- 1College of Biological and Pharmaceutical Engineering, West Anhui University, Lu’an, China
- 2Anhui Province Traditional Chinese Medicine Resource Protection and Sustainable Utilization Engineering Laboratory, Lu’an, China
- 3School of Pharmacy, Anhui University of Chinese Medicine, Hefei, China
Dendrobium huoshanense, a traditional medicinal and food homologous plant, belongs to the family Orchidaceae and has a long history of medicinal use. It is reported that the stem of D. huoshanense has a variety of bioactive ingredients such as polysaccharides, flavonoids, sesquiterpenes, phenols, etc. These bioactive ingredients make D. huoshanense remarkable for its pharmacological effects on anti-tumor, immunomodulation, hepatoprotective, antioxidant, and anticataract activities. In recent years, its rich pharmacological activities have attracted extensive attention. However, there is no systematic review focusing on the chemical compositions and pharmacological effects of D. huoshanense. Therefore, the present review aims to summarize current research on the chemical compositions and pharmacological activities of D. huoshanense. This study provides valuable references and promising ideas for further investigations of D. huoshanense.
Introduction
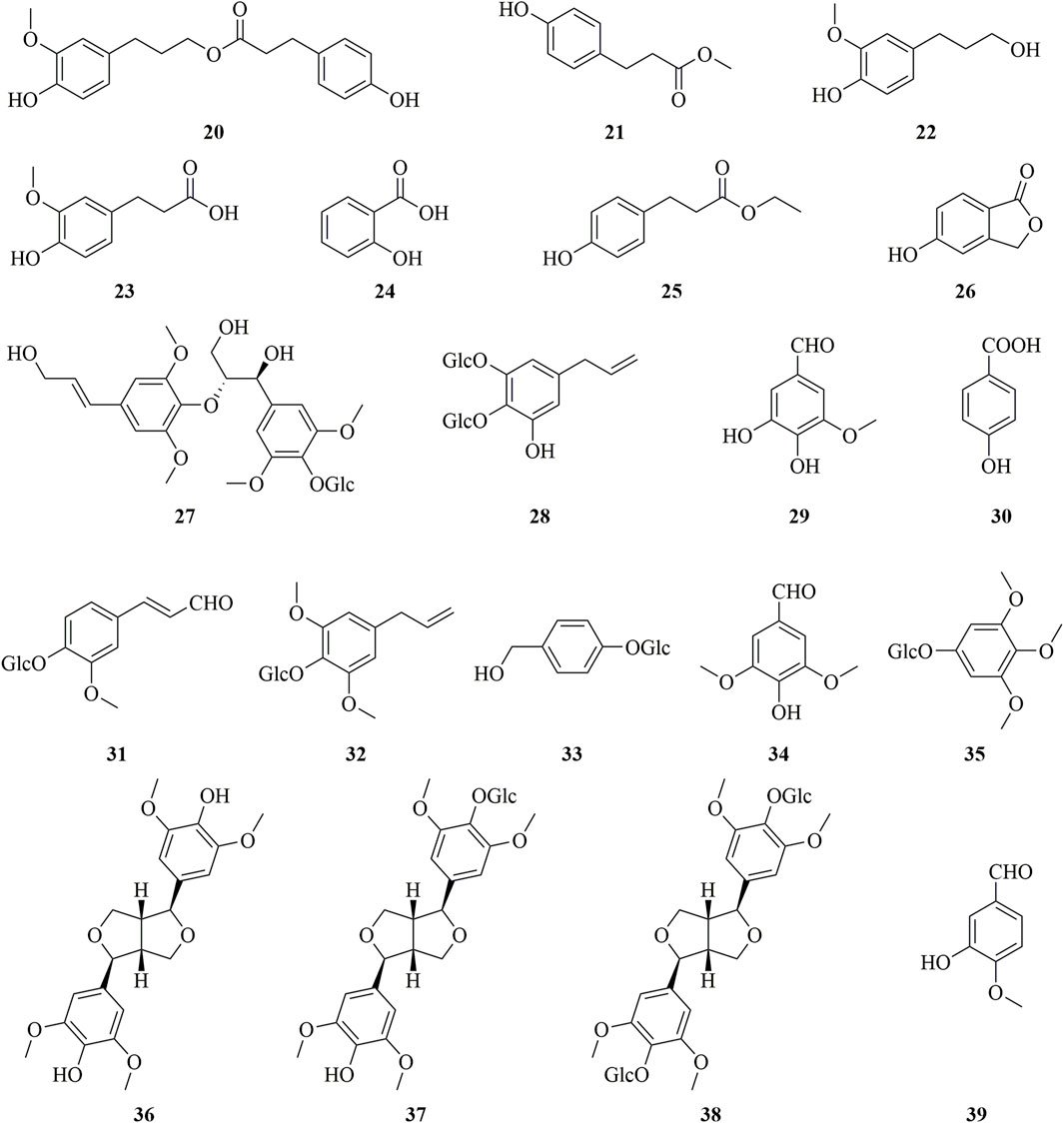
Dendrobium is a valuable traditional Chinese medicine with a long history of medicinal use (Li et al., 2022; Zhu et al., 2021; Niu et al., 2018). It was first recorded in “Shennong’s Herbal Classic” (Chen et al., 2021). However, there are various species in this genus, and their quality varies significantly (Chen et al., 2011). Dendrobium huoshanense C.Z.Tang et S.J.Cheng is an endemic epiphytic orchid species as well as a national geographical indication product of China (Figure 1) (Huang et al., 2007). This plant was first described in a local classic reference “Bai Cao Jing” (《百草镜》) and the distribution area of this plant is in the Da-bie Mountains and adjacent areas, especially in the Huoshan County town, Anhui province, China (Zhao et al., 2017; Yuan et al., 2019; Chen et al., 2020). Currently, the species has been included in the Pharmacopoeia of the Peoples Republic of China (2020 Edition) and approved to be used as food (Hao et al., 2018; Editorial Board of China Pharmacopoeia Committee 2020; Hao et al., 2021c).

FIGURE 1. The whole plant (A), fresh strips (B), and the commercial product named Fengdou (C) of D. huoshanense.
D. huoshanense is locally known as “Mihu,” and its stem has high medicinal value, which is sweet in taste and slightly cold in nature. It is commonly utilized for benefiting the stomach and producing body fluid, clearing heat, and nourishing yin (Hsieh et al., 2008; Wang et al., 2009; Dai et al., 2021). Previous studies have showed that D. huoshanense has various activities, such as immunoregulation, anti-oxidation, anti-cataract, anti-glycation, anti-aging, anti-cataract, antitumor, anti-rheumatoid arthritis, anti-atherosclerosis, anti-inflammation, hypoglycemic activity, and liver protection activities (Wu et al., 2009; Ohara et al., 2013; Deng et al., 2016; Zhang et al., 2020; Hao J.-W. et al., 2021; Zhu et al., 2022). The main components that play a therapeutic role are active substances such as flavonoids, sesquiterpenes, and especially polysaccharides, which is the index of the quality evaluation of D. huoshanense (Lee and Chen 2014; Liu et al., 2018; Yuan et al., 2018; Chen et al., 2019; Zhou et al., 2020; Hao et al., 2021a; Wang et al., 2021). These diverse chemical compositions and extensive pharmacological activities of D. huoshanense have attracted much attention, while posing great difficulties for further research.
At present, there is much literature summarizing the Dendrobium genus, but no literature systematically reviewing the status of the research on D. huoshanense. Therefore, this review systematically describes the current research status of a specific species, D. huoshanense, including the materia medica research, resource, phytochemistry, and pharmacology, to provide a reference for further research on D. huoshanense.
Materia Medica Research
Materia Medica Research on Origin
The habitats of D. huoshanense have been recorded in many important classical documents of traditional Chinese medicine. In the period of 220–450 AD, “Records of Famous Doctors” (《名医别录》) recorded that “Dendrobium grows on the stone beside the water in the valley of Lu’an”. It is found that Lu’an refers to the area of Huoshan in the Anhui province at that time, demonstrating that the earliest recorded origin of D. huoshanense is in the Huoshan County. “Bai Cao Jing” (《百草镜》) in the Qing Dynasty described that Dendrobium from the Lu’an and Huoshan Counties was called “Huoshan Shihu” and was the best, which was first documented with the name of D. huoshanense. In 1984, Tang et al. systematically studied several species of Dendrobium and analyzed their botanical traits (Tang and Cheng 1984). Moreover, “Mihu” produced in the Huoshan County was officially named Dendrobium huoshanense C.Z. Tang et S.J. Cheng.
At present, “Flora Reipublicae Popularis Sinicae” reported that “D. huoshanense is produced in southwestern Henan (Nanzhao) and southwestern Anhui (Huoshan) of China. It grows on tree trunks in mountain forests and on rocks in valleys. The type specimen was collected in Anhui (Huoshan) of China”. Through materia medica research and field investigations, Liu and wang et al. also clearly support the occurrence of D. huoshanense in Huoshan, Anhui province of China (Liu 1996; Wang and Peng 2004). In 2007, D. huoshanense was listed in the national geographic indication to protect products. In short, the habitats of D. huoshanense are mainly concentrated in Huoshan, Anhui province of China.
Materia Medica Research on Medicinal Use
D. huoshanense has been used in medicine for a long time. “Records of Famous Doctors” (《名医别录》) recorded that Dendrobium could nourish the essence, reinforce the kidneys, calm the stomach, build muscles, relieve foot and knee pain, and remove convulsions, while emphasizing that the origin of Dendrobium is the now Huoshan County. These are enough to show that the orchid plant recorded in “Records of Famous Doctors” (《名医别录》) refers to D. huoshanense (Tao 2013). The ancient medical reference “Bai Cao Jing” (《百草镜》) put forward the local name of D. huoshanense for the first time and stated that this plant is the best for medicinal use. The traits of D. huoshanense, including dwarf plantlets in a cluster and an upright or bending stem resembling grasshopper legs, are almost identical to those recorded in the “Shen Nong Ben Cao” (Zhao 1998). It can be seen that D. huoshanense has been used as high-quality Dendrobium for medicinal purposes for many generations. In addition, D. huoshanense is included in the Chinese Pharmacopoeia (2020 version), and its efficacy is recorded for benefiting the stomach and producing body fluids, clearing heat, and nourishing the yin (Editorial Board of China Pharmacopoeia Committee 2020).
