- 1Fujian Provincial Key Laboratory of Innovative Drug Target Research, State Key Laboratory of Cellular Stress Biology, School of Pharmaceutical Sciences, Xiamen University, Xiamen, China
- 2School of Population Medicine and Public Health, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Melbourne School of Population and Global Health, The University of Melbourne, Melbourne, VIC, Australia
- 4Department of Medical Oncology, Xiamen Key Laboratory of Antitumor Drug Transformation Research, The First Affiliated Hospital of Xiamen University, Xiamen, China
- 5Department of Clinical Medicine, Fujian Medical University, Fuzhou, China
Background: Epidermal growth factor receptor (EGFR) mutations are common in patients with non-small-cell lung cancer (NSCLC), particularly in Asian populations. Tyrosine kinase inhibitors (TKIs) are a first-line treatment in patients with mutant EGFR, but their use is often accompanied by drug resistance, which leads to disease progression. Chemotherapy and immunotherapy are the main treatment options after progression. The efficacy of immune checkpoint inhibitors (ICIs) and their combination therapy in patients with EGFR-TKI resistant is not clear. It is thus necessary to evaluate the efficacy of ICIs and ICI-based combination therapies in patients with EGFR-TKI-resistant NSCLC.
Methods: We searched for randomized controlled trials (RCTs) comparing ICI therapy alone or in combination versus other therapies using PubMed, the Cochrane Library, Web of Science, EMBASE, MEDLINE, ClinicalTrials.gov, and several international conference databases, from database inception to 10 March 2022. The hazard ratio (HR) and 95% confidence interval (95% CI) for median overall survival (OS) and median progression-free survival (PFS) were evaluated. Odds ratio (OR), risk ratio (RR), and 95% CI were used as effect indicators for objective response rate (ORR) and safety data.
Results: Seven eligible RCTs were included in the present meta-analysis. The results showed that neither ICIs nor combination therapy prolonged median OS in EGFR-TKI resistant NSCLC patients (HR = 1.04, 95% CI: 0.84–1.29, p = 0.73). However, compared with the control group, the patients treated with ICI-based combination therapy had better PFS (HR = 0.62, 95% CI: 0.45–0.86, p = 0.004) and ORR (OR = 1.84, 95% CI: 1.28–2.66, p = 0.001).
Conclusion: ICI monotherapy did not improve the OS or PFS of NSCLC patients previously treated with EGFR-TKIs, whereas patients treated with ICI-based combination therapy had better PFS compared with those receiving conventional chemotherapy, indicating that this therapy could be offered to patients with EGFR-mutant NSCLC after progression following TKI treatment. There was no significant difference in all-grade treatment-related adverse events (TRAEs) between the combination therapy group and the control group. However, a higher incidence of discontinuation due to TRAEs was observed; this requires attention in future studies. The results of this meta-analysis provide a reference for clinical practice and future trial design.
PROSPERO registration number: CRD42021282207
Introduction
Lung cancer is the main cause of cancer death worldwide. In 2020, the number of deaths due to lung cancer accounted for about 18% of all cancer-related deaths (Cancer Today 2021), and they were mainly concentrated in Europe, America, and Asia. Lung cancer can be divided into small-cell lung cancer and non-small-cell lung cancer (NSCLC). NSCLC is the main type of lung cancer in Western and Asian countries, accounting for about 85% of cases (Thai et al., 2021). Mutations in driving genes are often observed in patients with advanced NSCLC. Mutation of the epidermal growth factor receptor (EGFR) gene is a common mutation type in patients with advanced NSCLC (Sabari et al., 2021), with EGFR mutation rates of 14–20% in European and American populations and about 50% in Asian populations (Dearden et al., 2013; Midha et al., 2015; Zhang et al., 2016).
With the development of targeted therapies, tyrosine kinase inhibitors targeting EGFR (EGFR-TKIs) have become a common treatment for patients with EGFR-mutant advanced NSCLC. TKI treatment has shown significant clinical efficacy compared with traditional chemotherapy. Studies have shown that EGFR-TKIs significantly prolonged the progression-free survival (PFS) and overall survival (OS) of patients (Fukuoka et al., 2011; Zhou et al., 2011; Yang et al., 2015; Greenhalgh et al., 2016; Zhao et al., 2019; Ramalingam et al., 2020), and most of these TKIs have been adopted as standard first-line treatments. However, most patients with EGFR mutation develop drug resistance after receiving TKI treatment for 10–15 months (Wu and Shih, 2018; Wu et al., 2020). The main causes of drug resistance include T790M mutation, c797x mutation, Met amplification, Axl activation, and HER2 amplification (Lim et al., 2018; Westover et al., 2018). For drug-resistant patients with the EGFR T790M mutation, third-generation TKIs are the choice of treatment. However, for resistant patients without EGFR T790M mutation, continuing TKI treatment results in no benefit or even has negative effects (Soria et al., 2015). Traditional pemetrexed-based chemotherapy is still the main treatment for such patients. However, chemotherapy often has serious adverse effects and does not lead to any significant benefit with respect to PFS in patients with EGFR-TKI resistance. Therefore, there is a very limited choice of treatments for advanced NSCLC patients with EGFR mutations and drug resistance.
Immune checkpoint inhibitors (ICIs) are a recently developed type of cancer treatment that has emerged in the past 10 years. Some studies have shown that patients with advanced NSCLC without driver gene mutation can benefit from immunotherapy (Antonia et al., 2019; Mok et al., 2019; Wu et al., 2019; Garassino et al., 2020). Immunotherapy has been used as a first-line treatment for advanced NSCLC. Studies have also shown that activation of the EGFR pathway can induce expression of PD-L1 and related immunosuppressive factors, thereby reshaping the immune microenvironment of tumors (Chen et al., 2015). Mice with EGFR mutation showed a significant response to PD-1 inhibitor treatment (Akbay et al., 2013). EGFR-TKI treatment could also reduce PD-L1 expression and T cell inhibition in patients with NSCLC, resulting in immune enhancement (Hack et al., 2020). This suggests that a combination of a PD-1 inhibitor and an EGFR-TKI may have a better curative effect than TKI alone. However, the combination of PD-1 inhibitors and TKIs as a first-line treatment regimen was shown to have enhanced toxicity with no significant improvement in curative effect (Yang et al., 2019; Oxnard et al., 2020). Therefore, the combination of ICIs and TKIs is not suitable for patients with EGFR-positive NSCLC. Cell experiments have shown that the viability of EGFR-TKI-resistant cells can be decreased by PD-1 inhibitors, which suggests that immunotherapy could be a promising follow-up treatment for patients with EGFR-TKI resistance (Chen et al., 2015).
Whether immunotherapy is suitable for advanced NSCLC patients with EGFR mutation has become a hot-button issue. In studies mentioned above, NSCLC patients with EGFR mutation have generally not benefited from immunotherapy. However, some studies have shown that a small number of patients can benefit from immunotherapy. In addition, there have been few reports on the applications of ICI-related combination therapy in EGFR-TKI-resistant patients. Therefore, the efficacy of ICIs in patients with EGFR-TKI-resistant NSCLC is unclear and needs to be further explored.
