
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 29 August 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.942563
 Li Jiang1†
Li Jiang1† Shidong Wang1*
Shidong Wang1* Jinxi Zhao1*
Jinxi Zhao1* Chieh Chien2†
Chieh Chien2† Yaofu Zhang1
Yaofu Zhang1 Guanxun Su1
Guanxun Su1 Xiaoyu Chen1
Xiaoyu Chen1 Dechao Song1
Dechao Song1 Yu Chen1
Yu Chen1 Weijun Huang3
Weijun Huang3 Yonghua Xiao1
Yonghua Xiao1 Yandong Cao1
Yandong Cao1 Zixian Hu1
Zixian Hu1Objective: To compare the clinical efficacy and safety of SIX Traditional Chinese Patent Medicines (TCPM) recommended by guidelines in improving lipids for patients with prediabetes by network meta-analysis.
Methods: Randomized controlled trials of 6 TCPM in the treatment of prediabetes were searched systematically in various databases. After extracting effective data, the risk of bias was assessed using Review Manager 5.3 and Cochrane Collaboration Systems Evaluator’s Manual. Network meta-analysis was performed using STATA 15.0 based on the frequency statistical model. The effect size and credibility of the evidence for the intervention were summarized based on a minimal contextualized framework.
Results: A total of 27 studies involving 2,227 patients were included. Compared with lifestyle modification (LM), Shenqi + LM [SMD −0.49 (95% CI: −0.85, −0.12)] and Jinqi + LM [SMD −0.44 (95% CI: −0.81, −0.06)] showed statistically significant effect in lowering TG, Shenqi + LM [SMD −0.51 (95%CI: −0.86, −0.17)] and Jinqi + LM [SMD −0.44 (95%CI: −0.80, −0.08)] in lowering TC, Jinlida + LM [SMD −0.31 (95%CI: −0.59, −0.04)] in lowering LDL-C, Shenqi + LM [SMD 0.29 (95%CI: 0.06, 0.51)] and Jinqi + LM [SMD 0.16 (95%CI: 0.01, 0.31)] in increasing HDL-C.
Conclusion: For patients with prediabetes, Traditional Chinese patent medicine Jinqi and Shenqi combined with lifestyle modification were associated with a significant reduction in TG and TC, while Shenqi + LM was among the most effective. Jinlida + LM was among the least effective.
Systematic Review Registration: https://clinicaltrials.gov/, identifier PROSPERO(CRD42021279332).
Prediabetes refers to an intermediate stage of dysglycemia along the continuum from normo-glycemia to diabetes. It is characterized by mild impaired fasting blood glucose (IFG) and/or impaired glucose tolerance (IGT) and clinically assessed by fasting blood glucose (FBG), glycosylated hemoglobin level (HbA1C), and 2-h post-load blood glucose (2hBG) (Echouffo-Tcheugui and Selvin, 2021). The increasing prevalence of prediabetes globally is a major public health concern and exacerbates the growing epidemic of diabetes and its complications. According to the “Prevalence and Treatment of Diabetes in China, 2013–2018” issued by Chinese official institutions, the estimated prevalence of prediabetes was 35.7% in 2013 and 38.1% in 2018, bringing a huge potential burden to the health system (Xu et al., 2013). Dyslipidemia, the independent risk factor for coronary atherosclerosis, was shown to be a high-risk factor for cardiovascular disease associated with diabetes. Clinical studies have recently revealed that Traditional Chinese Patent Medicines (TCPM) played a positive role in improving the lipid profile and relieving symptoms of prediabetes. So, several TCPMs have been clearly recommended as the important intervention for prediabetes in Chinese domestic guidelines (Endocrinology and Metabolic Diseases Committee of the Chinese Physicians Association, Integrated Chinese and Western Medicine Branch, 2021; Chinese Diabetes Society, 2021), which are namely Shen qi jiang tang capsule/granule (Shenqi), Tian mai xiao ke tablet (Tianmai), Tian qi jiang tang capsule (Tianqi), Jin qi jiang tang tablet (Jinqi), Jin li da granule (Jinlida), and Tang mai kang granule (Tangmaikang) (Jiang et al., 2022).
In the traditional Chinese medicine (TCM) context, diabetes and pre-diabetes are both diseases caused by Fire toxin, which injures Qi and damages the normal function of the body. Qi is the essential substance to maintain fluid metabolism. Qi deficiency would cause clinical manifestations like thirst and fatigue in these patients (Pang and Ni, 2019). The included TCPM all followed the basic understanding of traditional context and contained mainly herbs that have the effect of clearing Fire and benefiting Qi, thus showing some positive significance in clinical research studies and getting recommended in experts’ advice and official guidelines (Xia et al., 2020). Until now, although there were some meta-analyses focusing on the clinical effectiveness and safety of TCPM for treating prediabetes (Pang et al., 2017; Pang et al., 2018; Jiang et al., 2022), the general shortcomings still existed: 1) the main outcome was blood glucose indicators, such as glycosylated hemoglobin, FBG, and PBG, with insufficient emphasis on lipids. 2) The number of literatures covered was limited, the sample size was insufficient, and the heterogeneity among different comparisons was large; 3) in recent add-on research studies, there was still a lack of comparative efficacy between the various types of TCPM.
Network meta-analysis (NMA) is a method which has been applied widely to assess all the available medications and rank different therapies through the transitivity of the same control within a consistent framework, even though when there was no direct comparison in head-to-head trials. Therefore, this study used the NMA to compare the effectiveness and safety of the aforementioned 6 TCPM in improving the lipid profile, in order to provide an evidence-based reference for clinical use in the supplementary therapy for prediabetes.
This article was reported following the guidelines of the Cochrane Multiple Interventions Methods Group and PRISMA Extension Statement (Hutton et al., 2015) and the checklist was shown in Supplementary File S1. The protocol for the research was submitted to the International Prospective Register of Systematic Reviews (PROSPERO) (CRD 42020180045). The web-based registration scheme could be found in Supplementary File S2. The introduction and discussion section of the article was elaborated with reference to the General requirements for developing, conducting, and reporting pharmacological research on medicinal plants and natural products (phytopharmacology) (Heinrich et al., 2020). The summary table describing the composition of the preparations and how these were reported in the original studies was structured following the principles described in the Four Pillars of Ethnopharmacology.
The research studies were considered if they matched the following criteria: 1)randomized controlled trials (RCTs) with available data on blood lipids, including triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C). LDL-C was chosen as the primary outcome for its independent predicting effect on the risk of ASCVD in individuals or populations. The others were secondary outcomes; 2) patients with prediabetes (≥18 years old) according to the various definitions indicated by authoritative academic institutions, including the ADA (American Diabetes Association, 2018), the WHO (WHO, 1999), and the Chinese Diabetes Society (CDS) (Chinese Diabetes Society, 2021); 3) treatments of interest in the experimental group contained 6 kinds of TCPM (Shenqi, Tianqi, Jinlida, Tianmai, Jinqi, and Tangmaikang). The TCPM is defined as the Chinese medical products made from herbal medicine and processed into certain dosage forms. The formula is formed according to prescribed prescriptions under the guidance of the theory of TCM. The manufacturing is put in line with specified pharmaceutical technology based on the PRC Pharmacopoeia’s monograph (Pang et al., 2017). Interventions in the control group were not limited (LM, placebo, oral hypoglycemic drugs, or their combination); 4) treatment duration of the study which includes HbA1c should be more than 12 weeks.
