
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 24 August 2022
Sec. Inflammation Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.950450
This article is part of the Research TopicInflammatory immune disease: Molecular Mechanisms, Translational Approaches and Therapeutics, Volume IIView all 51 articles
 Diqin Yan1,2†
Diqin Yan1,2† Huaying Fan3†
Huaying Fan3† Min Chen1,2
Min Chen1,2 Lin Xia1,4
Lin Xia1,4 Simin Wang1,4
Simin Wang1,4 Wenliang Dong1,2
Wenliang Dong1,2 Qian Wang1
Qian Wang1 Suping Niu3
Suping Niu3 Huiying Rao5
Huiying Rao5 Liming Chen1*
Liming Chen1* Xiaoyan Nie2*
Xiaoyan Nie2* Yi Fang1*
Yi Fang1*Background: Due to the lack of comprehensive evidence based on prospective studies, the efficacy and safety of Janus Kinase (JAK) inhibitors (including tofacitinib, ruxolitinib, baricitinib, ritlecitinib and brepocitinib) for alopecia areata (AA) are yet to be proved.
Methods: The systematic review and meta-analysis was performed pursuant to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline and registered on PROSPERO (CRD42022303007).
Results: Fourteen prospective studies (5 RCTs and 9 non-RCTs), enrolling a total of 1845 patients with AA, were included for quantitative analysis. In RCTs, oral JAK inhibitors resulted in higher good response rate compared with control (RR: 6.86, 95% CI: 2.91–16.16); topical JAK inhibitors did not show any difference compared with control (RR: 1.00, 95% CI: 0.31–3.18). In non-RCTs, the pooled rate of good response to oral, topical and sublingual JAK inhibitors were 63% (95% CI: 44%–80%), 28% (95% CI: 1%–72%) and 11% (95% CI: 1%–29%), respectively. The pooled recurrence rate in patients treated with JAK inhibitors was 54% (95% CI: 39%–69%), mainly due to the withdrawal of JAK inhibitors. In RCTs, no difference was found in the risk of experiencing most kind of adverse events; in non-RCTs, the reported adverse events with high incidence rate were mostly mild and manageable.
Conclusion: JAK inhibitors are efficacious and generally well-tolerated in treating AA with oral administration, whereas topical or sublingual administration lacks efficacy. Subgroup analyses indicate that baricitinib, ritlecitinib and brepocitinib seem to have equal efficacy for AA in RCTs; ruxolitinib (vs. tofacitinib) and AA (vs. AT/AU) are associated with better efficacy outcomes in non-RCT. Due to the high recurrence rate after withdrawal of JAK inhibitors, continuous treatment should be considered to maintain efficacy.
Systematic Review Registration: PROSPERO: CRD 42022303007
Alopecia areata (AA) is a common, inflammatory, non-scarring type of hair loss. AA presents most commonly as limited patches of hair loss (patchy AA) that can progress to loss of all scalp hairs (alopecia totalis, AT) or all body hairs (alopecia universalis, AU) (Strazzulla et al., 2018). The risk of progression from patchy AA to AT or AU is approximately 5%, and an extensive involvement portends a worse prognosis (Safavi et al., 1995; Tosti et al., 2006). As a common type of alopecia in human, the estimated prevalence of AA is approximately between 0.1% and 0.2%, second only to male and female pattern alopecia (Pratt et al., 2017). And the lifetime incidence risk of AA is approximately 2% (Mirzoyev et al., 2014). The chronic course and frequent relapse of AA can be distressing for patients, even leading to psychosocial disorder and reduction in quality of life. Therefore, the importance should be attached to the treatment of AA.
There are several treatment approaches available for the management of AA, including corticosteroids, minoxidil, topical immunotherapy, cyclosporine, methotrexate, etc (Meah et al., 2020). However, the response of AA patients to these treatments varies widely and adverse events occur frequently especially in systemic medications; few robust and well-designed clinical trials have evaluated and supported these therapies (Lai et al., 2019). Therefore, more effective and less toxic drugs for AA are needed.
As the molecular mechanisms of AA are further defined, targeted therapies including Janus kinase (JAK) inhibitors are considered to be a preferable treatment option. Genome-wide association studies and functional immunological studies have identified that CD8+NKG2D + T cells are the major effectors of AA pathogenesis, which promote the inflammation of hair follicles through interferon-γ (IFN-γ) and interleukin-15 (IL-15) signaling pathways. JAK/signal transducer and activator of transcription (STAT) is in the downstream molecular pathway of IFN-γ and IL-15 receptors (Petukhova et al., 2010; Betz et al., 2015), (Xing et al., 2014). Therefore, JAK inhibitors can blockade the signaling pathway of AA by inhibiting JAK/STAT activation, leading to the reverse of AA. Among the JAK inhibitors for AA, baricitinib is the first treatment approved for the indication of AA by the Food and Drug Administration (FDA) in 13 June 2022; tofacitinib and ruxolitinib were approved for the treatment of rheumatoid arthritis and other inflammatory disorders; ritlecitinib and brepocitinib are under investigation and not available for routine clinical use. Hence, clinical statistics regarding the efficacy and safety of JAK inhibitors are required to provide a better insight in this new treatment strategy.
Thus, we systematically reviewed the evidence 1) to evaluate the efficacy and safety of JAK inhibitors for AA, 2) to determine the relative efficacy of JAK inhibitors in different administration route (oral vs. topical vs. sublingual administration), and 3) to identify more factors influencing the good response to JAK inhibitors in AA patients.
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline and registered on PROSPERO (CRD42022303007) (Moher et al., 2009).
