
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 04 October 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.938915
This article is part of the Research TopicApplication of Plant Secondary Metabolites to Pain Neuromodulation, Volume IIIView all 6 articles
 Liqiong Yu1
Liqiong Yu1 Shiling Li1
Shiling Li1 Lili Pu1
Lili Pu1 Chunhong Yang1
Chunhong Yang1 Qian Shi1
Qian Shi1 Qi Zhao1
Qi Zhao1 Shengbu Meniga2
Shengbu Meniga2 Yue Liu2*
Yue Liu2* Yi Zhang2*
Yi Zhang2* Xianrong Lai1,2*
Xianrong Lai1,2*Rheumatoid arthritis (RA) is a severe inflammatory autoimmune disease characterized by the failed spontaneous resolution of inflammation. The induction of immune regulation and resolution of inflammatory pathways are effective in alleviating inflammation in RA. As the oldest medical system in the world, traditional Tibetan medicine (TTM) has a long history of preventing and treating RA. This review provides a comprehensive overview of medicinal plants with anti-RA activity in the TTM system, using classic books of Tibetan medicine, modern research literature, and drug standards. A total of 27 species have been found to be effective in treating RA, including Tinospora sinensis (Lour.) Merr., Terminalia chehula Retz., P. hookeri (C. B. Clarke) Hock.), and Aconitum pendulum Busch. Alkaloids, flavonoids, polyphenols, and terpenoids have turned out to be the major bioactive components for RA treatment. The inhibition of pro-inflammatory cytokine expression by mediating the NF-κB, MAPK, and JAK/STAT pathways is the core mechanism in RA treatment. In conclusion, this review provides key information and research perspectives for further research on the anti-RA effects of TTM.
Rheumatoid arthritis (RA) is a chronic autoimmune disease that mainly affects the lining of the synovial joints, bones, muscles, blood vessels, and related soft tissues or connective tissues. If the inflammation fails to resolve spontaneously, the disease will remain in patients throughout their lives (Deane and Holers, 2021). The early stages of RA tend to affect small joints, and as the disease progresses, symptoms can appear in the wrists, ankles, and knees, leading to irreversible joint disability, loss of function, and ultimately, considerable disability or premature death (Koga et al., 2021). Approximately 0.1–2.0% of the population worldwide suffered from progressive joint damage, permanent disability, or even shortened lifespan because of RA (Almutairi et al., 2021). In China, the prevalence of RA is higher in Tibet (4.86%) than in other areas of China (Zhang H. et al., 2020).
Many classic well-established agents (e.g., NSAIDs, DMARDs, and glucocorticoids) have their own limiting effects and adverse reactions, such as nausea, vomiting, abdominal discomfort, liver damage, and nephrotoxicity (Hoes et al., 2010; Thakur et al., 2018). Novel classes of biological agents, including TNF-α antagonists and IL-1 antagonists, provide a favorable opportunity for anti-RA with a lower risk of side effects and remarkable treatment results (Ding et al., 2015). However, these drugs are far more expensive than conventional ones, seriously aggravating financial burdens on patients. Thus, seeking inexpensive, effective, and safe anti-RA agents has been an area of great interest.
Owing to their unique geography, people in Tibetan areas are more prone to RA than people in other parts of China. Traditional Tibetan medicine (TTM) has a history of more than 3,800 years of understanding rheumatic diseases and corresponding treatments. To date, 3,105 kinds of natural medicines have been recorded in the Tibetan medicine system (Pan et al., 2021). Tibetan medicine has accumulated a wealth of experience in the treatment of chronic diseases, such as rheumatism, high-altitude polycythemia, cholecystitis, hepatitis, and gastritis (Fu et al., 2020). In fact, Tibetan medicine has been widely used in RA treatment, but these records are fragmented and lack a comprehensive and standardized summary. This situation is not conducive to the development of TTM for RA treatment. This study summarizes the traditional experience and potential value of Tibetan medicine in RA treatment by using a collection of Tibetan medicines for RA treatment from Tibetan medicine classics, such as the “Dictionary of Chinese Ethnic Medicine,” “Drug Standards of Tibetan Medicine,” “Chinese Tibetan Medicine,” and “Jing Zhu Materia Medica,” and sorting modern studies on the active ingredients, mechanisms, and therapeutic effects of TTMs for RA.
In the theoretical system of Tibetan medicine, RA is regarded as a kind of “Grum bu disease,” which belongs to the category of joint disease and is similar to the “arthralgia disease” of traditional Chinese medicine (Zhijia and Ciren, 2021). According to the Tibetan medical classic “blue glaze,” the occurrence and development of the disease is related to three factors (r-lung, mKhris-pa, and Bad-kan). Tibetan medicine states that the disease is due to living in a humid place for a long time and the excessive consumption of fatty, hot, sour, and spicy food, which disrupts the balance of the internal and external environments of the human body and causes stomach fire and dysfunction and the gradual transformation of Bad-kan into mKhirs-pa. Moreover, “yellow water” in the body causes lesions in the meridians, muscles, and joints, resulting in restricted joint movement, stiffness, redness and swelling, and other pathological manifestations of difficult diseases (Ge and Nima, 2021) (Figure 1). “Yellow water” is scattered in the skin, bones, and internal and external organs, exerting nourishing, lubricating, and other physiological effects, nourishing internal organs, and ensuring that the joints move freely. Under the influence of a variety of internal and external pathogenic factors, the human body has three factors of imbalance, resulting in the abnormal quality and quantity of “yellow water,” inducing “yellow water” disease (Bian et al., 2019). In modern medicine, the “yellow water” disease is mainly manifested in RA and skin diseases (Grum bu disease). Owing to the geographical environment, people’s living habits, and other factors in plateau areas, “Grum bu disease” is a common disease. Thus, its treatment has been systematically researched in Tibetan medicine. It is recorded that the treatment of RA mainly focuses on combinations of internal and external treatment and starts from the regulation of diet and living. The combination of Tibetan medicine with medicinal baths, fire moxibustion, and other characteristic external treatment methods, has achieved a unique clinical effect for the treatment of the “Grum bu disease” (Zhijia et al., 2021).
This study searched the “Treasure House of Tibetan Medicine Prescriptions,” “Jing Zhu Materia Medica,” “Dictionary of Chinese Ethnic Medicine,” “Drug Standards of Tibetan Medicine,” “Tibetan Medicine Annals,” and other Tibetan medicine monographs and drug standards, to obtain information on TTMs and their effects against RA. The names, original species, families, medicinal parts, and treated diseases were listed in detail. Plant names were mainly derived from references and verified by their Chinese names in the “Flora of China” (http://frps.eflora.cn/) and the “Medicinal Plant Names Services: Royal Botanic Gardens” (https://www.kew.org/). At the same time, this study performed a large-scale text mining of online Chinese databases (e.g., CNKI, Wanfang, and Weipu) and international databases (e.g., NCBI, Open Access Library, Science Direct, and Google Scholar) to obtain information about the active ingredients of Tibetan medicines for RA and their biological or pharmacological effects.
Studies on Tibetan medicines with anti-RA activities were reviewed. A total of 27 species of Tibetan medicinal plants were associated with RA treatment. The Latin names, Tibetan names, medicinal parts, and active parts of relevant modern research of these 27 Tibetan medicines for anti-RA are listed in Table 1. This Tibetan medicine is distributed in 18 families. The most common families are Gentianaceae, Ranunculaceae, and Asteraceae. Furthermore, among the plant parts, the most frequently used medicinal parts are whole grass, roots, leaves, and flowers. Alkaloids, flavonoids, phenolic acids, and other chemical components are the main materials responsible for the anti-RA effects of these TTMs. Immune-regulatory cytokines are important factors for anti-inflammatory effects in RA. The following sections will focus on the mechanisms related to immunomodulatory cytokines and anti-inflammation associated with the treatment of RA with TTMs and their compounds.
