
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 31 August 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.946548
This article is part of the Research Topic Medicinal Plants for Cardiovascular and Neurodegenerative Ageing-related Diseases: From Bench to Bedside, Volume II View all 6 articles
 Yuebo Song1,2
Yuebo Song1,2 Qiuyang Jia1,2
Qiuyang Jia1,2 Xiaorui Guan1,2
Xiaorui Guan1,2 Sugimoto Kazuo1,2,3
Sugimoto Kazuo1,2,3 Jia Liu1,2
Jia Liu1,2 Weisong Duan4,5
Weisong Duan4,5 Luda Feng1,2
Luda Feng1,2 Chi Zhang1*
Chi Zhang1* Ying Gao1,2*
Ying Gao1,2*Background: The effect of herbal medicine (HM) on amyotrophic lateral sclerosis (ALS) is controversial. Clinical trials investigating HMs continue; however, the use of HM is still questioned. We aimed to systematically review the literature pertaining to the effects and safety of HM in ALS.
Methods: Randomised controlled trials (RCTs) that investigated the efficacy of HMs in ALS patients compared to any types of controls were identified. Nine databases and six registers were searched from their inception dates to 25 March 2022. Per the PRISMA guidelines, trials were identified and extracted. The risk of bias was evaluated using the Cochrane’s tool. Certainty of evidence was assessed as per the GRADE criteria. Forest plots were constructed to assess the effect size and corresponding 95% CIs using fixed-effect models, and random-effect models were employed when required. The primary outcome was the activity limitation measured by validated tools, such as the revised ALS Functional Rating Scale.
Results: Twenty studies (N = 1,218) were eligible. Of these, only five studies were double-blinded, and two were placebo-controlled. Fourteen HMs (fifty-one single botanicals) were involved; Astragalus mongholicus Bunge, Atractylodes macrocephala Koidz., and Glycyrrhiza glabra L. were commonly used in nine, eight, and six trials, respectively. For delaying activity limitation, Jiweiling injection (MD, 2.84; 95% CI, 1.21 to 4.46; p = 0.0006) and Shenmai injection (SMD, 1.07; 0.69 to 1.45; p < 0.00001) were significantly more efficacious than Riluzole, but the evidence was low quality. For ameliorating motor neuron loss, Jiweiling injection [right abductor pollicis brevis (APB): MD, 32.42; 7.91 to 56.93; p = 0.01 and left APB: MD, 34.44; 12.85 to 56.03; p = 0.002] was favoured, but the evidence was very low quality. Nine studies reported one hundred and twenty-three adverse events, twenty-six of which occurred in the treatment groups and ninety-seven in the control groups.
Conclusion: Very low to low quality of evidence suggests that HMs seem to produce superior treatment responses for ALS without increased risk of adverse events. Additional studies with homogeneous participants, reduced methodological issues, and more efficient outcome measures are required to provide confirmatory evidence.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42021277443.
As one of the diseases that modifying therapies are urgently needed, the effects of novel treatments such as herbal medicine (HM) on amyotrophic lateral sclerosis (ALS) are being broadly investigated. However, the various responses to HMs were questioned continually. ALS is a fatal, neurodegenerative disorder characterized by the progression of focal muscle weakness and wasting until respiratory failure within 3–5 years (Brown and Al-Chalabi, 2017). Motor neuron loss triggers activity limitation and reduces the quality of life (QOL) of patients with ALS. Currently, only two disease-modifying drugs are approved by the Food and Drug Administration for ALS treatment. Riluzole only slightly prolongs survival (Miller et al., 2002), and Edaravone is efficacious merely in patients who meet strict eligibility criteria (Abe et al., 2017). Because of the modest benefits of current therapies, many patients with ALS resort to HMs.
According to a cross-sectional survey in China, the proportion of herbal users among patients with ALS exceeds 90% (Pan et al., 2013b), and the corresponding proportion is 40% in America (Vardeny and Bromberg, 2005). Nevertheless, the HMs get both praise and blame along with their widespread use. The effects of HMs have been continuously praised in ALS animal models and in vitro. Bojungikgi formula improved muscle and spinal cord function (Cai et al., 2019), Shenqi Fuzheng injection extended the overall survival and improved the pathological manifestations in the brain (Sugimoto et al., 2021), and Huoling Shengji formula significantly prolonged lifespan and prevented motor neuron loss (Zhou et al., 2018). Additionally, published clinical trials have enhanced the credibility of the experimental evidence. However, many RCTs of proposed HMs have failed to show positive results in the past 20 years. Furthermore, certain HMs have been linked with poorer prognosis in patients with ALS in a single-centre cohort study (Chen et al., 2015). This phenomenon confuses both the researchers and patients about whether the effect of HM is fair or whether it should be blamed for the design of RCTs for ALS.
Consequently, we performed a systematic review and meta-analysis of the published literature to explore whether HM may usefully improve the activity limitation and whether the safety evidence of HM for ALS can be established.
This systematic review was registered prospectively with PROSPERO: CRD42021277443. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Page et al., 2021). Ethical approval was not required for this study.
The RCTs were included irrespective of language and publication status.
Adults diagnosed with ALS, regardless of sex or ethnicity, were eligible. The diagnostic criteria based on all versions of consensus criteria including the El Escorial criteria (Brooks, 1994), the revised El Escorial criteria (Brooks et al., 2000), the Awaji algorithm (de Carvalho et al., 2008), or the Gold Coast criteria (Shefner et al., 2020) were acceptable. The adapted diagnostic criteria on the basis of these standards and commonly used in various countries were allowed.
HMs in any form were included. Our definition of HMs includes herbs, herbal materials, herbal preparations, and finished herbal products that contain active ingredient parts of plants, other plant materials, or combinations, according to the World Health Organization (The World Health Organization, 2021). The comparators could be as follows: placebo, other pharmacological interventions such as Riluzole or Edaravone, or non-pharmacological intervention such as acupuncture or massage, when these interventions were administered as comparators or equally to all arms in trials.
Any effect-related outcomes were measured. The primary outcome was activity limitation, measured with validated instruments such as the ALS Functional Rating Scale (ALSFRS) (The ALS CNTF treatment study (ACTS) phase I-II Study Group, 1996), revised ALSFRS (ALSFRS-R) (Cedarbaum et al., 1999), or the modified Norris Scale (Norris et al., 1974). The secondary outcomes included tracheostomy-free survival (Paganoni et al., 2014) or overall survival, loss of strength (respiratory muscles and limb muscles), QOL, functional status, motor neuron loss, measurements based on traditional medicine theory, and pharmacodynamic biomarkers. The tracheostomy-free survival is defined as time to death, tracheostomy, or permanent non-invasive positive pressure ventilation, which shows end-of-life care for patients with ALS (Paganoni et al., 2014). The deficits of respiratory muscles are commonly assessed via forced vital capacity (FVC), and limb muscles are quantitatively evaluated by hand-held dynamometry (HHD) or Medical Research Council Scale (MRC). The change of QOL captured using validated instruments, such as the ALS Assessment Questionnaire-40 (ALSAQ-40) (Jenkinson et al., 1999), the ALS Specific Quality of Life-revised (ALSSQOL-R) (Simmons et al., 2006), the MOS Item Short-form Health Survey (SF-36) (Ware and Sherbourne, 1992), or the Barthel index, during the treatment was evaluated. Validated scales assessing the functional status, such as the Appel ALS Score (AALSS) (Appel et al., 1987), were included. The motor neuron loss measured via motor unit number estimation (MUNE) or other neurophysiological tests was assessed. The MUNE is a measure of remaining motor units and, therefore, an indirect measure of motor neuron loss. Any data of pharmacodynamic biomarkers such as the neurofilament light chain were abstracted. For safety assessment, any adverse events (AEs) and serious adverse events (SAEs) were summarized.
