
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 15 August 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.922744
This article is part of the Research Topic Challenges and Solutions for The Incomplete Immune Restoration in HIV-infected Patients Under Antiretroviral Therapy View all 7 articles
 Adisu Birhanu Weldesenbet1
Adisu Birhanu Weldesenbet1 Biruk Shalmeno Tusa1*
Biruk Shalmeno Tusa1* Gebiso Roba Debele2
Gebiso Roba Debele2 Malede Mequanent Sisay3
Malede Mequanent Sisay3 Tadesse Awoke Ayele3
Tadesse Awoke Ayele3Background: Even though determining the time to anti-retroviral therapy (ART) adverse drug reaction and its predictors is a crucial step to overcome the negative consequences of the adverse drug reaction, there is limited information regarding the time to ART adverse drug reaction and its predictors. Therefore, this study aimed to determine the time to first ART adverse drug reaction and its predictors among adult HIV/AIDS patients on first-line antiretroviral therapy in West Hararghe Zone, Eastern Ethiopia.
Methods: An institution-based retrospective cohort study was conducted on 561 HIV/AIDS patients on first-line ART from September 2013–January 2019 at public hospitals in West Hararghe Zone, Eastern Ethiopia. Data were collected using checklists and document reviews, entered using Epi Info and analyzed in R software. A Cox proportional hazard model was fitted to identify predictors of the time to first ART adverse drug reaction. Model adequacy was checked using Cox Snell residuals. An adjusted hazard ratio with its confidence interval was used to show the presence and strength of association at a 95% confidence level.
Result: Most (90.74%) ART adverse drug reactions occurred within 1 year of initiation of ART. Overall, 54 patients developed ART adverse drug reactions with an incidence density of 3.5/100 persons-years of observations (95% CI: 2.7–4.6). The initial ART regimen (TDF, 3TC, EFV) [AHR = 0.3, 95% CI 0.1–0.7], fair adherence [AHR = 8.8, 95% CI 3.3–23.2], poor adherence [AHR = 7.8, 95% CI 3.1–19.5], moderate body mass index (BMI) at the baseline [AHR = 4.4, 95% CI 1.8–11.0], severe body mass index [AHR = 2.8, 95% CI 1.1–6.8], World Health Organization (WHO) stage II [AHR = 3.7, 95% CI 1.2–11.3] and WHO stage IV [AHR = 6.3, 95% CI 2.0–19.8] were significant predictors of the time to ART adverse drug reactions.
Conclusion: In conclusion, most of the ART adverse drug reactions occurred within 1 year of initiation of ART. The initial ART regimen (TDF, 3TC, EFV), adherence, HIV/AIDS stage, and BMI were risk factors for the time to ART adverse drug reaction. The incidence of the antiretroviral therapy adverse reaction was relatively low with early onset. Close monitoring of clients in clinical stage II and above is required and continuous assessment for improving the detection and management of adverse drug reactions is recommended. Patients with poor adherence need to get continuous counseling to improve their adherence status.
Globally, 36.9 million people are living with human immunodeficiency virus (HIV) and more than half of them are in Africa (WHO, 2019). At the end of 2017, 21.7 million (59%) patients were receiving antiretroviral therapy (ART). Consequently, acquired immunodeficiency syndrome (AIDS)-related mortality was reduced by around 51% globally (WHO, 2017). In Ethiopia, reports indicate that the overall prevalence of HIV/AIDS is 1.1% (Girum et al., 2018). Timely initiation of ART and appropriate monitoring of treatment effectiveness improve the life expectancy of people living with HIV/AIDS (Daniel Murrell, 2018).
Antiretroviral therapy-related adverse drug reactions are one of the challenges to the United Nations’ target set to end AIDS epidemics by 2030 (WHO, 2013). Antiretroviral therapy adverse drug reactions may cause discontinuation of all antiretroviral drugs and make HIV/AIDS patients susceptible to life-threatening chronic diseases (Carr and Cooper, 2000; Hicks et al., 2006).
Globally, the incidence of adverse drug reactions among patients on first-line ART ranges between 11 and 35.9% (Hogg et al., 1999; Bonfanti et al., 2000; Severe et al., 2010). In Ethiopia, a study from Debre Markos reported that 51.5% of patients on first-line ART experienced ART adverse drug reactions (Asrat et al., 2014). Furthermore, ART adverse drug reactions are common reasons for poor adherence to antiretroviral drugs (Duval et al., 2004; Masenyetse et al., 2015; Kindie et al., 2017).
