- 1Xiangya School of Pharmaceutical Sciences, Central South University, Changsha, China
- 2Hunan Provincial Key Laboratory of Cardiovascular Research, Xiangya School of Pharmaceutical Sciences, Central South University, Changsha, China
- 3Department of Pharmacy, Xiangya Hospital, Central South University, Changsha, China
- 4National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
- 5Geriatric Medical Center, People’s Hospital of Ningxia Hui Autonomous Region, Yinchuan, China
Cancer is one of the leading causes of death worldwide due to high morbidity and mortality. Many attempts and efforts have been devoted to fighting cancer. Owing to the significant role of the endoplasmic reticulum (ER) in cell function, inducing ER stress can be promising for cancer treatment. However, the sustained activation of cytoprotective unfolded protein response (UPR) presents a tremendous obstacle for drugs in inducing unsolved ER stress in tumor cells, especially small-molecule drugs with poor bioavailability. Therefore, many emerging nanodrugs inducing and amplifying ER stress have been developed for efficient cancer treatment. More importantly, the novel discovery of ER stress in immunogenic cell death (ICD) makes it possible to repurpose antitumor drugs for immunotherapy through nanodrug-based strategies amplifying ER stress. Therefore, this mini-review aims to provide a comprehensive summary of the latest developments of the strategies underlying nanodrugs in the treatment of cancer via manipulating ER stress. Meanwhile, the prospects of ER stress–inducing nanodrugs for cancer treatment are systematically discussed, which provide a sound platform for novel therapeutic insights and inspiration for the design of nanodrugs in treating cancer.
Introduction
Consistently, cancer treatment is a long-standing conundrum within the field of medicine. Currently, the main treatment modalities for cancer are still based on chemotherapy, radiotherapy, and surgery. However, these traditional treatments elicit significant side effects and even tissue damage. For example, chemotherapy often causes severe hepatotoxicity and nephrotoxicity (Zhao et al., 2018; Zhao et al., 2022a; Liu et al., 2022; Xiao et al., 2022), which are responsible for severe deleterious hepatic and renal dysfunctions in patients. Therapeutic strategies targeting key organelles have attracted considerable attention, on account of the important role of organelles in maintaining the normal physiological function of cells. The endoplasmic reticulum (ER) is a central organelle that carries out many important functions such as synthesizing and folding proteins, modifying secreted and transmembrane proteins, regulating lipid synthesis and metabolism, storing calcium, and mediating signal transduction (King and Wilson, 2020). In addition, ER is closely apposed and dynamically tethered to other organelles through membrane networks, such as the nucleus, mitochondria, and Golgi apparatus (Chen and Cubillos-Ruiz, 2021). Once the ER is injured, the physiology of the entire cell gets adversely affected. Therefore, the tightly regulated process of ER function is crucial for cell fate determination.
Many factors, such as intracellular reactive oxygen species (ROS) and nutrient deprivation, disturb ER functioning in inducing ER stress based on the accumulation of unfolded and misfolded proteins in the ER (Yao et al., 2017). The initial adaptive mechanisms such as unfolded protein response (UPR) in tumor cells provide a possibility to restore and maintain protein homeostasis, but unsolved ER stress still leads to cell death (Chen and Cubillos-Ruiz, 2021). Some clinical chemotherapeutics are found to be associated with ER stress induction, while the upregulation of UPR promotes resistance (Bahar et al., 2019). Therefore, many drugs have been proposed to induce and exacerbate severe ER stress in killing tumor cells as potential therapeutics. For example, molecular chaperone binding immunoglobulin (BiP), an ER stress sensor, is highly expressed in mediating tumor chemotherapy resistance by activating UPR to restore ER homeostasis in cancer cells (Hetz et al., 2020). Currently, many BiP inhibitors have been developed for killing tumors, such as KP1339 (Wernitznig et al., 2019) and HA15 (Cerezo et al., 2016).
However, the clinical application of these ER-targeted small-molecule drugs faces great bottlenecks, such as the lack of tumor targeting and strong side effects. The development of nanodrugs offers a possibility to address the dilemma in traditional drugs (Shi et al., 2021). The enhanced penetration and retention (EPR) effects mediate the accumulation of nanodrugs at tumor sites (Huang et al., 2022) to reduce the risk of toxicity in normal tissues. Moreover, nanodrugs can be modified with specific ligands targeting tumor cells or ER (Li et al., 2020) to further improve the enrichment of nanodrugs in tumor sites (Yang et al., 2022) and anticancer efficacy. Notably, nanodrugs can induce immunogenic cell death (ICD) to effectively improve immunotherapy by amplifying ER stress. Specifically, when the nanodrugs disrupt the ER, ER stress induces imbalances in calcium homeostasis, and calreticulin (CRT) transfers from the ER to the cell membrane to act as an “eat-me” signal, inducing inflammatory cell infiltration and enhancing tumor cell antigens presented.
To our knowledge, no similar reviews have been published in summarizing newly developed nanodrugs based on ER stress for cancer treatment. Herein, this review covers the recent progress in oncology therapeutics about nanodrugs for ER stress induction, recapitulating the design of nanodrugs that induce ER stress, together with the strategies of nanodrugs that amplify ER stress to repurpose antitumor drugs for cancer immunotherapy (Figure 1) (Table 1). Finally, the obstacles and prospects of ER stress–based nanodrugs for cancer treatment are discussed.
