- 1Nephrology, The Third Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Nephrology, YunYang County People’s Hospital, Chongqing, China
At present, there is no effective drug for the treatment of renal fibrosis; in particular, a safe and effective treatment for renal fibrosis should be established. Cordyceps has several medical effects, including immunoregulatory, antitumor, anti-inflammatory, and antioxidant effects, and may prevent kidney, liver, and heart diseases. Cordyceps has also been reported to be effective in the treatment of renal fibrosis. In this paper, we review the potential mechanisms of Cordyceps against renal fibrosis, focusing on the effects of Cordyceps on inflammation, oxidative stress, apoptosis, regulation of autophagy, reduction of extracellular matrix deposition, and fibroblast activation. We also discuss relevant published clinical trials and meta-analyses. Available clinical studies support the possibility that Cordyceps and related products provide benefits to patients with chronic kidney diseases as adjuvants to conventional drugs. However, the existing clinical studies are limited by low quality and significant heterogeneity. The use of Cordyceps and related products may be a potential strategy for the treatment of renal fibrosis. Randomized controlled trial studies with good methodological quality, favorable experimental design, and large sample size are needed to evaluate the efficacy and safety of Cordyceps.
Introduction
Renal fibrosis (RF) is a common outcome of the progression of various chronic kidney diseases (CKD) and the main pathological change in the progression of CKD to end-stage renal disease (Humphreys, 2018). It includes glomerulosclerosis (GS), renal interstitial fibrosis (RIF), and arteriosclerosis and perivascular fibrosis (Djudjaj and Boor, 2019). It shows histopathological features of excessive extracellular matrix (ECM) deposition, tubular atrophy, inflammatory cell infiltration, and loss of peritubular microvasculature. Subsequent structural destruction and functional impairment of the organ occurs, with scarring and sclerosis of the renal parenchyma. Cells involved in this event include renal tubular epithelial cells (TECs), endothelial cells (ECs), fibroblasts, pericytes, macrophages, and mast cells (Sun et al., 2016; Yan et al., 2021). In addition, cellular and molecular events such as inflammatory injury, oxidative stress, apoptosis, fibroblast activation, and epithelial–mesenchymal transition (EMT) are closely associated with RF (Liu et al., 2017; Nogueira et al., 2017). Despite significant progress in preclinical research of the mechanisms of RF and its therapeutic targets (Nogueira et al., 2017; Bai et al., 2021; Yan et al., 2021; Ruiz-Ortega et al., 2022), good target effects have not been demonstrated in clinical practice (Vincenti et al., 2017; Voelker et al., 2017). Therefore, there is still a lack of effective treatment that specifically targets RF. It is especially important to find safe and effective treatments for RF.
Chinese herbal medicines (CHMs) have been used for thousands of years in the treatment of kidney disease. Chinese herbs and fungi with potential kidney benefits include Cordyceps, Sairei-to, Rheum spp., Salvia miltiorrhiza and its components, and Magnesium lithospermate B (Wojcikowski et al., 2004). Anti-RF mechanisms of CHMs include anti-inflammatory and antioxidant effects, inhibition of EMT, reduction of ECM deposition, immune regulation, regulation of autophagy, inhibition of apoptosis, and control of hemorheology (Zhao et al., 2020). In this paper, we review the mechanisms of Cordyceps and related products in the treatment of RF (Table 1), as well as relevant clinical trials and meta-analyses (Table 2). We also discuss the possible nephrotoxicity of Cordyceps and the possible antifibrotic effects of the compounded formulation.
Cordyceps is a fungus that colonizes the larvae of moths. It has been used for centuries as a medicine in China, Japan, and other Asian countries. It has several species, including Cordyceps sinensis, Cordyceps militaris, and Cordyceps cicadae. Cordyceps has a variety of medicinal properties or bioactive compounds, including nucleosides, polysaccharides, cyclodepsipeptides, sterols, alkaloids, and phenolics (Olatunji et al., 2018), which show immunomodulatory, antitumor, anti-inflammatory, antioxidant, renoprotective, and other effects (Das et al., 2020). Cordyceps has shown potential promise as an adjunct to conventional medicine to decrease serum creatinine (Scr), increase creatinine clearance, reduce proteinuria, and alleviate CKD-related complications (Zhang et al., 2014). Based on the network pharmacology tools that are used to investigate the molecular mechanism of Cordyceps for the treatment of diabetic nephropathy (DN), seven active ingredients were screened from Cordyceps, 293 putative target genes were identified, and 85 overlapping targets matching DN were identified as potential therapeutic targets, such as tumor necrosis factor (TNF), mitogen-activated protein kinase 1, epidermal growth factor receptor (EGFR), angiotensin-converting enzyme, and Caspase-9. Cordyceps are involved in an inflammatory response, apoptosis, oxidative stress, insulin resistance, and other biological processes through these pathways (Li et al., 2021).
Mechanisms of Cordyceps and Related Products Against Renal Fibrosis
Cordyceps Relieves Inflammation
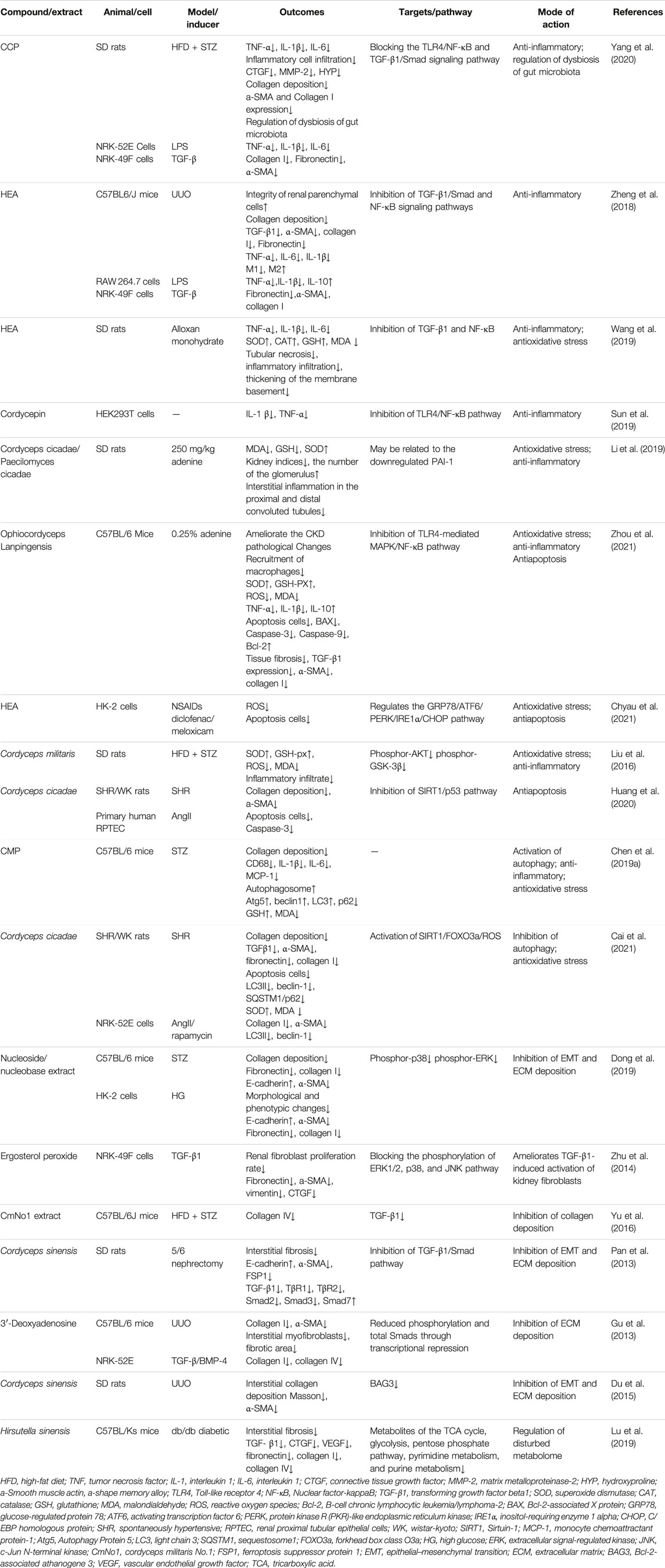
Inflammation is the initiator of RF, and persistent chronic inflammation is considered a hallmark feature of CKD (Meng, 2019). In response to pathogenic factors, damaged renal TECs recruit inflammatory cells to the renal interstitial region, and these inflammatory cells then produce large amounts of proinflammatory and profibrotic cytokines (Gewin et al., 2017). Various active ingredients in Cordyceps have anti-inflammatory effects (Phull et al., 2022). They have been shown to protect against liver fibrosis through anti-inflammatory effects (Xu et al., 2021) (Ying-Mei et al., 2020). Cordyceps extract attenuates renal histological changes, reduces Scr and blood urea nitrogen (BUN) levels, and decreases inflammatory factors in acute kidney injury (AKI), including renal ischemia/reperfusion injury (Han et al., 2020) and cisplatin-induced AKI (Deng et al., 2020). In RF related to streptozotocin (STZ), or alloxan monohydrate–induced DN, the active components of Cordyceps, Cordyceps cicadae polysaccharides (CCP) or N6-(2-hydroxyethyl) adenosine (HEA), are effective in reducing the expression of proinflammatory factors TNF-α, IL-1β, and IL-6 in serum and kidney and in reducing the expression of interstitial fibrosis-associated proteins α-SMA and collagen I (Wang et al., 2019; Yang et al., 2020). In vitro, CCP also reduces the release of lipopolysaccharide-induced proinflammatory factors and TGF-β-induced activation of fibroblasts (Yang et al., 2020). Unilateral ureteral obstruction (UUO) is a classical RF model, and Cordyceps extract HEA reduces fibrosis-associated proteins TGF-β1, α-SMA, collagen I, and fibronectin, and inflammatory factors TNF-α, IL-6, and IL-1β in renal tissue 14 days after UUO (Zheng et al., 2018). Zheng et al.’s (2018) study initially explored the effect of HEA on inflammatory cells and found that HEA blocked the accumulation of M1 macrophages and induced the accumulation of M2 macrophages in the kidney as reflected by the positive detection of the F4/80 antigen. The role of M2 macrophages in fibrosis is controversial (Ricardo et al., 2008) (Lin et al., 2010), but the M1/M2 macrophage ratio is an important stage in the development of RIF. However, this study did not further distinguish between the subtypes of M2.
