in the 1950s in China and now they are endangered with limited production. For a long time, the Ferula imported from Persia must be the close species Ferula assa-foetida L. or other species in Iran. Re-connection of the China–Iran bilateral authentification of the medicine may help to utilize the rich resources of Ferula in Iran. Third, some of the medicinal plants are not distributed in Iran or even in old Persia, but they were introduced by the Persia businessmen and brought to China. It is confirmed that Iran does not produce myrrh and for a long time and the great demand of the Chinese market might be provided by Persian commercial men, who obtained it from other places. It was until recent decades that China started importing the myrrh from Ethiopia and nearby countries. In this way, the Silk Road in the old times may have extended to Africa by Persia (Figure 1). We are now collecting more evidence to verify the connection of East Asia to the central Africa of the Silk Road.
There are 24 TCMs, consisting of 38 medicinal plants and four stones, studied in this study and were included in the 2020 edition of the Pharmacopoeia of the People’s Republic of China. The pharmacopoeia is a drug code established by the state to ensure the quality and safety of the medicine. Drugs recorded in the pharmacopoeia are commonly used as medicinal materials and are permitted in use in the clinical practice. In Table 3, these drugs were counted to provide reference for the use of these medicinal materials. Since ancient times, the Chinese had been actively absorbing outstanding foreign culture. The development of communication between China and abroad spreads Chinese civilization to the world and brings back the civilizations from all over the world. The CMM has played an important role in the TCM development. Until today, the inclusive thinking of Shizhen Li on foreign culture is something we should learn.
The introduction and cultivation of exotic drugs is an important part of the development history of Chinese herbal medicines. Persia was an important country on the Silk Road. Looking back to the date when Shizhen Li was writing the book and even earlier, the introduction of traditional medicine benefited both sides. With saffron as an example, even though it is now sporadically cultivated in China, much more is imported to China from Iran, perhaps continuously lasting over two thousand years. It is not because it cannot grow in China. It is because in the Chinese medicinal culture Persia produces the best quality saffron even though it is treated as a native medicine. The peaceful development of China and Persia has a positive impact on the politics, economy, and culture of the two territories. It is the best example and most valuable legacy of the Silk Road on the sharing of traditional medicinal resources. It shall inspire us to peacefully develop a collaborative relationship to share the advancement of the human civilization.
Author contributions
Conceived and designed the project: JS, YY, XZ, and XH. Analyzed data: XZ, XL, LZ JS, AY, MH, and FS, and XH. Writing of the manuscript: JS, YY, XZ, and XH.
Funding
The research was supported by the National Science and Technology Fundamental Resources Investigation Program of China (No. 2018FY100704 to XL) and National Key R&D Program of China (No. 2019YFE0108700 to XL). This research was supported by the Shengnongjia Academy of Forestry, Hubei, China (No. SAF202102), Hubei Technology Innovation Center for Agricultural Sciences—“2020 key technology research and demonstration project of safe and efficient production of genuine medicinal materials” (No. 2020-620-000-002-04), China-Bulgaria science and technology exchange meeting for traditional medicine (year of 2020) to XH.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1Here the common name, scientific name, Chinese phonics, and characters for each medicine are shown. The Chinese phonics and characters are from the CMM. The current name is shown in Table 1 and 3.
References
Adhikary, S., Choudhary, D., Ahmad, N., Kumar, S., Dev, K., Mittapelly, N., et al. (2017). Dried and free flowing granules of Spinacia oleracea accelerate bone regeneration and alleviate postmenopausal osteoporosis. Menopause 24 (6), 686–698. doi:10.1097/GME.0000000000000809
Ali, M. A., Wahed, M. I., Khatune, N. A., Rahman, B. M., Barman, R. K., and Islam, M. R. (2015). Antidiabetic and antioxidant activities of ethanolic extract of Semecarpus anacardium (Linn.) bark. BMC Complement. Altern. Med. 15, 138. doi:10.1186/s12906-015-0662-z
AlMadi, E. M., Almohaimede, A. A., Al-Obaida, M. I., and Awaad, A. S. (2019). Comparison of the antibacterial efficacy of Commiphora molmol and sodium hypochlorite as root canal irrigants against Enterococcus faecalis and Fusobacterium nucleatum. Evid. Based. Complement. Altern. Med. 2019, 6916795. doi:10.1155/2019/6916795
Alrashdi, A. S., Salama, S. M., Alkiyumi, S. S., Abdulla, M. A., Hadi, A. H., Abdelwahab, S. I., et al. (2012). Mechanisms of gastroprotective effects of ethanolic leaf extract of Jasminum sambac against HCl/Ethanol-Induced gastric mucosal injury in rats. Evid. Based. Complement. Altern. Med. 2012, 786426. doi:10.1155/2012/786426
AlRomaiyan, A., Huang, G. C., Jones, P., and Persaud, S. (2021). Commiphora myrrha stimulates insulin secretion from mouse and human islets of Langerhans. J. Ethnopharmacol. 264, 113075. doi:10.1016/j.jep.2020.113075
Antonisamy, P., Agastian, P., Kang, C. W., Kim, N. S., and Kim, J. H. (2019). Anti-inflammatory activity of rhein isolated from the flowers of Cassia fistula L. and possible underlying mechanisms. Saudi J. Biol. Sci. 26 (1), 96–104. doi:10.1016/j.sjbs.2017.04.011
Ataei, D., Hamidi-Esfahani, Z., and Ahmadi-Gavlighi, H. (2020). Enzymatic production of xylooligosaccharide from date (Phoenix dactylifera L.) seed. Food Sci. Nutr. 8 (12), 6699–6707. doi:10.1002/fsn3.1964
Avijgan, M., Kamran, A., and Abedini, A. (2016). Effectiveness of Aloe vera gel in chronic ulcers in comparison with conventional treatments. Iran. J. Med. Sci. 41 (3), S30.
Bagherzadeh Karimi, A., Elmi, A., Mirghafourvand, M., and Baghervand Navid, R. (2020). Effects of date fruit (Phoenix dactylifera L.) on labor and delivery outcomes: A systematic review and meta-analysis. BMC Pregnancy Childbirth 20 (1), 210. doi:10.1186/s12884-020-02915-x
Bailey, H. M., and Stein, H. H. (2020). Raw and roasted pistachio nuts (Pistacia vera L.) are 'good' sources of protein based on their digestible indispensable amino acid score as determined in pigs. J. Sci. Food Agric. 100 (10), 3878–3885. doi:10.1002/jsfa.10429
Barolo, M. I., Mostacero, N. R., and López, S. N. (2014). Ficus carica L (moraceae): An ancient source of food and health. Food Chem. 164, 119–127. doi:10.1016/j.foodchem.2014.04.112
Bieski, I. G. C., Leonti, M., Arnason, J. T., Ferrier, J., Rapinski, M., Violante, I. M. P., et al. (2015). Ethnobotanical study of medicinal plants by population of valley of Juruena region, legal Amazon, Mato Grosso, Brazil. J. Ethnopharmacol. 173, 383–423. doi:10.1016/j.jep.2015.07.025
Bouhlali, E. D. T., Hmidani, A., Bourkhis, B., Khouya, T., Harnafi, H., Filali-Zegzouti, Y., et al. (2020). Effect of phoenix dactylifera seeds (dates) extract in triton WR-1339 and high fat diet induced hyperlipidaemia in rats: A comparison with simvastatin. J. Ethnopharmacol. 259, 112961. doi:10.1016/j.jep.2020.112961
Bozorgi, H., Ghahremanfard, F., Motaghi, E., Zamaemifard, M., Zamani, M., and Izadi, A. (2021). Effectiveness of crocin of saffron (Crocus sativus L.) against chemotherapy-induced peripheral neuropathy: A randomized, double-blind, placebo-controlled clinical trial. J. Ethnopharmacol. 281, 114511. doi:10.1016/j.jep.2021.114511
Buso, P., Manfredini, S., Reza Ahmadi-Ashtiani, H., Sciabica, S., Buzzi, R., Vertuani, S., et al. (2020). Iranian medicinal plants: From ethnomedicine to actual studies. Medicina 56 (3), 97. doi:10.3390/medicina56030097
Cao, B., Wei, X. C., Xu, X. R., Zhang, H. Z., Luo, C. H., Feng, B., et al. (2019). Seeing the unseen of the combination of two natural resins, frankincense and myrrh: Changes in chemical constituents and pharmacological activities. Molecules 24 (17), 3076. doi:10.3390/molecules24173076
Cao, J. (2009). Study on the literature and quality of Psoralea corylifolia. Beijing: Beijing University of Chinese Medicine.