To improve the survival of HIV/AIDS patients on ART, information on factors associated with the time to ART adverse drug reactions and its predictors are needed. However, there is a paucity of evidence on the time to ART adverse drug reactions and its predictors among HIV/AIDS patients in Ethiopia. Although few studies were conducted on the time to ART adverse drug reactions, they did not assess the effect of the initial ART regimen, comorbidity, and adherence on the time to ART adverse drug reactions (Kindie et al., 2017; Asfaw and Enquselassie, 2018a). Therefore, the present study aimed to determine the time to ART adverse drug reactions and its predictors among HIV/AIDS patients at public hospitals in West Hararghe Zone, Eastern Ethiopia, from 2013–2019. The finding of the present study will be helpful for the timely identification and treatment of ART adverse drug reactions and to decrease the progression of adverse drug reactions and related complications.
An institution-based retrospective cohort study was conducted among adult HIV/AIDS patients on ART from September 2013–January 2019 at public hospitals in West Hararghe Zone of the Oromia region, Eastern Ethiopia. Among five hospitals in the zone, the study was conducted in Chiro Zonal Hospital and Gelemso General Hospital because of the availability of ART data. The two hospitals render services to around 2,300,000 people in the zone. In these hospitals, 1,711 patients were on ART at the time of the survey.
The study included all HIV/AIDS patients aged 18 years and more who started ART between 2013 and 2019 and registered in the ART registry log book from Chiro and Gelemso Hospitals. Patients whose date of treatment initiation was not recorded, transferred in from other treatment centers, patients who have been receiving ART for less than 3 months, and pregnant mothers were excluded from the study.
The sample size was determined by STATA software after considering Cox proportional hazard model assumptions. We assumed a probability of type I error (α) 0.05, power of the study of 80%, and withdrawal probability of 0.1 which are the proportions of the subjects anticipated to withdraw from the study.
By taking the hazard ratio (HR) for strong predictors of adverse drug reactions (tuberculosis (TB) co-infection (HR = 2.5), World Health Organization (WHO) stage (HR = 2.2), and opportunistic infection prophylaxis (OIP) (HR = 3.2)) from a study in Bahir Dar, Ethiopia (Kindie et al., 2017), the calculated sample size is 412, 556 and 256, respectively. Therefore, 556 was taken as a minimum adequate sample size. Among 1,711 patients on ART at the time of the survey, 561 HIV/AIDS patients met the inclusion criteria and all of them were included in the study.
Antiretroviral therapy (ATR) adverse drug reaction (ADR): The patient is said to have experienced ART adverse drug reactions if either hospitalization or discontinuation of treatment and switching of drugs to other ART regimen happened as a result of an undesired and excessive response to ART (Pharmacists ASoH-, 1995; Organization, 2008; Kindie et al., 2017). In the present study, at both hospitals, physicians’ diagnoses on patient cards and laboratory reports were used to check whether the patient developed ART adverse drug reactions.
In this study, baseline adherence (when the patient is first started on ART until the initial 30/60 dose was taken) was considered and accordingly classified in the following ways:
Good adherence: In the present study, if it is ≥ 95% or (<2 doses of 30 doses or <30 doses of 60 doses is missed) as documented by the ART healthcare provider (Nabukeera-Barungi et al., 2007; Chalker et al., 2009).
Fair adherence: The patient who missed 3–5 doses of 30 doses or 3–9 doses of 60 doses as documented by the ART healthcare provider is said to have fair adherence (Nabukeera-Barungi et al., 2007; Chalker et al., 2009).
Poor adherence: The patient who missed >6 doses of 30 doses or >9 doses of 60 doses as documented by the ART healthcare provider is said to have poor adherence (Nabukeera-Barungi et al., 2007; Chalker et al., 2009).
Time to ART adverse drug reaction: The time gap in days between being put on ART to the development of the first episode of ART adverse drug reactions.
Censored: Patients who did not experience ART adverse drug reactions during the follow-up period, died before experiencing ART adverse drug reactions within the study period, or discontinued ART before experiencing the event within the study period by reason not related to the event were counted as censored.
Data for this study were collected from patients’ routine records from the two hospitals. Data were extracted by reviewing the follow-up charts, ART registration log books, and patients’ intake forms. The health management information system card number was used to identify individual patient cards. The dependent variable for this study was the time to ART adverse drug reactions. The event of interest is the occurrence of the adverse drug reaction within the follow-up period. In the present study, the minimum follow-up period was 1 month. Patients were followed-up for a total of 5 years and 5 months.
Socio-demographic variables (age, sex, educational status, occupational status, place of residence, and marital status), baseline clinical variables (WHO HIV/AIDS stage, baseline CD4 count, body mass index (BMI), initial ART regimen, TB co-infection, OIP, baseline functional status, and adherence), and comorbidity were independent variables.
By conducting a preliminary review, the adequacy of the checklist was evaluated and variables which are unavailable on records were excluded from the checklist. Trained healthcare workers who are familiar with chronic diseases were assigned as data collectors. In addition, to ensure data quality, a filled checklist was checked for consistency and completeness. Strict supervision was applied by supervisors during data collection.
