
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 18 October 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.938239
Background and purpose: The TASTE trial indicated that patients with acute ischemic stroke (AIS) using edaravone dexborneol have a significantly higher proportion of 90-day good functional outcomes (mRS 0–1) than those using edaravone. This study compared the cost-effectiveness of the aforementioned interventions in treating AIS in the Chinese setting, aiming to inform treatment decisions in clinical practice.
Methods: A model combining a decision tree and a Markov model was developed to assess the cost-effectiveness of edaravone dexborneol versus edaravone for AIS over a 30-year time horizon from the Chinese healthcare system’s perspective. Both efficacy and safety data were extracted from the TASTE study. Local costs and utilities were derived from publications and open-access databases; both cost and effectiveness were discounted at a rate of 5% per year. Sensitivity analyses were conducted to ensure robustness and identify the main drivers of the result.
Results: Compared with edaravone, edaravone dexborneol for AIS was found to be cost-effective in the first year and highly cost-effective as the study time horizons extended. In the long term (30 years), edaravone dexborneol yielded a lifetime gain of 0.25 (0.07–0.45) quality-adjusted life years (QALYs) at an additional cost of CNY 2201.07 (-3,445.24–6,637.23), yielding an ICER of CNY 8823.41 per QALY gained under the willingness-to-pay (WTP) of 1.5 times per capita GDP (121,464 CNY). The result is robust in both deterministic and probabilistic sensitivity analysis (PSA) methods, with the advantage of the edaravone dexborneol strategy increasing over time. Specifically, the probability of edaravone dexborneol dominant dexborneol is 76.30%, 98.90%, and 99.50% over 1-, 5-, and 30-year time horizons.
Conclusion: Both short- and long-term economic analyses suggest that edaravone dexborneol is highly likely to be a cost-effective alternative to treat AIS compared with edaravone in China.
According to the Global Burden of Disease Study 2019, China bears the highest lifetime risk of stroke worldwide, with 28.76 million prevalent cases, 3.94 million new stroke cases, and 2.19 million deaths in 2019 (Wang et al., 2022). Stroke is also one of the leading causes of disability-adjusted life year (DALY) in China, estimated to be as high as 45.9 million in 2019 (Wang et al., 2022). Of all strokes, ischemic stroke accounts for 82% and results in over 30 billion CNY direct and indirect losses every year, placing a heavy burden on society, families, and individuals (Sun et al., 2021).
Existing research suggests that early reperfusion therapy, including intravenous thrombolysis (IVT) and endovascular therapy (EVT), is the most effective and cost-effective treatment for AIS (Lees et al., 2010; Pan et al., 2014; Berkhemer et al., 2015; Pan et al., 2018). However, early reperfusion therapy is not meeting clinical needs. According to a new study, the overall IVT rate for acute ischemic stroke (AIS) was 5.64%, and the EVT rate was 1.45% in China between 2019 and 2020 (Ye et al., 2022).
In addition to reperfusion therapy, neuroprotective agents, such as edaravone and (+)-borneol, are another effective treatment for stroke (Zhang et al., 2005; Watanabe et al., 2008; Liu et al., 2011; Wu et al., 2014). Edaravone dexborneol is a multi-target neuroprotective agent consisting of edaravone and dexborneol in a 4:1 ratio. A double-blind, randomized, controlled study [NCT02430350] among 1,200 patients with AIS showed that edaravone dexborneol has clear efficacy benefits (modified Rankin scale score (mRS) ≤1, 67.18% versus 58.97%; OR, 1.42 [95% CI, 1.12–1.81]; p = 0.004) and similar clinical safety compared to single-agent edaravone injection (Xu et al., 2021). Although edaravone dexborneol treatment showed superior clinical benefit, its cost-effectiveness in treating AIS awaits further investigation. To this end, this article evaluates the cost-effectiveness of edaravone dexborneol compared with edaravone for treating AIS patients from the Chinese healthcare payers’ perspective, aiming to inform policy and clinical practice.