Resource
Resource Distribution
Dendrobium is the second largest genus belonging to the family Orchidaceae, widely distributed in Asia, Europe, Oceania, and other regions (Liu JL. et al., 2020; Han et al., 2020; Hu et al., 2020; Wang et al., 2020). There are 105 species and two varieties of Dendrobium in China, which are produced in Anhui, Zhejiang, Jiangxi, Fujian, Taiwan, Hubei, Hunan, Guangdong, Guangxi, Hainan, Sichuan (including Chongqing), Guizhou, Yunnan, Tibet, etc. Among them, there are 39 species of Dendrobium with medicinal purposes, including D. huoshanense (Chiang et al., 2012; Ye et al., 2021). However, the natural distribution of D. huoshanense is relatively narrow, because of its preference for a cool and moist environment, and high environmental requirements (Liu et al., 2007; Qin et al., 2008; Niu et al., 2020). Wild D. huoshanense mainly grows as an epiphytic on the cliffs beside rivers and valleys at a slight altitude ranging between 200 and 1,200 m. In view of its strict requirements on the environment, D. huoshanense is currently endemic to the Da-bie Mountains, with Huoshan in the Anhui Province as the center of distribution (Zha et al., 2007d; Zheng et al., 2011; Wang et al., 2012; Wu et al., 2016). The cultivation industry of D. huoshanense is also in Huoshan, which has reached 8.0 million m2 with 350 tons of the annual production (including flower, fresh and dry materials of D. huoshanense).
Resource Conservation
Over the years, due to the unreasonable collection of D. huoshanense and the limitation of its own reproduction modes, the wild resources are on the verge of extinction. It has been listed in the National Key Protected Wild Plants as a “Class I protected species” (http://www.gov.cn/zhengce/zhengceku/2021-09/09/content_5636409.htm). In order to improve resource conservation and resolve the market demand, key measures must be taken. By mastering the distribution, reserves, and the native environment of D. huoshanense, the provenance protection base has been established and some local standards such as “Dendrobium huoshanense C. Z. Tang et S. J. Cheng” and “Technical regulation for the protection of the protospecies of Dendrobium huoshanense C. Z. Tang et S. J. Cheng” have been promulgated. In addition, the rapid breeding of D. huoshanense has been realized through modern biotechnology, which effectively alleviates the resource situation of D. huoshanense (Luo et al., 2003; Shi et al., 2003).
Scientific-based harvesting is also an important factor for resource protection and reserve. The suitable harvesting time for D. huoshanense is from November to June of the following year, and there are two ways for harvesting: “Cai Lao Liu Xin” and whole plant harvesting (Ren et al., 2014). “Cai Lao Liu Xin” is the method of harvesting certain stems with ages of more than 3 years old, while whole plants should be harvested from clumps of more than 20 months old. Furthermore, the cultivation modes of D. huoshanense have been systematically researched, including facility cultivation mode, under-forest cultivation mode, and simulative habitat cultivation mode, and provide powerful help for the conservation resources of D. huoshanense (Figure 2) (Yi et al., 2021). All in all, the resources of D. huoshanense have been effectively protected and rationally exploited.
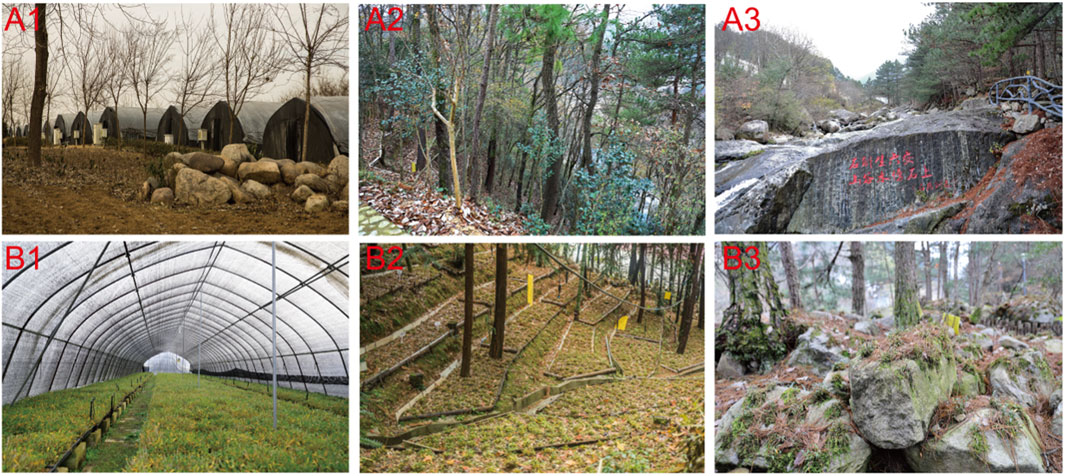
FIGURE 2. External (A1–A3) and internal (B1–B3) growth environments of D. huoshanense under the facility cultivation mode, under-forest cultivation mode, and simulative habitat cultivation mode, respectively.
Constituents From D. huoshanense
Modern biomedical researches show that D. huoshanense contains many active ingredients, such as polysaccharides, flavonoids, bibenzyls, phenols, sesquiterpenoids, etc. It is confirmed that these active ingredients are used singly or in combinations to make D. huoshanense of high medicinal value. The following sections will elaborate on the bioactivity present in between the ingredients and D. huoshanense.
Polysaccharides
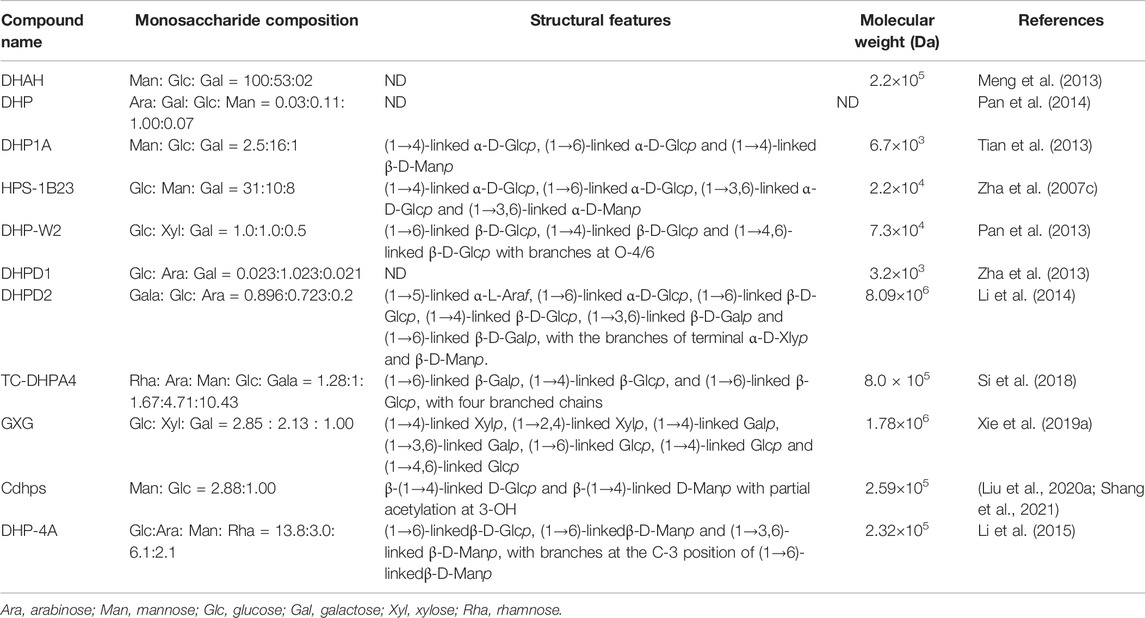
Polysaccharide is the main active ingredient of D. huoshanense, which is effective in anti-inflammation, anti-oxidant, anti-tumor, and immunoregulation, and is mainly composed of glucose, galactose, xylose, and arabinose and mannose, with the molecular weight distribution range of 3,200–8,090,000 Da (Zha et al., 2007b; Xie et al., 2018; Hao et al., 2019; Dai et al., 2020; Yi et al., 2021b; Zhang Y. et al., 2021; Wu et al., 2022). At present, a variety of D. huoshanense polysaccharides have been isolated, such as DHAH (Meng et al., 2013), DHP (Pan et al., 2014), DHP1A (Tian et al., 2013), HPS-1B23 (Zha et al., 2007c), DHP-W2 (Pan et al., 2013), DHPD1 (Zha et al., 2013), DHPD2 (Li et al., 2014), TC-DHPA4 (Si et al., 2018), GXG (Xie et al., 2019a), cDHPS (Liu B. et al., 2020; Shang et al., 2021), and DHP-4A (Li et al., 2015). Their structures are identified by GC-MS, HPLC-GPC, IR, NMR, HSQC, and HMBC, and the details are shown in Table 1.
Sesquiterpenoids
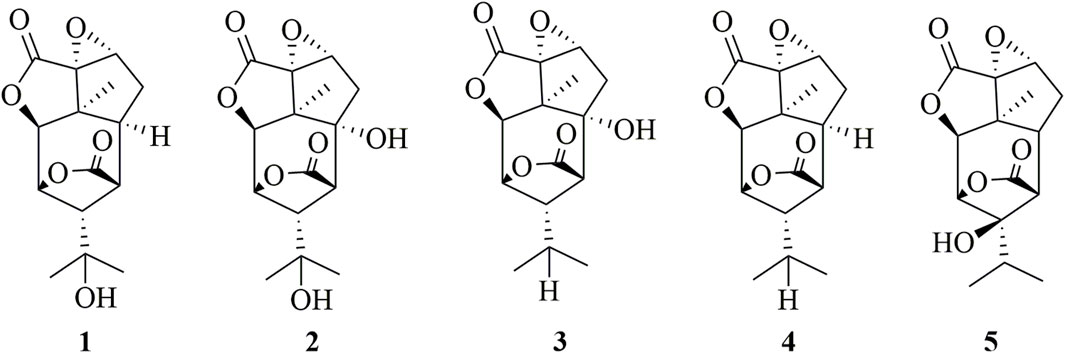
Sesquiterpenoids are essential chemical components, which are also the significant material basis for the pharmacological activity of Dendrobium (Fan et al., 2021). Sesquiterpenoids (1–5) have also been found in D. huoshanense and their structures have been elucidated through extensive spectroscopic analyses. The detailed structural information is shown in Figure 3 (Chen et al., 2022).
