
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 18 July 2022
Sec. Renal Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.917975
This article is part of the Research Topic Applications of Herbal Medicine to Control Chronic Kidney Disease: Volume II View all 30 articles
Chronic kidney disease (CKD) is a common and progressive disease that has become a major public health problem on a global scale. Renal fibrosis is a common feature in the pathogenesis of CKD, which is mainly related to the excessive accumulation and deposition of extracellular matrix caused by various inflammatory factors. No ideal treatment has yet been established. In recent years, based on the traditional Chinese medicine (TCM) theory of CKD and its molecular mechanism, clinical evidence or experimental studies have confirmed that a variety of Chinese materia medica (CMM) and their effective components can delay the progress of CKD. TCM believes that the pathogenesis of CKD is the deficiency in the root and excess in the branch, and the deficiency and excess are always accompanied by the disease. The strategies of TCM in treating CKD are mainly based on invigorating Qi, tonifying the kidneys, promoting blood circulation, removing stasis, eliminating heat and dampness, removing turbidity, and eliminating edema, and these effects are multitargeted and multifunctional. This review attempts to summarize the theories and treatment strategies of TCM in the treatment of CKD and presents the efficacy and mechanisms of several CMMs supported by clinical evidence or experimental studies. In addition, the relationship between the macroscopic of TCM and the microscopic of modern medicine and the problems faced in further research were also discussed.
In recent years, research on the mechanism and intervention strategies of chronic kidney disease (CKD) has become a hot spot in the nephrology field. CKD is defined by the Kidney Disease Outcomes Quality Initiative in terms of either kidney damage or decreased glomerular filtration rate (GFR, <60 ml/min per 1.73 m2) with or without evidence of kidney damage, for three or more months, regardless of the cause (National Kidney Foundation, 2002; Steven and Levin, 2013). It is characterized by increased inflammatory cell infiltration, tubular atrophy, tubulointerstitial fibrosis, and glomerulosclerosis, finally leading to some forms of end-stage renal disease (ESRD) or renal failure (Boor et al., 2010). However, in ESRD, the survival of patients depends on the renal replacement therapy or dialysis because of lack of kidney donors. The global burden of CKD study in 2017 showed that the global prevalence of CKD has exceeded 9%, accounting for 18.97% of CKD patients worldwide living in China (GBD Chronic Kidney Disease Collaboration, 2020). In 2017, CKD resulted in 1.2 million deaths, and the number has been projected to rise to 2.2 million (best-case scenario) and up to 4.0 million (worst-case scenario) by 2040 (Foreman et al., 2018). Therefore, CKD has become a major public health problem on a global scale. Delaying or preventing the progress of CKD has become an important challenge facing the clinical medicine community and the health departments of various countries.
Current therapy for CKD includes angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers, which act by decreasing proteinuria, lowering blood pressure, and thus retarding CKD progression (Levey and Coresh, 2012; Ruggenenti et al., 2012). However, the treatments are not sufficient for all patients and long-term medication may lead to a number of adverse effects such as hyperkalemia and acute kidney injury (Kidney Disease Outcomes Quality Initiative (K/DOQI), 2004). As an important branch of complementary and alternative medicine, traditional Chinese medicine (TCM) has been proved to protect people’s health for thousands of years. Preclinical studies or clinical trials have shown that Chinese materia medica (CMM), a form of TCM treatment, is promising in treating CKD, especially in the aspects of reducing proteinuria and adverse effects of western drugs, and reduces ESRD risk by 60% (Wojcikowski et al., 2006; Lin et al., 2015). As a large number of people use herbs for medicinal purposes, the safety of CMM has been questioned. The most well-known adverse effect is nephropathy induced by aristolochic acid, which resulted in ESRD and urothelial malignancy. The mechanism of nephrotoxicity induced by aristolochic acid has been clarified as mainly related to the induction of tubular cell apoptosis, the formation of aristolochic acid-DNA adducts, and the inhibition of mitochondrial ATP synthesis (Nortier and Vanherweghem, 2007; Han et al., 2019). Some studies suggest that nephrotoxic effects may be caused by incorrect use of CMMs or toxic CMMs or potentially toxic CMMs (Jha et al., 2013; Jha and Rathi, 2018). It is important to point out that the use of CMMs by physicians practicing TCM is based on the theory of TCM and they prescribe formulas on the basis of syndrome differentiation and treatment approach (Li L. et al., 2019). According to the ancient compatibility rule of “Jun-Chen-Zuo-Shi” (“monarch-minister-assistant-courier”), making a prescription with two or more CMMs can increase the medicinal effects and restrain the CMM’s toxic properties (Wei and Zheng, 2008). Therefore, the syndrome differentiation and treatment approach and compatibility theory ensure the efficacy and safety of CMMs.
To date, the pathophysiological mechanism of CKD has been reviewed in some studies (Chen et al., 2018; Lv et al., 2018; Rinschen and Saez-Rodriguez, 2021). Some evidence has shown that single CMM and CMM formulas possess a range of important pharmacological properties in improving CKD. In this review, we attempt to discuss the current knowledge of TCM for the treatment of CKD and its possible interventional mechanisms.
The advantages of TCM in the treatment of CKD are mainly reflected in the overall concept and syndrome differentiation. The concept of organs in TCM is different from that in modern medicine. As recorded in the “Yellow Emperor’s Canon of Internal Medicine,” the kidney is the place of true Yin and true Yang and the base of hiding and the place for storing the essence. In the TCM theory, the essence transforms Qi (the vital energy) and produces Blood (the body circulation) (Tu X. et al., 2013). Therefore, abnormalities of the kidney are believed to cause the disorder of the body. TCM classifies CKD into the categories of “edema,” “retention of urine,” and “kidney fatigue.” As CKD is characterized by severe proteinuria, the lesions are mainly in the spleen and kidneys. Proteinuria is the pathological product of human essence substance flowing down and leakage of the essence. A basic substance that constitutes the human body and sustains life activities is similar to “vital essence” in TCM, which emphasizes that these essences should be stored in the human body and should not be released. Damage to the kidney leads to a loss of vital substances, resulting in the deficiency of Qi and Yang. Furthermore, the kidney is called the viscera of water, and it is responsible for the body fluid. If the water is stagnant, dampness and heat will occur (Ding et al., 2014). Moreover, with the damage to the kidney essence, Qi cannot consolidate the Blood, resulting in unfavorable blood circulation and blood stasis (anticoagulation) formation (Li X. et al., 2019a).
Besides, the vital substances stored in the kidneys depend on the transportation and distribution of the spleen. The disorder of spleen transport is usually caused by improper diet, deficiency of endowment, and excessive fatigue, which lead to spleen Qi deficiency. Furthermore, with the development of spleen Qi deficiency, the development of disease, the decline of fire in the vital gate, and the loss of warm spleen, it will further lead to the deficiency of spleen Yang. The spleen cannot warm the grain and liquid, and then the transport of liquid and water is abnormal, which leads to water dampness. As mentioned in “Plain Questions,” with Yin and Yang in relative balance, the spirit can be cured. Thus, TCM believes that “spleen-kidney deficiency” is an internal condition; blood stasis, internal dampness, and heat are inextricably linked to the patient’s viscera (Shi and Shen, 1982; Nie, 2008; Wu and Ma, 2011; Su et al., 2013). The treatment principle for CKD is reinforcing deficiency and purging excess, and simultaneous treatment of the branch and the root to achieve “Yin and Yang in relative balance” (stabilization status).
The pathogen of CKD is mainly manifested as blood stasis, dampness heat (hygropyrexia), and turbid toxin (retained hazardous substances). Blood stasis syndrome is one of the most common CM syndromes among patients with primary glomerular disease (Li et al., 2009). “Blood stasis” in TCM covers glomerulosclerosis, an increase of the extraglomerular matrix, thickening of the basement membrane, adhesion of balloon, microthrombosis in the glomerulus, collapse or stenosis of the capillary lumen, compression and occlusion of vascular loops, and tubulointerstitial fibrosis and atrophy (Wu and Ma, 2011; Guo et al., 2019). TCM has the viewpoint that by removing excessive patterns of stagnated blood, the Blood and Qi can be invigorated, and then they promote blood circulation. This condition is suitable for promoting blood circulation and removing blood stasis, invigorating Qi to reduce swelling.
When the spleen and kidneys are deficient, water dampness is endogenous. Pathogenic dampness resides in the body, leading to heat from Yang, or heat from dietary intake, and eventually pathogenic dampness changes to heat from dampness. As the famous medical scientist Lingtai Xu inferred, there must be heat when there is dampness. In microscopic differentiation of syndromes, dampness heat in renal pathology often shows swelling of endothelial cells, formation of microthrombus, narrowing of the capillary lumen, and release of inflammatory mediators (Shen, 2008; Yu et al., 2016). The theory of lipid toxicity and abnormal hemodynamics can also refer to the syndrome differentiation of dampness heat. If the dampness heat is not treated, the mediating center function of the spleen and stomach will be further affected, the metabolites produced in the body will accumulate in the body, and the biological activity of many metabolites can further lead to the clinical uremic syndrome. These solutes are called uremic toxins, which are equivalent to the category of turbid toxin in TCM (Guo et al., 2019). Turbid toxin acts on the human body and leads to turbidities of cells, tissues, and organs, including hypertrophy, hyperplasia, atrophy, metaplasia, and canceration in modern pathology, as well as changes in inflammation, degeneration, apoptosis, and necrosis (Wang and Zhang, 2003; Wang Z. et al., 2010). The relationship between dampness heat and turbid toxin can be summarized as follows: dampness is the source of turbidity, turbidity is the further step of dampness, heat is the gradual toxin, and the toxin is the extreme of heat. It is important to promote the excretion or removal of dampness heat and turbid toxin in CKD, using methods such as the method of clearing heat, dispelling dampness, and removing turbidity.
According to the long-term clinical practice, different CMMs have different pharmacological effects and are endowed with different TCM efficacies. Previous studies have shown that in patients with CKD stage 3 with different TCM syndrome patterns, four different therapies including invigorating Qi and Blood, promoting blood flow, expelling wind-evil (a kind of exogenous pathogenic factors), and clearing heat and dissipating dampness (regulating the immune system and promoting urination) significantly improved the glomerular filtration rate and decreased proteinuria (Wang YJ. et al., 2012). This reflects the basic principle that TCM adopts different treatment strategies for different syndromes (Figure 1). Here, we summarize the strategies and mechanisms of TCM against CKD from different forms of medication, such as single CMM or CMM extracts, CMM pairs, and CMM formula.
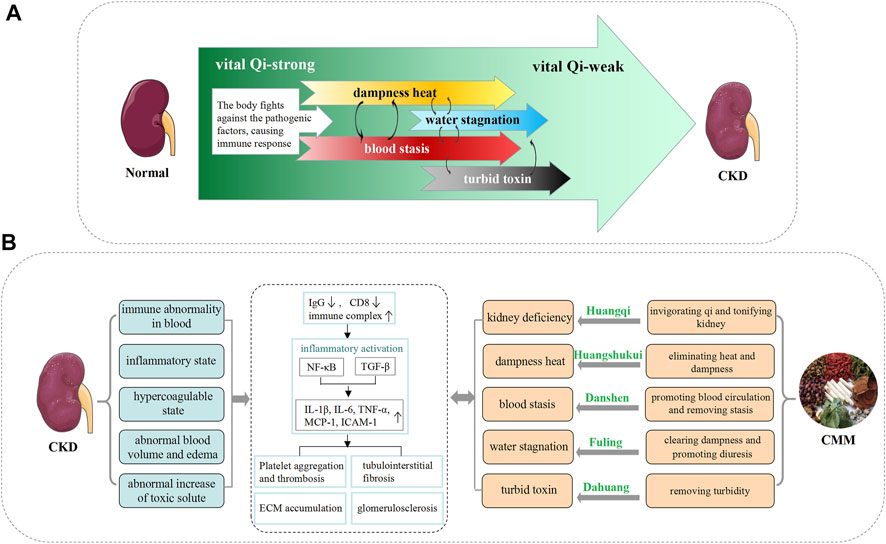
FIGURE 1. The corresponding relationship between the pathogenesis of the TCM theory and the modern medicine theory. (A) Characteristics of TCM pathogenesis in the process of chronic kidney disease. In the early stage of CKD, although the spleen and kidneys are insufficient, the vital Qi can still resist exogenous pathogens, and the pathogenesis is characterized by pathogenic excess (such as dampness heat and blood stasis). However, as the disease progresses to the middle stage, the vital Qi cannot overcome the pathogenic factors, various pathological products are formed, and its pathogenesis characteristics change into a mixture of deficiency and excess. When the disease progresses to the terminal stage, the vital Qi is exhausted, and the pathogenesis is characterized by a deficiency of vital Qi (leading to the accumulation of liquid and turbid toxin). In different stages of nephropathy, the priority of the deficiency and the excess are different. Deficiency and excess are cause and effect of each other, which leads to the continuous progress of CKD. (B) Cognition and treatment strategy of CKD in the TCM theory. On the left are the modern medical characteristics and the main pathological process of CKD. On the right is the treatment strategy of TCM using CMM, and the representative CMMs corresponding to each pathogenesis are listed.
The difference between TCM and modern medicine is that it has the significant property of being multicomponent. Single CMM is a unit that contains the least amount of components in TCM, and the pharmacodynamic material basis has been studied most deeply. With the continuous progress of research, the mechanism of single CMM or CMM extracts for CKD is gradually been clarified.
Astragali Radix (AR, Huangqi) is derived from the dry roots of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao or Astragalus membranaceus (Fisch.) Bge. In the clinical practice of TCM, AR is mainly used to invigorate Qi, manifested in two aspects, supplementing the Defense-Qi (which has the physiological functions of defending exogenous pathogenic factors, warming and nourishing the whole body) and replenishing the Middle-Qi (which is the function motivity of spleen and stomach) (Chen Z. et al., 2020). Therefore, as recorded in “Compendium of Materia Medica,” AR is known as the key CMM of tonics. The chemical studies of its ingredients are relatively clear, mainly including triterpenoid saponins (mostly cycloartane-type), flavonoids, and polysaccharides (Su et al., 2021).
The effects of AR on the reduction of proteinuria and serum creatinine have been studied in patients. After treatment with AR injection in 30 patients with chronic glomerulonephritis, the content of urine protein decreased obviously from 2328 ± 3157 to 1017 ± 765 mg/24 h (Shi et al., 2002). The effect of AR on reducing total cholesterol, triglyceride, and low-density lipoprotein (LDL) and increasing plasma total protein and albumin has also been confirmed in patients with CKD (Zhang et al., 2005). In addition to small clinical trials, a systematic review of 66 studies involving 4,785 diabetic kidney disease participants also demonstrated the benefits of AR injection or preparation in reducing albuminuria, proteinuria, and serum creatinine levels (Zhang L. et al., 2019). Furthermore, AR-containing preparations are effective in improving eGFR in patients with mild to moderate CKD (Yoshino et al., 2022).