The data were entered into Epi Info 7 and then exported to R statistical software 3.4.4 for analysis. Descriptive measures such as means and standard deviations were used to characterize the study population for quantitative variables whereas percentage and frequencies were used for categorical variables. The time to ART adverse drug reactions was estimated using the Kaplan–Meier (KM) method and the log-rank test was used to compare the survival time between groups of categorical variables. Proportional hazards assumption (PHA) was checked before fitting the Cox proportional hazard model by a cumulative log hazard plot. The factors significantly associated with ART adverse drug reactions in the bi-variable analysis at p-values less than 0.2 were included in the multivariable Cox regression model. The adjusted hazard ratio (AHR) with its confidence interval was used to show the presence and strength of association at a 95% confidence level. Overall adequacy of the model was checked using Cox Snell residuals.
A total of 561 HIV/AIDS patients were included in this study. The baseline age of the participants ranged from 18–64 years with a mean age of 33.4 (±8.9) years. Of the participants, 356 (63.5%) were females, 351 (62.6%) were urban dwellers, 139 (24.8) were non-government employed, and 304 (54.2%) were married (Table 1).
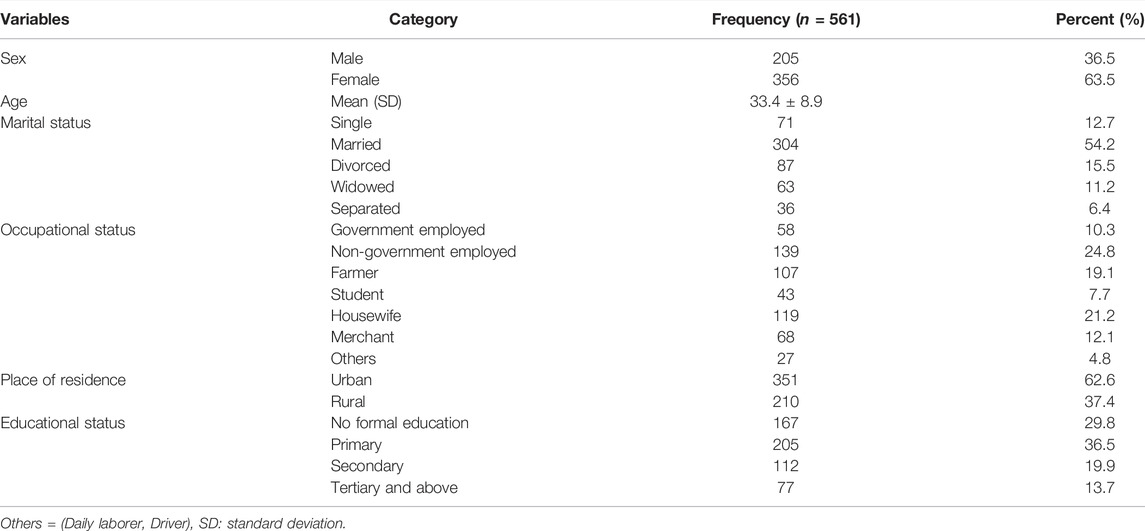
TABLE 1. Baseline socio-demographic characteristics of HIV/AIDS patients on ART in Eastern Ethiopia, 2013–2019.
Of the total participants, 158 (28.2%) and 26 (4.6%) were in WHO stage III and IV, respectively, at the baseline, 100 (17.8%) had TB co-infection, 494 (88.1%) received OIP, and 358 (63.8%) had a baseline CD4 count of more than 200. Poor adherence was reported by 48 (8.6) HIV/AIDS patients. The most commonly prescribed initial ART regimen is TDF, 3 TC, and EFV. The most common types of ART adverse drug reactions encountered were anemia (33.9%) and rash (26.4%) (Table 2).
The median survival time was not reached which means the study ended before 50% of the participants experienced ART adverse drug reactions.
The cumulative probability of survival without developing an adverse drug reaction at the end of one and a half years was 0.91 and 0.90 years, respectively, and the cumulative probability of survival by the end of the follow-up period was found to be 0.89. Regarding the timing of ART adverse drug reactions, most (90.7%) ART adverse drug reactions occurred within 1 year of initiation of ART.
The Kaplan–Meier survival estimate revealed that the hazards of developing ART adverse drug reactions were higher among patients who had TB co-infection and patients presented with poor baseline adherence status (Figure 1).