The abovementioned studies have explored the regulatory mechanism of Cordyceps extract against inflammation in RF by inhibiting the TGF-β1/Smad and TLR4/NF-κB signaling pathways (Zheng et al., 2018; Yang et al., 2020). Sun et al. found that p-TLR4, TLR4, p-NF-κB, NF-κB, IL-1 β, and TNF-α levels were effectively reduced by Cordycepin treatment in human embryonic kidney 293T cells. However, Cordycepin did not reduce the levels of these molecules when TLR4 was silenced. These results suggest that Cordycepin may affect the NF-κB signaling pathway through TLR4 (Sun et al., 2019). TLRs/NF-κB activate the downstream inflammasome NOD-like receptor family pyrin domain containing 3 (NLRP3) (Xue et al., 2019), which promotes the maturation of proinflammatory factors IL-1β and IL-18 by activating Caspase-1 (Liston and Masters, 2017). Multiple studies have shown that the NLRP3 inflammasome and its downstream pyroptosis and inflammation play an important role in the development of RF (Ma et al., 2022) (Ram et al., 2022; Wang et al., 2022). It has been shown that Ophiocordyceps sinensis causes inhibition of mRNA and protein expression of NLRP3 inflammasome and downstream effectors IL-1β and IL-18 in a rat model of DN (Wang et al., 2018). Thus, Cordyceps and related products are effective in relieving inflammation in RF through multiple mechanisms.
Cordyceps Attenuates Oxidative Stress
The kidney is a highly metabolically active organ with mitochondria rich in oxidative reactions and susceptible to oxidative stress damage. Oxidative stress and inflammation interact to play a key role in renal tissue destruction, irreversible loss of renal function, and progression of RF (Xu et al., 2015; Darby and Hewitson, 2016; Richter and Kietzmann, 2016). Normal cells produce small amounts of reactive oxygen species (ROS), which play an important physiological role. Free radical–scavenging enzymes and antioxidants maintain oxygen metabolism homeostasis by activating transcription factors, regulating physiologically active substances and inflammatory immunity, and promoting cell proliferation and differentiation. Oxidative stress occurs when the balance between ROS and reactive nitrogen species and the antioxidant defense system is disrupted, that is, when the production of pro-oxidants or ROS exceeds the endogenous antioxidant capacity (Sies et al., 2017). It has been shown that oxidative stress promotes the progression of RF (Aranda-Rivera et al., 2021); if oxidative stress is suppressed, RF can be attenuated (Liao et al., 2022; Lo et al., 2022). Antioxidant enzymes, such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GSH-Px), protect cells from damage caused by oxygen free radicals. Malondialdehyde (MDA) is one of the products of the reaction between lipids and oxygen free radicals, and it accumulates during oxidative stress. Cordyceps improves the redox properties of CKD by affecting the levels of NO, SOD, and MDA in serum. In rat models of DN (Liu et al., 2016; Wang et al., 2019) and membranous nephropathy (Song et al., 2016), both C. militaris and HEA showed excellent ability to attenuate oxidative stress, elevated SOD and GSH, and decreased MDA levels. Using diclofenac or meloxicam to induce oxidative stress, HEA intervention significantly reduced the level of ROS in human proximal tubular cells (HK-2) cells (Chyau et al., 2021). In rats with adenine-induced chronic renal failure, Cordyceps cicadae and Paecilomyces cicadae effectively reduced serum urea and creatinine levels, improved renal histopathology, inhibited oxidative stress, and enhanced antioxidant capacity (Li et al., 2019). In a CKD mouse model established by adenine gavage, Ophiocordyceps lanpingensis polysaccharides elevated SOD and GSH-PX, decreased ROS and MDA, improved histopathological staining, and decreased fibrosis-related proteins TGF-β1, α-SMA, and collagen I (Zhou et al., 2021). Cordyceps and related products have a favorable ability to attenuate oxidative stress, but the mechanisms involved are not well understood.
Cordyceps Inhibits Apoptosis
A variety of kidney injury factors may trigger apoptosis, including ischemia/reperfusion injury (Xu et al., 2019), cisplatin-induced kidney injury (Yang et al., 2018), and DN (Peng et al., 2015). Apoptosis (a programmed cell death) of glomerular ECs, podocytes, and TECs is closely associated with RF (Thomas et al., 1998; Docherty et al., 2006). Inhibition of apoptosis as a therapeutic target can alleviate fibrosis (Chen et al., 2021; Xia et al., 2021; Park et al., 2022). Cordyceps downregulates apoptosis in ischemia/reperfusion injury and Cyclosporine A–induced renal tubular dysfunction (Shahed et al., 2001; Chyau et al., 2014). Apoptosis is also considered an important mechanism in contrast-induced nephropathy (CIN). C. sinensis prevents CIN in diabetic rats by decreasing the expression of apoptosis-related proteins Caspase-3 and Bax and increasing the expression of antiapoptotic protein Bcl-2. Mechanistically, C. sinensis decreases the expression of JNK protein and increases the expression of ERK protein (Zhao et al., 2018). Renal tubular interstitial fibrosis is a typical pathological feature of hypertensive kidney injury. In a spontaneous hypertension rat model, C. cicadae reduces interstitial fiber deposition, α-SMA expression, apoptosis, and Caspase-3 activity by regulating the SIRT1/p53 signaling pathway (Huang et al., 2020). In vitro, the major renal damage caused by hepatitis B virus infection is hepatitis B virus X (HBx)–induced apoptosis of renal TECs, which is related to the increased Caspase-3 and Caspase-9 activity and increased PI3K/Akt pathway activity. C. sinensis attenuates all of these HBx-induced responses, at least in part by inhibiting the PI3K/Akt signaling pathway (He et al., 2020). Cordyceps and related products have been shown to inhibit apoptosis in vivo and in vitro, but the mechanisms involved have not been unified.
