
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pharmacol., 08 September 2022
Sec. Drug Metabolism and Transport
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.952804
 Zekang Ye1†
Zekang Ye1† Pengsheng Chen1,2†
Pengsheng Chen1,2† Chuchu Tan1†
Chuchu Tan1† Xiaoxuan Gong1†
Xiaoxuan Gong1† Ran Li1
Ran Li1 Zhou Dong1
Zhou Dong1 Inam Ullah1
Inam Ullah1 Chen Zhou3
Chen Zhou3 Sufeng Zhou3
Sufeng Zhou3 Lijun Xie3
Lijun Xie3 Xuemei Hou4
Xuemei Hou4 Zhihui Han4
Zhihui Han4 Qian Gu1
Qian Gu1 Jiazheng Ma1
Jiazheng Ma1 Jianzhen Teng1
Jianzhen Teng1 Yingdan Tang5
Yingdan Tang5 Zhuanxia Zhang4
Zhuanxia Zhang4 Haitang Hu4
Haitang Hu4 Quankun Zhuang3
Quankun Zhuang3 Juan Chen3
Juan Chen3 Bei Zhu3
Bei Zhu3 Feng Shao3,6*
Feng Shao3,6* Chunjian Li1*
Chunjian Li1*Background: Previous studies have suggested that proton pump inhibitors could impair the antiplatelet effect of clopidogrel. It is uncertain whether ilaprazole affects the antiplatelet effect of clopidogrel. This study aimed to determine the drug-drug interaction between ilaprazole and clopidogrel.
Methods: A randomized crossover trial of 40 healthy subjects was performed. Clopidogrel was administered alone or in combination with ilaprazole for 7 days. The maximal platelet aggregation (MPA) to 5 μmol/L adenosine diphosphate was measured by light transmission aggregometry and the platelet reactivity index (PRI) was determined by vasodilator-stimulated phosphoprotein P2Y12 assay. High on-treatment platelet reactivity (HOPR) was defined as a MPA of >40%. The inhibition of platelet aggregation (IPA) and PRI in the two phases were compared between two regimens after the last dosing.
Results: IPA was comparable between the two regimens at 0, 10 and 24 h (p > 0.05), but higher at 4 h in the clopidogrel alone regimen compared with that in the combined treatment regimen (75.66 ± 18.44% vs. 70.18 ± 17.67%, p = 0.031). The inhibition of PRI was comparable between the two regimens at 0 and 24 h. There were no significant differences in the area under the time-IPA% curve (AUC) or the incidence of HOPR at all time-points between the two regimens.
Conclusion: In healthy subjects, ilaprazole has limited effect on the pharmacodynamics of clopidogrel and it may not be clinically relevant.
Clinical Trial Registration: [www.chictr.org.cn], identifier [ChiCTR2000031482].
The introduction of clopidogrel was a milestone in the development of antiplatelet therapy. Dual antiplatelet therapy with clopidogrel and aspirin is associated with a significant reduction of adverse cardiovascular events in patients with coronary atherosclerosis disease, especially in those undergoing percutaneous coronary intervention (Bhatt et al., 2006). Clopidogrel is a prodrug that needs to be metabolized through the cytochrome P450 (CYP450) system to exert antiplatelet activity. CYP450 family 2 subfamily C member 19 genotypes (CYP2C19) contributes predominantly to the bioactivation of clopidogrel and consequently affects its therapeutic response (Kazui et al., 2010; Cattaneo, 2012).
Proton pump inhibitors (PPIs) have been adopted to prevent and treat gastrointestinal bleeding in patients on dual antiplatelet therapy with clopidogrel and aspirin (Levine et al., 2016). However, PPIs, such as omeprazole and esomeprazole, are mainly metabolized via CYP2C19 and CYP3A4 isoenzymes, which competitively affect the metabolism of clopidogrel (Li et al., 2004). Omeprazole can cause a 40% reduction of the clopi-H4 (the active clopidogrel metabolite) and consequently a significantly decreased antiplatelet effect while administered in combination of clopidogrel (Angiolillo et al., 2011). The U.S. Food and Drug Administration has issued warnings to concomitant use of omeprazole or esomeprazole with clopidogrel due to the drug-drug interaction (FDA, 2011; Scott et al., 2013).
Ilaprazole, the latest generation of benzimidazole PPI, is non-inferior to the traditional PPIs in inhibiting gastric acid secretion (Wang et al., 2019; Fan et al., 2019; Wang et al., 2011). The in vitro microsome tests have shown that ilaprazole is mainly metabolized by non-enzymatic degradation and partially by CYP3A4, but hardly by CYP2C19, which is significantly different from the current PPIs (Wang et al., 2011; Cho et al., 2012; Seo et al., 2012; Pu et al., 2018). It is unknown yet whether ilaprazole could interfere with the pharmacodynamics of clopidogrel. This study was designed to determine the impact of ilaprazole on the antiplatelet effect of clopidogrel in healthy volunteers.
This is an open-label randomized crossover study to assess the impact of ilaprazole on the antiplatelet effect of clopidogrel in healthy volunteers. Subjects were enrolled in the First Affiliated Hospital of Nanjing Medical University from 3 March 2021, to 5 June 2021. This study was registered at www.chiwww.chictr.org.cn (Unique Identifier: ChiCTR2000031482), which complied with the Declaration of Helsinki (64th, 2013) and was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (Approval Number: 2020-MD-030). All participants signed the consent form.
