- 1Institute of Integrated Traditional Chinese and Western Medicine, Beijing University of Chinese Medicine, Beijing, China
- 2The Affiliated Hospital to Changchun University of Chinese Medicine, Changchun, China
- 3Department of Endocrinology, Guang’anmen Hospital of China, Academy of Chinese Medical Sciences, Beijing, China
- 4Graduate College, Beijing University of Traditional Chinese Medicine, Beijing, China
- 5Gansu University of Chinese Medicine, Lanzhou, China
- 6Institute of Metabolic Diseases, Guang’anmen Hospital of China, Academy of Chinese Medical Sciences, Beijing, China
- 7Molecular Biology Laboratory, Guang’anmen Hospital of China Academy of Chinese Medical Sciences, Beijing, China
- 8School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
Background: Coronavirus disease 2019 (COVID-19) was declared a global pandemic in March 2020 by the World Health Organization (WHO). As of July 2, 2022, COVID-19 has caused more than 545 million infections and 6.3 million deaths worldwide, posing a significant threat to human health. Currently, there is still a lack of effective prevention and control strategies for the variation and transmission of SARS-CoV-2. Traditional Chinese medicine (TCM), which has a unique theoretical system, has treated various conditions for thousands of years. Importantly, recent studies have revealed that TCM contributed significantly to COVID-19. SanHanHuaShi (SHHS) granules, a Chinese herbal medicine, which has been included in Protocol for the Diagnosis and Treatment of Novel Coronavirus Disease 2019 (6th to 9th editions) issued by the National Health Commission of China and used to prevent and treat COVID-19 disease. A previous retrospective cohort study showed that SHHS could significantly reduce the severity of mild and moderate COVID-19. However, there is an absence of high-quality randomized controlled clinical studies to confirm the clinical effectiveness of SHHS. Therefore, a clinical study protocol and a statistical analysis plan were designed to investigate the efficacy and safety of SHHS for the prevention and treatment of COVID-19. This study will increase the integrity and data transparency of the clinical research process, which is of great significance for improving the practical application of SHHS granules in the future.
Methods and analysis: The study was designed as a 7-day, randomized, parallel controlled, open-label, noninferiority clinical trial of positive drugs. A total of 240 patients with mild and moderate COVID-19 will be enrolled and randomly assigned to receive SanHanHuaShi granules or LianHuaQingWen granules treatment in a 1:1 ratio. Disease classification, vital signs, SARS-CoV-2 nucleic acid testing, symptoms, medications, adverse events, and safety evaluations will be recorded at each visit. The primary outcome will be the clinical symptom recovery rate. Secondary outcomes will include the recovery time of clinical symptoms, negative conversion time of SARS-CoV-2 nucleic acid test negative conversion rate, hospitalization time, antipyretic time, rate of conversion to severe patients, and time and rate of single symptom recovery. Adverse incidents and safety assessments will be documented. All data will be analyzed using a predetermined statistical analysis plan, including our method for imputation of missing data, primary and secondary outcome analyses, and safety outcomes.
Discussion: The results of this study will provide robust evidence to confirm the effectiveness and safety of SHHS in the treatment of COVID-19.
Clinical Trial Registration: http://www.chictr.org.cn. Trial number: ChiCTR2200058080. Registered on 29 March 2022.
Introduction
The extremely contagious coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), has become a global public health concern. COVID-19 has a wide range of clinical manifestations, leading to dysfunction of multiple organs and systems throughout the body and even death (Harrison et al., 2020). The most common symptoms in COVID-19 patients include fever, cough, shortness of breath, fatigue, gastrointestinal problems, and taste and smell disorders (Wiersinga et al., 2020; To et al., 2021). Although people of all ages are vulnerable to infection with the highly transmissible SARS-COV-2, elderly patients or patients with comorbidities are more likely to develop severe or critical disease (Hu B et al., 2021). Therefore, early diagnosis and intervention are essential to control the onset and management of COVID-19. However, currently there is no specific drug with clear evidence of targeting SARS-CoV-2. Therefore, exploring effective prevention and control strategies or drugs against SARS-COV-2 remains an international concern.
The safety and effectiveness of traditional Chinese medicine (TCM) have been widely recognized and evaluated for thousands of years. In the early stages of the outbreak, TCM was incorporated into prevention and treatment strategies, and played an important role in the fight against COVID-19 not only in China but also internationally (Huang et al., 2020; Chu et al., 2021; Huang et al., 2021; Lyu et al., 2021; Wang and Yang, 2021). Owing to the synergistic effect of multiple components and multiple targets, Chinese herbal medicine has a comprehensive therapeutic effect in antiviral, anti-inflammatory, and immunomodulatory functions, thereby aiding in preventing and treating COVID-19 (Li P et al., 2021; Li BH et al., 2021; Ren et al., 2021). With the increase in the number of recovered patients from COVID-19, post-acute sequelae of SARS-CoV-2 infection (PASC) become an important public health problem that can have a long-term impact on pulmonary and multiple extrapulmonary tissues and organs (Wang and Yang, 1827). TCM has a potential advantage in preventing and improving PASC in patients with convalescent COVID-19 (Li et al., 2020; An et al., 2021; Chen et al., 2022).
“Three Chinese medicines and three Chinese recipes” have been included in the 2019 Protocol for Diagnosis and Treatment of Novel Coronavirus Disease 2019 issued by the National Health Commission (NHC) of China and are recommended for the treatment of COVID-19. Several clinical and mechanistic studies have shown that a variety of TCM herbal preparations have the potential to treat COVID-19 (Shen and Yin, 2021; Wu and Zhong, 2021; Yang et al., 2021). LianHuaQingWen (LHQW) capsules or granules have been approved by the NHC to treat COVID-19 and are used extensively worldwide. Multiple randomized controlled clinical trials and mechanistic studies have confirmed the potential advantages of LHQW in improving clinical symptoms and prognosis in patients with COVID-19 (Runfeng et al., 2020; Xiao et al., 2020; Hu K et al., 2021).