This study was conducted according to the latest Consolidated Health Economic Evaluation Reporting Standards (CHEERS 2022) reporting guidelines (Husereau et al., 2022). The decision tree and Markov model (Figure 1) were developed to simulate the 30-year cost-effectiveness of edaravone dexborneol versus edaravone. Our study was based on clinical data from the TASTE trial (NCT02430350), a phase III, randomized, double-blind, parallel, comparative study that enrolled 1,200 patients from May 2015 through December 2016 at 48 centers in China. With the development of disease, patients were assumed to transition among the four health states: no disability (mRS score 0–1), minor or moderate disability (mRS score 2–3), severe disability (mRS score 4–5), and dead (mRS score 6). Health states according to mRS categories at 3 months were derived from the clinical efficacy of the TASTE trial. Then, patients could undergo transitions annually for the remainder of their lifetime (30 years), and the cycle length was 1 year. At the end of each Markov cycle, patients either remained in their current health state, transitioned to a state of equal or greater disability due to a recurrent stroke, or died. A half-cycle correction was used for all states in every year.
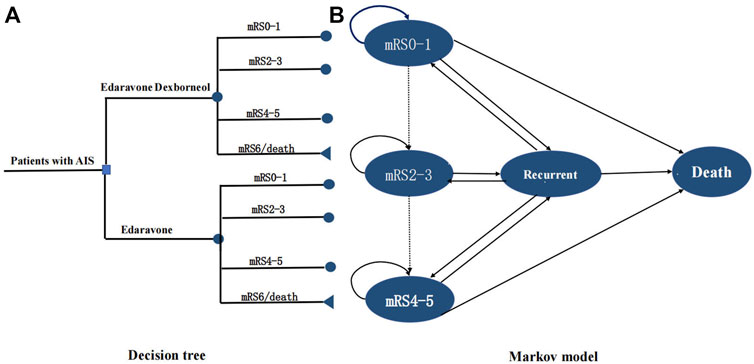
FIGURE 1. Model structure. (A) Short-term decision tree model and (B). long-term Markov state transition model. A patient with an acute ischemic stroke within 48 h after symptom onset entered the model at 63 y old receiving either edaravone dexborneol or edaravone, and transitioned between health states until death or 30 y. Patients may have a stable health state, transition to a state of equal or greater disability after recurrent stroke, or died. mRS indicates modified Rankin Scale.
We use incremental cost-effectiveness ratios (ICERs) to indicate cost-effectiveness and apply a willingness-to-pay (WTP) threshold of 1.5 times per capita GDP per QALY (121,464 CNY/QALY) (Cai et al., 2021). Both cost and effectiveness were discounted at a rate of 5% per year, as recommended by the China guidelines for pharmacoeconomic evaluations (China Market Press., 2020). We also report the net monetary benefits (NMBs), defined as expected QALY × WTP − cost, and this was used to compare strategies in the sensitivity analyses.
The model was developed using Microsoft Excel 2019.
The target population in the model was patients diagnosed as AIS (aged 35–80 years), with a National Institutes of Health Stroke Scale (NIHSS) score of between 4 and 24, and administered within 48 h of AIS onset. The characteristics of the target population in this model were assumed to be the same as those in the TASTE study.
The intervention group received an edaravone dexborneol intravenous infusion of 37.5 mg/dose (edaravone, 30 mg; (+)-borneol, 7.5 mg), once every 12 h, which was continued for 14 days. The control group received an edaravone intravenous infusion of 30 mg/dose, once every 12 h, which was continued for 14 days.
The clinical efficacy parameters of the edaravone dexborneol and edaravone groups were derived from the TASTE study (Xu et al., 2021). In each cycle of the Markov model, patients could either remain in the same health state, experience a recurrent stroke, or die. Recurrent rates of stroke and mortality rates of recurrent strokes in years after the first 90 days were estimated by the China National Stroke Registry (CNSR) (Pan et al., 2014). Based on the previous study, we further assumed an increase in stroke recurrence rates by 1.019-fold per life year (Pan et al., 2014). Recurrent stroke stages were considered tunnel health states unless the onset led to death (Briggs et al., 2006). The age-specific death rate was drawn from and adjusted according to the causes of death in 2020 reported in the China Health Statistics Yearbook 2021 (National Bureau of Statistics of China, 2022a; National Health Commission of the People’s Republic of China, 2021). Excess mortality risk due to stroke was incorporated into the model as the hazard rate ratio for each mRS health state by using the age-specific death rate multiplied by the hazard rate ratio for each mRS health state (Samsa et al., 1999) (Table 1).
