
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 29 August 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.938472
Objective: Malignant ascites (MA) is a common complication of terminal cancer, which seriously affects the life quality and prognosis of patients. Both hyperthermic intraperitoneal chemotherapy (HIPEC) and traditional Chinese medicine (TCM) preparations have achieved significant efficacy in the treatment of MA. The treatment strategy of TCM combined with HIPEC has been gradually promoted and applied in China. The purpose of this systematic review and meta-analysis was to assess the efficacy of TCM combined with HIPEC in the treatment of MA.
Methods: Randomized controlled trials (RCTs) of TCM combined with HIPEC for MA were searched from seven electronic databases. Two researchers used the Cochrane Collaboration’s tool to assess the risk of bias. Excel 2019 was used to establish a database for information extraction, RevMan 5.4 software was used to analyze the included test data, and STATA v16.0 was used to conduct Egger’s test to further detect publication bias.
Results: A total of 19 studies involving 1,504 patients were included in this meta-analysis. The results showed that compared with the single use of HIPEC, TCM combined with HIPEC could significantly improve the clinical efficacy (RR = 1.51, 95% CI [1.40, 1.63], p < 0.00001) and karnofsky performance status (KPS) score (MD = 8.16, 95% CI [6.46, 9.85], p < 0.00001), reduce the ascites volume (MD = −156.98, 95% CI [−213.71, −100.25], p < 0.00001). However, there was no statistical significance in reducing abdominal circumference between TCM combined with HIPEC and HIPEC alone (MD = −1.8, 95% CI [−4.57, −0.97], p = 0.2).
Conclusion: This study found that TCM combined with HIPEC had a beneficial therapeutic effect on MA. However, more standard, double-blind, multicenter RCTs are needed to further confirm the efficacy of TCM combined with HIPEC in the treatment of MA.
Systematic Review Registration: https://www.crd.york.ac.uk/, identifier CRD42022319993.
Malignant ascites (MA) is a common complication of peritoneal disseminated tumors such as ovarian cancer, breast cancer, intestinal cancer, gastric cancer and pancreatic cancer (Becker et al., 2006). Modern medicine believes that its mechanism is mostly related to the damage of abdominal wall serosa and secretion of certain mediators by metastatic cancer cells in the abdominal wall, resulting in increased peritoneal vascular permeability, excessive fluid production, and hypoproteinemia, which further leads to hydrodynamic imbalance, portal vein obstruction, subdiaphragmatic lymphatic vessel and vein return disorders (Zhan et al., 2016; Ito and Hanafusa, 2017). If no effective intervention measures are taken for patients with MA, symptoms such as abdominal distention, abdominal pain, nausea, vomiting, and even dyspnea may occur, seriously affecting the life quality and prognosis of patients (Maeda et al., 2015; Hodge and Badgwell, 2019).
Commonly used methods for the clinical treatment of MA include abdominal paracentesis drainage, diuretics, chemotherapy, immunotherapy, targeted therapy, etc. These treatment methods often have disadvantages such as side effects, high price and drug resistance, which limit the overall therapeutic effect. Hyperthermic intraperitoneal chemotherapy (HIPEC) is an emerging treatment method for peritoneal disseminated tumors in recent years, which has a wide range of indications, simple operation and remarkable efficacy, and has achieved certain efficacy in the control of MA (Robella et al., 2019). However, in actual clinical practice, due to the differences of individual efficacy, single use of HIPEC cannot completely and effectively control the condition of each patient. In clinical practice, appropriate and effective treatment plans need to be formulated according to the specific situation of patients.
Traditional Chinese medicine (TCM) is a medical system with unique theoretical style and characteristics of diagnosis and treatment formed gradually in long-term medical practice. Studies have shown that TCM treatment can effectively curb the generation of MA, alleviate the toxicity of chemotherapy, relieve the clinical symptoms of patients, improve the immune function and quality of life of patients, and prolong the survival period, and TCM treatment has few side effects and high safety (Kong, 2021). TCM is considered to be used in combination with HIPEC in patients with MA, which can improve the efficacy of drugs and reduce the side effects of drugs, and is widely used in the treatment of MA (Wang, 2020). Therefore, it is of great significance to further evaluate the clinical value of HIPEC combined with TCM on the basis of evidence-based medicine. This paper was based on a systematic review of randomized controlled trial (RCTS) of TCM including oral and external administration combined with HIPEC for MA. The purpose is to obtain evidence of the efficacy and safety of TCM adjuvant treatment of MA, and to provide evidence-based medical basis for its treatment.
This systematic review and meta-analysis followed the PRISMA guidelines and were registered in the PROSPERO database (https://www.crd.york.ac.uk/). The registration number is CRD42022319993.
We searched PubMed, Cochrane Library, Embase, China National Knowledge Infrastructure (CNKI), Wanfang Database, China Science and Technology Journal Database (VIP) and Sinomed. The search time was from establishment to 20 March 2022. The following search terms were used: “traditional Chinese medicine,” “herbs,” “ascites,” “peritoneal effusion,” “malignant ascites,” “malignant peritoneal effusion,” “hyperthermic perfusion,” “hyperthermic intraperitoneal chemotherapy,” “peritoneal hyperthermic perfusion chemotherapy.” Details of the search strategies were available in Supplementary Material. In addition, we searched conference literature and clinical registry data for relevant trials that might have been missing in web searches. This study was conducted independently by two researchers (JC and ZL).
Inclusion criteria were as follows: 1) Study design: All included studies were RCTs. 2) Participants: Malignant tumors were diagnosed by histopathology and/or cytology. At the same time, ascites was confirmed by imaging examination (B-ultrasound and/or computed tomography) and cancer cells were found in ascites cytology. 3) Interventions: The control group received HIPEC, in combination with or without intravenous chemotherapy, with no restrictions on the drugs for HIPEC, dosage or course of treatment. On the basis of the control group, the trial group was given oral or external TCM treatment, and there were not any restrictions on the prescription composition, dosage and course of treatment of TCM. 4) Outcomes: The main outcome was clinical efficacy. The efficacy was evaluated according to the malignant tumor related curative effect standards formulated by WHO (Xu, 2005). Complete remission (CR) is defined as complete absorption of ascites with a duration >4 weeks. Partial remission (PR) is the reduction of ascites ≥50% and the duration >4 weeks. Stable disease (SD) is a decrease in ascites <50% or increase in ascites <25%. Progression disease (PD) is a significant increase in ascites ≥25%. Clinical efficacy = CR + PR. The secondary outcomes were the comparison of ascites volume, abdominal circumference, and karnofsky performance status (KPS) score after treatment. The included studies reported at least one of the above outcomes.
Exclusion criteria were as follows: 1) The full text cannot be obtained through electronic retrieval, manual retrieval and author’s email. 2) Studies with the following patients: Patients with severe heart, liver, kidney and other organic diseases; patients complicated with blood system, immune system and serious infectious diseases; patients with severe peritoneal adhesion; ascites caused by other diseases; patients with contraindications to chemotherapy. 3) Drugs for HIPEC included non-chemotherapy drugs in the studies. 4) The intervention measures in the trial group included TCM injection. 5) Studies that lacked outcome data or cannot be analyzed.
Two researchers (JC and ZL) carried out the study selection and data extraction independently, and the third researcher (YL) resolved any disagreement. Using Excel 2019 establishing database of information extraction, we extracted the following information from the included studies: general information (the first author, year of publication), participants characteristics (age, gender, sample size, type of cancer), interventions (drugs for HIPEC, ways of using TCM, herbs in prescriptions, course of treatment), and outcomes.