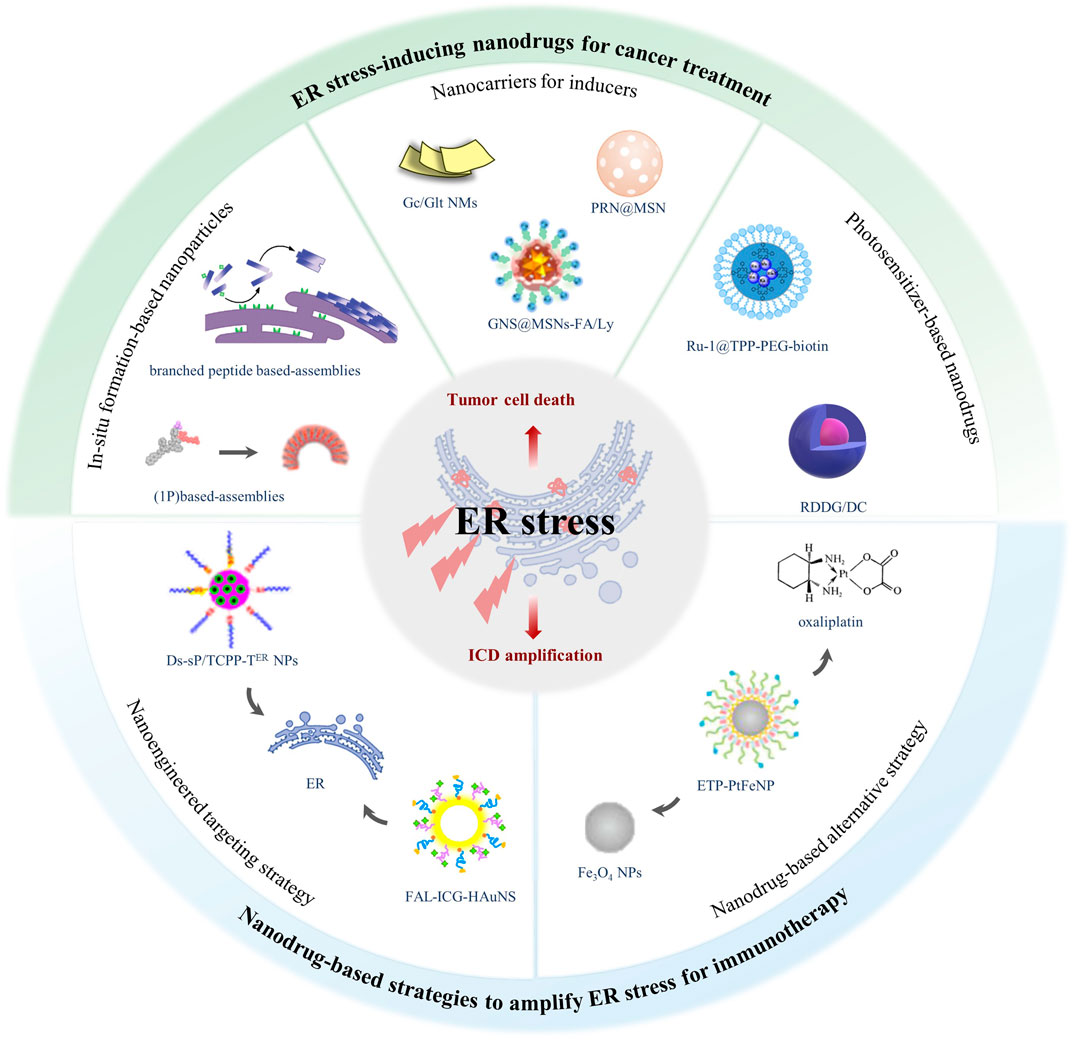
FIGURE 1. The scope and focus of this article. Accumulation of misfolded protein results in ER stress, which can induce antitumor effects by triggering tumor cell death and amplifying ICD. ER stress–inducing nanodrugs can treat cancer through activating unsolved ER stress. In addition, nanodrugs amplifying ER stress via ER-targeting strategy and combination strategy make it possible for traditional ICD inducers to initiate antitumor immunotherapy.