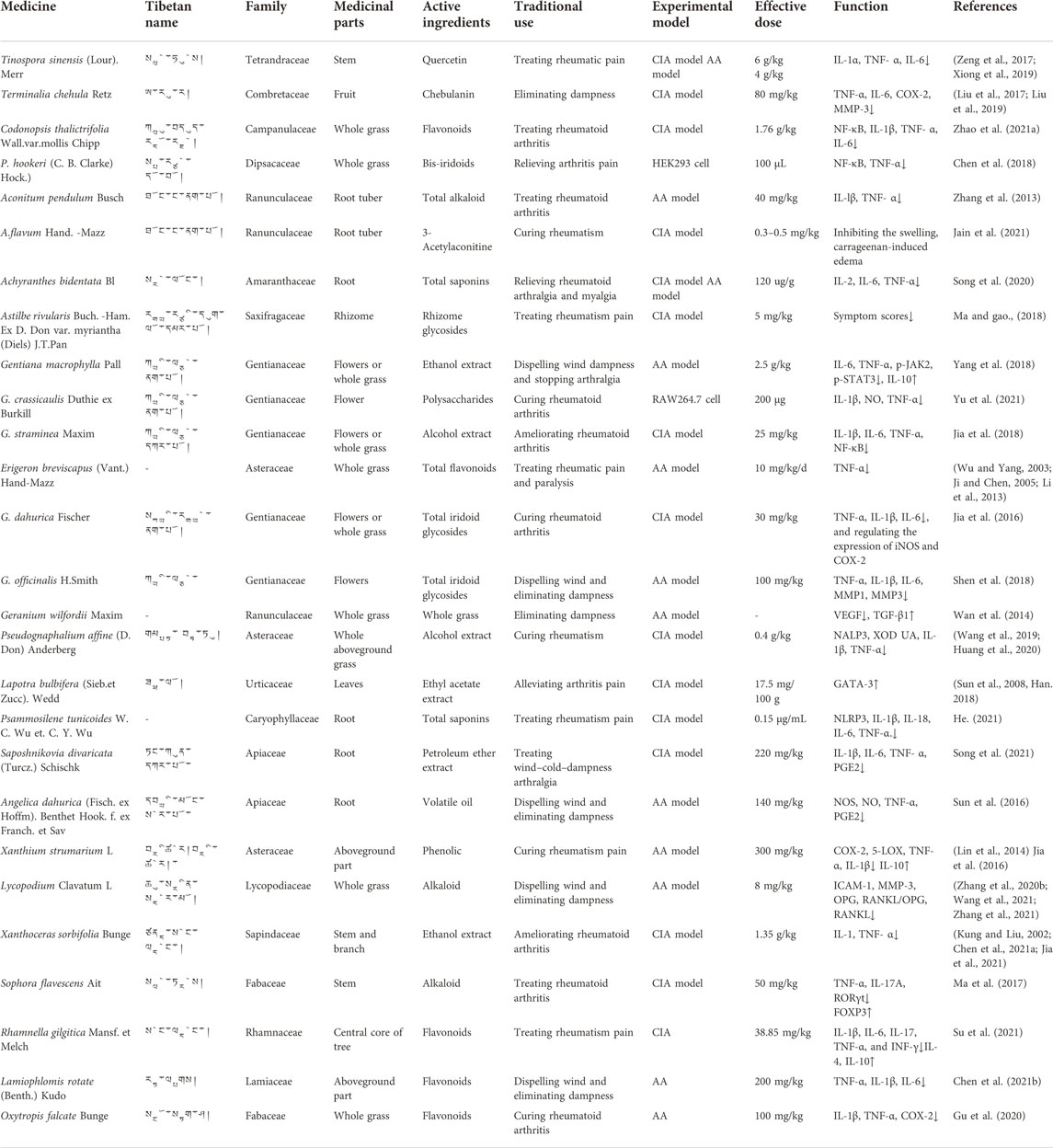
TABLE 1. Tibetan medicine for anti-RA (Tibetan medicine names are ranked from high to low in the frequency of use).
In addition, there are a total of 44 kinds of Tibetan medicine prescriptions for anti-rheumatism recorded in the “Treasure House of Tibetan Medicine Prescriptions” and “Tibetan Medicine Standards.” By reviewing modern pieces of literature, perhaps because of the complex formulations and diverse components of Tibetan medicinal prescriptions, we found only four prescriptions with modern pharmacological research on RA. These are Ershiwuwei Lvxue Pill, Shiwei Ruxiang Powder, Shibawei Dangshen Pills, and Twenty-Five Wei’er Tea Pills (Ding and Zhang, 2017; Zhu et al., 2017; Liu et al., 2021; Li C. Y. et al., 2022). Detailed information of these prescriptions is given in Supplementary Tables S1, S2.
Aconitine (AC) (Figure 3) is the main chemical component in Aconitum species, such as the famous TTM A. pendulum (Figure 2), which is considered to be the main active substance of Aconitum species and widely applied in the treatment of diverse diseases (Li L. et al., 2022). Low-dose AC (0.3 and 0.9 mg/kg) has good therapeutic potential in heart failure, myocardial infarction, and rheumatic diseases, and can inhibit pain response (Deng et al., 2021).

FIGURE 2. Tibetan medicines are commonly used to treat rheumatism (all the images are collected from the Flora of China, http://ppbc.iplant.cn) (A). (G) Maxim. (B). T. chehula Retz (C). G. macrophylla Pall. (D). A. pendulum Busch (E). P. affine (D. Don) Anderberg (F). C. thalictrifolia Wall. var.mollis Chipp. (G). T. sinensis (Lour.) Merr. (H). S. flavescens Ait. (I). X. strumarium L (J). X. sorbifolia Bunge (K). L. bulbifera (Sieb.et Zucc.) Wedd. (L) P. hookeri (C.B.Clarke) Hoeck (M). L. rotate (Benth.)Kudo.
3-Acetylaconitine (Figure 3) is a nitrogen-containing alkaloid, obtained from A. pendulum Busch and A. flavum Hand. -Mazz. (Ranunculaceae) (Li Z. et al., 2022). Pre-clinical research illustrated that 0.03 mg/kg 3-acetyl aconitine combined with Mor or KET has a significant synergic analgesic effect and its mechanism may be associated with NMDA receptors or endogenous opioid peptides (Yao et al., 2014).
Matrine (Figure 3), a tetracyclo-quinolizidine alkaloid, is the main bioactive compound in S. flavescens Ait. (Figure 2). Studies have proved that matrine reduces the levels of Th1 cytokines, such as IFN-γ, TNF-α, and IL-1β, and increases the levels of Th2 cytokines (IL-4 and IL-10) to balance the Th1/Th2 axis by regulating the NF-κB signaling pathway (Zhang et al., 2020). Moreover, matrine induces G0/G1 cell cycle arrest and inhibits the activation of the JAK/STAT signaling pathway, thereby increasing the rate of apoptosis in vitro (Yang et al., 2017).
Quercetin (Figure 3) is a natural coumarin compound found in many plants and in some TTMs. It is also the pharmacological basis of T. sinensis (Lour.) Merr. (Figure 2). In an AA model, quercetin promoted apoptosis in activated neutrophils. In addition, quercetin inhibited NET formation by suppressing ROS production and autophagy (Yuan et al., 2020). In a recent study, quercetin was shown to inhibit proliferation-induced IL-1 and the expression of matrix metalloproteinases and COX-2 by RA synovial fibroblasts.
Chalcones (Figure 3), 2,4-dihydroxy chalcone, is isolated from TTM O. falcate Bunge (Figure 2). (Zhang Q. et al., 2020). It was verified to inhibit NO production in LPS-induced RAW 264.7 cells with 91.91% (10 mol/L). In addition, the anti-inflammatory mechanism of 2,4-dihydroxychalcone may also reduce the production of arachidonic acid by inhibiting cyclooxygenase (COX-1 or COX-2), thus further preventing the synthesis and release of inflammation (Ur Rashid et al., 2019).
Chebulanin (Figure 3) is a natural polyphenol acid isolated from TTM T. chebula Retz (Figure 2), which was confirmed to possess anti-inflammatory and anti-arthritic effects by inhibiting the activation of the NF-κB and MAPK signaling pathways in a CIA model (Zhao et al., 2015). In addition, at 100 μM concentration, it could effectively ameliorate RA by inhibiting the nuclear translocation of p38 and p65 in LPS-stimulated macrophages (Liu et al., 2020).
Iridoid glucosides (Figure 3) widely exist in L. rotata (Benth.) (Figure 2) and other TTMs and have strong anti-inflammatory and anti-oxidant activities. Iridoid glucosides (40 mg/kg) could treat RA by inhibiting the production of the serum pro-inflammatory cytokines IL-1β, TNF-α, IL-6, IFN-γ, and IL-17, and increasing the production of the anti-inflammatory cytokine IL-10 (Zhao X. et al., 2021). Iridoid glucosides (30 mg/kg) from G. macrophylla Pall. could inhibit the production of TNF-α, IL-1β, and IL-6 and regulate the expression of iNOS and COX-2 (Jia et al., 2016). In addition, bis-iridoids A and bis-iridoids B (Figure 3) from P. hookeri (C.B.Clarke) Hoeck. (Figure 2) could reduce the production of NF-κB and pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 and down-regulate the expression of inducible nitric oxide synthase and COX-2 in response to lipopolysaccharide stimulation (Chen et al., 2018).