Two researchers (JC and ZL) independently assessed the risk of bias for included studies using the Cochrane Collaboration’s risk of bias tool (Higgins et al., 2011). The risk of bias assessment tool includes the following assessments: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. After the risk of bias assessment, each item can be classified as “high risk of bias,” “risk of unilateral bias” or “low risk of bias.” If there is a difference in the assessment results of two researchers, it can be resolved by consultation with the third researcher.
RevMan (Review Manager 5.4) statistical software provided by the Cochrane Collaboration was used for data analysis (Cochrane, 2012). Dichotomous data were expressed as risk ratio (RR) with 95% confidence interval (CI). Continuous data were expressed as mean difference (MD) with 95% CI. Chi-square and I-Square (I2) indexes were used for heterogeneity test. When p ≥ 0.05 and I2 ≤ 50%, the fixed-effect model was selected. Otherwise, the random-effect model was selected. We conducted subgroup analysis according to whether it was combined with intravenous chemotherapy, different ways of using TCM, different drugs for HIPEC, and different course of treatment to further verify the efficacy of TCM combined with HIPEC in the treatment of MA. p ≤ 0.05 was considered statistically significant, and all tests were two-sided tests. In addition, for a single outcome, if the number of studies analyzed exceeds 10, a funnel plot will be drawn to assess the existence of publication bias (Pérez and Rodrı́guez, 2006), and STATA v16.0 was used to conduct Egger’s test to further detect publication bias (Egger et al., 1997). Sensitivity analysis was performed by removing individual studies to assess the stability of the results. In addition, Excel software was used to analyze the frequency and medicinal properties of herbs. Pharmacopoeia of the People’s Republic of China (The Chinese pharmacopoeia, the first part, 2015) was the standard for the names and efficacy classifications of herbs in prescriptions, supplemented by the Traditional Chinese Pharmacology and Chinese Materia Medica (Qiu, 2006; Gao, 2010).
We retrieved 336 potentially relevant articles from seven electronic databases (PubMed, Cochrane Library, Embase, CNKI, Wanfang database, VIP and Sinomed). After we used EndNote software to delete duplicate articles, we retained 125 studies for further confirmation. Then, by reading titles and abstracts, 90 articles (83 unrelated studies, four retrospective studies, 2 case reports and one review) that did not meet the criteria for full text review were deleted. After reading the full text of the remaining 35 articles, 16 articles were further removed (1 participant did not meet the inclusion criteria, 12 interventions in the control and trial groups did not meet the inclusion criteria, and three lacked outcome data). Finally, we included 19 eligible studies for comprehensive analysis. The screening process was shown in Figure 1.
A total of 19 RCTs were included in this study, with a total of 1,504 patients, including 762 patients in the trial group and 742 patients in the control group. All studies were conducted in China between 2010 and 2021. Seven of the studies (Wen et al., 2015; Dai et al., 2016; Chen and Hua, 2017; Gao and Zhang, 2017; Zhang et al., 2018; Shao et al., 2019; Rui and Peng, 2021) included only ovarian cancer patients, one study (Zhang and Li, 2018) only included gastric cancer patients, nine studies (Cui and Wu, 2010; Wu et al., 2016; Pan et al., 2017; Zhang et al., 2017; Jiang and Hu, 2019; Cai and Zhu, 2020; Li et al., 2020; Mei et al., 2020; Li, 2021) included multiple tumor types, two studies (Li, 2020; Zhang et al., 2020) did not report the tumor type. The control group in five studies (Wu et al., 2016; Pan et al., 2017; Zhang et al., 2018; Shao et al., 2019; Rui and Peng, 2021) were treated with HIPEC combined with intravenous chemotherapy, and the trial group were treated with TCM on this basis, and the control group in remaining 14 studies (Cui and Wu, 2010; Wen et al., 2015; Dai et al., 2016; Chen and Hua, 2017; Gao and Zhang, 2017; Zhang et al., 2017; Zhang and Li, 2018; Jiang and Hu, 2019; Cai and Zhu, 2020; Li, 2020; Li et al., 2020; Mei et al., 2020; Zhang et al., 2020; Li, 2021) only received HIPEC, and the trial group added TCM on this basis. One study (Dai et al., 2016) used paclitaxel single agent for HIPEC, one study (Zhang and Li, 2018) used 5-fluorouracil and cisplatin for HIPEC, and the remaining 17 studies (Cui and Wu, 2010; Wen et al., 2015; Wu et al., 2016; Chen and Hua, 2017; Gao and Zhang, 2017; Pan et al., 2017; Zhang et al., 2017; Zhang et al., 2018; Jiang and Hu, 2019; Shao et al., 2019; Cai and Zhu, 2020; Li, 2020; Li et al., 2020; Mei et al., 2020; Zhang et al., 2020; Li, 2021; Rui and Peng, 2021) used cisplatin single agent for HIPEC. The trial group in 15 studies (Cui and Wu, 2010; Dai et al., 2016; Wu et al., 2016; Chen and Hua, 2017; Gao and Zhang, 2017; Pan et al., 2017; Zhang et al., 2018; Jiang and Hu, 2019; Shao et al., 2019; Cai and Zhu, 2020; Li, 2020; Mei et al., 2020; Zhang et al., 2020; Li, 2021; Rui and Peng, 2021) was treated with oral administration of TCM, the trial group in four studies (Wen et al., 2015; Zhang et al., 2017; Zhang and Li, 2018; Li et al., 2020) were treated with external application of TCM. Table 1 summarized the patient characteristics of the included studies, including age, gender, sample size, interventions, the course of treatment, outcomes, etc.
We used the Cochrane Collaboration’s risk of bias tool to assess the quality of studies. 1) Selection bias (Random sequence Generation): 11 studies (Dai et al., 2016; Zhang et al., 2017; Zhang et al., 2018; Zhang and Li, 2018; Jiang and Hu, 2019; Shao et al., 2019; Li, 2020; Li et al., 2020; Mei et al., 2020; Li, 2021; Rui and Peng, 2021) used a random table for random allocation, and one study (Cai and Zhu, 2020) used draw method for random allocation, and the risk of selection bias was considered “low.” Seven studies (Cui and Wu, 2010; Wen et al., 2015; Wu et al., 2016; Chen and Hua, 2017; Gao and Zhang, 2017; Pan et al., 2017; Zhang et al., 2020) claimed that randomization was used, but did not report details of how randomization was done, and the risk of selection bias was considered “unclear.” 2) Selection bias (allocation concealment): None of the studies reported allocation concealment, and the risk of selection bias was considered “unclear.” 3) Performance bias and detection bias: None of the studies showed whether blind method was used. However, considering that these studies used objective outcome indicators, the results were not interfered by researchers and participants. Therefore, the risk of performance bias and detection bias were considered “low.” 4) Attrition bias: There was no missing data in all included studies, so the risk of attrition bias was considered “low.” 5) Reporting bias and other bias: None of the studies had enough information to assess whether there was a risk of selective reporting and other bias, and therefore they were identified as “unclear risks.” All risk of bias assessment data was shown in Figure 2.
The frequency of each herb in the prescription for MA was ranked from high to low. The top three were atractylodes macrocephala Koidz, poria cocos (Schw.) Wolf, astragalus membranaceus (Fisch.) Bunge, as shown in the Table 2. We made statistics on the medicinal properties of herbs commonly used in MA. The top three were qi-invigorating herbs, herbs for inducing diuresis and excreting, heat-clearing herbs, as shown in the Table 3.
