
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 29 August 2022
Sec. Obstetric and Pediatric Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.922015
This article is part of the Research TopicMethods and Protocols in Obstetric and Pediatric Pharmacology: 2022View all 5 articles
Objective: To investigate the effect of dietary fiber intake during pregnancy on the prevention of gestational diabetes mellitus (GDM) in women who are overweight/obese prior to pregnancy.
Methods: This randomized controlled trial was conducted in Shanghai General Hospital from June 2021 to March 2022. A total of 98 women who reported BMI≥24 kg/m2 prior to pregnancy were recruited before their 20th gestational week, and randomly (simple random allocation) assigned to the fiber supplement group (12 g of dietary fiber power twice daily) and the control group (standard prenatal care) from 20 to 24+6 gestational weeks. Both groups received nutrition education and dietary advice during the study. GDM diagnosis was performed by an oral glucose tolerance test (OGTT) at 25–28 weeks’ gestation. Data are presented as means with SD, as medians with IQR, or as counts with percentages as appropriate. Comparisons were conducted using a t-test, Mann-Whitney U test, and χ2 test, respectively.
Results: The incidence of GDM was significantly reduced in the fiber supplement group compared with the control group: 8.3 vs. 24.0% (χ2 = 4.40, p = 0.036). At OGTT, the mean fasting plasma glucose in the fiber supplement group was significantly lower than before the intervention (4.57 ± 0.38 mmol/L vs. 4.41 ± 0.29 mmol/L, p < 0.01) but not in the control group (4.48 ± 0.42 mmol/L vs. 4.37 ± 0.58 mmol/L, p = 0.150). Compared with the control group, the TG and TG/HDL-C ratio levels in the intervention group were significantly higher than those in the control group (2.19 ± 0.54 mmol/L vs. 2.70 ± 0.82 mmol/L and 1.19 ± 0.49 vs.1.63 ± 0.63, respectively, all P<0.05). The body weight gain was significantly lower in the fiber supplement group than the control group (1.99 ± 1.09 kg vs. 2.53 ± 1.20kg, p = 0.022). None of the women randomized to the fiber supplement group experienced preterm birth (<37 weeks gestation) compared with 12.0% in the control group (p = 0.040). Excessive weight gain (total weight gain >11.5 kg for overweight, and >9.0 kg for obesity) occurred in 46.7% of women in the fiber supplement group compared with 68.0% in the control group (p = 0.035). There were no differences in other maternal and neonatal outcomes.
Conclusion: Increased dietary fiber intake in pregnant women who were overweight/obese prior to pregnancy may reduce the risk of GDM, excessive weight gain, and preterm birth, but it did not improve blood lipids.
Gestational diabetes mellitus (GDM) is defined as any glucose intolerance firstly recognized or onset in pregnancy. Considered one of the most common metabolic diseases experienced by women during pregnancy, GDM is associated with an increased risk of adverse maternal and neonatal outcomes, including macrosomia, neonatal hypertensive disorders, cesarean delivery, and an increased risk of developing type 2 diabetes later in life for the mother (McIntyre et al., 2019; Chen et al., 2021). It is estimated that approximately 14% of pregnancies are affected by GDM worldwide, and the prevalence is increasing as rates of overweight/obesity continue to rise among women of childbearing age (Stern et al., 2021).
Women with pre-pregnancy overweight/obesity (defined using ethnic-specific thresholds of BMI ≥24 kg/m2 for Chinese, and ≥25 kg/m2 for White, Black, and mixed), a higher risk factor for GDM, are associated with increased levels of inflammatory markers, which contribute directly to the development of insulin resistance and GDM(Yen et al., 2019; Godfrey et al., 2021). Chu and others reported ORs of developing GDM were 2.14 (95% CI: 1.82–2.53), 3.56 (95% CI: 3.05–4.21), and 8.56 (95% CI: 5.07–16.04) among overweight (BMI ≥25 kg/m2), obesity (BMI ≥30 kg/m2), and severely obesity (BMI ≥40 kg/m2), respectively, compared with normal-weight women (Chu et al., 2007). Lifestyle interventions involving exercise and a healthy diet were strongly recommended owing to medical treatments that pose potential threats to the fetus (Juan and Yang, 2020; Li et al., 2021). However, the DALI (Vitamin D and lifestyle intervention for GDM prevention) study group reported that among pregnant women with obesity, healthy eating and physical activity alone were unlikely to prevent GDM development, nor was it a cost-effective early intervention to decrease fasting glucose and insulin sensitivity (Simmons et al., 2017), emphasizing the need for new preventive approaches.
Dietary fiber consists of nondigestible carbohydrates and lignin, which are not digested and absorbed by the human body (Barber et al., 2020). Several studies show that increasing the intake of dietary fiber during pregnancy benefits a lot of women by reducing excessive weight gain, insulin resistance, and lowering the risk of glucose intolerance (Pretorius and Palmer, 2020; Jaworsky et al., 2021; Zhang et al., 2021). Fruits and vegetables, along with whole-grains, are excellent sources of dietary fiber, and the Food and Drug Administration (FDA) has also approved that foods high in fruits, vegetables, and whole-grains have multiple health benefits for pregnant women (Zerfu and Mekuria, 2019). The Chinese Dietary Reference Intakes (DRIs) 2013 recommends a minimum of 25 g/day of dietary fiber during pregnancy, while the average total dietary fiber intake in Chinese pregnant women (14.9 g/day) is far below the recommended daily intake (Liu F.-L. et al., 2015; Liu F. L. et al., 2015; Bailey et al., 2019; Zerfu and Mekuria, 2019; Chen et al., 2020; Roskjaer et al., 2021). It was based on the fact that a larger proportion of dietary fiber intake is essential to reduce the chance of developing metabolic complications during pregnancy. Therefore, we designed and conducted a randomized controlled trial in pre-pregnancy overweight/obese women to investigate whether intervention with dietary fiber supplement, compared with standard prenatal care, taken during pregnancy, would reduce the risk of GDM and improve maternal pregnancy outcomes.
We conducted a unicentric, clinic-based, randomized controlled trial between June 2021 and March 2022 at the Department of Obstetrics and Gynecology at the Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China. This study was approved by the Shanghai General Hospital of School of Medicine, Shanghai Jiao Tong University ethics committee (2020KY098) and was registered on Chinese Clinical Trial Registry website (ChiCTR2000036575).
