- 1Institute of Liver Diseases, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Shanghai Key Laboratory of Traditional Chinese Clinical Medicine, Shanghai, China
- 3Key Laboratory of Liver and Kidney Diseases, Ministry of Education, Shanghai, China
Background and Aims: Although different kinds of traditional Chinese medicines could reportedly improve the efficacy of antiviral therapy on liver fibrosis caused by HBV, the problem of clinicians on how to choose the appropriate treatment strategies for the patients fails to be solved. This study aims at comparing and ranking different traditional Chinese medicine (TCM) therapies in the treatment of liver fibrosis due to chronic hepatitis B (CHB).
Methods: Eight electronic databases were searched from their establishment to 17 Aug 2021. All included data and pooled odds ratio were used for network meta-analysis (NMA) and statistical analysis. The consistency was evaluated by the node-splitting analysis. The stability of results and source of heterogeneity were tested by sensitivity analysis. Different treatment strategies (regimens) in this network meta-analysis were ranked with the aid of surface under the cumulative ranking curve (SUCRA) probability value.
Results: A total of 29 articles with 3,106 sufferers were recruited in this NMA. Results of SUCRA value rankings indicated that Fuzheng Huayu therapy or combined with entecavir had preferable effects in improving the clinical efficacy, recovering the level of hyaluronic acid, IV-C, ALT, ALB, and TBil, relieving the TCM symptoms including hypochondriac pain and poor appetite, regaining the width of portal vein and thickness of spleen, and lessening side effects. Apart from these, Ziyin Shugan therapy or combined with ETV could also be suitable to regain the level of laminin, PC-III, and AST, relieve fatigue and HBV-DNA conversion.
Conclusion: This NMA confirmed the efficacy and safety of different treatment therapies for improving CHB liver fibrosis, including the serum biomarkers of live fibrosis and serum parameters for liver function, TCM symptoms, imaging indexes, HBV-DNA conversion rate, which offered the TCM practitioners crucial reference value on clinical medication.
Introduction
Chronic hepatitis B (CHB), characterized by persistent hepatitis B virus (HBV) infection, is a serious infectious disease with high morbidity and mortality (He et al., 2016; Luo et al., 2016). Liver fibrosis is characterized by excessive accumulation of the collagen extracellular matrix (ECM) (Suk and Kim, 2015). As is well known, CHB is one of the most important etiologies for liver fibrosis, which could develop into cirrhosis. It is reported that 3% of CHB sufferers have decompensated liver cirrhosis every year. This aggravation could shorten 5-years cumulative survival time for patients with CHB (Ren et al., 2020). Therefore, inhibiting or regressing liver fibrosis is as a crucial strategy for CHB as anti-HBV replication.
Currently, nucleos (t) ide analogs, such as entecavir (ETV), tenofovir dipivoxil, and adefovir dipivoxil, are mainly applied to clinical treatment, whose therapeutic mechanism is involved in the continuous suppression of the activity of HBV-DNA reverse transcriptase. Although these analogs, to some extent, can alleviate CHB fibrosis, they require the sufferers to take medicine for long time, and their efficacies of antifibrosis are limited. A research finding showed that the improvement rate of liver fibrosis was about 40% after 1 year of application of antiviral drugs (Schiff et al., 2008). Meanwhile, HBV patients with serious fibrosis received antiviral treatment for 5–10 years, and only about one-third of the patients failed to improve their liver fibrosis (Chang et al., 2010).
Due to the complexity of pathological mechanism of liver fibrosis, there are currently no FDA-approved biological or chemical antifibrosis drugs. However, traditional Chinese medicines (TCMs) have been accumulated in this field for a long time and have advantages at present. Accordingly, many new TCM drugs against liver fibrosis, such as Fuzheng Huayu prescription (FZHY prescription), Dahuang Zhechong pill (DHZC), and Anluo Huaxian pill (ALHX), have been developed (Wei et al., 2015; Wang and Peng, 2018; Gui et al., 2020; Wu et al., 2020). These TCM drugs are all taken orally twice or three times a day. So far, there have been evidence-based medical research methods to evaluate the efficacy and safety of these TCM drugs combined with current first-line drug ETV (Wei et al., 2015; Wang and Peng, 2018; Wang et al., 2018). Although these meta-analysis could prove that the application of TCM drugs combined with ETV could improve HBV sufferers’ liver function and liver fibrosis, the problem that which different treatment strategies should be chosen by clinicians need to be solved. As is well known, network meta-analysis (NMA) can compare and rank the efficacy and safety of different TCM drugs, which can help clinicians write an appropriate prescription. Moreover, a relevant publication has been published using an NMA to evaluate the efficacy of eight TCM drugs for HBV-related liver fibrosis (Wang et al., 2020). However, the conclusion that FZHY prescription combined with ETV is the best therapeutic strategy for HBV liver fibrosis is very broad to subdivide clinical efficacy, indexes of liver fibrosis and liver function, TCM symptoms, and imaging indexes. As for improving the HBV-DNA negative conversion rates, no TCM strategies showed absolute superiority because of insufficient articles. Recently, there are some new relevant publications published in databases. Considering these factors, we are about to conduct an NMA to evaluate the comparative effects and rankings of all known dominated TCM drugs on CHB liver fibrosis.
Materials and methods
This NMA was performed following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISRMA) statement (Liberati et al., 2009) (Supplementary Information S1).
Data sources and search strategy
We retrieved electronic databases of PubMed, MEDLINE Complete, OVID EMBASE, Scopus, Web of Science, Google Scholar, China National Knowledge Infrastructure (CNKI), and WanFang Data from their establishment to 17 Aug 2021. No language limitation was applied. The initial search strategies were performed as follows: “traditional Chinese medicine (TCM),” “Chinese medicine,” “herbs,” “herbal medicine,” “Fuzheng Huayu,” “Dahuang Zhechong,” “Anluo Huaxian,” “Biejia Ruangan,” “antiviral drugs/agents,” “entecavir,” “adefovir,” “chronic hepatits B liver fibrosis,” “chronic hepatits B,” “hepatitis B virus,” “liver fibrosis,” “hepatic fibrosis,” and “randomized controlled trials (RCTs).” Detailed information of all electronic databases is displayed in Supplementary Information S2.
Inclusion and exclusion criteria
Two investigators (Yun-kai Dai and Hai-na Fan) independently read the abstracts and full articles. Following the criteria (participants, interventions, comparisons, outcomes, and study design, PICOS), we included certain items in this research: RCTs; TCMs or nucleos (t) ide analogs in interventions; adults only; course of treatment >1 month; and Jadad score >2. Meanwhile, some items should be excluded: HBV negative; decompensated liver cirrhosis; no serum indicator of liver fibrosis in outcomes; meta-analyses or systematic reviews; conference summaries or abstracts only; case reports; single-sex researches; animal experiments or fundamental experiment studies; incomplete or error information; scientific and technological achievements; non-pharmaceutical therapy; and duplicates.
Data abstraction and quality evaluation
Two people (Yun-kai Dai and Yong-hong Hu), respectively, extracted relevant data and evaluated the methodological quality. Detailed information to be abstracted were listed as follows: first author and year of publication, patients’ age and gender, severity of the disease, courses of disease and treatment, interventions and outcomes (clinical efficacy, serum biomarkers of liver fibrosis, and serum parameters for liver function), administration route, and side effects. Missing information was remedied by getting in touch with the first or corresponding authors. Methodological quality evaluation of each literature was evaluated by means of the Cochrane Collaboration Recommendations assessment tool (Higgins et al., 2011). The assessment of risk of bias includes six domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcomes assessment, incomplete outcome data, and selective reporting. Each element of these domains was used to evaluate the included trials as low, unclear, or high risk. Meanwhile, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE), online guideline “https://gdt.gradepro.org/app/”, was applied to assess the evidence quality as high, moderate, low, and very low (Puhan et al., 2014).
Statistical analysis
Stata version 13.0 software was used to compare the efficacy and safety of different TCM drugs across the included studies. Based on the Bayesian framework and the Markov chain Monte Carlo (MCMC) method, WinBUGS version 1.4.3 was used for the evaluation and procession of research data a priori. In order to accommodate the model, three chains and non-informative uniform and normal priori distributions were applied (Ades et al., 2006; Sutton et al., 2008). After that, to gain their posterior distributions, 10 thinning intervals each chain and 50,000 iterations were all set. As for the simulation iterations, the top 20,000 were used for annealing in order to eliminate the impacts of the initial value, while the bottom 30,000 were applied to sampling. As for effect sizes, the standardized mean difference (SMD) was produced for continuous variable data, and the relative risk (RR) was pooled for dichotomous outcomes. Both of them, conducting a random effects model to minimize the risk, were used for the summarization of each comparison effect, with their corresponding 95% confidence intervals (CIs), and a network plot, where node sizes are representative of the number of sufferers, while connection sizes are related to the number of RCTs, was produced to examine the direct and indirect evidence involving in multiple-intervention comparisons. The Brooks–Gelman–Rubin statistic using the potential scale reduction factor (PSRF) value was conducted to assess model convergence. Meanwhile, the node-splitting analysis was calculated to evaluate the consistency. The inconsistency index statistic (I2) was used to quantify the heterogeneity between different treatment strategies. In order to investigate the stability of results, a sensitivity analysis was carried out. In addition, the surface under the cumulative ranking curve (SUCRA) was ranked to examine the efficacy and safety of all included strategies in each outcome.