Nineteen studies (Cui and Wu, 2010; Wen et al., 2015; Dai et al., 2016; Wu et al., 2016; Chen and Hua, 2017; Gao and Zhang, 2017; Pan et al., 2017; Zhang et al., 2017; Zhang et al., 2018; Zhang and Li, 2018; Jiang and Hu, 2019; Shao et al., 2019; Cai and Zhu, 2020; Li, 2020; Li et al., 2020; Mei et al., 2020; Zhang et al., 2020; Li, 2021; Rui and Peng, 2021) reported clinical efficacy with a total of 1,504 patients, including 762 patients in the trial group and 742 patients in the control group. Due to the low heterogeneity of the data (I2 = 5%, p = 0.4), a fixed-effect model was used. The results showed that the clinical efficacy of TCM combined with HIPEC (608/762) was significantly better than that of the single HIPEC (392/742) (RR = 1.51, 95% CI [1.40, 1.63], p < 0.00001, Figure 3).
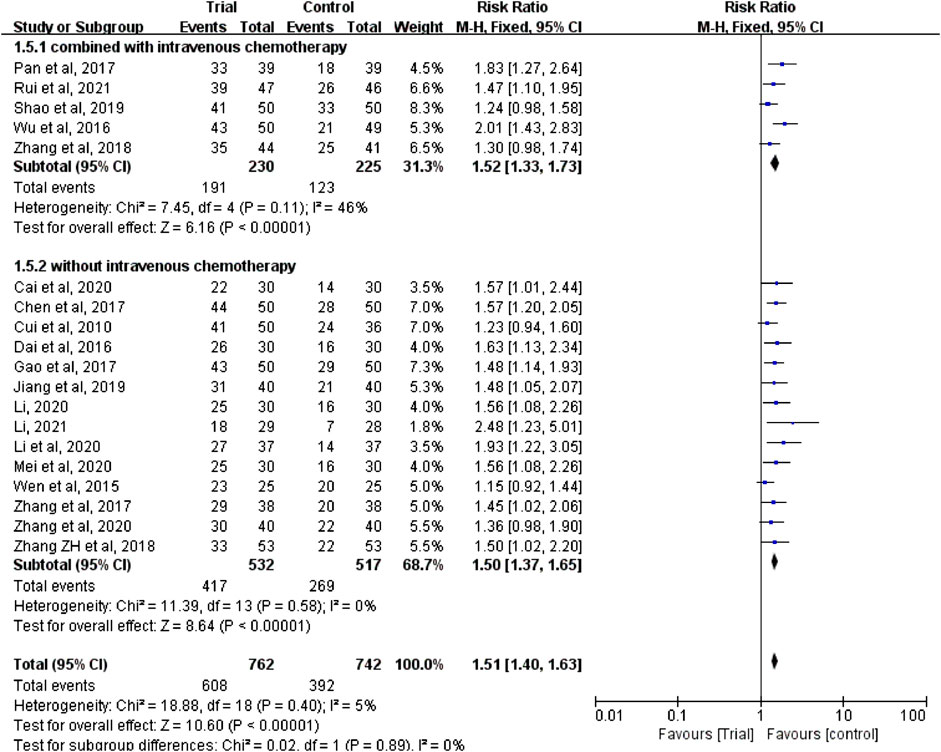
FIGURE 3. Subgroup analysis of clinical efficacy according to whether combined with intravenous chemotherapy.
In these studies, a subgroup analysis of clinical efficacy was performed according to whether or not it was combined with intravenous chemotherapy (Figure 3). The results showed that the heterogeneity was increased in the subgroup with intravenous chemotherapy (I2 = 46%) and the heterogeneity was decreased in the subgroup without intravenous chemotherapy (I2 = 0%), but with or without intravenous chemotherapy, the clinical efficacy of the trial group was better than that of the control group (RR = 1.52, 95% CI [1.33, 1.73], p < 0.00001; RR = 1.5, 95% CI [1.37, 1.65], p < 0.00001). A subgroup analysis of clinical efficacy was performed according to different ways of using TCM (oral or external) (Figure 4). The results showed that the heterogeneity was decreased (I2 = 0%) in the subgroup of oral administration of TCM (I2 = 0%) and heterogeneity was increased (I2 = 43%) in the subgroup of external application of TCM, but regardless of whether the TCM was used orally or externally, the clinical efficacy of the trial group was better than that of the control group (RR = 1.53, 95% CI [1.41, 1.67], p < 0.00001; RR = 1.41, 95% CI [1.20, 1.67], p < 0.0001). We also performed a subgroup analysis of clinical efficacy according to different drugs for HIPEC (cisplatin single drug or others) (Figure 5). The results showed that heterogeneity was increased in the subgroup of receiving cisplatin single drug for HIPEC (I2 = 14%) and decreased in the subgroup of receiving HIPEC without cisplatin single drug (I2 = 0%), but the clinical efficacy of the trial group was better than that of the control group no matter what kind of chemotherapy drugs were used for HIPEC (RR = 1.5, 95% CI [1.39, 1.63], p < 0.00001; RR = 1.55, 95% CI [1.19, 2.03], p = 0.001). In addition, we also performed subgroup analysis of clinical efficacy according to the different course of treatment (course of treatment ≤ 4 weeks or 4 weeks < course of treatment ≤ 8 weeks or course of treatment > 8 weeks) (Figure 6). The results showed that the heterogeneity was basically unchanged in the subgroup with a course of treatment ≤4 weeks (I2 = 4%). The heterogeneity was decreased in the subgroups with 4 weeks < course of treatment ≤8 weeks and course of treatment >8 weeks (I2 = 0%). When we analyzed the clinical efficacy of these three subgroups, we found that the clinical efficacy of the trial group was better than that of the control group (RR = 1.53, 95% CI [1.35, 1.72], p < 0.00001; RR = 1.4, 95% CI [1.22, 1.59], p = 0.00001; RR = 1.72, 95% CI [1.45, 2.03], p < 0.00001).
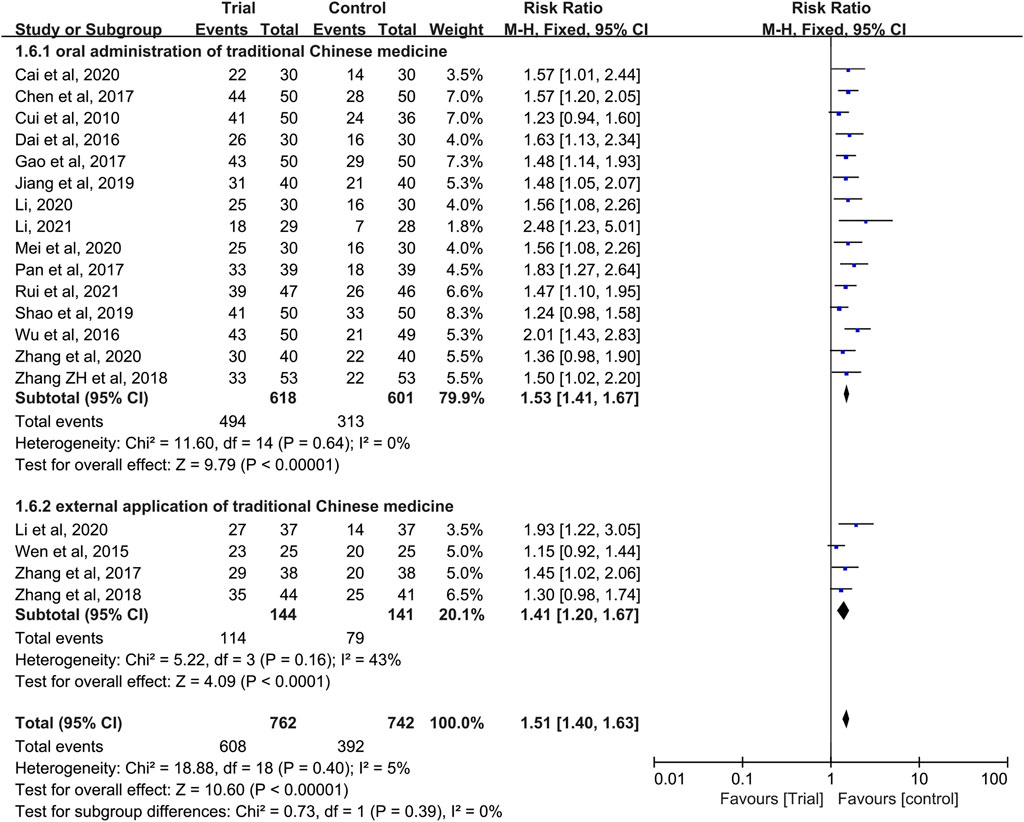
FIGURE 4. Subgroup analysis of clinical efficacy according to different ways of using traditional Chinese medicine.
