
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 22 July 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.945868
Background: Existing studies have shown that the relationship between anesthetic agents and non-small-cell lung cancer (NSCLC) prognosis remains controversial. Therefore, this retrospective cohort study was designed to investigate the effects of propofol or sevoflurane anesthesia on the long-term oncologic outcomes of NSCLC patients.
Methods: We identified 1,778 eligible patients (propofol-based total intravenous anesthesia (TIVA) group, n = 686; sevoflurane-based inhalation anesthesia (INHA) group, n = 1,092) out of 2,388 patients undergoing elective NSCLC surgery from June 2013 to June 2016 in the Harbin Medical University Cancer Hospital. The primary endpoints were five-year overall survival and recurrence-free survival. The secondary endpoints were independent risk factors of cancer recurrence or all-cause mortality. The data were analyzed with propensity score matching, Kaplan–Meier survival, and Cox multivariate analyses as appropriate.
Results: After propensity score matching, there were 672 patients in each group. The median follow-up period was 69 months (interquartile range: 68–70 months) for all patients. Five-year overall survival was 75.7% (95% confidence interval (CI) 72.4–79.1) in the TIVA group and 71.8% (68.4–75.4) in the INHA group (p = 0.160) (hazard ratio (HR), 0.86; 95% CI, 0.70–1.06; p = 0.158), and five-year recurrence-free survival was 68.5% (65.0–72.2) and 62.7% (59.1–66.5 (p = 0.108) (HR, 0.90; 95% CI, 0.75–1.08; p = 0.253), respectively. Subgroup analyses showed there were no significant difference in the overall survival or recurrence-free survival between the two groups in each TNM stage of NSCLC. The independent risk factors included age ≥60 years, male, blood transfusion, segmental/wedge resection and pneumonectomy, thoracotomy, postoperative complications, lung adenocarcinoma, TNM stages, high CEA and CYFRA211 levels, and postoperative radiotherapy.
Conclusions: Our data indicated no difference between the propofol-based TIVA and sevoflurane-based INHA in terms of five-year overall survival and recurrence-free survival after NSCLC surgery.
Lung cancer has the highest incidence and mortality among all malignant cancers worldwide (Sung et al., 2021) and its most common pathological type is non-small cell lung cancer (NSCLC), accounting for 85% of the total diagnosed cases (Oser et al., 2015). Although recent advances in therapeutic strategies, including surgery, radio- and targeted immune-therapy (Horowitz et al., 2015), the five-year survival of the operable NSCLC including all stages is still not optimal (Lu et al., 2019). This is likely due to the malignant nature of disease but risk factors including certain anesthetics/techniques use during the perioperative period that contributed cancer reoccurrence and patients’ death is emerging. Indeed, inhaled anesthetic isoflurane has been reported to increase hypoxia inducible factor (HIF), promoting angiogenesis through vascular endothelial growth factor (VEGF) signaling (Benzonana et al., 2013), likely accelerating cancer progression. The markers and mediators of angiogenesis, migration, invasion, proliferation, and even chemoresistance have been shown to be significantly enhanced by isoflurane through the potentiation of the tumorigenic PI3K/Akt/mTOR cell signaling pathway and upregulation of HIFs with these effects sustained for as long as 24 h after 2 h anesthetic exposure (Benzonana et al., 2013; Huang et al., 2014; Luo et al., 2015). In contrast, propofol has been shown to antagonize these same signaling pathways, as evidenced by a reduction in cellular levels of phosphorylated-Akt and HIF-1α protein synthesis. Furthermore, propofol also suppressed isoflurane’s aforementioned promalignant effects in vitro (Huang et al., 2014). Interestingly, propofol also encourages an anticancer environment by exerting little influence on NK cell and decreasing the synthesis of prostaglandin E2, a hormone that suppresses T-cell immunity (Inada et al., 2011). HIFs are ubiquitously expressed, evolutionarily conserved transcription factors that govern the cellular response to oxygen by activating transcriptional programs that promote adaptation and survival in conditions of hypoxia. It has been suggested that the HIF system plays a central role in cancer development (Unwith et al., 2015). In fact, tumors with high levels of HIF-1α and HIF-2α are significantly more malignant, metastatic, and resistant to radio- and chemo-therapy and are associated with a poorer prognosis (Semenza, 2003).
Furthermore, propofol at clinical concentrations regulated the biological behaviors of cancer cells, such as the proliferation, adhesion, invasion, metastasis, and tumor angiogenesis by mediating p38 MAPK, Wnt/β-catenin, and mTOR signaling pathways, and by upregulating or downregulating the expression of miR-372, lncRNA ANRIL, and circ-ERBB2 (Semenza, 2003; Zheng et al., 2020; Gao et al., 2021). In contrast, volatile anesthetics, such as isoflurane and sevoflurane promote the proliferation and migration of cancer cells in vitro (Benzonana et al., 2013), and increase the tumor load in vivo (Zhu et al., 2016). Sevoflurane directly suppresses cytokine release and the cytotoxicity of natural killer cells in experimental models, and potentially reduces the ability to destroy cancerous cells, which might increase the incidence of postoperative recurrence or metastasis (Jeon et al., 2020). In potentially keeping with this, a very recent clinical study has shown an association between volatile inhalational anesthesia and reduced long-term survival in cancer patients undergoing elective surgery (Wigmore et al., 2016). It must be stressed that the evidence available remains inadequate to substantiate any recommendations to alter the current clinical practice and more clinical study including trials are urgently needed (Perry et al., 2019).
Thus, we carried out a retrospective cohort study to assess whether the choice of anesthetics, propofol versus sevoflurane, influences the long-term survival after NSCLC surgery. Other risk factors related to postoperative recurrence or death were also analyzed in this study.
This retrospective cohort study is in compliance with the guidelines of the Declaration of Helsinki, and was approved by the Ethics Committee of Harbin Medical University Cancer Hospital. The informed consent was waived due to the study nature of the retrospective electronic medical record review and the data analyzed anonymously.