Chandan, B. K., Saxena, A. K., Shukla, S., Sharma, N., Gupta, D. K., Suri, K. A., et al. (2007). Hepatoprotective potential of Aloe barbadensis Mill. against carbon tetrachloride induced hepatotoxicity. J. Ethnopharmacol. 111 (3), 560–566. doi:10.1016/j.jep.2007.01.008
Chang, S. J., Chang, Y. C., Lu, K. Z., Tsou, Y. Y., and Lin, C. W. (2012). Antiviral activity of Isatis indigotica extract and its derived indirubin against Japanese encephalitis virus. Evid. Based. Complement. Altern. Med. 2012, 925830. doi:10.1155/2012/925830
Chen, J., and Chen, C. (1991). Some herbal textual comments on tianmingjing (Carpesium abrotanoides L.). China J. Chin. Materia Medica 16 (2), 67–69, 125.
Chen, W., Van Wyk, B. E., Vermaak, I., and Viljoen, A. M. (2012). Cape aloes—A review of the phytochemistry, pharmacology and commercialisation of Aloe ferox. Phytochem. Lett. 5 (1), 1–12. doi:10.1016/j.phytol.2011.09.001
Chen, H., Shao, J., Zhang, H., Jiang, M., Huang, L., Zhang, Z., et al. (2018a). Sequencing and analysis of Strobilanthes cusia (Nees) Kuntze chloroplast genome revealed the rare simultaneous contraction and expansion of the inverted repeat region in angiosperm. Front. Plant Sci. 9, 324. doi:10.3389/fpls.2018.00324
Chen, Z., Ni, W., Yang, C., Zhang, T., Lu, S., Zhao, R., et al. (2018b). Therapeutic effect of Amomum villosum on inflammatory bowel disease in rats. Front. Pharmacol. 9, 639. doi:10.3389/fphar.2018.00639
Chen, F., Yang, L., Huang, Y., Chen, Y., Sang, H., Duan, W., et al. (2020a). Isocorilagin, isolated from Canarium album (Lour.) Raeusch, as a potent neuraminidase inhibitor against influenza A virus. Biochem. Biophys. Res. Commun. 523 (1), 183–189. doi:10.1016/j.bbrc.2019.12.043
Chen, F., Yang, L., Zhai, L., Huang, Y., Chen, F., Duan, W., et al. (2020b). Methyl brevifolincarboxylate, a novel influenza virus PB2 inhibitor from Canarium Album (Lour.) Raeusch. Chem. Biol. Drug Des. 96 (5), 1280–1291. doi:10.1111/cbdd.13740
Chen, L. X., Lai, Y. F., Zhang, W. X., Cai, J., Hu, H., Wang, Y., et al. (2020c). Comparison of volatile compounds in different parts of fresh Amomum villosum Lour. from different geographical areas using cryogenic grinding combined HS-SPME-GC-MS. Chin. Med. 15, 97. doi:10.1186/s13020-020-00377-z
Chung, Y. C., Ko, J. H., Kang, H. K., Kim, S., Kang, C. I., Lee, J. N., et al. (2018). Antimelanogenic effects of Polygonum tinctorium flower extract from traditional jeju fermentation via upregulation of extracellular signal-regulated kinase and protein kinase B activation. Int. J. Mol. Sci. 19 (10), E2895. doi:10.3390/ijms19102895
Commission, C. P. (2020). Pharmacopoeia of the people's Republic of China. Beijing, China: China Medical Science Press.
Commission, C. P. (2017). Pharmacopoeia of the people's Republic of China. Beijing, China: China Medical Science Press.
Dey, P., Dutta, S., Chowdhury, A., Das, A. P., and Chaudhuri, T. K. (2017). Variation in phytochemical composition reveals distinct divergence of Aloe vera (L.) Burm. F. From other Aloe species: Rationale behind selective preference of Aloe vera in nutritional and therapeutic use. J. Evid. Based. Complement. Altern. Med. 22 (4), 624–631. doi:10.1177/2156587217698292
Dey, A., Dhadhal, S., Maharjan, R., Nagar, P. S., and Nampoothiri, L. (2022). Partially purified non-polar phytocomponents from Aloe barbadensis Mill. gel restores metabolic and reproductive comorbidities in letrozole-induced polycystic ovary syndrome rodent model- an "in-vivo" study. J. Ethnopharmacol. 291, 115161. doi:10.1016/j.jep.2022.115161
Dini, I., Falanga, D., Di Lorenzo, R., Tito, A., Carotenuto, G., Zappelli, C., et al. (2021). An extract from Ficus carica cell cultures works as an anti-stress ingredient for the skin. Antioxidants (Basel) 10 (4), 515. doi:10.3390/antiox10040515
Donkor, A. M., Donkor, M. N., and Kuubabongnaa, N. (2020). Evaluation of anti-infective potencies of formulated aloin A ointment and aloin A isolated from Aloe barbadensis Miller. BMC Chem. 14 (1), 8. doi:10.1186/s13065-020-0659-7
Fan, C., Wang, G., Qiu, Y., Zhao, Y., Zhang, J., Song, J., et al. (2021). The complete chloroplast genome sequence of Ferula sinkiangensis KM Shen, a precious and endangered traditional Chinese medicine. Mitochondrial DNA. B Resour. 6 (6), 1670–1672. doi:10.1080/23802359.2021.1927869
Faraji, A., Aghdaki, M., Hessami, K., Hosseinkhani, A., Roozmeh, S., Asadi, N., et al. (2021). Episiotomy wound healing by Commiphora myrrha (nees) Engl. And Boswellia carteri birdw. In primiparous women: A randomized controlled trial. J. Ethnopharmacol. 264, 113396. doi:10.1016/j.jep.2020.113396
Foshati, S., Nouripour, F., Sadeghi, E., and Amani, R. (2022). The effect of grape (Vitis vinifera) seed extract supplementation on flow-mediated dilation, blood pressure, and heart rate: A systematic review and meta-analysis of controlled trials with duration- and dose-response analysis. Pharmacol. Res. 175, 105905. doi:10.1016/j.phrs.2021.105905
Fu, J., Zeng, C., Zeng, Z., Wang, B., Wen, X., Yu, P., et al. (2016). Cinnamomum camphora seed kernel oil improves lipid metabolism and enhances β3-adrenergic receptor expression in diet-induced obese rats. Lipids 51 (6), 693–702. doi:10.1007/s11745-016-4147-8
Fu, F., Zhang, N., Wang, Y., Li, D., Abdelaala, W., and Xu, Z. (2022). In vitro pollen germination and pollen storage methods of Phoenix dactylifera L. Mol. Plant Breed. 20 (12), 4085–4093. doi:10.13271/j.mpb.020.004085
