- 1Department of Medical Oncology, Guangxi Medical University Cancer Hospital, Nanning, China
- 2Department of Hepatobiliary Surgery, Guangxi Medical University Cancer Hospital, Nanning, China
- 3Department of Gastrointestinal Surgery, Guangxi Medical University Cancer Hospital, Nanning, China
Background: Chemotherapy is the basic treatment for colorectal cancer (CRC). However, colorectal cancer cells often develop resistance to chemotherapy drugs, leading to recurrence and poor prognosis. More and more studies have shown that the Homologous recombination (HR) pathway plays an important role in chemotherapy treatment for tumors. However, the relationship between HR pathway, chemotherapy sensitivity, and the prognosis of CRC patients is still unclear.
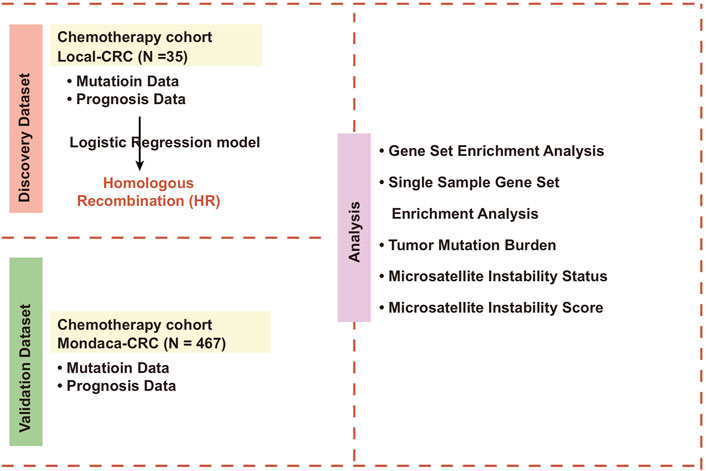
Methods: We collected 35 samples of CRC patients after chemotherapy treatment from Guangxi Medical University Cancer Hospital, then collected mutation data and clinical prognosis data from the group. We also downloaded Mondaca-CRC, TCGA-CRC cohorts for chemotherapy treatment.
Result: We found that HR mutant-type (HR-MUT) patients are less likely to experience tumor metastasis after receiving chemotherapy. Additionally, our univariate and multivariate cox regression models showed that HR-MUT can be used as an independent predictor of the prognosis of chemotherapy for CRC patients. The KM curve showed that patients with HR-MUT CRC had significantly prolonged overall survival (OS) time (log-rank p = 0.017; hazard ratio (HR) = 0.69). Compared to HR mutant-type (HR-WT), HR-MUT has a significantly lower IC50 value with several chemotherapeutic drugs. Pathway enrichment analysis further revealed that the HR-MUT displayed a significantly lower rate of DNA damage repair ability, tumor growth, metastasis activity, and tumor fatty acid metabolism activity than HR-WT, though its immune response activity was notably higher.
Conclusion: These findings indicate that HR-MUT may be a relevant marker for CRC patients receiving chemotherapy, as it is closely related to improving OS time and reducing chemotherapy resistance.
Introduction
Colorectal cancer, or colon cancer, is one of the most common types of malignant tumors found in the human digestive tract and is a seriously threat to human health. Its incidence rate ranks third amongst that of all malignant tumors worldwide, and its mortality rate ranks second (Miller et al., 2020; Liu et al., 2022a; Liu et al., 2022b). At present, besides surgery, chemotherapy is the main treatment for colon cancer. However, due to the high heterogeneity of colon cancer, the benefits of these treatments for difference patients may vary greatly. Moreover, the occurrence of chemotherapy resistance can result in failure of the final treatment of colon cancer patients (Bian et al., 2016; Fu et al., 2016). Therefore, it is of the utmost importance to investigate the specific mechanism of the occurrence and development of colon cancer and any accompanying chemotherapy resistance in order to develop new therapeutic targets, reverse chemotherapy resistance, and ultimately improve the prognosis of colon cancer patients.
Recent studies have shown that the molecular mechanisms of chemotherapy resistance mainly involve: 1) The reduction of the activation of drug precursors and the concentration of drugs in cells through drug efflux and inactivation; 2) Changes in drug action targets; 3) Disturbance of cell survival and apoptosis; 4) Hypoxia in the tumor microenvironment; 5) Changes in the extracellular mechanism; 5) Cytokines and other growth factors that maintain the activation of tumor survival-related pathways (Li et al., 2017).
Homologous recombination (HR) is a stable and error-free repair process that uses homologous sister chromatids as a template. HR determines the survival and fate of cells (Gonzalez and Stenzinger, 2021; Liu et al., 2021). Still, despite its reliability, the main cause of DNA damage in the S/G2 cell cycle is HR (Lisby and Rothstein, 2015). HR plays an important role in repairing cisplatin adducts, a DNA repair process that plays an important role in the chemotherapy resistance of tumor cells (Zdraveski et al., 2000). For example, HR-deficient Escherichia coli strains are known to show higher sensitivity to chemotherapeutic drugs than Nucleotide excision repair (NER)-deficient Escherichia coli strains (Zdraveski et al., 2000), while homologous recombination defect (HRD) is known to be related to chemotherapy resistance in ovarian cancer patients (Xiao et al., 2017). However, at present, the relationship between the mutation state of the HR pathway and the chemotherapy efficacy, prognosis, and chemotherapy sensitivity of CRC patients is unclear.
In this study, we use data collected from a sample of CRC patients who have been treated with chemotherapy from Guangxi Medical University Cancer Hospital to explore the relationship between the mutation state of HR pathway and the prognosis of the chemotherapy efficacy of CRC patients through curative effect analysis, prognosis analysis, and drug sensitivity analysis. Through this process, we found that HR-MUT CRC patients had significantly prolonged survival time and higher sensitivity to chemotherapy drugs. Therefore, HR-MUT may be a useful predictive marker for CRC patients receiving chemotherapy in future treatments.
Materials and Methods
CRC Cohort Collection
This study includes samples of primary CRC patients that underwent colon cancer resection and chemotherapy in Guangxi Medical University Cancer Hospital between 2015 to 2021. The sample patients were divided into patients with tumor metastasis and without tumor metastasis. We obtained the mutation data by targeted sequencing. We also collected the clinical data of the 35 patients, including their level of metastasis, sex, TNM stage, Eastern Cooperative Oncology Group Performance Status (ECOG), age, height, and weight. All participants signed a written informed consent agreement, and the work was approved by the Research Ethics Committee of Guangxi Medical University Cancer Hospital.
To expand our data, we downloaded a CRC cohort for chemotherapy (Mondaca-CRC; https://www.cbioportal.org/study/clinicalData?id=crc_apc_impact_2020) from the cbioportal web tool (Mondaca et al., 2020), as well as the entirety of the exon sequencing data and clinical data (including Tumor mutational burden (TMB), Microsatellite instability (MSI) scores, MSI status, gender, TNM stage, ECOG and age). We downloaded the RNA sequencing data, mutation data, and clinical prognosis data of TCGA-COAD and TCGA-READ from TCGA database (https://portal.gdc.cancer.gov/) using TCGAbiolinks R package (Colaprico et al., 2016). We then combined TCGA-COAD cohort and TCGA-READ cohort into TCGA-CRC cohort, which was used for our subsequent analysis. The TMB of TCGA-CRC was downloaded from the previous literature (Lin et al., 2020). Clinical basic information about Local-CRC, Mondaca-CRC and TCGA-CRC are defined in Supplementary Table S1–S3, respectively.
