- 1Department of Clinical Pharmacology and Therapeutics, Seoul National University College of Medicine and Hospital, Seoul, South Korea
- 2Department of Clinical Pharmacology and Therapeutics, Hanyang University Seoul Hospital, Seoul, South Korea
Aim: Sphingosine-1-phosphate receptor mediates the egress of lymphocytes from lymphoid organs, and its inhibition results in a decreased number of circulating lymphocytes. The aim of the current study was to investigate the safety and pharmacodynamic and pharmacokinetic characteristics of a novel sphingosine-1-phosphate receptor modulator, LC51-0255.
Methods: A phase 1 randomized, double-blind, placebo-controlled, multiple dosing, dose-escalation study was conducted on healthy Korean male subjects.
Results: After single and daily administration of LC51-0255 for 21 days, a dose-dependent decrease in lymphocyte count and heart rate was observed through 0.25–2 mg dose range of LC51-0255. The mean elimination half-life of LC51-0255 was 76–95 h. LC51-0255 was accumulated with a mean accumulation ratio of 5.17–6.64. During the study, LC51-0255 was generally well tolerated. The most common treatment-emergent adverse event was bradycardia. No clinically significant event of arrhythmia, including AV block, was observed. No clinically significant difference in blood pressure was observed between the dose groups. In other safety assessments, no clinically significant abnormalities were observed, except for bradycardia.
Conclusion: Daily administration of LC51-0255 in the range of 0.25–2 mg resulted in a dose-dependent reduction of lymphocyte counts and heart rate. LC51-0255 is generally safe and well tolerated in healthy volunteers.
Introduction
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid metabolite produced by various cell types to modulate diverse physiological responses via S1P receptors (S1PR) (Alvarez et al., 2007). The mechanism of S1P receptor modulator (S1PRM) action includes the inhibition of lymphocyte egress from secondary lymphoid organs to the periphery through S1P receptor 1 (S1PR1), resulting in a decreased number of circulating lymphocytes in the peripheral blood (Matloubian et al., 2004; Cyster, 2005).
Modulation of S1PR1 has established a new approach in the treatment of autoimmune diseases, such as multiple sclerosis (MS), inflammatory bowel disease (IBD), lupus, and psoriasis (Gonzalez-Cabrera et al., 2014; Dash et al., 2018). Fingolimod is a first-in-class drug that has been approved for the treatment of relapsing-remitting MS (Park and Im, 2017). Other drugs in this category include siponimod (approved for the treatment of MS), ozanimod (approved for the treatment of MS and ulcerative colitis), and several others, which are under development (Park and Im, 2017; Al-salama, 2019; Lamb, 2020).
LC51-0255 is a newly developed potent, selective, and orally available S1PRM. LC51-0255 showed selective activity on S1PR1 among S1PR subtypes through in vitro Ca2+ mobilization assays (Ministry of Healthy and Welfare, 2014). The half maximal effective concentration (EC50) of LC51-0255 on S1PR1 was 21.38 nM, while >10000 and 501.19 nM on S1PR3 and S1PR5, respectively (Supplementary Table S1) (Ministry of Healthy and Welfare, 2014). In an in vivo pharmacology study with a rat model, a dose-dependent reduction of peripheral lymphocyte was observed after single and multiple oral administration of LC51-0255 (Ministry of Healthy and Welfare, 2014). In the rat experimental autoimmune encephalomyelitis (EAE) model, a dose-dependent reduction of EAE severity was observed (Ministry of Healthy and Welfare, 2014). In addition, recovery of intestinal tissue and dose-dependent reduction of lymphocytes in a rat model of IBD have been shown (Kim J.A et al., unpublished data, 2016). In vitro G protein-coupled inwardly rectifying acetylcholine-regulated K+ channel assay suggested that LC51-0255 might have less cardiovascular risk compared to other S1PRM (Kim et al., 2019). In the first-in-human clinical trial, a single dose of LC51-0255 of up to 2 mg was well tolerated after single administration in healthy subjects (Lee et al., 2022). The lymphocyte counts and heart rates were reduced in a reversible and dose-dependent manner (Lee et al., 2022). Based on the results of preclinical and single administration studies, LC51-0255 is expected to be a novel treatment option for patients with autoimmune and chronic inflammatory conditions.
The aim of the current study was to investigate the safety, tolerability, pharmacokinetic (PK) characteristics, and pharmacodynamic (PD) characteristics of LC51-0255 after multiple ascending oral doses for 21 days in healthy Korean male subjects.
Materials and methods
Study design
A phase 1, randomized, double-blind, placebo-controlled, multiple dosing, and dose-escalation study was conducted on healthy Korean male subjects between March 2018 and July 2019. The doses of LC51-0255 selected for the study were 0.25, 0.5, 1, 1.5, and 2 mg. Eligible subjects were admitted to the Seoul National University Clinical Trials Center 2 days prior to the first dosing and were randomly assigned to either LC51-0255 or a matching placebo in a ratio of 8:2. Subjects received allocated doses of LC51-0255 or a matching placebo daily for 21 days. The inpatient clinical study was completed according to the predefined schedule, and the subjects were discharged 27 days after the first dosing (day 28). Subjects visited the clinical trial center on an outpatient basis on days 30, 32, 34, 36, 38, 40, and 42 for safety monitoring and blood sampling.