Nine databases, including MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang Data, SinoMed, National Institute of Informatics Support Academic Information Services (CiNii), and Korean Journal Database (KCL), were retrieved respectively from their inception dates to 25 March 2022. Relevant grey literature sources such as reports, dissertations, theses, and conference abstracts were identified to reduce the risk of publication bias.
The on-going trials and unpublished studies were searched via the following registers: ClinicalTrials.gov; the World Health Organization International Clinical Trials Registry Platform (ICTRP); Chinese Clinical Trial Registry (ChiCTR); EU Clinical Trials Register; Clinical Research Information Service (CRiS), Republic of Korea; Japan Primary Registries Network (JPRN).
The searched terms were as follow: “amyotrophic lateral sclerosis,” “motor neuron disease,” “Lou Gehrig’s disease,” “Charcot disease,” “phytotherapy,” “traditional medicine,” “medicinal plant,” “herbal medicine,” “plant extract,” “plant preparation,” “traditional Chinese medicine,” “Chinese drug,” “Chinese formul*,” “Chinese prescri*,” “kampo medicine,” “Chinese materia medica,” “japanese medicine,” “japanese drug,” “japanese formul*,” “japanese prescri*,” “korean medicine,” “korean drug,” “korean formul*,” “korean prescri*,” and “randomised controlled trial.” The search strategies are listed in Supplementary Appendix A1. To highly identify RCTs, the Cochrane sensitivity-maximizing filter for RCTs (2008 revision in Ovid format) was adopted (Lefebvre et al., 2021).
According to prespecified selection criteria, two authors (YBS and XRG) reviewed the titles and abstracts of retrieved articles after duplicates were removed. The articles that did not fulfil the inclusion criteria were removed. The remaining articles were screened with full text by the same two authors independently. Any disagreements in primary and full-text screening were discussed to be resolved. A third review author (CZ) was consulted if required. All exclusion reasons were recorded.
For eligible articles remaining after the primary and full-text screening, two authors (YBS and QYJ) extracted the eligibility criteria, study design, participants, interventions, comparators, outcomes, results, and other relevant information using standard data extraction templates. For studies reporting results at more than one time point, the final data of the intended treatment period was mainly extracted. The same scheme resolved the disagreements. The multiple publications of the same study were listed under the original article. The missing information from the included studies was obtained via contact with the authors to reduce the reporting biases. The reference lists of all relevant primary studies were checked for other potential studies.
In addition to identifying potential benefits, possible AEs were also extracted, including liver injury, kidney damage, gastrointestinal dysfunction, allergy, skin discomfort, cardiovascular events, and any SAEs.
Statistical analysis was performed using software provided by the Cochrane Collaboration (Review Manager 5.3). Relevant characteristics of studies were compared to assess which studies were eligible for each synthesis (Table 1). For continuous outcomes, the mean difference (MD) or standardized mean difference (SMD) with 95% confidence interval (CI) was calculated depending on the similarity of outcome measurements. MD would be selected when studies all reported the outcome using the same scale. The relative risk (RR) with a 95% CI was calculated for dichotomous outcomes. Trials were excluded from the synthesis when essential data were missing.
Both random-effect models and fixed-effect models were performed in meta-analysis when available. The results from both models were reported when significant heterogeneity existed, and the heterogeneity was tried to explain by subgroup if applicable. When there was no significant heterogeneity, the results of the fixed-effect model were reported. When the heterogeneity was substantial, both models were abandoned, and the meta-analysis was replaced by the qualitative summary. The heterogeneity was calculated with the I2 test. To visually display the results of syntheses, forest plots were constructed. We planned the subgroups classified by disease course and different durations of intervention. We projected to perform the sensitivity analysis using the following filters: certainty of ALS and risk of bias.
Two review authors (YBS and QYJ) assessed the risk of bias independently using the Cochrane Handbook for Systematic Reviews of Interventions (Revised Cochrane risk-of-bias tool for randomised trials) for eligible studies (Sterne et al., 2019). The bias domain of the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, selection of the reported result, and other biases were evaluated. A judgment of bias was made and divided into “low risk,” “high risk,” and “some concerns.” Unclear items in studies were further checked by contacting the corresponding authors. Any disagreements were discussed with a third reviewer (CZ). Funnel plots were constructed to evaluate the publication bias across studies when at least ten studies were included in the quantitative analysis synthesis.
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system was used by two reviewers (QYJ and YBS) to separately assess the certainty of evidence for each outcome. The discrepancies were resolved by discussion with a third reviewer (XRG).
A total of 489 records were retrieved. Of these trials, one was identified from the register and has been completed. 384 records remained after duplicates were removed. After titles and abstracts were screened, 331 records were excluded due to non-clinical trials, non-herbal interventions, or non-ALS participants. After reading the full text, 31 trials were excluded, and the reasons were listed in Supplementary Appendix A2. Before the manuscript was submitted, we updated retrieval, but no additional trials meeting inclusion criteria were found. Therefore, 20 RCTs were included. The PRISMA flowchart of study selection is presented in Figure 1.
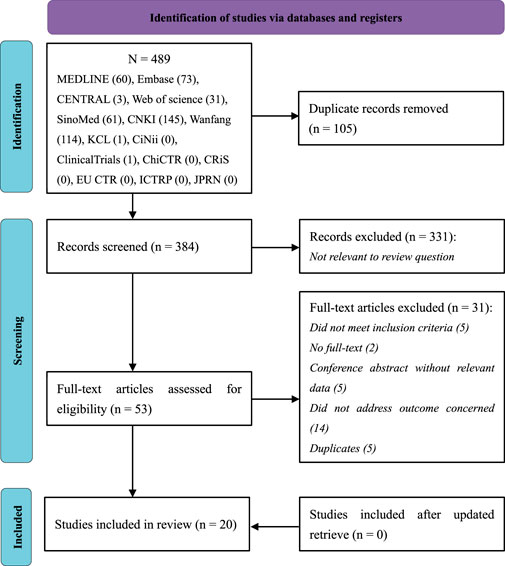
FIGURE 1. PRISMA flow diagram showing literature search results. PRISMA, Preferred reporting items for systematic reviews and meta-analysis.
The characteristics of 20 included RCTs are listed in Table 1. All included trials adopted parallel two-arm designs. Five studies were double-blinded (Ma, 2006; Pan, 2015; Zhu, 2016; Chico et al., 2018; Riva et al., 2019), and other studies were open-labelled. Three trials were published in English (Pan et al., 2013a; Chico et al., 2018; Riva et al., 2019), and others were reported in Chinese. Two included studies were conducted in Italy (Chico et al., 2018; Riva et al., 2019), and the rest were in China. Only three trials reported the sample size calculation (Fang, 2016; Zhu, 2016; Riva et al., 2019).
