- 1Clinical School of Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2State Key Laboratory of Southwestern Chinese Medicine Resources, School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3Affiliated Hospital of Chengdu University of Traditional Chinese Medicine-Sichuan Provincial Hospital of Traditional Chinese Medicine, Chengdu, China
Background: Renal fibrosis is a key pathological change that occurs in the progression of almost all chronic kidney diseases . CKD has the characteristics of high morbidity and mortality. Its prevalence is increasing each year on a global scale, which seriously affects people’s health and quality of life. Natural products have been used for new drug development and disease treatment for many years. The abundant natural products in R. ribes L. can intervene in the process of renal fibrosis in different ways and have considerable therapeutic prospects.
Purpose: The etiology and pathology of renal fibrosis were analyzed, and the different ways in which the natural components of R. ribes L. can intervene and provide curative effects on the process of renal fibrosis were summarized. Methods: Electronic databases, such as PubMed, Life Science, MEDLINE, and Web of Science, were searched using the keywords ‘R. ribes L.’, ‘kidney fibrosis’, ‘emodin’ and ‘rhein’, and the various ways in which the natural ingredients protect against renal fibrosis were collected and sorted out.
Results: We analyzed several factors that play a leading role in the pathogenesis of renal fibrosis, such as the mechanism of the TGF-β/Smad and Wnt/β-catenin signaling pathways. Additionally, we reviewed the progress of the treatment of renal fibrosis with natural components in R. ribes L. and the intervention mechanism of the crucial therapeutic targets.
Conclusion: The natural components of R. ribes L. have a wide range of intervention effects on renal fibrosis targets, which provides new ideas for the development of new anti-kidney fibrosis drugs.
1 Introduction
Renal fibrosis is a common pathological change in the end stage of all chronic kidney diseases (CKDs) (Loboda et al., 2016). CKD has high morbidity and mortality (Black et al., 2018). Primary glomerular disease, diabetes and hypertension are major contributors to CKD (Webster et al., 2017). With the increasing incidence of diabetes and hypertension and the aging of the population, the prevalence of CKD has increased (Morgado-Pascual et al., 2018). Excessive deposition of the extracellular matrix (ECM) is the basic feature of renal fibrosis (Ma and Meng, 2019). Inflammation, key cytokines (such as TGF-β, Wnt, etc.), the extracellular matrix, autophagy, apoptosis, and epigenetic modifications have all been found to be associated with renal fibrosis (Lv et al., 2018; Morgado-Pascual et al., 2018; Zhao et al., 2019). At present, it is vital and imminent to discover new anti-kidney fibrosis drugs. Since ancient times, many valuable medicines have been discovered in nature (Cragg and Newman, 2013). R. ribes L. is a heat-clearing medicine in Chinese herbal medicine. The Chinese medicine R. ribes L. has achieved good effects in the treatment of many diseases. According to multiple reports, natural components, such as emodin, rhein, and aloe-emodin, are contained in R. ribes L. These components can improve renal fibrosis by regulating the above signaling pathways and the processes of oxidative stress and inflammation (Isaka, 2018; Lv et al., 2018). With the development of analysis and calculation techniques, new methods for processing natural products with complex structures have emerged (Thomford et al., 2018). This provides technical assistance for drug development. We will review the intervention effects of the natural components in R. ribes L. on certain key signaling pathways, explain the mechanism of the natural components in R. ribes L. to treat renal fibrosis, and explore new ideas for the development of renal fibrosis drugs.
2 Overview of the process of renal fibrosis
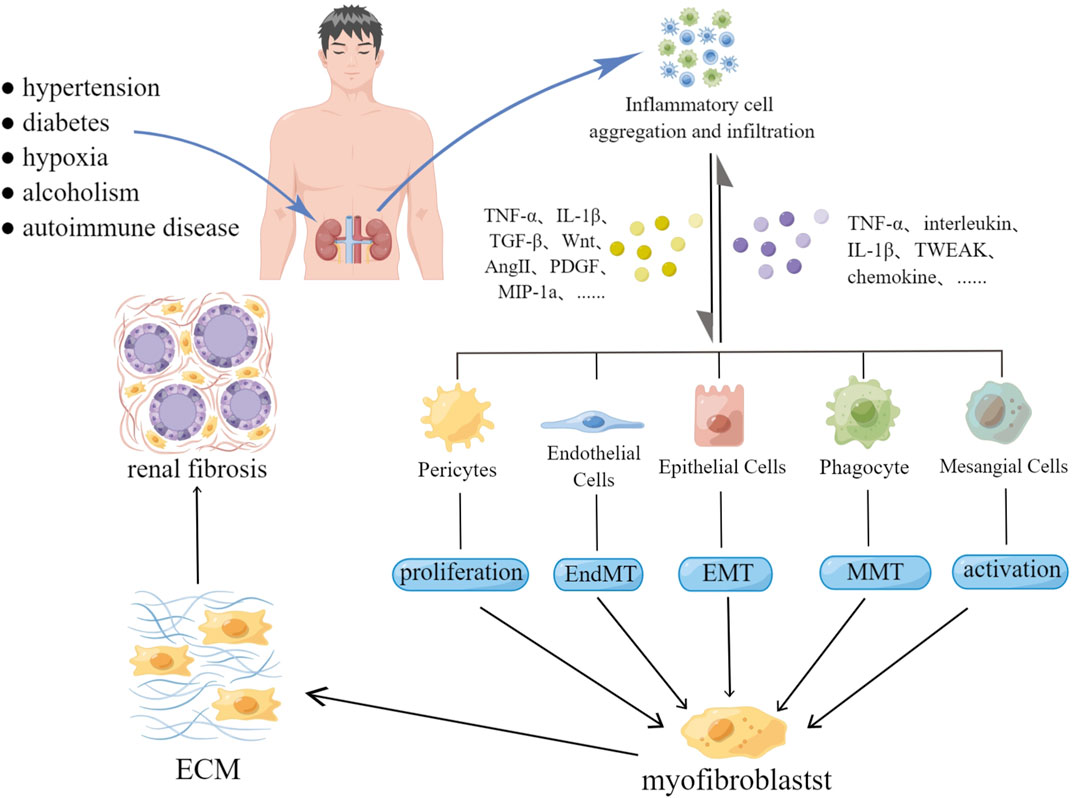
According to recent studies, the main cellular and molecular mechanisms involved in renal fibrosis include continuous stimulation of inflammatory cells, myofibroblast formation, massive extracellular matrix deposition (ECM), renal tubular atrophy and sparse microvascular networks (Liu, 2011). It is well known that the ECM is the most critical step in the renal fibrosis process, and myofibroblasts are the main component of the ECM. A large number of studies have shown that renal innate cells (including renal interstitial fibroblasts, pericytes, epithelial cells, and endothelial cells) are the main source of myofibroblasts (Sun Y. B. et al., 2016). In addition, studies have confirmed that some bone marrow-derived cells (such as phagocytes and T cells) can also be transformed into myofibroblasts under certain conditions (Meng et al., 2014). The above process is called cell phenotypic transformation. During kidney injury, fibroblasts activate and proliferate, becoming the main source of myofibroblasts (Liu, 2011). The transformation process of epithelial cells is called epithelial-mesenchymal transition (EMT). Refers to the phenotypic characteristics of myofibroblasts acquired by epithelial cells. These are characterized by increased expression of α-SMA, vimentin, and 1 type collagen (Cruz-Solbes and Youker, 2017). Similarly, endothelial cells can acquire mesenchymal or myofibroblast phenotypic characteristics after deadherence, and this process is termed the endothelial-mesenchymal transition (EndMT). The transformed endothelial cells show increased expression of FSP-1, ɑ-SMA, fibronectin, vimentin, and collagen types I and III (He et al., 2013). Pericytes are a type of mesenchymal cell that are partially or fully embedded in the capillary basement membrane. These cells play a role in maintaining capillary homeostasis and regulating blood flow. After renal injury, pericytes are separated from capillary endothelial cells through pericyte-myofibroblast transformation. This results in sparse capillaries and affects renal function (Kramann and Humphreys, 2014). The above process leads to renal interstitial fibrosis, podocyte lesions or glomerulosclerosis.
Inflammation is a key driver of renal fibrosis and plays a leading role in the transformation of cellular phenotypes (Meng et al., 2014). Long-term stimuli from damage to the body (such as proteinuria, hypertension, hyperglycemia, hypoxia, etc.) can lead to renal tissue damage, which in turn induces the inflammatory response of renal innate cells (Morgado-Pascual et al., 2018). Inflammatory cells, such as macrophages, lymphocytes, dendritic cells, and mast cells, aggregate to secrete cytokines and chemokines. These cytokines and chemokines aggregate inflammatory cells and induce the renal innate cell phenotype transformation. Damaged epithelial cells secrete cytokines, such as TGF-β and Wnt, and become proinflammatory cells, aggravating the inflammatory response. Activated endothelial cells and pericytes undergo phenotypic transformation. This causes capillary damage and the subsequent hypoxia, which further aggravate renal injury (Yuan et al., 2019). In addition, cytokines secreted by inflammatory cells, damaged epithelial cells, and myofibroblasts are also key factors in accelerating renal fibrosis. For example, TGF-β can promote renal fibrosis by activating the Smad or non-Smad signaling pathway. Wnt aggravates renal fibrosis by activating the Wnt/β-catenin signaling pathway. PDGF can promote the production of inflammatory mediators and accelerate the accumulation of ECM(Tan et al., 2014; Meng, 2019).
3 Clinical study of natural components in R. ribes L
As a traditional Chinese medicine, R. ribes L. is often used as a laxative to treat constipation (Gao et al., 2021). Modern clinical studies have demonstrate that R. ribes L. has considerable curative effects on some common clinical diseases. For example, the clinical studies of Wang et al. have indicated that R. ribes L. combined with early enteral nutrition can significantly improve gastrointestinal function, inhibit systemic inflammation, and reduce liver and kidney damage in patients with severe pancreatitis (Wan et al., 2014). In a clinical randomized controlled study by Liu et al., it was indicated that R. ribes L. can reduce blood lipid levels in patients with atherosclerosis and improve vascular endothelial function (Liu et al., 2007). To date, few clinical studies have confirmed that R. ribes L. has a therapeutic effect on renal fibrosis, but some clinical studies have confirmed that R. ribes L. has a certain intervention effect on renal disease. For example, clinical studies conducted by Irfan A Khan et al. confirmed that R. ribes L. has a significant effect on the conservative treatment of patients with advanced chronic kidney disease (Khan et al., 2014). The study by Li et al. confirmed that R. ribes L. can effectively prevent disease progression in patients with chronic renal failure (Li and Liu, 1991). Therefore, the author believes that the clinical treatment of R. ribes L. for renal fibrosis may also have certain potential.
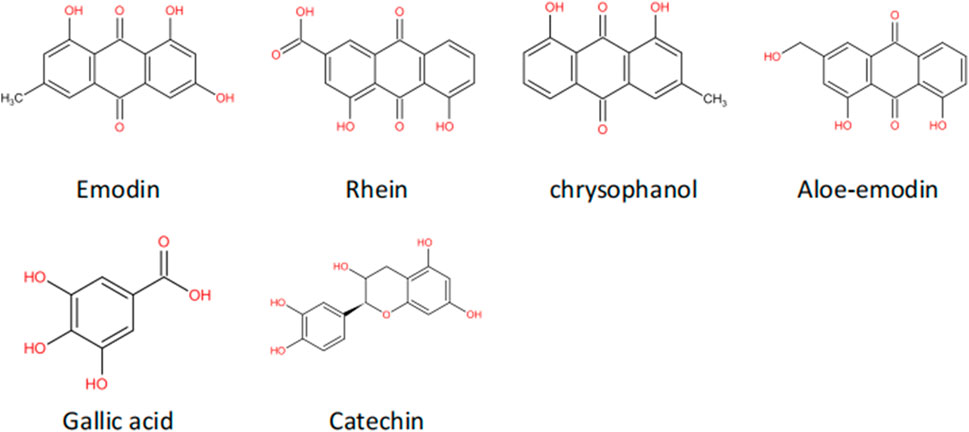
The most abundant chemical constituents in R. ribes L. are anthraquinone compounds, mainly rhein, chrysophanol, emodin and aloe-emodin (Aichner and Ganzera, 2015). Modern pharmacological studies have confirmed that the rich natural extracts in R. ribes L., such as emodin and rhein, have anti-tissue fibrosis effects (Sun H. et al., 2016; Zhao et al., 2019). This article will mainly discuss the feasibility of R. ribes L. as a clinical candidate drug in the treatment of renal fibrosis by describing the antagonistic effect of the anthraquinones in R. ribes L. on renal fibrosis.