Gad El-Hak, H. N., Mahmoud, H. S., Ahmed, E. A., Elnegris, H. M., Aldayel, T. S., Abdelrazek, H. M., et al. (2022). Methanolic Phoenix dactylifera L. Extract ameliorates cisplatin-induced hepatic injury in male rats. Nutrients 14 (5), 1025. doi:10.3390/nu14051025
Ge, C. Y., and Zhang, J. L. (2019). Bioactive sesquiterpenoids and steroids from the resinous exudates of Commiphora myrrha. Nat. Prod. Res. 33 (3), 309–315. doi:10.1080/14786419.2018.1448811
Ghasemi Ghehsareh, M., Salehi, H., Khosh-Khui, M., and Niazi, A. (2015). Application of ISSR markers to analyze molecular relationships in Iranian jasmine (Jasminum spp.) accessions. Mol. Biotechnol. 57 (1), 65–74. doi:10.1007/s12033-014-9802-9
Goodarzi, M. T., Khodadadi, I., Tavilani, H., and Abbasi Oshaghi, E. (2016). The role of Anethum graveolens L. (Dill) in the management of diabetes. J. Trop. Med. 2016, 1098916. doi:10.1155/2016/1098916
Guo, N., Jin, C., Shen, L., Wu, F., Lin, X., and Feng, Y. (2020). Chemical components, pharmacological actions, and clinical applications of Rhizoma Picrorhizae. Phytother. Res. 34 (5), 1071–1082. doi:10.1002/ptr.6591
Gupta, A., Marquess, A. R., Pandey, A. K., and Bishayee, A. (2022). Jackfruit (Artocarpus heterophyllus Lam.) in health and disease: A critical review. Crit. Rev. Food Sci. Nutr., 1–35. doi:10.1080/10408398.2022.2031094
Haghani, F., Arabnezhad, M. R., Mohammadi, S., and Ghaffarian-Bahraman, A. (2022). Aloe vera and streptozotocin-induced diabetes mellitus. Rev. Bras. Farmacogn. 32, 174–187. doi:10.1007/s43450-022-00231-3
He, L., Zen, H. S., and Pan, C. L. (2016). Research progress of aloe. Chin. J. Ethnomedicine Ethnopharmacy 25 (06), 47–48.
He, W., and Huang, B. (2011). Research progress in chemical constituents in dried fruits of Anethum graveolens and their pharmacological effects. Drugs Clin. 26 (6), 457–460.
He, Y., and Zhang, T. (2018). Talking about cinnabar again. J. Traditional Chin. Med. 36 (5), 27–31.
Hong, L., Xu, X., Chen, L., Li, B., Wu, D., Hu, M., et al. (2011). The anti-HSV-2 effect of alumen: In vitro and in vivo experimental studies. J. Huazhong Univ. Sci. Technol. Med. Sci. 31 (6), 828–833. doi:10.1007/s11596-011-0685-8
Hong, Y., Choi, Y. H., Han, Y. E., Oh, S. J., Lee, A., Lee, B., et al. (2021). Central administration of ampelopsin A isolated from Vitis vinifera ameliorates cognitive and memory function in a scopolamine-induced dementia model. Antioxidants (Basel) 10 (6), 835. doi:10.3390/antiox10060835
Hooshmand Moghadam, B., Rashidlamir, A., Attarzadeh Hosseini, S. R., Gaeini, A. A., and Kaviani, M. (2022). The effects of saffron (Crocus sativus L.) in conjunction with concurrent training on body composition, glycaemic status, and inflammatory markers in obese men with type 2 diabetes mellitus: A randomized double-blind clinical trial. Br. J. Clin. Pharmacol. 88, 3256–3271. doi:10.1111/bcp.15222
Hu, J., Liu, Y., Tang, X., Rao, H., Ren, C., Chen, J., et al. (2020). Transcriptome profiling of the flowering transition in saffron (Crocus sativus L.). Sci. Rep. 10 (1), 1–14. doi:10.1038/s41598-020-66675-6
Hu, Y., and Zhang, W. (1996). Import of foreign drugs in Tang dynasty. Jouranl Zhoukou Teach. Coll. 13 (1), 26–30. doi:10.13450/j.cnki.jzknu.1996.sl.008
Huang, B., and Fan, M. (2003). Research progress of tannin anti-caries. J. Mod. Stomatology 17 (5), 458–459.
Huang, B., and Xian, J. (1990). Investigation of exotic drugs in arcane essentials from the imperial library. Acta Chin. Med. Pharmacol. 22 (1), 51–52. doi:10.19664/j.cnki.1002-2392.1990.01.022
Huang, K., Chen, Y., Liang, K., Xu, X., Jiang, J., Liu, M., et al. (2022). Review of the chemical composition, pharmacological effects, pharmacokinetics, and quality control of Boswellia carterii. Evid. Based. Complement. Altern. Med. 2022, 6627104. doi:10.1155/2022/6627104
Huo, H., Sun, H., Zhang, Y., Chen, X., Yao, H., Ding, Z., et al. (2018). Advances in pharmacological effects and quality control of eaglewood. Eval. analysis drug-use Hosp. China 18 (2), 152–159. doi:10.14009/j.issn.1672-2124.2018.02.002
Hwang, Y. H., Lee, A., Kim, T., Jang, S. A., and Ha, H. (2021). Anti-osteoporotic effects of Commiphora myrrha and its poly-saccharide via osteoclastogenesis inhibition. Plants (Basel) 10 (5), 945. doi:10.3390/plants10050945
Ibrahim, S. R., Fadil, S. A., Fadil, H. A., Hareeri, R. H., Abdallah, H. M., and Mohamed, G. A. (2022). Ethnobotanical uses, phytochemical composition, biosynthesis, and pharmacological activities of Carpesium abrotanoides L. (Asteraceae). Plants 11 (12), 1598. doi:10.3390/plants11121598
Indu, S., Vijayalakshmi, P., Selvaraj, J., and Rajalakshmi, M. (2021). Novel triterpenoids from Cassia fistula stem bark depreciates STZ-induced detrimental changes in IRS-1/akt-mediated insulin signaling mechanisms in type-1 diabetic rats. Molecules 26 (22), 6812. doi:10.3390/molecules26226812
Iranshahy, M., and Iranshahi, M. (2011). Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin)-a review. J. Ethnopharmacol. 134 (1), 1–10. doi:10.1016/j.jep.2010.11.067
Jin, C., Wu, F., Zheng, X., Fang, X., Hu, J., and Feng, Y. (2019). Research progress on the chemical composition, analytical methodand pharmacological action of Rhizoma picrorhizae. Chin. J. New Drugs 28 (3), 292–302.