The inclusion criteria were patients who underwent an elective NSCLC surgery with age ≥18 years old, no distant metastasis assessed preoperatively, and the American Society of Anesthesiologists (ASA) physical status of I to III. The exclusion criteria included: pathological stage M1 or N3, unknown pathological type of lung cancer, unknown pathological stage, other malignancy, benign tumor, incomplete medical records (including follow-up failure), other inhalational but not sevoflurane anesthetics used, multiple operations, death within one month after surgery and incomplete resection.
The type of anesthesia used for including NSCLC operation was with the preference of anesthesiologists in Harbin Medical University Cancer Hospital as routine clinical practice. According to the type of anesthesia, patients were divided into propofol-based total intravenous anesthesia (TIVA) group or sevoflurane-based inhalational anesthesia (INHA) group. In this study, the TIVA group was set as the case group and the INHA group as the control group. Patients in both the groups were induced with 0.3 μg/kg sufentanil and 1–2.5 mg/kg propofol. In addition to sedatives and muscle relaxants, when necessary, the anesthesia maintenance was propofol 3 mg/kg/hr or sevoflurane 1–3% in combination with remifentanil 0.1–0.2 μg/kg/min. The INHA group was only given propofol during the induction period. The TIVA group received target-controlled infusion of propofol and remifentanil for anesthesia maintenance. All patients were treated with patient-controlled intravenous analgesia with a total 300 ml mixture of sufentanil (0.5 ug/mL) and flurbiprofen axetil (1 ug/mL) for postoperative pain control, and did not use additional regional anesthesia.
The data were harvested from the electronic medical records in patients who had lung cancer surgery in the Harbin Medical University Cancer Hospital between June 2013 and June 2016. Those were including age, sex, body mass index (BMI), smoking status, comorbidities at admission (hypertension, diabetes, cardiovascular disease, or cerebrovascular diseases), ASA physical status, anesthetics, intraoperative use of various drugs [dexmedetomidine, vasoactive drugs, opioids, or nonsteroidal anti-inflammatory drugs (NSAIDs)], perioperative blood transfusion, the type of operation (segmental/wedge resection, lobectomy, or pneumonectomy), video-assisted thoracic surgery (VATS), operation time, and complications after surgery. Tumor histological types (adenocarcinoma, squamous cell carcinoma, or other), tumor size, tumor-node-metastasis (TNM) cancer stages, tumor markers [squamous cell carcinoma antigen (SCC), carcinoembryonic antigen (CEA), and Cytokeratin-19 fragment 21-1 (CYFRA211)], chemotherapy or radiotherapy, cancer recurrence, and cancer related death were also recorded. The postoperative complications included anastomotic leakage, wound infection, pneumonia, malignant arrhythmia, acute myocardial infarction, heart failure, pulmonary embolism, cerebral infarction or hemorrhage, and acute renal failure. As more than 76% of patients were aged between 50 and 69 years old, patients were divided into four age groups (≤49, 50–59, 60–69, and ≥70 years old). According to the World Health Organization classifications, the BMI was divided into four categories (< 18.5 kg/m2, 18.5–24.9 kg/m2, 25.0–29.9 kg/m2, and ≥30.0 kg/m2). Because the number of patients with ASA grade I (n = 6) and III (n = 12) accounted for only 1% of the total, all patients were regarded as ASA grade II. All patients in this study received opioids for pain control and, thus, opioids were not included in the analysis as a variable.
The primary endpoints were five-year overall survival and recurrence-free survival after NSCLC surgery. The overall survival was defined as the period from the date of operation to the date of death or the last follow-up. Recurrence-free survival was defined as the period from the date of surgery to the date of recurrence, death, or the last follow-up. The dates of death and recurrence were obtained by the patients themselves or their relatives in the follow-up center of the Harbin Medical University Cancer Hospital. The deadline for the follow-up was 30 April 2021. Therefore, the duration and median of follow-up was between five and eight years. The secondary endpoints were independent risk factors for cancer recurrence or all-cause mortality.
The sample size of this study was the data from all patients undergoing NSCLC surgery in the Harbin Medical University Cancer Hospital from June 2013 to June 2016.
The data were described as the number with percentage for categorical variables and the mean with standard deviation or median with interquartile range for continuous variables, where appropriate. Shapiro–Wilk test was used to evaluate the normality of the distribution of continuous variables. The differences between the groups of continuous variables were compared with the independent sample t test or Wilcoxon rank sum test and the categorical variables were compared with the chi-square test.
The potential confounding effects of variables and the difference of baseline characteristics between the two groups were reduced by propensity score matching. Logistic regression analysis was used to estimate the propensity score of patients in the TIVA group. Two groups of patients were matched at a ratio of 1:1 by the nearest neighbor method with a caliper of 0.2 and an order of the largest propensity value (m.order = largest). The matched variables included age, sex, BMI, smoking status, hypertension, diabetes, cardiovascular disease, cerebrovascular disease, dexmetomidine, vasoactive drugs, NSAIDs, perioperative blood transfusion, type of operation, VATS, operation time, complications after surgery, tumor histologic types, TNM stages, SCC, CEA, CYFRA211, chemotherapy, and radiotherapy. The standardized mean difference (SMD) was used to evaluate the balance on baseline characteristics between the two groups when it was < 0.10, indicating a good balance.
In the propensity-matched cohort, Kaplan–Meier survival curve was used to evaluate the overall survival rate and recurrence-free survival rate, and the log-rank test was used to compare the two groups. The median follow-up time was calculated by Reverse Kaplan–Meier method. The Cox proportional hazard regression models were utilized to estimate the relationship between the anesthesia type and overall survival or recurrence-free survival, and to determine the independent risk factors for NSCLC recurrence or all-cause mortality. The results were expressed as hazard ratio (HR) and 95% confidence interval (CI). The variables with p < 0.05 in the results of univariate Cox regression analysis, which included all variables and other potential risk factors, were adjusted by the multivariate Cox regression analysis. Complementary log plots and Schoenfeld residuals test were used to evaluate the proportional hazard assumptions. In addition, subgroup analyses were performed for the TNM stages of NSCLC to evaluate the association between anesthesia type and cancer prognosis.