4 Pathogenesis of renal fibrosis
4.1 Inflammation
Inflammation is the basis of renal fibrosis. Autoimmune diseases, high blood pressure, diabetes, obesity, drug damage, and poor lifestyle habits (such as alcoholism) are all common causes of kidney damage (Schaeffner and Ritz, 2012; Kurts et al., 2013; Mennuni et al., 2014; Tonneijck et al., 2017; Lakkis and Weir, 2018). Stimulated by the damage caused by immune and nonimmune factors, the inflammatory response of the kidneys acts to promote regeneration and repair (Tecklenborg et al., 2018). However, at the same time, damaged tissues release a large number of cytokines and inflammatory mediators, which continuously activate inflammatory cells and renal innate cells. Additionally, they promote cell phenotypic transformation, myofibroblast accumulation, and ECM processes, resulting in kidney fibrosis (Black et al., 2019). The mechanism of action of inflammation is shown in (Figure 1).
In renal injury, both inflammatory cells and renal innate cells are the driving forces that exacerbate inflammation and fibrosis. The first driving force is the profibrotic and proinflammatory effects of inflammatory cells. After early kidney injury, under the action of chemokines and cytokines, inflammatory cells (such as T cells, macrophages, fibroblasts, and mast cells) are recruited to the kidney and release profibrotic factors and cytokines. This further aggravates the inflammatory response (Meng, 2019). Stimulated inflammatory cells secrete TNF-α, IL-1β, TGF-β, Wnt, Ang II, PDGF, collagen, MIP-1a and other factors. These factors are involved in renal fibrosis and aggravate the inflammatory response (Chesney and Bucala, 2000; Duffield, 2010; Tang et al., 2019). Under the action of chemokines, growth factors and a series of enzymes, mast cells release inflammatory factors and profibrotic factors and activate the Ang II signaling pathway to play a profibrotic role (Meng et al., 2014).
The second driving force is the role of renal innate cells. During renal injury, tubular epithelial cells transform into a secretory phenotype. They secrete and activate proinflammatory cytokines, such as TNF-α, interleukin, IL-1β, and TWEAK, and aggravate inflammatory cell infiltration. In addition, epithelial cells secrete chemokines and adhesion molecules to promote the aggregation of inflammatory cells, which is also one of the important causes of kidney inflammation aggravation (Liu et al., 2018). Continued stimulation induces the EMT with the aim of repairing damage through collagen production by myofibroblasts. Moreover, under the stimulation of factors such as Ang II, epithelial cells secrete TGF-β. Due to persistent inflammatory stimulation and the long-term effects of cytokines such as TGF-β, kidney fibrosis occurs (Macconi et al., 2014; Cruz-Solbes and Youker, 2017). The second contributor is the role of mesangial cells. Injured mesangial cells produce a variety of chemokines, cytokines, and proinflammatory mediators, which activate inflammation and damage the endothelial cell barrier (Schlöndorff and Banas, 2009). Finally, endothelial cells play a role. After kidney injury, endothelial cells activate inflammatory responses and recruit inflammatory cells. The accumulation of inflammatory cells increases hemodynamic resistance, causes local hypoxia and induces endothelial cell apoptosis. These effects eventually lead to increased capillary permeability and further aggravate hypoxia (Kelly et al., 2009; Guerrot et al., 2012).
Overall, renal injury is the beginning of renal fibrosis, and persistent inflammatory stimulation is the basis and driving force for the progression and aggravation of renal fibrosis.
After kidney injury, the kidney triggers an inflammatory response to promote repair and regeneration. Inflammatory cells secrete chemokines, inflammatory factors and cytokines, aggravating the inflammatory response. Simultaneously activate renal innate cells to secrete cytokines and pro-inflammatory mediators. Persistent inflammation and cytokines stimulate the proliferation and differentiation of pericytes and mesangial cells, and induce EMT, EndMT, and MMT, resulting in an increase and accumulation of fibroblasts. Initiates ECM and promotes renal fibrosis.
4.2 Formation of the fibrous matrix
4.2.1 The role of TGF-β
TGF-β is a growth factor that is secreted by most cells of the body. There are many different isoforms in the large TGF-β protein family. All of these members play an important role in regulating the growth, development and differentiation of cells in the body and are closely related to ligand secretion, ECM and presignaling activation (Massagué, 1998; Tzavlaki and Moustakas, 2020).
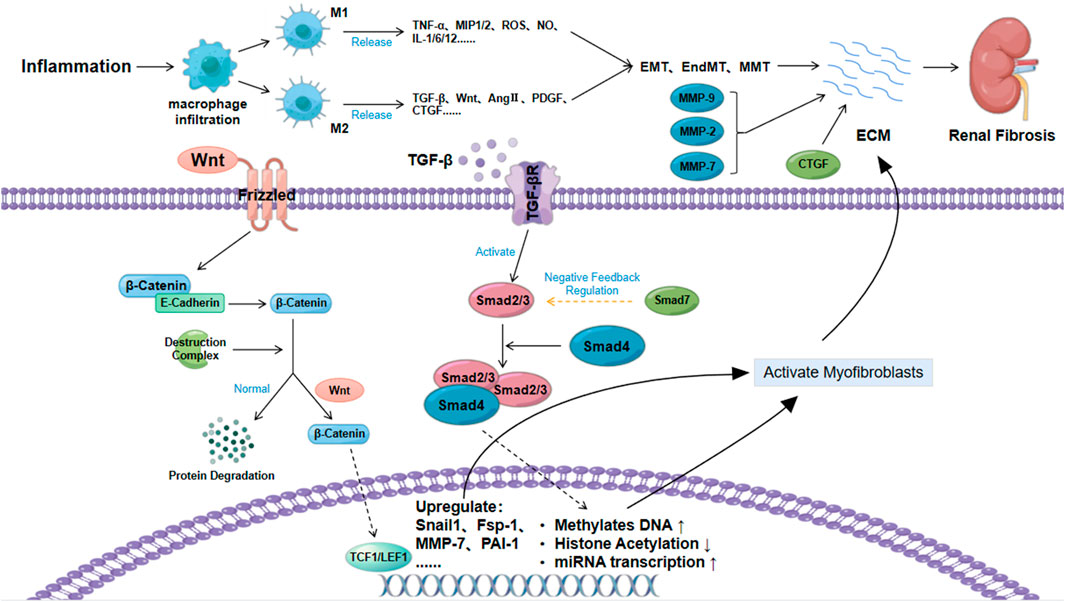
The TGF-β/Smad signaling pathway is closely related to diseases such as tissue fibrosis and cancer and has been a hot research topic in recent years (Blobe et al., 2000). TGF-β1 is the isoform that is most closely associated with tissue fibrosis (Tang R. et al., 2020). During renal fibrosis, TGF-β1 mainly activates downstream Smad3 signaling. After the binding of Smad3 and Smad4, they enter the nucleus and directly mediate renal fibrosis by promoting DNA methylation, reducing histone acetylation, and inducing miRNA transcription (Zhong et al., 2011; Tampe et al., 2014; Smith et al., 2019). In addition to the canonical TGF-β/Smad signaling pathway, TGF-β1 can also jointly mediate renal fibrosis by activating other signaling pathways, such as the MAPK and PI3K/Akt/mTOR pathways (Zhang, 2017; Nickel et al., 2018).
TGF-β also plays an important role in the phenotypic transformation of cells. Endothelial cells, epithelial cells, and macrophages can also transform into myofibroblasts under continuous stimulation of TGF-β1. This is a process that aggravates fibrous deposition and promotes renal fibrosis. Most studies have shown that TGF-β1 is mainly associated with EMT, and EndMT is mainly induced by TGF-β2 (Duffield, 2010; He et al., 2013; Cruz-Solbes and Youker, 2017). In addition, the secretion of TGF-β by mesangial cells under inflammatory stimulation induces mesangial cell proliferation and ECM, which further aggravates renal fibrosis (Zhao, 2019). In recent years, Wu et al. also confirmed that TGF-β1 can promote the differentiation of pericytes into myofibroblasts (Wu et al., 2013). TGF-β is also a key factor in activating myofibroblasts and inducing their proliferation and differentiation. The accumulation of fibroblasts causes ECM accumulation and renal fibrosis (Yuan et al., 2019).
4.2.2 The role of wnt
The secreted growth factor protein Wnt is closely related to cell renewal, proliferation and differentiation (Majidinia et al., 2018). The typical Wnt signaling pathway is the Wnt/β-catenin signaling pathway, which is relatively conserved (Steinhart and Angers, 2018). As a key nuclear effector of the Wnt signaling pathway, β-catenin exhibits different biological effects in the cell membrane, cytoplasm and nucleus (Valenta et al., 2012). β-Catenin forms a molecular complex with cadherin (usually E-cadherin), and the deadhesion of the E-cadherin/β-catenin complex plays an important role in the EMT (Tian et al., 2011). Normally, after E-cadherin/β-catenin deadhesion, free β-catenin is degraded (phosphorylated) by the destruction complex (Guo et al., 2012). Abnormal Wnt protein expression can increase the amount of free β-catenin by acting on the Twist and Slug genes to inhibit the transcription of E-cadherin. Then, it can block the degradation of β-catenin by acting on β-catenin to destroy the complex (Heuberger and Birchmeier, 2010; Valenta et al., 2012). The excessive accumulation of free β-catenin causes free β-catenin to translocate to the nucleus to activate TCF and LEF. This then induces the expression of fibronectin, Fsp-1, Snail genes, MMP-7, and PAI-1, promotes myofibroblast activation, accelerates ECM accumulation and promotes kidney fibrosis (Stemmer et al., 2008; Hao et al., 2011; Tan and Liu, 2012; Zuo and Liu, 2018). In addition to the Wnt proteins, TGF-β1, Akt and other factors can also induce renal fibrosis by activating β-catenin, as shown in Figure 2 (Fang et al., 2007; Wang et al., 2011).
4.2.3 The role of the extracellular matrix
The extracellular matrix not only acts as a scaffold to maintain cell stability but also plays a key role in inducing renal fibrosis. ECM is the main pathological factor of fibrosis. MMP plays an important role in the process of ECM accumulation. MMP is a metalloproteinase that can degrade collagen, and TIMP has an inhibitory effect on its activity (Bülow and Boor, 2019). Current studies have found that several subtypes of the MMP family, such as MMP-2, MMP-7, and MMP-9, are closely related to fibrosis (Cheng and Lovett, 2003; Wang et al., 2010; Ke et al., 2017). MMP-2 causes renal tubular damage and fibrosis by disrupting the integrity of the basement membrane. In addition, the synergistic effect of MMP-2 and TGF-β1 to promote the EMT has also been confirmed (Cheng and Lovett, 2003; Wozniak et al., 2021). MMP-7 can degrade E-cadherin, release β-catenin, reduce cell adhesion and accelerate the EMT (Wozniak et al., 2021). MMP-9 promotes the recruitment of macrophages and induces the EMT by cleaving osteopontin. In addition, MMP-9 also promotes the EndMT, which is mainly accomplished through the Notch signaling pathway (Zhao et al., 2017; Wozniak et al., 2021).
In addition to MMP, the role of CTGF in the cell matrix in fibrosis cannot be ignored, and this process is mainly accomplished by regulating other growth factor-mediated fibrosis-related signaling pathways (Toda et al., 2018). A large number of studies have confirmed that the expression of CTGF in patients with renal fibrosis is significantly increased. CTGF can not only promote EMT, recruit inflammatory cells, and induce ECM but also act as a downstream molecule of the TGF-β/Smad signaling pathway to increase renal fibrosis through multiple pathways (Yin and Liu, 2019). Fibrosis therapy treatments targeting CTGF have been shown to have certain potential (Phanish et al., 2010).