Similarly, the Hanshiyi formula recommended in the 2019 Protocol for the Diagnosis and Treatment of Novel Coronavirus Disease 2019 (6th to 9th editions) for patients with mild and moderate COVID-19 is also clinically effective prescription used in many regions of China. To further promote the study and utilize TCM against COVID-19, the Hanshiyi formula was produced as SanHanHuaShi (SHHS) granules after verification of the production process and improvement of quality standards by Jiangsu Kanion Pharmaceutical Co., Ltd. The composition and dosage of the SanHanHuaShi granules were the same as those of the Hanshiyi formula. SHHS is composed of 20 Chinese herbs, which play a role in releasing the exterior and dissipating cold, ventilating the lung and expelling pathogens, dispelling filth, and eliminating turbidity based on the theory of TCM (Han et al., 2020).
Through modern pharmaceutical technology, the Chinese medicine decoction is extracted, concentrated, dried and shaped into a granular formulation. The quality control analysis of SHHS granules utilizing HPLC is provided by Jiangsu Kanion Pharmaceutical Co., Ltd. The quality control data and preliminary identification of the main components are available in Additional file 1. The daily dosage of SHHS granules is as follows: Houpo (Magnolia officinalis Rehd.et Wils.): 3.13g (equally 15 g herb medicine, abbreviated as 15 g), Jiaobinglang (Areca catechu L.):1.88g (9g), Caoguo (Amomum tsao-ko Crevost et Lemaire): 9g (1.88g), Mahuang (Ephedra sinica Stapf):6g (1.25g), Kuxingren (Prunus armeniaca L.var.ansu Maxim.): 9g (1.88g), Qianghuo (Notopterygium incisum Ting ex H.T.Chang): 15g (3.13g), Shengjiang (Zingiber officinale Rosc.): 15g (3.13g), Guanghuoxiang (Pogostemon cablin(Blanco) Benth.): 15g (3.13g), Peilan (Eupatorium fortunei Turcz.): 9g (1.88g), Cangzhu (Atractylodes chinensis(DC.) Koidz.): 15g (3.13g), Fuling (Poria cocos(Schw.)Wolf): 45g (9.38g), Baizhu (Atractylodes macrocephala Koidz.): 30g (6.25g), Shigao (Gypsum Fibrosum): 15g (3.13g), Jiaoshanzha (Crataegus pinnatifida Bge.var.major N.E.Br.): 9g (1.88g), Jiaomaiya (Hordeum vulgare L.): 9g (1.88g), Jiaoshenqu (Massa Medicata Fermentata): 15g (3.13g), Dilong (Pheretima aspergillum (E.Perrier)): 15g (3.13g), Xuchangqing (Cynanchum paniculatum(Bge.)Kitag.): 15g (3.13g), Mianmaguanzhong (Dryopteris crassirhizoma Nakai), 9g (1.88g), Tinglizi (Descurainia sophia(L.)Webb.ex Prantl.):15g (3.13g).
A previous retrospective cohort study showed that SHHS could considerably reduce the incidence of mild and moderate COVID-19 turning into severe disease (Tian et al., 2020). Although this study suggests that SHHS can prevent and treat COVID-19, there are no further randomized controlled clinical trials to confirm this. Therefore, we hypothesized that SHHS plays a therapeutic role no less than LHQW in patients with mild and moderate COVID-19. Consequently, we designed a randomized, parallel-controlled, open-label clinical trial to demonstrate the efficacy and safety of SHHS in the treatment of COVID-19. The primary objective of this study research is to describe a precise clinical study protocol and a predetermined statistical analysis plan.