Results
Literature screening
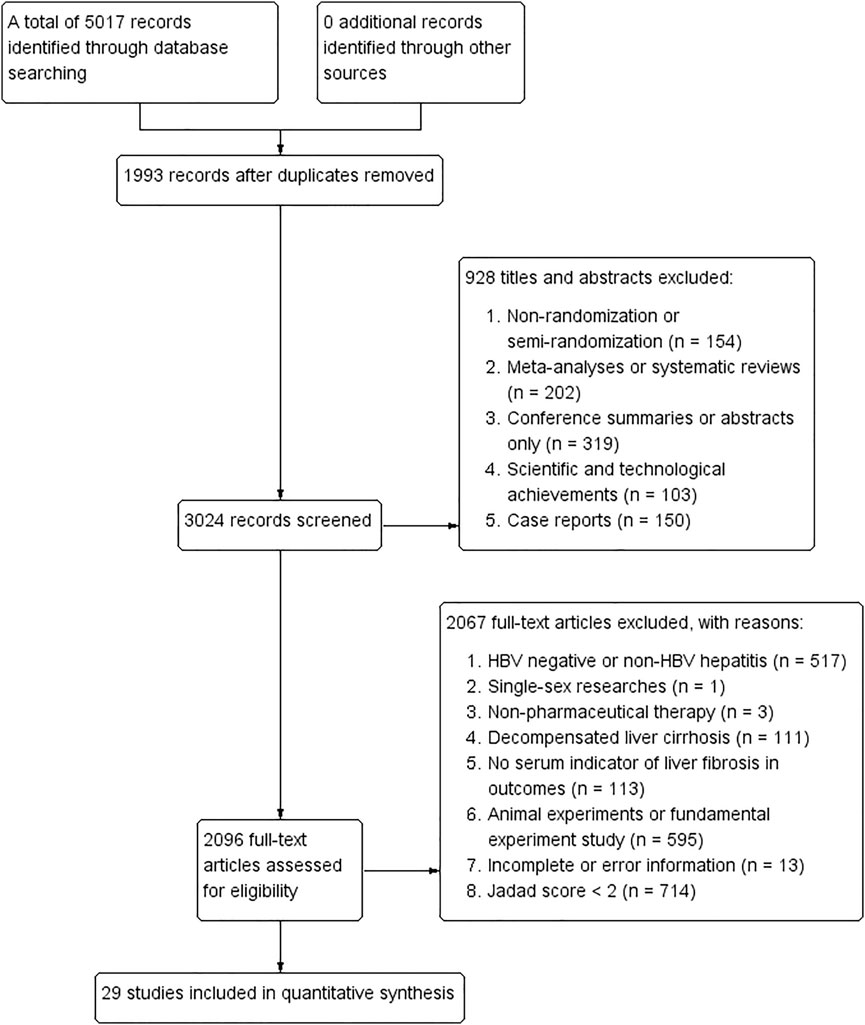
Following the inclusion and exclusion criteria in search strategies, a total of 5,017 publications were identified using five databases, of which 1,993 records were removed because of duplicates, 928 were excluded by browsing titles and abstracts, and 2,067 were removed by reading full-text articles. Finally, 29 articles were included in this NMA (Wang et al., 2004; Wang, 2005; Zhang et al., 2006; Wang et al., 2010; Zhang et al., 2016; Gu, 2018; Li, 2018; Xiao and Hu, 2018; Xu et al., 2018; Zhang et al., 2018; Zhao, 2018; Chen et al., 2019; Liang, 2019; Shi, 2019; Di and Xia, 2020; Li et al., 2020; Rong et al., 2020; Wang, 2020; Yin et al., 2020; Yu et al., 2020; Zhang, 2020; Zhang and Li, 2020; Du et al., 2021; Huang, 2021; Li et al., 2021; Wang, 2021; Zhang, 2021; Zhao, 2021; Zhu et al., 2021). Detailed information of study selection can be found in Figure 1, and characteristics of the included RCTs are concluded in Table 1. Ingredients of each formula and quality control measures in all included publications are shown in Table 2. Accordingly, the composition of the four representative formulas including “Fuzheng Huayu tablet/capsule,” “Dahuang Zhechong pill,” “Anluo Huaxian pill,” and “Biejia Ruangan tablet” is listed in Supplementary Information S3.
Risk of bias evaluation
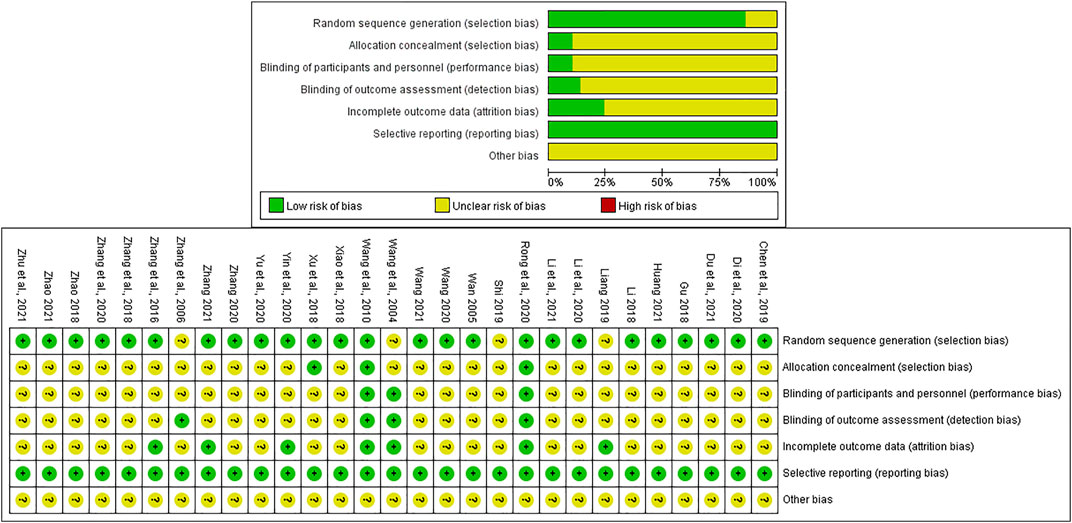
We evaluated the risk of bias of each included publication’s quality with the aid of the Cochrane risk of bias assessment tool (Savovic et al., 2014). 1) Random sequence generation: 23 articles (Wang, 2005; Wang et al., 2010; Zhang et al., 2016; Gu, 2018; Li, 2018; Xiao and Hu, 2018; Zhang et al., 2018; Zhao, 2018; Chen et al., 2019; Di and Xia, 2020; Li et al., 2020; Rong et al., 2020; Wang, 2020; Yin et al., 2020; Yu et al., 2020; Zhang, 2020; Zhang and Li, 2020; Du et al., 2021; Huang, 2021; Li et al., 2021; Zhang, 2021; Zhao, 2021; Zhu et al., 2021) used random number table and two (Xu et al., 2018; Wang, 2021) used random envelope, which were viewed as “low risk”. However, the remaining four (Wang et al., 2004; Zhang et al., 2006; Liang, 2019; Shi, 2019) only reported “randomization,” so they were assessed as “unclear risk.” 2) Allocation concealment: only 3 RCTs (Wang et al., 2010; Xu et al., 2018; Rong et al., 2020) mentioned it while the rest did not. Therefore, the three trials were regarded as “low risk” while the rest as “unclear risk.” 3) Blinding (including participants and personnel, outcome evaluation): only four publications (Wang et al., 2004; Zhang et al., 2006; Wang et al., 2010; Rong et al., 2020) conducted the blinding method, and 25 (Wang, 2005; Zhang et al., 2016; Gu, 2018; Li, 2018; Xiao and Hu, 2018; Xu et al., 2018; Zhang et al., 2018; Zhao, 2018; Chen et al., 2019; Liang, 2019; Shi, 2019; Di and Xia, 2020; Li et al., 2020; Wang, 2020; Yin et al., 2020; Yu et al., 2020; Zhang, 2020; Zhang and Li, 2020; Du et al., 2021; Huang, 2021; Li et al., 2021; Wang, 2021; Zhang, 2021; Zhao, 2021; Zhu et al., 2021) had insufficient information. Therefore, they were successively estimated as “low risk” and “unclear risk.” 4) Incomplete outcome data: only seven trials (Wang et al., 2004; Wang et al., 2010; Zhang et al., 2016; Liang, 2019; Rong et al., 2020; Yin et al., 2020; Zhang, 2021) described the situation of withdrawal or dropout leading to “low risk,” while the rest did not mention it resulting in “unclear risk.” 5) Selective reporting: all of the included studies were regarded as “low risk” due to the acquirement of the complete implementation scheme. 6) Other bias: considering that some certain unknown or unexpected biases could potentially influence the results of this NMA, the 29 articles were evaluated as “unclear risk.” The aforementioned detailed risk of bias evaluation is displayed in Figure 2.
Network evidence and analysis
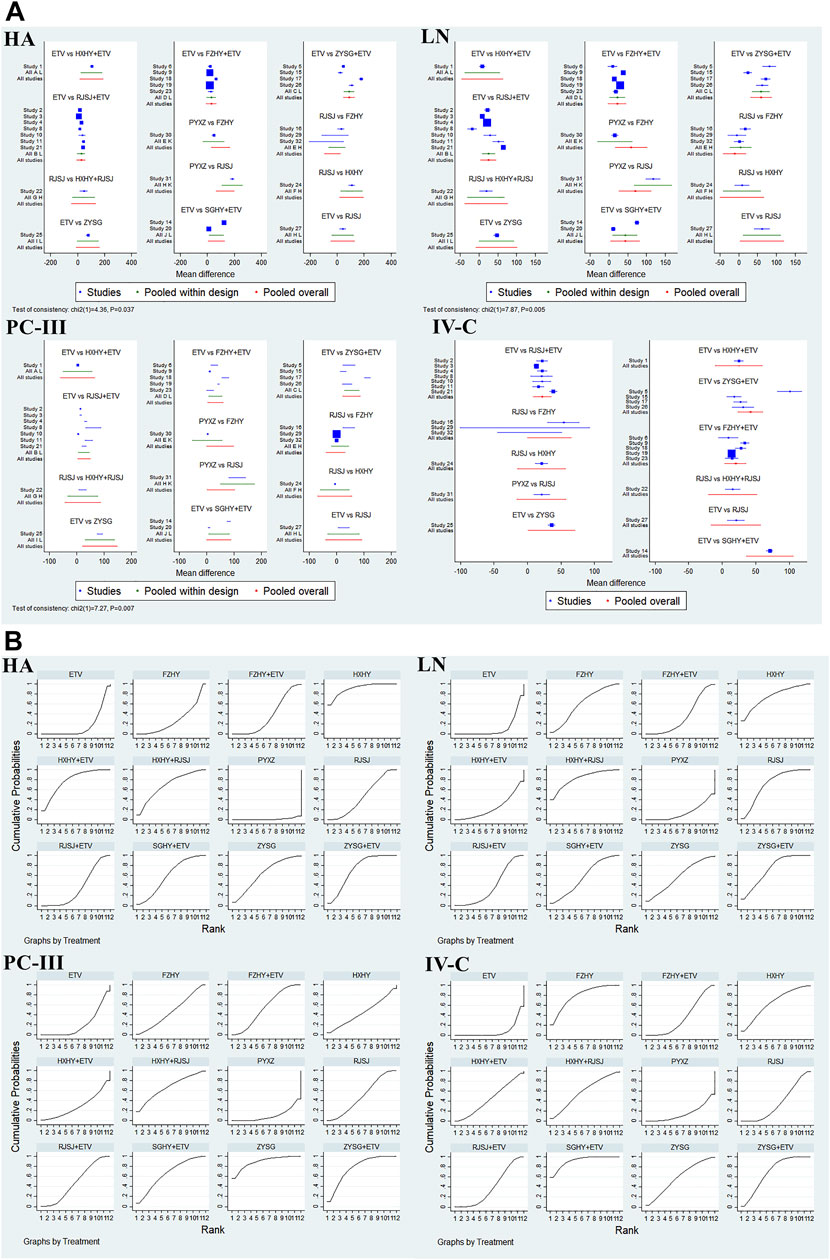
A total of 13 regimens in this NMA were as follows: Fuzheng Huayu therapy (FZHY), Huoxue Huayu therapy (HXHY), Ruanjian Sanjie therapy (RJSJ), Ziyin Shugan therapy (ZYSG), Shugan Huayu therapy (SGHY), Poyu Xiaozheng therapy (PYXZ), FZHY + ETV, HXHY + ETV, RJSJ + ETV, ZYSG + ETV, SGHY + ETV, HXHY + RJSJ, and ETV. Briefly speaking, primary outcomes included clinical efficacy, indexes of hepatic fibrosis (hyaluronic acid (HA), laminin (LN), pro-collagen type III (PC-III), or collagen-IV (IV-C)), and indexes of liver function (alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), or total bilirubin (TBil)). Secondary outcomes included total TCM symptom scores, TCM symptom scores (hypochondriac pain, fatigue, abdominal distension, or poor appetite), imaging indexes (thickness of spleen, length of spleen, width of portal vein, or the liver hardness), hepatitis B virus-deoxyribonucleic acid (HBV-DNA) conversion rate and the quantification of HBV-DNA, and rates of adverse reactions. Network evidence of all the outcomes can be found in Figure 3 (primary outcomes) and Figure 4 (secondary outcomes). As shown in Supplementary Information S4, results of node-splitting between direct and indirect evidence suggested consistency for the aforementioned primary and secondary endpoints (p > 0.05). Accordingly, the PSRF value with 1 or 1.01 proved no divergence and had a stable result. As a consequence, a consistent model was performed.