Endoplasmic Reticulum Stress–Inducing Nanodrugs for Cancer Treatment
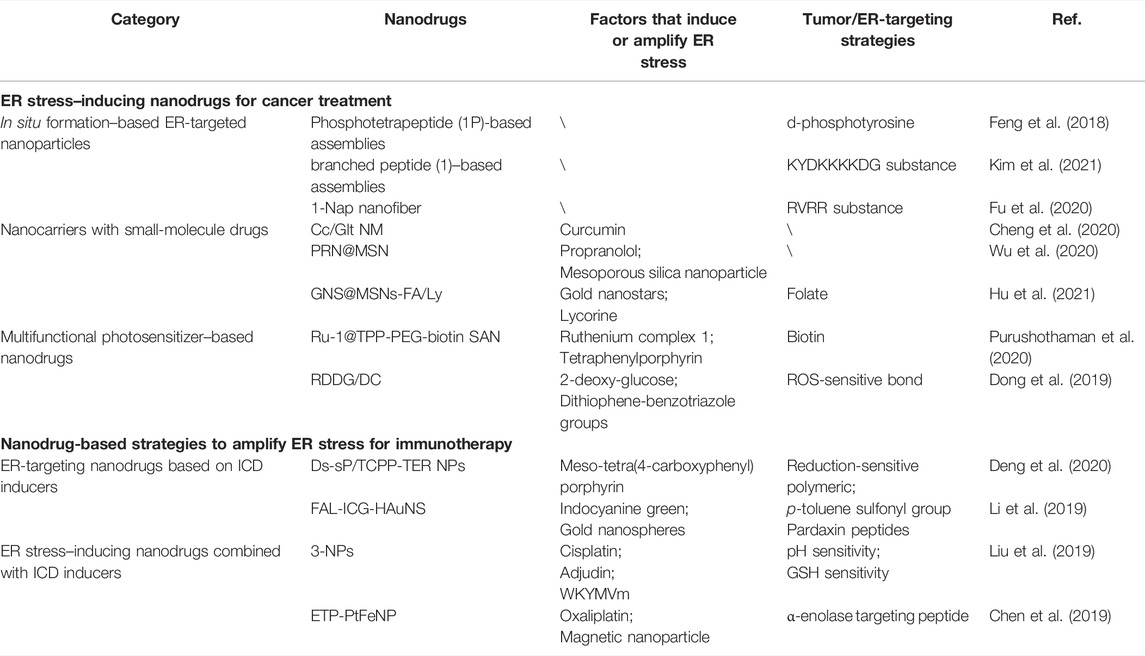
ER stress is a cellular condition characterized by the unsolved accumulation of misfolded proteins, which is detrimental to the organism. Misfolded proteins have a higher affinity to molecular chaperone BiP and activate sensors of ER stress via titrating BiP from sensors [PKR-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 (IRE1α)], termed UPR. Subsequently, the activation of sensors initiate cytoprotective mechanisms, including inducing transcription of the cell-protective molecule, directing the protein to the ubiquitin-proteasome system (UPS), promoting protective autophagy, and so on. Therefore, there are two strategies to induce ER stress as expected: promoting the production of misfolded proteins and inhibiting UPR-mediated protective effects (Marciniak et al., 2022). Currently, many emerging nanodrugs targeting ER have been developed for efficient cancer treatment. According to the pharmacological mechanism, these nanodrugs are mainly divided into the following three categories: the first category, the in situ formation-based ER-targeted nanoparticles to induce ER stress by enzyme-instructed self-assembly (EISA); the second category, nanocarriers for small-molecule drugs substantially induce ER stress by improving the targeting efficiency; the third category, photodynamic therapy (PDT)–based nanodrugs amplify ER stress by generating uncontrolled ROS.
In Situ Formation–Based Endoplasmic Reticulum–Targeted Nanoparticles
EISA technology is an effective method for in situ self-assembly, which is a benefit for organelles targeting and inducing stress (Ji et al., 2021). The overexpressed enzymes in tumor cells can achieve targeted enrichment and improve selectivity for some molecules containing specific amino acid sequence substrates for these enzymes. These specially designed molecules can self-assemble into ER-targeted nanoforms to induce ER stress through membrane lipid action in cancer cells after the substrate fragments are cleaved by these enzymes (Gao et al., 2020; Xiang et al., 2022a). Recently, Feng et al. (2018) adopted alkaline phosphatase (ALP), highly expressed in tumor cells (HeLa cells), to construct an EISA-related phosphotetrapeptide (1P)-based ER inducer for cancer therapy. The 1P precursor contained a d-phosphotyrosine as a specific substrate of ALP, and positively charged l-homoarginine, which targeted the ER. After the 1P precursor was catalyzed by ALP to generate 1P, 1P self-assembled on the surface of the cancer cell membrane to form unique crescent-shaped 1P nanoparticles. Subsequently, the 1P nanoparticles were selectively enriched in the ER to induce ER stress. Similarly, Kim et al. (2021) utilized trypsin-1, which was overexpressed in the ER of a high-grade serous ovarian cancer cell line (OVSAHO), to develop a trypsin-1 (PRSS1)–based branched peptide chain. After proteolysis, the branched peptide chains formed peptide assemblies to accumulate in the ER, upregulated ER stress-related proteins, and killed OVSAHO cells through various death pathways. In addition, furin that is highly expressed in many malignant tumor cells (Fu et al., 2020) has been used to treat cancer. Based on its location in the trans-Golgi network, furin-instruct EISA blocked the transport of ER to the Golgi apparatus to induce ER stress and cancer cell death.
Nanocarriers With Small-Molecule Drugs
Nanocarriers were also developed for ER-targeted small-molecule drugs to improve their efficacy in tumors (Wang et al., 2021). Cheng et al. (2020) encapsulated curcumin in gelatin-blended nanofibrous mat (Cc/Glt NM) to address the inherent insolubility, instability, poor absorption, and rapid systemic elimination of curcumin. Cc/Glt NM effectively entered cancer cells to release curcumin, which activated the BiP/p-PERK/p-elF2a pathway to induce ER stress. As expected, Cc/Glt NM significantly reduced the tumor volume in pancreatic adenocarcinoma (PDAC) tumor–bearing mice with enhanced ER stress levels after topical application. In addition, Wu et al. (2020) adopted mesoporous silica nanoparticle (MSN) to deliver propranolol (PRN), the first-line therapy for hemangiomas. PRN@MSN strongly inhibited the formation of microvessels in murine hemangioma models via increased ER stress, where MSN induced ER stress and PRN inhibited autophagy that was resistant to ER stress. Moreover, Hu et al. (2021) integrated gold nanostars (GNS) and the antineoplastic drug lycorine (Ly) into MSNs with modified tumor-targeted folate (FA). GNS@MSNs-FA/Ly exhibited highly specific tumor growth inhibition in osteosarcoma cell tumor–bearing mice without additional side effects, among which Ly promoted mitochondrial dysfunction to interfere with ER via endoplasmic reticulum–mitochondrial contact (Giacomello et al., 2020).