Flavonoids
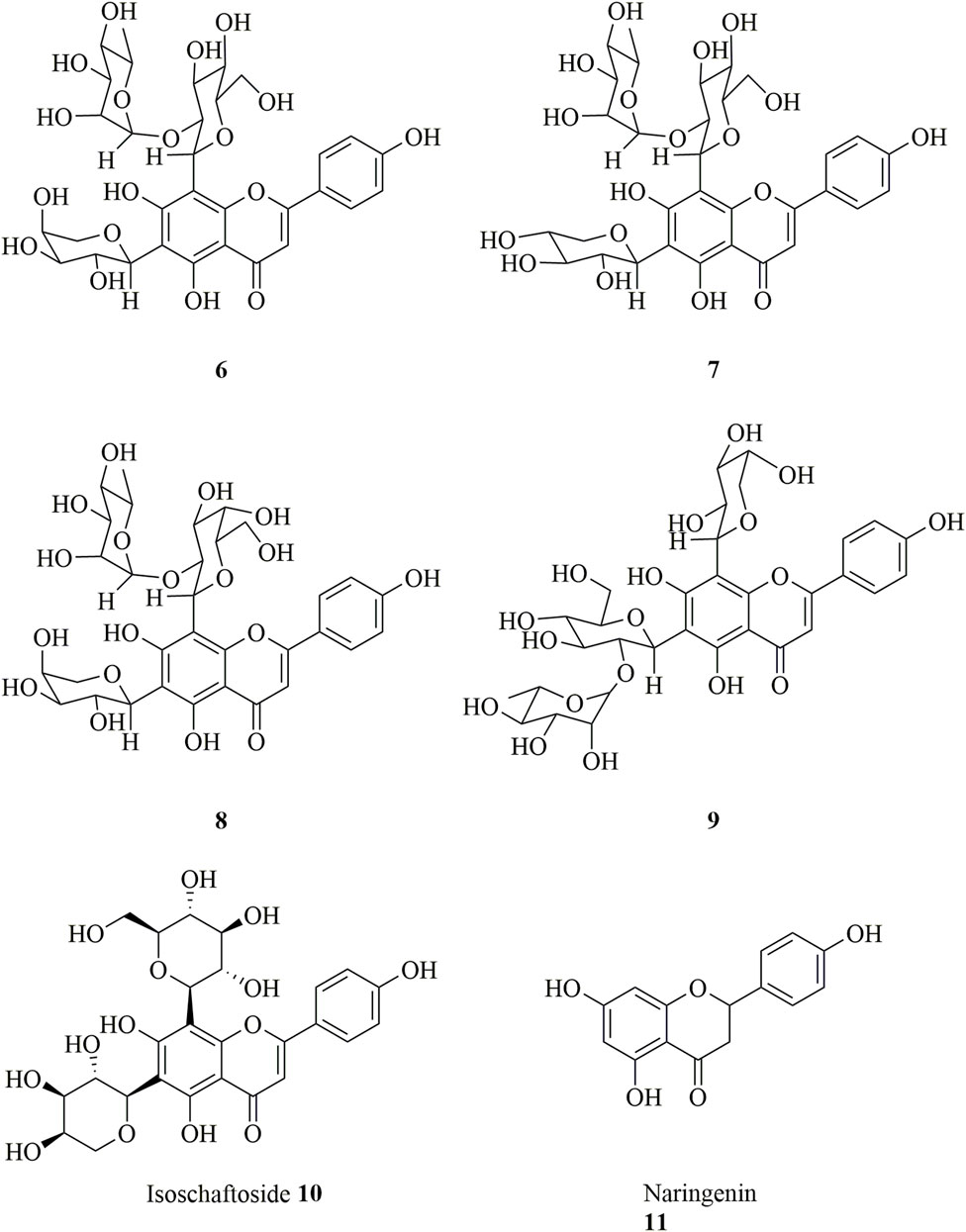
Flavonoids, which are the most widely distributed class of compounds in Chinese herbal medicine, are important chemical constituents of Dendrobium (Yuan et al., 2022). Flavonoids are also found in D. huoshanense, but only few flavonoids and flavonoid glycosides (6–11) have been isolated, and their structures are shown in Figure 4 (Chang et al., 2010; Zhao et al., 2021).
Bibenzyls
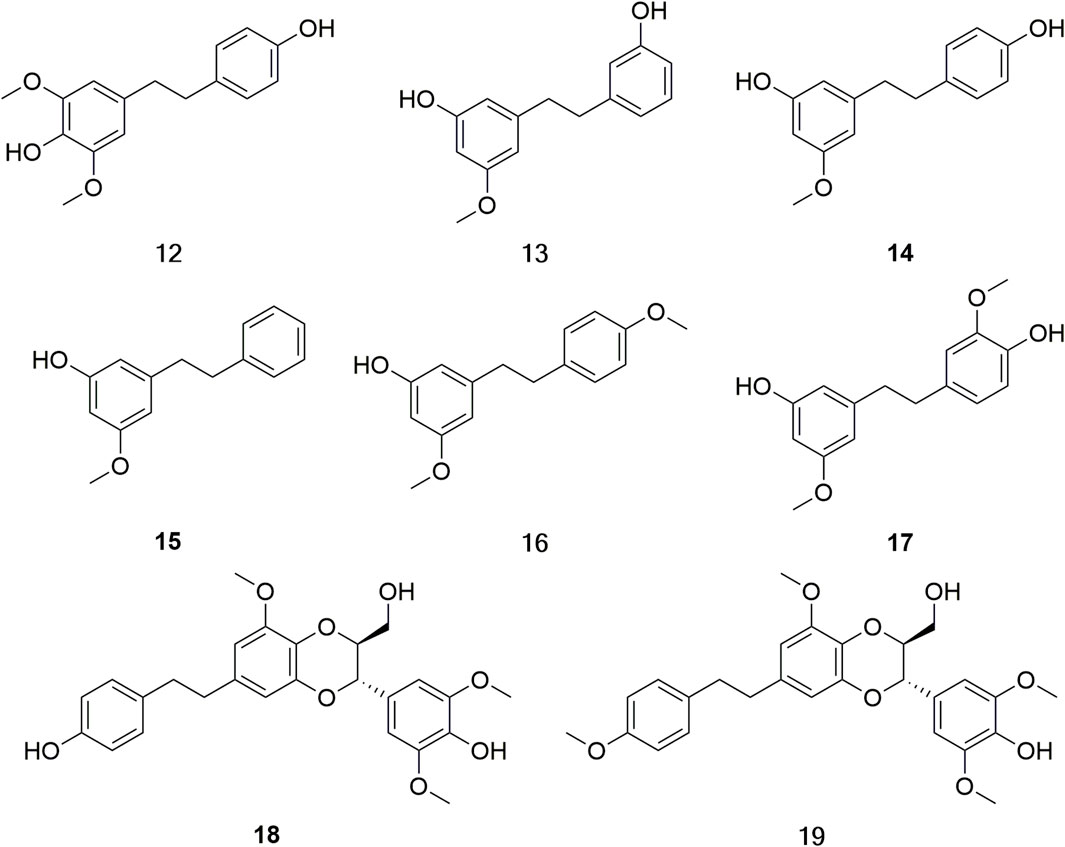
Bibenzyls, with a basic structural skeleton 1, 2-diphenylethane, are widely distributed in Dendrobium (Sun et al., 2021). Currently, few bibenzylates have been isolated from D. huoshanense. According to the literature statistics, eight bibenzylates (12–19) have been isolated and identified from D. huoshanense (Figure 5) (Li QM. et al., 2020; Zhao et al., 2021).
Phenols
Phenols are widely present in Dendrobium and have complex and diverse structures, but are not the main active components (Dong et al., 2020). D. huoshanense also contains a large number of phenols, and 20 phenols have been isolated. Their chemical structures are shown in Figure 6 (Chang et al., 2010; Zhao et al., 2021).
Other Compounds
In addition to the aforementioned chemical constituents, D. huoshanense also contains other types of chemical constituents (Chang et al., 2010; Zhao et al., 2021), including Malic acid, Dimethyl malate, N-phenylacetamide, Isopentyl butyrate, Shikimic acid, etc., and their chemical structures are shown in Figure 7.
Pharmacology
The active components in D. huoshanense are diverse and complex, and have many important effects. The pharmacological effects and possible mechanisms of action of D. huoshanense will be elaborated and reviewed below.
Antitumor Activity
As a special species of the Dendrobium genus, D. huoshanense has the ability to improve body function to prevent and treat tumor diseases. In the cell proliferation assay, the aqueous extract of D. huoshanense could effectively inhibit the growth of HeLa cells with a concentration range of 2–10 mg/ml (Zhang X. et al., 2021). Moreover, the inhibition rates of the aqueous extract of different plant ages on HeLa cells were varied. Among them, the aqueous extract of a 3 year old D. huoshanense possessed the highest anticancer activity, followed by a 2 year old, and the annuals were the least active.
In addition to the crude extract of D. huoshanense, polysaccharides and small molecule compounds isolated from D. huoshanense also have good antitumor activities. Luo et al. investigated the inhibitory effect of D. huoshanense polysaccharide cDHPS on gastric cancer and preliminarily explored its constitutive relationship. The results showed that cDHPS (at 0.2 mg/ml) could significantly inhibit tumor growth, induce tumor cell apoptosis, suppress tumor angiogenesis, and enhance a T cell immune response of murine forestomach carcinoma tumor-bearing mice (Liu B. et al., 2020; Liu et al., 2021). The structure–activity relationship investigation indicated that the molecular weight and the O-acetyl group of D. huoshanense polysaccharides greatly influenced the anti-gastric cancer activity.
Xu et al. isolated five picrotoxane-type sesquiterpenes (1–5) from D. huoshanense and examined their cytotoxicity activity on HL-60, MCF-7, SMMC-7721, and SW-480 human cancer cells (Chen et al., 2022). The results showed that compound 3 (Figure 3) showed significant effects on HL-60, MCF-7, SMMC-7721, and SW-480 cells with IC50s of 5.81, 6.49, 9.65, and 6.80 μM, respectively. Importantly, the cytotoxicity activity of compound 3 was comparable to 5-fluorouracil. To sum up, sesquiterpenes from D. huoshanense were worth further studying to find novel anticancer drugs.
Antioxidant Activity
Many traditional Chinese medicines have been reported to possess antioxidant activity (Muhammad et al., 2022). Compared with synthetic antioxidants, herbal components have the advantage of less toxicity, so they have attracted much attention. D. huoshanense, an important traditional Chinese medicine, has also shown a significant antioxidant activity. Luo et al. compared the antioxidant activity of crude polysaccharides of D. huoshanense with those crude polysaccharides of D. officinale, D. nobile, and D. chrysotoxum (Pan et al., 2014). The results showed that D. huoshanense polysaccharide had the strongest antioxidant activity and could significantly enhance the activities of antioxidative enzymes superoxidedismutase (SOD) and catalase (CAT) and increase the content of L-glutathione (GSH). In addition, refined D. huoshanense polysaccharides were isolated to investigate the antioxidant activity (Xu et al., 2019). It was found that a refined D. huoshanense polysaccharide could reduce malonaldehyde (MDA) levels, increase T-AOC levels, alleviate D-galactose-induced oxidative damage in mice, and exhibit a significant antioxidant activity.