All subjects provided their written informed consent prior to enrollment in the clinical study. The study protocol was reviewed and approved by the Ministry of Food and Drug Safety, Republic of Korea, and the Institutional Review Board of Seoul National University Hospital, Seoul (ClinicalTrials.gov identifier: NCT03174613). The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines of the International Council for Harmonization.
Subjects
Healthy Korean male volunteers aged 19–45 years with a body mass index (BMI) of 18.0 kg/m2 to 27.0 kg/m2 at the screening visit were eligible for enrollment in the study. Subjects with evidence or a history of clinically significant hepatic, renal, neurologic, immunologic, pulmonary, endocrine, hematologic, neoplastic, cardiovascular, or psychological disease were excluded from the study.
Determination of the LC51-0255 concentration
Plasma and urine concentrations of LC51-0255 were analyzed using a validated liquid chromatography with tandem mass spectrometry (LC-MS/MS) method (LC: Prominence UFLC, Shimadzu, Kyoto, Japan. MS: API 5000 for plasma and API 4000 for urine, SCIEX, Concord, Ontario, Canada) by International Scientific Standards, Inc. (Chuncheon, Republic of Korea). The bioanalytical methods were validated over the range of 0.3–1,000 ng/ml for plasma samples and 0.3–300 ng/ml for urine samples. The lower limits of quantification for both plasma and urine samples were 0.3 ng/ml.
Pharmacodynamics analysis
For absolute lymphocyte count (ALC) analysis, blood samples were collected at the following time points: day −1: 0, 2, 4, 6, 8, and 12 h; day 1: 0 (predose), 2, 4, 6, 8, and 12 h (postdose); day 2–day 20 (predose); day 21: 0 (predose), 2, 4, 6, 8, 12, 24, 36, 48, 60, 72, 96, 120, 144, 168, 216, 264, 312, 360, 408, 456, and 504 h (postdose).
The maximum effect (Emax), the area under the ALC–time curve from time zero to the last measurable point (AUEClast) and change of those parameters from the baseline (ΔEmax and ΔAUEClast), and the time to maximum effect (TEmax) and maximum change of ALC from the baseline (CFBmax) were calculated using the noncompartmental method of Phoenix WinNonlin® version 8.1 (Certara, Princeton, NJ, United States).
The baseline ALC of each subject was defined using the following formula:
For heart rate analysis, 24-h Holter monitoring was performed the day before the first dosing (day 1), on the first dosing (day 1), and last dosing (day 21). The hourly average heart rate (HR) and the area under the hourly HR–time curve (AUECHR) were calculated for each individual subject.
Pharmacokinetics analysis
For PK evaluation, serial blood samplings were performed at the following time points: day 1: 0 (predose), 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, and 24 h (postdose); day 4, 6, 8, 10, 12, 14, 16, 18, 19, and 20 (predose); and day 21: 0 (predose), 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 24, 36, 48, 72, 96, 120, 144, 168, 216, 264, 312, 360, 408, 456, and 504 h (postdose). Urine samples were collected at the following time points and intervals: day 1: 0 (predose), 0–24 h (postdose); day 21: 0 (predose), 0 to 24, 24 to 48, 48 to 72, 72 to 96, 96 to 120, 120 to 144, and 144–168 h postdose.
PK parameters were analyzed using a noncompartmental method using Phoenix WinNonlin® version 8.1 (Certara, Princeton, NJ, United States). The maximum steady-state plasma concentration of LC51-0255 (Cmax,ss), the time to reach maximum plasma concentration following administration of LC51-0255 at the steady state (Tmax,ss), the area under the plasma concentration–time curve during a dosing interval at steady state (AUCτ,ss), the terminal half-life (t1/2), the accumulation ratio calculated from AUCτ,ss and AUCτ after single dosing (Rac), the peak trough fluctuation over one dosing interval at steady state, apparent clearance at steady state (CLss/F), apparent volume of distribution during the terminal phase (Vz/F), the fraction of drug excreted into urine (fe), and renal clearance (CLR) were calculated.
Safety and tolerability assessment
Treatment-emergent adverse events (TEAEs), physical examinations, vital signs, 12-lead electrocardiogram (ECG), continuous ECG monitoring, 24-h Holter monitoring, clinical laboratory tests, pulmonary function tests, and ophthalmologic tests were performed to assess the safety and tolerability. To collect clinically significant events, the criterion for bradycardia was defined as below 40 beats per minute.
Exploratory assessment of immune cell subset counts
Immune cell subset counts in peripheral blood were measured by fluorescence-activated cell sorting analysis using FACSVerse (BD Biosciences, Franklin Lakes, NJ, United States) by Meditree Co., Ltd. (Seoul, Republic of Korea). The details of antibodies used are presented in Supplementary Table S2. Blood samples for analysis were collected at day 1 (predose), day 8 (4 h postdose), and day 21 (4 h postdose).
Statistical analysis
Descriptive statistics were used to summarize demographic, PK, PD, and safety data. All statistical analyses were performed using SAS® version 9.4 (SAS Institute, Inc., Cary, NC, United States). The Kruskal–Wallis test was performed to compare the demographic characteristics between the treatment groups. ANOVA using the general linear model and Dunnett’s method for multiple comparison tests was performed to compare PD parameters between the treatment groups. Pearson’s correlation coefficient was used to assess the correlation between PK and PD parameters.