Multifunctional Photosensitizer-Based Nanodrugs
PDT is one of the potential therapeutic strategies for cancers that generates cytotoxic ROS by the reaction of photosensitizers with oxygen (O2) under the light (Zhao et al., 2022b; Long et al., 2022). The ROS generated during PDT attack proteins to form toxic ROS-modified proteins, which can provoke ER stress by inducing the accumulation of toxic protein (Chen et al., 2021; Zhu et al., 2022) in the ER. In addition, excessive intracellular ROS affect ER-resident calcium channels (Görlach et al., 2015) and promote lipid peroxidation (Vladykovskaya et al., 2012), which also disturb the homeostasis of the ER. However, a single photosensitizer has limited utility to induce ER as well. Further amplification of ER stress is also essential for photosensitizers. Multifunctional nanodrugs can combine varying molecules and materials with photosensitizers into one nanoscale entity to bring the most efficient functionality against tumors.
Recently, Purushothaman et al. (2020) developed Ru-1@TPP-PEG-biotin self-assembled nanoparticles (Ru-1@TPP-PEG-biotin SAN) for cancer therapy. Ru-1@TPP-PEG-biotin SAN was loaded with ruthenium complex 1 (Ru-1, an inhibitor of chaperone GRP78 functions) as an ER stress inducer and photosensitizer. Ru-1@TPP-PEG-biotin SAN induced degradation of the lysosome and inhibition of autophagy, which benefited the release of Ru-1 and the induction of ER stress to cause strong cytotoxicity in MCF-7 and HepG2 cells. Combining photosensitizers and various ER stress inducers presented more potent and efficient antitumor effects than separately administering them. Dong et al. (2019) further developed a multifunctional photosensitizer-based nanodrug (RDDG/DC NPs) for treating breast cancer. The photosensitizers (polymers with dithiophene-benzotriazole groups, abbreviated with the letter C) and doxorubicin (a widely used chemotherapy drug, abbreviated with the letter D) were encapsulated in the ROS-sensitive dextran with 2-deoxy-glucose (2-DG, an ER stress inducer that interferes with N-linked glycosylation). The 2-DG and photosensitizers synthetically induced severe ER stress, which significantly increased CHOP mRNA (related to ER stress), and high cytotoxicity was observed in MCF-7 breast cancer cells treated with RDDG/DC NPs and light irradiation. Eventually, the well-fabricated multifunctional nanodrugs targeted the tumor site and exhibited the most potent suppressing effects on tumor growth under light illumination.
Nanodrug-Based Strategies to Amplify Endoplasmic Reticulum Stress for Immunotherapy
Antitumor immunotherapy, which boosts the body’s own immune system’s ability to recognize and attack tumor cells, represents one of the most promising advances in modern medicine (Wang et al., 2021). However, the immunosuppressive tumor microenvironment and poor tumor immunogenicity discourage tumor cells from immune attack, which confronts immunotherapy with enormous challenges (Dong et al., 2021). ICD is a special form of cell death, activating an immune response to recognize antigens of dead or dying tumor cells (Banstola et al., 2021). The expression and release of death-association molecular patterns (DAMPs), such as CRT and high mobility group box 1 (HMGB1), promote dendritic cell (DC) maturation and antigen presentation during ICD (Kroemer et al., 2022). More immunogen exposure to tumors’ signals potentiates an immune effect, which is beneficial for treating malignant tumors. However, most ICD inducers have poor antitumor immunity because ICD-related danger signaling is not their original pharmacology mechanism but a consequence of collateral ER stress effects.
ER stress has been shown to contribute to ICD. When ER stress occurs, abundant CRTs in the ER translocate to the cell surface as a signal for immune system recognition and antigen presentation, often referred to as the “eat me” signaling. Photosensitizers have the potential to be ICD inducers due to their ability to induce ER stress, but only a small amount of them have been applied in ER-associated ICD-induced research on account of their existing but limited influences on ER stress. Some promising strategies of nanodrugs to amplify ER stress benefit antitumor immunotherapy of these limited ICD inducers.
Endoplasmic Reticulum–Targeting Nanodrugs Based on Immunogenic Cell Death Inducers
A few ICD inducers, such as hypericin (an ER-target photosensitizer) (Lin et al., 2017), induce highly efficient ICD by directly and selectively targeting the ER. Therefore, engineering ER-targeting nanodrugs based on ICD inducers is an effective strategy for enhancing the ICD-associated antitumor immunity. Deng et al. (2020) modified meso-tetra(4-carboxyphenyl) porphyrin (TCPP) with N-tosylethylenediamine to form an ER-targeting photosensitizer TCPP-TER. Notably, the p-toluene sulfonyl group of TCPP-TER recognized the ATP-sensitive K+ channel (sulfonylurea receptor) on the ER. Then, TCPP-TER was loaded to reduction-sensitive polymer (Ds-sP) to respond to the tumor microenvironment. The dual-targeting of smart Ds-sP/TCPP-TER nanoparticles released TCPP-TER in tumor sites with high GSH levels and ensured the accumulation of TCPP-TER in the ER. The ROS generated from TCPP-TER under light exposure activated ER stress directly and increased the translocation of CRTs to the cell membrane in 4T1 cells (human breast cancer cell lines). The Ds-sP/TCPP-TER nanoparticles successfully inhibited the growth of primary and distant tumors along with evaluating the proportion of CD8+ T cells in tumor tissues.