Sample size was calculated based on a 66.7% reduction in GDM frequency with the use of dietary fiber supplement (from 36 to 12%), with a statistical power of 80% (α = 0.05), as well as allowing for 10% attrition, called for the recruitment of 102 women (92 completers) (Yan et al., 2019). In the end, a total of 104 pregnant women were recruited in the Shanghai General Hospital, and 98 of them completed the study. Simple randomization was applied with an allocation of 1:1 using Microsoft Excel 2019 random number generator in each group.
The inclusion criteria were as follows: 1) self-reported pre-pregnancy BMI ≥24 kg/m2(Wang et al., 2017); 2) <20 weeks of gestation; 3) singleton pregnancy; 4) not having diabetes mellitus; 5) do not current use of medications that might affect glucose metabolism (metformin, glucocorticoids, immunosuppressants, antipsychotics); 6) willingness or ability to provide written informed consent.
Participants in this study would be removed if one of the following occurred: 1) women request to be removed from the study or withdraw consent for any reason; 2) adverse events that made it difficult to continue; 3) compliance with intervention less than 50% of the time; or 4) women who lost contact during the follow-up.
The intervention was from 20 to 24+6 weeks of gestation, and all participants received standard prenatal care, including nutrition education and dietary advice by nutritionists based on the Chinese Dietary Guidelines for Pregnant Women (Wang et al., 2016). The guidelines recommend: 1) take iron-rich foods (20–50 g red meat/day), and iodized salt; 2) increase intake of milk (500 g/day), and the amounts of fish, poultry, eggs and lean meat increased by 50 g/day; 3) total carbohydrate daily intake≥130 g; 4) moderate physical activity (≥30 min/day) to maintain recommended gestational weight gain (overweight: 7–11.5 kg, and obesity: 5–9 kg). 5) smoking cessation and keep good spirits. In addition to the measures above mentioned, the fiber supplement group was given 1 bag (12 g) of soluble dietary fiber powder (Nutrasumma, Qingdao Nutrasumma Health Technology Co., Ltd.) twice daily, which contained 51.93 of kcal energy, 3.31 g of carbohydrates, and 9.78 g of dietary fiber. Furthermore, participants in both groups also completed food frequency questionnaires (FFQs) to assess dietary intake pre and post intervention. The doctors were responsible for timely management of any adverse reaction during the study. All pregnant women enrolled in the study underwent a 75 g oral glucose tolerance test (OGTT) at 25–28 weeks of gestation.
Flowchart 1. Enrollment of participants in the study.
Outcomes: The primary outcome measure was the incidence of GDM. Secondary outcomes included the leaves of blood glucose and lipids, diet changes, gestational weight gain, and maternal and neonatal outcomes.
Anthropometric measures: Pre-pregnancy BMI (kg/m2) was calculated using the self-reported data from participants. Body weight (kg) was measured while the participants were wearing light clothing at a gestational age of 20 and 25 weeks, and weight gain (kg) was evaluated at 25 weeks of gestation. Blood pressure (mmHg) was measured at the first two visits with an adequate armlet when the participants had been seated for at least 10 min, after which 3 blood pressure measurements were recorded at intervals of 10–15 min and the mean values were adopted. Data on maternal and neonatal outcomes were abstracted from the electronic medical record.
Biochemical variables: Blood samples were drawn between 07.30 and 09.00 a.m., after an overnight fast. Blood samples were collected and sent to be analyzed within 3 h. A biochemical autoanalyzer (ADVIA2400 Chemistry System, Siemens Healthcare Diagnostics Ltd., Germany) was adopted to analyze the plasma glucose (fasting plasma glucose [FPG], 1-h postprandial plasma glucose [1 hPG], 2-h postprandial plasma glucose [2 hPG]) and blood lipid profiles, including triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Serum glycated hemoglobin (HbA1c) was measured by high-pressure liquid chromatography using HLC-723G8 instruments (Tosoh, Tokyo, Japan). These biomarkers were mainly measured at baseline and at 25–28 weeks of gestation.
Diet evaluation: Energy and dietary fiber intakes were calculated from online FFQs (Zhang et al., 2015), including a total of 39 items from nine food groups, which are cereals (rice, pasta, roughage, bread, and crackers), vegetables (roots, stem, fruit, and leaf vegetables), fruits (fresh, canned, dried), meats and eggs (pork, beef, mutton, poultry, fish, and eggs), beans (soya-bean milk and other soy products.), nuts, milk and milk products (milk, yoghurt, and cheese), oil (liquid oil), and beverages (fresh fruit juice, juice drinks, carbonate drinks, and coffee). Participants were required to recall their usual frequency and portion size of consuming each food item in the past 5 weeks at 20 and 25 weeks of gestation. Food intake frequency was measured as per day, per week, per month, or never. Food models representing standard portion sizes of relevant food items were presented to participants to help them estimate their usual consumption. Subsequently, the mean daily total energy intake (including carbohydrate, protein, fat, and energy intake) and dietary fiber were evaluated using the Chinese Food Composition Table (Yang et al., 2009). Trained dietary interviewers helped all participants complete the FFQ, ensuring the accuracy of the data collected.
The diagnosis of GDM was based on a 75 g oral glucose tolerance test (OGTT) at 25–28 weeks of gestation, according to the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria (Weinert, 2010). Diagnosis of GDM was confirmed if one or more values were at or above the threshold level: fasting plasma glucose (FPG)≥5.1 mmo1/L; OGTT 1 h plasma glucose≥10.0 mmol/L; OGTT 2 h plasma glucose≥8.5 mmol/L.
Normal distribution of data was evaluated using the Shapiro-Wilk test. Continuous variables were shown as means with SD for normally distributed data, or as medians with IQR for non-normally distributed data, and categorical variables are presented as counts with percentages. The t-test was used to compare continuous variables with normal distribution, the Mann-Whitney U test was used to compare continuous variables with non-normal distribution, and χ two test or Fisher’s exact test for categorical variables, when applicable. Statistical significance was defined as a p value < 0.05. Statistical analyses were performed using SPSS statistical software (IBM, version 25.0 for Windows).
