- 1The First Clinical School of Guangzhou University of Chinese Medicine, Guangzhou, China
- 2The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 3School of Chinese Classics Guangzhou University of Chinese Medicine, Guangzhou, China
Background: Antibody-drug conjugate (ADC) is a promising therapy for solid cancer that has raised global concern. Although several papers have reviewed the current state of ADCs in different solid cancers, a quantitative analysis of the publications in this field is scarce.
Methods: Publications related to ADC in the field of solid cancer were obtained from the Web of Science Core Collection. Data analyses were performed with VOSviewer 1.6.9, HistCite 2.1, CiteSpace V and R package Bibliometrix.
Results: A total of 3,482 records were obtained in the holistic field and 1,197 in the clinical field. Steady growth in the number of publications was observed. The United States was the leading contributor in this field. Krop IE was the most influential author. The most productive institution was Genentech Inc., while Mem Sloan Kettering Canc Ctr was the most cited one. The most impactful journal was the Journal of Clinical Oncology. A total of 37 burst references and five burst references were identified between 2017–2022 in the holistic and clinical fields, respectively. Keywords analysis indicated that ADCs research mainly involved breast cancer, triple-negative breast cancer, ovarian cancer, small cell lung cancer, prostate cancer, gastric cancer, and urothelial carcinoma. ADC agents including trastuzumab emtansine, trastuzumab deruxtecan, sacituzumab govitecan, enfortumab vedotin, and rovalpituzumab tesirine were highly studied. Targets including HER2, trophoblast cell-surface antigen, mesothelin, delta-like ligand 3, and nectin-4 were the major concerns.
Conclusion: This study analyzed publications concerning ADCs in the field of solid cancer with bibliometric analysis. Further clinical trials of ADCs and designs of the next generation of ADCs are the current focuses of the field. Acquired resistance of ADCs and biomarkers for ADC therapy efficacy monitoring are future concerns.
1 Introduction
The continuous discovery of cytotoxic chemicals from the mid-20th century onward has facilitated the emergence of chemotherapy as the primary antitumor pharmacotherapy (Devita and Chu, 2008). However, its cytotoxicity can also damage normal cells due to a deficiency of specific targets and a precise drug delivery system. The appearance of targeted therapy compensated for the flaws of chemotherapy, and advantages such as the monoclonal antibody technique, identification of novel tumor markers, and antigens promoted the development of more targeted antitumor therapeutics (Liu, 2014). Nanotechnology and nanotherapeutics contribute to the combination of cytotoxic chemicals and antibodies, which are known as antibody-drug conjugates (ADCs).
An ADC consists of a monoclonal antibody (mAb) coupled to a cytotoxic compound (payload) via a linker (Chari, 2008). This unique structure of ADCs makes it possible for cytotoxic weapons to efficiently target tumor cells. The first ADC drug was approved for acute leukemia back in 2000 (Norsworthy et al., 2018). However, it was not until 2013 that ADC drugs achieved a breakthrough in the field of solid tumors. Ado-trastuzumab emtansine (T-DM1), which contains a monoclonal antibody targeting human epidermal growth factor receptor 2 (HER2) linked to a payload of microtubule inhibitor DM1 through a non-cleavable thioether linker, was approved for the treatment of HER2-positive breast cancer in 2013 (Boyraz et al., 2013). This has prompted unprecedented enthusiasm for developing ADC drugs as a transformative therapy for solid cancer. There is an increasing number of clinical or preclinical research on ADCs in the field of solid cancer with a rough estimate more than 30 ADCs, 15ADCs, 10 ADCs, 10 ADCs, and 5 ADCs in gastrointestinal malignancies, gynecological malignancies, lung cancer, HER2-positive breast cancer and hepatocellular carcinoma, respectively (Ferraro et al., 2021; Martín-Sabroso et al., 2021; Ricciuti et al., 2021; Singh et al., 2021; Murali et al., 2022). Although several reviews have summarized the current state of ADCs in different solid cancers (Lambert and Morris, 2017; Nagayama et al., 2017; Deonarain and Yahioglu, 2021), a quantitative analysis of publications in this field is scarce.
bibliometric analysis is a method that provides statistical analysis and quantitative to academic publications. Through bibliometric analysis, it is able to draw network knowledge maps, predict new trends and demonstrate the latest developments in a particular field (Guler et al., 2016). Currently, bibliometric analysis has been used in exploring the research trends of cancer drug therapy, such as immune checkpoint inhibitors (Gao et al., 2019). However, bibliometric studies concerning ADCs in solid cancer remain absent.
In the current study, a comprehensive bibliometric analysis was conducted to reveal the research status, research focus, and research trends of ADCs in the field of solid cancer.
2. Materials and Methods
2.1 Data Source and Collection
A comprehensive literature search was performed on the Web of Science Core Collection (WoSCC) database. The following search terms were combined to filter publications that were related to ADC and solid cancer (antibody-drug conjugate AND cancer) NOT hematologic cancer. We further combined it with “trial” and “meta-analysis” to obtain clinical publications. The final retrieval strategy is presented in Supplementary Table S1. The publication type was restricted to article and review. There was no limitation on the publication date, while the final retrieval was conducted in January 2022. Two researchers conducted the retrieval independently. Disagreements during the retrieval process were discussed with a third colleague or the entire academic team to achieve consensus.
2.2 Data Analysis and Tools
HistCite 2.1 software (New York, United States) was used to calculate the publications and citations of countries, institutions, authors, journals, targets, payloads and linkers related to ADC. The total local citation score (TLCS) is the number of citations to the author/journal/reference from papers within our data collection. Elements with a high TLCS are of significance to a given field. VOSviewer 1.6.9 software (Leiden University, Leiden, Netherlands) was used to depict network maps of journals, institutions, and countries and conducted a cluster analysis of high-frequency keywords. In the network maps, different nodes represent elements such as journals, institutions, countries, or keywords, while the size of nodes indicates the number of publications or the frequency of citation. Nodes in different colors represent different clusters or years and links between nodes reflect relationships such as collaboration or citation. The full counting method of VOSviewer and LinLog/modularity method was applied to analyze the network maps.
CiteSpace V is a full-featured bibliometric software designed by Chaomei Chen. CiteSpace V is characterized by revealing dynamics and hotspots in a given field through its function of burst analysis, which can detect topics that change dramatically over a while. Thus, a burst analysis for cited references was conducted to demonstrate the high influential references in the current field. Furthermore, a dual-map overlay of journals was used to analyze the scientific distribution and disciplinary evolution.
The R package Bibliometrix (Aria and Cuccurullo, 2017) was used for constructing a collaboration world map to reflect the geographical distribution of publications.
3. Results
3.1 Publication Language
In the holistic field, the 3,482 records retrieved were published in nine languages. Of the 3,482 records, 3,434 (98.62%) were published in English, 21 (0.60%) in French, 11 (0.32%) in German, 8 (0.23%) in Japanese, 2 (0.06%) in Chinese, Polish, Spanish, and 1 (0.03%) in Portuguese and Russian. In the clinical field, 1,197 records were retrieved and published in six languages. Of the 1,197 records, 1,174 (98.1%) were published in English, 13 (1.1%) in French, 6 (0.5%) in German, 2 (0.2%) in Japanese, and 1 (0.1%) in Portuguese and Spanish.
3.2 Publication Outputs
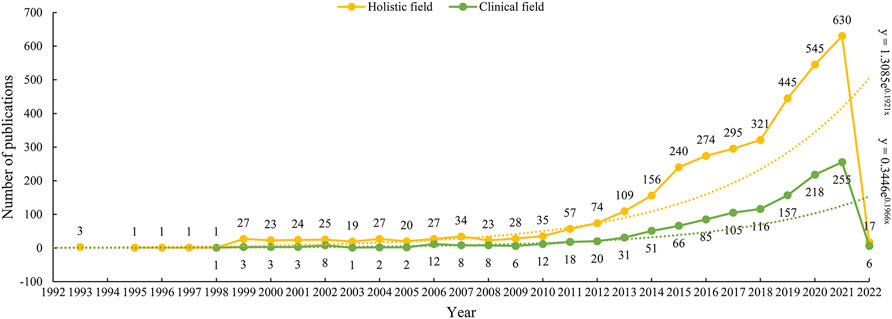
As we can see from Figure 1, the first three studies on ADC research in the field of solid cancer were all published in 1993. There was a silent stage where the annual publication stayed between 0–1 from 1994 to 1998 in the wake of the initial three studies. Between 1999 and 2009, it went into an exploratory stage, and the number of annual publications increased slightly. The number of annual publications started increasing rapidly in 2009. Especially after 2018, the number of annual publications gradually increased from 35 in 2010, 109 in 2013, 240 in 2015, 321 in 2018, to 630 in 2021. From 2018 to 2021, a total of 1941 papers were published, accounting for 55.74% of all the included studies. As for the outputs of clinical research, the publishing trend is similar to that of the holistic ADC field. The first paper on ADC clinical research was published in 1998, and its climax of publication was reached in 2021 with 255 papers coming out.
3.3 Countries and Institutions
A total of 75 countries have contributed to the publication of ADC research from 1993 to 2022. As can be seen from the data in Table 1, the top seven countries were the United States (1756, 50.43%), China (455, 13.07%), Germany (270, 7.75%), Italy (265, 7.61%), Japan (253, 7.27%), UK (240, 6.89%), France (233, 6.69%), while the other countries published less than 200 papers. The geographical distribution map is presented in Figure 2A, in which we can see a dense collaboration between the United States and Europe. A collaborative network map of the countries was also plotted. As shown in Figures 2B,C, the United States dominated country cooperation. As for clinical research, the top five countries with publications greater than 100 were the United States (636, 53.13%), Italy (135, 11.28%), China (150, 12.53%), Germany (118, 9.86%), and France (116, 9.69%).