The number of participants in each included trial ranged from 28 to 125, with a total of 1,218 investigated subjects (655 were in the treatment groups, and 563 were in the control groups). Of them, 838 (68.8%) participants were male sex. There were nine trials including subjects on the basis of both ALS diagnostic criteria and traditional medicine signs. Two trials did not describe inclusion and exclusion criteria (Su et al., 2006; Cai, 2011). One trial did not report whether baseline characteristics were well matched between groups or not (Su et al., 2006).
Among the twenty included trials, fourteen HMs (Supplementary Appendix A3) were investigated. Most were herbal preparations, and only four were patent herbal productions (Cannabinoids, Curcumin, Jiweiling injection, and Shenmai injection). One HM was delivered via an oromucosal spray (Riva et al., 2019), two were administered intravenously (Ma, 2006; Wang, 2007; Cai, 2011; Zhang, 2020), and the other eleven HMs were taken orally. The fourteen HMs contained fifty-one single botanicals (Supplementary Appendix A3); Astragalus mongholicus Bunge, Atractylodes macrocephala Koidz., Glycyrrhiza glabra L., Poria cocos (Schw.) Wolf., and Codonopsis pilosula (Franch.) Nannf. Were commonly used in nine, eight, six, six and five trials, respectively. One trial did not describe the procedure of medicine preparation. One trial investigated HM combined with acupuncture (Jin, 2013), and another combined with massage (Li P., 2019). The duration of treatments ranged from 1 month to 9 months, and most trials (nine trials) investigated participants for 3 months (Table 1).
Two studies compared HM with placebo (Chico et al., 2018; Riva et al., 2019). Eight studies compared HM with Riluzole (Ma, 2006; Wang, 2007; Wang et al., 2009; Xv et al., 2011; Pan et al., 2013a; Pan, 2015; Fang, 2016; Sui et al., 2016). Four studies conducted Riluzole add-on therapy for ALS (Su et al., 2006; Li et al., 2011; Jin, 2013; Wang et al., 2017). Five studies adopted conventional treatment add-on therapy (Cai, 2011; Bao et al., 2016; Wang, 2017; Li, 2019b; Zhang, 2020). One study used 1/10 dose of investigated medicine as a placebo, thus was classified as a dose-response controlled trial (Zhu, 2016) (Table 1).
The quality assessment of each study’s random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other bias are presented in Figures 2, 3. We were not able to detect publication bias for any analysis.
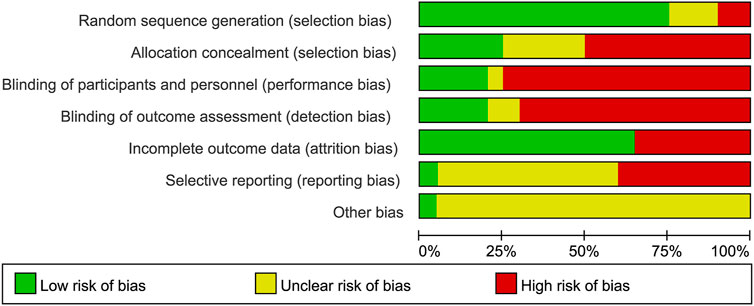
FIGURE 2. Judgments about each risk of bias item presented as percentages across all included studies.
A total of 19 studies (N = 1,045, Supplementary Appendix A4) measured activity limitation. Significant differences were found between intervention and controls.
Fourteen trials reported changes in activity limitation measured with the ALSFRS-R or the ALSFRS. As the modified Norris Scale is less sensitive than the ALSFRS-R, it has fallen out of favour (Paganoni et al., 2014). But five trials still employed it as the measure of activity limitation (Su et al., 2006; Wang et al., 2009; Li et al., 2011; Xv et al., 2011; Sui et al., 2016). Two trials used both the ALSFRS and modified Norris Scale (Ma, 2006; Pan, 2015).
Of the 19 trials, nine reported better effects of treatment than controls (Supplementary Appendix A4), among which four trials employed the ALSFRS-R (Jin, 2013; Zhu, 2016; Wang et al., 2017; Zhang, 2020); three trials measured with the ALSFRS (Ma, 2006; Wang, 2007; Pan, 2015), even though these trials were conducted after the ALSFRS-R (revised version of ALSFRS) has been applied in clinical studies regularly; one trial did not mention the accurate version of ALS Function Rating Scale (Cai, 2011); one trial measured with the modified Norris Scale (Li et al., 2011).
In pooling analysis, fixed-effect models were used and were good fits to the data. For delaying activity limitation, Jiweiling injection ([Araliaceae; Panax ginseng C. A. Mey.] and [Apiaceae; Angelica sinensis (Oliv.) Diels]) (MD, 2.84; 95% CI, 1.21 to 4.46; p = 0.0006) (Ma, 2006; Wang, 2007) and Shenmai injection ([Araliaceae; Panax ginseng C. A. Mey.] and [Asparagaceae; Ophiopogon japonicus (Thunb.) Ker Gawl.]) (SMD, 1.07; 0.69 to 1.45; p < 0.00001) (Cai, 2011; Zhang, 2020) were significantly more efficacious than controls (Figures 4, 5). However, the insufficient number of homogeneous trials did not allow the subgroup analysis and sensitivity analysis.
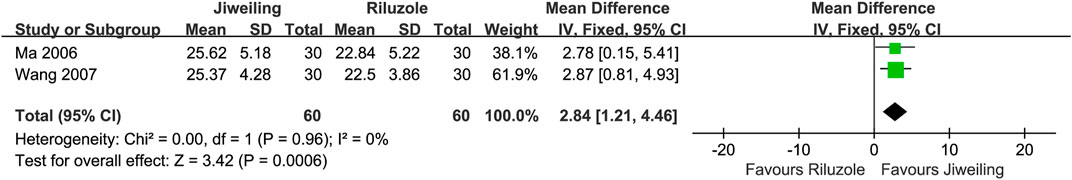
FIGURE 4. Effect of Jiweiling injection on activity limitation in patients with amyotrophic lateral sclerosis.
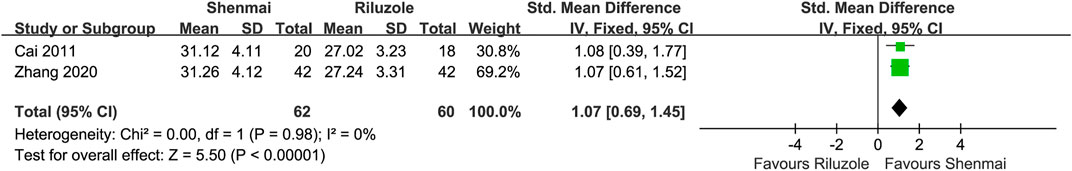
FIGURE 5. Effect of Shenmai injection on activity limitation in patients with amyotrophic lateral sclerosis.
Another 10 trials showed no statistically significant differences between treatments and controls. Four of them were measured with the ALSFRS-R (Pan et al., 2013a; Fang, 2016; Chico et al., 2018; Riva et al., 2019) and 2 with ALSFRS (Wang, 2017; Li P., 2019). In the Curcumin trial (Chico et al., 2018), the author also analysed the sub scale of the ALSFRS-R (question 10–12) for respiratory assessment, and the significant result was found. However, this article did not report any specific data for synthesis. Another three trials measured the modified Norris Scale (Su et al., 2006; Wang et al., 2009; Xv et al., 2011; Sui et al., 2016).
Only one trial (N = 58, follow-up for 18 months, Supplementary Appendix A4) reported tracheostomy-free survival but did not show a significant difference between treatment and control (Jin, 2013). This trial displayed the Kaplan-Meier survival curve but did not report the Hazard ratio and 95% CI.