Definition of the Abrupt State of HR Pathway
We downloaded the gene list of KEGG_HOMOLOGOUS_RECOMBINATION (Supplementary Table S4) from MsigDB database (Liberzon et al., 2011). Firstly, the synonymous mutations in the mutation data from Local-CRC, Mondaca-CRC and TCGA-CRC were deleted, retaining only the mutation data of non-synonymous mutation types. According to the gene list of KEGG_homologus_recombination (HR), we counted the mutation number of the HR pathway in each patient. When the number of gene mutations in a patient’s HR pathway was zero, we labeled it the HR wild-type (WT). Otherwise, they were referred to as the HR mutant-type (MUT).
Relationship Between HR Pathway Mutation and Prognosis or Curative Effect of CRC Patients
We used logistic regression analysis to explore the relationship between the mutation status of HR pathway and whether Local-CRC patients had metastasis after receiving chemotherapy. In Mondaca-CRC cohort, univariable and multivariate Cox regression analysis, as well as Kaplan-Meier analysis, were used to evaluate the influence of HR pathway mutation on the survival time of CRC patients after receiving chemotherapy.
Path Enrichment Analysis
Using clusterProfiler R package and gene set enrichment analysis (GSEA) (Yu et al., 2012), we analyzed the expression profile data of the HR-MUT and HR-WT groups and compared the enrichment scores and p values of the HR-MUT and HR-WT groups to those of GO-BP, GO-CC, GO-MF, KEGG, and REACTOME. We also used single sample GSEA (ssGSEA) to analyze the pathway score of each CRC patient (Lin et al., 2022).
Statistical Analysis
The Mann-Whitney U test was used to compare the differences between the continuity variables of the HR-MUT and HR-WT groups, while Fisher’ exact test was used to compare the differences in the classification variables of the HR-MUT and HR-WT groups. The log-rank test in combination with Kaplan-Meier analysis was used to calculate the p value. All statistical analysis and visual analysis in this study were based on R language. Here, the p value was bilateral and any p value of less than 0.05 was regarded as statistically significant.
Results
HR-MUT is Related to a Better Curative Effect and Prognosis in CRC Patients Receiving Chemotherapy
In order to explore the relationship between the mutation state of HR pathway and the chemotherapy efficacy and prognosis of CRC patients (Figure 1), we first analyzed whether HR-MUT can influence the presence of tumor metastasis in CRC patients after chemotherapy (Figure 2A). The results showed that HR-MUT might indeed cause less tumor metastasis in CRC patients after chemotherapy (OR < 0; p < 0.05). The results displayed in Figure 2B demonstrate how HR-WT was often found in CRC patients without tumor metastasis after chemotherapy (p < 0.05). Meanwhile, as shown in Figure 2C, CRC patients with HR-MUT often did not display any tumor metastasis after receiving chemotherapy (p < 0.05). For Mondaca-CRC patients, our univariable and multivariate Cox regression analysis showed that HR-MUT could be used as an independent predictor of chemotherapy prognosis for CRC patients (Figure 2D). Notably, the Kaplan-Meier curve showed that HR-MUT had significantly longer OS time than HR-WT (log-rank p = 0.017; HR = 0.69; Figure 2E).
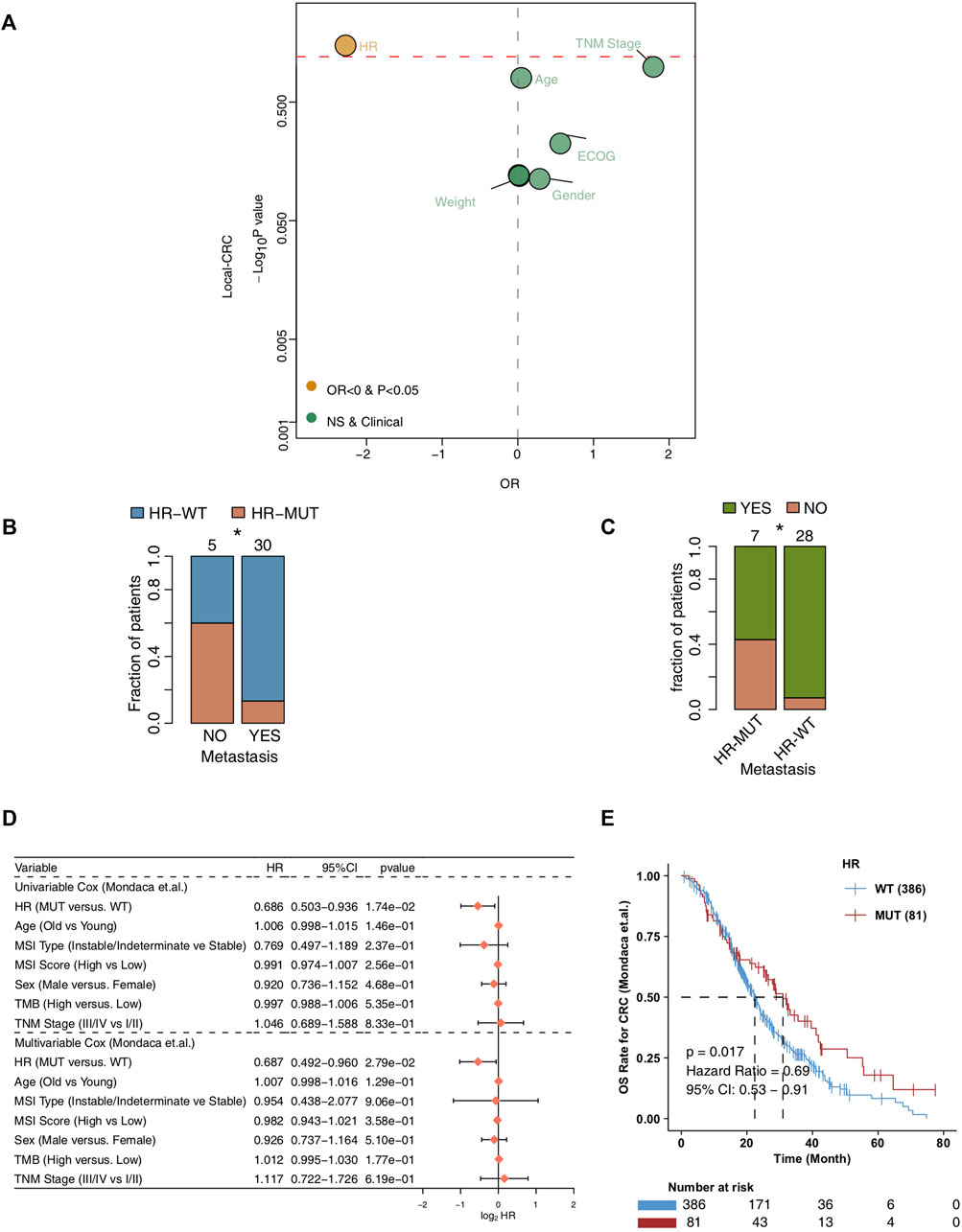
FIGURE 2. The prognosis value of the HR-MUT group (A). Logistic regression model of the HR-MUT group and clinical characteristics in the Local-CRC cohort (B). A comparison between the HR mutation statuses of the metastasis-yes group and metastasis-no group (C). A comparison between the tumor metastasis statuses of the HR-MUT and HR-WT groups (D). The univariable and multivariable cox regression model of the HR-MUT group and clinical characteristics in the Mondaca-CRC cohort (E). KM curve showed the HR-MUT CRC patients displayed a significant improvement in OS time compared with the HR-WT CRC patients in the Mondaca-CRC cohort (*p < 0.05).