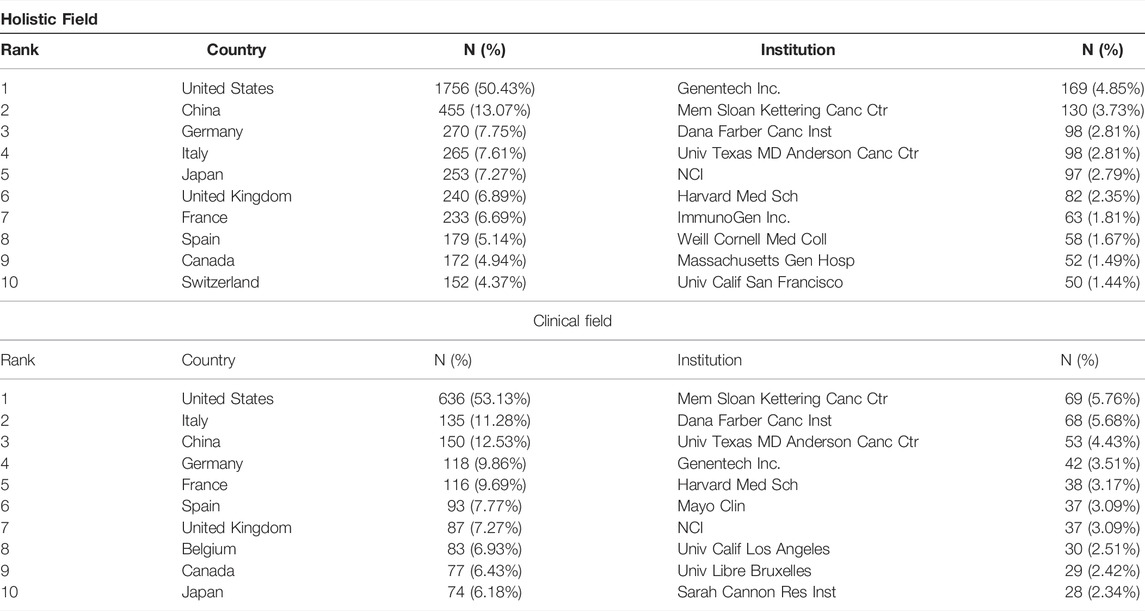
TABLE 1. The top 10 countries and institutions contributing to publications of ADC research in the solid cancer field [n (%)].
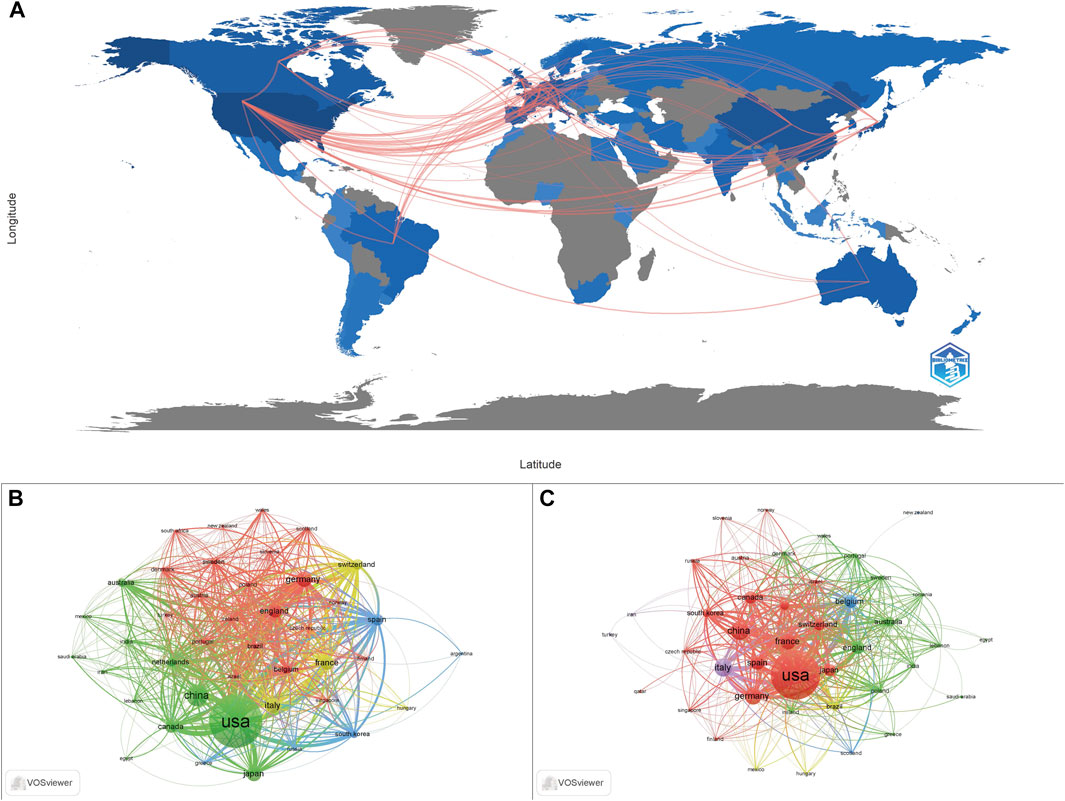
FIGURE 2. The geographical distribution of the publications and the country collaboration network map. (A) The geographical distribution of publications related to ADC research. (B) Country collaboration in the holistic ADC research field. (C) Country collaboration in the clinical ADC research field.
A total of 4,410 institutions contributed to the 3,482 papers. The top 10 institutions contributed 897 (25.76%) papers, and these institutions are all located in the United States (Table 1). Among the top 10 institutions, Genentech Inc. (169, 4.85%) published the highest number of papers, followed by Mem Sloan Kettering Canc Ctr (130, 3.73%), Dana Farber Canc Inst (98, 2.81%), Univ Texas MD Anderson Canc Ctr (98, 2.81%), NCI (97, 2.79%), and Harvard Med Sch (82, 2.35%). Mem Sloan Kettering Canc Ctr (69, 5.76%) has published the most clinical studies, followed by Dana Farber Canc Inst (68, 5.68%), Univ Texas MD Anderson Canc Ctr (53, 4.43%), and Genentech Inc., (42, 3.51%). Figure 3 presents the cooperation ship between institutions and indicates that Mem Sloan Kettering Canc Ctr, Dana Farber Canc Inst, and Univ Texas MD Anderson Canc Ctr are the dominating centers for launching clinical trials of ADC drugs. The overlay map (Figure 3B,D) suggests that Univ Texas MD Anderson Canc Ctr starting later but growing rapidly in the ADC research field compared to the other three institutions.
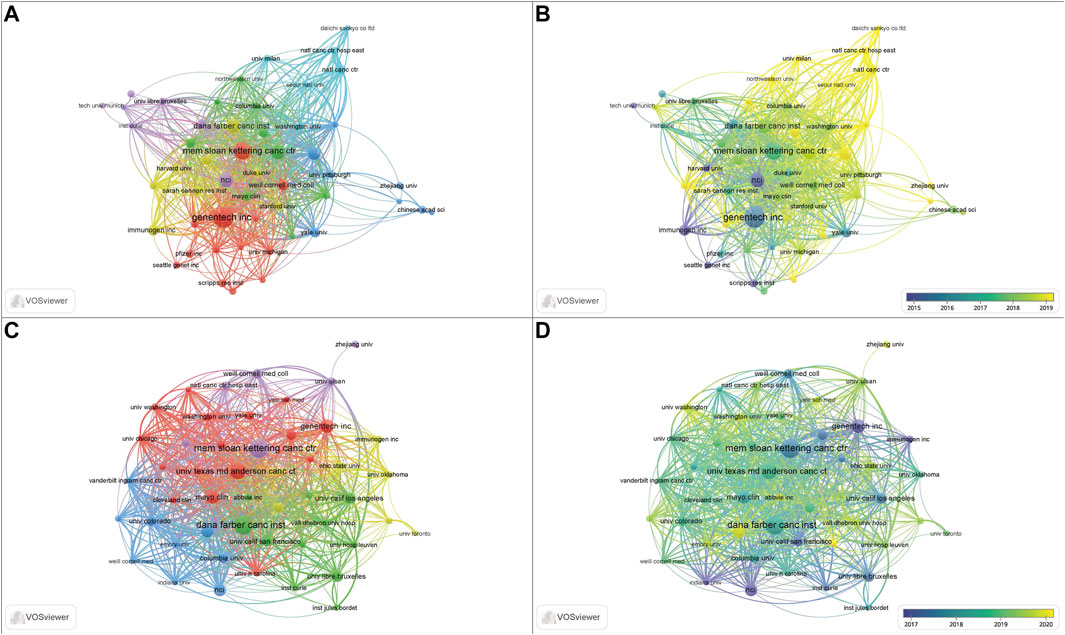
FIGURE 3. The collaboration and overlay map of institutions for ADC research in the solid cancer field. (A,B) The collaboration and overlay map of institutions for holistic ADC research, respectively. (C,D) The collaboration and overlay map of institutions for clinical ADC research, respectively.
3.4 Authors and Cited Authors
A total of 18,061 authors were obtained in the 3,482 publications. Table 2 shows the top 10 productive authors and the most cited authors. The top 10 authors contributed 239 papers. Krop IE (27 papers) published the highest number of papers, followed by Goldenberg DM and Zeglis BM (26 papers), Girish S, Lewis JS (25 papers), and Saint AD (23 papers). Among the top 10 cited authors, Krop, IE ranked first, with 2,333 local citations, followed by Guardino E (1415 local citations), Dieras V (1,267 local citations), and Sliwkowski MX (1,212 local citations), while the remaining authors had less than 1,200 local citations. As for clinical research, the most productive author was Goldenberg DM (19 papers), while the most cited author was Krop IE (696 local citations) followed by Kim SB (392 local citations) and Modi S (367 local citations).
