- 1Peking University First Hospital, Beijing, China
- 2Institute of Cardiovascular Disease, Peking University First Hospital, Beijing, China
Purpose: This study compared the effect of indobufen with that of aspirin on platelet function in patients with stable coronary heart disease after percutaneous coronary intervention (PCI).
Methods: Patients with stable coronary heart disease who had undergone PCI and received dual antiplatelet therapy (aspirin 100 mg + clopidogrel 75 mg once daily) for at least 12 months were allocated to receive indobufen 100 mg twice daily + clopidogrel 75 mg once daily, clopidogrel 75 mg once daily alone, indobufen 100 mg twice daily alone, and aspirin 100 mg once daily alone for 1 month each in an open-label crossover manner. Platelet function was assessed by using the rates of arachidonic acid (AA)-induced platelet aggregation (AA-PAR) and adenosine diphosphate (ADP)-induced platelet aggregation (ADP-PAR) measured by light transmission aggregometry, the platelet reactivity index measured by vasodilator-stimulated phosphoprotein (PRI-VASP), and the plasma and urinary thromboxane B2 (TXB2) concentrations recorded at baseline and during each treatment phase.
Results: Of 56 patients enrolled, 52 completed the study. The AA-PAR was lower in the indobufen alone group than in the aspirin alone group [5.21% (3.39, 7.98) vs. 5.27% (4.06, 6.60), p = 0.038], while biologically, a difference of 0.06% may represent no significant difference; there was no significant between-group difference in the plasma [531.16 pg/ml (203.89, 1035.06) vs. 373.93 pg/ml (194.04, 681.71), p = 0.251] or urinary [3951.97 pg/ml (2006.95, 6077.01) vs. 3610.48 pg/ml (1664.60, 6247.61), p = 0.717] TXB2 concentration. When the aspirin + clopidogrel group and indobufen + clopidogrel group were compared, similar results were found for AA-PAR [3.97% (3.05, 5.12) vs. 3.83% (3.10, 5.59), p = 0.947] and both plasma [849.47 pg/ml (335.96, 1634.54) vs. 455.41 pg/ml (212.47, 1489.60), p = 0.629], and urinary [4122.97 pg/ml (2044.96, 7459.86) vs. 3812.81 pg/ml (1358.95, 6021.07), p = 0.165] TXB2 concentrations. ADP-PAR was lower in the clopidogrel alone group than in the indobufen alone group (47.04% ± 16.89 vs. 61.7% ± 10.50, p < 0.001), as was PRI-VASP (66.53% ± 18.06 vs. 77.72% ± 19.87, p = 0.002).
Conclusion: These findings suggest that indobufen has antiplatelet effects similar to those of aspirin in patients with stable coronary heart disease after PCI, and may be an alternative for patients with aspirin intolerance after coronary stenting.
Introduction
Platelet activation and aggregation play a key role in the occurrence and development of atherosclerotic thrombosis (Davì and Patrono, 2007). Current guidelines recommend dual antiplatelet therapy combined with aspirin (acetylsalicylic acid) and an adenosine diphosphate (ADP) receptor antagonist for at least 6–12 months after percutaneous coronary intervention (PCI) followed by long-term maintenance on aspirin as antiplatelet monotherapy (Collet et al., 2021). This strategy is recommended for secondary prevention of thrombotic events, which can effectively reduce the risk of cardiovascular death, recurrent myocardial infarction and stroke and may improve the prognosis (Degrauwe et al., 2017).
Aspirin inhibits arachidonic acid (AA)-mediated platelet aggregation by irreversibly inhibiting the cyclo-oxygenase (COX) enzyme and is the cornerstone of primary and secondary prevention therapy for coronary artery disease (Antithrombotic Trialists’ Collaboration, 2002). However, aspirin is a major cause of iatrogenic gastrointestinal injury, including ulceration, erosion of the stomach, and upper gastrointestinal bleeding (Levy, 1974; Ibáñez et al., 2006), due in part to inhibition of synthesis of cytoprotective prostaglandins in the gastric mucosa (Miller and Jacobson, 1979). Furthermore, a proportion of patients are intolerant of aspirin, which can manifest as bronchospasm, urticaria/angioedema, or anaphylaxis (Stevenson, 2004), and are at high risk of discontinuation. Aspirin desensitization therapy has been found to be safe and may be effective (Bianco et al., 2016) but further confirmatory randomized controlled trials are needed. Therefore, alternative antiplatelet agents are required for this population.
Indobufen, a phenylbutyric acid derivative (Müller, 1991; Morocutti et al., 1997), is an antiplatelet agent that inhibits production of thromboxane and COX-dependent aggregation of platelets by reversible inhibition of COX-1 with less affecting production of prostacyclins. It can also reduce platelet adhesion and inhibit platelet aggregation induced by ADP (Bhana and McClellan, 2001). Previous studies have shown that indobufen is well tolerated, associated with a low incidence of adverse effects (Wiseman et al., 1992; Marzo et al., 2004), and has biochemical, functional, and clinical effects that are comparable with those of a standard dose of aspirin (Müller, 1991). In a group of patients with stable ischemia and aspirin intolerance undergoing coronary stent implantation, combined treatment with indobufen and thienopyridine resulted in a low rate of ischemic events (Latib et al., 2013). Another study suggested that combined antiplatelet treatment with clopidogrel + indobufen could be a good option in patients who are undergoing coronary stenting for acute coronary syndrome (ACS) and have aspirin hypersensitivity (Barillà et al., 2013). However, the evidence as to whether indobufen and aspirin have the same inhibitory effect on platelet aggregation has been inconsistent (De Caterina et al., 1996; Cipollone et al., 1997; Barillà et al., 2013; Lee et al., 2016; Yang et al., 2021). Recently, Yang et al. (2021) found significantly less suppression of AA-induced platelet aggregation in patients with coronary atherosclerosis who received indobufen 100 mg twice daily than in those who received aspirin 100 mg once daily but reported that both agents inhibited urinary 11-dehydrothromboxane B2 to a similar degree. Therefore, it remains uncertain whether the antiplatelet effect of indobufen is comparable with that of aspirin.
