
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 27 September 2022
Sec. Neuropharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.931384
This article is part of the Research Topic Cannabidiol Treatment in Neurotherapeutic Interventions, Volume II View all 10 articles
Cannabidiol is a promising potential therapeutic for neurodegenerative diseases, including Alzheimer’s disease (AD). Our laboratory has shown that oral CBD treatment prevents cognitive impairment in a male genetic mouse model of AD, the amyloid precursor protein 1 x presenilin 1 hemizygous (APPxPS1) mouse. However, as sex differences are evident in clinical populations and in AD mouse models, we tested the preventive potential of CBD therapy in female APPxPS1 mice. In this study, 2.5-month-old female wildtype-like (WT) and APPxPS1 mice were fed 20 mg/kg CBD or a vehicle via gel pellets daily for 8 months and tested at 10.5 months in behavioural paradigms relevant to cognition (fear conditioning, FC; cheeseboard, CB; and novel object recognition test, NORT) and anxiety-like behaviours (elevated plus maze, EPM). In the CB, CBD reduced latencies to find a food reward in APPxPS1 mice, compared to vehicle-treated APPxPS1 controls, and this treatment effect was not evident in WT mice. In addition, CBD also increased speed early in the acquisition of the CB task in APPxPS1 mice. In the EPM, CBD increased locomotion in APPxPS1 mice but not in WT mice, with no effects of CBD on anxiety-like behaviour. CBD had limited effects on the expression of fear memory. These results indicate preventive CBD treatment can have a moderate spatial learning-enhancing effect in a female amyloid-β-based AD mouse model. This suggests CBD may have some preventive therapeutic potential in female familial AD patients.
Recently, there has been increasing interest in cannabidiol (CBD), a non-intoxicating phytocannabinoid compound in the Cannabis sativa L. [Cannabaceae] plant, for the treatment of several neurodegenerative and psychiatric disorders. CBD possesses antioxidant, anti-apoptotic, neuroprotective, and anti-inflammatory properties [reviews: (Scuderi et al., 2009; Campos et al., 2016)]. This is particularly relevant for brain disorders characterised by neuroinflammation and cell death including neurodegenerative disorders such as Alzheimer’s disease (AD), which has no cure. Dementia affects over 55 million people globally, of which AD is the most common form (Wimo et al., 2015). AD is characterised by the presence of extracellular amyloid-beta (Aβ) plaques and intracellular neurofibrillary tangles consisting of hyperphosphorylated tau (Bloom, 2014); these are found in the neocortex (Aβ) and the transentorhinal cortex (tau) in early disease stages but spread throughout the brain as the disease progresses (Braak and Braak, 1991; Thal et al., 2002). Inflammatory markers [e.g., interleukin (IL)-1, IL-6, tumour necrosis factor (TNF)-α, and activated microglia] and markers for oxidative stress [e.g. oxidised proteins and oxidative modifications in nuclear and mitochondrial DNA (Gella and Durany, 2009; Chen and Zhong, 2014)] are also commonly found in AD postmortem brain tissue (McGeer et al., 2016) and are hypothesised to precede the development of Aβ and tau pathology (Holmes, 2013). Targeting inflammation is of increasing interest as an AD treatment approach (McGeer et al., 2016). The failure of anti-inflammatory therapies to date may be due to missing the therapeutic window (Rivers-Auty et al., 2020) or requiring multimodal drug strategies to target a complex disease (Karl et al., 2017). Considering the anti-inflammatory, anti-apoptotic, and neuroprotective properties of CBD, there is growing interest in its potential for the treatment of AD (Karl et al., 2017).
In vitro data indicate CBD can reduce AD-relevant pathology [reviews: (Karl et al., 2017; Watt and Karl, 2017)]. CBD inhibits tau hyperphosphorylation (Esposito et al., 2006a; Vallee et al., 2017), reduces full-length APP expression, and reduces Aβ peptide expression (Scuderi et al., 2014), suggesting CBD can reduce AD pathology in cell culture. CBD also improves cell survival and reduces the production of reactive oxygen species and nitric oxide production (Iuvone et al., 2004; Esposito et al., 2006b; Amini and Abdolmaleki, 2022), suggesting CBD can reduce Aβ-induced toxicity. CBD can also protect against cell viability loss induced by Aβ42 (Janefjord et al., 2014), which is a major component of amyloid plaques (Gu and Guo, 2013). CBD reduces microglial function and cytokine gene and protein expression after intracerebroventricular (i.c.v.) or hippocampal Aβ administration to mice (Esposito et al., 2007; Martin-Moreno et al., 2011) and can upregulate the immune system function and increase autophagy in AD models (Hao and Feng, 2021), which may be another mechanism by which CBD improves AD pathology. CBD may also have therapeutic effects in AD by acting on hippocampal long-term potentiation (LTP); pretreatment with CBD prevents the Aβ1-42 oligomer-induced reduction in hippocampal CA1 LTP in mice (Hughes and Herron, 2019), thereby reversing effects of AD pathology on synaptic plasticity.
Preclinical in vivo data suggest remedial CBD treatment via i. p. administration reverses cognitive impairment in pharmacological and genetic mouse models for Alzheimer’s disease [reviews: (Karl et al., 2017; Watt and Karl, 2017)]. For example, chronic CBD prevents learning and memory impairments in mice injected with Aβ intraventricularly (Martin-Moreno et al., 2011). Also, in a mouse model of familial AD (Cheng et al., 2014a; Aso et al., 2015; Coles et al., 2020; Watt et al., 2020a), i.e., mice hemizygous for amyloid precursor protein (APP) and presenilin 1 (PS1) genes (i.e. APPxPS1 mice), they are characterised by increased Aβ accumulation and accelerated plaque pathology from 4 months of age (Wang et al., 2003) and spatial learning and memory deficits from 7 to 8 months of age (Cao et al., 2007; Reiserer et al., 2007). Therapeutic effects of CBD in APPxPS1 mice have been found at different CBD doses [range of 5–50 mg/kg (Cheng et al., 2014a; Coles et al., 2020; Watt et al., 2020a)] and also when using CBD-enriched extracts (Aso et al., 2015). The mechanisms involved are not entirely clear. Chronic CBD has moderate effects on Aβ levels in the hippocampus (Watt et al., 2020a) and reduces the astrocytic response and cell surface adhesion molecule CCL4 mRNA expression in APPxPS1 mice (Aso et al., 2015). However, to date, remedial CBD treatment has not been shown to strongly affect other AD-relevant receptors and molecules in APPxPS1 mice, including brain-derived neurotrophic factor (BDNF), proliferator-activated receptor γ (PPARγ), ionised calcium-binding adaptor molecule 1 (IBA1) and various cytokines (Watt et al., 2020a).
In addition to the remedial effects (i.e., CBD administered when behavioural impairment is present), CBD has been found to prevent the development of AD-relevant behavioural impairments. When CBD is administered orally for 8 months from 2.5 months of age, CBD prevents the development of social recognition impairment in male APPxPS1 mice (Cheng et al., 2014c). In this study, there were also subtle effects of CBD on neuroinflammation and cholesterol in the cortex and dietary phytosterol retention in the cortex and hippocampus (Cheng et al., 2014c). This suggests CBD has potential preventive and pro-cognitive effects on AD in male animals.
Despite this, the potential preventive effects of CBD treatment on cognition in female APPxPS1 mice are unknown. This is a critical question as sex differences are evident in AD: there is a higher prevalence of AD in women, and women suffer greater cognitive deterioration than men at the same disease stage (Laws et al., 2018; Medeiros and Silva, 2019). Importantly, sex differences are also found in the APPxPS1 mouse model, e.g., social novelty recognition impairment is evident in male APPxPS1 mice but not in female mice, while spatial memory impairment is evident in female APPxPS1 mice but not in male APPxPS1 mice (Cheng et al., 2013; Cheng et al., 2014b). Female APPxPS1 mice also show greater amyloid burden and higher plaque number (Wang et al., 2003), as well as higher levels of phosphorylated tau and proinflammatory cytokines, more severe astrocytosis and microgliosis, and greater neuronal and synaptic degeneration than male mice at the same age (Jiao et al., 2016). These sex differences make the APPxPS1 mice an appropriate model to investigate potential sex differences in CBD’s efficacy for treating cognitive impairment in AD. Furthermore, remedial CBD treatment (i.e., after the development of cognitive deficits) affects different domains in male and female APPxPS1 mice: CBD improves social recognition, object recognition, and spatial reversal learning in male APPxPS1 mice (Cheng et al., 2014a; Watt et al., 2020a) but only object recognition deficits in female APPxPS1 mice (Coles et al., 2020). Indeed, there has been limited investigation of sex differences in CBD’s effects on anxiety-like behaviour and cognition, e.g., (Osborne et al., 2017; Osborne et al., 2019; Garcia-Baos et al., 2021), highlighting the importance of examining female and male animals. Thus, we sought to determine if preventive CBD affects different behavioural domains in male and female APPxPS1 mice. Finally, we assessed a preventative approach because treatment after symptom onset may be too late to limit ongoing neurodegenerative processes in AD (Lee et al., 2022), and thus, treatments with preventative potential could have significant clinical impact by limiting disease progression and symptom onset.