Healthy volunteers were screened according to the inclusion and exclusion criteria. The inclusion criteria were as follows: 1) subjects aged between 18 and 55 years; 2) subjects weighted ≥50 kg in males and ≥45 kg in females, with body mass index between 19 and 26 kg/m2; 3) subjects in healthy status assessed by medical history, laboratory test, electrocardiogram, and physical exam (Supplementary Table S1). The exclusion criteria were as follows: 1) allergic to the study drug; 2) abusing drug within 12 months or using drugs within the last 2 weeks; 3) intake of caffeine or xanthine for 48 h; 4) frequent smoking or drinking alcohol for 3 months; 5) pregnant and lactating women, or subjects who plan to give birth within 6 months; 6) with dysphagia or any gastrointestinal diseases; 7) participating in clinical studies within 3 months before screening; 8) with other conditions that made them unsuitable to be recruited at the discretion of the investigators. Subjects were required to avoid caffeine, alcohol, smoking, heavy exercise or any diet that could interfere with study drugs during the study.
We first enrolled 4 subjects for the trial test (Supplementary Figure S1), which was to evaluate the feasibility of the regimens and collect the preliminary data to calculate the sample size for the formal study. Four eligible subjects were recruited to receive clopidogrel 75 mg once daily for 7 days, and then clopidogrel 75 mg in combination with ilaprazole 10 mg once daily for 7 days (first Regimen A, then Regimen B; n = 2), or vice versa (first Regimen B, then Regimen A; n = 2), with a 10-day interval between the two regimens. Patients was allocated in different regimens as shown in Supplementary Figure S1.
The SAS software, version 9.4 (SAS Institute Inc., Cary, NC, United States) was used to program the randomization algorithm based on the blocked randomization (block size = 4, two arms) by Shanghai Zenith Data Technology Co., Ltd. After screening, 40 subjects were enrolled from 150 healthy volunteers, and randomized in a 1:1 ratio into two groups of AB and BA according to the cross-over design and the predetermined computer-generated random sequence (Figure 1). The drug doses and dosing regimens in the formal study were the same as the trial test. All participants were hospitalized in the clinical trial ward during the medication period (Figure 1).
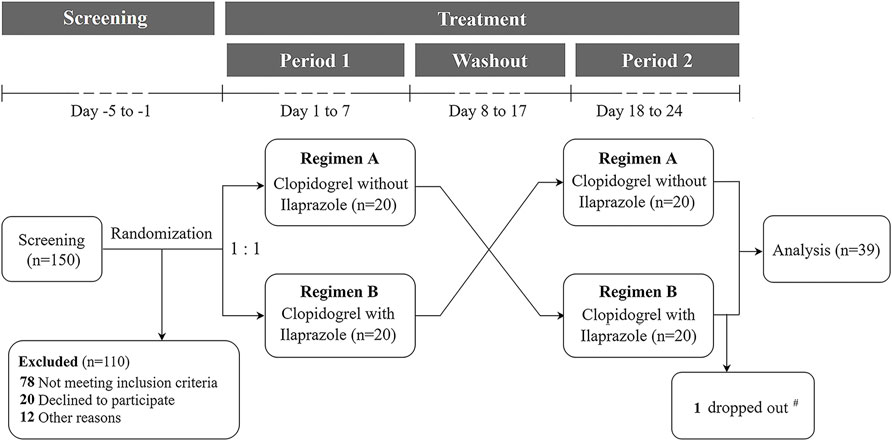
FIGURE 1. Flow diagram of the formal study. 150 subjects were screened and 40 were randomized in a 1:1 ratio into two protocols to receive clopidogrel 75 mg once daily for 7 days, and then clopidogrel 75 mg in combination with ilaprazole 10 mg once daily for 7 days (first Regimen A, then Regimen B; n = 20), or vice versa (first Regimen B, then Regimen A; n = 20), with a 10-day interval between the two regimens. # One subject dropped out on Day 18 after the first dosing of Regimen B.
Clopidogrel (PLAVIX®, 75 mg one tablet) was purchased from Sanofi Aventis (Paris, France), and ilaprazole (YILIAN®, 10 mg one tablet) was provided by Livzon Pharmaceutical Group Inc (Zhuhai, China). During the study period, all the drugs were stored in a dark environment of no more than 25°C under designated surveillance. After at least 10 h of fasting, the study drugs were orally administered with 240 ml of warm water in a sitting position at 8 a.m. It was confirmed that the drug had been properly taken by examining the patient’s oral cavity and drug containers. Subjects were not allowed to take other drugs during the study period except when adverse events happened and proper drugs were needed.
Venous blood was collected into three 2.7 ml vacutainer tubes (Becton, Dickinson and Company, Franklin Lakes, United States) containing 0.105 M buffered sodium citrate (3.2%) during the trial test as well as the formal study. The samples were collected before dosing (baseline) and at 0, 4, 10, and 24 h after the last dose of the study drug in each phase. Blood samples were transported, avoiding vigorous shaking, for platelet function assays in a thermostat (20–25°C).
Baseline venous blood was also collected for genotyping into a 2 ml EDTA tube which was frozen at -80 ± 10°C for further analysis.