3.5 Journals and Cited Journals
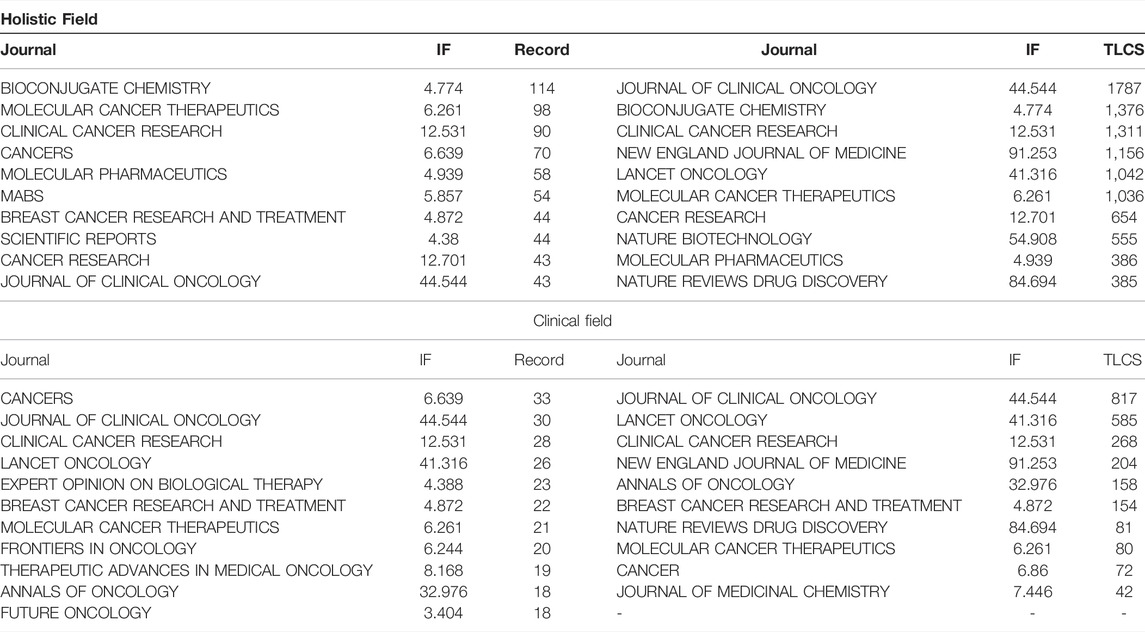
The 3,482 papers were published in 720 journals. Figure 4A,B presents the dual-map overlay of journals. The left side represents the map of citing journals and the right side represents the map of the cited journals. The label represents the subject covered by the journal. Colored curves represent paths of references, where each curve originates from the citing map and points to the cited map. There were four main citation paths in the holistic field and three main paths in the clinical field. Table 3 presents the top 10 journals and most cited journals. The top 10 journals contributed 658 (18.90%) papers. Bioconjugate Chemistry (114 papers) ranked first, followed by Molecular Cancer Therapeutics (98 papers), Clinical Cancer Research (90 papers), and Cancers (70 papers). As for clinical research, the top three productive journals are Cancers (33 papers), Journal of Clinical Oncology (30 papers), and Clinical Cancer Research (28 papers). The Journal of Clinical Oncology is the most-cited journal both in the holistic field (1787 local citations) and clinical field (817 local citations) of ADC research. Bioconjugate Chemistry (1,376 local citations) is the second cited journal in the holistic field, followed by Clinical Cancer Research (1,311 local citations) and New England Journal of Medicine (1,156 local citations). Lancet Oncology (585 local citations) is the second most cited journal in the clinical field, followed by Clinical Cancer Research (268 local citations), New England Journal of Medicine (204 local citations), and Annals of Oncology (158 local citations). Figure 4C,D shows the citation relationships between the journals.

FIGURE 4. The dual-map overlay of journals and network map of cited journals related to ADC research in the solid cancer field. (A,B). The dual-map overlay of journals for holistic and clinical research, respectively. (C,D). The network map of cited journals for holistic and clinical research, respectively.
3.6 Cited References and References With Citation Bursts
Table 4 presents the top 10 cited references from 2011 to 2022. These references suggest that T-DM1 for HER2-positive breast cancer is a focus of interest for researchers. There are eight clinical trials associated with the therapeutic effects evaluation of trastuzumab emtansine in HER2-positive breast cancer patients (Burris et al., 2011; Krop et al., 2012; Verma et al., 2012; Hurvitz et al., 2013; Krop et al., 2014; Diéras et al., 2017; Krop et al., 2017; Perez et al., 2017), and the most cited one was a phase 3 clinical trial, which demonstrated that T-DM1 prolonged progression-free survival of breast cancer patients who had been previously treated with trastuzumab and a taxane (Verma et al., 2012). Junttila et al. (2011) and Lorusso et al. (2011) reviewed the mechanisms and clinical progress of T-DM1, respectively. Trastuzumab deruxtecan is another trastuzumab-based ADC agent. Ogitani et al. (2016) evaluated its pharmacologic activities with HER2-positive cell lines and patient-derived xenograft models, while Modi et al. (2020) demonstrated its durable antitumor activity in HER2-positive metastatic breast cancer patients in the DESTINY-Breast01 trial. The second most cited paper was written by Beck et al. (2017), who reviewed the progresses of first- and second-generation ADCs as well as envisaged third-generation ADCs. Shen et al. (2012) assessed the impact of the conjugation site on ADCs.
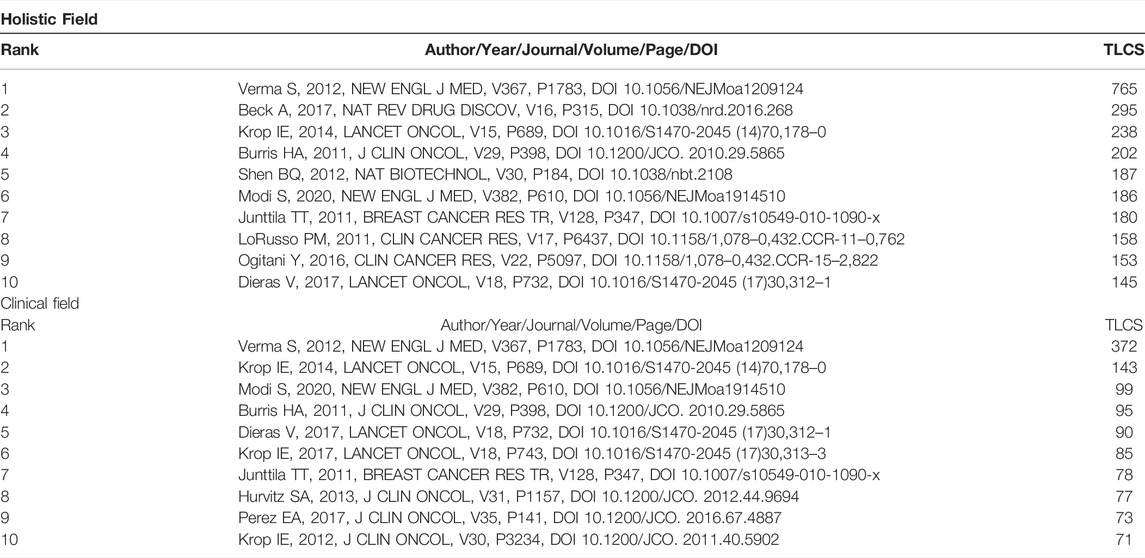
TABLE 4. The top 10 cited references related to ADC research in the solid cancer field from 2011–2022.
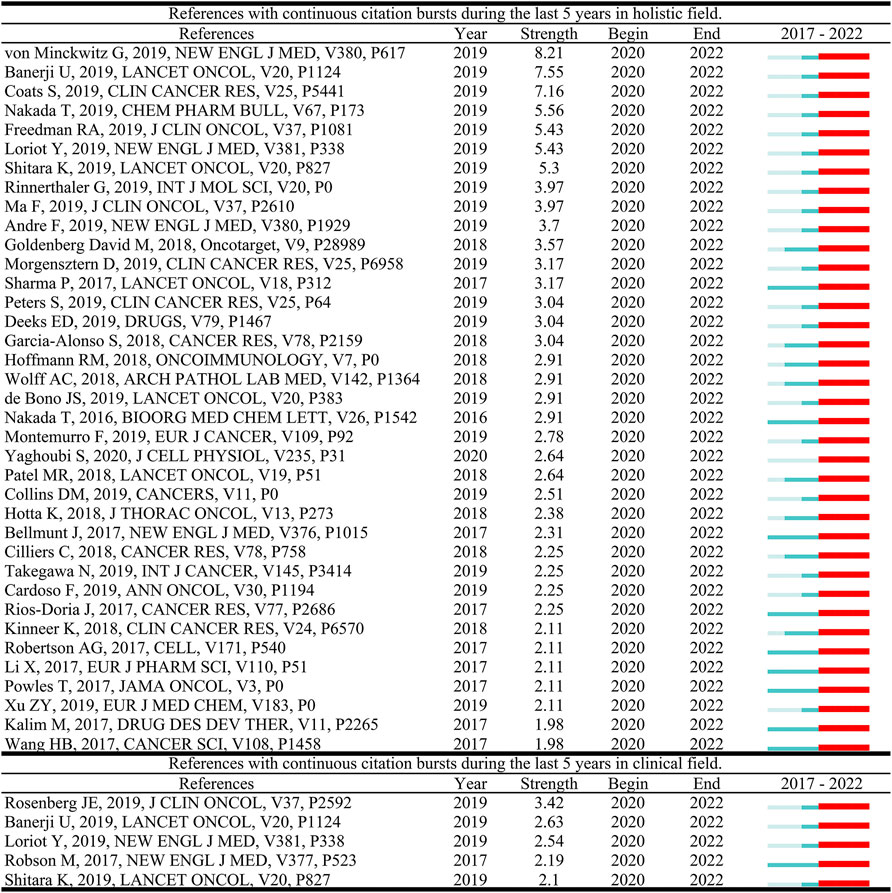
References with citation bursts are defined as those that are cited frequently over a while. In CiteSpace, the timespan was set as 2017–2022 and references with a burst termination date of 2022 remained. In Figure 5, the blue line represents the time interval. The time in which a reference was found to have a burst is displayed by a red line, indicating the first year and the last year of the duration of the burst. A total of 37 burst references and five burst references were detected in the holistic field and clinical field of ADC research, respectively. The reference with the strongest citation burst documented the efficacy of T-DM1 in the treatment of residual invasive HER2-positive breast cancer (Von Minckwitz et al., 2019), while in the clinical field, Rosenberg et al. (2019) reported the efficacy and safety data of enfortumab vedotin, an ADC drug that targets nectin-4, in the treatment of previously chemotherapy and immunotherapy treated urothelial carcinoma.