Methods
Study design
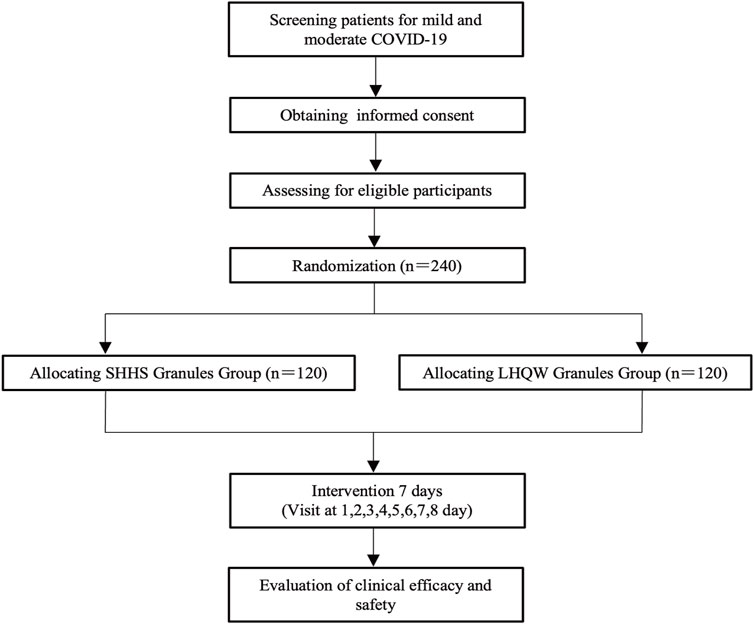
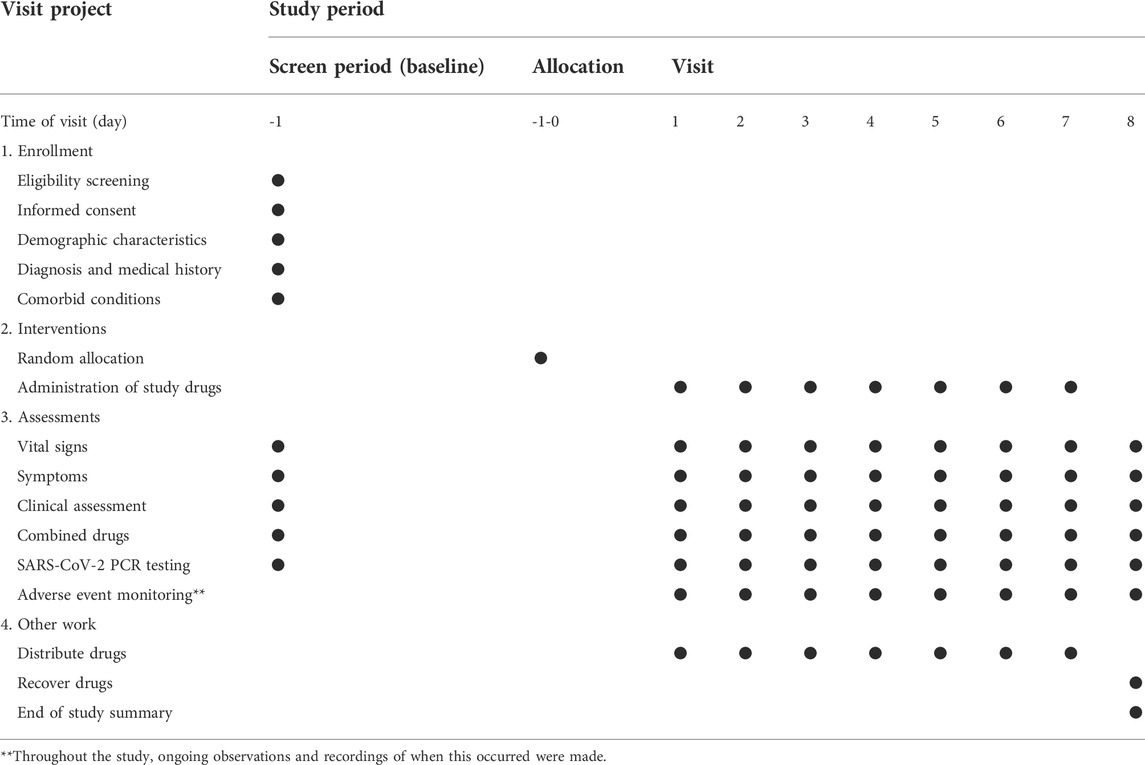
This study is a randomized, active parallel-controlled, open-label, and non-inferior phase 2 clinical trial in patients with mild and moderate COVID-19. A total of 240 patients who tested positive for SARS-CoV-2 will be enrolled in this study. The patients will be divided into two randomized groups and treated with granules of either SanHanHuaShi or LianHuaQingWen for 7 days. The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines will be followed (Chan et al., 2013). (Additional file 2). The study procedure is summarized in Figure 1. The details of the study schedule for enrollment, interventions, and assessments are presented in Table 1.
Recruitment of participants
This study will include 240 patients who test positive for COVID-19. The patients will be recruited from the Affiliated Hospital of Changchun University of Traditional Chinese Medicine (Jilin province, China). Recruiting advertising will be displayed online and on hospital notice boards. It will contain the study objectives, drugs, medical examinations, and the study eligibility conditions and application method. The study is open to individuals who meet the eligibility criteria and agree to participate. To obtain consent from prospective patients, investigators must outline the study objectives, requirements, and interventions and guarantee their understanding. Two resident physicians will evaluate the patients, each of whom will verify the participants’ study eligibility. Demographic data and reasons for non-participation of ineligible patients will also be documented. The recruitment process started in March 2022 and will last until March 2024. Patients will not participate in study design, recruitment, research procedures, analysis, or dissemination of findings. Medical examinations, laboratory tests, and therapies are provided at no cost to participants, and research may not reimburse them.
Inclusion criteria
Diagnostic criteria for COVID-19 are based on the Protocol for the Diagnosis and Treatment of Novel Coronavirus Disease 2019 (9th edition) released by the NHC of the People's Republic of China (General Office Of The National Health And Health Commission, 2022) and include individuals who qualified for eligibility meeting the following criteria: 1) clinically confirmed patients of COVID-19 according to the Protocol for Diagnosis and Treatment of Novel Coronavirus Pneumonia (9th edition) with laboratory confirmed SARS-CoV-2 infection as determined by RT-PCR; 2) patients judged to have mild or moderate COVID-19. (Patients with mild COVID-19 usually present with mild clinical symptoms, without radiological manifestations of pneumonia, whereas patients with moderate COVID-19 patients have a fever and respiratory symptoms, as well as radiological features of pneumonia); 3) presence of one or more symptoms of fever, cough, sore throat, and fatigue when entering the group; 4) the time of admission is not more than 24 hours; 5) patients of either sex who are 18 years or older, and 6) providing written informed consent before enrolling in the trial.
Exclusion criteria
Patients are ineligible for the study if they meet any of the following criteria: 1) show the early warning indicators of severe or critical condition; 2) use similar Chinese patent drugs before enrollment; 3) immunodeficiency diseases or use of immunosuppressants or glucocorticoids within the last 3 months; 4) pregnancy, lactation, or intention to become pregnant; 5) allergic constitution (referring to those allergic to more than two types of drugs or foods or the known ingredients of the investigational medications); 6) mental illness, or without self-knowledge ability, and 7) other conditions judged by the investigators.