The clinical studies were excluded with the following features: 1) Interventions in the experimental group contained oral hypoglycemic drugs. 2) Interventions in the experimental group included other TCM such as no-decoction pellets and water extracts. 3) The ingredients of applied TCPM were not completely stated or inconsistent with the description in Table 2. 4) Lipid-lowering drugs such as statins, fibrates, and monacolins were used in either group; 5) Common clinical practices in TCM such as acupuncture, cupping, Gua Sha and Tui Na were combined in either group; 6) Data of outcome indicators were still unavailable after contacting authors.
A comprehensive search strategy whose search string followed the PICOS method was developed to find peer-reviewed published studies. The English database like PubMed, EMBASE, Cochrane Library, Web of Science, and Clinical trials, and the Chinese database like Sinomed, CNKI, and WanFang. and VIP was used for article retrieval up to July 2022. Two prior systematic reviews (Pang et al., 2017; Pang et al., 2018) which were searched ended in September 2017 have been updated and referred to. Additionally, we also consulted with experts to identify candidate studies. The full electronic search strategy for Pubmed and CNKI was presented in Supplementary File S3.
Two independent reviewers (Li Jiang and Chieh Chien) screened the literature and extract data separately with reference to the Cochrane Handbook (Higgins et al., 2019). Information was checked and adjudicated independently by an additional investigator (Yaofu Zhang) until agreement was achieved. A pre-tested standard data extraction form will be designed specifically for this review. It contained the following base entries: the first author of the article, year of publication, sample size, age and gender distribution of subjects, diagnostic criteria, mode of intervention (including dose, frequency, usage), duration of intervention, lipid-related outcome indicators, the plan of randomization and concealment and specific reports of adverse reactions, etc. Δ indicates the change in indicators before and after the intervention.
The methodological quality of eligible RCTs was examined in Software Review Manager 5.3. The assessment tool standard covers 7 aspects: random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome; selective reporting; other biases. For each study, the aforementioned seven items were evaluated as “low bias”, “high bias”, and “unclear”. The differences were reviewed by the third member, and finally determined and drew the bias risk map after discussion. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework was used to assess the quality of evidence contributing to each estimated network, which namely have four grades, high, Moderate, low, and very low (Liu, 2022). The seven assessment domain of each contrast include the risk of bias, inconsistency, the indirectness, imprecision, publication bias, intransitivity, and incoherence in the GRADE framework for NMA (Puhan et al., 2014; Brignardello-Petersen et al., 2021).
We used frequentist network meta-analysis in software STATA 15.0 (Bhatnagar et al., 2014). For direct comparisons, the pooled analysis of the overall effect size for continuous variable outcome was presented as standardized mean difference (SMD) and an associated 95% confidence interval (CI) using the DerSimonian and Laird random-effects methods (DerSimonian and Laird, 1986). The assumption of consistency in the whole analytical network was conducted in a design-by-treatment approach. The loop-specific approach was used to evaluate the presence of inconsistency in network meta-analysis models locally. If no loop is formed, the node-splitting analysis can be applied (Caldwell et al., 2005). Heterogeneity was assessed by the Chi-squared test and the I2 index (Higgins et al., 2019), which was considered significant if I2 > 75% (Page et al., 2021). Sensitivity analyses, meta-regression, subgroup analyses, and a random-effects model were utilized if the heterogeneity was significant. Otherwise, the fixed-effects model was selected. Meta-regression was performed on the treatment duration (≤3, 3–6, ≥6 months), the type of TCPM, the control group (LM, LM + placebo, LM + oral hypoglycemic drugs), the diagnostic criteria (WHO, ADA, and CDS), the risk of bias (high, not high, unclear) and the baseline of LDL-C (ideal level: < 2.6, appropriate level: 2.6–3.4, pathologically elevated: 3.4 mmol/L) (Joint committee issued Chinese guideline for the management of dyslipidemia in adults, 2016). The forest plot and the league table was used to display the pairwise comparison in NMA. The surface under the cumulative ranking curve (SUCRA) would be used to estimate ranking probabilities for all treatments in order to create a treatment hierarchy (Salanti et al., 2011). The minimally contextualized framework was developed according to the result of the SUCRA and GRADE evaluation (Brignardello-Petersen et al., 2020). In addition, the correction funnel plot was drawn to assist in determining whether there is publication bias or a small sample effect among included literature.
A final total of 27 articles were included in the NMA. The process was shown in Figure 1. All trials were conducted in China and published between 2002 and 2018, corresponding to 2,227 adults (1,139 in the treatment group and 1,088 in the control group). Both groups were based on a lifestyle modification (LM), with the addition of TCPM in the treatment group and the addition of oral hypoglycemic drugs or placebo or blank to the control group. Among them, there were five articles related to Shenqi, two related to Tianmai, three related to Tianqi, five related to Jinqi, six related to Jinlida, and six related to Tangmaikang. LDL-C has been reported in 18 type of research, HDL-C in 17 researches, and TG, and TC in 27 researches. The basic characteristics of the included studies was shown in Table 1. The source, ingredients, quality control, and chemical analysis of the included TCPM were shown in Table 2. A total of 40 preclinical studies investigated the lipid or glucose-lowering effects of 6 TCPM in prediabetes or T2DM. The beneficial effects and potential mechanisms are summarized in Table 3.
The 27 included studies all used a randomized method, 17 using random number tables generated from random sequences, and two using systematic randomization (Mao, 2003; Wang et al., 2011a), and were therefore rated as “low risk”; one study was randomized according to the order of attendance, which was pseudo-randomized and rated as “high risk”. The remaining seven studies did not mention a specific randomized method and were therefore rated as “unclear risk”. Three studies were double-blinded with a placebo (Wei, 2009; Wang et al., 2011a; Chen, 2011) and were all rated as “low risk.” The remaining 23 studies did not report allocation concealment or blinded set-up and were therefore rated as “unclear risk.” Two studies (Yan et al., 2011; Zhang et al., 2011) reported missing visits and had incomplete outcome data, and were therefore rated as “high risk.” The remaining 25 studies had no missing data and were rated as “low risk.” None of the study protocols were first registered or could not be obtained, therefore the existence of selective reporting could not be determined and was reported as “unclear risk.” There exited no other bias in included literature. The bias assessment was shown in Figure 2.
The GRADE judgment was incorporated and the analyses showed the quality was low or very low for most of the comparisons. As for the primary outcome, LDL-C, no direct comparison showed significant results statistically in the total of eight comparisons, and all of them were evaluated as very low except Shenqi + LM vs. LM and Jinqi + LM vs. LM which were evaluated as low. For TG and TC, two direct comparisons showed significant results statistically (Shenqi + LM vs. LM and Jinqi + LM vs. LM) in a total of nine comparisons, and they were evaluated as moderate and low quality. For HDL-C, two direct comparisons showed significant results statistically (Tianmai + LM vs. placebo + LM and Jinqi + LM vs. LM) in the total of eight comparisons, and they were evaluated as very low and low. The detailed GRADE assessment was presented in Supplementary File S4.