All analyses were performed using R software version 4.1.2 (R Foundation for Statistical Computing, Austria). A two-tailed p < 0.05 was considered to be statistically significant.
Of 2,388 patients undergoing NSCLC surgery during the study period at the Harbin Medical University Cancer Hospital, 1,778 patients (TIVA group, n = 686 and INHA group, n = 1092) were finally included in the analyses (Figure 1). The median follow-up period was 69 months (interquartile range: 68–70 months).
The baseline characteristics of patients in the two groups for the total study cohort and the propensity-matched cohort (Table 1). In the total study cohort, there were differences between two groups in age, using NSAIDs drugs used, VATS, operation time, and adjuvant chemotherapy (SMD ≥0.10). After propensity score matching, there were 672 patients in each group. The SMD suggested that all variables were well-matched between the two groups (SMD <0.10) (Figure 2).
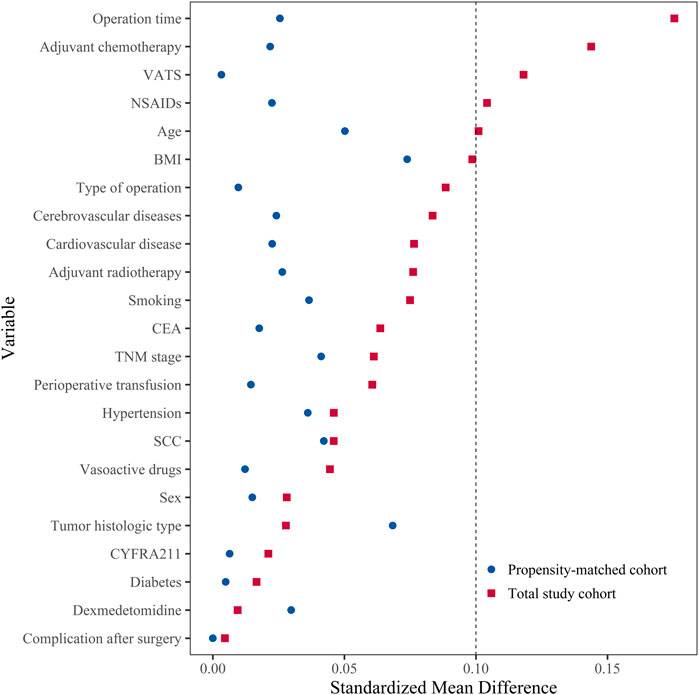
FIGURE 2. The distribution of standardized mean difference for variables included before and after matching. Standardized mean difference values <0.10 presented that the variable was well-matched and there was no difference between two groups. BMI, body mass index; CEA, carcinoembryonic antigen; CYFRA211, cytokeratin-19 fragment 21-1; NSAIDs, non-steroidal anti-inflammatory drugs; SCC, squamous cell carcinoma antigen; TNM, tumor-node-metastasis; VATS: video-assisted thoracic surgery.
The five-year overall survival rate was 75.7% (95% CI, 72.4–79.1) in the TIVA group and 71.8% (95% CI, 68.4–75.4) in the INHA group and the five-year recurrence-free survival rates were 68.5% (95% CI, 65.0–72.2) and 62.7% (95% CI, 59.1–66.5), respectively. There were no significant differences in the overall survival (p = 0.160) or recurrence-free survival (p = 0.108) between the two groups with log-rank test (Figures 3A,B).
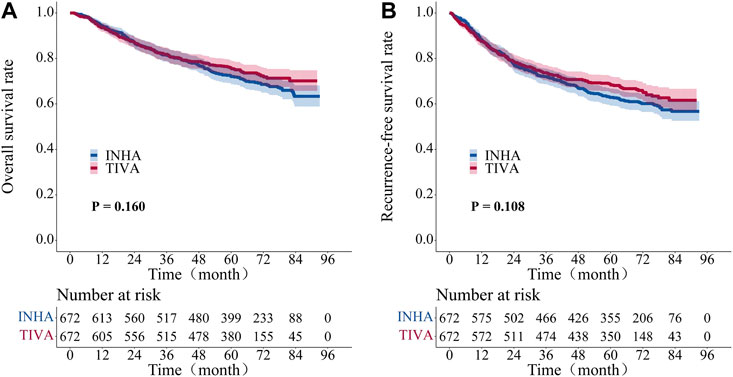
FIGURE 3. Kaplan–Meier survival curve for overall survival and recurrent-free survival in propensity-matched cohort patient. (A) overall survival rate. (B) recurrent-free survival rate. The Log-rank test was employed to evaluate the difference between the groups.
The Cox proportional hazards models for the overall survival and recurrence-free survival (Table 2) were constructed to compare the association between anesthesia type and cancer prognosis in the propensity-matched cohort. Multivariable Cox regression analyses showed no significant difference in the overall survival (HR, 0.86; 95% CI, 0.70–1.06; p = 0.158) and recurrence-free survival (HR, 0.90; 95% CI, 0.75–1.08; p = 0.253) between the TIVA and INHA groups. In addition, multivariable Cox regression analysis with inverse probability of treatment weighting also showed no significant association between the anesthesia type and overall survival (HR, 0.93; 95% CI, 0.76–1.12; p = 0.435) or recurrence-free survival (HR, 0.92; 95% CI: 0.78–1.09; p = 0.332).