In recent years, the immunomodulatory effect of AR has been gradually developed (Wang YP. et al., 2002; Chen Z. et al., 2020). A previous study has shown that AR significantly downregulated CD4 and upregulated CD8 in patients with chronic glomerulonephritis, suggesting that it has a regulatory effect on cellular immunology (Shi et al., 2002). Several pieces of evidence have accumulated showing the potential of AR in reducing water retention (Ma et al., 1996; Wang H. Y. et al., 2002). The therapeutic effect of AR on nephrotic syndrome (NS) may be demonstrated by reducing the expressions of arginine vasopressin (AVP) mRNA, AVP V2 receptor mRNA, and AVP-dependent aquaporin-2 (AQP2) mRNA, thereby eliminating edema. In addition, AR has the effect of ameliorating the blunted renal response to the atrial natriuretic peptide (Ma et al., 1999). It suggested that AR is different from diuretics in the treatment of edema. AR protects against oxidative stress-induced damage in proximal tubular epithelial cells via antiapoptotic and antiinflammatory mechanisms (Shahzad et al., 2016). It was demonstrated by the downregulation of pro-apoptotic Bax and upregulation of Bcl-XL, a decrease in nuclear factor kappa B (NF-κB, p65, p50), a decrease in tumor necrosis factor (TNF)-α, and an increase in transforming growth factor (TGF) β1.
As the main active ingredient of AR, astragaloside significantly improved the state of oxidative stress in podocytes cultured with adriamycin (ADR) and suppressed the cytoskeletal rearrangement, improving the migration ability of podocytes via upregulating the expression of matrix metalloproteinase 2 (MMP-2) and MMP-9 (Sai et al., 2018). The most studied astragaloside IV can inhibit high glucose-induced cell apoptosis through increasing hepatocyte growth factor production via its specific receptor c-met and inhibiting the phospho-p38 mitogen-activated protein kinase (MAPK) signal pathway (Mou et al., 2008; Wang Q. et al., 2014). Calycosin is another component of AR that can inhibit the phosphorylation of IKBα and NF-κB p65 in db/db mice and cultured mouse tubular epithelial cells (Zhang YY. et al., 2019).
Rhei Radix et Rhizoma (RRR, Dahuang), the dried roots and rhizomes of Rheum palmatum L., Rheum tanguticum Maxim. Ex Balf., or Rheum officinale Baill, is widely used as a laxative for many years. Pharmacological studies have confirmed its multiple activities, such as antibacterial, purgative, antifibrosis, regulating gastrointestinal, antiinflammatory, and antitumor activities (Xiang et al., 2020). In a systemic review of 18 randomized or quasirandomized trials including 1,322 patients with CKD, RRR showed significant positive effects on relieving symptoms, lowering serum creatinine, and adjusting disturbance of lipid metabolism (Li et al., 2004). In addition, in a prospective clinical trial of 151 patients with chronic renal failure (CRF), the frequency of end-stage renal failure in the RRR group was 52% lower than that in the ACE inhibitor group. Besides, a slope of progression as the progressive rate of decline in renal function was found to be more horizontal in the RRR-treated group (Li, 1996).
As shown by Zhang et al., treatment with the extracts of RRR reversed the abnormalities of the urinary metabolites in adenine-induced CKD animals and reduced the expression of histopathological inflammatory markers such as collagen (Col) I, Col III, and pro-fibrosis marker TGF-β1 (Zhang et al., 2015). Research on network pharmacology suggested that RRR could target multiple targets involved in the accumulation of extracellular matrix (ECM), the release of inflammatory factors, the balance of coagulation, and fibrinolysis, which showed a synergistic therapeutic effect (Xiang et al., 2015). Regarding the mechanism of RRR against renal fibrosis, some studies have shown that its nephroprotective effect was to reduce the expressions of TGF-β1, TGF-β receptor I (TGF-β RI), TGF-β RII, Smad2, p-Smad2, Smad3, p-Smad3, and Smad4, meanwhile increasing Smad7 (Zhang ZH. et al., 2018). In addition to improving renal fibrosis, RRR could also improve the intestinal barrier function in 5/6 nephrectomy (5/6Nx) rats by regulating systemic inflammation, intestinal barrier markers, and toll-like receptor 4-myeloid differential protein-88-NF-κB inflammatory response (Ji et al., 2020).
Rhein is the main active component of RRR. Recent studies have shown that in adenine mice or unilateral ureteral obstruction (UUO) mice, rhein effectively recovered Klotho promoter hypermethylation via reversing aberrant DNA methyltransferases expression, thus upregulating the expression of Klotho protein (Zhang et al., 2016; Zhang et al., 2017). Besides, the renal protective mechanism of rhein was also related to the activation of the sirtuin 3-forkhead box O3α signaling pathway to exert antioxidant capacity and the regulation of AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) signaling pathways to inhibit autophagy (Tu et al., 2017; Wu et al., 2020).
Abelmoschi Corolla (AC, Huangshukui), the flower of Abelmoschus manihot (L.) Medic., a single medicament of TCM for eliminating heat and dampness, has been widely used for the treatment of CKD in China. As a modern preparation extracted from AC, the Huangkui capsule has been approved by the China National Medical Products Administration (Z19990040). As reviewed by Sun et al., in approximately 2,000 CKD patients, AC showed to decrease proteinuria with stable kidney function during follow-up (Sun X. et al., 2022). Besides, after a comprehensive evaluation, the clinical value of the Huangkui capsule in treating CKD is class B and that for diabetic nephropathy (DN) and chronic nephritis is class A (Wang et al., 2022).
For the mechanistic studies, the Huangkui capsule has been shown to improve kidney inflammation and glomerular injury in ADR-induced nephropathy through inhibition of the p38 MAPK signaling pathway and AC inhibits ROS-ERK1/2-mediated NLRP3 inflammasome activation (Tu Y. et al., 2013; Li W. et al., 2019b). Recent research demonstrated that Huangkui capsule protection against renal fibrosis is dependent on the transient receptor potential channel 6 (TRPC6) pathway (Gu et al., 2020). In detail, the Huangkui capsule inhibited the expressions of a plethora of pro-inflammatory mediators in UUO mice by suppressing both canonical (Smad2/3) and noncanonical (MAPK) signaling pathways and the TRPC6/calcineurin A/nuclear factor of the activated T-cells signaling axis, which were possible through direct inhibition of TRPC6 activity in a heterologous expression system and indirect suppression of TRPC6 expression in both WT and TRPC6 knockout mice. Flavonoids, such as quercetin, isoquercitrin, hyperoside, gossypetin-8-O-β-D-glucuronide, and quercetin-3-O-glucoside, can inhibit the epithelial to mesenchymal transition in HK-2 cells (Cai et al., 2017). An et al. indicated that hyperoside pretreatment could significantly decrease albuminuria and prevent glomerular basement membrane (GBM) damage and oxidative stress in diabetes mellitus mice by decreasing podocyte heparanase expression (An et al., 2017). Liu et al. have revealed that quercetin ameliorated pyroptosis and injury in podocytes under HG conditions via adjusting METTL3-dependent m6A modification and regulating NLRP3-inflammasome activation and PTEN/PI3K/Akt signaling (Liu et al., 2021).
Salviae Miltiorrhizae Radix et Rhizoma (SM, Danshen) is a CMM derived from the root of Salvia miltiorrhiza Bunge. It exhibits various pharmacological activities, such as antioxidant activities, inhibiting the expression of adhesion molecules, antiplatelet aggregation, inhibiting mast cell degranulation, and inhibiting apoptosis and is commonly used for promoting blood circulation and removing stasis (Han et al., 2008). Previous studies have demonstrated that the injection of SM preparation could significantly reduce blood urea nitrogen and creatinine and improve renal function, probably by its antioxidant effects (You et al., 2012; Lu et al., 2015).
In the study of metabonomics in rats with CRF, 54 metabolites could be regulated by SM ethanol extract and water extract (Cai et al., 2018). Moreover, the extract of SM mentioned above significantly regulates the expressions of alpha-smooth muscle actin (α-SMA), FN, E-cadherin, p-ERK, NADPH1 oxidase 1 (NOX1), NOX2, NOX4, TGF-β, TGF-βRI, TGF-βRII, Smad2, Smad3, and Smad7 in HK-2 cells, that is, modulating the NADPH oxidase/ROS/ERK and TGF-β/Smad signaling pathways. A recent study also showed that SM can significantly regulate intestinal bacteria in CRF rats.
Several components isolated from SM have been used to treat kidney disease. The pharmacodynamic effects of salvianolic acid A on 5/6Nx rats showed that it could reduce proteinuria, improve renal function, and reduce renal tubulointerstitial fibrosis. The mechanism is closely related to its antiinflammatory activities through inhibition of the activation of the NF-κB and p38 MAPK signaling pathways (Zhang HF. et al., 2018). In addition, salvianolic acid A treatment improved glucocorticoid resistance of podocytes, partly by modulating the soluble urokinase plasminogen activator receptor (suPAR)/uPAR-αvβ3 signaling pathway (Li X. et al., 2021).
In general, the effect of SM on promoting blood circulation and removing stasis may be related to the increase in renal blood flow, the decrease of the expression of hypoxia-inducible factor (HIF)-1α and vascular endothelial growth factor (VEGF), and the recovery of expression of neuronal nitric oxide synthase (nNOS) by magnesium lithospermate B (Lin et al., 2019). On the other hand, the effects of magnesium lithospermate B on attenuating renal function, renal fibrosis, and inflammation were investigated in 5/6Nx rats, showing a reduction in the expressions of FN, Col III, Col IV, TNF-α, and monocyte chemoattractant protein-1 (MCP-1), which are associated with inhibition of TF activation (Wang et al., 2019). In addition, other studies have shown that inhibition of the mitochondrial apoptosis pathway, i.e., inhibition of mitochondrial Bax accumulation and release of cytochrome c, may also be the renal protective mechanism of magnesium lithospermate B.
Poria (Fuling) is the dry sclerotium derived from Poria cocos (Schw.) Wolf (Polyporaceae). The inner parts of Poria are commonly known as “Fuling” in Chinese. Triterpenoids are the main components of Poria, and it exhibits a variety of biological activities such as antiinflammatory, immunomodulatory, antitumor, antioxidant, and antibacterial activities (Ríos, 2011; Miao et al., 2016; Li X. et al., 2019). In TCM, Poria has the effects of strengthening the spleen, promoting diuresis, and eliminating edema and is mainly used to treat the disorder of fluid metabolism. In the ADR-induced NS rat model, Poria extract could significantly improve the levels of urine protein, creatinine, serum total cholesterol, and IL-4 cytokine and attenuate kidney injury (Zan et al., 2017). Likewise, Poria treatment significantly improves the disturbance of water metabolism in puromycin aminonucleoside (PAN)-induced NS rats by inhibiting the expression of the epithelial sodium channel and AQP2 (Lee et al., 2014). An in vitro study also revealed that Poria treatment inhibited the activation of Sgk1 and decreased the expression of TonEBP mRNA (Lee et al., 2012). Its effect on AQP2 might be related to the inhibition of PKA and the decrease of the cAMP content.
Because of the obvious diuretic effect of ethanol extract from the surface layer of Poria, Zhao et al. focused on the protective effect of triterpenoids from the surface layer of Poria on CKD (Zhao et al., 2012). Several studies have demonstrated that triterpenoids isolated from the surface layer of Poria have beneficial renal protective effects on renal fibrosis. Poricoic acid A is the main triterpenoid in the surface layer of Poria. It exhibited the suppression of fibrosis by stimulating AMPK and inhibiting Smad3, preventing abnormal ECM accumulation and remodeling, and promoting the deactivation of fibroblasts (Chen D. Q. et al., 2020). Poricoic acid A combined with melatonin has a protective effect on acute kidney injury (AKI)-to-CKD transition. On the 14th day of renal ischemia-reperfusion injury in rats, poricoic acid A and melatonin treatment significantly inhibited the expressions of α-SMA, Col I, and FN. Inhibition of the TGF-β/Smad, Wnt/β-catenin, and NF-κB/Nrf2 pathways may be the mechanisms of poricoic acid A against renal fibrosis (Chen D. Q. et al., 2019a; Chen D. Q. et al., 2019b). Interestingly, poricoic acid A and melatonin could upregulate growth arrest-specific 6 (Gas6)/Axl signaling to reduce oxidative stress and inflammation in the AKI disease status and further downregulate Gas6/Axl signaling to attenuate renal fibrosis in the CKD disease status (Chen D. Q. et al., 2019b). This may reveal the bidirectional regulation of TCM to some extent. As reported previously, some new poricoic acids such as poricoic acid ZC, poricoic acid ZD, poricoic acid ZE, poricoic acid ZG, poricoic acid ZH, poricoic acid ZI, poricoic acid ZM, and poricoic acid ZP significantly attenuated renal fibrosis though the same mechanisms as poricoic acid A in vivo and in vitro (Wang et al., 2018a; Wang et al., 2018b; Chen L. et al., 2019; Wang M. et al., 2020a).
CMM pairs (combination of two CMMs) are the most basic composition units, the simplest form of multi-CMM formula, and a centralized representative of CMM compatibility (Wang S. et al., 2012). It is not the superposition of any two CMMs but contains the wisdom and clinical experience of TCM doctors.
AR and Angelicae Sinensis Radix (AS, Danggui, the root of Angelica sinensis (Oliv.) Diels) are a common CMM pair used to treat kidney disease for years. The combination of the two CMMs is also known as Danggui Buxue Tang (DBT) with a weight ratio of 5:1. When the two CMMs are decocted together, it can help a better dissolution of effective components and the generation of new components. For example, with the increase in the ratio of AR, the dissolution of ferulic acid and ligustilide increased (Gao et al., 2007). Moreover, the results indicated that immunomodulatory, oesteotropic, and estrogenic effects were best exerted at the AR to AS ratio of 5:1.
According to what has been mentioned before, blood stasis always accompanies CKD. Studies have shown that the AR–AS pair also could promote recovery of blood flow, improve the microstructure dysfunction, enhance NO production via activating eNOS, and induce erythropoietin (EPO) mRNA expression and secretion of EPO protein (Meng et al., 2007; Gao et al., 2008). The mechanism acting on EPO might be related to increasing the expression of HIF-1α mRNA and promoting the protein translation of HIF-1α via a Raf/MEK/ERK-dependent signaling pathway (Zheng et al., 2010). However, some studies reported that the CMM pair could not reduce urinary protein (Lu et al., 1997; Li et al., 2000). It was suggested that the effect of the pair on preventing and treating glomerulosclerosis might not directly affect hemodynamics or filtration membrane permeability but is related to regulating lipid metabolism by upregulating the expression of hepatic LDL receptor gene and increasing the activities of serum lipoprotein and lecithin-cholesterol acyltransferase.