Jin, S., and Guo, X. (2011). History of introduction of foreign medicines from pre-qin to Sui, Tang and five dynasties. Chin. Med. Cult. 6 (1), 25–29. doi:10.16307/j.1673-6281.2011.01.007
Kataoka, M., Hirata, K., Kunikata, T., Ushio, S., Iwaki, K., Ohashi, K., et al. (2001). Antibacterial action of tryptanthrin and kaempferol, isolated from the indigo plant (Polygonum tinctorium Lour.), against Helicobacter pylori-infected Mongolian gerbils. J. Gastroenterol. 36 (1), 5–9. doi:10.1007/s005350170147
Keivanfar, M., Daris, S., Reisi, M., and Mehrkesh, M. (2020). The fractional excretion of sodium in patients with cystic fibrosis treated with oral sodium chloride. Am. J. Clin. Exp. Urol. 8 (6), 185–190.
Khan, H. B., Vinayagam, K. S., Renny, C. M., Palanivelu, S., and Panchanadham, S. (2013). Potential antidiabetic effect of the Semecarpus anacardium in a type 2 diabetic rat model. Inflammopharmacology 21 (1), 47–53. doi:10.1007/s10787-012-0136-6
Khazaeli, P., Goldoozian, R., and Sharififar, F. (2009). An evaluation of extracts of five traditional medicinal plants from Iran on the inhibition of mushroom tyrosinase activity and scavenging of free radicals. Int. J. Cosmet. Sci. 31 (5), 375–381. doi:10.1111/j.1468-2494.2009.00503.x
Khushboo, P. S., Jadhav, V. M., Kadam, V. J., and Sathe, N. S. (2010). Psoralea corylifolia Linn.-"Kushtanashini. Pharmacogn. Rev. 4 (7), 69–76. doi:10.4103/0973-7847.65331
Kim, K., Veerappan, K., Woo, N., Park, B., Natarajan, S., Chung, H., et al. (2021). Ulmus macrocarpa hance extract modulates intestinal microbiota in healthy adults: A randomized, placebo-controlled clinical trial. J. Microbiol. 59 (12), 1150–1156. doi:10.1007/s12275-021-1329-8
Kim, H. L., Lee, S. K., Min, D. E., Choi, B. K., and Lee, D. R. (2022). Anti-obesity effects of a mixture of Atractylodes macrocephala and Amomum villosum extracts on 3T3-L1 adipocytes and high-fat diet-induced obesity in mice. Molecules 27 (3), 906. doi:10.3390/molecules27030906
Kothari, P., Sinha, S., Sardar, A., Tripathi, A. K., Girme, A., Adhikary, S., et al. (2020). Inhibition of cartilage degeneration and subchondral bone deterioration by Spinacia oleracea in human mimic of ACLT-induced osteoarthritis. Food Funct. 11 (9), 8273–8285. doi:10.1039/d0fo01125h
Latha, S., Selvamani, P., and Prabha, T. (2021). Pharmacological uses of the plants belonging to the genus Commiphora. Cardiovasc. Hematol. Agents Med. Chem. 19 (2), 101–117. doi:10.2174/1871525718666200702125558
Lebda, M. A., Mostafa, R. E., Taha, N. M., Abd El-Maksoud, E. M., Tohamy, H. G., Al Jaouni, S. K., et al. (2021). Commiphora myrrh supplementation protects and cures ethanol-induced oxidative alterations of gastric ulceration in rats. Antioxidants 10 (11), 1836. doi:10.3390/antiox10111836
Lee, S., Kim, D. C., Baek, H. Y., Lee, K. D., Kim, Y. C., and Oh, H. (2018). Anti-neuroinflammatory effects of tryptanthrin from Polygonum tinctorium Lour. in lipopolysaccharide-stimulated BV2 microglial cells. Arch. Pharm. Res. 41 (4), 419–430. doi:10.1007/s12272-018-1020-8
Lee, D. Y., Li, Q. Y., Liu, J., and Efferth, T. (2021). Traditional Chinese herbal medicine at the forefront battle against COVID-19: Clinical experience and scientific basis. Phytomedicine 80, 153337. doi:10.1016/j.phymed.2020.153337
Lei, L., Wang, Q., Bai, X., and Deng, W. (2012). Study on anti-inflammatory and antipyretic effects of benzoin. Pharmacol. Clin. Chin. Materia Medica 28 (2), 109–110. doi:10.13412/j.cnki.zyyl.2012.02.043
Lei, Y. (2011). Silk Road Trade between Iran and China in middle ancient period. J. Humanit. (1), 138–142. doi:10.15895/j.cnki.rwzz.2011.01.028
Li, D., and Zhu, T. (2005). Historical Evolution and Development Prospect of exotic varieties of Chinese medicinal materials. Heilongjiang J. Traditional Chin. Med. (3), 56–58.
Li, J., Hu, S., and Li, H. (2013). Commodity types and historical sources of traditional Chinese medicine borneol. Mod. Chin. Med. 15 (6), 531–534. doi:10.13313/j.issn.1673-4890.2013.06.017
Li, L., Tian, W., Liu, Y., Li, Y., Cao, X., and Zhang, W. (2018). Research progress on chemical constituents and pharmacological effects of Amomum villosum. Prog. Mod. Biomed. 18 (22), 4390–4396. doi:10.13241/j.cnki.pmb.2018.22.044
Li, J., Lin, Z., Tang, X., Liu, G., Chen, Y., Zhai, X., et al. (2020). Oxyresveratrol extracted from Artocarpus heterophyllus Lam. inhibits tyrosinase and age pigments in vitro and in vivo. Food Funct. 11 (7), 6595–6607. doi:10.1039/d0fo01193b
Li, Q., Xia, L., Sun, C., Zhang, H., Zheng, M., Zhang, H., et al. (2021). Role of borneol induced autophagy in enhancing radiosensitivity of malignant glioma. Front. Oncol. 11, 749987. doi:10.3389/fonc.2021.749987
Li, D., Wang, R., Cheng, X., Yang, J., Yang, Y., Qu, H., et al. (2022a). Chemical constituents from the fruits of Piper longum L. and their vascular relaxation effect on rat mesenteric arteries. Nat. Prod. Res. 36 (2), 674–679. doi:10.1080/14786419.2020.1797726
Li, J., Wang, R., Wang, Y., Zeng, J., Xu, Z., Xu, J., et al. (2022b). Anti-inflammatory benzofuran neolignans from the fruits of Canarium album (Chinese olive). J. Agric. Food Chem. 70 (4), 1122–1133. doi:10.1021/acs.jafc.1c06457
Li, J., Ni, L., Li, B., Wang, M., Ding, Z., Xiong, C., et al. (2017). Coptis Chinensis affects the function of glioma cells through the down-regulation of phosphorylation of STAT3 by reducing HDAC3. BMC Complement. Altern. Med. 6, 524–529. doi:10.1186/s12906-017-2029-0
Lin, C. W., Tsai, F. J., Tsai, C. H., Lai, C. C., Wan, L., Ho, T. Y., et al. (2005). Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir. Res. 68 (1), 36–42. doi:10.1016/j.antiviral.2005.07.002
Lingaraju, G. M., Krishna, V., Joy Hoskeri, H., Pradeepa, K., and Venkatesh, and Babu, P. S. (2012). Wound healing promoting activity of stem bark extract of Semecarpus anacardium using rats. Nat. Prod. Res. 26 (24), 2344–2347. doi:10.1080/14786419.2012.656108
Liu, Y., and Kong, Z. K. (2007). Determination of FeAs in melanteritum and calcined melanteritum. Chin. J. Pharm. Analysis 27 (4), 595–596. doi:10.16155/j.0254-1793.2007.04.014
Liu, C., Xu, J., Gui, L., and Guo, Y. (2010). Studies on chemical constituents of carpesii fructus. Drug Eval. Res. 33 (3), 220–221.