Thus, the present study was designed to complement earlier behavioural research in our laboratory (Cheng et al., 2014c), to determine if 20 mg/kg CBD treatment given orally via gel pellets for 8 months prevents the development of the AD-relevant behavioural phenotype in APPxPS1 female mice.
APPxPS1 hemizygous mice on a congenic C57BL/6JxC3H/HeJ background were generated, as described previously (Cheng et al., 2013; Cheng et al., 2014a; Cheng et al., 2014b; Cheng et al., 2014c). These mice were originally described by Borchelt et al. (1997). They express the “humanized” mouse amyloid beta precursor protein gene modified at three amino acids to reflect the human residues and further modified to contain the K595N/M596L (also called K670N/M671L) mutations linked to familial Alzheimer’s. They also express a mutant human presenilin 1 carrying the exon-9-deleted variant (PSEN1dE9) associated with familial Alzheimer’s disease. These gene mutations are controlled by mouse prion protein promoter elements, directing transgene expression predominantly to CNS neurons.
Mice were bred at Australian BioResources (ABR: Moss Vale, NSW, Australia), where they were group housed in individually ventilated cages (Type Mouse Version 1: Airlaw, Smithfield, Australia) under a 12/12 h light/dark cycle with a dawn/dusk simulation. Mice were transported to the Neuroscience Research Australia animal facility (Randwick, Australia) at ∼10 weeks of age, where littermates were group housed (two to three mice per cage) in polysulfone cages (1144B: Techniplast, Rydalmere, Australia) with corn cob bedding (PuraCob Premium: Able Scientific, Perth, Australia) and tissues for nesting. Mice were kept under a 12:12 h light:dark schedule [light phase: white light (illumination: 210 lx); lights on 0700–1900 h]. Environmental temperature was automatically regulated at 21 ± 1°C, and relative humidity was 40–60%. Food (Gordon’s Rat and Mouse Maintenance Pellets: Gordon’s Specialty Stockfeeds, Yanderra, Australia) and water were provided ad libitum, except where specified.
Research and animal care procedures were approved by the University of New South Wales Animal Care and Ethics Committee in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. APPxPS1 mice and their non-transgenic wild type-like littermates (WT) were approximately 2.4 months of age at the onset of the study. The number of animals per group was as follows: 14 WT-vehicle, 16 APPxPS1-vehicle, 14 WT-CBD, and 12 APPxPS1-CBD.
Powdered CBD (CAS: 13956-29-1, THC Pharm GmbH, Frankfurt/Main, Germany) was used at a dose of 20 mg/kg body weight, based on previous work in our laboratory (Cheng et al., 2014a; Cheng et al., 2014c). CBD was administered in gel pellets to prevent the stress of chronic injections on behavioural and cognitive results; methods were identical to those published previously (Cheng et al., 2014c). Briefly, CBD or the vehicle were dissolved in a highly palatable, sweetened, and chocolate-flavoured gel pellet and administered at a volume of 8 ml/kg body weight. CBD was dissolved in gel pellets with a final composition of 2.0% ethanol, 2.0% Tween 80, 15.2% Splenda (Splenda Low Calorie Sweetener: Johnson & Johnson Pacific Pty, Broadway, Australia), 8.7% gelatine (Davis Gelatine: GELITA Australia Pty, Josephville, Australia), 20.1% chocolate flavouring (Queen Flavouring Essence Imitation Chocolate: Queen Fine Foods Pty, Alderley, Australia), and 52.0% water for irrigation. Vehicle gel pellets were identical but contained no CBD.
Mice were initially habituated to vehicle gel pellets in their home cages for 7 days prior to the start of treatment. Following this, mice were isolated within their home cages during treatment by placing a plastic divider in the home cage. Then, animals were given either a vehicle or a CBD gel pellet (treatments were quasi-randomized), which they consumed within 2–5 min. Mice did not need to be food-deprived to ensure they ate the gel pellet. A trained experimenter watched all the animals consume the gel pellets daily to ensure the correct dose was administered each day. The plastic divider was removed once the mice had consumed the gel pellets. Mice were treated daily for 8 months (i.e., from 2.5 to 10.5 months of age) late in the afternoon, to avoid potential effects of acute CBD on test outcomes.
Starting at 10 months of age, mice were tested with an inter-test interval of at least 48 h (Cheng et al., 2014c). We chose paradigms based on the baseline behavioural phenotype previously reported in these mice in our laboratory (Cheng et al., 2014b). This strategy was chosen rather than directly replicating the test biography of CBD-treated APPxPS1 male mice (Cheng et al., 2014c)] as female AD transgenic mice exhibit a different cognitive profile to males, i.e., only females exhibit impaired spatial memory (Cheng et al., 2014b), whereas only transgenic males show impaired social recognition memory (Cheng et al., 2013). All tests were conducted during the first 5 h of the light phase to minimize the effects of the circadian rhythm. All test apparatus was cleaned with 70% v/v ethanol in between test animals. Behavioural tests were conducted in the following order: fear conditioning, cheeseboard, elevated plus maze, and novel object recognition.
FC assesses hippocampal- and amygdala-dependent associative learning, and methods were identical to those published previously (Cheng et al., 2013; Cheng et al., 2014a; Cheng et al., 2014c). During conditioning, mice were placed into the test chamber (Model H10-11R-TC, Coulbourn Instruments, United States) for 2 min. An 80 dB conditioned stimulus (CS) was presented twice for 30 s with a co-terminating 0.4-mA 2-s foot shock (unconditioned stimulus; US) with an inter-pairing interval of 2 min. The test concluded 2 min later. The next day (context test), mice were returned to the apparatus for 7 min. On day 3 (cue test), animals were placed in an altered context for 9 min. After 2 min (pre-CS/baseline), the CS was presented continuously for 5 min. The test concluded after another 2 min, without the CS. Time spent freezing was measured by Any-MazeTM software.
Spatial memory was assessed in the CB using established methods (Cheng et al., 2014b; Coles et al., 2020; Watt et al., 2020a). Sweetened condensed milk, 1:4 in water, was used as a food reward, and mice were food-restricted during CB training and testing (access to food for 1–2 h, following completion of daily testing, mice kept at 85-90% of free feeding body weight). There were three trials per day, except at the probe, where there was one trial. All trials were 2 min, unless the food reward was located in <2 min, with a 20-min intertrial interval (ITI).
Mice were habituated to the blank side of the board for 2 days. Next, mice were trained for 7 days to locate a well containing a food reward. The latency of the mice to find the target well was recorded, and if the food reward was not located within 2 min, the mouse was gently guided to the well by the experimenter. Mice were considered to have learnt the task if the average latency of all three trials in 1 day was <20 s. After 7 days, our control group (WT VEH) met acquisition criteria. The next day, a probe trial was conducted to assess spatial reference memory. No wells were baited, and mice were given 2 min to explore the apparatus freely. To assess if animals could update their spatial learning contingencies, we conducted reversal learning, whereby the location of the food reward was changed. Mice completed 4 days of reversal training before the reversal probe trial (WT VEH mice met reversal criteria in 4 days), which was conducted 24 h after reversal training. During the reversal probe, no wells were baited and mice were given 2 min to explore the apparatus freely. Mice were returned to free feeding, following completion of the CB, and subsequent behavioural tests were conducted, and only once mice had returned to free feeding weight.