This study adopted the Chinese healthcare system’s perspective; therefore, only direct medical costs were considered, including the drug cost, cost of intravenous injection, hospitalization and post-hospitalization costs of AIS, and cost of recurrent stroke. Healthcare costs by mRS score were extracted from the China National Stroke Registry (CNSR) (Pan et al., 2014). The costs of edaravone dexborneol and edaravone were obtained from the mean bidding price from the Chinese drug bidding database on MENET (https://www.menet.com.cn/). The cost of intravenous injections was obtained from the mean price of medical services in 12 provinces across China to represent the national medical service price level (i.e., Beijing, Shanghai, Jiangsu, and Zhejiang). The costs of recurrent stroke were derived from the published literature (Xiang et al., 2021). All costs were discounted at 5% annually and inflated in 2021 Chinese Yuan 2020 using the medical care component of the consumer price index (Table 1).
Utility values and weights were derived from the study and ranged from 0.36 for patients with an mRS of 4–5 to 0.84 for those with an mRS of 0–1 (Table 1) (Pan et al., 2014). Cumulative outcomes of treatment algorithms were measured by quality-adjusted life years (QALYs), which were discounted at an annual rate of 5%.
Both deterministic sensitivity analyses and probabilistic sensitivity analyses were performed to ensure the robustness of the result. A one-way sensitivity analysis was performed to identify variables that significantly influence the INMB, including all variables within plausible ranges derived from the literature (Table 1). The uncertainty of effectiveness for edaravone dexborneol was calculated based on the proportions in the edaravone group and confidence intervals of the odds ratio of mRS 0–1 measured, as determined by the following formula: p2=(OR*p1)/(1+(OR−1)*p1). A tornado diagram was used to present the INMB by the one-way sensitivity analysis.
Furthermore, a Monte Carlo simulation was used to perform the probabilistic sensitivity analysis (PSA) with parameter inputs (efficacy, transition probabilities, costs, and utilities) sampled from fixed distributions. As transitions are multinomial, we assumed four mRS states followed the Dirichlet distribution, which is the multivariate generalization of the beta distribution with parameters equal to the number of categories in the multinomial distribution (Briggs et al., 2006). In addition, we assumed the probabilities and utilities followed a beta distribution, and costs followed a gamma distribution. The PSA performed 5,000 patient-level iterations, giving an incremental ICER scatterplot and a cost-effectiveness acceptability curve representing uncertainty.
This research is entirely based on secondary data sources and does not contain any ethic-related issues. There was no engagement of patients, the general public, or stakeholders in the design of the study.
Table 2 shows the results in both the short term (1 and 5 years) and long term (30 years). In the base case analysis, the edaravone dexborneol treatment gained 0.03 (0.01–0.06) QALYs at an additional cost of CNY 2648.20 (1,429.64–3,493.64) in the first year, yielding an ICER of CNY 79703.94 per QALY gained. The treatment was cost-effective in the first year, using the threshold of CNY 121,464 (1.5× GDP per capita of China in 2021) as the WTP per QALY. However, the edaravone dexborneol treatment became highly cost-effective as the study time horizons extended. In the short term (5 years), the edaravone dexborneol treatment gained 0.11 (0.03–0.22) QALYs at an additional cost of CNY 1967.44 (-1,544.82–4,499.32), yielding an ICER of CNY 16905.45 per QALY gained. In the long term (30 years), the edaravone dexborneol treatment gained 0.25 (0.07–0.45) QALYs at an additional cost of CNY 2201.07 (-3,445.24–6,637.23), yielding an ICER of CNY 8823.41 per QALY gained. The NMB of edaravone dexborneol was higher than that of edaravone at a willingness-to-pay threshold of 121,464 CNY/QALY, indicating that this option was preferred on cost-effectiveness grounds.