In order to further verify the relationship between HR-MUT and the sensitivity of chemotherapeutic drugs, we evaluated the IC50 value of several chemotherapeutic drugs using pRRophetic algorithm combined with the CRC patient expression data. From this analysis, we found that HR-MUT group displayed a significantly lower IC50 value with the chemotherapeutic drugs than the HR-WT group (Figure 3).
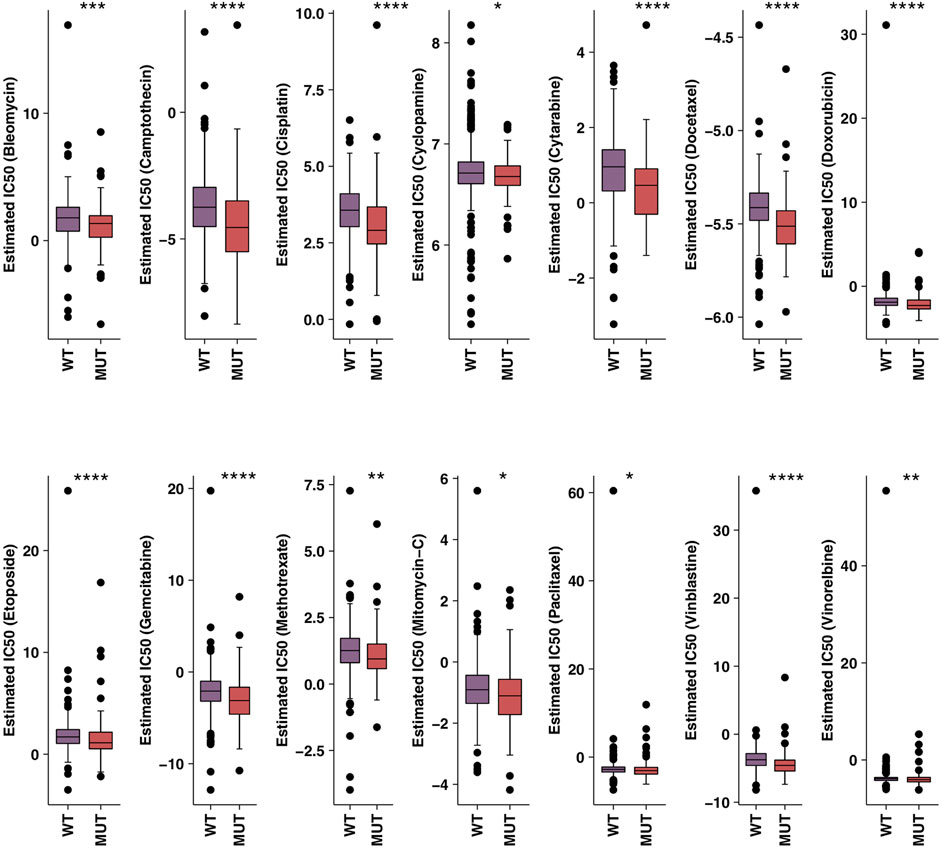
FIGURE 3. A comparison between the IC50 values of common chemotherapy drugs used on HR-MUT and the HR-WT CRC patients (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001).
HR-MUT is Associated With Higher Mutation Frequency, TMB, and MSIscore
Next, we compare the gene mutation frequencies of the HR-MUT and HR-WT groups to the top 20 mutation frequencies found in the other CRC cohorts. For Local-CRC, the HR-MUT group had significantly higher mutation frequency than the HR-WT group in Gli3 (42.9% vs. 3.6%), BRCA2 (42.9% vs. 0.0%), and ITGB2 (42.9% vs. 0.0%) (all p < 0.05; Figure 4A). In Mondaca-CRC, the HR-MUT group was notably higher than the HR-WT group in RNF43 (17.3% vs. 6.2%), ARID1A (21.0% vs. 4.9%), KMT2D (22.2% vs. 4.7%), PTPRS (18.5% vs. 4.7%), ERBB4 (12.3% vs. 4.1%), PTPRT (11.1% vs. 4.1%), NF1 (9.9% vs. 4.1%), and PTEN (11.1% vs. 3.6%), all of which had significantly increased mutation frequency (all p < 0.05; Figure 4B). Supplementary Figures S1A,B shows the mutual exclusion and co-occurrence of the top 20 gene mutations in the Local-CRC and Mondaca-CRC cohorts, respectively, while Supplementary Figures S2A,B show the gene mutation of HR-MUT patients in the Local-CRC Cohort and Mondaca-CRC Cohort, respectively.