Electronic searches were performed in PubMed, EMBASE and Cochrane library from inception to 17 June 2022, using MeSH or Emtree terms including ‘‘alopecia areata,’’ ‘‘JAK inhibitors,” ‘‘tofacitinib,’’ “ruxolitinib,” ‘‘baricitinib,’’ “ritlecitinib,” and “brepocitinib” and their synonyms. The detailed search strategy for each database is described in the Supplement. We searched the reference lists from retrieved full text articles and previous systematic reviews for further identification of potentially relevant studies. We also searched through PROSPERO for any related systematic reviews.
Studies were selected based on the following inclusion criteria: 1) studies enrolling human participants with AA/AT/AU; 2) studies in which patients were treated with JAK inhibitors; 3) studies reporting efficacy outcomes including scalp hair regrowth or recurrence rate, or safety outcomes including adverse events; 4) studies of prospective studies including RCTs, clinical trials and prospective cohort studies; 5) studies published in English. Studies were excluded based on the following exclusion criteria: 1) studies enrolling patients without scalp involvement, but only with nails, eyelashes or eyebrows involvement; 2) studies of observational studies, case series, case reports, repeated publications, abstracts, conference presentations, editorials and reviews.
Two authors independently reviewed the included articles and extracted data on the trial characteristics, baseline characteristics of participants, interventions, comparisons, efficacy and safety outcomes from each trial. Faced with the absence of data, we transformed or estimated measures of variance using the recommendations from the Cochrane Handbook for Systematic Reviews of Interventions (Akl et al., 2019). Any disagreement was resolved by discussion until consensus was reached or by consulting a third author.
The choice of outcomes was based on the usually reported primary and secondary outcomes in clinical trials of AA, AA investigational assessment guidelines (Olsen et al., 2004), and other systematic reviews of AA (Lee et al., 2018; Phan et al., 2019; Phan and Sebaratnam, 2019; Guo et al., 2020). The efficacy outcomes included good response [defined as 50% improvement in Severity of Alopecia Tool (SALT) scores (SALT50)], complete response [defined as 90% improvement in SALT scores (SALT90)], the percent change from baseline in SALT score and recurrence. The safety outcomes included the incidence rates of adverse events.
Two authors independently appraised risk of bias of each study using the Cochrane risk of bias tool for RCTs and ROBINS-I (Risk of Bias In Non-randomized Studies-of Interventions) for non-RCTs (including single-arm trials, non-randomized controlled trials and extension periods of RCT) (Higgins et al., 2011) (Sterne et al., 2016). Any disagreement was resolved by discussion until consensus was reached or by consulting a third author.
We conducted meta-analysis of each outcome using the available data for response rates, recurrence rate, and incidence rates of adverse events. All outcomes were reported with associated 95% confidence intervals (CI). Meta-analysis for RCTs and non-RCTs (including single-arm trials, non-randomized controlled trials and extension periods of RCT) were conducted separately. Heterogeneity of the included studies was calculated using Cochran Q statistic (significant at p < 0.1) and I2 test (significant at I2 > 50%). Overall, there was a significant heterogeneity, so a random effects model was used. Preplanned subgroup analysis was conducted according to administration route (oral vs. topical vs. sublingual administration), types of JAK inhibitors (baricitinib vs. ritlecitinib vs. brepocitinib, ruxolitinib vs. tofacitinib), treatment duration (<24 weeks vs. ≥24 weeks) and AA subtype (AA vs. AT/AU). All analyses were performed by the meta package (version 5.1-1) for R (version 4.1.1). p value < 0.05 was considered statistically significant.
Overall, 649 records were identified through three databases. After removing 208 duplicates, we excluded 290 records on the basis of the title and abstract. The remaining 151 potentially relevant reports were reviewed in full text. After detailed evaluation of these reports, 14 studies (5 RCTs and 9 non-RCTs) (Kennedy Crispin et al., 2016; Mackay-Wiggan et al., 2016; Almutairi et al., 2018; Jabbari et al., 2018; Liu et al., 2018; Olsen et al., 2020; King et al., 2021a; AlMarzoug et al., 2021; King et al., 2021b; Lai et al., 2021; Peeva et al., 2021; King et al., 2022), enrolling a total of 1,845 patients, were included for analysis (Figure 1).
Characteristics of included studies were described in Table 1. Among the included 5 RCTs, 3 compared oral baricitinib with placebo (King et al., 2021b; King et al., 2022), 1 compared oral ritlecitinib and brepocitinib with placebo (King et al., 2021a), and 1 compared topical ruxolitinib with placebo (Olsen et al., 2020). Among the included 9 non-RCTs, 7 single-arm clinical trials evaluated the efficacy and safety of oral/topical ruxolitinib and oral/topical/sublingual tofacitinib (Kennedy Crispin et al., 2016; Mackay-Wiggan et al., 2016; Jabbari et al., 2018; Liu et al., 2018; Olsen et al., 2020; AlMarzoug et al., 2021; Lai et al., 2021), 1 study of extension periods of RCT investigated the maintenance and withdrawal with oral ritlecitinib and brepocitinib (Peeva et al., 2021), and 1 non-randomized controlled trial compared oral ruxolitinib with oral tofacitinib (Almutairi et al., 2018).
Risk of bias assessment of included studies was described in Table 2. Given the limited number of included studies, we did not remove the studies with high risk of bias.