Platelet aggregation tests were performed by two investigators using a Chrono-log Model 700 aggregometer (Chrono-log Corporation, Havertown, PA). Each light transmission aggregation test was completed within 3 h of blood collection. Platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were prepared shortly after blood collection by spinning the sample at 200 g for 5 min in the centrifuge machine. The PRP was carefully removed, and the remaining blood was centrifuged at 2,465 g for 10 min to obtain PPP. The centrifuge temperature was maintained at 22°C. Platelet counts were adjusted by adding PPP to the PRP to achieve a count of 250 × 109/L. Then, 500 μL adjusted PRP was transferred into a test tube, and a 500 μL PPP was set as a control. ADP (with a final concentration of 5 μmol/L) was used as an agonist to induce platelet aggregation. The maximal platelet aggregation (MPA) rates were recorded within 8 min (Pi et al., 2019), and the inhibition of platelet aggregation (IPA) was calculated as follows:
The vasodilator-stimulated phosphoprotein (VASP) P2Y12 assay (Biocytex, Marseille, France) was performed using sodium citrate anticoagulated whole blood as per manufacturer instructions and was stored overnight at 25°C. The analysis was finished within 24 h of blood collection. The blood samples were incubated with PGE1 alone or with PGE1 and ADP simultaneously. After cellular permeabilization, VASP was labeled by indirect no-wash immunofluorescence using a specific monoclonal antibody (clone 16C2). Platelets were identified by flow cytometry in a FACSCalibur flow cytometer (Becton Dickinson), and the level of VASP-Ser239P was simultaneously determined by 16C2-FITC mean fluorescence intensity (MFI). The following formula was applied to calculate the platelet reactivity index (PRI) using corrected MFI (MFIc):
To compare the pharmacodynamic differences in different CYP2C19 genotypes when clopidogrel was co-administered alone or with ilaprazole, blood samples were transferred through the cold chain at -80 ± 10°C to Suzhou Hongxun Biotechnologies Co., LTD. The CYP2C19*2 (681, G > A) and CYP2C19*3 (636, G > A) were genotyped using Sanger sequencing via PCR amplification on an ABI 3730xl DNA Analyzer (Applied Biosystems) (Wang et al., 2021). Subjects with different CYP2C19 gene polymorphisms were defined as extensive metabolizers (EMs, CYP2C19*1/*1), intermediate metabolizers (IMs, CYP2C19*1/*2 and *1/*3) and poor metabolizers (PMs, CYP2C19*2/*2, *2/*3 and *3/*3) (Zhang et al., 2020).
Referring to the similar research on drug-drug interaction between clopidogrel and other PPIs (Funck-Brentano et al., 2013; Frelinger et al., 2012), the inter-group variation of IPA between regimens of clopidogrel alone and the combination of clopidogrel and omeprazole was about 15%. We presumed that the difference of IPA between the two regimens was 8.6% based on the results from the trial test. A total sample size of 36 participants (18 per group) was calculated to detect the prespecified effect size at a two-sided 0.05 significance level and a power of 90%. The sample size was adjusted for an anticipated 10% drop-out rate yielding a final sample size of 40 participants.
Statistical analysis was performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, United States). Continuous variables were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR) when data did not follow a normal distribution. Categorical variables were presented as frequencies and percentages. Subjects who participated in the formal trial were included in the final analysis according to the per-protocol set (PPS).
A generalized linear mixed-model approach was used to compare the pharmacodynamic indexes between the two treatment regimens. The estimated treatment difference, 95% confidential interval (CI) and p-value were adjusted with sequence, phase, and treatment as fixed factors, and subjects within the sequence as a random factor.
The sensitivity analysis was performed by adding the gender and CYP2C19 metabolizer into the generalized linear mixed-model. To evaluate the influence of gender and CYP2C19 metabolizer on the interaction between clopidogrel and ilaprazole, the interactions of gender, CYP2C19 metabolizer and treatment regimen were included in the generalized linear mixed-model.
Multilevel logistic regression models were used to compare the incidence of HOPR between the two treatment regimens. Pre-planned subgroup analysis was used to compare the effects of ilaprazole on clopidogrel among different CYP2C19 genotypes.
A two-sided p value of <0.05 was considered statistically significant for all analyses.
A total of 150 volunteers were consecutively screened, of whom 40 subjects who met the inclusion and exclusion criteria were included and randomized into the two study regimens. One subject dropped out the study on Day 18 after the first dosing of Regimen B. As a result, 39 subjects completed the study and were included in the final analyses (Figure 1). The demographic characteristics of the included subjects are shown in Table 1.
Baseline MPA was comparable between the two regimens of clopidogrel alone and clopidogrel plus ilaprazole (59.38 ± 21.69% vs. 64.97 ± 20.82%, p = 0.085). After taking the study drugs for 7 days, the MPA was significantly lower in the clopidogrel alone regimen compared with clopidogrel plus ilaprazole regimen at 0 h (19.21 ± 11.30% vs. 23.67 ± 12.99%, p = 0.001), 4 h (14.36 ± 10.22% vs. 18.49 ± 10.08%, p < 0.001), 10 h (16.51 ± 11.26% vs. 19.62 ± 10.83%, p < 0.001) and 24 h (19.28 ± 12.47% vs. 22.62 ± 11.73%, p < 0.001) (Supplementary Table S2).
The IPA was significantly higher at 4 h after 7-day administration of clopidogrel compared to coadministration of clopidogrel and ilaprazole (75.66 ± 18.44% vs. 70.18 ± 17.67%, p = 0.031). However, the IPA levels were comparable at 0 h (67.28 ± 16.80% vs. 62.88 ± 18.51%, p = 0.082), 10 h (72.12 ± 18.27% vs. 68.74 ± 17.14%, p = 0.183) and 24 h (66.96 ± 12.23% vs. 63.76 ± 19.97%, p = 0.181) between the two regimens (Figure 2; Supplementary Table S2).
