- 1Emergency Department, China-Japan Friendship Hospital, Beijing, China
- 2General Surgery Department, China-Japan Friendship Hospital, Beijing, China
- 3Cardiac Care Unit, The First Affiliated Hospital of Dalian Medical University, Dalian, China
- 4Intensive Care Unit, Zhejiang Provincial People’s Hospital, Hangzhou, China
- 5Cardiac Surgery Department, Qilu Hospital of ShanDong University, Jinan, China
- 6Cardiac Care Unit, XiangTan Central Hospital, Xiangtan, China
Background: This study assessed the effects of esmolol injection in patients with in-hospital cardiac arrest (IHCA) with refractory ventricular fibrillation (VF)/pulseless ventricular tachycardia (pVT).
Methods: From January 2018 to December 2021, 29 patients with IHCA with refractory shockable rhythm were retrospectively reviewed. Esmolol was administered after advanced cardiovascular life support (ACLS)-directed procedures, and outcomes were assessed.
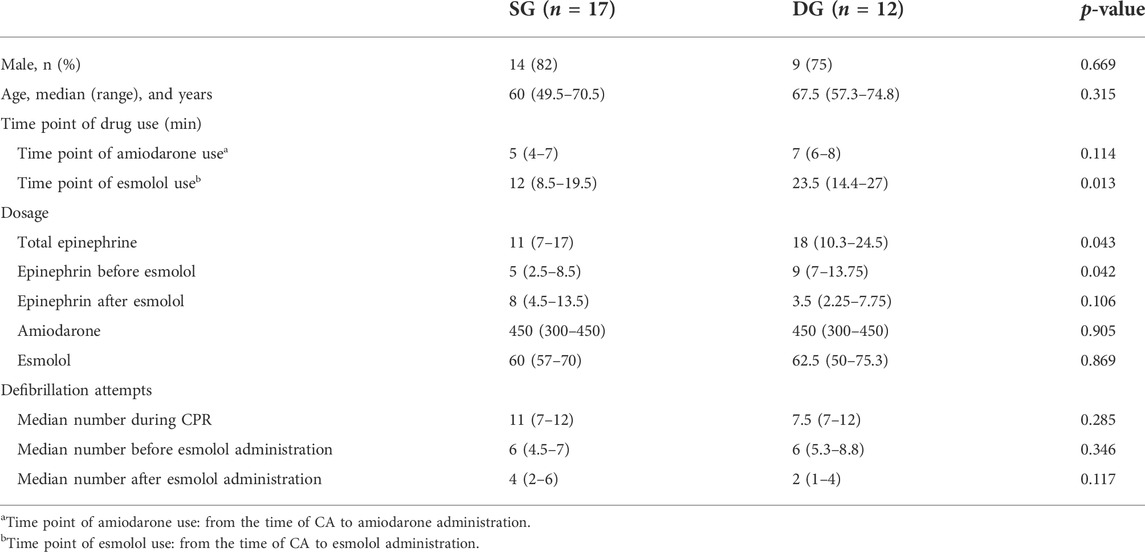
Results: Among the 29 cases, the rates of sustained return of spontaneous circulation (ROSC), 24-h ROSC, and 72-h ROSC were 79%, 62%, and 59%, respectively. Of those patients, 59% ultimately survived to discharge. Four patients with cardiac insufficiency died. The duration from CA to esmolol infusion was significantly shorter for patients in the survival group (SG) than for patients in the dead group (DG) (12 min, IQR: 8.5–19.5 vs. 23.5 min, IQR: 14.4–27 min; p = 0.013). Of those patients, 76% (22 of 29) started esmolol administration after the second dose of amiodarone. No significant difference was observed in the survival rate between this group and groups administered an esmolol bolus simultaneously or before the second dose of amiodarone (43% vs. 64%, p = 0.403). Of those patients, 31% (9 of 29) were administered an esmolol bolus for defibrillation attempts ≤ 5, while the remaining 69% of patients received an esmolol injection after the fifth defibrillation attempt. No significant differences were observed in the rates of ≥ 24-h ROSC (67% vs. 60%, p = 0.73), ≥ 72-h ROSC (67% vs. 55%, p = 0.56), and survival to hospital discharge (67% vs. 55%, p = 0.56) between the groups administered an esmolol bolus for defibrillation attempts ≤ 5 and defibrillation attempts > 5.
Conclusion: IHCA patients with refractory shockable rhythms receiving esmolol bolus exhibited a high chance of sustained ROSC and survival to hospital discharge. Patients with end-stage heart failure tended to have attenuated benefits from beta-blockers. Further large-scale, prospective studies are necessary to determine the effects of esmolol in patients with IHCA with refractory shockable rhythms.
1 Introduction
Despite extensive research on cardiac arrest (CA), the morbidity and mortality of in-hospital cardiac arrest (IHCA) remain high. Ventricular fibrillation (VF)/pulseless ventricular tachycardia (pVT) is estimated to be the initial rhythm in approximately 15.6% of cases of sudden death in China (Zeng et al., 2013; Shao et al., 2016). The current consensus is that short intervals from collapse to the first defibrillation attempt may significantly improve survival after CA due to VF/pVT (Valenzuela et al., 2000). However, VF/pVT that remains refractory to the first few delivered shocks is associated with a poorer prognosis (Herlitz et al., 1997a). Indeed, the mortality of refractory shockable rhythms is between 86% and 97% (Herlitz et al., 1997b; Allegra et al., 2001; Dorian et al., 2002).
In addition to high-quality cardiopulmonary resuscitation (CPR) and early defibrillation, current ACLS algorithms for the management of VF/pVT recommend the sequential administration of epinephrine. Epinephrine predominantly exerts its effects via α-adrenergic signaling to improve coronary perfusion pressure (CPP), which is associated with an increased incidence of return of spontaneous circulation (ROSC). Moreover, β-adrenergic properties are likely to reduce the threshold of fatal arrhythmia (Bourque et al., 2007; Gough and Nolan, 2018). Thus, the utility of epinephrine in patients with CA remains controversial.