Few studies have used platelet function tests to compare the antiplatelet effect of indobufen with that of aspirin in patients with stable coronary heart disease after PCI. The aim of this study was to compare the effects of indobufen on platelet function with those of aspirin when used alone and in combination with clopidogrel to provide more evidence regarding whether indobufen can be used as an alternative to aspirin.
Methods
The study protocol was approved by the Ethics Committee of Peking University First Hospital (approval number 2018-12) and performed in accordance with the tenets of the Declaration of Helsinki. Informed consent was obtained from each study participant.
Subjects
Patients with stable coronary heart disease who had undergone PCI at Peking University First Hospital and received aspirin 100 mg + clopidogrel 75 mg once daily for at least 12 months were recruited. All patients received standard medication for secondary prevention of coronary heart disease.
Patients were considered eligible for enrolment if they met the following criteria: able to sign an informed consent form; age 18–85 years; confirmed stable coronary heart disease after PCI; and currently receiving antiplatelet therapy in combination with aspirin 100 mg + clopidogrel 75 mg once daily. The following exclusion criteria were applied: ACS within the 12 months before screening; PCI within the 12 months before screening; receiving oral or intravenous anticoagulant therapy for another condition, such as atrial fibrillation, pulmonary embolism, lower limb venous thrombosis, or an artificial heart valve; an AA-induced platelet aggregation rate (AA-PAR) measured by light transmission aggregometry (LTA) to be more than 20% when treated with aspirin + clopidogrel in the previous 3 months; congestive heart failure or left ventricular ejection fraction <35%; forced expiratory volume in 1 s or forced vital capacity below the lower reference limit; bleeding tendency or severe lung disease; active pathological bleeding; history of intracranial hemorrhage; allergy to an indobufen formulation or any of its excipients; severe liver injury (elevation of transaminases up to 3-fold the upper reference limit); pregnancy, lactation, or planning pregnancy; hematological disease, a platelet count <100,000/mm3, or hemoglobin <10 g/dl; glycosylated hemoglobin >10%; history of drug abuse or alcoholism in the previous 2 years; use of non-steroidal anti-inflammatory drugs; and a creatinine clearance rate <30 ml/min.
Study interventions
Patients received sequential antiplatelet therapy consisting of indobufen 100 mg twice daily + clopidogrel 75 mg once daily for 1 month (V1), clopidogrel 75 mg once daily alone for 1 month (V2), indobufen 100 mg twice daily alone for 1 month (V3), and aspirin 100 mg once daily alone for 1 month (V4) in an open-label crossover manner (Figure 1). Platelet activity and adverse events were assessed at baseline (V0) and at the end of each treatment phase (V1, V2, V3, and V4) by laboratory investigations and clinical evaluation.
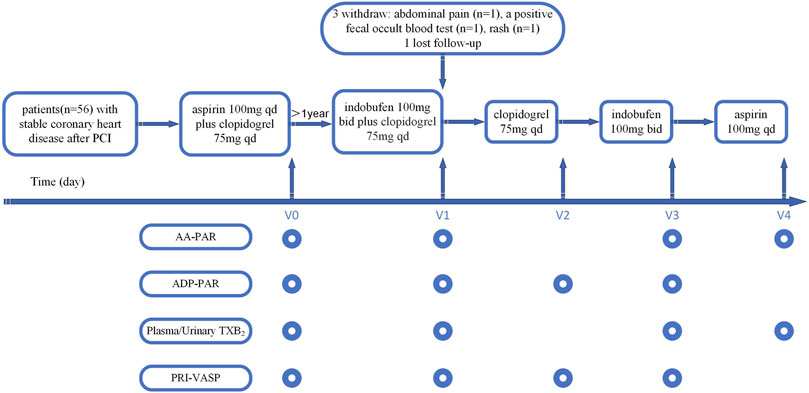
FIGURE 1. Study flow chart. AA-PAR = rate of arachidonic acid-induced platelet aggregation measured by light transmission aggregometry; ADP-PAR = rate of adenosine diphosphate-induced platelet aggregation measured by light transmission aggregometry; bid = twice daily; PCI = percutaneous coronary intervention; PRI-VASP = platelet reactivity index measured by vasodilator-stimulated phosphoprotein; qd = once daily; TXB2 = thromboxane B2; V0 = visit at baseline; V1 = visit at the first month; V2 = visit at the second month; V3 = visit at the third month; V4 = visit at the fourth month.
We measured platelet aggregation in response to AA and ADP using LTA. Vasodilator-stimulated phosphoprotein (VASP) levels were used to measure the platelet reactivity index (PRI-VASP, %) in response to ADP. Plasma and urinary TXB2 concentrations were used to reflect the plasma TXA2 level produced by platelets after being induced by AA. Platelet function tests were performed according to the antiplatelet agent(s) used in each phase (Figure 1).
Laboratory investigations, including a whole blood count and classification, routine urine and stool tests, a fecal occult blood test, coagulation function, and serum creatinine, alanine aminotransferase, and aspartate aminotransferase levels, were performed at the start and end of the study to evaluate the safety of the trial medications. Compliance and adverse events were assessed at each follow-up visit. Other non-steroidal anti-inflammatory drugs and non-investigational antiplatelet agents, such as glycoprotein IIb/IIIa receptor antagonists, were prohibited during the study period. The indobufen used in the study was provided as 200 mg tablets free of charge by Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd. (Hangzhou, China). Aspirin was purchased as 100 mg tablets from Bayer AG (Leverkusen, Germany), and clopidogrel as 75 mg tablets from Sanofi Pharmaceuticals (Paris, France). All the trial medications were commercially available with approved labelling.
