
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 15 July 2022
Sec. Experimental Pharmacology and Drug Discovery
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.925993
This article is part of the Research Topic Novel Pharmacological Approaches Targeting Mitochondrial Dysfunction in Diseases View all 5 articles
 Md. Ataur Rahman1,2,3†
Md. Ataur Rahman1,2,3† Sumaya Akter1†
Sumaya Akter1† Debra Dorotea4
Debra Dorotea4 Arpita Mazumder1
Arpita Mazumder1 Md. Naim Uddin1
Md. Naim Uddin1 Md. Abdul Hannan1,5
Md. Abdul Hannan1,5 Muhammad Jahangir Hossen6
Muhammad Jahangir Hossen6 Md. Selim Ahmed7
Md. Selim Ahmed7 Woojin Kim3,8
Woojin Kim3,8 Bonglee Kim2,3*
Bonglee Kim2,3* Md Jamal Uddin1,4*
Md Jamal Uddin1,4*Kidney diseases, including acute kidney injury (AKI) and chronic kidney disease (CKD), have become critical clinical, socioeconomic, and public health concerns worldwide. The kidney requires a lot of energy, and mitochondria act as the central organelle for the proper functioning of the kidney. Mitochondrial dysfunction has been associated with the pathogenesis of AKI and CKD. Natural products and their structural analogs have been sought as an alternative therapeutic strategy despite the challenges in drug discovery. Many studies have shown that small-molecule natural products can improve renal function and ameliorate kidney disease progression. This review summarizes the nephroprotective effects of small-molecule natural products, such as berberine, betulinic acid, celastrol, curcumin, salidroside, polydatin, and resveratrol. Treatment with small-molecule natural products was shown to attenuate renal oxidative stress and mitochondrial DNA (mtDNA) damage and restore mitochondrial biogenesis and dynamics in the kidneys against various injury stimuli. Therefore, small-molecule natural products should be recognized as multi-target therapeutics and promising drugs to prevent kidney diseases, particularly those with mitochondrial dysfunction.
Kidney diseases are a major escalating public health issue globally associated with serious clinical complications (Liu et al., 2019; Li Q. et al., 2021; Wang D.-W. et al., 2021). Kidney injury is broadly classified into acute kidney injury (AKI) and chronic kidney disease (CKD). AKI is characterized by the rapid loss of renal excretory function within a short duration (hours to days) (Wang D.-W. et al., 2021). The common etiologies of AKI include ischemia, obstructive nephropathy (ON), nephrotoxins, and sepsis (Martínez-Klimova et al., 2020; Wang D.-W. et al., 2021). AKI has been recognized as a crucial risk factor for the occurrence and progression of CKD (Jiang et al., 2020), which involves the gradual loss of kidney function with reduced glomerular filtration rate and enhanced urinary albumin excretion, resulting in end-stage renal disease (ESRD) (Liu et al., 2019; Li Q. et al., 2021). Despite the availability of advanced supportive management and diagnosis, AKI and CKD have high morbidity and mortality due to less effective therapies (Gao et al., 2020; Li Q. et al., 2021; Wang D.-W. et al., 2021). Therefore, it is imperative to explore more efficacious therapeutic strategies to treat and prevent kidney disease progression (Li Q. et al., 2021; Wang D.-W. et al., 2021).
Natural products and their structural analogs have made a major contribution to pharmacotherapy despite the challenges in drug discovery. Consumers are using naturally-derived substances in the form of herbal medications or nutraceuticals to avoid the potential adverse effects of the pharmaceutical drugs (Dias et al., 2012). According to the World Health Organization (WHO), 60% of the global population and 80% of the population of developing countries prefer herbal drugs for their healthcare needs (Chikezie, 2015). The natural product or herbal medicine contains multiple chemical compounds with therapeutic and nutritional value, determining its multi-target nature (Zhong et al., 2015; Li Q. et al., 2021).
Various studies suggest that herbal medicines such as berberine, betulinic acid, celastrol, curcumin, salidroside, polydatin, and resveratrol could improve renal function and slow kidney disease progression (Zhong et al., 2015). Mitochondria are one of the primary organelles responsible for energy production. They are essential for the kidney to eliminate waste in the blood and control the signaling transduction process, cell proliferation, cell cycle, cell growth, cell death, water, and electrolyte balance (Sun et al., 2014; Sun et al., 2019; Li Q. et al., 2021; Tang et al., 2021). Mitochondrial dysfunction is associated with AKI and CKD pathogenesis (Li Q. et al., 2021; Tang et al., 2021). It results in the breakdown of adenosine triphosphate (ATP), excessive production of reactive oxygen species (ROS), and release of proapoptotic proteins such as cytochrome c, inducing cell injury through apoptosis, inflammation, fibrosis, and oxidative damage to DNA and proteins (Sun et al., 2014).
There is increasing evidence that natural products can protect the kidneys against various toxic stimuli by maintaining mitochondrial fitness (Martínez-Klimova et al., 2020; Li Q. et al., 2021). Consequently, phytoconstituents such as flavonoids, terpenoids, steroids, and fatty acids have been the subject of significant research to determine their possible nephroprotective effects (Basist et al., 2022). Because of their ability to manipulate oxidative, inflammatory, and apoptotic variables, they are almost universally recognized as potentially useful for treating kidney-related conditions. This review discusses the molecular mechanisms of action of natural products that improve mitochondrial fitness and the pathological changes induced by their treatment.
A literature search was performed to collect original research articles published in English on therapeutic compounds for kidney disease and their mechanism of action in the Google Scholar, Web of Science, Scopus, and PubMed databases. Various search terms were used, including kidney disease, autophagy, apoptosis, natural compounds, kidney cancer, phytochemical, drug delivery system, targeted signaling pathway, and prospective role of kidney treatment. All figures were created using the Adobe Illustrator software (San Jose, CA, United States).
The kidney has the highest proportion of mitochondria of all tissues, providing the energy needed to maintain kidney functions, such as nutrient reabsorption, regulation of electrolytes and fluid balance, and acid-base homeostasis (Bhargava and Schnellmann, 2017). Mitochondrial DNA (mtDNA) health and the presence of mitochondrial biogenesis, mitochondrial dynamics, and oxidative stress is often required for mitochondria to perform their functions (Pedraza-Chaverri et al., 2016; Aparicio-Trejo et al., 2018). The mechanisms involved in mitochondrial dysfunction contributing to the pathogenesis of kidney diseases are discussed in the following sections (Figure 1).
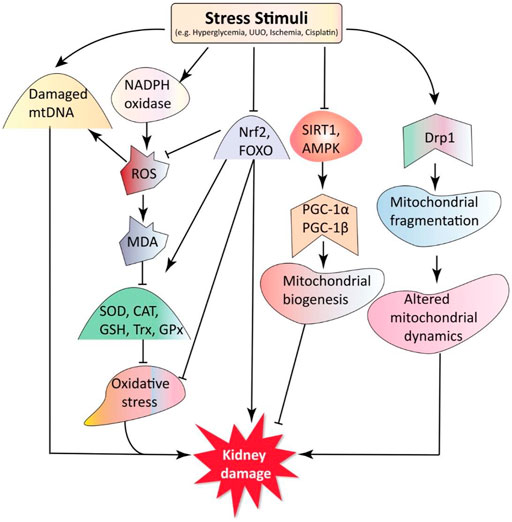
FIGURE 1. Mitochondria dysfunction in kidney diseases. Stress stimuli, such as hyperglycemia (HG), unilateral ureteric obstruction (UUO), cisplatin, and ischemia cause an imbalance between oxidative stress and antioxidants. Under stress stimuli, the expression of transcription factors nuclear factor erythroid 2-related factor 2 (Nrf-2) and forkhead box O (FOXO) decreases, activating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase leading to excessive ROS and malondialdehyde (MDA) production. This oxidative stress also reduces the transcription of antioxidants such as superoxide dismutase (SOD), catalase (CAT), glutathione synthetase (GSS), glutathione peroxidase (GPX), and thioredoxin (Trx). Sirtuin 1 (SIRT1) and AMP-activated protein kinase (AMPK) can activate peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), a master regulator of mitochondrial biogenesis. The activated dynamin-related protein 1 (Drp1) disturbs mitochondrial dynamics and causes mitochondria fragmentation. Moreover, various stress stimuli can induce mtDNA damage. Altogether, these cause changes in mitochondria structure and function, exacerbating kidney disease progression. Key: AMPK, AMP-activated protein kinase; CAT, catalase; DN, diabetic nephropathy; Drp1, dynamin-related protein 1; FOXO, forkhead-box class O; GSH, glutathione; GPx, glutathione peroxidase; GM, gentamycin; HN, hyperuricemic nephropathy; MDA, malondialdehyde; mtDNA, mitochondrial DNA; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; ROS, reactive oxygen species; SIRT1, silent mating type information regulation 2 homolog 1; SOD, superoxide dismutase; Trx, thioredoxin; UUO, unilateral ureteral obstruction.
Nitric oxide (NO) produced by endothelial nitric oxide synthase (eNOS) is believed to be responsible for protective effects such as the suppression of leukocyte and platelet activation and adhesion. Evidence supporting eNOS’s positive role includes lower eNOS expression in experimental models of glomerulonephritis and human biopsy specimens. This reduction in eNOS expression most likely reflects the necrosis of endothelial cells. The interaction of NO with superoxide anions or the myeloperoxidase (MPO) and hypochlorous acid (HOCL) system are two potential mechanisms that may lead to reduce NO production in acute glomerulonephritis (Heeringa et al., 2002).
Endothelial NO is an important vasodilator involved in various cardiovascular diseases (CVDs) and CKDs (Kumar et al., 2021). Renovascular hypertension is a leading cause of secondary hypertension, induced by atherosclerotic renovascular stenosis or fibromuscular dysplasia. Angiotensin II production, oxidative stress, and peroxynitrite generation reduce NO availability, causing hypertension, renal and endothelial dysfunction, and cardiac and vascular remodeling (Hiyoshi et al., 2005). eNOS uncoupling reduces NO availability in renovascular hypertension. NO donors and NO-derived metabolites reduce blood pressure and organ damage in experimental renovascular hypertension (Segawa et al., 2020). Therefore, understanding the function of reduced NO in renovascular hypertension stimulates the investigation of NO donors and molecules that can be converted into NO, which are potentially important to the future treatment of CVD and CKD (Pereira et al., 2022).