As indicated in Figure 2, the most significant driver of the INMB was the odds ratio of mRS 0–1 at day 90, followed by the discount rate, the utility of mRS 4–5, and the utility of mRS 0–1. The sensitivity results for all assumptive inputs were under the 121,464 CNY/QALY thresholds, which demonstrated the robustness and consistency of the model outcomes.
The PSA with a 30-year time horizon is shown in Figure 3. With 5,000 iterations, edaravone dexborneol treatment was 99.5% cost-effective at a willingness-to-pay threshold of 121,464 CNY/QALY. Within the 5-year short run, the probability of edaravone dexborneol being the cost-effective strategy ranges from 76.3% (first year) to 98.90% (fifth year), which still holds a distinct advantage over edaravone. The cost-effectiveness acceptability curves of both treatments are shown in Figure 4.
As the evidence of reperfusion therapies keeps accumulating in recent years, the exploration of neuroprotective agents faces clinical translation dilemmas. In the last 10 years, a series of clinical trials of several neuroprotective agents, such as SAINT I and II trials (Lees et al., 2006; Shuaib et al., 2007; Diener et al., 2008), ALIAS trials (Ginsberg et al., 2013; Martin et al., 2016), URICO-ICTUS trials (Yu et al., 1998; Squadrito et al., 2000; Chamorro et al., 2014; Onetti et al., 2015), FAST-MAG trial (Saver et al., 2015), ACTION trial (Elkins et al., 2017), and ESCAPE-NA1 trial (Hill et al., 2020), have failed to show clinical efficacy. Of note, all the drugs have a specific target or show a well-proven inhibitory effect on the pathway in preclinical studies. However, for AIS, many pathways of damage in the ischemic cascade progress simultaneously and might interact with each other. Hence, combination treatments targeting several pathways of ischemic injury may have advantages over the single-pathway strategy.
Edaravone has been shown to relieve both endothelial and neuronal cell injury in AIS (Lapchak, 2010). (+)-Borneol has been shown to have potent neuroprotective effects through multiple molecular pathways in ischemia and reperfusion injury (Liu et al., 2011). Edaravone dexborneol—a novel neuroprotectant with an optimal proportion of 4:1—has been shown to have clear efficacy benefits compared with edaravone (Xu et al., 2021). Based on the TASTE study, this economic evaluation developed a model combining a decision tree and a Markov model to evaluate the cost-effectiveness of edaravone dexborneol versus edaravone in China from the perspective of the Chinese healthcare system. The main findings of this study suggest that compared with edaravone, the edaravone dexborneol strategy was highly likely to be a cost-effective treatment for Chinese patients treated with edaravone dexborneol within 48 h of AIS onset.
According to the present sensitivity analysis, the top factor with great influence on INMB was the odds ratio of mRS 0–1 at day 90, which is similar to the findings of Pan et al. (2014). Patients in mRS 0–1 status require less hospitalization and prognostic costs, so a treatment strategy that can improve the proportion of patients with mRS 0–1 after acute treatment will be more cost-effective. We also found that the economic advantage of the edaravone dexborneol strategy is increasing over time. The reason might be that the edaravone dexborneol strategy is highly effective in the functional outcome (mRS 0–1) for AIS, which dramatically influences the long-term utility and costs.
The China Stroke Prevention Programme Committee (CSPPC) was established in 2011 to improve the quality of stroke prevention and treatment. The CSPPC launched the accreditation process for stroke centers in 2015 and started publishing stroke emergency maps in 2016 (Chao et al., 2021; Ye et al., 2022). Although the rates of IVT and EVT in China are restricted by poor infrastructure, inefficient systems, and a deficiency of specialists, the significant differences in technical level and medical equipment across different grades of hospitals and the time points of patient entry into the hospital may cause inequity for those who cannot receive these technologies. Unlike IVT and EVT, using edaravone dexborneol was less restricted by other conditions of the health system and the time points of patient entry to the hospital. Therefore, the edaravone dexborneol was a cost-effective strategy to improve the outcomes of AIS patients. These findings inform national health care plans and implementation of AIS management in China.