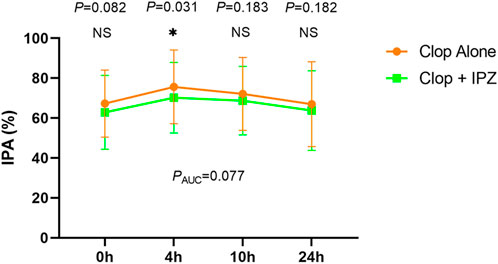
FIGURE 2. IPA-time curve after 7-day treatment of the two regimens. Data are expressed as the mean ± SD (n = 39). IPA = inhibition of platelet aggregation; Clop = clopidogrel; IPZ = ilaprazole. NS = not significant. AUC = the area under the time-IPA% curve. * represents p < 0.05.
There was no significant difference in the area under the time-IPA% curve (AUC) between the two regimens (1702.78 ± 430.75% h vs. 1,610.42 ± 410.29% h, PAUC = 0.077) (Figure 2). Besides, the incidences of HOPR were not significantly different between the two regimens at 0, 4, 10 and 24 h after 7-day administration of the study drugs (Table 2).
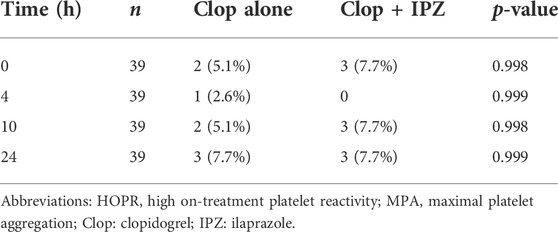
TABLE 2. HOPR status on basis of MPA after the regimen of clopidogrel alone compared with coadministration of clopidogrel with ilaprazole.
Before administration of the study drugs, baseline PRI was not significantly different between the clopidogrel alone and the coadministration regimens (91.16 ± 3.55% vs. 90.68 ± 3.79%, p = 0.527). The PRI was significantly lower in the regimen of clopidogrel alone compared to coadministration of clopidogrel and ilaprazole at 4 h (54.69 ± 22.21% vs. 59.60 ± 21.15%, p = 0.003) and 10 h (56.25 ± 19.95% vs. 61.38 ± 18.76%, p = 0.006). However, PRI was not statistically different between the two regimens at 0 h (63.22 ± 18.17% vs. 65.52 ± 19.58%, p = 0.527) and 24 h (60.33 ± 17.85% vs. 62.87 ± 17.54%, p = 0.076) (Table 3).
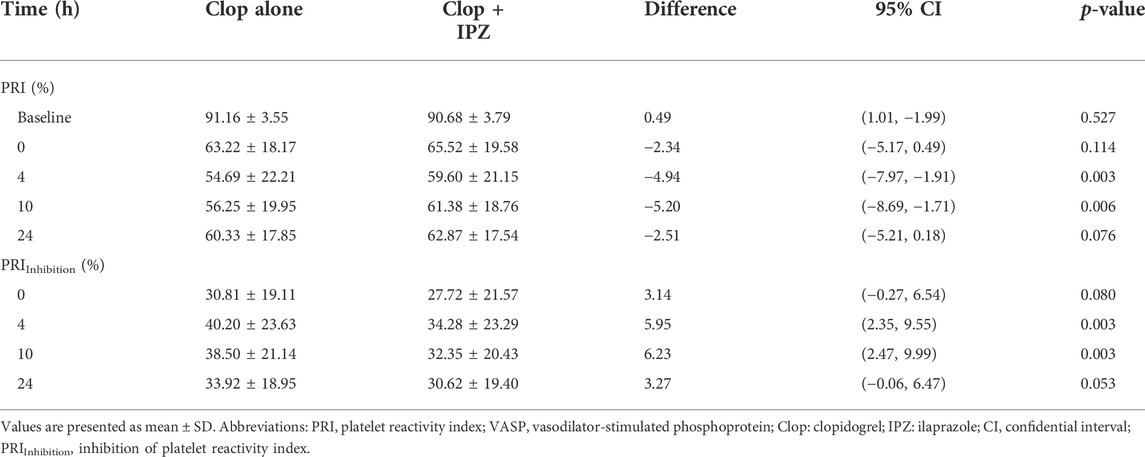
TABLE 3. PRI and PRIInhibition by VASP P2Y12 assay in subjects under clopidogrel treatment with or without ilaprazole.
Further analysis shows that PRIInhibition was significantly higher after 7-day administration of clopidogrel compared to coadministration of clopidogrel and ilaprazole at 4 h (40.20 ± 23.63% vs. 34.28 ± 23.29%, p = 0.003) and 10 h (38.50 ± 21.14% vs. 32.35 ± 20.43%, p = 0.003). However, the levels of PRIInhibition were similar at 0 h (30.81 ± 19.11% vs. 27.72 ± 21.57%, p = 0.080) and 24 h (33.92 ± 18.95% vs. 30.62 ± 19.40%, p = 0.053) in the two regimens (Table 3).
The CYP2C19 genotype analysis showed that there were 9 (23%) EMs, 22 (56.4%) IMs and 8 (20.5%) PMs in the participants. As the interaction between ilaprazole and clopidogrel was significant at 4 h after medication for both IPA and PRIInhibition (Supplementary Table S2; Table 3), the pharmacodynamics of clopidogrel at this timepoint were selected for the subgroup analysis by different CYP2C19 genotypes.
In IMs, the IPA was significantly higher at 4 h after medication in the clopidogrel alone regimen than that in the combination regimen (76.84 ± 13.66% vs. 71.49 ± 15.63%, p = 0.044). However, the IPA were comparable between the two regimens in both EMs (88.58 ± 13.20% vs. 76.53 ± 17.66%, p = 0.084) and PMs (57.85 ± 22.49% vs. 59.42 ± 20.42%, p = 0.573) (Figure 3; Supplementary Table S3).