In the end, a total of 52 women were randomized to the fiber supplement and 52 to the control group, and 48 and 50 completed the follow-up, respectively. (Flowchart 1). Demographic and clinical characteristics did not significantly differ between the fiber supplement group and the control group at baseline (Table 1).
OGTT, scheduled for all participants, was performed at a mean of 25.57 ± 1.00 weeks of gestation. Among women in the control group, 12 of 50 (24.0%) were diagnosed with GDM according to IADPSG criteria, whereas only four of 48 (8.3%) were diagnosed with GDM in the fiber supplement group. We found a statistically significant difference in the incidence of GDM between the groups (χ2 = 4.400, p = 0.036) (Table 3).
The comparison between the two groups showed no significant differences with the levels of FPG and HbA1c at the baseline and the end (all p > 0.05). After 5 weeks of intervention, HbA1c levels in both groups were slightly decreased, and FPG levels were only decreased slightly in the fiber supplement group compared to baseline values (all p < 0.05) (Table 2).
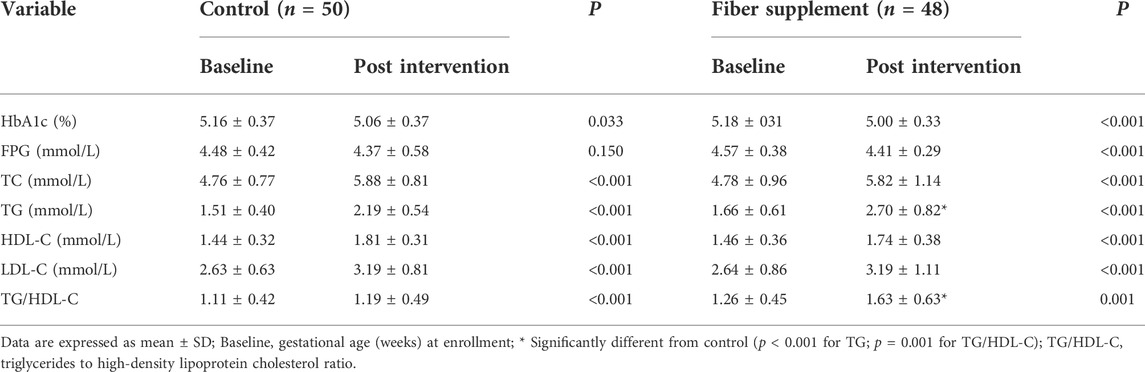
TABLE 2. Glucose and lipids levels in overweight/obese mothers in fiber supplement and control groups pre and post-intervention.
OGTT measured between 25 and 28 weeks of gestation revealed that 1 hPG, 2 hPG, the difference of lhPG and FPG, and IAUC did not differ between the two groups after intervention (p > 0.05). However, the value decreased from 1 hPG to 2 hPG was significantly lower in the control group (0.91 ± 1.18 mmol/L) compared with the fiber supplement group (1.49 ± 1.54 mmol/L) (p = 0.037), and the value increased from FPG to 2 hPG in control group (2.47 ± 1.60 mmol/L) was significantly (p = 0.042) higher than the fiber supplement group (1.90 ± 1.07 mmol/L). The results are shown in Table 3.
At baseline, no significant difference was found in the levels of TG, TC, HDL-C, and LDL-C between the two groups (all p > 0.05). After intervention, TG, TC, HDL-C, LDL-C, and TG/HDL-C levels in both groups significantly increased with the progression of gestational age (all p ≤ 0.001). The levels of TG and TG/HDL-C ratio in the fiber supplement group were significantly higher than those in the control group (2.19 ± 0.54 mmol/L vs. 2.70 ± 0.82 mmol/L and 1.19 ± 0.49 vs.1.63 ± 0.63, respectively, all p < 0.05). As seen in Table 2.
Dietary intake data during the study are displayed in Table 4. The daily calorie, carbohydrate, protein, fat, and the proportion of daily calories from protein and fat intake were not differ significantly between groups before and after the intervention (all p > 0.05). The proportion of daily calories from carbohydrates and the median daily intakes of dietary fiber in the two groups were similar before the intervention but statistically different during the intervention period (57.36 ± 4.31 vs. 55.25 ± 5.56 and 15.25 vs. 33.81g, respectively, all p < 0.05). The mean increase of total calories in the control group (261.57 kcal) was significantly higher than the fiber supplement group (192.78 kcal); the mean intake of carbohydrates increased in the control group was significantly higher than the fiber supplement group (24.56 ± 74.10 g vs. 0.06 ± 29.52g, p = 0.034). In addition, participants randomized to the control group had a higher increase in daily protein intake than the fiber supplement group (15.76 ± 8.03 g vs. 10.92 ± 13.06g, p = 0.031), and there was no difference in fat.