ECM deposition is the most critical pathological change in renal fibrosis. TGF-β, Wnt and extracellular matrix are the main factors affecting the ECM. TGF-β can directly synthesize fibrous matrix by inducing epigenetic modification and miRNA expression through the TGF-β/Smad signaling pathway, or it can induce the accumulation of myofibroblasts by inducing cell phenotypic transformation, and indirectly lead to ECM. Wnt can activate myofibroblast activation by blocking β-catenin degradation. The abnormal expression of metalloproteinases and CTGF in the extracellular matrix under the condition of renal injury is also an important reason for aggravating renal fibrosis.
4.3 Apoptosis and autophagy signal transduction
4.3.1 Regulation of apoptosis
Apoptosis is a type of programmed cell death that plays a key regulatory role in the development of kidney disease. Apoptosis can be regulated in two ways, endogenous and exogenous, both of which can cause different degrees of renal damage when unregulated (Savill, 1994). Its mechanism is shown in (Figure 3).
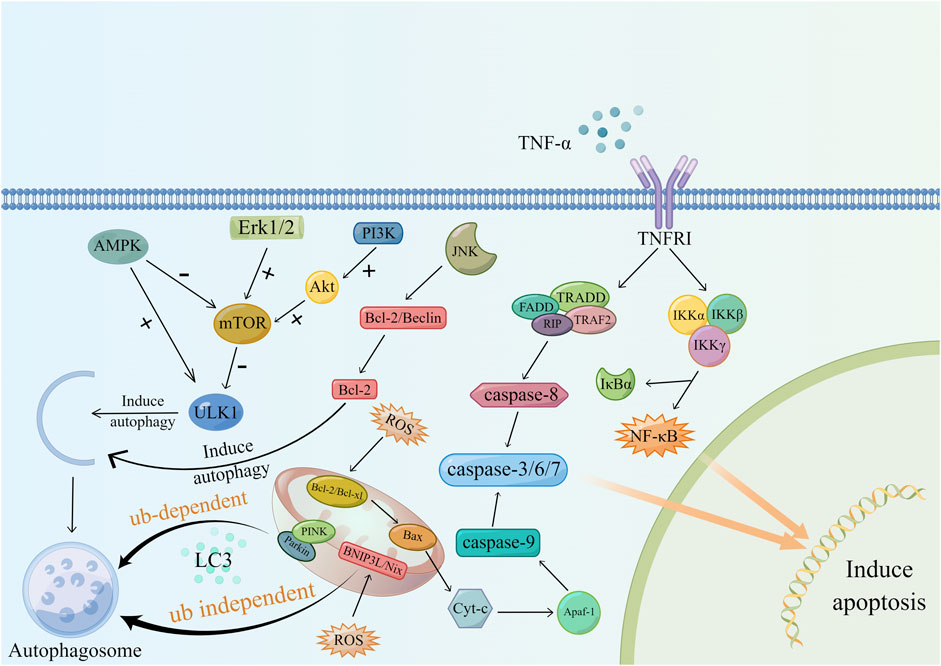
Animal experiments have demonstrated the correlation between renal fibrosis and apoptosis and that mitochondrial damage mediated by oxidative stress is one of the important causes of uncontrolled apoptosis (Xu et al., 2013). In CKD patients, changes in renal hemodynamics and the continuous stimulation of inflammation lead to increased mitochondrial anaerobic metabolism, lipid deposition in the cytoplasm, and changes in mitochondrial membrane permeability (Martínez-Klimova et al., 2020). The increased release of ROS from damaged mitochondria further increases mitochondrial membrane permeability. Cytochrome-C (cytochrome C), Smac/DIABLO and other proapoptotic factors are released, and then these factors activate caspase-9, caspase-3 and caspase-7 through anti-perinuclear factor-1 (Apaf-1) to initiate apoptosis (Yang et al., 2006). Studies have shown that excessive apoptosis in the renal parenchyma in the short term can cause glomerular and tubular atrophy, while long-term tubular atrophy provides a suitable microenvironment for the formation of fibrosis (Ortiz et al., 1996).
Second, another related factor that regulates apoptosis is TNF-α. Macrophages and renal tubular epithelial cells are major sources of TNF-α. Studies by R. Misseri et al. showed that elevated levels of TNF-α protein in obstructed kidneys can induce apoptosis in renal tubular cells (Misseri and Meldrum, 2005; Misseri et al., 2005). TNF-α-mediated apoptosis includes two regulatory pathways. After TNF-α binds to cell membrane surface receptors, it forms a complex with TRADD and then couples with FADD to activate caspase-8. In addition, TNF-α can also activate NF-κB through the IKK complex. However, NF-κB has a bidirectional regulatory effect on apoptosis, and its proapoptotic or antiapoptotic function depends on the surrounding environment of the cell (Chinnaiyan et al., 1995; Hsu et al., 1995; Misseri and Meldrum, 2005; Hayden and Ghosh, 2014).
4.3.2 Autophagy
Autophagy is a lysosomal degradation process that can occur in different types of kidney cells and plays an important role in maintaining cell stability (Zhao et al., 2019). Studies have shown that the regulation of autophagy in the process of renal fibrosis is bidirectional. Autophagy can mediate collagen degradation and inhibit renal fibrosis. Moreover, persistent activation of autophagy can lead to tubular atrophy and promote fibrosis (Kim et al., 2012; Livingston et al., 2016). Mitophagy is the molecular basis of cell-selective autophagy, and it includes ubiquitin (Ub)-dependent and Ub-independent pathways (Tang C. et al., 2020). During mitochondrial damage, PINK binds to Parkin and aggregates autophagy-related proteins, such as NBR1, through the polyubiquitination of Parkin. PINK then initiates autophagy by interacting with LC3. In the ubiquitin-independent pathway, mitochondrial hypoxia increases the expression of BNIP3L/Nix, which can also induce autophagy after binding to LC3 (Ding and Yin, 2012).
mTOR is a key regulator of autophagy, and its activation is related to signaling pathways mediated by factors such as MAPK, PI3K, and AMPK. In this process, the perception of the cell to changes in the nutritional and energy status plays a part in the selection role (Tang C. et al., 2020). AMPK, mTORC1, and ULK1 are known as the kinase triplet. When nutrients are abundant, mTOR is activated and inhibits ULK1 through phosphorylation. Then, it blocks the binding of AMPK to ULK1, thereby inhibiting autophagy. Upon starvation, ULK1 inhibition is released, and AMPK can activate autophagy by activating ULK1 (Kim and Guan, 2011; Dunlop and Tee, 2013). The signaling pathways related to MAPK and autophagy regulation include the MAPK/JNK signaling pathway and MAPK/Erk signaling pathway. JNK mainly relieves Beclin dependence and induces autophagy by destroying Bcl-2/Beclin and phosphorylating Bcl-2. Erk inhibits autophagy by activating mTOR. JNK and Erk have opposite regulatory effects on autophagy (Kim and Guan, 2015; Zhou Y. Y. et al., 2015). In addition, growth factors, such as insulin, can activate the PI3K/Akt/mTOR signaling pathway. Activated Akt can promote the dissociation of TSC1/2 and activate mTOR by targeting Rheb. Additionally, Akt can directly interact with mTOR to inhibit autophagy (Kim and Guan, 2015). The molecular mechanism of autophagy is shown in (Figure 3).
Hypoxia-induced mitochondrial damage underlies apoptosis and autophagy. After mitochondrial injury, pro-apoptotic factors and autophagy-related factors are expressed to initiate apoptosis and autophagy. In addition, activation of mTOR through MAPK/Erk, PI3K, AMPK and other related signaling pathways can promote autophagy. It has to be said that in the process of autophagy, JNK plays an inhibitory role.
4.4 Epigenetic modifications
4.4.1 Deoxyribonucleic acid methylation
DNA methylation generally occurs on CpG islands located in the DNA promoter region. In this process, a methyl group is covalently added to the C5 position of the cytosine residue to achieve gene transcription silencing through physical obstruction (Larkin et al., 2018). Under normal circumstances, DNA methylation is in a dynamic state, maintaining stable intracellular gene transcription. Abnormal DNA methylation patterns can occur in most kidney diseases, such as AKI, DN, CKD and DKD. Regardless of the type of kidney damage, the end result is the induction of end-stage renal disease and renal fibrosis (Ding et al., 2021). The process of DNA methylation is regulated by DNA methyltransferase 1 (DNMT1). The main mechanism of this process is to induce chromatin conformational changes, thereby hindering the entry of proteins required for transcription and inhibiting transcription (Wanner and Bechtel-Walz, 2017). In the analysis of the whole genome and specific genes of CKD patients, it was found that the DNA methylation sites of CKD patients were different from those of nonrenal insufficiency patients. These differentiated genes (such as NOS3, TGFB3, and CARS2) are involved in the process of destroying renal function and inducing fibrosis. In addition, hypermethylation of these specific genes (such as RASAL1, Klotho, etc.) can also directly lead to severe renal fibrosis, suggesting that the steady-state imbalance of DNA methylation directly affects renal fibrosis progression (Chen et al., 2013; Wing et al., 2014; Mao, 2015).
4.4.2 Histone modifications
Histones are highly conserved proteins in mammals and are the building blocks of nucleosomes. Histone modifications include acetylation, methylation, phosphorylation and other processes. Histone modifications include acetylation, methylation, phosphorylation and other processes. Renal fibrosis is mainly related to histone acetylation and methylation processes, which mainly occur on key amino acids in histone tails (Tampe and Zeisberg, 2014b; Ding et al., 2021).
Histone acetylation is generally associated with active gene transcription. It promotes gene transcription by reducing the net positive charge of histones and reducing the interaction between histones and DNA. This process is induced by histone acetyltransferases (HATs) and can be reversed by histone deacetylases (HADCs) (Morgado-Pascual et al., 2018). Most studies have found that the levels of HDAC1, HDAC2, and HDAC7 are increased in the kidneys of mice of the UUO model. This decreased histone acetylation and increased deacetylation. These effects resulted in gene transcriptional silencing and the induction of inflammation and renal fibrosis (Pang et al., 2009; Marumo et al., 2010). Various HDAC inhibitors (such as TSA, FK228, butyrate, MS-275, and valproic acid) can reverse the activation and proliferation of fibroblasts, tubular death and the EMT caused by abnormal HDAC expression. Fibrosis-related signaling pathways induced by factors such as TGF-β, EGRF, STAT3, and JNK/Notch2 can also be inhibited (Nie et al., 2020). This finding indicates that histone deacetylation is positively correlated with renal fibrosis and that reducing histone deacetylation can effectively alleviate renal fibrosis.
Another important genetic modification is histone methylation. Histone methylation can both activate and repress gene transcription, which is related to the position of the modified amino acid residues (Morgado-Pascual et al., 2018). In a study by Sun et al., TGF-β1 significantly increased the expression of ECM-related genes, such as collagen-1α1, CTGF, and PAI-1, by inducing histone lysine 4 methylation (H3K4me) and promoted fibrosis (Tampe and Zeisberg, 2014a).