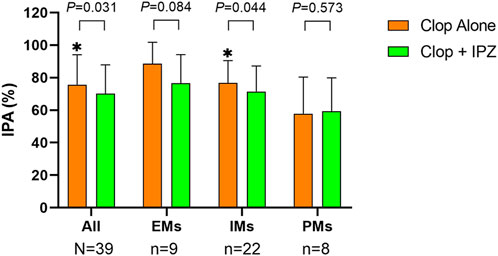
FIGURE 3. IPA at 4 h after 7-day treatment in different CYP2C19 genotypes. Upper boundaries of boxes represent means; upper whiskers represent standard deviations of IPA. IPA = inhibition of platelet aggregation; Clop = clopidogrel; IPZ = ilaprazole; EMs = extensive metabolizers (CYP2C19*1/*1); IMs = intermediate metabolizers (CYP2C19*1/*2 and *1/*3); PMs = poor metabolizers (CYP2C19*2/*2, *2/*3 and *3/*3). * represents p < 0.05.
PRIInhibition was significantly higher at 4 h in the regimen of clopidogrel alone compared to coadministration of clopidogrel and ilaprazole in both EMs (62.99 ± 18.58% vs. 52.69 ± 24.93%, p = 0.036) and IMs (38.14 ± 20.95% vs. 32.70 ± 21.46%, p = 0.037). However, it was comparable between the two regimens (20.20 ± 13.46% vs. 17.91 ± 10.18%, p = 0.251) in PMs (Supplementary Table S3).
After adjusted for gender and CYP2C19 metabolizer, the difference of IPA at 4 h (p = 0.031) as well as the difference of PRIInhibition at 4 h (p = 0.003) and 10 h (p = 0.003) between the two regimens kept statistically significant. However, only CYP2C19 metabolizer was significantly related to the IPA at 4 h (p = 0.014) and PRIInhibition at 4 h (p = 0.0004) and 10 h (p = 0.0006), while gender was not significantly related to either the IPA at 4 h (p = 0.402), or PRIInhibition at 4 h (p = 0.598) and 10 h (p = 0.934).
After adding gender, CYP2C19 metabolizer, and the interactions between these two factors and treatment regimen in the generalized linear mixed-model, the interactions between gender (p = 0.127), metabolizer (p = 0.069) and treatment regimen were not statistically significant for IPA at 4 h. Similarly, the interactions between gender, metabolizer and treatment regimen were also not significant for PRIInhibition at 4 h (p = 0.135 for gender and 0.252 for metabolizer) and 10 h (p = 0.135 for gender and 0.195 for metabolizer).
To the best of our knowledge, this is the first prospective study investigating the drug-drug interaction between clopidogrel and ilaprazole. We found that, after being treated for 7 days, the IPAs were comparable between the two regimens at all time-points of 0, 10 and 24 h, except that at 4 h.
Regarding the study design, the routine recommended doses of both clopidogrel and ilaprazole were adopted. Referring to previous studies (Thebault et al., 1999; Li et al., 2012; Shin et al., 2014), 7-day treatment and 10-day interval were chosen. As this study aimed to investigate the drug-drug interaction of clopidogrel and ilaprazole, healthy volunteers instead of patients were recruited, and the laboratory endpoint of IPA instead of clinical events was set as the primary endpoint.
After baseline adjustment, no statistical difference of IPAs was found between the two regimens at all time-points except that at 4 h. Besides, the areas under the time-IPA% curves were comparable between the two regimens. These results indicated that the interaction between clopidogrel and ilaprazole was limited. Our study revealed that the difference of IPA at 4 h was 5.52%, which was less than the impact of other PPIs reported in previous studies (Angiolillo et al., 2011; Frelinger et al., 2012). Frelinger, A.L. et al. Found that the decrease in IPA was 12.9%, 11.6% respectively at 24 h after 9 days of coadministration of omeprazole or esomeprazole with clopidogrel (Frelinger et al., 2012). Another study by Angiolillo, D.J. et al. reported that the IPA decreased by 7.7% at 2, 4 and 6 h after 5-day coadministration of clopidogrel and pantoprazole compared with clopidogrel alone (Angiolillo et al., 2011).
The MPAs were statistically higher at all time-points in the coadministration regimen compared with those in the clopidogrel alone regimen. However, a difference of MPA>10% was suggested to be the indication of clinically relevant effect in other studies (Angiolillo et al., 2011; Hochholzer et al., 2006). By comparison, none of the MPA difference was beyond this range in our study, which suggested that the differences of MPAs caused by ilaprazole might have no clinical impact.
The differences of PRIs between the two regimens were statistically significant at 4 h [−4.94 (−7.97, −1.91)] and 10 h [−5.20 (−8.69, −1.71)], respectively. However, as the equivalence range of (−15%, 15%) for PRI was recommended previously (Frelinger et al., 2012), our results suggested a limited impact of ilaprazole on the antiplatelet effect of clopidogrel.
It should be noted that HOPR has been regarded as a risk factor of major adverse cardiovascular events in coronary atherosclerosis disease patients (Ying et al., 2021). Our results demonstrated that the incidence of HOPR was not significantly increased in the coadministration regimen at any time-point. Besides, no significant increase of major adverse cardiovascular events was demonstrated when greater degrees of drug-drug interaction existed between other PPIs and clopidogrel (Bhatt et al., 2010; Lin et al., 2012).