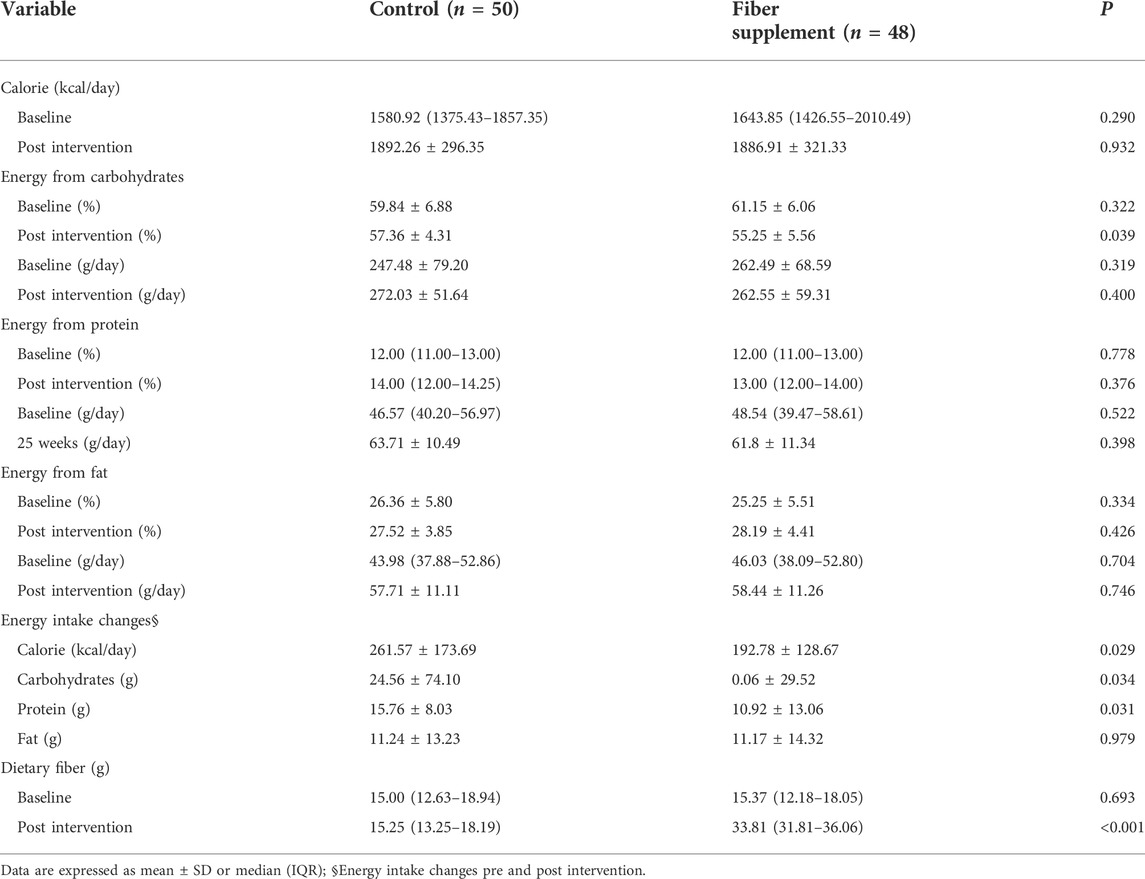
TABLE 4. Comparison of dietary intake within and between the group at the base and post intervention.
There were no significant differences in body weight before and after intervention between the two groups (all p > 0.05). However, after the intervention, women in the intervention group (1.99 ± 1.09 kg) showed significantly lower weight gain than the control group (2.53 ± 1.20 kg), and the difference was statistically significant (p = 0.022). As shown in Table 5.
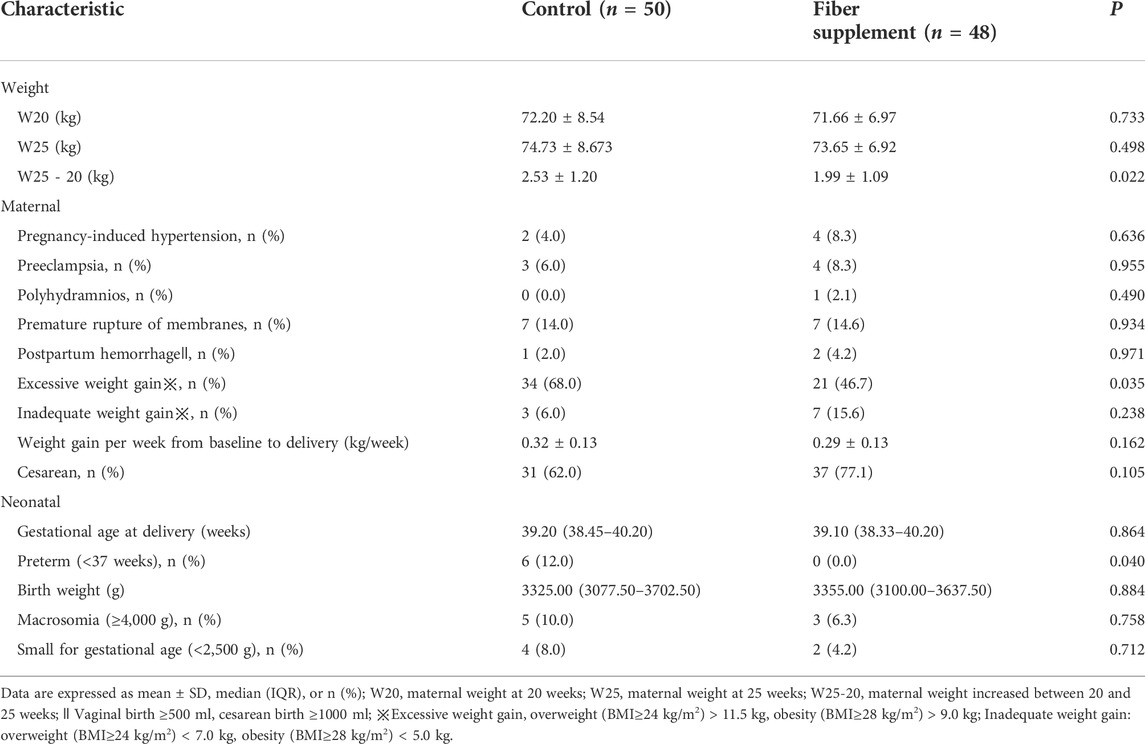
TABLE 5. Weight control and Outcomes of pregnancy complications, delivery events, and neonatal in fiber supplement and control groups.
Maternal and neonatal outcomes are presented in Table 5. In women taking dietary fiber, none experienced premature birth compared with 12.0% in the control group (p = 0.040), and 46.7% gained more than the recommended amount, which was significantly lower than 68.0% in the standard prenatal care group (p = 0.035). There were no other statistically significant differences in any maternal and neonatal outcome measures between the two groups.
Adherence to the intervention was good overall. 89.58% (43/48) taking 85% or more and 10.42% (5/48) between 50 and 80% of the provided dietary fiber powder. The compliance calculated from the returned dietary fiber powder indicated that a mean of 94.70% of the powder had been consumed.
Adverse effects of the dietary fiber powder were reported by five of 52 (9.62%) of the women; three of those reported some degree of diarrhea or bloating in the early 3–5 days of eating, and the other two women voluntary withdrew from the study due to mild to moderate abdominal pain.
The aim of the study was to evaluate the effect of dietary fiber supplement on prevention of GDM and improving maternal pregnancy outcomes. Our results demonstrated that intervention with dietary fiber supplement, from 20 to 24+6 gestational weeks in women with overweight/obesity prior to pregnancy, did lower the incidence of GDM, excessive weight gain, and preterm birth, but it did not confirm the positive effect of blood lipids.