Liu, Y. F., Chen, M. H., Guo, Q. L., Lin, S., Xu, C. B., Jiang, Y. P., et al. (2015). Antiviral glycosidic bisindole alkaloids from the roots of Isatis indigotica. J. Asian Nat. Prod. Res. 17 (7), 689–704. doi:10.1080/10286020.2015.1055729
Liu, Y. P., Guo, J. M., Yan, G., Zhang, M. M., Zhang, W. H., Qiang, L., et al. (2019). Anti-inflammatory and antiproliferative prenylated isoflavone derivatives from the fruits of Ficus carica. J. Agric. Food Chem. 67 (17), 4817–4823. doi:10.1021/acs.jafc.9b00865
Liu, S., Long, Y., Yu, S., Zhang, D., Yang, Q., Ci, Z., et al. (2021). Borneol in cardio-cerebrovascular diseases: Pharmacological actions, mechanisms, and therapeutics. Pharmacol. Res. 169, 105627. doi:10.1016/j.phrs.2021.105627
Lu, S., Zhang, T., Gu, W., Yang, X., Lu, J., Zhao, R., et al. (2018). Volatile oil of Amomum villosum inhibits nonalcoholic fatty liver disease via the gut-liver Axis. Biomed. Res. Int. 2018, 3589874. doi:10.1155/2018/3589874
Mala, K. N., Thomas, J., Syam, D. S., Maliakel, B., and Krishnakumar, I. M. (2018). Safety and efficacy of Ferula asafoetida in functional dyspepsia: A randomized, double-blinded, placebo-controlled study. Evid. Based. Complement. Altern. Med. 2018, 4813601. doi:10.1155/2018/4813601
Mandal, A., Jha, A. K., and Hazra, B. (2021). Plant products as inhibitors of coronavirus 3CL protease. Front. Pharmacol. 167, 583387. doi:10.3389/fphar.2021.583387
Mao, W., Yao, G., Chen, B., Pan, Q., Wang, H., Hu, G., et al. (2019). Characterization of the complete chloroplast genome of the Pistacia vera L. Mitochondrial DNA. B Resour. 4 (2), 4140–4141. doi:10.1080/23802359.2019.1692723
Massoud, A. M., El-Shazly, A. M., and Morsy, T. A. (2007). Mirazid (Commiphora molmol) in treatment of human heterophyiasis. J. Egypt. Soc. Parasitol. 37 (2), 395–410.
Meng, X. L., Ma, J. N., Guo, X. H., Liu, B. C., Cui, N. N., Li, K., et al. (2017). Processing of calamine with modern analytical techniques: Processed with huanglian decoction (黄连汤) and sanhuang decoction (三黄汤). Chin. J. Integr. Med. 23 (11), 850–857. doi:10.1007/s11655-017-2798-9
Meng, L., Jiao, Y., Zhou, X., Liang, C., Yan, K., Zhao, Y., et al. (2021). Leaf extract from Vitis vinifera L. reduces high fat diet-induced obesity in mice. Food Funct. 12 (14), 6452–6463. doi:10.1039/d1fo00460c
Mi, Y., Jiao, K., Xu, J. K., Wei, K., Liu, J. Y., Meng, Q. Q., et al. (2021). Kellerin from Ferula sinkiangensis exerts neuroprotective effects after focal cerebral ischemia in rats by inhibiting microglia-mediated inflammatory responses. J. Ethnopharmacol. 269, 113718. doi:10.1016/j.jep.2020.113718
Misra, P., Kumar, A., Khare, P., Gupta, S., Kumar, N., and Dube, A. (2009). Pro-apoptotic effect of the landrace Bangla Mahoba of Piper betle on Leishmania donovani may be due to the high content of eugenol. J. Med. Microbiol. 58 (8), 1058–1066. doi:10.1099/jmm.0.009290-0
Moghadam, B. H., Bagheri, R., Roozbeh, B., Ashtary-Larky, D., Gaeini, A. A., Dutheil, F., et al. (2021). Impact of saffron (Crocus Sativus Linn) supplementation and resistance training on markers implicated in depression and happiness levels in untrained young males. Physiol. Behav. 233, 113352. doi:10.1016/j.physbeh.2021.113352
Mohammadparast Tabas, P., Aramjoo, H., Yousefinia, A., Zardast, M., Abedini, M. R., and Malekaneh, M. (2021). Therapeutic and preventive effects of aqueous extract of date palm (Phoenix dactylifera L.) pits on ethylene glycol-induced kidney calculi in rats. Urol. J. 18 (6), 612–617. doi:10.22037/uj.v18i.6530
Mojtahedi, S., Hooshmand-Moghadam, B., Rosenkranz, S., Shourideh, Z., Amirshaghaghi, F., and Shabkhiz, F. (2022). Improvement of inflammatory status following saffron (Crocus sativus L.) and resistance training in elderly hypertensive men: A randomized controlled trial. Exp. Gerontol. 162, 111756. doi:10.1016/j.exger.2022.111756
Morrison, I. J., Zhang, J., Lin, J., Murray, J. E., Porter, R., Langat, M. K., et al. (2021). Potential chemopreventive, anticancer and anti-inflammatory properties of a refined artocarpin-rich wood extract of Artocarpus heterophyllus Lam. Sci. Rep. 11 (1), 6854. doi:10.1038/s41598-021-86040-5
Motai, T., and Kitanaka, S. (2005). Sesquiterpene chromones from Ferula fukanensis and their nitric oxide production inhibitory effects. J. Nat. Prod. 68 (12), 1732–1735. doi:10.1021/np058079e
Mwangi, R. W., Macharia, J. M., Wagara, I. N., and Bence, R. L. (2021). The medicinal properties of Cassia fistula L: A review. Biomed. Pharmacother. 144, 112240. doi:10.1016/j.biopha.2021.112240
Naziz, P. S., Das, R., and Sen, S. (2019). The scent of stress: Evidence from the unique fragrance of agarwood. Front. Plant Sci. 10, 840. doi:10.3389/fpls.2019.00840
Oh, K. S., Ryu, S. Y., Oh, B. K., Seo, H. W., Kim, Y. S., and Lee, B. H. (2008). Antihypertensive, vasorelaxant, and antioxidant effect of root bark of Ulmus macrocarpa. Biol. Pharm. Bull. 31 (11), 2090–2096. doi:10.1248/bpb.31.2090
Osanloo, M., Sereshti, H., Sedaghat, M. M., and Amani, A. (2017). Nanoemulsion of Dill essential oil as a green and potent larvicide against Anopheles stephensi. Environ. Sci. Pollut. Res. 25 (1), 6466–6473. doi:10.1007/s11356-017-0822-4
Oshaghi, E. A., Khodadadi, I., Tavilani, H., and Goodarzi, M. T. (2016). Effect of dill tablet (Anethum graveolens L) on antioxidant status and biochemical factors on carbon tetrachloride-induced liver damage on rat. Int. J. Appl. Basic Med. Res. 6 (2), 111–114. doi:10.4103/2229-516X.179019