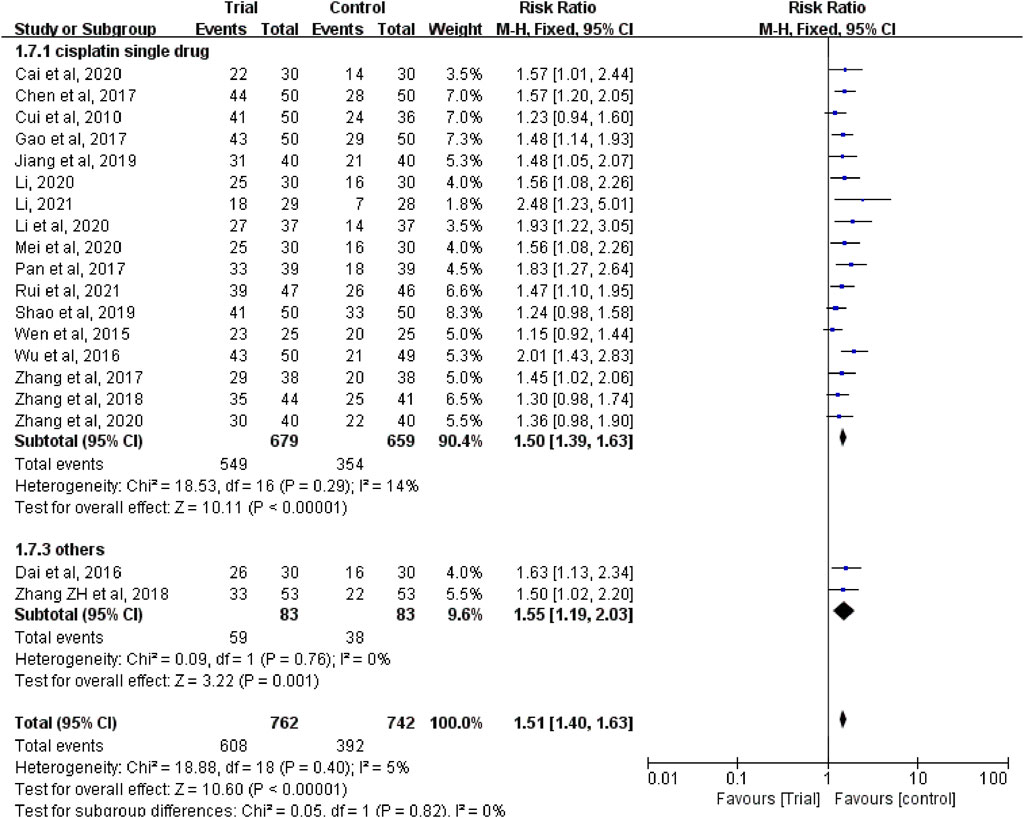
FIGURE 5. Subgroup analysis of clinical efficacy according to different drugs for hyperthermic intraperitoneal chemotherapy.
Three studies (Chen and Hua, 2017; Zhang et al., 2018; Shao et al., 2019) reported the ascites volume with a total of 285 patients, including 144 patients in the trial group and 141 patients in the control group. Owing to heterogeneity between studies (I2 = 98%, p < 0.00001), a random-effect model was used. The results showed that TCM combined with HIPEC could effectively reduce the ascites volume (MD = −156.98, 95% CI [−213.71, −100.25], p < 0.00001, Figure 7).
Abdominal circumference data can be clearly extracted from four studies (Zhang et al., 2017; Cai and Zhu, 2020; Li et al., 2020; Li, 2021), with a total of 267 patients, including 134 patients in the trial group and 133 patients in the control group. Due to heterogeneity between studies (I2 = 81%, p = 0.001), a random-effect model was used. The results showed that the result was not statistically significant (MD = −1.8, 95% CI [−4.57, −0.97], p = 0.2, Figure 8).
Eleven studies (Wen et al., 2015; Dai et al., 2016; Chen and Hua, 2017; Gao and Zhang, 2017; Zhang et al., 2017; Zhang et al., 2018; Li, 2020; Li et al., 2020; Mei et al., 2020; Li, 2021; Rui and Peng, 2021) reported the KPS score with a total of 815 patients, including 410 patients in the trial group and 405 patients in the control group. Due to heterogeneity between studies (I2 = 68%, p = 0.0006), a random-effect model was used. The results showed that the KPS score of TCM combined with HIPEC was significantly higher than that of single HIPEC (MD = 8.16, 95% CI [6.46, 9.85], p < 0.00001, Figure 9).
In these studies, subgroup analysis of KPS score was performed according to whether or not intravenous chemotherapy was combined (Figure 9). The results showed that the heterogeneity of the two groups was decreased (I2 = 0%; I2 = 30%), and KPS score of the trial group was higher than that of the control group (MD = 11.24, 95% CI [9.78, 12.69], p < 0.00001; MD = 7.15, 95% CI (5.74 to 8.56], p < 0.00001). A subgroup analysis of KPS score was performed according to different ways of using TCM (oral or external) (Figure 10). The results showed that the heterogeneity remained unaffected (I2 = 68%) in the subgroup of oral administration of TCM (I2 = 68%). Heterogeneity was decreased in the subgroup of external application of TCM (I2 = 0%), but no matter whether the TCM was used orally or externally, the KPS score of the trial group were higher than that of the control group (MD = 8.88, 95% CI [7.10, 10.66], p < 0.00001; MD = 5.10, 95% CI [2, 45, 7.75], p = 0.0002). In addition, we also performed subgroup analysis of KPS score according to the different course of treatment (course of treatment ≤4 weeks or 4 weeks < course of treatment ≤8 weeks or course of treatment >8 weeks) (Figure 11). The results showed that the heterogeneity was decreased in the subgroups with the course of treatment ≤ 4 weeks and 4 weeks < course of treatment ≤8 weeks (I2 = 26%; I2 = 33%). Heterogeneity was basically unchanged in the subgroup with a course of treatment >8 weeks (I2 = 60%). When we analyzed the clinical efficacy of these three subgroups, we found that the KPS score of the trial group was higher than that of the control group (MD = 6.17, 95% CI [4.32, 8.01], p < 0.00001; MD = 9.96, 95% CI [7.79, 12.13], p < 0.00001; MD = 9.91, 95% CI [7.48, 12.34], p < 0.00001).