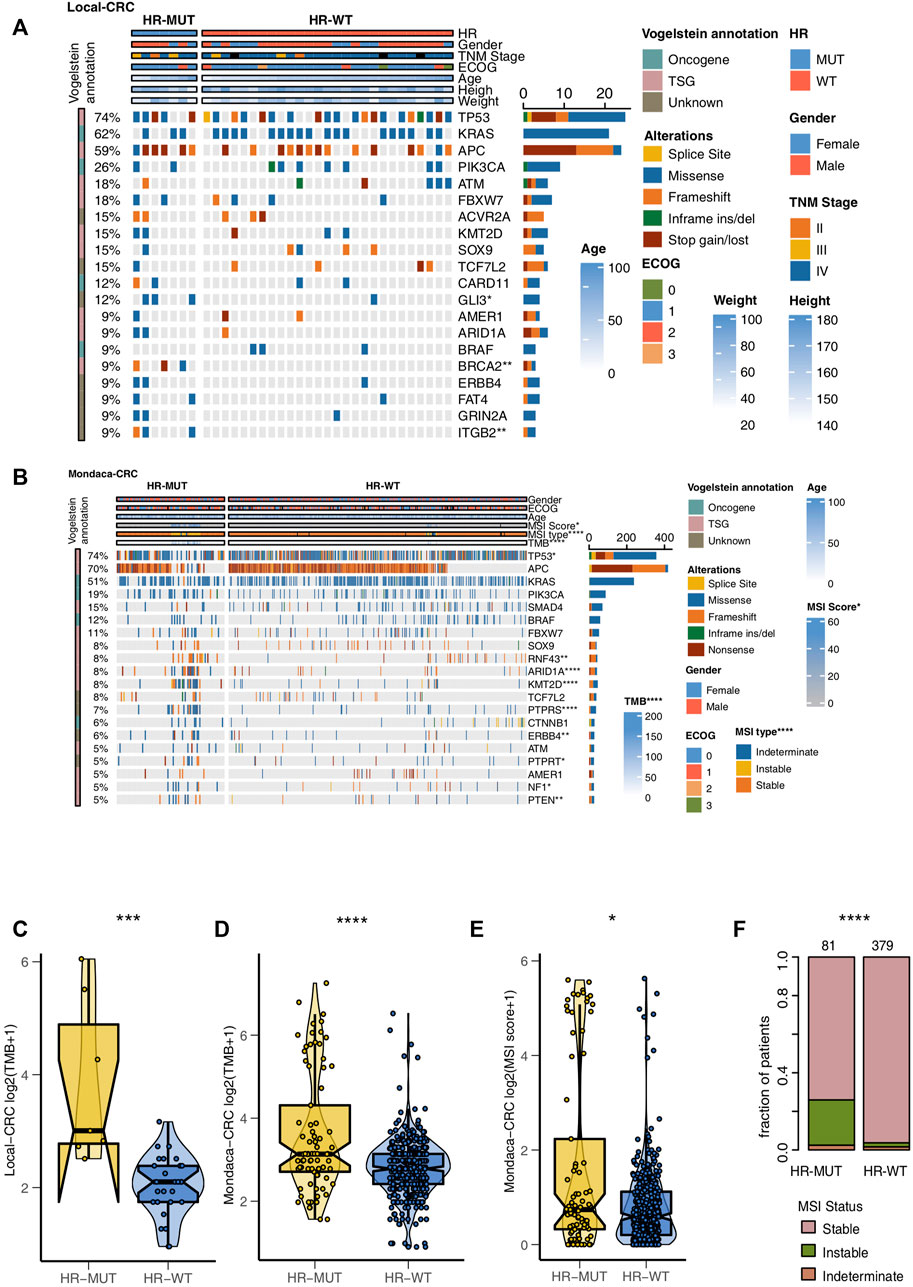
FIGURE 4. The mutation profiles of the HR-MUT and HR-WT CRC patients. The mutational landscape of the top mutated gene mutations in the Local-CRC cohort (A) and Mondaca-CRC cohort (B). A comparison of the TMB levels of the HR-MUT group and HR-WT group in the Local-CRC cohort (C) and Mondaca-CRC cohort (D). A comparison between the MSI scores (E) and MSI statuses (F) the HR-MUT and HR-WT groups in the Mondaca-CRC cohort (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001).
Compared with HR-WT, HR-MUT displayed a significantly higher level of TMB in Local-CRC and Mondaca-CRC (p = 2.55e-04, Figure 4C; P = 5e-05, Figure 4D). We also found that HR-MUT had a higher MSI score than HR-WT (p = 2.66e-02; Figure 4E). Similarly, HR-MUT had a higher prevelence of instable MSI status (p < 0.0001; Figure 4F).
HR-MUT is Related to the Less Active DNA Repair Ability of CRC Patients and Promoting Tumor Metastasis and Metabolism
In order to further explore the difference between HR-MUT and HR-WT in pathway activity, we utilized GSEA and ssGSEA. Compared with HR-WT, HR-MUT displayed a significant increase in immune activity, which is manifested by an increase in antigen presenting ability, infiltration and recruitment of CD4+ T cells, and production of INF-gamma (ES > 0, p < 0.05, Figure 5A; Supplementary Table S5). Additionally, HR-MUT had significantly reduced tumor metabolic activity, as well as fatty acid metabolic activity (ES < 0, p < 0.05; Figure 5B; Supplementary Table S5). Compared with HR-WT, HR-MUT also had significantly reduced WNT pathway activity (ES < 0, p < 0.05; Figure 5C; Supplementary Table S5).
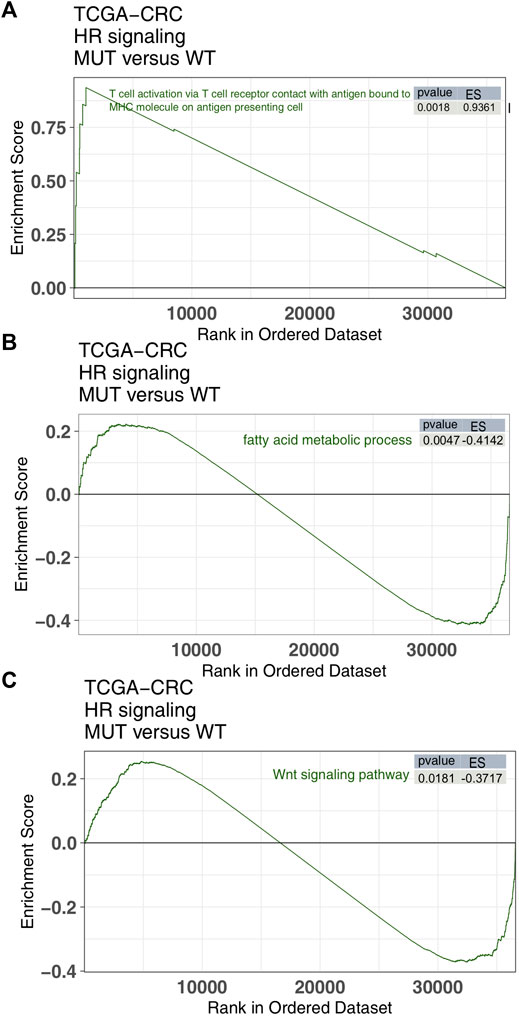
FIGURE 5. The GSEA results. A comparison of the enrichment scores of immune-related (A), tumor metabolism (B) and tumor metastasis/growth (C) found in the HR-MUT and HR-WT groups based on the GSEA algorithm.
In order to further verify the channel activity, we used the ssGSEA algorithm and obtained similar results (Figures 6A–D). From this, we found that HR-MUT had significantly higher immune response ability than HR-WT (Figure 6A), while HR-MUT had significantly lower DNA repair ability, tumor metabolism ability, and tumor metastasis ability (Figures 6B–D).
