
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 30 August 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.944342
This article is part of the Research TopicTherapeutic Drug Monitoring and Clinical Toxicology of Anti-Cancer Drugs, volume IIView all 13 articles
Introduction: Non-small cell lung cancer patients have gained therapeutic benefits from immune checkpoint inhibitors, although immune-related adverse events (irAEs) could be inevitable. Whether irAEs are associated with chronic diseases is still unclear, our study aims to clarify the distinct adverse events in NSCLC patients with concomitant hypertension.
Methods: Adverse event cases were searched and collected in the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database from January 2015 to December 2021. We performed disproportionality analysis to detect safety signals by calculating reporting odds ratios (ROR) and corresponding 95% confidence intervals (95% CIs), information component (IC), and the lower bound of the information component 95% credibility interval (IC025).
Results: Among 17,163 NSCLC patients under treatment with single-agent anti-programmed death-1/programmed death ligand-1 (PD-1/PD-L1) inhibitor (nivolumab, pembrolizumab, cemiplimab, durvalumab, atezolizumab, and avelumab), 497 patients had hypertension while 16,666 patients had no hypertension. 4,283 pulmonary AEs were reported, including 166 patients with hypertension and 4,117 patients without hypertension. Compared with patients without hypertension, patients with hypertension were positively associated with increased reporting of interstitial lung disease (ROR = 3.62, 95%CI 2.68–4.89, IC = 1.54, IC025 = 0.57) among patients receiving anti-PD-1 treatment. The median duration of onset from the time of initiation of anti-PD-1 administration was 28 days (IQR, 12.00–84.25).
Conclusion: Our pharmacovigilance analysis showed the profile of pulmonary toxicities in NSCLC patients with hypertension caused by anti-PD-1/PD-L1 inhibitors. Interstitial lung disease was the statistically significant reporting adverse event in patients with hypertension receiving anti-PD-1 treatment.
Immune checkpoint inhibitors (ICIs) that target the programmed death 1 receptor (PD-1) and programmed death-ligand 1 (PD-L1) have brought a durable long-term survival response to patients with malignant tumors. Nivolumab, pembrolizumab, cemiplimab, durvalumab, atezolizumab, and avelumab have been approved for non-small cell lung cancer (NSCLC). These approvals accelerated prescribing of these drugs in routine oncological practices. However, anti-tumor treatments also generate a series of unique dysimmune toxicities, which are termed as immune-related adverse events (irAEs) (Nishino et al., 2015; Tirumani et al., 2015; Michot et al., 2016). ICI-induced toxicities can cause suspension of the anti-tumor treatment, and some severe irAEs would impair life quality, even leading to death (Combs Scott and Pennell, 2017; Wang et al., 2018). Theoretically, irAEs can involve all organs and tissues (Champiat et al., 2016; Weber et al., 2017; Postow et al., 2018). Skin (Minkis et al., 2013; Abdel-Rahman et al., 2015), gastrointestinal tract (Di Giacomo et al., 2009; Gentile et al., 2013; Cheng et al., 2015), endocrine glands (Ryder et al., 2014; Albarel et al., 2015; Gaudy et al., 2015), and pulmonary system (Berthod et al., 2012; Barjaktarevic et al., 2013) are the most affected organs. The effective predictive biomarkers of irAEs are required to identify the risk for patients receiving anti-PD-1/PD-L1 administration. Patients with specific physical conditions are often at a high risk of irAEs. Therefore, before receiving immunotherapy, doctors need to carefully ask patients about their physical status. Patients with autoimmune disease (Kyi et al., 2014; Pedersen et al., 2014) and chronic infection (Sharma et al., 2013) are mentioned with a high risk of developing irAEs. Recently, biomarkers to predict irAEs have been reported, such as sex (Valpione et al., 2018), cytokines (Tarhini et al., 2015), autoantibodies (Duarte et al., 2018; Cortellini et al., 2019), TMB (Bomze et al., 2019), gut microbiome (Chaput et al., 2017), and multi-omics (Jing et al., 2020). However, the identification of candidate risk factors that prelude to irAEs is still a realm of highly unmet need.
Chronic conditions often lead to higher morbidity and mortality of malignant tumors. Aged patients with NSCLC are often associated with comorbidities, such as COPD, diabetes mellitus, hyperlipidemia, and hypertension. Hypertension, as a clinical factor, is the most frequently reported comorbidity in patients with malignancy, which has a reported prevalence of 38% (Piccirillo et al., 2004; Mouhayar and Salahudeen, 2011). Besides, hypertension is emerging as one of the most common side effects in NSCLC patients receiving immunotherapy (Garon et al., 2019). Its incidence increases significantly when combined with angiogenesis inhibitors including the anti-vascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab (Jain et al., 2006; Ranpura et al., 2010; Syrigos et al., 2011) and certain small molecular inhibitors of tyrosine kinase (sunitinib, sorafenib, and pazopanib) (Riely and Miller, 2007).
The number of patients with lung cancer complicated with chronic diseases is very large, and the safety of immunotherapy in this population should not be ignored. However, patients with comorbidities such as uncontrolled hypertension are often excluded from oncological clinical trials. Whether patients with hypertension have a higher risk of irAEs is a lack of knowledge. Therefore, we aimed to investigate the association between irAEs and hypertension. Herein, we investigated the characteristics and risk factors of pulmonary ICI-related AEs through the FAERS database. Numerous researches suggested that the use of angiogenesis inhibitors can increase the risk of hypertension in cancer patients (Wu et al., 2008; Ranpura et al., 2010). In order to exclude the interference of other drug factors, our study only included reports of pulmonary adverse reactions after receiving single-agent immunotherapy.