The average latency to find the reward was analysed as a general indication of learning, and this was used to determine when mice acquired the task (Cheng et al., 2014b; Coles et al., 2020). The first trial per day across training was also analysed to assess long-term reference memory (retention of ≥24 h), and the average of trials 2 and 3 each day across training was analysed to assess intermediate-term memory [retention falling between short-term (<2 min) and long-term (>24 h) memory] (Taglialatela et al., 2009; Coles et al., 2020). The average speed and distance were analysed throughout acquisition and reversal learning. At probe tests, the time spent in the different CB zones (i.e., board was separated into 8 equal zones, corresponding with the lines of wells) and the average speed and distance travelled were measured by Any-MazeTM software.
The EPM assesses the natural conflict between the tendency of mice to explore a novel environment and their avoidance of a brightly lit, elevated, and open area (Montgomery, 1955). Methods have been described previously (Cheng et al., 2013; Cheng et al., 2014b). The ‘+’ apparatus consisted of two alternate open arms (35 cm × 6 cm; without side walls) and two alternate enclosed arms (35 cm × 6 cm; height of enclosing walls 28 cm) connected by a central platform (6 cm × 6 cm), elevated 70 cm above the floor. Mice were placed at the centre of the ‘+’ of the grey PVC plus maze, facing an enclosed arm, and were allowed to explore the maze for 5 min. The time spent on open arms, entries into the open arms, and the distance travelled on the open and enclosed arms were recorded by AnyMaze™ tracking software.
The innate preference of a mouse for novelty and its ability to distinguish a novel object from a familiar object (Dere et al., 2007) are utilised in the NORT. The NORT was conducted over 3 days [methods: (Cheng et al., 2014a)]. Two 10-min trials were conducted per day, with a 1 h ITI. On day 1, mice were habituated to the empty arena during both trials. On day 2, mice were habituated to the empty arena during trial 1 and to two identical objects during trial 2. On the test day (day 3), mice were exposed to two identical objects in the training trial (objects distinct from day 2) and then one familiar and one novel object in the test trial. The objects used were a mini Rubik’s cube and a plastic garden hose nozzle. The objects and their locations were counterbalanced across genotypes and treatment groups. Time spent nosing and rearing on the objects was recorded by AnyMaze™ tracking software and confirmed by manual scoring. The percentage of time spent nosing the novel object indicated short-term object recognition memory (% novel object recognition) and was calculated using [(novel object nosing time/novel + familiar object nosing time) × 100]. The percentage of time spent nosing and rearing was combined to create an “exploration” score, and the percentage of novel object exploration was calculated in the same way as % nosing.
Data were analysed using SPSS Statistics 25 (IBM, NY, United States). Three- and two-way repeated measures (RM) analysis of variance (ANOVA) with within factors “minutes” (FC) or “cue” (FC) and between factors “genotype” (WT vs. APPxPS1) and “treatment” (VEH vs. CBD 20 mg/kg) was conducted. Where interactions were found, we conducted subsequent two- and one-way ANOVA split by the corresponding factor, as published previously (Long et al., 2012; Cheng et al., 2014a; Cheng et al., 2014c; Coles et al., 2020; Watt et al., 2020a). Post hoc effects are shown in figures only. Data from fear conditioning and cheeseboard were analysed with three-way ANOVA but are presented in separate graphs for visual clarity.
Data for the FC cue test were also analysed as total freezing in the 2 min prior to tone presentation, the 5 min during tone presentation, and the 2 min post-tone. Data for NORT, CB probe, and CB reversal probe tests were analysed using single-sample t-tests comparing data to the chance level for each test (Cheng et al., 2013; Cheng et al., 2014b; Coles et al., 2020). The chance level for NORT is 50% (1/2 objects), and for CB, it is 12.5% (1/8 zones). Data were presented as mean ± SEMs, and differences were regarded as statistically significant if p < 0.05.
Exclusions: FC: one WT CBD-treated mouse was excluded due to high baseline freezing (>2.5 SDs above the mean for that group). CB: three mice (1x WT VEH, 2x APPxPS1 CBD) were excluded from the CB analysis as their latency to find the food reward did not decrease across days (i.e., stayed at 120 s for the 7 days of training), so they did not engage with the paradigm.
There were no “genotype” or “treatment” differences in baseline freezing during conditioning (i.e., the first 2 min of the test), indicating baseline genotype or treatment differences did not confound the interpretation of subsequent analyses (all “treatment” and “genotype” p-values > 0.05; Table 1). During acquisition of fear conditioning, all mice increased their freezing behaviour as the test progressed, indicating acquisition of the tone-shock association [“minutes” F (6,306) = 40.3, p < 0.0001]. Although there was no overall effect of “treatment” on freezing [F (1,52) = 1.0, p = 0.3; no “treatment” interactions, all p-values > 0.05], a “minutes” by “genotype” interaction was detected [F (6,306) = 2.5, p = 0.02]. However, when split by “genotype”, both genotypes increased their freezing as the test progressed, irrespective of CBD treatment (all “time” p-values < 0.0001, no main “treatment” main effects, or interactions with ‘treatment’) (Figures 1A,B).
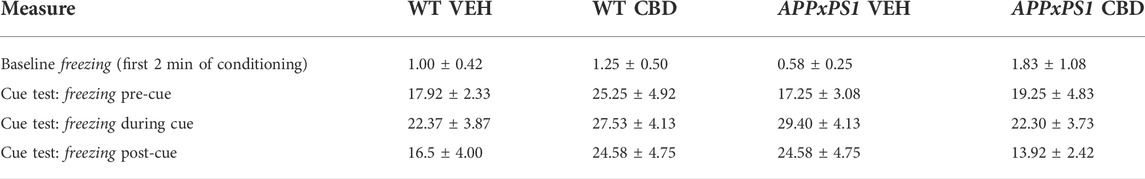
TABLE 1. Freezing during fear conditioning. Percentage of freezing within each time block [%] during the first 2 min on conditioning day and during the cue test.

FIGURE 1. Freezing time [s] during (A,B) acquisition of fear conditioning, (C,D) context test, and (E,F) cue test in APPxPS1 and WT female mice treated daily with 20 mg/kg CBD or VEH for 8 months. Interactions were present in (A, B) between “minutes” × “genotype” (p = 0.02) and in (E,F) between “minutes” × “treatment” (p = 0.02). Data were analysed using three-way RM ANOVA and presented as mean ± SEM in separate graphs for visual clarity. N = 14 WT VEH, 16 APPxPS1 VEH, 14 WT CBD, and 13 APPxPS1 CBD. Abbreviations: APPxPS1: amyloid precursor protein x presenilin 1; CBD: cannabidiol; VEH: vehicle; WT: wildtype-like.
In the context test, there were no effects of “genotype” [F (1,51) = 0.7, p = 0.4] or “treatment” [F (1,51) = 2.3, p = 0.1] on freezing in the shock-associated environment, and no interactions were detected (all p-values > 0.05) (Figures 1C,D). All mice, regardless of treatment or genotype, showed higher levels of freezing earlier in the test, which decreased as the test progressed [“minutes” F (6,306) = 6.8, p < 0.0001; no interactions] (Figures 1C,D).
During the cue test, there were no overall effects of “genotype” [F (1,51) = 0.1, p = 0.9] or “treatment” [F (1,51) = 0.1, p = 0.8]. There was an interaction between “minutes” × “treatment” [F (8,408) = 2.2, p = 0.02], suggesting CBD-treated animals froze less than VEH-treated animals, particularly in the 2nd half of the test, although follow-up analyses splitting by corresponding factors revealed no further significant differences (all p-values > 0.1) (Figures 1E,F). When data were analysed according to total time spent freezing pre-cue, during cue presentation, and post-cue, there were no effects of “genotype” or “treatment” and no interactions (all p-values > 0.05, Table 2).

TABLE 2. Open arm measures in the elevated plus maze test. Open arm entries [n] and the open arm distance ratio [%] in WT and APPxPS1 mice, following chronic treatment with a vehicle or 20 mg/kg CBD. Data presented as mean ± SEM.
Averaging latency to find the food reward from all three trials on each day, we found that all experimental groups reduced their latency during acquisition, indicating they learnt the location of the food reward [“days” F (6,294) = 102.1, p < 0.0001]. Generally, APPxPS1 mice had longer latencies than WT mice [“genotype” F (1,49) = 5.7, p = 0.02]. The latency improved across days to match control levels by the last 2 days of training [“days” × “genotype” F (6,294) = 2.5, p = 0.02]. CBD treatment did not influence the average latency to find the food reward during acquisition [“treatment” F (1,49) = 3.1, p = 0.09; no “treatment” interactions]. We explored these data further with two-way ANOVA split by “genotype”, which showed longer latencies in VEH-treated APPxPS1 mice than CBD-treated APPxPS1 mice [“treatment” F (1,24) = 5.1, p = 0.03] but not in WT mice [F (1,25) = 0.1, p = 0.9] (Figures 2A,B). Follow-up analyses split by “treatment” in WT mice revealed no further significant differences (all p-values > 0.1).