Cordyceps Regulates Autophagy
Autophagy is a “self-consuming” cell death pathway that degrades most cytoplasmic components by forming autophagosomes and autolysosomes (Yang and Klionsky, 2010). Basal autophagy in the kidney is critical for maintaining renal homeostasis, structure, and function. Namely, basal autophagy removes potentially dysfunctional organelles and long-lived proteins to maintain cellular homeostasis. In response to environmental and intracellular stress, autophagy may serve as an adaptive response to ensure cell survival. However, autophagy may also play a role in cellular dysfunction and organ lesions (Tang et al., 2020), such as AKI (Kaushal and Shah, 2016), AKI-CKD (Baisantry et al., 2016), and DN (Lenoir et al., 2015). The role of autophagy in RF is controversial. It has been reported that sustained activation of autophagy in renal tubular cells promotes interstitial fibrosis, and inhibition of autophagy with chloroquine or selective deletion of Atg7 in proximal tubules alleviates RF in UUO mice (Livingston et al., 2016). Several studies have also reported antifibrotic effects of autophagy, whereas inhibition of autophagy by 3-methyladenine exacerbated RF in a UUO rat model, suggesting that autophagy may exert antifibrotic effects by attenuating renal tubular cell injury (Kim et al., 2012). In STZ-induced DN mice, C. militaris polysaccharides (CMP) decrease collagen deposition in the kidney and increase the rate of autophagy; promote the expression of autophagy-specific protein Atg5, Beclin1, and LC3; and decrease the expression of p62 protein in the kidney. This suggests that CMP administration significantly enhances autophagy (Chen et al., 2019a). In a hypertensive nephropathy model, Cordyceps cicadae may attenuate the expression of fibrosis-associated proteins. It ameliorates RF induced by hypertension by downregulating autophagy-related proteins LC3II and Beclin-1, thereby significantly inhibiting autophagic vesicles and attenuating autophagic stress. This effect may be attributed to the regulation of SIRT1 pathway-mediated autophagic stress and is achieved by modulating the autophagy regulator forkhead box class O3a (FOXO3a) and oxidative stress (Cai et al., 2021). The regulatory effect of Cordyceps on autophagy is indisputable, but autophagy itself is diverse and can be divided into microautophagy, chaperone-mediated autophagy, and macroautophagy, and macroautophagy can be divided into nonselective autophagy and selective autophagy depending on whether the substrate is selective or not. Because autophagy is environment dependent and may play different roles at different stages of the disease, different cell types do not respond consistently to the autophagy activation, resulting in a bidirectional effect of cellular autophagy on the process of RF. The regulatory role of autophagy in fibrosis remains to be further investigated.
Cordyceps Reduces Extracellular Matrix Deposition and Fibroblast Activation
Aberrant activation and proliferation of fibroblasts are believed to be key causes of the progression of RF (Zhou et al., 2017). Activated fibroblasts, which promote the production and release of ECM collagens I, III, and IV and fibronectin (Sun et al., 2016), are the main source of ECM during scar tissue formation. Myofibroblasts in RF can be derived from mesenchymal fibroblasts, bone marrow–derived fibroblasts, renal TECs, ECs, pericytes, and macrophages. TECs can produce stroma through the EMT to fibroblasts and myofibroblasts (Yan et al., 2021). In this process, TGF-β is the main driving factor. TGF-β induces fibroblast activation and proliferation as well as excessive synthesis and accumulation of ECM through downstream signaling pathways such as SMAD, BMMP-7, and CTGF, thereby promoting fibrosis. Targeted inhibition of TGF-β and its downstream signaling pathways delay fibrosis (Meng et al., 2016; Walton et al., 2017; Ma and Meng, 2019). In STZ-induced diabetic mice and high glucose–exposed HK-2 cells, the nucleoside/nucleobase–rich extract from Cordyceps increased E-cadherin expression; decreased α-SMA, fibronectin, and collagen I expression; inhibited EMT and ECM deposition; and improved the fibrotic morphology of tissues and cells. Cordyceps extract effectively inhibited the phosphorylation of p38 and ERK, did not affect the phosphorylation level of JNK, and had a synergistic effect on fibrosis with p38 or ERK inhibitors (Dong et al., 2019). Ergosterol peroxide from Cordyceps cicadae improves TGF-β1-induced renal fibroblast activation via the mitogen-activated protein kinases signaling pathway (Zhu et al., 2014). In DN C57BL/6J mice and 5/6 nephrectomy rats, C. militaris (Yu et al., 2016) and C. sinensis (Pan et al., 2013) counteracted RF and reduced the expression of fibrosis-associated proteins by inhibiting the TGF-β1 pathway. Further exploring downstream mechanisms, 3′-deoxyadenosine interfered with TGF-β and bone morphogenetic protein signaling by downregulating Smads at the transcriptional level. In that way, it induced decreases in collagen I and α-SMA, mesenchymal myofibroblast number, and fibrotic area (Gu et al., 2013). Cordyceps and its extracts are potential therapeutic strategies against fibrosis via the TGF-β/Smad and other pathways.
Other Mechanisms
In addition to the above mechanisms, C. sinensis attenuates RF in the UUO model by inhibiting Bcl-2-associated athanogene 3 (BAG3), which has been reported to be involved in cell proliferation, apoptosis, adhesion and migration, and EMT processes (Du et al., 2015).
Recent studies have reported that gut microbiota mediates RF (Liu et al., 2021). CMP reduce renal injury by regulating intestinal flora imbalance (Song and Zhu, 2020). In a study of CCP to attenuate fibrosis in DN, high-throughput pyrophosphate sequencing of 16S rRNA indicated that CCP regulated dysbiosis of the intestinal flora by increasing the relative abundance and proliferation of probiotic bacteria, thereby exerting a beneficial effect on tubulointerstitial fibrosis in DN rats (Yang et al., 2020).
Normal energy metabolism is particularly important for maintaining the structure and function of the kidney, and metabolic reprogramming is generally considered a key process in activating fibrosis in different organs, as it is in the kidney (Barcena-Varela et al., 2021). The balance of fatty acid oxidation and glycolysis directly affects ECM production and degradation or indirectly affects RF through inflammation and hypoxia (Zhu et al., 2021). Metabolomic analysis has been used to explore the metabolic regulatory effects of C. sinensis in a db/db diabetic mice model. Hirsutella sinensis—the anamorph of the traditional Chinese medicine C. sinensis—attenuates RF, ameliorates system-wide disorders of glucolipid metabolism and amino acid deficiency, and regulates excessive activation energy and nucleotide metabolism (Lu et al., 2019). This suggests that Cordyceps may have a potential role in the regulation of renal metabolism and, consequently, may ameliorate fibrosis.
Clinical Studies of Cordyceps and Related Products in Treating Chronic Kidney Diseases
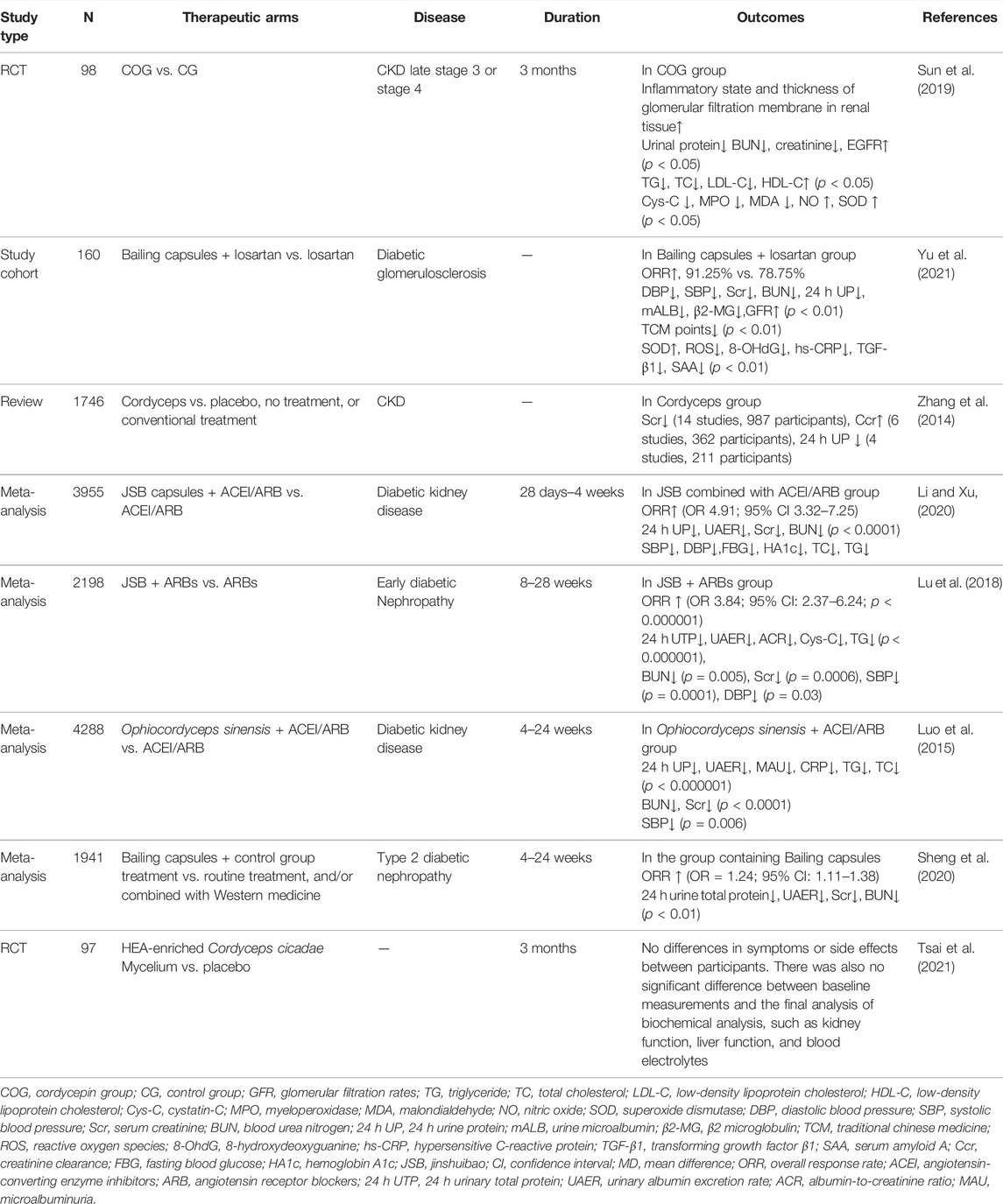
In this section, we review published clinical studies of Cordyceps and related products, including two randomized controlled trial (RCT) studies, one cohort study, one review, and four meta-analyses (Table 2).