Luo et al. have also proved that DHP1A obtained from D. huoshanense possesses a remarkable inhibition effect on the lipid peroxidation in vitro (Tian et al., 2013). At a concentration of 2.0 mg/ml, the inhibition rate of DHP1A reached 56.5%, which was higher than that of dextran (p < 0.01) and close to that of vitamin C. Furthermore, DHP1A could alleviate the hepatic oxidative stress caused by CCl4 in mice. These findings demonstrated that DHP1A exhibited a significant antioxidant activity and its mechanism of action might be anti-lipid peroxidation.
Immunomodulatory Activity
Immune function is the body’s resistance to diseases, improving the immunity of the body and enhancing the immune function is the key to prevent the occurrence of diseases and restore health. D. huoshanense has an excellent performance in immune regulation. For example, Wong et al. demonstrated that the administration of a crude polysaccharide of D. huoshanense (DH-PS) in mice not only induced the production of cytokines (IL-12 p40, IL-6, IL-10, and TNF-α) and chemokines (KC, RANTES, MCP-1, and MIP-1β), but also activated or amplified various immune cells, including NK cells/activated NK cells, NKT cells/activated NKT cells, regulatory T cells, B cells/activated B cells, CD4+ T cells/activated CD4+ T cells, and CD8+ T cells/activated CD8+ T cells (Lin et al., 2014). These results indicate that DH-PS regulated the immune function through stimulating cytokine secretions and promoting the expansions and/or activations of immune cells.
Luo et al. have investigated the immune activity of polysaccharides from D. huoshanense at different growth stages (Chen et al., 2012). The results showed that the polysaccharides obtained from different growth stages of D. huoshanense could promote the production of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) in mouse spleen cells, showing similar immune activities. Later, some authors confirmed that the water-soluble polysaccharides HPS-1B23, HPS1A, HPS1B, and HPS2B isolated from D. huoshanense exhibited immunomodulatory activities, through stimulating macrophages to secrete IFN-γ and TNF-α (Zha, Luo and Jiang 2007a; Zha et al., 2007c). Further study revealed that HPS2B23 activated macrophages by binding to toll-like receptor 4 and triggering nuclear factor-kappa B (NF-κB), mitogen-activated protein kinases (MAPKs), and phosphoinositide 3-kinase-Akt (PI3 K/Akt) signaling pathways (Xie et al., 2016). In addition, HPS-1B23 has also been shown to modulate the small intestinal immune system and the systemic immune system after oral administration (Zha et al., 2014). Luo et al. also fractionated the homogeneous polysaccharides DHP-4A and GXG from D. huoshanense, which showed significant immunomodulatory effects (Li et al., 2015; Xie et al., 2019b). In general, D. huoshanense polysaccharides have a significant activity in immunomodulation but the structure–activity relationship has not been systematically studied.
Protective Effects on Liver
The liver, which is the largest digestive and metabolic organ in the body, can metabolize endogenous or exogenous toxic substances and is highly susceptible to damage by various toxic substances. Therefore, it is especially necessary to improve the protection of the liver. In previous studies, our research team investigated the protective effect of D. huoshanense on liver injuries in mice. First, we compared the effect of D. huoshanense with the other four species of Dendrobium (D. officinale (Huoshan), D. officinale (Yunnan), D. moniliforme, and D. henanense) (Wang et al., 2017). It was found that the administration of freshly squeezed juices of Dendrobium at the dose of 1.25 and 7.5 g‧kg−1 for 2 weeks had a protective effect on CCl4-induced acute liver injury, but the effect was different, among which, D. huoshanense had the significant activity compared with others. Thereafter, the hepatoprotective effects of D. huoshanense with different cultivation patterns and growth years were also investigated. The results showed that D. huoshanense cultivated in under-forest cultivation planting had better protective effects on acute liver injuries induced by carbon tetrachloride, acetaminophen, and cyclophosphamide in mice than D. huoshanense cultivated in greenhouse (Li Z. Q. et al., 2020). In addition, D. huoshanense plants of different ages could reduce the acute liver injury induced by acetaminophen in mice, where the 2-year old D. huoshanense had the best efficacy (Li et al., 2021).
Luo et al. investigated that the protective effects of crude polysaccharides from D. huoshanense on CCl4-induced acute liver injuries in mice (Huang et al., 2013). The results showed that the crude polysaccharides with different doses (200, 100, and 50 mg‧kg−1) could reduce the activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the serum and the level of hepatic MDA, enhance the activity of hepatic SOD, inhibit the expression of TNF-α in hepatocytes, and alleviate liver tissue damage induced by CCl4, indicating that the D. huoshanense polysaccharide could protect mice from CCl4-induced acute liver injury by scavenging free radicals and inhibiting lipid peroxidation and the expression of TNF-α. Furthermore, Luo et al. further found that refined polysaccharides, galactoglucomannan form D. huoshanense, dose-dependently inhibited the activity of ALT, AST, and lactate dehydrogenase (LDH). At a dose of 200 mg‧kg−1‧day−1, galactoglucomannan significantly decreased the selenite-increased activities of ALT, AST, and LDH, and reduced the MDA levels and H2O2 to 59.5% and 34.6%, respectively (Pan et al., 2012). In addition, galactoglucomannan could reverse the selenium-induced decrease of the concentration of GSH in the liver and inhibit selenium-induced transforming growth factor β1 (TGF-β1) expression, showing significant potential to prevent liver injury and fibrosis. Interestingly, the daily supplementation of galactoglucomannan prevents ethanol-induced liver injury. The results of the proteomic analysis and metabolomic analysis indicated that galactoglucomannan might correct the perturbed metabolism pathways by ethanol exposure to protect the liver from ethanol-induced injuries (Wang et al., 2014; Wang et al., 2015).
DHP1A, a polysaccharide isolated from D. huoshanense, could also reduce the levels of ALT, AST, LDH, and 8-hydroxy-20-deoxyguanosine (8-OhdG) in the serum, exhibiting a strong hepatoprotective activity. Meanwhile, DHP1A also down-regulated the expression of TNF-α, interleukin-1β (IL-1β), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2), CD68, and phosphorylated IκBα (p-IκBα), demonstrating that the hepatoprotective activity of DHP1A was related with its anti-inflammatory activity (Tian et al., 2015).
Anticataract Activity
D. huoshanense is an important traditional Chinese medicine with a protective effect on the eyes and has long been used to prepare eye protection preparations, such as Shihu Yeguang pills (Editorial Board of China Pharmacopoeia Committee 2020; Horng et al., 2021). Therefore, D. huoshanense is of interest for the treatment of eye diseases. Luo et al. found that crude polysaccharides from D. huoshanense could inhibit the oxidation pathway by the down-regulation of inducible nitric oxide synthase (iNOS) gene expression and advanced glycation end product (AGEs) formations to suppress diabetic cataract, which is an important cause of blindness worldwide (Luo et al., 2008). Especially, at 200 mg.kg−1.day−1, the crude polysaccharide significantly reduced the level of nitric oxide (NO) (70.12 ± 1.2 μmol mg−1 protein) and the activity of iNOS (U mg−1 protein), and the fluorescence intensity of AGEs was remarkably inhibited, which showed that the D. huoshanense polysaccharide has potential for the prevention and cure of diabetic cataract. Another study showed that the D. huoshanense polysaccharide significantly improved the level of GSH, decreased the content of MDA, and increased the activities of glutathione peroxidase (GSH-PX), glutathione reductase (GR), glutathione S-transferase (GST), SOD, and CAT in the lens of diabetic rats, indicating that the D. huoshanense polysaccharide may also prevent the development of diabetic cataracts by ameliorating oxidative stress (Li et al., 2012). In order to investigate the core structure of the D. huoshanense polysaccharide against cataracts, the refined polysaccharide DHPD1 was enzymatically hydrolyzed with pectinase to obtain different fragments of oligosaccharides and their anti-cataract activities were evaluated with the apoptosis model of human lens epithelial cells induced by H2O2 (Zha et al., 2017). The results showed that DHPD1-24, composed of (1 → 5)-linked-Araf, (1 → 3,6)-linked-Manp, 1-linked-Glcp, (1 → 4)-linked-Glcp, (1 → 6)-linked-Glcp, (1 → 4,6)-linked-Glcp, (1 → 6)-linked-Galp, and 1-linked-Xylp in a molar ratio of 1.06:1.53:2.11:2.04:0.93:0.91:0.36:1.01, was the core structure of the D. huoshanense polysaccharide against cataract, and it could inhibit H2O2-induced human lens epithelial cell apoptosis through suppressing the MAPKs signaling pathway, which provided the foundation for further unraveling the structure–activity relationship of the D. huoshanense polysaccharide against cataract.
Antiglycation Activity
Glycation is a non-enzymatic reaction that inserts sugar chains into macromolecules such as proteins, DNA, and lipids, to form stable covalent structures. These bound products are involved in developing diabetes, metabolic syndrome, obesity, hypertension, atherosclerosis, and Alzheimer’s disease to result in body damage. Studies have shown that D. huoshanense polysaccharides have significant anti-glycation effects. For example, a polysaccharide, DHP-W2, isolated from D. huoshanense achieved 23% inhibition of protein glycation after 3 weeks of reaction at a concentration of 0.5 mg/ml, which was similar to that of vitamin C at 0.3 mg/ml (Inhibition of protein glycosylation, 28%) (Pan et al., 2013). Luo et al. isolated DHPD1 from D. huoshanense and prepared DHPD1 derivatives under enzymatic degradation conditions to investigate the inhibitory effects of molecular weight alteration of the D. huoshanense polysaccharide on protein glycation (Zha et al., 2013). The results indicated that the anti-glycation activity of the D. huoshanense polysaccharide reduced with the decrease of the molecular weight.
Furthermore, the sulfated DHPD1 with a degree of substitution of 1.473 was studied, displaying that the inhibition activity of sulfated DHPD1 on protein glycation at 1.0 mg/ml was 58.5%, which was 16.2% and 52.5% higher than the same dose of aminoguanidine and DHPD1, respectively (Qian et al., 2014). Luo et al. also extracted DHPD2 from D. huoshanense by fractionation on the DEAE-Cellulose column and dialysis (molecular weight cut off: 8,000 Da), and simultaneously prepared sulfated DHPD2 derivatives by chlorosulfonic acid-pyridine (CSA-Pyr) method (Li et al., 2014). The results of the anti-glycation assay showed that the activity of DHPD2 was enhanced after sulfation and was more favorable at C-2 and C-6 sulfations of the glycosyl residues.