The immunomodulatory effect of DBT is reflected in the immune activation in human T-lymphocytes and macrophages. Specifically, DBT could significantly induce the proliferation of cultured T-lymphocytes and the secretion of IL-2 and p-ERK and increase the phagocytosis of cultured macrophages (Gao et al., 2006). This is consistent with the view of the TCM theory that TCM with beneficial Qi participates in improving the defense ability of the immune system.
The antirenal fibrosis effect of the AR–AS pair has been extensively studied, and its mechanism mainly includes downregulating the expressions of TGF-β1 and connective tissue growth factor (CTGF), preventing the activation of c-Jun N-terminal kinase (JNK)-MAPK, and inhibiting the expression of NLRP3 inflammasomes (Huang et al., 2003; Wang et al., 2003; Wang et al., 2016). Integrated lipidomics, transcriptomics, and network pharmacology techniques were also used to reveal the mechanisms of DBT (Sun L. et al., 2022). Just as the AR–AS pair, astragaloside IV and ferulic acid have been proved to improve renal tubulointerstitial fibrosis and protect the function of vascular endothelium, which may be the pharmacological material basis of the pairs (Meng et al., 2011; Yin et al., 2014).
Sanqi oral solution (SQ), composed of AR and Notoginseng Radix et Rhizoma (Sanqi, the roots and rhizomes of Panax notoginseng (Burk.) F.H.Chen), is a patented hospital preparation, which was designed and developed by the famous nephrologist Nizhi Yang (Tian et al., 2019). Notoginseng Radix et Rhizoma is an important CMM related to Blood, with the features of promoting blood circulation, removing blood stasis, inducing blood clotting, and alleviating pain (Lei and Chiou, 1986; Cicero et al., 2003). Based on the efficacy of AR in invigorating healthy Qi and Notoginseng Radix et Rhizoma in eliminating pathogenic factors, the combination has been used for clinically treating CKD for over 20 years with good curative effect. In a rat model of membranous nephropathy, SQ successfully reduced proteinuria, increased serum albumin, and restored podocyte injury, which was associated with the inhibition of the NF-κB signaling pathway (Tian et al., 2019). Moreover, SQ could also exert renoprotective effects on renal I/R injury by inhibiting apoptosis and enhancing autophagy, combined with regulating ERK/mTOR pathways (Tian et al., 2020).
Since both have a distinct ability to regulate T-lymphocyte subsets (Yang Z. et al., 2007; Wan et al., 2013), SQ indicated regulation of lymphocyte subsets and reduction of macrophages infiltration in renal tissues (Wei et al., 2013). In vitro experiments showed that inhibition of mesangial cell proliferation by SQ may be related to inhibition of IL-β1 secretion and gene expression and promotion of IL-10 secretion and gene expression (Yang N. Z. et al., 2007). Thus, it was considered that mesangial cells were important target cells of SQ in preventing and treating chronic nephritis and delaying CKD progression.
CMM formula is the main form of clinical treatment of TCM, which is composed of two or more CMMs based on the theory of TCM. Under the appropriate dosage ratio, many CMMs in the formula can make full use of their advantages, avoid their disadvantages and play a synergistic role to realize the overall adjustment of TCM. CMM pairs are the smallest component units of a formula, and some representative CMM pairs have been described above. This section mainly discusses the characteristics of CMM formulas composed of multiple CMMs in the study of CKD and the details are summarized in Table 1.
Zhenwu Tang (ZWT) is a classical CMM formula originally from an ancient medicinal book “Treatise on Febrile Diseases.” It is composed of Aconiti Lateralis Radix Praeparata (Fuzi), Poria, Atractylodis Macrocephalae Rhizoma (Baizhu), Paeoniae Radix Alba (Baishao), and Zingiberis Rhizoma Recens (Shengjiang). ZWT has been widely used as a remedy for CKD, relieving the clinical manifestations of edema, dyuria, and oliguria through warming Kidney/Spleen Yang to promote diuresis. Clinical studies have indicated that ZWT improved the clinical curative effect, alleviated the scores of symptoms such as the limb chills, decreased proteinuria, and blood lipids, improved renal function, and alleviated serum levels of IL-8, IL-13, CTGF, and hepatocyte growth factor (Tian et al., 2017).
The warming Yang effect of ZWT is related to antiinflammation and antifibrosis. In improving the inflammatory response, ZWT significantly reduced the serum levels of AGEs and decreased the release of inflammatory mediators (TNF-α, IL-1β, and IL-6) (Wu J. et al., 2016). The mechanism is closely related to upregulating the expression of peroxisome proliferator-activated receptor-gamma (PPAR-γ) protein and IκB mRNA, downregulating the expressions of RAGE1, p-p65, and p-IκBα, and inhibiting the activation of the NF-κB/NLRP3 pathway (Wu J. et al., 2016; Liu et al., 2018; Liu et al., 2019; Li H. et al., 2020). Interestingly, ZWT blocked the activation of NLRP3 inflammasome and inhibited IL-1β and caspase-1, which is closely related to dramatically promoting the secretion of exosomes in renal tissues (Li H. et al., 2020). In ameliorating renal fibrosis, ZWT significantly inhibited renal mRNA and protein expression of Wnt4 and its downstream genes β-catenin and Axin (La et al., 2018). In addition, ZWT could ameliorate streptozotocin-induced proteinuria and podocyte injury by suppressing the hyperactivity of the renal renin–angiotensin system and modulating the slit diaphragm (Cai et al., 2010).
The diuretic effect of ZWT was tested in NS rats and kidney-Yang-deficient rats. It turns out that ZWT could attenuate ADR-induced renal edema, which was related to the downregulation of miR-92b expression and upregulation of AQP2 expression (Liang et al., 2019). The rat model of kidney-Yang deficiency was made by injecting hydrocortisone acetate. The results showed that ZWT could adjust the osmotic pressure set point, increase the secretion of plasma aldosterone, and reduce the secretion of antidiuretic hormone, thereby promoting the excretion of Na+ and K+ and keeping the balance of water and the electrolyte content (Liang et al., 1999).
According to the characteristics of chronic nephritis patients, such as kidney deficiency, spontaneous sweating, and susceptibility to exogenous evil, Zhan and Dai put forward the treatment of invigorating Qi and consolidating the superficial resistance (supportive treatment); on the other hand, patients with chronic nephritis are often combined with the exogenous wind-heat syndromes, damp-heat syndromes, and blood stasis syndromes, which should be treated by clearing heat and removing dampness, as well as promoting blood circulation and removing blood stasis (Zhan et al., 2003). Therefore, YiQi QingRe Gao (YQQRG), a CMM formula, was developed and has been applied in Guang’anmen Hospital for more than two decades.
As an empirical formula, YQQRG is composed of 12 CMMs. The prescription takes AR as the monarch medicine, combined with Atractylodis Macrocephalae Rhizoma and Saposhnikoviae Radix (Fangfeng), which is the composition of Yupingfeng San, to invigorate Qi, consolidate the superficial resistance, and eliminate evil or toxin in the outside; Lonicerae Japonicae Flos (Jinyinhua), Forsythiae Fructus (Lianqiao), Duchesneae Indicae (Shemei), Hedyotis Diffusa (Baihuasheshecao), and Imperatae Rhizoma (Baimaogen) can clear heat and remove dampness in the inside; Poria, Alismatis Rhizoma (Zexie), Leonuri Herba (Yimucao), and Dioscoreae Nipponica Rhizoma (Chuanshanlong) can promote blood circulation and diuresis and guide the evil of dampness, heat, and turbid to go out. In a previous clinical study, it was found that YQQRG could significantly reduce TCM symptom scores of patients with chronic glomerulonephritis, effectively decrease 24 h urine protein, and improve plasma albumin and immunoglobulin (Ig) G, IgA levels, and the effective rate of treatment was 90% (Zhan and Dai, 2003).
Animal experiments have shown that the formula inhibits the expression levels of inflammatory factors such as TNF-α, TNFR1, IL-1β, and MCP-1 in renal tubulointerstitium, downregulates the expression level of caspase-3 in the renal tissues, and upregulates the expression level of Bcl-2 to exert an antiapoptotic effect (Wen et al., 2015). In maintaining the glomerular filtration barrier, YQQRG could upregulate the expressions of podocyte-related proteins such as nephrin, podocin, and CD2AP in kidney tissues of rats with PAN nephropathy and inhibit their mRNA level in feedback (Zhan et al., 2014). Moreover, the main molecular chaperones of endoplasmic reticulum stress, such as glucose-regulated protein 78 (GRP78) and GRP94, and cytoskeletal regulatory proteins, such as α-actin-4, synaptopodin, desmin, and uPAR, were all inhibited by YQQRG (Yang et al., 2016a). An in vitro experiment indicated that treatment with serum-containing YQQRG inhibited LPS-induced rat mesangial cell proliferation, downregulated mRNA and protein levels of Wnt4 and TGF-β1, and reduced the aggregation of the mesangial matrix (Yang et al., 2016b). Dioscin might be the key component in YQQRG to exert the above effects.
Sun et al. consider that the pathogenesis of CKD is the deficiency of kidney collaterals caused by wind pathogen, mixed with blood stasis and dampness heat (Sun et al., 2008). Therefore, Qufeng Tongluo Recipe (QTR) was developed as an empirical formula. This prescription selects Zaocys (Wushaoshe) as the monarch medicine and Sinomenii Caulis (Qingfengteng) and Piperis Kadsurae Caulis (Haifengteng) as the minister medicines, and it takes the properties of insects’ moving and dredging collaterals (dredging the channel) and vines’ winding and spreading to achieve the effects of dispelling wind, eliminating dampness, and dredging collaterals. This is assisted by AR to invigorate Qi, dredge collaterals, promote diuresis, and reduce swelling and Taxilli Herba (Sangjisheng) to nourish the liver and kidneys and dispel wind and dampness. Rehmanniae Radix (Dihuang) is used as the courier to nourish Yin and generate fluid, which not only prevents the dryness of the herbs but also leads the herbs straight into the kidney meridian. All the CMMs are combined to achieve the effects of expelling wind, dredging collaterals, invigorating Qi, and tonifying the kidneys. In the clinical treatment of DN-complicated CRF, QTR showed the effect of reducing blood lipid, improving renal function, and reducing serum TGF-β1 level (Sun et al., 2004).
Animal experiments showed that QTR could improve kidney injury by regulating the expressions of cytoskeletal proteins (synaptopodin, α-actinin-4, and desmin) and slit diaphragm proteins (CD2AP) (Wang Z. et al., 2014a; Wang Z. et al., 2014b), increasing the anion site and HSPG expression in the GBM to protect the basement membrane damage (Sun et al., 2010; Ma et al., 2011). In addition to improving the glomerular filtration function, QTR could also inhibit the accumulation of ECM by reducing the expressions of Col IV, fibronectin, and laminin in kidney tissues and downregulate the expression of renal fibrosis-related proteins α-SMA and vimentin, thus playing an antifibrosis role (Wu et al., 2008; Ma et al., 2019). The results in vitro indicated that the inhibition of QTR on the proliferation of mesangial cells was related to the regulation of the cell cycle process and the inhibition of TGF expression (Sun et al., 2008; Wu et al., 2013). The regulation of cell cycle progression is reflected in the reduction of the protein and mRNA expression levels of cylinD1, cyclin-dependent kinase 2 (CDK2), and p21 and the increase of p27.
Uremic clearance granule (UCG) is the first CMM preparation in the field of treating early and mid-stage CRF. It contains 16 kinds of CMMs and exerts the effects of removing turbidity by catharsis, invigorating the spleen, dispelling dampness, promoting blood circulation, and removing blood stasis in TCM. In some small-scale clinical trials, UCG is effective in reducing serum creatinine levels, improving renal blood flow, and improving the state of microinflammation (Wu et al., 2004). In a multicenter clinical trial involving 300 patients with stage 3b-4 CKD, UCG significantly reduced serum creatinine, delayed the reduction of estimated GFR, and delayed the progression of CKD at 24 and 48 weeks after administration (Zheng et al., 2017; Zheng et al., 2019). Subsequently, it was recommended in the clinical application guide of Chinese patent medicine in the treatment of CKD stage 3–5 (nondialysis), version 2020 (The Standardization Project Team of The Clinical Application Guide of Chinese Patent Medicine in The Treatment of Dominant Diseases, 2021).
In the clinical application of UCG in the recent 30 years, its mechanism of action has been comprehensively studied. In terms of inhibiting the tubular epithelial to mesenchymal transition, UCG could significantly increase E-cadherin expression and suppress vimentin and α-SMA expression both in vivo and in vitro (Lu et al., 2013). On the effect of oxidative stress, UCG showed the effect of restoring mitochondrial regeneration (shown as restoring the expression of mitochondrial transcription factor A and peroxisome proliferator-activated receptor γ co-activator-1α), improving mitochondrial structure and function, and further inhibiting the apoptosis of tubular epithelial cells, which is related to the inhibition of the TGF-β1-ROS-MAPK pathway (Xu et al., 2015). Focusing on the role of TGF-β1 expression in renal fibrosis, it was proved that UCG downregulated the protein expressions of TGF-β1, TGF-β receptor I, receptor II and Smad2/3, and upregulated the protein expressions of SnoN and Smad7 (Huang et al., 2014; Wu W. et al., 2016). Further experiments revealed that regulation of TGF-β1 transcription and translation by UCG was due to induction of remethylation of the TGF-β1 promoter in CRF rats (Miao et al., 2010). In addition, the effect of UCG in correcting the imbalance of MMP-2/TIMP-1 in model rats has also been elucidated (Huang et al., 2014).
With the continuous research on UCG, the original prescription was simplified and recombined, and finally the second-generation product Huang Gan formula (HGF) was formed. HGF is composed of RRR, Zingiberis Rhizoma, Bupleuri Radix, Glycyrrhizae Radix et Rhizoma, and Aconiti Lateralis Radix Preparata. It can significantly improve the renal function of rats in CRF models, reduce oxidative stress injury, and delay the development of renal fibrosis (Xiao et al., 2014). At present, the mechanism of HGF is relatively limited. Mo et al. showed that HGF plays an antiinterstitial fibrosis role by inhibiting the Wnt/β-catenin pathway through depressing protein and mRNA expressions of Wnt1, β-catenin, transcription factor 4, and FN (Mo et al., 2015). Similar to UCG, HGF also shows the effect of antioxidative stress. The research indicated that the effect of HGF in advanced oxidation protein products-induced human mesangial cells was related to the inhibition of the JAK2/STAT3 pathway and the regulation of the balance of the advanced glycation end products receptor (Deng et al., 2017).
The above systematically summarizes the research strategies and mechanisms of TCM in treating CKD (the relevant mechanisms are summarized in Figure 2). At present, many small clinical studies have been published in Chinese journals to support the kidney protective effect of TCM on CKD, and there are many systematic reviews and meta-analyses. With the gradual deepening of research, more and more large-scale well-designed randomized controlled trials have been carried out. Table 2 summarizes the large-sample clinical trials on the prevention and treatment of various CKD with TCM in recent years, which further supports the effectiveness of TCM.