Many in vivo and in vitro studies have confirmed that TTM extracts can exert anti-RA therapeutic effects through different targets. G. straminea Maxim (Figure 2) is mainly distributed in Tibet, Sichuan, Qinghai, Gansu, Ningxia, and other places in China at altitudes of 2000–4950 m areas. In vitro studies have shown that the ethanol extract of G. straminea Maxim (2.5 g/kg) could reduce the levels of IL-1β, IL-6, and TNF-α inflammatory factors, inhibit NF-κB p65 protein expression in synovial tissue, and further reduce synovial inflammation (Wei. 2010; Bao et al., 2018). Similarly, the nontoxic ethanol extract of G. macrophylla Pall. (Figure 2) is widely used in the treatment of RA, cholecystitis, and cholelithiasis in China. Europe and other countries showed that it possesses significant anti-inflammatory effects in the AA model, which is demonstrated by inhibiting the expression of IL-6 and TNF-α, up-regulating the expression of IL-10, and down-regulating the relative protein expression levels of p-JAK2 and p-STAT3 (Wang et al., 2013; Lin et al., 2016). P. affine (D. Don) Anderberg (Figure 2) is a TTM, whose whole grass on the ground is its medicinal part. Its ethanol extract can alleviate joint inflammation by inhibiting the expression of NALP3 inflammasome-related proteins, restraining the activity of XOD, and reducing the production of UA, IL-1β, and TNF-α in the blood. The ethyl acetate extract of L. bulbifera (Sieb.et) Zucc. Wedd. (Figure 3) (5 mg/10 g) showed anti-inflammatory activity by negatively regulating the expression of T-bet in DC and T cells and positively regulating the expression of GATA-3, to affect the balance of Th1/Th2 cytokines in the body and alleviate RA (Wang, He et al., 2019; Huang, Yang et al., 2020). In the CIA model, the ethanol extract of X. sorbifolia Bunge (1.35 g/kg) could reduce the levels of IL-17 and TNF-α (Kung, Liu. 2002; Chen, Wu et al., 2021; Jia, Jia et al., 2021). The petroleum ether extract of S. divaricata (Turcz.) Schischk. (220 mg/kg) was considered a promising drug for RA treatment because it alleviates inflammatory response and RA symptoms in AA rats (Song, Li et al., 2021).
Apart from that, the combination of glycosides from A. rivularis (5 mg/kg) and methotrexate (1.5 mg/kg) could reduce symptoms and morbidity in clinical CIA rats, with no additional side effects and even slightly improved physical changes induced by low doses of MTX compared with monotherapy (Ma, Gao. 2018). The total saponin of A. bidentata Bl (120 μg/g) exerts its effect on RA by reversing the imbalance of Th17/Treg and inhibiting the secretion of inflammatory factors (Song, Xu et al., 2020). Similarly, the total saponin (0.15 μg/mL) from P. tunicoides could inhibit protein expression of NLRP3 in MH7A cells and reduce the production of inflammatory factors IL-6, IL-18, and IL-1β (Simon et al., 2021).
The pharmacological mechanisms of TTMs for RA are mainly focused on immunoregulation and anti-inflammation (Figures 4, 5). These pathways do not exist in isolation, but overlap and can influence each other. Cytokines and inflammatory pathways associated with inflammation in RA mainly influence inflammatory response.
In the inflammatory process of RA, the cascade of innate and adaptive immune responses is an important immunopathogenic mechanism in RA (Chen Z. et al., 2019). This development is driven by an excess of inflammatory cytokines and autoantibodies and maintained by epigenetic changes in fibroblast-like synoviocytes, thus supporting further inflammation (Harre and Schett, 2017). In the process, a large number of different immune cells, including neutrophils, B cells, macrophages, and T cells invade the synovial membrane and fluid. Synovial inflammation reflects subsequent immune activation characterized by the invasion of leukocytes by intrinsic immune cells (e.g. monocytes, macrophages, dendritic cells, and neutrophils) and adaptive immune cells (including Th1, Th2, Th17, and B cells and plasma cell lineage). Th1, Th2, Th17, and regulatory T (Treg) cells differentiate from CD4+ T cells (Bystrom et al., 2018; Zhang and Lee, 2018). Treg cells have been extensively studied in several autoimmune diseases (Chen S. J. et al., 2019). Th17 cells represent a distinct effector T-cell subset characterized by the expression of retinoic acid-related orphan receptor (ROR) γt and the production of IL-17 family members, IL-21 and IL-22. Under normal conditions, Th17/Treg cells are in dynamic equilibrium (Astry et al., 2011). It has been identified as a crucial event in the pathogenesis of RA.
In addition, within the innate immunity function that causes inflammation, macrophages play a pivotal role in RA because they are abundant in inflamed synovial tissues and cartilage–vascular opacification junctions (Kinne et al., 2000). They can differentiate into two distinct subpopulations (M1 or M2 phenotypes) with different physiological functions that depend on the microenvironment (Alivernini et al., 2020). M1 cells secrete pro-inflammatory cytokines, while M2 cells secrete anti-inflammatory cytokines. M1 macrophages produce pro-inflammatory cytokines such as TNFα, IL-1β, IL-6, IL-12, IL-23, and low levels of IL-10 and inflammatory enzymes in promoting acute RA (Kennedy et al., 2011). TNF-α is a major pro-inflammatory cytokine critical for immunity to infection. It stimulates inflammation, osteoclastogenesis, and subsequent joint tissue destruction and bone erosion within surrounding joints, which are the main features known to be associated with RA (Radner and Aletaha., 2015). M1 macrophages release inflammatory chemokines, including CXCL13, CXCL9, CXCL5, CXCL10, and CXCL8, to recruit leukocytes to inflammation sites, and these cells produce IL-1β, TNF-αI, IL-6, MMP, chemokine receptors, ROS, and inducible nitric oxide synthase in the joints, leading to joint destruction (Tardito et al., 2019). The main function of M2 macrophages is anti-inflammation. Therefore, in chronic inflammation, M2 macrophages remodel and repair tissues by producing IL-10 and IL-12, expressing CD163 and CD206, and releasing growth factors such as TGF-β and vascular endothelial growth factor (VEGF) (Fassio et al., 2019), (Tanaka et al., 2018). Therefore, reducing the level of pro-inflammatory factors, regulating the dynamic balance of Th17/Treg cells, and promoting the production of anti-inflammatory factors by M2 macrophages are effective methods for RA treatment. Pre-clinical research illustrated that A. bidentata Bl, L. bulbifera (Sieb.et Zucc.) Wedd, L. Clavatum L., and S. flavescens Ait. could regulate the imbalance of Treg and Th17 cells by reducing the levels of IL-2, IL-6, and TNF-α in the synovial tissues of CIA or AA model rats. (Yao. 2008, Han., 2018; Ma et al., 2017; Song et al., 2020). Moreover, many TTMs, T. sinensis (Lour.) Merr, T. chehula Retz, C. thalictrifolia Wall. var.mollis Chipp, and G. dahurica Fischer regulate cytokines by reducing the level of IL-6 and IL-1. At the same time, O. falcate Bunge, X. sorbifolia Bunge, X. strumarium L., and P. hookeri (C.B.Clarke) Hoeck. are potential drugs for RA treatment via stimulating the production of anti-inflammatory cytokines, such as IL-10, in M2 cells.
A class of proteins originally found in B lymphocytes, NF–κB, can specifically bind to κB sites on various gene enhancers or promoters to initiate gene transcription and plays an important role in cell growth, apoptosis, and inflammatory response (Yin et al., 2015). The NF-κB signaling pathway is a classical pathway regulating inflammatory responses and processes (Wang et al., 2018). At rest, NF-κB p50 binds to the heterodimer formed by p65 and IκB to form an NF-κB-IκB complex, which exists in the cytoplasm in an inactive state (Karwasra et al., 2019). When stimulated by upstream signals, IκB phosphorylation dissociates from the complex and undergoes degradation, inducing the activation of NF-κB p65 through phosphorylation. The activated NF-κB p65 exposes nuclear localization sequences, which can be redirected into the nucleus, bind to specific sites, initiate gene transcription, induce the production and release of a large number of inflammatory factors (such as TNF-α, IL-1β, and IL-6), and trigger an inflammatory cascade response, ultimately inducing the release of inflammatory factors, regulating oxidative stress, and accelerating inflammation progression (Hong et al., 2018; Xia, et al., 2018). NF-κB signaling pathway-related protein levels are significantly elevated in patients with RA and animal models (Li et al., 2020). Therefore, the NF-κB pathway is a major target in the development of therapeutic agents for RA. Modern studies have shown that the ethanolic extract of C. thalictrifolia Wall. var.mollis Chipp can significantly reduce the expression levels of p-NF-κB p65 and p-IκB proteins and significantly increase the expression levels of IκB proteins in the synovial tissues of CIA model rats and, thus, alleviates synovial pathological damage (Zhao et al., 2021). The glycosides of P. hookeri (C.B.Clarke) Hoeck can reduce the expression levels of TNF-α, IL-1β, IL-17, and NF-κB in AA rats, effectively reduce the swelling of the inflammatory side of the foot and plantar, and improve pathological changes in the synovial joints of AA rats (Ma et al., 2017; Chen et al., 2018).
