- 1Department of Radiology, The Second Hospital of Jilin University, Changchun, China
- 2Department of Blood Transfusion, Central Hospital of Changchun, Changchun, China
- 3Department ofGeneral Surgery, The Second Hospital of Jilin University, Changchun, China
Nanomaterials integrating a variety of excellent properties (such as controllable/suitable size, surface modifier, and multifunctionality) have attracted increasing attention in the biomedical field and have been considered a new generation of magnetic resonance imaging (MRI) contrast agents (CAs). In recent years, stimuli-responsive nanomaterials with specifically responsive ability have been synthesized as MRI CAs, which can significantly improve the diagnostic sensitivity and accuracy depending on their outstanding performance. Furthermore, the inherent tumor microenvironment (TME) of malignant tumor is considered to possess several unique features, such as low extracellular pH, redox condition, hypoxia, and high interstitial pressure, that are significantly different from healthy tissues. Hence, constructing nanomaterials for TME-responsive MRI as an emerging strategy is expected to overcome the current obstacles to precise diagnosis. This review focuses on recent advances of nanomaterials in their application of TME-responsive MRI that trigger the diagnostic function in response to various endogenous stimulations, including pH, redox, enzyme, and hypoxia. Moreover, the future challenges and trends in the development of nanomaterials serving as TME-responsive MRI CAs are discussed.
Introduction
Cancer has become a primary cause of death and a most serious threat to human health worldwide (Sung et al., 2021). Early and accurate diagnosis is crucial to conquering cancer, which can guide clinicians to design the most timely, suitable, and reasonable therapeutic strategies for cancer patients. Magnetic resonance imaging (MRI) as one of the most powerful non-invasive imaging instruments with advantages of non-radiation and excellent spatial resolution. It is widely used in the clinical diagnosis of cancer (Na et al., 2009). It can provide anatomical and functional information of organs and soft tissues, for instance, T1 MRI can display normal anatomy with excellent resolution and T2 MRI is adept at detecting tumors and inflammation lesions with outstanding soft tissue imaging. The introduction of contrast agents (CAs) further improves the sensitivity and capability of MRI to detect lesions, which offers unprecedented and comprehensive diagnostic information for clinicians (Lohrke et al., 2016). Gadolinium (Gd3+) chelate–based MRI CAs are most commonly used in clinics at present. However, these Gd-based CAs have their own limitations, such as very short internal circulation time, poor specificity, and relatively low relaxation efficiency. In order to overcome the above defects, it is necessary to use large doses of Gd-based CAs to increase the CA concentration in the target area (Raymond and Pierre, 2005). Afterward, high-dose injection of Gd3+ chelates may cause severe nephrogenic systemic fibrosis syndrome, as has been warned by the US Food and Drug Administration (FDA) (Penfield and Reilly, 2007; Perez-Rodriguez et al., 2009; Chen et al., 2012). Therefore, the comprehensive performance of currently available CAs in clinic is far from ideal. Research and development of innovative and efficient MRI CAs as a substitute for traditional Gd-based CAs is highly desirable and has become a very promising field.
Over the past decades, nanomaterials integrating a variety of excellent properties have been proposed as a new generation of CAs with great potential in MRI. As imaging CAs, nanomaterials have a series of attractive characteristics: 1) controllable and suitable size, which ensures sufficient circulation time in blood and clearance ability by kidneys or reticuloendothelial system (RES) (Longmire et al., 2011). 2) Surface modifiers, which allow them to be coated with biocompatible materials to improve their biocompatibility or conjugated with ligands to provide additional properties such as target-specific binding ability, barrier-penetrating ability, and long circulation time (Lim et al., 2015). 3) Multifunctionality, integrating multiple imaging modes into one platform, making them capable of providing comprehensive information for detecting lesions. The most frequently developed nanomaterials as T1 MRI CAs are Gd-based complexes (e.g., Gd2O3-, NaGdF4-, GdF3-, and Gd3+-doped nanoparticles [NPs]) (Liu et al., 2011; Kimura et al., 2012; Park et al., 2012; Passuello et al., 2012; Xing et al., 2014; Du et al., 2017) and paramagnetic manganese (Mn)-based composites (e.g., MnO NPs, MnO2 nanosheets, and Mn3O4 NPs) (Kim et al., 2011; Chen et al., 2014; Li et al., 2017), which can shorten their longitudinal relaxation time (T1) due to their excellent ability to interact with hydrogen protons in the surrounding water. Superparamagnetic iron oxide (SPIO) NPs are the other kind of popular CAs which can shorten transverse relaxation time (T2) because of their intrinsic magnetic properties, serving as T2 MRI CAs (Laurent et al., 2008; Tong et al., 2010). Despite the variety of nanomaterials with remarkable performance having been synthesized as MRI CAs which undeniably improved the sensitivity of detecting subtle lesions to a certain extent, there are still some shortages that cannot be neglected. For instance, 1) the majority of current MRI CAs possess the function of enhancing MR contrast signals “always on” regardless of their specific accumulation in target tumors or tissues, leading to poor target-to-background signal ratios. Therefore, it is difficult to distinguish the region of interest from surrounding normal tissues due to non-specific signal enhancement, which may lead to a false-positive diagnosis or missed diagnosis (Yoo and Pagel, 2008; Fu et al., 2021). 2) Dissatisfactory relaxivity causes insufficient sensitivity to detect early-stage tumors or tiny lesions, which may miss the optimal opportunity for treatment (Yu et al., 2014; Lee et al., 2015). 3) Inadequate internal circulation time results in narrow observation window of MRI, reduces diagnostic efficiency, and impedes their applications (Kielar et al., 2010; Ai, 2011; Shen et al., 2017).