The present study may have some limitations. First, our model focused on the influence of edaravone dexborneol for AIS, and functional status and costs as a result of other causes, such as congestive heart failure and myocardial infarction, were not included in the present model. Second, the utility data in this study were derived from literature studies, which are from the non-Chinese population. Third, this study was undertaken from the perspective of the Chinese healthcare system and did not include societal costs. However, given that the majority of the stroke population in the model has advanced age, the direct healthcare costs contribute far more than indirect costs. Fourth, adverse event costs were not calculated in this study due to the lack of corresponding adverse event cost data. However, given the low incidence of adverse events for both drugs, the impact of this cost on the final results is negligible Finally, patient adherence to secondary preventive treatment might have varied or decreased in the years after discharge, leading to underestimation or overestimation of the true cost-effectiveness of the intervention.
This study demonstrated that edaravone dexborneol was highly likely to be a cost-effective alternative to treat AIS compared with edaravone in both short- and long-term economic analyses in China. These results provide important practical information for clinical practice decision-making and the resource allocation and policy development for reimbursement by medical insurance in China in the future.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
This research is entirely based on secondary data sources and does not contain any ethic-related issues. There was no engagement of patients, the general public, or stakeholders in the design of the study.
FS and SH were responsible for the study design. FS built the model and conducted the statistical analysis. ZH prepared the manuscript. LW and HS collected the data. All the authors reviewed the model structure, data source, formula, and results. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Berkhemer, O. A., Fransen, P. S., Beumer, D., Van Den Berg, L. A., Lingsma, H. F., Yoo, A. J., et al. (2015). A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 372, 11–20. doi:10.1056/NEJMoa1411587
Briggs, A., Sculpher, M., and Claxton, K. (2006). Decision modelling for health economic evaluation. Oxford: Oup.
Cai, D., Shi, S., Jiang, S., Si, L., Wu, J., and Jiang, Y. (2021). Estimation of the cost-effective threshold of a quality-adjusted life year in China based on the value of statistical life. Eur. J. Health Econ. 23, 607–615. doi:10.1007/s10198-021-01384-z
Chamorro, Á., Amaro, S., Castellanos, M., Segura, T., Arenillas, J., Martí-Fábregas, J., et al. (2014). Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): A randomised, double-blind phase 2b/3 trial. Lancet. Neurol. 13 (5), 453–460. doi:10.1016/S1474-4422(14)70054-7
Chao, B. H., Yan, F., Hua, Y., Liu, J. M., Yang, Y., Ji, X. M., et al. (2021). Stroke prevention and control system in China: CSPPC-stroke program. Int. J. Stroke 16 (3), 265–272. doi:10.1177/1747493020913557
China Market Press (2020). China guidelines for pharmacoeconomic evaluations. Beijing, China: China Market Press, 175.
Diener, H., Lees, K. R., Lyden, P., Grotta, J., Davalos, A., Davis, S. M., et al. (2008). NXY-059 for the treatment of acute stroke: Pooled analysis of the SAINT I and II trials. Stroke 39 (6), 1751–1758. doi:10.1161/STROKEAHA.107.503334
Elkins, J., Veltkamp, R., Montaner, J., Johnston, S. C., Singhal, A. B., Becker, K., et al. (2017). Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): A randomised, placebo-controlled, double-blind phase 2 trial. Lancet. Neurol. 16 (3), 217–226. doi:10.1016/S1474-4422(16)30357-X