Withdrawal criteria and termination criteria
First, the investigator's decision to withdraw the patient is based on the following principles: 1) during the clinical trial, the patients develop diseases that are not suitable for further study; 2) patients with adverse events or serious adverse events are not suitable to continue the study, and 3) other conditions that the researchers believe will harm the health of the patients by continuing the study.
Second, patients can and should withdraw if they are unwilling to continue with the clinical trial. According to the provisions of the informed consent form, participants can withdraw at any time during the study. If patients have not explicitly proposed to withdraw but no longer accept medication and testing, as well as loss to follow-up, are regarded as 'withdrawal'. The reasons for withdrawal should be known and recorded as precisely as possible. Examples include consciously being unable to tolerate adverse events, inability to continue clinical research due to other reasons, or loss to follow-up without explaining the reasons. In electronic case report forms (eCRFs), the reasons for dropout will be recorded and the data analysis will include their last record.
Third, when reviewing the data prior to statistical analysis, it is up to the principal investigator (PI) and statisticians to judge whether an individual case is excluded. If one of the following events occurs, the two sides should consider whether the patient should be excluded based on the study's progress and the reasons for withdrawal and provide a detailed explanation: 1) After randomization, individuals not meeting the selection criteria or meeting the exclusion criteria of the research proposal are found; 2) The failure to take at least one dose of trial medication and the lack of any data post-randomization.
Fourth, the conditions for stopping early are as follows: 1) if a high incidence of serious adverse events is found during the research, the study should be suspended in time; 2) if during the research, significant mistakes are found in the research proposal or there are serious deviations in its implementation, making it difficult to evaluate the efficacy of drugs, the research should be stopped; 3) the ethics committee requests to suspend or terminate the research; 4) the administrative department cancels the research.
Sample size
The study hypothesis is SHHS group will be non-inferior to LHQW group. Clinicians and statisticians set the non-inferiority threshold at 10% and predicted that the recovery rate of clinical symptoms after 7 days of LHQW is about 87% and that of SHHS is 90% according to the literature reports on similar drugs. It is estimated that 94 patients for each group, ie. a total of 188 patients are needed to achieve the power of 80% at a one-sided statistically significant level of 0.025. Considering the possible dropout rate of 20%, 240 patients will be enrolled, that is 120 patients for each group.
Randomization and allocation
A permuted block randomization procedure will be used to allocate 240 eligible patients in a 1:1 ratio. Randomization numbers will be generated using the PROC PLAN process statement in the SAS statistical software package (version 9.4; SAS Inc., Cary, NC, USA). Patients will be assigned to either the SHHS or LHQW group. The Department of Statistics at the Nanjing Medical University will be responsible for randomization. Randomization code will be concealed in opaque envelopes and the allocation information will not be known before randomization procedure.
Interventions measures
Patients in the SHHS group will receive SHHS granules (10 g per bag), two bags at a time, three times a day, to be swallowed with warm water. The administration of LHQW granules (10 g per bag) will be the same as the SHHS group. The medication course was 7 days (the medication could be stopped in advance if the discharge standard was reached). It will be forbidden to receive other TCM for COVID-19 treatment during the treatment period, including TCM decoctions, granules, or proprietary Chinese medicine. The types, dosage, and cumulative time (including the start and end dates) of the combined therapy drugs should be recorded during the observation period. The intervention will be stopped if there are any severe adverse effects or withdrawal.
Blinding
The SHHS used in this study is provided by Jiangsu Kanion Pharmaceutical Co. Ltd. (Lianyungang City, Jiangsu Province, China). LianHuaQingWen granules were purchased from Shijiazhuang Yiling Pharmaceutical Co., Ltd. (Shijiazhuang, China). All medications are administered under good manufacturing practices. Even though the trial is open-label, the research assistants who allocate patients to groups will be unaware of their treatment allocation before grouping. Also, assessors and statisticians will be blinded to the group assignment when assessing the efficacy and analyzing the data. Before the analysis and evaluation, all samples and data are anonymized.
Adherence to study protocol
Protocol adherence during the treatment period will be assessed at each visit and will be based on medication adherence. Frequency distributions are used to describe the proportion of patients in both groups. Medication adherence in the two groups will be calculated and compared according to the ratio of the actual drug administered to the recommended dose. The drugs distributed to the patients and those not administered are documented every day.
Outcome measures
Baseline measures for eligibility
During the treatment period, CRFs will be completed with information about the demographic and clinical characteristics of the patients, including age, sex, height, weight, and medical and treatment histories.
Primary outcomes
The primary outcome is the rate of recovery from four types of patients' self-reported clinical symptoms, including fever, coughing, sore throat and fatigue. It specifically refers to the proportion of patients who met the criteria at the baseline for specific clinical symptoms and achieved recovery at the end of treatment on the seventh day.
Secondary outcomes
The secondary outcomes are the recovery time of patients self-reported clinical symptoms, SARS-CoV-2 nucleic acid testing negative conversion time, and negative conversion rate, hospitalization time, antipyretic time, the incidence of severe patients, single symptom disappearance time, and disappearance rate.
Criteria for judging the curative effect:
(1) Recovery time and cure rate of clinical symptoms: The recovery of clinical symptoms refers to the disappearance of the main symptoms, ie. fever, coughing, sore throat, and fatigue. The cure rate is defined as the proportion of patients whose clinical symptoms recovered after the end of treatment on the seventh day.