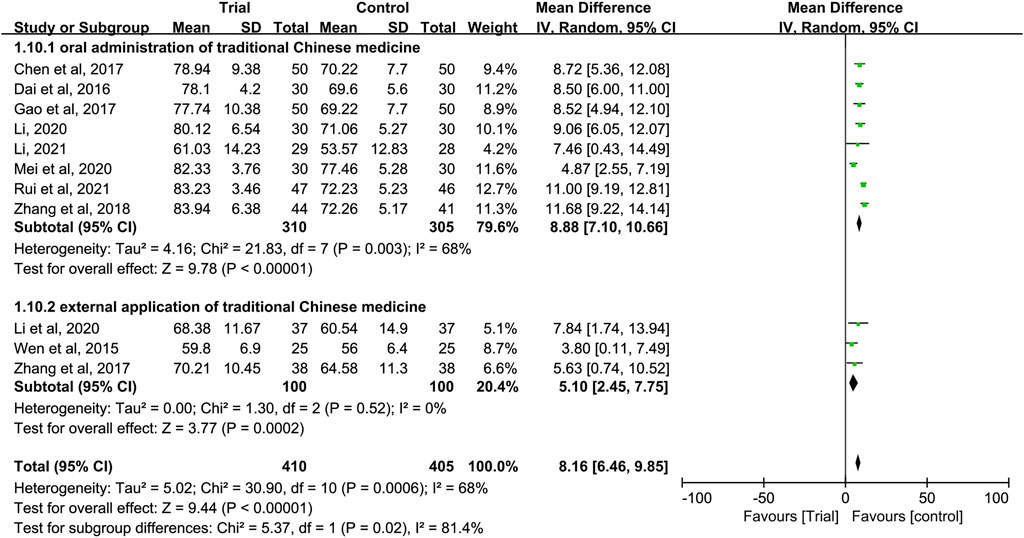
FIGURE 10. Subgroup analysis of the KPS according to different ways of using traditional Chinese medicine.
Of the 19 included studies, 16 studies (Cui and Wu, 2010; Wen et al., 2015; Dai et al., 2016; Wu et al., 2016; Chen and Hua, 2017; Gao and Zhang, 2017; Pan et al., 2017; Zhang et al., 2017; Zhang et al., 2018; Jiang and Hu, 2019; Shao et al., 2019; Li, 2020; Li et al., 2020; Mei et al., 2020; Zhang et al., 2020; Rui and Peng, 2021) reported adverse events. Among them, patients of 14 studies (Cui and Wu, 2010; Dai et al., 2016; Wu et al., 2016; Chen and Hua, 2017; Gao and Zhang, 2017; Pan et al., 2017; Zhang et al., 2017; Zhang et al., 2018; Jiang and Hu, 2019; Shao et al., 2019; Li et al., 2020; Mei et al., 2020; Zhang et al., 2020; Rui and Peng, 2021) in both trial group and the control group developed myelosuppression and gastrointestinal reaction. Patients of six studies (Cui and Wu, 2010; Chen and Hua, 2017; Gao and Zhang, 2017; Jiang and Hu, 2019; Shao et al., 2019; Rui and Peng, 2021) in both trial group and control group developed hepatic dysfunction, and some patients in both trial group and control group experienced adverse reactions such as renal dysfunction or alopecia, but further research found that the probability of adverse events in the trial group was generally less than that in the control group. In three studies (Zhang et al., 2018; Li, 2020; Mei et al., 2020), the patients in the control group had more adverse reactions such as hepatic and renal dysfunction, neurotoxicity, cardiotoxicity and fever than those in the trial group. In addition, only one study (Zhang et al., 2017) showed rash symptoms in the trial group. One study (Wen et al., 2015) did not mention specific adverse events. Three studies (Zhang and Li, 2018; Cai and Zhu, 2020; Li, 2021) did not mention adverse events.
The funnel plot of clinical efficacy (Figure 12A) was visually asymmetrically distributed, which might suggests publication bias. Egger’s test results (Figure 12B) were consistent with the funnel plot results (t = 4.73, p < 0.001), further proving the existence of publication bias in clinical efficacy. The KPS funnel plot (Figure 12C) was visually asymmetrically distributed, but the Egger’s test result (Figure 12D) was contrary (t = −1.27, p = 0.234), indicating that there was no obvious publication bias in the KPS studies. Since the number of RCTs included in the two studies of abdominal circumference and abdominal volume was both less than 10, the funnel plot-based publication bias test and Egger’s test were not performed. Sensitivity analyses of clinical efficacy, ascites volume and KPS were performed. After we excluded each study one by one, the results did not change significantly, indicating good stability of clinical efficacy, ascites volume, and KPS outcome data. However, when we performed a sensitivity analysis on abdominal circumference, we found that after excluding one study (Li, 2021), the results changed significantly, with P from 0.2 to <0.00001 and I2 from 81% to 0%.
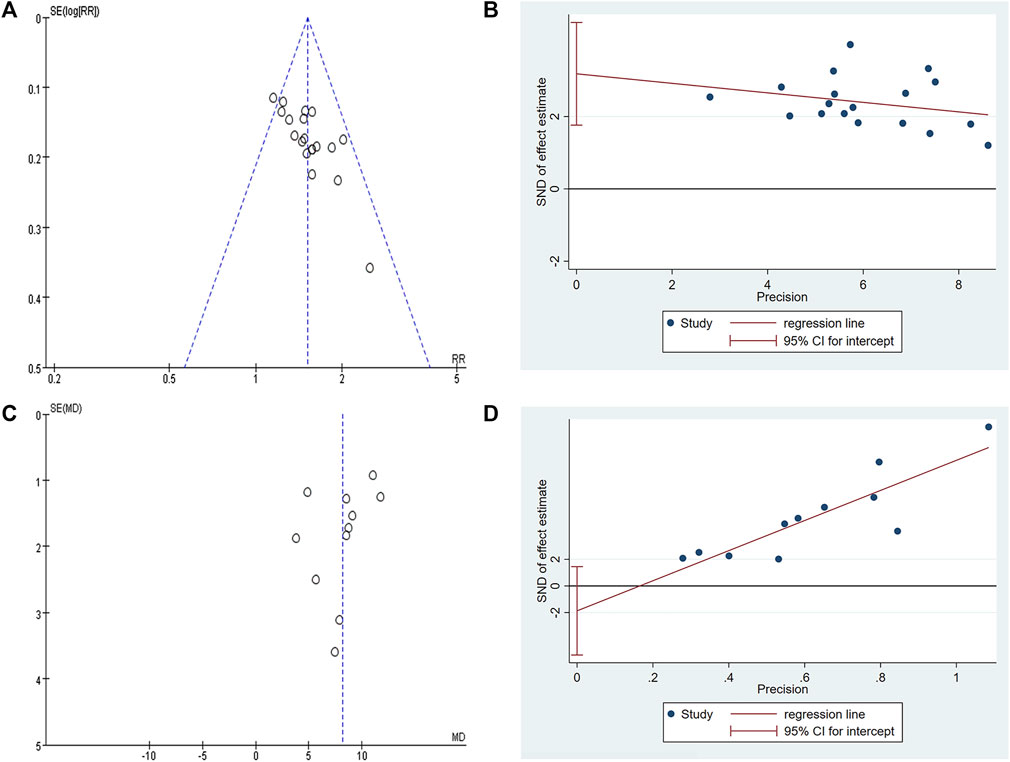
FIGURE 12. Publication bias plots. (A) Funnel plot of clinical efficacy; (B) Egger’s plot of clinical efficacy; (C) Funnel plot of KPS; (D) Egger’s plot of KPS.