Results
Study population
A total of 50 subjects were enrolled, and 46 subjects completed the study. Three subjects withdrew their consent after receiving LC51-0255 1 mg, 1.5 mg, and 2 mg, respectively, and discontinued from the study citing personal reasons. One subject was discontinued from the study by the investigator due to noncompliance with the study instructions after receiving LC51-0255 2 mg (Supplementary Figure S1).
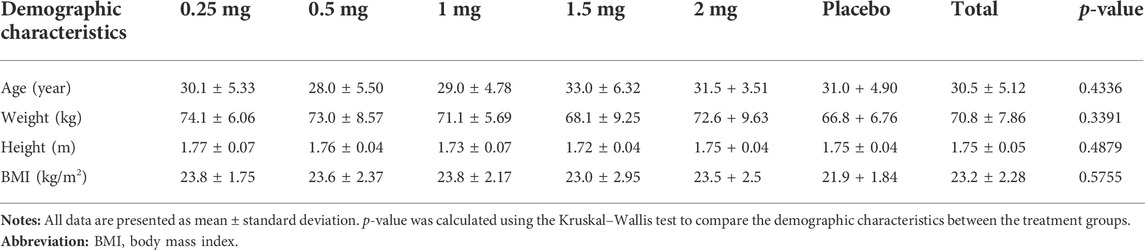
Mean ± standard deviation (SD) values of age, weight, height, and BMI were 30.5 ± 5.12 years, 70.8 ± 7.86 kg, 1.75 ± 0.05 m, and 23.2 ± 2.28 kg/m2, respectively (Table 1). No statistically significant difference in demographic characteristics was observed among the treatment groups (p > 0.05).
Pharmacodynamics
Absolute lymphocyte count
After single and daily administration of LC51-0255 for 21 days, a dose-dependent decrease of ALC was observed through 0.25–2 mg dose range of LC51-0255 (Figure 1). The mean ± SD values for the maximum change in ALC from the baseline (CFBmax) were −61.84 ± 14.70%, −76.70 ± 5.60%, −82.12 ± 3.68%, −85.30 ± 6.41%, and −87.98 ± 3.50% in the LC51-0255 0.25, 0.5, 1, 1.5, and 2 mg dose groups, respectively, which were significantly lower than that in the placebo group (−31.57 ± 11.17%) (Table 2). Other PD parameters (Emax and AUECτ) and baseline-corrected parameters were also significantly lower in all the dose groups than in the placebo group (Figure 2). All dose levels reached their maximum PD effect at 6 h postdose, and ALC recovered to the baseline within 14 days of drug discontinuation (Table 2).
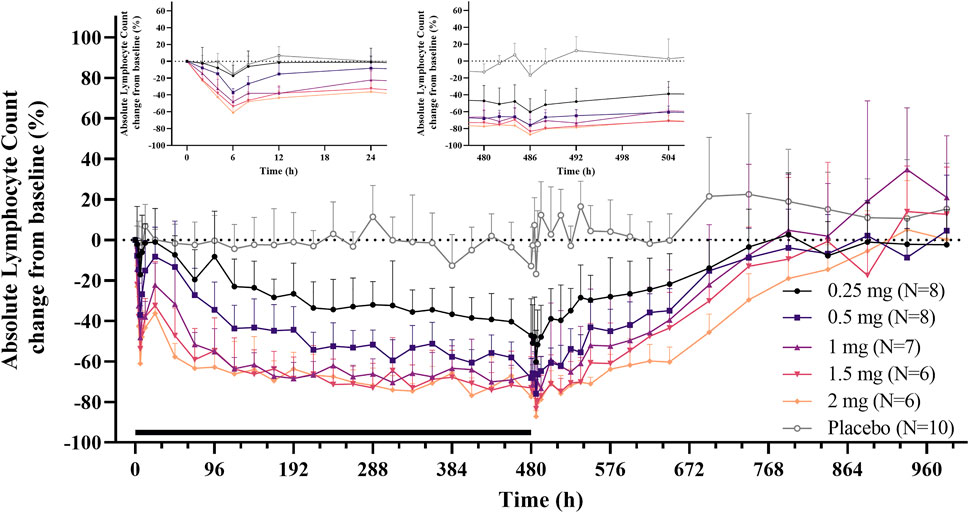
FIGURE 1. Mean absolute lymphocyte count (change from the baseline) profiles after daily oral administration of 0.25, 0.5, 1, 1.5, and 2 mg LC51-0255 for 21 days. Notes: Error bars denote the standard deviations. The black bar represents the duration of treatment. The inset graphs show the enlarged 24-h profiles after the first and last dose.