Additionally, Li et al. (2019) fabricated an ER target nanodrug, pardaxin (FAL) peptides–modified indocyanine green (ICG)–conjugated hollow gold nanospheres (FAL-ICG-HAuNS). The AuNS were an excellent carrier with both photothermal properties and ER stress–inducing function, which exerted a synergistic effect with photosensitizer ICG. FAL-ICG-HAuNS increased CHOP and CRTs on the cell surface under light exposure. The ER stress induced by FAL-ICG-HAuNS was ROS-dependent and was blocked by antioxidant vitamin C. To overcome the limitations of the hypoxia tumor microenvironment on PDT, the oxygen-delivering hemoglobin (Hb) liposome (FAL-Hb lipo) was adopted to provide sufficient O2. FAL-ICG-HAuNS inhibited tumor growth and prolonged survival time of CT-26 tumor–bearing mice and B16 tumor–bearing mice, which was reversed by depleting either CD4+ or CD8+ T cells, demonstrating the important role of ICD in tumor killing. In summary, the nanoengineered targeting strategy successfully enhanced ICD and related antitumor immunity by amplifying ER stress.
Endoplasmic Reticulum Stress–Inducing Nanodrugs Combined With Immunogenic Cell Death Inducers
Clinically available chemotherapeutic drugs, such as DOX, platinum-based drugs, cyclophosphamide, and so on, belong to the ICD inducers via collateral ER stress effects (Tham et al., 2020). The increasing targeting of ER benefits the ICD-inducing ability of these inducers. However, the increasing immunogenicity is accompanied by less cytotoxicity because they initiate cell death via non–ER-related targets (Xiang et al., 2022b). Therefore, combining different ER stress inducers and ICD inducers to form an alternative ICD inducer with strong antitumor efficacy is a promising strategy to promote ICD-associated immunogenicity and preserve original cytotoxicity.
Recently, Xu et al. (2019) combined cisplatin and adjudin (ADD) into a multi-responsive peptide-based prodrug platform for cancer therapy. ADD was a derivative of lonidamine with potent antitumor effects, and it significantly increased intracellular ROS levels by attacking the mitochondria. The combination of cisplatin and ADD along with tumor targeting from the nanoplatform amplified CRTs exposure, ATP secretion, and HMGB-1 release. To further improve antitumor immunity, WKYMVm were loaded to nanoparticles to form 3-NPs assembled from 2-(Nap)-FFKPt-2TPA-ADDGGGPLGVRG-WKYMVm-mPEG1000. WKYMVm was an agonist of formyl peptide receptor 1 (FRR-1) which facilitated DCs to touch and contact with dying tumor cells stably. More importantly, 3-NPs demonstrated minimal lung metastases surprising rate of tumor shrinkage, as high as 93.1% in the 4T1 orthotopic tumor model. Therefore, 3-NPs were efficient in treating triple-negative breast cancer (TNBC) and inhibiting tumor metastasis via provoking innate and adaptive anti-TNBC immunity. In addition, Chen et al. (2019) constructed a core-shell magnetic nanoparticle (FeNP) to load oxaliplatin (the third-generation star product of platinum-based drugs) with a modified tumor-targeting peptide (α-enolase-targeting peptide, ETP) (ETP-PtFeNP). The ETP-PtFeNP induced intracellular Fenton’s reaction and elicited ROS bursting (Chen et al., 2022), which was partially beneficial for ICD. ERS-mediated ICD activation was demonstrated by markedly elevated levels of CRT on the cell surface in the ETP-PtFeNP–treated group. ETP-PtFeNP eventually activated entire-body immunity and suppressed tumor growth in the 4T1 tumor–bearing balb/c mice.
Conclusion and Prospects
This article reviewed the tumor treatment strategies of ER stress–inducing nanodrugs. Nanodrugs are specifically enriched in tumor sites and achieve efficient treatment of cancer via the enhancement of ER stress. Moreover, a combination of various ER stress inducers in a nanoplatform can further amplify ER stress and demonstrate attractive antitumor effects. In addition, nanodrug-based strategies to amplify ER stress repurposed those antitumor drugs for immunotherapy and acquired amazing results, considering the importance of ER stress for ICD.
Although significant progress has been made in this emerging field, there are still some unsolved questions about these nanodrugs. First, ER exists in almost all cells, which represents the wide toxic side effects of ER-targeting nanodrugs. As such, precise tumor cell targeting is imperative. Second, many ROS-based drugs are limited by tumor environment hypoxia in tumor therapy, especially the strategy that requires direct utilization of O2 like PDT (Long et al., 2022). Although the work conducted by Li et al. (2019) provided a direction for breaking the restriction, there was still a long way to overcome this challenge. Finally, the ER targeting of many nanodrugs is achieved through the modification of targeting peptides, but the modification of macromolecules such as proteins increases the difficulty of synthesis. More superior ER targeting strategies, such as nanoliposome targeting ER, require further deep study (Shi et al., 2021).