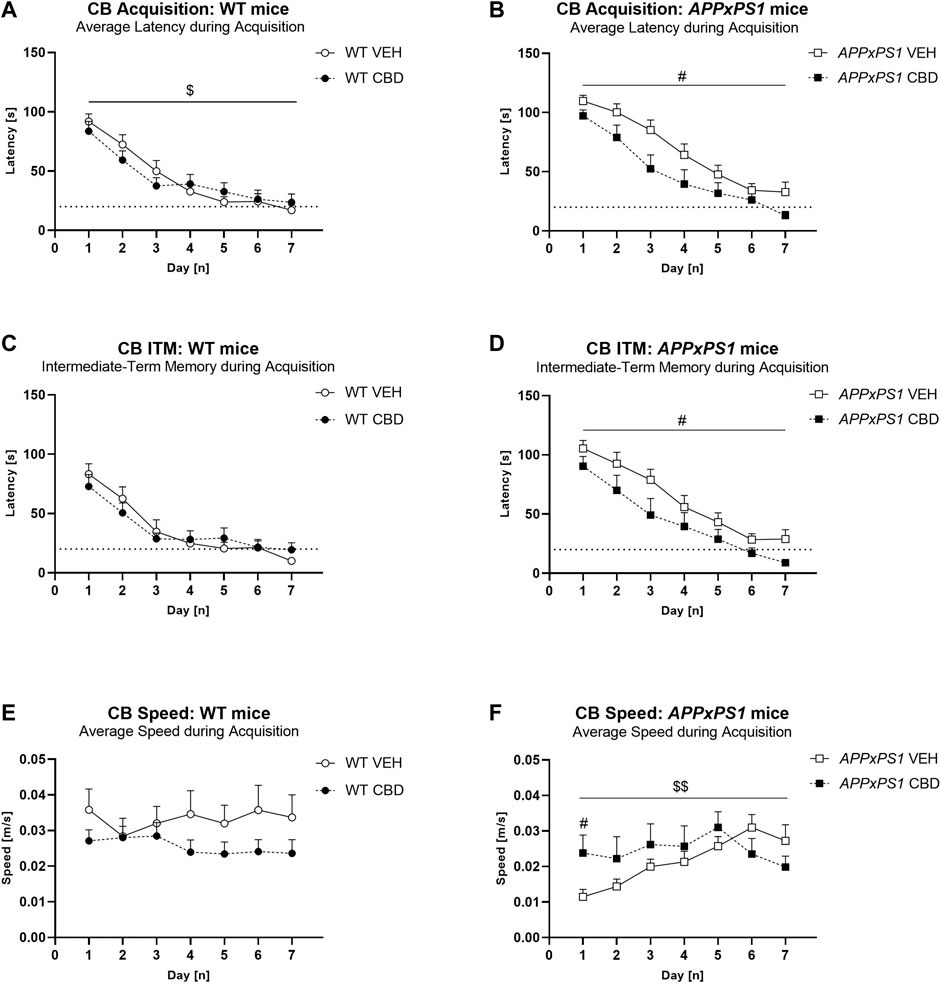
FIGURE 2. Long-term CBD improves average latency, intermediate-term memory latency, and speed during cheeseboard acquisition in APPxPS1 female mice. Latency [s] and speed [m/s] when finding the food reward in the cheeseboard in APPxPS1 and WT mice treated daily with 20 mg/kg CBD for 8 months. (A,B) Average latency [s] to find the food reward (averaged across 3 trials per day) during acquisition of the cheeseboard task. (C,D) Intermediate-term memory latency [s] (i.e., average latency for trials 2 and 3 of each day) during acquisition. (E,F) Average speed [m/s] (averaged across three trials per day) during cheeseboard acquisition. The dotted line in (A–D) indicates the 20-s cut-off threshold for acquisition. In (A,B), a “days” × “genotype” interaction (p = 0.02) was detected, and in (C,D), a “days“ × “genotype” interaction (p = 0.004) was detected. In (E,F), a “days“ × “treatment” interaction was detected; when split by “treatment,” there was a “days” × “genotype” interaction (p = 0.02) in VEH-treated mice. Splitting by “day” confirmed “genotype” differences on days 1–3 (p-values < 0.02). Data analysed using three-way RM ANOVA and presented as mean ± SEM in separate graphs for visual clarity. When data were split by the corresponding factor, significant “treatment” effects in APPxPS1 mice are indicated by hash symbols (#p < 0.05); interactions between “treatment” and “days” are indicated by ‘$’ ($p < 0.05; $$p < 0.01). N = 13 WT VEH, 16 APPxPS1 VEH, 14 WT CBD, and 10 APPxPS1 CBD. Abbreviations: APPxPS1: amyloid precursor protein x presenilin 1; CB: cheeseboard; CBD: cannabidiol; ITM: intermediate-term memory; VEH: vehicle; WT: wildtype-like.
Similarly, examination of intermediate-term memory revealed that APPxPS1 mice had longer latencies than WT mice [“genotype” F (1,49) = 8.0, p = 0.007], which was more prominent earlier in acquisition [“days” × “genotype” F (6,294) = 3.2, p = 0.004]. Overall, CBD had no effect on intermediate-term memory [“treatment” [F (1,49) = 2.8, p = 0.1; no “treatment” interactions]. Split by “genotype,” CBD reduced intermediate-term memory latencies specifically in APPxPS1 mice [“treatment” F (1,24) = 4.6, p = 0.04] but not in WT mice [F (1,25) = 0.1, p = 0.9] (Figures 2C,D). Follow-up ANOVA split by “days” revealed no further significant differences (all p-values > 0.1). Long-term memory was not different between the genotypes or treatment groups (all “genotype” or “treatment” main effects and interaction p-values > 0.05, Supplementary Figures S1A,B).
The speed of mice was also assessed. APPxPS1 mice were slower than WT controls across days [“days” × “genotype” F (6,294) = 2.6, p = 0.02], and CBD treatment affected speed as well [“days” × “treatment” F (6,294) = 3.2, p = 0.005] (Figures 2E,F). Split by “genotype,” in APPxPS1 mice, there was a “days” × “treatment” interaction [F (6,144) = 3.4, p = 0.003], suggesting APPxPS1 VEH mice were slower than CBD-treated APPxPS1 mice in the first half of acquisition, but APPxPS1 VEH mice were faster than APPxPS1 CBD mice by the end of training (Figure 2F). We split by “day” and confirmed “treatment” effects on day 1 only (p = 0.02). Similarly, split by “treatment”, VEH-treated APPxPS1 mice were initially slower than VEH-treated WT mice, but this reached WT levels by mid-training [“genotype” F (1,27) = 5.6, p = 0.03; “days” × “genotype” F (6,162) = 2.6, p = 0.02]. Splitting by “day” confirmed “genotype” differences on days 1–3 (p-values < 0.02). This speed difference was not evident in CBD-treated APPxPS1 mice (no “genotype” or “days” × “genotype” interaction, all p-values > 0.2). APPxPS1 VEH mice were slower than WT VEH or APPxPS1 CBD mice only on days 1–3 of acquisition (Figures 2E,F). No other significant differences were detected.
The distance travelled during acquisition is presented in the Supplementary Results section (see also Supplementary Figure S1).
At probe, all groups spent more time in the target zone than by chance [WT VEH: t = 2.7, df = 12, p = 0.03; APPxPS1 VEH: t = 3.8, df = 15, p = 0.002; WT CBD: t = 2.4, df = 13, p = 0.03; APPxPS1 CBD: t = 2.4, df = 9, p = 0.04] (Figure 3A).