Others
In addition to the bioactivities described previously, it was found that D. huoshanense also had important effects on anti-aging (Gu F. L. et al., 2021), anti-rheumatoid arthritis (Shang et al., 2021), anti-atherosclerosis (Fan et al., 2020), anti-inflammation (Ge et al., 2018; Gu FL. et al., 2021), hypoglycemic activity (Pan et al., 2014; Wang et al., 2019), regulation of intestinal flora (Xie et al., 2019a), and constipation caused by the spleen’s yin deficiency (Gan et al., 2019). However, its specific mechanism of action is still to be further explored. All in all, D. huoshanense is worthy for an in-depth study.
Conclusion and Future Prospects
As the best of Dendrobium, D. huoshanense has attracted increasing attention. Currently, the materia medica research and resource aspects of D. huoshanense have been evident. Furthermore, the D. huoshanense industry is vast, with an 8.0 million m2 promoted planting area and 350 tons of the annual production (including flower, fresh and dry materials of D. huoshanense) in Lu’an of the Anhui province. Therefore, this review systematically summarized the recent research on the chemical composition and pharmacological effects of D. huoshanense to provide references for further research on D. huoshanense, as well as help in a more in-depth understanding, development, and utilization of D. huoshanense. Importantly, this review reveals that many aspects of D. huoshanense warrant further investigation. The detailed discussion is as follows.
(i) Although efficient methods for the conservation resources of D. huoshanense have been obtained, further studies aiming at improving bioactive secondary metabolites in cultivated D. huoshanense should be actively performed, which are relevant to its pharmacological activity and beneficial for its commercialization.
(ii) Via different isolation methods, D. huoshanense polysaccharides with different structures can be obtained so that D. huoshanense polysaccharides are worthy of continued research.
(iii) Compared with other natural polysaccharides, the pharmacological effects of D. huoshanense polysaccharides are still under-researched, which need to be comprehensively studied to improve the medicinal value of D. huoshanense.
(iv) There are many studies on immunomodulation of the D. huoshanense polysaccharide, which shows desirable results, but its druggability has not been studied in depth.
(v) Compared with D. officinale, there are fewer studies on the small molecule chemical compositions of D. huoshanense, and there remains significant room for further research.
(vi) Many studies on the pharmacological activity and chemical compositions of D. huoshanense have not clearly marked the origin, including the cultivation mode and growth years, which is unfavorable to the systematic study of D. huoshanense and should be paid more attention.
These findings provide guidance for further research on D. huoshanense and encourage researchers to develop new functions and utilization.
Author Contributions
LG collated the literature and wrote the manuscript. FW and TH helped to classify the literature and prepare the table. CG and TX contributed to performing the arrangement of pictures and checking the chemical structures. BH and DL supervised and reviewed the manuscript. All authors have approved the final version.
Funding
This work was supported by the China Agriculture Research System (CARS-21), key projects of excellent young talents support program of Anhui universities (gxyqZD2020040), and Natural Science Foundation of Higher Education Institutions of Anhui Province (KJ2021A0951). We also acknowledged the funding sponsored by the Administration of Traditional Chinese Medicine of Anhui Province (2020ccyb09) and West Anhui University (WGKQ2021022, WXZR202030, WGKQ202001012).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Chang, C. C., Ku, A. F., Tseng, Y. Y., Yang, W. B., Fang, J. M., and Wong, C. H. (2010). 6,8-Di-C-glycosyl Flavonoids from Dendrobium Huoshanense. J. Nat. Prod. 73 (2), 229–232. doi:10.1021/np900252f
Chen, J., Hu, K.-X., Hou, X.-Q., and Guo, S.-X. (2011). Endophytic Fungi Assemblages from 10 Dendrobium Medicinal Plants (Orchidaceae). World J. Microbiol. Biotechnol. 27 (5), 1009–1016. doi:10.1007/s11274-010-0544-y
Chen, C., Wu, H. Q., Zha, X. Q., Pan, L. H., and Luo, J. P. (2012). Comparison on Physicochemical Properties and Immun Activities of Polysaccharides from Cultures at Different Development Stages of Dendrobium Huoshanense. Chin. Med. Mat. 35 (8), 1195–1199. doi:10.13863/j.issn1001-4454.2012.08.006
Chen, S. T., Dai, J., Jiang, X. P., Song, X. W., Chen, C. W., Chen, N. F., et al. (2019). Diversity and Difference of Endophytes in Dendrobium Huoshanense with Different Growth Years. Chin. J. Chin. Mat. Med. 44 (6), 1145–1150. doi:10.19540/j.cnki.cjcmm.2019.0022
Chen, S., Dai, J., Song, X., Jiang, X., Zhao, Q., Sun, C., et al. (2020). Endophytic Microbiota Comparison of Dendrobium Huoshanense Root and Stem in Different Growth Years. Planta Med. 86 (13/14), 967–975. doi:10.1055/a-1046-1022
Chen, W., Lu, J., Zhang, J., Wu, J., Yu, L., Qin, L., et al. (2021). Traditional uses, phytochemistry, pharmacology, and quality control of Dendrobium officinale Kimura et. Migo. Front. Pharmacol. 12, 726528. doi:10.3389/fphar.2021.726528
Xu, F., Chen, X., Hu, J., Zhao, H., Peng, D., Wu, D., et al. (2022). Cytotoxic Picrotoxane-type Sesquiterpenoid Lactones from Dendrobium Huoshanense. Rec. Nat. Prod. 16 (2), 144–149. doi:10.25135/rnp.260.21.04.2048
Chiang, C.-H., Yu, T.-A., Lo, S.-F., Kuo, C.-L., Peng, W.-H., and Tsay, H.-S. (2012). Molecular Authentication of Dendrobium Species by Multiplex Polymerase Chain Reaction and Amplification Refractory Mutation System Analysis. J. Amer. Soc. Hort. Sci. 137 (6), 438–444. doi:10.21273/JASHS.137.6.438
Dai, J., Han, X.-B., Chen, N.-F., Chen, C.-W., Chen, N.-D., Song, X.-W., et al. (2020). Effects of Different Treatments on Genes Related to Polysaccharide Content in Dendrobium Huoshanense. Braz. J. Bot. 43 (3), 531–539. doi:10.1007/s40415-020-00628-1
Dai, J., Wei, P. P., and Wang, Y. J. (2021). Highly Efficient N-Doped Carbon Quantum Dots for Detection of Hg2+ and Cd2+ Ions in Dendrobium Huoshanense. Int. J. Electrochem. Sci. 16 (7), 210716. doi:10.20964/2021.07.22
Deng, Y., Chen, L. X., Han, B. X., Wu, D. T., Cheong, K. L., Chen, N. F., et al. (2016). Qualitative and Quantitative Analysis of Specific Polysaccharides in Dendrobium Huoshanense by Using Saccharide Mapping and Chromatographic Methods. J. Pharm. Biomed. Anal. 129, 163–171. doi:10.1016/j.jpba.2016.06.051
Dong, X., Yang, J., Zhen, X. T., Chen, Y., Zheng, H., and Cao, J. (2020). Micellar Extraction with Vesicle Coated Multi-Walled Carbon Nanotubes to Assist the Dispersive Micro-solid-phase Extraction of Natural Phenols in Dendrobium. J. Pharm. Biomed. Anal. 188, 113461. doi:10.1016/j.jpba.2020.113461
Editorial Board of Chinese Pharmacopoeia Committee (2020). Chinese Pharmacopoeia. Beijing, China: China Medical Science Press, 94.