5 Modern pharmacological studies of natural components in R. ribes L
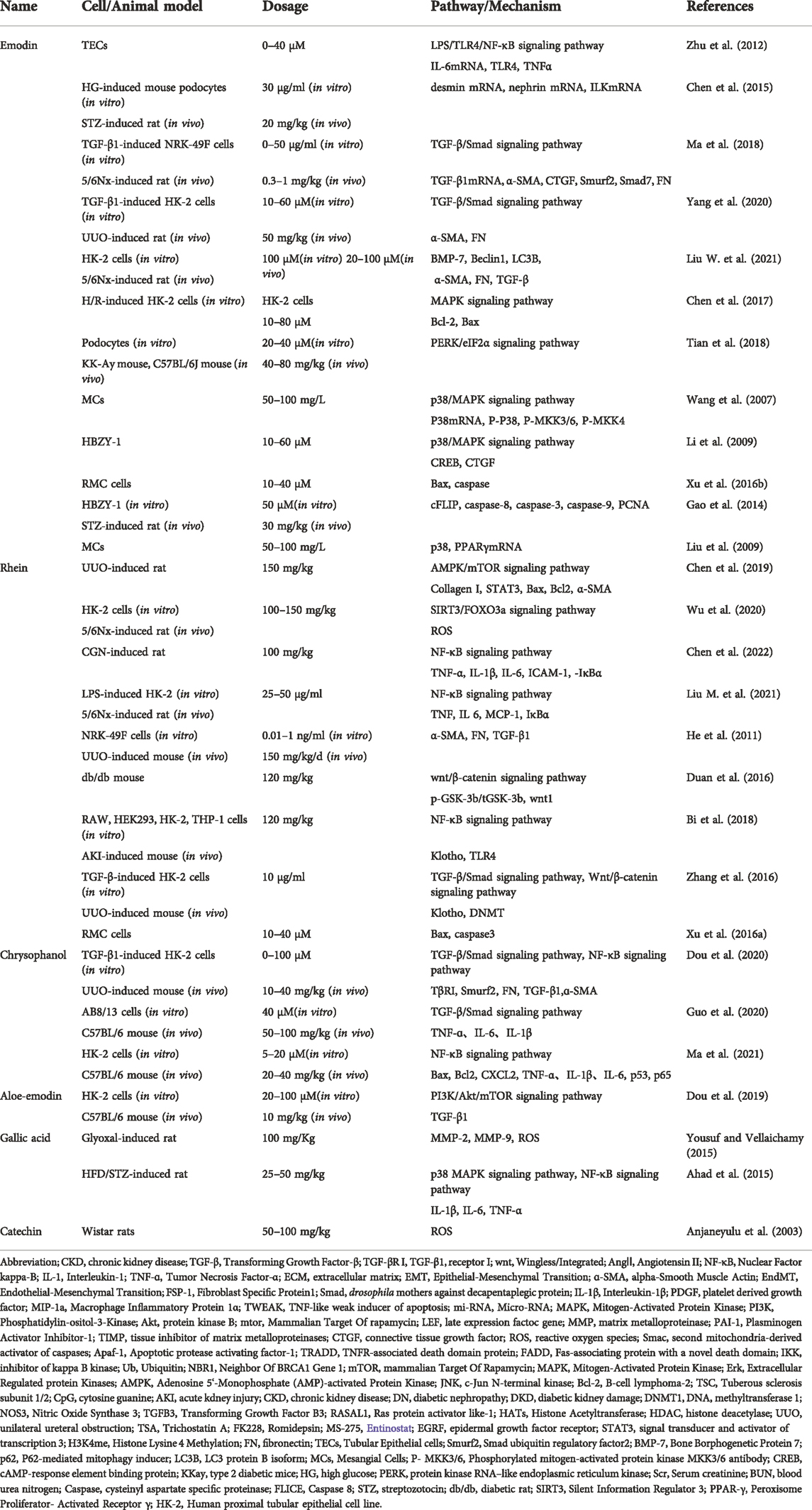
Emodin is generally extracted from the rhizomes of plants. Studies have found that emodin has anticancer, anti-inflammatory, antibacterial, and antioxidant effects (Dong et al., 2016; Cui et al., 2020). Its molecular formula is shown in Figure 4. In recent years, studies have confirmed that in addition to the above effects, emodin also has an anti-tissue fibrosis effect (Zheng et al., 2021). Rhein is another anthraquinone compound with good lipophilicity (Zhou Y. X. et al., 2015). Modern pharmacological studies have found that rhein has anti-inflammatory, antitumor and antifibrotic effects and has certain protective effects in the liver and kidneys. The molecular schematic diagram of rhein is shown in Figure 4 (Sun H. et al., 2016; Li et al., 2021). Chrysophanol is derived from Polygonaceae plants. According to reports in recent years, chrysophanol has anti-inflammatory, antioxidant, anticancer, and neuroprotective effects (Xie et al., 2019; Yusuf et al., 2019). Chrysophanol also shows considerable curative effects in the fight against renal fibrosis, especially its inhibitory effect on the TGF-β/Smad signaling pathway. Aloe-emodin is not only rich in R. ribes L. but is also an effective ingredient in Chinese medicinal materials such as aloe and fleece-flower root. It has anticancer, antiviral, antibacterial, antiparasitic and other pharmacological effects (Dong et al., 2020).
6 Inhibitory effects of the natural ingredients on renal fibrosis
6.1Emodin
6.1.1 Inhibition of the inflammatory response and podocyte injury
Zhu et al. reported that emodin at a concentration of 20 μM or 40 μM could inhibit lipopolysaccharide (LPS)-induced renal injury and increase the inflammatory response and interstitial fibrosis in mouse TECs. Moreover, emodin mediated the decrease in TNF-ɑ, IL-6 and TLR4 mRNA levels. This suggests that emodin can reduce inflammation and renal injury and inhibit renal fibrosis by inhibiting the LPS/TLR4/NF-κB signaling pathway (Zhu et al., 2012). A study by Chen et al. confirmed that emodin can inhibit high glucose-induced podocyte dysfunction and EMT. In addition, emodin decreased ILK and desmin expression in podocytes and increased renin expression. Therefore, emodin attenuates renal injury and restores podocyte function by inhibiting ILK expression (Chen et al., 2015).
6.1.2 Inhibition of TGF-β
Ma et al. confirmed that 1 mg/kg emodin could improve renal function in 5/6 nephrectomy rats. In addition, rhubarb was also detected to reduce the levels of fibrosis-related molecules, such as TGF-β1 mRNA and FN, α-SMA, CTGF and Smurf2 proteins, while upregulating the level of Smad7. It has been shown that emodin can inhibit renal fibrosis by inhibiting the TGF-β/Smad signaling pathway (Ma et al., 2018). Yang et al. found that 20 μM emodin inhibited TGF-β1-induced activation of FN and ɑ-SMA in HK2 cells. When emodin was used in combination with HGF, the expression of TGF-β1 in UUO mice and in HK2 cells was decreased. This indicates that the combined use of HGF and emodin can inhibit renal fibrosis by inhibiting the TGF-β/Smad signaling pathway, and the effect is better than that of emodin alone (Yang et al., 2020). Yang et al. confirmed that emodin can inhibit the expression of TGF-β1 and FN by inhibiting the NF-κB signaling pathway (Yang et al., 2013). In addition, the study of Liu Wei et al. confirmed that emodin can improve TGF-β1-induced EMT. Downregulation of TGF-β, FN and α-SMA levels inhibits renal fibrosis (Liu W. et al., 2021).
6.1.3 Regulation of autophagy and apoptosis
Liu Hong et al. confirmed that emodin could improve renal fibrosis, podocyte injury and epithelial cell apoptosis in streptozotocin (STZ)-treated rats. Experiments showed that emodin enhanced the protein expression of LC3-II/I, Beclin-1, and p-AMPK and inhibited the protein expression of p62 and p-mTOR. Therefore, emodin enhances podocyte autophagy, inhibits apoptosis, and alleviates renal fibrosis mainly by regulating the AMPK/mTOR signaling pathway (Liu H. et al., 2021). Liu Wei et al. confirmed that 100 μM emodin could inhibit and improve renal function in rats. Studies have shown that emodin induces the downregulation of p62 protein expression, while BMP-7, Beclin1, and LC3B protein expression is upregulated. It was demonstrated that emodin can clear injured renal cells by upregulating BMP-7-induced autophagy, alleviating EMT, maintaining renal homeostasis, and inhibiting fibrosis (Liu W. et al., 2021).
Chen et al. showed that emodin attenuates the hypoxia/reoxygenation (H/R)-induced loss of cell viability and apoptosis. Emodin induces an increase in Bcl-2 expression and a decrease in Bax expression. This reduces tubular apoptosis to protect the renal tubules from H/R damage (Chen et al., 2017). Tian et al. used KKay mice and found that 80 mg/kg/d emodin inhibited endoplasmic reticulum stress and podocyte apoptosis induced by HG. Furthermore, emodin treatment decreased the expression of endoplasmic reticulum kinase (P-PERK), P-eIF2α, ATF4 and CHOP. This suggests that emodin can antagonize the podocyte apoptosis that is induced by endoplasmic reticulum stress by inhibiting the PERK/eIF2α signaling pathway (Tian et al., 2018).
6.1.4 Restoration of normal mesangial cell function
Yang et al. reported that emodin could alleviate high glucose-induced renal mesangial cell proliferation and glomerulosclerosis. Decreased levels of p65 and increased levels of IκB were detected in the study. Emodin can inhibit the proliferation of mesangial cells by inhibiting the NF-κB signaling pathway (Yang et al., 2013). Wang Rong et al. found that emodin can improve IL-1β-induced mesangial cell proliferation and extracellular matrix deposition. Experiments demonstrated that the upstream kinase P-MKK3/6 activity of p38 was inhibited. Emodin reduces renal fibrosis by inhibiting the p38/MAPK signaling pathway (Wang et al., 2007). Similarly, Li et al. also proposed that emodin can inhibit the abnormal proliferation of rat mesangial cells mediated by HG. The levels of the p38/MAPK downstream protein factors CREB and CTGF were decreased. It was confirmed that emodin can inhibit the proliferation of mesangial cells, which is mainly achieved by inhibiting the p38/MAPK signaling pathway (Li et al., 2009). The study by Xu et al. showed that emodin can also inhibit the abnormal proliferation of mesangial cells that is induced by HG by upregulating the levels of Bax and caspase. Emodin can also improve renal fibrosis by inducing the apoptosis of abnormally proliferating mesangial cells (Xu et al., 2016b). Gao et al. proposed that the abnormal proliferation and apoptotic inhibition of mesangial cells caused by HG may be related to the activation of CFLIP. After emodin treatment, the level of CFLIP decreased, apoptosis inhibition was relieved, and the expression of FN decreased. It was proven that the protective effect of emodin on the kidney tissue of DN mice may be related to the inhibition of CFLIP expression (Gao et al., 2014). Liu et al. found that emodin ameliorated high glucose-induced mesangial cell contractility deficiency and increased the glomerular filtration rate. It was confirmed that emodin activates PPARγ mRNA and reduces the expression of P38. This finding indicates that emodin improves the function of mesangial cells and glomeruli in diabetic nephropathy by inhibiting the P38/MAPK signaling pathway (Liu et al., 2009).
6.2 Rhein
6.2.1 Inhibition of inflammation and oxidative stress
Rhein also has considerable efficacy in inhibiting kidney inflammation and oxidative stress. A study published in 2020 showed that rhein significantly inhibited the lipopolysaccharide-induced aggravation of kidney inflammation in 5/6 Nx rats and protected the function of damaged kidneys. Experiments detected that the expression of proinflammatory factors, such as TNF-ɑ, IL-6 and MCP-1, was inhibited. In particular, the level of phosphorylated IκBɑ was significantly reduced, and the mechanism by which rhein inhibits the inflammatory response and oxidative stress may act to block the activation of the NF-κB signaling pathway by inhibiting IκBɑ phosphorylation (Liu M. et al., 2021). Similarly, Chen et al. demonstrated that rhein attenuates the renal inflammatory response in CGN rats by inhibiting the activation of the NF-κB signaling pathway (Chen et al., 2022). Studies have reported that treatment with rhein in 5/6 Nx rats alleviated tubular damage, interstitial inflammation, and collagen deposition. By using SIRT3 knockout as a control experiment, it was confirmed that rhein inhibited Foxo3a expression and reduced ROS synthesis and oxidative stress. The pharmacological mechanism of rhein acts to inhibit the expression of the SIRT3/Foxo3a signaling pathway (Wu et al., 2020). The experiments of Chen Yakun et al. also found that UUO was accompanied by an increase in the phosphorylation level of STAT3, and the expression of ɑ-SMA and type I collagen was increased. The mRNA expression levels of STAT3 and ɑ-SMA were inhibited by rhein treatment. This also indicates that the inhibition of renal fibrosis by rhein may be related to the inhibition of STAT3 expression by rhein (Chen et al., 2019).
6.2.2 Inhibition of TGF-β and wnt
In a study by Ho et al., rhein attenuated the collagen deposition caused by interstitial injury in UUO mice. Rhein inhibits the activation of the TGF-β/Smad signaling pathway by inhibiting the expression of TGF-β1 and TβRI and improves the deformation of renal tubular epithelial cells, tubular atrophy, ECM deposition and renal fibrosis in mice (He et al., 2011). Rhein also intervenes in the Wnt/β-catenin signaling pathway. In a study by Duan et al., rhein inhibited the expression of Wnt protein, β-catenin and GSK3b in the kidneys of db/db mice. Rhein can also increase the level of renin in podocytes and inhibit the ablation of podocyte foot processes, thereby maintaining podocyte stability (Duan et al., 2016).
