- 1Chinese Evidence-Based Medicine Center, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2NMPA Key Laboratory for Real World Data Research and Evaluation in Hainan, Chengdu, Sichuan, China
- 3Sichuan Center of Technology Innovation for Real World Data, Chengdu, Sichuan, China
- 4Evidence-Based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, Gansu, China
- 5Department of Science and Technology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 6Evidence-Based Medicine Research Center, School of Basic Science, Jiangxi University of Traditional Chinese Medicine, Nanchang, Jiangxi, China
Background: Danhong injection is widely used for treating ischemic stroke in China. However, its effects on ischemic stroke patients when given along with Western medicines (i.e., the add-on effect) were not well-established.
Methods: We searched PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and three Chinese databases from inception to 20 July 2020 to identify randomized controlled trials (RCTs) that assessed the effects of Danhong injection as add-on therapy in patients with ischemic stroke. Pairs of trained reviewers independently screened for eligible studies, assessed risk of bias, and extracted the data. The outcomes were the National Institutes of Health Stroke Scale Score (NIHSS), Barthel index, activities of daily living (ADL), total cholesterol, and homocysteine (Hcy).
Results: Sixty-seven RCTs of 6594 patients with varying risk of bias were included. Compared with Western medicine alone, the addition of Danhong injection to Western medicine significantly lowered the NIHSS score (45 RCTs with 4565 patients; MD −4.21, 95% CI −4.96 to −3.46), total cholesterol (10 trials with 1019 patients; MD −1.14 mmol/L, 95% CI −1.57 to −0.72), and Hcy (four trials with 392 patients; MD −3.54 μmol/L, 95% CI −4.38 to −2.07). The addition of Danhong also increased the Barthel index (14 trials with 1270 patients; MD 8.71, 95% CI 3.68–13.74) and ADL (12 trials with 1114 patients; MD 14.48, 95% CI 9.04–19.92) scores. Subgroup analyses showed differential effects in the average cerebral blood flow rate by mean age of patients (<60 years: MD 0.74 cm/s, 95% CI 0.29–1.19; ≥60 years: MD 4.09 cm/s, 95% CI 2.02–6.16; interaction p = 0.002) and the NIHSS score by type of baseline Western medicines (interaction p < 0.00001).
Conclusion: The addition of Danhong injection to Western medicine may improve neurological function, self-care ability, and blood lipid level of ischemic stroke patients. However, given most included trials with unclear risk of bias, current evidence is not definitive, and more carefully designed and conducted trials are warranted to confirm our findings.
Systematic Review Registration: [https://www.crd.york.ac.uk/PROSPERO/], identifier [CRD42022298628].
Introduction
Ischemic stroke, defined as all thromboembolic and atherosclerotic events resulting in compromised blood flow to cerebral tissue and subsequent infarction (Collaborators, 2019), is a leading cause of death and long-term disability worldwide. Current recommendations for patients with ischemic stroke include antiplatelet, anticoagulants, volume expansion, vasodilators, neuroprotective agents, intravenous thrombolysis, and mechanical thrombectomy (Powers et al., 2019). In spite of these, patients with ischemic stroke continue to suffer from a high risk of disability and recurrence, and add-on treatments are often necessary (van der Meij and Wermer, 2021).
Danhong injection is a Chinese patent medicine extracted from Salvia miltiorrhiza Bunge (Lamiaceae; Salviae miltiorrhizae radix et rhizoma) (Danshen in Chinese) and Carthamus tinctorius L (Compositae; carthami oleum raffinatum) (Honghua in Chinese), which improve microcirculation, prevent platelet aggregation, decrease plasma viscosity, and boost the activity of fibrinogen dissolved. Danhong injection has been widely used for cardiovascular and cerebrovascular diseases (Xu et al., 2018; Feng et al., 2019). Evidence from randomized controlled trials (RCTs) with small sample sizes showed that the combination of Danhong injection and Western medicine was effective in improving total efficiency and hemodynamic outcomes among ischemic stroke patients (Liu et al., 2019; Li et al., 2020; Liu and Jin, 2020). Three systematic reviews also found that, compared with other Chinese botanical drug injections (e.g., Shuxuening injection), Danhong injection appeared to be beneficial in total clinical effectiveness rate and neurologic impairment (Wang et al., 2017; Liu et al., 2018; Liu et al., 2019b). However, these reviews included a relatively small number of RCTs and were limited with improper comparisons, inappropriate outcome selection, and analysis, as a result of which the resulting findings and recommendations were not definitive.
Most importantly, Danhong injection is routinely used as an add-on therapy for patients with ischemic stroke receiving Western medicine; its add-on effects on ischemic stroke patients still need further systematic assessment to inform current practice. Therefore, we conducted this systematic review and meta-analyses, which included a significantly larger number of newly published RCTs.
Methods
We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (Page et al., 2021) to conduct and report this systematic review and meta-analysis. This protocol was registered with the International Prospective Register of Systematic Reviews (CRD42022298628).
Eligibility criteria
We included randomized controlled trials (RCTs) that compared the efficacy and safety of Danhong injection combined with Western medicine therapy versus Western medicine alone in patients with ischemic stroke. Western medicine therapy in our study refers to the western standard of care, which contained a range of Western drugs, interventional therapy, and surgical treatment. There was no limitation on the dosages and courses of treatment. Eligible studies explicitly reported outcome data on the National Institute of Health Stroke Scale (NIHSS), the Fugl–Meyer assessment, the Barthel index, activities of daily living (ADL), intima-media thickness (IMT), cerebral blood flow, average cerebral blood flow rate, total cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), homocysteine (Hcy), D-dimer, and adverse drug reactions (ADRs)/adverse drug events (ADEs). We excluded RCTs that involved other Chinese botanical drugs.
Literature search
We searched PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), the China National Knowledge Infrastructure (CNKI) database, the Wanfang database, and the Chinese Scientific Journal Database to identify relevant studies from inception to 20 July 2020. The subject terms (e.g., MeSH terms) and free-text words were used to search for potentially eligible studies, and language restriction was imposed in English and Chinese. The specific retrieval strategy was provided in Supplementary Appendix S1.
Study process
Two reviewers (YM and KD) independently screened titles/abstracts and full texts for eligibility, assessed risk of bias, and collected data from each eligible study using a prespecified form. Reviewers dealt with discrepancies through discussion or, if required, adjudication by a third researcher (LL).
Risk of bias assessment
We used the modified Cochrane Risk of Bias Assessment Tool to assess the risk of bias of included RCTs (Higgins et al., 2011). The items contained random sequence generation (selection bias), allocation concealment (selection bias), blinding of patients and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias. The judgment for each item included a low/high/unclear risk of bias.
Data extraction
We collected the following information from included RCTs: general study characteristics (first author, year of publication, and total number of patients randomized); patient characteristics (age and sex); intervention and control characteristics [medications used across groups (baseline treatment), details of Danhong injection treatment and the control group (dose and type of Western medicines used), and course of treatment]; outcome data (mean and standard deviation of continuous variables, and number of patients with adverse drug reactions/adverse drug events in each group).
Statistical analysis
Reviewer Manager 5.3 (Cochrane Collaboration, Oxford, United Kingdom) was used to analyze and compare the efficacy outcomes of the Danhong injection groups versus the control groups. The mean difference (MD) was calculated for continuous variables; the effects of each outcome were estimated with 95% confidence intervals (95% CIs). We used Cochran’s chi-squared test and the I2 statistic to examine statistical heterogeneity among RCTs. Meta-analysis was calculated by a random-effects model.