One study recruited 98 patients with CKD3 or CKD4 and randomized them to the Cordycepin group (COG, patients received 100 mg of C. militaris per day) and the control group (CG, patients received dried chickweed herb placebo per day). After 3 months of treatment, C. militaris improved the inflammatory status and thickness of the glomerular filtration membrane of renal tissues. Compared with the CG group, the COG group had lower CKD biomarkers (p < 0.05); higher EGFR (p < 0.05); lower serum Cys-C, MPO, and MDA levels; and higher NO and SOD levels (p < 0.05). C. sinensis reduced protein and mRNA levels of the TLR4/NF-κB signaling pathway (p < 0.05). This suggests that C. militaris improves CKD by affecting the TLR4/NF-κB redox signaling pathway (Sun et al., 2019).
To evaluate the efficacy of the Cordyceps preparation Bailing capsule combined with losartan in the treatment of diabetic GS, a cohort that included 160 patients with diabetic GS was randomly divided into the observation and the CGs; the observation group was treated with losartan and Bailing capsules, and the CG was treated with losartan. The overall effective rate was higher in the observation group than in the CG (p < 0.05). After the treatment, the DBP, SBP, Scr, 24 h UP, BUN, mALB, and β2-MG levels were lower, and the GFR was higher in the observation group than in the CG (p < 0.01). The traditional Chinese medicine symptom scores were lower in the observation group than in the CG (p < 0.01). The observation group also showed higher serum SOD and lower ROS, 8-OHdG, hs-CRP, TGF-β1, and SAA levels than the control group (p < 0.01). Bailing capsules combined with losartan improved the efficacy; improved the blood and urine biochemical indexes, the renal function, and the clinical symptoms; and reduced the oxidative stress. However, this study has the disadvantages of small sample size, short follow-up time, and bias in case selection (Yu et al., 2021).
In another review, 22 studies involving 1,746 participants were included. C. sinensis was found to significantly reduce Scr, increase creatinine clearance, and reduce 24 h proteinuria in patients with CKD. All 22 studies were published in Chinese and conducted in Chinese hospitals. The quality of evidence from the included trials in the evaluation was suboptimal; namely, four trials had a high risk of bias because of the lack of clear description of randomization, allocation concealment, and blinding, whereas the remaining 18 trials had an unclear risk of bias. In some of the included trials, there was significant heterogeneity in the conventional treatment as a co-intervention. Since there was no blinding during the study, possible differences in the multiple combined interventions between the treatment and control groups may have introduced bias into the results. Therefore, the poor methodological quality and underreporting of the included studies meant that no definitive conclusions could be drawn regarding the possible effects of Cordyceps preparations in patients with CKD (Zhang et al., 2014).
Four meta-analyses on Cordyceps preparations (JinShuiBao capsule/Bailing capsule) for DN included 51 RCT studies consisting of 3,955 participants (Li and Xu, 2020), 26 RCT studies with 2,198 early DN participants (Lu et al., 2018), 60 studies with 4,288 participants (Luo et al., 2015), and 24 studies with 1,941 participants (Sheng et al., 2020). All four studies showed that the Cordyceps preparation combined with ACEI/ARB was superior to ACEI/ARB alone, as shown by increased overall response rate and decreased 24 h UTP, UAER, BUN, and Scr. None of the studies had serious publication bias. However, all of the participating patients were Chinese with no other ethnicity; most of the included RCTs did not report detailed methodologies; in addition, efficacy assessments were not standardized and rigorous. The low quality of the study design and significant heterogeneity reduced the credibility of the meta-analysis.
To assess the safety of C. cicadae, 97 healthy adults were randomized to the treatment group (n = 49) and the placebo group (n = 48) in a double-blind, randomized trial. The treatment group received 1.05 g of C. cicadae mycelium granules once a day after a meal for 3 months. The placebo group received 1.05 g granules with the same nutrition, but without additional C. cicadae mycelium. Blood samples were collected before and after the treatment for biochemical analysis, including renal function, liver function, blood lipid, and electrolyte levels. No differences in symptoms or side effects were observed between the participants in the trial. There were also no significant differences between the baseline measurements and the final biochemical analyses. The results of the trial indicate that it is safe to consume less than 1.05 g of HEA-rich mycelium per day (Tsai et al., 2021).
In addition to single drugs or extracts, there are many compound preparations of Chinese herbs; some of the compound formulations of Cordyceps also have renal protective effects. WH30+ is a Chinese herb preparation composed of Rheum palmatum, S. miltiorrhiza, C. sinensis, Leonurus sibiricus, Epihedium macranthum, Radix Astragali, and Radix Codonopsis Pilosulae. It has nephroprotective effects in both glycerol-induced acute renal failure and adenine-induced chronic renal failure rats (Ngai et al., 2005). Cordyceps-related formulations of Novel JY5 formula (Fu et al., 2021), CGA formula (Li et al., 2016; Tian et al., 2019), and Fuzheng Huayu formula (Chen et al., 2019b) all have good effects against liver fibrosis, suggesting that these formulations may also be potential novel therapeutic candidates for RF.
Conclusion and Perspective
In this study, we found that Cordyceps and related products could attenuate RF through multiple pathways and targets, but the mechanisms were not independent of each other. Targeting TLR4 could reduce inflammation and oxidative stress via NF-κB or activate the downstream TGF-β/Smad signaling pathway to promote ECM deposition and fibroblast activation. Acting on SIRT1 was important in the regulation of autophagy, oxidative stress, energy homeostasis, and apoptosis. Although multitargeting had certain advantages, it could also have more side effects. Toxic side effects associated with Cordyceps have been reported in animals and humans. C. militaris was administered to rats at doses of 0, 1, 2, and 3 g/kg per day. Nephrotoxicity characterized by renal tubular epithelial degeneration and necrosis occurred at high doses of 3 g/kg, and the male rats were more susceptible to nephrotoxicity than female rats (Zhou and Yao, 2013). Doan et al. reported 60 cases of apparent Cordyceps fungus poisoning occurring in southern Vietnam during the rainy seasons, between 2008 and 2015. All patients showed symptoms within 60 min of consuming cicada flowers. A 60-year-old male patient died after consuming five cicada flowers and ingesting approximately 200 ml of rice wine. Another patient ingested 15–16 cicada flowers; although he exhibited severe symptoms, he survived after 3 weeks of treatment. These observations suggest that there are individual differences in the doses of cicada flower poisoning (Doan et al., 2017). It is therefore crucial to establish more systematic, complete, and feasible pharmacological and toxicological research methods.
Based on the available preclinical studies, Cordyceps and related products are supported for the treatment of RF. However, the available clinical data are limited. The published study protocol has been improved (Hu et al., 2021); as such, future RCTs with good methodological quality, favorable experimental design, and large sample size are needed to explore the therapeutic effects of Cordyceps and related products on RF to provide more rigorous clinical study data.