6.2.3 Regulation of epigenetic modifications
Klotho is a joint-regulatory gene that is involved in renal fibrosis. It can inhibit key signaling pathways that mediate fibrosis, such as TGF-β/Smad and Wnt/β-catenin. In a study by Zhang et al., rhein attenuated UUO treatment and TGF-β1-induced DNMT aberrations. Klotho inhibition regulates fibrosis-related signaling pathways by reversing Klotho methylation, improves abnormal pathological changes in renal tissue, and slows the process of renal fibrosis (Zhang et al., 2016). Klotho also mediates toll-like receptor 4 (TLR4) degradation, thereby reducing lipopolysaccharide (LPS)-induced acute inflammation. Studies have shown that the expression of the NF-κB signaling pathway and cytokines in mice treated with rhein was reduced, mainly because rhein relieved the inhibitory effect of LPS on Klotho (Bi et al., 2018).
6.2.4 Apoptosis regulation
In the study of Chen et al., the expression of Bax was increased and the expression of Bcl2 was decreased in rats after UUO treatment. The ratio of Bax to Bcl2 was significantly decreased after treatment with emodin, indicating that emodin can regulate cell apoptosis (Chen et al., 2019). Xu et al. placed RMC cells in a high glucose environment and found that rhein can antagonize the abnormal proliferation of mesangial cells caused by HG. Rhein downregulated HG-induced collagen IV and laminin mRNA expression. Evidence suggests that this is achieved primarily by regulating apoptosis in abnormally proliferating mesangial cells (Xu et al., 2016a).
6.3 Chrysophanol
6.3.1 Inhibition of TGF-β
A study by Dou et al. found that chrysophanol can downregulate the mRNA expression of TβRI, Smurf2, TGF-β1, FN, ɑ-SMA, collagen type I and collagen type III both in vivo and in vitro, thereby inhibiting renal fibrosis. Further studies confirmed that chrysophanol inhibits the activation of the TGF-β/Smad signaling pathway by inhibiting the expression of TβRI and Smurf2 and promoting the interaction between Smad7 and Smurf2 and TβRI (Dou et al., 2020). Guo Chuan et al. placed AB8/13 cells in a high-glucose environment. They found that HG induced the expression of Smad2 and Smad3 in podocytes and increased the levels of TNF-ɑ, IL-6, and IL-1β. After chrysophanol treatment, the TGF-β/Smad signaling pathway was inhibited, and the above inflammation- and fibrosis-related factors were inhibited (Guo et al., 2020).
6.3.2 Inhibition of inflammation, apoptosis and oxidative stress
Another study demonstrated that chrysophanol alleviated cisplatin (CDDP)-induced acute kidney injury (AKI). Its pharmacological mechanism may primarily act through the inhibition of CDDP-induced oxidative stress, apoptosis and the NF-κB signaling pathway (Ma et al., 2021).
6.4 Aloe-emodin
6.4.1 PI3K/Akt/mTOR
To verify the anti-renal fibrosis effect of aloe-emodin, Dou et al. constructed a model of renal fibrosis in vitro and in vivo. Studies have found that aloe-emodin can reduce the protein levels of PI3K, Akt and mTOR in UUO mice, improve biochemical indicators such as Scr and BUN in UUO mice, and inhibit the mRNA expression of TGF-β1, collagen type I, collagen type IV and FN. In the in vitro model, aloe-emodin blocked the activation of the PI3K/Akt/mTOR signaling pathway in TGF-β1-treated HK2 cells. The above findings all prove that aloe-emodin can inhibit the PI3K/Akt/mTOR signaling pathway and improve renal fibrosis (Dou et al., 2019).
6.5 Gallic acid
In addition to the above natural components, there are also some natural products with a lower content in rhubarb that also have a considerable intervention effect on renal fibrosis. For example, gallic acid can reduce the expression of fibrosis-related molecules, such as collagen and MMP-2, in the kidneys of rats induced by glyoxal. It can also reduce the level of serum markers such as Scr, protect renal function, and inhibit renal fibrosis (Yousuf and Vellaichamy, 2015). In addition, studies have reported that gallic acid can improve renal dysfunction and inflammatory responses in diabetic nephropathy rats. It can also reduce IL-1β, IL-6, TNF-α levels, and improve renal fibrosis by inhibiting p38 MAPK signaling pathway and NF-κB signaling pathway (Ahad et al., 2015).
6.6 Catechin
Muragundla et al. found that catechin can reduce ROS activity. This indicates that catechin can protect against renal function damage caused by CsA by inhibiting oxidative stress. Thus, it can inhibit renal interstitial fibrosis and reduce the degree of renal tubular atrophy (Anjaneyulu et al., 2003).
The natural components in rhubarb (such as rhein, emodin, chrysophanol, aloe-emodin, gallic acid and catechin) can be used for TGF-β/Smad, LPS/TLR4/NF-κB, Wnt/β, respectively. -Catenin, PI3K/Akt/mTOR and other signaling pathways achieve the purpose of inhibiting oxidative stress, regulating apoptosis and autophagy, and reducing inflammation. Ultimately, the anti-fibrotic effect is achieved.
7 Discussion and outlook
In recent years, there have been many studies on the effectiveness of natural components in R. ribes L. against clinical diseases. For instance, emodin has high potential for the treatment of atherosclerosis, cardiovascular disease, liver disease and cancer and has the advantages of multitarget, multiefficacy, and broad pharmacological effects (Hsu and Chung, 2012; Hu et al., 2020; Luo et al., 2021). Rhein has shown inhibitory effects against a variety of cancers, both in in vitro and in vivo models, and rhein can modulate different signaling cascades in cancer cells (Henamayee et al., 2020). Chrysophanol can effectively prevent and treat central nervous system diseases, such as stroke and cerebral hemorrhage (Li et al., 2019). Furthermore, many studies based on animal models have confirmed that the natural components in R. ribes L. can delay the process of renal fibrosis. Therefore, we reviewed the research progress of the natural components in the Chinese herbal medicine R. ribes L. in inhibiting renal fibrosis in recent years, considered its advantages and disadvantages, and summarized and discussed how to exert the therapeutic potential of this drug.
The natural components in R. ribes L. can inhibit renal fibrosis through multiple pathways. For example, the main targets of emodin are the TGF-β/Smad signaling pathway, p38/MAPK signaling pathway, MMP and AMPK/mTOR signaling pathway. Through the above pathways, emodin can ultimately reduce the levels of FN and ɑ-SMA, reduce the ECM deposition, and inhibit the expression of apoptosis-related factors. Rhein can inhibit renal fibrosis through the TGF-β/Smad signaling pathway, Wnt/β-catenin signaling pathway, DNA methylation, apoptosis and autophagy. Chrysophanol mainly acts on inflammation and the TGF-β/Smad signaling pathway and inhibits podocyte ECM, effectively alleviating fibrosis. Aloe-emodin showed a good protective effect on podocyte autophagy, which was mainly achieved by inhibiting the PI3K/Akt/mTOR signaling pathway. Other natural ingredients, such as gallic acid and catechins, primarily inhibit oxidative stress and collagen deposition. In general, the above natural ingredients for the treatment of renal fibrosis reflect the advantages of multiple targets, multiple pathways, extensive effects, strong pertinence, and considerable curative effects and have certain potential for exploration The molecular mechanism of action of natural ingredients against renal fibrosis can be summarized in (Figure 5; Table 1).
Although studies have shown that the natural components of R. ribes L. have extensive protective effects on various targets of renal fibrosis, there are also certain limitations. For example, some natural ingredients have poor solubility, low bioavailability, poor intestinal absorption, strong dose dependence when intervening in some targets, hepatotoxicity and nephrotoxicity (Dong et al., 2016; Xie et al., 2019; Dong et al., 2020; Li et al., 2021). Most of the above conclusions are based on animal experiments, and there is a lack of their use in clinical practice. It is imminent to find efficient antifibrotic drugs. In the future, large-scale clinical studies can be carried out that focus on improving bioavailability and revealing the relationship between dose and nephrotoxicity.
Although modern pharmacological studies have proven that the natural components in R. ribes L. have certain toxicity to the liver and kidney, it is interesting that R. ribes L. does not show obvious toxicity in general. At a certain dose, it has a protective effect on the liver and kidney (Xiang et al., 2020; Zhuang et al., 2020). This may be related to factors such as the route of administration, individual differences, or interactions between several natural components. In addition, studies have found that the antifibrotic efficacy of some of the above natural products is significantly increased when combined. For example, curcumin and rhein showed synergistic effects when combined, and the therapeutic effect increased (He et al., 2020). The combined application of emodin and HGF increased the inhibitory effect on the TGF-β/Smad signaling pathway (Yang et al., 2020). This suggests that the combination of natural products with other antifibrotic drugs can increase efficacy and reduce toxicity. In terms of drug extraction, the subcritical water extraction method discovered in recent years has certain advantages in the extraction of natural products (Cheng et al., 2021). The application of this method to the extraction of anthraquinones may increase the extraction rate and utilization rate. In general, understanding the dose or combination of drugs may be the key to increasing the antifibrotic efficacy and reducing toxicity, and emerging extraction technology may have certain potential in improving bioavailability.
In conclusion, the extensive anti-renal fibrosis effect of the natural components in R. ribes L. provides information for the development of new drugs for renal fibrosis, and future in-depth research may find new targets and new ways to treat renal fibrosis.