Studies have investigated the interactions between multiple PPIs and clopidogrel. Lin SF and Przespolewski et al. found no interactions between lansoprazole, esomeprazole, pantoprazole, rabeprazole and clopidogrel in Asian patients or healthy male participants (Lin et al., 2020; Przespolewski et al., 2018). However, it was found that omeprazole and dexlansoprazole could affect the anti-platelet effect of clopidogrel (Lin et al., 2020; Furtado et al., 2016). Up to date, only one latest study retrospectively investigated the interaction between clopidogrel and ilaprazole in acute stroke patients, which proved that the combination therapy of ilaprazole does not interfere with the metabolism of clopidogrel [Lim et al., 2022]. It could be concluded that no robust interaction between clopidogrel and PPIs was found. Our results add data to a growing body of evidence indicating that the addition of a PPI may have a weak effect on clopidogrel’s antiplatelet properties, which may not be clinically relevant.
Our study proved that the CYP2C19 metabolizers were significantly related to the pharmacodynamics of clopidogrel, which was consistent with the previous study results (Xu et al., 2019). However, the interaction analysis demonstrated that the CYP2C19 metabolizers had no influence on the interaction between ilaprazole and clopidogrel. Additionally, we found that gender had neither significant effect on the pharmacodynamics of clopidogrel nor the interaction between ilaprazole and clopidogrel.
It is well known that about 50% of clopidogrel is absorbed from the intestine after administration. Once delivered to the liver, a number of CYP450 enzymes, including CYP2C19, CYP1A2, CYP2B6, CYP2C9 and CYP3A4, mediate the bioactivation of clopidogrel via a two-step process (Jiang et al., 2015). CYP2C19 contributes to the two steps of clopidogrel metabolism by 45%, 20% respectively, in which clopidogrel is metabolized to 2-oxo-clopidogrel and active metabolite (Kazui et al., 2010). Ilaprazole, however, is not metabolized by CYP2C19, but by non-enzymatic sulfoxide and partially oxidized by CYP3A4, which contributes to the second step of clopidogrel metabolism by 40% (Kazui et al., 2010; Pu et al., 2018; Funck-Brentano et al., 2013). Our study showed that ilaprazole caused changes in the pharmacodynamics of clopidogrel in a certain degree at some time-points. However, the underlying mechanism may not be related to CYP2C19. The real mechanism remains to be further clarified.
This was an open-label randomized crossover study, which has been carefully designed to control possible baseline conditions affecting clopidogrel and/or PPI metabolism. During the treatment period, subjects were hospitalized and confined to receive a uniform diet and refrained from factors that might affect or compromise clopidogrel’s efficacy, including caffeine, alcohol, smoking, and strenuous exercise.
First, no other PPIs were set as controls to compare the impacts of ilaprazole and other PPIs on the pharmacodynamics of clopidogrel. Second, our study was confined to young healthy subjects and the results need further validation in patients.
In healthy subjects, ilaprazole has limited effect on the pharmacodynamics of clopidogrel and it may not be clinically relevant.
The genotypes presented in the study are available in the Supplementary Table 4. Further inquiries for other data can be directly from the corresponding authors on reasonable request.
The original contributions presented in the study are publicly available. This data can be found here: https://www.ncbi.nlm.nih.gov/clinvar/ SCV00256845 and SCV002568452.
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
CL, FS, XH, and HH designed the research. PC, RL, ZD, QG, JM, JT, ZH, ZZ, BZ, and JC performed the research. ZY, IU, YT, and QZ analyzed the data. ZY, CT, XG, CZ, SZ, and LX wrote the manuscript. CL critically corrected the manuscript.
This work was supported by the Livzon Pharmaceutical Group Inc., and grants from the National Natural Science Funding of China (82170351), the Second Level of 333 High Level Talent Training Project in Jiangsu Province (BRA2019099), the Special Fund for Key R & D Plans (Social Development) of Jiangsu Province (BE2019754), and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutes (PAPD).
The authors would like to acknowledge Xinyu Xu in the Department of Endocrinology, the First Affiliated Hospital of Nanjing Medical University for her kindly offer of the flow cytometer for VASP P2Y12 assay. We also acknowledge the study participants, nurses and clinicians in the clinical research ward for their contributions to this study.
CL received funding from the Livzon Pharmaceutical Group Inc. XH, ZH, ZZ, and HH work in the Livzon Pharmaceutical Group Inc., who sponsored this study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.952804/full#supplementary-material
Supplementary Figure S1 | Flow diagram of the trial test. Four eligible subjects were recruited to receive clopidogrel 75 mg once daily for 7 days, and then clopidogrel 75 mg in combination with ilaprazole 10 mg once daily for 7 days (first Regimen A, then Regimen B; n=2), or vice versa (first Regimen B, then Regimen A; n=2), with a 10-day interval between the two regimens.