A good response was defined as the achievement of SALT50. Meta-analysis based on 5 RCTs and 8 non-RCTs evaluated the rate of good response to JAK inhibitors in patients with AA (Figure 2). In RCTs, JAK inhibitors were associated with an increase in the pooled good response rate compared with control (RR: 5.06, 95% CI: 1.87–13.70). Due to high heterogeneity, subgroup analysis was conducted based on the route of administration, and this difference was significant (p < 0.01). A significant difference was found in studies where JAK inhibitor was orally administered that the intervention group showed a higher good response rate compared with a controlled group (RR: 6.86, 95% CI: 2.91–16.16), yet such significance was not observed in the study where JAK inhibitor was topically administered (RR: 1.00, 95% CI: 0.31–3.18). In non-RCTs, the pooled rate of good response to JAK inhibitors in AA was 50% (95% CI: 30%–70%). From subgroup analysis, the pooled good response rate in studies where JAK inhibitor was orally administered was 63% (95% CI: 44%–80%), significantly higher than that in studies where participants were treated with topical (28%, 95% CI:1%–72%) and sublingual JAK inhibitors (11%, 95% CI: 1%–29%, p < 0.01).
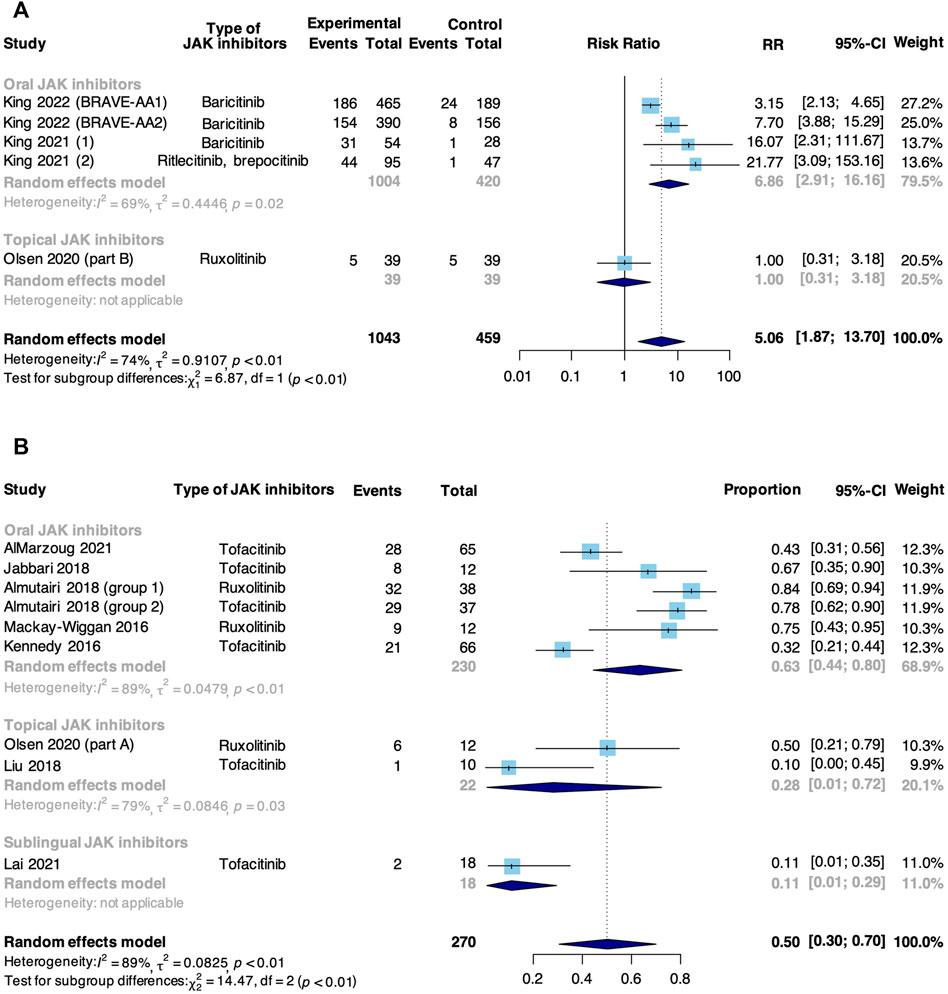
FIGURE 2. Forest plot of the pooled rate of good response to JAK inhibitors in patients with AA based on (A) RCTs and (B) non-RCTs.
A complete response was defined as the achievement of SALT90. Meta-analysis based on 5 RCTs and 4 non-RCTs evaluated the rate of complete response to JAK inhibitors in AA (Figure 3). In RCTs, JAK inhibitors were associated with an increase in the pooled complete response rate compared with control (RR: 9.57, 95% CI: 4.07–22.51). There was no significant difference in subgroup analysis based on the route of administration (p = 0.62). However, a significant difference was found in studies where JAK inhibitor was orally administered that the intervention group showed a higher complete response rate compared with a controlled group (RR: 11.13, 95% CI: 4.02–30.84), but not found in the study where JAK inhibitor was topically administered (RR: 5.00, 95% CI: 0.25–100.85). In non-RCTs, the pooled rate of complete response to JAK inhibitors in AA was 25% (95% CI: 15%–36%). From subgroup analysis, the complete response rate in studies where JAK inhibitor was orally administered (27%, 95% CI: 14%–42%) was higher than that in where JAK inhibitor was topically administered (17%, 95% CI: 2%–42%), but the difference was insignificant (p = 0.46).