3.7 Co-occurrence Keywords and Cluster Analysis
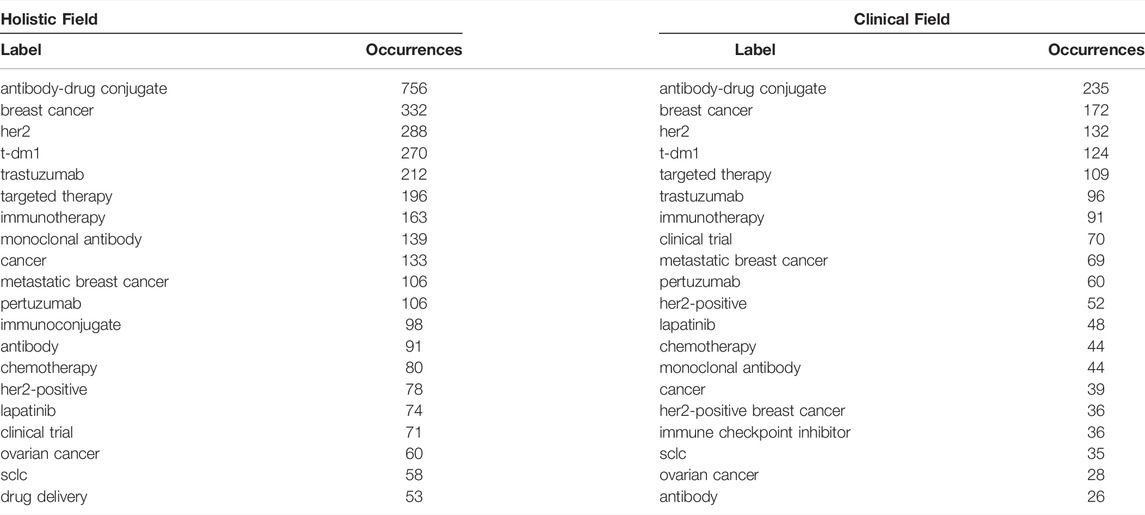
A total of 4,634 keywords were extracted from the 3,482 papers. Figure 6 shows the density map, co-occurrence map, and overlay map for the keyword. Table 5 presents the top 20 occurring keywords. Keywords with high occurrence in the clinical field were consistent with the holistic field. Antibody-drug conjugate, breast cancer, HER2, T-DM1, metastatic breast cancer, ovarian cancer, and small cell lung cancer were greatly concerned. As can be seen from Figure 6, the ADC research field has been extended to triple-negative breast cancer, prostate cancer, gastric cancer, bladder cancer, urothelial carcinoma, pancreatic cancer, colorectal cancer, melanoma, glioblastoma, and non-small-cell lung cancer. Multifarious ADC agents including T-DM1, trastuzumab deruxtecan, sacituzumab govitecan, enfortumab vedotin, and rovalpituzumab tesirine were observed. Several targets of ADCs are of eminent interest, such as HER2, trophoblast cell-surface antigen (trop2), delta-like ligand 3 (dll3), and nectin-4.
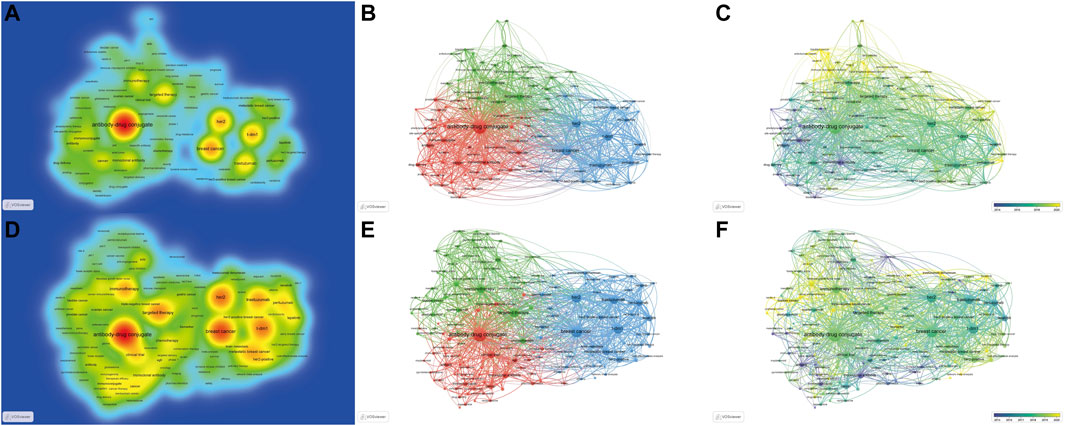
FIGURE 6. The density, co-occurrence, and overlay map of keywords. (The density, co-occurrence, and overlay map are displayed in the first to third columns, respectively.) (A–C). Maps for the holistic ADC research field. (D–F). Maps for the clinical ADC research field.
A clustering analysis was performed based on the co-occurrence of keywords and a total of 3 clusters were identified. As we can see from Figure 6B, the red cluster is the largest cluster, which contains 49 keywords, mainly including antibody-drug conjugate, immunoconjugate, monoclonal antibody, drug delivery, pharmacokinetics, prostate cancer, linker, and targeted delivery. The blue cluster includes 25 keywords, which primarily consist of breast cancer, HER2, T-DM1, metastatic breast cancer, gastric cancer, and trastuzumab deruxtecan. The green cluster is composed of targeted therapy, immunotherapy, clinical trial, ovarian cancer, small cell lung cancer, triple-negative breast cancer, bladder cancer, trop2, urothelial carcinoma, sacituzumab govitecan, non-small cell lung cancer, apoptosis, dll3, enfortumab vedotin, and biomarker et al.
An overlay map can present the dynamic development process of a given research field. The terms Immunoconjugate, conjunction, and monoclonal antibody indicate the focus of original research on ADC. Antibody-drug conjugate, HER2, breast cancer, T-DM1, target therapy, ovarian cancer, and small cell lung cancer are at intermediate, while gastric cancer, triple-negative breast cancer, bladder cancer, urothelial carcinoma, nectin-4, dll3, sacituzumab govitecan, and trastuzumab deruxtecan are novel topics.
The knowledge map for the clinical research field provides a similar keyword distribution to the holistic field, while we can obtain a more specific perspective of clinical research of ADC in the field of solid cancer from Figure 6D–F.
3.8 The Top Five Targets, Payloads and Linkers of ADC
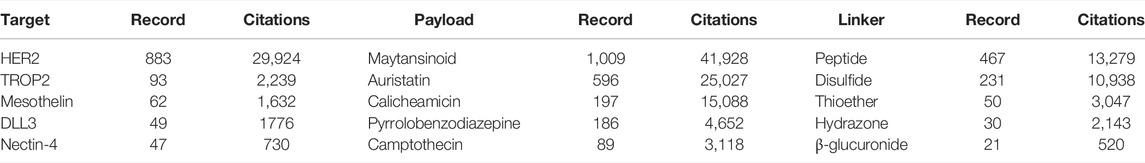
The composition of the ADC structure was retrieved on WoSCC and the top five targets, payloads, and linkers, including their publications and citations, are presented in Table 6. HER2 is the most investigated target with 883 publications and 29,924 citations in the ADC field, followed by trop2 (93 papers and 2,239 citations) and mesothelin (62 papers and 1,632 citations). The most studied payload is maytansinoid with 1,009 papers published and 41,928 citations, followed by auristatin (596 papers and 25,027 citations), and calicheamicin (197 papers and 15,088 citations). Peptide linker (467 papers and 13,279 citations) has the highest publications and citations, followed by disulfide linker (231 papers and 10,938 citations), and thioether linker (50 papers and 3,047 citations).
4 Discussion
In the current study, we conducted a bibliometric analysis to reveal the research trend and frontier topic of ADC research in the field of solid cancer for the first time. A pivotal step forward toward ADC was the invention of monoclonal antibodies, which can bind antigenic epitopes specifically (Köhler and Milstein, 1975). The concept of “magic bullet,” which means that chemicals specifically and efficiently target tumor cells, contributes to the emergence of ADC (Schwartz, 2004). There are currently three generations of ADC drugs. In the first generation of ADC drugs, antitumor agents such as mitomycin C, idarubicin, anthracyclines, N-acetylmaran, adriamycin, perillyl alkaloids, and methotrexate are conjugated to murine monoclonal antibodies or humanized monoclonal antibodies mainly through non-cleavable linkers (Ponziani et al., 2020). The conjugating approach is random, resulting in uncontrollable drug-to-antibody ratio (DAR) values, which influence the release of the payload (Deslignière et al., 2021). Gemtuzumab ozogamicin was a first-generation ADC drug, which was approved for acute myeloid leukemia in 2000 (Norsworthy et al., 2018). However, it was withdrawn by Pfizer in 2010 due to its limited efficacy and redundant toxicity (Beck et al., 2010). The second-generation of ADCs possess increased cytotoxic drug conjugation levels and more-stable linkers compared to the first-generation and have shown significant clinical efficacy and safety performance (Beck et al., 2017). For the second generation of ADC drugs, humanized monoclonal antibodies are prudently selected, payloads with enhancive toxicity such as microtubule protein inhibitors are introduced, and reduced hinge cysteine conjugation is engineered, which largely improves the efficacy, safety, and chemistry, manufacturing and controls properties (Donaghy, 2016). However, the DAR values range from 0 to 8 (Lyon et al., 2015). Brentuximab vedotin and T-DM1 both belong to the second-generation ADC drug, and they have been approved for lymphoma and breast cancer by the FDA (Nagayama et al., 2017). The third generation ADC drug ameliorates the shortcomings of the previous generations. The site-specific binding of small molecule drugs to monoclonal antibodies is designed to produce third-generation ADCs, bringing about DARs of 2 or 4 (Martin et al., 2018). This generation of ADC drugs has improved stability, pharmacokinetics, and anti-tumor activity (Beck et al., 2017). Based on previous knowledge, lessons learned are now being concentrated on the development of third-generation ADCs, and numerous ADCs for solid tumors are under investigation. Our results suggested that there was a significantly increasing number of publications in the field, especially from 2018.
The United States was the most productive country and dominated the countries’ collaboration. Genentech Inc., and Mem Sloan Kettering Canc Ctr were the most productive institutions in the holistic field and clinical field of the ADC research, respectively, and both of them are located in the United States. Genentech Inc.’s reputation in the ADC field is ascribed to the design of T-DM1, which has been approved for advanced and early breast cancer in 2013 and 2019 by the FDA (Boyraz et al., 2013; Von Minckwitz et al., 2019). Aside from that, they’ve studied T-DM1 as an adjuvant for breast cancer, as well as its cost-effectiveness and drug resistance in recent years (Boyer et al., 2021; Mamounas et al., 2021; Sussell et al., 2021). Genentech Inc., also devotes itself to studying the mechanisms, pharmacokinetics, and structures of ADCs and to developing novel ADC agents. The phase I dose-escalation study of DMUC4064A, an innovative ADC drug that targets MUC16 expressed in the ovarian cancer cell, was completed in 2021 (Liu et al., 2021). Mem Sloan Kettering Canc Ctr released the results of its first ADC clinical trial in 2008, the phase I trial of MLN2704, a prostate-specific membrane antigen-targeted immunoconjugate, in the treatment of prostate cancer (Galsky et al., 2008). Over the past few years, Mem Sloan Kettering Canc Ctr conducted clinical trials in various solid cancers, including epithelial cancer, ovarian cancer, urothelial carcinoma, and early-stage HER2-positive breast cancer. The results of the IMMU-132-01 trial, FORWARD I trial, EV-201 trial, and ATEMPT trial were disclosed in 2021 (Bardia et al., 2021; Moore et al., 2021; Ruddy et al., 2021; Yu et al., 2021). Furthermore, the most cited paper published by Mem Sloan Kettering Canc Ctr was the clinical reports of the DESTINY-Breast01 trial (Modi et al., 2020).