Fan, X., Han, J., Zhu, L., Chen, Z., Li, J., Gu, Y., et al. (2020). Protective activities of Dendrobium huoshanense C. Z. Tang et S. J. Cheng polysaccharide against high-cholesterol diet-induced atherosclerosis in zebrafish. Oxid. Med. Cell. Longev. 2020, 8365056. doi:10.1155/2020/8365056
Fan, W. W., Yang, D., Cheng, Z. Q., Xu, F. Q., Dong, F. W., Wei, X. Y., et al. (2021). Ten Picrotoxane-type Sesquiterpenoids from the Stems of Dendrobium Wardianum Warner. Phytochemistry 190, 112858. doi:10.1016/j.phytochem.2021.112858
Gan, J. H., Huang, Y. F., Peng, D. Y., Yu, N. J., Chen, W. D., Luo, J. P., et al. (2019). Therapeutic Effect and Mechanism of Three Kinds of Dendrobium on Constipation in Rats with Spleen Yin Deficiency. Chin. J. Chin. Mat. Med. 44 (12), 2600–2606. doi:10.19540/j.cnki.cjcmm.20190128.002
Ge, J. C., Zha, X. Q., Nie, C. Y., Yu, N. J., Li, Q. M., Peng, D. Y., et al. (2018). Polysaccharides from Dendrobium Huoshanense Stems Alleviates Lung Inflammation in Cigarette Smoke-Induced Mice. Carbohydr. Polym. 189, 289–295. doi:10.1016/j.carbpol.2018.02.054
Gu, F. L., He, X. M., and Huang, R. S. (2021a). Skin Antiaging Attributes of the Dendrobium Huoshanense Polysaccharides. Curr. Top. Nutraceut. R. 19 (2), 176–180. doi:10.37290/ctnr2641-452X.19:176–180
Gu, F. L., Huang, R. S., He, X. M., Chen, N. F., Han, B. X., and Deng, H. (2021b). Dendrobium Huoshanense Polysaccharides Prevent Inflammatory Response of Ulcerative Colitis Rat through Inhibiting the NF-Κb Signaling Pathway. Chem. Biodivers. 18 (7), e2100130. doi:10.1002/cbdv.202100130
Han, B., Jing, Y., Dai, J., Zheng, T., Gu, F., Zhao, Q., et al. (2020). A Chromosome-Level Genome Assembly of Dendrobium Huoshanense Using Long Reads and Hi-C Data. Genome Biol. Evol. 12 (12), 2486–2490. doi:10.1093/gbe/evaa215
Hao, J. W., Chen, N. D., Chen, C. W., Zhu, F. C., Qiao, D. L., Zang, Y. J., et al. (2018). Rapid Quantification of Polysaccharide and the Main Onosaccharides in Dendrobium Huoshanense by Near-Infrared Attenuated Total Reflectance Spectroscopy. J. Pharm. Biomed. Anal. 151, 331–338. doi:10.1016/j.jpba.2018.01.027
Hao, J.-W., Chen, N.-D., Fu, X.-C., and Zhang, J. (2019). Predicting the Contents of Polysaccharides and its Monosugars in Dendrobium Huoshanense by Partial Least Squares Regression Model Using Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy. Spectrosc. Lett. 52 (5), 297–305. doi:10.1080/00387010.2019.1630445
Hao, J. W., Chen, Y., Chen, N. D., and Qin, C. F. (2021a). Rapid Detection of Adulteration in Dendrobium Huoshanense Using NIR Spectroscopy Coupled with Chemometric Methods. J. AOAC Int. 104 (3), 854–859. doi:10.1093/jaoacint/qsaa138
Hao, J.-W., Liu, X.-Q., Zang, Y.-J., Chen, N.-D., Zhu, A.-l., Li, L.-F., et al. (2021b). Simultaneous Determination of 16 Important Biologically Active Phytohormones in Dendrobium Huoshanese by Pressurized Capillary Electrochromatography. J. Chromatogr. B 1171, 122612. doi:10.1016/j.jchromb.2021.122612
Hao, J. W., Zhu, A. L., Chen, N. D., Liu, X. Q., Li, Q., Xu, H. M., et al. (2021c). Simultaneous Analysis of 20 Free Amino Acids by a Single Marker Combined with an HPLC Fingerprint Evaluation of Dendrobium Huoshanense. J. Food Sci. 86 (11), 4828–4839. doi:10.1111/1750-3841.15931
Horng, C.-T., Ma, J.-W., and Shieh, P.-C. (2021). Improvement of Presbyopia Using a Mixture of Traditional Chinese Herbal Medicines, Including Cassiae Semen, Wolfberry, and Dendrobium Huoshanense. Evidence-Based Complementary Altern. Med. 2021, 9902211. doi:10.1155/2021/9902211
Hsieh, Y. S., Chien, C., Liao, S. K., Liao, S. F., Hung, W. T., Yang, W. B., et al. (2008). Structure and Bioactivity of the Polysaccharides in Medicinal Plant Dendrobium Huoshanense. Bioorg Med. Chem. 16 (11), 6054–6068. doi:10.1016/j.bmc.2008.04.042
Hu, L., Zhou, C., Huang, Y. C., Wang, Y., Wei, G., Liang, Z., et al. (2020). HPLC Coupled with Electrospray Ionization Multistage MS/MS and TLC Analysis of Flavones-C-Glycosides and Bibenzyl of Dendrobium Hercoglossum. J. Sep. Sci. 43 (20), 3885–3901. doi:10.1002/jssc.202000647
Huang, S., Zha, X. Q., Luo, J. P., and Yu, L. (2007). Studies on the Extraction of Active Polysaccharide from Dendrobium Huoshanense with Box-Behnken Method. Chin. Med. Mat. 30 (5), 591–594. doi:10.3321/j.issn:1001-4454
Huang, J., Li, S. L., Zhao, H. W., Pan, L. H., Sun, H. Q., and Luo, J. P. (2013). Protective Effects of Polysaccharides from Dendrobium Huoshanense on CCl4-Induced Acute Liver Injury in Mice. Chin. J. Chin. Mat. Med. 38 (4), 528–532.doi:10.4268/cjcmm20130413
Lee, P.-L., and Chen, J.-T. (2014). Plant Regeneration via Callus Culture and Subsequent In Vitro Flowering of Dendrobium Huoshanense. Acta Physiol. Plant. 36 (10), 2619–2625. doi:10.1007/s11738-014-1632-7
Li, X. F., Deng, Y. Y., Pan, L. H., Huang, J., and Luo, J. P. (2012). 93. Antioxidant Effect of Polysaccharide from Dendrobium Huoshanense on Lens Tissue of Diabetic Cataract Rats. Chin. Tradit. Pat. Med. 34 (03), 418–421.
Li, X. L., Xiao, J. J., Zha, X. Q., Pan, L. H., Asghar, M. N., and Luo, J. P. (2014). Structural Identification and Sulfated Modification of an Antiglycation Dendrobium Huoshanense Polysaccharide. Carbohydr. Polym. 106, 247–254. doi:10.1016/j.carbpol.2014.02.029
Li, F., Cui, S. H., Zha, X. Q., Bansal, V., Jiang, Y. L., Asghar, M. N., et al. (2015). Structure and Bioactivity of a Polysaccharide Extracted from Protocorm-like Bodies of Dendrobium Huoshanense. Int. J. Biol. Macromol. 72, 664–672. doi:10.1016/j.ijbiomac.2014.08.026
Li, Q. M., Jiang, H., Zha, X. Q., Wu, D. L., Pan, L. H., Duan, J., et al. (2020a). Anti-inflammatory Bibenzyls from the Stems of Dendrobium Huoshanense via Bioassay Guided Isolation. Nat. Prod. Res. 34 (4), 563–566. doi:10.1080/14786419.2018.1489394
Li, Z. Q., Zhou, H. Q., Ouyang, Z., Dai, J., Yue, Q., Wei, Y., et al. (2020b). Protective Effects of Differently Cultivated Dendrobium Huoshanense on Acute Liver Injury in Mice. Chin. Tradit. Pat. Med. 42 (05), 1155–1162.
Li, Z. Q., Zhou, H. Q., Ouyang, Z., Dai, J., Yue, Q., Wei, Y., et al. (2021). Comparison of Active Ingredients and Protective Effects of Dendrobium Huoshanense of Different Growth Years on Acute Liver Injury. Chin. J. Chin. Mat. Med. 46 (2), 298–305. doi:10.19540/j.cnki.cjcmm.20201023.102
Li, M., Trapika, I. G. S. C., Tang, S. Y. S., Cho, J.-L., Qi, Y., Li, C. G., et al. (2022). Mechanisms and Active Compounds Polysaccharides and Bibenzyls of Medicinal Dendrobiums for Diabetes Management. Front. Nutr. 8, 811870. doi:10.3389/fnut.2021.811870
Lin, J., Chang, Y. J., Yang, W. B., Yu, A. L., and Wong, C. H. (2014). The Multifaceted Effects of Polysaccharides Isolated from Dendrobium Huoshanense on Immune Functions with the Induction of Interleukin-1 Receptor Antagonist (IL-1ra) in Monocytes. Plos One 9 (4), e94040. doi:10.1371/journal.pone.0094040
Liu, S. Q., Li, X. J., Yu, Q. B., Xie, H., and Zhou, G. Y. (2007). Analysis on Genetic Stability in Different Development Stages of Dendrobium Huoshanense by RAPD. Chin. J. Chin. Mat. Med. 32 (10), 902–905.
Liu, L., Han, R., Yu, N., Zhang, W., Xing, L., Xie, D., et al. (2018). A Method for Extracting High-Quality Total RNA from Plant Rich in Polysaccharides and Polyphenols Using Dendrobium Huoshanense. Plos One 13 (5), e0196592. doi:10.1371/journal.pone.0196592
Liu, B., Shang, Z.-Z., Li, Q.-M., Zha, X.-Q., Wu, D.-L., Yu, N.-J., et al. (2020a). Structural Features and Anti-gastric Cancer Activity of Polysaccharides from Stem, Root, Leaf and Flower of Cultivated Dendrobium Huoshanense. Int. J. Biol. Macromol. 143, 651–664. doi:10.1016/j.ijbiomac.2019.12.041
Liu, J. L., Yu, N. J., Xing, L. H., Wang, R., Xu, J., Peng, D. Y., et al. (2020b). Simultaneous Determination and Analysis of Amino Acids and Nucleosides in Dendrobium by UHPLC-QTRAP-MS/MS. Chin. J. Chin. Mat. Med. 45 (16), 3890–3899. doi:10.19540/j.cnki.cjcmm.20200513.201
Liu, B., Li, Q. M., Shang, Z. Z., Zha, X. Q., Pan, L. H., and Luo, J. P. (2021). Anti-gastric Cancer Activity of Cultivated Dendrobium Huoshanense Stem Polysaccharide in Tumor-Bearing Mice: Effects of Molecular Weight and O-Acetyl Group. Int. J. Biol. Macromol. 192, 590–599. doi:10.1016/j.ijbiomac.2021.10.016
Liu, S. J. (1996). Textual Research of Dendrobium Huoshanense. Chin. Med. Mat. 19 (7), 373–375. doi:10.13863/j.issn1001-4454.1996.07.026
Luo, J. P., Zha, X. Q., and Jiang, S. T. (2003). Suspension Culture of Protocorn-like Bodies from the Endangered Medicinal Plant Dendrobium Huoshanense. Chin. J. Chin. Mat. Med. 28 (7), 611–614.