Overall, nanodrugs-induced/amplified ER stress is available to increase ICD and antitumor effects. Of whatever function as cytotoxic antitumor drugs or immunotherapeutic drugs, the ER stress-inducing nanodrugs are potential and promising for cancer treatment. At the same time, the ideas of nanodrugs-based strategies based on ER stress are also beneficial for the treatment of other diseases.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the National Natural Science Foundation of China, China (Nos. 81974508, 21974134), the Hunan Science Fund for Distinguished Young Scholar (No. 2021JJ10067), Innovation-Driven Project of Central South University (No. 202045005), Hunan Provincial Natural Science Foundation of China (No. 2021JJ31066), Key Research Project of Ningxia Hui Autonomous Region in 2021 (Major Project) (No. 2021BEG01001), and The Key Program of Ningxia Hui Autonomous Region Natural Science Foundation of China (No. 2022JJ21059).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bahar, E., Kim, J.-Y., and Yoon, H. (2019). Chemotherapy Resistance Explained through Endoplasmic Reticulum Stress-dependent Signaling. Cancers 11, 338. doi:10.3390/cancers11030338
Banstola, A., Poudel, K., Kim, J. O., Jeong, J.-H., and Yook, S. (2021). Recent Progress in Stimuli-Responsive Nanosystems for Inducing Immunogenic Cell Death. J. Control. Release 337, 505–520. doi:10.1016/j.jconrel.2021.07.038
Cerezo, M., Lehraiki, A., Millet, A., Rouaud, F., Plaisant, M., Jaune, E., et al. (2016). Compounds Triggering ER Stress Exert Anti-melanoma Effects and Overcome BRAF Inhibitor Resistance. Cancer Cell. 29, 805–819. doi:10.1016/j.ccell.2016.04.013
Chen, L., Huang, Q., Zhao, T., Sui, L., Wang, S., Xiao, Z., et al. (2021). Nanotherapies for Sepsis by Regulating Inflammatory Signals and Reactive Oxygen and Nitrogen Species: New Insight for Treating COVID-19. Redox Biol. 45, 102046. doi:10.1016/j.redox.2021.102046
Chen, Q., Li, N., Wang, X., Yang, Y., Xiang, Y., Long, X., et al. (2022). Mitochondria-Targeting Chemodynamic Therapy Nanodrugs for Cancer Treatment. Front. Pharmacol. 13, 847048. doi:10.3389/fphar.2022.847048
Chen, Q., Liu, L., Lu, Y., Chen, X., Zhang, Y., Zhou, W., et al. (2019). Tumor Microenvironment‐Triggered Aggregated Magnetic Nanoparticles for Reinforced Image‐Guided Immunogenic Chemotherapy. Adv. Sci. 6, 1802134. doi:10.1002/advs.201802134
Chen, X., and Cubillos-Ruiz, J. R. (2021). Endoplasmic Reticulum Stress Signals in the Tumour and its Microenvironment. Nat. Rev. Cancer 21, 71–88. doi:10.1038/s41568-020-00312-2
Cheng, T., Zhang, Z., Shen, H., Jian, Z., Li, J., Chen, Y., et al. (2020). Topically Applicated Curcumin/gelatin-Blended Nanofibrous Mat Inhibits Pancreatic Adenocarcinoma by Increasing ROS Production and Endoplasmic Reticulum Stress Mediated Apoptosis. J. Nanobiotechnol 18, 126. doi:10.1186/s12951-020-00687-2
Deng, H., Zhou, Z., Yang, W., Lin, L.-s., Wang, S., Niu, G., et al. (2020). Endoplasmic Reticulum Targeting to Amplify Immunogenic Cell Death for Cancer Immunotherapy. Nano Lett. 20, 1928–1933. doi:10.1021/acs.nanolett.9b05210
Dong, M., Xiao, X. Z., Su, Z. G., Yu, Z. H., Qian, C. G., Liu, J. H., et al. (2019). Small 15, e1900212. doi:10.1002/smll.201900212
Dong, Y., Zhang, S., Gao, X., Yin, D., Wang, T., Li, Z., et al. (2021). HIF1α Epigenetically Repressed Macrophages via CRISPR/Cas9-EZH2 System for Enhanced Cancer Immunotherapy. Bioact. Mater. 6, 2870–2880. doi:10.1016/j.bioactmat.2021.02.008
Feng, Z., Wang, H., Wang, S., Zhang, Q., Zhang, X., Rodal, A. A., et al. (2018). Enzymatic Assemblies Disrupt the Membrane and Target Endoplasmic Reticulum for Selective Cancer Cell Death. J. Am. Chem. Soc. 140, 9566–9573. doi:10.1021/jacs.8b04641
Fu, C., Zhan, J., Huai, J., Ma, S., Li, M., Chen, G., et al. (2020). Furin-instructed Molecular Self-Assembly Actuates Endoplasmic Reticulum Stress-Mediated Apoptosis for Cancer Therapy. Nanoscale 12, 12126–12132. doi:10.1039/d0nr00151a