Krop IE was the most productive and cited author in the holistic field, who has participated in several crucial clinical trials for T-DM1 in the treatment of HER2-positive breast cancer, such as the EMILIA (Verma et al., 2012) and TH3RESA (Krop et al., 2014) trials. Besides T-DM1, he has recently been involved in preliminary clinical trials of other HER2-targeted ADC agents, such as trastuzumab deruxtecan and MM-302. (Munster et al., 2018; Tamura et al., 2019). His latest research assessed the impact of HER2 heterogeneity in patients who had received T-DM1 and pertuzumab therapy, and the results indicated that HER2 heterogeneity was associated with resistance to HER2-targeted therapy (Filho et al., 2021). Goldenberg DM was the most productive author in the clinical field. He has been focusing on the development and clinical research of ADC drugs targeting trop2, whose initial study assessed the anti-tumor efficacy of an SN-38-anti-trop2 ADC in human cancer xenograft models and monkeys (Cardillo et al., 2011). In recent years, a mature anti-trop2 ADC drug named sacituzumab govitecan has been applied in the clinical research of triple-negative breast cancer (Bardia et al., 2019), NSCLC (Heist et al., 2017) and SCLC (Gray et al., 2017) by Goldenberg DM, as well as another anti-trop2 ADC drug named labetuzumab govitecan in colorectal cancer (Dotan et al., 2017).
Bioconjugate Chemistry, a journal aiming to present the preparation, properties, and applications of biomolecular conjugates, has the most papers published. The paper with the topmost citations in the past decade published by Bioconjugate Chemistry introduced a cell-free protein expression system designed for the rapid synthesis of ADCs through site-specific incorporation of the para-azidomethyl-L-phenylalanine (Zimmerman et al., 2014). The second most cited paper measured the surface expression of trop2 in a wide range of human solid cancers and the pharmacokinetics of sacituzumab govitecan (Cardillo et al., 2015). The Journal of Clinical Oncology was the most cited journal, which published its first clinical paper about ADC drugs in 1999 and kept presenting substantial clinical research. The results of the MARIANNE study were reported in 2017 in the Journal of Clinical Oncology, which immediately elicited eminent concerns (Perez et al., 2017).
References and keywords analysis reflect current and future research topics jointly. The monoclonal antibody in the ADC structure generally determines the range of applicability of an ADC. A total of five targets, including HER2, trop2, mesothelin, nectin-4, and dll3 were presented as high-occurrence keywords in our knowledge map. HER2 is a pivotal target for ADC drugs. The alteration of HER2 occurs in a wide range of solid cancers and it can cause increased downstream signaling, which further results in cell growth, metastasis, drug resistance, and angiogenesis (Zhao and Xia, 2020). Breast cancers are the main battleground for HER2-targeted ADC drugs, consequentially. There are already two ADC drugs, T-DM1 and trastuzumab deruxtecan, approved for HER2-positive breast cancers by FDA. However, breast cancer patients with low or negative HER2 expression remain challenging. The next generation of ADCs have the potential to be active in these populations due to their advanced pharmaceutical properties, such as trastuzumab duocarmazine, which showed notable clinical activity in HER2-low expression breast cancer patients in a phase 1 trial (Banerji et al., 2019; Ferraro et al., 2021). In addition, gastrointestinal cancer, NSCLC, endometrial cancer, and urothelial carcinoma also express HER2 on the cell surface, and early clinical trials have been conducted with HER2-targeted ADC drugs (Tsurutani et al., 2020). ADCs that target antigenic epitopes beyond HER2 have opened up a slew of new clinical possibilities for triple-negative breast cancer. Trop2 is a transmembrane glycoprotein that is overexpressed in a variety of solid cancers such as breast cancer, lung cancer, and urothelial carcinoma with minimal or no baseline expression in normal tissues. Previous studies have proved that trop2 is associated with tumor progression and the development of metastases (Ripani et al., 1998; Bignotti et al., 2012; Trerotola et al., 2013). Trop2-targeted ADC drugs mainly include sacituzumab govitecan, PF-06664178, and datopotamab deruxtecan, of which sacituzumab govitecan and datopotamab deruxtecan have presented appealing clinical efficacy in solid cancers, while PF-06664178 has failed in a phase I trial (Liao et al., 2021). The response rate of heavily pretreated metastatic triple-negative breast cancer patients who received sacituzumab govitecan-hziy was 33.3%, and the median overall survival was 13.0 months. Sacituzumab govitecan-hziy prolonged the duration of treatment when compared with the immediate previous antitumor therapy (5.1 vs 2.5 months) (Bardia et al., 2019). As a result, sacituzumab govitecan-hziy was granted accelerated approval for triple-negative breast cancer by the FDA in 2020. Mesothelin is a 40-kDa cell surface glycoprotein, which is highly expressed in several human cancers (Chang and Pastan, 1996), including lung cancer (∼50% of cases) (Ordóñez, 2003), ovarian cancer (∼70% of cases) (Hassan et al., 2005) and pancreatic/biliary adenocarcinomas (∼100% of cases) (Argani et al., 2001). Two mesothelin-targeted ADC drugs including Anetumab ravtansine and DMOT4039A have presented active anti-tumor effects in the preclinical studies and further clinical trials are in progress (Singh et al., 2021). Nectin-4 is a member of the nectin family and it is weakly expressed in normal tissues while highly expressed in various tumor cells, including urothelial, lung, breast, and ovarian cancers. Urothelial carcinoma patients with overexpressed nectin-4 had a significantly worse prognosis. Nectin-4-targeted ADC therapy was applied in urothelial carcinoma. Enfortumab vedotin was approved for heavily pretreated locally advanced or metastatic urothelial cancer patients by the FDA in 2019 (Moussa et al., 2021), and it is currently being tested for solid cancers in a phase II study (Bruce et al., 2020). DLL3 is an inhibitory notch ligand that is highly expressed in SCLC but minimally expressed in normal lung tissues (Saunders et al., 2015). Overexpression of DLL3 was associated with irrepressible migration and invasion of SCLC (Furuta et al., 2019). Therefore, DLL3 was regarded as an emerging target for SCLC. Rovalpituzumab tesirine is a DLL3-targeted ADC. Although rovalpituzumab tesirine represented satisfactory anti-tumor effects in preclinical models (Saunders et al., 2015), its phase 3 trial was halted due to shorter overall survival when compared with topotecan (Owen et al., 2019). Researchers are testing rovalpituzumab tesirine in different disease settings of SCLC, and a clinical study of rovalpituzumab tesirine as maintenance therapy for SCLC is ongoing (Owen et al., 2019). Tissue factor has been a promising topic in recent years, and one of our burst references detailed a clinical trial about tissue factor-targeted ADC drugs. The tissue factor is a transmembrane glycoprotein that functions not only as the main initiator of extrinsic coagulation pathways but also as a promoter of tumor progression. Tissue factor is overexpressed in a variety of solid cancers, such as cervical cancer (Cocco et al., 2011), NSCLC (Koomägi and Volm, 1998), endometrial cancer (Fadare et al., 2011), prostate cancer (Akashi et al., 2003) and ovarian cancer (Abu Saadeh et al., 2013). The phase 1–2 trial of tisotumab vedotin, a tissue factor targeted-ADC drug, indicated that tisotumab vedotin had encouraging preliminary antitumor activity in solid cancers with an objective response of 15.6% (De Bono et al., 2019).
Burst references indicated several future research concerns. Acquired resistance is a future challenge for ADC therapy. Garcia-Alonso et al. (2018) reviewed the mechanisms of resistance to ADCs and raised strategies to overcome resistance. Collins et al. (2019) introduced the progress of combination therapy, which is regarded as a potential approach to avoiding acquired resistance. Previous in vivo studies demonstrated that ADCs synergized with PD-1 antibodies to exert antitumor effects, which supported a combination treatment strategy of ADC therapy and PD-1 inhibitors (Rios-Doria et al., 2017). Kinneer et al. (2018) and Wang et al. (2017) discovered that the expression of SLC46A3 and the activity of V-ATPase in lysosomes could be used as biomarkers for predicting T-DM1 resistance, respectively. Studies were also conducted to identify biomarkers that can recognize the beneficiary population of ADC therapies. Takegawa et al. (2019) reported that colorectal cancer patients who expressed HER2 protein without HER2 amplification might be sensitive to trastuzumab deruxtecan. The accumulation of knowledge on the structure, mechanism, and pharmacokinetics of ADCs has facilitated the development of the next generation of ADCs. Yaghoubi et al. (2020) and Hoffmann et al. (2018) summarized the cytotoxic small molecule drugs that were potentially processed into payloads, and the characteristics of engineering antibodies in ADCs, respectively. Nakada et al. (2016) reviewed the progress of ADCs that contained exatecan derivative-based cytotoxic payloads. In addition, Kalim et al. (2017) illustrated endocytosis and intracellular trafficking of ADCs, which was the approach by that ADCs entered tumor cells, while Cilliers et al. (2018) demonstrated that the intratumoral distribution of ADCs plays a major role in ADC efficacy. Both of them have enlightened the future development of ADCs.
5 Conclusion
In this study, we conducted a bibliometric analysis to reveal the research trend of ADCs in the field of solid cancer. Publications associated with this field are increasing rapidly. Further clinical trials of ADCs and designs of the next-generation of ADCs are the current focuses of the field. Acquired resistance to ADCs and biomarkers for ADC therapy efficacy monitoring are future concerns.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
XQ designed the study. XQ, YL, WL, YW, and ZC collected the data. XQ, WL, and YL analyzed the data and drafted the manuscript. LL revised the final version of the manuscript. All the authors have read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to express their appreciation to Professor Chaomei Chen and his team for free access to CiteSpace.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.921385/full#supplementary-material
References
Abu Saadeh, F., Norris, L., O'toole, S., Mohamed, B. M., Langhe, R., O'leary, J., et al. (2013). Tumour Expresion of Tissue Factor and Tissue Factor Pathway Inhibitor in Ovarian Cancer- Relationship with Venous Thrombosis Risk. Thromb. Res. 132 (5), 627–634. doi:10.1016/j.thromres.2013.09.016
Akashi, T., Furuya, Y., Ohta, S., and Fuse, H. (2003). Tissue Factor Expression and Prognosis in Patients with Metastatic Prostate Cancer. Urology 62 (6), 1078–1082. doi:10.1016/s0090-4295(03)00768-4
Argani, P., Iacobuzio-Donahue, C., Ryu, B., Rosty, C., Goggins, M., Wilentz, R. E., et al. (2001). Mesothelin Is Overexpressed in the Vast Majority of Ductal Adenocarcinomas of the Pancreas: Identification of a New Pancreatic Cancer Marker by Serial Analysis of Gene Expression (SAGE). Clin. Cancer Res. 7 (12), 3862–3868.