Osuntokun, O. S., Olayiwola, G., Adekomi, D. A., Oyeyipo, I. P., and Ayoka, A. O. (2021). Proanthocyanidin from Vitis vinifera attenuates memory impairment due to convulsive status epilepticus. Epilepsy Behav. 124, 108333. doi:10.1016/j.yebeh.2021.108333
Ozcelik, B., Aslan, M., Orhan, I., and Karaoglu, T. (2005). Antibacterial, antifungal, and antiviral activities of the lipophylic extracts of Pistacia vera. Microbiol. Res. 160 (2), 159–164. doi:10.1016/j.micres.2004.11.002
Panda, V., Mistry, K., Sudhamani, S., Nandave, M., and Ojha, S. K. (2017). Amelioration of abnormalities associated with the metabolic syndrome by Spinacia oleracea (spinach) consumption and aerobic exercise in rats. Oxid. Med. Cell. Longev. 2017, 2359389. doi:10.1155/2017/2359389
Parsa, A. (1959a). Medicinal plants and drugs of plant origin in Iran. I. Plant. Food. Hum. Nutr. 5, 375–394. doi:10.1007/BF01099755
Parsa, A. (1959b). Medicinal plants and drugs of plant origin in Iran II. Plant. Food. Hum. Nutr. 6, 69–96. doi:10.1007/BF01151968
Pasrsa, A. (1960). Medicinal plants and drugs of plant origin in Iran IV. Plant. Food. Hum. Nutr. 7, 65–136. doi:10.1007/BF01260413
Paterniti, I., Impellizzeri, D., Cordaro, M., Siracusa, R., Bisignano, C., Gugliandolo, E., et al. (2017). The anti-inflammatory and antioxidant potential of pistachios (Pistacia vera L.) in vitro and in vivo. Nutrients 9 (8), E915. doi:10.3390/nu9080915
Perera, H. D. S. M., Samarasekera, J. K. R. R., Handunnetti, S. M., Weerasena, O. V. D. S. J., Weeratunga, H. D., Jabeen, A., et al. (2018). In vitro pro-inflammatory enzyme inhibition and anti-oxidant potential of selected Sri Lankan medicinal plants. BMC Complement. Altern. Med. 18 (1), 1–15. doi:10.1186/s12906-018-2335-1
Pu, Y., and Pu, H. (2012). Research progress in biological activity of sesquiterpene compounds. Occup. Health 28 (18), 2291–2293. doi:10.13329/j.cnki.zyyjk.2012.18.043
Qian, Q., Cai, L., Yang, S., Shao, L., Gong, L., and Zhou, X. (2019). Chemical constituents from Carpesium abrotanoides. Chin. Tradit. Pat. Med. 41 (11), 2671–2675. doi:10.3969/j.issn.1001-1528.2019.11.023
Ramadan, S., Hegab, A. M., Al-Awthan, Y. S., Al-Duais, M. A., Tayel, A. A., and Al-Saman, M. A. (2021). Comparison of the efficiency of Lepidium sativum, Ficus carica, and Punica granatum methanolic extracts in relieving hyperglycemia and hyperlipidemia of streptozotocin-induced diabetic rats. J. Diabetes Res. 2021, 6018835. doi:10.1155/2021/6018835
Ren, Y., Song, X., Tan, L., Guo, C., Wang, M., Liu, H., et al. (2020). A review of the pharmacological properties of psoralen. Front. Pharmacol. 11, 571535. doi:10.3389/fphar.2020.571535
Rho, J., Seo, C. S., Park, H. S., Wijerathne, C. U., Jeong, H. Y., Moon, O. S., et al. (2019). Ulmus macrocarpa Hance improves benign prostatic hyperplasia by regulating prostatic cell apoptosis. J. Ethnopharmacol. 233, 115–122. doi:10.1016/j.jep.2018.11.042
Roberts, J. L., and Moreau, R. (2016). Functional properties of spinach (Spinacia oleracea L.) phytochemicals and bioactives. Food Funct. 7 (8), 3337–3353. doi:10.1039/c6fo00051g
Rokaya, M. B., Parajuli, B., Bhatta, K. P., and Timsina, B. (2020). Neopicrorhiza scrophulariiflora (pennell) hong: A comprehensive review of its traditional uses, phytochemistry, pharmacology and safety. J. Ethnopharmacol. 247, 112250. doi:10.1016/j.jep.2019.112250
Sadeghi, M., Kabiri, S., Amerizadeh, A., Heshmat-Ghahdarijani, K., Masoumi, G., Teimouri-Jervekani, Z., et al. (2021). Anethum graveolens L. (Dill) effect on human lipid profile: An updated systematic Review. Curr. Probl. Cardiol., 101072. doi:10.1016/j.cpcardiol.2021.101072
Saeed, S. A., Farnaz, S., Simjee, R. U., and Malik, A. (1993). Triterpenes and B-sitosterol from piper betle: Isolation, antiplatelet and anti-inflammatory effects. Biochem. Soc. Trans. 21 (4), 462S. doi:10.1042/bst021462s
Sarkar, M., Gangopadhyay, P., Basak, B., Chakrabarty, K., Banerji, J., Adhikary, P., et al. (2000). The reversible antifertility effect of Piper betle Linn. on Swiss albino male mice. Contraception 62 (5), 271–274. doi:10.1016/s0010-7824(00)00177-3
Sarkhail, P., Navidpour, L., Rahimifard, M., Hosseini, N. M., and Souri, E. (2020). Bioassay-guided fractionation and identification of wound healing active compound from Pistacia vera L. hull extract. J. Ethnopharmacol. 248, 112335. doi:10.1016/j.jep.2019.112335
Sengar, N., Joshi, A., Prasad, S. K., and Hemalatha, S. (2015). Anti-inflammatory, analgesic and anti-pyretic activities of standardized root extract of Jasminum sambac. J. Ethnopharmacol. 160, 140–148. doi:10.1016/j.jep.2014.11.039
Seo, M. G., Jo, M. J., Hong, N. I., Kim, M. J., Shim, K. S., Shin, E., et al. (2021). Anti-inflammatory and anti-vascular leakage effects by combination of Centella asiatica and Vitis vinifera L. Leaf extracts. Evid. Based. Complement. Altern. Med. 2021, 7381620. doi:10.1155/2021/7381620
Sharifi-Rad, J., Kamiloglu, S., Yeskaliyeva, B., Beyatli, A., Alfred, M. A., Salehi, B., et al. (2020). Pharmacological activities of psoralidin: A comprehensive review of the molecular mechanisms of action. Front. Pharmacol. 11, 571459. doi:10.3389/fphar.2020.571459
Shi, R. (2014). A textual research on spinach into China. Yuejiang Acad. J. 6 (1), 139–148. doi:10.13878/j.cnki.yjxk.2014.01.002
Sotoudeh, R., Hadjzadeh, M. A., Gholamnezhad, Z., and Aghaei, A. (2019). The anti-diabetic and antioxidant effects of a combination of Commiphora mukul, Commiphora myrrha and Terminalia chebula in diabetic rats. Avicenna J. Phytomed. 9 (5), 454–464.