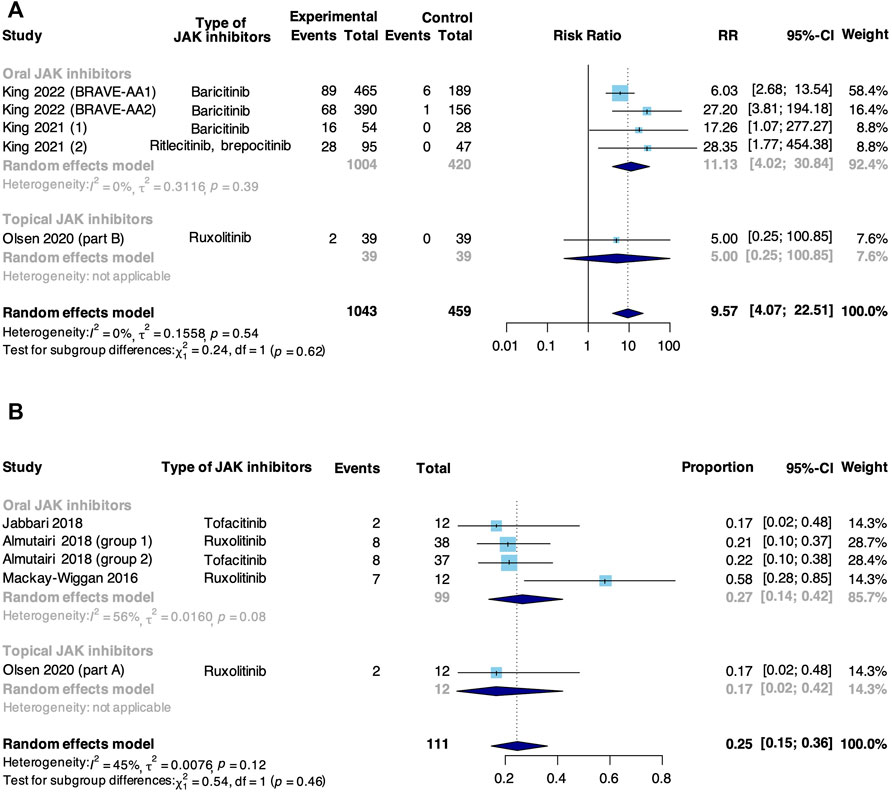
FIGURE 3. Forest plot of the pooled rate of complete response to JAK inhibitors in patients with AA based on (A) RCTs and (B) non-RCTs.
Meta-analysis based on 5 RCTs and 6 non-RCTs evaluated the percent change from baseline in SALT score in patients taking JAK inhibitors for AA (Figure 4). In RCTs, JAK inhibitors were associated with an increase in the percent change from baseline in SALT score compared with control (MD: 31.77, 95% CI: 19.86–43.67). The subgroup analysis revealed that there was a significant difference between oral (MD: 36.05, 95% CI: −31.69–40.42) and topical JAK inhibitors (MD: −0.30, 95% CI: −20.88 to 20.28, p < 0.01). In non-RCTs, the pooled percent change from baseline in SALT score was 53.17% (95% CI: 25.69%–80.64%). From subgroup analysis, there was a significant difference among oral (81.18%, 95% CI: 62.65%–99.70%), topical (10.89%, 95% CI: 1.70%–20.09%) and sublingual JAK inhibitors (15.57%, 95% CI: 4.76%–26.38%, p < 0.01).
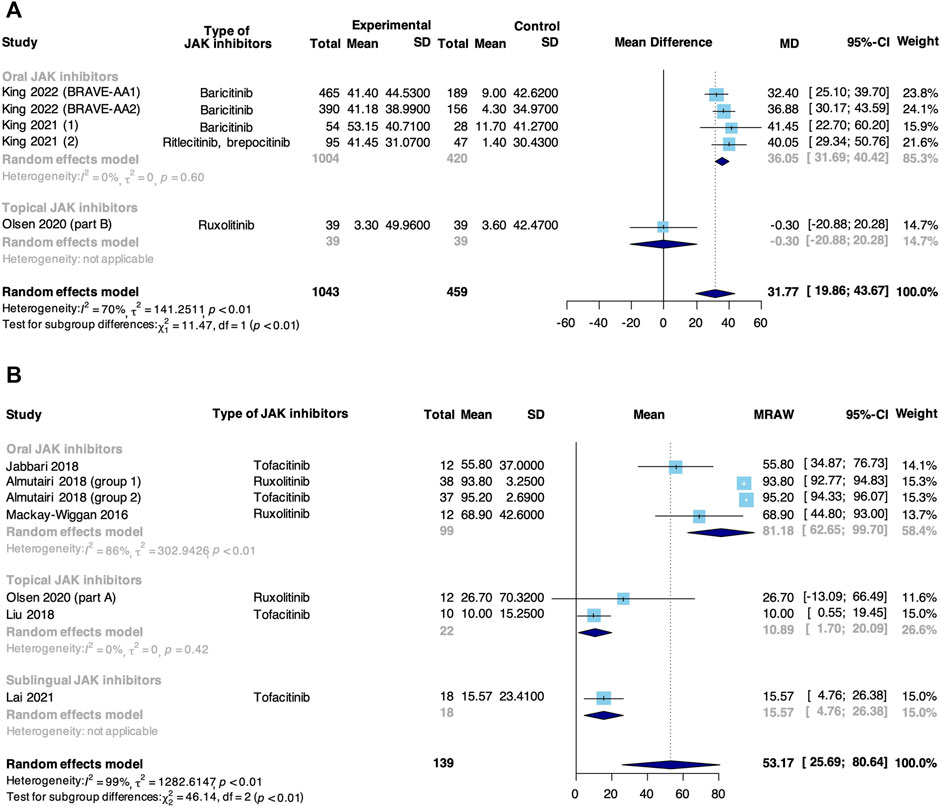
FIGURE 4. Forest plot of the percent change from baseline in SALT score in patients taking JAK inhibitors for AA based on (A) RCTs and (B) non-RCTs.