Author Contributions
WT and YW designed the work of review. HD, JD, ZW, and LL reviewed the literature available on this topic, WT and YW wrote the paper, WT and JY revised the manuscript. All authors approved the paper for publication. As the leader of the project team, JY won the research funding supporting this manuscript.
Funding
This project is supported by the Chongqing Talent Program Project (cstc2021ycjh-bgzxm0090).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aranda-Rivera, A. K., Cruz-Gregorio, A., Aparicio-Trejo, O. E., Ortega-Lozano, A. J., and Pedraza-Chaverri, J. (2021). Redox Signaling Pathways in Unilateral Ureteral Obstruction (UUO)-induced Renal Fibrosis. Free Radic. Biol. Med. 172, 65–81. doi:10.1016/j.freeradbiomed.2021.05.034
Bai, X., Nie, P., Lou, Y., Zhu, Y., Jiang, S., Li, B., et al. (2021). Pirfenidone Is a Renal Protective Drug: Mechanisms, Signalling Pathways, and Preclinical Evidence. Eur. J. Pharmacol. 911, 174503. doi:10.1016/j.ejphar.2021.174503
Baisantry, A., Bhayana, S., Rong, S., Ermeling, E., Wrede, C., Hegermann, J., et al. (2016). Autophagy Induces Prosenescent Changes in Proximal Tubular S3 Segments. J. Am. Soc. Nephrol. 27 (6), 1609–1616. doi:10.1681/asn.2014111059
Barcena-Varela, M., Paish, H., Alvarez, L., Uriarte, I., Latasa, M. U., Santamaria, E., et al. (2021). Epigenetic Mechanisms and Metabolic Reprogramming in Fibrogenesis: Dual Targeting of G9a and DNMT1 for the Inhibition of Liver Fibrosis. Gut 70 (2), 388–400. doi:10.1136/gutjnl-2019-320205
Cai, Y., Feng, Z., Jia, Q., Guo, J., Zhang, P., Zhao, Q., et al. (2021). Cordyceps Cicadae Ameliorates Renal Hypertensive Injury and Fibrosis through the Regulation of SIRT1-Mediated Autophagy. Front. Pharmacol. 12, 801094. doi:10.3389/fphar.2021.801094
Chen, C. M., Lin, C. Y., Chung, Y. P., Liu, C. H., Huang, K. T., Guan, S. S., et al. (2021). Protective Effects of Nootkatone on Renal Inflammation, Apoptosis, and Fibrosis in a Unilateral Ureteral Obstructive Mouse Model. Nutrients 13 (11). doi:10.3390/nu13113921
Chen, D. D., Xu, R., Zhou, J. Y., Chen, J. Q., Wang, L., Liu, X. S., et al. (2019a). Cordyceps Militaris Polysaccharides Exerted Protective Effects on Diabetic Nephropathy in Mice via Regulation of Autophagy. Food Funct. 10 (8), 5102–5114. doi:10.1039/c9fo00957d
Chen, J., Hu, Y., Chen, L., Liu, W., Mu, Y., and Liu, P. (2019b). The Effect and Mechanisms of Fuzheng Huayu Formula against Chronic Liver Diseases. Biomed. Pharmacother. 114, 108846. doi:10.1016/j.biopha.2019.108846
Chyau, C. C., Chen, C. C., Chen, J. C., Yang, T. C., Shu, K. H., and Cheng, C. H. (2014). Mycelia Glycoproteins from Cordyceps Sobolifera Ameliorate Cyclosporine-Induced Renal Tubule Dysfunction in Rats. J. Ethnopharmacol. 153 (3), 650–658. doi:10.1016/j.jep.2014.03.020
Chyau, C. C., Wu, H. L., Peng, C. C., Huang, S. H., Chen, C. C., Chen, C. H., et al. (2021). Potential Protection Effect of ER Homeostasis of N6-(2-Hydroxyethyl)adenosine Isolated from Cordyceps Cicadae in Nonsteroidal Anti-inflammatory Drug-Stimulated Human Proximal Tubular Cells. Int. J. Mol. Sci. 22 (4). doi:10.3390/ijms22041577
Darby, I. A., and Hewitson, T. D. (2016). Hypoxia in Tissue Repair and Fibrosis. Cell Tissue Res. 365 (3), 553–562. doi:10.1007/s00441-016-2461-3
Das, G., Shin, H. S., Leyva-Gómez, G., Prado-Audelo, M. L. D., Cortes, H., Singh, Y. D., et al. (2020). Cordyceps spp.: A Review on its Immune-Stimulatory and Other Biological Potentials. Front. Pharmacol. 11, 602364. doi:10.3389/fphar.2020.602364
Deng, J. S., Jiang, W. P., Chen, C. C., Lee, L. Y., Li, P. Y., Huang, W. C., et al. (2020). Cordyceps Cicadae Mycelia Ameliorate Cisplatin-Induced Acute Kidney Injury by Suppressing the TLR4/NF-Κb/MAPK and Activating the HO-1/Nrf2 and Sirt-1/AMPK Pathways in Mice. Oxid. Med. Cell Longev. 2020, 7912763. doi:10.1155/2020/7912763
Djudjaj, S., and Boor, P. (2019). Cellular and Molecular Mechanisms of Kidney Fibrosis. Mol. Asp. Med. 65, 16–36. doi:10.1016/j.mam.2018.06.002
Doan, U. V., Mendez Rojas, B., and Kirby, R. (2017). Unintentional Ingestion of Cordyceps Fungus-Infected Cicada Nymphs Causing Ibotenic Acid Poisoning in Southern Vietnam. Clin. Toxicol. (Phila) 55 (8), 893–896. doi:10.1080/15563650.2017.1319066
Docherty, N. G., O'Sullivan, O. E., Healy, D. A., Fitzpatrick, J. M., and Watson, R. W. (2006). Evidence that Inhibition of Tubular Cell Apoptosis Protects against Renal Damage and Development of Fibrosis Following Ureteric Obstruction. Am. J. Physiol. Ren. Physiol. 290 (1), F4–F13. doi:10.1152/ajprenal.00045.2005
Dong, Z., Sun, Y., Wei, G., Li, S., and Zhao, Z. (2019). A Nucleoside/Nucleobase-Rich Extract from Cordyceps Sinensis Inhibits the Epithelial-Mesenchymal Transition and Protects against Renal Fibrosis in Diabetic Nephropathy. Molecules 24 (22). doi:10.3390/molecules24224119
Du, F., Li, S., Wang, T., Zhang, H. Y., Zong, Z. H., Du, Z. X., et al. (2015). Cordyceps Sinensis Attenuates Renal Fibrosis and Suppresses BAG3 Induction in Obstructed Rat Kidney. Am. J. Transl. Res. 7 (5), 932–940.