Measurement of platelet function
The platelet aggregation rate (PAR, %) in response to AA and ADP was measured by an LTA device (Model 700, Chrono-Log Corporation, Havertown, PA, United States), and the results are reported as AA-PAR and ADP-PAR. All tests were performed by technicians in the Laboratory Department of Peking University First Hospital using standard operating procedures (Tang et al., 2015).
VASP was assessed in accordance with the standard protocol with labeled monoclonal antibodies by flow cytometry using the Platelet VASP-FCM kit (BioCytex Inc., Marseille, France) as previously described (Rollini et al., 2016). The results are reported as the platelet reactivity index (PRI-VASP, %), which was calculated after measuring VASP-P levels following stimulation with prostaglandin (PG) E1 (MFIcPGE1) and PGE1 + ADP (MFIcPGE1 + ADP). PRI was calculated as [(MFIcPGE1) − (MFIcPGE1 + ADP)/(MFIcPGE1)] × 100%.
Plasma and urinary TXB2 concentrations were measured using the Thromboxane B2 ELISA Kit-Monoclonal (Item No. 501020, Cayman Chemical, Ann Arbor, MI, United States) in accordance with the manufacturer’s instructions and confirmed using standard curves (R2 > 99%).
Study endpoints
The primary study endpoint was the difference in AA-PAR between the indobufen alone group and the aspirin alone group. The secondary endpoints were as follows: 1) differences in plasma and urinary TXB2 concentrations between the indobufen alone group and the aspirin alone group; 2) differences in AA-PAR and in plasma and urinary TXB2 concentrations between the aspirin + clopidogrel group and the indobufen + clopidogrel group; and 3) differences in ADP-PAR and PRI-VASP between the clopidogrel alone group and the indobufen alone group. Patient adherence to the antiplatelet regimen was monitored during follow-up, and all adverse events were recorded.
Acquisition of data and statistical analysis
A case report form was used for data collection and questioning purposes. Data were entered in duplicate into an Epidata database by two administrators working independently. Continuous data were summarized as the mean ± standard deviation or the median (interquartile range) and compared using the t-test and paired t-test as appropriate. Categorical variables are presented as the count and proportion (percent), and they were compared using the chi-squared and McNemar’s chi-squared test as appropriate. The statistical analysis was performed using EmpowerStats software (www.empowerstats.com) and SPSS 24.0 software. A two-sided p-value < 0.05 was considered statistically significant.
Results
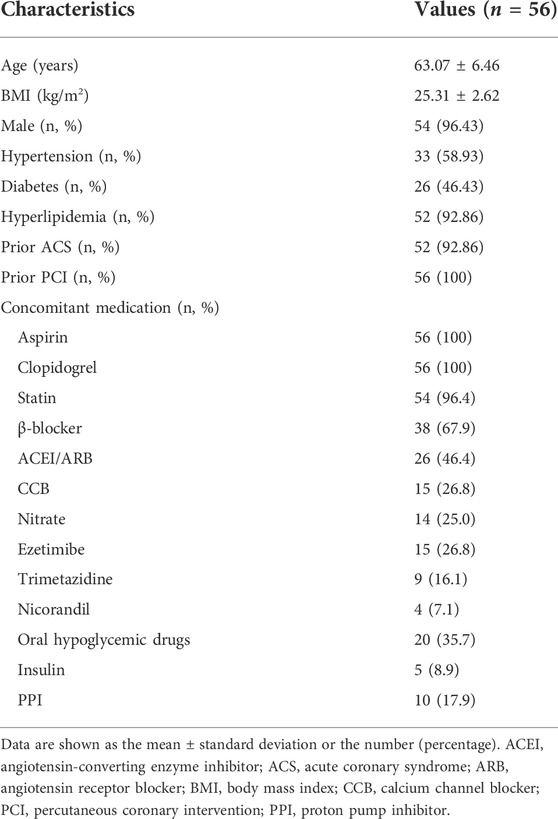
Fifty-six patients were enrolled in the study. All 52 patients who completed the study had undergone PCI for stable coronary heart disease and had received aspirin 100 mg + clopidogrel 75 mg once daily for at least 12 months. Table 1 shows the patient demographics, clinical characteristics, and medications used at baseline. Four patients dropped out of the study before visit 1 because of abdominal pain (n = 1), a positive fecal occult blood test (n = 1), rash (n = 1), or an unknown reason (n = 1).
Platelet aggregation
AA-PAR was found to be lower in the indobufen alone group than in the aspirin alone group [5.21% (3.39, 7.98) vs. 5.27% (4.06, 6.60), p = 0.038; Figure 2A] but was similar between the aspirin + clopidogrel group and indobufen + clopidogrel group [3.97% (3.05, 5.12) vs. 3.83% (3.10, 5.59), p = 0.947; Figure 2B]. ADP-PAR was lower in the clopidogrel alone group than in the indobufen alone group (47.04% ± 16.89 vs. 61.7% ± 10.50, p < 0.001), as was the PRI-VASP value (66.53 ± 18.06 vs. 77.72 ± 19.87, p = 0.002, Figure 2C).