The purpose of this meta-analysis was to evaluate the efficacy of ICIs alone and in combination therapies in patients with advanced NSCLC resistant to EGFR-TKI, so as to better address the problems associated with the use of ICIs in such patients.
Methods
The present systematic review and meta-analysis was conducted following PRISMA guidelines (Page et al., 2021). The protocol has been registered in PROSPERO with the registration number CRD42021282207.
Data sources and searches
We searched PubMed, the Cochrane Library, Web of Science, EMBASE, and MEDLINE. The retrieval time limit was from the establishment of each database to 10 March 2022. In addition to the above databases, in order to include unpublished studies and obtain more comprehensive relevant research data, we searched the clinical trial registration website (https://clinicaltrials.gov/), conference abstracts of American Society of Clinical Oncology, European Society for Medical Oncology, and World Lung Cancer Conference. The keywords searched included: “non-small cell lung cancer,” “immune checkpoint inhibitors,” “tyrosine kinase inhibitor,” “epithelial growth factor receptor,” and “randomized controlled trial”. The detailed inclusion and exclusion criteria are listed in Table 1.
Study selection and data extraction
The main purpose of this meta-analysis was to evaluate the efficacy of ICIs compared with traditional chemotherapy in patients with EGFR-TKI-resistant advanced NSCLC. The subjects were NSCLC patients with EGFR mutation who experienced disease progression after TKI treatment. For each included trial, we extracted the basic characteristics of the study and participants, and retrieved the objective response rate (ORR), hazard ratio (HR), and 95% CI for median OS and median PFS.
Two researchers (Q. X. and G. X.) independently screened the literature, and extracted and cross-checked the data. Discrepancies were resolved by the third researcher. Data types extracted from the trials were: trial phase, authors, year of publication, number of patients, number of patients with EGFR mutation, age of patients, gender of patients, number of smoking patients, treatment comparison, median OS, HR and 95% CI of median OS, median PFS, HR and 95% CI of median PFS, ORR, and safety data. All study periods and follow-up durations were eligible. Titles and abstracts were screened, and the full text of each potentially eligible article was evaluated for final inclusion.
Data synthesis and analysis
Statistical analysis in this study was performed using the Cochrane Review Manager (RevMan 5.4) software provided by the Cochrane Library Collaboration Network.
The risk of bias of the study was assessed using the Cochrane bias risk assessment tool (Higgins et al., 2011), which includes the following items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias.
For the included studies, HR and 95% CI were used as effect indicators for median OS and median PFS, odds ratio (OR) and 95% CI were used as effect indicators for ORR, and risk ratio (RR) and 95% CI were used for safety data. I2 index and q-statistics were used to evaluate the heterogeneity among studies. If I2<50%, p > 0.05, indicating that the research results may be homogeneous, the fixed effect model is used for analysis. In contrast, when I2>50%, p < 0.05, indicating substantial heterogeneity, the source of heterogeneity should be analyzed. If the heterogeneity still exists, the random effect model should be used.
Results
Search results
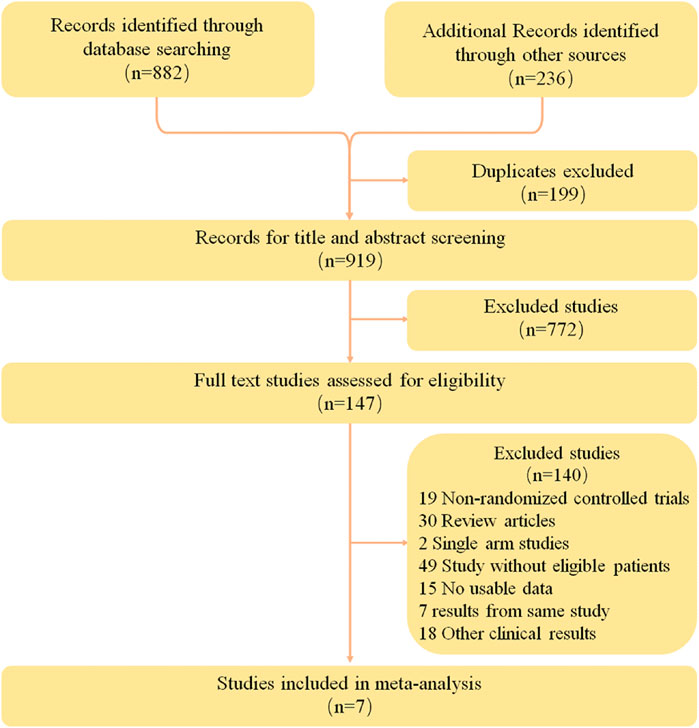
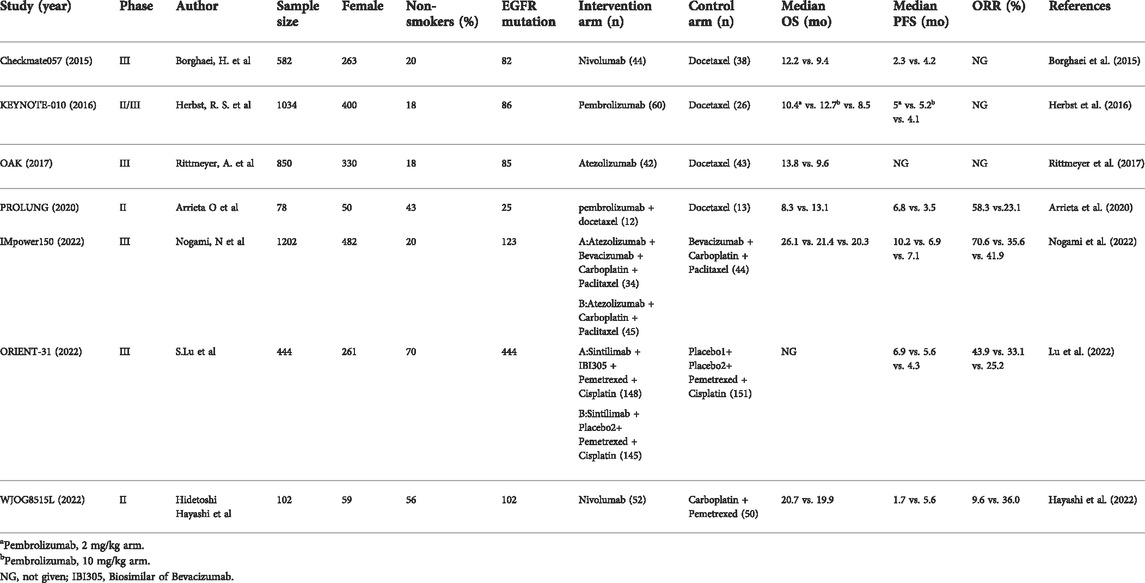
A total of 1118 relevant studies were retrieved according to the retrieval strategy. The literature was screened according to the exclusion criteria, and finally seven studies (Borghaei et al., 2015; Herbst et al., 2016; Rittmeyer et al., 2017; Arrieta et al., 2020; Hayashi et al., 2022; Lu et al., 2022; Nogami et al., 2022) were included. These studies involved a total of 4,292 patients, including 2,453 in the intervention groups and 1839 in the control groups. Data from one study (Lu et al., 2022) was obtained from relevant conference abstracts. The literature retrieval results and flow chart are shown in Figure 1, and the basic information of the included studies is shown in Table 2.