Seven studies (N = 738, Supplementary Appendix A4) assessed the loss of strength. Meta-analysis was replaced by the qualitative summary due to substantial heterogeneity [I2 = 75% in pooling analysis of respiratory function measured with FVC; I2 = 81% when measured with the vital capacity (VC)].
Five trials measured FVC (Ma, 2006; Wang, 2007; Li et al., 2011; Wang et al., 2017; Riva et al., 2019). Two of them showed statistical significance in delaying the decline of ventilatory muscle strength (Ma, 2006; Wang, 2007). These two trials also showed significant differences when measuring VC. Another three trials showed no statistical significance (Li et al., 2011; Wang et al., 2017; Riva et al., 2019).
Four trials evaluated MRC (Pan et al., 2013a; Wang et al., 2017; Chico et al., 2018; Riva et al., 2019), and all of them did not show significant effects. Of these, the Curcumin trial (Chico et al., 2018) also used HHD to measure the accurate grip force.
Seven trials (N = 394, Supplementary Appendix A4) evaluated the QOL. Three reported significant difference between treatment and control when measuring the ALSAQ-40 (Ma, 2006; Wang, 2007; Pan, 2015). Additionally, one trial measuring the subscale of ALSAQ-40 reported no significant effect (Fang, 2016).
Two trials employed the Barthel index; one showed a significant effect (Wang, 2017), and another showed no significant difference (Riva et al., 2019). One trial (Pan et al., 2013a) reported results on the subscale of the SF-36 and found no significant improvement.
Two trials (N = 138, Supplementary Appendix A4) assessed the functional status of ALS patients via the AALSS. Of them, one showed a statistically significant difference (Ma, 2006), and another reported no significant effect (Li, 2019b).
Nine trials (N = 560, Supplementary Appendix A4) recorded the change in symptoms and signs based on traditional medicine theory (Su et al., 2006; Wang et al., 2009; Xv et al., 2011; Jin, 2013; Bao et al., 2016; Sui et al., 2016; Zhu, 2016; Wang, 2017; Li, 2019b), and only one of them reported no significant improvement compared to control (Li, 2019b). However, most measurements used in these trials lack verification of reliability and validity.
Three trials (N = 170, Supplementary Appendix A4) reported the change in MUNE (Ma, 2006; Wang, 2007; Pan, 2015) and all showed statistical significance.
The MUNE detected from the right and left abductor pollicis brevis (APB) were pooled respectively in our analysis to reduce the heterogeneity, and the results favoured Jiweiling injection (right APB: MD, 32.42; 7.91 to 56.93; p = 0.01; left APB: MD, 34.44; 12.85 to 56.03; p = 0.002) compared to Riluzole (Figure 6). No subgroup or sensitivity analysis was conducted due to the insufficient number of trials.
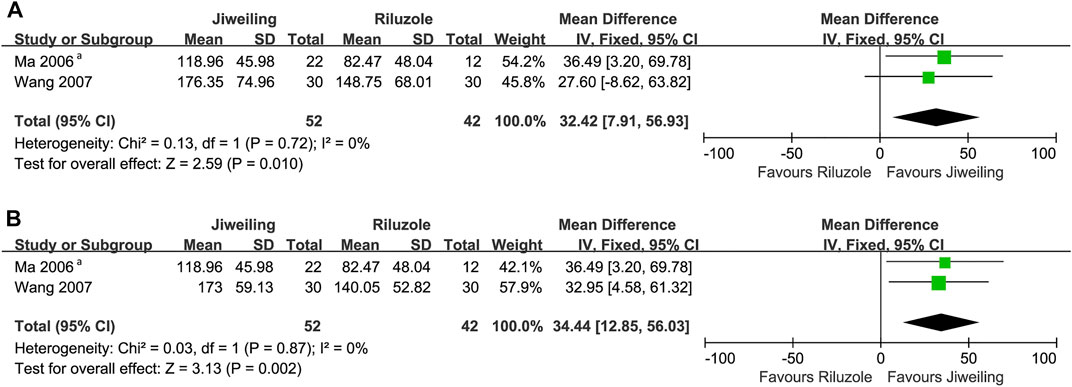
FIGURE 6. Effect of Jiweiling injection on MUNE in patients with amyotrophic lateral sclerosis. (A) The MUNE detected from the right abductor pollicis brevis in Wang 2007 was used. (B) The MUNE detected from the left abductor pollicis brevis in Wang 2007 was used. MUNE, motor unit number estimation. aWhich side of abductor pollicis brevis was tested was no noted in Ma 2006.
Two trials (Supplementary Appendix A4) evaluated motor neuron loss using the amplitude of compound muscle action potential (Su et al., 2006; Wang et al., 2009), and they did not show better effects than controls.
Another trial (N = 78, Supplementary Appendix A4) used other electrophysiologic parameters related to muscle denervation and showed no statistical significance (Li, 2019b).
Three trials (N = 221, Supplementary Appendix A4) measured five types of biofluid markers (a total of fourteen biomarkers): creatine kinase, oxidative stress biomarkers, neuron-specific enolase, amino acid, and immunoglobulin (Wang, 2007; Wang et al., 2009; Chico et al., 2018). The corresponding results of comparisons between treatments and controls are presented in Supplementary Appendix A4, and two trials reported significant differences (Wang, 2007; Chico et al., 2018). Unfortunately, no trials measured neurofilament, the most promising candidate biomarker at present.
A total of 14 studies (N = 845) evaluated the safety of treatments (Table 1). Of these, nine studies reported the occurrence of 123 adverse events (Table 2). 26 AEs happened in the treatment groups and 97 in the control groups. Of the 26 AEs, 2 SAEs (death) occurred in two trials that investigated Jiawei Sijunzi formula and Jianpi Yifei formula, respectively (Pan et al., 2013a; Fang, 2016). Two participants receiving Jianpi Yifei formula added on Riluzole were reported abnormal liver function (Wang et al., 2017). Gastrointestinal discomforts were found in a total of 17 individuals who were treated with Cannabinoids (N = 5) (Riva et al., 2019), Curcumin (N = 4) (Chico et al., 2018), Huoling Shengji formula (N = 3) (Sui et al., 2016), Jianpi Yifei formula plus Riluzole (N = 1) (Wang et al., 2017), and Jiawei Sijunzi formula (N = 4) (Pan et al., 2013a), respectively. Three participants taking Jianpi Yifei formula plus Riluzole developed skin allergies (Wang et al., 2017), and one patient receiving Curcumin had a skin rash (Chico et al., 2018). In addition, one patient who took Cannabinoids was reported cardiovascular disease.