Abundant evidence from animal studies indicates that blocking the beta effects of the high catecholamine concentrations provides significantly better outcomes during CA from refractory VF/pVT (Nademanee et al., 2000; Bourque et al., 2007; Jingjun et al., 2009; Bassiakou et al., 2009; Andersen and Granfeldt, 2018; Aves et al., 2020). Only a limited number of case reports (the largest number of cases of patients with CA was 15) (Driver et al., 2014; Lee et al., 2016) have reported the successful use of esmolol in OHCA patients with refractory shockable rhythms. Due to poor awareness among physicians using esmolol in patients with CA and the difficulty of including such patients, there has been a lack of clear application status of esmolol among in-hospital patients with refractory VF/pVT and a paucity of established standard protocols for managing these patients in China. Since 2018, our team has been investigating patients with CA with refractory shockable rhythms and has administered esmolol as a rescue therapy after the sequential administration of epinephrine and anti-arrhythmic agents. This study aimed to report on a retrospective series of 29 IHCA patients with refractory VF/pVT treated with esmolol as a supplementary agent at our institution and within the network of baseline investigation of patients with CA in China (BASIC, NCT03926325) between January 2018 and December 2021. Our findings may provide useful information for future clinical trials. To the best of our knowledge, this is the first case series analysis on the efficacy of esmolol administration for patients with IHCA with refractory shockable rhythms in China.
2 Patients and methods
2.1 Patient eligibility
The files of patients administered esmolol by intravenous injection for refractory VF/pVT between January 2018 and December 2021 were reviewed. We identified 29 CA patients from IHCA with refractory shockable rhythms defined as VF/pVT that persisted after at least three defibrillation attempts treated with esmolol at China-Japan Friendship Hospital, Beijing (10 patients), and all patients were included in the database of the BASIC network by other six institutions (The First Affiliated Hospital of Dalian Medical University, seven patients; Zhejiang Provincial People’s Hospital, three patients; The First Affiliated Hospital of Harbin Medical University, two patients; XiangTan Central Hospital, two patients; Qilu Hospital of Shandong University, four patients; The First Hospital of Lanzhou University, one patient). As all patients with COVID-19 in China are admitted to designated infectious disease hospitals or mobile cabin hospitals, which were not included in the database of the BASIC network, no patients with COVID-19 were included in this study. Patients with CA with refractory VF/pVT were included based on the following criteria: 1) patients aged ≥18 years; 2) detailed medical records were available, including records of ACLS procedures, electrocardiogram, lab tests, and radiological images; 3) the initial rhythm was VF or VT refractory to at least three defibrillation attempts, including defibrillation after administration of standard ACLS medications; 4) received esmolol during CA. Exclusion criteria were 1) esmolol was not administered during the period of CA (including those who received esmolol before or after CA); 2) medical records were insufficient to analyze treatment outcomes; 3) recent medication history of beta-blockers or anti-arrhythmic medications. Informed consent forms for the use of clinical data for medical research were signed by each patient or their family members. The study protocol was approved by the local ethics committee.
2.2 Clinical assessments
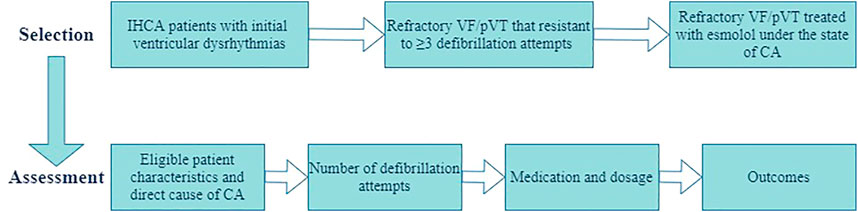
The patient selection and assessment are presented in Figure 1. Data collected from patient records included patient characteristics and direct cause of CA, reported epinephrine and anti-arrhythmic drug use, number of defibrillations, treatment modalities, duration of resuscitation, and outcomes. Response to treatment was assessed with the criteria for evaluating ROSC based on the 2015 American Heart Association guidelines. Efficacy assessment included sustained ROSC (≥20 min of spontaneous circulation without recurrence of CA), ≥24-h ROSC, ≥72-h ROSC, and survival to hospital discharge.
2.3 Treatment
Patients received esmolol after obtaining verbal informed consent from their proxies during the resuscitative effort. Written informed consent was obtained after the resuscitation. Esmolol was initially bolus injected intravenously to achieve a loading dose, followed by continuous infusion.
2.4 Statistical analysis
Descriptive statistics were used to summarize patient characteristics. Values are expressed as medians and interquartile ranges (IQRs). Intergroup differences were evaluated using the independent two-sample t test, chi-square test, or Fisher’s exact test. All p values that are equal to 0.05 or less were considered statistically significant. Analyses were performed using SPSS software (version 22.0; SPSS Inc., Chicago, IL, United States).