This study systematically analyzed the clinical evidence of TCM combined with HIPEC in the treatment of MA, so as to better guide clinical practice. Analysis of 19 RCTs showed that TCM combined with HIPEC had a significant effect on the treatment of MA, which could reduce ascites volume and improve KPS score. The results of the combined analysis of ascites volume showed that there was heterogeneity among different studies, but after excluding the study of Chen and Hua, 2017, we found that the heterogeneity became 0%, and the trial group could still effectively reduce ascites volume compared with the control group. This may be related to the fact that study of Chen and Hua, 2017 used cisplatin single agent for HIPEC, while the other two studies used paclitaxel intravenous chemotherapy combined with cisplatin HIPEC. In addition, the results of the combined analysis of abdominal circumference showed that there was heterogeneity among studies, but after excluding the study of Li, 2021, we found that the heterogeneity was decreased to 0%, and the trial group could effectively reduce the abdominal circumference compared with the control group. We analyzed the possible reasons as follows: 1) Some tumor patients might have a huge abdominal mass, which might cause the measurement of the abdominal circumference, thus affecting the trial data; 2) The study of Li, 2021 included patients diagnosed as spleen-kidney yang deficiency in TCM syndrome, which was different from the inclusion criteria of other studies. What’s more, we also found that there was heterogeneity in the analysis results of KPS score. Therefore, we conducted a subgroup analysis based on whether combined with intravenous chemotherapy or not, and the results showed that subgroup heterogeneity was significantly reduced, indicating that whether or not it was combined with intravenous chemotherapy is one of the important factors affecting heterogeneity. According to the subgroup analysis of different ways of using TCM, the results showed that the subgroup heterogeneity of oral administration of TCM did not change, while the subgroup heterogeneity of external application of TCM decreased dramatically. We speculated that it might be because the drugs for HIPEC in the subgroup of external application of TCM were cisplatin single drug, while the subgroup of oral administration of TCM used different drugs for HIPEC or combined with intravenous chemotherapy, resulting in the heterogeneity of this subgroup. According to the course of treatment, subgroup analysis showed that the heterogeneity of the treatment course ≤4 weeks and 4 weeks < treatment course ≤8 weeks decreased significantly, while the heterogeneity of the treatment course >8 weeks remained basically unchanged, which might be related to the small number of studies included in the subgroup >8 weeks. Combining the three subgroups analyses of the KPS score, the results showed that whether combined with intravenous chemotherapy, different ways of using TCM (oral or external) and different course of treatment were important factors influencing the heterogeneity of the results. In addition, our analysis found that the heterogeneity of clinical efficacy in each subgroup was less than 50%, and the trial group could significantly improve the clinical efficacy compared with the control group. The consistency of these results enhanced the reliability of the conclusion that TCM combined with HIPEC can effectively treat MA. Among the 19 included studies, 16 studies reported adverse events. After our observation and analysis, we believed that these adverse events might be caused by chemotherapy drugs, further analysis found that the probability of adverse events in the trial group was equal to or even lower than that in the control group, it could be considered that TCM combined with HIPEC might be safe. The results of visual evaluation of funnel plot and Egger’s test showed that there was publication bias in clinical efficacy. We believed that the possible reasons were as follows: 1) The sample size of the included RCTs was small; 2) Lack of negative results; 3) Except for six studies (Dai et al., 2016; Zhang et al., 2017; Jiang and Hu, 2019; Mei et al., 2020; Zhang et al., 2020; Rui and Peng, 2021) reported the sources of funding, other studies did not clearly report the sources of funding, and it was uncertain whether there was a conflict of interest. In the sensitivity analysis of abdominal circumference, we found that heterogeneity was decreased significantly after excluding a study of Li, 2021, and the results were statistically significant. The inconsistency of the results might be due to the inclusion of patients diagnosed as spleen-kidney yang deficiency in TCM syndrome in this study, which was different from other studies. It was also possible that the small sample size of included studies made the results unstable. In addition, the statistical analysis of the frequency and medicinal properties of herbs for the treatment of MA found that the frequency of qi-invigorating herbs was the highest, because MA is a symptom of deficiency in origin and excess in superficiality, and deficiency of qi leads to stagnation of qi, and qi deficiency is the root. The application of qi-invigorating herbs to promote qi can promote the operation of water and liquid, which is conducive to the elimination of MA. Herbs for inducing diuresis and excreting were second. In the treatment of MA, no matter whether it was deficient or excessive, no matter what type of syndrome it was, it was necessary to add herbs for inducing diuresis and excreting in the prescription to promote the discharge of water. From the analysis of the frequency of use of a single herb, the frequency of commonly used herbs was basically consistent with the properties of the herbs, suggesting the importance of qi-invigorating herbs in the treatment of MA, and most of the herbs that have both qi-invigorating and diuresis-promoting effects were used.
MA is mainly caused by intra-abdominal metastasis of advanced malignant tumors, and the disease is dangerous, with poor quality of life and short survival. Currently, there is not any standard treatment plan. Although the survival of patients with MA is limited, successful palliative treatment can greatly improve the prognosis of patients (Levine et al., 2007). Modern medical research believes that MA is the result of a combination of multiple factors, and the main mechanism can be summarized as the reduction of lymphatic reflux absorption and excessive fluid production (He et al., 2013). At present, WM treatment mainly starts from the treatment of primary disease and the treatment of MA, including restriction of water and sodium intake, use of diuretics, drainage of MA, and intravenous or intraperitoneal infusion of chemotherapy drugs (Cavazzoni et al., 2013). In recent years, anti-angiogenic drugs had been used in many studies to treat MA (Hsu et al., 2019), and Alfapump system had also been used for continuous low-flow ascites drainage through the bladder to reduce the need for repeated puncture (Fotopoulou et al., 2019). However, these treatments have limitations, such as high side effects of drugs, poor tolerance of treatment by patients, ow feasibility of promotion, and many factors can bring more difficulties. HIPEC is an emerging method for the treatment of MA in recent years, which can control the metastasis of cancer cells through the combination of intraperitoneal perfusion chemotherapy and hyperthermia (Turrini et al., 2012). Its principle is to perfuse the heated chemotherapy perfusate into the abdominal cavity, so that the intra-abdominal temperature rises to the effective treatment temperature and maintains it for a certain period of time. It promotes a large amount of heat absorption in the visceral peritoneum, parietal peritoneum and arterial beds of various organs in the abdominal cavity, using the difference in temperature tolerance between tumor cells and normal cells, it can cause the destruction of tumor blood vessels and block the blood supply of tumor cells, thereby inhibiting the proliferation of tumor cells and increasing the sensitivity of patients to chemotherapy drugs, so as to achieve the therapeutic purpose of inducing tumor cell apoptosis and effectively killing tumor cells without damaging normal cells (Cui et al., 2012; Glehen et al., 2014). In addition, studies have confirmed that HIPEC also has a significant effect on inhibiting the expression level of drug-resistant genes in cancer cells (Zhang et al., 2014). However, drugs for HIPEC have poor penetration, which can only act 2 mm below the tumor surface, and the adverse reactions such as gastrointestinal reaction, leukopenia and water and electrolyte disorders are inevitable during the treatment process (Li et al., 2012). TCM classifies MA in the category of tympanites, which belongs to dropsy in tympanites. Its pathogenesis is dysfunction of the liver, spleen and kidney, and stagnantion of pneuma, blood stasis and water retention are interlinked in the abdomen. The pathological characteristics are deficiency in origin and excess in superficiality (Wu et al., 2015). The initial stage is liver stagnation and spleen deficiency, dysfunction of the spleen in transport, deficiency of the kidney essence, further development of the disease leads to the kidney yang deficiency, resulting in the spleen-kidney deficiency, water and moisture retention are even worse. The liver storing blood, kidney storing essence, homogeny of liver and kidney, stagnation of liver qi causing heat-transmission and consumption of yin. Liver yin deficiency for a long time will extend kidney, so that the yin deficiency of liver and kidney aggravates the distention (Zhang et al., 2019). This disease is locally excess, and the whole body is deficient. This primary deficiency is a deficiency of spleen-yang and kidney-yang, deficiency of liver-yin and kidney-yin, and the treatment should be based on the principles of reinforcement and elimination in combination as well as strengthening the body resistance (Wu et al., 2015). HIPEC is equivalent to TCM therapy for eliminating the pathogen. In order to prevent and cure the injury of human body’s vital qi caused by HIPEC and better achieve the purpose of strengthening the body resistance to eliminate pathogenic factors, it is necessary to take it orally or externally with TCM for syndrome differentiation and treatment. What’s more, studies have pointed out that Chinese herbs for strengthening the body’s resistance can enhance the activity of natural killer cells in vivo, thus enhancing cellular immunity in the body, and can eliminate the release of cytotoxins by cancer cells, thereby improving the life quality of patients (Peng and Su, 2017).