We explored sources of heterogeneity with three prespecified subgroup hypotheses: length of treatment (≤2 vs. > 2 weeks; larger effect in trials with longer treatment), mean age of patients (<60 vs. ≥ 60 years old; larger effect in trials with younger patients), and types of baseline Western medicine treatment (conventional therapy vs. intravenous thrombolysis vs. anti-platelet aggregation vs. dilation of blood vessels vs. statins vs. neuroprotective agents vs. other therapies).
In addition, we used the funnel plot and Egger’s test for examining publication bias (Egger et al., 1997). We also used the Duval and Tweedie trim-and-fill method for exploring the influence of a single trial on publication bias (Duval and Tweedie, 2000).
Results
Search results
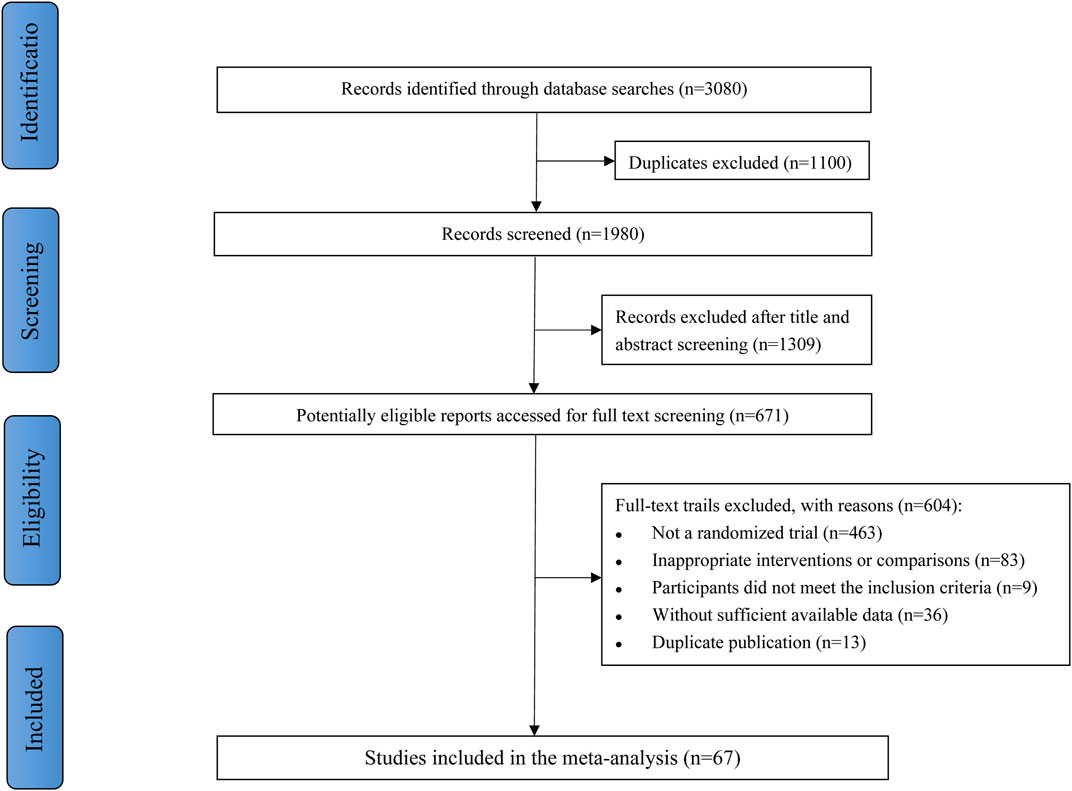
Figure 1 described the study selection process. Our search retrieved 3,080 citations during the initial detection by the search strategy. First, 1,100 duplicates were removed, and 1309 studies were excluded after screening titles and abstracts. Of 671 studies included for full further text screening, 604 studies were excluded for the following reasons: not a randomized trial (n = 463), inappropriate interventions or comparisons (n = 83), participants did not meet the inclusion criteria (n = 9), without sufficient available data (n = 36), or duplicate publication (n = 13). Finally, 67 RCTs were eligible for meta-analysis.
Patient characteristics
These 67 RCTs (Liu and Huang 2010; Xue eand Li, 2010; Ren et al., 2011; Luo et al., 2012; Shen, 2012; Su et al., 2012; Zou et al., 2013; Liang et al., 2014; Song and Yang 2014; Yin, 2014; Fan et al., 2015; Li, 2015; Ma et al., 2015; Zhang, 2015; Cao and Mei 2016; Feng et al., 2016; Wang, 2016; Wang et al., 2016; Wu and Bao 2016; Zeng et al., 2016; Li Q. et al., 2017; Liu F. et al., 2017; Li Y. et al., 2017; Liu Z. et al., 2017; Chen, 2017; Chen and Shi., 2017; Li, 2017; Liu, 2017; Ma, 2017; Ou and Liu 2017; Pen, 2017; Qiu et al., 2017; Wei et al., 2017; Yun et al., 2017; Zhang, 2017; Yang and Liu 2018a; Yang et al., 2018b; Dai, 2018; Fan et al., 2018; Ge, 2018; Li et al., 2018; Liu, 2018; Luo, 2018; Lv et al., 2018; Shi, 2018; Yang, 2018; Zhang et al., 2018; Liu Q. et al., 2019; Cao et al., 2019; Chai et al., 2019; Chen et al., 2019; Cheng, 2019; Liu Y. et al., 2019; Deng, 2019; Jin et al., 2019; Li and Tian 2019; Yuan, 2019; Zhang, 2019; Cao, 2020; Huo et al., 2020; Jiang et al., 2020; Jing and Yu, 2020; Kang et al., 2020; Li et al., 2020; Liu and Jin, 2020; Zhu and Qi 2020; Zhuang and Zhao., 2020) comprised 3293 patients in the combination treatment group and 3301 patients in the control group. The mean age of included patients ranged from 42.2 to 71.5 years, and the sample size ranged from 40 to 300 patients. All RCTs were conducted among Chinese populations in China. Patients in the combination treatment group received Danhong injection of 10–40 ml once or twice daily. There was no significant difference between the experiment group and the control group in baseline characteristics (Table 1).
Risk of bias
All RCTs reported methods of random sequence generation. Only three trials included (4%) blinded patients and caregivers (Liu and Huang 2010; Su et al., 2012; Liu, 2017). No details were identified in the domains of allocation concealment, selective outcome reporting, and other sources of bias; thus, we judged these items as unclear risk of bias (Supplementary Appendix 3, Table S1).