Sun, Q., Leng, J., Tang, L., Wang, L., and Fu, C. (2021). A comprehensive review of the chemistry, pharmacokinetics, pharmacology, clinical applications, adverse events, and quality control of Indigo Naturalis. Front. Pharmacol. 12, 664022. doi:10.3389/fphar.2021.664022
Sun, R., Feng, J., Wu, M., and Liu, Y. (2022). Herbal textual research on benzoinum in famous classical formulas. Chin. J. Exp. Traditional Med. Formulae. doi:10.13422/j.cnki.syfjx.20220456
Tabrez, S., Rahman, F., Ali, R., Alouffi, A. S., Alshehri, B. M., Alshammari, F. A., et al. (2021). Assessment of the antileishmanial potential of Cassia fistula leaf extract. ACS Omega 6 (3), 2318–2327. doi:10.1021/acsomega.0c05629
Tamura, S., Miyoshi, A., Kawano, T., Horii, T., Itagaki, S., and Murakami, N. (2020). Structure-activity relationship of anti-malarial allylpyrocatechol isolated from Piper betle. Chem. Pharm. Bull. 68 (8), 784–790. doi:10.1248/cpb.c20-00294
Tan, J., Zheng, M., Duan, S., Zeng, Y., Zhang, Z., Cui, Q., et al. (2018). Chemical profiling and screening of the marker components in the fruit of Cassia fistula by HPLC and UHPLC/LTQ-Orbitrap MS(n) with chemometrics. Molecules 23 (7), E1501. doi:10.3390/molecules23071501
Tan, X., and Peng, Y. (2014). Study on the collation of foreign drugs included in Compendium of Materia Medica. J. Chin. Med. Mater. 37 (11), 2099–2102. doi:10.13863/j.issn1001-4454.2014.11.047
Tang, C., Chen, J., Zhou, Y., Ding, P., He, G., Zhang, L., et al. (2021). Exploring antimicrobial mechanism of essential oil of Amomum villosum Lour through metabolomics based on gas chromatography-mass spectrometry in methicillin-resistant Staphylococcus aureus. Microbiol. Res. 242, 126608. doi:10.1016/j.micres.2020.126608
Tao, L., Zhuo, Y. T., Qiao, Z. H., Li, J., Tang, H. X., Yu, Q. M., et al. (2021). Prenylated coumarins from the fruits of Artocarpus heterophyllus with their potential anti-inflammatory and anti-HIV activities. Nat. Prod. Res. 36, 2526–2533. doi:10.1080/14786419.2021.1913590
Teng, J. (2007). Study on the application of mineral medicine in ancient books of Materia Medica. Beijing: People's Medical Publishing House.
Thabit, S., Handoussa, H., Roxo, M., El Sayed, N. S., Cestari de Azevedo, B., and Wink, M. (2018). Evaluation of antioxidant and neuroprotective activities of Cassia fistula (L.) using the Caenorhabditis elegans model. PeerJ 6, e5159. doi:10.7717/peerj.5159
Tian, J. (2012). Studies on antifungal activity, mechanism against food spoilage fungi and development of a natural preservative of essential oil from dill (Anethum graveolens L.). Wuhan: Wuhan University.
Trautmann, T., Bang, C., Franke, A., Vincent, D., Reinshagen, K., and Boettcher, M. (2020). The impact of oral sodium chloride supplementation on thrive and the intestinal microbiome in neonates with small bowel ostomies: A prospective cohort study. Front. Immunol. 11, 1421. doi:10.3389/fimmu.2020.01421
Vikrama Chakravarthi, P., Murugesan, S., Arivuchelvan, A., Sukumar, K., Arulmozhi, A., and Jagadeeswaran, A. (2022). Therapeutic antigout and antioxidant activity of Piper betle L. in gout-induced broilers. Br. Poult. Sci. 63, 324–331. doi:10.1080/00071668.2021.1998365
Vinayagam, K. S., Khan, H. B., Keerthiga, G., Palanivelu, S., and Panchanatham, S. (2012). Hypolipidemic effect of Semecarpus anacardium in high cholesterol fed hypercholesterolemic rats. Chin. J. Integr. Med. doi:10.1007/s11655-012-1252-2
Wan, L. (2014). The originations of spinach and jasmine and their migrations to China. J. Xinyang Agric. Coll. 24 (3), 124–126. doi:10.1021/acsomega.1c05305
Wang, T., Wang, X., Zhuo, Y., Si, C., Yang, L., Meng, L., et al. (2020). Antiviral activity of a polysaccharide from Radix Isatidis (Isatis indigotica Fortune) against Hepatitis B virus (HBV) in vitro via activation of JAK/STAT signal pathway. J. Ethnopharmacol. 257, 112782. doi:10.1016/j.jep.2020.112782
Wang, W., Jeong, C., Lee, Y., Park, C., Oh, E., Park, K. H., et al. (2022). Flavonoid glycosides from Ulmus macrocarpa inhibit osteoclast differentiation via the downregulation of NFATc1. ACS Omega 7 (6), 4840–4849. doi:10.1021/acsomega.1c05305
Wedenoja, S., Örmälä, T., Berg, U. B., Halling, S. F. E., Jalanko, H., Karikoski, R., et al. (2008). The impact of sodium chloride and volume depletion in the chronic kidney disease of congenital chloride diarrhea. Kidney Int. 74 (8), 1085–1093. doi:10.1038/ki.2008.401
Wintola, O. A., and Afolayan, A. J. (2014). The foliar anatomy and micromorphology of Aloe ferox Mill. (Asphodelaceae). Afr. J. Tradit. Complement. Altern. Med. 11 (2), 350–357. doi:10.4314/ajtcam.v11i2.21
Wong, L. W., Goh, C. B. S., and Tan, J. B. L. (2022). A systemic review for ethnopharmacological studies on Isatis indigotica fortune: Bioactive compounds and their therapeutic insights. Am. J. Chin. Med. 50 (1), 161–207. doi:10.1142/S0192415X22500069
Wu, G. (2019). A historical investigation on the spread of the name and concept of the silk road. Acad. Mon. 51 (5), 145–167. doi:10.19862/j.cnki.xsyk.2019.05.014
Wu, G., Dong, Z., Dong, J., Wei, L., Shi, R., Kang, S., et al. (2020a). Effects of Mongolian medicine Terminalia chebula Retz. on 6 CYP450 enzymes in rats. Int. J. Clin. Exp. Pathol. 13 (12), 3128–3138.