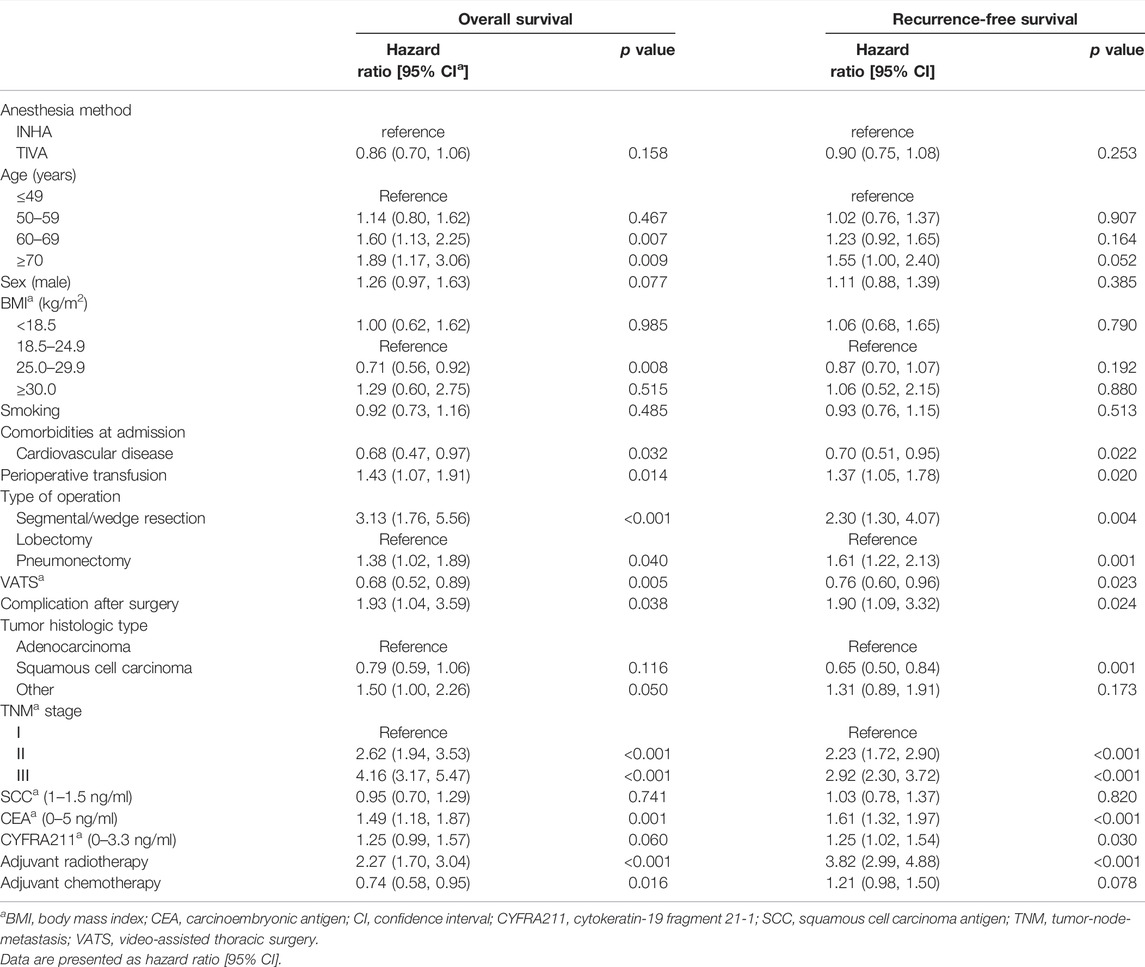
TABLE 2. Multivariable Cox regression analysis for overall survival and recurrence-free survival after propensity score matching.
The subgroup analyses showed that there were no significant differences in the overall survival or recurrence-free survival between the two groups in each TNM stage of NSCLC (Figure 4).
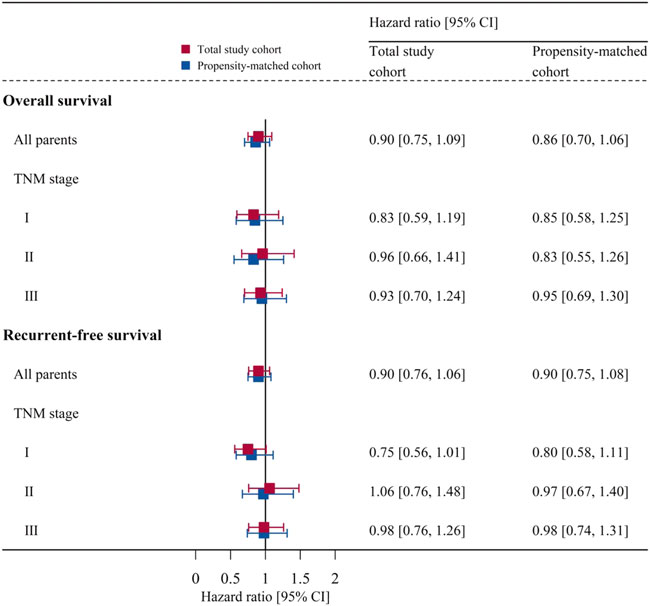
FIGURE 4. Subgroup analyses for each TNM stage of NSCLC: association of anesthesia type with overall survival or recurrent-free survival before and after matching. Data are presented as hazard ratio [95% CI]. CI, confidence interval; TNM, tumor-node-metastasis.
The independent risk factors for cancer recurrence or all-cause mortality included age ≥60 years old, male patients, perioperative blood transfusion, segmental/wedge resection and pneumonectomy, thoracotomy, postoperative complications, lung adenocarcinoma, TNM stages, elevated CEA and CYFRA211 values, and postoperative radiotherapy (Table 3).