(2) SARS-CoV-2 nucleic acid testing negative conversion time and negative conversion rate: Taking the first time of the two consecutive respiratory tract SARS-CoV-2 PCR negative tests (sampling interval at least 1 d) as the SARS-CoV-2 PCR testing negative time (the SARS-CoV-2 nucleic acid testing negative conversion time equals the SARS-CoV-2 PCR testing negative time–the diagnosis time of the patient).
(3) Hospitalization time: the time from patient admission to discharge. The discharge standard refers to the release from isolation or discharge criterion of the protocol for diagnosing and treating novel coronavirus pneumonia (9th edition).
(4) Antipyretic time: The body temperature is less than 37.4°C after treatment, for 24 h or more.
(5) The conversion rate of turning to severe patients: the proportion of patients who develop severe infection 7 days after taking medicine.
Safety outcomes and adverse events (AEs)
AEs will be continuously checked during the 7-day treatment period. AEs indices will include all adverse events. At any time, all information about adverse events, including their severity, duration, and correlation to SHHS granules, should be recorded in detail. The AEs was graded as mild, moderate, or severe. Mild AEs could be defined as situations that could be tolerated without any further medical therapies. Moderate AEs is pain or discomfort that patients can not tolerate and require medical treatments. Severe AEs means that, after receiving the medications used in the study, the patients have a dead, life-threatening, permanent, or severe disability or loss of function; the patients need hospitalization or extended hospitalization or lead to congenital abnormalities or birth defects. AEs must be reported to the research center, principal sponsor, and research medical ethics committee as soon as possible. At the same time, the National Medicinal Product Administration (NMPA) will be notified within 24 h. A serious adverse event must also be completed as soon as possible.
Data collection, management, and quality control
An electronic data capture (EDC) system will gather and manage data in this trial. The electronic case report form (eCRF) data matches the information in the original medical records, laboratory examination reports, and other sources. According to the research protocol, the eCRF should guarantee that all the data required for analysis are collected efficiently and precisely. To maintain the quality of the data and guarantee adherence to the protocol, the sponsor and investigators will establish quality control and quality assurance systems, respectively. Investigators and research assistants who participate in clinical research must have professional expertise, qualifications, and abilities in clinical research. They should explicitly explain the protocols for patient selection, data input, medication use, AEs reporting, and dropout documentation. They should conscientiously and patiently explain to the patients to fully understand and cooperate with the research to provide good patient compliance. Patients should be informed of possible adverse reactions to the medication and the coping approach to be taken in the event of adverse reactions.
It is necessary to protect clinical research data's authenticity, integrity, and privacy and to guarantee the traceability of clinical research data. For the questions existing in the data, the data administrator will fill in the concentrator data-ready queue (DRQ) and send a query to the investigators through the clinical monitor. Investigators should answer this question and provide feedback as soon as possible. The data administrator will modify the data according to the investigators' answers, confirm and enter them, and send the DRQ again if necessary. Data verification includes computerized program verification (edit check), manual verification, and data verification meetings. Any inconsistent data (data query) found in the verification shall be corrected in time. The data department shall issue a discrepancy report and submit it to the researcher for confirmation and changes. At the end of the study, the researchers and statistical analysts will review the data and lock them after confirming that they are correct. All electronic data will be stored in password-protected files on designated computers that researchers can access. The necessary documents for this study shall be archived and managed in relevant drug clinical research institutions and statistical units, respectively. The research data shall be kept until five years after the end of the study.
Statistical analysis plan
Statistical methods
The statistical analysis plan will comply with the guidelines for the content of statistical analysis plans in clinical trials (Gamble et al., 2017). (Additional File 3). After finishing the trial, all statistical analysis tests will be conducted using SAS (version 9.4; SAS Inc., Cary, NC, USA) and one-sided tests with a nominal alpha level of 0.025. All confidence intervals (Cls) will be stated at the 95 confidence level. To assess non-inferiority, the CI lower limit of the difference between groups will be compared with a predefined non-inferiority limit. Non-inferiority could only be declared when the 95% CI lower limit of the difference between groups is not larger than the non-inferiority threshold of 10%.
Continuous outcomes will be described by calculating the mean, standard deviation, median, and minimum and maximum values. The means and standard deviations are reported for normal continuous data. For non-normally distributed continuous variables, the median and interquartile ranges are given. The frequency and proportion of each category are displayed as counting indications.
Between-group comparisons are performed using an independent two-sample t-test (normality, homogeneity of variance) or the Wilcoxon rank-sum test (non-normality, heterogeneity of variance). The paired t-test is used for comparisons within normally distributed groups, and the paired rank-sum test is used for comparisons within non-normally distributed groups. For count metrics, the χ2 test or Fisher's exact probability will be required, whereas, for categorical variables, the Wilcoxon rank-sum test will be utilized. Repeated measured outcomes are applied to a generalized linear mixed model analysis.
In patients where baseline indicators are unbalanced, an adjustment to the efficacy contrast of variables such as age, sex, and concurrent therapy is required. The covariance approach is employed for continuous variables, and for count or rank data, logistic regression analysis is performed.
Definition of analysis sets
(1) Full analysis set (FAS): All participants randomly assigned to the group, use the medicine at least once, and have at least one visit will be analyzed using intention-to-treat analysis of the entire dataset.
(2) Per protocol set (PPS): This includes all participants who comply with the research protocol, which have no missing data on important baseline variables and primary outcome. Major violations on concomitant medicine and extremely lower compliance will be considered to be excluded from PPS.
(3) Safety set (SS): All randomized participants could be included in SS if they have used at least one dose of drugs and have completed at least a one-time safety assessment. Impution will not be conducted for safety outcomes in SS.