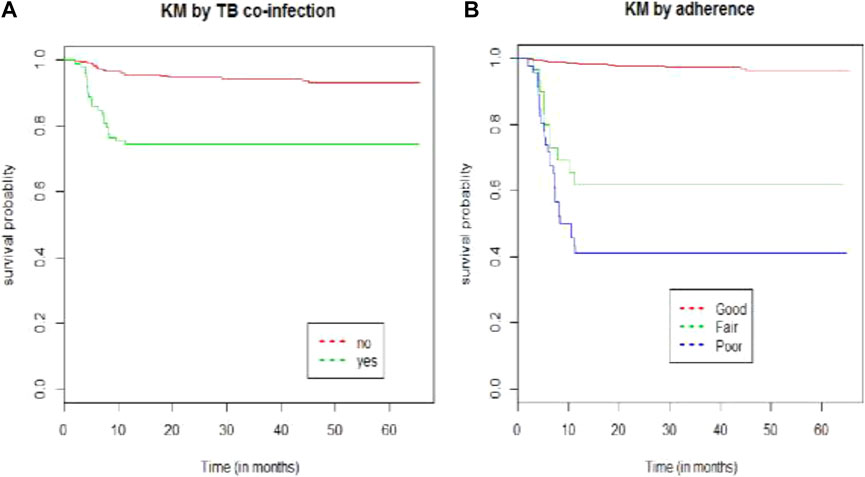
FIGURE 1. Kaplan–Meier survival curve by (A) TB co-infection and (B) adherence for HIV/AIDS patients on ART at public hospitals in Eastern Ethiopia from 2013–2019.
The median follow-up time was 31.3 months [inter-quartile range (IQR), (18.3, 47.5)]. Of the 561 participants, 54 (9.6%) (95% CI: 7.2–12.1%) patients had developed ART adverse drug reactions with 1,514.2 persons-years (PYs) of observations. The incidence density was 3.5/100 PYs with a 95% CI of 2.7, 4.6. About 24 (44.4%), 50 (92.6%), and 52 (96.3%) adverse drug reaction occurred within the first half, 1, and 2 years of the initiation of ART, respectively.
In the bi-variable Cox regression analysis, WHO RVI stage, adherence, BMI, initial ART regimen, comorbidity, TB co-infection, CD4 count, OIP, place of residence, and functional status were significantly associated with the time to ART adverse drug reaction at a p-value of 0.2. In the multivariable analysis, the initial ART regimen (TDF, 3TC, and EFV) [AHR = 0.3, 95% CI 0.1–0.7], fair adherence [AHR = 8.8, 95% CI 3.3–23.2], poor adherence [AHR = 7.8, 95% CI 3.1–19.5], moderate BMI at baseline [AHR = 4.4, 95% CI 1.8–11.0], severe BMI [AHR = 2.8, 95% CI 1.1–6.7], WHO stage II [AHR = 3.7, 95% CI 1.2–11.3], and WHO stage IV [AHR = 6.3, 95% CI 2.0–19.8] were significant predictors of the time to ART adverse drug reaction.
For HIV/AIDS patients whose baseline ART regimen was TDF, 3TC, and EFZ, the hazards of developing ART adverse drug reactions were decreased by 70% (AHR = 0.3, 95% CI [0.1–0.7]) at the baseline as compared with patients taking AZT, 3TC, and NVP at baseline while keeping other variables constant.
Adjusting for other variables in the model, the hazard of experiencing ART adverse reactions was 3.7 times [AHR = 3.7, 95% CI (1.2–11.3)] and 6.3 times higher [AHR = 6.3, 95% CI (2.0, 19.8)] for RVI patients with baseline WHO RVI stage II and stage IV, respectively, than those with baseline RVI stage I.
Adjusting for other variables, the hazards of experiencing ART adverse reactions were 4.4 and 2.8 times higher among patients with moderate [AHR = 4.4, 95% CI (1.8–11.0)] and severe [AHR = 2.8, 95%CI (1.1, 6.8)] baseline BMI, respectively, than those with normal BMI at baseline.
Adjusting for other variables, patients with fair and poor baseline adherence status were at 8.8 and 7.8 times higher risk of developing ART adverse reactions [AHR = 8.8, 95% CI (3.3, 23.2) and AHR = 7.8, 95% CI (3.1, 19.5)], respectively, than those patients with good baseline adherence (Table 3).