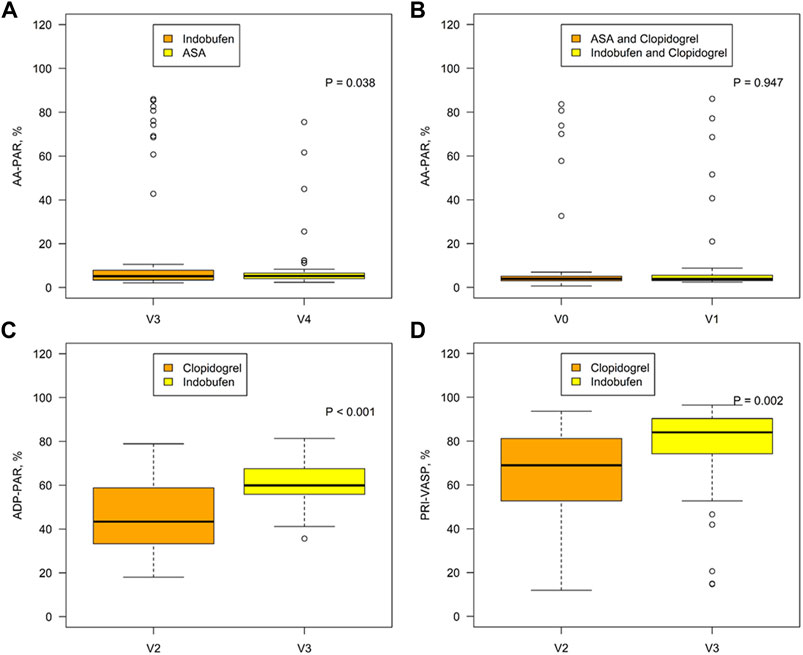
FIGURE 2. Comparison of the AA-PAR between the indobufen alone group (V3) and the aspirin alone group (V4) (A) and between the aspirin + clopidogrel group (V0) and the indobufen + clopidogrel group (V1) (B). Comparison of the ADP-PAR (C) and PRI-VASP (D) between the clopidogrel alone group (V2) and the indobufen alone group (V3). AA-PAR = rate of arachidonic acid-induced platelet aggregation; ADP-PAR = rate of adenosine diphosphate-induced platelet aggregation; PRI-VASP = platelet reactivity index measured by vasodilator-stimulated phosphoprotein.
Plasma and urinary TXB2 concentrations
There was no significant difference in plasma TXB2 concentration between indobufen and aspirin [531.16 pg/ml (203.89, 1035.06) vs. 373.93 pg/ml (194.04, 681.71), p = 0.251] or urinary TXB2 concentration [3951.97 pg/ml (2006.95, 6077.01) vs. 3610.48 pg/ml (1664.60, 6247.61), p = 0.717; Figures 3A,B]. The plasma and urinary TXB2 concentrations were similar in the aspirin + clopidogrel group and the indobufen + clopidogrel group [849.47 pg/ml (335.96, 1634.54) vs. 455.41 pg/ml (212.47, 1489.60), p = 0.629 and 4122.97 pg/ml (2044.96, 7459.86) vs. 3812.81 pg/ml (1358.95, 6021.07), p = 0.165; Figures 4A,B].
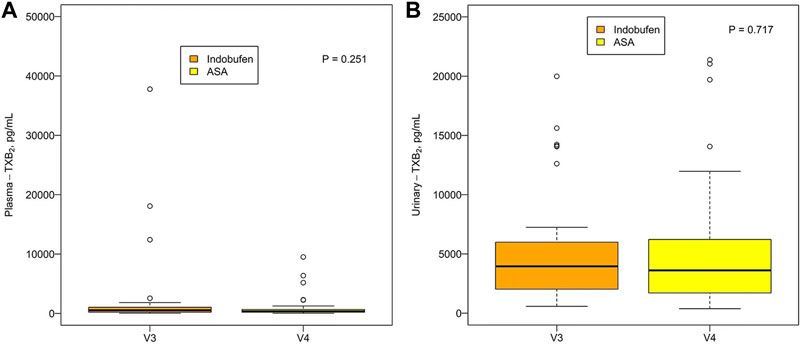
FIGURE 3. (A) Comparison of the (A) plasma and (B) urinary TXB2 concentration between the indobufen alone group (V3) and the aspirin alone group (V4). ASA = aspirin; TXB2 = thromboxane B2.
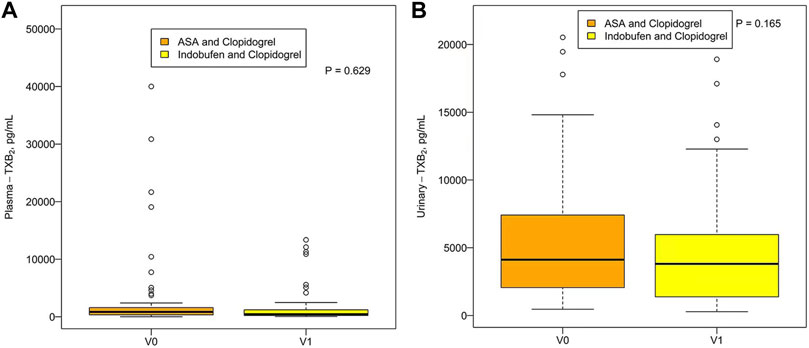
FIGURE 4. Plasma (A) and urinary (B) TXB2 concentrations in the aspirin + clopidogrel group (V0) and the indobufen + clopidogrel group (V1). ASA = aspirin; TXB2 = thromboxane B2.
Clinical outcomes
There was no significant difference in blood pressure, pulse rate, or laboratory test results between baseline and the end of the study (Supplementary Tables S1, S2). According to the Bleeding Academic Research Consortium consensus document (Mehran et al., 2011), there were no severe bleeding events during the study that met the criteria for type 2, 3, 4, or 5, including fatal or life-threatening bleeding, a clinically significant or obvious bleeding-related decrease in hemoglobin, or need for blood transfusion. Gastrointestinal discomfort and bleeding events that occurred during the study period are shown in Table 2. Two minor type 1 bleeding episodes were noted at visit 1, one in a patient who had a positive fecal occult blood test that became negative on re-examination and the other in a patient who developed mild epistaxis that recovered spontaneously. There were no serious or life-threatening adverse events that resulted in hospitalization, disability, dysfunction, deformity, or death.