Of the included patients, a total of 902 (21.01%) had known EGFR mutations and had received previous TKI treatment, with disease progression during or after treatment. Of the seven therapeutic regimens, three (Arrieta et al., 2020; Lu et al., 2022; Nogami et al., 2022) were combination therapy, and four (Borghaei et al., 2015; Herbst et al., 2016; Rittmeyer et al., 2017; Hayashi et al., 2022) were ICI monotherapy. In the control group, three studies (Borghaei et al., 2015; Herbst et al., 2016; Rittmeyer et al., 2017) used docetaxel monotherapy, one study (Hayashi et al., 2022) used carboplatin plus pemetrexed, one study (Lu et al., 2022) used placebo plus pemetrexed and cisplatin, and one study (Nogami et al., 2022) used bevacizumab plus carboplatin and paclitaxel.
Analysis of median OS
Six of the seven studies reported the median OS of patients, and one did not. According to the results of the heterogeneity test (I2 = 0, p = 0.95) the heterogeneity among the studies was low. The results were pooled and analyzed by a fixed effect model. The results showed that there was no significant difference in median OS between the intervention group and the control group (HR = 1.04, 95% CI: 0.84–1.29, p = 0.73) (Figure 2), regardless of whether the intervention was monotherapy or combination therapy.
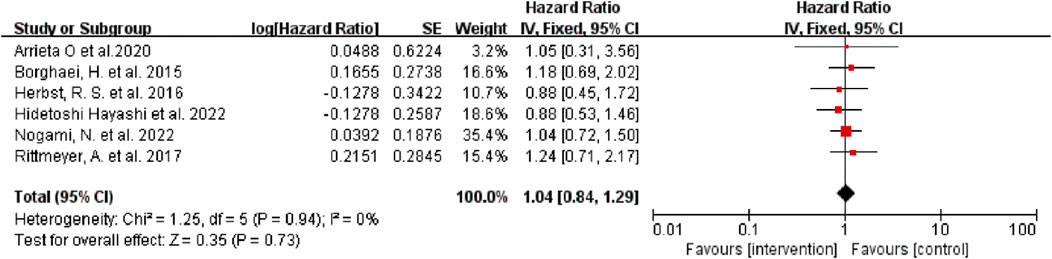
FIGURE 2. Forest Plot of Hazard Ratios Comparing OS in Patients Who Received Immune Checkpoint Inhibitors vs. Other Therapeutics.
Analysis of median PFS
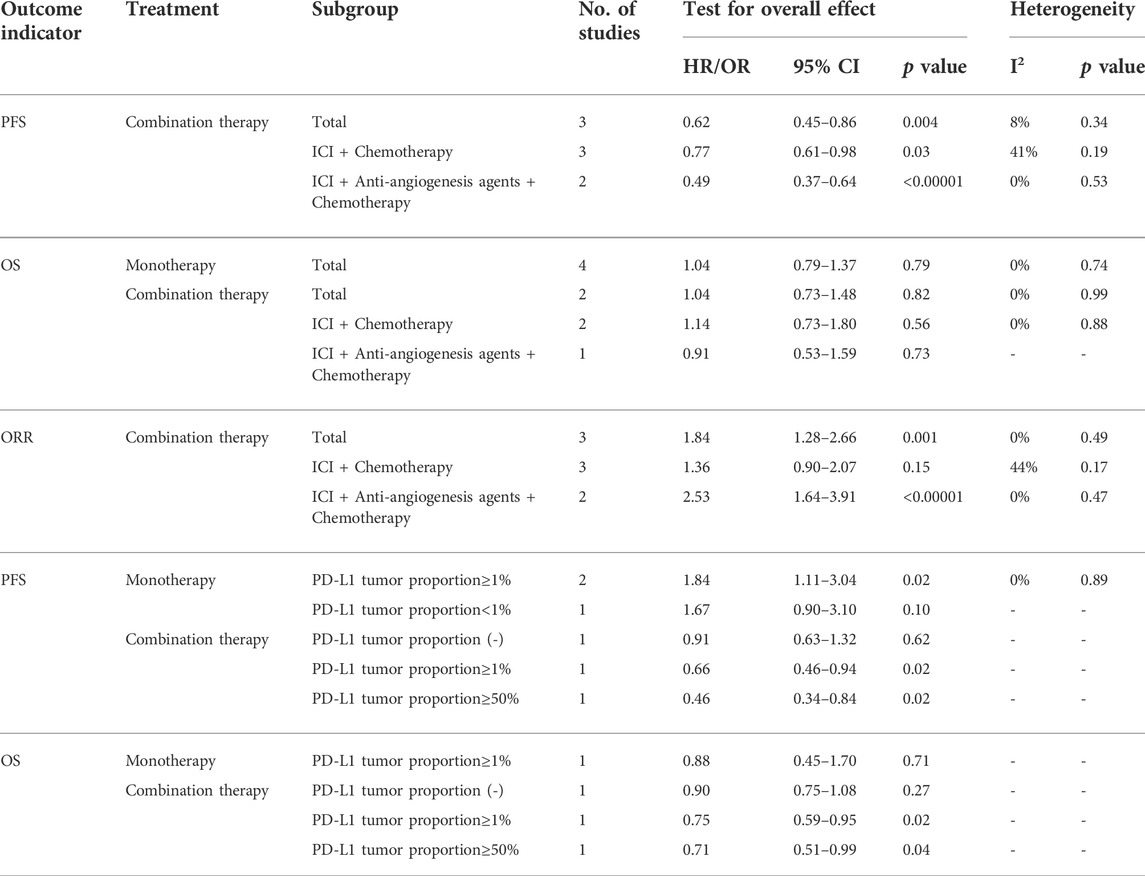
Six of the seven studies reported the median PFS of patients, and one did not. In the monotherapy group, there was no improvement in PFS, and traditional chemotherapy showed better efficacy (HR = 1.73, 95% CI:1.30–2.29, p = 0.0002) (Figure 3A). The pooled results showed that ICI-based combination therapy could prolong PFS, with a pooled HR of 0.62 (95% CI: 0.45–0.86, p = 0.004) (Figure 3B). Subgroup analysis according to the specific administration scheme showed that ICI plus chemotherapy was beneficial with respect to PFS. Notably, the effect of ICI plus anti-angiogenesis agents plus chemotherapy was better than ICI plus chemotherapy (HR = 0.49, 95% CI: 0.37–0.64, p < 0.00001) (Figure 3C).

FIGURE 3. Forest Plot of HR Comparing PFS in Patients Who Received ICIs vs. Other Therapeutics. (A) Summary Hazard Ratios in monotherapy group. (B) Summary Hazard Ratios in ICI-based combination therapy group. (C) Subgroup analysis according to administration scheme in ICI-based combination therapy group.