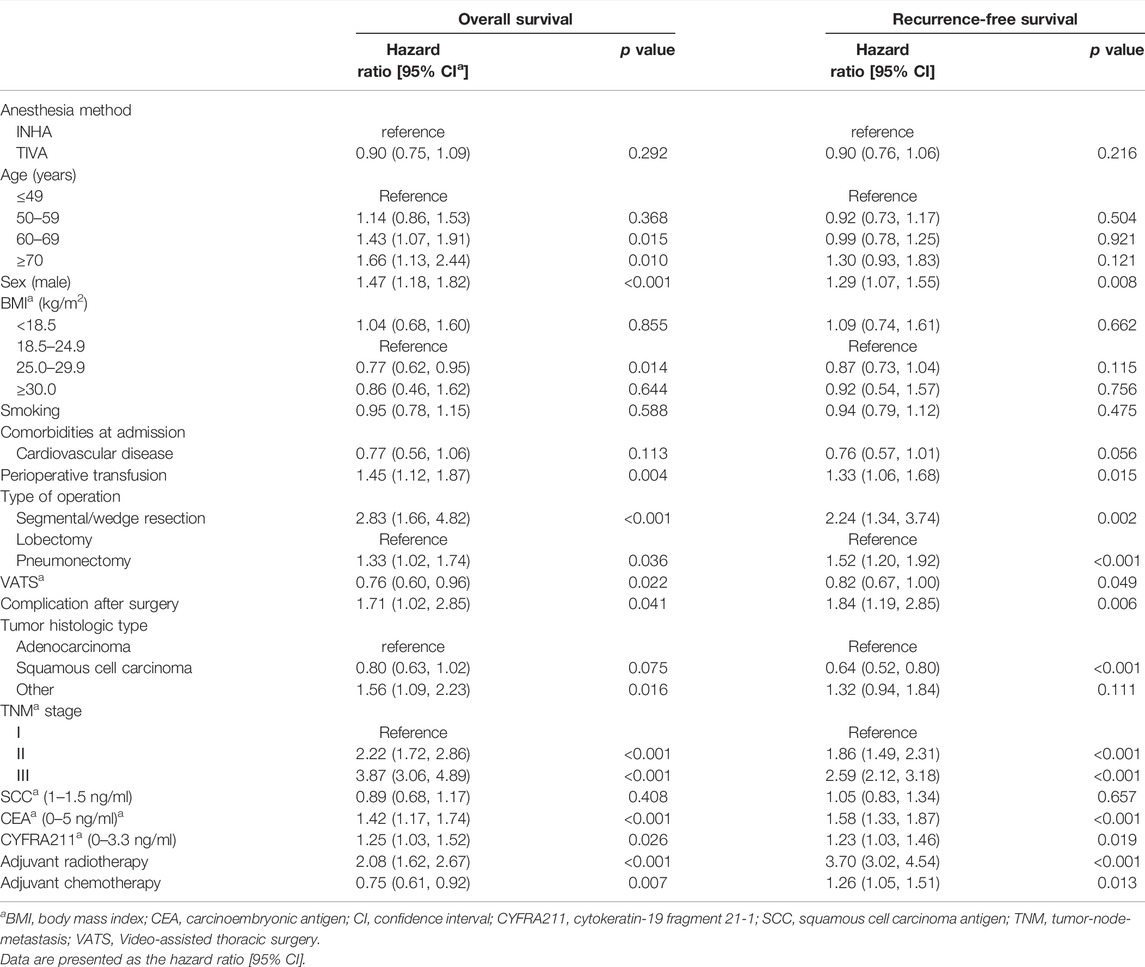
TABLE 3. Multivariable Cox regression analysis for overall survival and recurrence-free survival in the total study cohort.
This retrospective study indicated that the propofol-based TIVA was not beneficial to the prognosis of cancer patients than the sevoflurane-based INHA in patients with NSCLC. The independent risk factors included age ≥60 years old, male patients, TNM stage, elevated CEA and CYFRA211 values, perioperative blood transfusion, thoracotomy, wedge resection, postoperative complications, and postoperative radiotherapy.
Perioperative factors, such as surgery-induced neuroendocrine stress, injury-related inflammation, hyperglycaemia, and hypothermia responsible for immunosuppression, which can foster a potentially protumor environment that may facilitate distal seeding of circulating tumor cells, the growth of micrometastases into established clinical metastases, or both (Jeon et al., 2020). This kind of protumor environment may be further aggravated by the administration of anesthetics that themselves have a potential direct effect on the cancer cell biology and indirectly modulating effects. Indeed, propofol promoted NK cell cytotoxicity and potentiated the expression of CD28 on peripheral T-helper cells (Xu et al., 2020). In contrast, volatile anesthetics inhibited NK cell-mediated cytotoxicity, induced T-lymphocyte apoptosis, and upregulated tumorigenic modulator tumorigenic growth factors, VEGF, and HIF-1 (Buckley et al., 2014; Shi et al., 2015; Iwasaki et al., 2016; Lim et al., 2018). Sevoflurane caused the accumulation of intracellular reactive oxygen species by activating the PI3K and MAPK signaling pathways, and upregulating the expression of MMPs (Fan et al., 2019). Li et al. (2020) found that sevoflurane anesthesia led to significantly more lung metastasis than propofol in mice models with surgery of primary tumors. Similarly, a retrospective analysis by Hayasaka et al. (2021) revealed that in 230 patients, who had surgery with pathological stage I NSCLC, the five-year recurrence-free survival rates in the TIVA group was 91.7%, which was significantly higher than that in the INHA group (77.4%), indicating that propofol anesthesia may improve the prognosis of early NSCLC patients. However, in line with previous retrospective studies (Oh et al., 2018; de La Motte Watson et al., 2021), our retrospective analysis revealed that propofol-based TIVA was not associated with significantly better survival when compared to INHA in patients with surgically resected p-stage I-III NSCLC. In our study, the five-year recurrence-free survival rate was 68.5% in the TIVA group and 62.7% in the INHA group. Previous studies have found that the five-year cancer-specific survival of patients with lung cancer in the TIVA group was 68.1%, and that in the inhalation group was 70.8%. But patients who underwent thoracoscopic surgery were not included in this study (de La Motte Watson et al., 2021). There are several possible explanations for these findings. First, in our patients, propofol was also used in the inhalation group for anesthesia induction. The stress response and immunosuppression caused by the operation stimulated the progression of tumor cells during the perioperative period. Propofol was reported to have less immunosuppression and has a lower inflammatory response than volatile drugs during the perioperative period (Lee et al., 2022). This may reduce opioid use for pain relief and may have favorable to contribute outcomes (Moorthy et al., 2021). Therefore, the net effects of propofol and inhalational agent sevoflurane on the oncologic outcomes in our patients remain unknown and this was the case for many studies, even clinical trials (Ishikawa et al., 2020). Second, sevoflurane was found to suppress lung cancer cells while it promoted renal cancer cell growth in in vitro model (Ciechanowicz et al., 2018). If those findings were corroborated in clinical settings, then its differential effects may contribute no different outcomes between two anesthetics based anesthesia. Third, due to the retrospective nature of our study, we cannot comment on study power but it is very likely underpower per se. Finally, there are many factors affecting the oncological outcomes following surgery and anesthetic use is likely one of them and thus, negative findings are not surprised.