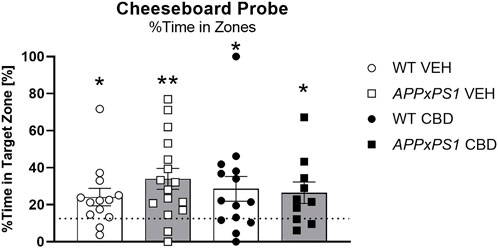
FIGURE 3. No effect of long-term CBD on recall of spatial memory. Percentage [%] of time spent in the target zone at A probe test in APPxPS1 and WT mice treated daily with 20 mg/kg CBD for 8 months. Data were analysed using a single sample t-test against chance levels, i.e., 12.5%, corresponding to 1/8 zones. Data presented as mean ± SEM. Significant t-tests against chance are indicated by asterisks (*p < 0.05; **p < 0.01). N = 13 WT VEH, 16 APPxPS1 VEH, 14 WT CBD, and 10 APPxPS1 CBD. Abbreviations: APPxPS1: amyloid precursor protein x presenilin 1; CBD: cannabidiol; VEH: vehicle; WT: wildtype-like.
Data for reversal learning and reversal probe are presented in the Supplementary Results section (see also Supplementary Figures S2–S4).
APPxPS1 mice showed more anxiety-like behaviour in the EPM, evidenced by a reduced percentage of time spent in the open arms [“genotype” F (1,51) = 4.3, p = 0.04] (Figure 4A). “CBD treatment” did not affect the percentage of open arm time [“treatment” F (1,51) = 0.09, p = 0.8; no interaction]. Open arm entries and open arm distance ratios were unaffected by the “genotype” or “treatment” (all main effect and interaction p-values > 0.05; Table 2). Although there was no overall effect of the “genotype” or “treatment” on the total distance travelled in the EPM, a “genotype” x “treatment” interaction [F (1,51) = 9.2, p = 0.004] indicates chronic CBD increased locomotion in APPxPS1 mice but not in WT mice (Figure 4B). This was confirmed when data were split by the “genotype”: CBD increased locomotion in APPxPS1 mice [“treatment” F (1,25) = 7.9, p = 0.009] but not WT mice [“treatment” F (1.26) = 2.4, p =0 .1]. Also, when data were split by “treatment,” there was a main effect of the “genotype” in CBD-treated mice [F (1,24) = 6.1, p = 0.02] but not VEH-treated mice [F (1,27) = 3.4, p = 0.08], suggesting greater distance travelled in CBD-treated APPxPS1 mice than CBD-treated WT mice (Figure 4B).

FIGURE 4. Increased locomotion but unaltered anxiety-like behaviour in APPxPS1 mice in the elevated plus maze test, following long-term CBD. (A) Distance travelled [m] and (B) percentage of open arm time [%] in the elevated plus maze test in APPxPS1 and WT mice treated daily with 20 mg/kg CBD for 8 months. A main effect of “genotype” (p = 0.04) was detected in (A). A “genotype” x “treatment” interaction (p = 0.004) was detected in (B). Data were analysed using two-way RM ANOVA and presented as mean ± SEM. When data were split by the corresponding factor, significant “genotype” effects in CBD-treated mice are indicated by asterisks (*p < 0.05, vs. WT CBD), and significant “treatment” effects in APPxPS1 mice are indicated by hash symbols (#p < 0.05, vs. APPxPS1 VEH). N = 14 WT VEH, 15 APPxPS1 VEH, 14 WT CBD, and 12 APPxPS1 CBD. Abbreviations: APPxPS1: amyloid precursor protein x presenilin 1; CBD: cannabidiol; VEH: vehicle; WT: wildtype-like.
The NORT data are presented in Supplementary Figure S5 as WT VEH-treated mice did not demonstrate novel object recognition (i.e. > 50% time nosing the novel object) [see a similar lack of object preference: (Kreilaus et al., 2019)] despite this protocol producing significant object novelty recognition in female APPxPS1 mice previously in our laboratory (Cheng et al., 2014b).
In the current study, we found that long-term preventative oral CBD improved spatial memory acquisition, which was accompanied by changes to speed and locomotion in female APPxPS1 mice. No effects of CBD treatment were detected on reversal learning or the recall of previously rewarded locations in AD transgenic mice. CBD reduced freezing, following the presentation of a discrete cue associated with footshock in both genotypes. Long-term CBD increased the distance travelled in the EPM in APPxPS1 females but did not affect anxiety-like behaviours in either genotype.
In the CB task, CBD improved the spatial learning of AD transgenic females. APPxPS1 VEH mice had longer average and intermediate-term memory latencies to find the reward location than APPxPS1 CBD mice. This effect was not evident in WT mice, suggesting CBD improved spatial learning specifically in AD-affected APPxPS1 mice but not at baseline (i.e., WT mice), potentially aligning with its low side effect profile (Gaston and Szaflarski, 2018). Interestingly, CBD increased speed and distance travelled by APPxPS1 mice in the early phases of CB learning (i.e., days 1–3), suggesting effects of CBD on spatial task acquisition may be linked to improved motor function. However, improved locomotion cannot account for all the spatial learning effects observed, as by the end of acquisition APPxPS1 VEH mice had similar speed yet still slower latencies than APPxPS1 CBD mice, suggesting APPxPS1 CBD mice moved more directly to the rewarded location rather than simply moving faster. Strengthening this argument, slower reversal latencies in APPxPS1 mice also did not correspond with slower speed.
The effects of CBD on motor function require further clarification as the CB and EPM are not traditionally utilised as primary measures for locomotor ability. There are currently no reports of improved locomotor activity by chronic CBD in mouse models of dementia (Cheng et al., 2014a, Cheng et al., 2014c, Coles et al., 2020, de Paula Faria et al., 2022; Khodadadi et al., 2021; Kreilaus et al., 2022; Watt et al., 2020b), and indeed, inconsistent effects of acute and chronic CBD on locomotor activity across a variety of neurological models have been found (reviewed in Calapai et al., 2022). Interestingly, locomotor impairment can occur in some individuals with AD (Scarmeas et al., 2004) and may be linked to PS1 mutations (Ryan et al., 2016), which may explain some of the locomotor changes observed here in APPxPS1 mice.
Despite improvements in spatial learning, CBD had no effect on the recall of spatial learning at probe or reversal probe. This reflects previous reports where chronic CBD did not affect spatial memory recall in the CB (Coles et al., 2020; Watt et al., 2020a). We also found no effect of CBD on reversal learning, suggesting oral preventive CBD may not improve performance once the task has been learnt and suggesting only specific cognitive domains may be ameliorated by preventative oral CBD.
The finding of improved spatial learning by CBD is similar to other reports investigating remedial CBD treatment in AD mouse models (i.e., treatment started after spatial learning deficits were present; Amini and Abdolmaleki, 2022; Coles et al., 2020; Martin-Moreno et al., 2011; Watt et al., 2020a). Importantly, ours is the first study to show that long-term CBD can prevent the development of some spatial learning deficits in female AD transgenic mice, suggesting CBD may have the potential to prevent cognitive impairment in both men (Cheng et al., 2014c) and women. Considering a preventative approach may limit the development or severity of AD pathology and symptoms, our results demonstrate some utility of preventive CBD, although the moderate nature of our findings suggests that preventive CBD may not be as effective as remedial CBD (see Amini and Abdolmaleki, 2022; Coles et al., 2020; Cheng et al., 2014a; Martin-Moreno et al., 2011; Watt et al., 2020a). It is also possible that a higher preventive oral CBD dose may have resulted in more pronounced effects on spatial learning. Nonetheless, by using an oral route of CBD administration in this study and previous work (Cheng et al., 2014c), we provide data which are highly clinically relevant as oral administration is clinically preferable to intravenous or intramuscular injections, and using an oral route significantly boosts the translational power of our findings.
Long-term oral CBD treatment reduced freezing in the cue test of all females, regardless of the genotype. Although it is well established that acute systemic CBD can impair fear memory consolidation (Han et al., 2022; Shallcross et al., 2019; review: Stern et al., 2018), including in female mice (Montoya et al., 2020), effects of chronic CBD on fear memory have had limited investigation, and chronic CBD does not affect fear memory acquisition (Cheng et al., 2014a; 2014c). Considering CBD-induced differences in freezing were very limited in this study, future research should consider evaluating the effects of long-term CBD on fear learning in female mice.