Consequentially, designing stimuli-responsive CAs with specifically responsive ability is expected to overcome the above obstacles. Compared with traditional CAs, these stimuli-responsive CAs have exhibited conspicuously enhanced contrasts between target lesions (especially the small lesions or early-stage tumors) and normal tissues. Many endogenous or exogenous stimuli have been exploited to serve as triggers for stimuli-responsive CAs previously, including endo-stimuli (internal) such as pH, redox, and enzymes, and exo-stimuli (external) such as light, temperature, ultrasound, and electric or magnetic fields (Kang et al., 2017; García-Hevia et al., 2019). In addition, the tumor microenvironment (TME) of various types of malignant tumors, affected by malignant proliferation and metabolism, is considered to possess several unique features such as low extracellular pH, redox conditions, hypoxia, and high interstitial pressure (Danhier et al., 2010; Joyce and Fearon, 2015; Zhu et al., 2022). Nanomaterial-based TME-responsive CAs have attracted widespread attention for personalized cancer diagnosis owing to their outstanding performances in tumor-specific imaging, which could provide more accurate information for precise diagnosis of cancer and optimize treatment strategies (Yang et al., 2018; Wang J. et al., 2021).
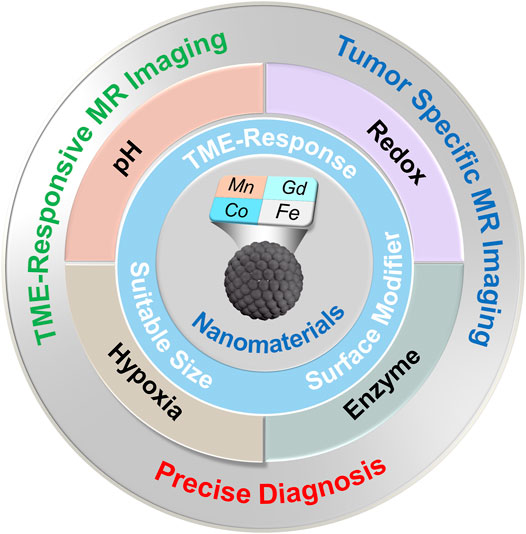
This review aims to summarize the recent advances and future prospects of nanomaterial-based CAs in their TME-responsive MRI applications rather than attempting to thoroughly contain the whole field (Figure 1). The inherent TME properties of tumors are significantly different from healthy normal tissues, which have been utilized in developing stimuli-responsive nano-MRI CAs for tumor-specific imaging. In this review, TME-responsive MRI CAs are classified according to types of endogenous stimulation, including pH, redox, enzyme, and hypoxia.
pH-Responsive MRI Contrast Agents
According to the Warburg effect, abnormal and rapid proliferation of tumor cells consumes a lot of oxygen and nutrients, leading to acidosis and a reduced pH in the neighboring microenvironment (Vaupel et al., 1989; Webb et al., 2011). Thus, the extracellular pH values in TME are typically acidic in many tumors, differing significantly from those of normal tissues and blood (Lee et al., 2007). The pH-responsive MRI CAs provide noninvasive contrast enhancement depending on pH, which could be applied to tumor-specific imaging. General designs of pH-responsive MRI CAs can be classified by imaging components.
Gd-Based pH-Responsive MRI CAs
Gd chelate is the most commonly used commercial MRI CA, so Gd-based pH-responsive MRI CAs have been widely studied. Zhu et al. attached pH-responsive block polymers on the surface of Gd-based NPs to achieve a surface modified Gd-metal organic frame (MOF) structure as a pH-responsive CA for MRI, which exhibited good performance in a series of characterization tests as well as imaging tests (Zhu et al., 2016). Its longitudinal relaxivity (r1) changed with the variation of environmental pH. The r1 value ranged from 6.6 mM−1 s−1 at pH 7.3 to 11.7 mM−1 s−1 at pH 6.6. To improve the biocompatibility and biodegradability of CAs, silkworm sericin (SS) was used to cross-link with Gd-based NPs by Huang et al. (Huang et al., 2021). A pH-responsive Gd-based NPs SS@GAH-GdCl3 with r1 values as 16.4 mM−1 s−1 at pH 5.8 and 9.2 mM−1 s−1 at pH 7.4 was obtained that showed a certain degree of increase with the decrease of pH value.
Mn-Based pH-Responsive MRI CAs
As an essential element, manganese participates in many cellular processes in vivo, which are of great importance to growth, development, and cellular homeostasis of the normal body (Aschner and Aschner, 2005; Erikson et al., 2005). Apart from Mn ions playing a key role in physiological processes owing to their redox and catalytic nature, Mn also possesses significant magnetic properties. Thus, Mn-based NPs and CAs have entered the field of MR imaging diagnosis and received extensive attention. In spite of some Mn-based CAs serving as regular T1 CAs and having achieved similar effects as clinical Gd-based CAs, a novel class of Mn-based nanomaterials has been designed with improved imaging sensitivity and is potentially expected to be substitutes for traditional CAs. These nanomaterials were constructed to respond to biological microenvironments, mainly to pH and redox potential variations. Chen et al. introduced pH-sensitive degradable manganese oxide (MnO) NPs into mesopores of hollow silica nanocapsules (HMCNs) through in-site redox reaction, then gained MnO-HMCNs as a pH-responsive CA for T1 MRI (Chen et al., 2012). Under weak acidic conditions, MnO dissolves into Mn ions, causing a great increase in r1 value (8.81 mM−1s−1), which is 11 times higher than that in neutral conditions and 2 times higher than commercial Gd-based CAs. To improve the diagnostic specificity and sensitivity, stimuli-responsive multiple-mode MRI CAs are extremely advisable for obtaining overall and detailed diagnostic information to complement the deficiencies of each other. Our group synthetized cobalt phosphides (Co-P) as core, coating with manganese dioxide (MnO2) nanosheets to obtain Co-P@mSiO2–MnO2 for pH-responsive T1/T2 dual-mode MRI-guided synergistic anticancer therapy (Jin et al., 2017). For imaging, Co-P core was applied to T2 MRI owing to its intrinsic magnetic properties, and biodegradable MnO2 was employed as pH-responsive T1 MRI CA. Under acidic conditions, the r1 value of Co-P@mSiO2–MnO2 was determined to be 9.05 mM−1s−1, the r2 value was 253.44 mM−1s−1 which increased approximately 1.5 times than under neutral conditions.