In addition, the JAK-STAT signaling pathway is a common pathway for multiple cytokine signaling cascades and is involved in the ligand-induced transcriptional activation of target genes (Xin et al., 2020). The binding of ligands to receptors induces JAK phosphorylation, which in turn promotes STAT phosphorylation, thereby regulating the transcription of target genes encoding pro-inflammatory cytokines and chemokines and directly contributing to RA tissue damage (Choe et al., 2013). STAT3 is activated throughout the course of RA, and the p-STAT3 level increases, which aggravates the inflammatory response by inhibiting FLS apoptosis and promoting T-cell survival and other biological effects (Park et al., 2014). Therefore, the targeted inhibition of p-STAT3 activity is a good research direction. The alcoholic extract (2.5 g/kg) of G. macrophylla Pall. inhibited the expression of IL-6 and TNF-α, up-regulated the expression of IL-10, and down-regulated the relative protein expression of p-JAK2 and p-STAT3 in the sera of AA rats, thus regulating the JAK2/STAT3 pathway and inhibiting related responses (Wang et al., 2013). In addition, the administration of 900 mg-kg-1-d-1 by gavage can inhibit the level of the anti-CCP antibody and TNF-α in the sera of rats with collagen-induced arthritis at an early stage, reduce the degree of joint swelling, alleviate the symptoms of synovitis, and protect the joints, showing effectiveness in early RA treatment (Lin et al., 2016). Meanwhile, the PI3K/Akt signaling pathway bridges the imbalance between FLS proliferation and apoptosis. The activation of PI3K increased the expression of chemokine SDF-1, thus increasing the number of osteoclast precursor cells and enhancing migration (Dinesh and Rasool., 2018). The expression of TNF-α-induced B lymphocyte-induced maturation protein 1 (Blimp 1) can be significantly inhibited by blocking the PI3K/Akt pathway, and thus, disturbing the PI3K/Akt pathway may be a novel approach for RA treatment. Through network pharmacology and experimental validation, T.sinensis (Lour.) Merr. can disrupt PI3K-Akt signaling pathway and mediate the expression of the major apoptotic genes of RA, such as synovial fibroblasts and B cells, regulate apoptosis, and alleviate RA (Meng, 2020).
The Ershiwuwei Lvxue Pill is included in the standard of the Tibetan Medicine Ministry (standard number WS3-BC-0149-95). It dispels wind, removes dampness, and dries yellow water and is thus used to treat arthritis, RA, and gout (Tibet Health Bureau et al., 1979b). Pharmacological studies have shown that the Ershiwuwei Lvxue Pill can reduce the levels of serum pro-inflammatory cytokines (TNF-α, IL-6, and IL-17), increase the level of anti-inflammatory cytokine IL-10, down-regulate the mRNA and protein expression levels of Bcl-2, and up-regulate Bax, SOCS1, and SOCS3 (Liu et al., 2021). A total of 180 cases were clinically randomized into three groups, and case-specific information is provided in Supplementary Tables S3. The blank control group received conventional treatment, whereas the test group received the Ershiwuwei Lvxue Pill and conventional treatment. The positive control group received compound Xuanju capsules and conventional treatment. The course of treatment was 12 weeks in all cases. Blood was drawn and sent for examination before and after treatment, and the time of morning stiffness, number of joint swelling, and number of joint pressure pain were determined. The total effective rate of the test group was 91.67%, while that of the positive control group was 88.3%. The combination of the Ershiwuwei Lvxue Pill with non-steroidal anti-inflammatory analgesic and rheumatism improving drugs for RA can significantly improve symptoms with few adverse effects (Lin et al., 2015).
Included in the standard of the Tibetan Medicine Ministry (standard number WS3-BC-0186–95; Tibet Health Bureau et al., 1979c), the Shibawei Dangshen Pill inhibits the expression of IL-17, NF-κB, and TNF-α in the synovial cells of CIA rats and improves joint inflammation. In addition, it increases the level of caspase-3 and decreases the Bcl-2/Bax ratio in the synovial tissues, thus promoting the apoptosis of synovial cells, inhibiting the abnormal proliferation of synovial membrane, and alleviating inflammation in the joints of CIA rats (Zhu et al., 2017). A total of 88 patients with RA (see Supplementary Tables S3 for case information) received one capsule of the Shisanwei Niaopeng Pill every morning on an empty stomach, three capsules of the Shibawei Dangshen Pill at noon, four capsules of the Twenty-Five Wei’er Tea Pill in the afternoon, and three capsules of the Ershiwuwei Lvxue Pill at night, all consumed with warm water. Dosage was reduced according to the severity of the disease. After the treatment, 55 of the 88 rheumatic patients were cured, and the treatments were effective in 29 cases and ineffective in four cases within 1 year. The total efficiency was 95.45% and no side effects or recurrence were found (Long., 2001).
Shiwei Ruxiang Powder is included in the standard of the Tibetan Medicine Ministry (standard number WS3-BC-0212-095). It dispels wind and reduces dampness and is thus used for eczema, RA, gout, and other rheumatic paralysis and “yellow water” disease (Tibet Health Bureau et al., 1979d). Pharmacological studies have shown that Shiwei Ruxiang Powder can regulate the balance of Th17/Treg cells by decreasing the serum level of IL-17A and increasing the level of TGF-β1 in CIA rats, which in turn can significantly reduce the AI score and paw thickness of swollen limbs in CIA rats to treat RA (Ding and Zhang, 2017). A total of 51 patients with RA were selected for clinical treatment with the Tibetan medicine Shiwei Ruxiang Powder. Specific information about the cases is given in Supplementary Tables S3. Clinical effects, symptoms, and changes in inflammatory factors before and after treatment were observed. The results showed that 31 cases were cured and the treatment was effective in 15 cases and ineffective in five cases. The total effective rate was 90.20%. After treatment, the joint pain index, joint swelling, and morning stiffness were significantly improved compared with those before treatment, and the levels of inflammatory factors such as IL-6, TNF-α, and hs-CRP decreased after treatment (Dou, 2017). The Tibetan medicine Shiwei Ruxiang Powder is effective in the treatment of RA, can quickly relieve clinical symptoms, reduce patients’ pain, and reduce the levels of inflammatory factors.
The Twenty-Five Wei’er Tea Pill is included in the standard of the Tibetan Medicine Ministry (standard number WS3-BC-0141–95). It dispels wind, alleviates paralysis, has anti-inflammatory effects, and causes pain relief. It is used for gout, RA, swollen and painful joint deformation, limb stiffness, and “yellow water” disease (Pharmacopoeia Committee of the Ministry of Health of PRC, 1995). The Twenty-Five Wei’er Tea Pill can decrease the serum levels of TNF-α and IL-6, increase the levels of IL-4 and IL-10, and modulate the pathways of histidine, phenylalanine, alanine, aspartate, and glutamate metabolism. A total of 80 patients with RA were equally divided into control and observation groups. The patients in the control group were treated with diclofenac sodium enteric dissolved tablets orally, whereas the patients in the observation group were treated with 25-year-old tea pills orally. The therapeutic effects of the two groups were observed and compared after one course of treatment. The results showed that the total effective rate of the observation group was 92.5%, while that of the control group was 77.5% (Zha, 2017).
This study summarized the theories of TTM regarding the knowledge and treatment of RA. The results show that the direct cause of RA in TTM is the imbalance of the internal and external environment of the body, which leads to the imbalance and dysfunction of the stomach fire, resulting in the gradual transformation of the Bad-kan prevalence into the mKhris-pa and “yellow water” in the body. Lesions appear in the meridians, muscles, and joints throughout the body (Figure 1). In addition, natural Tibetan medicines traditionally used in the Tibetan system for RA treatment were reviewed. The traditional applications and mechanisms of action of these Tibetan medicines for RA are summarized in Table 1. These Tibetan medicines were mainly distributed in 18 families, and the most commonly used family was Gentianaceae. Alkaloids and flavonoids are the main material bases of Tibetan medicines for RA. Their mechanism of action is mainly the immunomodulation and inhibition of inflammatory factors. The pharmacological effects and clinical outcomes of four Tibetan medicinal formulas for RA treatment were then summarized. Overall, the application of Tibetan medicine to RA treatment is a good strategy with satisfactory clinical efficacy and acceptable safety. Compared with expensive drug discovery models for chemical drugs, a drug screening method that originates from practice used in clinics and guided by the long history of Tibetan medicine theory may be advantageous.
However, the limitations of Tibetan medicine research should be considered. Although TTM has a long history of use in the treatment of RA and sufficient practical experience, modern assessments of clinical effectiveness have not been particularly adequate. For example, the overall efficiency of four Tibetan medicine formulas for the treatment of RA is above 90%, but few cases have been included in the study. Studies on the safety of some drugs are inadequate, and thus, their use may cause problems. For example, the Tibetan medicines A. pendulum Busch. and A. flavum Hand-Mazz. are drugs with great medicinal value for RA treatment, but at the same time, they are drugs with co-existing toxic effects. Their active ingredient, aconitine, causes poisoning when it exceeds 1 mg (Chan, 2009). These plants are dangerous to use as medicines if not processed and detoxified. Various processing and detoxification methods have been recorded in ancient books (Pu, 2008), such as processing with highland barley wine, frying with sand, and cooking. However, the specific attenuation mechanisms have not been studied in depth. Therefore, detailed studies on these attenuation processes and exploring the laws of material transformation are necessary.