Ginsberg, M. D., Palesch, Y. Y., Hill, M. D., Martin, R. H., Moy, C. S., Barsan, W. G., et al. (2013). High-dose albumin treatment for acute ischaemic stroke (ALIAS) Part 2: A randomised, double-blind, phase 3, placebo-controlled trial. Lancet. Neurol. 12 (11), 1049–1058. doi:10.1016/S1474-4422(13)70223-0
Hill, M. D., Goyal, M., Menon, B. K., Nogueira, R. G., Mctaggart, R. A., Demchuk, A. M., et al. (2020). Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): A multicentre, double-blind, randomised controlled trial. Lancet 395 (10227), 878–887. doi:10.1016/S0140-6736(20)30258-0
Husereau, D., Drummond, M., Augustovski, F., de Bekker-Grob, E., Briggs, A. H., Carswell, C., et al. (2022). Consolidated health economic evaluation reporting standards (CHEERS) 2022 explanation and elaboration: A report of the ISPOR CHEERS II good practices task force. Value Health. 25 (1), 10–31. doi:10.1016/j.jval.2021.10.008
Lapchak, P. A. (2010). A critical assessment of edaravone acute ischemic stroke efficacy trials: Is edaravone an effective neuroprotective therapy? Expert Opin. Pharmacother. 11 (10), 1753–1763. doi:10.1517/14656566.2010.493558
Lees, K. R., Bluhmki, E., Von Kummer, R., Brott, T. G., Toni, D., Grotta, J. C., et al. (2010). Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 375 (9727), 1695–1703. doi:10.1016/S0140-6736(10)60491-6
Lees, K. R., Zivin, J. A., Ashwood, T., Davalos, A., Davis, S. M., Diener, H. C., et al. (2006). NXY-059 for acute ischemic stroke. N. Engl. J. Med. 354 (6), 588–600. doi:10.1056/NEJMoa052980
Liu, R., Zhang, L., Lan, X., Li, L., Zhang, T., Sun, J., et al. (2011). Protection by borneol on cortical neurons against oxygen-glucose deprivation/reperfusion: Involvement of anti-oxidation and anti-inflammation through nuclear transcription factor κappaB signaling pathway. Neuroscience 176, 408–419. doi:10.1016/j.neuroscience.2010.11.029
Martin, R. H., Yeatts, S. D., Hill, M. D., Moy, C. S., Ginsberg, M. D., Palesch, Y. Y., et al. (2016). ALIAS (albumin in acute ischemic stroke) trials: Analysis of the combined data from parts 1 and 2. Stroke 47 (9), 2355–2359. doi:10.1161/STROKEAHA.116.012825
National Bureau of Statistics of China (2021). The 2010 population census of the People’s Republic of China. (in Chinese). Available at: http://www.stats.gov.cn/tjsj/pcsj/rkpc/6rp/indexch.html (Accessed April 17, 2022).
National Health Commission of the People’s Republic of China (2021). China health Statistics Yearbook 2021. Beijing: Peking Union Medical College Press. (in Chinese).
Onetti, Y., Dantas, A. P., Pérez, B., Cugota, R., Chamorro, A., Planas, A. M., et al. (2015). Middle cerebral artery remodeling following transient brain ischemia is linked to early postischemic hyperemia: A target of uric acid treatment. Am. J. Physiol. Heart Circ. Physiol. 308 (8), H862–H874. doi:10.1152/ajpheart.00001.2015
Pan, Y., Cai, X., Huo, X., Zhao, X., Liu, L., Wang, Y., et al. (2018). Cost-effectiveness of mechanical thrombectomy within 6 hours of acute ischaemic stroke in China. BMJ Open 8 (2), e018951. doi:10.1136/bmjopen-2017-018951
Pan, Y., Chen, Q., Zhao, X., Liao, X., Wang, C., Du, W., et al. (2014). Cost-effectiveness of thrombolysis within 4.5 hours of acute ischemic stroke in China. PLoS One 9 (10), e110525. doi:10.1371/journal.pone.0110525
Pan, Y., Zhang, L., Li, Z., Meng, X., Wang, Y., Li, H., et al. (2020). Cost-Effectiveness of a multifaceted quality improvement intervention for acute ischemic stroke in China. Stroke 51 (4), 1265–1271. doi:10.1161/STROKEAHA.119.027980