Adverse event reports are available on FAERS database which is submitted by healthcare professionals, consumers, and manufacturers. The FAERS database contains demographic information, drug information, patient outcomes, and preferred terms (PTs) coded for the adverse events. These PTs are categorized into their primary system organ classes (SOCs) in the MedDRA and SOCs are equivalent to systematic classification in other medical terms. Our study was designed as a retrospective pharmacovigilance study. 14,072,154 FAERS records from January 2015 to December 2021 were included. According to the FDA’s recommendation, duplicate reports were removed by case number in this study, with only the most recent case version adopted. After extraction and de-duplication of case reports, there were 112,764 unique reports for patients who used anti-PD-1 (nivolumab, pembrolizumab, and cemiplimab) or anti-PD-L1 (durvalumab, atezolizumab, avelumab), then we excluded adverse events caused by combined therapies, only 63,055 cases receiving monotherapy included. 17,163 cases of non-small cell lung cancer (lung adenocarcinoma, lung squamous cell carcinoma/squamous cell carcinoma of lung, adenosquamous cell lung cancer, large cell lung cancer, sarcoid carcinoma, and not specified type of NSCLC) were finally included in our study, including 4,283 respiratory, thoracic and mediastinal AE reports. Severe adverse events were defined as death, life-threatening, disability, hospitalization, required for intervention, or any other outcomes.
Disproportionality analysis was applied to measure safety signals for patients who used anti-PD-1/PD-L1 therapy with hypertension under study (Almenoff et al., 2007). We calculated reporting odds ratios (ROR), 95% confidence intervals (95% CIs) and the lower bound of a two-sided 95% interval of information component (IC025) to detect potential associations between hypertension and irAEs (Bate et al., 1998; Bate et al., 2002; Bate and Evans, 2009). The calculation formulas for ROR and 95% CI were as follows: ROR = (a/c)/(b/d), 95% CI = eln(ROR) ± 1.96SQRT(1/a + 1/b + 1/c + 1/d). a = Number of patients with hypertension who received anti-PD1/PD-L1 therapy and developed the target irAEs. b = No. of hypertensive patients receiving anti-PD1/PD-L1 therapy with other adverse effects. c = No. of patients without hypertension who received anti-PD1/PD-L1 therapy and developed the target irAEs. d = No. of patients without hypertension receiving anti-PD1/PD-L1 therapy with other adverse effects. The safety signal was considered to be statistically significant when the ROR was greater than 1.0, IC more than zero and IC025 > 0. We also calculated the time-to onset of adverse events. The formula of the time-to-onset of events was as follows: Time-to-onset = Event onset date–Therapy start date. The median and interquartile ranges (IQR) were also calculated to show the time to onset.
RStudio (version 4.1.1; Boston, MA, United States) was used for all statistical analyses and for generating graphs in our study.
From 2015 to 2021, a total of 17,163 records were extracted (Figure 1), 497 patients were also diagnosed with hypertension 16,666 patients were diagnosed without hypertension. 4,283 (24.95%) were reported as respiratory thoracic and mediastinal AEs after using ICI regimes. Among them, 166 NSCLC patients were also diagnosed with hypertension. All demographic and clinical characteristics of patients were presented in Table 1. In the hypertensive and non-hypertensive groups, the proportion of males was higher than that of females. In the hypertensive group, the proportion of men (80.12%) was higher than that (67.09%) of the non-hypertensive group. In addition, compared to those aged younger than 65 years, higher percentage of patients older than 65 years in both cohorts (74.7%, 52.5%). Due to the severity of pulmonary irAEs, death was the most frequent report. Death (n = 76) was the most common outcome in hypertension cohort. Furthermore, death accounted for a larger proportion in hypertensive patients than that in non-hypertensive patients.
The distribution of SOCs for NSCLC patients was shown in Table 2. In total, general disorders (n = 4,493) and pulmonary disorders (n = 4,283) had the largest number of AEs. For patients receiving nivolumab, cemiplimab, or atezolizumab, the main irAEs were general disorders. For patients taking pembrolizumab, durvalumab or avelumab, the number of pulmonary disorders was the largest.
The pulmonary signal spectrum of different anti-PD-1 therapies was shown in Figure 2 and Supplemetary Table S1. Cumulative event rates of irAEs since the initiation of ICI were shown in Figure 3. According to ROR and Bayesian confidence propagation neural network (BCPNN) algorithm, interstitial lung disease (ROR = 3.62, 95%CI 2.68–4.89, IC = 1.54, IC025 = 0.57) with median time-to-onset of 28 (12.00–84.25) days (Supplemetary Table S5), was the only one statistically positively associated with hypertension in patients receiving PD-1 inhibitors. Pneumonitis (ROR = 2.57, 95%CI 1.18–5.63, IC = 1.02, IC025 = −1.42) was not significantly correlated with hypertension in NSCLC patients receiving nivolumab. Interstitial lung disease (ROR = 3.04, 95%CI 2.19–4.23, IC = 1.25, IC025 = 0.20) was the mostly reported among the statistically significant reported adverse event in pembrolizumab subgroup. 54 patients with hypertension developed interstitial lung disease, with a disease severity rate of 100%, a mortality rate of 63%, and a hospitalization rate of 83%. Besides, we performed the disproportionality analysis of NSCLC patients without hypertension receiving anti-PD-1 treatment. The results demonstrated that no statistically significant signal was detected in the group without hypertension (Supplemetary Table S3,S4).