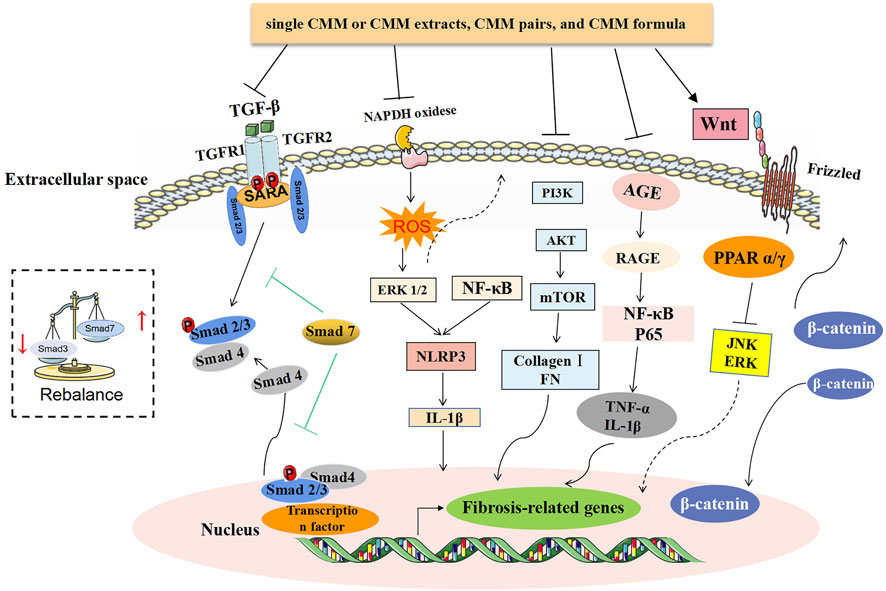
FIGURE 2. Summary of the related mechanism of treating CKD with CMM. TGF-β/Smad and Wnt/β-catenin signaling pathways are two antifibrotic mechanisms that have been confirmed by a variety of TCM studies. Among them, Smad3 is pathogenic in renal fibrosis but Smad7 plays a protective role by negatively regulating the phosphorylation of Smad2/3 and NF-κB-driven inflammatory response. For the dual functions of Smad4, the therapeutic effect of CMM is to inhibit Smad3-dependent renal fibrosis. Besides, the sustained excessive reaction of abnormal activation of the PI3K/Akt/mTOR signaling pathway can lead to an increase in Col I and FN and ultimately lead to renal fibrosis. In the occurrence of glomerular hyperperfusion, hyperfiltration, and hyperglycemia, it causes oxidative stress and AGE activation, followed by a series of inflammatory reactions. In addition to inhibiting the activation of AGE, the antiinflammatory effect of CMM is also manifested in the inhibition of ROS-ERK1/2 and NF-κB-mediated NLRP3 inflammasomes.
CKD is a common and progressive disease that has become a major public health problem on a global scale. However, the single target characteristic of modern medicine cannot meet the clinical requirements. According to the syndrome differentiation of diseases, CMM with multilink and multipath effects is selected, which is indeed effective for complex diseases. Treatment based on syndrome differentiation is a very important feature of TCM. The syndrome is a summary of the body’s pathological response at a certain stage in the development of the disease, including the location, cause, nature of the lesion, and relationship between healthy Qi and pathogenic factor, reflecting the essence of pathological changes at this stage (Chen and Lu, 2006). Only when doctors accurately identify the TCM symptoms of the disease can they issue an accurate prescription. Therefore, treatment based on syndrome differentiation comprehensively considers the various factors of the disease and provides a multilink and multipath control method for the prevention and treatment of CKD. In addition, under the guidance of syndrome differentiation and treatment, the prescriptions used by TCM physicians also conformed to the compatibility theory of “Jun-Chen-Zuo-Shi,” which clearly pointed out the roles played by each ingredient in the prescription (Luan et al., 2020). Under the guidance of these rules, TCM emphasizes the treatment of the variable related to systemic disease and different targets and allows some ingredients in prescriptions to be changed when symptoms and signs change, so TCM presents an ideal choice for the intervention of chronic diseases.
Modern medicine considers that in the course of the disease, renal fibrosis is a general characteristic of CKD. Although there is no “renal fibrosis” in the TCM theory, the progressive development of various kidney diseases can be manifested as a deficiency in the root and excess in the branch, and the change of syndromes can occur, such as “edema,” “retention of urine,” and “kidney fatigue” in TCM. This is consistent with the understanding of the disease process in modern medicine. The ancients believed that “most of the kidney diseases are of deficiency syndrome.” Modern physicians also agree that kidney deficiency should be considered as the pathological basis of kidney disease and even believe that there is no kidney disease without kidney deficiency (Liu et al., 2011a). Together with the views that kidney-Qi deficiency is a leading cause of renal fibrosis (Cao et al., 2015; Zhao, 2019), it is considered that the pathogenesis of root deficiency may be reflected in renal fibrosis. As summarized in this study, many single CMM and CMM formulas aiming at the pathogenesis of kidney deficiency of CKD have different compositions but all exhibit antifibrosis effects. In addition, these CMMs can improve renal fibrosis in different disease models (such as NS, UUO, 5/6Nx, and CRF), which embodies the theory of “same treatment for different diseases” in TCM. For example, ZWT can be used to treat DN, NS, CRF, and other diseases by improving renal fibrosis as described above. Since CMMs have antiinflammatory, antioxidation, antiapoptosis, and antifibrosis properties and play a role in the regulation of immunity, they can inhibit the TGF-β1/Smad, NF-κB, PI3K/Akt/mTOR, Wnt/β-catenin, ERK-1/2, p38 MAPK, JNK, and other pathways and activate the HGF/c-met pathway to exert an antifibrosis role.
In the process of the progression of CKD, in addition to the common pathology of renal fibrosis, it is also related to the infiltration of inflammatory factors, renal ischemia injury, hyperlipidemia, and the change of blood volume (Peng et al., 2005; Dou et al., 2009; Liu, 2011). Here, due to the reduced renal filtration function in CKD, a large number of potentially nephrotoxic components (such as complement factors, growth factors, and others) are abnormally filtered into the urine, causing these macromolecules to damage the tubulointerstitium, and these substances induce renal interstitial damage far more serious than that of urine albumin (Cravedi and Remuzzi, 2013). Therefore, causing proteinuria has a certain toxic effect. As mentioned above, the pathogenic excess in the pathogenesis of CKD mainly includes blood stasis, dampness heat, turbid toxin, and water stagnation. Therefore, both modern medicine and TCM can recognize the different characteristics in the pathogenesis of CKD, and their understanding of the characteristics of the disease can be explained by each other. Briefly, the microscopic manifestations of blood stasis can be described as the disorder of local blood microcirculation in the kidneys, resulting in ischemia and hypoxia of kidney tissues, necrosis, and apoptosis of endothelial cells as well as ischemic collapse and sclerosis of glomerular (Guo et al., 2019). The microscopic manifestations of dampness heat are the lesion of the capillary loop, the infiltration of interstitial inflammatory cells, the proliferation of glomerular cells, and segmental sclerosis or adhesion of glomerulus (Yu et al., 1992; Wang et al., 2008; Wang X. et al., 2015). Turbid toxin manifests as the abnormal increase of solutes with different molecular weights, such as inflammatory factors, urine protein, LDL, and oxidized LDL (Vanholder et al., 2003; Palmer, 2007). Water stagnation can be manifested as an abnormal increase in blood volume, leading to edema (Rodríguez-Iturbe et al., 2002; Ellison, 2017). Therefore, when blood stasis is present, SM or Shenkangling, which promotes blood circulation and removes blood stasis, is used to improve the expressions of nNOS, HIF-1α, and VEGF. When NS is accompanied by dampness heat syndrome, AC or Wulingsan, which has the effect of clearing dampness heat, can be selected to reduce the infiltration of inflammatory cells and the proliferation of glomerular cells. Likewise, ZWT, which regulates the distribution and expression of AQP2, can be used to eliminate edema and UCG, which inhibits the apoptosis of renal tubular epithelial cells and reduces the toxicity of proteinuria, can be used to reduce the turbidity. To sum up the above points, the use of different treatments at different stages of disease development is the idea of “different treatments for the same disease” in TCM.
It should be pointed out that in the course of disease development, different pathogenic factors can influence each other. Just as the infiltration of inflammatory cells is the microscopic manifestation of dampness heat, it is also the initiating factor of fibrosis. Besides, if the dampness heat is not removed, it will lead to the accumulation of solutes with different molecular weights and the formation of turbid toxin. Therefore, TCM can not only regulate the root of the disease (renal fibrosis), but also regulate other factors toward normal from different links and the balance of Yin and Yang.
However, there are still some issues that need to be resolved. 1) The current clinical evidence, even from the meta-analysis, is not enough to support the clinical application of CMMs in kidney disease. More large-scale clinical trials are needed to assess the efficacy, effectiveness, and toxicity of CMMs to optimize their therapeutic and preventive potential. 2) The therapeutic effects of CMMs are attributed to their unique multitarget and multipath effects that are difficult to separate from the different stages of CKD. Therefore, when to use CMM and what kind of efficacy of CMM should be selected mainly depend on clinicians’ self-experience. It is urgent to construct modern pharmacological indicators to characterize the conditions for the application of CMMs, to facilitate the globalization of the application of CMMs. 3) Different prescriptions of TCM have different therapeutic characteristics, but there are few specific indicators that can reflect the action characteristics of TCM. It is necessary to clarify the scientific connotation of TCM with clear pharmacological indexes. 4) The multitarget mechanism of the TCM therapy has not been fully elucidated, and most of them are based on a single pathway. However, the interaction relationship between different signaling pathways is not clear yet, and the cross-targets among the pathways are rarely studied. It is necessary to deeply explore the mechanism and the interaction relationship of TCM in a multidisciplinary way and visualize the multitargets and multilink network of CMMs. 5) Currently, some ingredients derived from CMMs also show good efficacy, but it is unclear which ingredient has the best efficacy or which one is the most effective among CMMs and their ingredients. A well-designed randomized controlled trial is needed to confirm the efficacy and safety of potential drugs.
In summary, TCM has made major progress in the treatment of CKD and has shown a beneficial role in delaying CKD in clinical, animal, and in vitro studies. The effectiveness of CMM is the result of long-term clinical practice under the guidance of the TCM theory, which seeks to “reinforce deficiency and purge excess” through the synergistic effect of multitargets and multipaths. CMMs can not only inhibit the activation of myofibroblasts, the infiltration of inflammatory cells, the transdifferentiation of epithelial cells to mesenchymal cells, and the excessive deposition of ECM to improve renal fibrosis through various mechanisms, but also improve blood flow, inhibit the proliferation of glomerular cells and the accumulation of solutes, and relieve water retention to achieve a relative balance between Yin and Yang, which highlights the advantages of holistic control of TCM and provides a good therapeutic strategy for clinical treatment of CKD. Further research should focus more attention to large-scale clinical trials and combine the TCM theory with the latest medical research to deeply explore its mechanism, which is of great significance for TCM treatment of CKD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YW and FX conceived the idea. YW and YF wrote the draft of the manuscript. MY and GS searched and classified the literature. ML drew the figures of the manuscript. FX, DY, and ZX revised the manuscript. All authors have read and approved the final version of the manuscript.
This study was supported by grants from the National Natural Science Foundation of China (Grant 81974536), Anhui Provincial Science and Technology Major Project (Grant 202203a07020006), the Scientifific Research Foundation of Education Department of Anhui Province (Grant KJ2021A0536), and the talents Program of Anhui University of Chinese Medicine (Grant 2022rcyb010).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
An, X., Zhang, L., Yuan, Y., Wang, B., Yao, Q., Li, L., et al. (2017). Hyperoside Pre-treatment Prevents Glomerular Basement Membrane Damage in Diabetic Nephropathy by Inhibiting Podocyte Heparanase Expression. Sci. Rep. 7 (1), 6413. doi:10.1038/s41598-017-06844-2
Boor, P., Ostendorf, T., and Floege, J. (2010). Renal Fibrosis: Novel Insights into Mechanisms and Therapeutic Targets. Nat. Rev. Nephrol. 6 (11), 643–656. doi:10.1038/nrneph.2010.120
Cai, H., Su, S., Li, Y., Zeng, H., Zhu, Z., Guo, J., et al. (2018). Protective Effects of Salvia Miltiorrhiza on Adenine-Induced Chronic Renal Failure by Regulating the Metabolic Profiling and Modulating the NADPH oxidase/ROS/ERK and TGF-β/Smad Signaling Pathways. J. Ethnopharmacol. 212, 153–165. doi:10.1016/j.jep.2017.09.021
Cai, H. D., Su, S. L., Qian, D. W., Guo, S., Tao, W. W., Cong, X. D., et al. (2017). Renal Protective Effect and Action Mechanism of Huangkui Capsule and its Main Five Flavonoids. J. Ethnopharmacol. 206, 152–159. doi:10.1016/j.jep.2017.02.046
Cai, Y., Chen, J., Jiang, J., Cao, W., and He, L. (2010). Zhen-Wu-tang, a Blended Traditional Chinese Herbal Medicine, Ameliorates Proteinuria and Renal Damage of Streptozotocin-Induced Diabetic Nephropathy in Rats. J. Ethnopharmacol. 131 (1), 88–94. doi:10.1016/j.jep.2010.06.004
Cao, H., Zhang, A., Sun, H., Zhou, X., Guan, Y., Liu, Q., et al. (2015). Metabolomics-proteomics Profiles Delineate Metabolic Changes in Kidney Fibrosis Disease. Proteomics 15 (21), 3699–3710. doi:10.1002/pmic.201500062
Cao, S., Huang, W., Yu, Z., Yang, X., Liu, N., Ma, T., et al. (2019). Effect of Shenyan Xiaobai Granule on Nephrin and Podocin of Adriamycin-Induced Renal Injury: A Randomised Controlled Trial. J. Ethnopharmacol. 244, 112104. doi:10.1016/j.jep.2019.112104
Chen, D. Q., Cao, G., Zhao, H., Chen, L., Yang, T., Wang, M., et al. (2019a). Combined Melatonin and Poricoic Acid A Inhibits Renal Fibrosis through Modulating the Interaction of Smad3 and β-catenin Pathway in AKI-To-CKD Continuum. Ther. Adv. Chronic Dis. 10, 2040622319869116. doi:10.1177/2040622319869116
Chen, D. Q., Feng, Y. L., Chen, L., Liu, J. R., Wang, M., Vaziri, N. D., et al. (2019b). Poricoic Acid A Enhances Melatonin Inhibition of AKI-To-CKD Transition by Regulating Gas6/AxlNFκB/Nrf2 axis. Free Radic. Biol. Med. 134, 484–497. doi:10.1016/j.freeradbiomed.2019.01.046
Chen, D. Q., Hu, H. H., Wang, Y. N., Feng, Y. L., Cao, G., and Zhao, Y. Y. (2018). Natural Products for the Prevention and Treatment of Kidney Disease. Phytomedicine 50, 50–60. doi:10.1016/j.phymed.2018.09.182
Chen, D. Q., Wang, Y. N., Vaziri, N. D., Chen, L., Hu, H. H., and Zhao, Y. Y. (2020). Poricoic Acid A Activates AMPK to Attenuate Fibroblast Activation and Abnormal Extracellular Matrix Remodelling in Renal Fibrosis. Phytomedicine 72, 153232. doi:10.1016/j.phymed.2020.153232
Chen, L., Cao, G., Wang, M., Feng, Y. L., Chen, D. Q., Vaziri, N. D., et al. (2019). The Matrix Metalloproteinase‐13 Inhibitor Poricoic Acid ZI Ameliorates Renal Fibrosis by Mitigating Epithelial‐Mesenchymal Transition. Mol. Nutr. Food Res. 63 (13), 1900132. doi:10.1002/mnfr.201900132
Chen, X. M., Chen, Y. P., Chen, J., Zhou, Z. L., He, Y. N., Li, P., et al. (2007). Multicentered, Randomized, Controlled Clinical Trial on Patients with IgA Nephropathy of Qi-Yin Deficiency Syndrome Type. Zhongguo Zhong Xi Yi Jie He Za Zhi 27 (2), 101–105.