VATS lobectomy have been performed increasingly, as an alternative to open thoracotomy in the early-stage NSCLC, and the advantages include smaller incisions, less postoperative pain and complications, and shorter length of hospitalization (Boffa et al., 2014; Oda et al., 2019). However, VATS removed fewer lymph nodes, especially N2 nodes, compared with thoracotomy, thus this concern would heighten the risk of leaving residual tumors at the surgical margin, and an insufficient lymph node dissection (Boffa et al., 2012; Decaluwé et al., 2016). In the study, VATS was associated with improved survival, due to better postoperative quality of life and overall survival rates compared to open thoracotomy. Despite there being no demonstrable significant difference in the postoperative complications in this study, this is most likely attributable to a combination of factors including a significantly shorter hospital stay, decreased postoperative pain, improved pulmonary toilet, and earlier chest tube removal (Yang et al., 2017). The study did not exclude patients who received the wedge resection of tumor. The pulmonary wedge resection causes a compression on the pulmonary tissue and the tumor, which may result in the shedding of tumor cells into the blood (Li et al., 2015). In addition, perioperative allogeneic blood transfusions have a dose-dependent relationship with a shorter disease-free time interval and early recurrence in patients with stage I–II NSCLC, and a peak effect occurred at six units (Latif et al., 2019). pRBC transfusions immunoedited in patients with NSCLC due to allogenic cells and antigens that divert and disrupt the balance between immune surveillance and cancer progression (Muszynski et al., 2017). Thus, the type of surgery and blood transfusions were independent factors of shorter long-term survival in patients undergoing NSCLC resection.
In the study, various variables were also found to be significantly associated with mortality including older age, TNM stage, and adjuvant radiotherapy. Our subgroup analyses showed that there were no significant differences in the overall survival or recurrence-free survival after the propensity-matched cohort, in each TNM stage of NSCLC. Studies showed that the risk of poor survival increases significantly with increasing age (Vogelaar et al., 2015), ASA physical status, and poor preoperative functional status (Wigmore et al., 2016), and neoadjuvant chemotherapy has prolonged the prognosis of tumor (Aloia et al., 2014).
There are limitations to our study. First, this is a single-center retrospective observational study, patients were not randomly allocated, and selection bias may exist, although Cox regression analysis was performed to reduce the potential confounding effect of each variable. The sample size was further reduced while balancing the baseline characteristics between the two groups via propensity score matching. Second, the longest follow-up period of our study was eight years, and there could have been advances in surgical, anesthetic, and neoadjuvant chemotherapy management. These changes were not adequately considered. Finally, patients in the both groups received propofol during anesthesia induction in our study, which may mask the effect of sevoflurane. All these factors need to be addressed in the future prospective multicenter study.
Our study showed no benefit for propofol-based TIVA, in comparison with sevoflurane-based INHA, in terms of five-year overall survival and recurrence-free survival after NSCLC surgery. Large-sample size, well-designed prospective multicenter studies are necessary to clarify this association of anesthetics and oncologic outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Harbin Medical University Cancer Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
ZG collected data and wrote the manuscript; JX conducted statistical analysis; MC wrote the manuscript; DM conceived the study concept and wrote the manuscript; and KW designed the study protocol and wrote the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors appreciate all assistance and support provided by the colleagues not listed here and their respective institutions.