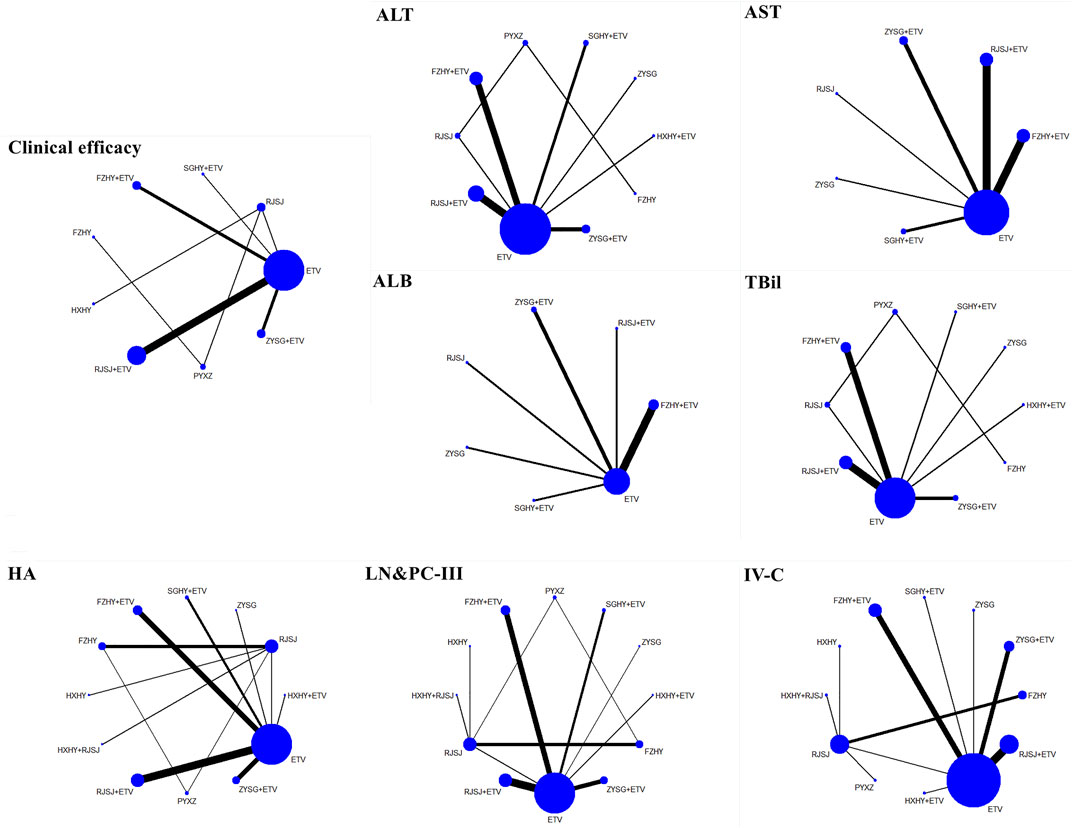
FIGURE 3. Network evidence of primary endpoints (clinical efficacy, indexes of hepatic fibrosis, and liver function) png.
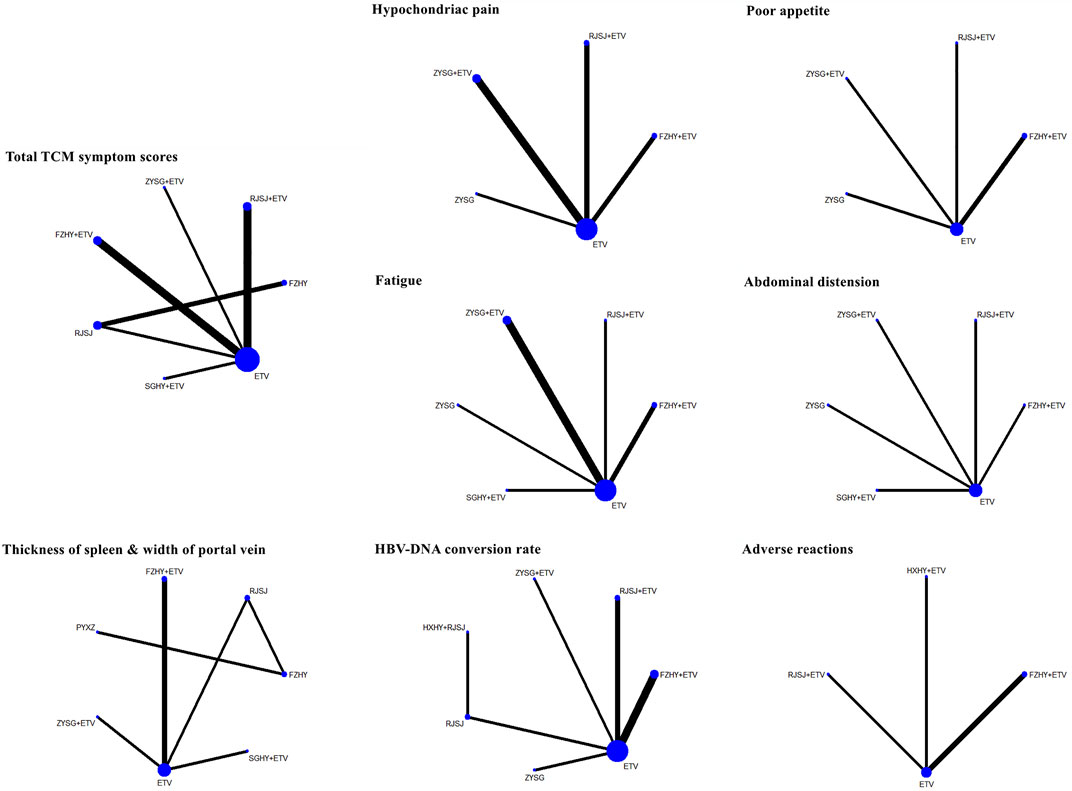
FIGURE 4. Network evidence of secondary endpoints (TCMs symptom scores, imaging indexes, HBV-DNA conversion rate, and adverse reactions) png.
Primary endpoints
Clinical efficacy
Clinical efficacy of liver fibrosis was researched by 18 publications involving in nine interventions (ETV, RJSJ + ETV, FZHY + ETV, ZYSG + ETV, SGHY + ETV, FZHY, RJSJ, PYXZ, and HXHY) (Wang, 2005; Zhang et al., 2006; Zhang et al., 2016; Zhao, 2018; Shi, 2019; Di and Xia, 2020; Li et al., 2020; Rong et al., 2020; Wang, 2020; Yin et al., 2020; Yu et al., 2020; Zhang, 2020; Du et al., 2021; Li et al., 2021; Wang, 2021; Zhang, 2021; Zhao, 2021; Zhu et al., 2021). As shown in Figure 5A, result of heterogeneity analysis indicated good homogeneity (p = 0.18 > 0.05), and result of the SUCRA plot (Figure 5B) suggested HXHY (91.2%) ranked first, followed by FZHY (67.4%) and ZYSG + ETV (67.4%). In addition, strong stability in the ranking of the nine interventions was observed in the sensitivity analysis (Figure 5C), and the asymmetry funnel plot of clinical efficacy was exhibited in Figure 5D.
