
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 16 September 2022
Sec. Inflammation Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.946030
This article is part of the Research Topic The Role of Natural Bioactive Molecules in Inflammatory Injury View all 8 articles
Autoimmune diseases a group of disorders elicited by unexpected outcome of lymphocytes self-tolerance failure, and the common members of which include multiple sclerosis, systemic lupus erythematosus, inflammatory bowel disease, rheumatoid arthritis, and type 1 diabetes mellitus, etc. The pathogenesis of autoimmune diseases is not fully understood and the current therapeutic regimen’s inefficacy in certain cases coupled with low rates of success, exorbitant financial burden, as well as numerous side effects, which do open new avenues for the role of natural products as novel therapeutic agents for auto-inflammatory disorders. Scutellaria baicalensis Georgi is a well-known and widely-recognized herbal medicine with certain ameliorative effect on diverse inflammation-involved dysfunction. Though recent advances do highlight its potential to be applied in the fight against autoimmune diseases, the specific mechanism and the related opinion on the exploring possibility are still limited which hampered the further progress. Here in this timeline review, we traced and collected the evidence of how Scutellaria baicalensis Georgi and its bioactive contents, namely baicalin, baicalein, wogonoside and wogonin affect autoimmune diseases. Moreover, we also discussed the clinical implications and therapeutic potential of Scutellaria baicalensis Georgi and its bioactive contents in autoimmune diseases treatment.
Immune system is generally noted as a double-edged sword due to its nature of either heal or even harm the physiological mechanism of human beings. Defined as dysfunction of antigenic recognition and immune cells elimination, autoimmune diseases (ADS) are undesired consequence caused by self-tolerance failure of lymphocytes, the chief drive of their development (Rosetti et al., 2022). By far, a series of ADS, either systemic or organ-specific, have been identified and described. The typical ones include multiple sclerosis, systemic lupus erythematosus (SLE), inflammatory bowel disease (IBD), rheumatoid arthritis (RA), type 1 diabetes mellitus (T1DM), autoimmune hepatitis and autoimmune orchitis (Wu et al., 2021), details shown in Table 1. Epidemiological data indicated ADS afflict approximately 10% of the population worldwide, the percentage increase of which was 3.7, 6.2, 6.3 and 7.1% for neurological, gastrointestinal, endocrinological and rheumatic autoimmune diseases, respectively (Rengasamy et al., 2019). By far, the underlying pathogenesis mechanism of ADS are not fully unveiled though it may be closely related to abnormal immune modulation, internal and external environmental factor (Boardman and Levings, 2022). In fact, the current therapeutic criteria for ADS includes janus kinase (JAK) inhibitors and several specific monoclonal antibodies which is somehow based on the manifestations that complemented with laboratory test (Tavakolpour, 2017; Wu et al., 2018; Tanaka et al., 2022). With a goal of introducing a new generation of treating regimen with fewer life-threatening side effect, there’s an urgent need to explore novel small-molecule alternative medicine from natural resources.
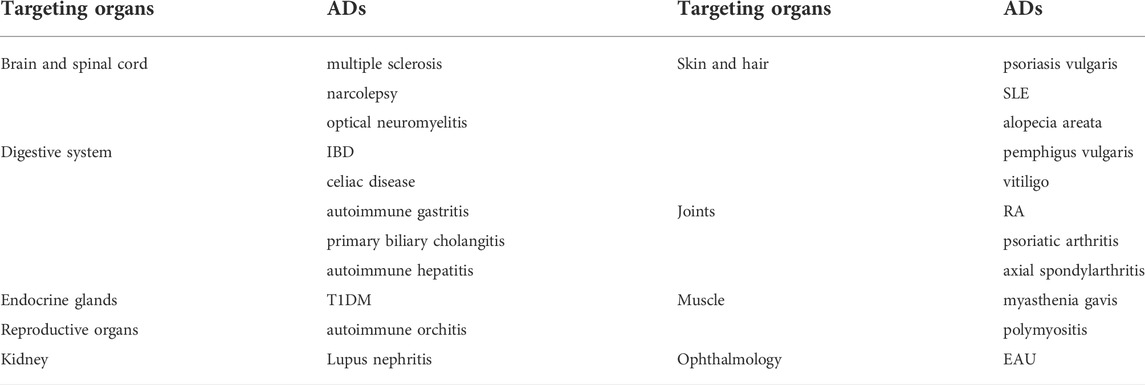
TABLE 1. Typical systemic or organ-specific ADS (Wu et al., 2021).
Baicalin (BAI)and its aglycone baicalein (BAE), as well as wogonin (WGI) and its aglycone wogonoside (WGS) are bioactive flavonoids which can be extracted from a well-known Chinses herb named Scutellaria baicalensis Georgi (SBG) originally documented in Shennong Bencao Jing (also known as the Divine Farmer’s Materia Medica) which was written between 200 and 250 AD (depicted in Figure 1). Another ancient medical book named Bencao Gangu (also known as the authoritative Materia Medica, accomplished in 1953) described SGB’s outstanding pharmacological properties against a range of disorders in details, and the author, Lishizhen, self-administrated SBG and reported it was cure for severe lung infection (Zhao et al., 2016). SBG was a representative medicinal plant with natural property of fire-purging and toxin-removing, which was attributed to its flavonoid contents that mentioned above. Clinically, when used in combination therapy, SBG demonstrated certain effect in treatment of non-small cell pulmonary carcinomas. Besides, modern evidence showed SGB potentiated positive outcome when applied to treat inflammatory disorders, respiratory tract infections, diarrhea, dysentery, liver dysfunctions, hypertension, hemorrhaging, as well as insomnia (Li-Weber, 2009). By far, systemic review which focused on the interaction between SBG and ADS is yet limited. On the other hand, several reviews concerning SBG’s small molecular contents have been released during the past decades, in which the general therapeutic and pharmacological effect (Huynh et al., 2020), prevention against cancer (Butt et al., 2021; Banik et al., 2022), controlling of diabetic cardiomyopathy (Khan and Kamal, 2019), protection against ischemia-induced neurodegeneration (Pan et al., 2021) as well as amelioration on ocular disorders (Xiao et al., 2014) of them were analyzed and discussed, while there’s no consensus on whether SBG and its compounds were promising candidates being capable of blocking ADS pathogenesis progress. Therefore, here in this timeline, we will concern about the virtue of SBG from the viewpoint of both in vivo and in vitro correlated with ADS immunologic pathologies, and meanwhile put forward synoptic outlook regarding these bioactive small-molecular compounds in SBG.
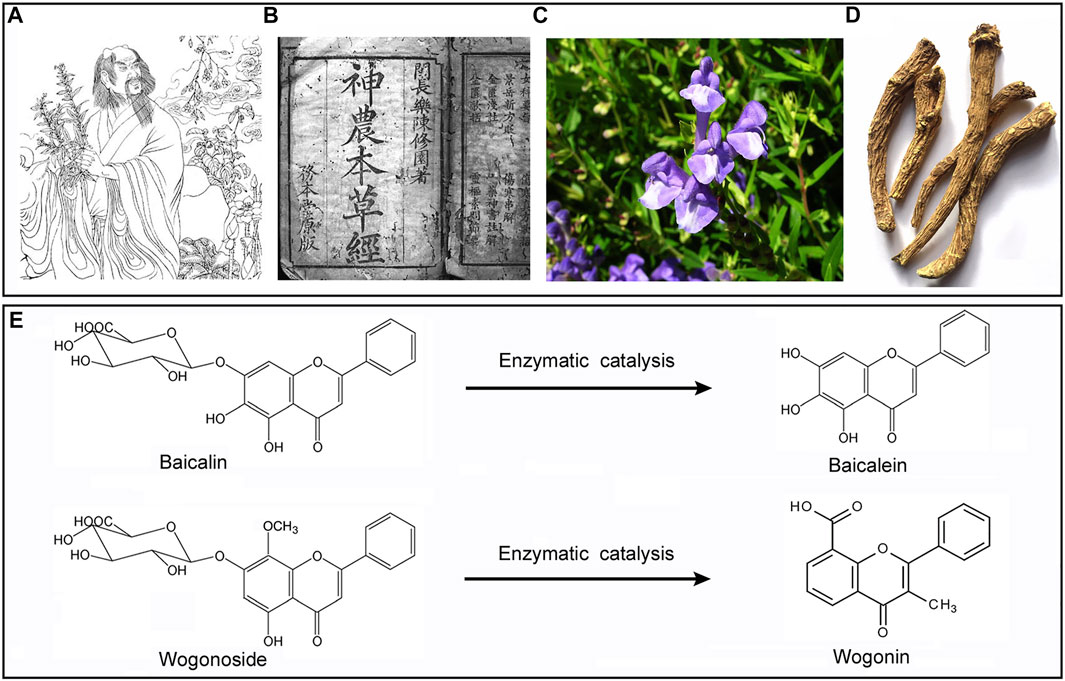
FIGURE 1. SBG was originally documented in Shennong’s (A) Shennong materia Medica (B). The plant (C), dry root (D), and the chemical structure of WGS/WGI (E).