In conclusion, this study provides the first compilation of data for the ethnomedicinal knowledge of TTM in RA treatment. Further studies on TTM will definitely extend its clinical applications. Tibetan medicines have complex formulations and diverse components. Therefore, while conducting scientific research and clinical trials, on the basis of controlling the quality and stability of Tibetan medicine, we should use multiple disciplines to clarify the pharmacodynamic 1basis of the substances and their mechanism of action, and combine novel technologies to explore some directions related to the diagnosis of RA. At the same time, clinical medicine combined with external treatment with Tibetan medicine is a potential and valuable treatment means, for example, medicated bath, acupuncture, and adjustment of diet and daily life.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
XL, YZ, and YL designed the work and provided financial support; LY carried out the collection of relevant literature; LP and CY sorted out the literature; SL, QS, QZ, and SM were involved in the revision of the data; and XL reviewed the manuscript. All authors wrote, discussed, and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (No. 82130113), the National Key R&D Program of China (2017YFC1703904), the China Postdoctoral Science Foundation (No. 2021MD703800), and the “Xinglin Scholars” Research Promotion Program of Chengdu University of Traditional Chinese Medicine (No. BSH2021009).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.938915/full#supplementary-material
RA, rheumatoid arthritis; TTM, traditional Tibetan medicine; CIA, collagen-induced arthritis; AA, adjuvant-induced arthritis; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; NF-κB, nuclear factor kappa-B; MAPK, mitogen-activated protein kinase; JAK/STAT, Janus kinase/signal transducer and activator of transcription; Nrf2, nuclear factor erythroid 2-related factor 2; COX-2, cyclooxygenase 2; MMP-3, matrix metalloproteinase 3; AAc, 3-acetylaconitine; FOXP3, forkhead box P3; RORγt, retinoic acid-related orphan receptor; PI3K, phosphatidylinositol-3-kinase; VEGF, vascular endothelial growth factor; NLRP3, nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing 3; NQO1, NAD(P)H quinone oxidoreductase 1; LTB4, leukotriene B4; NOS, nitric oxide synthase; IFN-γ, interferon-γ; NSAIDs, non-steroidal anti-inflammatory analgesics drugs; DMARDs, disease-modifying anti-rheumatic drugs; MTX, methotrexate.
Alivernini, S., MacDonald, L., Elmesmari, A., Finlay, S., Tolusso, B., Gigante, M. R., et al. (2020). Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nat. Med. 26 (8), 1295–1306. doi:10.1038/s41591-020-0939-8
Almutairi, K., Nossent, J., Preen, D., Keen, H., and Inderjeeth, C. (2021). The global prevalence of rheumatoid arthritis: A meta-analysis based on a systematic review. Rheumatol. Int. 41 (5), 863–877. doi:10.1007/s00296-020-04731-0
Arima, H., Koirala, S., Nema, K., Nakano, M., Ito, H., Poudel, K. M., et al. (2022). High prevalence of rheumatoid arthritis and its risk factors among Tibetan highlanders living in Tsarang, Mustang district of Nepal. J. Physiol. Anthropol. 41 (1), 12. doi:10.1186/s40101-022-00283-3
Astry, B., Harberts, E., and Moudgil, K, D. (2011). A cytokine-centric view of the pathogenesis and treatment of autoimmune arthritis. J. Interferon Cytokine Res. 31 (12), 927–940. doi:10.1089/jir.2011.0094
Bao, T., Zuo, M., Wang, M., and Wang, Y. (2018). Anti-inflammatory effects of ethanol extracts from Different parts of Gentiana straminea Maxim. J. Chin. Pharm. 29 (22), 3114–3118. doi:10.6039/j.issn.1001-0408.2018.22.20
Bian, B., Ciren, D., and Da, W. (2019). The cognition and treatment of rheumatic diseases in Tibetan medicine Tibet. Sci. Technol. 0 (8), 58–59+75. doi:10.3969/j.issn.1004-3403.2019.08.019
Bystrom, J., Clanchy, F. I., Taher, T. E., Mangat, P., Jawad, A. S., Williams, R. O., et al. (2018). TNFα in the regulation of Treg and Th17 cells in rheumatoid arthritis and other autoimmune inflammatory diseases. Cytokine 101, 4–13. doi:10.1016/j.cyto.2016.09.001
Chan, T. Y. (2009). Aconite poisoning. Clin. Toxicol. 47 (4), 279–285. doi:10.1080/15563650902904407
Chen, K., Wu, H., Fan, H., and Jia, L. (2021a). Effects of alcohol extract of Mongolian medicine Xanthoceras sorbifolia Bunge on serum IL-17 and TNF-α in CIA model rats. Chin. J. Ethnomed Ethnopharm 27 (08), 37–39. doi:10.16041/j.cnki.cn15-1175.2021.08.019
Chen, R., Yuan, M., Yang, Z., Huang, T., Chen, S., and Jiang, Y. (2021b). Study on effects and active ingredients of Tibetan medicine lamiophlomis rotata against rheumatoid arthritis. J. Chin. Pharm. 32 (5), 578–583. doi:10.6039/j.issn.1001-0408.2021.05.12
Chen, S. J., Lin, G. J., Chen, J. W., Wang, K. C., Tien, C. H., Hu, C. F., et al. (2019b). Immunopathogenic mechanisms and novel immune-modulated therapies in rheumatoid arthritis. Int. J. Mol. Sci. 20 (6), 1332. doi:10.3390/ijms20061332
Chen, Y., Yu, H., Guo, F., Wu, Y., and Li, Y. (2018). Antinociceptive and anti-inflammatory activities of a standardizedextract of bis-iridoids from P. hookeri. J. Ethnopharmacol. 216, 233–238. doi:10.1016/j.jep.2018.01.035
Chen, Z., Bozec, A., Ramming, A., and Schett, G. (2019a). Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 15 (1), 9–17. doi:10.1038/s41584-018-0109-2
Deane, K. D., and Holers, V. M. (2021). Rheumatoid arthritis pathogenesis, prediction, and prevention: An emerging paradigm shift. Arthritis Rheumatol. 73 (2), 181–193. doi:10.1002/art.41417
Deng, J., Han, J., Chen, J., Zhang, Y., Huang, Q., Wang, Y., et al. (2021). Comparison of analgesic activities of aconitine in different mice pain models. PLoS One 16 (4), e0249276. doi:10.1371/journal.pone.0249276
Dinesh, P., and Rasool, M. (2018). Berberine inhibits IL-21/IL-21R mediated inflammatory proliferation of fibroblast-like synoviocytes through the attenuation of PI3K/Akt signaling pathway and ameliorates IL-21 mediated osteoclastogenesis. Cytokine 106, 54–66. doi:10.1016/j.cyto.2018.03.005
Ding, J., and Zhang, C. (2017). Effect of Tibetan medicine Shiwei ruxiangsan on rats with rheumatoid arthritis. Med. J. Natl. Defending Forces Northwest China 38 (6), 368–371. doi:10.16021/j.cnki.1007-8622.2017.06.005
Ding, R., Li, P., Song, D., Zhang, X., and Bi, L. (2015). Predictors of response to TNF-α antagonist therapy in Chinese rheumatoid arthritis. Clin. Rheumatol. 34 (7), 1203–1210. doi:10.1007/s10067-015-2973-3
Dou, G, J. (2017). Clinical efficacy of Tibetan medicine Shiwei Ruxiang Powder in the treatment of rheumatoid arthritis. Mod. Wellness (12), 101–102. doi:10.3969/j.issn.1671-0223(x).2017.12.081
Fassio, A., Adami, G., Gatti, D., Orsolini, G., Giollo, A., Idolazzi, L., et al. (2019). Inhibition of tumor necrosis factor-alpha (TNF-alpha) in patients with early rheumatoid arthritis results in acute changes of bone modulators. Int. Immunopharmacol. 67, 487–489. doi:10.1016/j.intimp.2018.12.050
Fu, K., Xu, M., Zhou, Y., Li, X., Wang, Z., Liu, X., et al. (2020). The Status quo and way forwards on the development of Tibetan medicine and the pharmacological research of Tibetan materia Medica. Pharmacol. Res. 155, 104688. doi:10.1016/j.phrs.2020.104688
Ge, Z., and Nima, C. (2021). Literature study on the treatment of Grum bu disease (rheumatoid arthritis) with Tibetan medicine bath method. Yunnan J. Tradit. Chin. Med. 42 (07), 86–88. doi:10.3969/j.issn.1007-2349.2021.07.027
Gu, L., Huang, X., Luo, Y., Tian, L., Hu, M., and Lin, P. (2020). The effects of total flavonoids of Tibetan medicine lianxing jidou on cytokines and cyclooxygenase in adjuvant-induced arthritis rats. West. J. Traditional Chin. Med. 33 (3), 38–41. doi:10.12174/j.issn.1004-6852.2020.03.10
Han, H. (2018). Preliminary study on the basis and metabolism of anti - inflammatory substance of Lapotra bulbifera(Sieb.et Zucc). Beijing: Beijing University of Traditional Chinese Medicine.