Discussion
To our knowledge, this is the first study to compare the antiplatelet effect of indobufen with that of aspirin in patients with stable coronary heart disease after PCI using platelet function testing in both monotherapy and dual antiplatelet therapy scenarios. The major findings of this study were as follows: 1) AA-PAR was similar in the indobufen alone group with that in the aspirin alone group, though the p value was less than 0.05, a difference of 0.06% may represent no significant difference biologically; 2) there was no significant difference in the plasma or urinary TXB2 concentration between the indobufen alone group and the aspirin alone group; 3) there were no significant differences in AA-PAR or plasma or urinary TXB2 concentrations between the indobufen + clopidogrel group and the aspirin + clopidogrel group; 4) ADP-PAR and PRI-VASP values were higher in the indobufen alone group than in the clopidogrel alone group.
A platelet function test can reflect individual responsiveness to antiplatelet agents and help with formulation of antiplatelet treatment strategies. The methods commonly used to detect platelet function include LTA, the VerifyNow system, thromboelastography, flow cytometry to detect platelet VASP phosphorylation levels, and the plasma TXB2 level (Paniccia et al., 2015).
Indobufen and aspirin exert their antiplatelet effects mainly by inhibiting COX-1, thereby inhibiting synthesis of TXA2 and aggregation of platelets (Eikelboom et al., 2002). Therefore, we can assess their antiplatelet efficacy via the indirect effect of TXA2-induced platelet aggregation by adding AA to blood samples (Morocutti et al., 1997). LTA, developed by Born in the 1960s, is performed using platelet-rich plasma as the milieu. By adding a variety of agonists such as AA or ADP to platelet-rich plasma, the corresponding residual platelet response rate can be obtained and is usually expressed as the PAR. It is the most widely employed methodology for detecting disorders of platelet function and monitoring the effects of antiplatelet therapy. The residual platelet reactivity rate defined by ADP-LTA, AA-LTA, or both, has been associated with ischemic events in patients with ACS and in those with stable coronary artery disease (Breet et al., 2010). Furthermore, TXA2 is rapidly transformed by hydrolysis into TXB2, which is a biologically inactive and stable product (Wiseman et al., 1992; Latib et al., 2013). Serum and urinary TXB2 metabolites reflect biosynthesis of TXA2 and are useful for assessment of platelet function in various disease states, detection of defects in production of thromboxane, and monitoring the effects of antiplatelet therapy.
Previous studies have used platelet function to compare the antiplatelet effect of indobufen with that of aspirin. In 1996, De Caterina et al. (1996) showed that aspirin (300 mg/day for 1 week) and indobufen (200 mg twice a day for 1 week) reduced the plasma TXB2 level and inhibited the maximum extent of whole blood platelet aggregation to similar extents in patients with ischemic heart disease and in healthy volunteers. Cipollone et al. (1997) reported that urinary excretion of 11-dehydrothromboxane B2 was significantly lower in patients with unstable angina who received indobufen (200 mg twice a day) than in their counterparts who received aspirin (320 mg daily). Moreover, a study by Barillà et al. (2013) that included 42 consecutive patients with ACS and hypersensitivity to aspirin undergoing coronary stenting found that the maximum percent platelet aggregation in response to AA was lower in those who received clopidogrel 75 mg daily + indobufen 100 mg twice a day than in those who received clopidogrel 75 mg daily alone. In the study by Barillà et al., the plasma TXB2 level at 1 week and 1 month was also very low in the patients whose treatment included indobufen.
In a study by Lee et al. (2016) in which 20 healthy volunteers received aspirin (200 mg/day for 2 weeks) followed by a 4-week washout period and then indobufen (200 mg twice a day for 2 weeks), the percent inhibition of platelet aggregation assessed using AA as the agonist was similar at 4 h after the last dose of indobufen and aspirin but was significantly lower after the last dose of indobufen than after the last dose of aspirin at 12, 24, and 48 h.
All the above-mentioned studies showed that the inhibitory effect of indobufen on platelet aggregation was at least equivalent to that of aspirin and that the anti-aggregation effect diminished more rapidly after indobufen than after aspirin. However, as mentioned earlier, the results of the study by Yang et al. (2021) were different in that AA-induced platelet aggregation was significantly less suppressed in patients with coronary atherosclerosis who received indobufen 100 mg twice daily than in those who received aspirin 100 mg once daily. Furthermore, Yang et al. reported that the inhibitory effect on the plasma TXB2 level in healthy volunteers at 8 and 12 h after the final dose of indobufen was weaker than that after the final dose of aspirin. Therefore, more studies are needed to confirm whether the antiplatelet effect of indobufen is comparable with that of aspirin.
The present study had an open-label crossover design whereby each patient served as their own matched control to minimize the influence of interindividual differences in drug metabolism. Furthermore, the study population comprised patients with stable coronary heart disease after PCI; thus, our results could be used to guide antiplatelet therapy in patients with coronary heart disease. In terms of dose selection, in the Chinese population, the more likely dosage of indobufen would be 100 mg twice a day (Author Anonymous, 2019). Therefore, in our study, we chose this dosage for evaluation.
Our main finding in this study was that indobufen 100 mg twice daily inhibited AA-induced platelet aggregation detected by the LTA method to a significantly greater extent than aspirin 100 mg once daily, though a difference of 0.06% may represent no significant difference biologically, with no significant between-group difference in the plasma or urinary TXB2 concentration. Moreover, there was no difference in the platelet aggregation rate or thromboxane concentration between the indobufen and aspirin groups when clopidogrel was added. Overall, we found that the antiplatelet effects via the AA pathway were similar between indobufen and aspirin.