As mentioned in previous chapters, aberrant immune and inflammatory responses potentiate damage towards diverse parts of human body, substantial efforts have been spared to evaluate how inflammasome, either canonical or non-canonical, get involved in ADS’s etiology and pathogenesis in recent years. A significant body of literature has been published to indicate the bioactive beneficial effect of BAI, BAE, WGI and WGS at multiple organs and systems throughout the whole body. Since clinical features and molecular mechanism of ADS exert some certain differences, details on the ADS-ameliorative property of SBG’s small-molecular compounds will be described following the order of brain/spinal cord, skin/hair, gastrointestinal tract, joints, and others. Figure 2
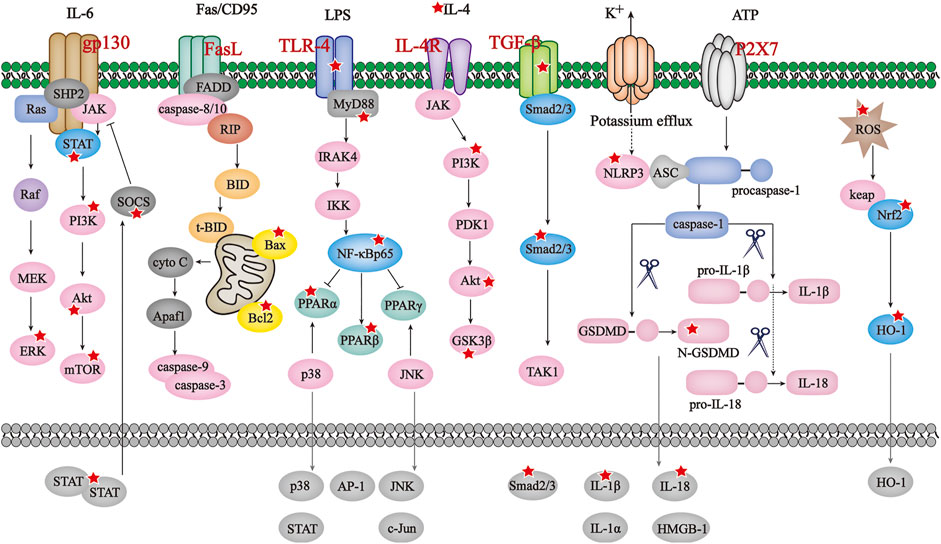
FIGURE 2. The principal pathways modulated by SBG and its compounds in ADS. JAK/STAT/mTOR, BAX/Bcl2/caspase-3/9, TLR4/NF-κB/PPAR, IL-4/PI3K/Akt/GSK3β, TGF-β/Smad2/3,NLRP3/caspase-1/GSDMD and ROS/Nrf2/HO-1 pathways are the principal signaling modulated by SBG and its compounds in ADS. The reported molecules regulated by SBG are marked with a red star.
The best-known ADS which occur at brain and spinal cord include multiple sclerosis, narcolepsy and optical neuromyelitis. Multiple sclerosis is characterized as localized demyelination and constant overactivated neuroinflammation can be observed during the progress. Likewise, abnormally activated astrocytes mediate transduction of eIF-2α/ATF4/CHOP signaling and downstream elevated caspase-11 noncanonical inflammasome. LW-213, a derivative of WGI, was reported to modulate eIF-2α/ATF4/CHOP axis in human cutaneous T-cell (Yu et al., 2021), and given the fact that WGI could pass the blood brain barrier to regulate eIF-2α/ATF4/CHOP in neurons (Chen et al., 2015), it is likely to ameliorate multiple sclerosis via inhibiting caspase-11. Experimental autoimmune encephalomyelitis (EAE) is a recognized murine model of multiple sclerosis, several canonical inflammasome, namely NLRP3 (Ren et al., 2021), AIM2 (Ma et al., 2021), caspase-8 (Zhang et al., 2018) and NLRC4 (Biliktu et al., 2020), have been ascertained to play pivotal roles in EAE pathogenesis. Accordingly, BAI exerted therapeutic effect on the symptoms in EAE mice model and this effect was associated with its modulatory effect on STAT/NF-κB, which was significantly abolished after the pharmacological ablation of SOCS3 (Zhang Y. et al., 2015). Similar results were observed in a previous study conducted by Zeng and colleagues, in which EAE animal model was built in proteolipid protein 139–151 in SJL/J mice, and reduction of IL-4 and IFN-γ was observed following BAI administration (Zeng et al., 2007). Also, BAE showed potential to ameliorate EAE, and the underlying mechanism was associated with its 12/15-lipoxygenase inhibition, downstream microglia PPARβ induction as well as MAPK/NF-κB blockage (Xu et al., 2013). Similarly, cuprizone model is also an established murine model of multiple sclerosis. BAE reversed cuprizone-induced increase in Iba1-positive microglia, GFAP-positive astrocytes, as well as the gene expressions of CD11b, GFAP, TNFα, IL-1β, and iNOS to suppress neuroinflammation (Hashimoto et al., 2017; Sanadgol et al., 2017). It is also worth mentioning that scutellarin (STR) inhibited cuprizone-induced inflammatory response in neural stem cells, thereby rescuing behavioral deficits in this mouse model of multiple sclerosis, even STR is not one of the highest-content compounds from SBG (Wang et al., 2016).
Despite of the temporary shortage of direct evidence indicating BAE small-molecule compounds’ effect to suppressed the above four canonical inflammasome to ameliorate EAE, a range of current reports have demonstrated that they exerted potent negative effect on disease took place in central nervous system via suppressing canonical inflammasomes. For example, WGI mediated mitochondria dysfunction in malignant neuroblastoma cells via inducing activation of caspase-4 (Ge et al., 2015), the human ortholog of caspase-11 functioning as targeting the pathological hallmark of amyotrophic ateral sclerosis through a route of driving TDP-43 cytoplasmic accumulation in the primate brains. WGS also down-regulated NLRP3 inflammasome activation to alleviate traumatic spinal cord injury-induced inflammation (Zhu et al., 2017). To date, no report on SBG and its principal flavonoids’ effect on narcolepsy and optical neuromyelitis have been released.
Skin autoimmune diseases, such as psoriasis vulgaris, alopecia areata and systemic lupus erythematosus (SLE) affects approximately 200 million people globally. BAI and ex vivo expanded Foxp3+ regulatory T cells were recently reported to be promising therapeutics strategy for SLE treatment since it was able to reduce Tfh cell differentiation, IL-21 production and mTOR activation while promote the differentiation of Foxp3+ regulatory T cell (Yang et al., 2019). On the other hand, BAE demonstrated reno-protective effect in a mouse model of lupus nephritis, which is regarded as a representative manifestation in SLE. Authors also shed the light of the underlying mechanisms, indicating the ROS production, Nrf2/heme-oxygenase (HO)-1, NLRP3 inflammasome, as well as NF-κB phosphorylation are the dominant pathways regulated by BAE (Li D. et al., 2019). Of notes, whether SBG or its flavonoids have any affection on psoriasis vulgaris and alopecia areata remain to be clarified.
Distinct from type 2 diabetes that caused by unhealthy lifestyles, T1DM is an undesirable consequence of autoimmune destruction (Syed, 2022). WGI demonstrated certain protective effect on diabetic renal injury, the mechanism involves its modulation on BCL-2-mediated autophagy and apoptosis and the inhibition of downstream pro-inflammatory cytokine production, which is likely to be associated with its inhibitory effect on osteopontin expression through enhancing PPARα activity (Zhang Y. M. et al., 2015; Liu et al., 2022). Besides, WGI’s ameliorative effect on STZ-induced symptoms like urinary albumin, histopathological damage and renal inflammation and fibrosis was related to its negative regulation on PI3K/Akt/NF-κB and TGF-β1/Smad3 (Zheng et al., 2020; Lei et al., 2021). Apart from T1DM secondary renal dysfunction, WGI was announced to alleviate diabetic cardiomyopathy through enhancing SOD1/2 and CAT while attenuating productions of ROS/MDA and proinflammatory cytokines (Khan et al., 2016).