Analysis of ORR
Four of the seven studies reported the median PFS of patients, and three did not. Only one article reported ORR in the monotherapy group, so it was impossible to calculate the ORR of monotherapy. We only conducted a meta-analysis on the ORR of the combination group (Figure 4).
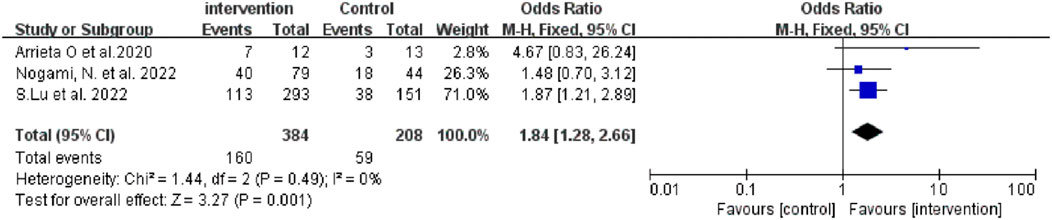
FIGURE 4. Forest Plot of OR Comparing ORR in Patients Who Received ICI-based combination therapy vs. Other Therapeutics.
The results showed that ICI-based combination therapy was associated with better ORR than the control treatment (OR = 1.84, 95% CI: 1.28–2.66, p = 0.001). Subgroup analysis showed that ICI plus chemistry was beneficial with respect to ORR, but this benefit was not statistically significant. ICI plus anti-angiogenesis agents plus chemotherapy had better efficacy than ICI plus chemotherapy (Table 3).
Analysis of safety
We investigated all-grade treatment-related adverse events (TRAEs), grade ≥3 TRAEs, and TRAEs leading to discontinuation of included studies. Compared with traditional chemotherapy, ICI monotherapy did not cause more serious adverse reactions, whether all-grade TRAEs (RR = 0.78, 95% CI: 0.75–0.82, p < 0.00001), grade ≥3 TRAEs (RR = 0.34, 95% CI: 0.24–0.47, p < 0.00001), or TRAEs leading to discontinuation (RR = 0.55, 95% CI: 0.33–0.90, p = 0.02) (Figure 5A). There was no significant difference in the frequency of all-grade TRAEs (RR = 1.01, 95% CI: 0.96–1.06, p = 0.70) and grade ≥3 TRAEs (RR = 1.01, 95% CI: 0.84–1.20, p = 0.95) between the combination therapy and chemotherapy groups; however, combination therapy was associated with more TRAEs leading to discontinuation (RR = 2.00, 95% CI: 1.18–3.40, p = 0.01) (Figure 5B).
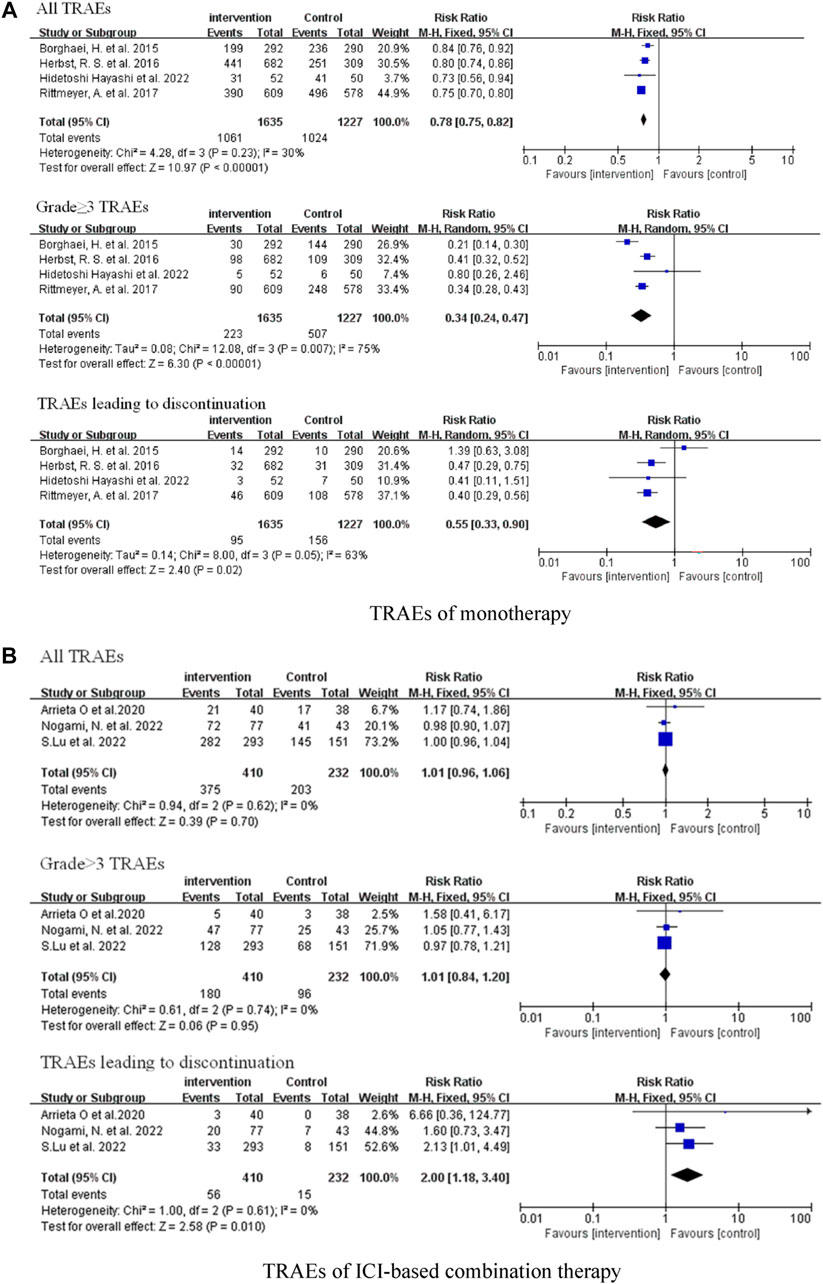
FIGURE 5. Forest Plot of Overall Comparison of TRAEs. (A) TRAEs of monotherapy. (B) TRAEs of ICI-based combination therapy.