Network evidence plots were made based on direct comparative relationships, as shown in Figure 3, with the node representing a certain intervention. The size of the node represented the total number of people in each study, and the thickness of the line represented the standard error of SMD or logarithmic OR. The relationship between the interventions did not form a closed loop and therefore no loop inconsistency testing was required. 27 studies (Zhou, 2002; Mao, 2003; Zhou and Chen, 2003; Chen, 2005; Shen et al., 2006; Chen and Wu, 2007; Gu, 2007; Lin and Hu, 2007; Wei, 2009; Cao and Jin, 2010; Tan, 2010; Wang et al., 2011a; Wang et al., 2011b; Chen, 2011; Salanti et al., 2011; Yan et al., 2011; Zhang et al., 2011; Tao et al., 2012; Tian and Li, 2012; Dong and Wang, 2015; Liu, 2015; Shi et al., 2016; Yin, 2016; Cai et al., 2017; Wang and Jiang, 2018; Zhao, 2018; Brignardello-Petersen et al., 2020) reported TG and TC with a total arm size of 54 and 2,227 patients. 18 studies (25, 26, 28–33, 35, 39–43, 47–49] reported LDL-C with a total arm size of 36 and 1,633 patients. 17 studies (Zhou, 2002; Mao, 2003; Zhou and Chen, 2003; Chen, 2005; Shen et al., 2006; Chen and Wu, 2007; Lin and Hu, 2007; Wei, 2009; Tan, 2010; Wang et al., 2011b; Chen, 2011; Yan et al., 2011; Zhang et al., 2011; Dong and Wang, 2015; Liu, 2015; Wang and Jiang, 2018; Zhao, 2018) reported HDL-C with a total arm size of 34 and 1,508 patients.
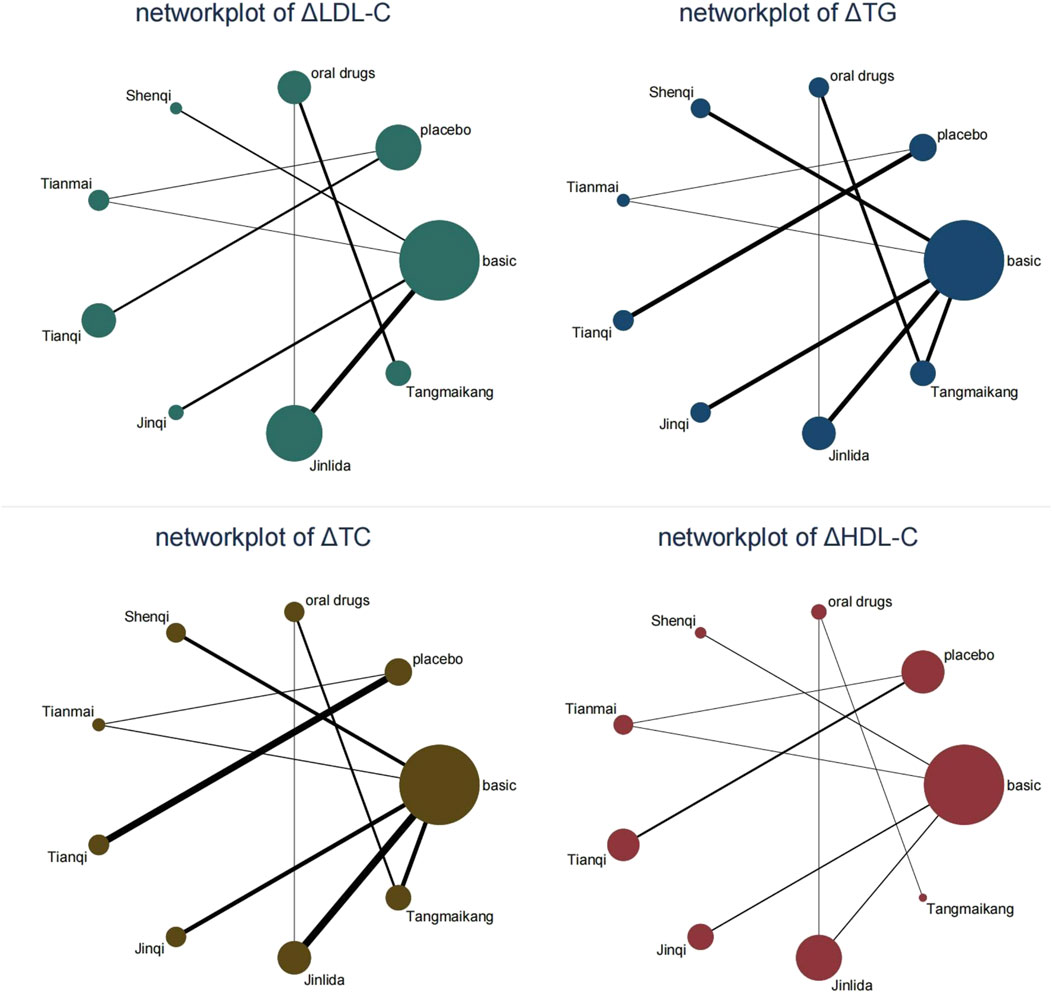
FIGURE 3. Network evidence plots. Legend: “basic” means the basic lifestyle modification without any drugs. “oral drug” means oral hypoglycemic drugs (metformin in the included studies) based on the lifestyle modification.
Compared with LM, Jinlida + LM [SMD −0.31 (95%CI: −0.59, −0.04)] showed a statistically significant effect in lowering LDL-C. Compared with placebo + LM, except for Tianqi + LM [SMD 0.05 (95%CI: −0.26, 0.37)], other 5 TCPM showed statistically significant effect in lowering LDL-C, with SMD fluctuating between −1.28 (95%CI: −2.11, −0.45) (Shenqi + LM) to −1.04 (95%CI: −2.04, −0.04) (Tangmaikang + LM). Detailed comparative results are shown in Figure 4 and Table 4A.
Compared with LM, Shenqi + LM and Jinqi + LM showed statistically significant effect in lowering TG [Shenqi -0.49 (−0.85, −0.12), Jinqi -0.44 (−0.81, −0.06)] and TC [Shenqi −0.51 (−0.86, −0.17), Jinqi 0.44 (−0.80, −0.08)]. Compared with placebo + LM, except for Tianqi + LM [SMD 0.20 ( 95% CI: −0.34, 0.74)], all TCPM showed statistically significant effect in lowering TG, with SMD fluctuating between [−1.48 (95% CI: −2.65, −0.31)] (Shenqi + LM) to [−1.10 (95% CI: −1.90, −0.30)] (Tianmai + LM). Except for Tianqi + LM [SMD 0.21 (95%CI: −0.32, 0.74)] and Jinlida + LM [SMD −1.07 (95%CI: −2.27, 0.14)], other 4 TCPM showed statistically significant effect in lowering TC, with SMD fluctuating between [−1.50 (95%CI: −2.30, −0.70)] (Tianmai + LM) to [−1.27 (95%CI: −2.51, −0.04)] (Tangmaikang + LM). None of the six TCPMs showed a statistical difference in TG or TC compared with oral hypoglycaemic drugs + LM. Detailed comparative results are shown in Figures 5, 6 and Table 4B.