Chen, Y., Deng, Y., Ni, Z., Chen, N., Chen, X., Shi, W., et al. (2013). Efficacy and Safety of Traditional Chinese Medicine (Shenqi Particle) for Patients with Idiopathic Membranous Nephropathy: a Multicenter Randomized Controlled Clinical Trial. Am. J. Kidney Dis. 62 (6), 1068–1076. doi:10.1053/j.ajkd.2013.05.005
Chen, Z., Liu, L., Gao, C., Chen, W., Vong, C. T., Yao, P., et al. (2020). Astragali Radix (Huangqi): A Promising Edible Immunomodulatory Herbal Medicine. J. Ethnopharmacol. 258, 112895. doi:10.1016/j.jep.2020.112895
Chen, Z. Q., and Lu, L. G. (2006). Integral Syndrome Differentiation, Local Syndrome Differentiation and Microcosmic Syndrome Differentiation----Thinking on the Modern TCM System of Syndrome Differentiation Dependent Treatment. Chin. J. Integr. Ttradit. West. Med. 26 (12), 1126–1127.
Cicero, A. F., Vitale, G., Savino, G., and Arletti, R. (2003). Panax notoginseng (Burk.) effects on fibrinogen and lipid plasma level in rats fed on a high-fat diet. Phytother. Res. 17 (2), 174–178. doi:10.1002/ptr.1262
Cravedi, P., and Remuzzi, G. (2013). Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. Br. J. Clin. Pharmacol. 76 (4), 516–523. doi:10.1111/bcp.12104
Deng, Q., Bu, C., Mo, L., Lv, B., Song, S., Xiao, X., et al. (2017). Huang Gan Formula Eliminates the Oxidative Stress Effects of Advanced Oxidation Protein Products on the Divergent Regulation of the Expression of AGEs Receptors via the JAK2/STAT3 Pathway. Evidence-Based Complementary Altern. Med. 2017, 4520916. doi:10.1155/2017/4520916
Ding, D., Yan, H., and Zhen, X. (2014). Effects of Chinese herbs in children with Henoch-Schonlein purpura nephritis: a randomized controlled trial. J. Tradit. Chin. Med. 34 (1), 15–22. doi:10.1016/s0254-6272(14)60048-0
Dou, C., Wan, Y., Sun, W., Zhagn, H., Chen, J., Shui, G., et al. (2009). Mechanism of Chinese herbal medicine delaying progression of chronic kidney disease. Zhongguo Zhong Yao Za Zhi 34 (8), 939–943.
Ellison, D. H. (2017). Treatment of Disorders of Sodium Balance in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 24 (5), 332–341. doi:10.1053/j.ackd.2017.07.003
Foreman, K. J., Marquez, N., Dolgert, A., Fukutaki, K., Fullman, N., McGaughey, M., et al. (2018). Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 392 (10159), 2052–2090. doi:10.1016/S0140-6736(18)31694-5
Gao, Q., Li, J., Cheung, J. K., Duan, J., Ding, A., Cheung, A. W., et al. (2007). Verification of the formulation and efficacy of Danggui Buxue Tang (a decoction of Radix Astragali and Radix Angelicae Sinensis): an exemplifying systematic approach to revealing the complexity of Chinese herbal medicine formulae. Chin. Med. 2, 12. doi:10.1186/1749-8546-2-12
Gao, Q. T., Cheung, J. K., Choi, R. C., Cheung, A. W., Li, J., Jiang, Z. Y., et al. (2008). A Chinese herbal decoction prepared from Radix Astragali and Radix Angelicae Sinensis induces the expression of erythropoietin in cultured Hep3B cells. Planta Med. 74 (4), 392–395. doi:10.1055/s-2008-1034322
Gao, Q. T., Cheung, J. K., Li, J., Chu, G. K., Duan, R., Cheung, A. W., et al. (2006). A Chinese herbal decoction, Danggui Buxue Tang, prepared from Radix Astragali and Radix Angelicae Sinensis stimulates the immune responses. Planta Med. 72 (13), 1227–1231. doi:10.1055/s-2006-947186
GBD Chronic Kidney Disease Collaboration (2020). Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395 (10225), 709–733. doi:10.1016/S0140-6736(20)30045-3
Geng, W., Wei, R., Liu, S., Tang, L., Zhu, H., Chen, P., et al. (2016). Shenhua Tablet inhibits mesangial cell proliferation in rats with chronic anti-Thy-1 nephritis. Biol. Res. 49, 17. doi:10.1186/s40659-016-0078-3
Gu, L. F., Ge, H. T., Zhao, L., Wang, Y. J., Zhang, F., Tang, H. T., et al. (2020). Huangkui Capsule Ameliorates Renal Fibrosis in a Unilateral Ureteral Obstruction Mouse Model Through TRPC6 Dependent Signaling Pathways. Front. Pharmacol. 11, 996. doi:10.3389/fphar.2020.00996
Guo, C., Li, S., and Rao, X. R. (2019). New Goals and Strategies of Chinese Medicine in Prevention and Treatment of Chronic Kidney Disease. Chin. J. Integr. Med. 25 (3), 163–167. doi:10.1007/s11655-019-3065-z
Han, J., Xian, Z., Zhang, Y., Liu, J., and Liang, A. (2019). Systematic Overview of Aristolochic Acids: Nephrotoxicity, Carcinogenicity, and Underlying Mechanisms. Front. Pharmacol. 10, 648. doi:10.3389/fphar.2019.00648
Han, J. Y., Fan, J. Y., Horie, Y., Miura, S., Cui, D. H., Ishii, H., et al. (2008). Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol. Ther. 117 (2), 280–295. doi:10.1016/j.pharmthera.2007.09.008
Huang, H. C., Min, Y. L., Li, J. Z., and Wang, H. Y. (2003). Comparative effects between Astragalus-Angelica mixture and enalapril on expression of connective tissue growth factor in rats with renal tubulointerstitial fibrosis. Chin. J. Nephrol. 19 (3), 133–136.
Huang, Y. R., Wei, Q. X., Wan, Y. G., Sun, W., Mao, Z. M., Chen, H. L., et al. (2014). Ureic clearance granule, alleviates renal dysfunction and tubulointerstitial fibrosis by promoting extracellular matrix degradation in renal failure rats, compared with enalapril. J. Ethnopharmacol. 155 (3), 1541–1552. doi:10.1016/j.jep.2014.07.048
Jha, V., Garcia-Garcia, G., Iseki, K., Li, Z., Naicker, S., Plattner, B., et al. (2013). Chronic kidney disease: global dimension and perspectives. Lancet 382 (9888), 260–272. doi:10.1016/S0140-6736(13)60687-X
Jha, V., and Rathi, M. (2018). Natural medicines causing acute kidney injury. Semin. Nephrol. 28 (4), 416–428. doi:10.1016/j.semnephrol.2008.04.010
Ji, C., Deng, Y., Yang, A., Lu, Z., Chen, Y., Liu, X., et al. (2020). Rhubarb Enema Improved Colon Mucosal Barrier Injury in 5/6 Nephrectomy Rats May Associate With Gut Microbiota Modification. Front. Pharmacol. 11, 1092. doi:10.3389/fphar.2020.01092
Ji, J., Tao, P., and He, L. (2019). Kangxianling decoction prevents renal fibrosis in rats with 5/6 nephrectomy and inhibits Ang II-induced ECM production in glomerular mesangial cells. J. Pharmacol. Sci. 139 (4), 367–372. doi:10.1016/j.jphs.2019.03.003
Jiang, M. Q., Wang, L., Cao, A. L., Zhao, J., Chen, X., Wang, Y. M., et al. (2015). HuangQi Decoction Improves Renal Tubulointerstitial Fibrosis in Mice by Inhibiting the Up-Regulation of Wnt/β-Catenin Signaling Pathway. Cell. Physiol. biochem. 36 (2), 655–669. doi:10.1159/000430128
Kidney Disease Outcomes Quality Initiative (K/DOQI) (2004). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am. J. Kidney Dis. 43 (5 Suppl. 1), S1–S290. doi:10.1053/j.ajkd.2004.03.003
La, L., Wang, L., Qin, F., Jiang, J., He, S., Wang, C., et al. (2018). Zhen-wu-tang ameliorates adenine-induced chronic renal failure in rats: regulation of the canonical Wnt4/beta-catenin signaling in the kidneys. J. Ethnopharmacol. 219, 81–90. doi:10.1016/j.jep.2017.12.013
Lee, S. M., Lee, Y. J., Yoon, J. J., Kang, D. G., and Lee, H. S. (2012). Effect of Poria cocos on hypertonic stress-induced water channel expression and apoptosis in renal collecting duct cells. J. Ethnopharmacol. 141 (1), 368–376. doi:10.1016/j.jep.2012.02.048
Lee, S. M., Lee, Y. J., Yoon, J. J., Kang, D. G., and Lee, H. S. (2014). Effect of Poria cocos on Puromycin Aminonucleoside-Induced Nephrotic Syndrome in Rats. Evidence-Based Complementary Altern. Med. 2014, 570420. doi:10.1155/2014/570420
Lei, X. L., and Chiou, G. C. (1986). Cardiovascular pharmacology of Panax notoginseng (Burk) F.H. Chen and Salvia miltiorrhiza. Am. J. Chin. Med. 14 (3-4), 145–152. doi:10.1142/S0192415X86000235
Levey, A. S., and Coresh, J. (2012). Chronic kidney disease. Lancet 379 (9811), 165–180. doi:10.1016/S0140-6736(11)60178-5
Li, H., Lu, R., Pang, Y., Li, J., Cao, Y., Fu, H., et al. (2020). Zhen-Wu-Tang Protects IgA Nephropathy in Rats by Regulating Exosomes to Inhibit NF-κB/NLRP3 Pathway. Front. Pharmacol. 11, 1080. doi:10.3389/fphar.2020.01080
Li, J., Yu, L., Li, N., and Wang, H. (2000). Astragalus mongholicus and Angelica sinensis compound alleviates nephrotic hyperlipidemia in rats. Chin. Med. J. Engl. 113 (4), 310–314.
Li, L. (1996). End-stage renal disease in China. Kidney Int. 49 (1), 287–301. doi:10.1038/ki.1996.41
Li, L., Yao, H., Wang, J., Li, Y., and Wang, Q. (2019). The Role of Chinese Medicine in Health Maintenance and Disease Prevention: Application of Constitution Theory. Am. J. Chin. Med. 47 (3), 495–506. doi:10.1142/S0192415X19500253
Li, M. M., Xu, F., Fu, S. P., Hou, J., Feng, Y., Xu, Z. P., et al. (2021). Protective Mechanism of Danggui Shaoyao san on Podocytes of Nephrotic Syndrome Rats Based on AngⅡ-TRPC6 Pathway. Chin. J. Exp. Tradit. Med. Form. 27 (19), 9–18. doi:10.13422/j.cnki.syfjx.20211936
Li, P., Chen, Y., Liu, J., Hong, J., Deng, Y., Yang, F., et al. (2015). Efficacy and safety of tangshen formula on patients with type 2 diabetic kidney disease: a multicenter double-blinded randomized placebo-controlled trial. PLoS One 10 (5), e0126027. doi:10.1371/journal.pone.0126027
Li, P., Lin, H., Ni, Z., Zhan, Y., He, Y., Yang, H., et al. (2020). Efficacy and safety of Abelmoschus manihot for IgA nephropathy: A multicenter randomized clinical trial. Phytomedicine 76, 153231. doi:10.1016/j.phymed.2020.153231
Li, P., Yan, J., Sun, Y., Burczynski, F. J., and Gong, Y. (2007). Chinese herbal formula Qilong-Lishui granule improves puromycin aminonucleoside-induced renal injury through regulation of bone morphogenetic proteins. Nephrol. Carlt. 12 (5), 466–473. doi:10.1111/j.1440-1797.2007.00828.x
Li, Q. P., Wei, R. B., Yang, X., Zheng, X. Y., Su, T. Y., Huang, M. J., et al. (2019). Protective Effects and Mechanisms of Shenhua Tablet () on Toll-Like Receptors in Rat Model of Renal Ischemia-Reperfusion Injury. Chin. J. Integr. Med. 25 (1), 37–44. doi:10.1007/s11655-017-2756-6
Li, S., Rao, X. R., Wang, S. X., Zhang, G. H., Li, X. M., Dai, X. W., et al. (2009). Study on the relationship between blood stasis syndrome and clinical pathology in 227 patients with primary glomerular disease. Chin. J. Integr. Med. 15 (3), 170–176. doi:10.1007/s11655-009-0170-4
Li, W., He, W., Xia, P., Sun, W., Shi, M., Zhou, Y., et al. (2019d). Total Extracts of Abelmoschus manihot L. Attenuates Adriamycin-Induced Renal Tubule Injury via Suppression of ROS-ERK1/2-Mediated NLRP3 Inflammasome Activation. Front. Pharmacol. 10, 567. doi:10.3389/fphar.2019.00567
Li, W., Gao, K., Zhao, J., Xia, P., Zhou, Y., Chen, J., et al. (2019a). Scientific Connotation of Chronic Kidney Disease with Kidney Deficiency and Dampness Stasis and the Mechanism of Yishen Qingli Huoxue Therapy. World Sci. Technol. Mod. Tradit. Chin. Med. 21, 1041–1047. doi:10.11842/wst.2019.06.001
Li, X., He, Y., Zeng, P., Liu, Y., Zhang, M., Hao, C., et al. (2019e). Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell Mol. Med. 23 (1), 4–20. doi:10.1111/jcmm.13564
Li, X., Qi, D., Wang, M.-y., Ji, K., Xie, Q.-l., Wang, Y., et al. (2021). Salvianolic acid A attenuates steroid resistant nephrotic syndrome through suPAR/uPAR-αvβ3 signaling Inhibition. J. Ethnopharmacol. 279, 114351. doi:10.1016/j.jep.2021.114351
Li, Z., Qing, P., Ji, L., Su, B. H., He, L., and Fan, J. M. (2004). Systematic review of rhubarb for chronic renal failure. Chin. Evid-Based Med. 7, 468–473.