Dietary fiber, the seventh most important dietary nutrient, plays a protective role in the improvement of glucose and lipid metabolism, weight control, and the regulation of intestinal flora during pregnancy (Zareei et al., 2018; Pretorius and Palmer, 2020; Tian et al., 2021). Compared to non-pregnant women, insulin sensitivity is decreased by approximately 50–60% in patients with GDM(Chiefari et al., 2017). Moreover, pregnant women with pre-pregnancy overweight/obesity have decreased insulin sensitivity as compared with lean or normal-weight women, which puts them at a higher risk of GDM(Johns et al., 2018). Dietary fiber has previously been associated with a reduced risk of GDM by several evidences (Goletzke et al., 2021; Pajunen et al., 2022). In our study, the incidence of GDM in women randomized to the fiber supplement group was significantly lower than in the control group. Consistent with the Australian longitudinal cohort study (3607 women; 12 years), women in the highest quartile of fiber intake had a 33% lower risk of GDM (p = 0.05) (Looman et al., 2018). Furthermore, Zhang et al. reported that women with the highest fiber intake in the first trimester or the second trimester, had an approximately 17%, or 18% lower risk of GDM, respectively (p ≤ 0.03) (Zhang et al., 2021).
An earlier meta-analysis demonstrated that fiber-rich diets benefit individuals with type 2 diabetes by lowering FPG and Hba1c levels (Post et al., 2012). Xie et al. observed a comparable impact of dietary fiber supplementation in improving glycemic control in type 2 diabetes, and they also found a convenient way to help individuals meet standard dietary fiber needs (Xie et al., 2021). After that, Cassidy et al. reported that dietary fiber, including β-glucan, inulin, guar gum, psyllium, resistant starch, and alginate, also had favorable effects on the regulation of postprandial plasma glucose (Cassidy et al., 2018). Our findings are consistent with prior research. After the intervention, women in the fiber supplement group had lower FPG and HbA1c levels than before the intervention. In addition, from the perspective of postprandial plasma glucose response, the value of the difference of 1 hPG and 2 hPG in the fiber supplement group was significantly higher than the control group, and the value of the difference of 2 hPG and FPG in the intervention group was significantly lower than the control group. These data may suggest that women demonstrated improved glucose tolerance following dietary fiber supplement. Moreover, a plethora of research has demonstrated that the mechanisms of glycemic control by fiber are strongly associated with the two predominant physicochemical properties of viscosity and fermentability (Gill et al., 2021; Malunga et al., 2021).
Lipid metabolism is essential for healthy pregnancy development, and women with GDM have increased concentrations of TG, TC, and LDL-C and lower levels of HDL-C (Wang et al., 2019). Wang et al. reported that the TG/HDL-c ratio could be a good marker to predict the risk of GDM(Wang et al., 2021). In addition, Observational studies have demonstrated that a daily fiber intake of 25 g or more can reduce the risk of cardiovascular disease by lowering blood lipids and cholesterol levels (Soliman, 2019; Kim et al., 2022). Similarly, Dehghan et al. conducted a randomized controlled clinical trial to determine the benefits of soluble fiber supplementation (10 g/day) on glycemic status and lipid profile in women with type 2 diabetes, and the results showed a significant reduction in FPG (8.50%), HbA1c (10.40%), TC (12.90%), TG (23.60%), LDL-C (35.30%), LDL-C/HDL-C ratio (16.25%),TC/HDL-C ratio (25.20%) and increased HDL-C (19.90%) (Dehghan et al., 2013). Discordantly, our study indicated that the TG and TG/HDL-C ratio levels in the fiber supplement group were statistically higher than those in the control group after the intervention, although the small differences were likely not clinically significant. A possible reason might be attributed to the blood lipids levels increases with the progression of gestational age mediated by human placental prolactin, and the dietary fiber might not be sufficient in inhibiting the physiological effects (Duttaroy and Basak, 2021). Furthermore, the phenomenon may also be owing to the limited sample size. This is a question that will need to be explored in future studies.
Gestational weight gain has been considered a potentially modifiable risk factor for GDM and other adverse pregnancy outcomes (Park et al., 2021). The Institute of Medicine (IOM) recommends that overweight/obese pregnant women gain less than 0.33 kg per week in the second and third trimesters (Khanolkar et al., 2020). Previous studies have demonstrated that increased dietary fiber intake contributes to satiety by increasing the volume of food in the stomach, hence reducing the calorie density of the meal and resulting in weight loss. (Atakan et al., 2021). Another study indicated that dietary fiber can be fermented by the gut bacteria into short-chain fatty acids (SCFAs), which stimulate the production of gut anorexic hormones such as glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) from the L cells to reduce energy intake (Muller et al., 2018; Lin and Li, 2021). The study by Guess and others also shown that a high intake of dietary fiber had a positive impact on weight control in pre-diabetic patients (Guess et al., 2015). Consistently, we also found that higher fiber consumption was conducive to a significantly lower increase in total calories, carbohydrates, and protein intake in the fiber supplement group, suggesting that fiber might be beneficial for improving satiety, which may reduce food intake. This may be why women who were intervened by dietary fiber gained less weight than the control group. More importantly, the excessive weight gain went down by 21.3%, among women in the fiber supplement group.
Overweight/obesity prior to pregnancy was associated with an increased risk of unfavorable birth outcomes such as pregnancy-induced hypertension, preeclampsia, polyhydramnios, preterm birth (<37 weeks), cesarean section, and fetal macrosomia (Hashim et al., 2019; Ayensu et al., 2020; Liu et al., 2020). Adequate dietary fiber intake has potential health benefits for maternal and neonatal health outcomes. A prospective cohort research revealed that the VPR (vegetable, fruit, and white rice) dietary pattern, high in fiber, during pregnancy is related with a lower risk of preterm birth (OR: 0.55; 95% CI: 0.26–1.17, p < 0.01) (Chia et al., 2016; Zhang et al., 2017). Another meta-analysis of twenty-one studies found that adherence to a healthy dietary pattern (intake of vegetables, fruits, legumes, whole grains) was significantly associated with lower odds of preterm birth (OR: 0.75; 95% CI: 0.57–0.93, p = 0 < 0.01) and preeclampsia (OR: 0.78; 95% CI: 0.70–0.86; p = 0.178) (Kibret et al., 2018). Findings from the present study are consistent with those outlined above. Our study indicated that women in the intervention group had significantly lower rates of preterm birth compared with the control group. The significant improvements in pregnancy outcomes in the fiber supplement group might be related to the fact that 8.3% of these pregnancies were exposed to GDM compared with 24.0% in the control group. In addition, the lower rates of excessive weight gain may also have contributed. This will need to be explored in future meta-analyses with a larger set of studies.