Compared with LM, Shenqi + LM [SMD 0.29 (95%CI: 0.06, 0.51)] and Jinqi + LM [SMD 0.16 (95%CI: 0.01, 0.31)] showed statistically significant effect in increasing HDL-C. Compared with placebo + LM, except for Tianqi + LM [SMD 0.02 (95%CI: −0.18, 0.22)], other 5 TCPM showed statistically significant effect in increasing HDL-C, with SMD fluctuating between [0.54 (95%CI: 0.22, 0.86)] (Tianmai + LM) to [0.78 (95%CI: 0.30, 1.25)] (Shenqi + LM). Detailed comparative results are shown in Figure 7 and Table 4A.
The treatment hierarchy was summarized and reported as the surface under the cumulative ranking curve (SUCRA) and mean ranks, which was shown in Supplementary File S5. The SUCRA plot of all TCPM in each outcome was shown in Supplementary File S6.
Considering all the crucial information and taking the simplicity and applicability across different contexts into account, the minimally contextualized framework was developed to draw a comprehensive conclusion regarding the effectiveness of interventions in the NMA. The LM was most closely connected to the other interventions and selected as the reference group, with an ineffective value, i.e., an absolute effect value of 0, as the decision threshold in outcomes. Interventions were classified as better, worse, and no different than the reference group based on whether the 95% confidence interval of the intervention vs. reference group effect size crossed the decision threshold. For all outcomes, the intervention has been divided into 2 categories, namely no different from the reference group (Category 0) and better or worse than the reference group (Category 1). Based on the same decision threshold, the interventions classified as more effective than the reference were compared against each other by examining whether the confidence or credible interval of their estimate of effect crosses the decision threshold. None intervention proved more effective than another Category 1 intervention, so there was no need to classify the intervention into a higher-level group. Then, the 8 interventions were separated into two main groups according to the certainty of the evidence, namely the group with high or moderate certainty evidence when compared with the reference, the and group with low or very low certainty evidence when compared with the reference. Last but not least, after examining the pairwise comparisons and ranking, those interventions ranked highest were ensured as among the most effective. As presented in Table 5.
Among the included studies, 10 reported adverse reactions. Eight reported gastrointestinal upset reactions in the treatment group (Zhou and Chen, 2003; Shen et al., 2006; Cao and Jin, 2010; Tan, 2010; Yan et al., 2011; Liu, 2015; Shi et al., 2016; Zhao, 2018), including diarrhea and bloating, and two reported no adverse reactions (Xiao et al., 2013; Yin, 2016). Six reported gastrointestinal upset reactions in the control group (Cao and Jin, 2010; Tan, 2010; Xiao et al., 2013; Liu, 2015; Shi et al., 2016; Yin, 2016), including nausea, vomiting, abdominal distention, and diarrhea. The adverse reactions were all mild and did not affect the treatment. No acute complications of diabetes such as hypoglycemia and ketoacidosis were reported, so the 6 TCPM could ameliorate dyslipidemia with a favorable safety profile.
There was no source of inconsistency for ΔLDL-C and ΔHDL-C. The global inconsistency test effect values (chi2) for ΔTG and ΔTC were 0.65 and 0.69 respectively, with no statistically significant difference (p ≥ 0.05). The local inconsistency test showed that there was no difference between each comparison (p ≥ 0.05). Therefore, no evidence of inconsistency existed in all networks. The detailed data of global and local inconsistency was presented in Supplementary File S7.
The Chi-squared test and the I2 index for the primary outcome LDL-C indicated a high degree of heterogeneity among the included literature (I2 = 78.3%). For the comparisons with significant heterogeneity, we conducted sensitivity analysis as presented in Figure 8. Although the exclusion of Zhang HF (2011) would increase the upper CI limit by −0.19 and the exclusion of Wang YR (2011) would decrease the lower CI limit by −0.46, the exclusion from any one study did not exceed the expected confidence interval (−0.42, −0.25), suggesting the stability of meta-analysis. Meta-regression was performed to further investigate the sources of heterogeneity as presented in Table 6. The results indicated heterogeneity might stem from the baseline of LDL-C among these six factors (p = 0.076). Thus, subgroup analysis was conducted for the baseline of LDL-C as presented in Figure 9. One study reported the ideal LDL-C levels at baseline (˂2.6 mmol/L), 13 studies reported the appropriate LDL-C levels at baseline (2.6–3.4 mmol/L) and 4 studies reported pathologically elevated LDL-C levels (˃3.4 mmol/L). The heterogeneity analysis suggested that there was acceptable heterogeneity in subgroups (I2 = 0, 52.8%, 65.8%). The combined results of SMD (95% CI) in fixed-effect model analysis were respectively −0.64 (−1.08, −0.20), −0.11 (−0.22, 0.01) (p = 0.013) and −0.97 (−1.21, −0.73) (p = 0.032).
A funnel plot for risk of publication bias was shown in Supplementary File S8. The visual inspection of the funnel plot indicated that the nodes representing the comparison studies were evenly distributed at the ends of the null line. The fitted line was skewed at a greater angle, suggesting a small sample of events and a small possibility of publication bias for all outcomes.
The test for inconsistency is a necessary process for NMA. It contains two major connotations of heterogeneity and consistency. Consistency refers to the agreement between direct and indirect comparisons and can be tested by a design-by-treatment approach or loop-specific approach. The heterogeneity refers to the difference between each pair of direct comparisons and includes clinical, methodological, and statistical differences. The I2 index gives the magnitude of statistical heterogeneity due to reasons other than the effect of chance (sampling error) (Higgins et al., 2019).
In this study, the consistency of direct and indirect comparisons was confirmed by the node-splitting analysis. Subsequently, the presence of non-negligible heterogeneity between included studies was confirmed in LDL-C. Meta-regression was thus performed for the different variables. Although none of the results were statistically different, the baseline values of LDL-C were the most likely source of heterogeneity, which was also consistent with the meta-regression of previous NMA (Zheng et al., 2020). The results of the subgroup analysis showed that the greater the baseline value, the more potent the lipid-lowering effect of TCPM if LDL-C did not reach the ideal value, which was consistent with previous results regarding the hypoglycaemic effect of TCPM (Jiang et al., 2021). Notably, among the 4 studies that reported pathologically elevated LDL-C levels, two studies using Shenqi (Tian WJ, Yan J) and one using jinlida (Wang SM). Both TCPMs were ranked highly in the NMA’s SUCRA, suggesting that the high ranking of the drugs in this NMA was associated with the high baseline of blood lipids. Therefore, the ranking result needs to be interpreted with caution when applied clinically.
Blood Lipids are the general term for lipids in blood plasma, including mainly total cholesterol (TC), triglycerides (TG), phospholipids, and free fatty acids. The level of lipoprotein cholesterol (LDL-C, HDL-C) reflects the level of lipoproteins (Kasper, 2016). Lipid monitoring and treatment are significant for the prevention of type 2 diabetes. On the one hand, prediabetes and diabetes are often associated with abnormalities in lipid metabolism. An analysis of the cross-sectional investigation of health and nutrition in the United States between 2011 and 2014 displayed that people with prediabetes (defined by ADA-FPG) were significantly more likely to have hyperlipidemia (51.2%) than the general population (Ali et al., 2018). On the other hand. Lipids are considered an essential hazard for the progression of prediabetes into diabetes and combined cardiovascular complications. A non-interventional cross-sectional study conducted in South Korea showed that prediabetes combined with abnormal lipid metabolism (high TG, low HDL-C) was 2.89 times more likely to develop cardiac complications later in life than patients with normal lipid metabolism (Kwon et al., 2021). Therefore, targeted measures should be taken to enhance the management of dyslipidemia in prediabetes.