Heterogeneity and bias of included studies
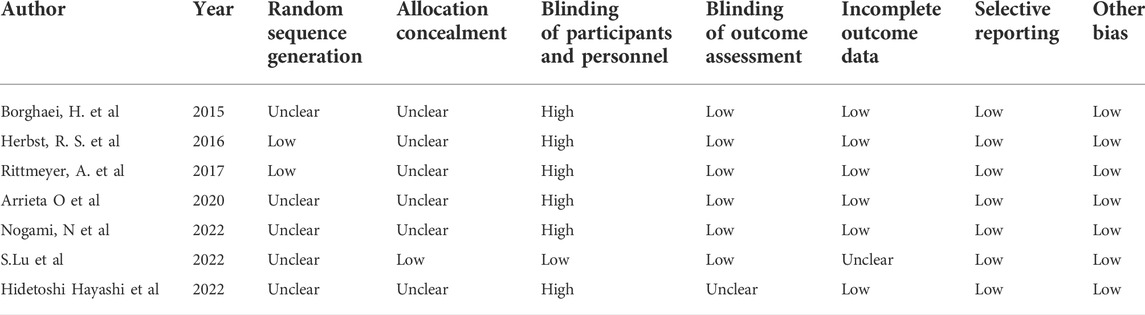
As shown in Figures 2–4, there was low heterogeneity with respect to median OS (I2 = 0%, p = 0.94), median PFS (I2 = 0%, p = 0.70 for monotherapy; I2 = 8%, p = 0.34 for combination therapy) and ORR (I2 = 0%, p = 0.49) among the included studies. The results obtained with the Cochrane risk-of-bias assessment tool for the seven enrolled RCTs are shown in Figure 6; Table 4.
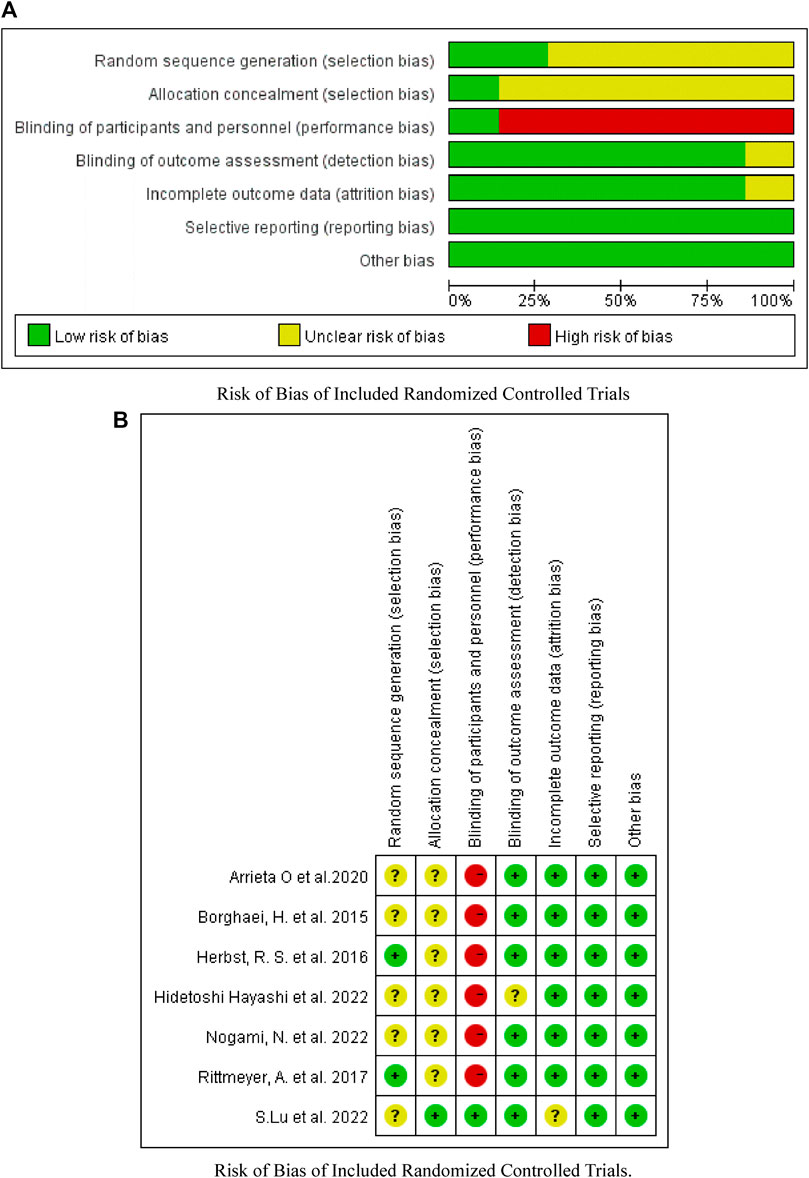
FIGURE 6. Assessment of bias risk. (A) Risk of Bias of Included Randomized Controlled Trials. (B) Risk of Bias of Included Randomized Controlled Trials.
Discussion
This meta-analysis showed that in NSCLC patients with disease progression or recurrence after TKIs treatment, immunotherapy as second-line or third-line treatment confers no benefit with respect to OS and PFS compared with chemotherapy. However, chemotherapy resulted in better PFS in the patients with TKI treatment progress, consistent with previous research results (Lee et al., 2017; Cavanna et al., 2019). ICI-based combination therapy showed better results as a second-line or third-line treatment with respect to PFS. A combination of a PD-1 inhibitor, antiangiogenic agents, and chemotherapeutic agents significantly improved the PFS and ORR of patients with EGFR mutation, suggesting that combination therapy may be an option for EGFR-TKI-resistant patients. Although combined immunotherapy and chemotherapy performed well in terms of PFS, there was no significant advantage in the ORR analysis. Although the analysis results tended to be combined therapy, it was not statistically significant. Owing to the limited number of studies included, further examination is needed in the future to determine the efficiency of the combination of immunotherapy and chemotherapy. Recent research has also investigated the efficiency of combinations of immunotherapy and chemotherapy. The ORR of toripalimab combined with carboplatin/pemetrexed in an intention-to-treat population was 54.8% (Zhang et al., 2019), and that of tislelizumab combined with albumin paclitaxel/carboplatin was 59.4% (Han et al., 2021). Immunotherapy combined with chemotherapy showed an advantage in terms of ORR in EGFR-mutant patients, but the final data remains to be investigated.
In order to further explore the efficacy of combined therapy, we systematically searched observational studies and found only three relevant studies. One of these studies compared the efficacy of chemotherapy plus ICIs with and without anti-angiogenic drugs and chemotherapy alone (Deng et al., 2021). However, the other two studies were comparisons between combination therapy and ICI monotherapy (Tian et al., 2021; Bai et al., 2022); these studies were inconsistent with the requirements of our control group and thus no further data analysis could be carried out. The results of Deng et al. showed that the combined treatment could prolong median PFS by 2.9 months (HR = 0.22; 95% CI: 0.05–0.93; p = 0.039), and the ORR was better (50% in combination group vs. 22% in chemotherapy group). Tian et al. found that the ICI-based combination therapy was better than ICI monotherapy, with median PFS values of five and 2.2 months and median OS values of 14.4 and 7 months, respectively. Bai et al. found that the ICI-based combination therapy prolonged the survival of patients, especially the regimen of immunotherapy plus chemotherapy plus antiangiogenetic agents. All of these studies showed that combination therapy was effective in patients with previous TKI progression, and the combination of immunotherapy plus chemotherapy plus antiangiogenetic agents was the most effective treatment.