3 Results
3.1 Patients
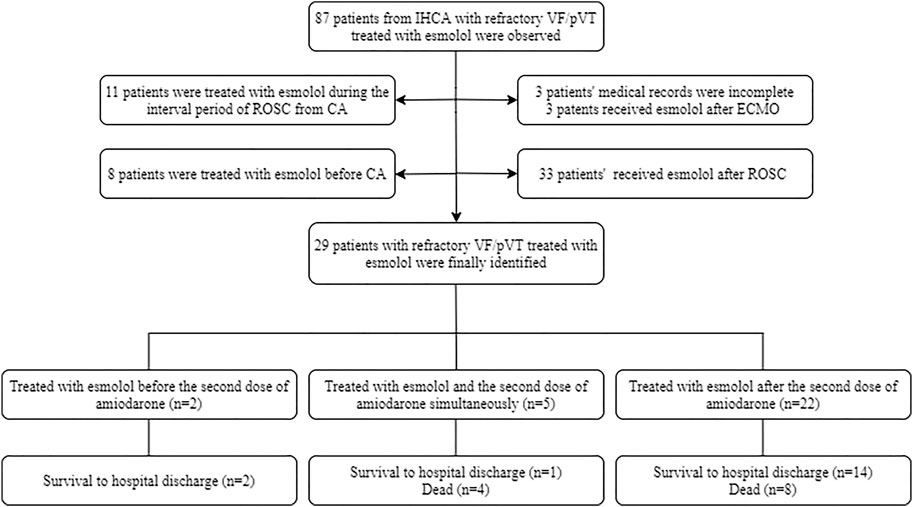
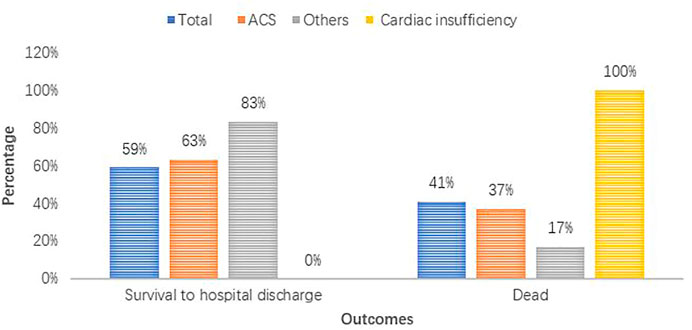
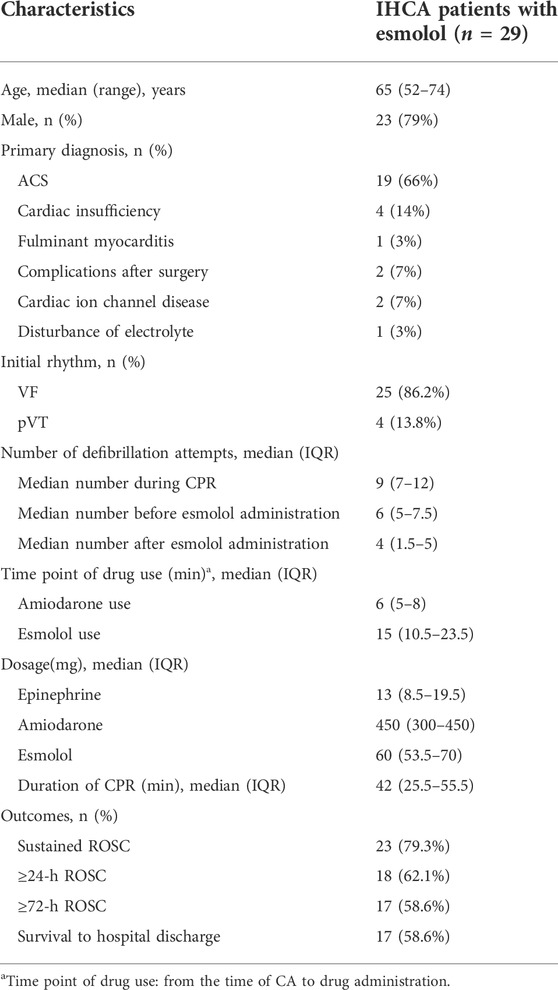
At the start of the study, a total of 87 patients from IHCA with refractory shockable rhythms treated with esmolol were observed. Of those patients, three opted to use esmolol after establishing extracorporeal membrane oxygenation (ECMO). Three patients had incomplete medical records, and 11 patients were treated with esmolol during the interval period of ROSC from CA. Eight patients selected esmolol therapy before CA, and 33 patients received esmolol after ROSC. In total, 29 patients with persistent or recurrent VF/pVT received esmolol treatment in the real absence of spontaneous circulation conditions, comprising 25 patients with persistent VF/pVT during CPR and four patients with recurrence after standard ACLS. Therefore, these 29 cases were included in the final analysis. The patient flowchart and enrolment are presented in Figure 2. Of the 29 cases, 15 (52%), 6 (21%), 3 (14%), 4 (10%), and 1 (3%) were reported from the emergency room (ER), cardiac intensive care unit (CCU), cardiology, intensive care unit (ICU), and operation room (OR), respectively (Supplementary Figure S1). At baseline, all 29 patients who were not previously treated with beta-blockers experienced IHCA and initially received standard manual CPR by first responders. Of these patients, 19 were diagnosed with acute coronary syndrome (ACS), four with cardiac insufficiency (two with ischemic cardiomyopathy and two with dilated cardiomyopathy), three had complications after valve replacement, two had cardiac ion channel disease, and one had severe electrolyte disturbance. Patient outcomes for different diagnoses are shown in Figure 3. The median age of patients who received esmolol during CPR was 65 years (IQR: 52–74 years), with an unequal sex distribution (79.3% were men and 20.7% were women). The median number of defibrillation attempts during CPR was nine (IQR: 7–12) times. The median number of defibrillations before and after esmolol administration was six (IQR: 5–7.5) and four (IQR: 1.5–5) times, respectively. The median time between CA and the first dose of traditional anti-arrhythmic agents was 6 (IQR: 5–8) min. The median time between CA and initiation of esmolol bolus was 15 min (IQR: 10.5–23.5 min). The median doses of epinephrine, amiodarone, and esmolol were 13 (IQR: 8.5–19.5) mg, 450 (IQR: 300–450) mg, and 60 (IQR: 53.5–70) mg, respectively. Patient characteristics are listed in Table 1. All patients had documented VF/pVT as their initial rhythm before starting esmolol treatment.