Increased LDL-C is a major risk factor for the development of atherosclerosis (Jacobson et al., 2015). LDL enters the vascular wall through the endothelium and is modified to oxidized low-density lipoprotein (Ox-LDL) in the subendothelial layer. The latter grows and fuses to form the lipid core of the atherosclerotic plaque. In general, LDL-C is parallel to TC, but TC levels are also influenced by HDL-C levels, so LDL-C was recommended as an indicator of ASCVD risk in the guideline (Joint committee issued Chinese guideline for the management of dyslipidemia in adults, 2016). Therefore, LDL-C was chosen as the primary outcome of this study.
The NMA analysis showed that no TCPM was among the most or least effective in reducing LDL-C based on the existing clinical studies. However, Shenqi + LM was among the most effective in reducing TG and TC, while Jinlida + LM was among the least effective.
Compared with other TCPM, Shenqi granule contains ginsenosides instead of Panax ginseng C.A.Mey. [Araliaceae], resulting in the richer active ingredient of ginseng in the formula. Roh et al. (2020) and Lu et al. (2020) have demonstrated that ginsenosides could improve lipid homeostasis in high-fat mice through various pathways, therefore significantly reducing body mass and serum levels of TC and TG. Meanwhile, pharmacological studies have shown that Shenqi granule could upregulate the expression of Bcl2 and relevant regulatory proteins and thus improve lipid metabolism (Zhang et al., 2019a). Network pharmacological prediction also found that Shenqi granule could participate in biological processes such as medium-density lipoprotein particle remodeling and RNA polymerase II promoter transcriptional regulation of glycolysis, leading to the reverse of the defective hepatic insulin signaling pathway and the improvement of lipid disorder (Shi et al., 2022). Thereby, Shenqi granule was shown superior efficacy in lowering TG and TC compared with other TCPM.
Although Jinlida also has been demonstrated its lipid-lowering effects in several preclinical studies (Table 3), the treatment duration of the clinical studies was far shorter than its in vivo experiments (10 weeks in Zhou HR and 15 weeks in Zhang H). In fact, it was also the shortest intervention time of the six TCPMs with an average intervention length of 3.5 weeks, which may explain its slightly weaker advantage of lowering lipids compared to other TCPMs.
This study was to compare the differences in the efficacy of 6 TCPM based on the network meta-analysis. Its strength lies in the first exploration of the clinical use of different TCPMs to improve lipid profiles in prediabetic patients. Based on direct and indirect evidence, we provided a preliminary comprehensive ranking of these drugs in terms of their effects on lipids levels, which could provide a basis for future clinical research.
However, the study has the following potential limitations: ① the included studies were all conducted on the Chinese mainland without information on race and ethnicity, and the findings need to be extended with caution given the cross-racial and ethnic differences in prevalence and genetics of prediabetes. ② the period of treatment in the included literature varied widely, ranging from 1 month to 12 months ③ Most studies did not describe or qualify the specific means and duration of lifestyle modification, so potential sources of heterogeneity between studies may be related to the rigorousness and duration of lifestyle interventions. ④ Adverse events were poorly reported, the extent of adverse effects was not differentiated, and safety needs further confirmation. All of the aforementioned shortcomings may affect the authenticity of the results, so the ranking results should be viewed with caution and clinical application needs to be considered in the context of actual circumstances, expert opinion, and guidelines.
For patients with prediabetes, Traditional Chinese patent medicine Jinqi and Shenqi combined with lifestyle modification were associated with a significant reduction in TG and TC, while Shenqi + LM was among the most effective. Jinlida + LM was among the least effective. Jinqi + LM might be among the most effective in reducing TG and TC. However, head-to-head comparisons between drugs and further mechanistic exploration are warranted.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
All included authors have made significant contributions to this article. LJ and CC designed and monitored the whole analysis. YZ and ZH provided methodological guidance on GRADE scoring during the revision process. XC and YC contributed to study selection and data extraction. YC and WH provided the software support. YX and DS contributed to the data analysis and paper writing. SW and JZ provided the project fund and were responsible for the data review.
This study was supported by the National Key R&D Program of China: The Study on syndrome differentiation standard of Yin deficiency syndrome in type 2 diabetes mellitus (2018YFC1704402), and the National Key Discipline of Chinese Medicine-Construction of Endocrine Discipline (JB041).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.942563/full#supplementary-material
Ali, M. K., Bullard, K. M., Saydah, S., Imperatore, G., and Gregg, E. W. (2018). Cardiovascular and renal burdens of prediabetes in the USA: Analysis of data from serial cross-sectional surveys, 1988-2014. Lancet. Diabetes Endocrinol. 6 (5), 392–403. doi:10.1016/S2213-8587(18)30027-5
American Diabetes Association (2018). 6. Glycemic targets: Standards of medical care in diabetes-2018. Diabetes care 41 (1), S55–S64. doi:10.2337/dc18-S006
An, J. Y., Qin, L., Yang, L. P., Li, X. Y., and Ma, Y. L. (2014). Study on thin-layer identification and quantitative determination of Zinlida granules[J]. Chin. J. Mod. Appl. Pharm. 31 (02), 213–217.
Bhatnagar, N., Lakshmi, P. V., and Jeyashree, K. (2014). Multiple treatment and indirect treatment comparisons: An overview of network meta-analysis. Perspect. Clin. Res. 5 (4), 154–158. doi:10.4103/2229-3485.140550
Brignardello-Petersen, R., Florez, I. D., Izcovich, A., Santesso, N., Hazlewood, G., Alhazanni, W., et al. (2020). GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. BMJ 371, m3900. doi:10.1136/bmj.m3900
Brignardello-Petersen, R., Guyatt, G. H., Mustafa, R. A., Chu, D. K., Hultcrantz, M., Schunemann, H. J., et al. (2021). GRADE guidelines 33: Addressing imprecision in a network meta-analysis. J. Clin. Epidemiol. 139, 49–56. doi:10.1016/j.jclinepi.2021.07.011
Cai, J., Dong, L. N., and Zhao, Z. G. (2017). Clinical effect of Jin li da granules on insulin resistance in prediabetic patients[J]. J. Hebei Traditional Chin. Med. Pharmacol. 32 (03), 32–33.
Caldwell, D. M., Ades, A. E., and Higgins, J. P. (2005). Simultaneous comparison of multiple treatments: Combining direct and indirect evidence. BMJ 331, 897–900. doi:10.1136/bmj.331.7521.897
Cao, F., Zhang, Q. B., and Jiang, W. H. (2009). Study on the quality standard of Tianqi hypoglycemic capsule[J]. Drug Stand. China 10 (05), 346–349.
Cao, H. X., and Jin, J. L. (2010). Clinical trial of Zhong hui tang mai kang in the intervention of pretype 2 diabetes mellitus[J]. Chin. J. Inf. Traditional Chin. Med. 17 (02), 8–9.
Chen, C. (2005). Intervention effect of Shen qi jiang tang Capsule on blood glucose and blood lipid levels in patients with decreased glucose tolerance [J]. Mod. J. Integr. Traditional Chin. West. Med. 2005 (13), 1681–1683.