Also in this model, BAI repaired T1DM-induced renal injury partly through modulating Klotho promoter methylation (a well-recognized endogenous inhibitor against renal fibrosis), as well as suppressing epithelial-to-mesenchymal transition (EMT) and microRNA-124/TLR4/NF-κB axis to repair renal fibrosis (Zhang S. et al., 2020; Zhang X. T. et al., 2020). Besides, several in-vitro studies verified the above results. For example, in TNF-α-stimulated pancreatic β-cell line Min6 which mimics β-cell destruction in TIDM, BAI reversed cell apoptosis and dysfunction via elevating miR-205 in a PI3K/AKT- and-NF-κB-dependent way (You et al., 2018). Endothelial dysfunction is commonly observed in T1DM. BAI was reported to alleviate endothelial impairment which was attributed to its role in reducing ROS via Akt/GSK3B/Fyn-mediated Nrf2 activation (Chen et al., 2018). A study conducted by Wang and colleagues revealed that cardiovascular system malformation induced by hyperglycemia was ameliorated by BAI administration due to its inhibition on ROS production and autophagy involving p62 ubiquitin, SOD, GSH-Px, MQAE and GABAA (Wang C. et al., 2018). Diabetic food ulcer is another complication of T1DM, the pathogenesis of which is proven to be correlated with elevated NO, MDA, p-ERK and p-HSP27 while decreased level of SOD, GSH, VEGF-c, Ang-1, Tie-2, TGF-β and Smad2/3. BAI administration reversed all the alterations and eventually promote the wound healing of food ulcer (Mao et al., 2021).
Accumulative evidence indicates BAE’s certain protective effect on T2DM (Li Y. et al., 2019; Wang Y. et al., 2022; Dong et al., 2022). The difference of T1DM and T2DM was firstly announced by Harold Percival Himsworth in 1936. ADS-associated T1DM patients completely lost their ability to produce insulin, while T2DM is a metabolic disease generally caused by insulin resistance and relative insulin deficiency, and is characterized by hyperglycemia (Ramirez et al., 2020; Lambrinoudaki et al., 2022). Of interest, current diabetic animal model seems to have no clear distinction between these two types, both of which were established by STZ injection. In brief, BAE, WGI and WGS prevented the progress of diabetic complications through regulating different pathways (Wang Y. et al., 2022). Mechanistically, for 1) Insulin resistance, BAE functioned as eliminating free radicals, restraining protein kinase C, enhancing α-Glucosidase activity and protecting β Cells, thus playing a role in reducing blood glucose, lipid and suppressing inflammatory reaction. WGI promoted GLUT4 protein level by activating PI3K-Akt pathway, and meanwhile facilitated glucose utilization via modulating PPAR-α pathway. WGS inactivated TLR4/NF-κB, NLRP3 and SOCS3to decrease IL-1 β, IL-6 and TNF-α secretion to trigger feedback regulation network. Aa for 2) Diabetic nephropaty, BAE rescued renal fibrosis via modulating AngII, TGF–β, α-SMA protein expression as well as p38MAPK/NF-κB transduction while WGS and WGI both negatively-regulated TLR4/NF-κB to protect renal tissue. Besides, for 3) Diabetic retinopathy, WGI restrained VEGF, bFGF, CTGF and TGF-β through ROS modulation, it also scavenged oxygen free radicals and reduced protein kinase C expression to potentiate vascular-protecting effect. Moreover, for 4) Diabetes peripheral neuropathy and cardiovascular diseases, BAE respectively regulated p38MAPK/Akt-Nrf2 pathway and AngII/SRP14 pathway to restore nerve conduction velocity and to repair endothelial cells peroxidation damage. Furthermore, WGI was reported to ameliorate hyperglycemia and hypoinsulinemia in T1DM mice through modulating p62DOK (Liu et al., 2018).
Rheumatoid arthritis (RA) is an autoimmune disease that brings about constant chronic joint inflammation with no cure yet. In mice model of collagen-induced arthritis (CIA) which is widely used to mimic joint inflammatory symptoms in human RA (Wu et al., 2022), BAI treatment alleviated radiographic and histologic abnormalities in the hind paw joints of CIA rats, and the underlying mechanism was associated with its modulation on TLR2/MYD88/NF-κB p65 signaling as well as the blockage of IL-1β, TNF-α and IL-6 production (Wang H. Z. et al., 2014; Xu et al., 2018; Bai et al., 2020). BAI also lowered relevant proinflammatory cytokines including TNF-α, IL-1β, IL-6, MMP-2, MMP-9, NO and COX-2 secretions in e synovial fluids and tissues of CIA rats to interfere JAK/STAT signaling transduction, which attributed to its restoring of pressure pain thresholds and clinical arthritis scores (Wang G. et al., 2018). In addition to collagen II, RA animal model established by complete Freund’s adjuvant (CFA) is also well-recognized because it shares similar experimental designs, immunological and pathological characteristics with RA in human beings (Huang et al., 2020). In this model, WGI reduced arthritic score and paw thickness-mediated by CFA and decreased IL-1β, IL-6, and TNF-α production via blocking MAPK and NF-κB signaling (Huang et al., 2020). Of notes, ICAM-1 was reported to occur in RA and meanwhile IL-17 are abundantly expressed in synovial fibroblasts. Since IL-17 not only mediates the synthesis of ICAM-1 and diverse cytokines (IL-6 and TNF-α, etc), but also accelerates collagen degradation via diminishing collagen and proteo glycan synthesis while enhancing bone erosion, its inhibition was believed to be an effective way to ameliorate RA. BAI rescued ankle swelling in Rats model of RA through negatively-regulating Th17 cell population expansion in spleen, and this effect was exerted through reducing the gene expression of RORgt, a dominant transcription factor in Th17 cell differentiation (Dinda et al., 2017; Sun and Gu, 2019).
Targeting immunological inflammation has been regarded as a promising way to prevent pathogenesis of experimental autoimmune uveitis (EAU), a sight-threatening ocular inflammatory disorder. Zhu and colleagues declared BAI demonstrated protective effect on EAU due to its inhibition on intraocular inflammatory process, as well as infiltrated inflammatory cells and transcriptions of proinflammatory cytokines like IL-17A, IFN-γ and TNF-α. Moreover, it is observed that BAI promoted regulatory T cells’ amounts and frequency while restrained those of Th1 and Th17. Similar results were obtained in in-vitro study which further drew a conclusion that BAI was a promising candidate for EAU treatment since it modulated reg/Teff balance as a inducer of aryl hydrocarbon receptor (Zhu et al., 2018). Besides, the pathogenesis of EAU could also be rescued by BAE administration with an underlying mechanism of IL-17 inhibition (Li et al., 2016). Research concerning the interaction between WGI, WGS, or SBG and EAU is yet limited.
Comprised with ulcerative colitis and Crohn’s disease, inflammatory bowel disease (IBD) is characterized as intestinal microbiota alterations which are linked with a wide range of autoimmune conditions (Spalinger et al., 2022). A recent released review concentrated on the up-to-date studies investigating the affection of baicalin on IBD, indicating BAI can be a promising therapeutic prospect as a potential supplementary agent due to it reversed IBD by virtue of anti-inflammation and antioxidant properties, immune regulation, as well as maintenance of intestinal barrier and intestinal flora balance (Wang X. et al., 2022). Liang and colleagues found that BAI, BAE, and the combination of the two flavonoids (those extracted either young or withered SBG) blocked NF-κB and MAPK signaling to alleviate clinical symptoms and signs of IBD with different potency, they also repaired the abnormal organ indices and normalized the blood biochemistry (Liang et al., 2019). Besides, a novel agent named PF2405 which enriched with BAE, oroxylin A and WGI had outstanding preventive and therapeutic activities in trinitrobenzene sulfonic acid or dextran sulfate sodium-induce colitis mice. Mechanically, PF2405 relieved the histopathological severity and meanwhile decreased the expression of COX-2, TNF-α, and IL-1β in colon tissue, and this effect was correlated with its abrogating to inflammatory response mediated by COX-2/MAPK signaling (Jiang et al., 2015). A study conducted by Zhu and colleagues shed the light of BAI ameliorated DSS-induced IBD via regulating macrophage polarization to the M2 phenotype. In specific, it dampens LPS-induced tumor necrosis factor α, IL-23 and IRF5 expression while enhancing IL-10, arginase-1 (Arg-1) and IRF4 expression in colon, to reverse macrophage subset redistribution (Zhu et al., 2016). Of notes, a range of ancient prescriptions that contains SBG also demonstrated certain ameliorative effect on IBD in addition to small-molecules from SBG, typical ones include Jiawei Gegen Qinlian decoction (Li et al., 2021), Banxia Xiexin decoction (Wang et al., 2021), Gegen Qinlian decoction (Xu et al., 2021) and Huangqin decoction (Chen et al., 2016), among which BAE, WGI, and BAI are the principal contents accounting for these bioactive effects, and the principal involved molecular pathways include NF-κB, NLRP3 inflammasome, MAPK, JAK/STAT3, IL-17, the Th17 cell differentiation and oxidative stress pathway.