Aria, M., and Cuccurullo, C. (2017). Bibliometrix: An R-tool for Comprehensive Science Mapping Analysis. Journal of Informetrics 11 (4), 959–975. doi:10.1016/j.joi.2017.08.007
Banerji, U., Van Herpen, C. M. L., Saura, C., Thistlethwaite, F., Lord, S., Moreno, V., et al. (2019). Trastuzumab Duocarmazine in Locally Advanced and Metastatic Solid Tumours and HER2-Expressing Breast Cancer: a Phase 1 Dose-Escalation and Dose-Expansion Study. Lancet Oncol. 20 (8), 1124–1135. doi:10.1016/S1470-2045(19)30328-6
Bardia, A., Mayer, I. A., Vahdat, L. T., Tolaney, S. M., Isakoff, S. J., Diamond, J. R., et al. (2019). Sacituzumab Govitecan-Hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 380 (8), 741–751. doi:10.1056/NEJMoa1814213
Bardia, A., Messersmith, W. A., Kio, E. A., Berlin, J. D., Vahdat, L., Masters, G. A., et al. (2021). Sacituzumab Govitecan, a Trop-2-Directed Antibody-Drug Conjugate, for Patients with Epithelial Cancer: Final Safety and Efficacy Results from the Phase I/II IMMU-132-01 Basket Trial. Ann. Oncol. 32 (6), 746–756. doi:10.1016/j.annonc.2021.03.005
Beck, A., Goetsch, L., Dumontet, C., and Corvaïa, N. (2017). Strategies and Challenges for the Next Generation of Antibody-Drug Conjugates. Nat. Rev. Drug Discov. 16 (5), 315–337. doi:10.1038/nrd.2016.268
Beck, A., Haeuw, J. F., Wurch, T., Goetsch, L., Bailly, C., and Corvaïa, N. (2010). The Next Generation of Antibody-Drug Conjugates Comes of Age. Discov. Med. 10 (53), 329–339. doi:10.1038/nri2747
Bignotti, E., Zanotti, L., Calza, S., Falchetti, M., Lonardi, S., Ravaggi, A., et al. (2012). Trop-2 Protein Overexpression Is an Independent Marker for Predicting Disease Recurrence in Endometrioid Endometrial Carcinoma. BMC Clin. Pathol. 12, 22. doi:10.1186/1472-6890-12-22
Boyer, J. Z., Phillips, G. D. L., Nitta, H., Garsha, K., Admire, B., Kraft, R., et al. (2021). Activity of Trastuzumab Emtansine (T-DM1) in 3D Cell Culture. Breast Cancer Res. Treat. 188 (1), 65–75. doi:10.1007/s10549-021-06272-x
Boyraz, B., Sendur, M. A., Aksoy, S., Babacan, T., Roach, E. C., Kizilarslanoglu, M. C., et al. (2013). Trastuzumab Emtansine (T-DM1) for HER2-Positive Breast Cancer. Curr. Med. Res. Opin. 29 (4), 405–414. doi:10.1185/03007995.2013.775113
Bruce, J. Y., Pusztai, L., Braiteh, F. S., Gorla, S. R., Wu, C., and Baranda, J. (2020). EV-202: A Phase II Study of Enfortumab Vedotin in Patients with Select Previously Treated Locally Advanced or Metastatic Solid Tumors. J. Clin. Oncol. 38 (15_Suppl. l), TPS3647. doi:10.1200/jco.2020.38.15_suppl.tps3647
Burris, H. A., Rugo, H. S., Vukelja, S. J., Vogel, C. L., Borson, R. A., Limentani, S., et al. (2011). Phase II Study of the Antibody Drug Conjugate Trastuzumab-DM1 for the Treatment of Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Breast Cancer after Prior HER2-Directed Therapy. J. Clin. Oncol. 29 (4), 398–405. doi:10.1200/JCO.2010.29.5865
Cardillo, T. M., Govindan, S. V., Sharkey, R. M., Trisal, P., Arrojo, R., Liu, D., et al. (2015). Sacituzumab Govitecan (IMMU-132), an Anti-trop-2/sn-38 Antibody-Drug Conjugate: Characterization and Efficacy in Pancreatic, Gastric, and Other Cancers. Bioconjug Chem. 26 (5), 919–931. doi:10.1021/acs.bioconjchem.5b00223
Cardillo, T. M., Govindan, S. V., Sharkey, R. M., Trisal, P., and Goldenberg, D. M. (2011). Humanized Anti-trop-2 IgG-SN-38 Conjugate for Effective Treatment of Diverse Epithelial Cancers: Preclinical Studies in Human Cancer Xenograft Models and Monkeys. Clin. Cancer Res. 17 (10), 3157–3169. doi:10.1158/1078-0432.CCR-10-2939
Chang, K., and Pastan, I. (1996). Molecular Cloning of Mesothelin, a Differentiation Antigen Present on Mesothelium, Mesotheliomas, and Ovarian Cancers. Proc. Natl. Acad. Sci. U. S. A. 93 (1), 136–140. doi:10.1073/pnas.93.1.136
Chari, R. V. (2008). Targeted Cancer Therapy: Conferring Specificity to Cytotoxic Drugs. Acc. Chem. Res. 41 (1), 98–107. doi:10.1021/ar700108g
Cilliers, C., Menezes, B., Nessler, I., Linderman, J., and Thurber, G. M. (2018). Improved Tumor Penetration and Single-Cell Targeting of Antibody-Drug Conjugates Increases Anticancer Efficacy and Host Survival. Cancer Res. 78 (3), 758–768. doi:10.1158/0008-5472.CAN-17-1638
Cocco, E., Varughese, J., Buza, N., Bellone, S., Glasgow, M., Bellone, M., et al. (2011). Expression of Tissue Factor in Adenocarcinoma and Squamous Cell Carcinoma of the Uterine Cervix: Implications for Immunotherapy with hI-Con1, a Factor VII-IgGFc Chimeric Protein Targeting Tissue Factor. BMC Cancer 11, 263. doi:10.1186/1471-2407-11-263
Collins, D. M., Bossenmaier, B., Kollmorgen, G., and Niederfellner, G. (2019). Acquired Resistance to Antibody-Drug Conjugates. Cancers (Basel) 11 (3), 394. doi:10.3390/cancers11030394
De Bono, J. S., Concin, N., Hong, D. S., Thistlethwaite, F. C., Machiels, J. P., Arkenau, H. T., et al. (2019). Tisotumab Vedotin in Patients with Advanced or Metastatic Solid Tumours (InnovaTV 201): a First-In-Human, Multicentre, Phase 1-2 Trial. Lancet Oncol. 20 (3), 383–393. doi:10.1016/S1470-2045(18)30859-3
Deonarain, M. P., and Yahioglu, G. (2021). Current Strategies for the Discovery and Bioconjugation of Smaller, Targetable Drug Conjugates Tailored for Solid Tumor Therapy. Expert Opin. Drug Discov. 16 (6), 613–624. doi:10.1080/17460441.2021.1858050
Deslignière, E., Ehkirch, A., Duivelshof, B. L., Toftevall, H., Sjögren, J., Guillarme, D., et al. (2021). State-of-the-Art Native Mass Spectrometry and Ion Mobility Methods to Monitor Homogeneous Site-specific Antibody-Drug Conjugates Synthesis. Pharm. (Basel) 14 (6), 498. doi:10.3390/ph14060498
Devita, V. T., and Chu, E. (2008). A History of Cancer Chemotherapy. Cancer Res. 68 (21), 8643–8653. doi:10.1158/0008-5472.CAN-07-6611
Diéras, V., Miles, D., Verma, S., Pegram, M., Welslau, M., Baselga, J., et al. (2017). Trastuzumab Emtansine versus Capecitabine Plus Lapatinib in Patients with Previously Treated HER2-Positive Advanced Breast Cancer (EMILIA): a Descriptive Analysis of Final Overall Survival Results from a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 18 (6), 732–742. doi:10.1016/S1470-2045(17)30312-1
Donaghy, H. (2016). Effects of Antibody, Drug and Linker on the Preclinical and Clinical Toxicities of Antibody-Drug Conjugates. Mabs 8 (4), 659–671. doi:10.1080/19420862.2016.1156829
Dotan, E., Cohen, S. J., Starodub, A. N., Lieu, C. H., Messersmith, W. A., Simpson, P. S., et al. (2017). Phase I/II Trial of Labetuzumab Govitecan (Anti-ceacam5/sn-38 Antibody-Drug Conjugate) in Patients with Refractory or Relapsing Metastatic Colorectal Cancer. J. Clin. Oncol. 35 (29), 3338–3346. doi:10.1200/JCO.2017.73.9011
Fadare, O., Renshaw, I. L., and Liang, S. X. (2011). Expression of Tissue Factor and Heparanase in Endometrial Clear Cell Carcinoma: Possible Role for Tissue Factor in Thromboembolic Events. Int. J. Gynecol. Pathol. 30 (3), 252–261. doi:10.1097/PGP.0b013e3181ff9234
Ferraro, E., Drago, J. Z., and Modi, S. (2021). Implementing Antibody-Drug Conjugates (ADCs) in HER2-Positive Breast Cancer: State of the Art and Future Directions. Breast Cancer Res. 23 (1), 84. doi:10.1186/s13058-021-01459-y
Filho, O. M., Viale, G., Stein, S., Trippa, L., Yardley, D. A., Mayer, I. A., et al. (2021). Impact of HER2 Heterogeneity on Treatment Response of Early-Stage HER2-Positive Breast Cancer: Phase II Neoadjuvant Clinical Trial of T-DM1 Combined with Pertuzumab. Cancer Discov. 11 (10), 2474–2487. doi:10.1158/2159-8290.CD-20-1557
Furuta, M., Kikuchi, H., Shoji, T., Takashima, Y., Kikuchi, E., Kikuchi, J., et al. (2019). DLL3 Regulates the Migration and Invasion of Small Cell Lung Cancer by Modulating Snail. Cancer Sci. 110 (5), 1599–1608. doi:10.1111/cas.13997
Galsky, M. D., Eisenberger, M., Moore-Cooper, S., Kelly, W. K., Slovin, S. F., Delacruz, A., et al. (2008). Phase I Trial of the Prostate-specific Membrane Antigen-Directed Immunoconjugate MLN2704 in Patients with Progressive Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 26 (13), 2147–2154. doi:10.1200/JCO.2007.15.0532