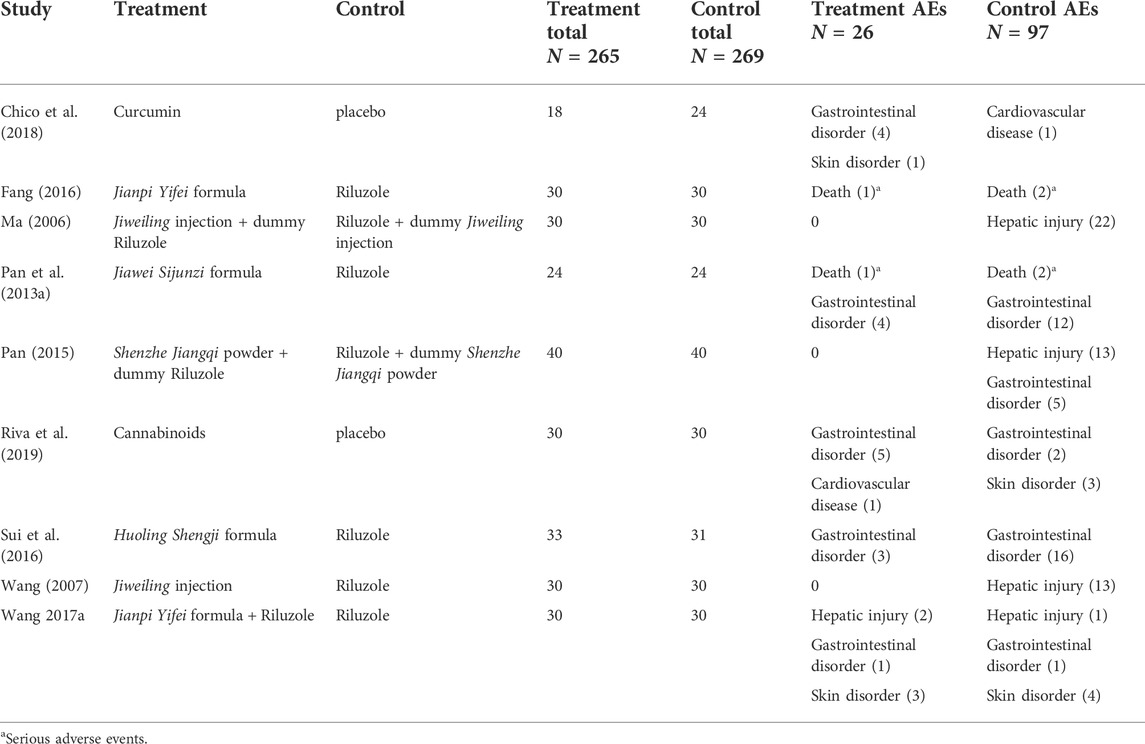
TABLE 2. Occurrence of adverse events in randomised clinical trials of herbal medicine for amyotrophic lateral sclerosis.
All outcome measurements for evaluation of function, survival, biofluid markers, and electrophysiological markers were rated using GRADE. All included evidence was very low to low quality (Table 3). The risk of bias and imprecision were the reasons for downgrading all outcomes.
Twenty studies of HM for ALS were included. Whether these clinical trials are futile under the circumstances that so many efforts have been made but the effects of HMs are still being questioned. We systematically appraised published RCTs of HM for ALS to address this issue. Our results suggest that HMs may be effective for improvement in activity limitation, muscle strengths, QOL, functional status, traditional medicine syndromes, and motor neuron loss for individuals with ALS. However, only low and very low quality of evidence was available, which restricts the confidence that can be placed in the findings. Results of the meta-analysis revealed significant improvement in activity limitation (Jiweiling injection and Shenmai injection) and motor neuron loss (Jiweiling injection) when patients were treated with finished herbal productions. Nevertheless, the less number of trials brought into analysis reduce the reliability of the results to some extent. In addition, there was insufficient evidence of HMs prolonging survival.
Of these included herbal medicines, several possible therapeutic mechanisms were reported. Antioxidant was associated with the neuroprotective actions of ALS in Shenmai injection (She et al., 2013), Curcumin (Zhang et al., 2014), Guilu Erxian glues (Xv et al., 2013), Huoling Shengji formula (Zhou, 2017), and Jianpi Yifei formula (She et al., 2013; Li, 2019). In addition, Jiweiling injection plays a role in inhibiting calcium toxicity and anti-apoptosis (Wang, 2005). Cannabinoids exert effects on anti-inflammatory and antioxidant (Alexander, 2016). The pharmacologic mechanisms of the other seven HMs lack reports. However, Astragalus mongholicus Bunge, with properties of anti-aggregation of proteins and anti-inflammation (Zhang et al., 2014), is the main herb of four of them (Bushen Jianpi Shugan formula, Fuyuan Shengji granule, Jiawei Sijunzi formula, and Yiqi Qiangji formula). And ginseng, which has a neuroprotective effect against neuroinflammation and oxidative stress (Cai and Yang, 2016), is the main herb of Shenzhe Jiangqi powder.
When faced with significant evidence with very low to low quality, critical analyses may benefit the future clinical trials of HM for ALS. In the context of disease heterogeneity in ALS, the restrictive inclusion criteria for phase Ⅱ and Ⅲ clinical trials are becoming an important consideration (Kiernan et al., 2021). The stratification of patients at the time of recruitment according to their characteristics enables patients to be matched with suitable therapies. Our included HM studies were mainly small sample clinical trials and thus should have a higher requirement of recruiting homogeneous subjects. Instead, participants with little restriction on diagnostic certainties and disease course were recruited and treated without stratification. Nowadays, several prognostic models and tools have been proposed to optimize ALS trials, especially in the recruitment process (Westeneng et al., 2018; van Eijk et al., 2019; van Eijk et al., 2021). Under the circumstances that ALS clinical trials with large sample sizes are difficult to be carried out, employing these models to select appropriate populations of patients sensitive to HMs makes sense.
Concerning the treatments, some trials did not describe the source and concentration of herbal reparations (Supplementary Appendix A3). Moreover, our meta-analysis showed statistical significance when data from finished herbal products were used. These findings suggest that unspecific constituents and manufacturing processes may hinder objective HM efficacy evaluations and thus reduce the repeatability of HM tested in further clinical trials. In addition, most HM trials only investigated patients for 3 months or less (Table 1). Such a short duration is not long enough to provide confirmatory evidence.
Regarding the comparators, in order to reduce unnecessary exposure to placebos, eight studies compared investigated HMs with Riluzole. However, such a design hampers the recognition of HM’s net effect. The master protocol (Kiernan et al., 2021), a strategy aiming to minimize unnecessary exposure to placebo and allowing for the simultaneous evaluation of multiple treatments with a shared placebo group, is a promising approach to address this issue. In addition, the Food and Drug Administration recommends the consideration of add-on designs in ALS clinical trials (The Food and Drug Administration, 2019), which is another choice to comply with the ethical criteria.
In terms of the selection of outcome measures, some limitations were identified. First, the ALSFRS-R/ALSFRS is the most widely accepted outcome measure of activity limitation in ALS patients (Gordon et al., 2007; Cudkowicz et al., 2014; Abe et al., 2017; Paganoni et al., 2020). However, about one-fourth of the included trials measuring activity limitation did not employ them but used the modified Norris Scale instead, which is less favourable at present. Furthermore, most HM clinical trials did not measure survival in consideration of disease heterogeneity, variation in the expected disease course, and high cost due to extended follow-up. However, the comparison of the design of clinical trials between Riluzole (Bensimon et al., 1994; Lacomblez et al., 1996) and Edaravone (Abe et al., 2017) shows that an increased life expectancy leaves little doubt about a treatment’s therapeutic potential. Thus, the benefits of measuring survival time in HM clinical trials may outweigh the disadvantages. Additionally, incorporation of optimized biomarkers into early-stage clinical trials is in prospect. Inspiringly, some of the included studies measured biofluid markers or electrophysiological markers and even drew significant conclusions, which benefits the understanding of pharmacological mechanisms. Rapid advances in the detection of the molecular biology and pathology of ALS are making the novel biomarker constantly emerge but also leading to the phenomenon that various biomarkers are employed without a uniform standard. Meanwhile, reliable biomarkers are badly needed for monitoring the response to treatment, but the consensus about the robust candidates has not yet been established, even though some high-quality publications have made recommendations (Grossman et al., 2014; Benatar et al., 2018; Magen et al., 2021), and certain biomarkers, such as the neurofilament light chain, have been already used in multicentre clinical trials (Paganoni et al., 2020). In addition, it is found that two studies (Ma, 2006; Wang, 2007) misunderstood the clinical meaning of the ALSAQ-40. Therefore, we abandoned the relevant data synthesis, even if they were homogenous and claimed to be effective.