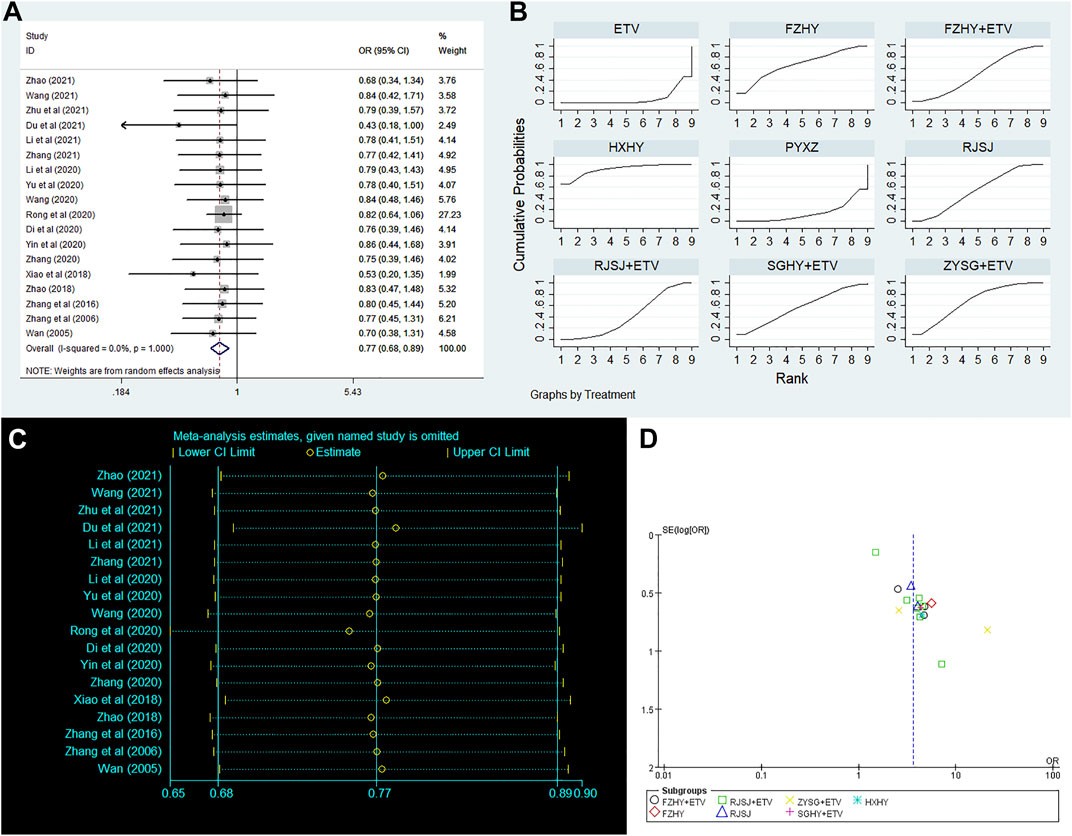
FIGURE 5. Clinical efficacy ((A) forest plot; (B) SUCRA plot; (C) sensitivity analysis; and (D) funnel plot) png.
Serum biomarkers of liver fibrosis (HA, LN, PC-III, and IV-C)
In this NMA, 28 RCTs with different treatments (FZHY + ETV, RJSJ + ETV, SGHY + ETV, HXHY + ETV, ZYSG + ETV, HXHY + RJSJ, FZHY, RJSJ, ZYSG, HXHY, PYXZ, and ETV) reported the HA, LN, and PC-III (Wang et al., 2004; Wang, 2005; Zhang et al., 2006; Wang et al., 2010; Zhang et al., 2016; Gu, 2018; Li, 2018; Xiao and Hu, 2018; Xu et al., 2018; Zhang et al., 2018; Zhao, 2018; Chen et al., 2019; Liang, 2019; Shi, 2019; Di and Xia, 2020; Li et al., 2020; Wang, 2020; Yin et al., 2020; Yu et al., 2020; Zhang, 2020; Zhang and Li, 2020; Du et al., 2021; Huang, 2021; Li et al., 2021; Wang, 2021; Zhang, 2021; Zhao, 2021; Zhu et al., 2021), and 26 with the same interventions described the IV-C (Wang et al., 2004; Wang, 2005; Wang et al., 2010; Zhang et al., 2016; Gu, 2018; Li, 2018; Xiao and Hu, 2018; Xu et al., 2018; Zhang et al., 2018; Zhao, 2018; Chen et al., 2019; Liang, 2019; Di and Xia, 2020; Li et al., 2020; Wang, 2020; Yin et al., 2020; Yu et al., 2020; Zhang, 2020; Zhang and Li, 2020; Du et al., 2021; Huang, 2021; Li et al., 2021; Wang, 2021; Zhang, 2021; Zhao, 2021; Zhu et al., 2021). Test of consistency including the four indexes of liver fibrosis showed good consistency (p < 0.05) (Figure 6A). As for the results of the SUCRA value (Figure 6B), the SUCRA plot of HA exhibited that FZHY (90.8%) ranked first, followed by HXHY + ETV (77.6%) and ZYSG + ETV (72.8%). The plot of LN indicated that HXHY + RJSJ (82.5%) ranked first, followed by HXHY (74.9%) and ZYSG + ETV (72.9%). The plot of PC-III suggested ZYSG (88.9%) ranked first, followed by ZYSG + ETV (77.0%) and HXHY + RJSJ (66.7%). The plot of IV-C showed that SGHY + ETV (92.0%) ranked first, followed by FZHY (79.0%) and ZYSG + ETV (69.6%).
Serum parameters for liver function (alanine aminotransferase, aspartate aminotransferase, albumin, and total bilirubin)
In terms of the four serum parameters of liver function, 21 researches with 10 various therapies (FZHY + ETV, RJSJ + ETV, SGHY + ETV, HXHY + ETV, ZYSG + ETV, FZHY, RJSJ, ZYSG, PYXZ, and ETV) reported the ALT (Wang, 2005; Zhang et al., 2006; Zhang et al., 2016; Li, 2018; Xiao and Hu, 2018; Zhang et al., 2018; Chen et al., 2019; Liang, 2019; Shi, 2019; Di and Xia, 2020; Li et al., 2020; Wang, 2020; Yin et al., 2020; Yu et al., 2020; Zhang, 2020; Du et al., 2021; Huang, 2021; Li et al., 2021; Wang, 2021; Zhang, 2021; Zhu et al., 2021). A total of 17 trials with seven treatments (FZHY + ETV, RJSJ + ETV, ZYSG + ETV, SGHY + ETV, RJSJ, ZYSG, and ETV) reported the AST (Zhang et al., 2016; Li, 2018; Xiao and Hu, 2018; Zhang et al., 2018; Chen et al., 2019; Liang, 2019; Shi, 2019; Di and Xia, 2020; Li et al., 2020; Wang, 2020; Yin et al., 2020; Zhang, 2020; Du et al., 2021; Li et al., 2021; Wang, 2021; Zhang, 2021; Zhu et al., 2021). A total of 11 publications reported the ALB (Zhang et al., 2006; Zhang et al., 2016; Xiao and Hu, 2018; Zhang et al., 2018; Liang, 2019; Di and Xia, 2020; Li et al., 2020; Yin et al., 2020; Yu et al., 2020; Zhang, 2020; Li et al., 2021). However, because one article was different from the other 10 in terms of evaluation criterion (Zhang et al., 2006), the remaining 10 with the same treatments as AST were used for subsequent analysis. In addition, there were 17 RCTs with the same as ALT reported the TBil (Wang, 2005; Zhang et al., 2006; Zhang et al., 2016; Zhang et al., 2018; Chen et al., 2019; Liang, 2019; Di and Xia, 2020; Li et al., 2020; Wang, 2020; Yin et al., 2020; Yu et al., 2020; Zhang, 2020; Huang, 2021; Li et al., 2021; Wang, 2021; Zhang, 2021; Zhu et al., 2021). Test of heterogeneity including the four indexes of liver function showed good homogeneity (p > 0.05) (Figure 7A). As for the results of the SUCRA value (Figure 7B), the optimal value of ALT was SGHY + ETV (87.3%), second was FZHY (84.0%), and third was ZYSG (69.9%). The top three of AST about the value were SGHY + ETV (83.4%), ZYSG + ETV (67.7%), and RJSJ (66.9%) in order. The SUCRA plot of ALB suggested ETV (95.6%) ranked first, followed by FZHY + ETV (81.2%) and RJSJ (46.5%). As for the TBil, the highest SUCRA value was found for ZYSG (95.1%), second was FZHY (92.8%), and third was RJSJ + ETV (60.4%).
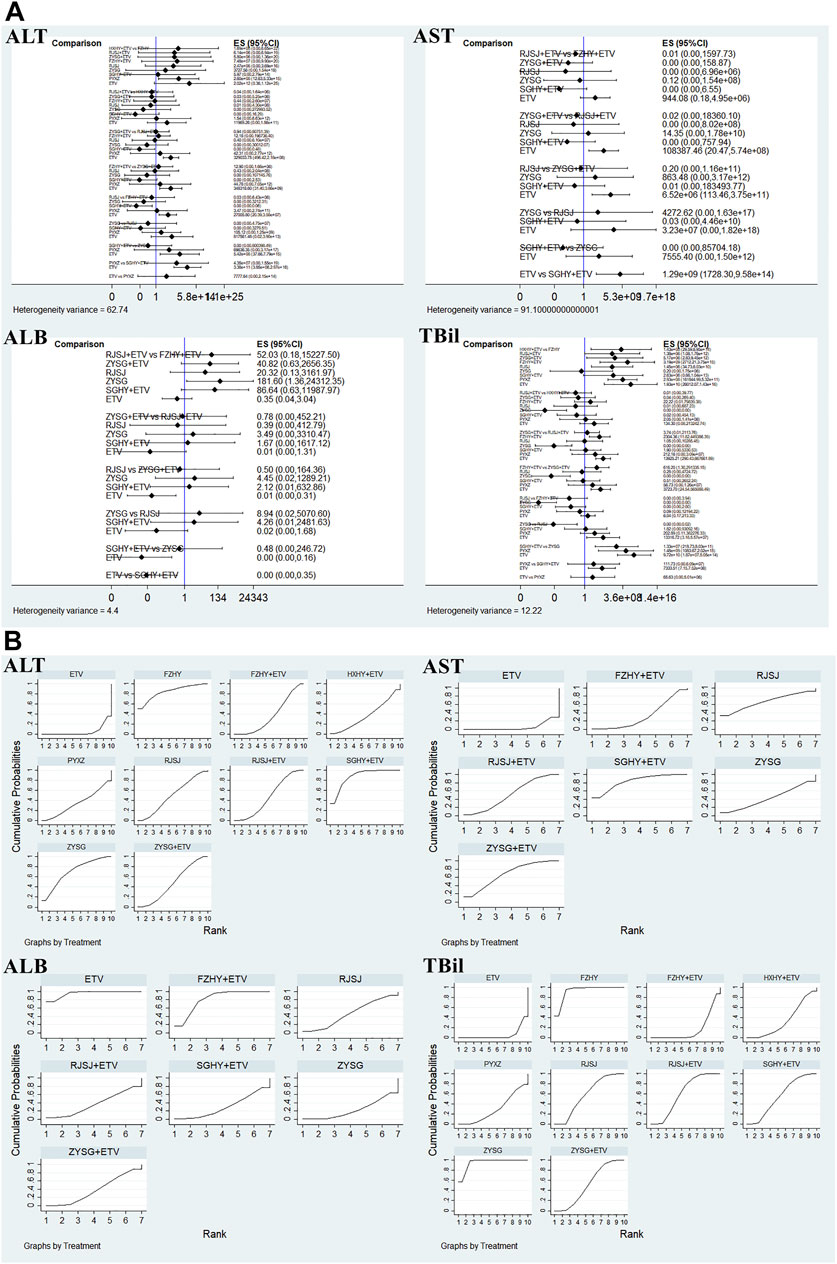
FIGURE 7. Four serum parameters for liver function ((A) test of heterogeneity and (B) SUCRA value) png.