In terms of safety, ICI monotherapy did not show more serious toxicity overall compared with chemotherapy. There was no significant difference in frequency of all-grade TRAEs and grade ≥3 TRAEs between the combination treatment group and the control group. However, the incidence of adverse events leading to discontinuation in the combined treatment group was higher than that in the control group. The health status of patients should thus be closely monitored. The TRAEs of grade 3 or higher in the combination therapy group were mainly neutropenia, anemia, hypertension, and leucopenia, and no additional safety signals were found.
Although studies have shown that PD-1/L1 inhibitors represent a promising treatment for patients with EGFR-TKI-resistant NSCLC, according to our meta-analysis, ICI monotherapy did not have a significant effect. The formation of blood vessels around the tumor is a possible reason for the lack of effectiveness of immunotherapy. Such neovascularization around the tumor forms a physical barrier that prevents the infiltration of immune cells into the tumor tissue. Tumor vascular endothelial growth factor (VEGF) also induces FasL expression and thus triggers T cell apoptosis; therefore, even if the ICI can activate more immune cells, it is difficult for these to have a considerable effect. Antiangiogenic agents can induce tumor vascular normalization by inhibiting VEGF, weakening the physical barrier of tumor angiogenesis, increasing the infiltration of immune cells, and further activating immune effector cells under the action of ICI, thereby improving the efficacy of immunotherapy. The infiltration of more immune effector cells promotes the release of interferon γ and vascular remodeling, which in turn improves the efficacy of angiogenic agents and forms a virtuous circle (Motz et al., 2014; Tian et al., 2017). Further, the addition of chemotherapy can accelerate the apoptosis of tumor cells and promote the release of related antigens, as well as reducing the number of immune suppressor cells, thereby regulating the tumor immune microenvironment together with antiangiogenic agents (Chen and Mellman, 2013); this is a possible mechanism underlying the effects of the combination of ICIs with antiangiogenic agents and chemotherapy.
A major advantage of this study is that it included data from all the latest relevant trials, including three outcome indicators from seven studies; it also included data on immune-related combination therapy for the first time. The study population was only composed of EGFR-mutant NSCLC patients who had received previous TKI treatment, which ensured the homogeneity of the study population to a certain extent. The results of this meta-analysis suggest that combination therapy may have a better therapeutic effect than immune monotherapy; the combination of ICIs and antiangiogenic agents in particular deserves further attention.
There were many limitations of this meta-analysis. Only a limited number of trials were included in this study. As the subject matter has only become a research hotspot in recent years, many trials are still in progress. Several intention-to-treat studies have shown that ICI monotherapy is not effective, decreasing the research focus on ICIs monotherapy. Most current research focuses on ICI combination therapy ICIs in EGFR-TKI-resistant patients; in particular, ICIs combined with chemotherapy and ICIs combined with antiangiogenic agents have become a research hotspot. We have summarized the relevant RCTs in progress (Table 5) to provide guidance for follow-up research. Only multi-arm RCTs were included in this study, and many single-arm trials were excluded, which led to a small sample size and limited data. In addition, it is undeniable that the therapeutic effect of ICIs is affected by PD-L1 tumor proportion. In the included studies, only one study reported OS in people with PD-L1 tumor proportion ≥1% in the monotherapy group. The results showed that the effect of ICI was still not as good as that of docetaxel. In the combination therapy group, one study reported that there was no significant difference between combined therapy and chemotherapy in a population without PD-L1 expression. When the PD-L1 tumor proportion score was ≥1%, the results were biased towards combination therapy, regardless of whether PD-L1 expression was high or low. Two studies reported PFS in people with PD-L1 expression ≥1% in the monotherapy group; chemotherapy still worked better than ICI monotherapy. In the combination group, one study reported data related to PD-L1 expression; the results were the same as those for OS (Table 3). In the phase II single-arm ATLANTIC study, NSCLC patients were divided according to their PD-L1 expression status (Garassino et al., 2018). In the EGFR mutation subgroup, the median OS of the population with PD-L1 expression <25% treated with durvalumab was 9.9 months (HR 5.9–10.8), whereas that of the population with PD-L1 expression ≥25% was 16.1 months (HR 6.2–33.2). Although the ORR of the EGFR mutation subgroup was lower than that of the total population, patients with higher PD-L1 expression had better median OS and higher ORR. In this meta-analysis, the EGFR mutation type, smoking status, and number of TKI treatment lines received in the past were unknown; investigation of PD-L1 expression was also limited. Although these factors may affect the treatment effect, it was impossible to investigate their impact owing to the limited reports in the literature. Owing to these limitations, more clinical trials are needed in the future to study relevant problems and obtain more detailed data for further summary, so as to elucidate the therapeutic effect of ICIs in NSCLC patients with EGFR mutation.
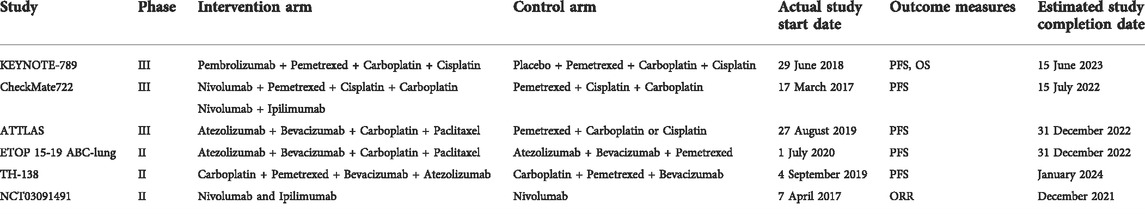
TABLE 5. Ongoing randomized controlled trials of immune checkpoint inhibitors in EGFR-TKI-resistant NSCLC.
In conclusion, compared with chemotherapy, ICI monotherapy as a second-line or third-line treatment did not significantly improve the survival of patients with EGFR-TKI resistant NSCLC according to the data analyzed. For patients with EGFR mutation, based on the reports included in this study, the expression state of PD-L1 does not have a significant impact on the effect of monotherapy. In PD-L1+ patients, combination therapy has shown good efficacy. Further investigation of the mechanisms of PD-L1 expression and drug resistance will help to guide subsequent immunotherapy. Combination therapy seems to play a better role than monotherapy; however, according to the results of our analysis, the efficacy of ICI combined with chemotherapy needs further study. The combination of ICIs with chemotherapy or antiangiogenic agents showed considerable efficacy. Owing to the limited sample size, the therapeutic value of this combination treatment scheme needs to be verified using more clinical trial data.
ICI-based combination therapy could be used as a follow-up treatment option for patients with EGFR-TKI-resistant NSCLC, especially the combination of ICIs with chemotherapy or antiangiogenic agents. There was no significant difference in all-grade TRAEs between the combination group and the control group, but it should be emphasized that combination therapy was related to a higher incidence of TRAEs leading to discontinuation, which requires close attention. Furthermore, the treatment cost of the combination of three agents is relatively high, which will lead to fewer patients taking it as a follow-up treatment option. How to optimize the treatment strategy of ICI combination therapy to reduce toxicity and economic burden is a difficult problem to be overcome; more and larger-scale research is needed. Finally, it is worth mentioning that the reliability of meta-analysis results and their applicability in clinical practice depend on critical thinking and objective judgment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
XQ and XG: Study concept and design; acquisition of data; statistical analysis. TL and WH: interpretation of data; drafting and critical review of the manuscript for important intellectual content. LZ, CW, and FY: conceptualization and design of the study; coordination and supervision of data collection; approval of the final version of the manuscript.