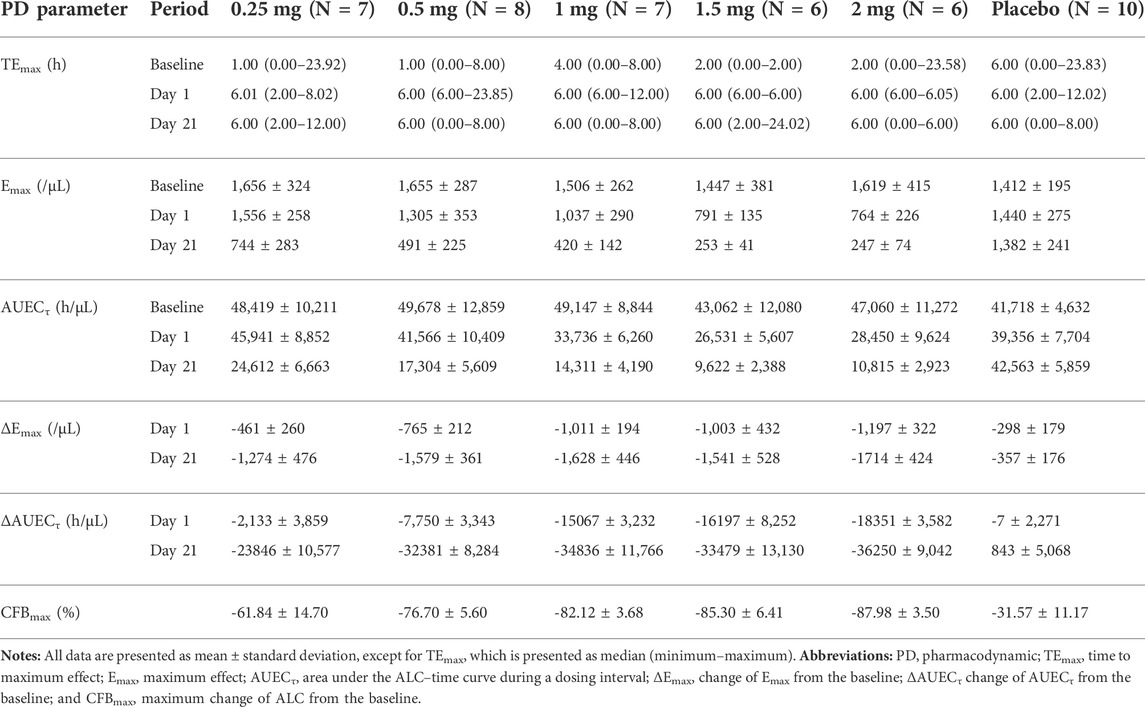
TABLE 2. Pharmacodynamic parameters of LC51-0255 after daily oral administration of 0.25, 0.5, 1, 1.5, 2 mg of LC51-0255 or placebo for 21 days.
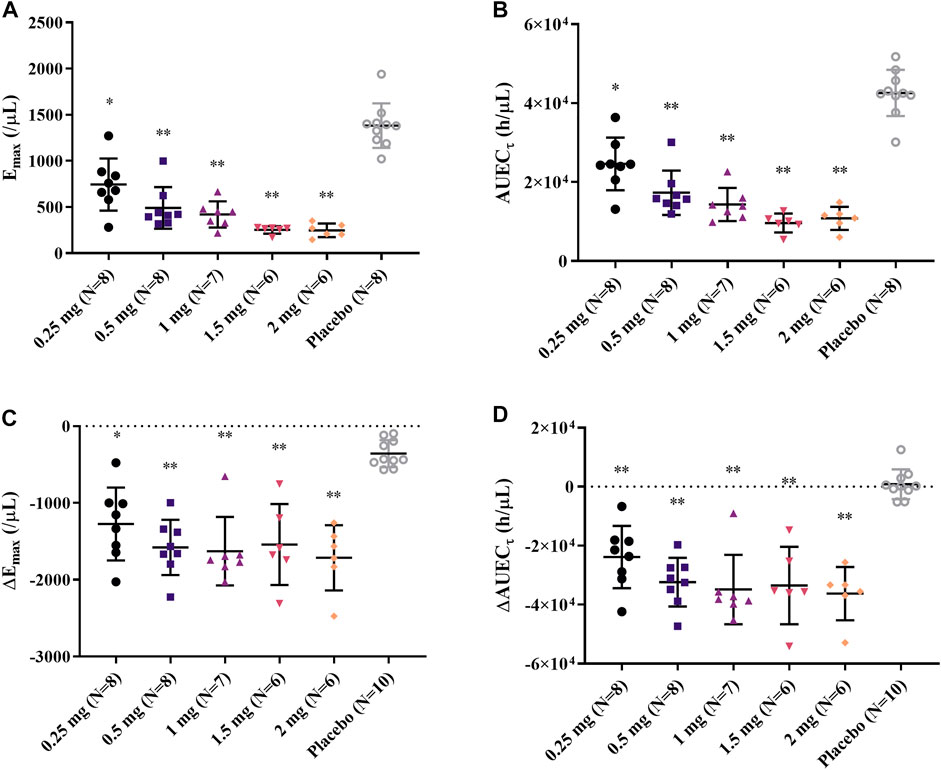
FIGURE 2. Individual (A) Emax, (B) AUECτ, (C) ΔEmax, and (D) ΔAUECτ after daily oral administration of 0.25, 0.5, 1, 1.5, and 2 mg LC51-0255 for 21 days. Notes: Middle line: mean value; upper and lower lines: standard deviations; ∗p < 0.05, versus placebo and ∗∗p < 0.0001, versus placebo.
Heart rate
A dose-dependent reduction in HR was observed after a single administration of LC51-0255 (0.25–2 mg) (Figure 3A). A statistically significant decrease in AUECHR compared to the placebo group was observed in the 2 mg dose group (Figure 4). After daily administration of LC51-0255 for 21 days, the reduction in HR was insignificant (Figure 3B). No dose group showed a statistically significant decrease in AUECHR compared to the placebo group (Figure 4).