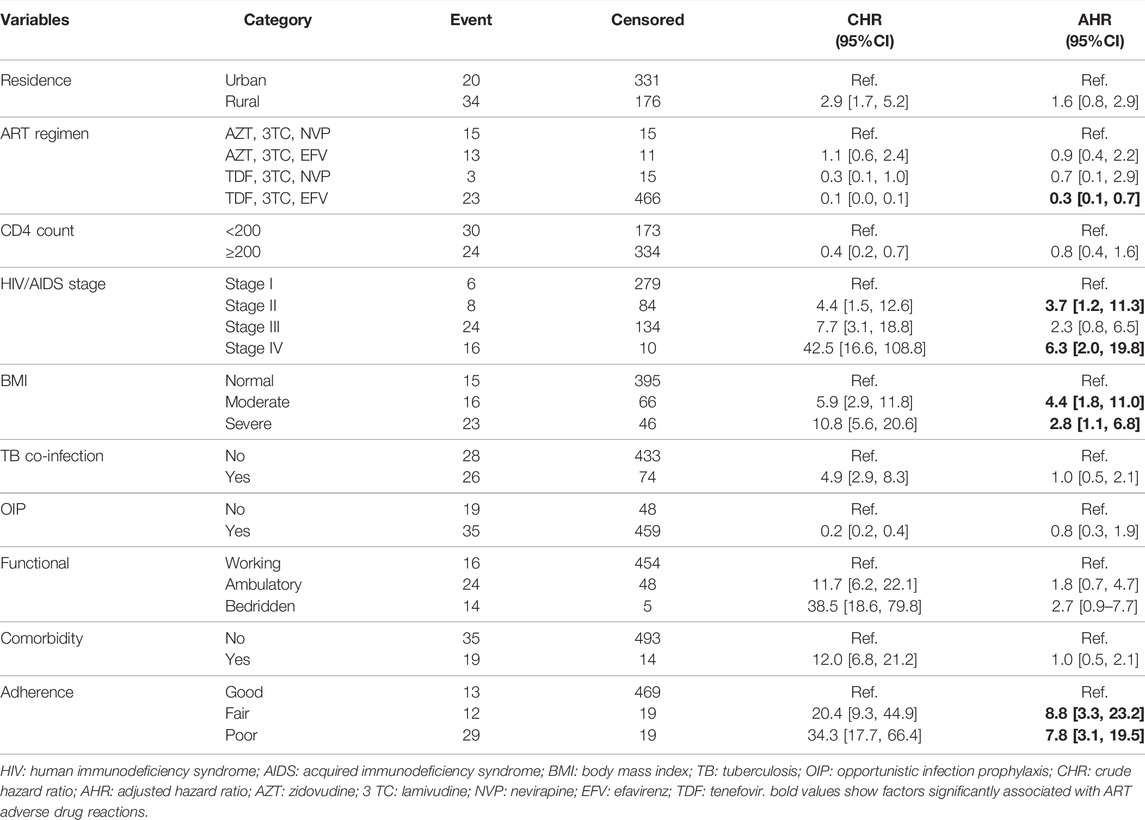
TABLE 3. Predictors of the time to ART ADR among HIV/AIDS patients on ART at public hospitals in Eastern Ethiopia, 2013–2019.
This study assessed the time to ART adverse drug reaction and its predictors among HIV/AIDS patients. A Cox proportional hazard model was applied to identify the predictors of ART adverse drug reactions. Factors observed to have a significant association with the time to ART adverse drug reactions among HIV/AIDS patients were WHO HIV/AIDS stage, BMI, adherence, and initial ART regimen.
The present study revealed that about 9.6% of the participants had ART adverse drug reactions with an incidence density of 3.5 per 100 PY 95% CI [2.7, 4.6]. This result is in line with previous studies conducted in Ethiopia (4.3/100PY) and Nigeria (4.6/100 PY) (Huang et al., 2012; Kindie et al., 2017). However, the finding of the present study was lower than another retrospective study conducted in India (10.11/100 PY) (Shet et al., 2014) and Hosanna, Ethiopia, which reported an incidence density of 4.88 per 100 person-days (Asfaw and Enquselassie, 2018b). The reasons for this discrepancy might be because of the difference in how the adverse drug reaction was measured and the follow-up period. In the later study, patients were followed-up for 2 years whereas in the present study, patients were followed-up for 5.5 years.
In the present study, most (92.6%) ART adverse drug reactions occurred within 1 year of initiation of ART which is supported by a study in Bahir Dar, Ethiopia (Kindie et al., 2017). The possible explanation for this is most HIV/AIDS patients are presented with complications and advanced HIV disease with severe immunodeficiency states which makes adverse drug reactions more likely. Moreover, an intrinsic metabolic intolerance when patients first started ART might also contribute to early ART adverse drug reactions (Shelburne et al., 2002).
In the present study, cumulative probability of survival without developing adverse drug reactions by the end of the follow-up period was 89% [95% CI 86–92%]. This result is supported by a previous study in Ethiopia (Kindie et al., 2017) and this promising survival probability might be because of an early screening program for TB and other associated comorbidities at the start of ART in the study area and most of the patients took opportunistic infection prophylaxis which might contribute to the survival of patients.
Patients with moderate and severe baseline BMI had higher risks of experiencing ART adverse drug reactions than those with a normal BMI at baseline and this is in agreement with a prospective study conducted in Ethiopia (Bezabhe et al., 2015). This might be because of the BMI’s effect on drug metabolism as it contributes to the efficacy of highly active antiretroviral therapy (HAART) (Li et al., 2019). Thus, HIV/AIDS patients with normal BMI are at a lower risk of developing ART adverse drug reactions.