Funding
This review was supported by the National Natural Science Foundation of China under Grant Nos. U1903119 and 82173779, the Pilot Project of Science and Technology of Fujian Province (2020Y0013), Guyuan Municipal Bureau of Science and Technology Planning Project (2020GYKYF036), and Xiamen Municipal Bureau of Science and Technology Planning Project (Grant Nos. 3502Z2021YJ11).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akbay, E. A., Koyama, S., Carretero, J., Altabef, A., Tchaicha, J. H., Christensen, C. L., et al. (2013). Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 3, 1355–1363. doi:10.1158/2159-8290.Cd-13-0310
Antonia, S. J., Borghaei, H., Ramalingam, S. S., Horn, L., De Castro Carpeño, J., Pluzanski, A., et al. (2019). Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: A pooled analysis. Lancet. Oncol. 20, 1395–1408. doi:10.1016/s1470-2045(19)30407-3
Arrieta, O., Barrón, F., Ramírez-Tirado, L. A., Zatarain-Barrón, Z. L., Cardona, A. F., Díaz-García, D., et al. (2020). Efficacy and safety of pembrolizumab plus docetaxel vs docetaxel alone in patients with previously treated advanced non–small cell lung cancer: The PROLUNG phase 2 randomized clinical trial. JAMA Oncol. 6, 856–864. doi:10.1001/jamaoncol.2020.0409
Bai, M., Wang, W., Gao, X., Wu, L., Jin, P., Wu, H., et al. (2022). Efficacy of immune checkpoint inhibitors in patients with EGFR mutated NSCLC and potential risk factors associated with prognosis: A single institution experience. Front. Immunol. 13, 832419. doi:10.3389/fimmu.2022.832419
Borghaei, H., Paz-Ares, L., Horn, L., Spigel, D. R., Steins, M., Ready, N. E., et al. (2015). Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373, 1627–1639. doi:10.1056/NEJMoa1507643
Cancer Today (2021). Cancer Today. Available at: https://gco.iarc.fr/today/home.
Cavanna, L., Citterio, C., and Orlandi, E. (2019). Immune checkpoint inhibitors in EGFR-mutation positive TKI-treated patients with advanced non-small-cell lung cancer network meta-analysis. Oncotarget 10, 209–215. doi:10.18632/oncotarget.26541
Chen, D. S., and Mellman, I. (2013). Oncology meets immunology: The cancer-immunity cycle. Immunity 39, 1–10. doi:10.1016/j.immuni.2013.07.012
Chen, N., Fang, W., Zhan, J., Hong, S., Tang, Y., Kang, S., et al. (2015). Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: Implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J. Thorac. Oncol. 10, 910–923. doi:10.1097/jto.0000000000000500
Dearden, S., Stevens, J., Wu, Y. L., and Blowers, D. (2013). Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann. Oncol. 24, 2371–2376. doi:10.1093/annonc/mdt205
Deng, H., Lin, X., Xie, X., Yang, Y., Wang, L., Wu, J., et al. (2021). Immune checkpoint inhibitors plus single-agent chemotherapy for advanced non-small-cell lung cancer after resistance to EGFR-TKI. Front. Oncol. 11, 700023. doi:10.3389/fonc.2021.700023
Fukuoka, M., Wu, Y. L., Thongprasert, S., Sunpaweravong, P., Leong, S. S., Sriuranpong, V., et al. (2011). Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J. Clin. Oncol. 29, 2866–2874. doi:10.1200/jco.2010.33.4235
Garassino, M. C., Cho, B. C., Kim, J. H., Mazières, J., Vansteenkiste, J., Lena, H., et al. (2018). Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): An open-label, single-arm, phase 2 study. Lancet. Oncol. 19, 521–536. doi:10.1016/s1470-2045(18)30144-x
Garassino, M. C., Cho, B. C., Kim, J. H., Mazières, J., Vansteenkiste, J., Lena, H., et al. (2020). Final overall survival and safety update for durvalumab in third- or later-line advanced NSCLC: The phase II ATLANTIC study. Lung Cancer 147, 137–142. doi:10.1016/j.lungcan.2020.06.032
Greenhalgh, J., Boland, A., Bates, V., Vecchio, F., Dundar, Y., Chaplin, M., et al. (2021). First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst. Rev., CD010383. doi:10.1002/14651858.CD010383.pub3
Hack, S. P., Zhu, A. X., and Wang, Y. (2020). Augmenting anticancer immunity through combined targeting of angiogenic and PD-1/PD-L1 pathways: Challenges and opportunities. Front. Immunol. 11, 598877. doi:10.3389/fimmu.2020.598877
Han, B., Tian, P., Zhao, Y., Yu, X., Guo, Q., Yu, Z., et al. (2021). 148P A phase II study of tislelizumab plus chemotherapy in EGFR mutated advanced non-squamous NSCLC patients failed to EGFR TKI therapies: First analysis. Ann. Oncol. 32, S1443–S1444. doi:10.1016/j.annonc.2021.10.167
Hayashi, H., Sugawara, S., Fukuda, Y., Fujimoto, D., Miura, S., Ota, K., et al. (2022). A randomized phase II study comparing nivolumab with carboplatin-pemetrexed for EGFR-mutated NSCLC with resistance to EGFR tyrosine kinase inhibitors (WJOG8515L). Clin. Cancer Res. 28, 893–902. doi:10.1158/1078-0432.Ccr-21-3194
Herbst, R. S., Baas, P., Kim, D. W., Felip, E., Pérez-Gracia, J. L., Han, J. Y., et al. (2016). Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 387, 1540–1550. doi:10.1016/s0140-6736(15)01281-7
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj 343, d5928. doi:10.1136/bmj.d5928
Lee, C. K., Man, J., Lord, S., Links, M., Gebski, V., Mok, T., et al. (2017). Checkpoint inhibitors in metastatic EGFR-mutated non–small cell lung cancer—a meta-analysis. J. Thorac. Oncol. 12, 403–407. doi:10.1016/j.jtho.2016.10.007
Lim, S. M., Syn, N. L., Cho, B. C., and Soo, R. A. (2018). Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer Treat. Rev. 65, 1–10. doi:10.1016/j.ctrv.2018.02.006
Lu, S., Wu, L., Jian, H., Cheng, Y., Wang, Q., Fang, J., et al. (2022). VP9-2021: ORIENT-31: Phase III study of sintilimab with or without IBI305 plus chemotherapy in patients with EGFR mutated nonsquamous NSCLC who progressed after EGFR-TKI therapy. Ann. Oncol. 33, 112–113. doi:10.1016/j.annonc.2021.10.007
Midha, A., Dearden, S., and McCormack, R. (2015). EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: A systematic review and global map by ethnicity (mutMapII). Am. J. Cancer Res. 5, 2892–2911.