3.2 Treatment
Most patients had received recommended doses of adrenaline and anti-arrhythmic agents according to the ACLS protocol but still had a rhythm of VF/pVT at the time of esmolol administration. Of those patients, seven (24%) were administered an esmolol bolus concurrently or before the second dose of amiodarone, while the remaining 22 (76%) were administered esmolol after the second dose of amiodarone. All patients received an esmolol loading dose and infusion at 500–1000 and 0–100 mcg/kg/min, respectively. The 29 patients were divided into a survival group (SG) and a dead group (DG) for further comparison (Table 2). Age, sex, anti-arrhythmic drug dose, and number of defibrillation attempts were similar between groups (Table 2). CPR to esmolol duration was significantly shorter in the SG than in the DG (12 min, IQR: 8.5–19.5 vs. 23.5 min, IQR: 14.4–27 min; p = 0.013). No difference was observed in amiodarone and esmolol doses between SG and DG. In contrast, the DG received higher dosages of total epinephrine (18 mg, IQR: 10.3–24.5 mg) and epinephrine before esmolol administration (9 mg, IQR: 7–13.75 mg) than the SG (total epinephrine was 11 mg, IQR: 7–17 mg; epinephrine before esmolol administration was 5 mg, IQR: 2.5–8.5 mg) throughout CPR (p < 0.05).
3.3 Efficacy of esmolol
In the 29 esmolol-treated patients, ≥72-h ROSC and survival to hospital discharge were both achieved in 17 cases (59%). Of those 29 cases, 18 (62%) exhibited ≥24-h ROSC. The majority of patients achieved at least 20 min ROSC (79%). The overall survival rate was 59%.
Of the 23 patients with sustained ROSC, the median interval from the start of esmolol bolus to the observation of ROSC was 15 (IQR: 10.5–23.5) min. All four patients with cardiac insufficiency ultimately died (100%). The corresponding numbers of patients diagnosed with ACS and others were 7 (37%) and 1 (17%), respectively. The proportion of acute coronary syndrome was higher in the SG (76% vs. 58%, p = 0.422). The characteristics of six patients who did not achieve sustained ROSC are presented in Supplementary Table S1. Of the six patients, three (50%) were finally diagnosed with ST-segment elevation myocardial infarction (STEMI), while the other three (50%) were diagnosed with cardiac insufficiency. Of those patients with cardiac insufficiency, 75% failed to achieve sustained ROSC during the first 20 min of resuscitation.
The groups with different timings of esmolol administration are presented in Figures 4 and 5. Of the seven patients who started esmolol administration concurrently or before the second dose of amiodarone, three (43%) achieved 72-h ROSC and survival to hospital discharge, two (29%) were diagnosed with cardiac insufficiency caused by dilated cardiomyopathy, and two were (29%) diagnosed with acute extensive anterior STEMI and eventually died. Of the 22 patients who started esmolol administration after the second dose of amiodarone, 14 (64%) achieved 72-h ROSC and survival to hospital discharge, and eight (36%) eventually died. Among the eight patients who died, five (63%), two (25%), and one (12%) suffered from large myocardial infarction, cardiac insufficiency, and postsurgical complications, respectively. No significant difference was observed in the survival rate between the two groups (43% vs. 64%, p = 0.403). Of those 29 patients, nine were administered an esmolol bolus when defibrillation attempts ≤ 5, while the remaining 20 received an esmolol injection after the fifth defibrillation attempt. In the group administered an esmolol bolus when defibrillation attempts ≤ 5, one patient died within 20 min, and three other patients died within 24 h. Of the nine patients in the group administered an esmolol bolus given when defibrillation attempts≤ 5, the median duration from CA to esmolol infusion was 8 (range: 6–24) min. Of the 20 patients administered an esmolol bolus after the fifth defibrillation attempt, 15 had sustained ROSC, 12 had ≥24-h ROSC, and 11 survived to discharge. The median interval from CA to esmolol infusion was significantly shorter for the group with defibrillation attempts ≤ 5 than for these 20 patients (p = 0.011). Patients who received an esmolol bolus when defibrillation attempts ≤ 5 exhibited a trend for higher rates of ≥ 24-h ROSC, ≥ 72-h ROSC, and survival to hospital discharge compared to the defibrillation attempts ≥ 5 group (67% vs. 60%, 67% vs. 55%, and 67% vs. 55%, respectively) (Figure 5), but these differences did not reach statistical significance due to the small sample size.
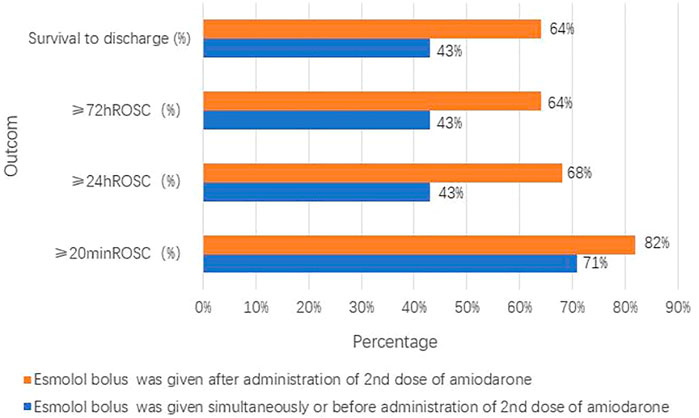
FIGURE 4. Groups with different timings of esmolol administration compared with the timing of amiodarone administration.
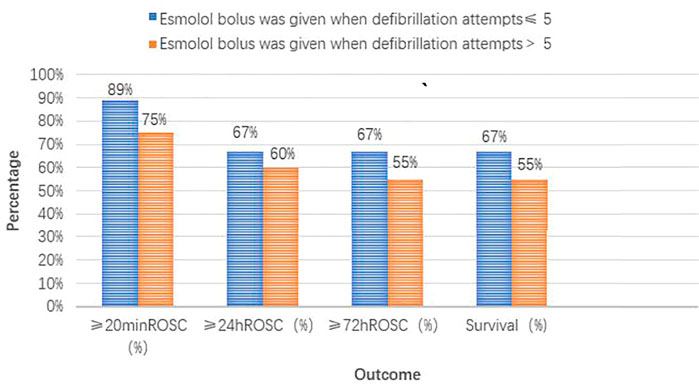
FIGURE 5. Different timing groups of esmolol administration (compared with defibrillation attempt timing).