Secondary endpoints
Traditional Chinese medicine symptom scores
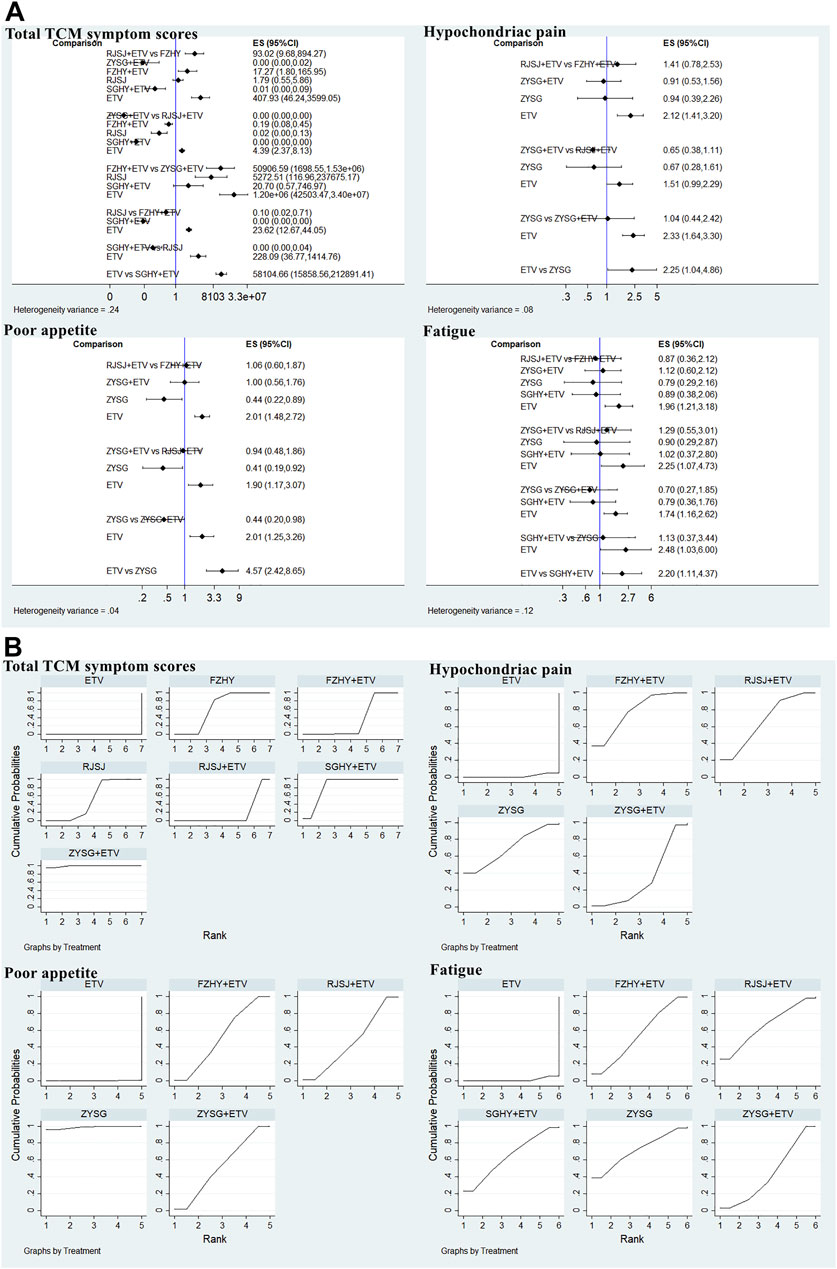
In this study, 11 publications with different therapies (FZHY + ETV, RJSJ + ETV, ZYSG + ETV, SGHY + ETV, FZHY, RJSJ, and ETV) calculated the total TCM symptom scores (Wang et al., 2004; Wang et al., 2010; Zhang et al., 2016; Li, 2018; Xiao and Hu, 2018; Chen et al., 2019; Shi, 2019; Wang, 2020; Du et al., 2021; Li et al., 2021; Zhao, 2021). Considering one article having a discrepancy in evaluation criterion in this scores (Zhang et al., 2006), it was not used for subsequent analysis. As for the hypochondriac pain, eight trials with interventions (FZHY + ETV, RJSJ + ETV, ZYSG + ETV, ZYSG, and ETV) mentioned it (Xu et al., 2018; Zhang et al., 2018; Liang, 2019; Li et al., 2020; Yin et al., 2020; Zhang, 2020; Zhang, 2021; Zhu et al., 2021). Five with the same therapies as hypochondriac pain reported poor appetite (Zhang et al., 2018; Liang, 2019; Li et al., 2020; Yin et al., 2020; Zhang, 2021). Eight RCTs with six treatments (FZHY + ETV, RJSJ + ETV, ZYSG + ETV, SGHY + ETV, ZYSG, and ETV) mentioned fatigue (Xu et al., 2018; Zhang et al., 2018; Liang, 2019; Di and Xia, 2020; Li et al., 2020; Yin et al., 2020; Zhang, 2020; Zhang, 2021). Five articles with the same treatments as fatigue reported abdominal distension (Zhang et al., 2018; Liang, 2019; Di and Xia, 2020; Yin et al., 2020; Zhang, 2021). Test of heterogeneity including total TCM symptom scores, hypochondriac pain, and fatigue showed good homogeneity (p > 0.05) (Figure 8A). In terms of the SUCRA value (Figure 8B), the highest value of total TCM symptom scores was observed for ZYSG + ETV (99.2%), followed by SGHY + ETV (84.2%) and FZHY (63.8%). The top three of hypochondriac pain about the value were FZHY + ETV (78.1%), ZYSG (70.0%), and RJSJ + ETV (67.2%) in order. The optimal value of poor appetite was ZYSG (98.8%), followed by ZYSG + ETV (52.8%) and FZHY + ETV (52.2%). As for the fatigue, ZYSG accounting for 71.3% ranked first, RJSJ + ETV for 65.5% ranked second, and SGHY + ETV for 64.3% ranked third.
Imaging indexes
Considering the included trials which reported the indexes of length of the spleen and liver hardness were inconsistent with each other in terms of evaluation criterion, so they were only qualitatively described. In this study, both thickness of the spleen and width of the portal vein were mentioned in seven included studies with seven therapies (FZHY + ETV, ZYSG + ETV, SGHY + ETV, FZHY, RJSJ, PYXZ, and ETV) (Zhang et al., 2006; Zhang et al., 2016; Xiao and Hu, 2018; Di and Xia, 2020; Li et al., 2020; Yin et al., 2020; Zhang and Li, 2020). Results of heterogeneity variance showed good homogeneity in thickness of the spleen (p > 0.05) except for the width of the portal vein (Figure 9A). As for the rankings of the SUCRA value (Figure 9B), result of thickness of the spleen showed that SGHY + ETV (97.3%) ranked first, FZHY + ETV (76.3%) was second, and FZHY (57.7%) was third. Result of the width of the portal vein exhibited that FZHY (80.4%) had the highest value, SGHY + ETV (77.6%) ranked second, and PYXZ (76.6%) ranked third.
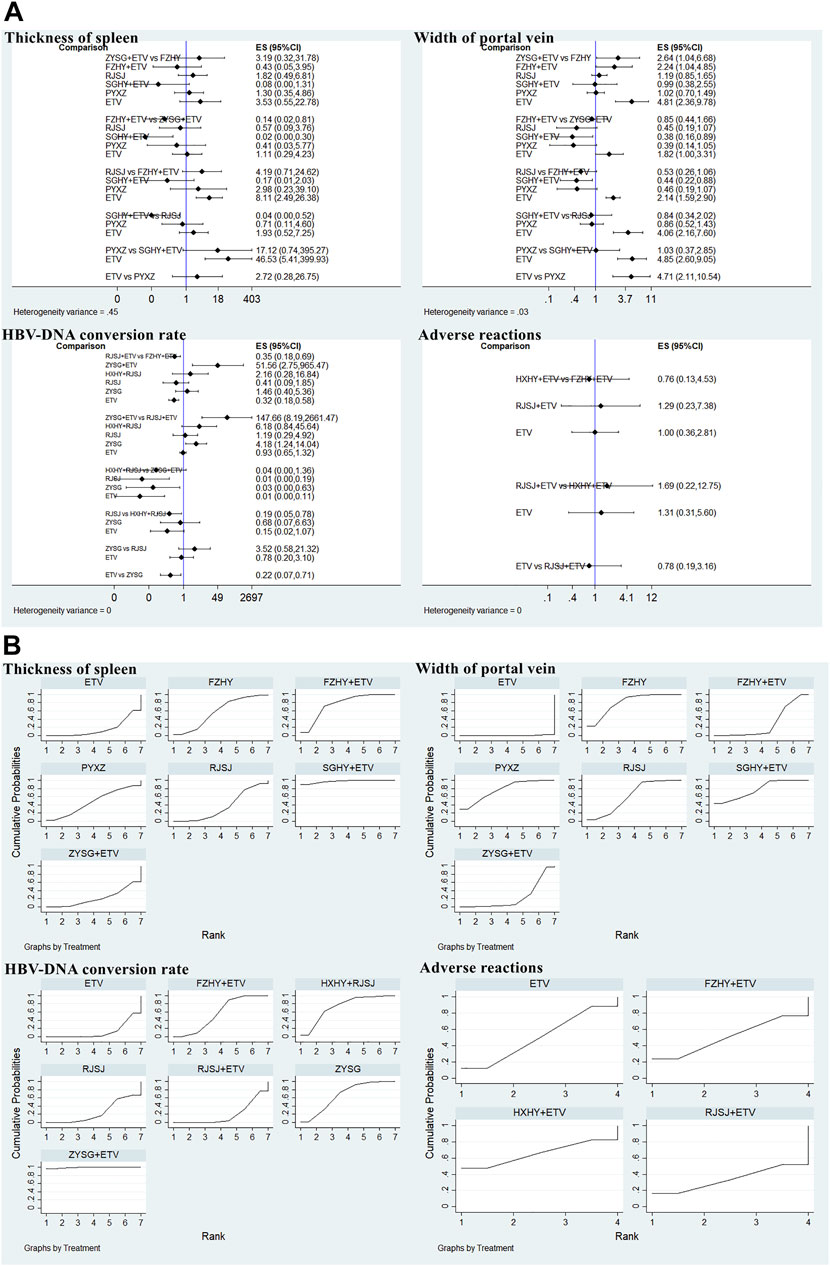
FIGURE 9. Two imaging indexes, HBV-DNA conversion rate, and adverse reactions ((A) test of heterogeneity and (B) SUCRA value) png.