Fe-Based pH-Responsive MRI CAs
Fe is another essential element in the human body, mainly existing as ferritin in blood circulation. Paramagnetic Fe3+ is regarded as a promising candidate as T1 MR CAs owing to its large number of unpaired electrons (Wu et al., 2014; Zhang et al., 2017). Qu et al. recently fabricated an Fe-based biomimetic melanin-like multifunctional nanoagent (amino-Fe-PDANPs) as a positive pH-responsive MRI CA (Qu et al., 2021). Polydopamine was employed as a pH-responsive sensitizer for imaging. The r1 value was 10.0 mM−1s−1 at pH 7.5, then increasing to 15.4 mM−1s−1 at pH 6.5. It has been proven that the approachability of water molecules to the paramagnetic centers is the critical factor for T1 signal enhancement (Taylor et al., 2008). Interestingly, Fe-based NPs are also extensively applied as T2 MR Cas. Their magnetic property reduces rapidly with decreasing size owing to the reduction of magnetic anisotropy and spin disordering on the surface of NPs (Jun et al., 2008). Liu et al. synthesized PEGylated ultrasmall superparamagnetic iron oxide nanoparticles (USPIONs) by incorporating the methods of microemulsion and biomineralization, and coating with CaCO3 to obtain PEG-USPIONs@CaCO3 as a pH-responsive T2-T1–switchable MRI CA (Liu et al., 2021). The USPIONs agglomerated compactly inside the nanostructure, resulting in the enhancement of the T2 signal. While exposed to the acidic conditions in the tumor microenvironment, the CaCO3-layer releases free USPIONs by degradation, then switching to T1 MRI signal enhancement. With the gradual decrease of pH value, the r2/r1 ratio reduces substantially. In brief, PEG-USPIONs@CaCO3 will change from T2 CA in the neutral pH condition to T1 CA in the acidic environment of tumors. Not coincidentally, He et al. reported an extremely small iron oxide nanoparticle (ESIONP)–based pH-responsive T1-T2–switchable MRI CA which exhibited good performance (He et al., 2020).
Redox-Responsive MRI Contrast Agents
In addition to weakly acidic pH, redox state as another characteristic biomarker of TME has drawn remarkable attention. Glutathione (GSH), among a variety of redox couples, is usually considered the most important thiol-disulfide redox buffer in charge of maintaining the balance of intracellular redox reactions (Do et al., 2014; Zhao et al., 2022). The concentration of GSH in tumor cells is much higher than that in normal tissues or blood (Cheng et al., 2011). Therefore, reduction-sensitive disulfide bonds and hydrogen peroxide (H2O2)–responsive boronated moieties were extensively used as redox-responsive MRI CAs, While Gd itself cannot be redoxed, many other metal ions have been developed, such as Mn, Fe, Co, Eu, and Cu.
Mn-Based Redox-Responsive MRI CAs
Recently, Wang et al. developed an intelligent redox-responsive nanoplatform (MUM NPs) via the coprecipitation process involving upconversion NPs (UCNPs) and aggregation-induced emission-active photosensitizers, as well as an in situ generation process of MnO2 as the outer shell (Wang Y. et al., 2021). MUM NPs exhibited high specificity to TME, rapidly exhausting intracellular GSH and efficiently generating Mn2+, which were instrumental in ROS preservation and T1 MRI enhancement, respectively. This nanoplatform performed well in redox-responsive MR imaging. In addition to single-mode stimuli-responsive MRI, Kim et al. designed a redox-responsive T1/T2 dual-mode MRI CA, integrating a superparamagnetic core (Fe3O4) and a paramagnetic shell (Mn3O4) into a core-shell structure through a seed-mediated growth process (Kim et al., 2016). Under the stimulation of tumors’ intracellular reducing environments by glutathione, the Mn3O4 shell will be decomposed into Mn2+ for T1 signal enhancement and allow Fe3O4 to interact with water protons for T2 signal enhancement. Under GSH-free conditions, the r1 was 2.4 mM−1s−1 and the r2 was 92.2 mM−1s−1. After activation in GSH solution, the r1 and r2 values increased to 16.1 and 258.6 mM−1s−1, illustrating that this nanoplatform can be qualified as redox-responsive T1/T2 dual-MRI CA.
Fe-Based Redox-Responsive MRI CAs
The yolk-shell type of GSH-responsive nanovesicles (NVs) were synthesized to encapsulate USPIO NPs and chemotherapy drugs by Liu et al.; the obtained USD NVs can respond to GSH-releasing drugs and activate T1 signal enhancement (Liu et al., 2020). The r1 value increased obviously to 3.1 mM−1s−1 in the presence of GSH. For switchable MRI, Cao et al. encapsulated citric acid–modified ESIONPs-CA in disulfide cross-linked poly (CBMA) nanogels, further introducing tumor-targeted c (RGD) ligand to obtain ICNs-RGD (Cao et al., 2020). With the stimulation of GSH, ICNs-RGD are rapidly degraded, and the agglomerated ESIONPs are dispersed evenly, which achieves switching from a T2 CA to a T1 one. ICNs-RGD exhibits a T2 contrast effect (dark) during its transport in the vessel, then switches to a T1 contrast effect (bright) after reaching the tumor region with a redox microenvironment. After stimulation by GSH, the r1 value was increased mildly from 5.56 to 7.40 mM−1s−1 and the r2 value drastically decreased from 103.01 to 14.36 mM−1s−1, which demonstrated that ICNs-RGD had the potential to be an efficient MRI CA in clinics.