Descriptive statistics
Participants' baseline characteristics will be reported in a baseline table for each randomization group, and baseline data and descriptive statistics will be assessed using standard measures in accordance with the recommendations of the consolidated standards of reporting trials (CONSORT) guidelines (Moher et al., 2010). (Table 2). The table describes the following variables: age, sex, height, weight, clinical symptoms, and medical and treatment histories. The sample is displayed in accordance with the entire analysis set. Baseline characteristics will be compared between the groups.
Imputation of missing data
Missing values of the primary and secondary outcomes will be carried forward from the latest observation data to the final results (LOCF method).
Analysis of primary outcome
The primary analysis will be conducted on the FAS and PPS, which is the rate of self-reported clinical symptoms, including fever, fatigue, and coughing recovery. The percentage of patients whose clinical symptoms disappeared on the seventh day will be summarized. CMH-Chisquare test will be applied to compare rates between groups and Clopper-Pearson method will be used to calculate the 95%CI for rate difference (Table 3).
Analysis of secondary outcomes
The secondary outcomes of the FAS and PPS will be analyzed, including the recovery time of clinical symptoms, SARS-CoV-2 nucleic acid testing negative conversion time and negative conversion rate, hospitalization time, antipyretic time, the conversion rate of turning to severe patients, disappearance time of single symptoms, and disappearance rate. All secondary outcomes will be compared between and within groups based on the distribution and changes at baseline and each visit point (Table 4).
Continuous data will be summarized using number, mean, standard deviation, minimum, maximum, median, upper and lower quartile. A t-test or Wilcoxon rank test will be applied to compare groups. ANCOVA will be considered to evaluate the influence of covariates. Chi-square test or Fisher exact test will be used to compare groups for categorical data. CMH-Chisquare test and logistic regression will be considered to explore the influence of covariates. Time-to-event data will be estimated by Kaplan-Meier method to compute the median and corresponding 95%CI. Log-rank test will be used for between-group comparison.
Analysis of safety outcomes
All participants in the safety set are examined during safety analysis. All AEs that occurred during the intervention period are recorded, including their type, severity, frequency, and relationship to the intervention medicine. The quantity and frequency of adverse events are calculated for both groups. Severe adverse events and consequent drug discontinuation are meticulously recorded. Safety analysis will use qualitative judgment to define the distribution, counting the number of participants and their fraction of abnormal indicators using cross-tabulation (Table 5). The correlation between AE and medications will be evaluated using standards from the 2011 Ministry of Health of the People's Republic of China's Measures for the Reporting and Monitoring of Adverse Drug Reactions.
Ethics and dissemination
The study protocol was approved by the Ethics Committee of the Affiliated Hospital of Changchun University of Traditional Chinese Medicine (no. CCZYFYLL2022). All participants will provide written informed consent. The results of this study will be published in peer-reviewed journals.
Discussion
The ongoing COVID-19 pandemic, which has been spreading for more than two years, has led to various variants of SARS-CoV-2 (Tao et al., 2021; Khandia et al., 2022). The increased transmissibility or severity of SARS-CoV-2 continually poses serious challenges to vaccine development and targeted therapeutic strategies (Dong et al., 2021; Lino et al., 2022). Timely intervention with TCM in COVID-19 has been shown to significantly relieve symptoms, shorten the course, and improve the prognosis of the disease An et al., 2021. The trial is a randomized, active-drug parallel-controlled, open-label, non-inferiority clinical trial carried out in China to evaluate the effectiveness of SHHS compared to LHQW among patients with COVID-19. This phase 2 clinical trial will provide clinical evidence of TCM herbal preparations for preventing and treating COVID-19. The SAP was developed to prepare for statistical analysis of all data after the completion of this study and locking of the database. Although this is a single-center and open-label study, the quality of evidence will be improved by strict quality control and transparent reporting. The findings of this study will have important implications both for Chinese and western clinical practices. Furthermore, if the non-inferior conclusion could be declared in our study, SHHS granules will be confirmed its effects not only for symptoms management of COVID-19 and reducing the severe incidence, but also for extended indications of virus pneumonia or influenza. All of these depend on integrated multi-source high-quality data including randomized controlled trials and observational studies in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Affiliated Hospital of Changchun University of Traditional Chinese Medicine. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YZ, JR, and JW conceived and initiated the overall design of the study protocol. YL, XC, HW, and CY helped draft the manuscript and shared the first authorship. XG wrote part of the statistical analysis. ZG provided the supplementary materials. LS, DL, and CT helped prepare the trial. YW, QD, HY, JL, KC, and JC reviewed the literature. LZ, ML, and LH critically revised the manuscript. All authors have refined and approved the final version of the manuscript.
Funding
This study was funded by the Special Project for Emergency of the Ministry of Science and Technology of PR. China (No. 2022YFC0868300), National Key Research and Development Program of China (No. 2021YFE0201100), and Special Project for Emergency Research on the Prevention and Control of COVID-19 by Traditional Chinese Medicine, supported by National Administration of Traditional Chinese Medicine (2022ZYLCYJ01-4). The funder has no role in the design of the study; collection, analysis, and interpretation of data; writing of the manuscript, or decision to submit the manuscript for publication.
Acknowledgments
All authors would like to thank Jiangsu Kanion Pharmaceutical Co., Ltd. (Lianyungang City, Jiangsu Province, China) for providing the research drugs used in this study. We would like to thank the experts who provided suggestions and ideas for the design of this study and clinicians and their colleagues for their contributions in establishing standardized operation procedures, recruiting, and following up with patients.