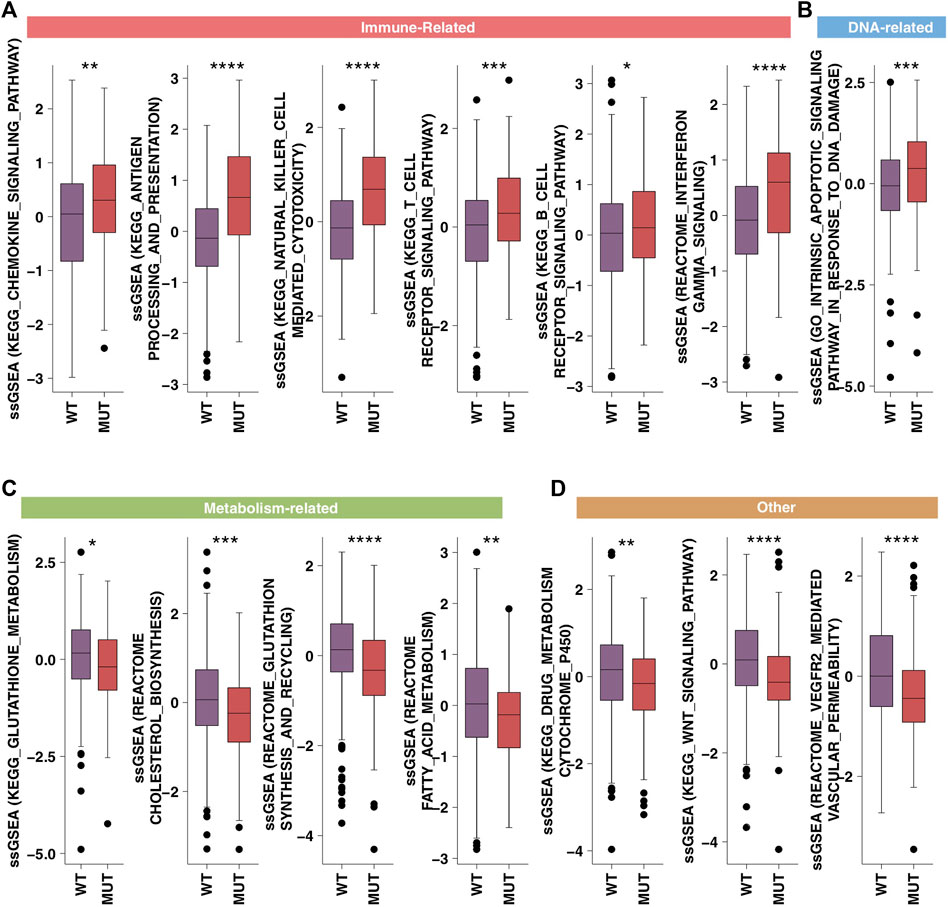
FIGURE 6. The ssGSEA results. A comparison between the ssGSEA scores in the category of immune-related (A), DNA-related (B), metabolism-related (C) and tumor metastasis/growth related (D) of the HR-MUT and HR-WT based on the ssGSEA algorithm (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001).
Discussion
In this study, we found that HR-MUT is related to the reduced occurrence of tumor metastasis in CRC patients who have received chemotherapy. Additionally, HR-MUT was related to a significantly longer OS time than HR-WT for CRC patients receiving chemotherapy. More importantly, univariate and multivariate cox regression models showed that HR-MUT acts as an independent prognostic marker for CRC patients receiving chemotherapy. From the data derived from the genome level analysis, we found that HR-MUT CRC patients had significantly increased TMB and MSI scores, as well as MSI-Instable status, in comparison to HR-WT CRC patients. Based on the pathway enrichment analysis, HR-MUT patients displayed a significant reduction in their DNA repair ability, tumor survival or metastasis related activity, and tumor metabolic activity. Conversely, HR-MUT patients displayed a much higher level of immune response activity. We also found that HR-MUT CRC patients had a longer survival time after receiving immunotherapy than HR-WT CRC patients. Therefore, we speculate that HR-MUT may be a valuable prognostic marker for CRC patients receiving chemotherapy.
The stronger immune response in the tumor microenvironment may be one of the potential mechanisms behind the better prognosis found in HR-MUT patients receiving chemotherapy. In the HR-MUT group, the activity of the interferon gamma production pathway was significantly higher than that of the HR-WT group. In this vein, studies have shown that although chemotherapeutic drugs can directly clear divided endothelial cells by inhibiting enzymes involved in DNA replication or microtubule metabolism, most of the vascular injuries may be caused by chemotherapy-induced inflammation (Kerbel and Kamen, 2004; Lee and Wu, 2015). This is due to the fact that in the process of tumor rejection induced by cyclopamine, immune downregulation is activated to produce interferon-γ (IFN-γ), thus inhibiting angiogenesis (Ibe et al., 2001). IFNγ-γ-mediated vascular inhibition is one of the important anti-tumor mechanisms of T cells (Qin and Blankenstein, 2000; Kammertoens et al., 2017), as it can reduce the expression of Dll4 signaling pathway in endothelial cells, destroy the connection between endothelial cells, and resist angiogenesis in tumor tissue (Deng et al., 2014). However, in a study on early treatment of tumors that combined IFNγ with chemotherapy, it was found that IFNγ could in fact increase the mortality rate (Alberts et al., 2008; Zarogoulidis et al., 2013). As for the activity of IL-6 production, the rates in HR-MUT were significantly lower than that in HR-WT. Further research on this topic has revealed that IL-6 activates the STAT3 signaling pathway of tumor cells and promotes the expression of VEGF, thereby inducing angiogenesis (Wei et al., 2003). We also found that the activation of macrophages in the HR-MUT group was significantly lower than that in the HR-WT group. Earlier research on this shows that macrophages are one of the main cell types in the tumor microenvironment, and they can promote cell survival and chemotherapy resistance by releasing interleukin -17 (Guo et al., 2017). Similarly, according to an earlier study by Ruffell. et al., it was found that M2 macrophage inhibited the production of IL-12 by dendritic cells through the over-secretion of IL-10, therefore blocking the immune response of CD8+T cells (Ruffell et al., 2014).
Tumor metabolic reprogramming may also be one of the important mechanisms involved in CRC chemotherapy resistance. In our study, we found that lipid metabolism was significantly down-regulated in HR-MUT. Beyond our own results, other studies have confirmed that lipid metabolism rearrangement is involved in the regulation of the CRC cell chemotherapy resistance mechanism (Fiucci et al., 2000; Cotte et al., 2018), plays a role in the occurrence and development of CRC (Hwang et al., 2014; Liu et al., 2016; Mihajlovic et al., 2019; Liu et al., 2020), and has an impact on the prognosis (de Figueiredo Junior et al., 2018). Research has also found that lipid droplets (LD) levels in CRC cells are significantly higher than in those of normal cells, indicating that it may play a key role in the development of CRC (Accioly et al., 2008). Many studies have confirmed that lipid metabolism-related enzymes participate in the occurrence and development of CRC (Liu et al., 2020), regulate cell proliferation and invasion (Sánchez-Martínez et al., 2015; Nath and Chan, 2016), and participate in drug resistance (Fiucci et al., 2000; Cotte et al., 2018). Because of this, fatty acids, phospholipids, and cholesterol are synthesized actively, and their concentrations are significantly increased in tumor tissues (Janardhan et al., 2006; Lupu and Menendez, 2006). With this in mind, some anti-tumor drugs have been developed to significantly reduce cell cholesterol levels by blocking different steps of cholesterol biosynthesis (Eliassen et al., 2005; Gorin et al., 2012; Luu et al., 2013). Except for lipid metabolism, we found that the activity of glutathione synthesis in HR-MUT group was significantly lower than that in HR-WT group. Glutamine metabolism can also promote drug resistance in tumors by influencing the post-translational modification of proteins. Treatment with glutamine analogue DON can increase the sensitivity of pancreatic cancer to gemcitabine. In past studies, researchers have found that DON can inhibit hexosamine pathways, which leads to a change in the glycosylation level of protein groups in the cell as while. This ultimately affects the sensitivity of tumors to chemotherapy (Chen et al., 2017). Additionally, glutamine metabolism can affect the drug resistance of tumors epigenetically. In KRAS mutant colon cancer cells, SLC25A22 promotes the accumulation of succinic acid, a metabolite of glutamine in the tricarboxylic acid cycle, which further increases the local methylation degree of DNA and then promotes the activation of Wnt signaling pathway. This sequence of events leads to tolerance of colon cancer cells to chemotherapeutic drugs (Wong et al., 2020).