This study has certain limitations. First, the methodological quality of the included studies was not high, and they reported inadequate information on allocation sequence generation, allocation concealment, and blinding. Second, all the included studies were conducted in China, and no foreign countries were involved. Therefore, it is difficult to verify whether the efficacy of TCM combined with HIPEC in the treatment of MA is applicable to populations in other regions. Third, there might be heterogeneity among most of the included studies: 1) different characteristics of participants, such as age, gender, tumor type, etc.; 2) different temperature, course, drugs and dose of HIPEC; 3) the prescription composition and course of treatment of TCM were different. Fourth, there was a potential publication bias in the clinical efficacy of this study, which might affect the reliability of the study results. Fifth, although most studies reported adverse events, it was not clear whether the adverse events were caused by HIPEC or TCM. In addition, some studies did not report data on adverse events in the trial and control groups, and some studies lacked data that could be analyzed. Despite these limitations, this study is the first to systematically evaluate the efficacy of TCM combined with HIPEC in MA, which may be helpful to clinicians.
The results of this study indicated that TCM combined with HIPEC can not only improve the efficacy of MA, but also improve the quality of life of patients. However, due to the small sample size and the risk of bias of included studies, further standard, double-blind, multicenter RCTs are needed to confirm the clinical value of TCM combined with HIPEC in the treatment of MA. In addition, whether the ways of using TCM will affect the clinical efficacy of MA is the focus of future research.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
ZL and JC initiated this study and participated in its design. ZL, JC, and YL performed study selection, data extraction, and data analysis. The manuscript was drafted by ZL and revised by ZL and JC. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.938472/full#supplementary-material
CNKI, China national knowledge infrastructure; CI, confidence interval; CR, complete remission; HIPEC, hyperthermic intraperitoneal chemotherapy; I2, I-square; KPS, karnofsky performance status; MA, malignant ascites; MD, mean difference; PD, progression disease; PR, partial remission; RCTs, randomized controlled trials; RR, risk ratio; SD, stable disease; TCM, traditional Chinese medicine; VIP, Chinese scientific journal database; WM, western medicine.
Becker, G., Galandi, D., and Blum, H. E. (2006). Malignant ascites: systematic review and guideline for treatment. Eur. J. Cancer 42, 589–597. doi:10.1016/j.ejca.2005.11.018
Cai, X. C., and Zhu, X. M. (2020). Improvement of therapeutic effect of jianpilishui decoction combined with intraperitoneal hyperthermic perfusion chemotherapy for malignant ascites: a randomized controlled study. Pract. Clin. J. Integr. Traditional Chin. West. Med. 20, 5–7. doi:10.13638/j.issn.1671-4040.2020.13.002
Cavazzoni, E., Bugiantella, W., Graziosi, L., Franceschini, M., and Donini, A. (2013). Malignant ascites: Pathophysiology and treatment. Int. J. Clin. Oncol. 18, 1–9. doi:10.1007/s10147-012-0396-6
Chen, S. Q., and Hua, Y. H. (2017). Clinical observation on the curative effect of wenyang Supplementing Qi traditional Chinese medicine in the treatment of secondary peritoneal effusion of advanced ovarian cancer. J. Emerg. Traditional Chin. Med. 26, 1487–1489. doi:10.3969/j.issn.1004-745X.2017.08.055
Cui, H. H., and Wu, Z. T. (2010). Clinical research of intra-abdominal catheter drainage hot perfusion chemotherapy and shipi drink in treating cancerous ascites. China J. Chin. Med. 25, 824–825. doi:10.16368/j.issn.1674-8999.2010.05.104
Cui, S., Ba, M., Tang, Y., Liu, J., Wu, Y., Wang, B., et al. (2012). B ultrasound - guided hyperthermic intraperitoneal perfusion chemotherapy for the treatment of malignant ascites. Oncol. Rep. 28, 1325–1331. doi:10.3892/or.2012.1913
Dai, L. Q., Ding, Q., and Liu, H. (2016). Clinical study of zhenwutan combined with hyperthermic intraperitoneal chemotherapy of paclitaxel in treating epithelial ovarian cancer with malignant ascites in middle and late period. Guid. J. Traditional Chin. Med. Pharmacol. 22, 37–38+41. doi:10.13862/j.cnki.cn43-1446/r.2016.04.012
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi:10.1136/bmj.315.7109.629
Fotopoulou, C., Berg, T., Hausen, A., Hennig, R., Jalan, R., Malagóet, M., et al. (2019). Continuous low flow ascites drainage through the urinary bladder via the Alfapump system in palliative patients with malignant ascites. BMC Palliat. Care 18, 109. doi:10.1186/s12904-019-0497-3
Gao, M., and Zhang, X. W. (2017). Clinical observation of wenyang Jianpi Decoction assisted peritoneal heat perfusion chemotherapy in the treatment of secondary peritoneal effusion of advanced ovarian cancer. Mod. J. Integr. Tradit. Chin. West. Med. 26, 2579–2581. doi:10.3969/j.issn.1008-8849.2017.23.025
Glehen, O., Passot, G., Villeneuve, L., Vaudoyer, D., Bin-Dorel, S., Boschetti, G., et al. (2014). GASTRICHIP:D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC cancer 14, 183. doi:10.1186/1471-2407-14-183
He, Y. F., Luo, H. Q., and Hu, B. (2013). Advances of medical management in malignant ascites. Chin. Clin. Oncol. 18, 472–474. doi:10.3969/j.issn.1009-0460.2013.05.020
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Hodge, C., and Badgwell, B. D. (2019). Palliation of malignant ascites. J. Surg. Oncol. 120, 67–73. doi:10.1002/jso.25453
Hsu, J. F., Lee, Y. L., Chang, H. L., Wei, P. J., Shen, Y. T., Lin, C. M., et al. (2019). Clinical efficacy of concurrent bevacizumab for malignant ascites in nonsquamous cell carcinoma of the lung. Asia. Pac. J. Clin. Oncol. 15, e126–e131. doi:10.1111/ajco.13131
Ito, T., and Hanafusa, N. (2017). CART: Cell-Free and concentrated ascites reinfusion therapy against malignancy-related ascites. Transfus. Apher. Sci. 56, 703–707. doi:10.1016/j.transci.2017.08.018
Jiang, S. S., and Hu, L. Q. (2019). Observation on 40 cases of malignant peritoneal effusion treated by Wenyang Lishui Huayu prescription combined with peritoneal heat perfusion chemotherapy. Zhejiang J. Tradit. Chin. Med. 54, 832. doi:10.13633/j.cnki.zjtcm.2019.11.032
Kong, Z. P. (2021). Research overview of TCM treatment of malignant ascites. J. Pract. Tradit. Chin. Med. 37, 512–514.
Levine, E. A., Stewart, J. H., Russell, G. B., Geisinger, K. R., Loggie, B. L., and Shen, P. (2007). Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: experience with 501 procedures. J. Am. Coll. Surg. 204, 943–953; discussion 953-955. doi:10.1016/j.jamcollsurg.2006.12.048
Li, Y., Zhou, Y. F., Xie, C. H., Peng, C. W., Huang, C. Q., Yang, X. J., et al. (2012). Cytoreductive surgery plus hyperthermic intra-peritoneal chemotherapy for peritoneal carcinomatosis from gastric cancer. Chin. J. Clin. Oncol. 39, 1734–1740. doi:10.3969/j.issn.1000-8179.2012.22.012
Li, Z. R., Li, W. J., and Li, Y. W. (2020). Clinical observation on the treatment of malignant peritoneal effusion with drainage plaster combined with peritoneal heat perfusion chemotherapy. Hebei J. Tradit. Chin. Med. 42, 1536–1539. doi:10.3969/j.issn.1002-2619.2020.10.021
Li, Y. Y. (2020). Observation on the curative effect of peritoneal heat perfusion combined with Fuzheng Yaxue Decoction on malignant ascites. Diabetes World 17, 44.