Hepatitis B virus-deoxyribonucleic acid conversion rate
There were nine trials with seven treatments (FZHY + ETV, RJSJ + ETV, ZYSG + ETV, HXHY + ETV, RJSJ, ZYSG, and ETV), which reported this endpoint (Zhang et al., 2016; Gu, 2018; Zhang et al., 2018; Liang, 2019; Li et al., 2020; Rong et al., 2020; Yin et al., 2020; Yu et al., 2020; Li et al., 2021), and there were heterogeneity existed in the test of heterogeneity (p < 0.05) (Figure 9A). In terms of rankings, ZYSG + ETV (99.1%) had the highest SUCRA value, followed by HXHY + RJSJ (73.0%) and ZYSG (66.3%) (Figure 9B).
Adverse reactions
Four RCTS with four interventions (FZHY + ETV, RJSJ + ETV, HXHY + ETV, and ETV) mentioned adverse reactions (Li et al., 2020; Huang, 2021; Li et al., 2021; Zhu et al., 2021). Because heterogeneity variance was zero, heterogeneity was observed in this outcome (Figure 9A). Result of SUCRA rankings indicated that HXHY + ETV (65.4%) ranked first, followed by FZHY + ETV (50.3%) and ETV (50.2%) (Figure 9B).
Other endpoints
In this NMA, Ishak fibrosis scores were reported by two trials with RJSJ + ETV (Rong et al., 2020; Zhu et al., 2021). Liver stiffness in FibroScan before and after treatment was reported by four RCTs with two treatments (FZHY + ETV and RJSJ + ETV) (Li et al., 2020; Rong et al., 2020; Wang, 2020; Yu et al., 2020). Fibrosis index based on the 4 factor (FIB-4) was mentioned by only one document with ZYSG + ETV (Du et al., 2021). Aspartate aminotransferase-to-platelet ratio index (APRI) was mentioned by two articles with two interventions (ZYSG + ETV and RJSJ) (Zhang et al., 2016; Du et al., 2021). Considering few publications and discrepancy in assessment criteria, these indexes of hepatic histopathology were only qualitatively described. But results showed whatever treatment strategies did patients choose, TCM drugs or combined with ETV were more effective than single usage of ETV.
Rating evidence of quality by grading of recommendations assessment, development, and evaluation system
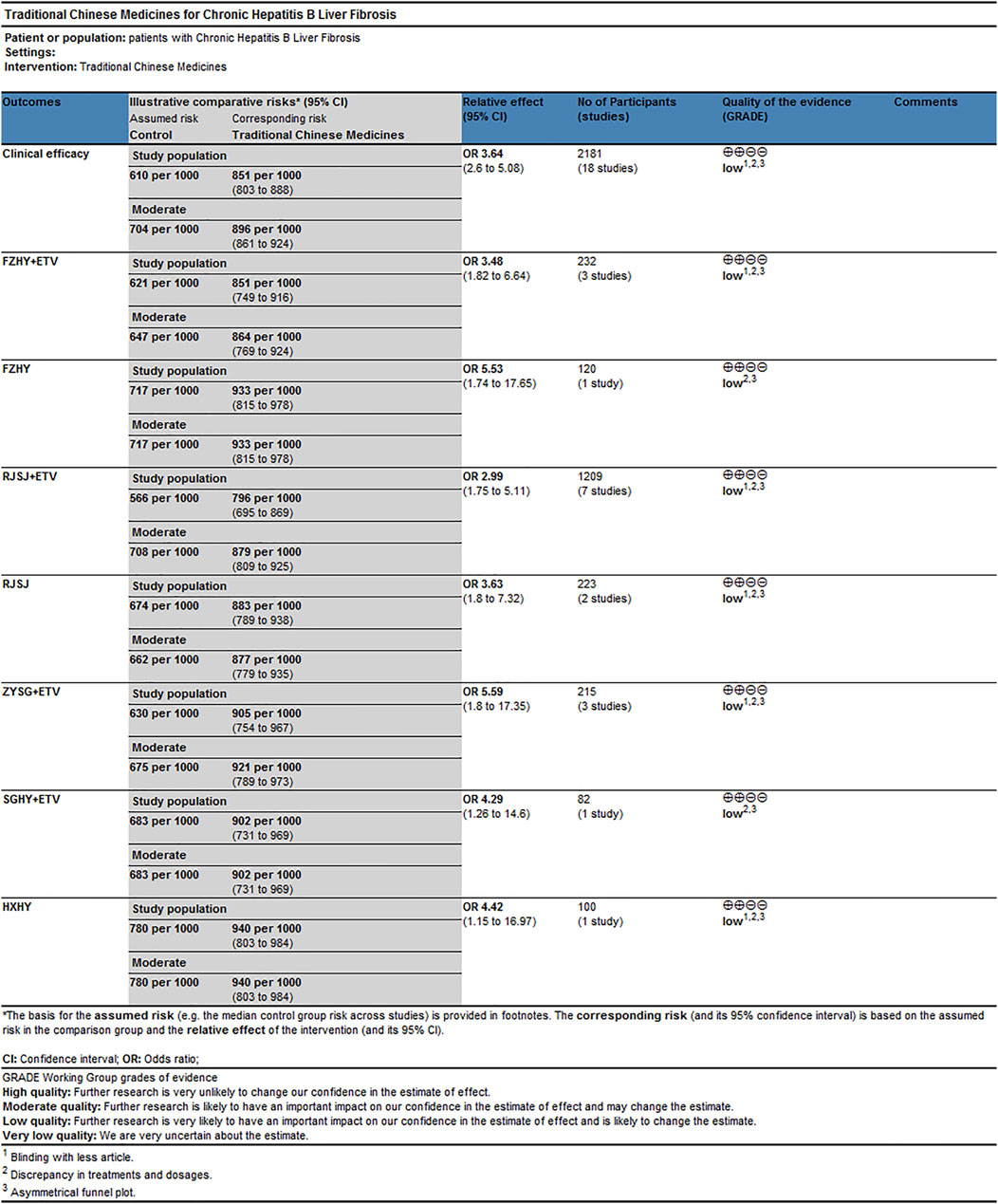
As for the clinical efficacy, the reasons for downgrading or upgrading the quality of evidence were as follows (Figure 10): 1) blinding with fewer articles; 2) discrepancy in treatments and dosages; and 3) asymmetrical funnel plot. Result of quality of estimates showed “Low.” They could be associated with risk of bias and publication bias.
Discussion
NMA allows the development of credible ranking systems of the likely efficacy and safety of various treatment strategies to help clinicians to make decisions even in the absence of head-to-head RCTs (Salanti et al., 2011). The liver biopsy is the gold standard for the diagnosis and prognosis of liver fibrosis. However, because of few RCTs involving in TCM treatments with description of Ishak fibrosis scores, qualitative description was conducted in this study. Nevertheless, our findings indicated that FZHY or combined with ETV might be a better choice to improve the clinical efficacy, recover serum biomarkers of liver fibrosis (including HA and IV-C), and serum parameters for liver function (ALT, ALB, and TBil), lowered the total TCM symptom scores, relieved hypochondriac pain and poor appetite, regained the width of portal vein and thickness of spleen, and reduced the occurrence of adverse reactions. In addition, ZYSG or combined with ETV could also recover the level of LN, PC-III, and AST and improve fatigue and the HBV-DNA conversion rate. As for the safety of TCM drugs, our results showed no serious adverse event during the period of treatment. TCM drugs or combined with ETV also suggested low risk of side effects. Therefore, their safety should be worth affirming.
Hepatic fibrosis is a key pathological process of chronic hepatitis to cirrhosis and presents in most chronic liver diseases. It can also cause structural and functional abnormalities of the liver, which seriously threatens the health of patients. Currently, HBV infection is still the main cause of chronic liver disease in China. HBV-mediated immune response leads to repeated liver cell damage and inflammatory response, promoting the progression of cirrhosis and even hepatocellular carcinoma (Dandri and Locarnini, 2012). Although the nucleos (t) ide analogs inhibited HBV-DNA and also reduced liver fibrosis for CHB patients, not all patients experienced improvement in liver fibrosis (Marcellin et al., 2013; Xu et al., 2015). The reason for this phenomenon may be associated with the following three aspects. First, activated hepatic stellate cell (HSC) secreted a variety of cytokines to maintain the continuity of activation. Second, disrupted liver micro-environment can lead to complex pathological effects between cell and cell and between cell and matrix. Third, the deposition of ECM degrades less. Therefore, except antiviral therapy, antifibrotic treatment is also necessary for CHB hepatic fibrosis (Sun et al., 2018).
Many TCM products against liver fibrosis have been widely applied in practice. On one hand, FZHY prescription, a representative one of FZHY therapy, can effectively improve liver fibrosis. Its mechanism included inhibiting the activation of HSC(Jiang et al., 2003), protecting liver cells from peroxidation and apoptosis (Hu et al., 1997), regulating hepatic ECM metabolism and angiogenesis (Liu et al., 2019), and adjusting differential expression at the molecular biological level of liver fibrosis (Xie et al., 2013). On the other hand, DHZC, a representative one of PYXZ, can reduce serum levels of transforming growth factor β1 and tumor necrosis factor-α by downregulating protein levels of phosphatidylinositol 3-kinase (PI3K) and phosphorylated Akt in the rat model with liver fibrosis, and in vitro experiment further confirmed that it was capable of suppressing HSC proliferation via downregulating PI3K/Akt (Gong et al., 2020). In addition to the earlier, a meta-analysis showed DHZC also improved CHB-related liver fibrosis by reducing serum biomarkers just like HA, LN, PC-III, and IV-C (Wei et al., 2015). Other than that, the combined use of ALHX (a representative one of RJSJ) with ETV could improve liver histology of CHB and boost the improvement rate of liver fibrosis (Jiang et al., 2012; Miao et al., 2019). All the aforementioned herbal formulas have been put into clinical application for many years and have obtained satisfactory treatment results for the patients.
There were several limitations, which need to be noticed in this NMA. First, all the RCTs included were conducted in China, which caused geographically limited distribution and thus the universality of therapeutic schedules should be cautious. Second, although we tried our best to set a classification criteria for different treatment strategies in case of heterogeneity, different documents with different sample sizes had various methods of administration (such as q.d., b.i.d., t.i.d.), which could be one of the sources of heterogeneity. Meanwhile, disproportion on quantity among treatment strategies may fluctuate the strength of evidence. Third, in the absence of the implementation of blinding or allocation concealment, this inadequate information could be the source of moderate methodological quality and “Low” quality of estimates. Finally, although the gender ratio of included patients remained balanced, it is noteworthy that in some RCTs, age differences among them may have contributed to the heterogeneity.