Aloia, T. A., Zimmitti, G., Conrad, C., Gottumukalla, V., Kopetz, S., and Vauthey, J. N. (2014). Return to Intended Oncologic Treatment (RIOT): A Novel Metric for Evaluating the Quality of Oncosurgical Therapy for Malignancy. J. Surg. Oncol. 110, 107–114. doi:10.1002/jso.23626
Benzonana, L. L., Perry, N. J., Watts, H. R., Yang, B., Perry, I. A., Coombes, C., et al. (2013). Isoflurane, a Commonly Used Volatile Anesthetic, Enhances Renal Cancer Growth and Malignant Potential via the Hypoxia-Inducible Factor Cellular Signaling Pathway In Vitro. Anesthesiology 119, 593–605. doi:10.1097/ALN.0b013e31829e47fd
Boffa, D. J., Kosinski, A. S., Paul, S., Mitchell, J. D., and Onaitis, M. (2012). Lymph Node Evaluation by Open or Video-Assisted Approaches in 11,500 Anatomic Lung Cancer Resections. Ann. Thorac. Surg. 94, 347–353. doi:10.1016/j.athoracsur.2012.04.059
Boffa, D. J., Dhamija, A., Kosinski, A. S., Kim, A. W., Detterbeck, F. C., Mitchell, J. D., et al. (2014). Fewer Complications Result from a Video-Assisted Approach to Anatomic Resection of Clinical Stage I Lung Cancer. J. Thorac. Cardiovasc. Surg. 148, 637–643. doi:10.1016/j.jtcvs.2013.12.045
Buckley, A., McQuaid, S., Johnson, P., and Buggy, D. J. (2014). Effect of Anaesthetic Technique on the Natural Killer Cell Anti-tumour Activity of Serum from Women Undergoing Breast Cancer Surgery: A Pilot Study. Br. J. Anaesth. 113 (Suppl. 1), i56–62. doi:10.1093/bja/aeu200
Ciechanowicz, S., Zhao, H., Chen, Q., Cui, J., Mi, E., Mi, E., et al. (2018). Differential Effects of Sevoflurane on the Metastatic Potential and Chemosensitivity of Non-small-cell Lung Adenocarcinoma and Renal Cell Carcinoma In Vitro. Br. J. Anaesth. 120, 368–375. doi:10.1016/j.bja.2017.11.066
de La Motte Watson, S., Puxty, K., Moran, D., Morrison, D. S., Sloan, B., Buggy, D., et al. (2021). Association between Anesthetic Dose and Technique and Oncologic Outcomes after Surgical Resection of Non-small Cell Lung Cancer. J. Cardiothorac. Vasc. Anesth. 35, 3265–3274. doi:10.1053/j.jvca.2021.03.030
Decaluwé, H., Stanzi, A., Dooms, C., Fieuws, S., Coosemans, W., Depypere, L., et al. (2016). Central Tumour Location Should Be Considered when Comparing N1 Upstaging between Thoracoscopic and Open Surgery for Clinical Stage I Non-small-cell Lung Cancer. Eur. J. Cardiothorac. Surg. 50, 110–117. doi:10.1093/ejcts/ezv489
Fan, L., Wu, Y., Wang, J., He, J., and Han, X. (2019). Sevoflurane Inhibits the Migration and Invasion of Colorectal Cancer Cells through Regulating ERK/MMP-9 Pathway by Up-Regulating miR-203. Eur. J. Pharmacol. 850, 43–52. doi:10.1016/j.ejphar.2019.01.025
Gao, J., Ding, C., Zhou, J., Wu, G., Han, Z., Li, J., et al. (2021). Propofol Suppresses Lung Cancer Tumorigenesis by Modulating the Circ-ERBB2/miR-7-5p/FOXM1 Axis. Thorac Cancer 12, 824–834. doi:10.1111/1759-7714.13856
Hayasaka, K., Shiono, S., Miyata, S., Takaoka, S., Endoh, M., and Okada, Y. (2021). Prognostic Significance of Propofol-Based Intravenous Anesthesia in Early-Stage Lung Cancer Surgery. Surg. Today 51, 1300–1308. doi:10.1007/s00595-020-02216-y
Horowitz, M., Neeman, E., Sharon, E., and Ben-Eliyahu, S. (2015). Exploiting the Critical Perioperative Period to Improve Long-Term Cancer Outcomes. Nat. Rev. Clin. Oncol. 12, 213–226. doi:10.1038/nrclinonc.2014.224
Huang, H., Benzonana, L. L., Zhao, H., Watts, H. R., Perry, N. J., Bevan, C., et al. (2014). Prostate Cancer Cell Malignancy via Modulation of HIF-1α Pathway with Isoflurane and Propofol Alone and in Combination. Br. J. Cancer 111, 1338–1349. doi:10.1038/bjc.2014.426
Inada, T., Kubo, K., and Shingu, K. (2011). Possible Link between Cyclooxygenase-Inhibiting and Antitumor Properties of Propofol. J. Anesth. 25, 569–575. doi:10.1007/s00540-011-1163-y
Ishikawa, M., Sakamoto, A., and Ma, D. (2020). Recurrence of Breast Cancer after Anaesthesia. Lancet 396, 375–376. doi:10.1016/S0140-6736(20)30488-8
Iwasaki, M., Zhao, H., Jaffer, T., Unwith, S., Benzonana, L., Lian, Q., et al. (2016). Volatile Anaesthetics Enhance the Metastasis Related Cellular Signalling Including CXCR2 of Ovarian Cancer Cells. Oncotarget 7, 26042–26056. doi:10.18632/oncotarget.8304
Jeon, S., Kim, H. K., Kwon, J. Y., Baek, S. H., Ri, H. S., Choi, H. J., et al. (2020). Role of Sevoflurane on Natural Killer Group 2, Member D-Mediated Immune Response in Non-small-cell Lung Cancer: An In Vitro Study. Med. Sci. Monit. 26, e926395. doi:10.12659/MSM.926395
Latif, M. J., Tan, K. S., Molena, D., Huang, J., Bott, M. J., Park, B. J., et al. (2019). Perioperative Blood Transfusion Has a Dose-dependent Relationship with Disease Recurrence and Survival in Patients with Non-small Cell Lung Cancer. J. Thorac. Cardiovasc. Surg. 157, 2469. doi:10.1016/j.jtcvs.2018.12.109
Lee, S., Pyo, D. H., Sim, W. S., Lee, W. Y., and Park, M. (2022). Early and Long-Term Outcomes after Propofol-And Sevoflurane-Based Anesthesia in Colorectal Cancer Surgery: A Retrospective Study. J. Clin. Med. 11, 2648. doi:10.3390/jcm11092648
Li, F., Jiang, G., Chen, Y., and Wang, J. (2015). Curative Effects of Different Sequences of Vessel Interruption during the Completely Thoracoscopic Lobectomy on Early Stage Non-small Cell Lung Cancer. Ann. Thorac. Cardiovasc. Surg. 21, 536–543. doi:10.5761/atcs.oa.15-00044