Author contributions
YW: A major contributor to this manuscript. Wrote first draft, downloaded bibliography and created diagrams. AL, FY: Analyzed literature and organized data CQ, CH: Collecting data ZH: Organize Abbreviations and Import References XM, HZ: Guided and revised article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahad, A., Ahsan, H., Mujeeb, M., and Siddiqui, W. A. (2015). Gallic acid ameliorates renal functions by inhibiting the activation of p38 MAPK in experimentally induced type 2 diabetic rats and cultured rat proximal tubular epithelial cells. Chem. Biol. Interact. 240, 292–303. doi:10.1016/j.cbi.2015.08.026
Aichner, D., and Ganzera, M. (2015). Analysis of anthraquinones in rhubarb (Rheum palmatum and Rheum officinale) by supercritical fluid chromatography. Talanta 144, 1239–1244. doi:10.1016/j.talanta.2015.08.011
Anjaneyulu, M., Tirkey, N., and Chopra, K. (2003). Attenuation of cyclosporine-induced renal dysfunction by catechin: possible antioxidant mechanism. Ren. Fail. 25 (5), 691–707. doi:10.1081/jdi-120024285
Bi, F., Chen, F., Li, Y., Wei, A., and Cao, W. (2018). Klotho preservation by Rhein promotes toll-like receptor 4 proteolysis and attenuates lipopolysaccharide-induced acute kidney injury. J. Mol. Med. 96 (9), 915–927. doi:10.1007/s00109-018-1644-7
Black, L. M., Lever, J. M., and Agarwal, A. (2019). Renal inflammation and fibrosis: a double-edged sword. J. Histochem. Cytochem. 67 (9), 663–681. doi:10.1369/0022155419852932
Black, L. M., Lever, J. M., Traylor, A. M., Chen, B., Yang, Z., Esman, S. K., et al. (2018). Divergent effects of AKI to CKD models on inflammation and fibrosis. Am. J. Physiol. Ren. Physiol. 315 (4), F1107–f1118. doi:10.1152/ajprenal.00179.2018
Blobe, G. C., Schiemann, W. P., and Lodish, H. F. (2000). Role of transforming growth factor beta in human disease. N. Engl. J. Med. 342 (18), 1350–1358. doi:10.1056/NEJM200005043421807
Bülow, R. D., and Boor, P. (2019). Extracellular matrix in kidney fibrosis: more than just a scaffold. J. Histochem. Cytochem. 67 (9), 643–661. doi:10.1369/0022155419849388
Chen, H., Huang, R. S., Yu, X. X., Ye, Q., Pan, L. L., Shao, G. J., et al. (2017). Emodin protects against oxidative stress and apoptosis in HK-2 renal tubular epithelial cells after hypoxia/reoxygenation. Exp. Ther. Med. 14 (1), 447–452. doi:10.3892/etm.2017.4473
Chen, J., Zhang, X., Zhang, H., Lin, J., Zhang, C., Wu, Q., et al. (2013). Elevated Klotho promoter methylation is associated with severity of chronic kidney disease. PLoS One 8 (11), e79856. doi:10.1371/journal.pone.0079856
Chen, Q., Guo, H., Hu, J., and Zhao, X. (2022). Rhein inhibits NF-κB signaling pathway to alleviate inflammatory response and oxidative stress of rats with chronic glomerulonephritis. Appl. Bionics Biomech. 2022, 9671759. doi:10.1155/2022/9671759
Chen, T., Zheng, L. Y., Xiao, W., Gui, D., Wang, X., and Wang, N. (2015). Emodin ameliorates high glucose induced-podocyte epithelial-mesenchymal transition in-vitro and in-vivo. Cell. Physiol. Biochem. 35 (4), 1425–1436. doi:10.1159/000373963
Chen, Y., Mu, L., Xing, L., Li, S., and Fu, S. (2019). Rhein alleviates renal interstitial fibrosis by inhibiting tubular cell apoptosis in rats. Biol. Res. 52 (1), 50. doi:10.1186/s40659-019-0257-0
Cheng, S., and Lovett, D. H. (2003). Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am. J. Pathol. 162 (6), 1937–1949. doi:10.1016/S0002-9440(10)64327-1
Cheng, Y., Xue, F., Yu, S., Du, S., and Yang, Y. (2021). Subcritical water extraction of natural products. Molecules 26 (13), 4004. doi:10.3390/molecules26134004
Chesney, J., and Bucala, R. (2000). Peripheral blood fibrocytes: mesenchymal precursor cells and the pathogenesis of fibrosis. Curr. Rheumatol. Rep. 2 (6), 501–505. doi:10.1007/s11926-000-0027-5
Chinnaiyan, A. M., O'Rourke, K., Tewari, M., and Dixit, V. M. (1995). FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 81 (4), 505–512. doi:10.1016/0092-8674(95)90071-3
Cragg, G. M., and Newman, D. J. (2013). Natural products: a continuing source of novel drug leads. Biochim. Biophys. Acta 1830 (6), 3670–3695. doi:10.1016/j.bbagen.2013.02.008
Cruz-Solbes, A. S., and Youker, K. (2017). Epithelial to mesenchymal transition (EMT) and endothelial to mesenchymal transition (EndMT): role and implications in kidney fibrosis. Results Probl. Cell Differ. 60, 345–372. doi:10.1007/978-3-319-51436-9_13
Cui, Y., Chen, L. J., Huang, T., Ying, J. Q., and Li, J. (2020). The pharmacology, toxicology and therapeutic potential of anthraquinone derivative emodin. Chin. J. Nat. Med. 18 (6), 425–435. doi:10.1016/S1875-5364(20)30050-9
Ding, H., Zhang, L., Yang, Q., Zhang, X., and Li, X. (2021). Epigenetics in kidney diseases. Adv. Clin. Chem. 104, 233–297. doi:10.1016/bs.acc.2020.09.005
Ding, W. X., and Yin, X. M. (2012). Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol. Chem. 393 (7), 547–564. doi:10.1515/hsz-2012-0119
Dong, X., Fu, J., Yin, X., Cao, S., Li, X., Lin, L., et al. (2016). Emodin: a review of its pharmacology, toxicity and pharmacokinetics. Phytother. Res. 30 (8), 1207–1218. doi:10.1002/ptr.5631
Dong, X., Zeng, Y., Liu, Y., You, L., Yin, X., Fu, J., et al. (2020). Aloe-emodin: a review of its pharmacology, toxicity, and pharmacokinetics. Phytother. Res. 34 (2), 270–281. doi:10.1002/ptr.6532
Dou, F., Ding, Y., Wang, C., Duan, J., Wang, W., Xu, H., et al. (2020). Chrysophanol ameliorates renal interstitial fibrosis by inhibiting the TGF-β/Smad signaling pathway. Biochem. Pharmacol. 180, 114079. doi:10.1016/j.bcp.2020.114079
Dou, F., Liu, Y., Liu, L., Wang, J., Sun, T., Mu, F., et al. (2019). Aloe-emodin ameliorates renal fibrosis via inhibiting PI3K/Akt/mTOR signaling pathway in vivo and in vitro. Rejuvenation Res. 22 (3), 218–229. doi:10.1089/rej.2018.2104
Duan, S., Wu, Y., Zhao, C., Chen, M., Yuan, Y., Xing, C., et al. (2016). The wnt/β-catenin signaling pathway participates in rhein ameliorating kidney injury in DN mice. Mol. Cell. Biochem. 411 (1-2), 73–82. doi:10.1007/s11010-015-2569-x
Duffield, J. S. (2010). Macrophages and immunologic inflammation of the kidney. Semin. Nephrol. 30 (3), 234–254. doi:10.1016/j.semnephrol.2010.03.003
Dunlop, E. A., and Tee, A. R. (2013). The kinase triad, AMPK, mTORC1 and ULK1, maintains energy and nutrient homoeostasis. Biochem. Soc. Trans. 41 (4), 939–943. doi:10.1042/BST20130030
Fang, D., Hawke, D., Zheng, Y., Xia, Y., Meisenhelder, J., Nika, H., et al. (2007). Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J. Biol. Chem. 282 (15), 11221–11229. doi:10.1074/jbc.M611871200
Gao, C. C., Li, G. W., Wang, T. T., Gao, L., Wang, F. F., Shang, H. W., et al. (2021). Rhubarb extract relieves constipation by stimulating mucus production in the colon and altering the intestinal flora. Biomed. Pharmacother. 138, 111479. doi:10.1016/j.biopha.2021.111479
Gao, J., Wang, F., Wang, W., Su, Z., Guo, C., and Cao, S. (2014). Emodin suppresses hyperglycemia-induced proliferation and fibronectin expression in mesangial cells via inhibiting cFLIP. PLoS One 9 (4), e93588. doi:10.1371/journal.pone.0093588
Guerrot, D., Dussaule, J. C., Kavvadas, P., Boffa, J. J., Chadjichristos, C. E., and Chatziantoniou, C. (2012). Progression of renal fibrosis: the underestimated role of endothelial alterations. Fibrogenes. Tissue Repair 5 (1), S15. doi:10.1186/1755-1536-5-S1-S15
Guo, C., Wang, Y., Piao, Y., Rao, X., and Yin, D. (2020). Chrysophanol inhibits the progression of diabetic nephropathy via inactivation of TGF-β pathway. Drug Des. Devel. Ther. 14, 4951–4962. doi:10.2147/DDDT.S274191
Guo, Y., Xiao, L., Sun, L., and Liu, F. (2012). Wnt/beta-catenin signaling: a promising new target for fibrosis diseases. Physiol. Res. 61 (4), 337–346. doi:10.33549/physiolres.932289
Hao, S., He, W., Li, Y., Ding, H., Hou, Y., Nie, J., et al. (2011). Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J. Am. Soc. Nephrol. 22 (9), 1642–1653. doi:10.1681/ASN.2010101079
Hayden, M. S., and Ghosh, S. (2014). Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 26 (3), 253–266. doi:10.1016/j.smim.2014.05.004
He, D., Lee, L., Yang, J., and Wang, X. (2011). Preventive effects and mechanisms of rhein on renal interstitial fibrosis in obstructive nephropathy. Biol. Pharm. Bull. 34 (8), 1219–1226. doi:10.1248/bpb.34.1219
He, J., Xu, Y., Koya, D., and Kanasaki, K. (2013). Role of the endothelial-to-mesenchymal transition in renal fibrosis of chronic kidney disease. Clin. Exp. Nephrol. 17 (4), 488–497. doi:10.1007/s10157-013-0781-0
He, X., Li, G., Chen, Y., Xiao, Q., Yu, X., Yu, X., et al. (2020). Pharmacokinetics and pharmacodynamics of the combination of rhein and curcumin in the treatment of chronic kidney disease in rats. Front. Pharmacol. 11, 573118. doi:10.3389/fphar.2020.573118
Henamayee, S., Banik, K., Sailo, B. L., Shabnam, B., Harsha, C., Srilakshmi, S., et al. (2020). Therapeutic emergence of rhein as a potential anticancer drug: a review of its molecular targets and anticancer properties. Molecules 25 (10), E2278. doi:10.3390/molecules25102278
Heuberger, J., and Birchmeier, W. (2010). Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb. Perspect. Biol. 2 (2), a002915. doi:10.1101/cshperspect.a002915
Hsu, H., Xiong, J., and Goeddel, D. V. (1995). The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 81 (4), 495–504. doi:10.1016/0092-8674(95)90070-5
Hsu, S. C., and Chung, J. G. (2012). Anticancer potential of emodin. Biomed. (Taipei) 2 (3), 108–116. doi:10.1016/j.biomed.2012.03.003
Hu, N., Liu, J., Xue, X., and Li, Y. (2020). The effect of emodin on liver disease -- comprehensive advances in molecular mechanisms. Eur. J. Pharmacol. 882, 173269. doi:10.1016/j.ejphar.2020.173269
Isaka, Y. (2018). Targeting TGF-β signaling in kidney fibrosis. Int. J. Mol. Sci. 19 (9), E2532. doi:10.3390/ijms19092532
Ke, B., Fan, C., Yang, L., and Fang, X. (2017). Matrix metalloproteinases-7 and kidney fibrosis. Front. Physiol. 8, 21. doi:10.3389/fphys.2017.00021
Kelly, K. J., Burford, J. L., and Dominguez, J. H. (2009). Postischemic inflammatory syndrome: a critical mechanism of progression in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 297 (4), F923–F931. doi:10.1152/ajprenal.00205.2009
Khan, I. A., Nasiruddin, M., Haque, S. F., and Khan, R. A. (2014). Evaluation of rhubarb supplementation in stages 3 and 4 of chronic kidney disease: a randomized clinical trial. Int. J. Chronic Dis. 2014, 789340. doi:10.1155/2014/789340
Kim, J., and Guan, K. L. (2011). Regulation of the autophagy initiating kinase ULK1 by nutrients: roles of mTORC1 and AMPK. Cell Cycle 10 (9), 1337–1338. doi:10.4161/cc.10.9.15291
Kim, S. I., Na, H. J., Ding, Y., Wang, Z., Lee, S. J., and Choi, M. E. (2012). Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-β1. J. Biol. Chem. 287 (15), 11677–11688. doi:10.1074/jbc.M111.308460
Kim, Y. C., and Guan, K. L. (2015). mTOR: a pharmacologic target for autophagy regulation. J. Clin. Invest. 125 (1), 25–32. doi:10.1172/JCI73939
Kramann, R., and Humphreys, B. D. (2014). Kidney pericytes: roles in regeneration and fibrosis. Semin. Nephrol. 34 (4), 374–383. doi:10.1016/j.semnephrol.2014.06.004
Kurts, C., Panzer, U., Anders, H. J., and Rees, A. J. (2013). The immune system and kidney disease: basic concepts and clinical implications. Nat. Rev. Immunol. 13 (10), 738–753. doi:10.1038/nri3523
Lakkis, J. I., and Weir, M. R. (2018). Obesity and kidney disease. Prog. Cardiovasc. Dis. 61 (2), 157–167. doi:10.1016/j.pcad.2018.07.005
Larkin, B. P., Glastras, S. J., Chen, H., Pollock, C. A., and Saad, S. (2018). DNA methylation and the potential role of demethylating agents in prevention of progressive chronic kidney disease. FASEB J. 32 (10), 5215–5226. doi:10.1096/fj.201800205R
Li, G. M., Chen, J. R., Zhang, H. Q., Cao, X. Y., Sun, C., Peng, F., et al. (2021). Update on pharmacological activities, security, and pharmacokinetics of rhein. Evid. Based. Complement. Altern. Med. 2021, 4582412. doi:10.1155/2021/4582412
Li, L. S., and Liu, Z. H. (1991). Clinical and experimental studies of rheum on preventing progression of chronic renal failure. Zhong Xi Yi Jie He Za Zhi 11 (7), 392–396.