In addition to immune response and tumor metabolism reprogramming, pathways related to tumor growth and metastasis may also be involved in CRC chemotherapy resistance. Based on GSEA and ssGSEA, we found that the WNT pathway activity was significantly upregulated in HR-WT. According to Nagaraj et al., the expression levels of β-catenin and survivin in A549 sensitive and drug-resistant cells were higher than those in sensitive cells after cisplatin treatment (Nagaraj et al., 2015). Furthermore, Novetsky et al.‘s research indicated that inhibiting the activity of GSK-3β in cells can activate the WNT pathway, which mediates chemotherapy resistance (Novetsky et al., 2013). Zhang et al. found that interfering with β-catenin using siRNA can improve the sensitivity of tumor cells to chemotherapeutic drugs, further proving that the WNT pathway plays an important role in chemotherapeutic drug resistance (Zhang et al., 2015). In our own research, we found that VEGFR2 pathway was significantly activated in HR-WT. Similarly, Mann et al. inhibited the phosphorylation of VEGFR2 in tumor cells and observed that while blood vessels in the tumor remained normal, the sensitivity of the tumor cells to chemotherapeutic drugs such as cyclophosphamide and cisplatin was enhanced (Stockmann et al., 2008).
Conclusion
In summary, our research shows that HR-MUT is associated with less tumor metastasis in CRC patients after receiving chemotherapy. The HR-MUT CRC patients had significantly improved OS time and, as such, HR-MUT could be used as a predictor of chemotherapy prognosis in the future treatment plan. Additionally, HR-MUT patients had higher TMB levels, MSI scores, and immune response activity, while they also had lower tumor survival, metastasis pathway activity, and tumor metabolic reprogramming activity. The activity of these pathways may greatly increase the sensitivity of HR-MUT patients to chemotherapy drugs. Therefore, HR-MUT may be used as a new predictive marker for CRC patients who are receiving chemotherapy treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Guangxi Medical University Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization, YL and RL; Formal analysis, YL and XL; Visualization, YL and XL; Writing–original draft, YZ, GW, JY, SL, XH, ML, MX, JZ, QL, YH, SL, YL, and RL; Writing–review and editing, YL, XL, YZ, GW, JY, SL, XH, ML, MX, JZ, QL, YH, SL, YL, and RL. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.920939/full#supplementary-material
Supplementary Figure S1 | The co-occurrence and mutually exclusive occurrence of the top 20 mutated genes in the Local-CRC (A) and the Mondaca-CRC (B) cohorts.
Supplementary Figure S2 | The mutation landscape of the HR mutations of the HR-MUT CRC patients in the Local-CRC (A) and the Mondaca-CRC (B) cohorts.
Supplementary Table S1 | The clinical characteristics of the Local-CRC cohort.
Supplementary Table S2 | The clinical characteristics of the Mondaca-CRC cohort.
Supplementary Table S3 | The clinical characteristics of the TCGA-CRC cohort.
Supplementary Table S4 | The gene set of the HR signaling used in this study.
Supplementary Table S5 | The GSEA result of the enrichment scores between the HR-MUT and HR-WT groups based on the GSEA algorithm.
Abbreviation
CRC, colorectal cancer; ECOG, Eastern Cooperative Oncology Group Performance Status; GSEA, gene set enrichment analysis; HR, Homologous recombination; HR-MUT, HR mutant-type; HR-WT, HR wild-type; IC50, half maximal inhibitory concentration; HRD, homologous recombination defect; LD, lipid droplets; IFN-γ, interferon-γ; MSI: Microsatellite instability; NER, Nucleotide excision repair; OS, overall survival; TMB, Tumor mutational burden, ssGSEA, single sample gene set enrichment analysis.
References
Accioly, M. T., Pacheco, P., Maya-Monteiro, C. M., Carrossini, N., Robbs, B. K., Oliveira, S. S., et al. (2008). Lipid Bodies Are Reservoirs of Cyclooxygenase-2 and Sites of Prostaglandin-E2 Synthesis in Colon Cancer Cells. Cancer Res. 68, 1732–1740. doi:10.1158/0008-5472.CAN-07-1999
Alberts, D. S., Marth, C., Alvarez, R. D., Johnson, G., Bidzinski, M., Kardatzke, D. R., et al. (2008). Randomized Phase 3 Trial of Interferon Gamma-1b Plus Standard Carboplatin/Paclitaxel Versus Carboplatin/paclitaxel Alone for First-Line Treatment of Advanced Ovarian and Primary Peritoneal Carcinomas: Results from a Prospectively Designed Analysis of Progression-Free Survival. Gynecol. Oncol. 109, 174–181. doi:10.1016/j.ygyno.2008.01.005
Bian, Z., Jin, L., Zhang, J., Yin, Y., Quan, C., Hu, Y., et al. (2016). LncRNA-UCA1 Enhances Cell Proliferation and 5-Fluorouracil Resistance in Colorectal Cancer by Inhibiting miR-204-5p. Sci. Rep. 6, 23892. doi:10.1038/srep23892
Chen, R., Lai, L. A., Sullivan, Y., Wong, M., Wang, L., Riddell, J., et al. (2017). Disrupting Glutamine Metabolic Pathways to Sensitize Gemcitabine-Resistant Pancreatic Cancer. Sci. Rep. 7, 7950. doi:10.1038/s41598-017-08436-6
Colaprico, A., Silva, T. C., Olsen, C., Garofano, L., Cava, C., Garolini, D., et al. (2016). TCGAbiolinks: An R/Bioconductor Package for Integrative Analysis of TCGA Data. Nucleic Acids Res. 44, e71. doi:10.1093/nar/gkv1507
Cotte, A. K., Aires, V., Fredon, M., Limagne, E., Derangère, V., Thibaudin, M., et al. (2018). Lysophosphatidylcholine Acyltransferase 2-Mediated Lipid Droplet Production Supports Colorectal Cancer Chemoresistance. Nat. Commun. 9, 322. doi:10.1038/s41467-017-02732-5