Like clopidogrel, indobufen can inhibit platelet aggregation induced by ADP Bianco et al. (2016). In an in vitro and in vivo study, Li et al. (2021) showed that indobufen + clopidogrel had a higher inhibitory effect on ADP induced platelet aggregation than aspirin + clopidogrel. However, in the study by Barillà et al. (2013), there was no difference in the maximum inhibition rate of ADP-induced aggregation of platelets between the group that received clopidogrel 75 mg daily + indobufen 100 mg twice a day and the group that received clopidogrel 75 mg daily alone, suggesting that indobufen does not increase the inhibition of ADP-induced platelet aggregation further.
To date, few studies have compared the antiplatelet effects of indobufen alone with those of clopidogrel alone. Among the currently available methods used to assess platelet function, the most established and clinically validated ones used to explore ADP-induced platelet aggregation are VerifyNow P2Y12, LTA, and VASP (Rollini et al., 2016). In our study, we performed LTA and VASP assays to compare the ability of indobufen to inhibit platelet aggregation with that of clopidogrel. The PRI-VASP is used to assess P2Y12 receptor blockade and is specific and reproducible. This test is highly specific for the P2Y12 receptor pathway and correlates with the concentration of active metabolites. According to the PRI, platelet reactivity is divided into low (LPR), optimal (OPR), or high (HPR), and the respective cut-off values for the LPR, OPR, and HPR categories are <16%, 16%–50%, and >50%. Several studies have found that HPR is associated with a greater risk of ischemic complications while LPR has been associated with a greater likelihood of bleeding events (Bonello et al., 2012; Tantry et al., 2013; Aradi et al., 2014; Aradi et al., 2015). In our study, both ADP-PAR and PRI-VASP were higher in the indobufen alone group than in the clopidogrel alone group. PRI-VASP was 77.72% ± 19.87% in the indobufen group and 66.53% ± 18.06% in the clopidogrel group. Therefore, our study also suggests that indobufen inhibits platelet aggregation by inhibiting the ADP pathway but not as well as clopidogrel.
A more relevant issue was to look at non-response to the anti-platelet agents. In LTA non-response to AA in aspirin-treated patients is usually set at 20% (Hankey and Eikelboom, 2006). There were five non-responders in aspirin plus clopidogrel group and six non-responders in indobufen plus clopidogrel group (Supplementary Table S3), and the chi-square test showed that there was no significant difference between the two groups (p = 1.000). There were 10 non-responders in indobufen alone group and four non-responders in aspirin alone group (Supplementary Table S3), and the chi-square test showed no significant difference between the two groups as well (p = 0.114). Non-adherence to the treatment has been previously shown to be the major cause of aspirin non-response in patients (Peace et al., 2010). Similarly, our statistical results showed that in the indobufen plus clopidogrel group and indobufen alone groups, the responders were more compliant and showed lower plasma and urinary TXB2 concentrations (Supplementary Table S4). It looks like that non-adherence to the indobufen may be the major cause of indobufen non-response in patients. Furthermore, there were 2, 1, 4, and 0 non-inhibiters (>80% LTA) in aspirin plus clopidogrel group, indobufen plus clopidogrel group, indobufen alone group and aspirin alone group respectively. It looks like that four of the non-responders to indobufen show no inhibition at all while none on aspirin show such high levels. Once clopidogrel is added in this removes all differences. This suggests that even with AA there is a small component of aggregation that is ADP-dependent. This is a similar finding to what was previously reported by Cox et al. (2006).
There were no serious complications requiring hospitalization or medical intervention or any life-threatening complications, including serious bleeding, throughout the study. Bleeding events and gastrointestinal discomfort occurred infrequently, which suggests that indobufen would be a safe antiplatelet therapy in patients with coronary heart disease after PCI.
Our study has several potential limitations. First, the small sample size reduced the power of the study to detect significant differences between groups. More prospective multicenter studies are needed to evaluate the efficacy and safety of indobufen in patients with stable coronary heart disease after PCI. Second, we assessed platelet function by testing the urinary TXB2 concentration rather than urinary 11-dehydrothromboxane B2, which is the major product of TXB2 found in urine and provides a better indirect assessment of the ability of platelets to form TXA2. Third, the open-label design of this study introduces the possibility of bias, even though the adjudication of endpoints was performed in a blinded manner by the study evaluators. Fourth, the study was not powered for clinical outcomes. Therefore, we cannot draw any conclusions regarding the efficacy and safety of the treatment regimens used. Fifth, the most of the patients were male and it may bias the experimental results, and given that we only included Chinese patients, our results may not be generalizable to all populations. Last but not least, during the study, aspirin and clopidogrel were purchased and taken by patients themselves, so we did not recycle aspirin and clopidogrel, and lacked relevant compliance evaluation. However, we recovered the drugs after indobufen combined with clopidogrel and indobufen alone treatment, and evaluated the drug use compliance, which reached 97.69% ± 9.27% and 96.49% ± 17.48% respectively. Therefore, the overall compliance of indobufen treatment is relatively ideal. We cannot compare the compliance between indobufen treatment and non indobufen treatment, which is the limitation of this study, and we will correct it in the future study.
The findings of this study suggest that the antiplatelet effect of indobufen is equivalent to that of aspirin in patients with stable coronary heart disease after PCI. Indobufen may be an option for patients with aspirin severe hypersensitivity, resistance or intolerance after coronary stent implantation. More multicenter studies are needed to evaluate the efficacy and safety of this new antiplatelet agent in patients with coronary disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Peking University First Hospital (approval number 2018-12). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Q-PS and X-YL: contributed equally to this work. Q-PS and BoZ: designed and executed the study. JZ and Q-FX: tested the platelet function. X-YL, JZ, Q-FX, J-HL, and Y-KL: collected the data. Q-PS: analyzed the data. Q-PS, X-YL, BiZ, X-GW, JJ, and BoZ: discussed the results. Q-PS and X-YL: wrote the original manuscript. BoZ: reviewed and edited the manuscript.