Under normal physiological conditions, mitochondria serve as the primary intracellular location for the production of ROS and oxidants (Satoh et al., 2011; Xu et al., 2012). Through a process called the respiratory chain, mitochondria can produce ATP in Complex V, where a low concentration of superoxide anions is produced in Complexes I and III. While low ROS levels are important for cell function, high levels are toxic to mitochondria and cells. Mitochondrial damage can increase the release of cytochrome C into the cytosol, activating excessive ROS production and leading to oxidative stress and activation of the inflammatory response, resulting in CKD progression. In addition, reduced expression of antioxidant genes induces mitochondrial oxidative stress (Satoh et al., 2011; Xu et al., 2012; Hui et al., 2017). Excessive ROS can cause mtDNA damage, resulting in mutations in the next generation of mitochondria, decreasing the efficiency of the respiratory chain, reducing ATP production, and damaging proteins and lipids. In addition, ROS can induce cell apoptosis by causing cytochrome C release, leading to mitochondrial dysfunction (Ruiz et al., 2013).
Mitochondrial dysfunction is marked by an increase in mitochondrial ROS (mtROS), a decrease in matrix metalloproteinases (MMPs), and increases in calcium influx, damage, and release of cytochrome C (Zhou et al., 2021). Electrons that leak out of the electron transport chain (ETC) combine with oxygen to make O2−, which superoxide dismutases (SODs) catalyze into hydrogen peroxide (H2O2). Reduced MMP levels and mitochondrial calcium overload are important signs of mitochondrial dysfunction, which has a significant effect on oxidative stress, inflammation, neurodegeneration, and apoptosis (Rahman et al., 2020). Mutations and releases of mtDNA break down the mitochondrial respiratory chain, increase the mtROS levels, and speed up mitochondrial dysfunction (Tang et al., 2021). Therefore, mitochondrial dysfunction plays an important role in the development of AKI, aberrant kidney repair, and CKD.
Mitochondrial oxidative stress stimulates the glomerulosclerosis and pathogenesis of diabetic kidney disease (DKD) (Zhang et al., 2019; Ogura et al., 2020). Additionally, renal ischemia-reperfusion, hyperglycemia (HG), cis-diamminedichloroplatinum (cisplatin), cadmium (Cd), and gentamicin (GM) induce oxidative stress and contribute to kidney injuries through the production of excess ROS (Visnagri et al., 2015; Adil et al., 2016; Ortega-Domínguez et al., 2017; ALTamimi et al., 2021). Oxidative stress stimulates NO production by NOS. NO reacts with superoxide radicals and produces cytotoxic ROS such as peroxynitrite (ONOO−), which causes nitrosative stress and oxidizes lipids, DNA, and proteins, resulting in changes to cell signaling and oxidative damage leading to cell necrosis and apoptosis (Kim et al., 2018).
Additionally, NO is crucial in the control of kidney, cardiovascular, and metabolic systems, both in normal and diseased states, creating interest in the development of therapeutic technologies that can alter NO bioactivity (Bulboacă et al., 2021). Innovative pharmacological and nutritional approaches that increase NO bioactivity and reduce oxidative stress may represent viable therapeutics for preventing and treating kidney disease (Carlström, 2021).
Changes in mitochondrial structure and function lead to decreased ATP production capacity in aging cells due to mitochondrial dysfunction. However, while mitochondrial oxidative stress decreases with age (Satoh et al., 2011; Kim et al., 2018), inflammatory signaling molecules can stimulate the production of free radicals to enhance oxidative stress (Zhang et al., 2019; Huang et al., 2020). p66Shc is a prooxidant that accumulates in the kidneys of diabetic patients that induces ROS production by increasing the expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) and inhibiting the expression of the endogenous antioxidant enzymes, including manganese superoxide dismutase (MnSOD) and glutathione synthetase (GSS) (ALTamimi et al., 2021). NOXs are enzymes that primarily serve the purpose of catalyzing ROS production. Using NADPH as an electron donor, NOXs can catalyze the transfer of electrons from NADPH to oxygen, resulting in the production of either superoxide or H2O2 (Lee et al., 2020). Under normal conditions, the expression level of NADPH oxidase 4 (NOX4) is highest in kidney tubular cells but also present at lower levels in endothelial cells, cardiomyocytes, and other cell types (Lee et al., 2020).
Enzymes with antioxidant activity such as GSS, SODs, and catalase (CAT) function as free radical scavengers to protect cells against the harmful effect of ROS. ROS can also activate nuclear respiratory factor-like 2 (Nrf-2), which translocates to the nucleus and binds to antioxidant-responsive elements (AREs) to activate the transcription of genes encoding antioxidant enzymes, such as NADPH quinone oxidoreductase (NQO-1), heme oxygenase-1 (HO-1), SOD1, and SOD2 (Kim et al., 2018). The forkhead-box class O (FOXO) family includes FOXO1 and FOXO3 and function as transcription factors that inhibit ROS production and lipid peroxidation by enhancing the expression of ROS scavenging and antioxidant enzymes such as thioredoxin (Trx), thioredoxin reductases (TXNRDs), GSS, SODs, CAT, glutathione peroxidases 1/2 (GPX1/2) (ALTamimi et al., 2021).
Endothelial dysfunction, inflammation, and oxidative stress are associated with CKD and CVD (Jin et al., 2021), where they have similar roles (Kumar et al., 2022). Both CVD and CKD are associated with endothelial dysfunction, inflammation, and oxidative stress (Streeter et al., 2013). As kidney disease progresses, as measured by the glomerular filtration rate (GFR) and the amount of protein in the urine, the risk of CVD and chronic renal failure increases (Stenvinkel et al., 2008), leading to ESRD, where therapies are required to replace the kidneys or even a kidney transplant (Ravarotto et al., 2018). Traditional and non-traditional risk factors have a significant effect on the life expectancy of CKD patients. The interconnectivity of molecular mechanisms in oxidative stress, inflammation, and endothelial dysfunction, is the primary shared factor in determining CKD, high blood pressure, CVD, and cardiovascular-renal remodeling (Wang and Gao, 2022). Therefore, summarizing our existing understanding and identifying prospective future research pathways and therapeutic options for intervention and our current state of knowledge would help treat oxidative stress-related CKD.
Mitochondrial homeostasis is tightly regulated by balancing mitochondrial biogenesis, fission, fusion, and mitophagy. These processes are required to maintain mitochondria structure and function, particularly mitochondrial energetics (Yuan et al., 2012; Zhang et al., 2019). Mitochondrial biogenesis is regulated by various transcriptional coactivators and corepressors. The peroxisome proliferator-activated receptor-γ coactivator (PGC) family includes 1α (PGC-1α) and 1β (PGC-1β) and PGC-1-related coactivator (PRC), which act as key upstream transcriptional regulators of mitochondrial biogenesis (Martínez-Klimova et al., 2020; Qin et al., 2020). PGC-1α is known to be a prominent regulator of oxidative phosphorylation, the tricarboxylic acid (TCA) cycle, and fatty acid metabolism in the kidneys (Svensson et al., 2016). Decreased mitochondrial biogenesis exacerbates oxidative stress in kidneys with unilateral ureteral obstruction (UUO) (Qin et al., 2019; Martínez-Klimova et al., 2020). Pan-peroxisome proliferator-activated receptors (PPARs) agonist bezafibrate was found to increase PGC-1α expression, leading to stimulation of mitochondrial biogenesis (Martínez-Klimova et al., 2020). Since PGC-1α is activated by signals with high-energy demands, it is mostly expressed in the kidney tissues as renal cells require much energy to perform normal functions (Qin et al., 2019; Martínez-Klimova et al., 2020). Other markers of mitochondrial biogenesis include transcription factor A of mitochondria (TFAM), nuclear factor erythroid 2-related factor 1 (Nrf-1), and Nrf-2 (Qin et al., 2019). Mitochondrial biogenesis is an extremely complex process requiring mtDNA replication and the synthesis, import, and incorporation of proteins and lipids into the existing mitochondrial reticulum. PGC-1α coactivates the transcriptional activity of Nrf-1, which binds to the TFAM promoter, a direct mediator of mtDNA replication (Yuan et al., 2012).
Histone deacetylase sirtuin 1 (SIRT1) has emerged as a critical regulator of mitochondrial function via energy-sensing pathways that stimulate mitochondrial biogenesis (Li Y. et al., 2021). Sirtuin 1 (SIRT1) and 3 (SIRT3) are protein deacetylases that play a role in regulating mitochondrial processes, including biogenesis (Ahn et al., 2008). Nicotinamide adenine dinucleotide (NAD+) has been found to activate SIRT1, which then activates its downstream targets, including PGC-1α (Whitaker et al., 2016). SIRT3 is specifically located in the mitochondria, and its activation also stimulates mitochondrial biogenesis (Kong et al., 2010). The suppression of PGC-1α by a mutant histone deacetylase 5 (HDAC5) causes morphological alterations to mitochondria and inhibits mitochondrial enzymes (Yuan et al., 2012). Reduced PGC-1α expression causes mitochondrial dysfunction, reduces cell viability, and induces cell apoptosis and dedifferentiation, resulting in kidney failure (Qin et al., 2020).
Recent studies have explored how sirtuins change and their roles in various kidney and heart diseases to identify sirtuin activators and inhibitors that may help prevent these diseases (Wang W. et al., 2021). Several compounds that target sirtuins are promising drug candidates for various kidney and heart diseases (Kumar et al., 2020). However, large, well-designed clinical trials are still required to determine their effectiveness and safety. Because SIRT1 deacetylates its targets with the assistance of coenzyme NAD+, it is connected to cellular energy metabolism and redox status through various signaling and survival pathways (Kitada et al., 2013). SIRT1 can control lipid metabolism, autophagy, blood pressure, and salt balance in the kidneys and decrease renal cell death, inflammation, and fibrosis (Chen et al., 2020). In addition, it can prevent renal cell apoptosis. Therefore, the activation of SIRT1 in the kidney may be a potential therapeutic target that can increase resistance to many of the causative factors contributing to renal disorder development, such as diabetic nephropathy (DN).