Chronic CBD had no effect on anxiety-like behaviours in the EPM, and this corresponds with previous reports. Although the anxiolytic-like effects of acute systemic CBD are well established [reviews: (Blessing et al., 2015; Wright et al., 2020)], the anxiolytic-like effects of chronic CBD are less clear. Chronic low-dose CBD (up to 30 mg/kg) does not affect anxiety-like behaviour in the EPM in APPxPS1 male mice (Cheng et al., 2014a; Cheng et al., 2014c) or in outbred rats and mice (Schiavon et al., 2016; Murphy et al., 2017; Gall et al., 2020). However, high-dose chronic CBD (30-100 mg/kg i. p. or subcutaneous, s. c.) can decrease anxiety-like behaviour in the EPM in mice (Long et al., 2012; Fogaca et al., 2018; Patra et al., 2020). It is possible that higher doses of CBD are necessary for anxiolytic-like effects, following long-term administration. Also, most studies use systemic injections (i.p. or s.c.) to evaluate the anxiolytic effects of CBD (Long et al., 2012; Schiavon et al., 2016; Murphy et al., 2017; Fogaca et al., 2018; Gall et al., 2020; Patra et al., 2020), and it is unknown if the oral route may alter CBD’s effects on anxiety-like behaviour.
The mechanisms by which CBD exerts pro-cognitive effects are poorly understood, but recent reports suggest potential mechanisms. Chronic CBD can enhance the immune response and increase hippocampal autophagy in APPxPS1 mice (Hao and Feng, 2021). An enhanced immune response by CBD may also drive increased microglial migration and reduced nitrite generation (Martin-Moreno et al., 2011), which can facilitate Aβ phagocytosis and decrease hippocampal Aβ plaque load, thus improving cognition in APPxPS1 mice (Watt et al., 2020a; Hao and Feng, 2021). Alternatively, it is possible that CBD ameliorates hippocampal synaptic plasticity deficits to improve spatial learning as CBD pretreatment prevents Aβ1–42-mediated LTP deficits in mouse hippocampal slices (Hughes and Herron, 2019). Examining the brain pathology in these mice to determine the mechanism/s of CBD in this instance would be a valuable focus for future research studies.
It is possible there are sex differences in the effects of CBD on cognition in APPxPS1 mice. In the present study, long-term CBD reversed spatial learning impairment in female APPxPS1 mice, while in male APPxPS1 mice, long-term CBD reversed social recognition impairment (Cheng et al., 2014c). It should be noted that male and female APPxPS1 mice show deficits in different cognitive behavioural domains (Cheng et al., 2013; Cheng et al., 2014b; Richetin et al., 2017), and this is why the behavioural tests conducted in the present study were not identical to those conducted in male APPxPS1 mice treated with long-term oral CBD (Cheng et al., 2014c). Nonetheless, it is possible that CBD could have sex-specific effects on cognition, and this may be related to sex-specific differences in hippocampal dendritic spine density. Hippocampal dendritic spine density is reduced in female APPxPS1 mice compared to WT female mice, where this effect is not as pronounced in male APPxPS1 mice (Richetin et al., 2017). Dendritic spine density is associated with spatial memory function (Mahmmoud et al., 2015), and CBD can ameliorate stress-induced reductions in the hippocampal spine density in mice (Fogaca et al., 2018). Thus, in female APPxPS1 mice, CBD may increase the hippocampal dendritic spine density to improve spatial memory.
A final consideration for the current study is that of the administration route. This study and others (Cheng et al., 2014c) gave 20 mg/kg CBD orally, whereas other work has administered 20 mg/kg CBD i. p. (Cheng et al., 2014a). In mice, i. p. administration leads to a faster peak brain concentration of CBD than oral administration (Deiana et al., 2012), and the plasma concentration of i. v. CBD is consistently higher than oral CBD for up to 24 h post-administration (Xu et al., 2019). The bioavailability of i. v. or i. p. CBD is close to 100% (Zgair et al., 2016; Xu et al., 2019), whereas oral CBD is 8.6% (Xu et al., 2019). This suggests a faster and more potent effect of i. p. CBD than oral CBD even at the same CBD dose, which may explain why the effects of oral CBD are not as pronounced as for i. p. CBD, e.g., i. p. CBD reversed both object and social memory impairment in male APPxPS1 mice (Cheng et al., 2014a), but oral CBD only reversed social memory impairment in male mice (Cheng et al., 2014c).
In conclusion, we found moderate effects of long-term oral CBD treatment on the acquisition of spatial learning by CBD in a female mouse model of familial AD. This suggests that preventive CBD may help limit some cognitive impairment in women with AD.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the University of New South Wales Animal Care and Ethics Committee.
DC and TK designed the research. DC ran all the experiments. DC, RC, and CS conducted the data analysis. RC wrote the manuscript. RC and TK edited the manuscript.
TK was supported by two project grants from the National Health and Medical Research Council (NHMRC: #1102012 and #1141789) and the NHMRC dementia research team initiative (#1095215). RC and TK were supported by the Ainsworth Medical Research Innovation Fund. In addition, RC was supported by the Rebecca Cooper Medical Research Foundation (Project Grant PG2020883). DC received an Australian Postgraduate Award scholarship from the University of New South Wales and a supplementary scholarship provided by Neuroscience Research Australia.
We thank JacKee Low and Warren Logge (Neuroscience Research Australia, Randwick, Australia) for assisting in the treatment of our test mice and the staff of Australian BioResources and Adam Bryan at Neuroscience Research Australia for taking care of our test mice. We thank Jerry Tanda for critical comments on the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor HL declared a past collaboration with the author TK.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.931384/full#supplementary-material.
Amini, M., and Abdolmaleki, Z. (2022). The effect of cannabidiol coated by nano-chitosan on learning and memory, hippocampal CB1 and CB2 levels, and amyloid plaques in an Alzheimer's disease rat model. Neuropsychobiology 81 (3), 171–183. doi:10.1159/000519534
Aso, E., Sanchez-Pla, A., Vegas-Lozano, E., Maldonado, R., and Ferrer, I. (2015). Cannabis-based medicine reduces multiple pathological processes in AβPP/PS1 mice. J. Alzheimers Dis. 43, 977–991. doi:10.3233/JAD-141014
Blessing, E. M., Steenkamp, M. M., Manzanares, J., and Marmar, C. R. (2015). Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics 12, 825–836. doi:10.1007/s13311-015-0387-1
Bloom, G. S. (2014). Amyloid-beta and tau: The trigger and bullet in alzheimer disease pathogenesis. JAMA Neurol. 71, 505–508. doi:10.1001/jamaneurol.2013.5847
Borchelt, D. R., Ratovitski, T., van Lare, J., Lee, M. K., Gonzales, V., Jenkins, N. A., et al. (1997). Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron 19 (4), 939–945. doi:10.1016/s0896-6273(00)80974-5
Braak, H., and Braak, E. (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259. doi:10.1007/BF00308809
Calapai, F., Cardia, L., Calapai, G., Di Mauro, D., Trimarchi, F., Ammendolia, I., et al. (2022). Effects of cannabidiol on locomotor activity. Life (Basel) 12 (5), 652. doi:10.3390/life12050652
Campos, A. C., Fogaca, M. V., Sonego, A. B., and Guimaraes, F. S. (2016). Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 112, 119–127. doi:10.1016/j.phrs.2016.01.033
Cao, D., Lu, H., Lewis, T. L., and Li, L. (2007). Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J. Biol. Chem. 282 (50), 36275–36282. doi:10.1074/jbc.M703561200