Samsa, G. P., Reutter, R. A., Parmigiani, G., Ancukiewicz, M., Abrahamse, P., Lipscomb, J., et al. (1999). Performing cost-effectiveness analysis by integrating randomized trial data with a comprehensive decision model: Application to treatment of acute ischemic stroke. J. Clin. Epidemiol. 52 (3), 259–271. doi:10.1016/s0895-4356(98)00151-6
Saver, J. L., Starkman, S., Eckstein, M., Stratton, S. J., Pratt, F. D., Hamilton, S., et al. (2015). Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N. Engl. J. Med. 372 (6), 528–536. doi:10.1056/NEJMoa1408827
Shuaib, A., Lees, K. R., Lyden, P., Grotta, J., Davalos, A., Davis, S. M., et al. (2007). NXY-059 for the treatment of acute ischemic stroke. N. Engl. J. Med. 357 (6), 562–571. doi:10.1056/NEJMoa070240
Squadrito, G. L., Cueto, R., Splenser, A. E., Valavanidis, A., Zhang, H., Uppu, R. M., et al. (2000). Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch. Biochem. Biophys. 376 (2), 333–337. doi:10.1006/abbi.2000.1721
Sun, T., Chen, S., Wu, K., Sun, M., Zhang, X., and You, C. (2021). Trends in incidence and mortality of stroke in China from 1990 to 2019. Front. Neurol. 12, 759221. doi:10.3389/fneur.2021.759221
Wang, Y., Li, Z., Gu, H., Zhai, Y., Zhou, Q., Jiang, Y., et al. (2022). China stroke Statistics 2019: A report from the national center for healthcare quality management in neurological diseases, China national clinical research center for neurological diseases, the Chinese stroke association, national center for chronic and non-communicable disease control and prevention, Chinese center for disease control and prevention and institute for global neuroscience and stroke collaborations. Stroke Vasc. Neurol. 5, 211–239. doi:10.1136/svn-2020-000457
Watanabe, T., Tahara, M., and Todo, S. (2008). The novel antioxidant edaravone: From bench to bedside. Cardiovasc. Ther. 26 (2), 101–114. doi:10.1111/j.1527-3466.2008.00041.x
Wu, H., Tang, Y., Gao, L., Sun, W., Hua, Y., Yang, S., et al. (2014). The synergetic effect of edaravone and borneol in the rat model of ischemic stroke. Eur. J. Pharmacol. 740, 522–531. doi:10.1016/j.ejphar.2014.06.035
Xiang, Y., Yang, N., Guo, Z., Zhou, L., Guo, J. J., and Hu, M. (2021). Cost-effectiveness analysis of ginkgolide injection in the treatment of ischemic stroke based on a randomized clinical trial. J. Altern. Complement. Med. 27 (4), 331–341. doi:10.1089/acm.2020.0455
Xu, J., Wang, A., Meng, X., Yalkun, G., Xu, A., Gao, Z., et al. (2021). Edaravone dexborneol versus edaravone alone for the treatment of acute ischemic stroke: A phase III, randomized, double-blind, comparative trial. Stroke 52 (3), 772–780. doi:10.1161/STROKEAHA.120.031197
Ye, Q., Zhai, F., Chao, B., Cao, L., Xu, Y., Zhang, P., et al. (2022). Rates of intravenous thrombolysis and endovascular therapy for acute ischaemic stroke in China between 2019 and 2020. Lancet Reg. Health. West. pac. 21, 100406. doi:10.1016/j.lanwpc.2022.100406
Yu, Z. F., Bruce Keller, A. J., Goodman, Y., and Mattson, M. P. (1998). Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J. Neurosci. Res. 53 (5), 613–625. doi:10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1
Keywords: cost effectiveness, acute ischemic stroke, edaravone, edaravone dexborneol, economic evaluation
Citation: Shi F, He Z, Wang L, Su H and Han S (2022) Cost-effectiveness of edaravone dexborneol versus edaravone for the treatment of acute ischemic stroke in China: Based on the TASTE study. Front. Pharmacol. 13:938239. doi: 10.3389/fphar.2022.938239
Received: 10 May 2022; Accepted: 03 October 2022;
Published: 18 October 2022.
Edited by:
Joseph Kiambi Mworia, Kenyatta University, KenyaReviewed by:
Mohammad Tasavon Gholamhoseini, Kerman University of Medical Sciences, IranCopyright © 2022 Shi, He, Wang, Su and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Han, aGFuc2hlbmdAYmptdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.