Mitochondrial structure and morphology are crucial for maintaining optimal ATP production. Mitochondrial morphology is tightly regulated by a series of processes encompassing fission, fusion, and mitophagy (Qin et al., 2019; Zhang et al., 2020). Mitochondrial fission occurs at the specific sites where mitochondria interconnect with the endoplasmic reticulum (ER), and four adapter proteins, fission protein 1 (Fis1), mitochondrial fission factor (MFF), and the mitochondrial elongation factors 1 and 2 (Mief1 and Mief2), mediates the fission (Martínez-Klimova et al., 2020). Mitochondria undergo fission to alter their number and shape to adjust to fluctuations in metabolic demands. Mitochondrial fission is controlled by dynamin-related protein 1 (Drp1), which moves to the mitochondrial outer membrane (MOM) following phosphorylation at position S616. Drp1 binds to its receptors Fis1 and mitochondrial dynamics protein 49/51 (MiD49/51) and creating large oligomers, fragmenting mitochondrial tubules, and leading to mitochondrial fragmentation (Ni et al., 2017; Qin et al., 2019; Haschler et al., 2021).
In contrast, mitochondrial fusion preserves mitochondria from mitophagy, induces ATP production, regulates the appropriate mtDNA distribution, and increases mitochondria size and metabolic components (Molina-Jijón et al., 2016; Qin et al., 2019; Haschler et al., 2021). Fusion is controlled by anchor proteins mitofusin 1/2 (MFN1/2) and optic atrophy 1 (OPA1). MFN1/2 regulates mitochondrial outer membrane fusion, and OPA1 regulates mitochondrial inner membrane fusion (Molina-Jijón et al., 2016; Hui et al., 2017; Haschler et al., 2021). Various stressors, such as enhanced ROS production, minimized cellular ATP, and obstructions in cellular respiration caused by oxidative damage, can disrupt mitochondrial dynamics (Ni et al., 2017). During metabolic or environmental stresses such as ATP depletion (Molina-Jijón et al., 2016), mitochondrial dynamic balance switches to fission, characterized by a fragmented morphology, excess ROS production, and reduced energy metabolism and mitochondrial membrane potential (MMP) (Qin et al., 2019). Excessive mitochondrial fission and mitochondrial dysfunction in podocytes under metabolic or environmental stresses lead to DKD progression (Ni et al., 2017; Qin et al., 2019). Under hyperglycemic conditions, Drp1 interacts with Bcl-2-associated X-protein (Bax), resulting in the release of mitochondrial apoptotic proteins such as cytochrome C into the cytoplasm. These proapoptotic proteins then activate the caspase signaling cascade, leading to cellular apoptosis (Ni et al., 2017; Qin et al., 2019).
Increased levels of mtDNA damage have been associated with mitochondrial dysfunction and alteration of mitochondrial morphology in the kidney (Satoh et al., 2011; Yu et al., 2018). Mitochondrial fission, chemotherapeutic agent cisplatin, and HG have been found to stimulate excess ROS production, causing mtDNA damage (Xu et al., 2012). mtDNA fragmentation due to cisplatin is regulated by DNAse I and endonuclease G. After being passively shifted to nuclei, DNAse I initiates the breakdown of single DNA strands (ssDNA) that are more sensitive to endonuclease G digestion (Miller et al., 2010). HG disrupts the mitochondrial respiratory chain, leading to MMP hyperpolarization, reduced mtDNA content, and decreased ATP production (Xu et al., 2012). Oxidative stress causes mtDNA damage (Qin et al., 2019), and the oxidative damage of mtDNA is associated with the aging phenotype in renal cells (Satoh et al., 2011). mtDNA mutation can also exacerbate ROS production due to impaired oxidative phosphorylation (OXPHOS), eventually leading to mitochondrial dysfunction (Wang D.-W. et al., 2021).
Mitochondrial dysfunction plays a critical role in CKD progression, including DN, ON, kidney aging, drug-induced AKI, ischemia-reperfusion injury (IRI)-induced AKI, and chemical-induced AKI. Some small molecule natural products have been reported to mediate renoprotection and improve the various types of CKD. Here, we provide a comprehensive overview of the protective effects of small molecule natural products against mitochondrial dysfunction in the kidney. The experimental and disease models, pathobiology involved, protective effects, and molecular markers altered by these compounds are summarized in Tables 1, 2 and Figure 2.
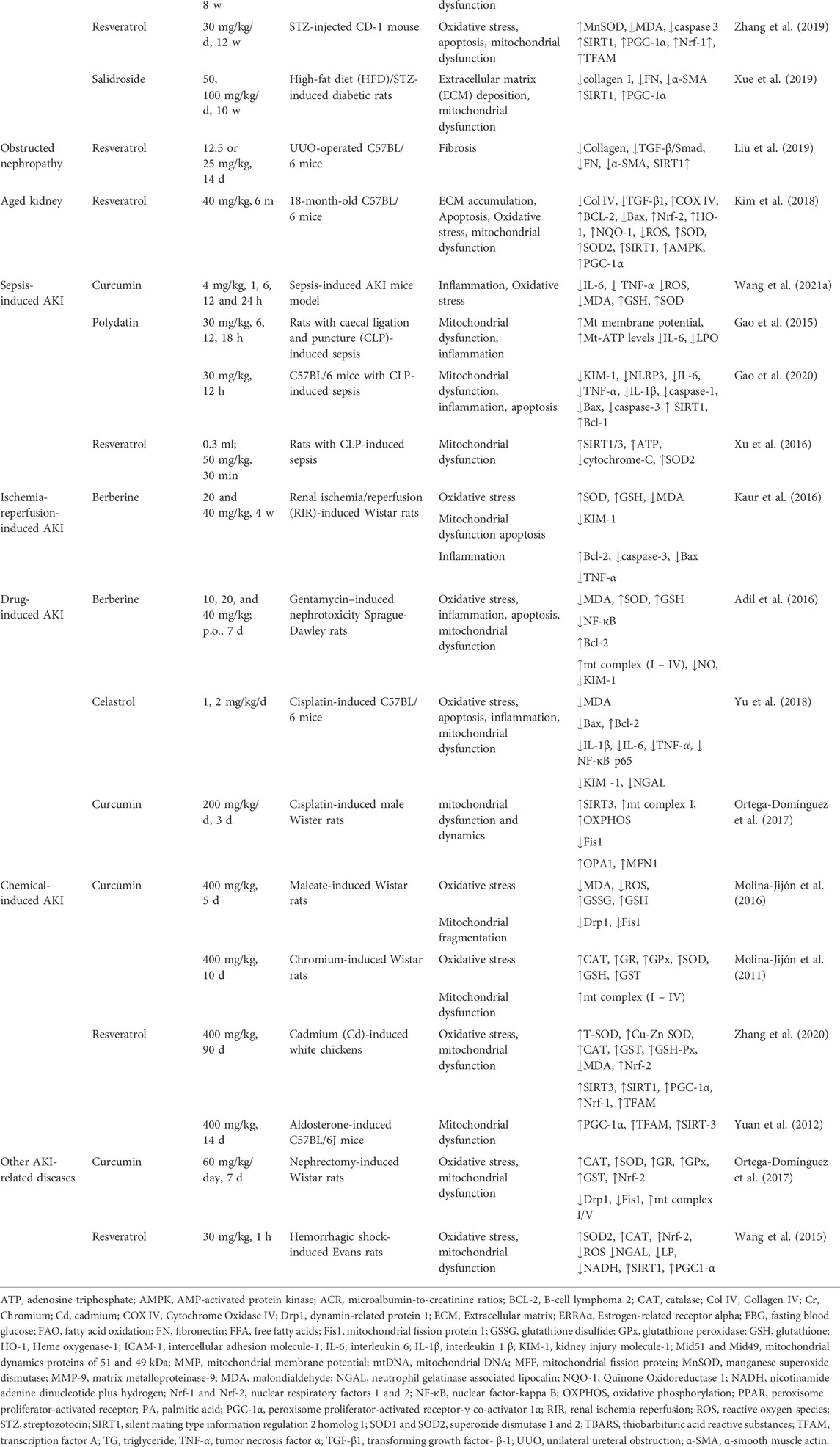
TABLE 1. Kidney protective effects are provided by phytochemicals targeting mitochondrial fitness in animal models.
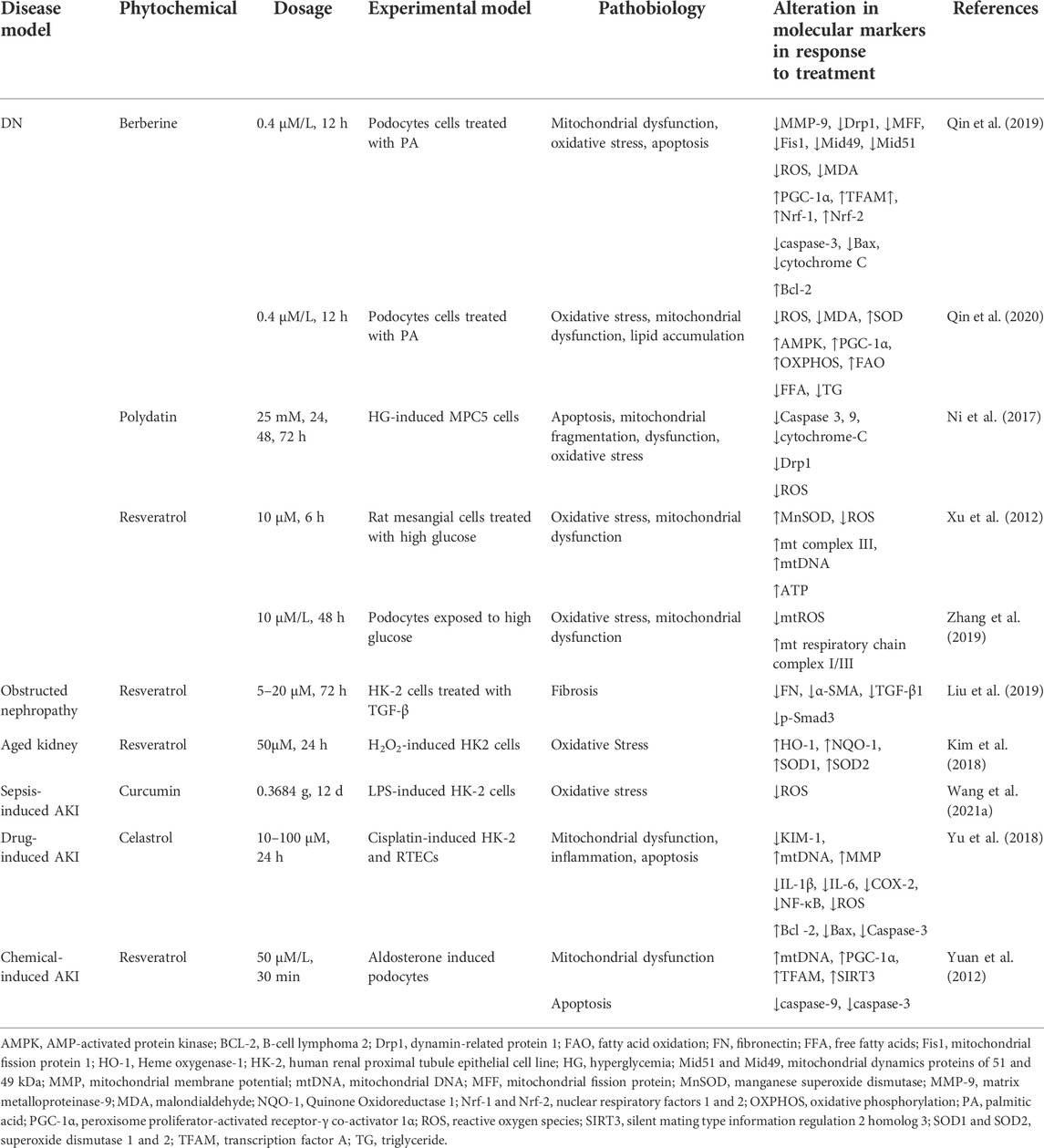
TABLE 2. Kidney protective effects are provided by phytochemicals targeting the mitochondrial fitness in cellular models.