Fu, Y., Xiao, Z., Tian, X., Liu, W., Xu, Z., Yang, T., et al. (2021). The Novel Chinese Medicine JY5 Formula Alleviates Hepatic Fibrosis by Inhibiting the Notch Signaling Pathway. Front. Pharmacol. 12, 671152. doi:10.3389/fphar.2021.671152
Gewin, L., Zent, R., and Pozzi, A. (2017). Progression of Chronic Kidney Disease: Too Much Cellular Talk Causes Damage. Kidney Int. 91 (3), 552–560. doi:10.1016/j.kint.2016.08.025
Gu, L., Johno, H., Nakajima, S., Kato, H., Takahashi, S., Katoh, R., et al. (2013). Blockade of Smad Signaling by 3'-deoxyadenosine: a Mechanism for its Anti-fibrotic Potential. Lab. Invest. 93 (4), 450–461. doi:10.1038/labinvest.2013.4
Han, F., Dou, M., Wang, Y., Xu, C., Li, Y., Ding, X., et al. (2020). Cordycepin Protects Renal Ischemia/reperfusion Injury through Regulating Inflammation, Apoptosis, and Oxidative Stress. Acta Biochim. Biophys. Sin. (Shanghai) 52 (2), 125–132. doi:10.1093/abbs/gmz145
He, P., Lei, J., Miao, J. N., Wu, D., and Wang, C. (2020). Cordyceps Sinensis Attenuates HBx-induced C-ell A-poptosis in HK-2 C-ells through S-uppressing the PI3K/Akt P-athway. Int. J. Mol. Med. 45 (4), 1261–1269. doi:10.3892/ijmm.2020.4503
Hu, X., Wang, J., Yang, H., Ji, S., Li, Y., Xu, B., et al. (2021). Bailing Capsule Combined with α-ketoacid Tablets for Stage 3 Chronic Kidney Disease. Med. Baltim. 100 (20), e25759. doi:10.1097/md.0000000000025759
Huang, Y. S., Wang, X., Feng, Z., Cui, H., Zhu, Z., Xia, C., et al. (2020). Cordyceps Cicadae Prevents Renal Tubular Epithelial Cell Apoptosis by Regulating the SIRT1/p53 Pathway in Hypertensive Renal Injury. Evid. Based Complement. Altern. Med. 2020, 7202519. doi:10.1155/2020/7202519
Humphreys, B. D. (2018). Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 80, 309–326. doi:10.1146/annurev-physiol-022516-034227
Kaushal, G. P., and Shah, S. V. (2016). Autophagy in Acute Kidney Injury. Kidney Int. 89 (4), 779–791. doi:10.1016/j.kint.2015.11.021
Kim, W. Y., Nam, S. A., Song, H. C., Ko, J. S., Park, S. H., Kim, H. L., et al. (2012). The Role of Autophagy in Unilateral Ureteral Obstruction Rat Model. Nephrol. Carlt. 17 (2), 148–159. doi:10.1111/j.1440-1797.2011.01541.x
Lenoir, O., Jasiek, M., Hénique, C., Guyonnet, L., Hartleben, B., Bork, T., et al. (2015). Endothelial Cell and Podocyte Autophagy Synergistically Protect from Diabetes-Induced Glomerulosclerosis. Autophagy 11 (7), 1130–1145. doi:10.1080/15548627.2015.1049799
Li, L., Zhang, T., Li, C., Xie, L., Li, N., Hou, T., et al. (2019). Potential Therapeutic Effects of Cordyceps Cicadae and Paecilomyces Cicadae on Adenine-Induced Chronic Renal Failure in Rats and Their Phytochemical Analysis. Drug Des. Devel Ther. 13, 103–117. doi:10.2147/dddt.S180543
Li, X. M., Peng, J. H., Sun, Z. L., Tian, H. J., Duan, X. H., Liu, L., et al. (2016). Chinese Medicine CGA Formula Ameliorates DMN-Induced Liver Fibrosis in Rats via Inhibiting MMP2/9, TIMP1/2 and the TGF-β/Smad Signaling Pathways. Acta Pharmacol. Sin. 37 (6), 783–793. doi:10.1038/aps.2016.35
Li, Y., and Xu, G. (2020). Clinical Efficacy and Safety of Jinshuibao Combined with ACEI/ARB in the Treatment of Diabetic Kidney Disease: A Meta-Analysis of Randomized Controlled Trials. J. Ren. Nutr. 30 (2), 92–100. doi:10.1053/j.jrn.2019.03.083
Li, Y., Wang, L., Xu, B., Zhao, L., Li, L., Xu, K., et al. (2021). Based on Network Pharmacology Tools to Investigate the Molecular Mechanism of Cordyceps Sinensis on the Treatment of Diabetic Nephropathy. J. Diabetes Res. 2021, 1–12. doi:10.1155/2021/8891093
Liao, X., Lv, X., Zhang, Y., Han, Y., Li, J., Zeng, J., et al. (2022). Fluorofenidone Inhibits UUO/IRI-Induced Renal Fibrosis by Reducing Mitochondrial Damage. Oxid. Med. Cell Longev. 2022, 2453617. doi:10.1155/2022/2453617
Lin, S. L., Li, B., Rao, S., Yeo, E. J., Hudson, T. E., Nowlin, B. T., et al. (2010). Macrophage Wnt7b Is Critical for Kidney Repair and Regeneration. Proc. Natl. Acad. Sci. U. S. A. 107 (9), 4194–4199. doi:10.1073/pnas.0912228107
Liston, A., and Masters, S. L. (2017). Homeostasis-altering Molecular Processes as Mechanisms of Inflammasome Activation. Nat. Rev. Immunol. 17 (3), 208–214. doi:10.1038/nri.2016.151
Liu, C., Song, J., Teng, M., Zheng, X., Li, X., Tian, Y., et al. (20162016). Antidiabetic and Antinephritic Activities of Aqueous Extract ofCordyceps militarisFruit Body in Diet-Streptozotocin-Induced Diabetic Sprague Dawley Rats. Oxidative Med. Cell. Longev. 2016, 1. doi:10.1155/2016/9685257
Liu, J. R., Miao, H., Deng, D. Q., Vaziri, N. D., Li, P., and Zhao, Y. Y. (2021). Gut Microbiota-Derived Tryptophan Metabolism Mediates Renal Fibrosis by Aryl Hydrocarbon Receptor Signaling Activation. Cell Mol. Life Sci. 78 (3), 909–922. doi:10.1007/s00018-020-03645-1
Liu, M., Ning, X., Li, R., Yang, Z., Yang, X., Sun, S., et al. (2017). Signalling Pathways Involved in Hypoxia-Induced Renal Fibrosis. J. Cell Mol. Med. 21 (7), 1248–1259. doi:10.1111/jcmm.13060
Livingston, M. J., Ding, H. F., Huang, S., Hill, J. A., Yin, X. M., and Dong, Z. (2016). Persistent Activation of Autophagy in Kidney Tubular Cells Promotes Renal Interstitial Fibrosis during Unilateral Ureteral Obstruction. Autophagy 12 (6), 976–998. doi:10.1080/15548627.2016.1166317
Lo, Y. H., Yang, S. F., Cheng, C. C., Hsu, K. C., Chen, Y. S., Chen, Y. Y., et al. (2022). Nobiletin Alleviates Ferroptosis-Associated Renal Injury, Inflammation, and Fibrosis in a Unilateral Ureteral Obstruction Mouse Model. Biomedicines 10 (3). doi:10.3390/biomedicines10030595
Lu, Q., Li, C., Chen, W., Shi, Z., Zhan, R., and He, R. (2018). Clinical Efficacy of Jinshuibao Capsules Combined with Angiotensin Receptor Blockers in Patients with Early Diabetic Nephropathy: A Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2018, 6806943. doi:10.1155/2018/6806943
Lu, Z., Li, S., Sun, R., Jia, X., Xu, C., Aa, J., et al. (2019). Hirsutella Sinensis Treatment Shows Protective Effects on Renal Injury and Metabolic Modulation in Db/db Mice. Evid. Based Complement. Altern. Med. 2019, 4732858. doi:10.1155/2019/4732858
Luo, Y., Yang, S. K., Zhou, X., Wang, M., Tang, D., Liu, F. Y., et al. (2015). Use of Ophiocordyceps Sinensis (Syn. Cordyceps Sinensis) Combined with Angiotensin-Converting Enzyme Inhibitors (ACEI)/angiotensin Receptor Blockers (ARB) versus ACEI/ARB Alone in the Treatment of Diabetic Kidney Disease: a Meta-Analysis. Ren. Fail 37 (4), 614–634. doi:10.3109/0886022x.2015.1009820
Ma, T. T., and Meng, X. M. (2019). TGF-β/Smad and Renal Fibrosis. Adv. Exp. Med. Biol. 1165, 347–364. doi:10.1007/978-981-13-8871-2_16