4 Discussion
There is currently a lack of effective treatments for refractory VF/pVT, which is considered a critical status (Shih et al., 2007; Sakai et al., 2010; Reynolds et al., 2013; Soar et al., 2019). Our study demonstrated that administration of an esmolol bolus to patients with IHCA with refractory shockable rhythms may result in good clinical outcomes.
4.1 Medicinal agents in advanced cardiovascular life support
4.1.1 Epinephrine
The PARAMEDIC-2 study results (Perkins et al., 2018) resulted in the emergence of controversy regarding the benefits of epinephrine for CA patients with both shockable and non-shockable initial rhythms (Tang et al., 1995; Niemann James and Garner, 2005; Bourque et al., 2007; Eifling et al., 2011; Gao and Sapp, 2013; Gough and Nolan, 2018; Jasmeet, 2020). The possibility of a refractory shockable rhythm depends predominantly on excessive endogenous catecholamines, sympathetic activation, and deleterious effects of β-adrenoceptor stimulation (Tang et al., 1995; Eifling et al., 2011; Gao and Sapp, 2013). In this case series of refractory VF/pVT, we observed that total epinephrine dosage and dosage of epinephrine before esmolol administration were significantly higher in the DG (p < 0.05). These data highlight the difficulties of managing individuals with refractory VF/pVT arrest due to a poor response to conventional resuscitation maneuvers including cumulated dosage of epinephrine. Therefore, aggressive therapies for CA are urgently needed.
4.1.2 Anti-arrhythmic agents
Current guidelines recommend intravenous anti-arrhythmic agents (amiodarone and lidocaine) for the management of VF/pVT. However, our study demonstrated that 22 of 29 patients with CA (76%) did not respond to the second dose of amiodarone (Figure 1). Moreover, both the ARREST (Kudenchu et al., 1999) and ALIVE trials (Dorian et al., 2002) failed to confirm the effects of these agents on long-term survival. In these circumstances, clinicians often face challenges due to limited evidence-based treatment options.
4.1.3 Other interventions
Several studies (Hassan et al., 2002; Harayama et al., 2014) have suggested new medicinal agents as an adjunct to existing ACLS procedures. However, none of these proposed drugs such as magnesium sulfate and nifekalant is sufficient to reverse a “shock-resistant” critical state. Notably, using extracorporeal CPR (ECPR) has recently been reported as a promising intervention in patients with refractory VF/pVT (Siao et al., 2015; Stub et al., 2015). However, ECPR is not routinely available and is restricted due to cost, complications, local medical conditions, and patients’ characteristics.
4.2 β-blockade administration
Since the mid-1990s, studies (Ditchey et al., 1992; Ditchey and Slinker, 1994; Killingsworth et al., 2004; Bassiakou et al., 2008) have reported that the administration of propranolol/esmolol/atenolol during CPR significantly decreased the fibrillating myocardium contraction force, thus reducing myocardial oxygen requirements. Moreover, several animal studies (Theochari et al., 2008; Jingjun et al., 2009; de Oliveira et al., 2012; Laura et al., 2021) have confirmed that higher rates of temporary ROSC, sustained ROSC, and neuroprotection were achieved using β-blockade. However, few studies (Driver et al., 2014; Lee et al., 2016) focusing on the use of esmolol in real clinical scenarios are available. In addition, concerns (Laurent et al., 2002; Hwang et al., 2019) regarding potential adverse negative inotropic effects associated with β-blockers on post-resuscitation myocardial function have emerged. In relation to these deleterious effects, Strohmenger et al. (1999) reported that myocardial function indices were lower in the epinephrine group than in the placebo group only in the first 5 min after ROSC. The distinguishing feature of esmolol is its rapid onset and short duration of action, which allows for a rapid return to pre-arrest cardiac function. Thus, most clinical cases (Nademanee et al., 2000; Driver et al., 2014; Lee et al., 2016; Gottlieb et al., 2019; Manogaran and Yang, 2020) have attempted to use esmolol with a 300–500 mcg/kg loading dose after the failure of standard CPR.
Despite the rarity of such situations (Gottlieb et al., 2019; Manogaran and Yang, 2020) in real-world settings, we retrospectively collected data on 29 patients with IHCA treated with esmolol. In our study, 74% (17 of 23) of patients with sustained ROSC survived long-term, which is consistent with the rate of 75% (3 of 4) reported by Driver et al. (2014) but was higher than the findings of Lee et al. (2016) (3 of 9; 33%). This difference may be related to the occurrence location of CA, absence or presence of witness by the first responder, and quality of basic CPR, since cases in our study were all witnessed IHCA cases provided from large tertiary hospitals. Overall, we observed a 59% survival to discharge rate, which was higher than the reported average survival (Sakai et al., 2010). Moreover, over two-thirds of patients had experienced more than five defibrillation attempts before receiving an esmolol bolus in our study. This indicates that esmolol was used as definite salvage therapy in our study. With regard to the significantly higher number of defibrillation attempts in our study, the outcome of higher survival rates is promising.
4.3 Administration time of β-blockade
Despite the small sample size of both groups, the interval from CA to esmolol infusion was shorter in the SG than in the DG. This may be related to the higher number of defibrillation attempts, resulting in temporary or permanent myocardial damage (Walcott et al., 2003; Lin et al., 2006). These data indicate that the optimal time of esmolol bolus may be appropriately shifted to an earlier timepoint in cases of poor prognosis caused by prolonged cardioplegia state and ischemia/hypoxia injury. Moreover, no difference was identified in sustained ROSC and survival rate between the group administered an esmolol bolus simultaneously or before the second dose of amiodarone and the group administered an esmolol bolus after the second dose of amiodarone. This indicates that the failure to respond to traditional anti-arrhythmia agents prior to esmolol bolus administration did not preclude sustained ROSC or survival to discharge.