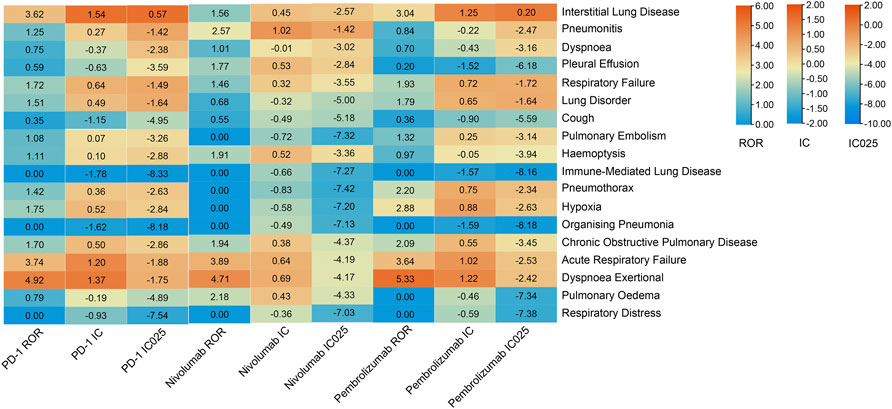
FIGURE 2. Safety signals of anti-PD-1 treatment in the NSCLC group with hypertension. ROR, reporting odds ratios; IC, information component; IC025, the lower limit of the 95% confidence interval of IC. ROR was greater than 1.0, the lower limit of 95% CI was above 1.0, IC more than zero and IC025 > 0. The value of each column is represented by a different color, the more orange the color, the larger the value. A signal is defined as ROR >1.0, IC > 0, and IC025 > 0.
The safety signal spectrum of different anti-PD-L1 treatments was presented in Figure 4 and Supplemetary Table S2. Using the ROR algorithm, haemoptysis (ROR 3.23, 95%CI 1.12–9.31) and acute respiratory failure (ROR 5.63, 95%CI 1.57–20.17) were mostly reported among the statistically significant reported adverse events in patients receiving PD-L1 inhibitors. However, when we used the Bayesian algorithm to estimate drug safety signals, neither of these achieved statistical significance (IC = 0.19, IC025 = −1.55; IC = 0.28, IC025 = −1.89). The median (IQR) time from therapy start to the onset of interstitial lung disease, pneumonitis, dyspnoea, pleural effusion and respiratory failure were 55 (29.00–58.00) days, 31 (14.00–67.50) days, 14 (11.00–14.00) days, 35 (20.50–47.25) days, 28.5 (27.25–39.25) days (Figure 3; Supplemetary Table S5).
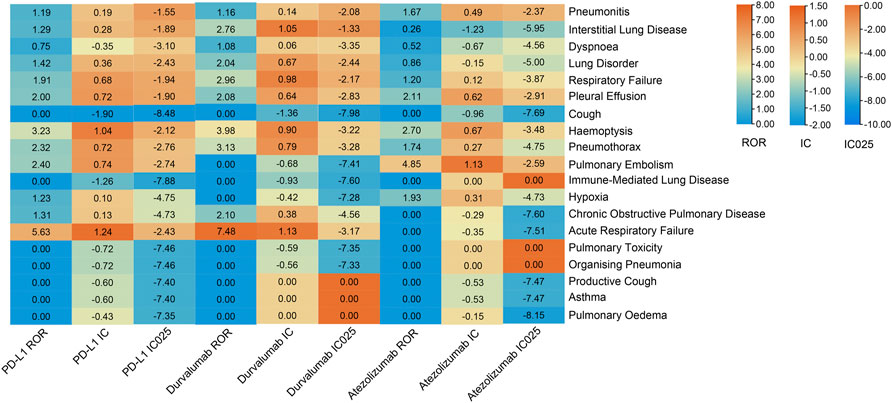
FIGURE 4. Safety signals of anti-PD-L1 therapy in the NSCLC group with hypertension. ROR, reporting odds ratios; IC, information component; IC025, the lower limit of the 95% confidence interval of IC. The value of each column is represented by a different color, the more orange the color, the larger the value. A signal is defined as ROR >1.0, IC > 0, and IC025 > 0.