Enzyme-Responsive MRI Contrast Agents
Because of their unique substrate specificity and high selectivity as well as efficient catalysis in biochemical reactions, enzymes play an indispensable role in most biological and metabolic processes, which can be associated with a series of pathological changes, such as tumors, inflammation, and so on (Zhang et al., 2014; Wang et al., 2019). In particular, cathepsin B and matrix metalloproteinases (MMPs) with elevated expression in the tumor environment participate in numerous biological processes associated with cancer, such as progression, metastasis, and angiogenesis. These tumor-associated enzymes can be regarded as stimulators for enzyme-responsive imaging or treatment of cancer (Hu et al., 2012). By means of one step reaction, Sun et al. synthesized hyperbranched poly (oligo-(ethylene glycol) methacrylate)-Gd complexes (HB-POEGMA-Gd and HB-POEGMA-cRGD-Gd), which employed lysosomal cathepsin B as a stimuli-response component to realize enzyme-responsive T1 CA (Sun et al., 2016). Their r1 values were 12.25 and 14.65 mM−1s−1, respectively. Recently, Yan et al. reported a Gd-based enzyme-responsive MRI and NIR fluorescence imaging CA (P-CyFF-Gd) with alkaline phosphatase (ALP) as a model enzyme (Yan et al., 2019). Upon ALP activation, r1 increased from 8.9 to 20.1 mM−1s−1, which demonstrated that P-CyFF-Gd can be qualified as a new generation of enzyme-responsive T1 CA. Another Gd-based enzyme-responsive T1 CA was designed through a self-assembly approach with caspase-3/7 as an activator by Ye et al. (Ye et al., 2014). The r1 value significantly increased from 10.2 mM−1s−1 before activation to 19.0 mM−1s−1 after activation. For enzyme-responsive T2 imaging, Gallo et al. constructed Fe-based MMP, enzyme-activatable, and tumor-specific targeting NPs, which were tethered with CXCR4-targeted peptide ligands for targeting tumors (Gallo et al., 2014). Upon MMP activation, the structure of NPs changes to that of a self-assembled superparamagnetic cluster network through a cycloaddition reaction, resulting in T2 signal enhancement.
Hypoxia-Responsive MRI Contrast Agents
The malignant proliferation of tumors depletes a large amount of oxygen, resulting in the hypoxia of the tumor environment. As an inevitable feature of tumors, hypoxia is considered to be the obvious causative factor of therapeutic resistance and metastasis (Gatenby and Gillies, 2008). Therefore, exploiting novel nanotheranostics to achieve accurate diagnosis of hypoxia and timely treatment simultaneously has drawn great attention in biomedical research as well as clinical studies. The Gd complex of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid with a 2-nitroimidazole attached to one carboxyl group by an amide linkage was synthesized by Rojas-Quijano et al.; the r1 value was determined to be 6.38 mM−1s−1. The MR imaging results demonstrated that the nitroimidazole-derivant modified nanoprobe could be qualified as a hypoxia-responsive T1 CA to distinguish hypoxic from normoxic tissues. For Mn-based CAs, Song et al. constructed a rattle-structured NP consisting of a UCNP core, a hollow mesoporous silica shell, and hypoxia-sensitive MnO2 nanosheet modification (Song et al., 2018). MnO2 could be disintegrated into Mn2+ to achieve hypoxia-responsive T1 signal enhancement, and the r1 value is 1.137 mM−1s−1 after activation. O’Neill et al. studied the possibility of Co-based bioreductive pro-drugs working as a hypoxia-responsive MRI CA. Once in the hypoxic environment of tumors, the diamagnetic Co(III) ions of the CA will be reduced to ions of paramagnetic Co(II), ones which could significantly shorten the T2 relaxation to realize a T2 signal enhancement.
Discussion
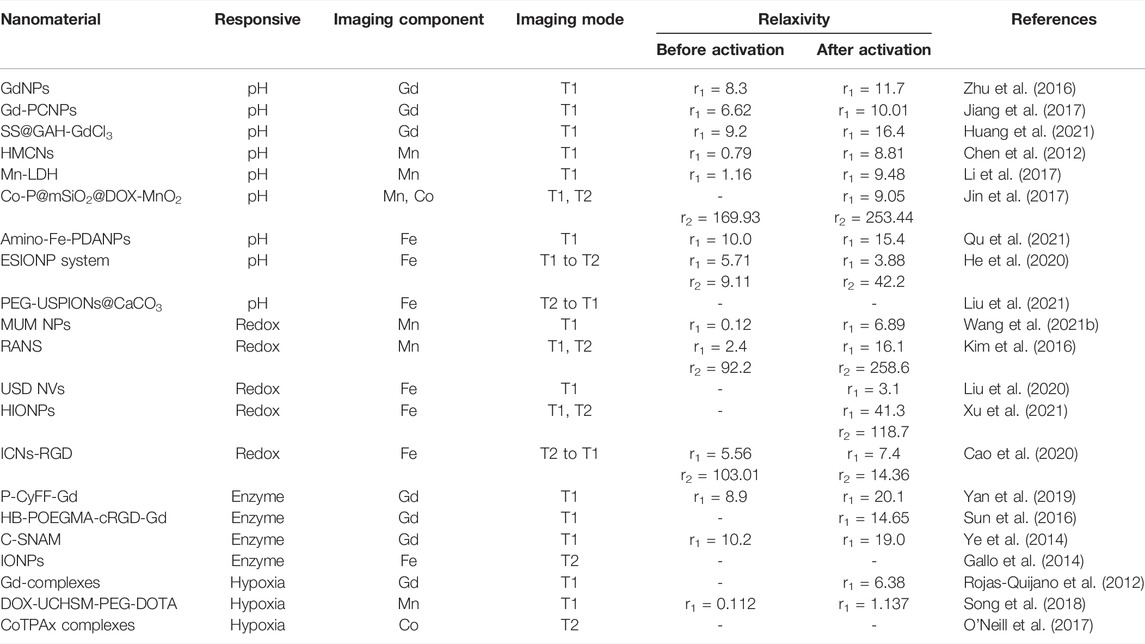
TME-responsive MRI CAs have been widely developed for accurate diagnosis of cancer because of their outstanding properties. The recent work on nanomaterials for TME-responsive MRI is summarized in Table 1. Although great efforts have been devoted to exploring a variety of TME-responsive MRI CAs in the biomedical field, there are still some problems that need to be solved for accelerating their clinical transformation. For instance, 1) up to date, most studies of stimuli-responsive CAs based on nanomaterials stay in the stage of concept verification as well as the research of laboratory application; systematic and in-depth researches are needed to promote their clinical application. 2) The safety concerns of these CAs need to be investigated more thoroughly, including their long-term biocompatibility, pharmacokinetics, biodistribution, biodegradability, and excretion, which are vitally important for their clinical transformation. 3) Despite numerous stimuli-responsive CAs exhibiting good performance, nanomaterials with better properties (such as high relaxivity, adequate circulation time, and appropriate stimuli-response function) should be explored to further improve the imaging effect and diagnostic efficiency. We believe that ingenious design and construction of TME-responsive MRI CAs will greatly promote the development of early and accurate cancer diagnosis in the future.