National institute of health stroke scale analysis
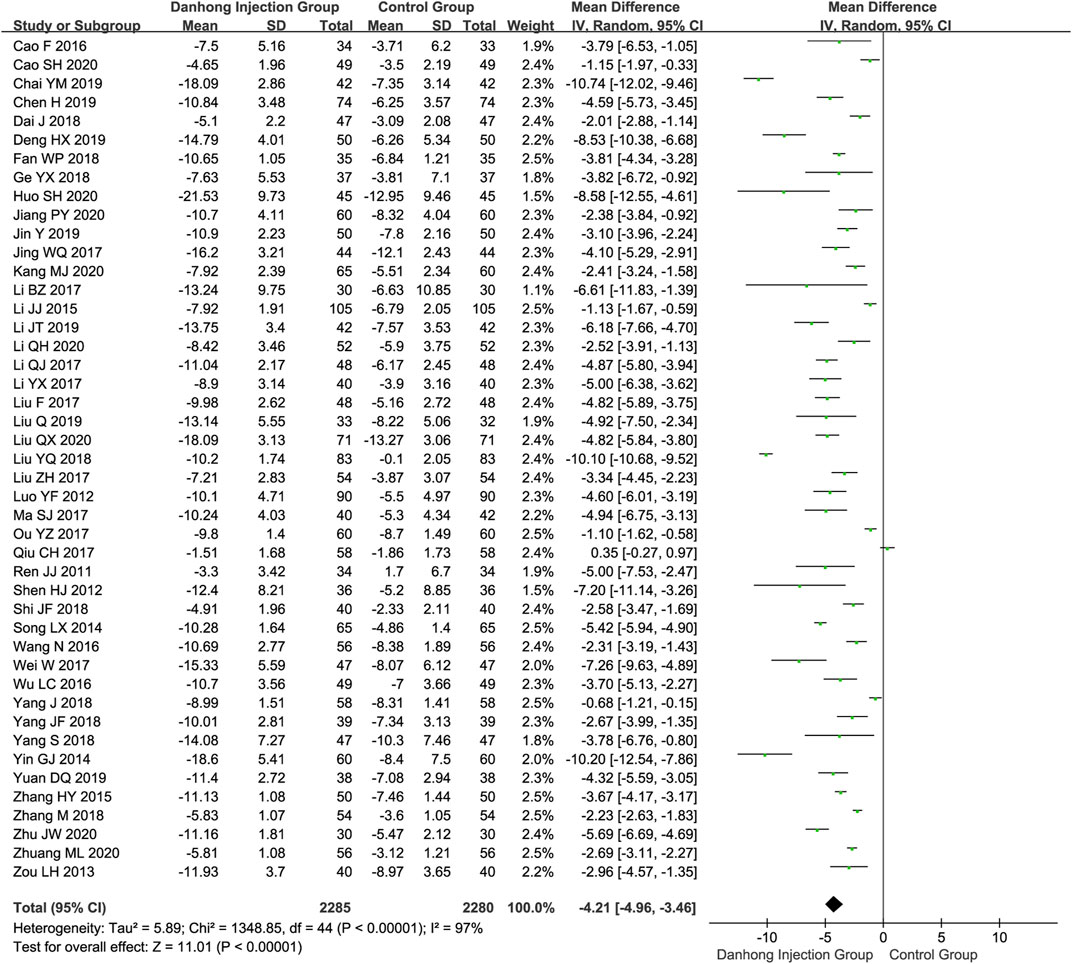
In total, 45 RCTs (Ren et al., 2011; Luo et al., 2012; Shen, 2012; Zou et al., 2013; Song and Yang 2014; Yin, 2014; Li, 2015; Zhang, 2015; Cao and Mei 2016; Wang et al., 2016; Wu and Bao, 2016; Li Q. et al., 2017; Liu F. et al., 2017; Li Y. et al., 2017; Liu Z. et al., 2017; Li, 2017; Ma, 2017; Ou and Liu 2017; Qiu et al., 2017; Wei et al., 2017; Yang and Liu, 2018a; Yang et al., 2018b; Dai, 2018; Fan et al., 2018; Ge, 2018; Liu, 2018; Shi, 2018; Yang, 2018; Zhang et al., 2018; Liu Q. et al., 2019; Chai et al., 2019; Chen et al., 2019; Deng, 2019; Jin et al., 2019; Li and Tian 2019; Yuan, 2019; Cao, 2020; Huo et al., 2020; Jiang et al., 2020; Jing and Lian, 2020; Kang et al., 2020; Liu and Jin, 2020; Zhu and Qi, 2020; Zhuang and Zhao, 2020) involving 4565 patients reported the NIHSS score. For Danhong injection combined with the Western medicine group, the NIHSS score significantly changed −10.50 (95% CI: −11.50 to −9.51), and the control group changed −6.22 (95% CI: −7.05 to −5.39). Meta-analysis showed that Dahong injection combined with Western medicine had a better effect in improving neurological impairment than Western medicine alone (NIHSS score MD −4.21, 95% CI −4.96 to −3.46) (Figure 2).
Self-care ability analysis
Six RCTs (Ma et al., 2015; Chen and Shi, 2017; Ma, 2017; Luo, 2018; Liu Y. et al., 2019; Yuan, 2019) involving 552 patients showed that Danhong injection combined with Western medicine could significantly improve the Fugl–Meyer assessment than Western medicine alone (MD 14.28, 95% CI 9.47–19.09) (Figure 3). Compared with Western medicine alone, Danhong injection plus Western medicine suggested a significant improvement in the Barthel index (14 trials with 1,270 patients; MD 8.71, 95% CI 3.68–13.74) and the ADL score (12 trials with 1,114 patients; MD 14.48, 95% CI 9.04–19.92) (Supplementary Figures S1, S2, Appendix 2).
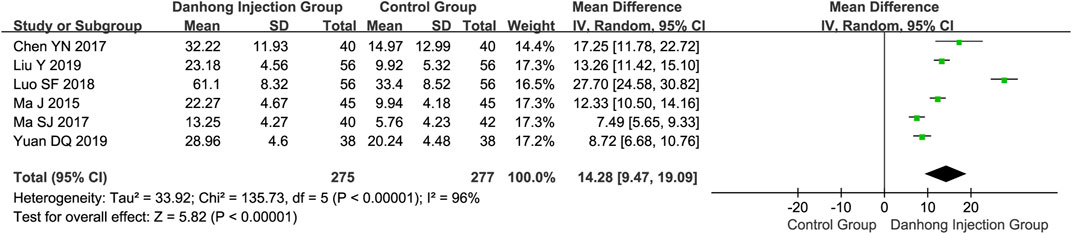
FIGURE 3. Meta-analysis of Fugl–Meyer Assessment between DHI + WM and WM in patients with ischemic stroke.
Hemodynamic outcomes analysis
Meta-analysis showed that compared with Western medicine alone, the combination therapy of Danhong injection and Western medicine could significantly lower the IMT (three trials with 284 patients; MD −0.23 mm, 95% CI −0.26 to −0.20), increase the cerebral blood flow (two trials with 200 patients; MD 1.16 ml/s, 95% CI 0.64–1.68), and average cerebral blood flow rate (five trials with 508 patients; MD 3.05 cm/s, 95% CI 1.61–4.50) (Supplementary Figures S3–S5, Appendix 2).
Blood lipid analysis
The combination therapy of Danhong injection and Western medicine could significantly lower total cholesterol (10 trials with 1019 patients; MD −1.14 mmol/L, 95% CI −1.57 to −0.72) (Figure 4), triglycerides (eight trials with 869 patients; MD −1.00 mmol/L, 95% CI −1.69 to −0.31), and LDL (five trials with 441 patients; MD −0.91 mmol/L, 95% CI −1.33 to −0.49), and increase the level of HDL (three trials with 236 patients; MD 0.31 mmol/L, 95% CI 0.22–0.40) (Supplementary Figures S6–S8, Appendix 2).
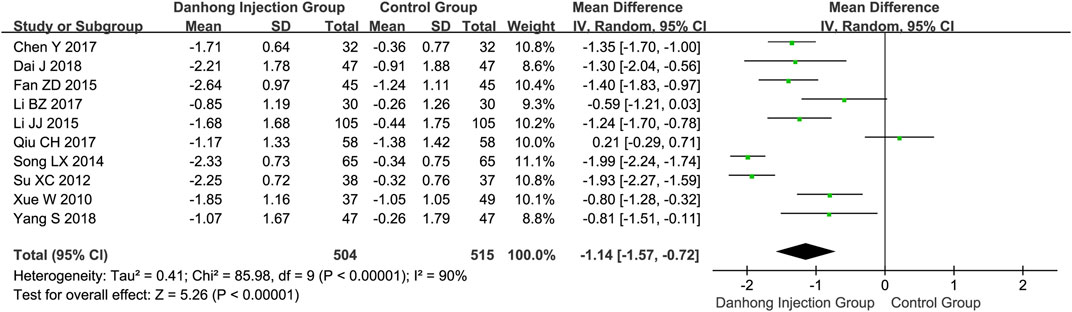
FIGURE 4. Meta-analysis of total cholesterol between DHI + WM and WM in patients with ischemic stroke.