Luo, J.-P., Deng, Y.-Y., and Zha, X.-Q. (2008). Mechanism of Polysaccharides from Dendrobium Huoshanense on Streptozotocin-Induced Diabetic Cataract. Pharm. Biol. 46 (4), 243–249. doi:10.1080/13880200701739397
Meng, L. Z., Lv, G. P., Hu, D. J., Cheong, K. L., Xie, J., Zhao, J., et al. (2013). Effects of Polysaccharides from Different Species of Dendrobium (Shihu) on Macrophage Function. Molecules 18 (5), 5779–5791. doi:10.3390/molecules18055779
Muhammad, F., Liu, Y., Zhou, Y., Yang, H., and Li, H. (2022). Antioxidative Role of Traditional Chinese Medicine in Parkinson's Disease. J. Ethnopharmacol. 285, 114821. doi:10.1016/j.jep.2021.114821
Niu, Z., Pan, J., Xue, Q., Zhu, S., Liu, W., and Ding, X. (2018). Plastome-wide Comparison Reveals New SNV Resources for the Authentication of Dendrobium Huoshanense and its Corresponding Medicinal Slice (Huoshan Fengdou). Acta Pharm. Sin. B 8 (3), 466–477. doi:10.1016/j.apsb.2017.12.004
Niu, Z., Hou, Z., Wang, M., Ye, M., Zhang, B., Xue, Q., et al. (2020). A Comparative Plastomics Approach Reveals Available Molecular Markers for the Phylogeographic Study of Dendrobium Huoshanense, an Endangered Orchid with Extremely Small Populations. Ecol. Evol. 10 (12), 5332–5342. doi:10.1002/ece3.6277
Ohara, K., Lin, C. C., Yang, P. J., Hung, W. T., Yang, W. B., Cheng, T. J., et al. (2013). Synthesis and Bioactivity of β-(1→4)-linked Oligomannoses and Partially Acetylated Derivatives. J. Org. Chem. 78 (13), 6390–6411. doi:10.1021/jo4005266
Pan, L. H., Lu, J., Luo, J. P., Zha, X. Q., and Wang, J. H. (2012). Preventive Effect of a Galactoglucomannan (GGM) from Dendrobium Huoshanense on Selenium-Induced Liver Injury and Fibrosis in Rats. Exp. Toxicol. Pathol. 64 (7-8), 899–904. doi:10.1016/j.etp.2011.04.001
Pan, L.-H., Feng, B.-J., Wang, J.-H., Zha, X.-Q., and Luo, J.-P. (2013). Structural Characterization and Anti-glycation Activity In Vitro of a Water-Soluble Polysaccharide from Dendrobium Huoshanense. J. Food Biochem. 37 (3), 313–321. doi:10.1111/j.1745-4514.2011.00633.x
Pan, L. H., Li, X. F., Wang, M. N., Zha, X. Q., Yang, X. F., Liu, Z. J., et al. (2014). Comparison of Hypoglycemic and Antioxidative Effects of Polysaccharides from Four Different Dendrobium Species. Int. J. Biol. Macromol. 64, 420–427. doi:10.1016/j.ijbiomac.2013.12.024
Qian, X. P., Zha, X. Q., Xiao, J. J., Zhang, H. L., Pan, L. H., and Luo, J. P. (2014). Sulfated Modification Can Enhance Antiglycation Abilities of Polysaccharides from Dendrobium Huoshanense. Carbohydr. Polym. 101, 982–989. doi:10.1016/j.carbpol.2013.10.035
Qin, Z., Zhao, T., Qiu, J., Lin, Y., and Cai, Y. (2008). Germination and Propagartors of Artificial Seeds of Dendrobium Huoshanense. Chin. J. Biotechnol. 24 (5), 803–809.
Ren, J., Wang, J., Ding, Z. C., Hu, J. R., and Yang, Z. Q. (2014). Study on Good Agricultural Practice (GAP) for Dendrobium Huoshanense. J. Agric. 4 (6), 72–76.
Shang, Z.-Z., Qin, D.-Y., Li, Q.-M., Zha, X.-Q., Pan, L.-H., Peng, D.-Y., et al. (2021). Dendrobium Huoshanense Stem Polysaccharide Ameliorates Rheumatoid Arthritis in Mice via Inhibition of Inflammatory Signaling Pathways. Carbohydr. Polym. 258, 117657. doi:10.1016/j.carbpol.2021.117657
Shi, W., Luo, J. P., and Huang, X. Y. (2003). Effect of Growth Regulators on Rooting of Regenerated Shoots from Dendrobium Huoshanense Protocorm-like Bodies. Chin. Tradit. Herb. Drugs 34 (10), 955–957.
Si, H. Y., Chen, N. F., Chen, N. D., Huang, C., Li, J., and Wang, H. (2018). Structural characterisation of a water-soluble polysaccharide from tissue-cultured Dendrobium huoshanense C.Z. Tang et S.J. Cheng. Nat. Prod. Res. 32 (3), 252–260. doi:10.1080/14786419.2017.1350670
Sun, J., Liu, J., Liu, Y., Chen, R., Li, Y., Cen, S., et al. (2021). Dengratiols A-D, Four New Bibenzyl Derivatives from Dendrobium Gratiossimum. Fitoterapia 152, 104926. doi:10.1016/j.fitote.2021.104926
Tang, Z. Z., and Cheng, S. J. (1984). A Study on the Raw Plants for the Chinese Traditional Medicine “HUOSHAN SHI-HU”. Bull. Bot. Res. 4 (3), 141–146.
Tian, C. C., Zha, X. Q., Pan, L. H., and Luo, J. P. (2013). Structural Characterization and Antioxidant Activity of a Low-Molecular Polysaccharide from Dendrobium Huoshanense. Fitoterapia 91, 247–255. doi:10.1016/j.fitote.2013.09.018
Tian, C. C., Zha, X. Q., and Luo, J. P. (2015). A Polysaccharide from Dendrobium Huoshanense Prevents Hepatic Inflammatory Response Caused by Carbon Tetrachloride. Biotechnol. Biotechnol. Equip. 29 (1), 132–138. doi:10.1080/13102818.2014.987514
Wang, D. Q., and Peng, H. S. (2004). Confusion of Name and Origin of Dendrobium Huoshanense. Chin. J. Chin. Mat. Med. 29 (12), 80–81.
Wang, Y., Luo, J. P., Wei, Z. J., and Zhang, J. C. (2009). Molecular Cloning and Expression Analysis of a Cytokinin Oxidase (DhCKX) Gene in Dendrobium Huoshanense. Mol. Biol. Rep. 36 (6), 1331–1338. doi:10.1007/s11033-008-9316-2
Wang, H., Chen, N. F., Zheng, J. Y., Wang, W. C., Pei, Y. Y., and Zhu, G. P. (2012). Isolation and Characterization of Eleven Polymorphic Microsatellite Loci for the Valuable Medicinal Plant Dendrobium Huoshanense and Cross-Species Amplification. Int. J. Mol. Sci. 13 (12), 16779–16784. doi:10.3390/ijms131216779
Wang, X. Y., Luo, J. P., Chen, R., Zha, X. Q., and Wang, H. (2014). The Effects of Daily Supplementation of Dendrobium Huoshanense Polysaccharide on Ethanol-Induced Subacute Liver Injury in Mice by Proteomic Analysis. Food Funct. 5 (9), 2020–2035. doi:10.1039/c3fo60629e
Wang, X. Y., Luo, J. P., Chen, R., Zha, X. Q., and Pan, L. H. (2015). Dendrobium Huoshanense Polysaccharide Prevents Ethanol-Induced Liver Injury in Mice by Metabolomic Analysis. Int. J. Biol. Macromol. 78, 354–362. doi:10.1016/j.ijbiomac.2015.04.024
Wang, K., Sui, D. J., Wang, C. S., Yang, L., Ouyang, Z., Chen, N. F., et al. (2017). Protective Effects of Five Different Types of Dendrobium on CCl4-Induced Liver Injury in Mice. Chin. J. Chin. Mat. Med. 42 (10), 1945–1950. doi:10.19540/j.cnki.cjcmm.2017.0082
Wang, H. Y., Li, Q. M., Yu, N. J., Chen, W. D., Zha, X. Q., Wu, D. L., et al. (2019). Dendrobium Huoshanense Polysaccharide Regulates Hepatic Glucose Homeostasis and Pancreatic β-cell Function in Type 2 Diabetic Mice. Carbohydr. Polym. 211, 39–48. doi:10.1016/j.carbpol.2019.01.101
Wang, Z., Zhao, M., Cui, H., Li, J., and Wang, M. (2020). Transcriptomic Landscape of Medicinal Dendrobium Reveals Genes Associated with the Biosynthesis of Bioactive Components. Front. Plant Sci. 11, 391. doi:10.3389/fpls.2020.00391
Wang, Y., Dai, J., Chen, R., Song, C., Wei, P., Wang, Y., et al. (2021). Long Noncoding RNA-Based Drought Regulation in the Important Medicinal Plant Dendrobium Huoshanense. Acta Physiol. Plant. 43 (11), 144. doi:10.1007/s11738-021-03314-1
Wu, S. J., Liu, Y. S., Chen, T. W., Ng, C. C., Tzeng, W. S., and Shyu, Y. T. (2009). Differentiation of Medicinal Dendrobium Species (Orchidaceae) Using Molecular Markers and Scanning Electron Microscopy. J. Food Drug Anal. 17 (6), 474–488. doi:10.1097/JCP.0b013e3181bef8a6
Wu, C., Gui, S., Huang, Y., Dai, Y., Shun, Q., Huang, K., et al. (2016). Characteristic Fingerprint Analysis of Dendrobium Huoshanense by Ultra-high Performance Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry. Anal. Methods 8 (18), 3802–3808. doi:10.1039/c6ay00232c
Wu, J., Meng, X., Jiang, W., Wang, Z., Zhang, J., Meng, F., et al. (2022). Qualitative Proteome-wide Analysis Reveals the Diverse Functions of Lysine Crotonylation in Dendrobium Huoshanense. Front. Plant Sci. 13, 822374. doi:10.3389/fpls.2022.822374
Xie, S. Z., Hao, R., Zha, X. Q., Pan, L. H., Liu, J., and Luo, J. P. (2016). Polysaccharide of Dendrobium Huoshanense Activates Macrophages via Toll-like Receptor 4-mediated Signaling Pathways. Carbohydr. Polym. 146, 292–300. doi:10.1016/j.carbpol.2016.03.059
Xie, S. Z., Ge, J. C., Li, F., Yang, J., Pan, L. H., Zha, X. Q., et al. (2018). Digestive Behavior of Dendrobium Huoshanense Polysaccharides in the Gastrointestinal Tracts of Mice. Int. J. Biol. Macromol. 107, 825–832. doi:10.1016/j.ijbiomac.2017.09.047
Xie, S. Z., Liu, B., Ye, H. Y., Li, Q. M., Pan, L. H., Zha, X. Q., et al. (2019a). Dendrobium Huoshanense Polysaccharide Regionally Regulates Intestinal Mucosal Barrier Function and Intestinal Microbiota in Mice. Carbohydr. Polym. 206, 149–162. doi:10.1016/j.carbpol.2018.11.002
Xie, S. Z., Shang, Z. Z., Li, Q. M., Zha, X. Q., Pan, L. H., and Luo, J. P. (2019b). Dendrobium Huoshanense Polysaccharide Regulates Intestinal Lamina Propria Immune Response by Stimulation of Intestinal Epithelial Cells via Toll-like Receptor 4. Carbohydr. Polym. 222, 115028. doi:10.1016/j.carbpol.2019.115028