Furthermore, other flaws in methodology could also hinder the recognition of the efficacy of HMs. Fifteen studies employed an open-labelled design because of common difficulties in imitating the smell and appearance of dummy herbal products, especially the peroral dosage form, which increased the information bias. Notably, most included studies claimed to be RCTs. However, the investigators did not sufficiently describe details related to the sample size calculation, randomization process, implementation of blinding, or measurement outcomes. Hence, reporting in detail according to the Consolidated Standards of Reporting Trials (CONSORT) Statement is urgently needed. Additionally, one study used a 10th of the dose of the investigated medicine as the placebo. Such a methodological flaw dramatically reduced the reliability of the study’s evidence.
Some inherent limitations in HM clinical trials mentioned above are hard to be remedied in the short run. A registration study with heterogeneous populations and long-term follow-up can bring researchers and patients objective and comprehensive knowledge about disease trajectory and even effectiveness of HMs via statistical methods. Such a study has been established in mainland China to investigate the properties of ALS patients who take HM (CARE-TCM) (Song et al., 2022). In addition, we had noticed that a new HM called TJ-68 (Shaoyao Gancao formula) was expected to be tested in an N-of-1 study and the RCT of Huoling Shengji formula was on-going while this manuscript was drafted. More evidence derived from well-designed trials may update our understanding of HM for ALS.
Some evidence shows more extensive involvement of pathological changes in ALS than previously recognized, such as cognitive dysfunction, emotional instability, and insomnia (Sedda, 2014; Boentert, 2020; Pender et al., 2020). It is found that these symptoms related to the extra-motor system are not rare concomitant behaviours and directly impact QOL even though they hardly threaten survival. This systematic review aimed to appraise the effects of HMs on motor system symptoms. Further reviews summarizing the therapeutic effects of HMs for extra-motor system symptoms are needed.
Nine of twenty studies reported AEs, and two reported SAEs (Table 2). The occurrences of AEs, such as liver dysfunction, and SAEs, such as death, were more frequent in the control groups.
This systematic review had some limitations. First, a large number of potential descriptors for HM hinder the design of a search strategy, even though we have used the Cochrane sensitivity-maximizing filter for RCTs to highly identify clinical trials. Second, the less number of trials with a small sample size included in the meta-analysis reduces the reliability of the pooled results. Third, the lack of data from on-going trials may alter the results dramatically.
HMs may play a role in delaying decline in function, and the evidence for the role in extending survival was insufficient. The very low to low quality of evidence requires further RCTs that have adequate methods, use placebos as controls, select appropriate participants, and employ efficient outcome measures.
We reviewed the results of measurements based on traditional medicine theory, which was not stated at registration.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
YG, CZ, and YS designed the study and organized the team. CZ and LF provided methodological direction. YG, SK, and JL gave clinical suggestions. YS, QJ, and XG conducted study selection, data extraction, assessment of the risk of bias, and evaluation of the evidence certainty. YS completed the report writing and analysis. WD revised the language. CZ revised the draft. YG supervised this project. All authors have read and approved this manuscript.
This study was funded by the National Foreign Expert Project (Grant No. QN2021110001L), the Chinese Medicine Inheritance and Innovation Talent Project Leading Talent Support Program of National Traditional Chinese Medicine (Grant No. 2018, 12), the Beijing University of Chinese Medicine Project (Grant No. 2019-JYB-XS-165), and the Beijing University of Chinese Medicine Project (Grant No. 2020-tsxk-001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.946548/full#supplementary-material
Abe, K., Aoki, M., Tsuji, S., Itoyama, Y., Sobue, G., Togo, M., et al. (2017). Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 16 (7), 505–512. doi:10.1016/s1474-4422(17)30115-1
Alexander, S. P. (2016). Therapeutic potential of cannabis-related drugs. Prog. Neuropsychopharmacol. Biol. Psychiatry 64, 157–166. doi:10.1016/j.pnpbp.2015.07.001
Appel, V., Stewart, S. S., Smith, G., and Appel, S. H. (1987). A rating scale for amyotrophic lateral sclerosis: Description and preliminary experience. Ann. Neurol. 22 (3), 328–333. doi:10.1002/ana.410220308
Bao, J., Fu, M., Shen, B., and Zhu, X. (2016). Treatment of amyotrophic lateral sclerosis with Jiawei Sijunzi decoction. Her. Med. 35 (S1), 43–45. doi:10.3870/j.issn.1004-0781.2016.z1.021
Benatar, M., Wuu, J., Andersen, P. M., Lombardi, V., and Malaspina, A. (2018). Neurofilament light: A candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann. Neurol. 84 (1), 130–139. doi:10.1002/ana.25276
Bensimon, G., Lacomblez, L., and Meininger, V. (1994). A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N. Engl. J. Med. 330 (9), 585–591. doi:10.1056/NEJM199403033300901
Boentert, M. (2020). Sleep and sleep disruption in amyotrophic lateral sclerosis. Curr. Neurol. Neurosci. Rep. 20 (7), 25. doi:10.1007/s11910-020-01047-1
Brooks, B. R. (1994). El escorial World federation of neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on motor neuron diseases/amyotrophic lateral sclerosis of the World federation of neurology research group on neuromuscular diseases and the El escorial "clinical limits of amyotrophic lateral sclerosis" workshop contributors. J. Neurol. Sci. 124, 96–107. doi:10.1016/0022-510x(94)90191-0
Brooks, B. R., Miller, R. G., Swash, M., and Munsat, T. L. (2000). El escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 1 (5), 293–299. doi:10.1080/146608200300079536
Brown, R. H., and Al-Chalabi, A. (2017). Amyotrophic lateral sclerosis. N. Engl. J. Med. 377 (2), 162–172. doi:10.1056/NEJMra1603471
Cai, M., Lee, S. H., and Yang, E. J. (2019). Bojungikgi-tang improves muscle and spinal cord function in an amyotrophic lateral sclerosis model. Mol. Neurobiol. 56 (4), 2394–2407. doi:10.1007/s12035-018-1236-0
Cai, M., and Yang, E. J. (2016). Ginsenoside Re attenuates neuroinflammation in a symptomatic ALS animal model. Am. J. Chin. Med. 44 (2), 401–413. doi:10.1142/s0192415x16500233
Cai, R. (2011). Shenmai injection combined with comprehensive therapy for motor neuron disease: A clinical review of 38 cases. J. Math. Med. 24 (05), 534–535.