Liang, C. L., Zhang, P. C., Wu, J. B., Liu, B. H., He, Y., Lu, R. R., et al. (2019). Zhen-wu-tang attenuates Adriamycin-induced nephropathy via regulating AQP2 and miR-92b. Biomed. Pharmacother. 109, 1296–1305. doi:10.1016/j.biopha.2018.10.146
Liang, H., Li, S., and Guo, F. (1999). Experimental Research On the Diuresis of Zhenwu Tang. J. Beijing Univ. Tradit. Chin. Med. 22 (2), 68–70.
Lin, M. Y., Chiu, Y. W., Chang, J. S., Lin, H. L., Lee, C. T., Chiu, G. F., et al. (2015). Association of prescribed Chinese herbal medicine use with risk of end-stage renal disease in patients with chronic kidney disease. Kidney Int. 88 (6), 1365–1373. doi:10.1038/ki.2015.226
Lin, P., Wu, M., Qin, J., Yang, J., Ye, C., and Wang, C. (2019). Magnesium lithospermate B improves renal hemodynamics and reduces renal oxygen consumption in 5/6th renal ablation/infarction rats. BMC Nephrol. 20 (1), 49. doi:10.1186/s12882-019-1221-5
Liu, B., He, Y., Lu, R., Zhou, J., Bai, L., Zhang, P., et al. (2018). Zhen-wu-tang protects against podocyte injury in rats with IgA nephropathy via PPARγ/NF-κB pathway. Biomed. Pharmacother. 101, 635–647. doi:10.1016/j.biopha.2018.02.127
Liu, B., Lu, R., Li, H., Zhou, Y., Zhang, P., Bai, L., et al. (2019). Zhen-wu-tang ameliorates membranous nephropathy rats through inhibiting NF-κB pathway and NLRP3 inflammasome. Phytomedicine 59, 152913. doi:10.1016/j.phymed.2019.152913
Liu, B.-H., Tu, Y., Ni, G.-X., Yan, J., Yue, L., Li, Z.-L., et al. (2021). Total Flavones of Abelmoschus manihot Ameliorates Podocyte Pyroptosis and Injury in High Glucose Conditions by Targeting METTL3-Dependent m6A Modification-Mediated NLRP3-Inflammasome Activation and PTEN/PI3K/Akt Signaling. Front. Pharmacol. 12, 667644. doi:10.3389/fphar.2021.667644
Liu, H., Sun, W., Gu, L. B., Tu, Y., Yu, B. Y., and Hu, H. (2017). Huaiqihuang Granules () reduce proteinuria by enhancing nephrin expression and regulating necrosis factor κB signaling pathway in adriamycin-induced nephropathy. Chin. J. Integr. Med. 23 (4), 279–287. doi:10.1007/s11655-015-2293-0
Liu, J., Gao, L.-d., Fu, B., Yang, H.-t., Zhang, L., Che, S.-q., et al. (2022). Efficacy and safety of Zicuiyin decoction on diabetic kidney disease: A multicenter, randomized controlled trial. Phytomedicine 100, 154079. doi:10.1016/j.phymed.2022.154079
Liu, X., Yan, J., Ma, C., and Liu, W. (2011a). Research of TCM Etiology and Pathogenesis of Renal Interstitial Fibrosis. Liaoning J. Tradit. Chin. Med. 38 (12), 2373–2376. doi:10.13192/j.ljtcm.2011.12.71.liuxq.023
Liu, Y. (2011). Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 7 (12), 684–696. doi:10.1038/nrneph.2011.149
Liu, Y. M., Liu, R. H., Liu, W. J., Liu, L., Wu, Z. K., and Chen, Y. Y. (2010). Effects of Chinese herbal medicine Yiqi Huoxue Formula on TGF-β/smad signal transduction pathway and connective tissue growth factor in rats with renal interstitial fibrosis. Zhong Xi Yi Jie He Xue Bao 8 (12), 1165–1173. doi:10.3736/jcim20101209
Lu, J. R., Han, H. Y., Chen, J., Xiong, C. X., Wang, X. H., Hu, J., et al. (2014). Protective Effects of Bu-Shen-Huo-Xue Formula against 5/6 Nephrectomy-Induced Chronic Renal Failure in Rats. Evid. Based Complement. Altern. Med. 2014, 589846. doi:10.1155/2014/589846
Lu, X., Jin, Y., Ma, L., and Du, L. (2015). Danshen (Radix Salviae Miltiorrhizae) reverses renal injury induced by myocardial infarction. J. Tradit. Chin. Med. 35 (3), 306–311. doi:10.1016/s0254-6272(15)30102-3
Lu, Y., Li, J. Z., and Zheng, X. (1997). Effect of Astragalus Angelica mixture on serum lipids and glomerulosclerosis in rats with nephrotic syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi 17 (8), 478–480.
Lu, Z. Y., Liu, S. W., Xie, Y. S., Cui, S. Y., Liu, X. S., Geng, W. J., et al. (2013). Inhibition of the tubular epithelial-to-mesenchymal transition In Vivo and In Vitro by the Uremic Clearance Granule (). Chin. J. Integr. Med. 19 (12), 918–926. doi:10.1007/s11655-013-1654-9
Luan, X., Zhang, L. J., Li, X. Q., Rahman, K., Zhang, H., Chen, H. Z., et al. (2020). Compound-based Chinese medicine formula: From discovery to compatibility mechanism. J. Ethnopharmacol. 254, 112687. doi:10.1016/j.jep.2020.112687
Luo, L. P., Suo, P., Ren, L. L., Liu, H. J., Zhang, Y., and Zhao, Y. Y. (2021). Shenkang Injection and Its Three Anthraquinones Ameliorates Renal Fibrosis by Simultaneous Targeting IƙB/NF-ƙB and Keap1/Nrf2 Signaling Pathways. Front. Pharmacol. 12, 800522. doi:10.3389/fphar.2021.800522
Lv, W., Booz, G. W., Fan, F., Wang, Y., and Roman, R. J. (2018). Oxidative Stress and Renal Fibrosis: Recent Insights for the Development of Novel Therapeutic Strategies. Front. Physiol. 9, 105. doi:10.3389/fphys.2018.00105
Ma, J., Fan, S., Chen, J., Gu, Y., and Lin, S. (1999). Messenger RNA expressions of vasopressin system and aquaporin-2 in adriamycin-induced nephrotic rats and effects of astragalus membranaceus. Chin. Med. J. Engl. 112 (12), 1068–1072.
Ma, J., Peng, A., Chen, J., Gu, Y., Lu, L., and Lin, S. (1996). Different protective effects of Astragalus membranaceus on vasopressin system in experimental nephrotic syndrome and congestive heart failure. Chin. J. Nephrol. 12, 15–19.
Ma, Q., Li, R., Tie, X., Wang, Z., and Sun, W. (2019). Effects of Qufeng Tongluo Prescription on Renal Interstitial Fibrosis- associated Protein α-SMA and Vimentin Expression of UUO Rat. Acta Chin. Med. 34 (8), 1665–1669. doi:10.16368/j.issn.1674-8999.2019.08.394
Ma, Q. Y., Sun, W. S., Ren, Y. Y., and Wang, Z. (2011). Effects of Qufengtongluo recipe on proteinuria and glomerular filtration membrane in rats with adriamycin-induced nephropathy. Nan Fang. Yi Ke Da Xue Xue Bao 31 (1), 11–16.
Ma, X. H., and He, L. Q. (2014a). Effect and mechanism of jianpi qinghua recipe on renal functions of adriamycin-induced nephropathic rats from the angle of inhibiting renal fibrosis. Zhongguo Zhong Xi Yi Jie He Za Zhi 34 (6), 733–738. doi:10.7661/CJIM.2014.06.0733
Ma, X. H., and He, L. Q. (2014b). Effects of Jianpi Qinghua Decoctions on immune inflammatory injury in adriamycin-induced nephropathic rats MA. Sichuan Da Xue Xue Bao Yi Xue Ban. 45 (1), 19–23. doi:10.13464/j.scuxbyxb.2014.01.005
Mao, W., Yang, N., Zhang, L., Li, C., Wu, Y., Ouyang, W., et al. (2021). Bupi Yishen Formula Versus Losartan for Non-Diabetic Stage 4 Chronic Kidney Disease: A Randomized Controlled Trial. Front. Pharmacol. 11, 627185. doi:10.3389/fphar.2020.627185
Meng, L., Qu, L., Tang, J., Cai, S. Q., Wang, H., and Li, X. (2007). A combination of Chinese herbs, Astragalus membranaceus var. mongholicus and Angelica sinensis, enhanced nitric oxide production in obstructed rat kidney. Vasc. Pharmacol. 47 (2-3), 174–183. doi:10.1016/j.vph.2007.06.002
Meng, L. Q., Tang, J. W., Wang, Y., Zhao, J. R., Shang, M. Y., Zhang, M., et al. (2011). Astragaloside IV synergizes with ferulic acid to inhibit renal tubulointerstitial fibrosis in rats with obstructive nephropathy. Br. J. Pharmacol. 162 (8), 1805–1818. doi:10.1111/j.1476-5381.2011.01206.x
Miao, H., Zhao, Y. H., Vaziri, N. D., Tang, D. D., Chen, H., Chen, H., et al. (2016). Lipidomics Biomarkers of Diet-Induced Hyperlipidemia and Its Treatment with Poria cocos. J. Agric. Food Chem. 64 (4), 969–979. doi:10.1021/acs.jafc.5b05350
Miao, X. H., Wang, C. G., Hu, B. Q., Li, A., Chen, C. B., and Song, W. Q. (2010). TGF-beta1 immunohistochemistry and promoter methylation in chronic renal failure rats treated with Uremic Clearance Granules. Folia histochem. Cytobiol. 48 (2), 284–291. doi:10.2478/v10042-010-0001-7
Mo, L., Xiao, X., Song, S., Miao, H., Liu, S., Guo, D., et al. (2015). Protective effect of Huang Gan formula in 5/6 nephrectomized rats by depressing the Wnt/β-catenin signaling pathway. Drug Des. devel. Ther. 9, 2867–2881. doi:10.2147/DDDT.S81157
Mou, S., Ni, Z. H., and Zhang, Q. Y. (2008). Expression of c-met in human kidney fibroblasts induced by high glucose In Vitro and the regulation of Radix Astragali. Zhong Xi Yi Jie He Xue Bao 6 (5), 482–487. doi:10.3736/jcim20080510
National Kidney Foundation (2002). K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis. 39 (2 Suppl. 1), S1–S266.
Nie, L. (2008). Clinical experience in TCM treatment of refractory nephrotic syndrome. J. Tradit. Chin. Med. 28 (1), 46–48. doi:10.1016/s0254-6272(08)60013-8
Nortier, J. L., and Vanherweghem, J. L. (2007). For patients taking herbal therapy--lessons from aristolochic acid nephropathy. Nephrol. Dial. Transpl. 22 (6), 1512–1517. doi:10.1093/ndt/gfm167
Palmer, B. F. (2007). Proteinuria as a therapeutic target in patients with chronic kidney disease. Am. J. Nephrol. 27 (3), 287–293. doi:10.1159/000101958
Peng, A., Gu, Y., and Lin, S. Y. (2005). Herbal treatment for renal diseases. Ann. Acad. Med. Singap. 34 (1), 44–51.
Qin, T., Wu, Y., Liu, T., and Wu, L. (2021). Effect of Shenkang on renal fibrosis and activation of renal interstitial fibroblasts through the JAK2/STAT3 pathway. BMC Complement. Med. Ther. 21 (1), 12. doi:10.1186/s12906-020-03180-3
Rinschen, M. M., and Saez-Rodriguez, J. (2021). The tissue proteome in the multi-omic landscape of kidney disease. Nat. Rev. Nephrol. 17 (3), 205–219. doi:10.1038/s41581-020-00348-5
Ríos, J.-L. (2011). Chemical Constituents and Pharmacological Properties ofPoria cocos. Planta Med. 77 (7), 681–691. doi:10.1055/s-0030-1270823
Rodríguez-Iturbe, B., Herrera-Acosta, J., and Johnson, R. J. (2002). Interstitial inflammation, sodium retention, and the pathogenesis of nephrotic edema: a unifying hypothesis. Kidney Int. 62 (4), 1379–1384. doi:10.1111/j.1523-1755.2002.kid561.x
Ruggenenti, P., Cravedi, P., and Remuzzi, G. (2012). Mechanisms and treatment of CKD. J. Am. Soc. Nephrol. 23 (12), 1917–1928. doi:10.1681/ASN.2012040390
Sai, Y. P., Song, Y. C., Chen, X. X., Luo, X., Liu, J., and Cui, W. J. (2018). Protective effect of astragalosides from Radix Astragali on adriamycin-induced podocyte injury. Exp. Ther. Med. 15 (5), 4485–4490. doi:10.3892/etm.2018.5933
Shahzad, M., Small, D. M., Morais, C., Wojcikowski, K., Shabbir, A., and Gobe, G. C. (2016). Protection against oxidative stress-induced apoptosis in kidney epithelium by Angelica and Astragalus. J. Ethnopharmacol. 179, 412–419. doi:10.1016/j.jep.2015.12.027
Shen, Q. (2008). Kidney Disease of Traditional Chinese Medicine. Shanghai: Shanghai Pujiang Education Press, 53.
Shi, J. F., Zhu, H. W., Zhang, C., Bian, F., Shan, J. P., and Lu, W. I. (2002). Therapeutic effect of Astragalus on patients with chronic glomerulonephritis. Acta Univ. Med. Second. Shanghai 22 (3), 245–248.