Author Contributions
JL and NX conceived the topic and structure of the article. LJ conducted the literature research and drafted the manuscript. CY and JW prepared the figures, tables, and manuscript amendments. JL and NX reviewed and refined the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 32000953), the Natural Science Foundation of Jilin Province (Grant No. 20210101287JC), the Financial Department of Jilin Province (Grant No. 2019SCZT045), the Fundamental Research Funds for the Central Universities (Grant No. 415010200060), and the Education Project of Jilin University (#419070600046 and 45121031D024).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ai, H. (2011). Layer-by-layer Capsules for Magnetic Resonance Imaging and Drug Delivery. Adv. Drug Deliv. Rev. 63 (9), 772–788. doi:10.1016/j.addr.2011.03.013
Aschner, J. L., and Aschner, M. (2005). Nutritional Aspects of Manganese Homeostasis. Mol. Asp. Med. 26 (4-5), 353–362. doi:10.1016/j.mam.2005.07.003
Cao, Y., Mao, Z., He, Y., Kuang, Y., Liu, M., Zhou, Y., et al. (2020). Extremely Small Iron Oxide Nanoparticle-Encapsulated Nanogels as a Glutathione-Responsive T1 Contrast Agent for Tumor-Targeted Magnetic Resonance Imaging. ACS Appl. Mater Interfaces 12 (24), 26973–26981. doi:10.1021/acsami.0c07288
Chen, Y., Ye, D., Wu, M., Chen, H., Zhang, L., Shi, J., et al. (2014). Break-up of Two-Dimensional MnO2 Nanosheets Promotes Ultrasensitive pH-Triggered Theranostics of Cancer. Adv. Mater 26 (41), 7019–7026. doi:10.1002/adma.201402572
Chen, Y., Yin, Q., Ji, X., Zhang, S., Chen, H., Zheng, Y., et al. (2012). Manganese Oxide-Based Multifunctionalized Mesoporous Silica Nanoparticles for pH-Responsive MRI, Ultrasonography and Circumvention of MDR in Cancer Cells. Biomaterials 33 (29), 7126–7137. doi:10.1016/j.biomaterials.2012.06.059
Cheng, R., Feng, F., Meng, F., Deng, C., Feijen, J., and Zhong, Z. (2011). Glutathione-responsive Nano-Vehicles as a Promising Platform for Targeted Intracellular Drug and Gene Delivery. J. Control Release 152 (1), 2–12. doi:10.1016/j.jconrel.2011.01.030
Danhier, F., Feron, O., and Préat, V. (2010). To Exploit the Tumor Microenvironment: Passive and Active Tumor Targeting of Nanocarriers for Anti-cancer Drug Delivery. J. Control Release 148 (2), 135–146. doi:10.1016/j.jconrel.2010.08.027
Do, Q. N., Ratnakar, J. S., Kovács, Z., and Sherry, A. D. (2014). Redox- and Hypoxia-Responsive MRI Contrast Agents. ChemMedChem. 9 (6), 1116–1129. doi:10.1002/cmdc.201402034
Du, F., Zhang, L., Zhang, L., Zhang, M., Gong, A., Tan, Y., et al. (2017). Engineered Gadolinium-Doped Carbon Dots for Magnetic Resonance Imaging-Guided Radiotherapy of Tumors. Biomaterials 121, 109–120. doi:10.1016/j.biomaterials.2016.07.008
Erikson, K. M., Syversen, T., Aschner, J. L., and Aschner, M. (2005). Interactions between Excessive Manganese Exposures and Dietary Iron-Deficiency in Neurodegeneration. Environ. Toxicol. Pharmacol. 19 (3), 415–421. doi:10.1016/j.etap.2004.12.053
Fu, S., Cai, Z., and Ai, H. (2021). Stimulus‐Responsive Nanoparticle Magnetic Resonance Imaging Contrast Agents: Design Considerations and Applications. Adv. Healthc. Mat. 10 (5), 2001091. doi:10.1002/adhm.202001091
Gallo, J., Kamaly, N., Lavdas, I., Stevens, E., Nguyen, Q. D., Wylezinska-Arridge, M., et al. (2014). CXCR4-targeted and MMP-Responsive Iron Oxide Nanoparticles for Enhanced Magnetic Resonance Imaging. Angew. Chem. Int. Ed. Engl. 53 (36), 9550–9554. doi:10.1002/anie.201405442
García-Hevia, L., Bañobre-López, M., and Gallo, J. (2019). Recent Progress on Manganese-Based Nanostructures as Responsive MRI Contrast Agents. Chem. Eur. J. 25 (2), 431–441. doi:10.1002/chem.201802851
Gatenby, R. A., and Gillies, R. J. (2008). A Microenvironmental Model of Carcinogenesis. Nat. Rev. Cancer 8 (1), 56–61. doi:10.1038/nrc2255
He, Y., Mao, Z., Zhang, Y., Lv, H., Yan, J., Cao, Y., et al. (2020). Tumor Acid Microenvironment-Triggered Self-Assembly of ESIONPs for T1/T2 Switchable Magnetic Resonance Imaging. ACS Appl. Bio Mat. 3 (11), 7752–7761. doi:10.1021/acsabm.0c00958
Hu, J., Zhang, G., and Liu, S. (2012). Enzyme-responsive Polymeric Assemblies, Nanoparticles and Hydrogels. Chem. Soc. Rev. 41 (18), 5933–5949. doi:10.1039/C2CS35103J