Gao, Y., Shi, S., Ma, W., Chen, J., Cai, Y., Ge, L., et al. (2019). Bibliometric Analysis of Global Research on PD-1 and PD-L1 in the Field of Cancer. Int. Immunopharmacol. 72, 374–384. doi:10.1016/j.intimp.2019.03.045
García-Alonso, S., Ocaña, A., and Pandiella, A. (2018). Resistance to Antibody–Drug Conjugates. Cancer Res. 78 (9), 2159–2165. doi:10.1158/0008-5472.CAN-17-3671
Gray, J. E., Heist, R. S., Starodub, A. N., Camidge, D. R., Kio, E. A., Masters, G. A., et al. (2017). Therapy of Small Cell Lung Cancer (SCLC) with a Topoisomerase-I-Inhibiting Antibody-Drug Conjugate (ADC) Targeting Trop-2, Sacituzumab Govitecan. Clin. Cancer Res. 23 (19), 5711–5719. doi:10.1158/1078-0432.CCR-17-0933
Guler, A. T., Waaijer, C. J., and Palmblad, M. (2016). Scientific Workflows for Bibliometrics. Scientometrics 107, 385–398. doi:10.1007/s11192-016-1885-6
Hassan, R., Kreitman, R. J., Pastan, I., and Willingham, M. C. (2005). Localization of Mesothelin in Epithelial Ovarian Cancer. Appl. Immunohistochem. Mol. Morphol. 13 (3), 243–247. doi:10.1097/01.pai.00000141545.36485.d6
Heist, R. S., Guarino, M. J., Masters, G., Purcell, W. T., Starodub, A. N., Horn, L., et al. (2017). Therapy of Advanced Non-small-cell Lung Cancer with an SN-38-Anti-Trop-2 Drug Conjugate, Sacituzumab Govitecan. J. Clin. Oncol. 35 (24), 2790–2797. doi:10.1200/JCO.2016.72.1894
Hoffmann, R. M., Coumbe, B. G. T., Josephs, D. H., Mele, S., Ilieva, K. M., Cheung, A., et al. (2018). Antibody Structure and Engineering Considerations for the Design and Function of Antibody Drug Conjugates (ADCs). Oncoimmunology 7 (3), e1395127. doi:10.1080/2162402X.2017.1395127
Hurvitz, S. A., Dirix, L., Kocsis, J., Bianchi, G. V., Lu, J., Vinholes, J., et al. (2013). Phase II Randomized Study of Trastuzumab Emtansine versus Trastuzumab Plus Docetaxel in Patients with Human Epidermal Growth Factor Receptor 2-positive Metastatic Breast Cancer. J. Clin. Oncol. 31 (9), 1157–1163. doi:10.1200/JCO.2012.44.9694
Junttila, T. T., Li, G., Parsons, K., Phillips, G. L., and Sliwkowski, M. X. (2011). Trastuzumab-DM1 (T-DM1) Retains All the Mechanisms of Action of Trastuzumab and Efficiently Inhibits Growth of Lapatinib Insensitive Breast Cancer. Breast Cancer Res. Treat. 128 (2), 347–356. doi:10.1007/s10549-010-1090-x
Kalim, M., Chen, J., Wang, S., Lin, C., Ullah, S., Liang, K., et al. (2017). Intracellular Trafficking of New Anticancer Therapeutics: Antibody-Drug Conjugates. Drug Des. Devel Ther. 11, 2265–2276. doi:10.2147/DDDT.S135571
Kinneer, K., Meekin, J., Tiberghien, A. C., Tai, Y. T., Phipps, S., Kiefer, C. M., et al. (2018). SLC46A3 as a Potential Predictive Biomarker for Antibody-Drug Conjugates Bearing Noncleavable Linked Maytansinoid and Pyrrolobenzodiazepine Warheads. Clin. Cancer Res. 24 (24), 6570–6582. doi:10.1158/1078-0432.CCR-18-1300
Köhler, G., and Milstein, C. (1975). Continuous Cultures of Fused Cells Secreting Antibody of Predefined Specificity. Nature 256 (5517), 495–497. doi:10.1038/256495a0
Koomägi, R., and Volm, M. (1998). Tissue-factor Expression in Human Non-small-cell Lung Carcinoma Measured by Immunohistochemistry: Correlation between Tissue Factor and Angiogenesis. Int. J. Cancer 79 (1), 19–22. doi:10.1002/(sici)1097-0215(19980220)79:1<19::aid-ijc4>3.0.co;2-z
Krop, I. E., Kim, S. B., González-Martín, A., Lorusso, P. M., Ferrero, J. M., Smitt, M., et al. (2014). Trastuzumab Emtansine versus Treatment of Physician's Choice for Pretreated HER2-Positive Advanced Breast Cancer (TH3RESA): a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 15 (7), 689–699. doi:10.1016/S1470-2045(14)70178-0
Krop, I. E., Kim, S. B., Martin, A. G., Lorusso, P. M., Ferrero, J. M., Badovinac-Crnjevic, T., et al. (2017). Trastuzumab Emtansine versus Treatment of Physician's Choice in Patients with Previously Treated HER2-Positive Metastatic Breast Cancer (TH3RESA): Final Overall Survival Results from a Randomised Open-Label Phase 3 Trial. Lancet Oncol. 18 (6), 743–754. doi:10.1016/S1470-2045(17)30313-3
Krop, I. E., Lorusso, P., Miller, K. D., Modi, S., Yardley, D., Rodriguez, G., et al. (2012). A Phase II Study of Trastuzumab Emtansine in Patients with Human Epidermal Growth Factor Receptor 2-positive Metastatic Breast Cancer Who Were Previously Treated with Trastuzumab, Lapatinib, an Anthracycline, a Taxane, and Capecitabine. J. Clin. Oncol. 30 (26), 3234–3241. doi:10.1200/JCO.2011.40.5902
Lambert, J. M., and Morris, C. Q. (2017). Antibody-Drug Conjugates (ADCs) for Personalized Treatment of Solid Tumors: A Review. Adv. Ther. 34 (5), 1015–1035. doi:10.1007/s12325-017-0519-6
Liao, S., Wang, B., Zeng, R., Bao, H., Chen, X., Dixit, R., et al. (2021). Recent Advances in Trophoblast Cell-Surface Antigen 2 Targeted Therapy for Solid Tumors. Drug Dev. Res. 82 (8), 1096–1110. doi:10.1002/ddr.21870
Liu, J., Burris, H., Wang, J. S., Barroilhet, L., Gutierrez, M., Wang, Y., et al. (2021). An Open-Label Phase I Dose-Escalation Study of the Safety and Pharmacokinetics of DMUC4064A in Patients with Platinum-Resistant Ovarian Cancer. Gynecol. Oncol. 163 (3), 473–480. doi:10.1016/j.ygyno.2021.09.023
Liu, J. K. (2014). The History of Monoclonal Antibody Development - Progress, Remaining Challenges and Future Innovations. Ann. Med. Surg. (Lond) 3 (4), 113–116. doi:10.1016/j.amsu.2014.09.001
Lorusso, P. M., Weiss, D., Guardino, E., Girish, S., and Sliwkowski, M. X. (2011). Trastuzumab Emtansine: a Unique Antibody-Drug Conjugate in Development for Human Epidermal Growth Factor Receptor 2-positive Cancer. Clin. Cancer Res. 17 (20), 6437–6447. doi:10.1158/1078-0432.CCR-11-0762
Lyon, R. P., Bovee, T. D., Doronina, S. O., Burke, P. J., Hunter, J. H., Neff-Laford, H. D., et al. (2015). Reducing Hydrophobicity of Homogeneous Antibody-Drug Conjugates Improves Pharmacokinetics and Therapeutic Index. Nat. Biotechnol. 33 (7), 733–735. doi:10.1038/nbt.3212
Mamounas, E. P., Untch, M., Mano, M. S., Huang, C. S., Geyer, C. E., Von Minckwitz, G., et al. (2021). Adjuvant T-DM1 versus Trastuzumab in Patients with Residual Invasive Disease after Neoadjuvant Therapy for HER2-Positive Breast Cancer: Subgroup Analyses from KATHERINE. Ann. Oncol. 32 (8), 1005–1014. doi:10.1016/j.annonc.2021.04.011
Martin, C., Kizlik-Masson, C., Pèlegrin, A., Watier, H., Viaud-Massuard, M. C., and Joubert, N. (2018). Antibody-drug Conjugates: Design and Development for Therapy and Imaging in and beyond Cancer, LabEx MAbImprove Industrial Workshop, July 27-28, 2017, Tours, France. Mabs 10 (2), 210–221. doi:10.1080/19420862.2017.1412130
Martín-Sabroso, C., Lozza, I., Torres-Suárez, A. I., and Fraguas-Sánchez, A. I. (2021). Antibody-Antineoplastic Conjugates in Gynecological Malignancies: Current Status and Future Perspectives. Pharmaceutics 13 (10), 1705. doi:10.3390/pharmaceutics13101705
Modi, S., Saura, C., Yamashita, T., Park, Y. H., Kim, S. B., Tamura, K., et al. (2020). Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 382 (7), 610–621. doi:10.1056/NEJMoa1914510
Moore, K. N., Oza, A. M., Colombo, N., Oaknin, A., Scambia, G., Lorusso, D., et al. (2021). Phase III, Randomized Trial of Mirvetuximab Soravtansine versus Chemotherapy in Patients with Platinum-Resistant Ovarian Cancer: Primary Analysis of FORWARD I. Ann. Oncol. 32 (6), 757–765. doi:10.1016/j.annonc.2021.02.017
Moussa, M., Papatsoris, A., Abou Chakra, M., and Dellis, A. (2021). Profile of Enfortumab Vedotin in the Treatment of Urothelial Carcinoma: The Evidence to Date. Drug Des. Devel Ther. 15, 453–462. doi:10.2147/DDDT.S240854
Munster, P., Krop, I. E., Lorusso, P., Ma, C., Siegel, B. A., Shields, A. F., et al. (2018). Safety and Pharmacokinetics of MM-302, a HER2-Targeted Antibody-Liposomal Doxorubicin Conjugate, in Patients with Advanced HER2-Positive Breast Cancer: a Phase 1 Dose-Escalation Study. Br. J. Cancer 119 (9), 1086–1093. doi:10.1038/s41416-018-0235-2
Murali, M., Kumar, A. R., Nair, B., Pavithran, K., Devan, A. R., Pradeep, G. K., et al. (2022). Antibody-drug Conjugate as Targeted Therapeutics against Hepatocellular Carcinoma: Preclinical Studies and Clinical Relevance. Clin. Transl. Oncol. 24 (3), 407–431. doi:10.1007/s12094-021-02707-5