Autoimmune hepatitis (AIH) is another autoimmune disease occurring in digestive system. Abnormal apoptosis in activated lymphocytes accelerates the development of AIH. In AIH animal model established by concanavalin A, BAE was observed to reduce Bcl-2/Bax ration and meanwhile restraining caspase-9/-3 activation to further induce apoptosis in activated lymphocytes and eventually ameliorate AIH (Zhang et al., 2013). Of interest, SBG extract itself was reported to be a AIH model inducer when intraperitoneally-administrated to mice (Wang J. Y. et al., 2014). The reason for these controversial results may be related to the potential opposite effect of SBG constituents on AIH. Given the fact that reports on how AIH and constituents affect AIH remain limited, further studies concerning the effect of BAI, WGI and WGS on AIH are desperate to figure out the underlying mechanisms.
Due to the limitation of current therapeutic strategies which remain simplistic and reductionist understanding of ADS pathogenesis, it is urgent to explore novel alternative inhibitors with natural sources. In clinical, considerable chemical-modified agents show straightforward ameliorative effect on ADS by relieving symptoms broad-actively, while not disease-specifically (Wu et al., 2021). Of notes, adverse reactions such as infusion reactions, human anti-chimeric antibodies production, and neutropenia cannot be avoided in ADS-relieving approaches. Meanwhile, combining similarities among distinct ADS indeed brings about a grey zone for diagnosis by young clinicians since not all ADS share similar symptoms (Wu et al., 2018). The new generation of biological drugs emerged in past decade are targeted more efficient, more specific, and safer, which provides better options for ADS treatment. The representative ones include inhibitors targeting JAK and some species of monoclonal antibodies. However, those biological agents as well increase the financial burden for ADS patients (Tavakolpour, 2017). Thus, the current therapeutic regimen’s inefficacy in certain cases coupled with low rates of success as well as numerous side effects, which do open new avenues for the role of natural products as novel therapeutic agents for auto-inflammatory disorders. SBG is one of the most important and high-frequently used traditional Chinese medicine where dried roots preparation of ‘Huang-qin’, as well as bioactive constituents, particularly of those flavonoids, are applied for liver and pulmonary complaints and as alternative and complementary treatment for various of carcinoma, such as prostate, bladder, breast, and liver cancer (Bonham et al., 2005; Zhao Q. et al., 2019; Cao et al, 2020; Kong et al., 2021; Yao et al., 2021). However, no particular attention concerning how SBG affect ADS in a systematic way has been paid by far. By combing through the current reported evidence, even the impact of SBG on ADS symptoms is also broad-active like chemical-modified drugs, it has characteristic of multi-component and multi-targeting which potentiates mutual-reinforcement at both pharmacokinetic and pharmacodynamics level as displayed in Figure 2 and Table 2, and this goal is achieved through 1) superimposing potency on one certain target, 2) expanding the scope of efficacy or 3) promoting the intracellular transport of SBG flavonoids via affecting membrane transport proteins like P-gp and organic cation transporters (Wu et al., 2016). Compared with the chemical-modified agents currently used in clinic to reverse ADS, SBG oral preparation and its bioactive ingredients have advantage of relatively low toxicity of only causing symptoms such as stomach discomfort and muscle soreness, which suggest that it a promising candidate to be widely applied in clinical practices (Zhao T. et al., 2019). Indistinct diagnosis of different types of ADS has been being an enormous challenge for young clinicians. To cope with the diverse symptoms, patients may receive drug combination which is likely to lead to the accumulation of adverse reactions. As other traditional Chinese medicine, the characteristic of SBG is multi-component and multi-targeting which makes it possible to avoid toxicity superposition since no obvious adverse reaction is observed in oral preparation of SBG (Zhao Q. et al., 2019). In fact, SGB has a wide distribution in many provinces of China including Heilongjiang, Liaoning, Inner Mongolia, Hebei, Henan, Gansu, Shanxi, Shandong, and Sichuan, etc. Besides, it is also abundant in other countries like Soviet Union in Eastern Siberia, Mongolia, North Korea, and Japan. It is noteworthy that SBG grows on sunny grassy slopes as well as desert lands at an altitude of 60–2000 m, the nature of easily-accessible allows it to be finanial-friendly to ADS patients.
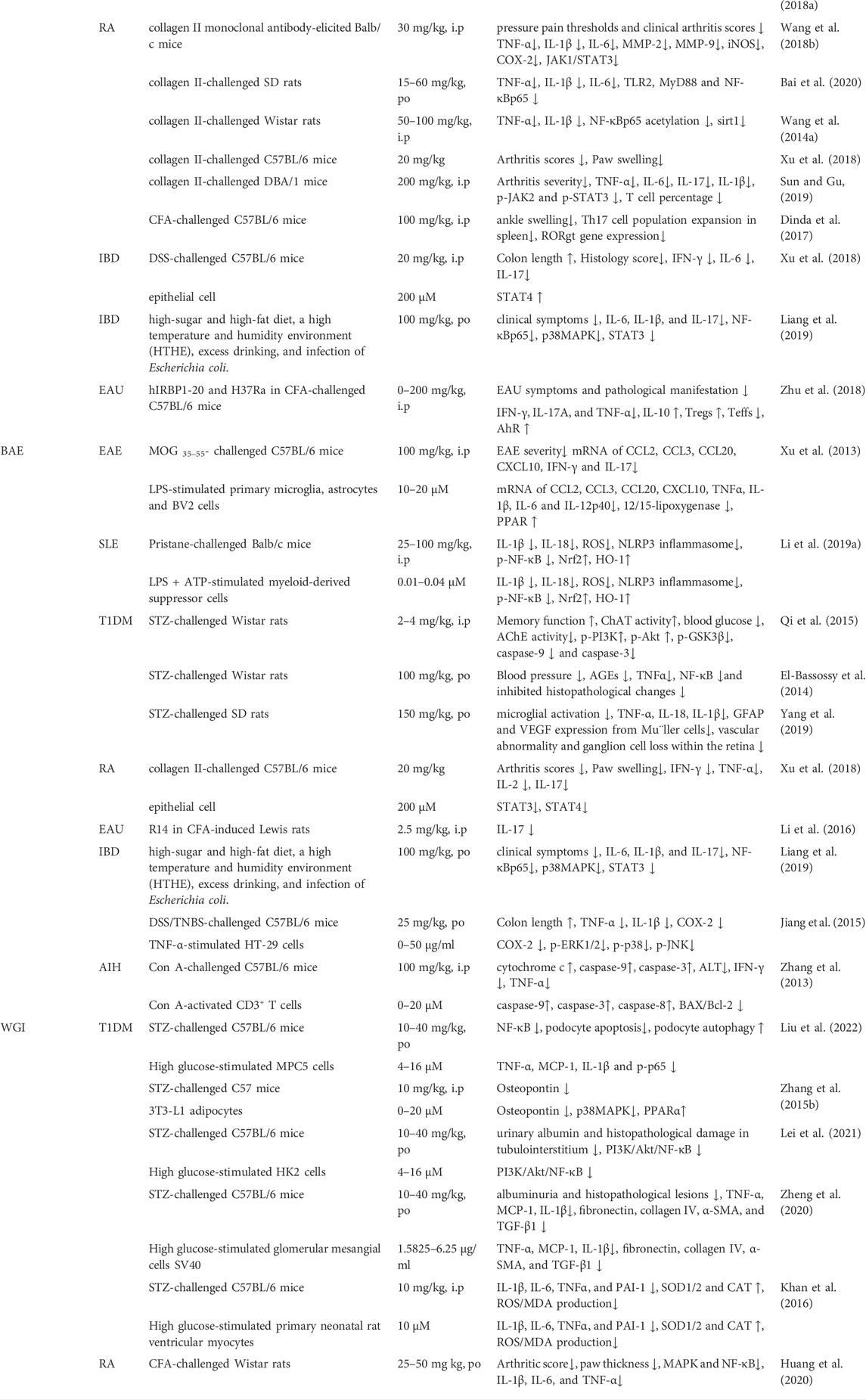
TABLE 2. JAK/STAT/mTOR, BAX/Bcl2/caspase-3/9, TLR4/NF-κ/PPAR, IL-4/PI3K/Akt/GSK3β, TGF-β/Smad2/3,NLRP3/caspase-1/GSDMD and ROS/Nrf2/HO-1 pathways are the principal signaling modulated by SBG and its compounds in ADS. The reported molecules regulated by SBG are marked with a red star.JAK/STAT/mTOR, BAX/Bcl2/caspase-3/9, TLR4/NF-κB/PPAR, IL-4/PI3K/Akt/GSK3β, TGF-β/Smad2/3,NLRP3/caspase-1/GSDMD and ROS/Nrf2/HO-1 pathways are the principal signaling modulated by SBG and its compounds in ADS. The reported molecules regulated by SBG are marked with a red star.