Li, X., Chu, S., Liu, Y., and Chen, N. (2019). Neuroprotective effects of anthraquinones from rhubarb in central nervous system diseases. Evid. Based. Complement. Altern. Med. 2019, 3790728. doi:10.1155/2019/3790728
Li, X., Liu, W., Wang, Q., Liu, P., Deng, Y., Lan, T., et al. (2009). Emodin suppresses cell proliferation and fibronectin expression via p38MAPK pathway in rat mesangial cells cultured under high glucose. Mol. Cell. Endocrinol. 307 (1-2), 157–162. doi:10.1016/j.mce.2009.03.006
Liu, B. C., Tang, T. T., Lv, L. L., and Lan, H. Y. (2018). Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int. 93 (3), 568–579. doi:10.1016/j.kint.2017.09.033
Liu, H., Wang, Q., Shi, G., Yang, W., Zhang, Y., Chen, W., et al. (2021). Emodin ameliorates renal damage and podocyte injury in a rat model of diabetic nephropathy via regulating AMPK/mTOR-Mediated autophagy signaling pathway. Diabetes Metab. Syndr. Obes. 14, 1253–1266. doi:10.2147/DMSO.S299375
Liu, M., Wang, L., Wu, X., Gao, K., Wang, F., Cui, J., et al. (2021). Rhein protects 5/6 nephrectomized rat against renal injury by reducing inflammation via NF-κB signaling. Int. Urol. Nephrol. 53 (7), 1473–1482. doi:10.1007/s11255-020-02739-w
Liu, W., Gu, R., Lou, Y., He, C., Zhang, Q., and Li, D. (2021). Emodin-induced autophagic cell death hinders epithelial-mesenchymal transition via regulation of BMP-7/TGF-β1 in renal fibrosis. J. Pharmacol. Sci. 146 (4), 216–225. doi:10.1016/j.jphs.2021.03.009
Liu, Y. (2011). Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 7 (12), 684–696. doi:10.1038/nrneph.2011.149
Liu, Y. F., Yu, H. M., Zhang, C., Yan, F. F., Liu, Y., Zhang, Y., et al. (2007). Treatment with rhubarb improves brachial artery endothelial function in patients with atherosclerosis: a randomized, double-blind, placebo-controlled clinical trial. Am. J. Chin. Med. 35 (4), 583–595. doi:10.1142/S0192415X07005089
Liu, Y., Jia, L., Liu, Z. C., Zhang, H., Zhang, P. J., Wan, Q., et al. (2009). Emodin ameliorates high-glucose induced mesangial p38 over-activation and hypocontractility via activation of PPARgamma. Exp. Mol. Med. 41 (9), 648–655. doi:10.3858/emm.2009.41.9.071
Livingston, M. J., Ding, H. F., Huang, S., Hill, J. A., Yin, X. M., and Dong, Z. (2016). Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 12 (6), 976–998. doi:10.1080/15548627.2016.1166317
Loboda, A., Sobczak, M., Jozkowicz, A., and Dulak, J. (2016). TGF-β1/Smads and miR-21 in renal fibrosis and inflammation. Mediat. Inflamm. 2016, 8319283. doi:10.1155/2016/8319283
Luo, N., Fang, J., Wei, L., Sahebkar, A., Little, P. J., Xu, S., et al. (2021). Emodin in atherosclerosis prevention: pharmacological actions and therapeutic potential. Eur. J. Pharmacol. 890, 173617. doi:10.1016/j.ejphar.2020.173617
Lv, W., Booz, G. W., Wang, Y., Fan, F., and Roman, R. J. (2018). Inflammation and renal fibrosis: recent developments on key signaling molecules as potential therapeutic targets. Eur. J. Pharmacol. 820, 65–76. doi:10.1016/j.ejphar.2017.12.016
Ma, L., Li, H., Zhang, S., Xiong, X., Chen, K., Jiang, P., et al. (2018). Emodin ameliorates renal fibrosis in rats via TGF-β1/Smad signaling pathway and function study of Smurf 2. Int. Urol. Nephrol. 50 (2), 373–382. doi:10.1007/s11255-017-1757-x
Ma, S., Xu, H., Huang, W., Gao, Y., Zhou, H., Li, X., et al. (2021). Chrysophanol relieves cisplatin-induced nephrotoxicity via concomitant inhibition of oxidative stress, apoptosis, and inflammation. Front. Physiol. 12, 706359. doi:10.3389/fphys.2021.706359
Ma, T. T., and Meng, X. M. (2019). TGF-β/Smad and renal fibrosis. Adv. Exp. Med. Biol. 1165, 347–364. doi:10.1007/978-981-13-8871-2_16
Macconi, D., Remuzzi, G., and Benigni, A. (2014). Key fibrogenic mediators: old players. Renin-angiotensin system. Kidney Int. Suppl. 4 (1), 58–64. doi:10.1038/kisup.2014.11
Majidinia, M., Aghazadeh, J., Jahanban-Esfahlani, R., and Yousefi, B. (2018). The roles of Wnt/β-catenin pathway in tissue development and regenerative medicine. J. Cell. Physiol. 233 (8), 5598–5612. doi:10.1002/jcp.26265
Mao, Y. (2015). Hypermethylation of RASAL1: a key for renal fibrosis. EBioMedicine 2 (1), 7–8. doi:10.1016/j.ebiom.2014.10.016
Martínez-Klimova, E., Aparicio-Trejo, O. E., Gómez-Sierra, T., Jiménez-Uribe, A. P., Bellido, B., and Pedraza-Chaverri, J. (2020). Mitochondrial dysfunction and endoplasmic reticulum stress in the promotion of fibrosis in obstructive nephropathy induced by unilateral ureteral obstruction. Biofactors 46 (5), 716–733. doi:10.1002/biof.1673
Marumo, T., Hishikawa, K., Yoshikawa, M., Hirahashi, J., Kawachi, S., and Fujita, T. (2010). Histone deacetylase modulates the proinflammatory and -fibrotic changes in tubulointerstitial injury. Am. J. Physiol. Ren. Physiol. 298 (1), F133–F141. doi:10.1152/ajprenal.00400.2009
Massagué, J. (1998). TGF-beta signal transduction. Annu. Rev. Biochem. 67, 753–791. doi:10.1146/annurev.biochem.67.1.753
Meng, X. M. (2019). Inflammatory mediators and renal fibrosis. Adv. Exp. Med. Biol. 1165, 381–406. doi:10.1007/978-981-13-8871-2_18
Meng, X. M., Nikolic-Paterson, D. J., and Lan, H. Y. (2014). Inflammatory processes in renal fibrosis. Nat. Rev. Nephrol. 10 (9), 493–503. doi:10.1038/nrneph.2014.114
Mennuni, S., Rubattu, S., Pierelli, G., Tocci, G., Fofi, C., and Volpe, M. (2014). Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J. Hum. Hypertens. 28 (2), 74–79. doi:10.1038/jhh.2013.55
Misseri, R., Meldrum, D. R., Dinarello, C. A., Dagher, P., Hile, K. L., Rink, R. C., et al. (2005). TNF-alpha mediates obstruction-induced renal tubular cell apoptosis and proapoptotic signaling. Am. J. Physiol. Ren. Physiol. 288 (2), F406–F411. doi:10.1152/ajprenal.00099.2004
Misseri, R., and Meldrum, K. K. (2005). Mediators of fibrosis and apoptosis in obstructive uropathies. Curr. Urol. Rep. 6 (2), 140–145. doi:10.1007/s11934-005-0083-5
Morgado-Pascual, J. L., Marchant, V., Rodrigues-Diez, R., Dolade, N., Suarez-Alvarez, B., Kerr, B., et al. (2018). Epigenetic modification mechanisms involved in inflammation and fibrosis in renal pathology. Mediat. Inflamm. 2018, 2931049. doi:10.1155/2018/2931049
Nickel, J., Ten Dijke, P., and Mueller, T. D. (2018). TGF-β family co-receptor function and signaling. Acta Biochim. Biophys. Sin. 50 (1), 12–36. doi:10.1093/abbs/gmx126
Nie, L., Liu, Y., Zhang, B., and Zhao, J. (2020). Application of histone deacetylase inhibitors in renal interstitial fibrosis. Kidney Dis. 6 (4), 226–235. doi:10.1159/000505295
Ortiz, A., González Cuadrado, S., Lorz, C., and Egido, J. (1996). Apoptosis in renal diseases. Front. Biosci. 1, d30–47. doi:10.2741/a114
Pang, M., Kothapally, J., Mao, H., Tolbert, E., Ponnusamy, M., Chin, Y. E., et al. (2009). Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am. J. Physiol. Ren. Physiol. 297 (4), F996–f1005. doi:10.1152/ajprenal.00282.2009
Phanish, M. K., Winn, S. K., and Dockrell, M. E. (2010). Connective tissue growth factor-(CTGF, CCN2)--a marker, mediator and therapeutic target for renal fibrosis. Nephron. Exp. Nephrol. 114 (3), e83–92. doi:10.1159/000262316
Savill, J. (1994). Apoptosis and the kidney. J. Am. Soc. Nephrol. 5 (1), 12–21. doi:10.1681/ASN.V5112
Schaeffner, E., and Ritz, E. (2012). Alcohol and kidney damage: a janus-faced relationship. Kidney Int. 81 (9), 816–818. doi:10.1038/ki.2012.14
Schlöndorff, D., and Banas, B. (2009). The mesangial cell revisited: no cell is an island. J. Am. Soc. Nephrol. 20 (6), 1179–1187. doi:10.1681/ASN.2008050549
Smith, E. R., Wigg, B., Holt, S., and Hewitson, T. D. (2019). TGF-β1 modifies histone acetylation and acetyl-coenzyme A metabolism in renal myofibroblasts. Am. J. Physiol. Ren. Physiol. 316 (3), F517–F529. doi:10.1152/ajprenal.00513.2018
Steinhart, Z., and Angers, S. (2018). Wnt signaling in development and tissue homeostasis. Development 145 (11), dev146589. doi:10.1242/dev.146589
Stemmer, V., de Craene, B., Berx, G., and Behrens, J. (2008). Snail promotes Wnt target gene expression and interacts with beta-catenin. Oncogene 27 (37), 5075–5080. doi:10.1038/onc.2008.140
Sun, H., Luo, G., Chen, D., and Xiang, Z. (2016). A comprehensive and system review for the pharmacological mechanism of action of rhein, an active anthraquinone ingredient. Front. Pharmacol. 7, 247. doi:10.3389/fphar.2016.00247
Sun, Y. B., Qu, X., Caruana, G., and Li, J. (2016). The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation. 92 (3), 102–107. doi:10.1016/j.diff.2016.05.008
Tampe, B., Tampe, D., Müller, C. A., Sugimoto, H., LeBleu, V., Xu, X., et al. (2014). Tet3-mediated hydroxymethylation of epigenetically silenced genes contributes to bone morphogenic protein 7-induced reversal of kidney fibrosis. J. Am. Soc. Nephrol. 25 (5), 905–912. doi:10.1681/ASN.2013070723
Tampe, B., and Zeisberg, M. (2014a). Contribution of genetics and epigenetics to progression of kidney fibrosis. Nephrol. Dial. Transpl. 29 (4), iv72–79. doi:10.1093/ndt/gft025
Tampe, B., and Zeisberg, M. (2014b). Evidence for the involvement of epigenetics in the progression of renal fibrogenesis. Nephrol. Dial. Transpl. 29 (1), i1–i8. doi:10.1093/ndt/gft361
Tan, R. J., and Liu, Y. (2012). Matrix metalloproteinases in kidney homeostasis and diseases. Am. J. Physiol. Ren. Physiol. 302 (11), F1351–F1361. doi:10.1152/ajprenal.00037.2012
Tan, R. J., Zhou, D., Zhou, L., and Liu, Y. (2014). Wnt/β-catenin signaling and kidney fibrosis. Kidney Int. Suppl. 4 (1), 84–90. doi:10.1038/kisup.2014.16
Tang, C., Livingston, M. J., Liu, Z., and Dong, Z. (2020). Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 16 (9), 489–508. doi:10.1038/s41581-020-0309-2
Tang, P. M., Nikolic-Paterson, D. J., and Lan, H. Y. (2019). Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 15 (3), 144–158. doi:10.1038/s41581-019-0110-2