Hcy analysis
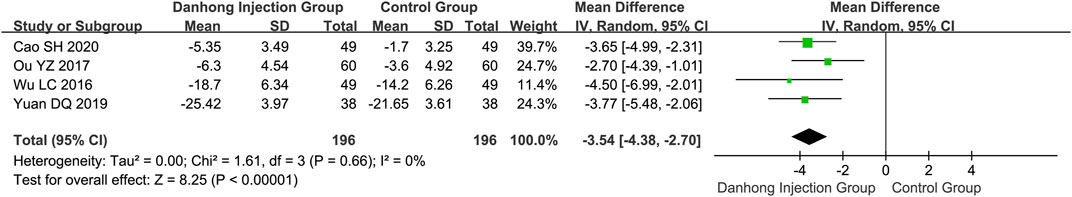
Four RCTs (Wu and Bao, 2016; Ou and Liu 2017; Yuan, 2019; Cao, 2020) with a total of 392 individuals reported the data of serum Hcy level. The results revealed a significant lowering effect on Hcy in the Danhong injection combination therapy (MD −3.54 μmol/L, 95% CI −4.38 to −2.07) (Figure 5).
D-dimer analysis
Meta-analysis of five trials with a total of 612 patients showed no statistically significant difference in D-dimer (MD −0.12 mg/L, 95% CI −0.61–0.37, p = 0.64) between treatment groups (Supplementary Figure S9, Appendix 2).
Subgroup analysis
Subgroup analysis by mean age showed differential effects in average cerebral blood flow rate (<60 years old: MD 0.74 cm/s, 95% CI 0.29–1.19; ≥60 years old: MD 4.09 cm/s, 95% CI 2.02–6.16; interaction p = 0.002). The analysis by length of treatment showed differential effects in ADL (≤2 weeks: MD 15.08, 95% CI 9.11–21.05; >2 weeks: MD 8.18, 95% CI 6.21–10.15; interaction p = 0.03). Subgroup analysis by the type of baseline Western medicines showed differential effects (conventional therapy: MD −3.79, 95% CI −4.83 to −2.76; intravenous thrombolysis: MD −2.19, 95% CI −3.52 to −0.86; anti-platelet aggregation: MD −3.79, 95% CI −5.06 to −2.51; dilation of blood vessels: MD −4.23, 95% CI −5.85 to −2.62; statins: MD −2.87, 95% CI −4.07 to −1.67; neuroprotective agents: MD −5.88, 95% CI −8.90 to −2.87; other therapies: MD −10.20, 95% CI −12.54 to −7.86; interaction p < 0.00001).
As for the Fugl-Meyer assessment, the Barthel index, total cholesterol, triglycerides, LDL, Hcy, and D-dimer, subgroup analyses by length of treatment, mean age of patients, and types of baseline Western medicine treatment showed no statistically differential effects.
Safety
Of the 67 included trials, 16 studies (Liu and Huang 2010; Shen, 2012; Wang, 2016; Li Q. et al., 2017; Li Y. et al., 2017; Chen and Shi, 2017; Wei et al., 2017; Zhang, 2017; Yang ad Liu 2018a; Ge, 2018; Li et al., 2018; Liu, 2018; Lv et al., 2018; Zhang et al., 2018; Li and Tian, 2019; Zhuang and Zhao, 2020) reported at least one case of ADRs, 12 studies (Xue and Li, 2010; Su et al., 2012; Song and yang, 2014; Cao and Mei, 2016; Wu and Bao, 2016; Luo, 2018; Cao et al., 2019; Cheng, 2019; Zhang, 2019; Cao, 2020; Jiang et al., 2020; Kang et al., 2020) reported no ADRs/ADEs during the study, and 39 RCTs did not mention the information of ADRs/ADEs.
The main ADRs that occurred during the trials were gastrointestinal reactions (4.48% vs. 4.76% in Danhong injection plus Western medicine and Western medicine alone group, respectively, p = 0.76), dizziness (3.69% vs. 2.74% in each group, p = 0.61), and skin rash (8.53% vs. 7.31% in each group, p = 0.56). ADRs that occurred during the trials in either group are shown in Supplementary Appendix 3, Table S2.
Assessment of publication bias
The funnel plots for NIHSS, Barthel index, and ADL were near asymmetric, and no important publication bias was detected by Egger’s test (Egger’s test p = 0.055, p = 0.342 and p = 0.998, respectively). Egger’s test of total cholesterol suggested potential publication bias (Egger’s test p = 0.019). The sensitivity analysis by the trim-and-fill method showed that the results were robust despite the potential bias (trim-and-fill method adjusted MD −1.14 mmol/L, 95% CI −1.57 to −0.72) (Supplementary Figures S10, S11, Appendix 2).
Discussion
Findings and interpretations
In this systematic review and meta-analysis of 67 randomized controlled trials, we found that adding Danhong injection to Western medicine could significantly improve neurological function (e.g., improving NIHSS, the Fugl–Meyer assessment), self-care ability (e.g., improving the Barthel index and ADL scores), hemodynamic outcomes (cerebral blood flow, average cerebral blood flow rate), blood lipids, and Hcy in patients with ischemic stroke. Furthermore, patients of different ages and receiving different baseline Western medicines may have different benefits in hemodynamic status and neurological function.
NIHSS is a stroke-specific quantitative scale with excellent reliability and validity for ischemic stroke outcomes and measures stroke-related neurological deficits such as the level of consciousness, language function, visual field, eye movement sensory function, and coordination (Muir et al., 1996; Kasner et al., 1999; Boone et al., 2012). In our study, we found that NIHSS scores were significantly decreased in patients receiving Danhong injection plus Western medicine. The minimal clinically important difference (MCID) of the NIHSS score in stroke patients was considered an increase or decrease of two points, that is, a change in the NIHSS score of more than two points suggests clinical importance of the differences (Chang and Xu., 2011; Neurology Chapter of China Association of Chinese Medicine et al., 2020; Zeitlberger et al., 2021). The change of the NIHSS score in our study was 4.21 points between the experiment group and the control group, which suggested that Danhong injection added to Western medicine would be more beneficial in reducing the severity of ischemic stroke and improving neurological status than Western medicine alone for ischemic stroke patients. Overactivation of inflammatory factors and aggregation of platelets cause thrombosis and can lead to vascular occlusion, resulting in cerebrovascular ischemia, and then contribute to neurological deficit and physical dysfunction (Franks et al., 2010; Xu et al., 2016; Komurcu et al., 2020). Since Danhong injection could inhibit platelet aggregation and boost the activity of fibrinogen dissolved, the add-on effects of Danhong injection and Western medicine play a key role in improving NIHSS.
The Fugl–Meyer assessment is considered one of the most comprehensive and evaluative measures for assessment of recovery in poststroke patients (Gladstone et al., 2002). As approximately 85% of patients with stroke present with arm weakness (Dawson et al., 2016), our study found that the scores in the Fugl–Meyer assessment were significantly increased in patients treated by the integrated treatment with Danhong injection and Western medicine, which suggests that Danhong injection plus Western medicine have the potential of enhancing limb function in stroke recovery.