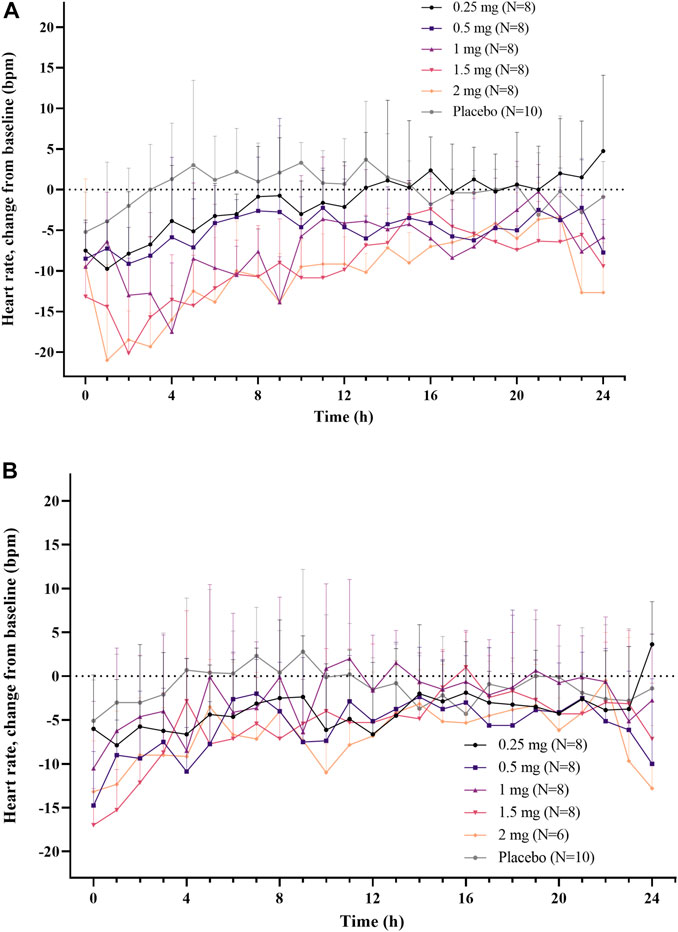
FIGURE 3. Mean hourly average heart rate (change from the baseline) after (A) single oral administration and (B) daily oral administration of 0.25, 0.5, 1, 1.5, and 2 mg of LC51-0255 for 21 days. Note: Error bars denote the standard deviations.
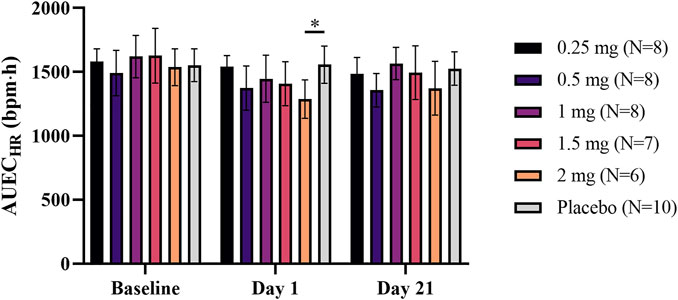
FIGURE 4. Mean area under effect curve of hourly average heart rate at the baseline, after single and daily oral administration of 0.25, 0.5, 1, 1.5, and 2 mg of LC51-0255 for 21 days. Notes: Error bars denote the standard deviations; ∗p < 0.05, LC51-0255 2 mg versus placebo at day 1.
Pharmacokinetics
After daily administration of LC51-0255 for 21 days, the peak plasma concentration at the steady state was observed at 4–4.5 h postdose, and the mean elimination half-life was 76–95 h (Figure 5; Table 3). LC51-0255 accumulated considerably with a mean accumulation ratio of 5.17–6.64 across the dose groups. Systemic exposure to LC51-0255 was proportional to the dose. The fraction excreted into urine ranged from 0.01 to 0.3% across the dose levels, indicating that renal excretion was not the major route of elimination.
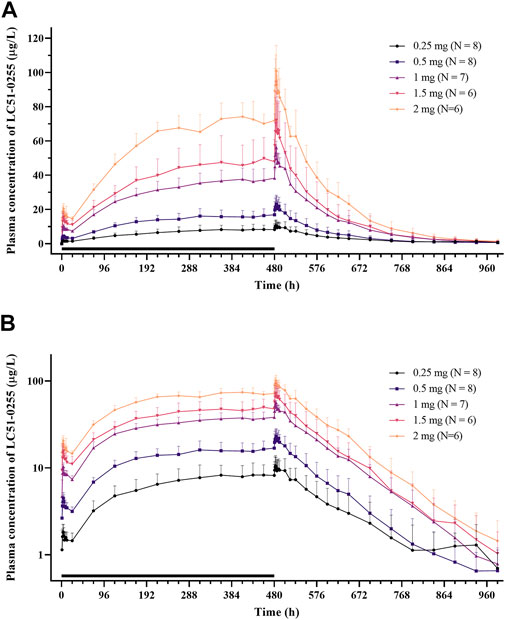
FIGURE 5. Mean plasma concentration–time profiles of LC51-0255 in (A) linear scale and (B) log linear scale after daily oral administration of 0.25, 0.5, 1, 1.5, and 2 mg of LC51-0255 for 21 days. Notes: Error bars denote the standard deviations. The black bar represents the duration of treatment.