In fact, up to 40 constituents have been isolated from SBG, among which BAI, BAE, WGS, and WGI are used as quality control indicators for SBG and its related preparations, and these four flavonoids are accounted for SBG’s bioactive properties and the key players in SBG’s clinical therapeutic effects (Wang Y. et al., 2022). To date, there has been no large-scale investigation of the actual clinical use of SBG, not only the distribution but also the frequency of use. Liao and colleagues traced an available robust healthcare system with complete database records, indicating SBG was recommended to combat a range of chronic diseases including respiratory inflammatory disorders, headache, sleep impairments, hepatitis, diarrhoea, vomiting, haemorrhage, hypertension, and gastrointestinal discomfort (Liao et al., 2021), These clinical applications mentioned above can dramatically be linked to the modern concept of anti-infection or anti-inflammation, which is specified in the Chinese Pharmacopoeia, paving the development of SBG as a natural anti-inflammatory, antibiotic, and anti-tumor drug (Chen et al., 2018). For the flavonoids of SBG, BAI has anti-microbial activity by exerting potent synergistic effect with penicillin G/amoxicillin against 20 clinical penicillinase-producing S. aureus strains. Meanwhile, the clinical trial of WGI as an anti-cancer agent candidate has recently been approved by the State Drug Administration of China (Wang Z. L. et al., 2018). However, clinical studies concerning whether and how SBG affect ADS are warranted to confirm the possible beneficial therapeutic outcome and the underlying mechanisms.
Although the issue of bioavailability is always being questioned for SBG’s flavonoids or even the majorities of small-molecular compounds with natural resource, SBG and its flavonoids were reported to have ameliorative effect on disorders occurred in multiple systems, thereby suggesting those flavonoids were able to reach targeted tissues via bloodstream and crossing the blood-brain barrier to perform therapeutic effect on ADS took place in diverse systems illustrated in Figure 3. The commercial interest and the increasing demand of SBG contributed to the appearance of huge gap of its development. Given the evidence provided by available scientific validation, SBG and its principal flavonoids, namely BAI, BAE, WGI and WGS, are potential candidates since they block abnormal auto-inflammatory response which occurred in a wide range of ADS including multiple sclerosis, EAU, SLE, T1DM, RA, IBD, as well as AIH. As discussed in previous chapters in this review, SBG and its principal flavonoids modulates diverse mechanistic routes to maintain their inflammatory-preventing and immune-regulatory actions. Notably, pathways of JAK/STAT/mTOR, BAX/Bcl2/caspase-3/9, TLR4/NF-κB/PPAR, IL-4/PI3K/Akt/GSK3β, TGF-β/Smad2/3, NLRP3/caspase-1/GSDMD and ROS/Nrf2/HO-1 are the key ones modulated by SBG. Nevertheless, there is still a long way before fully uncover the underlying molecular of SBG ameliorates ADS. In recent years, an increasing body of evidence ascertained canonical and noncanonical inflammasome activation pave the way for the development and exacerbation of ADS. During the process of ADS pathogenesis, immune tolerance dysfunction is believed to bring about unexpected pro-inflammatory debris accumulation and gargantuan cytokines and chemokines release, which further fuels cytokine storm and cause the secondary damage to tissues and organs (Fajgenbaum and June, 2020). The certain involvement of inflammasome in ADS progress has been revealed in the past decades while there are still many underlying mechanisms remaining to be clarified. Generally, inflammasomes can be divided into canonical ones (such as NLRP3, AIM2 and NLRP1, etc) and noncanonical caspase-11. The former category is architecturally assembled by a sensor (also known as recognition receptor), an adaptor and a protease effector in response to a wide range of exogenous and endogenous stimuli. Noncanonical caspase-11 inflammasome activation, on the other hand, directly triggered by LPS that gains access to cytosol with a dispensable role played by TLR-4 (Abu Khweek and Amer, 2020; De Carvalho Ribeiro and Szabo, 2022). Numerous evidence emerged in recent years indicate that canonical and noncanonical inflammasomes are of equal importance when induing pyroptotic cell death, the consequence of which is one of the principal reasons accounting for ADS exacerbation (Wu et al., 2021). Herein, targeting either canonical or noncanonical inflammasome thereby is an attracting way to calm down cytokine storm thereby preventing development and exacerbation of ADS. Unfortunately, studies concerning whether and how SBG and its flavonoids affect inflammsomes/pyroptosis pathways are still limited, future investigations are encouraged to focus on the interactions between SBG and various kind of inflammasomes such as AIM2, NLRP1 and caspase-11. In addition, Neutrophil extracellular traps (NETs), the fibrous networks which protrude from the membranes of activated neutrophils was reported to play a central role in ADS pathogenesis (Lee et al., 2017), it will be of interest to ascertain whether NETs is a target of SBG in future studies.
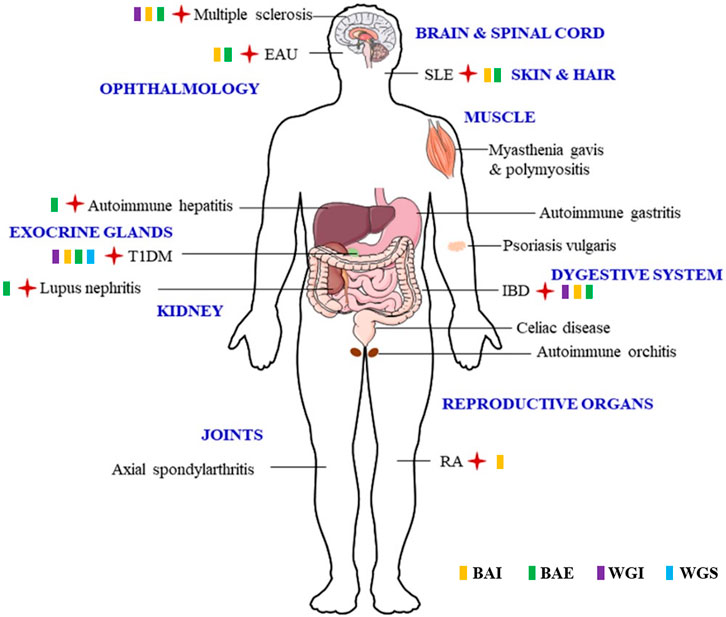
FIGURE 3. The verified ADS affected by SBG and its flavonoids are highlighted by red cross, and the effective flavonoid is marked by relevant symbols.
In almost all ADS cases, there is a definite sex difference in prevalence, whereby females are generally more frequently affected than that of males (almost three more times), and organ vulnerability as well as reproductive capacity are most vital factors facilitating this distinction (Hodson, 2021; Ngo et al., 2014). However, the current studies concerned more about the molecular mechanism while hardly conducted experiments to compared the sex difference in the therapeutic effect of SBG on ADS. Further investigations may be needed to address such questions.
To sum up, to introduce a new generation of treating regimen for ADs with fewer life-threatening side effect, it is desperate to explore novel small-molecule alternative medicine from natural resources. With advantages of multi-targeting and broadly-active with low adverse reactions and less financial burden, SBG and its flavonoids are promising candidates for the treatment of diverse ADS including multiple sclerosis, systemic lupus erythematosus, type 2 diabetes and the complications, rheumatoid arthritis, autoimmune uveitis, inflammatory bowel disease, and autoimmune hepatitis. Nevertheless, bioavailability issue need resolving and detailed information on the underlying molecular mechanism and gender difference in this process need to be addressed and supplemented before SBG and its flavonoids can be translated from bench to bedside.
JW: Draft the original manuscript, and prepare the figures; SC and JZ: Search and analyze the literatures; JW: Revise and finalize the article.
This work is supported by the National Natural Science Foundation of China (82104491), the “Xinglin Scholar” Scientific Research Promotion Plan of Chengdu University of Traditional Chinese Medicine (BSH2020024), and the Postdoctoral Science Foundation of China (2021M693789).