Some limitations should be noted in our study. Firstly, the study was a unicentric, pilot study, and the sample size in the two groups was small, thus, the ability to assess accuracy may be limited. Secondly, estimation of dietary intake was self-reported by pregnant women, and subjective bias could be a concern. Finally, our intervention was only conducted from 20 to 24+6 weeks of gestation and did not continue to gestational age at delivery. Thus, we failed to demonstrate a positive effect of dietary fiber on the incidence of pregnancy-induced hypertension, preeclampsia, polyhydramnios, cesarean section, macrosomia, and neonatal distress respiratory syndrome.
To sum up, our trial showed that supplement with dietary fiber during pregnancy among Chinese women who are overweight/obese prior to pregnancy was associated with a 15.7% decreased rate of GDM. In addition, dietary fiber also played a protective role in preventing excessive weight gain and preterm birth. However, we did not find its benefits in lowering blood lipids.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Shanghai General Hospital of School of Medicine, Shanghai Jiao Tong University ethics committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
X-MX conceived the idea and conceptualized the study. Data collection and data curation were all down to YS and LC. D-CC and Y-NC analyzed the data. D-YZ drafted the initial manuscript, then W-YL and Y-XY reviewed the manuscript. All authors read and approved the submitted version. D-YZ, D-CC and Y-NC contributed equally to this work.
Project name: To promote clinical skills and clinical innovation ability of municipal hospitals Three-year action plan project. Project number: SHDC2020CR 2060B. SETTING: Shanghai Shenkang Hospital Development Center.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.922015/full#supplementary-material
Atakan, M. M., Kosar, S. N., Guzel, Y., Tin, H. T., and Yan, X. (2021). The role of exercise, diet, and cytokines in preventing obesity and improving adipose tissue. Nutrients 13 (5), 1459. doi:10.3390/nu13051459
Ayensu, J., Annan, R., Lutterodt, H., Edusei, A., and Peng, L. S. (2020). Prevalence of anaemia and low intake of dietary nutrients in pregnant women living in rural and urban areas in the Ashanti region of Ghana. PLoS One 15 (1), e0226026. doi:10.1371/journal.pone.0226026
Bailey, R. L., Pac, S. G., Fulgoni, V. L., Reidy, K. C., and Catalano, P. M. (2019). Estimation of total usual dietary intakes of pregnant women in the United States. JAMA Netw. Open 2 (6), e195967. doi:10.1001/jamanetworkopen.2019.5967
Barber, T. M., Kabisch, S., Pfeiffer, A. F. H., and Weickert, M. O. (2020). The health benefits of dietary fibre. Nutrients 12 (10), 3209. doi:10.3390/nu12103209
Cassidy, Y. M., McSorley, E. M., and Allsopp, P. J. (2018). Effect of soluble dietary fibre on postprandial blood glucose response and its potential as a functional food ingredient. J. Funct. Foods 46, 423–439. doi:10.1016/j.jff.2018.05.019
Chen, M., Fan, B., Liu, S., Imam, K., Xie, Y., Wen, B., et al. (2020). The in vitro effect of fibers with different degrees of polymerization on human gut bacteria. Front. Microbiol. 11, 819. doi:10.3389/fmicb.2020.00819
Chen, X., Zhang, Y., Chen, H., Jiang, Y., Wang, Y., Wang, D., et al. (2021). Association of maternal folate and vitamin B12 in early pregnancy with gestational diabetes mellitus: A prospective cohort study. Diabetes Care 44 (1), 217–223. doi:10.2337/dc20-1607
Chia, A. R., de Seymour, J. V., Colega, M., Chen, L. W., Chan, Y. H., Aris, I. M., et al. (2016). A vegetable, fruit, and white rice dietary pattern during pregnancy is associated with a lower risk of preterm birth and larger birth size in a multiethnic asian cohort: The growing up in Singapore towards healthy outcomes (GUSTO) cohort study. Am. J. Clin. Nutr. 104 (5), 1416–1423. doi:10.3945/ajcn.116.133892
Chiefari, E., Arcidiacono, B., Foti, D., and Brunetti, A. (2017). Gestational diabetes mellitus: An updated overview. J. Endocrinol. Invest. 40 (9), 899–909. doi:10.1007/s40618-016-0607-5
Chu, S. Y., Callaghan, W. M., Kim, S. Y., Schmid, C. H., Lau, J., England, L. J., et al. (2007). Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 30 (8), 2070–2076. doi:10.2337/dc06-2559a
Dehghan, P., Pourghassem Gargari, B., and Asgharijafarabadi, M. (2013). Effects of high performance inulin supplementation on glycemic status and lipid profile in women with type 2 diabetes: A randomized, placebo-controlled clinical trial. Health promot. Perspect. 3 (1), 55–63. doi:10.5681/hpp.2013.007
Duttaroy, A. K., and Basak, S. (2021). Maternal fatty acid metabolism in pregnancy and its consequences in the feto-placental development. Front. Physiol. 12, 787848. doi:10.3389/fphys.2021.787848
Gill, S. K., Rossi, M., Bajka, B., and Whelan, K. (2021). Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 18 (2), 101–116. doi:10.1038/s41575-020-00375-4
Godfrey, K. M., Barton, S. J., El-Heis, S., Kenealy, T., Nield, H., Baker, P. N., et al. (2021). Myo-inositol, probiotics, and micronutrient supplementation from preconception for glycemia in pregnancy: NiPPeR international multicenter double-blind randomized controlled trial. Diabetes Care 44 (5), 1091–1099. doi:10.2337/dc20-2515