Li, R., Huang, Y., and Lin, J. (2020). Distinct Effects of General Anesthetics on Lung Metastasis Mediated by IL-6/JAK/STAT3 Pathway in Mouse Models. Nat. Commun. 11, 642. doi:10.1038/s41467-019-14065-6
Lim, J. A., Oh, C. S., Yoon, T. G., Lee, J. Y., Lee, S. H., Yoo, Y. B., et al. (2018). The Effect of Propofol and Sevoflurane on Cancer Cell, Natural Killer Cell, and Cytotoxic T Lymphocyte Function in Patients Undergoing Breast Cancer Surgery: An In Vitro Analysis. BMC Cancer 18, 159. doi:10.1186/s12885-018-4064-8
Lu, D., Wang, Z., Liu, X., Feng, S., Dong, X., Shi, X., et al. (2019). Differential Effects of Adjuvant EGFR Tyrosine Kinase Inhibitors in Patients with Different Stages of Non-small-cell Lung Cancer after Radical Resection: An Updated Meta-Analysis. Cancer Manag. Res. 11, 2677–2690. doi:10.2147/CMAR.S187940
Luo, X., Zhao, H., Hennah, L., Ning, J., Liu, J., Tu, H., et al. (2015). Impact of Isoflurane on Malignant Capability of Ovarian Cancer In Vitro. Br. J. Anaesth. 114, 831–839. doi:10.1093/bja/aeu408
Moorthy, A., Eochagáin, A. N., and Buggy, D. J. (2021). Can Acute Postoperative Pain Management after Tumour Resection Surgery Modulate Risk of Later Recurrence or Metastasis? Front. Oncol. 11, 802592. doi:10.3389/fonc.2021.802592
Muszynski, J. A., Spinella, P. C., Cholette, J. M., Acker, J. P., Hall, M. W., Juffermans, N. P., et al. (2017). Transfusion-related Immunomodulation: Review of the Literature and Implications for Pediatric Critical Illness. Transfusion 57, 195–206. doi:10.1111/trf.13855
Oda, R., Okuda, K., Osaga, S., Watanabe, T., Sakane, T., Tatematsu, T., et al. (2019). Long-term Outcomes of Video-Assisted Thoracoscopic Surgery Lobectomy vs. Thoracotomy Lobectomy for Stage IA Non-small Cell Lung Cancer. Surg. Today 49, 369–377. doi:10.1007/s00595-018-1746-4
Oh, T. K., Kim, K., Jheon, S., Lee, J., Do, S. H., Hwang, J. W., et al. (2018). Long-Term Oncologic Outcomes for Patients Undergoing Volatile versus Intravenous Anesthesia for Non-small Cell Lung Cancer Surgery: A Retrospective Propensity Matching Analysis. Cancer Control 25, 1073274818775360. doi:10.1177/1073274818775360
Oser, M. G., Niederst, M. J., Sequist, L. V., and Engelman, J. A. (2015). Transformation from Non-small-cell Lung Cancer to Small-Cell Lung Cancer: Molecular Drivers and Cells of Origin. Lancet Oncol. 16, e165–72. doi:10.1016/S1470-2045(14)71180-5
Perry, N. J. S., Buggy, D., and Ma, D. (2019). Can Anesthesia Influence Cancer Outcomes after Surgery? JAMA Surg. 154, 279–280. doi:10.1001/jamasurg.2018.4619
Semenza, G. L. (2003). Targeting HIF-1 for Cancer Therapy. Nat. Rev. Cancer 3, 721–732. doi:10.1038/nrc1187
Shi, Q. Y., Zhang, S. J., Liu, L., Chen, Q. S., Yu, L. N., Zhang, F. J., et al. (2015). Sevoflurane Promotes the Expansion of Glioma Stem Cells through Activation of Hypoxia-Inducible Factors In Vitro. Br. J. Anaesth. 114, 825–830. doi:10.1093/bja/aeu402
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Unwith, S., Zhao, H., Hennah, L., and Ma, D. (2015). The Potential Role of HIF on Tumour Progression and Dissemination. Int. J. Cancer 136, 2491–2503. doi:10.1002/ijc.28889
Vogelaar, F. J., Abegg, R., van der Linden, J. C., Cornelisse, H. G., van Dorsten, F. R., Lemmens, V. E., et al. (2015). Epidural Analgesia Associated with Better Survival in Colon Cancer. Int. J. Colorectal Dis. 30, 1103–1107. doi:10.1007/s00384-015-2224-8
Wigmore, T. J., Mohammed, K., and Jhanji, S. (2016). Long-term Survival for Patients Undergoing Volatile versus IV Anesthesia for Cancer Surgery: A Retrospective Analysis. Anesthesiology 124, 69–79. doi:10.1097/ALN.0000000000000936
Xu, Y., Pan, S., Jiang, W., Xue, F., and Zhu, X. (2020). Effects of Propofol on the Development of Cancer in Humans. Cell Prolif. 53, e12867. doi:10.1111/cpr.12867
Yang, H. X., Woo, K. M., Sima, C. S., Bains, M. S., Adusumilli, P. S., Huang, J., et al. (2017). Long-term Survival Based on the Surgical Approach to Lobectomy for Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-Assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann. Surg. 265, 431–437. doi:10.1097/SLA.0000000000001708
Zheng, X., Dong, L., Zhao, S., Li, Q., Liu, D., Zhu, X., et al. (2020). Propofol Affects Non-small-cell Lung Cancer Cell Biology by Regulating the miR-21/PTEN/AKT Pathway In Vitro and In Vivo. Anesth. Analg. 131, 1270–1280. doi:10.1213/ANE.0000000000004778
Keywords: propofol, sevoflurane, survival, risk factors, non-small-cell lung cancer
Citation: Gao Z, Xu J, Coburn M, Ma D and Wang K (2022) Postoperative Long-Term Outcomes and Independent Risk Factors of Non-Small-Cell Lung Cancer Patients With Propofol versus Sevoflurane Anesthesia: A Retrospective Cohort Study. Front. Pharmacol. 13:945868. doi: 10.3389/fphar.2022.945868
Received: 17 May 2022; Accepted: 22 June 2022;
Published: 22 July 2022.
Edited by:
William K. K. Wu, The Chinese University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Ying Zhi Liu, The Chinese University of Hong Kong, Hong Kong SAR, ChinaCopyright © 2022 Gao, Xu, Coburn, Ma and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daqing Ma, ZC5tYUBpbXBlcmlhbC5hYy51aw==; Kun Wang, aHlkd2FuZ2t1bkBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.