Mok, T. S. K., Wu, Y-L., Kudaba, I., Kowalski, D. M., Cho, B. C., Turna, H. Z., et al. (2019). Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 393, 1819–1830. doi:10.1016/s0140-6736(18)32409-7
Motz, G. T., Santoro, S. P., Wang, L. P., Garrabrant, T., Lastra, R. R., Hagemann, I. S., et al. (2014). Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 20, 607–615. doi:10.1038/nm.3541
Nogami, N., Barlesi, F., Socinski, M. A., Reck, M., Thomas, C. A., Cappuzzo, F., et al. (2022). IMpower150 final exploratory analyses for atezolizumab plus bevacizumab and chemotherapy in Key NSCLC patient subgroups with EGFR mutations or metastases in the liver or brain. J. Thorac. Oncol. 17, 309–323. doi:10.1016/j.jtho.2021.09.014
Oxnard, G. R., Yang, J. C., Yu, H., Kim, S. W., Saka, H., Horn, L., et al. (2020). Tatton: A multi-arm, phase ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann. Oncol. 31, 507–516. doi:10.1016/j.annonc.2020.01.013
Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. Bmj 372, n160. doi:10.1136/bmj.n160
Ramalingam, S. S., Vansteenkiste, J., Planchard, D., Cho, B. C., Gray, J. E., Ohe, Y., et al. (2020). Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 382, 41–50. doi:10.1056/NEJMoa1913662
Rittmeyer, A., Barlesi, F., Waterkamp, D., Park, K., Ciardiello, F., von Pawel, J., et al. (2017). Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 389, 255–265. doi:10.1016/s0140-6736(16)32517-x
Sabari, J. K., Heymach, J. V., and Sandy, B. (2021). Hitting the right spot: Advances in the treatment of NSCLC with uncommon EGFR mutations. J. Natl. Compr. Canc. Netw. 19, S1–s11. doi:10.6004/jnccn.2021.0200
Soria, J. C., Wu, Y. L., Nakagawa, K., Kim, S. W., Yang, J. J., Ahn, M. J., et al. (2015). Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): A phase 3 randomised trial. Lancet. Oncol. 16, 990–998. doi:10.1016/s1470-2045(15)00121-7
Thai, A. A., Solomon, B. J., Sequist, L. V., Gainor, J. F., and Heist, R. S. (2021). Lung cancer. Lancet 398, 535–554. doi:10.1016/s0140-6736(21)00312-3
Tian, L., Goldstein, A., Wang, H., Ching Lo, H., Sun Kim, I., Welte, T., et al. (2017). Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544, 250–254. doi:10.1038/nature21724
Tian, T., Yu, M., Li, J., Jiang, M., Ma, D., Tang, S., et al. (2021). Front-line ICI-based combination therapy post-TKI resistance may improve survival in NSCLC patients with EGFR mutation. Front. Oncol. 11, 739090. doi:10.3389/fonc.2021.739090
Westover, D., Zugazagoitia, J., Cho, B. C., Lovly, C. M., and Paz-Ares, L. (2018). Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann. Oncol. 29, i10–i19. doi:10.1093/annonc/mdx703
Wu, L., Ke, L., Zhang, Z., Yu, J., and Meng, X. (2020). Development of EGFR TKIs and options to manage resistance of third-generation EGFR TKI osimertinib: Conventional ways and immune checkpoint inhibitors. Front. Oncol. 10, 602762. doi:10.3389/fonc.2020.602762
Wu, S. G., and Shih, J. Y. (2018). Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol. Cancer 17, 38. doi:10.1186/s12943-018-0777-1
Wu, Y-L., Lu, S., Cheng, Y., Zhou, C., Wang, J., Mok, T., et al. (2019). Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J. Thorac. Oncol. 14, 867–875. doi:10.1016/j.jtho.2019.01.006
Yang, J. C., Wu, Y. L., Schuler, M., Sebastian, M., Popat, S., Yamamoto, N., et al. (2015). Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet. Oncol. 16, 141–151. doi:10.1016/s1470-2045(14)71173-8
Yang, J. C-H., Shepherd, F. A., Kim, D-W., Lee, G-W., Lee, J. S., Chang, G-C., et al. (2019). Osimertinib plus durvalumab versus osimertinib monotherapy in EGFR t790m-positive NSCLC following previous EGFR TKI therapy: CAURAL brief report. J. Thorac. Oncol. 14, 933–939. doi:10.1016/j.jtho.2019.02.001
Zhang, J., Zhou, C., Zhao, Y., Mu, X., Zhou, J., Bao, Z., et al. (2019). MA11.06 A PII study of toripalimab, a PD-1 mAb, in combination with chemotherapy in EGFR+ advanced NSCLC patients failed to prior EGFR TKI therapies. J. Thorac. Oncol. 14, S292. doi:10.1016/j.jtho.2019.08.587
Zhang, Y. L., Yuan, J. Q., Wang, K. F., Fu, X. H., Han, X. R., Threapleton, D., et al. (2016). The prevalence of EGFR mutation in patients with non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget 7, 78985–78993. doi:10.18632/oncotarget.12587
Zhao, Y., Liu, J., Cai, X., Pan, Z., Liu, J., Yin, W., et al. (2019). Efficacy and safety of first line treatments for patients with advanced epidermal growth factor receptor mutated, non-small cell lung cancer: Systematic review and network meta-analysis. Bmj 367, l5460. doi:10.1136/bmj.l5460
Zhou, C., Wu, Y. L., Chen, G., Feng, J., Liu, X. Q., Wang, C., et al. (2011). Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet. Oncol. 12, 735–742. doi:10.1016/s1470-2045(11)70184-x
Keywords: NSCLC, immune checkpoint inhibitors, EGFR mutation, TKIs, meta-analysis
Citation: Qian X, Guo X, Li T, Hu W, Zhang L, Wu C and Ye F (2022) Efficacy of immune checkpoint inhibitors in EGFR-Mutant NSCLC patients with EGFR-TKI resistance: A systematic review and meta-analysis. Front. Pharmacol. 13:926890. doi: 10.3389/fphar.2022.926890
Received: 25 April 2022; Accepted: 25 July 2022;
Published: 22 August 2022.
Edited by:
Lele Song, Eighth Medical Center of the General Hospital of the Chinese People’s Liberation Army, ChinaReviewed by:
Le-Tian Huang, Shengjing Hospital of China Medical University, ChinaLeli Zeng, Sun Yat-sen University, China
Copyright © 2022 Qian, Guo, Li, Hu, Zhang, Wu and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Zhang, dG9ueTE5ODIxMTBAZ21haWwuY29t; Caisheng Wu, d3Vjc2hAeG11LmVkdS5jbg==; Feng Ye, eWVmZW5nZG9jdG9yQHhtdS5lZHUuY24=
†These authors have contributed equally to this work
 Xiaoyu Qian1†
Xiaoyu Qian1† Lin Zhang
Lin Zhang Caisheng Wu
Caisheng Wu Feng Ye
Feng Ye