Cedarbaum, J. M., Stambler, N., Malta, E., Fuller, C., Hilt, D., Thurmond, B., et al. (1999). The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS study group (phase III). J. Neurol. Sci. 169 (1-2), 13–21. doi:10.1016/s0022-510x(99)00210-5
Chico, L., Ienco, E. C., Bisordi, C., Lo Gerfo, A., Petrozzi, L., Petrucci, M., et al. (2018). Amyotrophic lateral sclerosis and oxidative stress: A double-blind therapeutic trial after curcumin supplementation. CNS Neurol. Disord. Drug Targets 17 (10), 767–779. doi:10.2174/1871527317666180720162029
Cudkowicz, M. E., Titus, S., Kearney, M., Yu, H., Sherman, A., Schoenfeld, D., et al. (2014). Safety and efficacy of ceftriaxone for amyotrophic lateral sclerosis: A multi-stage, randomised, double-blind, placebo-controlled trial. Lancet. Neurol. 13 (11), 1083–1091. doi:10.1016/S1474-4422(14)70222-4
de Carvalho, M., Dengler, R., Eisen, A., England, J. D., Kaji, R., Kimura, J., et al. (2008). Electrodiagnostic criteria for diagnosis of ALS. Clin. Neurophysiol. 119 (3), 497–503. doi:10.1016/j.clinph.2007.09.143
Fang, Z. (2016). Master. Guangzhou: Guangzhou University of Chinese Medicine. Assessment of bulbar palsy in amyotrophic lateral sclerosis patients and evaluation of the effect of Jianpiyifei Decoction
Gordon, P. H., Moore, D. H., Miller, R. G., Florence, J. M., Verheijde, J. L., Doorish, C., et al. (2007). Efficacy of minocycline in patients with amyotrophic lateral sclerosis: A phase III randomised trial. Lancet. Neurol. 6 (12), 1045–1053. doi:10.1016/S1474-4422(07)70270-3
Grossman, M., Elman, L., McCluskey, L., McMillan, C. T., Boller, A., Powers, J., et al. (2014). Phosphorylated tau as a candidate biomarker for amyotrophic lateral sclerosis. JAMA Neurol. 71 (4), 442–448. doi:10.1001/jamaneurol.2013.6064
Jenkinson, R., Fitzpatrick, B., Brennan, C., and SwashM., M. (1999). Evidence for the validity and reliability of the ALS assessment questionnaire: The ALSAQ-40. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 1 (1), 33–40. doi:10.1080/146608299300080022
Jin, P. (2013). Clinical observation on treating amyotrophic lateral sclerosis by Guilu Erxian glues. Clin. J. Chin. Med. 5 (24), 28–30. doi:10.3969/j.issn.1674-7860.2013.24.012
Kiernan, M. C., Vucic, S., Talbot, K., McDermott, C. J., Hardiman, O., Shefner, J. M., et al. (2021). Improving clinical trial outcomes in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 17 (2), 104–118. doi:10.1038/s41582-020-00434-z
Lacomblez, L., Bensimon, G., Leigh, V., Guillet, P. N., and Meininger, P. (1996). Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet 347 (9013), 1425–1431. doi:10.1016/S0140-6736(96)91680-3
Lefebvre, C., Glanville, J., Briscoe, S., Littlewood, A., Marshall, C., Metzendorf, M-I., et al. (2021). Cochrane Handbook for systematic reviews of interventions.Chapter 4: Searching for and selecting studies
Li, C., Hu, L., Kong, L., Gao, J., Zhu, X., and Zhi, H. (2011). Efficacy of Fuyuanshengji Granule on the short period prognosis of the patients with amyotrophic lateral sclerosis. J. Neurology Neurorehabilitation 8 (02), 61–64.
Li, H. Z. (2019a). Effect of Jianpi Yifei Formula on glutamate induced spinal cord neuron injury. Guangzhou: Master, Guangzhou University of traditional Chinese Medicine.
Li, P. (2019b). Effects of Jianpi Yifei formula combined with massage on neurological function and electromyography in patients with amyotrophic lateral sclerosis. J. Sichuan Traditional Chin. Med. 37 (8), 147–149.
Ma, W. (2006). Master. Shijiazhuang: Hebei Medical University.The clinical study of Jiweiling injection in dealing with amyotrophic lateral sclerosis
Magen, I., Yacovzada, N. S., Yanowski, E., Coenen-Stass, A., Grosskreutz, J., Lu, C. H., et al. (2021). Circulating miR-181 is a prognostic biomarker for amyotrophic lateral sclerosis. Nat. Neurosci. 24 (11), 1534–1541. doi:10.1038/s41593-021-00936-z
Norris, F. H., Calanchini, P. R., Fallat, R. J., Panchari, S., and Jewett, B. (1974). The administration of guanidine in amyotrophic lateral sclerosis. Neurology 24 (8), 721–728. doi:10.1212/wnl.24.8.721
Paganoni, S., Cudkowicz, M., and Berry, J. D. (2014). Outcome measures in amyotrophic lateral sclerosis clinical trials. Clin. Investig. (Lond) 4 (7), 605–618. doi:10.4155/cli.14.52
Paganoni, S., Macklin, E. A., Hendrix, S., Berry, J. D., Elliott, M. A., Maiser, S., et al. (2020). Trial of sodium phenylbutyrate-taurursodiol for amyotrophic lateral sclerosis. N. Engl. J. Med. 383 (10), 919–930. doi:10.1056/NEJMoa1916945
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Pan, C. (2015). Clinical study on treatment of Chong meridian qi adversely ascending type amyotrophic lateral sclerosis by Shenzhe Jiangqi Powder Master. Shijiazhuang: Hebei Medical Univeristy.
Pan, W., Chen, X., Bao, J., Bai, Y., Lu, H., Wang, Q., et al. (2013b). The use of integrative therapies in patients with amyotrophic lateral sclerosis in shanghai, China. Evid. Based. Complement. Altern. Med. 2013, 613596. doi:10.1155/2013/613596
Pan, W., Su, J., Bao, J., Wang, J., Zhu, D., Cai, X., et al. (2013a). Open randomized clinical trial on JWSJZ Decoction for the treatment of ALS patients. Evid. Based. Complement. Altern. Med. 2013, 347525. doi:10.1155/2013/347525
Pender, N., Pinto-Grau, M., and Hardiman, O. (2020). Cognitive and behavioural impairment in amyotrophic lateral sclerosis. Curr. Opin. Neurol. 33 (5), 649–654. doi:10.1097/wco.0000000000000862
Riva, N., Mora, G., Sorarù, G., Lunetta, C., Ferraro, O. E., Falzone, Y., et al. (2019). Safety and efficacy of nabiximols on spasticity symptoms in patients with motor neuron disease (CANALS): A multicentre, double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. Neurol. 18 (2), 155–164. doi:10.1016/s1474-4422(18)30406-x
Sedda, A. (2014). Disorders of emotional processing in amyotrophic lateral sclerosis. Curr. Opin. Neurol. 27 (6), 659–665. doi:10.1097/wco.0000000000000147
She, J., Xin, Y. F., and Xuan, Y. X. (2013). Research Progress on the material basis of pharmacological action of Shenmai Injection. Her. Med. 32 (04), 497–500.