Harre, U., and Schett, G. (2017). Cellular and molecular pathways of structural damage in rheumatoid arthritis. Semin. Immunopathol. 39 (4), 355–363. doi:10.1007/s00281-017-0634-0
He, Z. (2021). Based on MH7A cell proliferation, migration, apoptosis and NLRP3 inflammasome to explore the mechanism of the treatment of RA. Yunnan: Yunnan University of Traditional Chinese Medicine.
Hoes, J, N., Jacobs, J, W., Buttgereit, F., and Bijlsma, J, W. (2010). Current view of glucocorticoid co-therapy with DMARDs in rheumatoid arthritis. Nat. Rev. Rheumatol. 6 (12), 693–702. doi:10.1038/nrrheum.2010.179
Hong, H., Zeng, Y., Jian, W., Li, L., Lin, L., Mo, Y., et al. (2018). CDK7 inhibition suppresses rheumatoid arthritis inflammation via blockage of NF-κB activation and IL-1β/IL-6 secretion. J. Cell. Mol. Med. 22 (2), 1292–1301. doi:10.1111/jcmm.13414
Huang, L., Yang, Q., and Xia, J. (2020). Therapeutic effect and mechanism of Geranium wilfordii Maxim on gouty arthritis in rats. Her. Med. 39 (11), 1477–1481. doi:10.3870/j.issn.1004-0781.2020.11.004
Jain, S., Vaidya, A., Gupta, P. K., Rosenholm, J. M., and Bansal, K. K. (2021). Antiarthritic activities of herbal isolates: A comprehensive review. Coatings 11 (11), 1329. doi:10.3390/coatings11111329
Ji, H., and Chen, Y. (2005). Effect of Dengzhanxin injection on related indexes of active rheumatoid arthritis. Inf. Tradit. Chin. Med. (07), 75–76. doi:10.3969/j.issn.1005-5304.2005.07.043
Jia, L., Jia, W., Liu, J., and Chen, K. (2021). Effects of Mongolian medicine Extract of Xanthoceras sorbifolia Bunge on neovascularization in CIA rats. Chin. Chin. J. Clin. Pharmacol. 37 (17), 2308–2311. doi:10.13699/j.cnki.1001-6821.2021.17.015
Jia, N., Chu, W., Li, Y., Ding, L., Duan, J., Cui, J., et al. (2016). Iridoid glycosides from the flowers of Gentiana macrophylla Pall. ameliorate collagen-induced arthritis in rats. J. Ethnopharmacol. 189, 1–9. doi:10.1016/j.jep.2016.05.027
Jia, N., Cui, J., and Wen, A. (2018). Effects of ethanol extract of Gentiana straminea Maxim. on NF-κBp65 expression in synovial tissue of CIA model mice. J. Chin. Pharm. 29 (15), 2082–2085. doi:10.6039/j.issn.1001-0408.2018.15.15
Karwasra, R., Singh, S., Sharma, D., Sharma, S., Sharma, N., and Khanna, K. (2019). Pomegranate supplementation attenuates inflammation, joint dysfunction via inhibition of NF-κB signaling pathway in experimental models of rheumatoid arthritis. J. Food Biochem. 43 (8), e12959. doi:10.1111/jfbc.12959
Kennedy, A., FearonV eale, U. D. J., and Godson, C. (2011). Macrophages in synovial inflammation. Front. Immunol. 2, 52. doi:10.3389/fimmu.2011.00052
Kinne, R. W., Bräuer, R., Stuhlmüller, B., Palombo-Kinne, E., and Burmester, G. R. (2000). Macrophages in rheumatoid arthritis. Arthritis Res. 2 (3), 189–202. doi:10.1186/ar86
Koga, T., Kawakami, A., and Tsokos, G. C. (2021). Current insights and future prospects for the pathogenesis and treatment for rheumatoid arthritis. Clin. Immunol. 225, 108680. doi:10.1016/j.clim.2021.108680
Kung, R., and Liu, Y. (2002). Effect and mechanism of n-butanol extract of Xanthoceras sorbifolia Bunge on AA rats. Chin. J. New Drugs Clin. Remed (04), 229–231+270. doi:10.19378/j.issn.1003-9783.2002.04.013
Li, C. Y., Zhou, Z., Xu, T., Wang, N. Y., Tang, C., Tan, X. Y., et al. (2022a). Aconitum pendulum and Aconitum flavum: A narrative review on traditional uses, phytochemistry, bioactivities and processing methods. J. Ethnopharmacol. 292, 115216. doi:10.1016/j.jep.2022.115216
Li, J., Tang, R. S., Shi, Z., and Li, J. Q. (2020). Nuclear factor-κB in rheumatoid arthritis. Int. J. Rheum. Dis. 23 (12), 1627–1635. doi:10.1111/1756-185X.13958
Li, L., Zhang, L., Liao, T., Zhang, C., Chen, K., and Huang, Q. (2022b). Advances on pharmacology and toxicology of aconitine. Fundam. Clin. Pharmacol. 36, 601–611. doi:10.1111/fcp.12761
Li, Z., Liu, C., and Yu, G. (2013). Effects of Erigeron breviscapus injection on tumor necrosis factor-A and leptin expression in serum of adjuvant arthritis rats. Heilongjiang Med. Sci. 36 (02), 74–75.
Li, Z., Nie, L., Li, Y., Yang, L., Jin, L., Du, B., et al. (2022c). Traditional Tibetan medicine twenty-five wei'er tea pills ameliorate rheumatoid arthritis based on chemical crosstalk between gut microbiota and the host. Front. Pharmacol. 13, 828920. doi:10.3389/fphar.2022.828920
Lin, B., Zhao, Y., Han, P., Yue, W., Ma, X.-Q., Rahman, K., et al. (2014). Anti-arthritic activity of Xanthium strumarium L. extract on complete Freund׳ s adjuvant induced arthritis in rats. J. Ethnopharmacol. 155 (1), 248–255. doi:10.1016/j.jep.2014.05.023
Lin, H., Ding, X, J., Chen, L, F., and Yu, Q. (2015). Clinical research of ershiwuweilvxue powder in treatment of rheumatoid arthritis. J. Hubei Univ. Chin. Med. 17 (2), 24–26. doi:10.3969/j.issn.1008-987x.2015.02.07
Lin, Y., Lin, W., and Deng, L. (2016). Regulation of Gentiana macrophylla Pall. on JAK2/STAT3 signaling pathway in adjuvant arthritis rats. Clin. J. Tradit. Chin. Med. 28 (06), 848–852. doi:10.16448/j.cjtcm.2016.0301
Liu, C., Zhao, Q., Zhong, L., Li, Q., Li, R., Li, S., et al. (2021). Tibetan medicine Ershiwuwei Lvxue Pill attenuates collagen-induced arthritis via inhibition of JAK2/STAT3 signaling pathway. J. Ethnopharmacol. 2021, 113820. doi:10.1016/j.jep.2021.113820
Liu, F., Feng, X, X., Zhu, S, L., Huang, H, Y., Chen, Y, D., Pan, Y, F., et al. (2018). Sonic hedgehog signaling pathway mediates proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis via MAPK/ERK signaling pathway. Front. Immunol. 9, 2847. doi:10.3389/fimmu.2018.02847
Liu, F., Liu, Y., and Zan, S. (2019). Preliminary study on the main active components and their mechanism of action of Terminalia chehula Retz in treating rheumatoid arthritis. PLA Army Mil. Med. Univ. 41 (22), 2238–2245. doi:10.16016/j.1000-5404.201907047
Liu, F., Liu, Y., Zhan, S., Lv, J., Sun, F., Weng, B., et al. (2020). Chebulanin exerts its anti-inflammatory and anti-arthritic effects via inhibiting NF-κB and MAPK activation in collagen-induced arthritis mice. Int. Immunopharmacol. 88, 106823. doi:10.1016/j.intimp.2020.106823
Liu, F., Zhang, P., and Zhao, H. (2017). Study on pharmacological basis and pharmacological action of Terminalia chehula Retz against rheumatoid arthritis. J. Chin. Pharm. 28 (25), 3575–3578. doi:10.6039/j.issn.1001-0408.2017.25.31
Liu, J, M. (2009). Evaluation of clinical efficacy of internal and external treatment of osteoarthritis with Niuxi. World Health Dig. 6 (26), 268–269. doi:10.3969/j.issn.1672-5085.2009.26.306
Long, B. (2001). Clinical observation of 88 cases of rheumatic diseases treated by Tibetan medicine. Sel. Med. Artic. 20 (1), 64–65. doi:10.3969/j.issn.1673-6575.2001.01.059
Ma, A., Yang, Y., Wang, Q., Wang, Y., Wen, J., and Zhang, Y. (2017). Anti-inflammatory effects of oxymatrine on rheumatoid arthritis in rats via regulating the imbalance between Treg and Th17 cells. Mol. Med. Rep. 15 (6), 3615–3622. doi:10.3892/mmr.2017.6484
Ma, Y., Gao, Z., Xu, F., Liu, L., Luo, Q., Shen, Y., et al. (2018). A novel combination of astilbin and low-dose methotrexate respectively targeting A2AAR and its ligand adenosine for the treatment of collagen-induced arthritis. Biochem. Pharmacol. 153, 269–281. doi:10.1016/j.bcp.2018.01.033
Meng, T. (2020). A study on the mechanism of anti-rheumatoid arthritis effect of broad vine based on network pharmacology. Central South University for Nationalities.