Hypertension is one of the common chronic degenerative diseases, that involves remodeling and inflammation of arterial walls, and has an intricate relationship with cancer. Both of them share some same risk factors including smoking, diabetes mellitus, and physical inactivity (Battistoni et al., 2015; Ameri et al., 2018). Adjunctive therapies concurrently administered with antineoplastic agents can promote the development of hypertension or worsen previously controlled hypertension (Tonia et al., 2012; Cohen et al., 2019). Meanwhile, high blood pressure increases the risk of cancer development (Sanfilippo et al., 2014; Seretis et al., 2019). Dyer et al. (1977) firstly pointed out that hypertension might be a risk factor for cancer mortality, which was confirmed by other studies (Stocks et al., 2012; Berger et al., 2016; Harding et al., 2016) that hypertension could accelerate the biological process of aging which favors carcinogenesis. The metabolic disorders of hypertension increase oxidative stress and result in an irreversible proinflammatory state that reduces intracellular antioxidant capacity and predisposes it to malignant transformation (Federico et al., 2007). As hypertension is the most prevalent comorbidity in patients diagnosed with cancer (Piccirillo et al., 2004), patients with lung cancer coexisting with hypertension do not affect anti-tumor responses, nor does it affect the survival time (Yan et al., 2018). Common antihypertensive drugs, such as renin–angiotensin system inhibitors (RASi), angiotensin-converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARBs) and direct renin inhibitors have no impact on clinical outcomes with anti-PD1/PD-L1 inhibitors (Bangalore et al., 2011; Cui et al., 2019).
Immunotherapeutic agents that target immune checkpoint pathways have shown great promise. Despite extensive research efforts, few biomarkers had a high accuracy and ubiquity to predict irAEs. Patients often receive additional concomitant therapies, which bring a lot of confounding factors to the risk of irAEs to immunotherapy. Concomitant medications in the treatment of malignant tumors have different effects on response to immunotherapy (Arbour et al., 2018; Fuca et al., 2019). Some reports found that antibiotics had detrimental efficacy and toxicity effects on ICIs. In fact, compare with patients without receiving extra agents, patients receiving baseline concomitant medication had worse outcomes (Cortellini et al., 2020). We have already known that antibiotics could increase the risk of irAE by changing the gut microbiome (Pinato et al., 2019). Not only is hypertension a common adverse reaction, frequently reported in clinical trials, but also a common comorbidity in patients with non-small cell lung cancer. However, the safety of antineoplastic therapy in these patients with hypertension has been rarely reported. As a common adverse reaction, the incidence of arterial hypertension is associated with the clinical outcome of antiangiogenetic-targeted treatment modalities in patients with tumors. (Scartozzi et al., 2009). As there were growing reports on the relationship between the occurrence of irAEs and tumor response, the anti-tumor treatments of ICI were associated with a reduced incidence of irAEs (Teraoka et al., 2017; Sato et al., 2018). We speculated that there is a potential link between high blood pressure and adverse reactions.
Although the causative pathogenic mechanism of hypertension-associated irAEs was poorly understood, studies have suggested that activation or reactivation of tissue-resident autoreactive T cells is thought to be a dominant prime factor in the development of irAEs (June et al., 2017; Dougan et al., 2021). Shared antigens between the specified organs and vessels could lead to de novo T cell activation and precipitate unwanted effects. High blood pressure caused endothelial dysfunction and vascular oxidative stress, leading to vasoconstriction. Neoantigens generated and then T cells were activated by binding specific antigens presented in major histocompatibility complex molecules on specific antigen-presenting cells, thereby activating of the adaptive immune system (Vinh et al., 2010). Activated T cells infiltrated blood vessels and produced cytokines, which promoted endothelial dysfunction and low-grade chronic inflammation (Idris-Khodja et al., 2014). Beyond increased perivascular immune cells accumulation and intravascular infiltration, circulating levels of certain cytokines and chemokines are abnormally elevated. Multiple chemokines recruited and stimulated the infiltration of T cells and monocytes and macrophages during hypertension (Guzik et al., 2007; Moore et al., 2015; Mikolajczyk et al., 2016). Besides, elevated circulatory levels of cytokines, C-reactive proteins, and immunoglobulins in patients with hypertension have also been reported. furthermore, autoreactive antibodies to vascular wall antigens have been detected (Martinez Amenos et al., 1985; Blake et al., 2003; Alexander et al., 2019). Recent investigations demonstrated that circulating antibody levels are elevated in both essential and pregnancy-related hypertension (Dib et al., 2012; Chan et al., 2014). Together, these studies indicated that T cells could be activated when self-peptides are presented through epitopes spread by antigen-presenting cells. Pre-existing autoreactive T cells have already existed and be kept in check through immune checkpoint molecules. When receiving immune checkpoint inhibitors, immune cells were over-activated, resulting in a low-level inflammatory response in tumor patients being amplified, further leading to immune-related adverse reactions.
To our knowledge, irAEs after receiving PD-1/PD-L1 inhibitors have never been reported in the context of cancer patients under chronic diseases. According to real-world data, we found a high reporting frequency of respiratory AEs associated with PD-1/PD-L1 inhibitors. Meanwhile, every PD-1/PD-L1 inhibitor has respective profiles of toxicities. Our study showed statistical evidence regarding the association between pulmonary irAEs and hypertension, which needs to be interpreted cautiously and further verified in pharmacology and clinical aspects. Beyond that, there may be some other potential mechanisms that could affect the safety of immunotherapies. Chronic diseases, particularly in aged patients, have an indirect causative effect on the occurrence of irAE. They need to pay attention to pulmonary adverse reactions during immunotherapy. Our study could help to recognize and manage irAEs in clinical practice. Further observational studies are required to establish the safety of ICIs in hypertensive patients.