Chen, Q., Su, A. L., and Huang, Z. T. (2017). Effect of Tangmaikang granules on CRP and organ index in rats with type 2 diabetes mellitus with Qi and Yin deficiency and stasis. Guangming J. Chin. Med. J. 32 (23), 3393–3396.
Chen, Q., and Wu, J. L. (2007). Intervention treatment of Jin qi jiang tang tablet in obese patients with elevated fasting glucose[J]. Mod. J. Integr. Traditional Chin. West. Med. 2007 (17), 2370–2371.
Chen, X. Y. (2011). Influence of comprehensive intervention of Traditional Chinese medicine on low glucose tolerance combined with metabolic syndrome (Qi and Yin deficiency and heat syndrome)[D]. Beijing: Beijing University of Chinese Medicine.
Chinese Diabetes Society (2021). Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin. J. Endocrinol. Metabolism 37 (04), 311–398.
Chinese Pharmacopoeia Commission (2020). The pharmacopoeia of the People's Republic of China 2020 edition[S]. Beijing: China Press of Traditional Chinese Medicine, 1219–1220.
DerSimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Control. Clin. Trials 7 (3), 177–188. doi:10.1016/0197-2456(86)90046-2
Dong, C. L., and Wang, S. Y. (2015). Clinical observation on intervention of Tian mai xiao ke Tablet in prediabetes[J]. J. Hebei Med. Univ. 36 (09), 1061–1064.
Echouffo-Tcheugui, J. B., and Selvin, E. (2021). Prediabetes and what it means: The epidemiological evidence. Annu. Rev. Public Health 42, 59–77. doi:10.1146/annurev-publhealth-090419-102644
Endocrinology and Metabolic Diseases Committee of the Chinese Physicians Association, Integrated Chinese and Western Medicine Branch (2021). Guideline for the diagnosis and treatment of prediabetes based on combination of disease and syndrome. World Chin. Med. 16 (04), 533–538.
Feng, G. J., Luo, D., and Ran, F. Y. (2021). Content determination of catalpol in shenqi Jiangtang capsules by RP-HPLC [J]. China Pharm. 30 (9), 63–65.
Fu, B. S., Liu, Y., and Wang, Y. X. (2022). Determination of polysaccharides with hypoglycemic effect in Zinlida granules[J]. J. Chengdu Med. Coll. 17 (02), 166–169.
Gao, L. H., Liu, Q., Liu, S. N., Chen, Z. y., Li, C. n., Lei, L., et al. (2014). A refined-JinQi-JiangTang tablet ameliorates prediabetes by reducing insulin resistance and improving beta cell function in mice. J. Ethnopharmacol. 151 (1), 675–685. doi:10.1016/j.jep.2013.11.024
Gu, J. W. (2007). Therapeutic effect of Tang mai kang on impaired fasting glucose[J]. Liaoning J. Traditional Chin. Med. 2007 (09), 1260–1261.
He, C. J., Peng, F., and Liu, T. (2018). Analysis of chemical composition of Tianqi hypoglycemic capsules based on UPLC-LTQ Orbitrap HRMS[J]. Chin. Traditional Herb. Drugs 49 (09), 2019–2025.
Heinrich, M., Appendino, G., Efferth, T., Furst, R., Izzo, A. A., and Kayser, O. (2020). Best practice in research - overcoming common challenges in phytopharmacological research. J. Ethnopharmacol. 246, 112230. doi:10.1016/j.jep.2019.112230
Higgins, J., Thomas, J., and Chandler, J. (2019). Cochrane Handbook for systematic reviews of interventions version 6.0. Chicester, England: The Cochrane Collaboration.
Hong, X. L., Xiao, X. Y., and Chen, F. S. (2014). The influence of tang mai kang granule intervention on the outcome of patients with pre-diabetes[J]. Clin. J. Chin. Med. 6 (02), 6–7.
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 162 (11), 777–784. doi:10.7326/M14-2385
Jacobson, T. A., Ito, M. K., Maki, K. C., Orringer, C. E., Bays, H. E., Jones, P. H., et al. (2015). National lipid association recommendations for patient-centered management of dyslipidemia: Part 1--full report. J. Clin. Lipidol. 9 (2), 129–169. doi:10.1016/j.jacl.2015.02.003
Jiang, L., Wang, S., Zhao, J., Huang, W., Li, J., Xiao, Y., et al. (2021). Qingre yiqi method along with oral hypoglycemic drugs in treating adults with type 2 diabetes mellitus: A systematic review and meta-analysis. Evid. Based. Complement. Altern. Med. 2021, 4395228. doi:10.1155/2021/4395228
Jiang, L., Zhang, Y., Zhang, H., Chen, Y., Huang, W., Xiao, Y., et al. (2022). Comparative efficacy of 6 traditional Chinese patent medicines combined with lifestyle modification in patients with prediabetes: A network meta-analysis. Diabetes Res. Clin. Pract. 188, 109878. doi:10.1016/j.diabres.2022.109878
Jin, H., Mou, J. J., and Xia, N. (2018). UPLC-ESI-MS study of blood-incorporated components of Jinqi jiangtang tablets[J]. Drug Eval. Res. 41 (12), 2227–2231.
Jin, X., Zhang, H. X., and Cui, W. W. (2015). Effect of jinlida on DGAT1 in skeletal muscle in fat-induced insulin resistance ApoE -/- mice. Zhong Yao Cai. J. 38 (6), 1237–1241. Chinese. PMID: 26762066.
Joint committee issued Chinese guideline for the management of dyslipidemia in adults (2016). 2016 Chinese guideline for the management of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi 44 (10), 833–853. doi:10.3760/cma.j.issn.0253-3758.2016.10.005
Kasper, Ji L. N. (2016). Harrison's internal medicine-endocrine and metabolic diseases. 19th ed. Beijing: Peking University Medical Press, 310–324.
Kwon, H. S., Song, K. H., Yu, J. M., Kim, D. S., Shon, H. S., Ahn, K. J., et al. (2021). Framingham risk score assessment in subjects with pre-diabetes and diabetes: A cross-sectional study in Korea. J. Obes. Metab. Syndr. 30, 261–270. doi:10.7570/jomes20137
Li, X., Lian, F. M., Guo, D., Fan, L., Tang, J., Peng, J. B., et al. (2013). The rs1142345 in TPMT affects the therapeutic effect of traditional hypoglycemic herbs in prediabetes. Evid. Based. Complement. Altern. Med. 2013, 327629. doi:10.1155/2013/327629
Lin, J. H., and Hu, H. Z. (2007). Effect of Shen qi jiang tang Granules on blood glucose and lipid in patients with abnormal glucose tolerance[J]. Mod. Hosp. 2007 (08), 83–84.
Liu, J. P. (2022). GRADE Methods in traditional medicine. Integr. Med. Res. 11 (2), 100836. doi:10.1016/j.imr.2022.100836
Liu, Q., Liu, S., Gao, L., Sun, S., Huan, Y., Li, C., et al. (2017). Anti-diabetic effects and mechanisms of action of a Chinese herbal medicine preparation JQ-R in vitro and in diabetic KKAy mice. Acta Pharm. Sin. B 7 (4), 461–469. doi:10.1016/j.apsb.2017.04.010
Liu, W. J. (2015). Effect of Jin li da granule on impaired glucose regulation of Qi and Yin deficiency[D]. Fujian, China: Fujian University of Traditional Chinese Medicine.