Adherence status is another significant predictor of the time to ART adverse drug reaction. Patients with fair and poor adherence are at higher risk of experiencing ART adverse drug reactions than those with good adherence status, which is supported by a previous study in South Africa (Masenyetse et al., 2015). A possible explanation for this might be that patients who miss their prescribed doses cannot consistently maintain virologic success and are prone to develop ART adverse drug reactions (Bangsberg et al., 2001).
According to our finding, the hazard of experiencing ART adverse drug reactions is higher among HIV/AIDS patients with baseline WHO HIV/AIDS stages II and IV than those with stage I. This result is in line with previous studies (Eluwa et al., 2012; Shet et al., 2014; Weldegebreal et al., 2016; Bhuvana and Hema, 2017; Kindie et al., 2017) which reported that patients with advanced stages of the disease are at a higher risk of ART adverse drug reactions. This might be related to the inability to resist side effects in patients with advanced stages of disease and possible drug interactions since immune-mediated adverse drug reactions are by far more common in HIV/AIDS patients (Peter et al., 2019).
Hazards of developing ART adverse drug reactions for HIV/AIDS patients whose baseline ART regimen was TDF, 3TC, and EFV are lower than those patients taking AZT, 3TC, and NVP at baseline. This finding is in agreement with previous studies in Uganda (van Leth et al., 2004) and Ethiopia (Weldegebreal et al., 2016) and the reason for this might be due to the higher toxicity profile of AZT and NVP-containing regimen (Wester et al., 2010; Masenyetse et al., 2015).
This study is not without limitations. The main drawback of the present study was the absence of important variables which might have an effect on the time to ART adverse drug reaction. Since the present study was based on retrospective data obtained from patient medical records, important variables such as the socio-economic status, behavioral variables (smoking and alcohol drinking status), and clinical variables (viral load and hemoglobin) which are assumed to be associated with ART adverse drug reactions were not obtained in the record.
In conclusion, most ART adverse drug reactions occurred within 1 year of initiation of ART. The initial ART regimen (TDF, 3TC, and EFV), adherence, HIV/AIDS stage, and body mass index were risk factors for the time to ART adverse drug reaction. The incidence of antiretroviral therapy adverse drug reaction was relatively low with early onset. Close monitoring of clients in clinical stage II and above is required and continuous assessment for improving the detection and management of adverse drug reactions is recommended. Patients with poor adherence need to get continuous counseling to improve their adherence status.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by ethical clearance and the letter of cooperation for selected hospitals was obtained from the Institutional Review Board of the University of Gondar. The support letter was obtained from the medical director of the hospitals to access medical records of patients. Data were handled confidentially using an anonymous medical registration number as an identification. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
AW contributed to the conceptualization, design, analysis, and writing and editing of the manuscript. BT, GD, TA, and MS contributed to data curation, analysis, investigation, and writing and editing of the manuscript. All authors reviewed the manuscript during preparation, provided critical feedback, and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank University of Gondar for funding and giving ethical clearance to conduct this research. Our special gratitude also goes to Chiro and Gelemso hospital administrations, data collectors and supervisors for their support during data collection period.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.922744/full#supplementary-material
AHR, Adjusted Hazard Ratio; AIDS, Acquired Immunodeficiency Syndrome; ART, Antiretroviral Therapy; BMI, Body Mass Index; CHR, Crude Hazard Ratio; CI, Confidence Interval; HAART, Highly Active Antiretroviral Therapy; HIV, Human Immunodeficiency Virus; HR, Hazard Ratio; KM: Kaplan Meier; PHA, Proportional Hazard Assumptions; PY, Person-Year; TB, Tuberculosis; WHO, World Health Organization.
Asfaw, L., and Enquselassie, F. (2018). Time to Major Adverse Reactions of Anti-Retroviral Drugs and its Predictors Among Cohort of Patients Receiving Antiretroviral Therapy in Hosanna Hospital, Hosanna, Ethiopia: Retrospective Cohort Study. Int. J. Virol. AIDS 5, 045.doi:10.23937/2469-567X/1510045
Asfaw, L., and Enquselassie, F. (2018). Time to Major Adverse Reactions of Anti-Retroviral Drugs and its Predictors Among Cohort of Patients Receiving Antiretroviral Therapy in Hosanna Hospital, Hosanna, Ethiopia. Retrosp. Cohort Study 5, 045. doi:10.23937/2469-567X/1510045
Asrat, M., Hailu, G., Gedefaw, M., and Tesfa, M. (2014). Prevalence and Associated Factors of Art Adverse Effect Among PLWH on Art in Debre Markose Referal Hospital, North East Ethiopia, 2013. Fam. Med. Med. Sci. Res. 3 (130), 2. doi:10.4172/2327-4972.1000130
Bangsberg, D. R., Perry, S., Charlebois, E. D., Clark, R. A., Roberston, M., Zolopa, A. R., et al. (2001). Non-adherence to Highly Active Antiretroviral Therapy Predicts Progression to AIDS. Aids 15 (9), 1181–1183. doi:10.1097/00002030-200106150-00015
Bezabhe, W. M., Bereznicki, L. R., Chalmers, L., Gee, P., Kassie, D. M., Bimirew, M. A., et al. (2015). Adverse Drug Reactions and Clinical Outcomes in Patients Initiated on Antiretroviral Therapy: a Prospective Cohort Study from Ethiopia. Drug Saf. 38 (7), 629–639. doi:10.1007/s40264-015-0295-7
Bhuvana, K., and Hema, N. (2017). A Prospective Observational Study of Adverse Drug Reactions to Antiretroviral Therapy: Type and Risk Factors in a Tertiary Care Teaching Hospital. Int. J. Basic & Clin. Pharmacol. 3 (2), 380–384.