We acknowledged several limitations in our study beyond its retrospective and observative nature, with reporting bias, missing data, and confounding bias on the FAERS database, specific grades of hypertension, and cancer outcomes. We would prospectively assess the physical condition of NSCLC patients and investigated interactions between hypertension and irAEs in our center to validate our results. In addition, we need to further analyze the clinical outcomes in NSCLC patients with hypertension.
NSCLC patients with hypertension receiving PD-1/PD-L1 inhibitors have higher reporting odds of pulmonary adverse events. Clinicians should pay special attention to the occurrence of interstitial lung disease when using immunotherapy for these patients, and should intervene in time if lung disease occurs. Other adverse events such as pneumonitis and haemoptysis, which were highly reported without significance by the Bayesian IC algorithm, should not be ignored in clinical practice.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
JC: the conception and design of the study; acquisition of data, analysis of data, drafting the article. YW: Manuscript revision, interpretation of data. XC: analysis and interpretation of data. YL: acquisition of data. CS: the conception and design of the study. All authors read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (Grant number: 82072568) and Shanghai Shenkang Hospital Development Center (Grant Number: SHDC12020110).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.944342/full#supplementary-material
Abdel-Rahman, O., ElHalawani, H., and Fouad, M. (2015). Risk of cutaneous toxicities in patients with solid tumors treated with immune checkpoint inhibitors: A meta-analysis. Future Oncol. 11, 2471–2484. doi:10.2217/fon.15.118
Albarel, F., Gaudy, C., Castinetti, F., Carre, T., Morange, I., Conte-Devolx, B., et al. (2015). Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur. J. Endocrinol. 172, 195–204. doi:10.1530/EJE-14-0845
Alexander, M. R., Norlander, A. E., Elijovich, F., Atreya, R. V., Gaye, A., Gnecco, J. S., et al. (2019). Human monocyte transcriptional profiling identifies IL-18 receptor accessory protein and lactoferrin as novel immune targets in hypertension. Br. J. Pharmacol. 176, 2015–2027. doi:10.1111/bph.14364
Almenoff, J. S., Pattishall, E. N., Gibbs, T. G., DuMouchel, W., Evans, S. J. W., and Yuen, N. (2007). Novel statistical tools for monitoring the safety of marketed drugs. Clin. Pharmacol. Ther. 82, 157–166. doi:10.1038/sj.clpt.6100258
Ameri, P., Canepa, M., Anker, M., Belenkov, Y., Bergler-Klein, J., Cohen-Solal, A., et al. (2018). Cancer diagnosis in patients with heart failure: Epidemiology, clinical implications and gaps in knowledge. Eur. J. Heart Fail. 20, 879–887. doi:10.1002/ejhf.1165
Arbour, K. C., Mezquita, L., Long, N., Rizvi, H., Auclin, E., Ni, A., et al. (2018). Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J. Clin. Oncol. 36, 2872–2878. doi:10.1200/JCO.2018.79.0006
Bangalore, S., Kumar, S., Kjeldsen, S. E., Makani, H., Grossman, E., Wetterslev, J., et al. (2011). Antihypertensive drugs and risk of cancer: Network meta-analyses and trial sequential analyses of 324 168 participants from randomised trials. Lancet. Oncol. 12, 65–82. doi:10.1016/S1470-2045(10)70260-6
Barjaktarevic, I. Z., Qadir, N., Suri, A., Santamauro, J. T., and Stover, D. (2013). Organizing pneumonia as a side effect of ipilimumab treatment of melanoma. Chest 143, 858–861. doi:10.1378/chest.12-1467
Bate, A., and Evans, S. J. W. (2009). Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol. Drug Saf. 18, 427–436. doi:10.1002/pds.1742
Bate, A., Lindquist, M., Edwards, I. R., Olsson, S., Orre, R., Lansner, A., et al. (1998). A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54, 315–321. doi:10.1007/s002280050466
Bate, A., Lindquist, M., Edwards, I. R., and Orre, R. (2002). A data mining approach for signal detection and analysis. Drug Saf. 25, 393–397. doi:10.2165/00002018-200225060-00002
Battistoni, A., Mastromarino, V., and Volpe, M. (2015). Reducing cardiovascular and cancer risk: How to address global primary prevention in clinical practice. Clin. Cardiol. 38, 387–394. doi:10.1002/clc.22394
Berger, S. M., Gislason, G., Moore, L. L., Andersson, C., Torp-Pedersen, C., Denis, G. V., et al. (2016). Associations between metabolic disorders and risk of cancer in Danish men and women - a nationwide cohort study. Bmc Cancer 16, 133. doi:10.1186/s12885-016-2122-7
Berthod, G., Lazor, R., Letovanec, I., Romano, E., Noirez, L., Stalder, J. M., et al. (2012). Pulmonary sarcoid-like granulomatosis induced by ipilimumab. J. Clin. Oncol. 30, E156–E159. doi:10.1200/JCO.2011.39.3298