Goletzke, J., De Haene, J., Stotland, N. E., Murphy, E. J., Perez-Rodriguez, M., and King, J. C. (2021). Effect of a low-glycemic load diet intervention on maternal and pregnancy outcomes in obese pregnant women. Nutrients 13 (3), 748. doi:10.3390/nu13030748
Guess, N. D., Dornhorst, A., Oliver, N., Bell, J. D., Thomas, E. L., and Frost, G. S. (2015). A randomized controlled trial: The effect of inulin on weight management and ectopic fat in subjects with prediabetes. Nutr. Metab. 12, 36. doi:10.1186/s12986-015-0033-2
Hashim, M., Radwan, H., Hasan, H., Obaid, R. S., Al Ghazal, H., Al Hilali, M., et al. (2019). Gestational weight gain and gestational diabetes among Emirati and arab women in the united Arab Eumirates: Results from the MISC cohort. BMC Pregnancy Childbirth 19 (1), 463. doi:10.1186/s12884-019-2621-z
Jaworsky, K., Ebersole, J. L., Planinic, P., and Basu, A. (2021). Associations of diet with cardiometabolic and inflammatory profiles in pregnant women at risk for metabolic complications. Int. J. Environ. Res. Public Health 18 (21), 11105. doi:10.3390/ijerph182111105
Johns, E. C., Denison, F. C., Norman, J. E., and Reynolds, R. M. (2018). Gestational diabetes mellitus: Mechanisms, treatment, and complications. Trends Endocrinol. Metab. 29 (11), 743–754. doi:10.1016/j.tem.2018.09.004
Juan, J., and Yang, H. (2020). Prevalence, prevention, and lifestyle intervention of gestational diabetes mellitus in China. Int. J. Environ. Res. Public Health 17 (24), 9517. doi:10.3390/ijerph17249517
Khanolkar, A. R., Hanley, G. E., Koupil, I., and Janssen, P. A. (2020). IOM guidelines for gestational weight gain: How well do they predict outcomes across ethnic groups? Ethn. Health 25 (1), 110–125. doi:10.1080/13557858.2017.1398312
Kibret, K. T., Chojenta, C., Gresham, E., Tegegne, T. K., and Loxton, D. (2018). Maternal dietary patterns and risk of adverse pregnancy (hypertensive disorders of pregnancy and gestational diabetes mellitus) and birth (preterm birth and low birth weight) outcomes: A systematic review and meta-analysis. Public Health Nutr. 22 (3), 506–520. doi:10.1017/S1368980018002616
Kim, H. L., Chung, J., Kim, K. J., Kim, H. J., Seo, W. W., Jeon, K. H., et al. (2022). Lifestyle modification in the management of metabolic syndrome: Statement from Korean society of CardioMetabolic syndrome (KSCMS). Korean Circ. J. 52 (2), 93–109. doi:10.4070/kcj.2021.0328
Li, N., Yang, Y., Cui, D., Li, C., Ma, R. C. W., Li, J., et al. (2021). Effects of lifestyle intervention on long-term risk of diabetes in women with prior gestational diabetes: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 22 (1), e13122. doi:10.1111/obr.13122
Lin, X., and Li, H. (2021). Obesity: Epidemiology, pathophysiology, and therapeutics. Front. Endocrinol. 12, 706978. doi:10.3389/fendo.2021.706978
Liu, F.-L., Zhang, Y.-M., Parés, G. V., Reidy, K. C., Zhao, W.-Z., Zhao, A., et al. (2015a). Nutrient intakes of pregnant women and their associated factors in eight cities of China: A cross-sectional study. Chin. Med. J. 128 (13), 1778–1786. doi:10.4103/0366-6999.159354
Liu, F. L., Zhang, Y. M., Pares, G. V., Reidy, K. C., Zhao, W. Z., Zhao, A., et al. (2015b). Nutrient intakes of pregnant women and their associated factors in eight cities of China: A cross-sectional study. Chin. Med. J. 128 (13), 1778–1786. doi:10.4103/0366-6999.159354
Liu, L., Wang, H., Zhang, Y., Niu, J., Li, Z., and Tang, R. (2020). Effect of pregravid obesity on perinatal outcomes in singleton pregnancies following in vitro fertilization and the weight-loss goals to reduce the risks of poor pregnancy outcomes: A retrospective cohort study. PLoS One 15 (2), e0227766. doi:10.1371/journal.pone.0227766
Looman, M., Schoenaker, D., Soedamah-Muthu, S. S., Geelen, A., Feskens, E. J. M., and Mishra, G. D. (2018). Pre-pregnancy dietary carbohydrate quantity and quality, and risk of developing gestational diabetes: The Australian longitudinal study on women's health. Br. J. Nutr. 120 (4), 435–444. doi:10.1017/S0007114518001277
Malunga, L. N., Ames, N., Zhouyao, H., Blewett, H., and Thandapilly, S. J. (2021). Beta-glucan from barley attenuates post-prandial glycemic response by inhibiting the activities of glucose transporters but not intestinal brush border enzymes and amylolysis of starch. Front. Nutr. 8, 628571. doi:10.3389/fnut.2021.628571
McIntyre, H. D., Catalano, P., Zhang, C., Desoye, G., Mathiesen, E. R., and Damm, P. (2019). Gestational diabetes mellitus. Nat. Rev. Dis. Prim. 5 (1), 47. doi:10.1038/s41572-019-0098-8
Muller, M., Canfora, E. E., and Blaak, E. E. (2018). Gastrointestinal transit time, glucose homeostasis and metabolic health: Modulation by dietary fibers. Nutrients 10 (3), 275. doi:10.3390/nu10030275
Pajunen, L., Korkalo, L., Koivuniemi, E., Houttu, N., Pellonpera, O., Mokkala, K., et al. (2022). A healthy dietary pattern with a low inflammatory potential reduces the risk of gestational diabetes mellitus. Eur. J. Nutr. 61 (3), 1477–1490. doi:10.1007/s00394-021-02749-z
Park, S., Chon, S., Park, S. Y., Yun, S., Baik, S. H., Woo, J. T., et al. (2021). Association of aryl hydrocarbon receptor transactivating activity, a potential biomarker for persistent organic pollutants, with the risk of gestational diabetes mellitus. Sci. Rep. 11 (1), 3185. doi:10.1038/s41598-021-82794-0