Huang, Z., Wang, Y., Wu, M., Li, W., Zuo, H., Xiao, B., et al. (2021). Sericin-based Gadolinium Nanoparticles as Synergistically Enhancing Contrast Agents for pH-Responsive and Tumor Targeting Magnetic Resonance Imaging. Mater. Des. 203, 109600. doi:10.1016/j.matdes.2021.109600
Jiang, D., Zhang, X., Yu, D., Xiao, Y., Wang, T., Su, Z., et al. (2017). Tumor-Microenvironment Relaxivity-Changeable Gd-Loaded Poly(L-lysine)/Carboxymethyl Chitosan Nanoparticles as Cancer-Recognizable Magnetic Resonance Imaging Contrast Agents. J. Biomed. Nanotechnol. 13 (3), 243–254. doi:10.1166/jbn.2017.2346
Jin, L., Liu, J., Tang, Y., Cao, L., Zhang, T., Yuan, Q., et al. (2017). MnO2-Functionalized Co-P Nanocomposite: A New Theranostic Agent for pH-Triggered T1/T2 Dual-Modality Magnetic Resonance Imaging-Guided Chemo-Photothermal Synergistic Therapy. ACS Appl. Mater Interfaces 9 (48), 41648–41658. doi:10.1021/acsami.7b10608
Joyce, J. A., and Fearon, D. T. (2015). T Cell Exclusion, Immune Privilege, and the Tumor Microenvironment. Science 348 (6230), 74–80. doi:10.1126/science.aaa6204
Jun, Y. W., Lee, J. H., and Cheon, J. (2008). Chemical Design of Nanoparticle Probes for High-Performance Magnetic Resonance Imaging. Angew. Chem. Int. Ed. Engl. 47 (28), 5122–5135. doi:10.1002/anie.200701674
Kang, T., Li, F., Baik, S., Shao, W., Ling, D., and Hyeon, T. (2017). Surface Design of Magnetic Nanoparticles for Stimuli-Responsive Cancer Imaging and Therapy. Biomaterials 136, 98–114. doi:10.1016/j.biomaterials.2017.05.013
Kielar, F., Tei, L., Terreno, E., and Botta, M. (2010). Large Relaxivity Enhancement of Paramagnetic Lipid Nanoparticles by Restricting the Local Motions of the Gd(III) Chelates. J. Am. Chem. Soc. 132 (23), 7836–7837. doi:10.1021/ja101518v
Kim, M. H., Son, H. Y., Kim, G. Y., Park, K., Huh, Y. M., and Haam, S. (2016). Redoxable Heteronanocrystals Functioning Magnetic Relaxation Switch for Activatable T1 and T2 Dual-Mode Magnetic Resonance Imaging. Biomaterials 101, 121–130. doi:10.1016/j.biomaterials.2016.05.054
Kim, T., Cho, E. J., Chae, Y., Kim, M., Oh, A., Jin, J., et al. (2011). Urchin-shaped Manganese Oxide Nanoparticles as pH-Responsive Activatable T1 Contrast Agents for Magnetic Resonance Imaging. Angew. Chem. Int. Ed. Engl. 50 (45), 10589–10593. doi:10.1002/anie.201103108
Kimura, Y., Kamisugi, R., Narazaki, M., Matsuda, T., Tabata, Y., Toshimitsu, A., et al. (2012). Size-controlled and Biocompatible Gd2 O3 Nanoparticles for Dual Photoacoustic and MR Imaging. Adv. Healthc. Mater 1 (5), 657–660. doi:10.1002/adhm.201200103
Laurent, S., Forge, D., Port, M., Roch, A., Robic, C., Vander Elst, L., et al. (2008). Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 108 (6), 2064–2110. doi:10.1021/cr068445e
Lee, E. S., Oh, K. T., Kim, D., Youn, Y. S., and Bae, Y. H. (2007). Tumor pH-Responsive Flower-like Micelles of poly(L-Lactic Acid)-B-Poly(ethylene Glycol)-B-poly(L-Histidine). J. Control Release 123 (1), 19–26. doi:10.1016/j.jconrel.2007.08.006
Lee, Y. J., Lee, J. M., Lee, J. S., Lee, H. Y., Park, B. H., Kim, Y. H., et al. (2015). Hepatocellular Carcinoma: Diagnostic Performance of Multidetector CT and MR Imaging-A Systematic Review and Meta-Analysis. Radiology 275 (1), 97–109. doi:10.1148/radiol.14140690
Li, B., Gu, Z., Kurniawan, N., Chen, W., and Xu, Z. P. (2017). Manganese-Based Layered Double Hydroxide Nanoparticles as a T1 -MRI Contrast Agent with Ultrasensitive pH Response and High Relaxivity. Adv. Mater 29 (29), 1. doi:10.1002/adma.201700373
Lim, E. K., Kim, T., Paik, S., Haam, S., Huh, Y. M., and Lee, K. (2015). Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem. Rev. 115 (1), 327–394. doi:10.1021/cr300213b
Liu, D., Zhou, Z., Wang, X., Deng, H., Sun, L., Lin, H., et al. (2020). Yolk-shell Nanovesicles Endow Glutathione-Responsive Concurrent Drug Release and T1 MRI Activation for Cancer Theranostics. Biomaterials 244, 119979. doi:10.1016/j.biomaterials.2020.119979
Liu, W., Yin, S., Hu, Y., Deng, T., and Li, J. (2021). Microemulsion-Confined Biomineralization of PEGylated Ultrasmall Fe3O4 Nanocrystals for T2-T1 Switchable MRI of Tumors. Anal. Chem. 93 (42), 14223–14230. doi:10.1021/acs.analchem.1c03128