Blake, G. J., Rifai, N., Buring, J. E., and Ridker, P. M. (2003). Blood pressure, C-reactive protein, and risk of future cardiovascular events. Circulation 108, 2993–2999. doi:10.1161/01.CIR.0000104566.10178.AF
Bomze, D., Ali, O. H., Bate, A., and Flatz, L. (2019). Association between immune-related adverse events during anti-PD-1 therapy and tumor mutational burden. JAMA Oncol. 5, 1633–1635. doi:10.1001/jamaoncol.2019.3221
Champiat, S., Lambotte, O., Barreau, E., Belkhir, R., Berdelou, A., Carbonnel, F., et al. (2016). Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann. Oncol. 27, 559–574. doi:10.1093/annonc/mdv623
Chan, C. T., Lieu, M., Toh, B. H., Kyaw, T. S., Bobik, A., Sobey, C. G., et al. (2014). Antibodies in the pathogenesis of hypertension. Biomed. Res. Int. 2014, 504045. doi:10.1155/2014/504045
Chaput, N., Lepage, P., Coutzac, C., Soularue, E., Le Roux, K., Monot, C., et al. (2017). Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 28, 1368–1379. doi:10.1093/annonc/mdx108
Cheng, R., Cooper, A., Kench, J., Watson, G., Bye, W., McNeil, C., et al. (2015). Ipilimumab-induced toxicities and the gastroenterologist. J. Gastroenterol. Hepatol. 30, 657–666. doi:10.1111/jgh.12888
Cohen, J. B., Geara, A. S., Hogan, J. J., and Townsend, R. R. (2019). Hypertension in cancer patients and survivors epidemiology, diagnosis, and management. JACC. CardioOncol. 1, 238–251. doi:10.1016/j.jaccao.2019.11.009
Combs Scott, S., and Pennell, N. (2017). PS02.09 Use of systemic corticosteroids during the first month of nivolumab therapy in patients with advanced non-small cell lung cancer. J. Thorac. Oncol. 12, S1567. doi:10.1016/j.jtho.2017.09.047
Cortellini, A., Buti, S., Santini, D., Perrone, F., Giusti, R., Tiseo, M., et al. (2019). Clinical outcomes of patients with advanced cancer and pre-existing autoimmune diseases treated with anti-programmed death-1 immunotherapy: A real-world transverse study. Oncologist 24, E327–E337. doi:10.1634/theoncologist.2018-0618
Cortellini, A., Tucci, M., Adamo, V., Stucci, L. S., Russo, A., Tanda, E. T., et al. (2020). Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J. Immunother. Cancer 8, e001361. doi:10.1136/jitc-2020-001361
Cui, Y., Wen, W., Zheng, T., Li, H., Gao, Y.-T., Cai, H., et al. (2019). Use of antihypertensive medications and survival rates for breast, colorectal, lung, or stomach cancer. Am. J. Epidemiol. 188, 1512–1528. doi:10.1093/aje/kwz106
Di Giacomo, A. M., Danielli, R., Guidoboni, M., Calabro, L., Carlucci, D., Miracco, C., et al. (2009). Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: Clinical and immunological evidence from three patient cases. Cancer Immunol. Immunother. 58, 1297–1306. doi:10.1007/s00262-008-0642-y
Dib, H., Tamby, M. C., Bussone, G., Regent, A., Berezne, A., Lafine, C., et al. (2012). Targets of anti-endothelial cell antibodies in pulmonary hypertension and scleroderma. Eur. Respir. J. 39, 1405–1414. doi:10.1183/09031936.00181410
Dougan, M., Luoma, A. M., Dougan, S. K., and Wucherpfennig, K. W. (2021). Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell 184, 1575–1588. doi:10.1016/j.cell.2021.02.011
Duarte, J. D., Parakh, S., Andrews, M. C., Woods, K., Pasam, A., Tutuka, C., et al. (2018). Autoantibodies may predict immune-related toxicity: Results from a phase I study of intralesional Bacillus calmette-guerin followed by ipilimumab in patients with advanced metastatic melanoma. Front. Immunol. 9, 411. doi:10.3389/fimmu.2018.00411
Dyer, A., Berkson, D., Stamler, J., Lindberg, H., and Stevens, E. (1975). High blood-pressure: A risk factor for cancer mortality? Lancet (London, Engl. 305, 1051–1056. doi:10.1016/s0140-6736(75)91826-7
Federico, A., Morgillo, F., Tuccillo, C., Ciardiello, F., and Loguercio, C. (2007). Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 121, 2381–2386. doi:10.1002/ijc.23192
Fuca, G., Galli, G., Poggi, M., Lo Russo, G., Proto, C., Imbimbo, M., et al. (2019). Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. Esmo Open 4, e000457. doi:10.1136/esmoopen-2018-000457
Garon, E. B., Hellmann, M. D., Rizvi, N. A., Carcereny, E., Leighl, N. B., Ahn, M. J., et al. (2019). Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE-001 study. J. Clin. Oncol. 37, 2518–2527. doi:10.1200/JCO.19.00934
Gaudy, C., Clevy, C., Monestier, S., Dubois, N., Preau, Y., Mallet, S., et al. (2015). Anti-PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care 38, E182–E183. doi:10.2337/dc15-1331