4.4 Etiologies of in-hospital cardiac arrest with refractory shockable rhythms
The final diagnosis of 29 cases needs to be highlighted. ACS was the most common etiology in our study, accounting for 66% of cases. This result is in accordance with the study of 5,516 sudden death autopsy cases in China recently reported by Zhao et al. (2020). Among our cases, more than 70% of the etiologies could be attributed to ACS and cardiac ion channel disease. A potential explanation is that shock-resistant rhythms occur most often in individuals with coronary artery disease and inherited arrhythmic syndromes (Polentini et al., 2006; Peichl et al., 2021). Of the four cardiac insufficiency cases, two (50%) failed to achieve sustained ROSC and the other two did not survive to discharge, suggesting that end-stage heart failure is associated with poorer clinical outcomes and may not be a reasonable indication for beta-blocker use (Park et al., 2022). These results highlight candidates that would gain the most benefit from esmolol administration during CA.
4.5 Limitations
This study has several limitations. It was a retrospective observation, and the population sample was small. Furthermore, clinicians in China may hesitate to take the risk of using beta-blockers in patients with a complete absence of spontaneous circulation. Selection bias and unduly favorable outcomes may have occurred due to non-consecutive enrollment. Moreover, the optimal esmolol administration timepoint and precise patient population that would benefit remain inconclusive.
5 Conclusion
Refractory VF/pVT, resistant to conventional CPR, is associated with a high mortality rate. In our observation of 29 IHCA patients with refractory VF/pVT who received esmolol, the success rates of sustained ROSC, 24 h ROSC, 72 h ROSC, and survival to hospital discharge were 79%, 62%, 59%, and 59%, respectively. However, there were no significant differences in the rates of ≥24-h ROSC, ≥72-h ROSC, and survival to hospital discharge between the group administered an esmolol bolus when defibrillation attempts ≤ 5 and the group with defibrillation attempts > 5. Patients with end-stage heart failure tended to have attenuated benefits from beta-blockers. Further prospective studies of β-blockade in patients with CA are warranted.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the institutional review board and research ethics committee of China-Japan Friendship Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
GqZ contributed to the conception and design of the study. RuL, GcZ, SY, and RH contributed to the writing and data collection. JY, XW, and RyL contributed to funding acquisition and methodology. LS, GC, and WG contributed to validation and software. GL and YW contributed to the visualization.
Funding
This study was supported by the National High Level Hospital Clinical Research Funding of China (2022-NHLHCRF-YS-03-02). Improvement of standardized training quality for residents programme of China-Japan friendship hospital (2022-JY-03).
Acknowledgments
We are grateful to the National Health Commission, which funded this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.930245/full#supplementary-material
References
Allegra, J., Lavery, R., Cody, R., Birnbaum, G., Brennan, J., HArtmAn, A., et al. (2001). Magnesium sulfate in the treatment of refractory ventricular fibrillation in the prehospital setting. Resuscitation 49, 245–249. doi:10.1016/s0300-9572(00)00375-0
Andersen, L. W., and Granfeldt, A. (2018). Epinephrine in cardiac arrest: Insights from observational studies. Resuscitation 131, e1. doi:10.1016/j.resuscitation.2018.07.028
Aves, T., Chopra, A., Patel, M., and Lin, S. (2020). Epinephrine for out-of-hospital cardiac arrest: An updated systematic review and meta-analysis. Crit. Care Med. 48, 225–229. doi:10.1097/CCM.0000000000004130
Bassiakou, E., Xanthos, T., Koudouna, E., Goulas, S., Prapa, V., Papadimitriou, D., et al. (2008). Atenolol in combination with epinephrine improves the initial outcome of cardiopulmonary resuscitation in a swine model of ventricular fibrillation. Am. J. Emerg. Med. 26, 578–584. doi:10.1016/j.ajem.2007.09.010
Bassiakou, E., Xanthos, T., and Papadimitriou, L. (2009). The potential beneficial effects of beta adrenergic blockade in the treatment of ventricular fibrillation. Eur. J. Pharmacol. 616, 1–6. doi:10.1016/j.ejphar.2009.06.019
Bourque, D., Daoust, R., Huard, V., and Charneux, M. (2007). beta-Blockers for the treatment of cardiac arrest from ventricular fibrillation? Resuscitation 75, 434–444. doi:10.1016/j.resuscitation.2007.05.013
de Oliveira, F. C., Feitosa-Filho, G. S., and Ritt, L. E. (2012). Use of beta-blockers for the treatment of cardiac arrest due to ventricular fibrillation/pulseless ventricular tachycardia: A systematic review. Resuscitation 83, 674–683. doi:10.1016/j.resuscitation.2012.01.025
Ditchey, R. V., and Slinker, B. K. (1994). Phenylephrine plus propranolol improves the balance between myocardial oxygen supply and demand during experimental cardiopulmonary resuscitation. Am. Heart J. 127, 324–330. doi:10.1016/0002-8703(94)90120-1
Ditchey, R. V., Goto, Y., and Lindenfeld, J. (1992). Myocardial oxygen requirements during experimental cardiopulmonary resuscitation. Cardiovasc. Res. 26, 791–797. doi:10.1093/cvr/26.8.791
Dorian, P., Cass, D., Schwartz, B., Cooper, R., Gelaznikas, R., and Barr, A. (2002). Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation. N. Engl. J. Med. 346, 884–890. doi:10.1056/NEJMoa013029
Driver, B. E., Debaty, G., Plummer, D. W., and Smith, S. W. (2014). Use of esmolol after failure of standard cardiopulmonary resuscitation to treat patients with refractory ventricular fibrillation. Resuscitation 85 (10), 1337–1341. doi:10.1016/j.resuscitation.2014.06.032
Eifling, M., Razavi, M., and Massumi, A. (2011). The evaluation and management of electrical storm. Tex. Heart Inst. J. 38, 111–121.