Further subgroup analysis was conducted with the good response rate (Table 3). In RCTs, a significant difference was found in terms of administration route (oral vs. topical administration, p < 0.01), and no significant difference was observed in terms of types of oral JAK inhibitors (baricitinib vs. ritlecitinib vs. brepocitinib, p = 0.55). In non-RCTs, oral administration (vs. topical and sublingual administration, p < 0.01), oral ruxolitinib (vs. oral tofacitinib, p = 0.02), topical ruxolitinib (vs. topical tofacitinib, p = 0.03) and AA (vs. AT/AU, p = 0.04) were associated with better response outcomes, with statistical significance; no significant difference was found in terms of treatment duration (≥24 weeks vs. <24 weeks, p = 0.28).
Meta-analysis based on 5 non-RCTs evaluated the recurrence rate in patients treated with JAK inhibitors (Figure 5). The pooled recurrence rate was 54% (95% CI: 39%–69%). The main cause of recurrence was the withdrawal of JAK inhibitors.
Meta-analysis based on 5 RCTs and 5 non-RCTs evaluated the safety of JAK inhibitors in patients with AA (Table 4). The types and reporting of adverse events varied across different studies. In RCTs, there was no significant difference between JAK inhibitors and placebo in the risk of experiencing treatment-emergent adverse event (TEAE, RR: 1.05, 95% CI: 0.96–1.14), serious AE (RR: 1.61, 95% CI: 0.70–3.68), upper respiratory tract infection (URTI, RR: 1.12, 95% CI: 0.76–1.67), headache (RR: 1.13, 95% CI: 0.72–1.77) and nasopharyngitis (RR: 1.00, 95% CI: 0.64–1.58). Acne was more common with baricitinib than with placebo (RR: 3.48, 95% CI: 1.55 to 7.82, p < 0.01). In non-RCTs, the highest risk was observed for URTI (37.05%), followed by diarrhea (19.65%), acne (9.31%), urinary tract infection (UTI, 6.98%), headache (6.33%) and folliculitis (4.48%).
In this systematic review and meta-analysis, 14 prospective studies (5 RCTs and 9 non-RCTs), including a total of 1845 participants with AA, were enrolled for syntheses. Overall, our results confirm that oral JAK inhibitors can be a promising option for the treatment of AA, which is corroborated as the JAK inhibitor was first approved for treatment of AA by FDA.
The efficacy outcomes demonstrated, based on both RCTs and non-RCTs, that oral JAK inhibitors could induce hair regrowth significantly in terms of all efficacy outcomes (including good response rate, complete response rate and the percent change from baseline in SALT score). On the contrary, there was no significant difference in efficacy outcomes between topical JAK inhibitors and placebo control based on RCTs; topical and sublingual JAK inhibitors induced minimal hair regrowth in terms of all efficacy outcomes based on non-RCTs, and the improvement was too little to be clinical meaningful or to be distinguished from the spontaneous remission and placebo effect. Our results were in line with previous study. Olsen et al. reported potential efficacy of topical ruxolitinib in part A (an open-label and single-arm clinical trial), but there was no significant difference in hair regrowth between topical ruxolitinib group and control group in part B (an RCT) (Olsen et al., 2020). The different findings between the two parts could be explained by the fact that the spontaneous remission of AA and placebo effect were mistakenly attributed to topical ruxolitinib in non-RCT, whereas the placebo control eliminated such biases in RCT, thus revealing the true response to topical ruxolitinib. Therefore, the finding of part B that topical ruxolitinib did not have a significant effect for AA was more convincing.
Cytokine receptors are paired with different JAKs [including JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2)], which are activated upon cytokine binding. JAK2 mediates IFN-γ receptor signaling, JAK3 mediates γc cytokine receptor signaling, TYK2 mediates IFN-α/β receptor signaling, and JAK1 mediates these three cytokine receptor signaling pathways (O'Shea et al., 2013). Among 5 types of JAK inhibitors included in this study, tofacitinib is a JAK1/3 inhibitor, ruxolitinib and baricitinib are JAK1/2 inhibitors, ritlecitinib is a JAK3 selective inhibitor, and brepocitinib is a JAK1/TYK2 inhibitor (Xing et al., 2014; King et al., 2021a; King et al., 2021b). According to the results of subgroup analysis based on types of JAK inhibitors, there was no significant difference observed among baricitinib, ritlecitinib and brepocitinib in RCTs. In non-RCTs, ruxolitinib was associated with better response outcomes, compared with tofacitinib. But the results of subgroup analysis need further verification because of inadequate reporting data and limited number of participants. Additionally, due to the limited types of selective JAK inhibitors included, it is hard to identify the relative contribution of JAK1, JAK2, JAK3, and TYK2 inhibition to the therapeutic effect on AA. However, some other studies demonstrated that IFN-γ (via JAK1/2) and γc cytokine (via JAK1/3) signaling pathways play key roles in AA pathogenesis, but the role of IFN-α/β (via JAK1/TYK2) in AA remains undefined. Besides, JAK2 is essential for the function of hematopoiesis-related cytokines, including erythropoietin, thrombopoietin, growth hormone, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (Neubauer et al., 1998). Hence, the blockade of JAK2 may lead to potential side effect, including anemia, thrombocytopenia, and neutropenia. Dai et al. found that JAK1 and JAK3 selective inhibitors robustly induced hair regrowth and decreased AA-associated inflammation, whereas JAK2 selective inhibitors failed to restore hair growth in C3H/HeJ mice with AA (Dai et al., 2021). Furthermore, unlike JAK1, which is broadly expressed in many tissues, the expression of JAK3 is mainly restricted to lymphocytes (Elwood et al., 2017), so that the inhibition of JAK3 signaling may be sufficient to reverse AA. Overall, JAK1 or JAK3 (especially JAK3) selective inhibitors may be a wise choice for AA, for they are theoretically related to less hematologic toxicity and more precise efficacy.