Liu, Y., Song, A., Zang, S., Wang, C., Song, G., Li, X., et al. (2015). Jinlida reduces insulin resistance and ameliorates liver oxidative stress in high-fat fed rats. J. Ethnopharmacol. 162, 244–252. doi:10.1016/j.jep.2014.12.040
Lu, H., Yuan, X., Zhang, Y., Han, M., Liu, S., Han, K., et al. (2020). HCBP6 deficiency exacerbates glucose and lipid metabolism disorders in non-alcoholic fatty liver mice. Biomed. Pharmacother. 129, 110347. doi:10.1016/j.biopha.2020.110347
Lv, Y. J., Li, C. P., and Tan, X. F. (2017). The effect of Jinqi Jiangtang tablet on expressions of IL-17 and IL-23 in kidney of diabetic rats. Tianjin Med. J. 45 (3), 249–253. Chinese.
Mao, L. H. (2003). Clinical observation on the intervention of Jin qi jiang tang tablet in the treatment of hypoglycemic tolerance in the elderly[J]. J. Esophageal Dis. 2003 (03), 200–202.
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 10 (1), 89. doi:10.1186/s13643-021-01626-4
Pang, B., Lian, F. M., Zhao, X. Y., Zhao, X. M., Jin, D., Lin, Y. Q., et al. (2017). Prevention of type 2 diabetes with the traditional Chinese patent medicine: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 131, 242–259. doi:10.1016/j.diabres.2017.07.020
Pang, B., and Ni, Q. (2019). Application of classical formula in treatment of diabetes. Zhongguo Zhong Yao Za Zhi 44 (18), 3895–3898. doi:10.19540/j.cnki.cjcmm.20190416.504
Pang, B., Ni, Q., Lin, Y. Q., Wang, Y. T., Zheng, Y. J., Zhao, X. M., et al. (2018). Traditional Chinese patent medicine for treating impaired glucose tolerance: A systematic review and meta-analysis of randomized controlled trials. J. Altern. Complement. Med. 24 (7), 634–655. doi:10.1089/acm.2017.0302
Puhan, M. A., Schünemann, H. J., Murad, M. H., Li, T., Brignardello-Petersen, R., Singh, J. A., et al. (2014). A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 349, g5630. doi:10.1136/bmj.g5630
Qian, Q., Liu, X., He, W., An, Y., Chen, Q., Wu, J., et al. (2012). TG accumulation inhibitory effects of Jinqi formula by AMPK signaling pathway. J. Ethnopharmacol. 143 (1), 41–48. doi:10.1016/j.jep.2012.05.052
Roh, E., Hwang, H. J., Kim, J. W., Hong, S. H., Lee, Y. B., Kim, J. A., et al. (2020). Ginsenoside Mc1 improves liver steatosis and insulin resistance by attenuating ER stress. J. Ethnopharmacol. 259, 112927. doi:10.1016/j.jep.2020.112927
Salanti, G., Ades, A. E., and Ioannidis, J. P. (2011). Graphical methods and numerical summaries for presenting results from multiple treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 64, 163–171. doi:10.1016/j.jclinepi.2010.03.016
Shen, Y. D., Tao, L. W., and Lu, H. (2006). Preliminary clinical observation of Tangmaikang intervention in patients with impaired glucose regulation[A]. Diabetes Branch of China Association of Chinese Medicine. Compilation of papers of the ninth National Conference of Traditional Chinese medicine diabetes[C]. Hebei, China: Diabetes Branch of China Association of Chinese Medicine, 5.
Shi, S., Sun, M., Liu, Y., Jiang, J., and Li, F. (2022). Insight into Shenqi Jiangtang Granule on the improved insulin sensitivity by integrating in silico and in vivo approaches. J. Ethnopharmacol. 282, 114672. doi:10.1016/j.jep.2021.114672
Shi, Y. L., Liu, W. J., Zhang, X. F., Su, W. J., Chen, N. N., Lu, S. H., et al. (2016). Effect of Chinese Herbal Medicine Jin li da Granule in Treatment of Patients with Impaired Glucose Tolerance. Chin. Med. J. 129 (19), 2281–2286. doi:10.4103/0366-6999.190676
Tan, P. (2010). Intervention trial of Jin qi jiang tang tablet on pre-type 2 diabetes mellitus[J]. J. Xinxiang Med. Univ. 27 (01), 69–71.
Tao, L. W., Lu, H., and Tao, F. (2012). Clinical comparative study of Tang mai kang and intensive lifestyle on short-term intervention in patients with impaired glucose regulation[J]. Chin. General Pract. 15 (33), 3897–3899.
Tian, W. Z., and Li, H. M. (2012). Observation of therapeutic effect of Shen qi jiang tang granule on hypoglycemia[J]. Ningxia Med. J. 34 (09), 917–918.
Wang, C., Dai, X., Zhang, D., Liu, Z., and Huang, Q. (2018). Jinlida granules improve dysfunction of hypothalamic-pituitary-thyroid Axis in diabetic rats induced by STZ. Biomed. Res. Int. 2018, 4764030. doi:10.1155/2018/4764030
Wang, E. Y., Chen, L., and Ma, H. (2016). Effects of Jinqi hypoglycemic tablets formulation on the in vivo pharmacokinetics of berberine in rats[J]. Chin. Traditional Herb. Drugs 47 (23), 4231–4234.
Wang, N., Li, T., and Han, P. (2016). The effect of Tianmai xiaoke pian on insulin resistance through PI3-K/AKT signal pathway. J. Diabetes Res. 2016, 9261259. doi:10.1155/2016/9261259
Wang, Q., Li, B. B., and Huang, W. J. (2021). Analysis of chemical constituents of s jiangtang granule based on UPLC-Q/TOF MS and GC-MS[J]. Chin. Traditional Herb. Drugs 52 (6), 1568–1581.
Wang, S. M., and Jiang, Q. Q. (2018). Clinical observation on the treatment of abnormal glucose tolerance with Jin li da granule combined with lifestyle intervention[J]. Hebei J. Traditional Chin. Med. 40 (06), 864–867.
Wang, X. H., Liu, T., and Yin, Z. (2011). Effect of jin li da on prognosis of elderly patients with coronary heart disease with low glucose tolerance[J]. Contemp. Med. 17 (35), 152–153.
Wang, Y. R., Tong, X. L., and Xiao, X. H. (2011). The rapeutic effect and mechanism of Tian qi jiang tang capsule on patients with impaired glucose tolerance[J]. Chin. J. Diabetes 19 (07), 525–528.
Wei, Y. (2009). Clinical observation on intervention of hypoglycemia with Tian qi jiang tang capsule[D]. Zhejiang: Zhejiang University.
WHO (1999). Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO consultation. Part 1: Diagnosis and classification of diabetes mellitus. Geneva, Switzerland: WHO, 1–50.