Liu, Y., Ai, K., Yuan, Q., and Lu, L. (2011). Fluorescence-enhanced Gadolinium-Doped Zinc Oxide Quantum Dots for Magnetic Resonance and Fluorescence Imaging. Biomaterials 32 (4), 1185–1192. doi:10.1016/j.biomaterials.2010.10.022
Lohrke, J., Frenzel, T., Endrikat, J., Alves, F. C., Grist, T. M., Law, M., et al. (2016). 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives. Adv. Ther. 33 (1), 1–28. doi:10.1007/s12325-015-0275-4
Longmire, M. R., Ogawa, M., Choyke, P. L., and Kobayashi, H. (2011). Biologically Optimized Nanosized Molecules and Particles: More Than Just Size. Bioconjug Chem. 22 (6), 993–1000. doi:10.1021/bc200111p
Na, H. B., Song, I. C., and Hyeon, T. (2009). Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mat. 21 (21), 2133–2148. doi:10.1002/adma.200802366
O'Neill, E. S., Kaur, A., Bishop, D. P., Shishmarev, D., Kuchel, P. W., Grieve, S. M., et al. (2017). Hypoxia-Responsive Cobalt Complexes in Tumor Spheroids: Laser Ablation Inductively Coupled Plasma Mass Spectrometry and Magnetic Resonance Imaging Studies. Inorg. Chem. 56 (16), 9860–9868. doi:10.1021/acs.inorgchem.7b01368
Park, Y. I., Kim, H. M., Kim, J. H., Moon, K. C., Yoo, B., Lee, K. T., et al. (2012). Theranostic Probe Based on Lanthanide-Doped Nanoparticles for Simultaneous In Vivo Dual-Modal Imaging and Photodynamic Therapy. Adv. Mater 24 (42), 5755–5761. doi:10.1002/adma.201202433
Passuello, T., Pedroni, M., Piccinelli, F., Polizzi, S., Marzola, P., Tambalo, S., et al. (2012). PEG-capped, Lanthanide Doped GdF3 Nanoparticles: Luminescent and T2 Contrast Agents for Optical and MRI Multimodal Imaging. Nanoscale 4 (24), 7682–7689. doi:10.1039/c2nr31796f
Penfield, J. G., and Reilly, R. F. (2007). What Nephrologists Need to Know about Gadolinium. Nat. Clin. Pract. Nephrol. 3 (12), 654–668. doi:10.1038/ncpneph0660
Perez-Rodriguez, J., Lai, S., Ehst, B. D., Fine, D. M., and Bluemke, D. A. (2009). Nephrogenic Systemic Fibrosis: Incidence, Associations, and Effect of Risk Factor Assessment-Rreport of 33 Cases. Radiology 250 (2), 371–377. doi:10.1148/radiol.2502080498
Qu, J., Guillory, D., Cheah, P., Tian, B., Zheng, J., Liu, Y., et al. (2021). Synthesis of Biomimetic Melanin-like Multifunctional Nanoparticles for pH Responsive Magnetic Resonance Imaging and Photothermal Therapy. Nanomater. (Basel) 11 (8). doi:10.3390/nano11082107
Raymond, K. N., and Pierre, V. C. (2005). Next Generation, High Relaxivity Gadolinium MRI Agents. Bioconjug Chem. 16 (1), 3–8. doi:10.1021/bc049817y
Rojas-Quijano, F. A., Tircsó, G., Tircsóné Benyó, E., Baranyai, Z., Tran Hoang, H., Kálmán, F. K., et al. (2012). Synthesis and Characterization of a Hypoxia-Sensitive MRI Probe. Chemistry 18 (31), 9669–9676. doi:10.1002/chem.201200266
Shen, Z., Chen, T., Ma, X., Ren, W., Zhou, Z., Zhu, G., et al. (2017). Multifunctional Theranostic Nanoparticles Based on Exceedingly Small Magnetic Iron Oxide Nanoparticles for T1-Weighted Magnetic Resonance Imaging and Chemotherapy. ACS Nano 11 (11), 10992–11004. doi:10.1021/acsnano.7b04924
Song, R., Zhang, M., Liu, Y., Cui, Z., Zhang, H., Tang, Z., et al. (2018). A Multifunctional Nanotheranostic for the Intelligent MRI Diagnosis and Synergistic Treatment of Hypoxic Tumor. Biomaterials 175, 123–133. doi:10.1016/j.biomaterials.2018.05.018
Sun, L., Li, X., Wei, X., Luo, Q., Guan, P., Wu, M., et al. (2016). Stimuli-Responsive Biodegradable Hyperbranched Polymer-Gadolinium Conjugates as Efficient and Biocompatible Nanoscale Magnetic Resonance Imaging Contrast Agents. ACS Appl. Mater Interfaces 8 (16), 10499–10512. doi:10.1021/acsami.6b00980
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Taylor, K. M., Kim, J. S., Rieter, W. J., An, H., Lin, W., and Lin, W. (2008). Mesoporous Silica Nanospheres as Highly Efficient MRI Contrast Agents. J. Am. Chem. Soc. 130 (7), 2154–2155. doi:10.1021/ja710193c
Tong, S., Hou, S., Zheng, Z., Zhou, J., and Bao, G. (2010). Coating Optimization of Superparamagnetic Iron Oxide Nanoparticles for High T2 Relaxivity. Nano Lett. 10 (11), 4607–4613. doi:10.1021/nl102623x
Vaupel, P., Kallinowski, F., and Okunieff, P. (1989). Blood Flow, Oxygen and Nutrient Supply, and Metabolic Microenvironment of Human Tumors: a Review. Cancer Res. 49 (23), 6449–6465.