Gentile, N. M., D'Souza, A., Fujii, L. L., Wu, T. T., and Murray, J. A. (2013). Association between ipilimumab and celiac disease. Mayo Clin. Proc. 88, 414–417. doi:10.1016/j.mayocp.2013.01.015
Guzik, T. J., Hoch, N. E., Brown, K. A., McCann, L. A., Rahman, A., Dikalov, S., et al. (2007). Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. J. Exp. Med. 204, 2449–2460. doi:10.1084/jem.20070657
Harding, J. L., Sooriyakumaran, M., Anstey, K. J., Adams, R., Balkau, B., Brennan-Olsen, S., et al. (2016). Hypertension, antihypertensive treatment and cancer incidence and mortality: A pooled collaborative analysis of 12 Australian and New Zealand cohorts. J. Hypertens. 34, 149–155. doi:10.1097/HJH.0000000000000770
Idris-Khodja, N., Mian, M. O. R., Paradis, P., and Schiffrin, E. L. (2014). Dual opposing roles of adaptive immunity in hypertension. Eur. Heart J. 35, 1238–1244. -+. doi:10.1093/eurheartj/ehu119
Jain, R. K., Duda, D. G., Clark, J. W., and Loeffler, J. S. (2006). Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat. Clin. Pract. Oncol. 3, 24–40. doi:10.1038/ncponc0403
Jing, Y., Liu, J., Ye, Y. Q., Pan, L., Deng, H., Wang, Y. S., et al. (2020). Multi-omics prediction of immune-related adverse events during checkpoint immunotherapy. Nat. Commun. 11, 4946. doi:10.1038/s41467-020-18742-9
June, C. H., Warshauer, J. T., and Bluestone, J. A. (2017). Is autoimmunity the Achilles' heel of cancer immunotherapy? Nat. Med. 23, 540–547. doi:10.1038/nm.4321
Kyi, C., Carvajal, R. D., Wolchok, J. D., and Postow, M. A. (2014). Ipilimumab in patients with melanoma and autoimmune disease. J. Immunother. Cancer 2, 35–44. doi:10.1186/s40425-014-0035-z
Martinez Amenos, A., Buendia, E., Carreras, L., Font, I., Mestre, M., Rama, H., et al. (1985). Humoral and cellular immunological abnormalities in hypertensive patients. J. Clin. Hypertens. 1, 153–160.
Michot, J. M., Bigenwald, C., Champiat, S., Collins, M., Carbonnel, F., Postel-Vinay, S., et al. (2016). Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 54, 139–148. doi:10.1016/j.ejca.2015.11.016
Mikolajczyk, T. P., Nosalski, R., Szczepaniak, P., Budzyn, K., Osmenda, G., Skiba, D., et al. (2016). Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension. Faseb J. 30, 1987–1999. doi:10.1096/fj.201500088R
Minkis, K., Garden, B. C., Wu, S. H., Pulitzer, M. P., and Lacouture, M. E. (2013). The risk of rash associated with ipilimumab in patients with cancer: A systematic review of the literature and meta-analysis. J. Am. Acad. Dermatology 69, E121–E128. doi:10.1016/j.jaad.2012.12.963
Moore, J. P., Vinh, A., Tuck, K. L., Sakkal, S., Krishnan, S. M., Chan, C. T., et al. (2015). M2 macrophage accumulation in the aortic wall during angiotensin II infusion in mice is associated with fibrosis, elastin loss, and elevated blood pressure. Am. J. Physiol. Heart Circ. Physiol. 309, H906–H917. doi:10.1152/ajpheart.00821.2014
Mouhayar, E., and Salahudeen, A. (2011). Hypertension in cancer patients. Tex. Heart Inst. J. 38, 263–265.
Nishino, M., Tirumani, S. H., Ramaiya, N. H., and Hodi, F. S. (2015). Cancer immunotherapy and immune-related response assessment: The role of radiologists in the new arena of cancer treatment. Eur. J. Radiol. 84, 1259–1268. doi:10.1016/j.ejrad.2015.03.017
Pedersen, M., Andersen, R., Norgaard, P., Jacobsen, S., Thielsen, P., Straten, P. T., et al. (2014). Successful treatment with Ipilimumab and Interleukin-2 in two patients with metastatic melanoma and systemic autoimmune disease. Cancer Immunol. Immunother. 63, 1341–1346. doi:10.1007/s00262-014-1607-y
Piccirillo, J. F., Tierney, R. M., Costas, I., Grove, L., and Spitznagel, E. L. (2004). Prognostic importance of Comorbidity in a hospital-based cancer registry. Jama-Journal Am. Med. Assoc. 291, 2441–2447. doi:10.1001/jama.291.20.2441
Pinato, D. J., Gramenitskaya, D., Altmann, D. M., Boyton, R. J., Mullish, B. H., Marchesi, J. R., et al. (2019). Antibiotic therapy and outcome from immune-checkpoint inhibitors. J. Immunother. Cancer 7, 287. doi:10.1186/s40425-019-0775-x
Postow, M. A., Sidlow, R., and Hellmann, M. D. (2018). Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. Overseas. Ed. 378, 158–168. doi:10.1056/nejmra1703481
Ranpura, V., Pulipati, B., Chu, D., Zhu, X. L., and Wu, S. H. (2010). Increased risk of high-grade hypertension with bevacizumab in cancer patients: A. Meta-analysis. Am. J. Hypertens. 23, 460–468. doi:10.1038/ajh.2010.25
Riely, G., and Miller, V. A. (2007). Vascular endothelial growth factor trap in non-small cell lung cancer. Clin. Cancer Res. 13, 4623S–4627S. doi:10.1158/1078-0432.ccr-07-0544