Conflict of interest
The authors declare the study is free of any business or financial relationships that could be interpreted as potential conflicts of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.936925/full#supplementary-material
References
An, YW, Yuan, B, Wang, JC, Wang, C, Liu, TT, Song, S, and Liu, HQ (2021). Clinical characteristics and impacts of traditional Chinese medicine treatment on the convalescents of COVID-19. Int. J. Med. Sci. 18 (3), 646–651. doi:10.7150/ijms.52664
An, X, Zhang, Y, Duan, L, Jin, D, Zhao, S, Zhou, R, et al. (2021). The direct evidence and mechanism of traditional Chinese medicine treatment of COVID-19. Biomed. Pharmacother. 137, 646–111267.
Chan, AW, Tetzlaff, JM, Altman, DG, Laupacis, A, Gøtzsche, PC, Krleža-Jerić, K, Hróbjartsson, A, Mann, H, Dickersin, K, Berlin, JA, et al. (2013). SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann. Intern. Med. 158 (3), 200–207. doi:10.7326/0003-4819-158-3-201302050-00583
Chen, Y, Liu, C, Wang, T, Qi, J, Jia, X, Zeng, X, Bai, J, Lu, W, Deng, Y, Zhong, B, et al. (2022). Efficacy and safety of Bufei Huoxue capsules in the management of convalescent patients with COVID-19 infection: a multicentre, double-blind, and randomised controlled trial. J. Ethnopharmacol. 284, 114830. doi:10.1016/j.jep.2021.114830
Chu, L, Huang, F, Zhang, M, Huang, B, and Wang, Y (2021). Current status of traditional Chinese medicine for the treatment of COVID-19 in China. Chin. Med. 16 (1), 63. doi:10.1186/s13020-021-00461-y
Dong, Y, Dai, T, Wang, B, Zhang, L, Zeng, LH, Huang, J, Yan, H, Zhang, L, and Zhou, F (2021). The way of SARS-CoV-2 vaccine development: success and challenges. Signal Transduct. Target. Ther. 6 (1), 387. doi:10.1038/s41392-021-00796-w
Gamble, C, Krishan, A, Stocken, D, Lewis, S, Juszczak, E, Doré, C., et al. (2017). Guidelines for the Content of Statistical Analysis Plans in Clinical Trials. JAMA 318 (23), 2337–2343. doi:10.1001/jama.2017.18556
Han, L, Wei, XX, Zheng, YJ, Zhang, LL, Wang, XM, Yang, HY, Ma, X, Zhao, LH, and Tong, XL (2020). Potential mechanism prediction of Cold-Damp Plague Formula against COVID-19 via network pharmacology analysis and molecular docking. Chin. Med. 15, 78. doi:10.1186/s13020-020-00360-8
Harrison, AG, Lin, T, and Wang, P (2020). Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 41 (12), 1100–1115. doi:10.1016/j.it.2020.10.004
Hu, B, Guo, H, Zhou, P, and Shi, ZL (2021). Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 19 (3), 141–154. doi:10.1038/s41579-020-00459-7
Hu, K, Guan, WJ, Bi, Y, Zhang, W, Li, L, Zhang, B, Liu, Q, Song, Y, Li, X, Duan, Z, et al. (2021). Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 85, 153242. doi:10.1016/j.phymed.2020.153242
Huang, F, Li, Y, Leung, EL, Liu, X, Liu, K, Wang, Q, Lan, Y, Li, X, Yu, H, Cui, L, et al. (2020). A review of therapeutic agents and Chinese herbal medicines against SARS-COV-2 (COVID-19). Pharmacol. Res. 158, 104929. doi:10.1016/j.phrs.2020.104929
Huang, K, Zhang, P, Zhang, Z, Youn, JY, Wang, C, Zhang, H, and Cai, H (2021). Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: efficacies and mechanisms. Pharmacol. Ther. 225, 107843. doi:10.1016/j.pharmthera.2021.107843
Khandia, R, Singhal, S, Alqahtani, T, Kamal, MA, El-Shall, NA, Nainu, F, Desingu, PA, and Dhama, K (2022). Emergence of SARS-CoV-2 Omicron (B.1.1.529) variant, salient features, high global health concerns and strategies to counter it amid ongoing COVID-19 pandemic. Environ. Res. 209, 112816. doi:10.1016/j.envres.2022.112816
Li, Q, Wang, H, Li, X, Zheng, Y, Wei, Y, Zhang, P, Ding, Q, Lin, J, Tang, S, Zhao, Y, et al. (2020). The role played by traditional Chinese medicine in preventing and treating COVID-19 in China. Front. Med. 14 (5), 681–688. doi:10.1007/s11684-020-0801-x
Li, P, Hu, S, Qian, C, Yao, Y, Li, LY, Yang, JF, Yang, L, Yang, CC, Zhou, H, Wang, SX, et al. (2021). The Therapeutic Effect of Traditional Chinese Medicine on Inflammatory Diseases Caused by Virus, Especially on Those Caused by COVID-19. Front. Pharmacol. 12, 650425. doi:10.3389/fphar.2021.650425
Li, BH, Li, ZY, Liu, MM, Tian, JZ, and Cui, QH (2021). Progress in Traditional Chinese Medicine Against Respiratory Viruses: a Review. Front. Pharmacol. 12, 743623. doi:10.3389/fphar.2021.743623
Lino, A, Cardoso, MA, Martins-Lopes, P, and Gonçalves, HMR (2022). Omicron - The new SARS-CoV-2 challenge? Rev. Med. Virol. 32, e2358. doi:10.1002/rmv.2358