Post, R. E., Mainous, A. G., King, D. E., and Simpson, K. N. (2012). Dietary fiber for the treatment of type 2 diabetes mellitus: A meta-analysis. J. Am. Board Fam. Med. 25 (1), 16–23. doi:10.3122/jabfm.2012.01.110148
Pretorius, R. A., and Palmer, D. J. (2020). High-fiber diet during pregnancy characterized by more fruit and vegetable consumption. Nutrients 13 (1), 35. doi:10.3390/nu13010035
Roskjaer, A. B., Asbjornsdottir, B., Tetens, I., Larnkjaer, A., Molgaard, C., and Mathiesen, E. R. (2021). Dietary intake of carbohydrates in pregnant women with type 1 diabetes-A narrative review. Food Sci. Nutr. 9 (1), 17–24. doi:10.1002/fsn3.1982
Simmons, D., Devlieger, R., van Assche, A., Jans, G., Galjaard, S., Corcoy, R., et al. (2017). Effect of physical activity and/or healthy eating on GDM risk: The DALI lifestyle study. J. Clin. Endocrinol. Metab. 102 (3), 903–913. doi:10.1210/jc.2016-3455
Soliman, G. A. (2019). Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients 11 (5), 1155. doi:10.3390/nu11051155
Stern, C., Schwarz, S., Moser, G., Cvitic, S., Jantscher-Krenn, E., Gauster, M., et al. (2021). Placental endocrine activity: Adaptation and disruption of maternal glucose metabolism in pregnancy and the influence of fetal sex. Int. J. Mol. Sci. 22 (23), 12722. doi:10.3390/ijms222312722
Tian, T., Zhang, X., Luo, T., Wang, D., Sun, Y., and Dai, J. (2021). Effects of short-term dietary fiber intervention on gut microbiota in young healthy people. Diabetes Metab. Syndr. Obes. 14, 3507–3516. doi:10.2147/DMSO.S313385
Wang, C., Wei, Y., Zhang, X., Zhang, Y., Xu, Q., Sun, Y., et al. (2017). A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am. J. Obstet. Gynecol. 216 (4), 340–351. doi:10.1016/j.ajog.2017.01.037
Wang, D., Ding, W., Ding, C., Chen, H., Zhao, W., Sun, B., et al. (2021). Higher peripheral cholesterol and a positive correlation with risk for large-for-gestational-age neonates in pre-pregnancy underweight women. Front. Endocrinol. 12, 760934. doi:10.3389/fendo.2021.760934
Wang, J., Li, Z., and Lin, L. (2019). Maternal lipid profiles in women with and without gestational diabetes mellitus. Med. Baltim. 98 (16), e15320. doi:10.1097/MD.0000000000015320
Wang, S. S., Lay, S., Yu, H. N., and Shen, S. R. (2016). Dietary guidelines for Chinese residents (2016): Comments and comparisons. J. Zhejiang Univ. Sci. B 17 (9), 649–656. doi:10.1631/jzus.B1600341
Weinert, L. S. (2010). International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: Comment to the international association of diabetes and pregnancy study groups consensus panel. Diabetes Care 33 (7), e97; author reply e98–e98. doi:10.2337/dc10-0544
Xie, Y., Gou, L., Peng, M., Zheng, J., and Chen, L. (2021). Effects of soluble fiber supplementation on glycemic control in adults with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 40 (4), 1800–1810. doi:10.1016/j.clnu.2020.10.032
Yan, B., Yu, Y., Lin, M., Li, Z., Wang, L., Huang, P., et al. (2019). High, but stable, trend in the prevalence of gestational diabetes mellitus: A population-based study in xiamen, China. J. Diabetes Investig. 10 (5), 1358–1364. doi:10.1111/jdi.13039
Yang, Y., Wang, G., and Pan, X. (2009). China food composition, 42. Beijing: Peking University Medical Press, 795–799.
Yen, I. W., Lee, C. N., Lin, M. W., Fan, K. C., Wei, J. N., Chen, K. Y., et al. (2019). Overweight and obesity are associated with clustering of metabolic risk factors in early pregnancy and the risk of GDM. PLoS One 14 (12), e0225978. doi:10.1371/journal.pone.0225978
Zareei, S., Homayounfar, R., Naghizadeh, M. M., Ehrampoush, E., and Rahimi, M. (2018). Dietary pattern in pregnancy and risk of gestational diabetes mellitus (GDM). Diabetes Metab. Syndr. 12 (3), 399–404. doi:10.1016/j.dsx.2018.03.004
Zerfu, T. A., and Mekuria, A. (2019). Pregnant women have inadequate fiber intake while consuming fiber-rich diets in low-income rural setting: Evidences from Analysis of common "ready-to-eat" stable foods. Food Sci. Nutr. 7 (10), 3286–3292. doi:10.1002/fsn3.1188
Zhang, H., Qiu, X., Zhong, C., Zhang, K., Xiao, M., Yi, N., et al. (2015). Reproducibility and relative validity of a semi-quantitative food frequency questionnaire for Chinese pregnant women. Nutr. J. 14, 56. doi:10.1186/s12937-015-0044-x
Zhang, X., Gong, Y., Della Corte, K., Yu, D., Xue, H., Shan, S., et al. (2021). Relevance of dietary glycemic index, glycemic load and fiber intake before and during pregnancy for the risk of gestational diabetes mellitus and maternal glucose homeostasis. Clin. Nutr. 40 (5), 2791–2799. doi:10.1016/j.clnu.2021.03.041
Keywords: gestational diabetes mellitus, dietary fiber, overweight/obesity, prevention, pregnancy, prospective study
Citation: Zhang D-Y, Cheng D-C, Cao Y-N, Su Y, Chen L, Liu W-Y, Yu Y-X and Xu X-M (2022) The effect of dietary fiber supplement on prevention of gestational diabetes mellitus in women with pre-pregnancy overweight/obesity: A randomized controlled trial. Front. Pharmacol. 13:922015. doi: 10.3389/fphar.2022.922015
Received: 17 April 2022; Accepted: 02 August 2022;
Published: 29 August 2022.
Edited by:
Kathleen Job, University of Utah, United StatesReviewed by:
Stefania Triunfo, University of Milan, ItalyCopyright © 2022 Zhang, Cheng, Cao, Su, Chen, Liu, Yu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian-Ming Xu, eHV4bTExQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.