Pan, L., Gao, J., Han, Y., Shi, Y., Tang, X., Pu, L., et al. (2021). The treatment of cholecystitis and cholelithiasis by Tibetan medicine. Evid. Based. Complement. Altern. Med. 2021, 9502609. doi:10.1155/2021/9502609
Pang, X, F. (2002). Observation of curative effect of modified decoction of qinjiao on rheumatoid arthritis. Guangxi J. Traditional Chin. Med. 25 (1), 11–12.
Park, J, S., Lee, J., Lim, M, A., Kim, E, K., Kim, S, M., Ryu, J, G., et al. (2014). JAK2-STAT3 blockade by AG490 suppresses autoimmune arthritis in mice via reciprocal regulation of regulatory T Cells and Th17 cells. J. Immunol. 192 (9), 4417–4424. doi:10.4049/jimmunol.1300514
Pu, Y, M. (2008). Preliminary study on the quality evaluation of the Tibetan medicine "reduce toxicity and keep effectiveness" by Aconitum pendulum Busch. Chengdu: Chengdu Unversity of Traditional Chinese Medicine. doi:10.7666/d.d167953
Radner, H., and Aletaha, D. (2015). Anti-TNF in rheumatoid arthritis: An overview. Wien. Med. Wochenschr. 165 (1-2), 3–9. doi:10.1007/s10354-015-0344-y
Sha, H, J. (2004). “TNF-α, ET-1, no,” in Treatment of Erigerontis with Leflunomide on RA and relationship between curative effect and IL-1β (Hebei Medical University). doi:10.7666/d.y603322
Shen, J., Zhang, Y., and Wu, G. (2018). Therapeutic effect of total iridoid glycosides from Gentiana officinalis H.Smith on rat model of osteoarthritis. Chin. J. Clin. Pharm. 34 (03), 85–89.
Shi, F., Li, S, Q., and Shi, H, Q. (2018). Regulation of Oxymatrine on the immunologic balance of rheumatoid arthritis and its effect on inflammatory level. Hainan Med. J. 29 (21), 2997–3000. doi:10.3969/j.issn.1003-6350.2018.21.011
Simon, L. S., Taylor, P. C., Choy, E. H., Sebba, A., Quebe, A., Knopp, K. L., et al. (2021). The jak/STAT pathway: A focus on pain in rheumatoid arthritis. Semin. Arthritis Rheum. 51 (1), 278–284. doi:10.1016/j.semarthrit.2020.10.008
Song, X., Li, B., Zhang, Y., and Ge, W. (2021). Effects of parsnip petroleum ether extract on saposhnikovia divaricata (turcz.) Schischk of P38 MAPK and AQP-1 expression in CIA rats. Chin. J. Clin. Pharm. 37 (05), 80–85. doi:10.13412/j.cnki.zyyl.2021.05.015
Song, X., Xu, B., and Zhang, H. (2020). Effects of total saponins of Achyranthes aspera var.indica L on Th17/Treg balance and IL-2, IL-6 and TNF-α in synovial tissues of rats with rheumatoid arthritis. Tradit Chin Med Res. 33 (03), 70–73. doi:10.3969/j.issn.1001-6910.2019.03.28
Su, J., Li, Q., Liu, J., Wang, H., Li, X., Wüntrang, D., et al. (2021). Ethyl acetate extract of Tibetan medicine Rhamnella gilgitica ameliorated type II collagen-induced arthritis in rats via regulating JAK-STAT signaling pathway. J. Ethnopharmacol. 267, 113514. doi:10.1016/j.jep.2020.113514
Sun, J., Xu, W., and Wang, Y. (2008). Determination of iridoid glycosides in Gentiana officinalis H.Smith by HPLC. Anal. Lab. 12, 51–54. doi:10.3969/j.issn.1000-0720.2008.12.012
Sun, S., Cong, L., and Guo, H. (2016). Effects of total volatile oil of Angelica dahurica on adjuvant arthritis in rats. Chin. J. Gerontol. 36 (22), 3. doi:10.3969/j.issn.1005-9202.2016.22.022
Tanaka, T., Narazaki, M., and Kishimoto, T. (2018). Interleukin (IL-6) immunotherapy. Cold Spring Harb. Perspect. Biol. 10 (8), a028456. doi:10.1101/cshperspect.a028456
Tardito, S., Martinelli, G., Soldano, S., Paolino, S., Pacini, G., Patane, M., et al. (2019). Macrophage M1/M2 polarization and rheumatoid arthritis: A systematic review. Autoimmun. Rev. 18 (11), 102397. doi:10.1016/j.autrev.2019.102397
Thakur, S., Riyaz, B., Patil, A., Kaur, A., Kapoor, B., and Mishra, V. (2018). Novel drug delivery systems for NSAIDs in management of rheumatoid arthritis: An overview. Biomed. Pharmacother. 106, 1011–1023. doi:10.1016/j.biopha.2018.07.027
Ur Rashid, H., Xu, Y., Ahmad, N., Muhammad, Y., and Wang, L. (2019). Promising anti-inflammatory effects of chalcones via inhibition of cyclooxygenase, prostaglandin E(2), inducible NO synthase and nuclear factor κb activities. Bioorg. Chem. 87, 335–365. doi:10.1016/j.bioorg.2019.03.033
Wan, Y., Wang, H., and Feng, D. (2014). Effects of Geranium wilfordii Maxim. extract on expression of VEGF and TGF-β1 in serum of AA rats. Hubei Agric. Sci. 53 (09), 2111–2113. doi:10.14088/j.cnki.issn0439-8114.2014.09.055
Wang, B., Wang, F., Song, L., and Pang, T. (2021). Comparative analysis of efficacy and hepatotoxicity of Lycopodium Clavatum L and methotrexate in rats with rheumatoid arthritis. Mod. Med. Clin. 36 (05), 861–865. doi:10.7501/j.issn.1674-5515.2021.05.001
Wang, F., Liu, C, X., Tian, G, Z., Geng, C, W., Cui, R, X., and Lv, C, H. (2015). Clinical study on the treatment of wind-cold and damp paralysis type knee osteoarthritis by adding reduction to. Fangfeng Tang (7), 866–867. doi:10.3969/j.issn.1000-7369.2015.07.052
Wang, G., Wang, L., and Wang, J. (2013). Effects of ethanol extract of Gentiana macrophylla Pall on anti-CCP and TNF-α in serum of CIA rats. Chin. J. Tradit. Chin. Med. 19 (19), 302–305. doi:10.11653/syfj2013190302
Wang, L., He, C., and Liu, C. (2019). Research progress on the active constituents and their efficacy in Geranium wilfordii Maxim. Jiangxi Acta Agricu 31 (10), 63–69. doi:10.19386/j.cnki.jxnyxb.2019.10.11
Wang, Q., Zhou, X., Zhao, Y., Xiao, J., Lu, Y., Shi, Q., et al. (2018). Polyphyllin I ameliorates collagen-induced arthritis by suppressing the inflammation response in macrophages through the NF-κB pathway. Front. Immunol. 9, 2091. doi:10.3389/fimmu.2018.02091
Wei, S. (2010). Study on chemical constituents bioactivity and determination of iridoid content of Gentiana macrophylla. Beijing: Beijing University of Chemical Technology. doi:10.7666/d.y1672901
Wu, Y., and Yang, Y. (2003). Therapeutic effect of Dengzhanxin injection on active rheumatoid arthritis. Guangzhou Med. J. 12, 1367–1368. doi:10.13820/j.cnki.gdyx.2003.12.069
Xia, Z. B., Yuan, Y. J., Zhang, Q. H., Li, H., Dai, J. L., and Min, J. K. (2018). Salvianolic acid B suppresses inflammatory mediator levels by downregulating NF-κB in a rat model of rheumatoid arthritis. Med. Sci. Monit. 24, 2524–2532. doi:10.12659/msm.907084
Xian, B. (2016). 40 cases of rheumatoid arthritis swelling and pain treated with external application of "Cujing Qinjiao" powder. J. Med. Pharm. Chin. Minorities 22 (525), 42. doi:10.3969/j.issn.1006-6810.2016.05.017
Xin, P., Xu, X., Deng, C., Liu, S., Wang, Y., Zhou, X., et al. (2020). The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 80, 106210. doi:10.1016/j.intimp.2020.106210