Wang, J., Sui, L., Huang, J., Miao, L., Nie, Y., Wang, K., et al. (2021a). MoS2-based Nanocomposites for Cancer Diagnosis and Therapy. Bioact. Mater 6 (11), 4209–4242. doi:10.1016/j.bioactmat.2021.04.021
Wang, X., Wang, X., Jin, S., Muhammad, N., and Guo, Z. (2019). Stimuli-Responsive Therapeutic Metallodrugs. Chem. Rev. 119 (2), 1138–1192. doi:10.1021/acs.chemrev.8b00209
Wang, Y., Li, Y., Zhang, Z., Wang, L., Wang, D., and Tang, B. Z. (2021b). Triple‐Jump Photodynamic Theranostics: MnO 2 Combined Upconversion Nanoplatforms Involving a Type‐I Photosensitizer with Aggregation‐Induced Emission Characteristics for Potent Cancer Treatment. Adv. Mater. 33 (41), 2103748. doi:10.1002/adma.202103748
Webb, B. A., Chimenti, M., Jacobson, M. P., and Barber, D. L. (2011). Dysregulated pH: a Perfect Storm for Cancer Progression. Nat. Rev. Cancer 11 (9), 671–677. doi:10.1038/nrc3110
Wu, M., Meng, Q., Chen, Y., Xu, P., Zhang, S., Li, Y., et al. (2014). Ultrasmall Confined Iron Oxide Nanoparticle MSNs as a pH-Responsive Theranostic Platform. Adv. Funct. Mat. 24 (27), 4273–4283. doi:10.1002/adfm.201400256
Xing, H., Zhang, S., Bu, W., Zheng, X., Wang, L., Xiao, Q., et al. (2014). Ultrasmall NaGdF4 Nanodots for Efficient MR Angiography and Atherosclerotic Plaque Imaging. Adv. Mater 26 (23), 3867–3872. doi:10.1002/adma.201305222
Xu, X., Zhou, X., Xiao, B., Xu, H., Hu, D., Qian, Y., et al. (2021). Glutathione-Responsive Magnetic Nanoparticles for Highly Sensitive Diagnosis of Liver Metastases. Nano Lett. 21 (5), 2199–2206. doi:10.1021/acs.nanolett.0c04967
Yan, R., Hu, Y., Liu, F., Wei, S., Fang, D., Shuhendler, A. J., et al. (2019). Activatable NIR Fluorescence/MRI Bimodal Probes for In Vivo Imaging by Enzyme-Mediated Fluorogenic Reaction and Self-Assembly. J. Am. Chem. Soc. 141 (26), 10331–10341. doi:10.1021/jacs.9b03649
Yang, G., Zhang, R., Liang, C., Zhao, H., Yi, X., Shen, S., et al. (2018). Manganese Dioxide Coated WS2 @Fe3 O4/sSiO2 Nanocomposites for pH-Responsive MR Imaging and Oxygen-Elevated Synergetic Therapy. Small 14 (2), 1. doi:10.1002/smll.201702664
Ye, D., Shuhendler, A. J., Pandit, P., Brewer, K. D., Tee, S. S., Cui, L., et al. (2014). Caspase-responsive Smart Gadolinium-Based Contrast Agent for Magnetic Resonance Imaging of Drug-Induced Apoptosis. Chem. Sci. 4 (10), 3845–3852. doi:10.1039/C4SC01392A
Yoo, B., and Pagel, M. D. (2008). An Overview of Responsive MRI Contrast Agents for Molecular Imaging. Front. Biosci. 13, 1733–1752. doi:10.2741/2796
Yu, M. H., Kim, J. H., Yoon, J. H., Kim, H. C., Chung, J. W., Han, J. K., et al. (2014). Small (≤1-cm) Hepatocellular Carcinoma: Diagnostic Performance and Imaging Features at Gadoxetic Acid-Enhanced MR Imaging. Radiology 271 (3), 748–760. doi:10.1148/radiol.14131996
Zhang, H., Li, L., Liu, X. L., Jiao, J., Ng, C. T., Yi, J. B., et al. (2017). Ultrasmall Ferrite Nanoparticles Synthesized via Dynamic Simultaneous Thermal Decomposition for High-Performance and Multifunctional T1 Magnetic Resonance Imaging Contrast Agent. ACS Nano 11 (4), 3614–3631. doi:10.1021/acsnano.6b07684
Zhang, H. W., Wang, L. Q., Xiang, Q. F., Zhong, Q., Chen, L. M., Xu, C. X., et al. (2014). Specific Lipase-Responsive Polymer-Coated Gadolinium Nanoparticles for MR Imaging of Early Acute Pancreatitis. Biomaterials 35 (1), 356–367. doi:10.1016/j.biomaterials.2013.09.046
Zhao, T., Wu, W., Sui, L., Huang, Q., Nan, Y., Liu, J., et al. (2022). Reactive Oxygen Species-Based Nanomaterials for the Treatment of Myocardial Ischemia Reperfusion Injuries. Bioact. Mater 7, 47–72. doi:10.1016/j.bioactmat.2021.06.006
Zhu, L., Yang, Y., Farquhar, K., Wang, J., Tian, C., Ranville, J., et al. (2016). Surface Modification of Gd Nanoparticles with pH-Responsive Block Copolymers for Use as Smart MRI Contrast Agents. ACS Appl. Mater Interfaces 8 (7), 5040–5050. doi:10.1021/acsami.5b12463
Keywords: nanomaterials, tumor microenvironment, stimuli-responsive, MRI, diagnosis of cancer
Citation: Jin L, Yang C, Wang J, Li J and Xu N (2022) Recent Advances in Nanotheranostic Agents for Tumor Microenvironment–Responsive Magnetic Resonance Imaging. Front. Pharmacol. 13:924131. doi: 10.3389/fphar.2022.924131
Received: 20 April 2022; Accepted: 13 May 2022;
Published: 22 June 2022.
Edited by:
Kelong Ai, Central South University, ChinaReviewed by:
Qiong Huang, Central South University, ChinaZhen Liu, Beijing University of Chemical Technology, China
Copyright © 2022 Jin, Yang, Wang, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiannan Li, am5saUBjaWFjLmFjLmNu; Nannan Xu, bm54dUBqbHUuZWR1LmNu
 Longhai Jin
Longhai Jin Chenyi Yang2
Chenyi Yang2 Jiannan Li
Jiannan Li Nannan Xu
Nannan Xu