Angiolillo, D. J., Gibson, C. M., Cheng, S., Ollier, C., Nicolas, O., Bergougnan, L., et al. (2011). Differential effects of omeprazole and pantoprazole on the pharmacodynamics and pharmacokinetics of clopidogrel in healthy subjects: Randomized, placebo-controlled, crossover comparison studies. Clin. Pharmacol. Ther. 89 (1), 65–74. doi:10.1038/clpt.2010.219
Bhatt, D. L., Cryer, B. L., Contant, C. F., Cohen, M., Lanas, A., Schnitzer, T. J., et al. (2010). Clopidogrel with or without omeprazole in coronary artery disease. N. Engl. J. Med. 363 (20), 1909–1917. doi:10.1056/NEJMoa1007964
Bhatt, D. L., Fox, K. A., Hacke, W., Berger, P. B., Black, H. R., Boden, W. E., et al. (2006). Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N. Engl. J. Med. 354 (16), 1706–1717. doi:10.1056/NEJMoa060989
Cattaneo, M. (2012). Response variability to clopidogrel: Is tailored treatment, based on laboratory testing, the right solution? J. Thromb. Haemost. 10 (3), 327–336. doi:10.1111/j.1538-7836.2011.04602.x
Cho, H., Choi, M. K., Cho, D. Y., Yeo, C. W., Jeong, H. E., Shon, J. H., et al. (2012). Effect of CYP2C19 genetic polymorphism on pharmacokinetics and pharmacodynamics of a new proton pump inhibitor, ilaprazole. J. Clin. Pharmacol. 52 (7), 976–984. doi:10.1177/0091270011408611
Fan, L., Xianghong, Q., Ling, W., Ying, H., Jielai, X., and Haitang, H. (2019). Ilaprazole compared with rabeprazole in the treatment of duodenal ulcer: A randomized, double-blind, active-controlled, multicenter study. J. Clin. Gastroenterol. 53 (9), 641–647. doi:10.1097/MCG.0000000000001186
FDA (2011). PLAVIX (clopidogrel bisulfate) tablets Labeling Revision. 2011 [cited; Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020839s055lbl.pdf.
Frelinger, A. L., Lee, R. D., Mulford, D. J., Wu, J., Nudurupati, S., Nigam, A., et al. (2012). A randomized, 2-period, crossover design study to assess the effects of dexlansoprazole, lansoprazole, esomeprazole, and omeprazole on the steady-state pharmacokinetics and pharmacodynamics of clopidogrel in healthy volunteers. J. Am. Coll. Cardiol. 59 (14), 1304–1311. doi:10.1016/j.jacc.2011.12.024
Funck-Brentano, C., Szymezak, J., Steichen, O., Ducint, D., Molimard, M., Remones, V., et al. (2013). Effects of rabeprazole on the antiplatelet effects and pharmacokinetics of clopidogrel in healthy volunteers. Arch. Cardiovasc. Dis. 106 (12), 661–671. doi:10.1016/j.acvd.2013.09.002
Furtado, R. H. M., Giugliano, R. P., Strunz, C. M. C., Filho, C. C., Ramires, J. A. F., Filho, R. K., et al. (2016). Drug interaction between clopidogrel and ranitidine or omeprazole in stable coronary artery disease: A double-blind, double dummy, randomized study. Am. J. Cardiovasc. Drugs 16 (4), 275–284. doi:10.1007/s40256-016-0172-5
Gurbel, P. A., Bliden, K. P., Saucedo, J. F., Suarez, T. A., DiChiara, J., Antonino, M. J., et al. (2009). Bivalirudin and clopidogrel with and without eptifibatide for elective stenting: Effects on platelet function, thrombelastographic indexes, and their relation to periprocedural infarction results of the CLEAR PLATELETS-2 (clopidogrel with eptifibatide to arrest the reactivity of platelets) study. J. Am. Coll. Cardiol. 53 (8), 648–657. doi:10.1016/j.jacc.2008.10.045
Hochholzer, W., Trenk, D., Bestehorn, H. P., Fischer, B., Valina, C. M., Ferenc, M., et al. (2006). Impact of the degree of peri-interventional platelet inhibition after loading with clopidogrel on early clinical outcome of elective coronary stent placement. J. Am. Coll. Cardiol. 48 (9), 1742–1750. doi:10.1016/j.jacc.2006.06.065
Jiang, X. L., Samant, S., Lesko, L. J., and Schmidt, S. (2015). Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clin. Pharmacokinet. 54 (2), 147–166. doi:10.1007/s40262-014-0230-6
Kazui, M., Nishiya, Y., Ishizuka, T., Hagihara, K., Farid, N. A., Okazaki, O., et al. (2010). Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab. Dispos. 38 (1), 92–99. doi:10.1124/dmd.109.029132
Levine, G. N., Bates, E. R., Bittl, J. A., Brindis, R. G., Fihn, S. D., Fleisher, L. A., et al. (2016). 2016 ACC/AHA guideline Focused Update on duration of Dual Antiplatelet therapy in patients with coronary artery disease: A report of the American college of cardiology/American heart association Task Force on Clinical practice guidelines. J. Am. Coll. Cardiol. 68 (10), 1082–1115. doi:10.1016/j.jacc.2016.03.513
Li, C., Hirsh, J., Xie, C., Johnston, M. A., and Eikelboom, J. W. (2012). Reversal of the anti-platelet effects of aspirin and clopidogrel. J. Thromb. Haemost. 10 (4), 521–528. doi:10.1111/j.1538-7836.2012.04641.x
Li, X. Q., Andersson, T. B., Ahlstrom, M., and Weidolf, L. (2004). Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab. Dispos. 32 (8), 821–827. doi:10.1124/dmd.32.8.821
Lim, I. H., Lee, S. J., Shin, B. S., and Kang, H. G. (2022). Ilaprazole and clopidogrel resistance in acute stroke patients. Biomedicines (6), 1366. doi:10.3390/biomedicines10061366