Xiong, H., Ding, X., Wang, H., Jiang, H., Wu, X., Tu, C., et al. (2019). Tibetan medicine Kuan-Jin-Teng exerts anti-arthritic effects on collagen-induced arthritis rats via inhibition the production of pro-inflammatory cytokines and down-regulation of MAPK signaling pathway. Phytomedicine 57, 271–281. doi:10.1016/j.phymed.2018.12.023
Yang, X., Zhao, F, L., Wang, Q., Lai, H., and Mi, X, Q. (2018). Analysis of clinical effect of Baizhi topical application combined with conventional drugs in the treatment of rheumatoid arthritis. J. North Pharm. 15 (80), 38–39. doi:10.3969/j.issn.1672-8351.2018.08.029
Yang, Y., Dong, Q., and Li, R. (2017). Matrine induces the apoptosis of fibroblast-like synoviocytes derived from rats with collagen-induced arthritis by suppressing the activation of the JAK/STAT signaling pathway. Int. J. Mol. Med. 39 (2), 307–316. doi:10.3892/ijmm.2016.2843
Yao, W., Chen, X., and Zhang, Y. (2014). Preliminary study on the analgesic effect and the mechanism of 3-acetyl aconitine. J. Ningxia Med. 36 (5), 3. doi:10.13621/j.1001-5949.2014.05.0388
Yao, Y. (2008). Study on the therapeutic effect and mechanism of effective parts of Lapotra bulbifera(Sieb.et Zucc.)Wedd on rheumatoid arthritis. Huazhong: Huazhong University of Science and Technology. doi:10.7666/d.d064358
Ye, F., Yang, H, B., and Xu, Y, C. (2007). Clinical observation of 69 cases of arthritis deformans treated with duyiwei capsule. World Chin. Med. 2 (6), 339–340. doi:10.3969/j.issn.1673-7202.2007.06.007
Yin, G., Wang, Y., Cen, X, M., Yang, M., Liang, Y., and Xie, Q. B. (2015). Lipid peroxidation-mediated inflammation promotes cell apoptosis through activation of NF-κB pathway in rheumatoid arthritis synovial cells. Mediat. Inflamm. 2015, 460310. doi:10.1155/2015/460310
Yu, J, P. (2005). The efficacy of topical treatment of rheumatoid arthritis in 30 cases with Xanthoceras sorbifolia Bunge. Jiangxi J. Traditional Chin. Med. 36 (2), 31.
Yu, S., Zeng, R., Han, Z., Zeng, Y., Yang, L., Qu, Y., et al. (2021). Isolation, purification, structure characterization, and anti-inflammatory activity of Gentiana crassicaulis polysaccharides. Chin. Herb. Med. 52 (03), 635–642. doi:10.7501/j.issn.0253-2670.2021.03.005
Yuan, K., Zhu, Q., Lu, Q., Jiang, H., Zhu, M., Li, X., et al. (2020). Quercetin alleviates rheumatoid arthritis by inhibiting neutrophil inflammatory activities. J. Nutr. Biochem. 84, 108454. doi:10.1016/j.jnutbio.2020.108454
Zeng, C., Wu, F., and Dai, W. (2017). The effect of Kuanjin Teng on ankle histopathology in rheumatoid arthritis model rats. Nat. Prod. Res. Dev. 29 (01), 147–151. doi:10.16333/j.1001-6880.2017.1.028
Zha, S. (2017). Twenty five catechu pill in the treatment of rheumatoid arthritis curative effect is discussed in this paper. Smart Healthc. 3 (5), 67–68. doi:10.19335/j.cnki.2096-1219.2017.05.28
Zhang, A., and Lee, Y, C. (2018). Mechanisms for joint pain in rheumatoid arthritis (RA): From cytokines to central sensitization. Curr. Osteoporos. Rep. 16 (5), 603–610. doi:10.1007/s11914-018-0473-5
Zhang, H., Chen, L., Sun, X., Yang, Q., Wan, L., and Guo, C. (2020a). Matrine: A promising natural product with various pharmacological activities. Front. Pharmacol. 11, 588. doi:10.3389/fphar.2020.00588
Zhang, M, R., Jiang, K., Yang, J, L., and Shi, Y, P. (2020b). Flavonoids as key bioactive components of Oxytropis falcata bunge, a traditional anti-inflammatory and analgesic Tibetan medicine. Nat. Prod. Res. 34 (23), 3335–3352. doi:10.1080/14786419.2019.1574786
Zhang, Q., Liu, Q., Lin, C., Baima, Y., Li, H., Gong, H., et al. (2020c). The prevalence of rheumatoid arthritis in middle-aged and elderly people living in Naqu City, Tibet, Autonomous Region of China. J. Orthop. Surg. Res. 15 (1), 338. doi:10.1186/s13018-020-01883-4
Zhang, Y., Bi, Y., and Jiang, H. (2021). Effects of total alkaloids of Lycopodium Clavatum L on proliferation and il-17A and TGF-β levels of spleen lymphocytes in rats with rheumatoid arthritis. Tradit. West Med. 30 (31), 3426–3430. doi:10.3969/j.issn.1008-8849.2021.31.002
Zhang, Y., Liu, J., and Wu, J. (2013). Study on the therapeutic mechanism of Aconitum pendulum Busch on AA rats. Zhengzhou, China: Chinese Anatomy Society annual Meeting.
Zhang, Y., Xie, X., Ao, P., and Liu, Y. (2020d). Effects of Lycopodium Clavatum L on ICAM-1, MMP-3 and OPG/RANKL/RANK signaling pathways in synovium of ankle joint in rats with rheumatoid arthritis. Inf. Tradit. Chin. Med. 48 (11), 10–14. doi:10.19664/j.cnki.1002-2392.200186
Zhao, C. (2014). Observation on clinical therapertic effect of Tie Bang Chui acesodyne plaster for treatment of knee osteoarthritis. China Pract. Med. (7), 11–13.
Zhao, J., Guo, R., and Luobu, Z. (2021a). Effect of Codonopsis tralictrifolia extract on collagen-induced arthritis model rats and its mechanism. J. Chin. Pharm. 32 (08), 967–973. doi:10.6039/j.issn.1001-0408.2021.08.12
Zhao, X., Jiang, S., Dong, Q., Dang, J., Liu, Z., Han, H., et al. (2021b). Anti-rheumatoid arthritis effects of iridoid glucosides from Lamiophlomis rotata (Benth.) kudo on adjuvant-induced arthritis in rats by OPG/RANKL/NF-κB signaling pathways. J. Ethnopharmacol. 266, 113402. doi:10.1016/j.jep.2020.113402
Zhao, Y., Liu, F., Liu, Y., Zhou, D., Dai, Q., and Liu, S. (2015). Anti-arthritic effect of chebulanin on collagen-induced arthritis in mice. PLoS One 10 (9), e0139052. doi:10.1371/journal.pone.0139052
Zhijia, G., and Ciren, N. (2021). The research progress with characteristic therapy of Tibetan medicine in grum bu disease(rheumatoid arthritis). Asia-Pacific Tradit. Med. 17 (11), 27–29. doi:10.11954/ytctyy.202111008
Keywords: rheumatoid arthritis, traditional Tibetan medicine, bioactive components, immunomodulation, perspectives
Citation: Yu L, Li S, Pu L, Yang C, Shi Q, Zhao Q, Meniga S, Liu Y, Zhang Y and Lai X (2022) Traditional Tibetan medicine: therapeutic potential in rheumatoid arthritis. Front. Pharmacol. 13:938915. doi: 10.3389/fphar.2022.938915
Received: 08 May 2022; Accepted: 15 August 2022;
Published: 04 October 2022.
Edited by:
Adolfo Andrade-Cetto, National Autonomous University of Mexico, MexicoReviewed by:
Asadollah Mohammadi, Kurdistan University of Medical Sciences, IranCopyright © 2022 Yu, Li, Pu, Yang, Shi, Zhao, Meniga, Liu, Zhang and Lai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Liu, MTA1MTE0MDE4QHFxLmNvbQ==; Yi Zhang, emhhbmd5aUBjZHV0Y20uZWR1LmNu; Xianrong Lai, MTA1MTE0MDE4QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.