We also give our special thanks to Wanshi Editor for the help in the preparation of Figure 3.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abu Khweek, A., and Amer, A. O. (2020). Pyroptotic and non-pyroptotic effector functions of caspase-11. Immunol. Rev. 297 (1), 39–52. doi:10.1111/imr.12910
Bai, L., Bai, Y., Yang, Y., Zhang, W., Huang, L., Ma, R., et al. (2020). Baicalin alleviates collagen-induced arthritis and suppresses TLR2/MYD88/NF-κB p65 signaling in rats and HFLS-RAs. Mol. Med. Rep. 22 (4), 2833–2841. doi:10.3892/mmr.2020.11369
Banik, K., Khatoon, E., Harsha, C., Rana, V., Parama, D., Thakur, K. K., et al. (2022). Wogonin and its analogs for the prevention and treatment of cancer: A systematic review. Phytother. Res. 36, 1854–1883. doi:10.1002/ptr.7386
Biliktu, M., Senol, S. P., Temiz-Resitoglu, M., Guden, D. S., Horat, M. F., Sahan-Firat, S., et al. (2020). Pharmacological inhibition of soluble epoxide hydrolase attenuates chronic experimental autoimmune encephalomyelitis by modulating inflammatory and anti-inflammatory pathways in an inflammasome-dependent and -independent manner. Inflammopharmacology 28 (6), 1509–1524. doi:10.1007/s10787-020-00691-w
Boardman, D. A., and Levings, M. K. (2022). Emerging strategies for treating autoimmune disorders with genetically modified Treg cells. J. Allergy Clin. Immunol. 149 (1), 1–11. doi:10.1016/j.jaci.2021.11.007
Bonham, M., Posakony, J., Coleman, I., Montgomery, B., Simon, J., and Nelson, P. S. (2005). Characterization of chemical constituents in Scutellaria baicalensis with antiandrogenic and growth-inhibitory activities toward prostate carcinoma. Clin. Cancer Res. 11 (10), 3905–3914. doi:10.1158/1078-0432.CCR-04-1974
Butt, G., Ozbey, U., Malik, D. E., Attar, R., Youssef, L., and Farooqi, A. A. (2021). Regulation of cell signaling pathways by Wogonin in different cancers: Mechanistic review. Cell. Mol. Biol. 67 (2), 1–7. doi:10.14715/cmb/2021.67.2.1
Cao, Y., Cao, W., Qiu, Y., Zhou, Y., Guo, Q., Gao, Y., et al. (2020). Oroxylin A suppresses ACTN1 expression to inactivate cancer-associated fibroblasts and restrain breast cancer metastasis. Pharmacol. Res. 159, 104981. doi:10.1016/j.phrs.2020.104981
Chen, F., Wu, R., Zhu, Z., Yin, W., Xiong, M., Sun, J., et al. (2015). Wogonin protects rat dorsal root ganglion neurons against tunicamycin-induced ER stress through the PERK-eIF2α-ATF4 signaling pathway. J. Mol. Neurosci. 55 (4), 995–1005. doi:10.1007/s12031-014-0456-7
Chen, G., Chen, X., Niu, C., Huang, X., An, N., Sun, J., et al. (2018a). Baicalin alleviates hyperglycemia-induced endothelial impairment 1 via Nrf2. J. Endocrinol. 18-0457, R1. doi:10.1530/JOE-18-0457
Chen, G., Yang, Y., Hu, C., Cheng, X., Xu, Y., Cai, X., et al. (2016). Protective effects of Huangqin Decoction against ulcerative colitis and associated cancer in mice. Oncotarget 7 (38), 61643–61655. doi:10.18632/oncotarget.11426
De Carvalho Ribeiro, M., and Szabo, G. (2022). Role of the inflammasome in liver disease. Annu. Rev. Pathol. 17, 345–365. doi:10.1146/annurev-pathmechdis-032521-102529
Dinda, B., Dinda, S., DasSharma, S., Banik, R., Chakraborty, A., and Dinda, M. (2017). Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 131, 68–80. doi:10.1016/j.ejmech.2017.03.004
Dong, Y., Sui, L., Yang, F., Ren, X., Xing, Y., and Xiu, Z. (2022). Reducing the intestinal side effects of acarbose by baicalein through the regulation of gut microbiota: An in vitro study. Food Chem. 394, 133561. doi:10.1016/j.foodchem.2022.133561
El-Bassossy, H. M., Hassan, N. A., Mahmoud, M. F., and Fahmy, A. (2014). Baicalein protects against hypertension associated with diabetes: effect onvascular reactivity and stiffness. Phytomedicine 21 (12), 1742–1745. doi:10.1016/j.phymed.2014.08.012
Fajgenbaum, D. C., and June, C. H. (2020). Cytokine storm. N. Engl. J. Med. 383 (23), 2255–2273. doi:10.1056/NEJMra2026131
Ge, W., Yin, Q., and Xian, H. (2015). Wogonin induced mitochondrial dysfunction and endoplasmic reticulum stress in human malignant neuroblastoma cells via ire1α-dependent pathway. J. Mol. Neurosci. 56 (3), 652–662. doi:10.1007/s12031-015-0530-9
Hashimoto, M., Yamamoto, S., Iwasa, K., Yamashina, K., Ishikawa, M., Maruyama, K., et al. (2017). The flavonoid Baicalein attenuates cuprizone-induced demyelination via suppression of neuroinflammation. Brain Res. Bull. 135, 47–52. doi:10.1016/j.brainresbull.2017.09.007
Huang, Y., Guo, L., Chitti, R., Sreeharsha, N., Mishra, A., Gubbiyappa, S. K., et al. (2020). Wogonin ameliorate complete Freund's adjuvant induced rheumatoid arthritis via targeting NF-κB/MAPK signaling pathway. Biofactors 46 (2), 283–291. doi:10.1002/biof.1585
Huynh, D. L., Ngau, T. H., Nguyen, N. H., Tran, G. B., and Nguyen, C. T. (2020). Potential therapeutic and pharmacological effects of wogonin: An updated review. Mol. Biol. Rep. 47 (12), 9779–9789. doi:10.1007/s11033-020-05972-9
Jiang, W. Y., Seo, G. S., Kim, Y. C., Sohn, D. H., and Lee, S. H. (2015). PF2405, standardized fraction of Scutellaria baicalensis, ameliorates colitis in vitro and in vivo. Arch. Pharm. Res. 38 (6), 1127–1137. doi:10.1007/s12272-015-0553-3
Khan, S., and Kamal, M. A. (2019). Can wogonin be used in controlling diabetic cardiomyopathy? Curr. Pharm. Des. 25 (19), 2171–2177. doi:10.2174/1381612825666190708173108
Khan, S., Zhang, D., Zhang, Y., Li, M., and Wang, C. (2016). Wogonin attenuates diabetic cardiomyopathy through its anti-inflammatory and anti-oxidative properties. Mol. Cell. Endocrinol. 428, 101–108. doi:10.1016/j.mce.2016.03.025
Kong, N., Chen, X., Feng, J., Duan, T., Liu, S., Sun, X., et al. (2021). Baicalin induces ferroptosis in bladder cancer cells by downregulating FTH1. Acta Pharm. Sin. B 11 (12), 4045–4054. doi:10.1016/j.apsb.2021.03.036
Lambrinoudaki, I., Paschou, S. A., Armen, i. E., and Goulis, D. G. (2022). The interplay between diabetes mellitus and menopause: Clinical implications. Nat. Rev. Endocrinol. doi:10.1038/s41574-022-00708-0
Lee, K. H., Kronbichler, A., Park, D. D., Park, Y., Moon, H., Kim, H., et al. (2017). Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun. Rev. 16 (11), 1160–1173. doi:10.1016/j.autrev.2017.09.012
Lei, L., Zhao, J., Liu, X. Q., Chen, J., Qi, X. M., Xia, L. L., et al. (2021). Wogonin alleviates kidney tubular epithelial injury in diabetic nephropathy by inhibiting PI3K/Akt/NF-κB signaling pathways. Drug Des. devel. Ther. 15, 3131–3150. doi:10.2147/DDDT.S310882
Li, D., Shi, G., Wang, J., Zhang, D., Pan, Y., Dou, H., et al. (2019a). Baicalein ameliorates pristane-induced lupus nephritis via activating Nrf2/HO-1 in myeloid-derived suppressor cells. Arthritis Res. Ther. 21 (1), 105. doi:10.1186/s13075-019-1876-0
Li, M., Chen, X., Liu, J., Wang, D., Gan, L., Lv, X., et al. (2016). Treatment of experimental autoimmune uveoretinitis with different natural compounds. Mol. Med. Rep. 13 (6), 4654–4658. doi:10.3892/mmr.2016.5096
Li, Q., Cui, Y., Xu, B., Wang, Y., Lv, F., Li, Z., et al. (2021). Main active components of Jiawei Gegen Qinlian decoction protects against ulcerative colitis under different dietary environments in a gut microbiota-dependent manner. Pharmacol. Res. 170, 105694. doi:10.1016/j.phrs.2021.105694
Li, Y., Chen, Q., Ran, D., Wang, H., Du, W., Luo, Y., et al. (2019b). Changes in the levels of 12/15-lipoxygenase, apoptosis-related proteins and inflammatory factors in the cortex of diabetic rats and the neuroprotection of baicalein. Free Radic. Biol. Med. 134, 239–247. doi:10.1016/j.freeradbiomed.2019.01.019
Li-Weber, M. (2009). New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat. Rev. 35 (1), 57–68. doi:10.1016/j.ctrv.2008.09.005
Liang, S., Deng, X., Lei, L., Zheng, Y., Ai, J., Chen, L., et al. (2019). The comparative study of the therapeutic effects and mechanism of baicalin, baicalein, and their combination on ulcerative colitis rat. Front. Pharmacol. 10, 1466. doi:10.3389/fphar.2019.01466
Liao, C. C., Liao, K. R., Lin, C. L., and Li, J. M. (2021). The effectiveness of Scutellaria baicalensis on migraine: Implications from clinical use and experimental proof. Evid. Based. Complement. Altern. Med. 2021, 8707280. doi:10.1155/2021/8707280
Liu, X. Q., Jiang, L., Li, Y. Y., Huang, Y. B., Hu, X. R., Zhu, W., et al. (2022). Wogonin protects glomerular podocytes by targeting Bcl-2-mediated autophagy and apoptosis in diabetic kidney disease. Acta Pharmacol. Sin. 43 (1), 96–110. doi:10.1038/s41401-021-00721-5
Liu, Y., Han, C., and Wang, X. (2018). Study on the intervention effect of wogonin on type 1 diabetic mice and its effect on liver P62DOK expression level. Chin. J. Diabetes. 26, 3.