Li, Z. J. (2021). Clinical observation on the treatment of malignant ascites with spleen-kidney-Yang deficiency by Wenyang Lishi Prescription combined with peritoneal circulation heat perfusion chemotherapy. Anhui: Anhui University of Traditional Chinese Medicine. doi:10.26922/d.cnki.ganzc.2021.000221
Maeda, H., Kobayashi, M., and Sakamoto, J. (2015). Evaluation and treatment of malignant ascites secondary to gastric cancer. World J. Gastroenterol. 21, 10936–10947. doi:10.3748/wjg.v21.i39.10936
Mei, Y. G., Liu, M. F., and He, S. X. (2020). Clinical observation of Yishen Sanjie Pill combined with peritoneal heat perfusion chemotherapy in the treatment of malignant ascites secondary to advanced gynecological malignant tumor. Contemp. Med. 26, 142–143. doi:10.3969/j.issn.1009-4393.2020.11.060
Pan, P., Zhong, M. W., Ye, H. Q., and Li, Q. Z. (2017). Observation on the curative effect of peritoneal heat perfusion chemotherapy combined with Fuzhengquxie Anti-cancer prescription in treating malignant ascites. J. Pract. Traditional Chin. Med. 33, 1171–1172. doi:10.3969/j.issn.1004-2814.2017.10.041
Peng, Z. Q., and Su, L. (2017). Clinical study on invigorating spleen for diuresis and activating blood circulation prescription combined with deep hyperthermia in the treatment of malignant ascites. Guangming J. Chin. Med. 32, 840–842. doi:10.3969/j.issn.1003-8914.2017.06.036
Pérez, S. P., and Rodrı́guez, M. D. (2006). Practical considerations on detection of publication bias. Gac. Sanit. 20, 10–16. doi:10.1157/13101085
Robella, M., Vaira, M., Cinquegrana, A., and Simone, M. D. (2019). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: morbidity and postoperative outcomes. Minerva Chir. 74, 195–202. doi:10.23736/S0026-4733.18.07649-6
Rui, Y., and Peng, M. N. (2021). Clinical efficacy and safety of danghuang kangai decoction combined with intracavitary hyperthermic perfusion chemotherapy for ovarian cancer with peritoneal effusion. Chin. Arch. Tradit. Chin. Med. 39, 227–230. doi:10.13193/j.issn.1673-7717.2021.10.054
Shao, L. L., Zhang, L. Y., Tian, Y. Z., and Liu, Z. L. (2019). Clinical observation on the treatment of advanced ovarian cancer complicated with malignant peritoneal effusion by self-designed detoxification and cancer suppression decoction combined with peritoneal heat perfusion chemotherapy. Zhejiang J. Integr. Tradit. Chin. West. Med. 29, 635–638. doi:10.3969/j.issn.1005-4561.2019.08.007
The Chinese pharmacopoeia, The first part (2015). The Chinese pharmacopoeia, the first part. Beijing: China Medical Science Press.
Turrini, O., Lambaudie, E., Faucher, M., Viret, F., Blache, J. L., Houvenaeghel, G., et al. (2012). Initial experience with hyperthermic intraperitoneal chemotherapy. Arch. Surg. 147, 919–923. doi:10.1001/archsurg.2012.988
Wang, P. P. (2020). Systematic evaluation and mesh meta-analysis of Chinese medicine injection combined with cisplatin in the treatment of cancer ascites. Beijing: Beijing University of Chinese Medicine. doi:10.26973/d.cnki.gbjzu.2020.000328
Wen, G. X., Ruan, H. J., Yuan, Y., Wang, Y. Y., and Huang, H. (2015). Clinical observation of self-made tumor prescription combined with peritoneal heat perfusion chemotherapy in the treatment of ovarian cancer ascites. Chin. Manip. Rehabilitation Med. 6, 71–72.
Wu, F. Z., Lou, Y. N., and Jia, L. Q. (2015). Analysis of the TCM syndromes of malignant ascites. China J. Traditional Chin. Med. Pharm. 30, 3112–3115.
Wu, Y. D., Li, Y. Y., Zhao, Y. P., Mao, S. H., and Zhang, L. L. (2016). Clinical effect of peritoneal heat perfusion chemotherapy combined with Traditional Chinese medicine on malignant ascites. Hebei Med. 22, 1734–1735. doi:10.3969/j.issn.1006-6233.2016.10.065
Xu, G. W. (2005). Clinical diagnosis and treatment guidelines: tumour fascicule. Beijing: People's Medical Publishing House, 322–323.
Zhan, N., Dong, W. G., and Wang, J. (2016). The clinical significance of vascular endothelial growth factor in malignant ascites. Tumour Biol. 37, 3719–3725. doi:10.1007/s13277-015-4198-0
Zhang, Z. H., and Li, P. (2018). Effect of external application of Shipi Xiaoshui Ointment combined with abdominal heat perfusion chemotherapy on patients with ascites with peritoneal metastasis of advanced gastric cancer. Guid. J. Tradit. Chin. Med. Pharmacol. 24, 68–70. doi:10.13862/j.cnki.cn43-1446/r.2018.05.019
Zhang, X. L., Shi, H. J., Wang, J. P., Tang, H. S., Wu, Y. B., Fang, Z. Y., et al. (2014). MicroRNA-218 is upregulated in gastric cancer after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy and increases chemosensitivity to cisplatin. World J. Gastroenterol. 20, 11347–11355. doi:10.3748/wjg.v20.i32.11347
Zhang, F. L., Li, P., and Zhang, M. (2017). Clinical research of longxie xiaoshui ointment for external application plus hyperthermic intraperitoneal chemotherapy in treatment of patients with malignant ascites. Liaoning J. Tradit. Chin. Med. 44, 2090–2093. doi:10.13192/j.issn.1000-1719.2017.10.025
Zhang, Y. P., Zhang, J. Q., and Hong, Y. G. (2018). Clinical observation and study of mechanism of applying wenyang lishui decoction combined with intraperitoneal chemotherapy in the treatment of ovarian cancer patients with ascites hyperthermic perfusion. J. Sichuan Traditional Chin. Med. 36, 157–160.
Zhang, Y., Wang, R. P., Wang, B., and Zhang, Z. (2019). Discussion on the application of huoxuelishui method in cancer ascites from the perspective of " when blood does not move, it becomes water. Glob. Tradit. Chin. Med. 12, 1699–1701. doi:10.3969/j.issn.1674-1749.2019.11.020
Keywords: hyperthermic intraperitoneal chemotherapy, malignant ascites, traditional Chinese medicine, efficacy, meta-analysis
Citation: Lin Z, Chen J and Liu Y (2022) The efficacy of traditional chinese medicine combined with hyperthermic intraperitoneal chemotherapy for malignant ascites: A systematic review and meta-analysis. Front. Pharmacol. 13:938472. doi: 10.3389/fphar.2022.938472
Received: 07 May 2022; Accepted: 25 July 2022;
Published: 29 August 2022.
Edited by:
Alessandra Durazzo, Council for Agricultural Research and Economics, ItalyReviewed by:
Sohan Lal Solanki, Tata Memorial Hospital, IndiaCopyright © 2022 Lin, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhixian Lin, ODQxNjc1OTcyQHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.