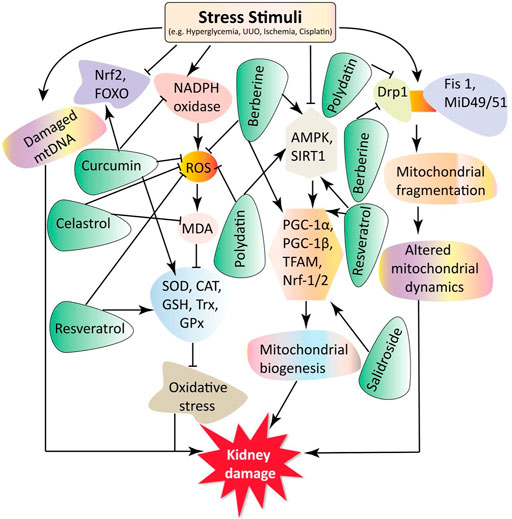
FIGURE 2. This schematic representation indicates that stress stimuli like HG, UUO, cisplatin, and ischemia regulate various pathological conditions involving oxidative stress, mitochondrial biogenesis, mtDNA damage, and altered mitochondrial dynamics. These ultimately lead to renal damage. The small molecule rotenone reduces mtDNA damage and ROS production. In addition, celastrol reduces the ROS and MDA oxidative stress markers. Polydatin, berberine, and resveratrol decrease ROS production. Salidroside increases SIRT1 and PGC-1α expression, resulting in mitochondrial biogenesis, enhancing and protecting the kidneys. Curcumin and resveratrol increase the transcription of antioxidants markers SOD, CAT, GSS, GPX, and Trx. Moreover, berberine and resveratrol increase the expression of mitochondrial biogenesis markers PGC-1α, TFAM, Nrf-1, and Nrf-2. Furthermore, polydatin decreases the expression of mitochondrial fission protein Drp1 and preserves kidney function. Key: DN, diabetic nephropathy; Drp1, dynamin-related protein 1; GSH, glutathione; GPx, glutathione peroxidase; GM, gentamycin; HN, hyperuricemic nephropathy; MDA, malondialdehyde; mtDNA, mitochondrial DNA; Nrf-1 and Nrf-2, nuclear respiratory factors 1 and 2; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α; ROS, reactive oxygen species; SIRT1, silent mating type information regulation 2 homologs 1; SOD, superoxide dismutase; Trx, thioredoxin; TFAM, transcription factor A of mitochondria.
DN is a microvascular complication of diabetes that occurs in patients with type 1 and type 2 diabetes (Kwon et al., 2017; Ni et al., 2017; Sohn et al., 2017; Huang et al., 2020) that leads to mitochondrial dysfunction in podocytes (Qin et al., 2019; Qin et al., 2020; ALTamimi et al., 2021). Podocytes are terminally differentiated and highly specialized glomerular epithelial cells that contain high numbers of mitochondria (Ni et al., 2017; Qin et al., 2019). Damaged podocytes act as a marker of DKD progression (Ni et al., 2017; ALTamimi et al., 2021). HG and lipotoxicity during diabetes are known to contribute to mitochondrial dysfunction (Zhang et al., 2019). ROS production, mostly of mitochondrial origin, induces DN initiation and development in patients. NOX and uncoupling of eNOS are the other sources of ROS in diabetic patients (Zhang et al., 2019; ALTamimi et al., 2021). Administration of berberine to db/db mice decreased free fatty acid (FFA) and triglyceride (TG) levels and attenuated pathohistological changes, such as basement membrane thickening, mesangial expansion, and glomerulosclerosis, resulting in protection against podocyte apoptosis (Qin et al., 2019). In cultured mouse podocytes exposed to palmitic acid (PA), berberine treatment was found to suppress the activation of Drp1-mediated mitochondria fission. Therefore, mitochondrial ROS production, dysfunction, and fragmentation were attenuated with berberine treatment (Qin et al., 2019). Furthermore, berberine was also shown to protect glomerular podocytes against PA-induced oxidative damage by increasing SOD activity and reducing excessive mtROS and MDA. It also preserves mitochondrial function by activating AMP-activated protein kinase (AMPK), enhancing PGC-1α expression. Therefore, berberine treatment was shown to induce mitochondrial biogenesis and restore oxidative phosphorylation and fatty acid oxidation (FAO) (Qin et al., 2020).
Curcumin treatment was also found to protect the kidneys against streptozotocin (STZ)-induced diabetic injury. Curcumin also restores the ROS-antioxidant balance under diabetic stress conditions. Furthermore, it decreased ROS levels, reduced the nuclear activity of the nuclear factor-kappa B (NF-κB), downregulated protein kinase CβII (PKCβII), NOX, and p66Shc, and decreased the activation of p66Shc. Moreover, it increased the transcript level of MnSOD and GSS, the protein levels of B-cell lymphoma-2 (Bcl-2) and MnSOD, and the nuclear levels of Nrf-2 and FOXO3A (ALTamimi et al., 2021). Curcumin also lowered adenine-induced high blood pressure, urinary albumin, the inflammatory cytokines interleukins 1 (IL-1) and 6 (IL-6), tumor necrosis factor α (TNF-α), cystatin C (CST3), and adiponectin (ADIPOQ) (Kumar et al., 2021). In addition, it increased sclerostin levels in the blood and reduced oxidative stress in kidney homogenates (Ali et al., 2018). This study also showed that curcumin had anti-hyperuricemic and anti-inflammatory effects by stopping the activation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome in the kidney (Chen et al., 2019). Curcumin also makes the intestinal alkaline phosphatase and tight junction proteins more active and fixes the leakiness of the gut. This action lowers the number of inflammatory biomolecules in the blood, and curcumin in the intestine may explain its low bioavailability, which may have anti-inflammatory effects in vivo and benefit CKD (Ghosh et al., 2014). Therefore, curcumin could potentially be used to treat hyperuricemia and the inflammation associated with renal treatment.
Polydatin was also found to attenuate mitochondrial fragmentation by suppressing Drp1 activity in KKAy mice. Polydatin also inhibited podocyte apoptosis by suppressing Drp1 and attenuating mitochondrial dysfunction and ROS production in HG-induced MPC5 cells (Ni et al., 2017). Resveratrol treatment in podocytes exposed to high glucose resulted in conserved mitochondrial function, reduced excessive mitochondrial ROS production, improved respiratory chain complex I and III activity, and inhibited the release of cytochrome C (Zhang et al., 2019). In diabetic mice, resveratrol treatment decreased MDA levels and increased MnSOD activity in the renal cortex. In addition, resveratrol mechanistically protected glomerular podocytes by enhancing SIRT1/PGC-1α signaling, suppressing mitochondrial oxidative stress in the diabetic milieu (Zhang et al., 2019). In high glucose-treated rat mesangial cells, resveratrol treatment was also shown to increase MnSOD activity and decrease mitochondrial complex III activity. Consequently, mitochondrial function was improved, indicated by restored MMP, ATP production, and mtDNA content (Xu et al., 2012). Finally, salidroside treatment in STZ-induced mice maintained mitochondrial function by increasing mtDNA copy number and SIRT1 and PGC-1α expression, inhibiting kidney fibrosis (Xue et al., 2019).
The common pathogenesis of obstructed nephropathy involves fibrosis, inflammation, and apoptosis (Manucha and Valles, 2012; You et al., 2019). Fibrosis refers to extracellular matrix (ECM) accumulation in glomerular and tubulointerstitial tissue, inducing renal dysfunction. It involves multiple signaling pathways such as transforming growth factor β (TGF-β) and suppressor of mothers against decapentaplegic (Smad) and the assembly of fibrotic markers such as collagen I, fibronectin (FN), and α-smooth muscle actin (α-SMA) (Liu et al., 2019; You et al., 2019). Mice with UUO are a common model of progressive CKD, characterized by the development of tubulointerstitial fibrosis (Manucha and Valles, 2012; You et al., 2019; Martínez-Klimova et al., 2020). During renal injury, the injured tubular cells are associated with interstitial macrophages and myofibroblasts that produce cytokines such as TNF-α, interleukin 1β (IL-1β), and intercellular adhesion molecule-1 (ICAM-1) that induce an inflammatory response in the kidney (Manucha and Valles, 2012). Mitochondria-derived oxidative stress also induces an inflammatory response in obstructive kidney disease (Sun et al., 2014). Mitochondrial dysfunctions or abnormalities are observed in obstructed kidneys, resulting in inflammation exacerbation, oxidative stress, and eventually kidney fibrosis development (Sun et al., 2014; Martínez-Klimova et al., 2020).