Shi, S. Z., and Shen, Z. Y. (1982). The regimen of tonifying and warming kidney-yang. Clinical application and experimental studies. J. Tradit. Chin. Med. 2 (4), 307–316. doi:10.19852/j.cnki.jtcm.1982.04.012
Stevens, P. E., and Levin, A. (2013). Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 158 (11), 825–830. doi:10.7326/0003-4819-158-11-201306040-00007
Su, H. F., Shaker, S., Kuang, Y., Zhang, M., Ye, M., and Qiao, X. (2021). Phytochemistry and cardiovascular protective effects of Huang-Qi (Astragali Radix). Med. Res. Rev. 41 (4), 1999–2038. doi:10.1002/med.21785
Su, K., Zhu, F., Guo, L., Zhu, Y., Li, W., and Xiong, X. (2013). Retrospective study on Professor Zhongying Zhou's experience in Traditional Chinese Medicine treatment on diabetic nephropathy. J. Tradit. Chin. Med. 33 (2), 262–267. doi:10.1016/s0254-6272(13)60137-5
Sun, L., Yang, Z., Zhao, W., Chen, Q., Bai, H., Wang, S., et al. (2022). Integrated lipidomics, transcriptomics and network pharmacology analysis to reveal the mechanisms of Danggui Buxue Decoction in the treatment of diabetic nephropathy in type 2 diabetes mellitus. J. Ethnopharmacol. 283, 114699. doi:10.1016/j.jep.2021.114699
Sun, W. S., Ren, Y. Y., Zhao, Y. L., and Ma, Q. Y. (2010). Effects of qufengtongluo recipe on expressions of HSPG mRNA and protein in adriamycin-induced nephropathy rats. Sichuan Da Xue Xue Bao Yi Xue Ban. 41 (2), 212–217. doi:10.13464/j.scuxbyxb.2010.02.005
Sun, W. S., Wang, Z., Wang, J., Ma, Q. Y., Zhang, X. Q., and Wu, X. L. (2008). Effects of Qufeng Tongluo Recipe on proliferation of and TGF-beta1 and IL-6 mRNA expressions in glomerular mesangial cells from rats. Zhong Xi Yi Jie He Xue Bao 6 (9), 915–920. doi:10.3736/jcim20080908
Sun, W. S., Wu, X. L., and Qiao, C. L. (2004). Clinical Study on Effect of Tongluo Capsule in Treating Diabetic Nephropathy Caused Chronic Renal Failure. Chin. J. Integr. Ttradit. West. Med. 24 (8), 704–706.
Sun, X., Li, P., Lin, H., Ni, Z., Zhan, Y., Cai, G., et al. (2022). Efficacy and safety of Abelmoschus manihot in treating chronic kidney diseases: A multicentre, open-label and single-arm clinical trial. Phytomedicine 99, 154011. doi:10.1016/j.phymed.2022.154011
The Standardization Project Team of The Clinical Application Guide of Chinese Patent Medicine in The Treatment of Dominant Diseases (2021). 2020 Version of The Clinical Application Guide of Chinese Patent Medicine in The Treatment of Chronic Kidney Disease Stage 3-5 (Non Dialysis). Chin. J. Integr. Ttradit. West. Med. 41 (3), 261–272. doi:10.7661/j.cjim.20210114.257
Tian, R., Wang, L., Chen, A., Huang, L., Liang, X., Wang, R., et al. (2019). Sanqi oral solution ameliorates renal damage and restores podocyte injury in experimental membranous nephropathy via suppression of NFκB. Biomed. Pharmacother. 115, 108904. doi:10.1016/j.biopha.2019.108904
Tian, R., Wang, P., Huang, L., Li, C., Lu, Z., Lu, Z., et al. (2020). Sanqi Oral Solution Ameliorates Renal Ischemia/Reperfusion Injury via Reducing Apoptosis and Enhancing Autophagy: Involvement of ERK/mTOR Pathways. Front. Pharmacol. 11, 537147. doi:10.3389/fphar.2020.537147
Tian, Z., Wang, B., and Zhang, L. (2017). Clinical Study on Modified Zhenwu Decoction in Treatment of Chronic Nephropathy (III-IV). Acta Chin. Med. 32 (9), 1757–1760. doi:10.16368/j.issn.1674-8999.2017.09.463
Tu, X., Liu, F., Jordan, J. B., Ye, X. F., Fu, P., Wang, F., et al. (2013). 'Huang Qi Elixir' for proteinuria in patients with diabetic nephropathy: a study protocol for a randomized controlled pilot trial. Trials 14, 223. doi:10.1186/1745-6215-14-223
Tu, Y., Gu, L., Chen, D., Wu, W., Liu, H., Hu, H., et al. (2017). Rhein Inhibits Autophagy in Rat Renal Tubular Cells by Regulation of AMPK/mTOR Signaling. Sci. Rep. 7, 43790. doi:10.1038/srep43790
Tu, Y., Sun, W., Wan, Y. G., Che, X. Y., Pu, H. P., Yin, X. J., et al. (2013). Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, ameliorates adriamycin-induced renal inflammation and glomerular injury via inhibiting p38MAPK signaling pathway activity in rats. J. Ethnopharmacol. 147 (2), 311–320. doi:10.1016/j.jep.2013.03.006
Vanholder, R., De Smet, R., Glorieux, G., Argilés, A., Baurmeister, U., Brunet, P., et al. (2003). Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int. 63 (5), 1934–1943. doi:10.1046/j.1523-1755.2003.00924.x
Wan, C. P., Gao, L. X., Hou, L. F., Yang, X. Q., He, P. L., Yang, Y. F., et al. (2013). Astragaloside II triggers T cell activation through regulation of CD45 protein tyrosine phosphatase activity. Acta Pharmacol. Sin. 34 (4), 522–530. doi:10.1038/aps.2012.208
Wang, D., Wu, T., Xie, T., Peng, W., Wang, Y., Yuan, M., et al. (2013). Syndrome differentiation-based treatment with traditional Chinese medicine for proteinuria in patients with chronic kidney disease: a randomized multicenter trial. Nan Fang. Yi Ke Da Xue Xue Bao 33 (4), 502–506.
Wang, H. Y., Li, J. Z., Pan, J. S., Zou, W. Z., Li, X. M., Zhang, Y. K., et al. (2002). The effect of Astragali and Angelica on nephrotic syndrome and its mechanisms of action. J. Peking. Univ. Health Sci. 34, 542–552.
Wang, L., Cao, A. L., Chi, Y. F., Ju, Z. C., Yin, P. H., Zhang, X. M., et al. (2015). You-gui Pill ameliorates renal tubulointerstitial fibrosis via inhibition of TGF-β/Smad signaling pathway. J. Ethnopharmacol. 169, 229–238. doi:10.1016/j.jep.2015.04.037
Wang, L., Ma, J., Guo, C., Chen, C., Yin, Z., Zhang, X., et al. (2016). Danggui Buxue Tang Attenuates Tubulointerstitial Fibrosis via Suppressing NLRP3 Inflammasome in a Rat Model of Unilateral Ureteral Obstruction. Biomed. Res. Int. 2016, 9368483. doi:10.1155/2016/9368483
Wang, L., Chen, J., Zhuang, Y., Zheng, Z., and Yao, L. (2008). Study on the relationship between damp-heat syndrome and clinical pathology in IgA nephropathy patients. Chin. Arch. Tradit. Chin. Med. 1, 178–180. doi:10.13193/j.archtcm.2008.01.180.wanglp.013
Wang, M., Chen, D. Q., Chen, L., Cao, G., Zhao, H., Liu, D., et al. (2018a). Novel inhibitors of the cellular renin-angiotensin system components, poricoic acids, target Smad3 phosphorylation and Wnt/β-catenin pathway against renal fibrosis. Br. J. Pharmacol. 175 (13), 2689–2708. doi:10.1111/bph.14333
Wang, M., Chen, D. Q., Chen, L., Liu, D., Zhao, H., Zhang, Z. H., et al. (2018b). Novel RAS Inhibitors Poricoic Acid ZG and Poricoic Acid ZH Attenuate Renal Fibrosis via a Wnt/β-Catenin Pathway and Targeted Phosphorylation of smad3 Signaling. J. Agric. Food Chem. 66 (8), 1828–1842. doi:10.1111/bph.1433310.1021/acs.jafc.8b00099
Wang, M., Hu, H. H., Chen, Y. Y., Chen, L., Wu, X. Q., and Zhao, Y. Y. (2020a). Novel poricoic acids attenuate renal fibrosis through regulating redox signalling and aryl hydrocarbon receptor activation. Phytomedicine 79, 153323. doi:10.1016/j.phymed.2020.153323
Wang, M., Yang, J., and Wang, C. (2020b). Shen Shuai II Recipe Attenuates Apoptosis in 5/6 Renal Ablation/Infarction Rats by Inhibiting p53 and the Mitochondrial Pathway of Apoptosis. Oxid. Med. Cell. Longev. 2020, 7083575. doi:10.1155/2020/7083575
Wang, M., Yang, L., Yang, J., Zhou, Y., and Wang, C. (2019). Magnesium lithospermate B attenuates renal injury in 5/6 renal ablation/infarction rats by mitochondrial pathway of apoptosis. Biomed. Pharmacother. 118, 109316. doi:10.1016/j.biopha.2019.109316
Wang, Q., Shao, X., Xu, W., Qi, C., Gu, L., Ni, Z., et al. (2014). Astragalosides IV inhibits high glucose-induced cell apoptosis through HGF activation in cultured human tubular epithelial cells. Ren. Fail. 36 (3), 400–406. doi:10.3109/0886022X.2013.867798
Wang, Q. L., Tao, Y. Y., Xie, H. D., Liu, C. H., and Liu, P. (2020). Fuzheng Huayu recipe, a traditional Chinese compound herbal medicine, attenuates renal interstitial fibrosis via targeting the miR-21/PTEN/AKT axis. J. Integr. Med. 18 (6), 505–513. doi:10.1016/j.joim.2020.08.006
Wang, Q. L., Yuan, J. L., Tao, Y. Y., Zhang, Y., Liu, P., and Liu, C. H. (2010). Fuzheng Huayu recipe and vitamin E reverse renal interstitial fibrosis through counteracting TGF-beta1-induced epithelial-to-mesenchymal transition. J. Ethnopharmacol. 127 (3), 631–640. doi:10.1016/j.jep.2009.12.011
Wang, R., Li, X. M., Li, J. Z., and Wang, H. Y. (2003). The effect of astragali and angelica on renal cell transdifferentiation and MAPK pathway in chronic puromycin aminonucleoside nephrosis (PAN). Chin. Pharm. Bull. 19, 1069–1074.
Wang, S., Hu, Y., Tan, W., Wu, X., Chen, R., Cao, J., et al. (2012). Compatibility art of traditional Chinese medicine: from the perspective of herb pairs. J. Ethnopharmacol. 143 (2), 412–423. doi:10.1016/j.jep.2012.07.033
Wang, X. T., Wei, M., and Wang, X. (2011). Inhibitory effect of chailing decoction on renal tubular epithelial phenotype transformation in rats with cyclosporine A-induced nephropathy. Zhongguo Zhong Xi Yi Jie He Za Zhi 31 (1), 94–98.
Wang, X., Wan, T., Zhao, Z., Wang, W., Li, Y., Yang, B., et al. (2015). Study on Pathological Indicators in TCM Microcosmic Syndrome Differentiation in Dampness-heat Syndrome of IgA Nephropathy. Chin. J. Inf. Tradit. Chin. Med. 22 (8), 31–35.
Wang, Y. J., He, L. Q., Sun, W., Lu, Y., Wang, X. Q., Zhang, P. Q., et al. (2012). Optimized project of traditional Chinese medicine in treating chronic kidney disease stage 3: a multicenter double-blinded randomized controlled trial. J. Ethnopharmacol. 139 (3), 757–764. doi:10.1016/j.jep.2011.12.009
Wang, Y. J., and Zhang, M. O. (2003). Phlegm stagnancy and blood stasis is the groundwork of micro-mass in kidney. Chin. J. Integr. Ttradit. West. Med. Nephrol. 1, 1–3. doi:10.3736/jcim20030110
Wang, Y. P., Li, X. Y., Song, C. Q., and Hu, Z. B. (2002). Effect of astragaloside IV on T, B lymphocyte proliferation and peritoneal macrophage function in mice. Acta Pharmacol. Sin. 23, 263–266.
Wang, Z., Liu, J. T., Sun, W. S., Li, R. P., and Wang, Y. (2014a). Effect of Qufeng Tongluo Recipe on expression of desmin and CD2AP proteins in adriamycin-induced nephropathy rats: an experimental research. Zhongguo Zhong Xi Yi Jie He Za Zhi 34 (2), 203–208. doi:10.7661/CJIM.2014.02.0203
Wang, Z. F., Zhang, Q., and Xie, Y. M. (2022). Clinical comprehensive evaluation of Huangkui Capsules in treatment of chronic kidney diseases. China J. Chin. Mat. Med. 47 (6), 1484–1492. doi:10.19540/j.cnki.cjcmm.20211124.501
Wang, Z., Li, D., Du, Y., Wang, Y., Zhang, W., Liu, Q., et al. (2010). Pathogenic theory of Turbidity-toxin and modern TCM etiology. J. Tradit. Chin. Med. 51 (1), 11–13. doi:10.13288/j.11-2166/r.2010.01.004
Wang, Z., Sun, W., Liu, J., Li, R., and Wang, Y. (2014b). Effects of Qufeng Tongluo recipe on expression of Synaptopodin and α-Actinin- 4 proteins in adriamycin- induced nephropathic rats. J. Shanxi Med. Univ. 45 (11), 1022–1027. doi:10.13753/j.issn.1007-6611.2014.11.005
Wei, F.-n., Chen, Z.-l., Yang, H.-f., Han, L., Ding, H.-m., Deng, S.-g., et al. (2013). Effect of Sanqi Oral Liquid (三芪口服液) on the expressions of CD4+, CD8+ and CD68+ cells in 5/6 nephrectomized rats with chronic renal failure. Chin. J. Integr. Med. 19 (8), 589–595. doi:10.1007/s11655-012-1233-5
Wei, G., and Zheng, X. (2008). A survey of the studies on compatible law of ingredients in Chinese herbal prescriptions. J. Tradit. Chin. Med. 28 (3), 223–227. doi:10.1016/s0254-6272(08)60051-5
Wen, Y., Zhan, Y., Liu, H., Zhao, T., Yang, L., Zhang, H., et al. (2015). Yi Qi Qing Re Gao formula ameliorates puromycin aminonucleoside-induced nephrosis by suppressing inflammation and apoptosis. BMC Complement. Altern. Med. 15, 155. doi:10.1186/s12906-015-0673-9
Wojcikowski, K., Johnson, D. W., and Gobe, G. (2006). Herbs or natural substances as complementary therapies for chronic kidney disease: ideas for future studies. J. Lab. Clin. Med. 147 (4), 160–166. doi:10.1016/j.lab.2005.11.011
Wu, D., and Ma, H. (2011). Treatment According to"Deficiency","Blood Stasis" for Chronic Kidney Disease. Chin. Arch. Tradit. Chin. Med. 29 (6), 1232–1234. doi:10.13193/j.archtcm.2011.06.42.wudj.007
Wu, F., Zhang, P. Q., Wang, X. Q., Nie, L. F., Fu, X. J., Peng, W., et al. (2015). Multi-center randomized control study on the effects of syndrome differentiated traditional Chinese medicine therapy on CKD 1-2 with chronic nephritis proteinuria. Sichuan Da Xue Xue Bao Yi Xue Ban. 46 (1), 145–148. doi:10.13464/j.scuxbyxb.2015.01.032
Wu, H. L., Lin, H. C., Ruan, X. L., Deng, C. H., She, Y. P., and Fang, J. A. (2004). Observation of the curative efficiency of Uremic Clearance Granules in 118 patients with chronic renal failure. Chin. J. Integr. Tradit. West Nephrol. 5, 21–24.