PPAR-α, a transcription factor that regulates diverse aspects of lipid metabolism, plays a key role in the regulation of hepatic lipid metabolism (Kersten et al., 1999; Li and Glass, 2004). Our study showed that the combination of Danhong injection and Western medicine had a noteworthy effect on reducing blood lipids (e.g., total cholesterol, triglycerides, and LDL). Our study also showed that Danhong injection plus Western medicine had a marked impact on increasing cerebral blood flow and average cerebral blood flow rate, which can be explained with the fact that Danhong injection could improve hemodynamic indices such as high shear viscosity, low shear viscosity, hematocrit, and platelet aggregation rate (Jiang and Lian, 2015).
Our study found the most common ADRs in these reported RCTs were gastrointestinal reactions including nausea, flatulence, and vomiting, which are a consequence of the drug’s normal pharmacological effects (Li et al., 2015). About 58.2% of RCTs did not mention the information of ADRs in this study; thus, we were unable to make a conclusion on safety so far. More credible evidence is warranted to confirm the safety of the add-on effect of this drug in the future.
Comparison with other studies
Three previous systematic reviews and meta-analyses have assessed the effect of Danhong injection on the treatment of ischemic stroke (Wang et al., 2017; Liu et al., 2018; Liu et al., 2019b). In terms of efficacy, all these three studies compared Danhong injection with other Chinese botanical drug injections such as Shuxuening injection, Yinxingdamo injection, and so on. Instead of making a claim of comparative effectiveness between traditional Chinese medicines, we aimed to address whether the addition of traditional Chinese medicine to routinely used medications would improve the effects on ischemic stroke, which was a question that needed strong evidence for conclusion. As it turned out, our study has provided a reliable assessment of the effects.
Strengths and limitations
Our study has several strengths. First, we used rigorous methods to search, screen, and collect data from eligible RCTs. Second, we included several newly published RCTs to summarize the efficacy and safety of Danhong injection in patients with ischemic stroke, aiming to provide a more comprehensive, up-to-date, and new level of clinical evidence. Third, we included a large number of RCTs and used both patients who reported important outcomes (e.g., NIHSS, Fugl-Meyer Assessment scores) and other objective outcomes (e.g., blood lipids, hemodynamic outcomes) to comprehensively assess the efficacy and safety of Danhong injection in patients with ischemic stroke. Fourth, we conducted three prespecified subgroup analyses to explore sources of heterogeneity.
However, our study also has some limitations. The included RCTs may be at a risk of high risk of bias. As a result, the findings may be susceptible to the bias. Additional well-designed and rigorous trials with long-term outcomes may be needed to further consolidate our findings.
Conclusion
In summary, the available evidence suggests that Danhong injection plus Western medicine may improve the effects of neurological function, self-care ability, hemodynamic status, blood lipids, and Hcy for patients with ischemic stroke. However, given that most included trials presented with unclear risk of bias, current evidence is inconclusive and should be explained with caution. Therefore, more carefully designed and conducted trials are needed to confirm the add-on effects of Danhong injection.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
XS and LL conceived the study. YM and LL had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. YM and KD conducted the literature searches and extracted the data. YM and LL conducted the analysis, interpreted the data, and drafted the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by the National Key R&D Program of China (Grant No. 2019YFC170 9804 and 2017YFC1700406), the National Natural Science Foundation of China (Grant No. 719 04134), Sichuan Youth Science and Technology Innovation Research Team (Grant No. 2020 JDTD0015), China Center for Evidence Based Traditional Chinese Medicine (Grant No. 2020YJSZX-3), and 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (Grant No. ZYYC08003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.937369/full#supplementary-material
Abbreviations
ADEs, adverse drug events; ADL, activities of daily living; ADRs, adverse drug reactions; CIs, confidence intervals; CNKI, China national knowledge infrastructure database; Hcy, homocysteine; HDL, high-density lipoprotein; IMT, intima-media thickness; LDL, low-density lipoprotein; MCID, minimal clinically important difference; MD, mean difference; NIHSS, national institutes of health stroke scale score; NR, not reported; PRISMA, preferred reporting items for systematic reviews and meta-analyses; RCTs, randomized controlled trials
References
Boone, M., Chillon, J. M., Garcia, P. Y., Canaple, S., Lamy, C., Godefroy, O., et al. (2012). NIHSS and acute complications after anterior and posterior circulation strokes. Ther. Clin. Risk Manag. 8, 87–93. doi:10.2147/TCRM.S28569
Cao, F., and Mei, B. (2016). The clinical effect of Danhong injection in the treatment of branch atheromatous disease. Stroke Nerv. Dis. 23, 442. doi:10.3969/j.issn.1007-0478.2016.06.014
Cao, S. (2020). Efficacy of Danhong injection combined with alteplase in the treatment of acute cerebral infarction. Chin. J. Integr. Med. Cardio/Cerebrovascular Dis. 18, 827. doi:10.12102/j.issn.1672-1349.2020.05.032
Cao, Y., Huang, H., Duan, D., Song, Y., and Wang, Y. (2019). Clinical efficacy and safety analysis of Danhong injection in treating acute cerebral infarction. HEBEI Med. 25, 676. doi:10.3969/j.issn.1006-6233.2019.04.039
Chai, Y., Zhang, Y., Shi, Y., Kang, L., and Zhu, Z. (2019). Efficacy of the combination of ozagrel sodium, Danhong injection and aspirin (ASA) on acute cerebral infarction and the effect on patients' ADL scores. Guizhou Med. J. 43, 1922. doi:10.3969/j.issn.1000-744X.2019.12.031
Chang, S., and Xu, Y. (2011). NIHSS's Re-evaluation. Neural Inj. Funct. Reconstr. 6, 305. doi:10.3870/sjsscj.2011.04.016
Chen, H., Zhang, F., Wang, W., and Lv, R. (2019). Efficacy of dan hong injection in the adjuvant treatment of acute cerebral infarction and its effect on oxidative stress. Mod. J. Integr. Traditional Chin. West. Med. 28, 1986
Chen, Y., and Shi, X. (2017). Clinical effect of Danhong injection combined with rehabilitation exercise on acute cerebral infarction and its effect on motor function. Chin. J. Biochem. Pharm. 10, 147. doi:10.3969/j.issn.1005-1678.2017.10.059
Chen, Y. (2017). The efficacy of Danhong injection in the treatment of patients with cerebral infarction. Med. Front. 7, 64. doi:10.3969/j.issn.2095-1752.2017.13.044
Cheng, S. (2019). Effect of Danhong injection combined with edaravone injection in the treatment of senile cerebral infarction. China Mod. Med. 26, 71. doi:10.3969/j.issn.1674-4721.2019.28.021
Collaborators, G. B. D. S. (2019). Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. Neurol. 18, 439–458. doi:10.1016/S1474-4422(19)30034-1
Dai, J. (2018). Clinical efficacy of Danhong injection in patients with acute cerebral infarction and it's influence on NIHSS and ADL scores. Clin. Med., 22. doi:10.19347/j.cnki.2096-1413.201818009
Dawson, J., Pierce, D., Dixit, A., Kimberley, T. J., Robertson, M., Tarver, B., et al. (2016). Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke 47, 143–150. doi:10.1161/STROKEAHA.115.010477
Deng, H. (2019). Danhong injection combined with edaravone injection on clinical efficacy study of patients with cerebral infarction. Clin. Res. 27, 101. doi:10.3969/j.issn.1004-8650.2019.01.055
Duval, S., and Tweedie, R. (2000). Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463. doi:10.1111/j.0006-341x.2000.00455.x
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi:10.1136/bmj.315.7109.629
Fan, W., Huang, X., Ma, F., Xu, M., and Wan, Q. (2018). Clinical efficacy of Danhong injection in the treatment of acute cerebral infarction. Prog. Mod. Biomed. 18, 1772–1775.