Shefner, J. M., Al-Chalabi, A., Baker, M. R., Cui, L. Y., de Carvalho, M., Eisen, A., et al. (2020). A proposal for new diagnostic criteria for ALS. Clin. Neurophysiol. 131 (8), 1975–1978. doi:10.1016/j.clinph.2020.04.005
Simmons, Z., Felgoise, S. H., Bremer, B. A., Walsh, S. M., Hufford, D. J., Bromberg, M. B., et al. (2006). The ALSSQOL: Balancing physical and nonphysical factors in assessing quality of life in ALS. Neurology 67 (9), 1659–1664. doi:10.1212/01.wnl.0000242887.79115.19
Song, Y., Li, M., Sugimoto, K., Han, Y., Liu, J., Ma, B., et al. (2022). China amyotrophic lateral sclerosis registry of patients with Traditional Chinese Medicine (CARE-TCM): Rationale and design. J. Ethnopharmacol. 284, 114774. doi:10.1016/j.jep.2021.114774
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Su, G., Zhang, J., and Hong, Y. (2006). Treatment of 25 cases of amyotrophic lateral sclerosis with Yiqi Qiangji Decoction. Chin. J. Integr. Med. Cardio-Cerebrovascular Dis. 4 (05), 452–453.
Sugimoto, K., Liu, J., Li, M., Song, Y., Zhang, C., Zhai, Z., et al. (2021). Neuroprotective effects of Shenqi Fuzheng injection in a transgenic SOD1-g93a mouse model of amyotrophic lateral sclerosis. Front. Pharmacol. 12, 701886. doi:10.3389/fphar.2021.701886
Sui, S., Wang, Y., Zhi, H., Hong, Y., and Feng, Y. (2016). Clinical observation of Huoling Shengji formula in the treatment of amyotrophic lateral sclerosis. Acad. J. Shanghai Univ. Traditional Chin. Med. 30 (02), 23–26. doi:10.16306/j.1008-861x.2016.02.006
The ALS CNTF treatment study (ACTS) phase I-II Study Group (1996). The Amyotrophic Lateral Sclerosis Functional Rating Scale. Assessment of activities of daily living in patients with amyotrophic lateral sclerosis. The ALS CNTF treatment study (ACTS) phase I-II Study Group. Arch. Neurol. 53 (2), 141–147. doi:10.1001/archneur.1996.00550020045014
The Food and Drug Administration (2019). Amyotrophic lateral sclerosis-developing drugs for treatment guidance for industry. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/amyotrophic-lateral-sclerosis-developing-drugs-treatment-guidance-industry.
The World Health Organization (2021). Traditional Chinese medicine could make "Health for one" true. Available at: https://www.who.int/intellectualproperty/studies/Jia.pdf?ua=1.
van Eijk, R. P. A., Nikolakopoulos, S., Roes, K. C. B., Kendall, L., Han, S. S., Lavrov, A., et al. (2021). Innovating clinical trials for amyotrophic lateral sclerosis: Challenging the established order. Neurology 97 (11), 528–536. doi:10.1212/WNL.0000000000012545
van Eijk, R. P. A., Nikolakopoulos, S., Roes, K. C. B., Middelkoop, B. M., Ferguson, T. A., Shaw, P. J., et al. (2019). Critical design considerations for time-to-event endpoints in amyotrophic lateral sclerosis clinical trials. J. Neurol. Neurosurg. Psychiatry 90 (12), 1331–1337. doi:10.1136/jnnp-2019-320998
Vardeny, O., and Bromberg, M. (2005). The use of herbal supplements and alternative therapies by patients with amyotrophic lateral sclerosis (ALS). J. Herb. Pharmacother. 5 (3), 23–31. doi:10.1080/j157v05n03_03
Wang, A., Li, X., Ren, Z., Hou, X., Lu, M., and Du, B. (2017). Therapeutic effect of strengthening spleen and tonifying lung on amyotrophic lateral sclerosis. World Chin. Med. 12 (06), 1364–1367.
Wang, J., Gao, J., Guo, Y., Qin, B., Ren, H., Xv, W., et al. (2009). Clinical study of the effect of Fuyuan Shengji Granuleon symptoms of the patients with amyotrophic lateral sclerosis. J. Neurology Neurorehabilitation 6 (03), 173–175.
Wang, J. M. (2005). Neuroprotective effect of Jiweiling injection on motor neuron disease. Doctor: Hebei Medical University.
Wang, W. (2017). Clinical observation on the treatment of motor neuron disease by nourishing kidney and strengthening spleen (liver-kidney yin deficiency type) Master. Beijing: Henan University of Chinese Medicine.
Wang, X. (2007). The clinical study of Jiweiling injection in dealing with amyotrophic lateral sclerosis bulbar paralysis Master. Shijiazhuang: Hebei Medical University.
Ware, J. E., and Sherbourne, C. D. (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 30 (6), 473–483. doi:10.1097/00005650-199206000-00002
Westeneng, H.-J., Debray, T. P. A., Visser, A. E., van Eijk, R. P. A., Rooney, J. P. K., Calvo, A., et al. (2018). Prognosis for patients with amyotrophic lateral sclerosis: Development and validation of a personalised prediction model. Lancet. Neurol. 17 (5), 423–433. doi:10.1016/s1474-4422(18)30089-9
Xv, W., Ren, H., and Zhi, H. (2011). Clinical observation on the treatment of amyotrophic lateral sclerosis by Tonifying the kidney, strengthening the spleen and soothing the liver. Acad. J. Shanghai Univ. Traditional Chin. Med. 25 (05), 46–49. doi:10.16306/j.1008-861x.2011.05.014
Xv, Y., Zhong, Y. Z., and Lin, S. Y. (2013). Experimental study and clinical application of Guilu Erxian glue. Heilongjiang J. Traditional Chin. Med. 42 (06), 72–73.
Zhang, N. (2020). Effect of Shenmai Injection on patients with amyotrophic lateral sclerosis. Med. J. Chin. People's Health 32 (8), 49–50. doi:10.3969/j.issn.1672-0369.2020.08.019
Zhang, X., Hong, Y. L., Xu, D. S., Feng, Y., Zhao, L. J., Ruan, K. F., et al. (2014). A review of experimental research on herbal compounds in amyotrophic lateral sclerosis. Phytother. Res. 28 (1), 9–21. doi:10.1002/ptr.4960
Zhou, Q. M. (2017). Neuroprotective therapy in amyotrophic lateral sclerosis model mice. Doctor: Shanghai Jiaotong University.
Zhou, Q., Wang, Y., Zhang, J., Shao, Y., Li, S., Wang, Y., et al. (2018). Fingerprint analysis of Huolingshengji Formula and its neuroprotective effects in SOD1G93A mouse model of amyotrophic lateral sclerosis. Sci. Rep. 8 (1), 1668. doi:10.1038/s41598-018-19923-9
Keywords: amyotrophic lateral sclerosis, herbal medicine, motor neuron disease, meta-analysis, alternative and complementary medicine, systematic review
Citation: Song Y, Jia Q, Guan X, Kazuo S, Liu J, Duan W, Feng L, Zhang C and Gao Y (2022) Herbal medicine for amyotrophic lateral sclerosis: A systematic review and meta-analysis. Front. Pharmacol. 13:946548. doi: 10.3389/fphar.2022.946548
Received: 17 May 2022; Accepted: 23 August 2022;
Published: 31 August 2022.
Edited by:
Yue Liu, Xiyuan Hospital, ChinaReviewed by:
Zhe Kang Law, National University of Malaysia, MalaysiaCopyright © 2022 Song, Jia, Guan, Kazuo, Liu, Duan, Feng, Zhang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Gao, Z2FveWluZzk3M0AxNjMuY29t; Chi Zhang, c2FnYTYxOEAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.