Ma, Z., Wang, F., Wang, H., Sun, T., Sun, W., and Xu, Q. (2022). Quercetin Ameliorates Renal Tubulointerstitial Transformation and Renal Fibrosis by Regulating NLRP3 in Obstructive Nephropathy. Minerva Med.. doi:10.23736/s0026-4806.22.08104-6
Meng, X. M. (2019). Inflammatory Mediators and Renal Fibrosis. Adv. Exp. Med. Biol. 1165, 381–406. doi:10.1007/978-981-13-8871-2_18
Meng, X. M., Nikolic-Paterson, D. J., and Lan, H. Y. (2016). TGF-β: the Master Regulator of Fibrosis. Nat. Rev. Nephrol. 12 (6), 325–338. doi:10.1038/nrneph.2016.48
Ngai, H. H., Sit, W. H., and Wan, J. M. (2005). The Nephroprotective Effects of the Herbal Medicine Preparation, WH30+, on the Chemical-Induced Acute and Chronic Renal Failure in Rats. Am. J. Chin. Med. 33 (3), 491–500. doi:10.1142/s0192415x05003089
Nogueira, A., Pires, M. J., and Oliveira, P. A. (2017). Pathophysiological Mechanisms of Renal Fibrosis: A Review of Animal Models and Therapeutic Strategies. Vivo 31 (1), 1–22. doi:10.21873/invivo.11019
Olatunji, O. J., Tang, J., Tola, A., Auberon, F., Oluwaniyi, O., and Ouyang, Z. (2018). The Genus Cordyceps: An Extensive Review of its Traditional Uses, Phytochemistry and Pharmacology. Fitoterapia 129, 293–316. doi:10.1016/j.fitote.2018.05.010
Pan, M. M., Zhang, M. H., Ni, H. F., Chen, J. F., Xu, M., Phillips, A. O., et al. (2013). Inhibition of TGF-β1/Smad Signal Pathway Is Involved in the Effect of Cordyceps Sinensis against Renal Fibrosis in 5/6 Nephrectomy Rats. Food Chem. Toxicol. 58, 487–494. doi:10.1016/j.fct.2013.04.037
Park, J. Y., Yoo, K. D., Bae, E., Kim, K. H., Lee, J. W., Shin, S. J., et al. (2022). Blockade of STAT3 Signaling Alleviates the Progression of Acute Kidney Injury to Chronic Kidney Disease through Antiapoptosis. Am. J. Physiol. Ren. Physiol. 322 (5), F553–f572. doi:10.1152/ajprenal.00595.2020
Peng, J., Li, X., Zhang, D., Chen, J. K., Su, Y., Smith, S. B., et al. (2015). Hyperglycemia, P53, and Mitochondrial Pathway of Apoptosis Are Involved in the Susceptibility of Diabetic Models to Ischemic Acute Kidney Injury. Kidney Int. 87 (1), 137–150. doi:10.1038/ki.2014.226
Phull, A. R., Ahmed, M., and Park, H. J. (2022). Cordyceps Militaris as a Bio Functional Food Source: Pharmacological Potential, Anti-inflammatory Actions and Related Molecular Mechanisms. Microorganisms 10 (2). doi:10.3390/microorganisms10020405
Ram, C., Gairola, S., Syed, A. M., Kulhari, U., Kundu, S., Mugale, M. N., et al. (2022). Biochanin A Alleviates Unilateral Ureteral Obstruction-Induced Renal Interstitial Fibrosis and Inflammation by Inhibiting the TGF-β1/Smad2/3 and NF-kB/NLRP3 Signaling axis in Mice. Life Sci. 298, 120527. doi:10.1016/j.lfs.2022.120527
Ricardo, S. D., van Goor, H., and Eddy, A. A. (2008). Macrophage Diversity in Renal Injury and Repair. J. Clin. Invest. 118 (11), 3522–3530. doi:10.1172/jci36150
Richter, K., and Kietzmann, T. (2016). Reactive Oxygen Species and Fibrosis: Further Evidence of a Significant Liaison. Cell Tissue Res. 365 (3), 591–605. doi:10.1007/s00441-016-2445-3
Ruiz-Ortega, M., Lamas, S., and Ortiz, A. (2022). Antifibrotic Agents for the Management of CKD: A Review. Am. J. Kidney Dis. S0272-6386 (21), 01051–01059. doi:10.1053/j.ajkd.2021.11.010
Shahed, A. R., Kim, S. I., and Shoskes, D. A. (2001). Down-regulation of Apoptotic and Inflammatory Genes by Cordyceps Sinensis Extract in Rat Kidney Following Ischemia/reperfusion. Transpl. Proc. 33 (6), 2986–2987. doi:10.1016/s0041-1345(01)02282-5
Sheng, X., Dong, Y., Cheng, D., Wang, N., and Guo, Y. (2020). Efficacy and Safety of Bailing Capsules in the Treatment of Type 2 Diabetic Nephropathy: a Meta-Analysis. Ann. Palliat. Med. 9 (6), 3885–3898. doi:10.21037/apm-20-1799
Sies, H., Berndt, C., and Jones, D. P. (2017). Oxidative Stress. Annu. Rev. Biochem. 86, 715–748. doi:10.1146/annurev-biochem-061516-045037
Song, J., Wang, Y., Liu, C., Huang, Y., He, L., Cai, X., et al. (2016). Cordyceps Militaris Fruit Body Extract Ameliorates Membranous Glomerulonephritis by Attenuating Oxidative Stress and Renal Inflammation via the NF-Κb Pathway. Food Funct. 7 (4), 2006–2015. doi:10.1039/c5fo01017a
Song, Q., and Zhu, Z. (2020). Using Cordyceps Militaris Extracellular Polysaccharides to Prevent Pb2+-Induced Liver and Kidney Toxicity by Activating Nrf2 Signals and Modulating Gut Microbiota. Food Funct. 11 (10), 9226–9239. doi:10.1039/d0fo01608j
Sun, T., Dong, W., Jiang, G., Yang, J., Liu, J., Zhao, L., et al. (2019). Cordyceps Militaris Improves Chronic Kidney Disease by Affecting TLR4/NF-Κb Redox Signaling Pathway. Oxid. Med. Cell Longev. 2019, 7850863. doi:10.1155/2019/7850863
Sun, Y. B., Qu, X., Caruana, G., and Li, J. (2016). The Origin of Renal Fibroblasts/myofibroblasts and the Signals that Trigger Fibrosis. Differentiation 92 (3), 102–107. doi:10.1016/j.diff.2016.05.008
Tang, C., Livingston, M. J., Liu, Z., and Dong, Z. (2020). Autophagy in Kidney Homeostasis and Disease. Nat. Rev. Nephrol. 16 (9), 489–508. doi:10.1038/s41581-020-0309-2
Thomas, G. L., Yang, B., Wagner, B. E., Savill, J., and El Nahas, A. M. (1998). Cellular Apoptosis and Proliferation in Experimental Renal Fibrosis. Nephrol. Dial. Transpl. 13 (9), 2216–2226. doi:10.1093/ndt/13.9.2216
Tian, H., Liu, L., Li, Z., Liu, W., Sun, Z., Xu, Y., et al. (2019). Chinese Medicine CGA Formula Ameliorates Liver Fibrosis Induced by Carbon Tetrachloride Involving Inhibition of Hepatic Apoptosis in Rats. J. Ethnopharmacol. 232, 227–235. doi:10.1016/j.jep.2018.11.027
Tsai, Y. S., Hsu, J. H., Lin, D. P., Chang, H. H., Chang, W. J., Chen, Y. L., et al. (2021). Safety Assessment of HEA-Enriched Cordyceps Cicadae Mycelium: A Randomized Clinical Trial. J. Am. Coll. Nutr. 40 (2), 127–132. doi:10.1080/07315724.2020.1743211
Vincenti, F., Fervenza, F. C., Campbell, K. N., Diaz, M., Gesualdo, L., Nelson, P., et al. (2017). A Phase 2, Double-Blind, Placebo-Controlled, Randomized Study of Fresolimumab in Patients with Steroid-Resistant Primary Focal Segmental Glomerulosclerosis. Kidney Int. Rep. 2 (5), 800–810. doi:10.1016/j.ekir.2017.03.011
Voelker, J., Berg, P. H., Sheetz, M., Duffin, K., Shen, T., Moser, B., et al. (2017). Anti-TGF-β1 Antibody Therapy in Patients with Diabetic Nephropathy. J. Am. Soc. Nephrol. 28 (3), 953–962. doi:10.1681/asn.2015111230
Walton, K. L., Johnson, K. E., and Harrison, C. A. (2017). Targeting TGF-β Mediated SMAD Signaling for the Prevention of Fibrosis. Front. Pharmacol. 8, 461. doi:10.3389/fphar.2017.00461
Wang, C., Hou, X. X., Rui, H. L., Li, L. J., Zhao, J., Yang, M., et al. (2018). Artificially Cultivated Ophiocordyceps Sinensis Alleviates Diabetic Nephropathy and its Podocyte Injury via Inhibiting P2X7R Expression and NLRP3 Inflammasome Activation. J. Diabetes Res. 2018, 1390418. doi:10.1155/2018/1390418