Ryder, M., Callahan, M., Postow, M. A., Wolchok, J., and Fagin, J. A. (2014). Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: A comprehensive retrospective review from a single institution. Endocr. Relat. Cancer 21, 371–381. doi:10.1530/ERC-13-0499
Sanfilippo, K. M., McTigue, K. M., Fidler, C. J., Neaton, J. D., Chang, Y., Fried, L. F., et al. (2014). Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension 63, 934–941. doi:10.1161/HYPERTENSIONAHA.113.02953
Sato, K., Akamatsu, H., Murakami, E., Sasaki, S., Kanai, K., Hayata, A., et al. (2018). Corrigendum to “Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab” [Lung Cancer 115 (2018) 71–74]. Lung Cancer 126, 230–231. doi:10.1016/j.lungcan.2018.11.007
Scartozzi, M., Galizia, E., Chiorrini, S., Giampieri, R., Berardi, R., Pierantoni, C., et al. (2009). Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann. Oncol. 20, 227–230. doi:10.1093/annonc/mdn637
Seretis, A., Cividini, S., Markozannes, G., Tseretopoulou, X., Lopez, D. S., Ntzani, E. E., et al. (2019). Association between blood pressure and risk of cancer development: A systematic review and meta-analysis of observational studies. Sci. Rep. 9, 8565. doi:10.1038/s41598-019-45014-4
Sharma, A., Thompson, J. A., Repaka, A., and Mehnert, J. M. (2013). Ipilimumab administration in patients with advanced melanoma and hepatitis B and C. J. Clin. Oncol. 31, E370–E372. doi:10.1200/JCO.2012.47.1946
Stocks, T., Van Hemelrijck, M., Manjer, J., Bjorge, T., Ulmer, H., Hallmans, G., et al. (2012). Blood pressure and risk of cancer incidence and mortality in the metabolic syndrome and cancer project. Hypertension 59, 802–810. doi:10.1161/HYPERTENSIONAHA.111.189258
Syrigos, K. N., Karapanagiotou, E., Boura, P., Manegold, C., and Harrington, K. (2011). Bevacizumab-induced hypertension pathogenesis and management. BioDrugs. 25, 159–169. doi:10.2165/11590180-000000000-00000
Tarhini, A. A., Zahoor, H., Lin, Y., Malhotra, U., Sander, C., Butterfield, L. H., et al. (2015). Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J. Immunother. Cancer 3, 39–46. doi:10.1186/s40425-015-0081-1
Teraoka, S., Fujimoto, D., Morimoto, T., Kawachi, H., Ito, M., Sato, Y., et al. (2017). Early immune-related adverse events and association with outcome in advanced non-small cell lung cancer patients treated with nivolumab: A prospective cohort study. J. Thorac. Oncol. 12, 1798–1805. doi:10.1016/j.jtho.2017.08.022
Tirumani, S. H., Ramaiya, N. H., Keraliya, A., Bailey, N. D., Ott, P. A., Hodi, F. S., et al. (2015). Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol. Res. 3, 1185–1192. doi:10.1158/2326-6066.CIR-15-0102
Tonia, T., Mettler, A., Robert, N., Schwarzer, G., Seidenfeld, J., Weingart, O., et al. (2012). Erythropoietin or darbepoetin for patients with cancer. Cochrane database Syst. Rev. 12:CD003407. doi:10.1002/14651858.cd003407.pub5
Valpione, S., Pasquali, S., Campana, L. G., Piccin, L., Mocellin, S., Pigozzo, J., et al. (2018). Sex and interleukin-6 are prognostic factors for autoimmune toxicity following treatment with anti-CTLA4 blockade. J. Transl. Med. 16, 94–10. doi:10.1186/s12967-018-1467-x
Vinh, A., Chen, W., Blinder, Y., Weiss, D., Taylor, W. R., Goronzy, J. J., et al. (2010). Inhibition and genetic ablation of the B7/CD28 T-cell costimulation Axis prevents experimental hypertension. Circulation 122, 2529–2537. doi:10.1161/CIRCULATIONAHA.109.930446
Wang, D. Y., Salem, J.-E., Cohen, J. V., Chandra, S., Menzer, C., Ye, F., et al. (2018). Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 4, 1721–1728. doi:10.1001/jamaoncol.2018.3923
Weber, J. S., Hodi, F. S., Wolchok, J. D., Topalian, S. L., Schadendorf, D., Larkin, J., et al. (2017). Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 35, 785–792. doi:10.1200/JCO.2015.66.1389
Wu, S. H., Chen, J. J., Kudelka, A., Lu, J., and Zhu, X. L. (2008). Incidence and risk of hypertension with sorafenib in patients with cancer: A systematic review and meta-analysis. Lancet Oncol. 9, 117–123. doi:10.1016/s1470-2045(08)70003-2
Keywords: pharmacovigilance, immune checkpoint inhibitor, hypertension, NSCLC, FAERS
Citation: Chen J, Wen Y, Chu X, Liu Y and Su C (2022) Pulmonary adverse events associated with hypertension in non-small cell lung cancer patients receiving PD-1/PD-L1 inhibitors. Front. Pharmacol. 13:944342. doi: 10.3389/fphar.2022.944342
Received: 15 May 2022; Accepted: 04 August 2022;
Published: 30 August 2022.
Edited by:
Miao Yan, Second Xiangya Hospital, Central South University, ChinaReviewed by:
Xiaojing Guo, Second Military Medical University, ChinaCopyright © 2022 Chen, Wen, Chu, Liu and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunxia Su, c3VzdV9tYWlsQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.