Wu, Q., He, X., Zhou, S., Shi, F., and Lu, Y. (2020b). Role of PEPT1in the transport of cinnabar in Caco-2 cells. Toxicol. Vitro 63, 104747. doi:10.1016/j.tiv.2019.104747
Xia, K., Yang, T., An, L. Y., Lin, Y. Y., Qi, Y. X., Chen, X. Z., et al. (2020). The relationship between pistachio (Pistacia vera L) intake and adiposity: A systematic review and meta-analysis of randomized controlled trials. Med. Baltim. 99 (34), e21136. doi:10.1097/MD.0000000000021136
Xiao, L., and Yu, S. (1986). Introduction of Foreign Drugs and development of traditional Chinese medicine. Fujian J. Traditional Chin. Med. (5), 56–57. doi:10.13260/j.cnki.jfjtcm.003760
Xiao, S., Yu, H., Xie, Y., Guo, Y., Fan, J., and Yao, W. (2021). The anti-inflammatory potential of Cinnamomum camphora (L.) J.Presl essential oil in vitro and in vivo. J. Ethnopharmacol. 267, 113516. doi:10.1016/j.jep.2020.113516
Xie, Q., Li, J., Dong, T., Yuan, J., Lu, D., Ma, R., et al. (2022). Neuroprotective effects of synthetic borneol and natural borneol based on the neurovascular unit against cerebral ischaemic injury. J. Pharm. Pharmacol. 74 (2), 236–249. doi:10.1093/jpp/rgab167
Yang, Z., Wang, Y., Zheng, Z., Zhao, S., Zhao, J., Lin, Q., et al. (2013). Antiviral activity of Isatis indigotica root-derived clemastanin B against human and avian influenza A and B viruses in vitro. Int. J. Mol. Med. 31 (4), 867–873. doi:10.3892/ijmm.2013.1274
Yang, L., Guo, P., Liu, A., Sui, S., Shi, S., Guo, S., et al. (2019). Aquilariaenes AH, eight new diterpenoids from Chinese eaglewood. Fitoterapia 133, 180–185. doi:10.1016/j.fitote.2019.01.010
Ye, B., Wang, S., and Zhang, L. (2011). Studies on the detoxification effects and acute toxicity of a mixture of cis-sec-butyl-1-propoenyl disulphide and trans-sec-butyl-1-propoenyl disulphide isolated from crude essential oil of Ferula sinkiangensis KM Shen, a Chinese traditional herbal medicine. Nat. Prod. Res. 25 (12), 1161–1170. doi:10.1080/14786419.2010.550027
Yeh, Y. T., Chiang, A. N., and Hsieh, S. C. (2017). Chinese olive (Canarium album L.) fruit extract attenuates metabolic dysfunction in diabetic rats. Nutrients 9 (10), E1123. doi:10.3390/nu9101123
Yin, Y., Huang, X., Wang, J., Dai, J., Liang, H., and Dai, Y. (2009). Studies on the chemical constituents of the stems of Piper betle. J. Chin. Med. Mater. 32 (6), 887–890. doi:10.13863/j.issn1001-4454.2009.06.050
Yin, H., Dan, W. J., Fan, B. Y., Guo, C., Wu, K., Li, D., et al. (2019). Anti-inflammatory and alpha-glucosidase inhibitory activities of labdane and norlabdane diterpenoids from the rhizomes of Amomum villosum. J. Nat. Prod. 82 (11), 2963–2971. doi:10.1021/acs.jnatprod.9b00283
You, D., Jeong, Y., Yoon, S. Y., Kim, S. A., Lo, E., Kim, S. W., et al. (2021). Entelon((R)) (Vitis vinifera seed extract) prevents cancer metastasis via the downregulation of interleukin-1 alpha in triple-negative breast cancer cells. Molecules 26 (12), 3644. doi:10.3390/molecules26123644
Yu, H., Li, T. N., Ran, Q., Huang, Q. W., and Wang, J. (2021). Strobilanthes cusia (nees) Kuntze, a multifunctional traditional Chinese medicinal plant, and its herbal medicines: A comprehensive review. J. Ethnopharmacol. 265, 113325. doi:10.1016/j.jep.2020.113325
Yue, J., Zhang, S., Zheng, B., Raza, F., Luo, Z., Li, X., et al. (2021). Efficacy and mechanism of active fractions in fruit of Amomum villosum lour. For gastric cancer. J. Cancer 12 (20), 5991–5998. doi:10.7150/jca.61310
Zhan, J., Lu, S., Qu, G., Meng, Z., Yang, L., Cao, Q., et al. (2007). Analysis volatile components of dill by GC-MS. Fine Chem. 24 (8), 805–808. doi:10.13550/j.jxhg.2007.08.016
Zhang, L., Wang, J., Wang, L., Huang, D., and Zheng, X. (2014). The progress of Anxixiang research. Pharm. Clin. Chin. Materia Medica 5 (3), 61–64.
Zhang, H., Lu, J., Zhou, L., Jiang, L., and Zhou, M. (2015). Antioxidant and antitumor effects of ferula sinkiangensis K. M. Shen. Int. J. Clin. Exp. Med. 8 (11), 20845–20852.
Zhang, G., Yan, X., Xia, J., Zhao, J., Ma, M., Yu, P., et al. (2021). Assessment of the effect of ethanol extracts from Cinnamomum camphora seed kernel on intestinal inflammation using simulated gastrointestinal digestion and a Caco-2/RAW264.7 co-culture system. Food Funct. 12 (19), 9197–9210. doi:10.1039/d1fo01293b
Zhang, Z., and Zheng, J. (2016). Rethinking of study on Compendium of Materia Medica. J. Traditional Chin. Med. 57 (22), 1896–1900. doi:10.13288/j.11-2166/r.2016.22.002
Zhao, H., and Zhang, R. (2015). Herbalogical textual research of Wuyi. China J. Chin. Materia Medica 40 (22), 4510–4513. doi:10.4268/cjcmm20152234
Zhou, B., Yang, Z., Feng, Q., Liang, X., Li, J., Zanin, M., et al. (2017). Aurantiamide acetate from baphicacanthus cusia root exhibits anti-inflammatory and anti-viral effects via inhibition of the NF-κB signaling pathway in Influenza A virus-infected cells. J. Ethnopharmacol. 199, 60–67. doi:10.1016/j.jep.2017.01.038
Zhu, K., Fan, H., Zeng, S., Nie, S., Zhang, Y., Tan, L., et al. (2021). Polysaccharide from Artocarpus heterophyllus Lam. (jackfruit) pulp modulates gut microbiota composition and improves short-chain fatty acids production. Food Chem. 364, 130434. doi:10.1016/j.foodchem.2021.130434
Zhuang, C., and Ling, Y. (1980). Textual research on exotic drugs in past dynasties. J. Chengdu Univ. Traditional Chin. Med. (6), 1–5.
Zin, N. N. I. N. M., Rahimi, W. N. A. W. M., and Bakar, N. A. (2019). A review of Quercus infectoria (olivier) galls as a resource for anti-parasitic agents: In vitro and in Vivo studies. Malays. J. Med. Sci. 26 (6), 19–34. doi:10.21315/mjms2019.26.6.3
Keywords: Compendium of Materia Medica, Persian, Iran, Chinese herbal medicine, the Silk Road, traditional Chinese medicine
Citation: Shi J, Yang Y, Zhou X, Zhao L, Li X, Yusuf A, Hosseini MSMZ, Sefidkon F and Hu X (2022) The current status of old traditional medicine introduced from Persia to China. Front. Pharmacol. 13:953352. doi: 10.3389/fphar.2022.953352
Received: 26 May 2022; Accepted: 24 August 2022;
Published: 14 September 2022.
Edited by:
Gokhan Zengin, Selcuk University, TurkeyReviewed by:
Md Aziz Rahman, Rajshahi University, BangladeshSengul Uysal, Erciyes University, Turkey
Copyright © 2022 Shi, Yang, Zhou, Zhao, Li, Yusuf, Hosseini, Sefidkon and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuebo Hu, xuebohu@mail.hzau.edu.cn
†These authors contribute equally to the work
 Jinmin Shi
Jinmin Shi