Xia, S., Gao, B., Chen, S., Lin, X., Zhang, P., Chai, Y., et al. (2020). Verification of the efficacy and safety of qi-replenishing Chinese medicine in treating prediabetes: A meta-analysis and literature review. Evid. Based. Complement. Altern. Med. 2020, 7676281. doi:10.1155/2020/7676281
Xiao, X. Y., Hou, X. L., and Qian, X. H. (2013). Clinical study of Tang mai kang granule in the treatment of prediabetes[J]. China Med. Pharm. 3 (01), 19–20+39.
Xu, Y., Wang, L., He, J., Bi, Y., Li, M., Wang, T., et al. (2013). Prevalence and control of diabetes in Chinese adults. JAMA 310 (9), 948–959. doi:10.1001/jama.2013.168118
Yan, J., He, L. Y., and He, Z. J. (2011). Clinical observation of Shen qi jiang tang granule in treatment of 25 cases of prediabetes[J]. J. Guangdong Med. Univ. 29 (04), 445–446.
Yan, M. X., Liao, X., and Ke, H. N. (2014). HPLC fingerprinting study of polysaccharide components of Shenqi Jiangtang tablets[J]. J. Guangdong Pharm. Univ. 30 (05), 574–577.
Yin, Y. (2016). Comparison of the clinical effect of Jin li da granule and metformin in the treatment of prediabetes (deficiency of Qi and Yin)[D]. Yunnan, China: Yunnan University of Chinese Medicine, 33.
Zang, S. S., Song, A., Liu, Y. X., Wang, C., Song, G. Y., Li, X. L., et al. (2015). Chinese medicine Jinlida (JLD) ameliorates high-fat-diet induced insulin resistance in rats by reducing lipid accumulation in skeletal muscle. Int. J. Clin. Exp. Med. 8 (3), 4620–4634.
Zhang, H., Chen, R., Xu, C., Zhang, Y., Tian, Q., and Wang, B. (2021). Simultaneous determination of saponins and lignans in rat plasma by UPLC- MS/MS and its application to a pharmacokinetic study of shenqi jiangtang granule. Curr. Drug Metab. 22 (3), 224–231. doi:10.2174/1389200222666210203182232
Zhang, H., Hao, Y., Wei, C., Yao, B., Liu, S., Zhou, H., et al. (2019). Chinese medicine Jinlida granules improve high-fat-diet induced metabolic disorders via activation of Brown adipose tissue in mice. Biomed. Pharmacother. 114, 108781. doi:10.1016/j.biopha.2019.108781
Zhang, H., Zhang, X. J., and Jiang, H. J. (2017). Analysis of chemical constituents in shenqi jiangtang granules by UPLC-Q-TOF MS/MS[J]. Chin. Tradit. Pat. Med. 39 (10), 2101–2108.
Zhang, H. F., Tang, Y. G., and He, Y. J. (2011). Study on the intervention of Tian mai xiao ke Tablet on abnormal glucose tolerance[J]. Chin. J. Exp. Traditional Med. Formulae 17 (21), 266–268.
Zhang, Q., Xiao, X., Li, M., Li, W., Yu, M., Zhang, H., et al. (2014). miR-375 and miR-30d in the effect of chromium-containing Chinese medicine moderating glucose metabolism. J. Diabetes Res. 2014, 862473. doi:10.1155/2014/862473
Zhang, Q., Xiao, X., Zheng, J., Li, M., Yu, M., Ping, F., et al. (2019). Shenqi jiangtang granule ameliorates kidney function by inhibiting apoptosis in a diabetic rat model. Evid. Based. Complement. Altern. Med. 2019, 3240618. doi:10.1155/2019/3240618
Zhang, Q., Xiao, X. H., Li, M., Li, W. H., Yu, M., Zhang, H. B., et al. (2014). Chromium-containing traditional Chinese medicine, Tianmai Xiaoke Tablet improves blood glucose through activating insulin-signaling pathway and inhibiting PTP1B and PCK2 in diabetic rats. J. Integr. Med. 12 (3), 162–170. doi:10.1016/S2095-4964(14)60020-0
Zhang, S. X., Sun, H., Sun, W. J., Jiao, G. Z., and Wang, X. J. (2010). Proteomic study of serum proteins in a type 2 diabetes mellitus rat model by Chinese traditional medicine Tianqi Jiangtang Capsule administration. J. Pharm. Biomed. Anal. 53 (4), 1011–1014. doi:10.1016/j.jpba.2010.06.033
Zhang, Y., and Zhao, Q. P. (2011). HPLC-DAD fingerprinting of Tang mai kang granules[J]. Chin. J. Exp. Traditional Med. Formulae 17 (19), 101–104.
Zhao, H. L., Sui, Y., Qiao, C. F., Yip, K. Y., Leung, R. K. K., Tsui, S. K. W., et al. (2012). Sustained antidiabetic effects of a berberine-containing Chinese herbal medicine through regulation of hepatic gene expression. Diabetes 61 (4), 933–943. doi:10.2337/db11-1164
Zhao, Q. (2018). Evaluation of therapeutic effect of Shen qi jiang tang granule on patients with abnormal glucose tolerance[J]. Zhenjiang Clin. Medcine J. 20 (02), 299–300.
Zhao, Q. P., and Zhang, Y. (2011). HPLC-ELSD fingerprints of Tang mai kang granules[J]. Chin. J. Exp. Traditional Med. Formulae 17 (20), 76–78.
Zheng, Q., Yang, H., Sun, L., Wei, R., Fu, X., Wang, Y., et al. (2020). Efficacy and safety of HIF prolyl-hydroxylase inhibitor vs epoetin and darbepoetin for anemia in chronic kidney disease patients not undergoing dialysis: A network meta-analysis. Pharmacol. Res. 159, 105020. doi:10.1016/j.phrs.2020.105020
Zhou, D. Y., and Chen, H. P. (2003). Effect of Jin qi Jiang tang tablet on non-obese patients with hypoglycemia[J]. Chin. Traditional Herb. Drugs 2003 (05), 68–69.
Zhou, H. R., Wang, T. X., Hao, Y. Y., Hou, Y. L., Wei, C., Yao, B., et al. (2022). Jinlida granules reduce obesity in db/db mice by activating beige adipocytes. Biomed. Res. Int. 2022, 4483009. doi:10.1155/2022/4483009
Keywords: traditional Chinese patent medicine, guidelines, prediabetes, dyslipidemia, network meta-analysis
Citation: Jiang L, Wang S, Zhao J, Chien C, Zhang Y, Su G, Chen X, Song D, Chen Y, Huang W, Xiao Y, Cao Y and Hu Z (2022) Treatment options of traditional Chinese patent medicines for dyslipidemia in patients with prediabetes: A systematic review and network meta-analysis. Front. Pharmacol. 13:942563. doi: 10.3389/fphar.2022.942563
Received: 12 May 2022; Accepted: 21 July 2022;
Published: 29 August 2022.
Edited by:
Anthony Booker, University of Westminster, United KingdomReviewed by:
Vahidreza Ostadmohammadi, Kashan University of Medical Sciences, IranCopyright © 2022 Jiang, Wang, Zhao, Chien, Zhang, Su, Chen, Song, Chen, Huang, Xiao, Cao and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shidong Wang, MjQ4NjIwNTQwOEBxcS5jb20=, d3NkMzEyMkAxMjYuY29t; Jinxi Zhao, emhhb2p4ODg4QDEyNi5jb20=
†These authors have contributed equally to this work and share the first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.