There was a contradiction among the results of subgroup analysis, recurrence and safety assessment. The results of subgroup analysis based on treatment duration showed that no significant difference was found between the treatment duration ≥24 weeks and <24 weeks. Paradoxically, the recurrence assessment indicated that approximately a half of patients treated with JAK inhibitors experienced disease relapse, and the main cause of recurrence was the withdrawal of JAK inhibitors. Peeva et al. reported 16 of 29 (55%) relapsed patients receiving re-treatment with JAK inhibitors achieved primary endpoint again (Peeva et al., 2021). Therefore, several studies suggested that to maintain hair regrowth, continuous treatment should be considered in patients who are tolerated and responsive to JAK inhibitors (Kennedy Crispin et al., 2016; Almutairi et al., 2018; Peeva et al., 2021). Unfortunately, to our knowledge there is no consensus on the optimal interval or duration of maintenance treatment. In addition, although the safety assessment reflects that JAK inhibitors are safe, the long-term safety is still in doubt because of limited experience with JAK inhibitors for the treatment of AA. According to the molecular mechanism of JAK inhibitors, immunosuppression will increase the risk of infection (O'Shea et al., 2004). Some studies on the safety of JAK inhibitors in rheumatic disease indicated that JAK inhibitors were associated with a decrease in neutrophil count and an increased risk of viral infection, particularly herpes zoster (Winthrop, 2017; Harigai, 2019). Based upon the above, the acceptable benefit-risk ratio can be obtained by early identifying strong responders, slow responders and non-responders to JAK inhibitors and then respectively applying optimal courses of treatment. AA disease activity index (ALADIN) score and AA responsiveness to JAK/STAT inhibitors (AARSIN) score were developed to effectively stratify AA patients based on disease phenotype, which may be useful as predictive biomarkers for response to JAK inhibitors (Xing et al., 2014; Kennedy Crispin et al., 2016; Mackay-Wiggan et al., 2016; Jabbari et al., 2018). Kennedy et al. stratified AA patients by AARSIN score, and 2 patients in the slow responder group who continued tofacitinib for an additional 3 months achieved SALT50, which demonstrated that longer treatment course or more potent JAK inhibitors could be beneficial to slow responders (Kennedy Crispin et al., 2016).
Different from the previous systematic reviews (Phan and Sebaratnam, 2019; Guo et al., 2020), which were mainly based on observational studies of low-quality, we included multiple varieties of JAK inhibitors evaluated in prospective studies (including RCTs, single-arm clinical trials, non-randomized controlled trials and extension periods of RCT) so that the more comprehensive evidence on the efficacy and safety of JAK inhibitors were obtained. To appraise the risk of bias of each study, we used the Cochrane risk of bias tool for RCTs and ROBINS-I for non-RCTs separately (Higgins et al., 2011; Sterne et al., 2016). Considering the differences of methodology and quality between RCTs and non-RCTs, we performed meta-analysis for them respectively.
Due to inadequate data reporting, we did not include several relevant trials in meta-analysis (King et al., 2021c; Ko et al., 2021; Senna et al., 2021). The publication language was restricted to English so that some relevant trials could have been missed. Although we included updated information based on prospective studies, better evidence could have been provided if there were more robust and well-designed RCTs comparing JAK inhibitors with negative or positive control. One of the major limitations of this review was the high heterogeneity of the studies, which could result from the inclusion of three routes of administration. For this reason, a random effects model was used and subgroup analyses were conducted to reduce heterogeneity.
JAK inhibitors are efficacious and generally well-tolerated in treating AA with oral administration, whereas topical or sublingual administration lacks efficacy. Subgroup analyses indicate that baricitinib, ritlecitinib and brepocitinib seem to have equal efficacy for AA in RCTs; ruxolitinib (vs. tofacitinib) and AA (vs. AT/AU) are associated with better efficacy outcomes in non-RCT. Given the high recurrence rate after withdrawal of JAK inhibitors, continuous treatment should be considered to maintain efficacy.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
YF and DY conceived this review. DY, HF, and MC selected records of studies. QW, SN, and HR extracted data. LX, SW, and WD appraised risk of bias of each study. DY, HF, LC, and XN performed statistical analyses. DY, HF, and MC drafted the manuscript. XN and YF helped for the language editing and proofreading. All authors contributed to the manuscript and approved its publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.950450/full#supplementary-material
Akl, E. A., Altman, D. G., Aluko, P., Askie, L. M., and Young, C. (2019). Cochrane Handbook for systematic Reviews of interventions. Cochrane Handbook for Systematic Reviews of Interventions.