de Figueiredo Junior, A. G., Serafim, P. V. P., de Melo, A. A., Felipe, A. V., Lo Turco, E. G., da Silva, I. D. C. G., et al. (2018). Analysis of the Lipid Profile in Patients with Colorectal Cancer in Advanced Stages. Asian Pac J. Cancer Prev. 19, 1287–1293. doi:10.22034/APJCP.2018.19.5.1287
Deng, J., Liu, X., Rong, L., Ni, C., Li, X., Yang, W., et al. (2014). Ifnγ-Responsiveness of Endothelial Cells Leads to Efficient Angiostasis in Tumours Involving Down-Regulation of Dll4. J. Pathol. 233, 170–182. doi:10.1002/path.4340
Eliassen, A. H., Colditz, G. A., Rosner, B., Willett, W. C., and Hankinson, S. E. (2005). Serum Lipids, Lipid-Lowering Drugs, and the Risk of Breast Cancer. Arch. Intern Med. 165, 2264–2271. doi:10.1001/archinte.165.19.2264
Fiucci, G., Czarny, M., Lavie, Y., Zhao, D., Berse, B., Blusztajn, J. K., et al. (2000). Changes in Phospholipase D Isoform Activity and Expression in Multidrug-Resistant Human Cancer Cells. Int. J. Cancer 85, 882–888. doi:10.1002/(sici)1097-0215(20000315)85:6<882::aid-ijc24>3.0.co;2-e
Fu, Q., Cheng, J., Zhang, J., Zhang, Y., Chen, X., Xie, J., et al. (2016). Downregulation of YEATS4 by miR-218 Sensitizes Colorectal Cancer Cells to L-OHP-Induced Cell Apoptosis by Inhibiting Cytoprotective Autophagy. Oncol. Rep. 36, 3682–3690. doi:10.3892/or.2016.5195
Gonzalez, D., and Stenzinger, A. (2021). Homologous Recombination Repair Deficiency (HRD): From Biology to Clinical Exploitation. Genes Chromosom. Cancer 60, 299–302. doi:10.1002/gcc.22939
Gorin, A., Gabitova, L., and Astsaturov, I. (2012). Regulation of Cholesterol Biosynthesis and Cancer Signaling. Curr. Opin. Pharmacol. 12, 710–716. doi:10.1016/j.coph.2012.06.011
Guo, B., Li, L., Guo, J., Liu, A., Wu, J., Wang, H., et al. (2017). M2 Tumor-Associated Macrophages Produce Interleukin-17 to Suppress Oxaliplatin-Induced Apoptosis in Hepatocellular Carcinoma. Oncotarget 8, 44465–44476. doi:10.18632/oncotarget.17973
Hwang, W. C., Kim, M. K., Song, J. H., Choi, K. Y., and Min, do. S. (2014). Inhibition of Phospholipase D2 Induces Autophagy in Colorectal Cancer Cells. Exp. Mol. Med. 46, e124. doi:10.1038/emm.2014.74
Ibe, S., Qin, Z., Schüler, T., Preiss, S., and Blankenstein, T. (2001). Tumor Rejection by Disturbing Tumor Stroma Cell Interactions. J. Exp. Med. 194, 1549–1559. doi:10.1084/jem.194.11.1549
Janardhan, S., Srivani, P., and Sastry, G. N. (2006). Choline Kinase: An Important Target for Cancer. Curr. Med. Chem. 13, 1169–1186. doi:10.2174/092986706776360923
Kammertoens, T., Friese, C., Arina, A., Idel, C., Briesemeister, D., Rothe, M., et al. (2017). Tumour Ischaemia by Interferon-γ Resembles Physiological Blood Vessel Regression. Nature 545, 98–102. doi:10.1038/nature22311
Kerbel, R. S., and Kamen, B. A. (2004). The Anti-Angiogenic Basis of Metronomic Chemotherapy. Nat. Rev. Cancer 4, 423–436. doi:10.1038/nrc1369
Lee, J. G., and Wu, R. (2015). Erlotinib-Cisplatin Combination Inhibits Growth and Angiogenesis Through C-MYC and HIF-1α in EGFR-Mutated Lung Cancer In Vitro and In Vivo. Neoplasia 17, 190–200. doi:10.1016/j.neo.2014.12.008
Li, L., Shang, J., Zhang, Y., Liu, S., Peng, Y., Zhou, Z., et al. (2017). MEG3 Is a Prognostic Factor for CRC and Promotes Chemosensitivity by Enhancing Oxaliplatin-Induced Cell Apoptosis. Oncol. Rep. 38, 1383–1392. doi:10.3892/or.2017.5828
Liberzon, A., Subramanian, A., Pinchback, R., Thorvaldsdóttir, H., Tamayo, P., and Mesirov, J. P. (2011). Molecular Signatures Database (MSigDB) 3.0. Bioinformatics 27, 1739–1740. doi:10.1093/bioinformatics/btr260
Lin, A., Zhang, J., and Luo, P. (2020). Crosstalk between the MSI Status and Tumor Microenvironment in Colorectal Cancer. Front. Immunol. 11, e15236–2039. doi:10.1200/JCO.2020.38.15_suppl.e1523610.3389/fimmu.2020.02039
Lin, A., Qi, C., Wei, T., Li, M., Cheng, Q., Liu, Z., et al. (2022). CAMOIP: A Web Server for Comprehensive Analysis on Multi-Omics of Immunotherapy in Pan-Cancer. Brief. Bioinform 23. doi:10.1093/bib/bbac129
Lisby, M., and Rothstein, R. (2015). Cell Biology of Mitotic Recombination. Cold Spring Harb. Perspect. Biol. 7, a016535. doi:10.1101/cshperspect.a016535
Liu, M., Du, K., Jiang, B., and Wu, X. (2020). High Expression of Phospholipase D2 Induced by Hypoxia Promotes Proliferation of Colon Cancer Cells Through Activating NF-κ Bp65 Signaling Pathway. Pathol. Oncol. Res. 26, 281–290. doi:10.1007/s12253-018-0429-1
Liu, M., Fu, Z., Wu, X., Du, K., Zhang, S., and Zeng, L. (2016). Inhibition of Phospholipase D2 Increases Hypoxia-Induced Human Colon Cancer Cell Apoptosis Through Inactivating of the PI3K/AKT Signaling Pathway. Tumour Biol. 37, 6155–6168. doi:10.1007/s13277-015-4348-4
Liu, Z., Guo, C., Dang, Q., Wang, L., Liu, L., Weng, S., et al. (2022). Integrative Analysis from Multi-Center Studies Identities a Consensus Machine Learning-Derived lncRNA Signature for Stage II/III Colorectal Cancer. EBioMedicine 75, 103750. doi:10.1016/j.ebiom.2021.103750
Liu, Z., Guo, C., Li, J., Xu, H., Lu, T., Wang, L., et al. (2021). Somatic Mutations in Homologous Recombination Pathway Predict Favourable Prognosis After Immunotherapy Across Multiple Cancer Types. Clin. Transl. Med. 11, e619. doi:10.1002/ctm2.619
Liu, Z., Liu, L., Weng, S., Guo, C., Dang, Q., Xu, H., et al. (2022). Machine Learning-Based Integration Develops an Immune-Derived lncRNA Signature for Improving Outcomes in Colorectal Cancer. Nat. Commun. 13, 816. doi:10.1038/s41467-022-28421-6
Lupu, R., and Menendez, J. A. (2006). Pharmacological Inhibitors of Fatty Acid Synthase (FASN)–Catalyzed Endogenous Fatty Acid Biogenesis: A New Family of Anti-Cancer Agents? Curr. Pharm. Biotechnol. 7, 483–493. doi:10.2174/138920106779116928