Interestingly, a study showed that the renoprotection effect of resveratrol occurred at specific concentrations. Low concentrations of resveratrol (5–20 μM) inhibited pro-fibrotic signaling and improved renal function by increasing SIRT1 expression in the TGF-β-induced human tubular epithelial cell (TEC) cell line HK-2. However, these protective effects were not observed with higher concentrations of resveratrol (≥40 μM), which induced mitochondrial dysfunction by increasing ROS and mtROS levels and decreasing mitochondrial length and density, ATP production, and PGC-1α expression (Liu et al., 2019). Consistent with the in vitro results, the renoprotective effects of resveratrol treatment in UUO-operated C57BL/6 mice were observed at lower doses (≤25 mg/kg). However, higher concentrations of resveratrol enhanced pro-fibrotic factors and reduced TFAM expression (Liu et al., 2019).
Age-related renal changes represent glomerulosclerosis, interstitial fibrosis, arteriosclerosis, and tubular atrophy (Lee et al., 2019), resulting from various potential biological aging processes, including the expression of senescence genes, hormonal changes, increased oxidative stress, and mitochondrial damage (Kim et al., 2018). Normal kidney aging is associated with the slow development of functional and structural changes (Satoh et al., 2011). The inactivation of SIRT1 and the activation of the renin-angiotensin system, oxidative stress, and mitochondrial dysfunction are associated with aging (Kim et al., 2018). A recent study has assessed the potential use of resveratrol in delaying kidney aging (Uddin et al., 2021). Administration of resveratrol at 40 mg/kg for 6 months in 24-month-old C57BL/mice prevented aging-related kidney damage by activating Nrf-2 and SIRT1 signaling and ROS suppression. Resveratrol treatment also prevented oxidative stress by increasing SOD levels in H2O2-induced HK2 cells (Kim et al., 2018).
Certain drugs can alter intraglomerular hemodynamics and activate inflammation in renal tubular cells, resulting in AKI and tubulointerstitial disease (Shahrbaf and Assadi, 2015). Gentamycin (GM) is widely recognized as an aminoglycoside antibiotic that can induce nephrotoxicity (Cui et al., 2019). GM activates the intrinsic apoptosis pathway by stimulating the release of cytochrome C. GM also enhances oxidative stress by increasing the production of superoxide anion and hydroxyl radicals and lowering the expression of antioxidant genes such as SOD, GSS, and mitochondrial enzymes NADH dehydrogenase and cytochrome C oxidase. Moreover, GM inhibits respiratory complex I and IV and ATP synthesis, inducing mitochondrial dysfunction (Adil et al., 2016; Cui et al., 2019; Khaksari et al., 2021). Berberine treatment in GM-induced nephrotoxicity in rats significantly increased SOD, GSS, and Bcl-2 expression and mitochondrial enzyme activity. Berberine also reduced ROS and MDA levels and inflammatory and tubular injury markers such as kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), and NF-κB in GM-induced mice (Adil et al., 2016).
Cisplatin nephrotoxicity is commonly characterized by oxidative stress, inflammation, and apoptotic cell death in tubules (Miller et al., 2010). Cisplatin alters mitochondrial bioenergetics by lowering mitochondrial oxygen consumption and ATP levels, which are associated with oxidative stress-induced mtDNA damage. Moreover, cisplatin disrupted mitochondria dynamics, indicated by induced Fis1 and decreased SIRT3 expression (Ortega-Domínguez et al., 2017). GM-induced oxidant-antioxidant imbalance also altered membrane lipid composition via lipid peroxidation, indicated by increased MDA levels (Randjelovic et al., 2017). Celastrol treatment in GM-induced nephrotoxicity improved mitochondrial function, reduced oxidative stress, and attenuated tubular injury, apoptosis, and inflammation (Yu et al., 2018). Furthermore, celastrol was found to improve mitochondrial function by maintaining MMP and OXPHOS activities in cisplatin-treated renal tubular epithelial RTC cells (Yu et al., 2018). Another phytochemical, curcumin, may also prevent nephrotoxicity by upregulating Fis1 and SIRT3 in cisplatin-induced Wister rats (Ortega-Domínguez et al., 2017).
Sepsis refers to uncontrolled and adverse host reactions to microbial infection and is a leading cause of mortality and complex illness worldwide (Xu et al., 2016). Sepsis progression is accompanied by multiple organ dysfunction (Gao et al., 2015; Xu et al., 2016). During sepsis-induced AKI (SI-AKI), cytokine release, oxidative stress, and apoptosis are major pathological features in the development of organ dysfunction (Manucha and Valles, 2012; Gao et al., 2015, 2020; Uddin et al., 2018). The kidney is affected during sepsis, and AKI is a general feature during sepsis pathogenesis which is associated with high mortality rates (Xu et al., 2016; Gao et al., 2020; Wang D.-W. et al., 2021). In response to sepsis, renal tubular cells have decreased oxygen consumption, indicating extreme mitochondrial dysfunction (Xu et al., 2016). Mitochondrial dysfunction increases ROS production in renal tubular epithelial cells during AKI progression. Mitochondrial outer membranes deteriorate, and mitochondrial edema occurs, resulting in proapoptotic cytochrome C release and eventually cellular apoptosis activation (Manucha and Valles, 2012; Wang D.-W. et al., 2021).
In lipopolysaccharides (LPS)-induced HK-2 cells, curcumin treatment restored mitochondrial function and decreased ROS levels (Wang D.-W. et al., 2021). In addition, curcumin treatment in SI-AKI mice suppressed the activation of inflammatory cytokines and enhanced mitochondrial protection by attenuating ROS and MDA levels and increasing GSS and SOD activity (Wang D.-W. et al., 2021), while resveratrol treatment improved mitochondrial function by increasing SIRT1 and SIRT3 expression and ATP production. Moreover, resveratrol treatment suppressed mitochondrial cytochrome c and upregulation of SOD2, contributing to the inhibition of tubular apoptosis (Xu et al., 2016).
Polydatin treatment also decreased mitochondrial dysfunction in the sepsis-induced rat model (Gao et al., 2015). Another mechanistic study in sepsis-induced C57BL/6 mice showed that polydatin-induced Parkin translocation and mitophagy were mediated by SIRT1. Moreover, mitochondrial dysfunction, mitochondrial-dependent apoptosis, and NLRP3 inflammasome activation were attenuated in polydatin-treated mice (Gao et al., 2020).
Renal ischemia-reperfusion is the most common cause of AKI (Haschler et al., 2021), and its pathophysiology includes renal vasoconstriction, inflammation, apoptosis, tubular and glomerular damage (Visnagri et al., 2015), and mitochondrial dysfunction (Haschler et al., 2021). The deteriorating effects of ischemia-reperfusion on mitochondria include increased ROS production, decreased antioxidants, modification of pyridine nucleotide ratios, fluctuating Ca2+ concentration, and enhanced inorganic phosphate in the matrix (Visnagri et al., 2015). MMP and the ATP pool are the major determinants of mitochondrial architecture, which are decreased during ischemia, inducing mitochondrial fragmentation (Haschler et al., 2021). In renal ischemia-reperfusion (RIR)-induced Wistar rats, berberine treatment protected kidneys against oxidative stress and mitochondrial dysfunction. Moreover, upregulated anti-apoptotic protein Bcl-2 and downregulated apoptotic proteins caspase-3 (Casp3) and Bax were found in berberine-treated mice (Visnagri et al., 2015).
Chemical-induced AKI has emerged as a significant concern (Li et al., 2019) Maleate-induced nephrotoxicity is associated with ATP breakdown, enhanced ROS production, and GSH depletion. Curcumin treatment in maleate-induced Wistar rats suppressed oxidative stress and attenuated mitochondrial fragmentation by downregulating Drp1 and Fis1 (Molina-Jijón et al., 2016). Furthermore, curcumin treatment prevented potassium dichromate (K2Cr2O7)-induced mitochondrial dysfunction by increasing MMP, complexes I-III and V, and CAT, glucocorticoid receptor (GR), GPX, SOD, GSS, and glutathione S-transferase (GST), reducing oxidative stress (Molina-Jijón et al., 2011).
Cadmium (Cd) is a toxic metallic compound that can accumulate in the kidney. Cd accumulates across the renal cortex and medulla, inducing kidney injury. Oxidative stress occurs due to acute or subchronic Cd action, impairing the antioxidant defense system and causing nephrotoxicity. Cd exposure also induces mitochondrial structural damage and MMP loss. Resveratrol treatment suppressed Cd-induced oxidative stress and upregulated mitochondrial function-related factors SIRT3, SIRT1, PGC-1α, Nrf-1, and TFAM. Furthermore, resveratrol ameliorated excessive mitochondria fission and enhanced mitochondria fusion, suppressing PTEN-induced kinase (PINK1)/Parkin-mediated mitophagy (Zhang et al., 2020).
The mineralocorticoid hormone aldosterone mediated nephrotoxicity through upregulation of mitochondria ROS production and reductions in MMP, ATP production, and mtDNA copy number. Aldosterone also induces CASP3 and caspase 9 (CASP9) mediated apoptosis in aldosterone-instigated podocytes (Yuan et al., 2012). Administration of 400 mg/kg resveratrol for 14 days in aldosterone-induced C57BL/6J mice showed improvement in mitochondria function. Treatment with 50 μM/L resveratrol for 30 min in aldosterone-induced podocytes increased mtDNA copy number and PGC-1α, TFAM, and SIRT3 expression. Apoptotic proteins CASP3 and CASP9 were also downregulated in response to the treatment (Yuan et al., 2012).
Therapeutic enzymes have also provided opportunities to explore novel therapeutic targets for CVD and CKD to discover new treatment regimens (Kumar et al., 2022). CVDs are the primary cause of death in CKD, suggesting that angiotensin inhibition caused a time-dependent increase in heart-ankle pulse wave velocity in non-diabetic CKD. In addition, it has been suggested that suppression of angiotensin led to an improvement in patient prognosis in non-diabetic CKD characterized by mild to moderate renal impairment (Mimura et al., 2008). Inhibition of some enzymes and activation of others are important regulatory strategies that can be used to slow or stop the progression of various kidney abnormalities (Granata et al., 2022). Extracellular superoxide dismutase (EC-SOD) is protective in CKD progression via reducing NOX activity and oxidative stress through β-catenin signaling in various kidney injuries (Tan et al., 2015).