Lin, C. F., Shen, L. J., Wu, F. L., Bai, C. H., and Gau, C. S. (2012). Cardiovascular outcomes associated with concomitant use of clopidogrel and proton pump inhibitors in patients with acute coronary syndrome in Taiwan. Br. J. Clin. Pharmacol. 74 (5), 824–834. doi:10.1111/j.1365-2125.2012.04250.x
Lin, S. F., Lin, P. C., Chang, C. C., Chang, W. L., and Chu, F. Y. (2020). Investigation of the interaction between proton pump inhibitors and clopidogrel using VerifyNow P2Y12 assay. Med. Baltim. 99 (50), e23695. doi:10.1097/MD.0000000000023695
Pi, L., Xu, Y., Fu, L., Zhang, L., Liu, Y., Zhou, H., et al. (2019). A PEAR1 polymorphism (rs12041331) is associated with risk of coronary artery aneurysm in Kawasaki disease. Ann. Hum. Genet. 83 (1), 54–62. doi:10.1111/ahg.12285
Przespolewski, E. R., Westphal, E. S., Rainka, M., Smith, N. M., Bates, V., and Gengo, F. M. (2018). Evaluating the effect of six proton pump inhibitors on the antiplatelet effects of clopidogrel. J. Stroke Cerebrovasc. Dis. 27 (6), 1582–1589. doi:10.1016/j.jstrokecerebrovasdis.2018.01.011
Pu, J., Wang, F., Tang, W., and Zhu, M. (2018). Biotransformation of ilaprazole in human liver microsomes and human: Role of CYP3A4 in ilaprazole clearance and drug-drug interaction. Drug Metab. Dispos. 46 (10), 1453–1461. doi:10.1124/dmd.118.081570
Scott, S. A., Sangkuhl, K., Stein, C. M., Hulot, J. S., Mega, J. L., Roden, D. M., et al. (2013). Clinical pharmacogenetics implementation consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin. Pharmacol. Ther. 94 (3), 317–323. doi:10.1038/clpt.2013.105
Seo, K. A., Lee, S. J., Kim, K. B., Bae, S. K., Liu, K. H., Kim, D. H., et al. (2012). Ilaprazole, a new proton pump inhibitor, is primarily metabolized to ilaprazole sulfone by CYP3A4 and 3A5. Xenobiotica. 42 (3), 278–284. doi:10.3109/00498254.2011.622416
Shin, J. S., Lee, J. Y., Cho, K. H., Park, H. L., Kukulka, M., Wu, J. T., et al. (2014). The pharmacokinetics, pharmacodynamics and safety of oral doses of ilaprazole 10, 20 and 40 mg and esomeprazole 40 mg in healthy subjects: A randomised, open-label crossover study. Aliment. Pharmacol. Ther. 40 (5), 548–561. doi:10.1111/apt.12860
Thebault, J. J., Kieffer, G., Lowe, G. D., Nimmo, W. S., and Cariou, R. (1999). Repeated-dose pharmacodynamics of clopidogrel in healthy subjects. Semin. Thromb. Hemost. 25 (2), 9–14.
Wang, C., Ma, X., and Tang, L. (2021). Isolation and characterization of twelve polymorphic microsatellite markers in the endangered Hopea hainanensis (Dipterocarpaceae). Ecol. Evol. 11 (1), 4–10. doi:10.1002/ece3.7077
Wang, H., Shao, F., Liu, X., Xu, W., Ou, N., Qin, X., et al. (2019). Efficacy, safety and pharmacokinetics of ilaprazole infusion in healthy subjects and patients with esomeprazole as positive control. Br. J. Clin. Pharmacol. 85 (11), 2547–2558. doi:10.1111/bcp.14076
Wang, L., Zhou, L., Lin, S., Hu, H., and Xia, J. (2011). A new PPI, ilaprazole compared with omeprazole in the treatment of duodenal ulcer: A randomized double-blind multicenter trial. J. Clin. Gastroenterol. 45 (4), 322–329. doi:10.1097/MCG.0b013e3181e88515
Xu, K., Ye, S., Zhang, S., Yang, M., Zhu, T., Kong, D., et al. (2019). Impact of platelet endothelial aggregation receptor-1 genotypes on platelet reactivity and early cardiovascular outcomes in patients undergoing percutaneous coronary intervention and treated with aspirin and clopidogrel. Circ. Cardiovasc. Interv. 12 (5), e007019. doi:10.1161/CIRCINTERVENTIONS.118.007019
Ying, L., Wang, J., Li, J., Teng, J., Zhang, X., Ullah, I., et al. (2021). Intensified antiplatelet therapy in patients after percutaneous coronary intervention with high on-treatment platelet reactivity: The OPTImal management of antithrombotic agents (OPTIMA)-2 trial. Br. J. Haematol. 196, 424–432. doi:10.1111/bjh.17847
Keywords: clopidogrel, ilaprazole, drug-drug interaction, maximal platelet aggregation, platelet reactivity index
Citation: Ye Z, Chen P, Tan C, Gong X, Li R, Dong Z, Ullah I, Zhou C, Zhou S, Xie L, Hou X, Han Z, Gu Q, Ma J, Teng J, Tang Y, Zhang Z, Hu H, Zhuang Q, Chen J, Zhu B, Shao F and Li C (2022) Effects of ilaprazole on the steady-state pharmacodynamics of clopidogrel in healthy volunteers: An open-label randomized crossover study. Front. Pharmacol. 13:952804. doi: 10.3389/fphar.2022.952804
Received: 26 May 2022; Accepted: 26 July 2022;
Published: 08 September 2022.
Edited by:
Stefania Tacconelli, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Sojeong Yi, United States Food and Drug Administration, United StatesCopyright © 2022 Ye, Chen, Tan, Gong, Li, Dong, Ullah, Zhou, Zhou, Xie, Hou, Han, Gu, Ma, Teng, Tang, Zhang, Hu, Zhuang, Chen, Zhu, Shao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Shao, c2hhb2ZlbmduakAxNjMuY29t; Chunjian Li, bGlqYXlAbmptdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.