Wang, M. Z., Wang, J., Cao, D. W., Tu, Y., Liu, B. H., Yuan, C. C., et al. (2022). Fucoidan Alleviates Renal Fibrosis in Diabetic Kidney Disease via Inhibition of NLRP3 Inflammasome-Mediated Podocyte Pyroptosis. Front. Pharmacol. 13, 790937. doi:10.3389/fphar.2022.790937
Wang, X., Qin, A., Xiao, F., Olatunji, O. J., Zhang, S., Pan, D., et al. (2019). N6 -(2-Hydroxyethyl)-Adenosine from Cordyceps Cicadae Protects against Diabetic Kidney Disease via Alleviation of Oxidative Stress and Inflammation. J. Food Biochem. 43 (2), e12727. doi:10.1111/jfbc.12727
Wojcikowski, K., Johnson, D. W., and Gobé, G. (2004). Medicinal Herbal Extracts-Rrenal Friend or Foe? Part Two: Herbal Extracts with Potential Renal Benefits. Nephrol. Carlt. 9 (6), 400–405. doi:10.1111/j.1440-1797.2004.00355.x
Xia, W. P., Chen, X., Ru, F., He, Y., Liu, P. H., Gan, Y., et al. (2021). Knockdown of lncRNA XIST Inhibited Apoptosis and Inflammation in Renal Fibrosis via microRNA-19b-Mediated Downregulation of SOX6. Mol. Immunol. 139, 87–96. doi:10.1016/j.molimm.2021.07.012
Xu, D., Wang, B., Chen, P. P., Wang, Y. Z., Miao, N. J., Yin, F., et al. (2019). c-Myc Promotes Tubular Cell Apoptosis in Ischemia-Reperfusion-Induced Renal Injury by Negatively Regulating C-FLIP and Enhancing FasL/Fas-Mediated Apoptosis Pathway. Acta Pharmacol. Sin. 40 (8), 1058–1066. doi:10.1038/s41401-018-0201-9
Xu, G., Luo, K., Liu, H., Huang, T., Fang, X., and Tu, W. (2015). The Progress of Inflammation and Oxidative Stress in Patients with Chronic Kidney Disease. Ren. Fail 37 (1), 45–49. doi:10.3109/0886022x.2014.964141
Xu, Y., Zhang, D., Yang, H., Liu, Y., Zhang, L., Zhang, C., et al. (2021). Hepatoprotective Effect of Genistein against Dimethylnitrosamine-Induced Liver Fibrosis in Rats by Regulating Macrophage Functional Properties and Inhibiting the JAK2/STAT3/SOCS3 Signaling Pathway. Front. Biosci. (Landmark Ed. 26 (12), 1572–1584. doi:10.52586/5050
Xue, Y., Enosi Tuipulotu, D., Tan, W. H., Kay, C., and Man, S. M. (2019). Emerging Activators and Regulators of Inflammasomes and Pyroptosis. Trends Immunol. 40 (11), 1035–1052. doi:10.1016/j.it.2019.09.005
Yan, H., Xu, J., Xu, Z., Yang, B., Luo, P., and He, Q. (2021). Defining Therapeutic Targets for Renal Fibrosis: Exploiting the Biology of Pathogenesis. Biomed. Pharmacother. 143, 112115. doi:10.1016/j.biopha.2021.112115
Yang, C., Guo, Y., Huang, T. S., Zhao, J., Huang, X. J., Tang, H. X., et al. (2018). Asiatic Acid Protects against Cisplatin-Induced Acute Kidney Injury via Anti-apoptosis and Anti-inflammation. Biomed. Pharmacother. 107, 1354–1362. doi:10.1016/j.biopha.2018.08.126
Yang, J., Dong, H., Wang, Y., Jiang, Y., Zhang, W., Lu, Y., et al. (2020). Cordyceps Cicadae Polysaccharides Ameliorated Renal Interstitial Fibrosis in Diabetic Nephropathy Rats by Repressing Inflammation and Modulating Gut Microbiota Dysbiosis. Int. J. Biol. Macromol. 163, 442–456. doi:10.1016/j.ijbiomac.2020.06.153
Yang, Z., and Klionsky, D. J. (2010). Eaten Alive: a History of Macroautophagy. Nat. Cell Biol. 12 (9), 814–822. doi:10.1038/ncb0910-814
Ying-Mei, K. E., Min, J., Shu-Bo, Z., Hong, Y. U., Juan, W., and Feng, G. E. (2020). Component Analysis of Ophiocordyceps Lanpingensis Polysaccharides and Study on Alleviation of Hepatic Fibrosis in Mice by Polysaccharides. Zhongguo Zhong Yao Za Zhi 45 (21), 5256–5264. doi:10.19540/j.cnki.cjcmm.20200628.401
Yu, S. H., Dubey, N. K., Li, W. S., Liu, M. C., Chiang, H. S., Leu, S. J., et al. (2016). Cordyceps Militaris Treatment Preserves Renal Function in Type 2 Diabetic Nephropathy Mice. PLoS One 11 (11), e0166342. doi:10.1371/journal.pone.0166342
Yu, W., Duan, S., and Yu, Z. (2021). The Effect of Bailing Capsules Combined with Losartan to Treat Diabetic Glomerulosclerosis and the Combination's Effect on Blood and Urine Biochemistry. Am. J. Transl. Res. 13 (6), 6873–6880.
Zhang, H. W., Lin, Z. X., Tung, Y. S., Kwan, T. H., Mok, C. K., Leung, C., et al. (2014). Cordyceps Sinensis (A Traditional Chinese Medicine) for Treating Chronic Kidney Disease. Cochrane Database Syst. Rev. 12, Cd008353. doi:10.1002/14651858.CD008353.pub2
Zhao, K., Gao, Q., Zong, C., Ge, L., and Liu, J. (2018). Cordyceps Sinensis Prevents Contrast-Induced Nephropathy in Diabetic Rats: its Underlying Mechanism. Int. J. Clin. Exp. Pathol. 11 (12), 5571–5580.
Zhao, M., Yu, Y., Wang, R., Chang, M., Ma, S., Qu, H., et al. (2020). Mechanisms and Efficacy of Chinese Herbal Medicines in Chronic Kidney Disease. Front. Pharmacol. 11, 619201. doi:10.3389/fphar.2020.619201
Zheng, R., Zhu, R., Li, X., Li, X., Shen, L., Chen, Y., et al. (2018). N6-(2-Hydroxyethyl) Adenosine from Cordyceps Cicadae Ameliorates Renal Interstitial Fibrosis and Prevents Inflammation via TGF-β1/Smad and NF-Κb Signaling Pathway. Front. Physiol. 9, 1229. doi:10.3389/fphys.2018.01229
Zhou, D., Fu, H., Zhang, L., Zhang, K., Min, Y., Xiao, L., et al. (2017). Tubule-Derived Wnts Are Required for Fibroblast Activation and Kidney Fibrosis. J. Am. Soc. Nephrol. 28 (8), 2322–2336. doi:10.1681/asn.2016080902
Zhou, S., He, Y., Zhang, W., Xiong, Y., Jiang, L., Wang, J., et al. (2021). Ophiocordyceps Lanpingensis Polysaccharides Alleviate Chronic Kidney Disease through MAPK/NF-κB Pathway. J. Ethnopharmacol. 276, 114189. doi:10.1016/j.jep.2021.114189
Zhou, X., and Yao, Y. (2013). Unexpected Nephrotoxicity in Male Ablactated Rats Induced by Cordyceps Militaris: The Involvement of Oxidative Changes. Evid. Based Complement. Altern. Med. 2013, 786528. doi:10.1155/2013/786528
Zhu, R., Zheng, R., Deng, Y., Chen, Y., and Zhang, S. (2014). Ergosterol Peroxide from Cordyceps Cicadae Ameliorates TGF-Β1-Induced Activation of Kidney Fibroblasts. Phytomedicine 21 (3), 372–378. doi:10.1016/j.phymed.2013.08.022
Keywords: cordyceps, renal fibrosis, Chinese herbal medicine, chronic kidney disease, mechanism
Citation: Tan W, Wang Y, Dai H, Deng J, Wu Z, Lin L and Yang J (2022) Potential Therapeutic Strategies for Renal Fibrosis: Cordyceps and Related Products. Front. Pharmacol. 13:932172. doi: 10.3389/fphar.2022.932172
Received: 29 April 2022; Accepted: 01 June 2022;
Published: 08 July 2022.
Edited by:
Zhiyong Guo, Second Military Medical University, ChinaReviewed by:
Lianli Shen, Shanghai University of Traditional Chinese Medicine, ChinaNoha Mohammed Saeed, Egyptian Russian University, Egypt
Chengguo Wei, Mount Sinai Hospital, United States
Copyright © 2022 Tan, Wang, Dai, Deng, Wu, Lin and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jurong Yang, eWpyOTIzQDE2My5jb20=
†These authors share first authorship
 Wei Tan
Wei Tan Yunyan Wang1†
Yunyan Wang1† Junhui Deng
Junhui Deng Lirong Lin
Lirong Lin Jurong Yang
Jurong Yang