Gao, D., and Sapp, J. L. (2013). Electrical storm: Definitions, clinical importance, and treatment. Curr. Opin. Cardiol. 28, 72–79. doi:10.1097/HCO.0b013e32835b59db
Gottlieb, M., Dyer, S., and Peksa, G. D. (2019). Beta-blockade for the treatment of cardiac arrest due to ventricular fibrillation or pulseless ventricular tachycardia: A systematic review and meta-analysis. Resuscitation 146, 118–125. doi:10.1016/j.resuscitation.2019.11.019
Gough, C. J. R., and Nolan, J. P. (2018). The role of adrenaline in cardiopulmonary resuscitation. Crit. Care 22, 139. doi:10.1186/s13054-018-2058-1
Harayama, N., Nihei, S., Nagata, K., Isa, Y., Goto, K., Aibara, K., et al. (2014). Comparison of nifekalant and amiodarone for resuscitation of out-of-hospital cardiopulmonary arrest resulting from shock-resistant ventricular fibrillation. J. Anesth. 28, 587–592. doi:10.1007/s00540-013-1775-5
Hassan, T. B., Jagger, C., and Barnett, D. B. (2002). A randomised trial to investigate the efficacy of magnesium sulphate for refractory ventricular fibrillation. Emerg. Med. J. 19, 57–62. doi:10.1136/emj.19.1.57
Herlitz, J., Bang, A., Holmberg, M., and Axelsson, A. (1997a). Rhythm changes during resuscitation from ventricular fibrillation in relation to delay until defibrillation, number of shocks delivered and survival. Resuscitation 34, 17–22. doi:10.1016/s0300-9572(96)01064-7
Herlitz, J., Ekstrom, L., Wennerblom, B., Axelsson, A., Bang, A., Lindkvist, J., et al. (1997b). Lidocaine in out of-hospital ventricular fibrillation. Does it improve survival? Resuscitation 33, 199–205. doi:10.1016/s0300-9572(96)01018-0
Hwang, C. W., Gamble, G., Marchick, M., and Becker, T. K. (2019). A case of refractory ventricular fibrillation successfully treated with low-dose esmolol. BMJ Case Rep. 12 (3), e228208. doi:10.1136/bcr-2018-228208
Jasmeet, S (2020). Epinephrine for cardiac arrest: Knowns, unknowns and controversies. Curr. Opin. Crit. Care 26, 590–595. doi:10.1097/MCC.0000000000000763
Jingjun, L., Yan, Z., Jie, W., Dongdong, Z., Guosheng, L., and Mingwei, B. (2009). Effect and mechanism of esmolol given during cardiopulmonary resuscitation in a porcine ventricular fibrillation model. Resuscitation 80, 1052–1059. doi:10.1016/j.resuscitation.2009.06.003
Killingsworth, C. R., Wei, C. C., Dell’Italia, L. J., Ardell, J. L., Kingsley, M. A., Smith, W. M., et al. (2004). Short-acting beta-adrenergic antagonist esmolol given at reperfusion improves survival after prolonged ventricular fibrillation. Circulation 109, 2469–2474. doi:10.1161/01.CIR.0000128040.43933.D3
Kudenchu, k. P. J., Cobb, L. A., Copass, M. K., Cummins, R. O., Doherty, A. M., Fahrenbruch, C. E., et al. (1999). Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. N. Engl. J. Med. 341, 871–878. doi:10.1056/NEJM199909163411203—
Laura, R, Francesca, N, Giuseppe, R, Fumagalli, F., Boccardo, A., Olivari, D., et al. (2021). Esmolol during cardiopulmonary resuscitation reduces neurological injury in a porcine model of cardiac arrest. Sci. Rep. 11 (1), 10635. doi:10.1038/s41598-021-90202-w
Laurent, I., Monchi, M., Chiche, J. D., Joly, L. M., Spaulding, C., Bourgeois, B., et al. (2002). Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J. Am. Coll. Cardiol. 40, 2110–2116. doi:10.1016/s0735-1097(02)02594-9
Lee, Y. H., Lee, K. J., Min, Y. H., Ahn, H. C., Sohn, Y. D., Lee, W. W., et al. (2016). Refractory ventricular fibrillation treated with esmolol. Resuscitation 107, 150–155. doi:10.1016/j.resuscitation.2016.07.243
Lin, C. C., Chiu, T. F., Fang, J. Y., Kuan, J. T., and Chen, J. C. (2006). The influence of cardiopulmonary resuscitation without defibrillation on serum levels of cardiac enzymes: A time course study of out-of-hospital cardiac arrest survivors. Resuscitation 68, 343–349. doi:10.1016/j.resuscitation.2005.07.018
Manogaran, M., and Yang, S. S. (2020). Data for beta-blockade in ACLS-A trial sequential analysis. Resuscitation 150, 191–192. doi:10.1016/j.resuscitation.2020.02.019
Nademanee, K., Taylor, R., Bailey, W. E., Rieders, D. E., and Kosar, E. M. (2000). Treating electrical storm: Sympathetic blockade versus advanced cardiac life support-guided therapy. Circulation 102, 742–747. doi:10.1161/01.cir.102.7.742
Niemann James, T., and Garner, D. (2005). Post-resuscitation plasma catecholamines after prolonged arrest in a swine model. Resuscitation 65 (1), 97–101. doi:10.1016/j.resuscitation.2004.09.012
Park, C. S., Park, J. J., Hwang, I. Ch, Park, J. H., and Cho, G. Y. (2022). Myocardial strain to identify benefit from beta-blockers in patients with heart failure with reduced ejection fraction. Esc. Heart Fail. 9, 1248–1257. doi:10.1002/ehf2.13800
Peichl, Pr, Rafaj, A., and Kautzner, J. (2021). Management of ventricular arrhythmias in heart failure: Current perspectives. Heart Rhythm O2 2, 796–806. doi:10.1016/j.hroo.2021.08.007