Hemorrhagic shock is a common cause of mortality in significantly injured patients and could contribute to AKI, causing mitochondrial dysfunction by increasing ROS production. In the five-sixths nephrectomy (5/6NX) model, remnant nephrons show metabolic and hemodynamic responses to compensate for the renal mass loss, where the remnant nephrons undergo hypertrophy and hyperfunction. This condition contributes to ROS overproduction that induces mitochondrial dysfunction and CKD progression (Aparicio-Trejo et al., 2017). Treatment with resveratrol mitigates oxidative stress by decreasing mitochondrial ROS production and increasing SOD2 and CAT activity in hemorrhagic shock-induced Evans rats. Moreover, resveratrol treatment increased SIRT1 and PGC-1α expression, restoring mitochondrial function (Wang et al., 2015). Administration of curcumin also suppressed oxidative stress by increasing CAT, SOD, GR, GPX, GST, and Nrf-2 expression in nephrectomy-induced Wistar rats. Curcumin significantly protected mitochondrial function by decreasing Drp1 and Fis1 expression and increasing the activities of mitochondrial complexes I and V (Aparicio-Trejo et al., 2017).
It has been shown that edible phytochemicals and their main chemical components from Camellia sinensis (green tea), Rubus idaeus (raspberry), Rubia cordifolia (common madder), Pistacia lentiscus (mastic), Petroselinum crispum (parsley), Punica granatum (pomegranate), Urtica dioica (stinging nettle), Solanum xanthocarpum (yellow-fruit nightshade), Dolichos biflorus (horse gram), and Nigella sativa (black cumin) have received considerable interest in treating CKD (Nirumand et al., 2018; Hannan et al., 2021). In addition, other phytochemicals such as the antioxidant polyphenols catechin, epicatechin, epigallocatechin-3-gallate, diosmin, rutin, quercetin, hyperoside, and curcumin have been found to help prevent urolithiasis (Maditz et al., 2013). The main mechanisms through which these plants and their isolated phytonutrients help treat urolithiasis are their diuretic, antispasmodic, and antioxidant effects and their ability to stop crystals from forming, growing, and sticking together (Bland and Editor, 2017). Therefore, eating plants and polyphenols may help prevent and treat kidney stones. Further research is required to ensure that these compounds are safe and effective.
Maintaining mitochondrial function has emerged as a potential strategy for ameliorating various human diseases, including kidney injury. This review highlighted how small molecule natural products could protect the kidneys from mitochondrial oxidative stress and mtDNA damage and improve mitochondrial biogenesis and dynamics in various kidney diseases, such as DN, HN, AKI, and CKD. Mitochondrial-targeted therapy is highly recommended to obtain maximum benefits, exploiting modern approaches such as nano-guided drug delivery. Further studies on the mechanism of action and therapeutic effects of small molecule natural products are required to enhance the viability of alternative therapeutic strategies in kidney diseases.
MAR editing and writing-original draft preparation. SA conceptualization, data curation, editing and writing-original draft preparation. MJU designed the outlines and drafted the manuscript. AM, DD, and MNU wrote the initial draft of the manuscript. MJH, MSA, and WK visualization. MAH and BK reviewed the scientific content described in the manuscript. All authors read and approved the final submitted version of the manuscript.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1I1A2066868), the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A5A2019413). Additionally, this work acknowledges the National Research Foundation (No. 2020R1I1A1A01072879 and 2015H1D3A1062189), and the Brain Pool program funded by the Ministry of Science and ICT through the National Research Foundation (No. 2020H1D3A2A02110924), South Korea.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adil, M., Kandhare, A. D., Dalvi, G., Ghosh, P., Venkata, S., Raygude, K. S., et al. (2016). Ameliorative effect of berberine against gentamicin-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Ren. Fail. 38, 996–1006. doi:10.3109/0886022X.2016.1165120
Ahn, B. H., Kim, H. S., Song, S., Lee, I. H., Liu, J., Vassilopoulos, A., et al. (2008). A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. U. S. A. 105, 14447–14452. doi:10.1073/pnas.0803790105
Ali, B. H., Al-Salam, S., Al Suleimani, Y., Al Kalbani, J., Al Bahlani, S., Ashique, M., et al. (2018). Curcumin ameliorates kidney function and oxidative stress in experimental chronic kidney disease. Basic Clin. Pharmacol. Toxicol. 122, 65–73. doi:10.1111/bcpt.12817
ALTamimi, J. Z., AlFaris, N. A., Al-Farga, A. M., Alshammari, G. M., BinMowyna, M. N., and Yahya, M. A. (2021). Curcumin reverses diabetic nephropathy in streptozotocin-induced diabetes in rats by inhibition of PKCβ/p66Shc axis and activation of FOXO-3a. J. Nutr. Biochem. 87, 108515. doi:10.1016/j.jnutbio.2020.108515
Aparicio-Trejo, O. E., Tapia, E., Molina-Jijón, E., Medina-Campos, O. N., Macías-Ruvalcaba, N. A., León-Contreras, J. C., et al. (2017). Curcumin prevents mitochondrial dynamics disturbances in early 5/6 nephrectomy: Relation to oxidative stress and mitochondrial bioenergetics. BioFactors 43, 293–310. doi:10.1002/biof.1338
Aparicio-Trejo, O. E., Tapia, E., Sánchez-Lozada, L. G., and Pedraza-Chaverri, J. (2018). Mitochondrial bioenergetics, redox state, dynamics and turnover alterations in renal mass reduction models of chronic kidney diseases and their possible implications in the progression of this illness. Pharmacol. Res. 135, 1–11. doi:10.1016/j.phrs.2018.07.015
Basist, P., Parveen, B., Zahiruddin, S., Gautam, G., Parveen, R., Khan, M. A., et al. (2022). Potential nephroprotective phytochemicals: Mechanism and future prospects. J. Ethnopharmacol. 283, 114743. doi:10.1016/j.jep.2021.114743
Bhargava, P., and Schnellmann, R. G. (2017). Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 13, 629–646. doi:10.1038/nrneph.2017.107
Bland, J., and Editor, A. (2017). Chronic kidney disease: The gut-kidney connection? Integr. Med. A Clin. J. 16, 14.
Bulboacă, A. E., Porfire, A., Bolboacă, S. D., Nicula, C. A., Feștilă, D. G., Roman, A., et al. (2021). Protective effects of liposomal curcumin on oxidative stress/antioxidant imbalance, metalloproteinases 2 and -9, Histological changes and renal function in experimental nephrotoxicity induced by gentamicin. Antioxidants (Basel) 10, 325. doi:10.3390/antiox10020325
Carlström, M. (2021). Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat. Rev. Nephrol. 17, 575–590. doi:10.1038/s41581-021-00429-z
Chen, C., Zhou, M., Ge, Y., and Wang, X. (2020). SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 187, 111215. doi:10.1016/j.mad.2020.111215
Chen, Y., Li, C., Duan, S., Yuan, X., Liang, J., and Hou, S. (2019). Curcumin attenuates potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Biomed. Pharmacother. 118, 109195. doi:10.1016/j.biopha.2019.109195
Chikezie, P. C. (2015). Herbal medicine: Yesterday, today and tomorrow. Altern. Integr. Med. 04, 1000195. doi:10.4172/2327-5162.1000195
Cui, J., Tang, L., Hong, Q., Lin, S., Sun, X., Cai, G., et al. (2019). N-Acetylcysteine ameliorates gentamicin-induced nephrotoxicity by enhancing autophagy and reducing oxidative damage in miniature pigs. Shock 52, 622–630. doi:10.1097/SHK.0000000000001319
Dias, D. A., Urban, S., and Roessner, U. (2012). A historical overview of natural products in drug discovery. Metabolites 2, 303–336. doi:10.3390/metabo2020303
Gao, Y., Dai, X., Li, Y., Li, G., Lin, X., Ai, C., et al. (2020). Role of parkin-mediated mitophagy in the protective effect of polydatin in sepsis-induced acute kidney injury. J. Transl. Med. 18, 114. doi:10.1186/s12967-020-02283-2
Gao, Y., Zeng, Z., Li, T., Xu, S., Wang, X., Chen, Z., et al. (2015). Polydatin inhibits mitochondrial dysfunction in the renal tubular epithelial cells of a rat model of sepsis-induced acute kidney injury. Anesth. Analg. 121, 1251–1260. doi:10.1213/ANE.0000000000000977
Ghosh, S. S., Gehr, T. W., and Ghosh, S. (2014). Curcumin and chronic kidney disease (CKD): Major mode of action through stimulating endogenous intestinal alkaline phosphatase. Molecules 19, 20139–20156. doi:10.3390/molecules191220139
Granata, S., Votrico, V., Spadaccino, F., Catalano, V., Netti, G. S., Ranieri, E., et al. (2022). Oxidative stress and ischemia/reperfusion injury in kidney transplantation: Focus on ferroptosis, mitophagy and new antioxidants. Antioxidants (Basel) 11, 769. doi:10.3390/antiox11040769