In conclusion, evidence from this NMA showed FZHY or combined with ETV had preferable effects in improving the clinical efficacy, recovering the level of serum biomarkers of live fibrosis (HA, IV-C) and serum parameters for liver function (ALT, ALB, and TBil), relieving the TCM symptoms (hypochondriac pain and poor appetite), regaining the width of portal vein and thickness of the spleen, and lessening side effects. Apart from these, ZYSG or combined with ETV could also be suitable to regain the level of serum biomarkers of live fibrosis (LN and PC-III) and serum parameters for liver function (AST), relieve the TCM symptom (fatigue) and HBV-DNA conversion. However, more relevant high-quality clinical articles should be acquired to strengthen the strength of existing evidence in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
Conceived and designed the study: CL and Z-mZ. Performed the experiment: Y-kD and H-nF. Analyzed the data: Y-kD, H-nF, and Y-hH. Wrote the manuscript: Y-kD. Study supervision: CL and Z-mZ. All authors approved the final manuscript as submitted.
Funding
This study was supported by the National Science and Technology Major Project (2014ZX10005001); Shanghai Key Specialty of Traditional Chinese Clinical Medicine (No. shslczdzk01201); and Siming Youth Fund of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (SGKJ-202104).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JY declared a shared parent affiliation with the authors to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.943063/full#supplementary-material
Abbreviations
TCMs, traditional Chinese medicines; CHB, chronic hepatitis B; NMA, network meta-analysis; SUCRA, surface under the cumulative ranking curve; HBV, hepatitis B virus; ECM, extracellular matrix; ETV, entecavir; FZHY prescription, Fuzheng Huayu prescription; DHZC, Dahuang Zhechong pill; ALHX, Anluo Huaxian pill; PRISRMA, the Preferred Reporting Items for Systematic Review and Meta-Analysis; CNKI, China National Knowledge Infrastructure; RCTs, randomized controlled trials; GRADE, the Grading of Recommendations Assessment, Development, and Evaluation; MCMC, the Markov chain Monte Carlo; SMD, standardized mean difference; RR, relative risk; CIs, confidence intervals; FZHY, Fuzheng Huayu therapy; HXHY, Huoxue Huayu therapy; RJSJ, Ruanjian Sanjie therapy; ZYSG, Ziyin Shugan therapy; SGHY, Shugan Huayu therapy; PYXZ, Poyu Xiaozheng therapy; HA, hyaluronic acid; LN, laminin; PC-III, pro-collagen type III; IV-C, collagen-IV; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALB, albumin; TBil, total bilirubin; HBV-DNA, hepatitis B virus-deoxyribonucleic acid; APRI, aminotransferase-to-platelet ratio index; HSC, hepatic stellate cell; and PI3K, phosphatidylinositol 3-kinase.
References
Ades, A. E., Sculpher, M., Sutton, A., Abrams, K., Cooper, N., Welton, N., et al. (2006). Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics 24 (1), 1–19. doi:10.2165/00019053-200624010-00001
Chang, T. T., Liaw, Y. F., Wu, S. S., Schiff, E., Han, K. H., Lai, C. L., et al. (2010). Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic Hepatitis B. Hepatology 52 (3), 886–893. doi:10.1002/hep.23785
Chen, H. P., He, Z. B., Ying, L. J., and Tan, M. (2019). Clinical study on fuzheng huayu capsules for liver fibrosis in chronic hepatitis B. J. New Chin. Med. 51 (11), 122–124. doi:10.13457/j.cnki.jncm.2019.11.036
Dandri, M., and Locarnini, S. (2012). New insight in the pathobiology of Hepatitis B virus infection. Gut 61 (1), i6–i17. doi:10.1136/gutjnl-2012-302056
Di, S. J., and Xia, Q. (2020). Clinical effects of Heluo Shugan Tablets combined with entecavir on patients with Hepatitis B virus-related active compensated liver cirrhosis. Chin. Tradit. Pat. Med. 42 (6), 1486–1489. doi:10.3969/j.issn.1001-1528.2020.06.017
Du, J. J., Liu, H. W., Yan, H. M., and Yu, S. D. (2021). On the effect of Jiawei Yiguan Decoction in treating hepatic fibrosis of liver-kidney yin deficiency type. Jilin J. Chin. Med. 41 (1), 62–65. doi:10.13463/j.cnki.jlzyy.2021.01.018
Gong, Z., Lin, J., Zheng, J., Wei, L., Liu, L., Peng, Y., et al. (2020). Dahuang Zhechong pill attenuates CCl4-induced rat liver fibrosis via the PI3K-Akt signaling pathway. J. Cell. Biochem. 121 (2), 1431–1440. doi:10.1002/jcb.29378
Gu, B. Y. (2018). Clinical observation on modified gexia zhuyu decoction in the treatment of liver fibrosis in chronic hepatitis B 60 cases. Guangming J. Chin. Med. 33 (7), 968–970. doi:10.3969/j.issn.1003-8914.2018.07.027
Gui, H. L., Zhao, C. Q., Wang, Y., Gu, H. T., Wang, W. J., Cai, W., et al. (2020). Histological outcome of fuzheng huayu plus entecavir combination therapy in chronic hepatitis B patients with significant liver fibrosis. J. Clin. Transl. Hepatol. 8 (3), 277–284. doi:10.14218/JCTH.2020.00004
He, X., Hong, Y., Wang, X., Zhang, X., Long, J., Li, H., et al. (2016). Identification and clinical significance of an elevated level of serum aminoacylase-1 autoantibody in patients with Hepatitis B virus-related liver cirrhosis. Mol. Med. Rep. 14 (5), 4255–4262. doi:10.3892/mmr.2016.5740
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Hu, Y. Y., Liu, C., Liu, P., Gu, H. T., Ji, G., and Wang, X. L. (1997). Anti-fibrosis and anti-peroxidation of lipid effects of Fuzhenghuayu decoction on rat liver induced by CCl4. Chin. J. New Gastroenterol. 5 (8), 485–486.
Huang, R. G. (2021). Effect of modified Gexia Zhuyu Decoction on liver function and liver fibrosis index in patients with chronic Hepatitis B. Mod. Med. Health Res. 5 (4), 120–121.
Jiang, C., Liu, C., and Liu, C. (2003). Inhibiting effect of Fuzhenghuayu capsule on the activation of hepatic stellate cells in rats. Chin. J. Integr. Tra West Med. Dig. 11 (5), 280–283.
Jiang, Y. F., Ma, J., He, B., Li, N. P., Tang, W., and Gong, G. Z. (2012). The therapeutic effect of Anluohuaxian capsule combined with adefovir dipivoxil on patients with chronic Hepatitis B and influence on hepatic histology. Zhonghua Gan Zang Bing Za Zhi 20 (5), 344–347. doi:10.3760/cma.j.issn.1007-3418.2012.05.008
Li, C., Li, G. M., and Gao, P. (2020). Clinical study on peitu huazheng tang assisted for hepatitis B in decompensated cirrhosis. J. New Chin. Med. 52 (8), 80–84. doi:10.13457/j.cnki.jncm.2020.08.024
Li, W. H. (2018). Effects of Compound Bijiaruangan Tablet on liver function and inflammatory factors of chronic Hepatitis B patients with blood stasis and collateral obstruction syndrome. Shaanxi J. Traditional Chin. Med. 39 (3), 325–327. doi:10.3969/j.issn.1000-7369.2018.03.017
Li, Y. C., Hu, W. Y., Xu, X. W., and Chen, H. (2021). Therapeutic effect of Huoxue Ruanjian Fuzheng Recipe combined with antiviral therapy on liver fibrosis in patients with HBeAg-negative CHB of qi deficiency and blood stasis syndrome. Chin. Mod. Dr. 59 (6), 23–27.
Liang, Y. L. (2019). Forty-eight cases of liver fibrosis treated with capsules for reinforcing healthy qi and removing blood stasis in combination with conventional western medicine. Henan Tradit. Chin. Med. 39 (4), 579–582. doi:10.16367/j.issn.1003-5028.2019.04.0144
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gotzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 339, b2700. doi:10.1136/bmj.b2700
Liu, H. L., Lv, J., Zhao, Z. M., Xiong, A. M., Tan, Y., Glenn, J. S., et al. (2019). Fuzhenghuayu Decoction ameliorates hepatic fibrosis by attenuating experimental sinusoidal capillarization and liver angiogenesis. Sci. Rep. 9 (1), 18719. doi:10.1038/s41598-019-54663-4
Luo, X., Huang, Y., Chen, Y., Tu, Z., Hu, J., Tavis, J. E., et al. (2016). Association of hepatitis B virus covalently closed circular DNA and human APOBEC3B in hepatitis B virus-related hepatocellular carcinoma. Plos One 11 (6), e0157708. doi:10.1371/journal.pone.0157708
Marcellin, P., Gane, E., Buti, M., Afdhal, N., Sievert, W., Jacobson, I. M., et al. (2013). Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic Hepatitis B: A 5-year open-label follow-up study. Lancet 381 (9865), 468–475. doi:10.1016/S0140-6736(12)61425-1
Miao, L., Yang, W. N., Dong, X. Q., Zhang, Z. Q., Xie, S. B., Zhang, D. Z., et al. (2019). Combined anluohuaxianwan and entecavir treatment significantly improve the improvement rate of liver fibrosis in patients with chronic Hepatitis B virus infection. Zhonghua gan zang bing za zhi = Zhonghua ganzangbing zazhi = Chin. J. hepatology 27 (7), 521–526. doi:10.3760/cma.j.issn.1007-3418.2019.07.009
Puhan, M. A., Schunemann, H. J., Murad, M. H., Li, T., Brignardello-Petersen, R., Singh, J. A., et al. (2014). A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 349, g5630. doi:10.1136/bmj.g5630
Ren, A., Li, Z., Zhou, X., Zhang, X., Huang, X., Deng, R., et al. (2020). Evaluation of the alpha-fetoprotein model for predicting recurrence and survival in patients with hepatitis B virus (HBV)-Related cirrhosis who received liver transplantation for hepatocellular carcinoma. Front. Surg. 7, 52. doi:10.3389/fsurg.2020.00052
Rong, G., Chen, Y., Yu, Z., Li, Q., Bi, J., Tan, L., et al. (2020). Synergistic effect of biejia-ruangan on fibrosis regression in patients with chronic hepatitis B treated with entecavir: A multicenter, randomized, double-blind, placebo-controlled trial. J. Infect. Dis. 225, 1091–1099. doi:10.1093/infdis/jiaa266
Salanti, G., Ades, A. E., and Ioannidis, J. P. (2011). Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 64 (2), 163–171. doi:10.1016/j.jclinepi.2010.03.016
Savovic, J., Weeks, L., Sterne, J. A., Turner, L., Altman, D. G., Moher, D., et al. (2014). Evaluation of the Cochrane collaboration's tool for assessing the risk of bias in randomized trials: Focus groups, online survey, proposed recommendations and their implementation. Syst. Rev. 3, 37. doi:10.1186/2046-4053-3-37
Schiff, E., Simsek, H., Lee, W. M., Chao, Y. C., Sette, H. J., Janssen, H. L., et al. (2008). Efficacy and safety of entecavir in patients with chronic Hepatitis B and advanced hepatic fibrosis or cirrhosis. Am. J. Gastroenterol. 103 (11), 2776–2783. doi:10.1111/j.1572-0241.2008.02086.x
Shi, X. L. (2019). Clinical study of ShuganTongluo decoction combined with entecavir in the treatment of hepatitis B fibrosis. Chin. J. Misdiagn 14 (6), 277–280.