Bonfanti, P., Valsecchi, L., Parazzini, F., Carradori, S., Pusterla, L., Fortuna, P., et al. (2000). Incidence of adverse reactions in HIV patients treated with protease inhibitors: a cohort study. Coordinamento Italiano Studio Allergia e Infezione da HIV (CISAI) Group. J. Acquir Immune Defic. Syndr. 23 (3), 236–245. doi:10.1097/00126334-200003010-00004
Carr, A., and Cooper, D. A. (2000). Adverse Effects of Antiretroviral Therapy. Lancet 356 (9239), 1423–1430. doi:10.1016/S0140-6736(00)02854-3
Chalker, J., Andualem, T., Tadeg, H., Gitau, L., Ntaganira, J., Obua, C., et al. Developing Standard Methods to Monitor Adherence to Antiretroviral Medicines and Treatment Defaulting in Resource-Poor Settings. 2009;1:4–8.
Daniel Murrell, M. (2018). The Comprehensive Guide to HIV and AIDS. Addise Ababa: Federal Ministry of Health.
Duval, X., Journot, V., Leport, C., Chêne, G., Dupon, M., Cuzin, L., et al. (2004). Incidence of and Risk Factors for Adverse Drug Reactions in a Prospective Cohort of HIV-Infected Adults Initiating Protease Inhibitor-Containing Therapy. Clin. Infect. Dis. 39 (2), 248–255. doi:10.1086/422141
Eluwa, G. I., Badru, T., Agu, K. A., Akpoigbe, K. J., Chabikuli, O., and Hamelmann, C. (2012). Adverse Drug Reactions to Antiretroviral Therapy (ARVs): Incidence, Type and Risk Factors in Nigeria. BMC Clin. Pharmacol. 12 (1), 7. doi:10.1186/1472-6904-12-7
Girum, T., Wasie, A., and Worku, A. (2018). Trend of HIV/AIDS for the Last 26 years and Predicting Achievement of the 90-90-90 HIV Prevention Targets by 2020 in Ethiopia: a Time Series Analysis. BMC Infect. Dis. 18 (1), 320. doi:10.1186/s12879-018-3214-6
Hicks, C. B., Cahn, P., Cooper, D. A., Walmsley, S. L., Katlama, C., Clotet, B., et al. (2006). Durable Efficacy of Tipranavir-Ritonavir in Combination with an Optimised Background Regimen of Antiretroviral Drugs for Treatment-Experienced HIV-1-Infected Patients at 48 Weeks in the Randomized Evaluation of Strategic Intervention in Multi-Drug reSistant Patients with Tipranavir (RESIST) Studies: an Analysis of Combined Data from Two Randomised Open-Label Trials. Lancet 368 (9534), 466–475. doi:10.1016/S0140-6736(06)69154-X
Hogg, R. S., Yip, B., Kully, C., Craib, K. J., O'shaughnessy, M. V., Schechter, M. T., et al. (1999). Improved Survival Among HIV-Infected Patients after Initiation of Triple-Drug Antiretroviral Regimens. CMAJ 160 (5), 659–665. doi:10.1001/jama.279.6.450
Huang, M. W., Yang, T. T., Ten, P. R., Su, P. W., Wu, B. J., Chan, C. H., et al. (2012). Effects of Paliperidone Extended Release on the Symptoms and Functioning of Schizophrenia. BMC Clin. Pharmacol. 12 (1), 1–11. doi:10.1186/1472-6904-12-1
Kindie, E., Alamrew Anteneh, Z., and Worku, E. (2017). Time to Development of Adverse Drug Reactions and Associated Factors Among Adult HIV Positive Patients on Antiretroviral Treatment in Bahir Dar City, Northwest Ethiopia. PloS one 12 (12), e0189322. doi:10.1371/journal.pone.0189322
Li, X., Ding, H., Geng, W., Liu, J., Jiang, Y., Xu, J., et al. (2019). Predictive Effects of Body Mass Index on Immune Reconstitution Among HIV-Infected HAART Users in China. BMC Infect. Dis. 19 (1), 373. doi:10.1186/s12879-019-3991-6
Masenyetse, L. J., Manda, S. O., and Mwambi, H. G. (2015). An Assessment of Adverse Drug Reactions Among HIV Positive Patients Receiving Antiretroviral Treatment in South Africa. AIDS Res. Ther. 12 (1), 6. doi:10.1186/s12981-015-0044-0
Nabukeera-Barungi, N., Kalyesubula, I., Kekitiinwa, A., Byakika-Tusiime, J., and Musoke, P. (2007). Adherence to Antiretroviral Therapy in Children Attending Mulago Hospital, Kampala. Ann. Trop. Paediatr. 27 (2), 123–131. doi:10.1179/146532807X192499
Organization, W. H. (2008). Medicines: Safety of Medicines–Adverse Drug Reactions. Geneva: World Health Organization.