Acknowledgments
We thank Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.950719/full#supplementary-material
References
Antithrombotic Trialists' Collaboration (2002). Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324 (7329), 71–86. doi:10.1136/bmj.324.7329.71
Aradi, D., Kirtane, A., Bonello, L., Gurbel, P. A., Tantry, U. S., Huber, K., et al. (2015). Bleeding and stent thrombosis on P2Y12-inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur. Heart J. 36 (27), 1762–1771. doi:10.1093/eurheartj/ehv104
Aradi, D., Storey, R. F., Komocsi, A., Trenk, D., Gulba, D., Kiss, R. G., et al. (2014). Expert position paper on the role of platelet function testing in patients undergoing percutaneous coronary intervention. Eur. Heart J. 35 (4), 209–215. doi:10.1093/eurheartj/eht375
Author Anonymous, (2019). Committee of experts on rational drug use of national health commission of the P.R.China.Rational medication guidelines for thrombolytic therapy for acute ST-segment elevation myocardial infarction (2nd edition). Chin. J. Front. Med. Sci. Version) 11 (1), 40–65. doi:10.12037/YXQY.2019.01-07
Barillà, F., Pulcinelli, F. M., Mangieri, E., Torromeo, C., Tanzilli, G., Dominici, T., et al. (2013). Clopidogrel plus indobufen in acute coronary syndrome patients with hypersensitivity to aspirin undergoing percutaneous coronary intervention. Platelets 24 (3), 183–188. doi:10.3109/09537104.2012.686072
Bhana, N., and McClellan, K. J. (2001). Indobufen: an updated review of its use in the management of atherothrombosis. Drugs Aging 18 (5), 369–388. doi:10.2165/00002512-200118050-00007
Bianco, M., Bernardi, A., D'Ascenzo, F., Cerrato, E., Omede, P., Montefusco, A., et al. (2016). Efficacy and safety of available protocols for aspirin hypersensitivity for patients undergoing percutaneous coronary intervention: a survey and systematic review. Circ. Cardiovasc. Interv. 9 (1), e002896. doi:10.1161/CIRCINTERVENTIONS.115.002896
Bonello, L., Mancini, J., PansieriM., , MaiLLard, L., Rossi, P., ColletF., , et al. (2012). Relationship between post-treatment platelet reactivity and ischemic and bleeding events at 1-year follow-up in patients receiving prasugrel. J. Thromb. Haemost. 10 (10), 1999–2005. doi:10.1111/j.1538-7836.2012.04875.x
Breet, N. J., van Werkum, J. W., Bouman, H. J., Kelder, J. C., Ruven, H. J. T., Bal, E. T., et al. (2010). Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. Jama 303 (8), 754–762. doi:10.1001/jama.2010.181
Cipollone, F., Patrignani, P., Greco, A., Panara, M. R., Padovano, R., CuccurulloF., , et al. (1997). Differential suppression of thromboxane biosynthesis by indobufen and aspirin in patients with unstable angina. Circulation 96 (4), 1109–1116. doi:10.1161/01.cir.96.4.1109
Collet, J. P., Thiele, H., Barbato, E., Barthélémy, O., Bauersachs, J., Bhatt, D. L., et al. (2021). 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 42 (14), 1289–1367. doi:10.1093/eurheartj/ehaa575
Cox, D., Maree, A. O., Dooley, M., Conroy, R., Byrne, M. F., and Fitzgerald, D. J. (2006). Effect of enteric coating on antiplatelet activity of low-dose aspirin in healthy volunteers. Stroke 37 (8), 2153–2158. doi:10.1161/01.STR.0000231683.43347.ec
Davì, G., and Patrono, C. (2007). Platelet activation and atherothrombosis. N. Engl. J. Med. 357 (24), 2482–2494. doi:10.1056/NEJMra071014
De Caterina, R., Giannessi, D., Bernini, W., Lazzerini, G., LavezzariM., , Stragliotto, E., et al. (1996). A prostacyclin-sparing effect of indobufen vs. aspirin. Thromb. Haemost. 75 (3), 510–514. doi:10.1055/s-0038-1650306
Degrauwe, S., Pilgrim, T., Aminian, A., Noble, S., Meier, P., and Iglesias, J. F. (2017). Dual antiplatelet therapy for secondary prevention of coronary artery disease. Open Heart 4 (2), e000651. doi:10.1136/openhrt-2017-000651
Eikelboom, J. W., Hirsh, J., Weitz, J. I., Johnston, M., Yi, Q., and Yusuf, S. (2002). Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation 105 (14), 1650–1655. doi:10.1161/01.cir.0000013777.21160.07
Hankey, G. J., and Eikelboom, J. W. (2006). Aspirin resistance. Lancet 367 (9510), 606–617. doi:10.1016/S0140-6736(06)68040-9
Ibáñez, L., Vidal, X., VendreLL, L., Moretti, U., and Laporte, J. R. (2006). Upper gastrointestinal bleeding associated with antiplatelet drugs. Aliment. Pharmacol. Ther. 23 (2), 235–242. doi:10.1111/j.1365-2036.2006.02759.x
Latib, A., Ielasi, A., Ferri, L., Chieffo, A., Godino, C., Carlino, M., et al. (2013). Aspirin intolerance and the need for dual antiplatelet therapy after stent implantation: a proposed alternative regimen. Int. J. Cardiol. 165 (3), 444–447. doi:10.1016/j.ijcard.2011.08.080