Suk, K. T., and Kim, D. J. (2015). Staging of liver fibrosis or cirrhosis: The role of hepatic venous pressure gradient measurement. World J. Hepatol. 7 (3), 607–615. doi:10.4254/wjh.v7.i3.607
Sun, X., Peng, Y., Lyu, J., Zhao, Z., Yang, T., Tao, Y., et al. (2018). Characteristics of target population and action mechanisms of the integrative medicine on regression of HBV induced hepatic fibrosis relating to liver immune microenvironment. World Chin. Med. 13 (11), 2906–2910. doi:10.3969/j.issn.1673-7202.2018.11.058
Sutton, A., Ades, A. E., Cooper, N., and Abrams, K. (2008). Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoeconomics 26 (9), 753–767. doi:10.2165/00019053-200826090-00006
Wan, C. J. (2005). Treatment of 87 cases of mild and moderate chronic Hepatitis B liver fibrosis with Ruangan Kangxianyin. Traditional Chin. Med. J. 4 (5), 42–44. doi:10.14046/j.cnki.zyytb.2002.2005.05.015
Wang, J., and Peng, F. C. (2018). Meta-analysis on indirect comparison of fuzheng huayu jiaonang and anluo huaxian wan in treatment of chronic Hepatitis B with liver fibrosis. Zhongguo Zhong Yao Za Zhi 43 (7), 1492–1500. doi:10.19540/j.cnki.cjcmm.2018.0057
Wang, L. C., Lei, B. J., Liu, L., Liu, C., Zhang, R. M., and Li, T. Q. (2004). Jiuweirougan granule in the treatment of chronic hepatitis with hepatic fibrosis: A double-blind randomized controlled trial. Chin. J. Evidence-Based Med. 4 (11), 778–782.
Wang, L. C., Lei, B. J., Liu, L., Liu, C., Zhang, R. M., Li, T. Q., et al. (2010). Jiuweirougan granule in the treatment of chronic hepatitis B with hepatic fibrosis: A double blind randomized controlled study. West China Med. J. 25 (10), 1801–1804.
Wang, S. L. (2021). Effect of Compound Bijiaruangan Tablet combined with entecavir in the treatment of Hepatitis B cirrhosis and its effect on liver function and liver fibrosis indexes. Chin J Clin. Ration. Drug Use 14 (2), 93–94. doi:10.15887/j.cnki.13-1389/r.2021.04.038
Wang, T., Jin, W., Huang, Q., Li, H., Zhu, Y., Liu, H., et al. (2020). Clinical efficacy and safety of eight traditional Chinese medicine combined with entecavir in the treatment of chronic hepatitis B liver fibrosis in Adults: A network meta-analysis. Evid. Based. Complement. Altern. Med. 2020, 7603410. doi:10.1155/2020/7603410
Wang, T., Zhou, X., Liu, H., Wang, J., Zhang, P., Zhu, Y., et al. (2018). Fuzheng huayu capsule as an adjuvant treatment for HBV-related cirrhosis: A systematic review and meta-analysis. Phytother. Res. 32 (5), 757–768. doi:10.1002/ptr.6009
Wang, Y. J. (2020). Case-control study of anluo huaxian pill in treating liver fibrosis. Chin. J. Ration. Drug Use 6 (17), 49–55.
Wei, F., Lang, Y., Gong, D., and Fan, Y. (2015). Effect of Dahuang zhechong formula on liver fibrosis in patients with chronic Hepatitis B: A meta-analysis. Complement. Ther. Med. 23 (1), 129–138. doi:10.1016/j.ctim.2014.12.011
Wu, M., Zhou, Y., Qin, S. L., Lin, L. J., Ping, J., Tao, Z., et al. (2020). Fuzheng huayu capsule attenuates hepatic fibrosis by inhibiting activation of hepatic stellate cells. Evid. Based. Complement. Altern. Med. 2020, 3468791. doi:10.1155/2020/3468791
Xiao, L. H., and Hu, X. Y. (2018). Clinical observation of Huayu yanggan prescription combined with entecavir in the treatment of liver fibrosis after Hepatitis B. Chin. J. Integr. Traditional West. Med. Liver Dis. 28 (3), 137–139. doi:10.3969/j.issn.1005-0264.2018.03.003
Xie, H., Tao, Y., Lv, J., Liu, P., and Liu, C. (2013). Proteomic analysis of the effect of fuzheng huayu recipe on fibrotic liver in rats. Evid. Based. Complement. Altern. Med. 2013, 972863. doi:10.1155/2013/972863
Xu, B., Lin, L., Xu, G., Zhuang, Y., Guo, Q., Liu, Y., et al. (2015). Long-term lamivudine treatment achieves regression of advanced liver fibrosis/cirrhosis in patients with chronic Hepatitis B. J. Gastroenterol. Hepatol. 30 (2), 372–378. doi:10.1111/jgh.12718
Xu, X. T., Liu, L. N., Qiao, F., Shao, M., and Sun, Z. G. (2018). Effect of Shugan Jianpi Decoction combined with Western medicine on liver fibrosis and cellular immune function in patients with chronic Hepatitis B. J. Chin. Med. Mater. 41 (9), 2227–2229. doi:10.13863/j.issn.1001-4454.2018.09.046
Yin, Y. T., Wang, J. C., Liao, J., Zhang, B. B., Xu, Y. J., Wen, B., et al. (2020). Clinical study of shugan jianpi formula combined with entecavir tablets in the treatment of hepatitis B cirrhosis with ganyupixu syndrome. Pharmacol. Clin. Chin. Materia Medica 36 (4), 187–191. doi:10.13412/j.cnki.zyyl.2020.04.025
Yu, C. W., Meng, C. X., Wang, Y., and Pan, L. Y. (2020). Clinical efficacy of anluo huaxian pills combined with western medicine in the treatment of hepatitis B cirrhosis. China J. Pharm. Econ. 15 (12), 57–61. doi:10.12010/j.issn.1673-5846.2020.12.013
Zhang, D., Fan, Z. D., Zhu, S. M., Chen, D. G., and Zhou, Q. (2018). Clinical effect of shugan jianpi granule on chronic hepatitis B with liver stagnation and spleen deficiency and its influence on quality of life of patients. Chin. Archiv. Tradi. Chin. Med. 36 (7), 1696–1699. doi:10.13193/j.issn.1673-7717.2018.07.043
Zhang, L., Zeng, J., Li, J., and Liu, X. L. (2016). Clinical observation of Ruangan Huaxian Decoction on treatment of 50 cases of chronic Hepatitis B with hepatic fibrosis. China J. Traditional Chin. Med. Pharm. 31 (12), 5382–5385.
Zhang, P. P., and Li, R. D. (2020). Clinical study of Xiongqi granule in treating liver fibrosis of chronic Hepatitis B. Clin. J. Chin. Med. 12 (12), 42–43. doi:10.3969/j.issn.1674-7860.2020.12.018
Zhang, W. N. (2020). Blood-nourishing and liver-softening decoction combined with entecavir in the treatment of liver fibrosis of chronic hepatitis B. Henan Tradit. Chin. Med. 40 (1), 100–103. doi:10.16367/j.issn.1003-5028.2020.01.0025
Zhang, X. B., Dang, Z. Q., and Yu, J. Z. (2006). 60 cases of Hepatitis B liver fibrosis treated by Shenjia Ronggan pill. Traditional Chin. Med. Res. 19 (10), 26–28.
Zhang, Z. W. (2021). Clinical observation of anluo Huaxian pill combined with entecavir in the treatment of chronic Hepatitis B liver fibrosis. J. Pract. Tradi. Chin. Med. 37 (7), 1124–1125.
Zhao, X. S. (2018). Clinical observation on treating liver fibrosis of chronic Hepatitis B by modified Gexia Zhuyu Decoction. J. North Pharm. 15 (1), 115–116.
Zhao, Y. (2021). Effects of Erjia Ruanjian Capsule combined with Entecavir dispersive tablets on liver fibrosis indexes and inflammatory factors in patients with compensatory stage of Hepatitis B cirrhosis. Chin. Naturop. 29 (12), 90–93. doi:10.19621/j.cnki.11-3555/r.2021.1232
Keywords: traditional Chinese medicines, chronic hepatitis B liver fibrosis, randomized controlled trials, clinical medication, surface under the cumulative ranking curve
Citation: Dai Y-k, Fan H-n, Hu Y-h, Zhao Z-m and Liu C (2022) Comparison on different traditional Chinese medicine therapies for chronic hepatitis B liver fibrosis. Front. Pharmacol. 13:943063. doi: 10.3389/fphar.2022.943063
Received: 13 May 2022; Accepted: 13 July 2022;
Published: 10 August 2022.
Edited by:
Adolfo Andrade-Cetto, National Autonomous University of Mexico, MexicoReviewed by:
Jianye Yuan, Shanghai University of Traditional Chinese Medicine, ChinaBangxing Han, West Anhui University, China
Manuela Neuman, University of Toronto, Canada
Copyright © 2022 Dai, Fan, Hu, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenghai Liu, Y2hlbmdoYWkubGl1QG91dGxvb2suY29t; Zhi-min Zhao, emhpbWluemhhb0BzaHV0Y20uZWR1LmNu
 Yun-kai Dai
Yun-kai Dai Hai-na Fan1
Hai-na Fan1 Yong-hong Hu
Yong-hong Hu Zhi-min Zhao
Zhi-min Zhao Chenghai Liu
Chenghai Liu