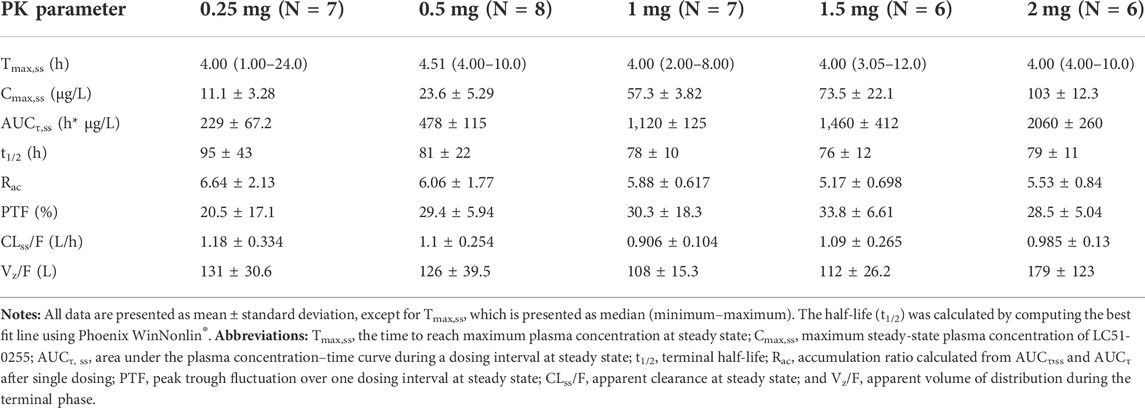
TABLE 3. Pharmacokinetic parameters of LC51-0255 after daily oral administration of 0.25, 0.5, 1, 1.5, or 2 mg of LC51-0255 for 21 days.
Relationship between pharmacokinetics and pharmacodynamics
Baseline-corrected PD parameters (ΔEmax and ΔAUECτ) were significantly correlated with selected PK parameters (Cmax and AUCτ) after the first administration of LC51-0255 (Supplementary Figure S2). After daily administration for 21 days, the correlation was weaker compared to that after the first day (Supplementary Figure S3).
Safety and tolerability
Overall, 89 TEAEs were reported in 34 subjects (68.0%). The most common TEAE was bradycardia, which occurred 14 times in a total of 8 subjects (16%) (Supplementary Table S3). No clinically significant event of arrhythmia, including AV block, was observed. No clinically significant difference in blood pressure was observed between the dose groups (Supplementary Figure S4).
One serious adverse event (SAE) was reported in the LC51-0255 1 mg dose group. One subject was diagnosed with colonic diverticulitis on day 21, which required intravenous antibiotics for treatment. The SAE resolved without sequelae.
In the clinical laboratory test, pulmonary function test, and ophthalmologic test, no clinically significant abnormalities or changes were observed. In ECGs, Holter monitoring, and vital signs, no clinically significant abnormalities were observed, except for bradycardia.
Explorative analysis of immune cell subset counts
A dose-dependent decrease in immune cell subset counts (CD3+, CD4+, CD8+, CD14+, and CD19+) was observed across all the dose groups. The reduction in CD4+ (helper T cells) counts was the most significant, while the reduction in CD14+ (myeloid cells) counts was relatively mild. The CD4+/CD8+ ratio was also reduced in a dose-dependent manner (Supplementary Figure S5).
Discussion
This study investigated the effect of LC51-0255 on ALC and HR, along with PK, safety, and tolerability profiles of LC51-0255 after daily oral administration for 21 days in a dose range of 0.25–2 mg.
As expected from other S1P agonists such as fingolimod, multiple administrations of LC51-0255 resulted in a lower nadir ALC (Emax) than that after a single administration (Kovarik et al., 2004). The Emax value ranged from 744/μL to 247/μL after daily administration of LC51-0255 for 21 days, with a tendency to decrease at higher doses (i.e., greater absolute ΔEmax values). The maximum change from the baseline exceeded 80% at LC51-0255 dose higher than 1 mg and was comparable to the standard dose of fingolimod (Francis et al., 2014). Three subjects (one in the 1.5 mg dose group and two in the 2 mg dose group) were reported to have severe lymphopenia (ALC <200 cells/μL) after LC51-0255 treatment. As severe lymphopenia after S1PRM therapy was manageable and was not related to SAEs in the previous clinical trials, the risk of opportunistic infection after administration of LC51-0255 was regarded as minimal (Chun et al., 2021).
In the case of HR, the first dose-related reduction of HR was observed, which is a well-known class effect (Dimarco et al., 2010). S1PR-dependent activation of G-protein-coupled inwardly rectifying potassium channels of atrial myocytes leads to a negative chronotropic effect and delayed atrioventricular conduction (Camm et al., 2014). LC51-0255 was thought to be less prone to the first-dose effect due to a long half-life, an effect called “built-in up-titration,” which was observed in S1PRMs with long half-lives, such as fingolimod (Juif et al., 2016). First, dose-related bradycardia observed in the study was mostly asymptomatic and was not clinically significant. Therefore, the need for an up-titration regimen was considered minimal.