Ma, C., Li, S., Hu, Y., Ma, Y., Wu, Y., Wu, C., et al. (2021). AIM2 controls microglial inflammation to prevent experimental autoimmune encephalomyelitis. J. Exp. Med. 218 (5), e20201796. doi:10.1084/jem.20201796
Mao, X., Li, Z., Li, B., and Wang, H. (2021). Baicalin regulates mRNA expression of VEGF-c, Ang-1/Tie2, TGF-β and Smad2/3 to inhibit wound healing in streptozotocin-induced diabetic foot ulcer rats. J. Biochem. Mol. Toxicol. 35 (11), e22893. doi:10.1002/jbt.22893
Ngo, S. T., Steyn, F. J., and McCombe, P. A. (2014). Gender differences in autoimmune disease. Front. Neuroendocrinol. 35 (3), 347–369. doi:10.1016/j.yfrne.2014.04.004
Pan, L., Cho, K. S., Yi, I., To, C. H., Chen, D. F., and Do, C. W. (2021). Baicalein, baicalin, and wogonin: Protective effects against ischemia-induced neurodegeneration in the brain and retina. Oxid. Med. Cell. Longev. 2021, 8377362. doi:10.1155/2021/8377362
Qi, Z., Xu, Y., Liang, Z., Li, S., Wang, J., Wei, Y., Dong, B., et al. (2015). Baicalein alters PI3K/Akt/GSK3β signaling pathway in rats with diabetes-associated cognitive deficits. Int. J. Clin. Exp. Med. 8 (2), 1993–2000.
Ramirez, D. G., Abenojar, E., Hernandez, C., Lorberbaum, D. S., Papazian, L. A., Passman, S., et al. (2020). Contrast-enhanced ultrasound with sub-micron sized contrast agents detects insulitis in mouse models of type1 diabetes. Nat. Commun. 11 (1), 2238. doi:10.1038/s41467-020-15957-8
Ren, G. M., Li, J., Zhang, X. C., Wang, Y., Xiao, Y., Zhang, X. Y., et al. (2021). Pharmacological targeting of NLRP3 deubiquitination for treatment of NLRP3-associated inflammatory diseases. Sci. Immunol. 6 (58), eabe2933. doi:10.1126/sciimmunol.abe2933
Rengasamy, K. R. R., Khan, H., Gowrishankar, S., Lagoa, R. J. L., Mahomoodally, F. M., Khan, Z., et al. (2019). The role of flavonoids in autoimmune diseases: Therapeutic updates. Pharmacol. Ther. 194, 107–131. doi:10.1016/j.pharmthera.2018.09.009
Rosetti, F., Madera-Salcedo, I. K., Rodríguez-Rodríguez, N., and Crispín, J. C. (2022). Regulation of activated T cell survival in rheumatic autoimmune diseases. Nat. Rev. Rheumatol. 18 (4), 232–244. doi:10.1038/s41584-021-00741-9
Sanadgol, N., Zahedani, S. S., Sharifzadeh, M., Khalseh, R., Barbari, G. R., and Abdollahi, M. (2017). Recent updates in imperative natural compounds for healthy brain and nerve function: A systematic review of implications for multiple sclerosis. Curr. Drug Targets 18 (13), 1499–1517. doi:10.2174/1389450118666161108124414
Spalinger, M. R., Shawki, A., Chatterjee, P., Canale, V., Santos, A., Sayoc-Becerra, A., et al. (2022). Autoimmune susceptibility gene PTPN2 is required for clearance of adherent-invasive Escherichia coli by integrating bacterial uptake and lysosomal defence. Gut 71 (1), 89–99. doi:10.1136/gutjnl-2020-323636
Sun, F., and Gu, W. (2019). Baicalin attenuates collagen-induced arthritis via inhibition of JAK2-STAT3 signaling and regulation of Th17 cells in mice. J. Cell Commun. Signal. 13 (1), 65–73. doi:10.1007/s12079-018-0475-1
Syed, F. Z. (2022). Type 1 diabetes mellitus. Ann. Intern. Med. 175 (3), ITC33–ITC48. doi:10.7326/AITC202203150
Tanaka, Y., Luo, Y., O'Shea, J. J., and Nakayamada, S. (2022). Janus kinase-targeting therapies in rheumatology: A mechanisms-based approach. Nat. Rev. Rheumatol. 18 (3), 133–145. doi:10.1038/s41584-021-00726-8
Tavakolpour, S. (2017). Towards personalized medicine for patients with autoimmune diseases: Opportunities and challenges. Immunol. Lett. 190, 130–138. doi:10.1016/j.imlet.2017.08.002
Wang, C., Song, Y., Wang, X., Mao, R., and Song, L. (2018a). Baicalin ameliorates collagen-induced arthritis through the suppression of janus kinase 1 (JAK1)/Signal transducer and activator of transcription 3 (STAT3) signaling in mice. Med. Sci. Monit. 24, 9213–9222. doi:10.12659/MSM.910347
Wang, G., Liang, J., Gao, L. R., Si, Z. P., Zhang, X. T., Liang, G., et al. (2018b). Baicalin administration attenuates hyperglycemia-induced malformation of cardiovascular system. Cell Death Dis. 9 (2), 234. doi:10.1038/s41419-018-0318-2
Wang, H. Z., Wang, H. H., Huang, S. S., Zhao, H., Cao, Y. G., Wang, G. Z., et al. (2014a). Inhibitory effect of baicalin on collagen-induced arthritis in rats through the nuclear factor-κB pathway. J. Pharmacol. Exp. Ther. 350 (2), 435–443. doi:10.1124/jpet.114.215145
Wang, J. Y., Lee, C. Y., Pan, P. J., Chang, W. C., Chiu, J. H., Chen, W. S., et al. (2014b). Herb-induced autoimmune-like hepatitis in C57BL/6J mice. Liver Int. 34 (4), 583–593. doi:10.1111/liv.12266
Wang, W. W., Lu, L., Bao, T. H., Zhang, H. M., Yuan, J., Miao, W., et al. (2016). Scutellarin alleviates behavioral deficits in a mouse model of multiple sclerosis, possibly through protecting neural stem cells. J. Mol. Neurosci. 58 (2), 210–220. doi:10.1007/s12031-015-0660-0
Wang, W., Xu, C., Li, X., Wang, Z., Yang, J., Shen, Y., et al. (2021). Exploration of the potential mechanism of Banxia Xiexin Decoction for the effects on TNBS-induced ulcerative colitis rats with the assistance of network pharmacology analysis. J. Ethnopharmacol. 277, 114197. doi:10.1016/j.jep.2021.114197
Wang, X., Xie, L., Long, J., Liu, K., Lu, J., Liang, Y., et al. (2022a). Therapeutic effect of baicalin on inflammatory bowel disease: A review. J. Ethnopharmacol. 283, 114749. doi:10.1016/j.jep.2021.114749
Wang, Y., Hu, T., Wei, J., Yin, X., Gao, Z., and Li, H. (2022b). Inhibitory activities of flavonoids from Scutellaria baicalensis Georgi on amyloid aggregation related to type 2 diabetes and the possible structural requirements for polyphenol in inhibiting the nucleation phase of hIAPP aggregation. Int. J. Biol. Macromol. 215, 531–540. doi:10.1016/j.ijbiomac.2022.06.107
Wang, Z. L., Wang, S., Kuang, Y., Hu, Z. M., Qiao, X., and Ye, M. (2018c). A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 56 (1), 465–484. doi:10.1080/13880209.2018.1492620
Wu, H., Liao, J., Li, Q., Yang, M., Zhao, M., and Lu, Q. (2018). Epigenetics as biomarkers in autoimmune diseases. Clin. Immunol. 196, 34–39. doi:10.1016/j.clim.2018.03.011
Wu, J., Feng, Z., Chen, L., Li, Y., Bian, H., Geng, J., et al. (2022). TNF antagonist sensitizes synovial fibroblasts to ferroptotic cell death in collagen-induced arthritis mouse models. Nat. Commun. 13 (1), 676. doi:10.1038/s41467-021-27948-4