Chen, Z., and Zhong, C. (2014). Oxidative stress in Alzheimer's disease. Neurosci. Bull. 30, 271–281. doi:10.1007/s12264-013-1423-y
Cheng, D., Logge, W., Low, J. K., Garner, B., and Karl, T. (2013). Novel behavioural characteristics of the APP(Swe)/PS1ΔE9 transgenic mouse model of Alzheimer's disease. Behav. Brain Res. 245, 120–127. doi:10.1016/j.bbr.2013.02.008
Cheng, D., Low, J. K., Logge, W., Garner, B., and Karl, T. (2014a). Chronic cannabidiol treatment improves social and object recognition in double transgenic APPswe/PS1E9 mice. Psychopharmacol. Berl. 231, 3009–3017. doi:10.1007/s00213-014-3478-5
Cheng, D., Low, J. K., Logge, W., Garner, B., and Karl, T. (2014b). Novel behavioural characteristics of female APPSwe/PS1ΔE9 double transgenic mice. Behav. Brain Res. 260, 111–118. doi:10.1016/j.bbr.2013.11.046
Cheng, D., Spiro, A. S., Jenner, A. M., Garner, B., and Karl, T. (2014c). Long-term cannabidiol treatment prevents the development of social recognition memory deficits in Alzheimer's disease transgenic mice. J. Alzheimers Dis. 42, 1383–1396. doi:10.3233/JAD-140921
Coles, M., Watt, G., Kreilaus, F., and Karl, T. (2020). Medium-dose chronic cannabidiol treatment reverses object recognition memory deficits of APPSwe/PS1ΔE9 transgenic female mice. Front. Pharmacol. 11, 587604. doi:10.3389/fphar.2020.587604
de Paula Faria, D., Estessi de Souza, L., Duran, F. L. S., Buchpiguel, C. A., Britto, L. R., Crippa, J. A. S., et al. (2022). Cannabidiol treatment improves glucose metabolism and memory in streptozotocin-induced Alzheimer's disease rat model: A proof-of-concept study. Int. J. Mol. Sci. 23 (3), 1076. doi:10.3390/ijms23031076
Deiana, S., Watanabe, A., Yamasaki, Y., Amada, N., Arthur, M., Fleming, S., et al. (2012). Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ⁹-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacol. Berl. 219, 859–873. doi:10.1007/s00213-011-2415-0
Dere, E., Huston, J. P., and De Souza Silva, M. A. (2007). The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci. Biobehav. Rev. 31, 673–704. doi:10.1016/j.neubiorev.2007.01.005
Esposito, G., De Filippis, D., Carnuccio, R., Izzo, A. A., and Iuvone, T. (2006a). The marijuana component cannabidiol inhibits beta-amyloid-induced tau protein hyperphosphorylation through Wnt/beta-catenin pathway rescue in PC12 cells. J. Mol. Med. 84, 253–258. doi:10.1007/s00109-005-0025-1
Esposito, G., De Filippis, D., Maiuri, M. C., De Stefano, D., Carnuccio, R., and Iuvone, T. (2006b). Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in beta-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-kappaB involvement. Neurosci. Lett. 399, 91–95. doi:10.1016/j.neulet.2006.01.047
Esposito, G., Scuderi, C., Savani, C., Steardo, L., De Filippis, D., Cottone, P., et al. (2007). Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br. J. Pharmacol. 151, 1272–1279. doi:10.1038/sj.bjp.0707337
Fogaca, M. V., Campos, A. C., Coelho, L. D., Duman, R. S., and Guimaraes, F. S. (2018). The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: Role of neurogenesis and dendritic remodeling. Neuropharmacology 135, 22–33. doi:10.1016/j.neuropharm.2018.03.001
Gall, Z., Farkas, S., Albert, A., Ferencz, E., Vancea, S., Urkon, M., et al. (2020). Effects of chronic cannabidiol treatment in the rat chronic unpredictable mild stress model of depression. Biomolecules 10, E801. doi:10.3390/biom10050801
Garcia-Baos, A., Puig-Reyne, X., Garcia-Algar, O., and Valverde, O. (2021). Cannabidiol attenuates cognitive deficits and neuroinflammation induced by early alcohol exposure in a mice model. Biomed. Pharmacother. 141, 111813. doi:10.1016/j.biopha.2021.111813
Gaston, T. E., and Szaflarski, J. P. (2018). Cannabis for the treatment of epilepsy: An update. Curr. Neurol. Neurosci. Rep. 18 (11), 73. doi:10.1007/s11910-018-0882-y
Gella, A., and Durany, N. (2009). Oxidative stress in Alzheimer disease. Cell adh. Migr. 3, 88–93. doi:10.4161/cam.3.1.7402
Gu, L., and Guo, Z. (2013). Alzheimer's Aβ42 and Aβ40 peptides form interlaced amyloid fibrils. J. Neurochem. 126, 305–311. doi:10.1111/jnc.12202
Han, X., Song, X., Song, D., Xie, G., Guo, H., Wu, N., et al. (2022). Comparison between cannabidiol and sertraline for the modulation of post-traumatic stress disorder-like behaviors and fear memory in mice. Psychopharmacol. Berl. 239 (5), 1605–1620. doi:10.1007/s00213-022-06132-6
Hao, F., and Feng, Y. (2021). Cannabidiol (CBD) enhanced the hippocampal immune response and autophagy of APP/PS1 Alzheimer's mice uncovered by RNA-seq. Life Sci. 264, 118624. doi:10.1016/j.lfs.2020.118624
Holmes, C. (2013). Review: Systemic inflammation and Alzheimer's disease. Neuropathol. Appl. Neurobiol. 39, 51–68. doi:10.1111/j.1365-2990.2012.01307.x
Hughes, B., and Herron, C. E. (2019). Cannabidiol reverses deficits in hippocampal LTP in a model of Alzheimer's disease. Neurochem. Res. 44, 703–713. doi:10.1007/s11064-018-2513-z
Iuvone, T., Esposito, G., Esposito, R., Santamaria, R., Di Rosa, M., and Izzo, A. A. (2004). Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J. Neurochem. 89, 134–141. doi:10.1111/j.1471-4159.2003.02327.x
Janefjord, E., Maag, J. L., Harvey, B. S., and Smid, S. D. (2014). Cannabinoid effects on beta amyloid fibril and aggregate formation, neuronal and microglial-activated neurotoxicity in vitro. Cell. Mol. Neurobiol. 34, 31–42. doi:10.1007/s10571-013-9984-x
Jiao, S. S., Bu, X. L., Liu, Y. H., Zhu, C., Wang, Q. H., Shen, L. L., et al. (2016). Sex dimorphism profile of Alzheimer's disease-type pathologies in an APP/PS1 mouse model. Neurotox. Res. 29, 256–266. doi:10.1007/s12640-015-9589-x
Karl, T., Garner, B., and Cheng, D. (2017). The therapeutic potential of the phytocannabinoid cannabidiol for Alzheimer's disease. Behav. Pharmacol. 28, 142–160. doi:10.1097/FBP.0000000000000247
Khodadadi, H., Salles, E. L., Jarrahi, A., Costigliola, V., Khan, M. B., and Yu, J. C. (2021). Cannabidiol ameliorates cognitive function via regulation of IL-33 and TREM2 upregulation in a murine model of Alzheimer's disease. J. Alzheimers Dis. 80 (3), 973–977. doi:10.3233/JAD-210026
Kreilaus, F., Chesworth, R., Eapen, V., Clarke, R., and Karl, T. (2019). First behavioural assessment of a novel Immp2l knockdown mouse model with relevance for Gilles de la Tourette syndrome and Autism spectrum disorder. Behav. Brain Res. 374, 112057. doi:10.1016/j.bbr.2019.112057
Kreilaus, F., Przybyla, M., Ittner, L., and Karl, T. (2022). Cannabidiol (CBD) treatment improves spatial memory in 14-month-old female TAU58/2 transgenic mice. Behav. Brain Res. 425, 113812. doi:10.1016/j.bbr.2022.113812
Laws, K. R., Irvine, K., and Gale, T. M. (2018). Sex differences in Alzheimer's disease. Curr. Opin. Psychiatry 31, 133–139. doi:10.1097/YCO.0000000000000401
Lee, J., Howard, R. S., and Schneider, L. S. (2022). The current landscape of prevention trials in dementia. Neurotherapeutics 19 (1), 228–247. doi:10.1007/s13311-022-01236-5
Long, L. E., Chesworth, R., Huang, X. F., Wong, A., Spiro, A., Mcgregor, I. S., et al. (2012). Distinct neurobehavioural effects of cannabidiol in transmembrane domain neuregulin 1 mutant mice. PLoS One 7, e34129. doi:10.1371/journal.pone.0034129
Mahmmoud, R. R., Sase, S., Aher, Y. D., Sase, A., Groger, M., Mokhtar, M., et al. (2015). Spatial and working memory is linked to spine density and mushroom spines. PLoS One 10, e0139739. doi:10.1371/journal.pone.0139739
Martin-Moreno, A. M., Reigada, D., Ramirez, B. G., Mechoulam, R., Innamorato, N., Cuadrado, A., et al. (2011). Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: Relevance to Alzheimer's disease. Mol. Pharmacol. 79, 964–973. doi:10.1124/mol.111.071290