Tang, R., Xiao, X., Lu, Y., Li, H., Zhou, Q., Kwadwo Nuro-Gyina, P., et al. (2020). Interleukin-22 attenuates renal tubular cells inflammation and fibrosis induced by TGF-β1 through Notch1 signaling pathway. Ren. Fail. 42 (1), 381–390. doi:10.1080/0886022X.2020.1753538
Tecklenborg, J., Clayton, D., Siebert, S., and Coley, S. M. (2018). The role of the immune system in kidney disease. Clin. Exp. Immunol. 192 (2), 142–150. doi:10.1111/cei.13119
Thomford, N. E., Senthebane, D. A., Rowe, A., Munro, D., Seele, P., Maroyi, A., et al. (2018). Natural products for drug discovery in the 21st century: innovations for novel drug discovery. Int. J. Mol. Sci. 19 (6), E1578. doi:10.3390/ijms19061578
Tian, N., Gao, Y., Wang, X., Wu, X., Zou, D., Zhu, Z., et al. (2018). Emodin mitigates podocytes apoptosis induced by endoplasmic reticulum stress through the inhibition of the PERK pathway in diabetic nephropathy. Drug Des. Devel. Ther. 12, 2195–2211. doi:10.2147/DDDT.S167405
Tian, X., Liu, Z., Niu, B., Zhang, J., Tan, T. K., Lee, S. R., et al. (2011). E-cadherin/β-catenin complex and the epithelial barrier. J. Biomed. Biotechnol. 2011, 567305. doi:10.1155/2011/567305
Toda, N., Mukoyama, M., Yanagita, M., and Yokoi, H. (2018). CTGF in kidney fibrosis and glomerulonephritis. Inflamm. Regen. 38, 14. doi:10.1186/s41232-018-0070-0
Tonneijck, L., Muskiet, M. H., Smits, M. M., van Bommel, E. J., Heerspink, H. J., van Raalte, D. H., et al. (2017). Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J. Am. Soc. Nephrol. 28 (4), 1023–1039. doi:10.1681/ASN.2016060666
Tzavlaki, K., and Moustakas, A. (2020). TGF-β signaling. Biomolecules 10 (3), E487. doi:10.3390/biom10030487
Valenta, T., Hausmann, G., and Basler, K. (2012). The many faces and functions of β-catenin. EMBO J. 31 (12), 2714–2736. doi:10.1038/emboj.2012.150
Wan, B., Fu, H., Yin, J., and Xu, F. (2014). Efficacy of rhubarb combined with early enteral nutrition for the treatment of severe acute pancreatitis: a randomized controlled trial. Scand. J. Gastroenterol. 49 (11), 1375–1384. doi:10.3109/00365521.2014.958523
Wang, D., Dai, C., Li, Y., and Liu, Y. (2011). Canonical Wnt/β-catenin signaling mediates transforming growth factor-β1-driven podocyte injury and proteinuria. Kidney Int. 80 (11), 1159–1169. doi:10.1038/ki.2011.255
Wang, R., Wan, Q., Zhang, Y., Huang, F., Yu, K., Xu, D., et al. (2007). Emodin suppresses interleukin-1beta induced mesangial cells proliferation and extracellular matrix production via inhibiting P38 MAPK. Life Sci. 80 (26), 2481–2488. doi:10.1016/j.lfs.2007.04.010
Wang, X., Zhou, Y., Tan, R., Xiong, M., He, W., Fang, L., et al. (2010). Mice lacking the matrix metalloproteinase-9 gene reduce renal interstitial fibrosis in obstructive nephropathy. Am. J. Physiol. Ren. Physiol. 299 (5), F973–F982. doi:10.1152/ajprenal.00216.2010
Wanner, N., and Bechtel-Walz, W. (2017). Epigenetics of kidney disease. Cell Tissue Res. 369 (1), 75–92. doi:10.1007/s00441-017-2588-x
Webster, A. C., Nagler, E. V., Morton, R. L., and Masson, P. (2017). Chronic kidney disease. Lancet 389 (10075), 1238–1252. doi:10.1016/S0140-6736(16)32064-5
Wing, M. R., Devaney, J. M., Joffe, M. M., Xie, D., Feldman, H. I., Dominic, E. A., et al. (2014). DNA methylation profile associated with rapid decline in kidney function: findings from the CRIC study. Nephrol. Dial. Transpl. 29 (4), 864–872. doi:10.1093/ndt/gft537
Wozniak, J., Floege, J., Ostendorf, T., and Ludwig, A. (2021). Key metalloproteinase-mediated pathways in the kidney. Nat. Rev. Nephrol. 17 (8), 513–527. doi:10.1038/s41581-021-00415-5
Wu, C. F., Chiang, W. C., Lai, C. F., Chang, F. C., Chen, Y. T., Chou, Y. H., et al. (2013). Transforming growth factor β-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am. J. Pathol. 182 (1), 118–131. doi:10.1016/j.ajpath.2012.09.009
Wu, X., Liu, M., Wei, G., Guan, Y., Duan, J., Xi, M., et al. (2020). Renal protection of rhein against 5/6 nephrectomied-induced chronic kidney disease: role of SIRT3-FOXO3α signalling pathway. J. Pharm. Pharmacol. 72 (5), 699–708. doi:10.1111/jphp.13234
Xiang, H., Zuo, J., Guo, F., and Dong, D. (2020). What we already know about rhubarb: a comprehensive review. Chin. Med. 15, 88. doi:10.1186/s13020-020-00370-6
Xie, L., Tang, H., Song, J., Long, J., Zhang, L., and Li, X. (2019). Chrysophanol: a review of its pharmacology, toxicity and pharmacokinetics. J. Pharm. Pharmacol. 71 (10), 1475–1487. doi:10.1111/jphp.13143
Xu, S., Lv, Y., Zhao, J., Wang, J., Wang, G., and Wang, S. (2016a). The inhibitory effect of rhein on proliferation of high glucose-induced mesangial cell through cell cycle regulation and induction of cell apoptosis. Pharmacogn. Mag. 12 (2), S257–S263. doi:10.4103/0973-1296.182158
Xu, S., Lv, Y., Zhao, J., Wang, J., Zhao, X., and Wang, S. (2016b). Inhibitory effects of Shenkang injection and its main component emodin on the proliferation of high glucose-induced renal mesangial cells through cell cycle regulation and induction of apoptosis. Mol. Med. Rep. 14 (4), 3381–3388. doi:10.3892/mmr.2016.5631
Xu, Y., Ruan, S., Wu, X., Chen, H., Zheng, K., and Fu, B. (2013). Autophagy and apoptosis in tubular cells following unilateral ureteral obstruction are associated with mitochondrial oxidative stress. Int. J. Mol. Med. 31 (3), 628–636. doi:10.3892/ijmm.2013.1232
Yang, F., Deng, L., Li, J., Chen, M., Liu, Y., Hu, Y., et al. (2020). Emodin retarded renal fibrosis through regulating HGF and TGFβ-smad signaling pathway. Drug Des. Devel. Ther. 14, 3567–3575. doi:10.2147/DDDT.S245847
Yang, J., Zeng, Z., Wu, T., Yang, Z., Liu, B., and Lan, T. (2013). Emodin attenuates high glucose-induced TGF-β1 and fibronectin expression in mesangial cells through inhibition of NF-κB pathway. Exp. Cell Res. 319 (20), 3182–3189. doi:10.1016/j.yexcr.2013.10.006
Yang, X., Shen, Y., Zhu, Z., and Deng, A. (2006). Apoptosis signaling pathway in a subtotal nephrectomy rat model. J. Huazhong Univ. Sci. Technol. Med. Sci. 26 (4), 425–428. doi:10.1007/s11596-006-0412-z
Yin, Q., and Liu, H. (2019). Connective tissue growth factor and renal fibrosis. Adv. Exp. Med. Biol. 1165, 365–380. doi:10.1007/978-981-13-8871-2_17
Yousuf, M. J., and Vellaichamy, E. (2015). Protective activity of gallic acid against glyoxal -induced renal fibrosis in experimental rats. Toxicol. Rep. 2, 1246–1254. doi:10.1016/j.toxrep.2015.07.007
Yuan, Q., Tan, R. J., and Liu, Y. (2019). Myofibroblast in kidney fibrosis: origin, activation, and regulation. Adv. Exp. Med. Biol. 1165, 253–283. doi:10.1007/978-981-13-8871-2_12
Yusuf, M. A., Singh, B. N., Sudheer, S., Kharwar, R. N., Siddiqui, S., Abdel-Azeem, A. M., et al. (2019). Chrysophanol: a natural anthraquinone with multifaceted biotherapeutic potential. Biomolecules 9 (2), E68. doi:10.3390/biom9020068
Zhang, Q., Yin, S., Liu, L., Liu, Z., and Cao, W. (2016). Rhein reversal of DNA hypermethylation-associated Klotho suppression ameliorates renal fibrosis in mice. Sci. Rep. 6, 34597. doi:10.1038/srep34597
Zhang, Y. E. (2017). Non-smad signaling pathways of the TGF-β family. Cold Spring Harb. Perspect. Biol. 9 (2), a022129. doi:10.1101/cshperspect.a022129
Zhao, J. H. (2019). Mesangial cells and renal fibrosis. Adv. Exp. Med. Biol. 1165, 165–194. doi:10.1007/978-981-13-8871-2_9
Zhao, X. C., Livingston, M. J., Liang, X. L., and Dong, Z. (2019). Cell apoptosis and autophagy in renal fibrosis. Adv. Exp. Med. Biol. 1165, 557–584. doi:10.1007/978-981-13-8871-2_28
Zhao, Y., Qiao, X., Tan, T. K., Zhao, H., Zhang, Y., Liu, L., et al. (2017). Matrix metalloproteinase 9-dependent Notch signaling contributes to kidney fibrosis through peritubular endothelial-mesenchymal transition. Nephrol. Dial. Transpl. 32 (5), 781–791. doi:10.1093/ndt/gfw308
Zheng, Q., Li, S., Li, X., and Liu, R. (2021). Advances in the study of emodin: an update on pharmacological properties and mechanistic basis. Chin. Med. 16 (1), 102. doi:10.1186/s13020-021-00509-z
Zhong, X., Chung, A. C., Chen, H. Y., Meng, X. M., and Lan, H. Y. (2011). Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J. Am. Soc. Nephrol. 22 (9), 1668–1681. doi:10.1681/ASN.2010111168
Zhou, Y. X., Xia, W., Yue, W., Peng, C., Rahman, K., and Zhang, H. (2015). Rhein: a review of pharmacological activities. Evid. Based. Complement. Altern. Med. 2015, 578107. doi:10.1155/2015/578107
Zhou, Y. Y., Li, Y., Jiang, W. Q., and Zhou, L. F. (2015). MAPK/JNK signalling: a potential autophagy regulation pathway. Biosci. Rep. 35 (3), e00199. doi:10.1042/BSR20140141
Zhu, X. L., Wang, Y. J., Yang, Y., Yang, R. C., Zhu, B., Zhang, Y., et al. (2012). Suppression of lipopolysaccharide-induced upregulation of toll-like receptor 4 by emodin in mouse proximal tubular epithelial cells. Mol. Med. Rep. 6 (3), 493–500. doi:10.3892/mmr.2012.960
Zhuang, T., Gu, X., Zhou, N., Ding, L., Yang, L., and Zhou, M. (2020). Hepatoprotection and hepatotoxicity of Chinese herb rhubarb (dahuang): how to properly control the "general (jiang jun)" in Chinese medical herb. Biomed. Pharmacother. 127, 110224. doi:10.1016/j.biopha.2020.110224
Keywords: renal fibrosis, R. ribes L., inflammation, ECM, TGF-β, emodin
Citation: Wang Y, Yu F, Li A, He Z, Qu C, He C, Ma X and Zhan H (2022) The progress and prospect of natural components in rhubarb (Rheum ribes L.) in the treatment of renal fibrosis. Front. Pharmacol. 13:919967. doi: 10.3389/fphar.2022.919967
Received: 14 April 2022; Accepted: 03 August 2022;
Published: 29 August 2022.
Edited by:
Yanggang Yuan, Nanjing Medical University, ChinaCopyright © 2022 Wang, Yu, Li, He, Qu, He, Ma and Zhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Ma, dG9ieW1heGlhb0AxNjMuY29t; Huakui Zhan, emhhbmh1YWtAcXEuY29t
 Yangyang Wang
Yangyang Wang Fangwei Yu
Fangwei Yu Ao Li
Ao Li Zijia He1
Zijia He1 Caiyan Qu
Caiyan Qu Xiao Ma
Xiao Ma