The correlation between PK parameters (Cmax and AUCτ) and the baseline-corrected PD parameters (ΔEmax and ΔAUECτ) was observed after the first administration but was weaker after repeated administration. It was considered to be due to the range of exposure which was relatively wider while the effect was rapidly saturated after repeated administration of LC51-0255.
During the study, one case of diverticulitis was reported as an SAE. Multiple genetic and environmental factors might contribute to the etiology of diverticulitis (Reichert and Lammert, 2015). As reduction of lymphocytes might have affected the occurrence and progression of the condition, the causal relationship between diverticulitis and LC51-0255 was not ruled out. Besides bradycardia, which was predicted from the clinical studies of fingolimod, no case of clinically significant arrhythmia was reported during the study.
In contrast to fingolimod, a nonselective S1P modulator, LC51-0255 did not show agonistic activity for S1PR3 in preclinical studies. Therefore, the risk of adverse events related to vasoconstriction (hypertension, headache, and stroke) which may be associated with S1PR3 was predicted to be low (Willis and Cohen, 2013; Camm et al., 2014; Stepanovska et al., 2020). Although the duration of the study was relatively short and the number of subjects was considered small to fully evaluate the frequency of such vasoconstrictive events, no event of stroke or clinically significant change in blood pressure was observed during the study. In addition, the ratio of a subject with headache was comparable between the placebo group (2/10, 20%) and the LC51-0255 treated group (5/40, 12.5%). Further evaluation of S1PR3-related adverse events is required in future studies.
Exploratory analysis of the immune cell subsets suggested that LC51-0255 affected CD4+ T cells the most, as the greatest decrease was observed. The CD4+/CD8+ cell ratio was also significantly reduced after the daily administration of LC51-0255. The extent of reduction increased with the dose of LC51-0255 and the length of dosing. Similar changes in lymphocyte subsets were observed in fingolimod-treated MS patients (Rudnicka et al., 2015). Cases of viral infections in patients treated with fingolimod might be correlated with impaired cellular immunity to suppress viral infection caused by such imbalances in lymphocyte subsets (Uccelli et al., 2011; Gross et al., 2012; Ratchford et al., 2012).
Restriction of the study subject to Korean ethnicity was one of the limitations of the study. As fingolimod did not show a marked difference in PK, lymphocyte trafficking, and HR responses between ethnic groups, the conclusion drawn from the study was considered sufficiently robust (Kovarik et al., 2007). Further studies should consider including subjects from various ethnic groups to generalize the result across ethnic groups.
In conclusion, daily administration of LC51-0255 in the range of 0.25–2 mg to healthy volunteers resulted in a dose-dependent reduction in lymphocyte counts. The decrease in HR after the first administration was also dose dependent; however, it was insignificant after repeated dosing. PK features, including a long half-life, favored the once-daily dosing regimen. LC51-0255 was generally safe and well tolerated during the study. The result of the study warrants further evaluation of the efficacy and safety of LC51-0255 for patients with autoimmune and chronic inflammatory diseases.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Seoul National University Hospital, Seoul, Republic of Korea. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: SL and KY. Formal analysis: IH, SL, and JO. Investigation: IH and SL. Resources: JO, SL, I-JJ, and K-SY. Project administration: K-SY. Manuscript writing: IH. All authors reviewed and approved the submitted manuscript.
Funding
This study was sponsored by LG Chem Ltd., Seoul, Republic of Korea, the manufacturer of LC51-0255. The study was conducted at the Clinical Trials Center, Seoul National University Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.930615/full#supplementary-material
References
Al-salama, Z. T. (2019). Siponimod: First global approval. Drugs 79, 1009–1015. doi:10.1007/s40265-019-01140-x
Alvarez, S. E., Milstien, S., and Spiegel, S. (2007). Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol. Metab. 18, 300–307. doi:10.1016/j.tem.2007.07.005
Camm, J., Hla, T., Bakshi, R., and Brinkmann, V. (2014). Cardiac and vascular effects of fingolimod: Mechanistic basis and clinical implications. Am. Heart J. 168, 632–644. doi:10.1016/j.ahj.2014.06.028
Chun, J., Giovannoni, G., and Hunter, S. F. (2021). Sphingosine 1-phosphate receptor modulator therapy for multiple sclerosis: Differential downstream receptor signalling and clinical profile effects. Drugs 81, 207–231. doi:10.1007/s40265-020-01431-8
Cyster, J. G. (2005). Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu. Rev. Immunol. 23, 127–159. doi:10.1146/annurev.immunol.23.021704.115628
Dash, R. P., Rais, R., and Srinivas, N. R. (2018). Ponesimod, a selective sphingosine 1-phosphate (S1P1) receptor modulator for autoimmune diseases: Review of clinical pharmacokinetics and drug disposition. Xenobiotica. 48, 442–451. doi:10.1080/00498254.2017.1329568
Dimarco, J., O’Connor, P., Cohen, J., Francis, G., Collins, W., and Zhang-Auberson, L. (2010). First-dose effect of fingolimod: Pooled safety data from two phase 3 studies (TRANSFORMS and FREEDOMS)(P830). Mult. Scler. 3, 629–638. doi:10.1016/j.msard.2014.05.005
Francis, G., Kappos, L., O'Connor, P., Collins, W., Tang, D., Mercier, F., et al. (2014). Temporal profile of lymphocyte counts and relationship with infections with fingolimod therapy. Mult. Scler. 20, 471–480. doi:10.1177/1352458513500551
Gonzalez-Cabrera, P. J., Brown, S., Studer, S. M., and Rosen, H. (2014). S1P signaling: New therapies and opportunities. F1000Prime Rep. 6, 109. doi:10.12703/P6-109
Gross, C. M., Baumgartner, A., Rauer, S., and Stich, O. (2012). Multiple sclerosis rebound following herpes zoster infection and suspension of fingolimod. Neurology 79, 2006–2007. doi:10.1212/WNL.0b013e3182735d24
Juif, P. E., Kraehenbuehl, S., and Dingemanse, J. (2016). Clinical pharmacology, efficacy, and safety aspects of sphingosine-1-phosphate receptor modulators. Expert Opin. Drug Metab. Toxicol. 12, 879–895. doi:10.1080/17425255.2016.1196188
Kim, K. C., Kim, J. W., Hong, P. S., and Seo, D. O. (2019). “Nonclinical safety assessment of LC51-0255, an oral sphingosine-1-phosphate 1 receptor (S1P1) modulator with a favorable profile,” in United European Gastroenterology Week 2019, Barcelona, Spain, October 19–October 23.