McGeer, P. L., Rogers, J., and Mcgeer, E. G. (2016). Inflammation, antiinflammatory agents, and Alzheimer's disease: The last 22 years. J. Alzheimers Dis. 54, 853–857. doi:10.3233/JAD-160488
Medeiros, A. M., and Silva, R. H. (2019). Sex differences in Alzheimer's disease: Where do we stand? J. Alzheimers Dis. 67, 35–60. doi:10.3233/JAD-180213
Montgomery, K. C. (1955). The relation between fear induced by novel stimulation and exploratory behavior. J. Comp. Physiol. Psychol. 48, 254–260. doi:10.1037/h0043788
Montoya, Z. T., Uhernik, A. L., and Smith, J. P. (2020). Comparison of cannabidiol to citalopram in targeting fear memory in female mice. J. Cannabis Res. 2 (1), 48. doi:10.1186/s42238-020-00055-9
Murphy, M., Mills, S., Winstone, J., Leishman, E., Wager-Miller, J., Bradshaw, H., et al. (2017). Chronic adolescent d9-tetrahydrocannabinol treatment of male mice leads to long-term cognitive and behavioral dysfunction, which are prevented by concurrent cannabidiol treatment. Cannabis Cannabinoid Res. 2, 235–246. doi:10.1089/can.2017.0034
Osborne, A. L., Solowij, N., Babic, I., Huang, X. F., and Weston-Green, K. (2017). Improved social interaction, recognition and working memory with cannabidiol treatment in a prenatal infection (poly I:C) rat model. Neuropsychopharmacology 42 (7), 1447–1457. doi:10.1038/npp.2017.40
Osborne, A. L., Solowij, N., Babic, I., Lum, J. S., Huang, X. F., Newell, K. A., et al. (2019). Cannabidiol improves behavioural and neurochemical deficits in adult female offspring of the maternal immune activation (poly I:C) model of neurodevelopmental disorders. Brain Behav. Immun. 81, 574–587. doi:10.1016/j.bbi.2019.07.018
Patra, P. H., Serafeimidou-Pouliou, E., Bazelot, M., Whalley, B. J., Williams, C. M., and Mcneish, A. J. (2020). Cannabidiol improves survival and behavioural co-morbidities of Dravet syndrome in mice. Br. J. Pharmacol. 177, 2779–2792. doi:10.1111/bph.15003
Reiserer, R. S., Harrison, F. E., Syverud, D. C., and McDonald, M. P. (2007). Impaired spatial learning in the APPSwe + PSEN1DeltaE9 bigenic mouse model of Alzheimer's disease. Genes Brain Behav. 6 (1), 54–65. doi:10.1111/j.1601-183X.2006.00221.x
Richetin, K., Petsophonsakul, P., Roybon, L., Guiard, B. P., and Rampon, C. (2017). Differential alteration of hippocampal function and plasticity in females and males of the APPxPS1 mouse model of Alzheimer's disease. Neurobiol. Aging 57, 220–231. doi:10.1016/j.neurobiolaging.2017.05.025
Rivers-Auty, J., Mather, A. E., Peters, R., Lawrence, C. B., and Brough, D. (2020). Anti-inflammatories in Alzheimer's disease-potential therapy or spurious correlate? Brain Commun. 2, fcaa109. doi:10.1093/braincomms/fcaa109
Ryan, N. S., Nicholas, J. M., Weston, P. S. J., Liang, Y., Lashley, T., Guerreiro, R., et al. (2016). Clinical phenotype and genetic associations in autosomal dominant familial Alzheimer's disease: A case series. Lancet. Neurol. 15 (13), 1326–1335. doi:10.1016/S1474-4422(16)30193-4
Scarmeas, N., Hadjigeorgiou, G. M., Papadimitriou, A., Dubois, B., Sarazin, M., Brandt, J., et al. (2004). Motor signs during the course of Alzheimer disease. Neurology 63 (6), 975–982. doi:10.1212/01.wnl.0000138440.39918.0c
Schiavon, A. P., Bonato, J. M., Milani, H., Guimaraes, F. S., and Weffort De Oliveira, R. M. (2016). Influence of single and repeated cannabidiol administration on emotional behavior and markers of cell proliferation and neurogenesis in non-stressed mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 64, 27–34. doi:10.1016/j.pnpbp.2015.06.017
Scuderi, C., Filippis, D. D., Iuvone, T., Blasio, A., Steardo, A., and Esposito, G. (2009). Cannabidiol in medicine: A review of its therapeutic potential in CNS disorders. Phytother. Res. 23, 597–602. doi:10.1002/ptr.2625
Scuderi, C., Steardo, L., and Esposito, G. (2014). Cannabidiol promotes amyloid precursor protein ubiquitination and reduction of beta amyloid expression in SHSY5YAPP+ cells through PPARγ involvement. Phytother. Res. 28, 1007–1013. doi:10.1002/ptr.5095
Shallcross, J., Hamor, P., Bechard, A. R., Romano, M., Knackstedt, L., and Schwendt, M. (2019). The divergent effects of CDPPB and cannabidiol on fear extinction and anxiety in a predator scent stress model of PTSD in rats. Front. Behav. Neurosci. 13, 91. doi:10.3389/fnbeh.2019.00091
Stern, C. A. J., de Carvalho, C. R., Bertoglio, L. J., and Takahashi, R. N. (2018). Effects of cannabinoid drugs on aversive or rewarding drug-associated memory extinction and reconsolidation. Neuroscience 370, 62–80. doi:10.1016/j.neuroscience.2017.07.018
Taglialatela, G., Hogan, D., Zhang, W. R., and Dineley, K. T. (2009). Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav. Brain Res. 200, 95–99. doi:10.1016/j.bbr.2008.12.034
Thal, D. R., Rub, U., Orantes, M., and Braak, H. (2002). Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58, 1791–1800. doi:10.1212/wnl.58.12.1791
Vallee, A., Lecarpentier, Y., Guillevin, R., and Vallee, J. N. (2017). Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer's disease. Acta Biochim. Biophys. Sin. 49, 853–866. doi:10.1093/abbs/gmx073
Wang, J., Tanila, H., Puolivali, J., Kadish, I., and Van Groen, T. (2003). Gender differences in the amount and deposition of amyloidbeta in APPswe and PS1 double transgenic mice. Neurobiol. Dis. 14, 318–327. doi:10.1016/j.nbd.2003.08.009
Watt, G., Chesworth, R., Przybyla, M., Ittner, A., Garner, B., Ittner, L. M., et al. (2020b). Chronic cannabidiol (CBD) treatment did not exhibit beneficial effects in 4-month-old male TAU58/2 transgenic mice. Pharmacol. Biochem. Behav. 196, 172970. doi:10.1016/j.pbb.2020.172970
Watt, G., and Karl, T. (2017). In vivo evidence for therapeutic properties of cannabidiol (CBD) for Alzheimer's disease. Front. Pharmacol. 8, 20. doi:10.3389/fphar.2017.00020
Watt, G., Shang, K., Zieba, J., Olaya, J., Li, H., Garner, B., et al. (2020a). Chronic treatment with 50 mg/kg cannabidiol improves cognition and moderately reduces Aβ40 levels in 12-month-old male AβPPswe/PS1ΔE9 transgenic mice. J. Alzheimers Dis. 74, 937–950. doi:10.3233/JAD-191242
Wimo, A., Ali, G.-C., Guerchet, M., Prince, M., Prina, M., and Wu, Y.-T. (2015). World alzheimer report 2015. The global impact of dementia. London, UK: Alzheimer's Disease International.
Wright, M., Di Ciano, P., and Brands, B. (2020). Use of cannabidiol for the treatment of anxiety: A short synthesis of pre-clinical and clinical evidence. Cannabis Cannabinoid Res. 5, 191–196. doi:10.1089/can.2019.0052
Xu, C., Chang, T., Du, Y., Yu, C., Tan, X., and Li, X. (2019). Pharmacokinetics of oral and intravenous cannabidiol and its antidepressant-like effects in chronic mild stress mouse model. Environ. Toxicol. Pharmacol. 70, 103202. doi:10.1016/j.etap.2019.103202
Keywords: Alzheimer’s disease, behaviour, cannabidiol (CBD), spatial memory, female, amyloid precursor protein, presenilin 1
Citation: Chesworth R, Cheng D, Staub C and Karl T (2022) Effect of long-term cannabidiol on learning and anxiety in a female Alzheimer’s disease mouse model. Front. Pharmacol. 13:931384. doi: 10.3389/fphar.2022.931384
Received: 28 April 2022; Accepted: 05 September 2022;
Published: 27 September 2022.
Edited by:
Huazheng Liang, Translational Research Institute of Brain and Brain-Like Intelligence affiliated to Tongji University School of Medicine, ChinaReviewed by:
Oliver Wirths, University Medical Center Göttingen, GermanyCopyright © 2022 Chesworth, Cheng, Staub and Karl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tim Karl, dC5rYXJsQHdlc3Rlcm5zeWRuZXkuZWR1LmF1
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.