Fan, Z., Chen, H., and Jiang, C. (2015). Clinical eficacy of Danhong injection on cerebral ischemic stroke and its influence on blood lipid metabolism and nerve functio. Chin. J. Evidenced Based Cardiovasc Med. 7, 809. doi:10.3969/j.issn.1674-4055.2015.06.27
Feng, Q., Xu, M., Zhang, W., Huang, J., Liu, T., HuanGPU, L., et al. (2016). Clinical study on intra-arterial thrombolysis with rt-PA and dan red injection for acute cerebral infarction. J. Emerg. Traditional Chin. Med. 25, 239. doi:10.3969/j.issn.1004-745X.2016.02.016
Feng, X., Li, Y., Wang, Y., Li, L., Little, P. J., Xu, S. W., et al. (2019). Danhong injection in cardiovascular and cerebrovascular diseases: pharmacological actions, molecular mechanisms, and therapeutic potential. Pharmacol. Res. 139, 62–75. doi:10.1016/j.phrs.2018.11.006
Franks, Z. G., Campbell, R. A., Weyrich, A. S., and Rondina, M. T. (2010). Platelet-leukocyte interactions link inflammatory and thromboembolic events in ischemic stroke. Ann. N. Y. Acad. Sci. 1207, 11–17. doi:10.1111/j.1749-6632.2010.05733.x
Ge, Y., Zhang, Q., Jiao, Z., Li, H., Bai, G., and Wang, H. (2018). Adipose-derived stem cells reduce liver oxidative stress and autophagy induced by ischemia-reperfusion and hepatectomy injury in swine. Life Sci. 30, 62–69. doi:10.1016/j.lfs.2018.10.054
Gladstone, D. J., Danells, C. J., and Black, S. E. (2002). The fugl-meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil. Neural Repair 16, 232–240. doi:10.1177/154596802401105171
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). Cochrane bias methods, G. & cochrane statistical methods, G The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Huo, S., Guo, Y., and Li, J. (2020). The effect of Danhong injection combined with calf serum deprotein injection for the treatment of elderly acute cerebral infarction. Clin. Med. 40, 104–105.
Jiang, P., Wang, S., and Guo, C. (2020). The treatment of acute cerebral infarction with Danhong injection. J. Henan Med. Coll. 32, 42. doi:10.3969/j.issn.1008-9276.2020.01.014
Jiang, Y., and Lian, Y. J. (2015). Effects of Danhong injection on hemodynamics and the inflammation-related NF-κB signaling pathway in patients with acute cerebral infarction. Genet. Mol. Res. 14, 16929–16937. doi:10.4238/2015.December.14.21
Jin, Y., Jin, L., Liao, Y., and Liu, F. (2019). Dan hong injection combined with recombinant tissue-type fibrinogen activator on the neurological coagulation function of patients with acute cerebral infarction and vascular endothelial function level in patients with acute cerebral infarction. Chin. Remedies Clin. 19, 3400. doi:10.11655/zgywylc2019.19.068
Jing, W., and Yu, J. (2020). Study on the clinical efficacy of danhong injection in the adjuvant treatment of acute cerebral infarction. Chian Pract. Med. 12, 138–139.
Kang, M., Wen, C., Liu, Y., Sun, J., and Zhang, B. (2020). Effect of Danhong injection and tirofiban on acute cerebral infarction and associated NF-κB inflammation signal pathway. Chin. J. Ration. Drug Use 5, 56. doi:10.3969/j.issn.2096-3327.2020.5.013
Kasner, Scott E., Chalela, Julio A., Luciano, Jean M., Cucchiara, Brett L., Raps, Eric C., Mcgarvey, MicH. A. E. L. L., et al. (1999). reliability and validity of estimating the NIH stroke scale score from medical records. Stroke 30, 1534–1537. doi:10.1161/01.str.30.8.1534
Kersten, S., Seydoux, J., Peters, J. M., Gonzalez, F. J., Desvergne, B., and Wahli, W. (1999). Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Invest. 103, 1489–1498. doi:10.1172/JCI6223
Komurcu, H. F., Gozke, E., Dogan Ak, P., Kalyoncu Aslan, I., Salt, I., and Ozgenc Bi Er, C. I. (2020). Changes in neutrophil, lymphocyte, platelet ratios and their relationship with NIHSS after rtPA and/or thrombectomy in ischemic stroke. J. Stroke Cerebrovasc. Dis. 29, 105004. doi:10.1016/j.jstrokecerebrovasdis.2020.105004
Li, A. C., and Glass, C. K. (2004). PPAR- and LXR-dependent pathways controlling lipid metabolism and the development of atherosclerosis. J. Lipid Res. 45, 2161–2173. doi:10.1194/jlr.R400010-JLR200
Li, B., Li, X. Y., Zhong, W., Shao, C., Wang, Z. Q., Yuan, W., et al. (2017). Impact of CD137-CD137L signaling mediated exocytosis of autophagosome within vascular smooth muscle cells on the formation of atherosclerotic calcification. Zhonghua Xin Xue Guan Bing Za Zhi 26, 49–56. doi:10.3760/cma.j.issn.0253-3758.2017.01.010
Li, J. (2015). An investigation of therapeutic efect of Danhong injection combined with edaravone for treatment of patients with acute cerebral infarction. Chin. J. Integr. Traditional West. Med. Intensive Crit. Care 22, 178. doi:10.3969/j.issn.1008-9691.2015.02.017
Li, J., and Tian, Y. (2019). Effect of dan hong injection combined with eurefrin in the treatment of acute ischemic stroke. Henan Med. Res. 28, 2630. doi:10.3969/j.issn.1004-437X.2019.14.064
Li, Q., He, Z., and Li, Q. (2017a). Observation on efficacy of Danhong injection combined with clopidogrel in treatment of acute cerebral infarction. Eval. Analysis Drug-use Hosp. China 17, 1336. doi:10.14009/j.issn.1672-2124.2017.10.014
Li, Q., Hou, S., Yang, H., Su, Y., Tu, Z., Bi, Q., et al. (2020). The effect of dan hong injection combined with butylphthalide injection on acute cerebral infarction patients effects of BDNF, NPY and NSE. Chin. J. Integr. Med. Cardio/Cerebrovascular Dis. 18, 1148. doi:10.12102/j.issn.1672-1349.2020.07.030
Li, X., Huang, S., Yu, T., and Lan, X. (2018). Efficacy of Danhong injection combined with atorvastatin calcium for diabetes mellitus complicated with cerebral infarction. Guangxi Med. J. 40, 1009. doi:10.11675/j.issn.0253-4304.2018.09.03
Li, X. L., Tang, J. F., Li, W. X., Li, C. X., Zhao, T., Zhao, B. C., et al. (2015). Postmarketing safety surveillance and reevaluation of Danhong injection: Clinical study of 30888 cases. Evid Based Complement Alternat Med, 610846. doi:10.1155/2015/610846
Li, Y., Li, Y., Zhang, S., Wang, H., and CaI, D. (2017b). Clinical efficacy of the edaravone combined with Danhong injection in the treatment of acute cerebral infarction and its influence on serum cytokine level. J. Guangdong Med. Univer. 35 469. doi:10.3969/j.issn.1005-4057.2017.05.003
Liang, Z., Zhang, H., Qi, D., and Wen, M. (2014). Effect of dan hong injection on serum of acute ischemic stroke patients inflammatory factors in acute ischemic stroke patients. J. Emerg. Traditional Chin. Med. 23, 2115. doi:10.3969/j.issn.1004-745X.2014.11.065
Liu, F., Li, Y., Liu, G., Wang, W., and Zhang, Q. (2017a). Effects of Danhong injection on the serum blood uric acid and bilirubin levels of patients with acute cerebral infarction. Prog. Mod. Biomed. 17, 1321. doi:10.13241/j.cnki.pmb.2017.07.031
Liu, H. (2017). Effects of Danhong injection on cerebral infarction in patients with type 2 diabetes mellitus effects of vasodilatory function and endothelial tPA and NO reserve release. Chin. J. Integr. Med. Cardio/Cerebrovascular Dis. 15, 3177. doi:10.3969/j.issn.1672-1349.2017.24.030
Liu, Q., and Jin, B. (2020). Effect of Danhong injection combined with edaravone in the treatment of patients with acute cerebral infarction and its influence on cytokines, cerebral hemodynamics and vascular endothelial function. Chin. J. Prim. Med. Pharm. 27, 423–427.