AlMarzoug, A., AlOrainy, M., AlTawil, L., AlHayaza, G., AlAnazi, R., AlIssa, A., et al. (2021). Alopecia areata and tofacitinib: A prospective multicenter study from a Saudi population. Int. J. Dermatol. 61, 886–894. doi:10.1111/ijd.15917
Almutairi, N., Nour, T. M., and Hussain, N. H. (2018). Janus kinase inhibitors for the treatment of severe alopecia areata: An open-label comparative study. Basel, Switzerland: Dermatology. doi:10.1159/000494613
Betz, R. C., Petukhova, L., Ripke, S., Huang, H., Menelaou, A., Redler, S., et al. (2015). Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat. Commun. 6, 5966. doi:10.1038/ncomms6966
Dai, Z., Chen, J., Chang, Y., and Christiano, A. M. (2021). Selective inhibition of JAK3 signaling is sufficient to reverse alopecia areata. JCI Insight 6 (7), 142205. doi:10.1172/jci.insight.142205
Elwood, F., Witter, D. J., Piesvaux, J., Kraybill, B., Bays, N., Alpert, C., et al. (2017). Evaluation of JAK3 biology in autoimmune disease using a highly selective, irreversible JAK3 inhibitor. J. Pharmacol. Exp. Ther. 361 (2), 229–244. doi:10.1124/jpet.116.239723
Guo, L., Feng, S., Sun, B., Jiang, X., and Liu, Y. (2020). Benefit and risk profile of tofacitinib for the treatment of alopecia areata: A systemic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 34 (1), 192–201. doi:10.1111/jdv.15937
Harigai, M. (2019). Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology 58 (1), i34–i42. doi:10.1093/rheumatology/key287
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj 343, d5928. doi:10.1136/bmj.d5928
Jabbari, A., Sansaricq, F., Cerise, J., Chen, J. C., Bitterman, A., Ulerio, G., et al. (2018). An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type Alopecia areata, totalis, and universalis. J. Invest. Dermatol. 138 (7), 1539–1545. doi:10.1016/j.jid.2018.01.032
Kennedy Crispin, M., Ko, J. M., Craiglow, B. G., Li, S., Shankar, G., Urban, J. R., et al. (2016). Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI insight 1 (15), e89776. doi:10.1172/jci.insight.89776
King, B., Guttman-Yassky, E., Peeva, E., Banerjee, A., Sinclair, R., Pavel, A. B., et al. (2021a). A phase 2a randomized, placebo-controlled study to evaluate the efficacy and safety of the oral Janus kinase inhibitors ritlecitinib and brepocitinib in alopecia areata: 24-week results. J. Am. Acad. Dermatol. 85 (2), 379–387. doi:10.1016/j.jaad.2021.03.050
King, B., Ko, J., Forman, S., Ohyama, M., Mesinkovska, N., Yu, G., et al. (2021b). Efficacy and safety of the oral Janus kinase inhibitor baricitinib in the treatment of adults with alopecia areata: Phase 2 results from a randomized controlled study. J. Am. Acad. Dermatol. 85 (4), 847–853. doi:10.1016/j.jaad.2021.05.050
King, B., Kwon, O., Mesinkovska, N., Ko, J., Dutronc, Y., Wu, W., et al. (2021c). LB785 Efficacy and safety of baricitinib in adults with Alopecia Areata: Phase 3 results from a randomized controlled trial (BRAVE-AA1). J. Investigative Dermatology 141 (9), B18. doi:10.1016/j.jid.2021.07.049
King, B., Ohyama, M., Kwon, O., Zlotogorski, A., Ko, J., Mesinkovska, N. A., et al. (2022). Two phase 3 trials of baricitinib for alopecia areata. N. Engl. J. Med. 386 (18), 1687–1699. doi:10.1056/NEJMoa2110343
Ko, J., Roberts, J., Hordinsky, M., Taylor, S., Mostaghimi, A., Chiasserini, C., et al. (2021). 27604 Response to baricitinib in the treatment of patients with early and late onset alopecia areata in the phase 2 portion of BRAVE-AA1 randomized controlled trial. J. Am. Acad. Dermatology 85 (3), AB155. doi:10.1016/j.jaad.2021.06.634
Lai, V. W. Y., Bokhari, L., and Sinclair, R. (2021). Sublingual tofacitinib for alopecia areata: A roll-over pilot clinical trial and analysis of pharmacokinetics. Int. J. Dermatol. 60 (9), 1135–1139. doi:10.1111/ijd.15657
Lai, V. W. Y., Chen, G., Gin, D., and Sinclair, R. (2019). Systemic treatments for alopecia areata: A systematic review. Australas. J. Dermatol. 60 (1), e1–e13. doi:10.1111/ajd.12913
Lee, S., Kim, B. J., Lee, Y. B., and Lee, W.-S. (2018). Hair regrowth outcomes of contact immunotherapy for patients with alopecia areata: A systematic review and meta-analysis. JAMA Dermatol. 154 (10), 1145–1151. doi:10.1001/jamadermatol.2018.2312
Liu, L. Y., Craiglow, B. G., and King, B. A. (2018). Tofacitinib 2% ointment, a topical Janus kinase inhibitor, for the treatment of alopecia areata: A pilot study of 10 patients. J. Am. Acad. Dermatol. 78 (2), 403403–403404. doi:10.1016/j.jaad.2017.10.043
Mackay-Wiggan, J., Jabbari, A., Nguyen, N., Cerise, J. E., Clark, C., Ulerio, G., et al. (2016). Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI insight 1 (15), e89790. doi:10.1172/jci.insight.89790
Meah, N., Wall, D., York, K., Bhoyrul, B., Bokhari, L., Sigall, D. A., et al. (2020). The Alopecia Areata Consensus of Experts (ACE) study: Results of an international expert opinion on treatments for alopecia areata. J. Am. Acad. Dermatol. 83 (1), 123–130. doi:10.1016/j.jaad.2020.03.004