Hannan, M. A., Rahman, M. A., Sohag, A. A. M., Uddin, M. J., Dash, R., Sikder, M. H., et al. (2021). Black cumin (Nigella sativa L.): A comprehensive review on phytochemistry, health benefits, molecular pharmacology, and safety. Nutrients 13, 1784. doi:10.3390/nu13061784
Haschler, T. N., Horsley, H., Balys, M., Anderson, G., Taanman, J. W., Unwin, R. J., et al. (2021). Sirtuin 5 depletion impairs mitochondrial function in human proximal tubular epithelial cells. Sci. Rep. 11, 15510. doi:10.1038/s41598-021-94185-6
Heeringa, P., Steenbergen, E., and Van Goor, H. (2002). A protective role for endothelial nitric oxide synthase in glomerulonephritis. Kidney Int. 61, 822–825. doi:10.1046/j.1523-1755.2002.00227.x
Hiyoshi, H., Yayama, K., Takano, M., and Okamoto, H. (2005). Angiotensin type 2 receptor-mediated phosphorylation of eNOS in the aortas of mice with 2-kidney, 1-clip hypertension. Hypertension 45, 967–973. doi:10.1161/01.HYP.0000164571.77710.19
Huang, S., Tan, M., Guo, F., Dong, L., Liu, Z., Yuan, R., et al. (2020). Nepeta angustifolia C. Y. Wu improves renal injury in HFD/STZ-induced diabetic nephropathy and inhibits oxidative stress-induced apoptosis of mesangial cells. J. Ethnopharmacol. 255, 112771. doi:10.1016/j.jep.2020.112771
Hui, Y., Lu, M., Han, Y., Zhou, H., Liu, W., Li, L., et al. (2017). Resveratrol improves mitochondrial function in the remnant kidney from 5/6 nephrectomized rats. Acta Histochem 119, 392–399. doi:10.1016/j.acthis.2017.04.002
Jiang, M., Bai, M., Lei, J., Xie, Y., Xu, S., Jia, Z., et al. (2020). Mitochondrial dysfunction and the AKI to CKD transition. Am. J. Physiol. Physiol. 319, F1105–F1116. doi:10.1152/ajprenal.00285.2020
Jin, J. Y., Wei, X. X., Zhi, X. L., Wang, X. H., and Meng, D. (2021). Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol. Sin. 42, 655–664. doi:10.1038/s41401-020-00518-y
Kaur, A., Kaur, T., Singh, B., Pathak, D., Singh Buttar, H., and Pal Singh, A. (2016). Curcumin alleviates ischemia reperfusion-induced acute kidney injury through NMDA receptor antagonism in rats. Ren. Fail. 38, 1462–1467. doi:10.1080/0886022X.2016.1214892
Khaksari, M., Esmaili, S., Abedloo, R., and Khastar, H. (2021). Palmatine ameliorates nephrotoxicity and hepatotoxicity induced by gentamicin in rats. Arch. Physiol. Biochem. 127, 273–278. doi:10.1080/13813455.2019.1633354
Kim, E. N., Lim, J. H., Kim, M. Y., Ban, T. H., Jang, I. A., Yoon, H. E., et al. (2018). Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging (Albany NY) 10, 83–99. doi:10.18632/aging.101361
Kitada, M., Kume, S., Takeda-Watanabe, A., Kanasaki, K., and Koya, D. (2013). Sirtuins and renal diseases: Relationship with aging and diabetic nephropathy. Clin. Sci. (Lond) 124, 153–164. doi:10.1042/CS20120190
Kong, X., Wang, R., Xue, Y., Liu, X., Zhang, H., Chen, Y., et al. (2010). Sirtuin 3, a new target of PGC-1α, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One 5, e11707. doi:10.1371/journal.pone.0011707
Kumar, G., Dey, S. K., and Kundu, S. (2020). Functional implications of vascular endothelium in regulation of endothelial nitric oxide synthesis to control blood pressure and cardiac functions. Life Sci. 259, 118377. doi:10.1016/j.lfs.2020.118377
Kumar, G., Dey, S. K., and Kundu, S. (2021). Herbs and their bioactive ingredients in cardio-protection: Underlying molecular mechanisms and evidences from clinical studies. Phytomedicine 92, 153753. doi:10.1016/j.phymed.2021.153753
Kumar, G., Saini, M., and Kundu, S. (2022). Therapeutic enzymes as non-conventional targets in cardiovascular impairments: A comprehensive review. Can. J. Physiol. Pharmacol. 100, 197–209. doi:10.1139/cjpp-2020-0732
Kwon, G., Uddin, M. J., Lee, G., Jiang, S., Cho, A., Lee, J. H., et al. (2017). A novel pan-nox inhibitor, APX-115, protects kidney injury in streptozotocin-induced diabetic mice: Possible role of peroxisomal and mitochondrial biogenesis. Oncotarget 8, 74217–74232. doi:10.18632/oncotarget.18540
Lee, G., Uddin, M. J., Kim, Y., Ko, M., Yu, I., and Ha, H. (2019). PGC-1α, a potential therapeutic target against kidney aging. Aging Cell 18, e12994. doi:10.1111/acel.12994
Lee, S. R., An, E. J., Kim, J., and Bae, Y. S. (2020). Function of NADPH oxidases in diabetic nephropathy and development of Nox inhibitors. Biomol. Ther. Seoul. 28, 25–33. doi:10.4062/biomolther.2019.188
Li, Q., Xing, C., and Yuan, Y. (2021a). Mitochondrial targeting of herbal medicine in chronic kidney disease. Front. Pharmacol. 12, 632388. doi:10.3389/fphar.2021.632388
Li, W., Yang, Y., Li, Y., Zhao, Y., and Jiang, H. (2019). Sirt5 attenuates cisplatin-induced acute kidney injury through regulation of Nrf2/HO-1 and Bcl-2. Biomed. Res. Int. 2019, 4745132. doi:10.1155/2019/4745132
Li, Y., Hepokoski, M., Gu, W., Simonson, T., and Singh, P. (2021b). Targeting mitochondria and metabolism in acute kidney injury. J. Clin. Med. 10, 3991. doi:10.3390/jcm10173991
Liu, S., Zhao, M., Zhou, Y., Wang, C., Yuan, Y., Li, L., et al. (2019). Resveratrol exerts dose-dependent anti-fibrotic or pro-fibrotic effects in kidneys: A potential risk to individuals with impaired kidney function. Phytomedicine 57, 223–235. doi:10.1016/j.phymed.2018.12.024
Maditz, K. H., Gigliotti, J. C., and Tou, J. C. (2013). Evidence for a role of proteins, lipids, and phytochemicals in the prevention of polycystic kidney disease progression and severity. Nutr. Rev. 71, 802–814. doi:10.1111/nure.12085
Manucha, W., and Vallés, P. G. (2012). Apoptosis modulated by oxidative stress and inflammation during obstructive nephropathy. Inflamm. Allergy Drug Targets 11, 303–312. doi:10.2174/187152812800958997
Martínez-Klimova, E., Aparicio-Trejo, O. E., Gómez-Sierra, T., Jiménez-Uribe, A. P., Bellido, B., and Pedraza-Chaverri, J. (2020). Mitochondrial dysfunction and endoplasmic reticulum stress in the promotion of fibrosis in obstructive nephropathy induced by unilateral ureteral obstruction. BioFactors 46, 716–733. doi:10.1002/biof.1673
Miller, R. P., Tadagavadi, R. K., Ramesh, G., and Reeves, W. B. (2010). Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2, 2490–2518. doi:10.3390/toxins2112490
Mimura, T., Takenaka, T., Kanno, Y., Moriwaki, K., Okada, H., and Suzuki, H. (2008). Vascular compliance is secured under angiotensin inhibition in non-diabetic chronic kidney diseases. J. Hum. Hypertens. 22, 38–47. doi:10.1038/sj.jhh.1002264
Molina-Jijón, E., Tapia, E., Zazueta, C., El Hafidi, M., Zatarain-Barrón, Z. L., Hernández-Pando, R., et al. (2011). Curcumin prevents Cr(VI)-induced renal oxidant damage by a mitochondrial pathway. Free Radic. Biol. Med. 51, 1543–1557. doi:10.1016/j.freeradbiomed.2011.07.018
Molina-Jijón, E., Aparicio-Trejo, O. E., Rodríguez-Muñoz, R., León-Contreras, J. C., del Carmen Cárdenas-Aguayo, M., Medina-Campos, O. N., et al. (2016). The nephroprotection exerted by curcumin in maleate-induced renal damage is associated with decreased mitochondrial fission and autophagy. BioFactors 42, 686–702. doi:10.1002/biof.1313
Ni, Z., Tao, L., Xiaohui, X., Zelin, Z., Jiangang, L., Zhao, S., et al. (2017). Polydatin impairs mitochondria fitness and ameliorates podocyte injury by suppressing Drp1 expression. J. Cell. Physiol. 232, 2776–2787. doi:10.1002/jcp.25943
Nirumand, M. C., Hajialyani, M., Rahimi, R., Farzaei, M. H., Zingue, S., Nabavi, S. M., et al. (2018). Dietary plants for the prevention and management of kidney stones: Preclinical and clinical evidence and molecular mechanisms. Int. J. Mol. Sci. 19, 765. doi:10.3390/ijms19030765
Ogura, Y., Kitada, M., Xu, J., Monno, I., and Koya, D. (2020). CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD+/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats. Aging (Albany NY) 12, 11325–11336. doi:10.18632/aging.103410
Ortega-Domínguez, B., Aparicio-Trejo, O. E., García-Arroyo, F. E., León-Contreras, J. C., Tapia, E., Molina-Jijón, E., et al. (2017). Curcumin prevents cisplatin-induced renal alterations in mitochondrial bioenergetics and dynamic. Food Chem. Toxicol. 107, 373–385. doi:10.1016/j.fct.2017.07.018
Pedraza-Chaverri, J., Sánchez-Lozada, L. G., Osorio-Alonso, H., Tapia, E., and Scholze, A. (2016). New pathogenic concepts and therapeutic approaches to oxidative stress in chronic kidney disease. Oxidative Med. Cell. Longev. 2016, 1–21. doi:10.1155/2016/6043601
Pereira, B. P., do Vale, G. T., and Ceron, C. S. (2022). The role of nitric oxide in renovascular hypertension: From the pathophysiology to the treatment. Naunyn. Schmiedeb. Arch. Pharmacol. 395, 121–131. doi:10.1007/s00210-021-02186-z