Perkins, G. D., Ji, C., Deakin, C. D., Quinn, T., Nolan, J. P., Scomparin, C., et al. (2018). A randomized trial of epinephrine in out of hospital cardiac arrest. N. Engl. J. Med. 3 379, 711–721. doi:10.1056/NEJMoa1806842
Polentini, M. S., Pirrallo, R. G., and McGill, W. (2006). The changing incidence of ventricular fibrillation in Milwaukee, Wisconsin (1992–2002). Prehosp. Emerg. Care 10, 52–60. doi:10.1080/10903120500366961
Reynolds, J. C., Frisch, A., Rittenberger, J. C., and Callaway, C. W. (2013). Duration of resuscitation efforts and functional outcome after out-of-hospital cardiac arrest: When should we change to novel therapies? Circulation 128, 2488–2494. doi:10.1161/CIRCULATIONAHA.113.002408
Sakai, T., Iwami, T., Tasaki, O., Kawamura, T., Hayashi, Y., Rinka, H., et al. (2010). Incidence and outcomes of out-of-hospital cardiac arrest with shock-resistant ventricular fibrillation: Data from a large population-based cohort. Resuscitation 81, 956–961. doi:10.1016/j.resuscitation.2010.04.015
Shao, F., Li, C. S., Liang, L. R., Qin, J., Ding, N., Fu, Y., et al. . Incidence and outcome of adult in-hospital cardiac arrest in Beijing, China. Resuscitation. 2016;102:51–56. doi:10.1016/j.resuscitation.2016.02.002
Shih, C. L., Lu, T. C., Jerng, J. S., Lin, C. C., Liu, Y. P., Chen, W. J., et al. (2007). A web-based Utstein style registry system of in-hospital cardiopulmonary resuscitation in Taiwan. Resuscitation 72, 394–403. doi:10.1016/j.resuscitation.2006.07.020
Siao, F. Y., Chiu, C. C., Chiu, C. W., Chen, Y. C., Chen, Y. L., Hsieh, Y. K., et al. (2015). Managing cardiac arrest with refractory ventricular fibrillation in the emergency department: Conventional cardiopulmonary resuscitation versus extracorporeal cardiopulmonary resuscitation. Resuscitation 92, 70–76. doi:10.1016/j.resuscitation.2015.04.016
Soar, J., Maconochie, I., Wyckoff, M. H., Olasveengen, T. M., Singletary, E. M., Greif, R., et al. 2019, 2019 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 145:95–150. doi:10.1016/j.resuscitation.2019.10.016
Strohmenger, H. U., Wenzel, V., Eberhard, R., Guth, B. D., Lurie, K. G., and Lindner, K. H. (1999). Effects of the specific bradycardic agent zatebradine on hemodynamic variables and myocardial blood flow during the early post resuscitation phase in pigs. Resuscitation 42, 211–220. doi:10.1016/s0300-9572(99)00093-3
Stub, D., Bernard, S., Pellegrino, V., Smith, K., Walker, T., Sheldrake, J., et al. (2015). Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation 86, 88–94. doi:10.1016/j.resuscitation.2014.09.010
Tang, W., Weil, M. H., Sun, S., NocM., , Yang, L., and Gazmuri, R. J. (1995). Epinephrine increases the severity of post resuscitation myocardial dysfunction. Circulation 92, 3089–3093. doi:10.1161/01.cir.92.10.3089
Theochari, E., Xanthos, T., Papadimitriou, D., Demestiha, T., Condilis, N., Tsirikos-Karapanos, N., et al. (2008). Selective beta blockade improves the outcome of cardiopulmonary resuscitation in a swine model of cardiac arrest. Ann. Ital. Chir. 79, 409–414. doi:10.2319/0003-3219(2008)078[1153:QVAO]2.0.CO;2
Valenzuela, T. D., Roe, D. J., Nichol, G., Clark, L. L., Spaite, D. W., and Hardman, R. G. (2000). Outcomes of rapid defibrillation by security officers after cardiac arrest in casinos. N. Engl. J. Med. 343 (17), 1206–1209. doi:10.1056/NEJM200010263431701
Walcott, G. P., Killingsworth, C. R., and Ideker, R. E. (2003). Do clinically relevant transthoracic defibrillation energies cause myocardial damage and dysfunction? Resuscitation 59, 59–70. doi:10.1016/s0300-9572(03)00161-8
Zeng, J., Qian, S., Zheng, M., Wang, Y., Zhou, G., and Wang, H. (2013). The epidemiology and resuscitation effects of cardiopulmonary arrest among hospitalized children and adolescents in beijing: An observational study. Resuscitation 84 (12), 1685–1690. doi:10.1016/j.resuscitation.2013.08.007
Keywords: cardiac arrest, ventricular fibrillation, pulseless ventricular tachycardia, esmolol, return of spontaneous circulation (ROSC)
Citation: Lian R, Zhang G, Yan S, Sun L, Gao W, Yang J, Li G, Huang R, Wang X, Liu R, Cao G, Wang Y and Zhang G (2022) The first case series analysis on efficacy of esmolol injection for in-hospital cardiac arrest patients with refractory shockable rhythms in China. Front. Pharmacol. 13:930245. doi: 10.3389/fphar.2022.930245
Received: 27 April 2022; Accepted: 06 September 2022;
Published: 30 September 2022.
Edited by:
Marek Postuła, Medical University of Warsaw, PolandReviewed by:
Eduardo Sternick, Biocor Institute, BrazilDragana Lončar-Stojiljković, Institute for Cardiovascular Diseases Dedinje, Serbia
Copyright © 2022 Lian, Zhang, Yan, Sun, Gao, Yang, Li, Huang, Wang, Liu, Cao, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoqiang Zhang, emhhbmdjaG9uZzIwMDNAdmlwLnNpbmEuY29t
 Rui Lian1
Rui Lian1 Guoqiang Zhang
Guoqiang Zhang