Luu, W., Sharpe, L. J., Gelissen, I. C., and Brown, A. J. (2013). The Role of Signalling in Cellular Cholesterol Homeostasis. IUBMB Life 65, 675–684. doi:10.1002/iub.1182
Mihajlovic, M., Gojkovic, T., Vladimirov, S., Miljkovic, M., Stefanovic, A., Vekic, J., et al. (2019). Changes in Lecithin: Cholesterol Acyltransferase, Cholesteryl Ester Transfer Protein and Paraoxonase-1 Activities in Patients with Colorectal Cancer. Clin. Biochem. 63, 32–38. doi:10.1016/j.clinbiochem.2018.11.010
Miller, K. D., Fidler-Benaoudia, M., Keegan, T. H., Hipp, H. S., Jemal, A., and Siegel, R. L. (2020). Cancer Statistics for Adolescents and Young Adults, 2020. CA Cancer J. Clin. 70, 443–459. doi:10.3322/caac.2159010.3322/caac.21637
Mondaca, S., Walch, H., Nandakumar, S., Chatila, W. K., Schultz, N., and Yaeger, R. (2020). Specific Mutations in APC, but Not Alterations in DNA Damage Response, Associate with Outcomes of Patients with Metastatic Colorectal Cancer. Gastroenterology 159, 1975–1978. doi:10.1053/j.gastro.2020.07.041
Nagaraj, A. B., Joseph, P., Kovalenko, O., Singh, S., Armstrong, A., Redline, R., et al. (2015). Critical Role of Wnt/β-Catenin Signaling in Driving Epithelial Ovarian Cancer Platinum Resistance. Oncotarget 6, 23720–23734. doi:10.18632/oncotarget.4690
Nath, A., and Chan, C. (2016). Genetic Alterations in Fatty Acid Transport and Metabolism Genes Are Associated with Metastatic Progression and Poor Prognosis of Human Cancers. Sci. Rep. 6, 18669. doi:10.1038/srep18669
Novetsky, A. P., Thompson, D. M., Zighelboim, I., Thaker, P. H., Powell, M. A., Mutch, D. G., et al. (2013). Lithium Chloride and Inhibition of Glycogen Synthase Kinase 3β as a Potential Therapy for Serous Ovarian Cancer. Int. J. Gynecol. Cancer 23, 361–366. doi:10.1097/IGC.0b013e31827cfecb
Qin, Z., and Blankenstein, T. (2000). CD4+ T Cell-Mediated Tumor Rejection Involves Inhibition of Angiogenesis that Is Dependent on IFN Gamma Receptor Expression by Nonhematopoietic Cells. Immunity 12, 677–686. doi:10.1016/s1074-7613(00)80218-6
Ruffell, B., Chang-Strachan, D., Chan, V., Rosenbusch, A., Ho, C. M., Pryer, N., et al. (2014). Macrophage IL-10 Blocks CD8+ T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell 26, 623–637. doi:10.1016/j.ccell.2014.09.006
Sánchez-Martínez, R., Cruz-Gil, S., Gómez de Cedrón, M., Álvarez-Fernández, M., Vargas, T., Molina, S., et al. (2015). A Link Between Lipid Metabolism and Epithelial-Mesenchymal Transition Provides a Target for Colon Cancer Therapy. Oncotarget 6, 38719–38736. doi:10.18632/oncotarget.5340
Stockmann, C., Doedens, A., Weidemann, A., Zhang, N., Takeda, N., Greenberg, J. I., et al. (2008). Deletion of Vascular Endothelial Growth Factor in Myeloid Cells Accelerates Tumorigenesis. Nature 456, 814–818. doi:10.1038/nature07445
Wei, L. H., Kuo, M. L., Chen, C. A., Chou, C. H., Lai, K. B., Lee, C. N., et al. (2003). Interleukin-6 Promotes Cervical Tumor Growth by VEGF-Dependent Angiogenesis via a STAT3 Pathway. Oncogene 22, 1517–1527. doi:10.1038/sj.onc.1206226
Wong, C. C., Xu, J., Bian, X., Wu, J. L., Kang, W., Qian, Y., et al. (2020). In Colorectal Cancer Cells with Mutant KRAS, SLC25A22-Mediated Glutaminolysis Reduces DNA Demethylation to Increase WNT Signaling, Stemness, and Drug Resistance. Gastroenterology 159, 2163–e6. doi:10.1053/j.gastro.2020.08.016
Xiao, M., Cai, J., Cai, L., Jia, J., Xie, L., Zhu, Y., et al. (2017). Let-7e Sensitizes Epithelial Ovarian Cancer to Cisplatin Through Repressing DNA Double Strand Break Repair. J. Ovarian Res. 10, 24. doi:10.1186/s13048-017-0321-8
Yu, G., Wang, L. G., Han, Y., and He, Q. Y. (2012). clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS 16, 284–287. doi:10.1089/omi.2011.0118
Zarogoulidis, K., Ziogas, E., Boutsikou, E., Zarogoulidis, P., Darwiche, K., Kontakiotis, T., et al. (2013). Immunomodifiers in Combination with Conventional Chemotherapy in Small Cell Lung Cancer: A Phase II, Randomized Study. Drug Des. Devel. Ther. 7, 611–617. doi:10.2147/DDDT.S43184
Zdraveski, Z. Z., Mello, J. A., Marinus, M. G., and Essigmann, J. M. (2000). Multiple Pathways of Recombination Define Cellular Responses to Cisplatin. Chem. Biol. 7, 39–50. doi:10.1016/s1074-5521(00)00064-8
Zhang, B., Yang, Y., Shi, X., Liao, W., Chen, M., Cheng, A. S., et al. (2015). Proton Pump Inhibitor Pantoprazole Abrogates Adriamycin-Resistant Gastric Cancer Cell Invasiveness via Suppression of Akt/GSK-Β/β-Catenin Signaling and Epithelial-Mesenchymal Transition. Cancer Lett. 356, 704–712. doi:10.1016/j.canlet.2014.10.016
Keywords: colorectal cancer, chemotherapy, biomarker, prognosis, homologous recombination
Citation: Lin Y, Liao X, Zhang Y, Wu G, Ye J, Luo S, He X, Luo M, Xie M, Zhang J, Li Q, Huang Y, Liao S, Li Y and Liang R (2022) Homologous Recombination Pathway Alternation Predicts Prognosis of Colorectal Cancer With Chemotherapy. Front. Pharmacol. 13:920939. doi: 10.3389/fphar.2022.920939
Received: 15 April 2022; Accepted: 23 May 2022;
Published: 06 June 2022.
Edited by:
Clare Y. Slaney, Peter MacCallum Cancer Centre, AustraliaReviewed by:
Xinwei Han, Zhengzhou University, ChinaXiaohua Li, Sixth People’s Hospital of Chengdu, China
Copyright © 2022 Lin, Liao, Zhang, Wu, Ye, Luo, He, Luo, Xie, Zhang, Li, Huang, Liao, Li and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Liang, bGlhbmdyb25nQGd4bXUuZWR1LmNu; Yongqiang Li, bGl5b25ncWlhbmdAZ3htdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Yan Lin
Yan Lin Xiaoli Liao1†
Xiaoli Liao1† Jiazhou Ye
Jiazhou Ye Rong Liang
Rong Liang