Liu, Q., Peng, G., and Lin, H. (2019a). Effect of Danhong injection on TCM syndrome score, NIHSS integral and inflammatory factor in patients with acute cerebral infarction. Chian Pract. Med. 14, 130. doi:10.14163/j.cnki.11-5547/r.2019.16.071
Liu, S., Wang, K., Duan, X., Wu, J., Zhang, D., Liu, X., et al. (2019b). Efficacy of danshen class injection in the treatment of acute cerebral infarction: a bayesian network meta-analysis of randomized controlled trials. Evid. Based. Complement. Altern. Med., 5814749. doi:10.1155/2019/5814749
Liu, S., Wu, J. R., Zhang, D., Wang, K. H., Zhang, B., Zhang, X. M., et al. (2018). Comparative efficacy of Chinese herbal injections for treating acute cerebral infarction: a network meta-analysis of randomized controlled trials. BMC Complement. Altern. Med. 18, 120. doi:10.1186/s12906-018-2178-9
Liu, W., and Huang, G. (2010). Clinical efficacy of fasudil hydrochloride combined with Danhong injection in the treatment of acute ischemic cerebrovascular disease. Strait Pharm. J. 22, 158. doi:10.3969/j.issn.1006-3765.2010.06.078
Liu, Y., Chen, X., and Xu, P. (2019c). Effect of Danhong injection combined with edaravone in the treatment of acute cerebral infarction. Contemp. Med. Symp. 17, 113. doi:10.3969/j.issn.2095-7629.2019.22.079
Liu, Y. (2018). Effects of edaravone combined with Danhong injection on diabetes complicated with cerebral infarction. Clin. Med. 38, 84. doi:10.19528/j.issn.1003-3548.2018.08.035
Liu, Z., Wang, H., Liu, L., Feng, C., Liu, Y., and Liu, X. (2017b). Influence of Danhong injection on endothelial progenitor CellsInflammatory factor and neural function in patients with acute cerebral infarction. Int. J. Laboratory Med. 38, 3265. doi:10.3969/j.issn.1673-4130.2017.23.017
Luo, S. (2018). Treatment of acute ischemic stroke with Danhong injection combined with butylphthalide soft capsule for the efficacy of post-stroke and the effect on cognitive function. Mod. Pract. Med. 30, 1171. doi:10.3969/j.issn.1671-0800.2018.09.025
Luo, Y., Xu, D., and Xu, X. (2012). 90 cases of acute cerebral infarction treated with Danhong injection combined with ozagrel. Chin. J. Prim. Med. Pharm. 19, 3136. doi:10.3760/cma.j.issn.1008-6706.2012.20.067
Lv, Q., Shi, F., Shan, K., and Jing, J. (2018). The effect of recombinant tissue plasminogen activator combined with Danhong injection on seruln cytokines and inflammatory factor levels in patients with acute cerebral infarction. Stroke Nerv. Dis. 25, 635. doi:10.3969/j.issn.1007-0478.2018.06.003
Ma, J., Yu, N., Wu, H., Chen, M., Ren, Z., and Zhao, D. (2015). Effect of Danhong injection on nerve function and hemorheology for patients with acute ischemic stroke. Chin. J. Exp. Traditional Med. Formulae 21, 204. doi:10.13422/j.cnki.syfjx.2015200204
Ma, S. (2017). Dan hong injection combined with clopidogrel for the treatment of efficacy and effect on blood rheology in patients with ischemic stroke. Cardiovasc. Dis. J. Integr. Traditional Chin. West. Med. 5, 100. doi:10.3969/j.issn.2095-6681.2017.24.080
Muir, K. W., Weir, C. J., Murray, G. D., Povey, C., and Lees, K. R. (1996). Comparison of neurological scales and scoring systems for acute stroke prognosis. stroke 27, 1817–1820. doi:10.1161/01.str.27.10.1817
Neurology Chapter Of China Association Of Chinese Medicine, (2020). Neurology committee of guangdong provincial association of Chinese medicine & stroke committee of guangdong provincial association of chinese integrative medicine 2020. evidence-Based practice guideline on integrative medicine for stroke 2019. Chin. J. Evidence-based Med. 20, 901. doi:10.7507/1672-2531.202001075
Ou, Y., and Liu, Q. (2017). Clinical efect of Danhong injection in treating acute cerebral infarction. J. Mod. Health 33, 1601. doi:10.3969/j.issn.1009-5519.2017.11.001
Page, M. J., Mckenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Pen, Y. (2017). The clinical efficacy of Danhong injection combined with conventional therapy for the treatment of diabetes cmbined with cerebral ifarction in patients clinical research, 72.