Lee, J. Y., Sung, K. C., and Choi, H. I. (2016). Comparison of aspirin and indobufen in healthy volunteers. Platelets 27 (2), 105–109. doi:10.3109/09537104.2015.1042853
Levy, M. (1974). Aspirin use in patients with major upper gastrointestinal bleeding and peptic-ulcer disease. a report from the boston collaborative drug surveillance program, boston university medical center. N. Engl. J. Med. 290 (21), 1158–1162. doi:10.1056/NEJM197405232902102
Li, F., Xu, D., Hou, K., Gou, X., Lv, N., Fang, W., et al. (2021). Pretreatment of indobufen and aspirin and their combinations with clopidogrel or ticagrelor alleviates inflammasome mediated pyroptosis via inhibiting NF-κB/NLRP3 pathway in ischemic stroke. J. Neuroimmune Pharmacol. 16 (4), 835–853. doi:10.1007/s11481-020-09978-9
Marzo, A., FumagallI, I., Giusti, A., and Lowenthal, D. T. (2004). Endoscopic evaluation of the effects of indobufen and aspirin in healthy volunteers. Am. J. Ther. 11 (2), 98–102. doi:10.1097/00045391-200403000-00004
Mehran, R., Rao, S. V., Bhatt, D. L., Gibson, C. M., Caixeta, A., Eikelboom, J., et al. (2011). Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research Consortium. Circulation 123 (23), 2736–2747. doi:10.1161/CIRCULATIONAHA.110.009449
Miller, T. A., and Jacobson, E. D. (1979). Gastrointestinal cytoprotection by prostaglandins. Gut 20 (1), 75–87. doi:10.1136/gut.20.1.75
Morocutti, C., Amabile, G., FattappostaF., , Nicolosi, A., Matteoli, S., TrappoliniM., , et al. (1997). Indobufen versus warfarin in the secondary prevention of major vascular events in nonrheumatic atrial fibrillation. SIFA (Studio Italiano Fibrillazione Atriale) Investigators. Stroke 28 (5), 1015–1021. doi:10.1161/01.str.28.5.1015
Müller, B. (1991). Pharmacology of thromboxane A2, prostacyclin and other eicosanoids in the cardiovascular system. Therapie 46 (3), 217–221.
Paniccia, R., Priora, R., Liotta, A. A., and Abbate, R. (2015). Platelet function tests: a comparative review. Vasc. Health Risk Manag. 11, 133–148. doi:10.2147/VHRM.S44469
Peace, A., Tedesco, T., Kenny, D., Conroy, R. M., Foley, D., Cox, D., et al. (2010). The role of weight and enteric coating on aspirin response in cardiovascular patients. J. Thromb. Haemost. 8 (10), 2323–2325. doi:10.1111/j.1538-7836.2010.03997.x
Rollini, F., Franchi, F., Singh, K., Cho, J. R., Bhatti, M., DeGroat, C., et al. (2016). Impact of timing from blood sampling to pharmacodynamic assessment on measures of platelet reactivity in patients treated with P2Y(12) receptor inhibitors. Thromb. Haemost. 116 (6), 1060–1069. doi:10.1160/TH16-05-0377
Stevenson, D. D. (2004). Aspirin and NSAID sensitivity. Immunol. Allergy Clin. North Am. 24 (3), 491–505. doi:10.1016/j.iac.2004.03.001
Tang, X. F., Han, Y. L., Zhang, J. H., Wang, J., Zhang, Y., Xu, B., et al. (2015). Comparing of light transmittance aggregometry and modified thrombelastograph in predicting clinical outcomes in Chinese patients undergoing coronary stenting with clopidogrel. Chin. Med. J. 128 (6), 774–779. doi:10.4103/0366-6999.152611
Tantry, U. S., Bonello, L., Aradi, D., Price, M. J., Jeong, Y. H., Angiolillo, D. J., et al. (2013). Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J. Am. Coll. Cardiol. 62 (24), 2261–2273. doi:10.1016/j.jacc.2013.07.101
Wiseman, L. R., Fitton, A., and Buckley, M. M. (1992). Indobufen. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in cerebral, peripheral and coronary vascular disease. Drugs 44 (3), 445–464. doi:10.2165/00003495-199244030-00009
Keywords: indobufen, platelet aggregation rate, antiplatelet therapy, coronary heart disease, percutaneous coronary intervention
Citation: Shi Q-P, Luo X-Y, Zhang B, Wang X-G, Zhao J, Xie Q-F, Liu J-H, Liu Y-K, Jiang J and Zheng B (2022) Effect of indobufen vs. aspirin on platelet accumulation in patients with stable coronary heart disease after percutaneous coronary intervention: An open-label crossover study. Front. Pharmacol. 13:950719. doi: 10.3389/fphar.2022.950719
Received: 23 May 2022; Accepted: 18 July 2022;
Published: 16 August 2022.
Edited by:
Pedro D’Orléans-Juste, Université de Sherbrooke, Sherbrooke, CanadaReviewed by:
Dermot Cox, Royal College of Surgeons in Ireland, IrelandYing Zhang, Xiyuan Hospital, China
Copyright © 2022 Shi, Luo, Zhang, Wang, Zhao, Xie, Liu, Liu, Jiang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Zheng, emhlbmdib3BhdHJpY2tAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Qiu-Ping Shi
Qiu-Ping Shi Xing-Yu Luo
Xing-Yu Luo Bin Zhang
Bin Zhang Xin-Gang Wang
Xin-Gang Wang Jing Zhao
Jing Zhao Qiu-Fen Xie
Qiu-Fen Xie Jia-Hui Liu
Jia-Hui Liu Yao-Kun Liu
Yao-Kun Liu Jie Jiang
Jie Jiang Bo Zheng
Bo Zheng