Xu, H.-J., Hao, X.-L., Qiao, D.-L., Xia, L.-B., Chen, R., He, X.-M., et al. (2019). Effects of Dendrobium Huoshanense Polysaccharides on Antioxidant Capacity, Mucosal Barrier Integrity and Inflammatory Responses in an Aging Rat Ileal Model. Biotechnol. Biotechnol. Equip. 33 (1), 1444–1452. doi:10.1080/13102818.2019.1674187
Ye, M., Wang, X., Zhou, Y., Huang, S., and Liu, A. (2021). Genetic diversity and population structure of cultivated Dendrobium huoshanense (CZ Tang et SJ Cheng) using SNP markers generated from GBS analysis. Pak. J. Bot. 53 (5), 1683–1690. doi:10.30848/pjb2021-5(19)
Yi, S. Y., Kang, C. Z., Wang, W., Song, X. W., Xu, T., Lu, H. B., et al. (2021). Comparison of Planting Modes of Dendrobium Huoshanense and Analysis of Advantages of Simulated Cultivation. Chin. J. Chin. Mat. Med. 46 (8), 1864–1868. doi:10.19540/j.cnki.cjcmm.20210225.101
Yi, Y., Liu, L., Zhou, W., Peng, D., Han, R., and Yu, N. (2021). Characterization of GMPP from Dendrobium Huoshanense Yielding GDP-D-Mannose. Open Life Sci. 16 (1), 102–107. doi:10.1515/biol-2021-0015
Yuan, Y., Yu, M., Jia, Z., Song, X., Liang, Y., and Zhang, J. (2018). Analysis of Dendrobium Huoshanense Transcriptome Unveils Putative Genes Associated with Active Ingredients Synthesis. BMC Genomics 19, 978. doi:10.1186/s12864-018-5305-6
Yuan, Y., Yu, M., Zhang, B., Liu, X., and Zhang, J. (2019). Comparative Nutritional Characteristics of the Three Major Chinese Dendrobium Species with Different Growth Years. Plos One 14 (9), e0222666. doi:10.1371/journal.pone.0222666
Yuan, Y., Zuo, J., Zhang, H., Zu, M., Yu, M., and Liu, S. (2022). Transcriptome and Metabolome Profiling Unveil the Accumulation of Flavonoids in Dendrobium Officinale. Genomics 114 (3), 110324. doi:10.1016/j.ygeno.2022.110324
Zha, X.-Q., Luo, J.-P., and Jiang, S.-T. (2007a). Induction of Immunomodulating Cytokines by Polysaccharides fromDendrobium Huoshanense. Pharm. Biol. 45 (1), 71–76. doi:10.1080/13880200601028420
Zha, X.-Q., Luo, J.-P., Jiang, S.-T., and Wang, J.-H. (2007b). Enhancement of Polysaccharide Production in Suspension Cultures of Protocorm-like Bodies from Dendrobium Huoshanense by Optimization of Medium Compositions and Feeding of Sucrose. Process Biochem. 42 (3), 344–351. doi:10.1016/j.procbio.2006.09.008
Zha, X.-Q., Luo, J.-P., Luo, S.-Z., and Jiang, S.-T. (2007c). Structure Identification of a New Immunostimulating Polysaccharide from the Stems of Dendrobium Huoshanense. Carbohydr. Polym. 69 (1), 86–93. doi:10.1016/j.carbpol.2006.09.005
Zha, X.-Q., Luo, J.-P., Jiang, S.-T., and Wang, Y. (2007d). Carbon and Nitrogen Metabolism duringDendrobium Huoshanenseprotocorm-like Body Development in Suspension Culture. Plant Biosyst. - Int. J. Deal. all Aspects Plant Biol. 141 (1), 62–68. doi:10.1080/11263500601153768
Zha, X. Q., Li, X. L., Zhang, H. L., Cui, S. H., Liu, J., Wang, J. H., et al. (2013). Pectinase Hydrolysis of Dendrobium Huoshanense Polysaccharide and its Effect on Protein Nonenzymatic Glycation. Int. J. Biol. Macromol. 61, 439–447. doi:10.1016/j.ijbiomac.2013.08.008
Zha, X. Q., Zhao, H. W., Bansal, V., Pan, L. H., Wang, Z. M., and Luo, J. P. (2014). Immunoregulatory Activities of Dendrobium Huoshanense Polysaccharides in Mouse Intestine, Spleen and Liver. Int. J. Biol. Macromol. 64, 377–382. doi:10.1016/j.ijbiomac.2013.12.032
Zha, X. Q., Deng, Y. Y., Li, X. L., Wang, J. F., Pan, L. H., and Luo, J. P. (2017). The Core Structure of a Dendrobium Huoshanense Polysaccharide Required for the Inhibition of Human Lens Epithelial Cell Apoptosis. Carbohydr. Polym. 155, 252–260. doi:10.1016/j.carbpol.2016.08.087
Zhang, C. C., Gao, Z., Luo, L. N., Liang, H. H., and Xiang, Z. X. (2020). Comparative Analysis of Active Components and Transcriptome between Autotetraploid and Diploid of Dendrobium Huoshanense. Chin. J. Chin. Mat. Med. 45 (23), 5669–5676. doi:10.19540/j.cnki.cjcmm.20200816.101
Zhang, X., Li, Z. Q., Yue, Q., Zou, Y. M., Ouyang, Z., and Han, B. X. (2021a). Comparison of Anti-inflammatory and Antitumor Effects of Dendrobium Huoshanense of Different Growth Years. Chin. Wild Plant Resour. 40 (05), 24–29. doi:10.3969/j.issn.1006-9690.2021.05.005
Zhang, Y., He, H., Chen, Z., Huang, Y., Xiang, G., Li, P., et al. (2021b). Merging Reagent Modulation and Remote Anchimeric Assistance for Glycosylation: Highly Stereoselective Synthesis of α˗Glycans up to a 30˗mer. Angew. Chem. Int. Ed. 60 (22), 12597–12606. doi:10.1002/anie.202103826
Zhao, Y., Han, B., Peng, H., Wang, X., Chu, S., Dai, J., et al. (2017). Identification of "Huoshan Shihu" Fengdou: Comparative Authentication of the Daodi Herb Dendrobium Huoshanense and its Related Species by Macroscopic and Microscopic Features. Microsc. Res. Tech. 80 (7), 712–721. doi:10.1002/jemt.22856
Zhao, H. S., Xu, F. Q., and Chen, X. X. (2021). Chemical constituents of Dendrobium huoshanense C. Z. Tang et S. J. Cheng. Nat. Prod. Res. Dev. 33 (09), 1491–1498. doi:10.16333/j.1001-6880.2021.9.006
Zhao, X. M. (1998). A Supplement to Compendium of Materia Medica. Beijing: China Press of Traditional Chinese Medicine.
Zheng, J., Chen, N., Wang, H., Gao, P., Shao, J., and Zhu, G. (2011). Isolation and Analysis of Polymorphic Microsatellite Loci in Dendrobium Huoshanense. Chin. J. Chin. Mat. Med. 36 (21), 2926–2931.doi:10.4268/cjcmm20112106
Zhou, P., Pu, T., Gui, C., Zhang, X., and Gong, L. (2020). Transcriptome Analysis Reveals Biosynthesis of Important Bioactive Constituents and Mechanism of Stem Formation of Dendrobium Huoshanense. Sci. Rep. 10 (1), 2857. doi:10.1038/s41598-020-59737-2
Zhu, A. L., Hao, J. W., Liu, L., Wang, Q., Chen, N. D., Wang, G. L., et al. (2021). Simultaneous Quantification of 11 Phenolic Compounds and Consistency Evaluation in Four Dendrobium Species Used as Ingredients of the Traditional Chinese Medicine Shihu. Front. Nutr. 8, 771078. doi:10.3389/fnut.2021.771078
Zhu, Y., Kong, Y., Hong, Y., Zhang, L., Li, S., Hou, S., et al. (2022). Huoshanmycins A‒C, New Polyketide Dimers Produced by Endophytic Streptomyces Sp. HS-3-L-1 from Dendrobium Huoshanense. Front. Chem. 9, 807508. doi:10.3389/fchem.2021.807508
Glossary
AGEs advanced glycation end products
AGEs advanced glycation end products
ALT alanine aminotransferase
AST aspartate aminotransferase
CAT catalase
CSA-Pyr method chlorosulfonic acid-pyridine method
D. chrysotoxum Dendrobium chrysotoxum
D. huoshanense Dendrobium huoshanense
D. moniliforme Dendrobium moniliforme
D. nobile Dendrobium nobile
D. officinale Dendrobium officinale
GC-MS gas chromatography-mass spectrometry
GSH L-glutathione
GSH-PX glutathione peroxidase
GST glutathione S-transferase
GR glutathione reductase
HMBC heteronuclear multiple bond correlation
H2O2 Hydrogen peroxide
HPLC-GPC high performance gel permeation chromatography
HSQC heteronuclear singular quantum correlation
IFN-γ interferon-γ
IL-1β interleukin-1β
IL-12 p40 interleukin 12 p40
IL-10 interleukin 10
IL-6 interleukin 6
iNOS inducible nitric oxide synthase
IR infrared radiation
LDH lactate dehydrogenase
MAPKs mitogen-activated protein kinases
MCP-1 monocyte chemoattractant protein-1
MDA malonaldehyde
MIP-2 macrophage inflammatory protein-2
NF-κB nuclear factor-kappa B
NK cells natural killer cells
NKT cells natural killer T cells
NMR nuclear magnetic resonance
NO nitric oxide
8-OhdG 8-hydroxy-20-deoxyguanosine
PI3 K/Akt phosphoinositide 3-kinase-akt
p-IκBα phosphorylated IκBα
SOD superoxidedismutase
TGF-β1 transforming growth factor β1
TNF-α tumor necrosis factor-α
T-AOC total antioxidant capacity
Keywords: Dendrobium huoshanense, phytochemistry, pharmacology, materia medica research, resource distribution
Citation: Gao L, Wang F, Hou T, Geng C, Xu T, Han B and Liu D (2022) Dendrobium huoshanense C.Z.Tang et S.J.Cheng: A Review of Its Traditional Uses, Phytochemistry, and Pharmacology. Front. Pharmacol. 13:920823. doi: 10.3389/fphar.2022.920823
Received: 15 April 2022; Accepted: 30 May 2022;
Published: 12 July 2022.
Edited by:
Michael Heinrich, University College London, United KingdomReviewed by:
Mark Arcebal Kling Naive, Jose Rizal Memorial State University, PhilippinesParomik Bhattacharyya, Institute of Himalayan Bioresource Technology (CSIR), India
Jian-Ping Luo, Hefei University of Technology, China
Destario Metusala, National Research and Innovation, Agency, Indonesia
Copyright © 2022 Gao, Wang, Hou, Geng, Xu, Han and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leilei Gao, Z2FvbGVpbGVpMTk4OUB5ZWFoLm5ldA==; Bangxing Han, aGFuYngxOTc4QHNpbmEuY29t; Dong Liu, bGl1ZG9uZzMwMDBAMTI2LmNvbQ==
 Leilei Gao1,2*
Leilei Gao1,2* Bangxing Han
Bangxing Han