Wu, J., Hu, Y., Xiang, L., Li, S., Yuan, Y., Chen, X., et al. (2016). San-huang-xie-xin-tang constituents exert drug-drug interaction of mutual reinforcement at both pharmacodynamics and pharmacokinetic level: A review. Front. Pharmacol. 7, 448. doi:10.3389/fphar.2016.00448
Wu, J., Sun, J., and Meng, X. (2021). Pyroptosis by caspase-11 inflammasome-Gasdermin D pathway in autoimmune diseases. Pharmacol. Res. 165, 105408. doi:10.1016/j.phrs.2020.105408
Xiao, J. R., Do, C. W., and To, C. H. (2014). Potential therapeutic effects of baicalein, baicalin, and wogonin in ocular disorders. J. Ocul. Pharmacol. Ther. 30 (8), 605–614. doi:10.1089/jop.2014.0074
Xu, J., Liu, J., Yue, G., Sun, M., Li, J., Xiu, X., et al. (2018). Therapeutic effect of the natural compounds baicalein and baicalin on autoimmune diseases. Mol. Med. Rep. 18 (1), 1149–1154. doi:10.3892/mmr.2018.9054
Xu, J., Zhang, Y., Xiao, Y., Ma, S., Liu, Q., Dang, S., et al. (2013). Inhibition of 12/15-lipoxygenase by baicalein induces microglia pparβ/δ: A potential therapeutic role for CNS autoimmune disease. Cell Death Dis. 4 (4), e569. doi:10.1038/cddis.2013.86
Xu, L., Zhang, J., Wang, Y., Zhang, Z., Wang, F., and Tang, X. (2021). Uncovering the mechanism of Ge-Gen-Qin-Lian decoction for treating ulcerative colitis based on network pharmacology and molecular docking verification. Biosci. Rep. 41 (2), BSR20203565. doi:10.1042/BSR20203565
Yang, J., Yang, X., Yang, J., and Li, M. (2019). Baicalin ameliorates lupus autoimmunity by inhibiting differentiation of Tfh cells and inducing expansion of Tfr cells. Cell Death Dis. 10 (2), 140. doi:10.1038/s41419-019-1315-9
Yao, J., Wang, J., Xu, Y., Guo, Q., Sun, Y., Liu, J., et al. (2021). CDK9 inhibition blocks the initiation of PINK1-PRKN-mediated mitophagy by regulating the SIRT1-FOXO3-BNIP3 axis and enhances the therapeutic effects involving mitochondrial dysfunction in hepatocellular carcinoma. Autophagy 10, 1879–1897. doi:10.1080/15548627.2021.2007027
You, W., Wang, K., Yu, C., and Song, L. (2018). Baicalin prevents tumor necrosis factor-α-induced apoptosis and dysfunction of pancreatic β-cell line Min6 via upregulation of miR-205. J. Cell. Biochem. 119 (10), 8547–8554. doi:10.1002/jcb.27095
Yu, X. X., Zhu, M. Y., Wang, J. R., Li, H., Hu, P., Qing, Y. J., et al. (2021). LW-213 induces cell apoptosis in human cutaneous T-cell lymphomas by activating PERK-eIF2α-ATF4-CHOP axis. Acta Pharmacol. Sin. 42 (2), 290–300. doi:10.1038/s41401-020-0466-7
Zeng, Y., Song, C., Ding, X., Ji, X., Yi, L., and Zhu, K. (2007). Baicalin reduces the severity of experimental autoimmune encephalomyelitis. Braz. J. Med. Biol. Res. 40 (7), 1003–1010. doi:10.1590/s0100-879x2006005000115
Zhang, C. J., Jiang, M., Zhou, H., Liu, W., Wang, C., Kang, Z., et al. (2018). TLR-stimulated IRAKM activates caspase-8 inflammasome in microglia and promotes neuroinflammation. J. Clin. Invest. 128 (12), 5399–5412. doi:10.1172/JCI121901
Zhang, S., Xu, L., Liang, R., Yang, C., and Wang, P. (2020a). Baicalin suppresses renal fibrosis through microRNA-124/TLR4/NF-κB axis in streptozotocin-induced diabetic nephropathy mice and high glucose-treated human proximal tubule epithelial cells. J. Physiol. Biochem. 76 (3), 407–416. doi:10.1007/s13105-020-00747-z
Zhang, X. T., Wang, G., Ye, L. F., Pu, Y., Li, R. T., Liang, J., et al. (2020b). Baicalin reversal of DNA hypermethylation-associated Klotho suppression ameliorates renal injury in type 1 diabetic mouse model. Cell Cycle 19 (23), 3329–3347. doi:10.1080/15384101.2020.1843815
Zhang, Y., Li, X., Ciric, B., Ma, C. G., Gran, B., Rostami, A., et al. (2015a). Therapeutic effect of baicalin on experimental autoimmune encephalomyelitis is mediated by SOCS3 regulatory pathway. Sci. Rep. 5, 17407. doi:10.1038/srep17407
Zhang, Y. M., Li, M. X., Tang, Z., and Wang, C. H. (2015b). Wogonin suppresses osteopontin expression in adipocytes by activating PPARα. Acta Pharmacol. Sin. 36 (8), 987–997. doi:10.1038/aps.2015.37
Zhang, Y., Shan, L., Hua, Y., Wang, D., Zeng, H., Liu, R., et al. (2013). Baicalein selectively induces apoptosis in activated lymphocytes and ameliorates concanavalin a-induced hepatitis in mice. PLoS One 8 (7), e69592. doi:10.1371/journal.pone.0069592
Zhao, Q., Yang, J., Cui, M. Y., Liu, J., Fang, Y., Yan, M., et al. (2019a). The reference genome sequence of Scutellaria baicalensis provides insights into the evolution of wogonin biosynthesis. Mol. Plant 12 (7), 935–950. doi:10.1016/j.molp.2019.04.002
Zhao, Q., Zhang, Y., Wang, G., Hill, L., Weng, J. K., Chen, X. Y., et al. (2016). A specialized flavone biosynthetic pathway has evolved in the medicinal plant, Scutellaria baicalensis. Sci. Adv. 2 (4), e1501780. doi:10.1126/sciadv.1501780
Zhao, T., Tang, H., Xie, L., Zheng, Y., Ma, Z., Sun, Q., et al. (2019b). Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 71 (9), 1353–1369. doi:10.1111/jphp.13129
Zheng, Z. C., Zhu, W., Lei, L., Liu, X. Q., and Wu, Y. G. (2020). Wogonin ameliorates renal inflammation and fibrosis by inhibiting NF-κB and TGF-β1/smad3 signaling pathways in diabetic nephropathy. Drug Des. devel. Ther. 14, 4135–4148. doi:10.2147/DDDT.S274256
Zhu, W., Chen, X., Yu, J., Xiao, Y., Li, Y., Wan, S., et al. (2018). Baicalin modulates the Treg/Teff balance to alleviate uveitis by activating the aryl hydrocarbon receptor. Biochem. Pharmacol. 154, 18–27. doi:10.1016/j.bcp.2018.04.006
Zhu, W., Jin, Z., Yu, J., Liang, J., Yang, Q., Li, F., et al. (2016). Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype. Int. Immunopharmacol. 35, 119–126. doi:10.1016/j.intimp.2016.03.030
Keywords: scutellaria baicalensis, autoimmune diseases, inflammatory response, inflammasome, inflammatory injury
Citation: Wang J, Chen S, Zhang J and Wu J (2022) Scutellaria baicalensis georgi is a promising candidate for the treatment of autoimmune diseases. Front. Pharmacol. 13:946030. doi: 10.3389/fphar.2022.946030
Received: 17 May 2022; Accepted: 15 August 2022;
Published: 16 September 2022.
Edited by:
Muhammad Ishfaq, Huanggang Normal University, ChinaReviewed by:
Fazlullah Khan, Capital University of Science and Technology, PakistanCopyright © 2022 Wang, Chen, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiasi Wu, d3VqaWFzaUBjZHV0Y20uZWR1LmNu
†These authors contribute equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.