Powers, W. J., Rabinstein, A. A., Ackerson, T., Adeoye, O. M., BaMBAKIDIS, N. C., Becker, K., et al. (2019). Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke 50, e344–e418. doi:10.1161/STR.0000000000000211
Qiu, C., Zang, Y., Wang, Q., Wang, C., and Zhao, C. (2017). Effect of Danhong injection in the treatment of senile acute cerebral infarction and its effect on blood LipidBlood rheology and high sensitivity C-reactive protein. Mod. Med. J. 45, 71. doi:10.3969/j.issn.1671-7562.2017.01.017
Ren, J., Zhang, A., and Cao, F. (2011). Effect of dan hong injection on plasma IL-6 and TNF-α concentrations in ACI patients. J. Radioimmunology 24, 570. doi:10.3969/j.issn.1008-9810.2011.05.055
Shen, H. (2012). Efficacy of Danhong injection combined with edaravone in the treatment of cerebral infarction. Guide China Med. 10, 580. doi:10.15912/j.cnki.gocm.2012.24.483
Shi, J. (2018). Observation on the clinical efficacy of Danhong injection combined with alprostadil in the treatment of acute cerebral infarction. Chin. Community Dr. 34, 101. doi:10.3969/j.issn.1007-614x.2018.13.057
Song, L., and Yang, M. (2014). The application value in dan hong injection combined with conventional treatment in patients with acute cerebral infarction. Mod. J. Integr. Traditional Chin. West. Med. 23, 1522. doi:10.3969/j.issn.1008-8849.2014.14.016
Su, X., Luo, S., and Long, J. (2012). The efficacy of Danhong injection in treating 38 cases of cerebral infarction. Guangxi Med. J. 34, 1543
Van Der Meij, A., and Wermer, M. J. H. (2021). Vagus nerve stimulation: a potential new treatment for ischaemic stroke. Lancet 397, 1520–1521. doi:10.1016/S0140-6736(21)00667-X
Wang, K., Zhang, D., Wu, J., Liu, S., Zhang, X., and Zhang, B. (2017). A comparative study of Danhong injection and Salvia miltiorrhiza injection in the treatment of cerebral infarction: A systematic review and meta-analysis. Med. Baltim. 96, e7079. doi:10.1097/MD.0000000000007079
Wang, L. (2016). Curative effect early use of Danhong injection senile cerebral infarction. J. Shandong Med. Coll. 38, 258. doi:10.3969/j.issn.1674-0947.2016.04.007
Wang, N., Guo, H., Liu, P., Guo, S., and Yang, Q. (2016). Levels of IL-8,Fibulin-5 and P-selectin in patients with acute cerebral infarction and the intervention effect of Danhong injection. J. Liaoning Univ. Traditional Chin. Med. 18, 177. doi:10.13194/j.issn.1673-842x.2016.11.055
Wei, W., Wang, S., and Li, N. (2017). The effect of Danhong injection on the improvement of nerve function and hemorheology in patients with acute cerebral infarction. Med. Innovation China 14, 27. doi:10.3969/j.issn.1674-4985.2017.36.008
Wu, L., and Bao, D. (2016). Clinical observation on the adjuvant treatment of acute cerebral infarction with Danhong injection. J. New Chin. Med. 48, 20. doi:10.13457/j.cnki.jncm.2016.04.007
Xu, W., Zhang, Y., Yu, Y., Li, B., Liu, J., Wang, P., et al. (2018). Dose-dependent target diversion of danhong injection on the Glu-GLT-1/Gly-GlyRα dynamic balance module of cerebral ischemia. Pharmacol. Res. 135, 80–88. doi:10.1016/j.phrs.2018.07.020
Xu, X. R., Carrim, N., Neves, M. A., Mckeown, T., Stratton, T. W., Coelho, R. M., et al. (2016). Platelets and platelet adhesion molecules: novel mechanisms of thrombosis and anti-thrombotic therapies. Thromb. J. 14, 29. doi:10.1186/s12959-016-0100-6
Xue, W., and Li, L. (2010). Effection of Danhong injection on atherosclerotic plaque in carotid artery of ischemic stroke. Med. J. Chin. People's Health 22, 644. doi:10.3969/j.issn.1672-0369.2010.06.002
Yang, J. (2018). Effect of Danhong injection combined with atorvastatin calcium on NIHSS score and ability of daily living in lacunar cerebral infarction. Inn. Mong. Med. J. 50, 997. doi:10.16096/j.cnki.nmgyxzz.2018.50.08.055
Yang, J., and Liu, Q. (2018a). Effect of dan hong injection combined with clopidogrel on coagulation, LPO, sICAM-1 and IGF-1 levels in patients with acute cerebral infarction. Chin. J. Integr. Med. Cardio/Cerebrovascular Dis. 16, 1119. doi:10.12102/j.issn.1672-1349.2018.08.039
Yang, S., Yang, M., Lei, J., and Yang, X. (2018b). Combined treatment of edaravone and Danhong injection ameliorates brain infarction by regulating the expression of inflammation-related factors. Immunol. J. 34, 247–251.
Yin, G. (2014). Clinical study on effect of danhong injection combined with hyperbaric oxygen on neural cytokine of cerebral infarction patients. J. Hunan Univ. Chin. Med. 34, 49. doi:10.3969/j.issn.1674-070X.2014.05.014.049.03
Yuan, D. (2019). Clinical efficacy of danhong combined with edaravone injection in the treatment of acute cerebral infarction and its influence on serum related factors. Chin. Foreign Med. Res. 17, 130. doi:10.14033/j.cnki.cfmr.2019.36.055
Yun, h., li, j., and hu, F. (2017). Effect of dan hong injection on cerebral hemodynamics and hemorheology in patients with cerebral infarction. Hainan Med. J. 28, 1059. doi:10.3969/j.issn.1003-6350.2017.07.010
Zeitlberger, A. M., Flynn, M. C., Hollenstein, M., and Hundsberger, T. (2021). Assessment of neurological function using the national Institute of health stroke scale in patients with gliomas. Neurooncol. Pract. 8, 699–705. doi:10.1093/nop/npab046
Zeng, Y., Liao, J., and Chen, D. (2016). Dan red injection adr mentioned joint effects of neural function in patients with cerebral infarction injection treatment research. Drugs Clin. 13, 45–52.
Zhang, H., Shang, Y. X., Wei, B., and Xiang, Y. (2015). Expression of leptin and its receptor in lungs of asthmatic BALB/c mice and effect of budesonide on their expression. Intern. Med. China 10, 623–628.
Zhang, M., Li, M., Zheng, Y., Tang, L., and Long, S. (2018). Effect of Danhong injection combined with alprostadil on cerebral vascular reserve and neurological impairment in patients with ischemic cerebral infarction. J. North Sichuan Med. Coll. 33, 777. doi:10.3969/j.issn.1005-3697.2018.05.037
Zhang, W. (2017). The clinical study of Danhong injection combined with edaravone in the treatment of acute ischemic stroke. Chin. J. Prim. Med. Pharm. 24, 1050. doi:10.3760/cma.j.issn.1008-6706.2017.07.023
Zhang, Y., Yang, S., Zou, Y., Yan, X., Wu, H., Zhou, M., et al. (2019). NK cell predicts the severity of acute graft-versus-host disease in patients after allogeneic stem cell transplantation using antithymocyte globulin (ATG) in pretreatment scheme. BMC Immunol. 31, 46–47. doi:10.1186/s12865-019-0326-8
Zhu, J., and Qi, J. (2020). Effect of Danhong injection in the treatment of acute cerebral infarction and its effect on patients' neurological function and inflammatory factor levels. Clin. Med. 40, 95. doi:10.19528/j.issn.1003-3548.2020.02.035
Zhuang, M., and Zhao, X. (2020). Analysis of Danhong injection in the treatment of type 2 diabetes with cerebral infarction. Diabetes New World, 60
Keywords: Danhong injection, ischemic stroke, meta-analysis, systematic review, randomized controlled trial
Citation: Ma Y, Deng K, Liu J, Ma B, Mei F, Hui W, Luo X, Yao M, Liu Y, Qin X, Zhou X, Zou K, Li L and Sun X (2022) The add-on effects of Danhong injection among patients with ischemic stroke receiving Western medicines: A systematic review and meta-analysis. Front. Pharmacol. 13:937369. doi: 10.3389/fphar.2022.937369
Received: 06 May 2022; Accepted: 08 July 2022;
Published: 23 August 2022.
Edited by:
Yue Liu, Xiyuan Hospital, ChinaReviewed by:
Sunita Nair, Consultant, Mumbai, IndiaKuo Yang, Beijing Jiaotong University, China
Haitong Wan, Zhejiang Chinese Medical University, China
Copyright © 2022 Ma, Deng, Liu, Ma, Mei, Hui, Luo, Yao, Liu, Qin, Zhou, Zou, Li and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Li, bGlsaW5nQHdjaHNjdS5jbg==
 Yu Ma1,2,3
Yu Ma1,2,3 Fan Mei
Fan Mei Xiaochao Luo
Xiaochao Luo Xu Zhou
Xu Zhou Xin Sun
Xin Sun