Lyu, M, Fan, G, Xiao, G, Wang, T, Xu, D, Gao, J, Ge, S, Li, Q, Ma, Y, Zhang, H, et al. (2021). Traditional Chinese medicine in COVID-19. Acta Pharm. Sin. B 11 (11), 3337–3363. doi:10.1016/j.apsb.2021.09.008
Moher, D, Hopewell, S, Schulz, KF, Montori, V, Gøtzsche, PC, Devereaux, PJ, Elbourne, D, Egger, M, and Altman, DG (2010). CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 340, c869. doi:10.1136/bmj.c869
Ren, W, Liang, P, Ma, Y, Sun, Q, Pu, Q, Dong, L, Luo, G, Mazhar, M, Liu, J, Wang, R, et al. (2021). Research progress of traditional Chinese medicine against COVID-19. Biomed Pharmacother 137, 111310. doi:10.1016/j.biopha.2021.111310
Runfeng, L, Yunlong, H, Jicheng, H, Weiqi, P, Qinhai, M, Yongxia, S, Chufang, L, Jin, Z, Zhenhua, J, Haiming, J, et al. (2020). Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol. Res. 156, 104761. doi:10.1016/j.phrs.2020.104761
Shen, X, and Yin, F (2021). The mechanisms and clinical application of Traditional Chinese Medicine Lianhua-Qingwen capsule. Biomed Pharmacother 142, 111998. doi:10.1016/j.biopha.2021.111998
Tao, K, Tzou, PL, Nouhin, J, Gupta, RK, de Oliveira, T, Kosakovsky Pond, SL, Fera, D, and Shafer, RW (2021). The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 22 (12), 757–773. doi:10.1038/s41576-021-00408-x
Tian, J, Yan, S, Wang, H, Zhang, Y, Zheng, Y, Wu, H, Li, X, Gao, Z, Ai, Y, Gou, X, et al. (2020). Hanshiyi Formula, a medicine for Sars-CoV2 infection in China, reduced the proportion of mild and moderate COVID-19 patients turning to severe status: a cohort study. Pharmacol. Res. 161, 105127. doi:10.1016/j.phrs.2020.105127
To, KK, Sridhar, S, Chiu, KH, Hung, DL, Li, X, Hung, IF, Tam, AR, Chung, TW, Chan, JF, Zhang, AJ, et al. (2021). Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. Emerg. Microbes Infect. 10 (1), 507–535. doi:10.1080/22221751.2021.1898291
Wang, Z, and Yang, L (1827). Post-Acute Sequelae of SARS-CoV-2 Infection: a Neglected Public Health Issue. Front. Public Health 10, 908757. doi:10.3389/fpubh.2022.908757
Wang, Z, and Yang, L (2021). Chinese herbal medicine: fighting SARS-CoV-2 infection on all fronts. J. Ethnopharmacol. 270, 113869. doi:10.1016/j.jep.2021.113869
Wiersinga, WJ, Rhodes, A, Cheng, AC, Peacock, SJ, and Prescott, HC (2020). Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): a Review. JAMA 324 (8), 782–793. doi:10.1001/jama.2020.12839
Wu, Y, and Zhong, P (2021). Clinical Progress on Management of Pneumonia Due to COVID-19 With Chinese Traditional Patent Medicines. Front. Pharmacol. 12, 655063. doi:10.3389/fphar.2021.655063
Xiao, M, Tian, J, Zhou, Y, Xu, X, Min, X, Lv, Y, Peng, M, Zhang, Y, Yan, D, Lang, S, et al. (2020). Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol. Res. 161, 105126. doi:10.1016/j.phrs.2020.105126
Keywords: SanHanHuaShi Granules, Traditional Chinese Medicine, COVID-19, effectiveness, protocol, Statistical analysis plan
Citation: Liu Y, Chen X, Wang H, Yao C, Gou X, Gao Z, Sun L, Liu D, Tang C, Wei Y, Ding Q, Yang H, Lin J, Chen K, Chen J, Zhao L, Li M, Han L, Wang J, Ren J and Zhang Y (2022) Effectiveness and safety analysis of SanHanHuaShi granules for the treatment of coronavirus disease 2019: Study protocol and statistical analysis plan for a randomized, parallel-controlled, open-label clinical trial. Front. Pharmacol. 13:936925. doi: 10.3389/fphar.2022.936925
Received: 10 May 2022; Accepted: 11 July 2022;
Published: 16 August 2022.
Edited by:
Sheyu Li, West China Hospital, Sichuan University, ChinaReviewed by:
Zhonglei Wang, Qufu Normal University, ChinaMei Wang, Liaoning University of Traditional Chinese Medicine, China
Zoltán Köntös, IOI Investment Zrt., Hungary
Liyan Yang, Qufu Normal University, China
Copyright © 2022 Liu, Chen, Wang, Yao, Gou, Gao, Sun, Liu, Tang, Wei, Ding, Yang, Lin, Chen, Chen, Zhao, Li, Han, Wang, Ren and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Wang, amlhbi13MjIyQDE2My5jb20=; Jixiang Ren, cmVuangyMDAzQDE2My5jb20=; Ying Zhang, eWluZ3poYW5nQGJ1Y20uZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Yangyang Liu1,2†
Yangyang Liu1,2† Chensi Yao
Chensi Yao Xiaowen Gou
Xiaowen Gou Zezheng Gao
Zezheng Gao Cheng Tang
Cheng Tang Yu Wei
Yu Wei Qiyou Ding
Qiyou Ding Haoyu Yang
Haoyu Yang Keyu Chen
Keyu Chen Linhua Zhao
Linhua Zhao Min Li
Min Li Lin Han
Lin Han Jian Wang
Jian Wang Jixiang Ren
Jixiang Ren Ying Zhang
Ying Zhang