Qin, X., Jiang, M., Zhao, Y., Gong, J., Su, H., Yuan, F., et al. (2020). Berberine protects against diabetic kidney disease via promoting PGC-1α-regulated mitochondrial energy homeostasis. Br. J. Pharmacol. 177, 3646–3661. doi:10.1111/bph.14935
Qin, X., Zhao, Y., Gong, J., Huang, W., Su, H., Yuan, F., et al. (2019). Berberine protects glomerular podocytes via inhibiting Drp1-mediated mitochondrial fission and dysfunction. Theranostics 9, 1698–1713. doi:10.7150/thno.30640
Rahman, M. A., Rahman, M. H., Biswas, P., Hossain, M. S., Islam, R., Hannan, M. A., et al. (2020). Potential therapeutic role of phytochemicals to mitigate mitochondrial dysfunctions in Alzheimer's disease. Antioxidants 10, 23. doi:10.3390/antiox10010023
Randjelovic, P., Veljkovic, S., Stojiljkovic, N., Sokolovic, D., and Ilic, I. (2017). Gentamicin nephrotoxicity in animals: Current knowledge and future perspectives. EXCLI J. 16, 388–399. doi:10.17179/excli2017-165
Ravarotto, V., Simioni, F., Pagnin, E., Davis, P. A., and Calò, L. A. (2018). Oxidative stress - Chronic kidney disease - Cardiovascular disease: A vicious circle. Life Sci. 210, 125–131. doi:10.1016/j.lfs.2018.08.067
Ruiz, S., Pergola, P. E., Zager, R. A., and Vaziri, N. D. (2013). Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 83, 1029–1041. doi:10.1038/ki.2012.439
Satoh, M., Fujimoto, S., Horike, H., Ozeki, M., Nagasu, H., Tomita, N., et al. (2011). Mitochondrial damage-induced impairment of angiogenesis in the aging rat kidney. Lab. Invest. 91, 190–202. doi:10.1038/labinvest.2010.175
Segawa, Y., Hashimoto, H., Maruyama, S., Shintani, M., Ohno, H., Nakai, Y., et al. (2020). Dietary capsaicin-mediated attenuation of hypertension in a rat model of renovascular hypertension. Clin. Exp. Hypertens. 42, 352–359. doi:10.1080/10641963.2019.1665676
Shahrbaf, F. G., and Assadi, F. (2015). Drug-induced renal disorders. J. Ren. Inj. Prev. 4, 57–60. doi:10.12861/jrip.2015.12
Sohn, M., Kim, K., Uddin, M. J., Lee, G., Hwang, I., Kang, H., et al. (2017). Delayed treatment with fenofibrate protects against high-fat diet-induced kidney injury in mice: The possible role of AMPK autophagy. Am. J. Physiol. Physiol. 312, F323–F334. doi:10.1152/ajprenal.00596.2015
Stenvinkel, P., Carrero, J. J., Axelsson, J., Lindholm, B., Heimbürger, O., and Massy, Z. (2008). Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin. J. Am. Soc. Nephrol. 3, 505–521. doi:10.2215/CJN.03670807
Streeter, J., Thiel, W., Brieger, K., and Miller, F. J. (2013). Opportunity Nox: The future of NADPH oxidases as therapeutic targets in cardiovascular disease. Cardiovasc. Ther. 31, 125–137. doi:10.1111/j.1755-5922.2011.00310.x
Sun, J., Zhang, J., Tian, J., Virzì, G. M., Digvijay, K., Cueto, L., et al. (2019). Mitochondria in sepsis-induced AKI. J. Am. Soc. Nephrol. 30, 1151–1161. doi:10.1681/ASN.2018111126
Sun, Y., Zhang, Y., Zhao, D., Ding, G., Huang, S., Zhang, A., et al. (2014). Rotenone remarkably attenuates oxidative stress, inflammation, and fibrosis in chronic obstructive uropathy. Mediat. Inflamm. 2014, 670106. doi:10.1155/2014/670106
Svensson, K., Schnyder, S., Cardel, B., and Handschin, C. (2016). Loss of renal tubular PGC-1α exacerbates diet-induced renal steatosis and age-related urinary sodium excretion in mice. PLoS One 11, e0158716. doi:10.1371/journal.pone.0158716
Tan, R. J., Zhou, D., Xiao, L., Zhou, L., Li, Y., Bastacky, S. I., et al. (2015). Extracellular superoxide dismutase protects against proteinuric kidney disease. J. Am. Soc. Nephrol. 26, 2447–2459. doi:10.1681/ASN.2014060613
Tang, C., Cai, J., Yin, X. M., Weinberg, J. M., Venkatachalam, M. A., and Dong, Z. (2021). Mitochondrial quality control in kidney injury and repair. Nat. Rev. Nephrol. 17, 299–318. doi:10.1038/s41581-020-00369-0
Uddin, M. J., Farjana, M., Moni, A., Hossain, K. S., Hannan, M. A., and Ha, H. (2021). Prospective pharmacological potential of Resveratrol in delaying kidney aging. Int. J. Mol. Sci. 22, 8258. doi:10.3390/ijms22158258
Uddin, M. J., Pak, E. S., and Ha, H. (2018). Carbon monoxide releasing molecule-2 protects mice against acute kidney injury through inhibition of ER stress. Korean J. Physiol. Pharmacol. 22, 567–575. doi:10.4196/kjpp.2018.22.5.567
Visnagri, A., Kandhare, A. D., and Bodhankar, S. L. (2015). Renoprotective effect of berberine via intonation on apoptosis and mitochondrial-dependent pathway in renal ischemia reperfusion-induced mutilation. Ren. Fail. 37, 482–493. doi:10.3109/0886022X.2014.996843
Wang, D.-W., Li, S. J., Tan, X. Y., Wang, J. H., Hu, Y., Tan, Z., et al. (2021a). Engineering of stepwise-targeting chitosan oligosaccharide conjugate for the treatment of acute kidney injury. Carbohydr. Polym. 256, 117556. doi:10.1016/j.carbpol.2020.117556
Wang, H., Guan, Y., Karamercan, M. A., Ye, L., Bhatti, T., Becker, L. B., et al. (2015). Resveratrol rescues kidney mitochondrial function following hemorrhagic shock. Shock 44, 173–180. doi:10.1097/SHK.0000000000000390
Wang, W., Li, J., and Cai, L. (2021b). Research progress of sirtuins in renal and cardiovascular diseases. Curr. Opin. Nephrol. Hypertens. 30, 108–114. doi:10.1097/MNH.0000000000000660
Wang, Y., and Gao, L. (2022). Inflammation and cardiovascular disease associated with hemodialysis for end-stage renal disease. Front. Pharmacol. 13, 800950. doi:10.3389/fphar.2022.800950
Whitaker, R. M., Corum, D., Beeson, C. C., and Schnellmann, R. G. (2016). Mitochondrial biogenesis as a pharmacological target: A new approach to acute and chronic diseases. Annu. Rev. Pharmacol. Toxicol. 56, 229–249. doi:10.1146/annurev-pharmtox-010715-103155
Xu, S., Gao, Y., Zhang, Q., Wei, S., Chen, Z., Dai, X., et al. (2016). SIRT1/3 activation by resveratrol attenuates acute kidney injury in a septic rat model. Oxidative Med. Cell. Longev. 2016, 1–12. doi:10.1155/2016/7296092
Xu, Y., Nie, L., Yin, Y. G., Tang, J. L., Zhou, J. Y., Li, D. D., et al. (2012). Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicol. Appl. Pharmacol. 259, 395–401. doi:10.1016/j.taap.2011.09.028
Xue, H., Li, P., Luo, Y., Wu, C., Liu, Y., Qin, X., et al. (2019). Salidroside stimulates the Sirt1/PGC-1α axis and ameliorates diabetic nephropathy in mice. Phytomedicine 54, 240–247. doi:10.1016/j.phymed.2018.10.031
You, Y. K., Luo, Q., Wu, W. F., Zhang, J. J., Zhu, H. J., Lao, L., et al. (2019). Petchiether A attenuates obstructive nephropathy by suppressing TGF-β/Smad3 and NF-κB signalling. J. Cell. Mol. Med. 23, 5576–5587. doi:10.1111/jcmm.14454
Yu, X., Meng, X., Xu, M., Zhang, X., Zhang, Y., Ding, G., et al. (2018). Celastrol ameliorates cisplatin nephrotoxicity by inhibiting NF-κB and improving mitochondrial function. EBioMedicine 36, 266–280. doi:10.1016/J.EBIOM.2018.09.031
Yuan, Y., Huang, S., Wang, W., Wang, Y., Zhang, P., Zhu, C., et al. (2012). Activation of peroxisome proliferator-activated receptor-γ coactivator 1α ameliorates mitochondrial dysfunction and protects podocytes from aldosterone-induced injury. Kidney Int. 82, 771–789. doi:10.1038/ki.2012.188
Zhang, Q., Zhang, C., Ge, J., Lv, M. W., Talukder, M., Guo, K., et al. (2020). Ameliorative effects of resveratrol against cadmium-induced nephrotoxicity via modulating nuclear xenobiotic receptor response and PINK1/Parkin-mediated Mitophagy. Food Funct. 11, 1856–1868. doi:10.1039/C9FO02287B
Zhang, T., Chi, Y., Kang, Y., Lu, H., Niu, H., Liu, W., et al. (2019). Resveratrol ameliorates podocyte damage in diabetic mice via SIRT1/PGC-1α mediated attenuation of mitochondrial oxidative stress. J. Cell. Physiol. 234, 5033–5043. doi:10.1002/jcp.27306
Zhong, Y., Menon, M. C., Deng, Y., Chen, Y., and He, J. C. (2015). Recent advances in traditional Chinese medicine for kidney disease. Am. J. Kidney Dis. 66, 513–522. doi:10.1053/j.ajkd.2015.04.013
Keywords: kidney diseases, mitochondrial dysfunction, small molecule natural products, traditional medicine, renoprotective effect
Citation: Rahman MA, Akter S, Dorotea D, Mazumder A, Uddin MN, Hannan MA, Hossen MJ, Ahmed MS, Kim W, Kim B and Uddin MJ (2022) Renoprotective potentials of small molecule natural products targeting mitochondrial dysfunction. Front. Pharmacol. 13:925993. doi: 10.3389/fphar.2022.925993
Received: 22 April 2022; Accepted: 27 June 2022;
Published: 15 July 2022.
Edited by:
Yasmina Mohammed Abd EL-Hakim, Zagazig University, EgyptReviewed by:
Marcos Roberto De Oliveira, Federal University of Rio Grande do Sul, BrazilCopyright © 2022 Rahman, Akter, Dorotea, Mazumder, Uddin, Hannan, Hossen, Ahmed, Kim, Kim and Uddin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bonglee Kim, Ym9uZ2xlZWtpbUBraHUuYWMua3I=; Md Jamal Uddin, aGFzYW44MDA5MjBAZ21haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.