Peter, J., Choshi, P., and Lehloenya, R. J. (2019). Drug Hypersensitivity in HIV Infection. Curr. Opin. Allergy Clin. Immunol. 19 (4), 272–282. doi:10.1097/ACI.0000000000000545
Pharmacists ASoH-, S. (1995). ASHP Guidelines on Adverse Drug Reaction Monitoring and Reporting. American Society of Hospital Pharmacy. Am. J. Health Syst. Pharm. 52 (4), 417–419. doi:10.1093/ajhp/52.4.417
Severe, P., Juste, M. A., Ambroise, A., Eliacin, L., Marchand, C., Apollon, S., et al. (2010). Early versus Standard Antiretroviral Therapy for HIV-Infected Adults in Haiti. N. Engl. J. Med. 363 (3), 257–265. doi:10.1056/NEJMoa0910370
Shelburne, S. A., Hamill, R. J., Rodriguez-Barradas, M. C., Greenberg, S. B., Atmar, R. L., Musher, D. W., et al. (2002). Immune Reconstitution Inflammatory Syndrome: Emergence of a Unique Syndrome during Highly Active Antiretroviral Therapy. Med. Baltim. 81 (3), 213–227. doi:10.1097/00005792-200205000-00005
Shet, A., Antony, J., Arumugam, K., Kumar Dodderi, S., Rodrigues, R., and DeCosta, A. (2014). Influence of Adverse Drug Reactions on Treatment Success: Prospective Cohort Analysis of HIV-Infected Individuals Initiating First-Line Antiretroviral Therapy in India. PloS one 9 (3), e91028. doi:10.1371/journal.pone.0091028
van Leth, F., Conway, B., Laplumé, H., Martin, D., Fisher, M., Jelaska, A., et al. (2004). Quality of Life in Patients Treated with First-Line Antiretroviral Therapy Containing Nevirapine And/or Efavirenz. Antivir. Ther. 9 (5), 721–728.
Weldegebreal, F., Mitiku, H., and Teklemariam, Z. (2016). Magnitude of Adverse Drug Reaction and Associated Factors Among HIV-Infected Adults on Antiretroviral Therapy in Hiwot Fana Specialized University Hospital, Eastern Ethiopia. Pan Afr. Med. J. 24 (1), 255. doi:10.11604/pamj.2016.24.255.8356
Wester, C. W., Thomas, A. M., Bussmann, H., Moyo, S., Makhema, J. M., Gaolathe, T., et al. (2010). Non-nucleoside Reverse Transcriptase Inhibitor Outcomes Among Combination Antiretroviral Therapy-Treated Adults in Botswana. AIDS 24 Suppl 1 (Suppl. 1), S27–S36. doi:10.1097/01.aids.0000366080.91192.55
WHO (2013). Global Update on HIV Treatment 2013: Results, Impact and Opportunities. Geneva, Switzerland: World Health Organization.
Keywords: ART, ADR, hiv/aids, predictors, adults, Ethiopia
Citation: Weldesenbet AB, Tusa BS, Debele GR, Sisay MM and Ayele TA (2022) Time to First Line Antiretroviral Therapy Adverse Drug Reaction and its Predictors Among Adult HIV/AIDS Patients on Treatment in Eastern Ethiopia. Front. Pharmacol. 13:922744. doi: 10.3389/fphar.2022.922744
Received: 18 April 2022; Accepted: 16 June 2022;
Published: 15 August 2022.
Edited by:
Marcus Tolentino Silva, University of Sorocaba, BrazilReviewed by:
Rannakoe Lehloenya, University of Cape Town, South AfricaCopyright © 2022 Weldesenbet, Tusa, Debele, Sisay and Ayele. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biruk Shalmeno Tusa, YmlydWtzaGFsbWVubzI3QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.