Kovarik, J. M., Schmouder, R., Barilla, D., Riviere, G. J., Wang, Y., Hunt, T., et al. (2004). Multiple-dose FTY720: Tolerability, pharmacokinetics, and lymphocyte responses in healthy subjects. J. Clin. Pharmacol. 44, 532–537. doi:10.1177/0091270004264165
Kovarik, J., Slade, A., Voss, B., Schmidli, H., Riviere, G., Picard, F., et al. (2007). Ethnic sensitivity study of fingolimod in white and Asian subjects. Int. J. Clin. Pharmacol. Ther. 45, 98–109. doi:10.5414/cpp45098
Matloubian, M., Lo, C. G., Cinamon, G., Lesneski, M. J., Xu, Y., Brinkmann, V., et al. (2004). Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360. doi:10.1038/nature02284
Park, S. J., and Im, D. S. (2017). Sphingosine 1-phosphate receptor modulators and drug discovery. Biomol. Ther. 25, 80–90. doi:10.4062/biomolther.2016.160
Ratchford, J. N., Costello, K., Reich, D. S., and Calabresi, P. A. (2012). Varicella-zoster virus encephalitis and vasculopathy in a patient treated with fingolimod. Neurology 79, 2002–2004. doi:10.1212/WNL.0b013e3182735d00
Reichert, M. C., and Lammert, F. (2015). The genetic epidemiology of diverticulosis and diverticular disease: Emerging evidence. United Eur. Gastroenterol. J. 3, 409–418. doi:10.1177/2050640615576676
Rudnicka, J., Czerwiec, M., Grywalska, E., Siwicka-Gieroba, D., Walankiewicz, M., Grafka, A., et al. (2015). Influence of fingolimod on basic lymphocyte subsets frequencies in the peripheral blood of multiple sclerosis patients - preliminary study. Cent. Eur. J. Immunol. 40, 354–359. doi:10.5114/ceji.2015.54599
Stepanovska, B., Zivkovic, A., Enzmann, G., Tietz, S., Homann, T., Kleuser, B., et al. (2020). Morpholino analogues of fingolimod as novel and selective S1P1 ligands with in vivo efficacy in a mouse model of experimental antigen-induced encephalomyelitis. Int. J. Mol. Sci. 21, 6463. doi:10.3390/ijms21186463
Uccelli, A., Ginocchio, F., Mancardi, G. L., and Bassetti, M. (2011). Primary varicella zoster infection associated with fingolimod treatment. Neurology 76, 1023–1024. doi:10.1212/WNL.0b013e31821043b5
Ministry of Healthy and Welfare (2014). (Pre-clinical trial) Development of novel oral agents for multiple sclerosis. Available: https://scienceon.kisti.re.kr/commons/util/originalView.do?cn=TRKO201600004461&dbt=TRKO&rn=.
Willis, M. A., and Cohen, J. A. (2013). Seminars in neurology. Thieme Medical Publishers, 037–044.Fingolimod therapy for multiple sclerosis
Keywords: autoimmune disease, immunosuppression, lymphocyte count, phase I, randomized controlled trial, sphingosine-1-phosphate receptor modulator
Citation: Hwang I, Lee SW, Oh J, Lee S, Jang I-J and Yu K-S (2022) Dose-dependent reduction of lymphocyte count and heart rate after multiple administration of LC51-0255, a novel sphingosine-1-phosphate receptor 1 modulator, in healthy subjects. Front. Pharmacol. 13:930615. doi: 10.3389/fphar.2022.930615
Received: 10 May 2022; Accepted: 13 July 2022;
Published: 22 August 2022.
Edited by:
Simona Federica Spampinato, University of Catania, ItalyReviewed by:
Martin Sebastian Winkler, University Medical Center Göttingen, GermanyAshok Kumar, All India Institute of Medical Sciences Bhopal, India
Copyright © 2022 Hwang, Lee, Oh, Lee, Jang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyung-Sang Yu, a3N5dUBzbnUuYWMua3I=
 Inyoung Hwang
Inyoung Hwang Sang Won Lee2
Sang Won Lee2 SeungHwan Lee
SeungHwan Lee Kyung-Sang Yu
Kyung-Sang Yu