Mirzoyev, S. A., Schrum, A. G., Davis, M. D. P., and Torgerson, R. R. (2014). Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990-2009. J. Invest. Dermatol. 134 (4), 1141–1142. doi:10.1038/jid.2013.464
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Bmj 339, b2535. doi:10.1136/bmj.b2535
Neubauer, H., Cumano, A., Müller, M., Wu, H., Huffstadt, U., and Pfeffer, K. (1998). Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell 93 (3), 397–409. doi:10.1016/s0092-8674(00)81168-x
O'Shea, J. J., Kontzias, A., Yamaoka, K., Tanaka, Y., and Laurence, A. (2013). Janus kinase inhibitors in autoimmune diseases. Ann. Rheum. Dis. 72 (2), ii111–ii115. doi:10.1136/annrheumdis-2012-202576
O'Shea, J. J., Pesu, M., Borie, D. C., and Changelian, P. S. (2004). A new modality for immunosuppression: Targeting the JAK/STAT pathway. Nat. Rev. Drug Discov. 3 (7), 555–564. doi:10.1038/nrd1441
Olsen, E. A., Hordinsky, M. K., Price, V. H., Roberts, J. L., Shapiro, J., Canfield, D., et al. (2004). Alopecia areata investigational assessment guidelines--Part II. National Alopecia Areata Foundation. J. Am. Acad. Dermatol. 51 (3), 440–447. doi:10.1016/j.jaad.2003.09.032
Olsen, E. A., Kornacki, D., Sun, K., and Hordinsky, M. K. (2020). Ruxolitinib cream for the treatment of patients with alopecia areata: A 2-part, double-blind, randomized, vehicle-controlled phase 2 study. J. Am. Acad. Dermatol. 82 (2), 412–419. doi:10.1016/j.jaad.2019.10.016
Peeva, E., Guttman-Yassky, E., Banerjee, A., Sinclair, R., Cox, L. A., Zhu, L., et al. (2021). Maintenance, withdrawal and re-treatment with ritlecitinib and brepocitinib in patients with alopecia areata in a single-blind extension of a phase 2a randomized clinical trial. J. Am. Acad. Dermatol. 87, 390–393. doi:10.1016/j.jaad.2021.12.008
Petukhova, L., Duvic, M., Hordinsky, M., Norris, D., Price, V., Shimomura, Y., et al. (2010). Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 466 (7302), 113–117. doi:10.1038/nature09114
Phan, K., Ramachandran, V., and Sebaratnam, D. F. (2019). Methotrexate for alopecia areata: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 80 (1), 120–127. e122. doi:10.1016/j.jaad.2018.06.064
Phan, K., and Sebaratnam, D. F. (2019). JAK inhibitors for alopecia areata: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 33 (5), 850–856. doi:10.1111/jdv.15489
Pratt, C. H., King, L. E., Messenger, A. G., Christiano, A. M., and Sundberg, J. P. (2017). Alopecia areata. Nat. Rev. Dis. Prim. 3 (1), 17011. doi:10.1038/nrdp.2017.11
Safavi, K. H., Muller, S. A., Suman, V. J., Moshell, A. N., and Melton, L. J. (1995). Incidence of alopecia areata in olmsted county, Minnesota, 1975 through 1989. Mayo Clin. Proc. 70 (7), 628–633. doi:10.4065/70.7.628
Senna, M. M., McMichael, A. J., Mayo, T. T., Mackay-Wiggan, J., Glashofer, M., Sun, L., et al. (2021). 26143 Time to scalp hair, eyebrow, and eyelash improvement in patients with alopecia areata treated with baricitinib in the phase 2 portion of the phase 2/3 BRAVE-AA1 study. J. Am. Acad. Dermatology 85 (3), AB85. doi:10.1016/j.jaad.2021.06.365
Sterne, J. A., Hernán, M. A., Reeves, B. C., Savović, J., Berkman, N. D., Viswanathan, M., et al. (2016). ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. Bmj 355, i4919. doi:10.1136/bmj.i4919
Strazzulla, L. C., Wang, E. H. C., Avila, L., Lo Sicco, K., Brinster, N., Christiano, A. M., et al. (2018). Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J. Am. Acad. Dermatol. 78 (1), 1–12. doi:10.1016/j.jaad.2017.04.1141
Tosti, A., Bellavista, S., and Iorizzo, M. (2006). Alopecia areata: A long term follow-up study of 191 patients. J. Am. Acad. Dermatol. 55 (3), 438–441. doi:10.1016/j.jaad.2006.05.008
Winthrop, K. L. (2017). The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 13 (4), 234–243. doi:10.1038/nrrheum.2017.23
Keywords: alopecia areata, JAK inhibitors, janus kinase inhibitors, tofacitinib, ruxolitinib, baricitinib, meta-analysis, systematic review
Citation: Yan D, Fan H, Chen M, Xia L, Wang S, Dong W, Wang Q, Niu S, Rao H, Chen L, Nie X and Fang Y (2022) The efficacy and safety of JAK inhibitors for alopecia areata: A systematic review and meta-analysis of prospective studies. Front. Pharmacol. 13:950450. doi: 10.3389/fphar.2022.950450
Received: 22 May 2022; Accepted: 20 July 2022;
Published: 24 August 2022.
Edited by:
Tao Xu, Anhui Medical University, ChinaReviewed by:
Wei Hu, The Second Hospital of Anhui Medical University, ChinaCopyright © 2022 Yan, Fan, Chen, Xia, Wang, Dong, Wang, Niu, Rao, Chen, Nie and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liming Chen, Y2xtMTAwM0AxNjMuY29t; Xiaoyan Nie, bmlleHlAcGt1LmVkdS5jbg==; Yi Fang, cGhhc2Vpc3R1ZHlAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.