Nagayama, A., Ellisen, L. W., Chabner, B., and Bardia, A. (2017). Antibody-Drug Conjugates for the Treatment of Solid Tumors: Clinical Experience and Latest Developments. Target Oncol. 12 (6), 719–739. doi:10.1007/s11523-017-0535-0
Nakada, T., Masuda, T., Naito, H., Yoshida, M., Ashida, S., Morita, K., et al. (2016). Novel Antibody Drug Conjugates Containing Exatecan Derivative-Based Cytotoxic Payloads. Bioorg Med. Chem. Lett. 26 (6), 1542–1545. doi:10.1016/j.bmcl.2016.02.020
Norsworthy, K. J., Ko, C. W., Lee, J. E., Liu, J., John, C. S., Przepiorka, D., et al. (2018). FDA Approval Summary: Mylotarg for Treatment of Patients with Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia. Oncologist 23 (9), 1103–1108. doi:10.1634/theoncologist.2017-0604
Ogitani, Y., Aida, T., Hagihara, K., Yamaguchi, J., Ishii, C., Harada, N., et al. (2016). DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 22 (20), 5097–5108. doi:10.1158/1078-0432.CCR-15-2822
Ordóñez, N. G. (2003). Application of Mesothelin Immunostaining in Tumor Diagnosis. Am. J. Surg. Pathol. 27 (11), 1418–1428. doi:10.1097/00000478-200311000-00003
Owen, D. H., Giffin, M. J., Bailis, J. M., Smit, M. D., Carbone, D. P., and He, K. (2019). DLL3: an Emerging Target in Small Cell Lung Cancer. J. Hematol. Oncol. 12 (1), 61. doi:10.1186/s13045-019-0745-2
Perez, E. A., Barrios, C., Eiermann, W., Toi, M., Im, Y. H., Conte, P., et al. (2017). Trastuzumab Emtansine with or without Pertuzumab versus Trastuzumab Plus Taxane for Human Epidermal Growth Factor Receptor 2-Positive, Advanced Breast Cancer: Primary Results from the Phase III MARIANNE Study. J. Clin. Oncol. 35 (2), 141–148. doi:10.1200/JCO.2016.67.4887
Ponziani, S., Di Vittorio, G., Pitari, G., Cimini, A. M., Ardini, M., Gentile, R., et al. (2020). Antibody-Drug Conjugates: The New Frontier of Chemotherapy. Int. J. Mol. Sci. 21 (15), 5510. doi:10.3390/ijms21155510
Ricciuti, B., Lamberti, G., Andrini, E., Genova, C., De Giglio, A., Bianconi, V., et al. (2021). Antibody-drug Conjugates for Lung Cancer in the Era of Personalized Oncology. Semin. Cancer Biol. 69, 268–278. doi:10.1016/j.semcancer.2019.12.024
Rios-Doria, J., Harper, J., Rothstein, R., Wetzel, L., Chesebrough, J., Marrero, A., et al. (2017). Antibody-Drug Conjugates Bearing Pyrrolobenzodiazepine or Tubulysin Payloads Are Immunomodulatory and Synergize with Multiple Immunotherapies. Cancer Res. 77 (10), 2686–2698. doi:10.1158/0008-5472.CAN-16-2854
Ripani, E., Sacchetti, A., Corda, D., and Alberti, S. (1998). Human Trop-2 Is a Tumor-Associated Calcium Signal Transducer. Int. J. Cancer 76 (5), 671–676. doi:10.1002/(sici)1097-0215(19980529)76:5<671:aid-ijc10>3.0.co;2-7
Rosenberg, J. E., O'donnell, P. H., Balar, A. V., Mcgregor, B. A., Heath, E. I., Yu, E. Y., et al. (2019). Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma after Platinum and Anti-programmed Death 1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 37 (29), 2592–2600. doi:10.1200/JCO.19.01140
Ruddy, K. J., Zheng, Y., Tayob, N., Hu, J., Dang, C. T., Yardley, D. A., et al. (2021). Chemotherapy-related Amenorrhea (CRA) after Adjuvant Ado-Trastuzumab Emtansine (T-DM1) Compared to Paclitaxel in Combination with Trastuzumab (TH) (TBCRC033: ATEMPT Trial). Breast Cancer Res. Treat. 189 (1), 103–110. doi:10.1007/s10549-021-06267-8
Saunders, L. R., Bankovich, A. J., Anderson, W. C., Aujay, M. A., Bheddah, S., Black, K., et al. (2015). A DLL3-Targeted Antibody-Drug Conjugate Eradicates High-Grade Pulmonary Neuroendocrine Tumor-Initiating Cells In Vivo. Sci. Transl. Med. 7 (302), 302ra136. doi:10.1126/scitranslmed.aac9459
Schwartz, R. S. (2004). Paul Ehrlich's Magic Bullets. N. Engl. J. Med. 350 (11), 1079–1080. doi:10.1056/NEJMp048021
Shen, B. Q., Xu, K., Liu, L., Raab, H., Bhakta, S., Kenrick, M., et al. (2012). Conjugation Site Modulates the In Vivo Stability and Therapeutic Activity of Antibody-Drug Conjugates. Nat. Biotechnol. 30 (2), 184–189. doi:10.1038/nbt.2108
Singh, D., Dheer, D., Samykutty, A., and Shankar, R. (2021). Antibody Drug Conjugates in Gastrointestinal Cancer: From Lab to Clinical Development. J. Control Release 340, 1–34. doi:10.1016/j.jconrel.2021.10.006
Sussell, J., Singh Jhuti, G., Antao, V., Herrera-Restrepo, O., Wehler, E., and Bilir, S. P. (2021). Cost-effectiveness Analysis of Ado-Trastuzumab Emtansine (T-DM1) for the Adjuvant Treatment of Patients with Residual Invasive HER2+ Early Breast Cancer in the United States. Am. J. Clin. Oncol. 44 (7), 340–349. doi:10.1097/COC.0000000000000816
Takegawa, N., Tsurutani, J., Kawakami, H., Yonesaka, K., Kato, R., Haratani, K., et al. (2019). [fam-] Trastuzumab Deruxtecan, Antitumor Activity Is Dependent on HER2 Expression Level rather Than on HER2 Amplification. Int. J. Cancer 145 (12), 3414–3424. doi:10.1002/ijc.32408
Tamura, K., Tsurutani, J., Takahashi, S., Iwata, H., Krop, I. E., Redfern, C., et al. (2019). Trastuzumab Deruxtecan (DS-8201a) in Patients with Advanced HER2-Positive Breast Cancer Previously Treated with Trastuzumab Emtansine: a Dose-Expansion, Phase 1 Study. Lancet Oncol. 20 (6), 816–826. doi:10.1016/S1470-2045(19)30097-X
Trerotola, M., Cantanelli, P., Guerra, E., Tripaldi, R., Aloisi, A. L., Bonasera, V., et al. (2013). Upregulation of Trop-2 Quantitatively Stimulates Human Cancer Growth. Oncogene 32 (2), 222–233. doi:10.1038/onc.2012.36
Tsurutani, J., Iwata, H., Krop, I., Jänne, P. A., Doi, T., Takahashi, S., et al. (2020). Targeting HER2 with Trastuzumab Deruxtecan: A Dose-Expansion, Phase I Study in Multiple Advanced Solid Tumors. Cancer Discov. 10 (5), 688–701. doi:10.1158/2159-8290.CD-19-1014
Verma, S., Miles, D., Gianni, L., Krop, I. E., Welslau, M., Baselga, J., et al. (2012). Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 367 (19), 1783–1791. doi:10.1056/NEJMoa1209124
Von Minckwitz, G., Huang, C. S., Mano, M. S., Loibl, S., Mamounas, E. P., Untch, M., et al. (2019). Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 380 (7), 617–628. doi:10.1056/NEJMoa1814017
Wang, H., Wang, W., Xu, Y., Yang, Y., Chen, X., Quan, H., et al. (2017). Aberrant Intracellular Metabolism of T-DM1 Confers T-DM1 Resistance in Human Epidermal Growth Factor Receptor 2-positive Gastric Cancer Cells. Cancer Sci. 108 (7), 1458–1468. doi:10.1111/cas.13253
Yaghoubi, S., Karimi, M. H., Lotfinia, M., Gharibi, T., Mahi-Birjand, M., Kavi, E., et al. (2020). Potential Drugs Used in the Antibody-Drug Conjugate (ADC) Architecture for Cancer Therapy. J. Cell Physiol. 235 (1), 31–64. doi:10.1002/jcp.28967
Yu, E. Y., Petrylak, D. P., O'donnell, P. H., Lee, J. L., Van Der Heijden, M. S., Loriot, Y., et al. (2021). Enfortumab Vedotin after PD-1 or PD-L1 Inhibitors in Cisplatin-Ineligible Patients with Advanced Urothelial Carcinoma (EV-201): a Multicentre, Single-arm, Phase 2 Trial. Lancet Oncol. 22 (6), 872–882. doi:10.1016/S1470-2045(21)00094-2
Zhao, J., and Xia, Y. (2020). Targeting HER2 Alterations in Non-small-cell Lung Cancer: A Comprehensive Review. JCO Precis. Oncol. 4, 411–425. doi:10.1200/PO.19.00333
Keywords: antibody-drug conjugate, solid cancer, bibliometric analysis, citespace, VOSviewer, histcite, bibliometrix
Citation: Qi X, Li Y, Liu W, Wang Y, Chen Z and Lin L (2022) Research Trend of Publications Concerning Antibody-Drug Conjugate in Solid Cancer: A Bibliometric Study. Front. Pharmacol. 13:921385. doi: 10.3389/fphar.2022.921385
Received: 15 April 2022; Accepted: 01 June 2022;
Published: 20 June 2022.
Edited by:
Kwong Tsang, Precision Biologics, Inc., United StatesReviewed by:
Pierpaolo Correale, Azienda ospedaliera “Bianchi-Melacrino-Morelli”, ItalyShahryar Khoshtinat Nikkhoi, Rutgers, The State University of New Jersey, United States
Copyright © 2022 Qi, Li, Liu, Wang, Chen and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lizhu Lin, bGlubGl6aHVAZ3p1Y20uZWR1LmNu
 Xiangjun Qi
Xiangjun Qi Yanlong Li1
Yanlong Li1 Wei Liu
Wei Liu