Gao, J., Zhan, J., and Yang, Z. (2020). Enzyme‐Instructed Self‐Assembly (EISA) and Hydrogelation of Peptides. Adv. Mat. 32, 1805798. doi:10.1002/adma.201805798
Giacomello, M., Pyakurel, A., Glytsou, C., and Scorrano, L. (2020). The Cell Biology of Mitochondrial Membrane Dynamics. Nat. Rev. Mol. Cell. Biol. 21, 204–224. doi:10.1038/s41580-020-0210-7
Görlach, A., Bertram, K., Hudecova, S., and Krizanova, O. (2015). Calcium and ROS: A Mutual Interplay. Redox Biol. 6, 260–271. doi:10.1016/j.redox.2015.08.010
Hetz, C., Zhang, K., and Kaufman, R. J. (2020). Mechanisms, Regulation and Functions of the Unfolded Protein Response. Nat. Rev. Mol. Cell. Biol. 21, 421–438. doi:10.1038/s41580-020-0250-z
Hu, H., Yang, W., Liang, Z., Zhou, Z., Song, Q., Liu, W., et al. (2021). Amplification of Oxidative Stress with Lycorine and Gold-Based Nanocomposites for Synergistic Cascade Cancer Therapy. J. Nanobiotechnol 19, 221. doi:10.1186/s12951-021-00933-1
Huang, J., Huang, Q., Liu, M., Chen, Q., and Ai, K. (2022). Emerging Bismuth Chalcogenides Based Nanodrugs for Cancer Radiotherapy. Front. Pharmacol. 13, 844037. doi:10.3389/fphar.2022.844037
Ji, S., Li, J., Duan, X., Zhang, J., Zhang, Y., Song, M., et al. (2021). Targeted Enrichment of Enzyme‐Instructed Assemblies in Cancer Cell Lysosomes Turns Immunologically Cold Tumors Hot. Angew. Chem. Int. Ed. 60, 26994–27004. doi:10.1002/anie.202110512
Kim, B. J., Fang, Y., He, H., and Xu, B. (2021). Adv. Healthc. Mater 10, e2000416. doi:10.1002/adhm.202000416
King, A. P., and Wilson, J. J. (2020). Endoplasmic Reticulum Stress: an Arising Target for Metal-Based Anticancer Agents. Chem. Soc. Rev. 49, 8113–8136. doi:10.1039/d0cs00259c
Kroemer, G., Galassi, C., Zitvogel, L., and Galluzzi, L. (2022). Immunogenic Cell Stress and Death. Nat. Immunol. 23, 487–500. doi:10.1038/s41590-022-01132-2
Li, J., Gao, H., Liu, R., Chen, C., Zeng, S., Liu, Q., et al. (2020). Endoplasmic Reticulum Targeted AIE Bioprobe as a Highly Efficient Inducer of Immunogenic Cell Death. Sci. China Chem. 63, 1428–1434. doi:10.1007/s11426-020-9846-4
Li, W., Yang, J., Luo, L., Jiang, M., Qin, B., Yin, H., et al. (2019). Targeting Photodynamic and Photothermal Therapy to the Endoplasmic Reticulum Enhances Immunogenic Cancer Cell Death. Nat. Commun. 10, 3349. doi:10.1038/s41467-019-11269-8
Lin, S., Yang, L., Shi, H., Du, W., Qi, Y., Qiu, C., et al. (2017). Endoplasmic Reticulum-Targeting Photosensitizer Hypericin Confers Chemo-Sensitization towards Oxaliplatin through Inducing Pro-death Autophagy. Int. J. Biochem. Cell. Biol. 87, 54–68. doi:10.1016/j.biocel.2017.04.001
Liu, M., Huang, Q., Zhu, Y., Chen, L., Li, Y., Gong, Z., et al. (2022). Harnessing Reactive Oxygen/nitrogen Species and Inflammation: Nanodrugs for Liver Injury. Mater. Today Bio 13, 100215. doi:10.1016/j.mtbio.2022.100215
Liu, P., Xiang, Y., Liu, X., Zhang, T., Yang, R., Chen, S., et al. (2019). Molecules 24. doi:10.3390/molecules24030647
Long, X., Zhang, X., Chen, Q., Liu, M., Xiang, Y., Yang, Y., et al. (2022). Nucleus-Targeting Phototherapy Nanodrugs for High-Effective Anti-cancer Treatment. Front. Pharmacol. 13, 905375. doi:10.3389/fphar.2022.905375
Marciniak, S. J., Chambers, J. E., and Ron, D. (2022). Pharmacological Targeting of Endoplasmic Reticulum Stress in Disease. Nat. Rev. Drug Discov. 21, 115–140. doi:10.1038/s41573-021-00320-3
Purushothaman, B., Lee, J., Hong, S., and Song, J. M. (2020). Multifunctional TPP-PEG-Biotin Self-Assembled Nanoparticle Drug Delivery-Based Combination Therapeutic Approach for Co-targeting of GRP78 and Lysosome. J. Nanobiotechnol 18, 102. doi:10.1186/s12951-020-00661-y
Shi, Y., Wang, S., Wu, J., Jin, X., and You, J. (2021). Pharmaceutical Strategies for Endoplasmic Reticulum-Targeting and Their Prospects of Application. J. Control. Release 329, 337–352. doi:10.1016/j.jconrel.2020.11.054
Tham, M. J. R., Babak, M. V., and Ang, W. H. (2020). PlatinER: A Highly Potent Anticancer Platinum(II) Complex that Induces Endoplasmic Reticulum Stress Driven Immunogenic Cell Death. Angew. Chem. Int. Ed. 59, 19070–19078. doi:10.1002/anie.202008604
Vladykovskaya, E., Sithu, S. D., Haberzettl, P., Wickramasinghe, N. S., Merchant, M. L., Hill, B. G., et al. (2012). Lipid Peroxidation Product 4-Hydroxy-Trans-2-Nonenal Causes Endothelial Activation by Inducing Endoplasmic Reticulum Stress. J. Biol. Chem. 287, 11398–11409. doi:10.1074/jbc.m111.320416
Wang, J., Sui, L., Huang, J., Miao, L., Nie, Y., Wang, K., et al. (2021). MoS2-based Nanocomposites for Cancer Diagnosis and Therapy. Bioact. Mater. 6, 4209–4242. doi:10.1016/j.bioactmat.2021.04.021
Wernitznig, D., Kiakos, K., Del Favero, G., Harrer, N., Machat, H., Osswald, A., et al. (2019). First-in-class Ruthenium Anticancer Drug (KP1339/IT-139) Induces an Immunogenic Cell Death Signature in Colorectal Spheroids In Vitro. Metallomics 11, 1044–1048. doi:10.1039/c9mt00051h
Wu, H., Wang, X., Liang, H., Zheng, J., Huang, S., and Zhang, D. (2020). Enhanced Efficacy of Propranolol Therapy for Infantile Hemangiomas Based on a Mesoporous Silica Nanoplatform through Mediating Autophagy Dysfunction. Acta Biomater. 107, 272–285. doi:10.1016/j.actbio.2020.02.033
Xiang, Y., Chen, L., Liu, C., Yi, X., Li, L., and Huang, Y. (2022). Small 18, e2104591. doi:10.1002/smll.202104591
Xiang, Y., Li, N., Liu, M., Chen, Q., Long, X., Yang, Y., et al. (2022). Nanodrugs Detonate Lysosome Bombs. Front. Pharmacol. 13, 909504. doi:10.3389/fphar.2022.909504
Xiao, Z., Huang, Q., Yang, Y., Liu, M., Chen, Q., Huang, J., et al. (2022). Emerging Early Diagnostic Methods for Acute Kidney Injury. Theranostics 12, 2963–2986. doi:10.7150/thno.71064
Xu, C., Yu, Y., Sun, Y., Kong, L., Yang, C., Hu, M., et al. (2019). Transformable Nanoparticle-Enabled Synergistic Elicitation and Promotion of Immunogenic Cell Death for Triple-Negative Breast Cancer Immunotherapy. Adv. Funct. Mater. 29, 1905213. doi:10.1002/adfm.201905213
Yang, Y., Huang, Q., Xiao, Z., Liu, M., Zhu, Y., Chen, Q., et al. (2022). Nanomaterial-based Biosensor Developing as a Route toward In Vitro Diagnosis of Early Ovarian Cancer. Mater. Today Bio 13, 100218. doi:10.1016/j.mtbio.2022.100218
Yao, Y., Lu, Q., Hu, Z., Yu, Y., Chen, Q., and Wang, Q. K. (2017). A Non-canonical Pathway Regulates ER Stress Signaling and Blocks ER Stress-Induced Apoptosis and Heart Failure. Nat. Commun. 8, 133. doi:10.1038/s41467-017-00171-w
Zhao, H., Huang, J., Miao, L., Yang, Y., Xiao, Z., Chen, Q., et al. (2022). Toward Urease-free Wearable Artificial Kidney: Widened Interlayer Spacing MoS2 Nanosheets with Highly Effective Adsorption for Uremic Toxins. Chem. Eng. J. 438, 135583. doi:10.1016/j.cej.2022.135583
Zhao, R., Liu, X., Yang, X., Jin, B., Shao, C., Kang, W., et al. (2018). Nanomaterial-Based Organelles Protect Normal Cells against Chemotherapy-Induced Cytotoxicity. Adv. Mat. 30, 1801304. doi:10.1002/adma.201801304
Zhao, T., Wu, W., Sui, L., Huang, Q., Nan, Y., Liu, J., et al. (2022). Reactive Oxygen Species-Based Nanomaterials for the Treatment of Myocardial Ischemia Reperfusion Injuries. Bioact. Mater. 7, 47–72. doi:10.1016/j.bioactmat.2021.06.006
Keywords: endoplasmic reticulum stress, tumor, immunogenic cell death, nanodrugs, photodynamic therapy
Citation: Xiang Y, Liu M, Yang Y, Wang Y, Qiu Y, Tu S, Jiang Y, Nan Y, Zhang X and Huang Q (2022) Nanodrugs Manipulating Endoplasmic Reticulum Stress for Highly Effective Antitumor Therapy. Front. Pharmacol. 13:949001. doi: 10.3389/fphar.2022.949001
Received: 20 May 2022; Accepted: 09 June 2022;
Published: 12 July 2022.
Edited by:
Zeming Liu, The Huazhong University of Science and Technology, ChinaReviewed by:
Wu-Yi Sun, Anhui Medical University, ChinaJianhua Liu, The Second Affiliated Hospital of Jilin University, China
Copyright © 2022 Xiang, Liu, Yang, Wang, Qiu, Tu, Jiang, Nan, Zhang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiong Huang, cWlvbmdodWFuZ0Bjc3UuZWR1LmNu; Xiaojie Zhang, ODAzMzc1QGNzdS5lZHUuY24=
†These authors have contributed equally to this work
 Yuting Xiang1,2†
Yuting Xiang1,2† Qiong Huang
Qiong Huang