Wu, J., Liu, B., Liang, C., Ouyang, H., Lin, J., Zhong, Y., et al. (2016). Zhen-wu-tang attenuates cationic bovine serum albumin-induced inflammatory response in membranous glomerulonephritis rat through inhibiting AGEs/RAGE/NF-κB pathway activation. Int. Immunopharmacol. 33, 33–41. doi:10.1016/j.intimp.2016.01.008
Wu, W., Huang, Y. R., Wan, Y. G., Yang, H. M., Mao, Z. M., Yang, J. J., et al. (2016). Effects and mechanisms of UCG ameliorating renal interstitial fibrosis by regulating TGF-β1/SnoN/Smads signaling pathway in renal failure rats. Zhongguo Zhong Yao Za Zhi 41 (12), 2291–2297. doi:10.4268/cjcmm20161220
Wu, X., Liu, M., Wei, G., Guan, Y., Duan, J., Xi, M., et al. (2020). Renal protection of rhein against 5/6 nephrectomied-induced chronic kidney disease: role of SIRT3-FOXO3α signalling pathway. J. Pharm. Pharmacol. 72 (5), 699–708. doi:10.1111/jphp.13234
Wu, X. L., An, P., Ye, B. Y., Shi, X. M., Sun, W. S., Fu, R. G., et al. (2013). Qufeng Tongluo Prescription () inhibits mesangial cell proliferation and promotes apoptosis through regulating cell cycle progression. Chin. J. Integr. Med. 19 (12), 927–934. doi:10.1007/s11655-013-1655-8
Wu, X. L., Sun, W. S., Zhang, W. G., and Wang, Z. (2008). Mechanism of the effect of Tongluo Recipe against glomerular sclerosis in rats. Nan Fang. Yi Ke Da Xue Xue Bao 28 (7), 1198–1201.
Xiang, H., Zuo, J., Guo, F., and Dong, D. (2020). What we already know about rhubarb: a comprehensive review. Chin. Med. 15, 88. doi:10.1186/s13020-020-00370-6
Xiang, L., Jiang, P., Zhou, L., Sun, X., Bi, J., Cui, L., et al. (2016). Additive Effect of Qidan Dihuang Grain, a Traditional Chinese Medicine, and Angiotensin Receptor Blockers on Albuminuria Levels in Patients with Diabetic Nephropathy: A Randomized, Parallel-Controlled Trial. Evidence-Based Complementary Altern. Med. 2016, 1064924. doi:10.1155/2016/1064924
Xiang, Z., Sun, H., Cai, X., Chen, D., and Zheng, X. (2015). The study on the material basis and the mechanism for anti-renal interstitial fibrosis efficacy of rhubarb through integration of metabonomics and network pharmacology. Mol. Biosyst. 11 (4), 1067–1078. doi:10.1039/c4mb00573b
Xiao, X., Mo, L., Song, S., Qin, M., and Yang, X. (2014). Compound Huang Gan delays chronic renal failure after 5/6 nephrectomy in rats. Nan Fang. Yi Ke Da Xue Xue Bao 34 (11), 1661–1667.
Xu, Y. F., Ruan, S. W., Lin, J. M., and Zhang, Z. (2015). Yishen Jiangzhuo Granules affect tubulointerstitial fibrosis via a mitochondrion-mediated apoptotic pathway. Chin. J. Integr. Med. 21 (12), 928–937. doi:10.1007/s11655-015-2078-5
Yang, L., Sun, X., Zhan, Y., Liu, H., Wen, Y., Mao, H., et al. (2016b). Yi Qi Qing Re Gao-containing serum inhibits lipopolysaccharide-induced rat mesangial cell proliferation by suppressing the Wnt pathway and TGF-β1 expression. Exp. Ther. Med. 11 (4), 1410–1416. doi:10.3892/etm.2016.3027
Yang, L., Liu, Y., Zhan, Y., Liu, H., Sun, X., Zhang, H., et al. (2016a). Yiqiqingregao Protects Podocyte Injury in Puromycin Aminonucleoside Nephrosis Rat Model by Inhibition of Endoplasmic Reticulum Stress. Chin. J. Integr. Tradit. West. Nephrol. 17 (8), 667–671.
Yang, L., Wang, M., Zhou, Y., Yang, J., Ye, C., and Wang, C. (2021). Shen Shuai II Recipe Attenuates Renal Interstitial Fibrosis by Improving Hypoxia via the IL-1β/c-Myc Pathway. Evidence-Based Complementary Altern. Med. 2021, 1–13. doi:10.1155/2021/5539584
Yang, N. Z., Zhong, D., Zhao, D. X., Liu, M. P., and Liu, X. S. (2007). Effects of Tongmai Oral Solution on cell proliferation and interleukin-β1, interleukin-10 production in mesangial cells. Chin. J. Integr. Tradit. West. Nephrol. 8, 444–447.
Yang, Z., Chen, A., Sun, H., Ye, Y., and Fang, W. (2007). Ginsenoside Rd elicits Th1 and Th2 immune responses to ovalbumin in mice. Vaccine 25 (1), 161–169. doi:10.1016/j.vaccine.2006.05.075
Yin, Y., Qi, F., Song, Z., Zhang, B., and Teng, J. (2014). Ferulic acid combined with astragaloside IV protects against vascular endothelial dysfunction in diabetic rats. Biosci. Trends 8 (4), 217–226. doi:10.5582/bst.2014.01081
Yoshino, T., Horiba, Y., Mimura, M., and Watanabe, K. (2022). Oral Astragalus Root Supplementation for Mild to Moderate Chronic Kidney Disease: A Self-Controlled Case-Series. Front. Pharmacol. 13, 775798. doi:10.3389/fphar.2022.775798
You, Z., Xin, Y., Liu, Y., Han, B., Zhang, L., Chen, Y., et al. (2012). Protective effect of Salvia miltiorrhizae injection on N(G)-nitro-D-arginine induced nitric oxide deficient and oxidative damage in rat kidney. Exp. Toxicol. Pathol. 64 (5), 453–458. doi:10.1016/j.etp.2010.10.013
Yu, D., Yang, R., Lin, Y., Zhou, D., and Wang, J. (2009). Impact of Fangjihuangqi Decoction on Proteinuria and Podocyte in Nephrotic Rats Induced by Adriamycin. Chin. J. Integr. Tradit. West. Nephrol. 10 (4), 295–298.
Yu, J., Xiong, N., Yu, C., Gong, L., Wang, J., Wang, N., et al. (1992). Clinical analysis and experimental study on damp-heat pathology of nephropathy. Chin. J. Integr. Tradit. West. Med. 12 (8), 458–460.
Yu, K. N., Ni, Z. H., Wang, N. S., Peng, W., Wang, Y., Zhang, C. M., et al. (2016). A Clinical Multicenter Randomized Controlled Study on JianpiQinghua Decoction in Treating Stage 3 Chronic Kidney Disease with A Syndrome Type of Dampness-heat due to Spleen Deficiency. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 38 (6), 686–695. doi:10.3881/j.issn.1000-503X.2016.06.010
Zan, J. F., Shen, C. J., Zhang, L. P., and Liu, Y. W. (2017). Effect of Poria cocos hydroethanolic extract on treating adriamycin-induced rat model of nephrotic syndrome. Chin. J. Integr. Med. 23 (12), 916–922. doi:10.1007/s11655-016-2643-6
Zhan, Y. L., Dai, X. W., Li, X. Y., Li, S., and Rao, X. R. (2003). Renoprotective Effect of Yiqiqingre Extract in Adriamycin Nephritic Rats. Chin. J. Integr. Tradit. West Nephrol. 4 (3), 135–138.
Zhan, Y. L., and Dai, X. W. (2003). Treatment of 30 cases with chronic nephritis with replenishing qi, removing blood stasis, clearing heat and toxin methods. J. Tradit. Chin. Med. 44 (12), 922–924.
Zhan, Y., Yang, L., Wen, Y., Liu, H., Zhang, H., Zhu, B., et al. (2014). Yi qi qing re gao attenuates podocyte injury and inhibits vascular endothelial growth factor overexpression in puromycin aminonucleoside rat model. Evidence-Based Complementary Altern. Med. 2014, 375986. doi:10.1155/2014/375986
Zhang, D., Yang, Y., Gao, C., Yang, L., and Xu, H. (2005). The Efficacy of Astragalus Injection on Patients with Primary Chronic Glomerular Disease. Chin. J. Integr. Ttradit. West. Med. Nephrol. 6 (11), 643–645.
Zhang, H. F., Wang, Y. L., Gao, C., Gu, Y. T., Huang, J., Wang, J. H., et al. (2018). Salvianolic acid A attenuates kidney injury and inflammation by inhibiting NF-κB and p38 MAPK signaling pathways in 5/6 nephrectomized rats. Acta Pharmacol. Sin. 39 (12), 1855–1864. doi:10.1038/s41401-018-0026-6
Zhang, L., Li, P., Xing, C. Y., Zhao, J. Y., He, Y. N., Wang, J. Q., et al. (2014). Efficacy and safety of Abelmoschus manihot for primary glomerular disease: a prospective, multicenter randomized controlled clinical trial. Am. J. Kidney Dis. 64 (1), 57–65. doi:10.1053/j.ajkd.2014.01.431
Zhang, L., Shergis, J. L., Yang, L., Zhang, A. L., Guo, X., Zhang, L., et al. (2019). Astragalus membranaceus (Huang Qi) as adjunctive therapy for diabetic kidney disease: An updated systematic review and meta-analysis. J. Ethnopharmacol. 239, 111921. doi:10.1016/j.jep.2019.111921
Zhang, Q., Liu, L., Lin, W., Yin, S., Duan, A., Liu, Z., et al. (2017). Rhein reverses Klotho repression via promoter demethylation and protects against kidney and bone injuries in mice with chronic kidney disease. Kidney Int. 91 (1), 144–156. doi:10.1016/j.kint.2016.07.040
Zhang, Q., Yin, S., Liu, L., Liu, Z., and Cao, W. (2016). Rhein reversal of DNA hypermethylation-associated Klotho suppression ameliorates renal fibrosis in mice. Sci. Rep. 6, 34597. doi:10.1038/srep34597
Zhang, Y. Y., Tan, R. Z., Zhang, X. Q., Yu, Y., and Yu, C. (2019). Calycosin Ameliorates Diabetes-Induced Renal Inflammation via the NF-κB Pathway In Vitro and In Vivo. Med. Sci. Monit. 25, 1671–1678. doi:10.12659/MSM.915242
Zhang, Z. H., Li, M. H., Liu, D., Chen, H., Chen, D. Q., Tan, N. H., et al. (2018). Rhubarb Protect Against Tubulointerstitial Fibrosis by Inhibiting TGF-β/Smad Pathway and Improving Abnormal Metabolome in Chronic Kidney Disease. Front. Pharmacol. 9, 1029. doi:10.3389/fphar.2018.01029
Zhang, Z. H., Wei, F., Vaziri, N. D., Cheng, X. L., Bai, X., Lin, R. C., et al. (2015). Metabolomics insights into chronic kidney disease and modulatory effect of rhubarb against tubulointerstitial fibrosis. Sci. Rep. 5, 14472. doi:10.1038/srep14472
Zhao, H. (2019). Treatment of renal fibrosis based on pathogenesis of healthy qi deficiency with insidious turbidity. J. Guangzhou Univ. Tradit. Chin. Med. 36 (7), 1098–1101. doi:10.13359/j.cnki.gzxbtcm.2019.07.033
Zhao, J., Sun, W., Chen, J., Sun, Z., Chen, D., Cao, C., et al. (2020). Efficacy and Safety of Chinese Herbal Formula Granules in Treating Chronic Kidney Disease Stage 3: A Multicenter, Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Evid. Based Complement. Altern. Med. 2020, 4073901. doi:10.1155/2020/4073901
Zhao, J., Wang, L., Cao, A. L., Jiang, M. Q., Chen, X., Wang, Y., et al. (2016). HuangQi Decoction Ameliorates Renal Fibrosis via TGF-β/Smad Signaling Pathway In Vivo and In Vitro. Cell. Physiol. biochem. 38 (5), 1761–1774. doi:10.1159/000443115
Zhao, Y. Y., Feng, Y. L., Du, X., Xi, Z. H., Cheng, X. L., and Wei, F. (2012). Diuretic activity of the ethanol and aqueous extracts of the surface layer of Poria cocos in rat. J. Ethnopharmacol. 144 (3), 775–778. doi:10.1016/j.jep.2012.09.033
Zheng, K. Y., Choi, R. C., Xie, H. Q., Cheung, A. W., Guo, A. J., Leung, K. W., et al. (2010). The expression of erythropoietin triggered by danggui buxue tang, a Chinese herbal decoction prepared from radix Astragali and radix Angelicae Sinensis, is mediated by the hypoxia-inducible factor in cultured HEK293T cells. J. Ethnopharmacol. 132 (1), 259–267. doi:10.1016/j.jep.2010.08.029
Zheng, Y., Cai, G. Y., He, L. Q., Lin, H. L., Cheng, X. H., Wang, N. S., et al. (2017). Efficacy and Safety of Niaoduqing Particles for Delaying Moderate-to-severe Renal Dysfunction: A Randomized, Double-blind, Placebo-controlled, Multicenter Clinical Study. Chin. Med. J. Engl. 130 (20), 2402–2409. doi:10.4103/0366-6999.216407
Zheng, Y., Wang, N. S., Liu, Y. N., He, L. Q., Jian, G. H., Liu, X. S., et al. (2019). Effects of Niaoduqing Particles () on Delaying Progression of Renal Dysfunction: A Post-trial, Open-Label, Follow-up Study. Chin. J. Integr. Med. 25 (3), 168–174. doi:10.1007/s11655-018-2998-y
Zhong, J., Zhao, N., Liu, C., Hu, J., Zhang, W., Xiong, C., et al. (2018). Research on the intervention of Yishen Huoxue prescription to renal fibrosis through the signal regulation by microRNA-126 to VEGF-Notch. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 30 (10), 991–995. doi:10.3760/cma.j.issn.2095-4352.2018.010.018
Zhong, Y., Wang, K., Zhang, X., Cai, X., Chen, Y., and Deng, Y. (2015). Nephrokeli, a Chinese Herbal Formula, May Improve IgA Nephropathy through Regulation of the Sphingosine-1-Phosphate Pathway. PloS one 10 (1), e0116873. doi:10.1371/journal.pone.0116873
Keywords: chronic kidney disease, renal fibrosis, traditional Chinese medicine, Chinese materia medica, reinforcing deficiency and purging excess, intervention mechanism
Citation: Wang Y, Feng Y, Li M, Yang M, Shi G, Xuan Z, Yin D and Xu F (2022) Traditional Chinese Medicine in the Treatment of Chronic Kidney Diseases: Theories, Applications, and Mechanisms. Front. Pharmacol. 13:917975. doi: 10.3389/fphar.2022.917975
Received: 11 April 2022; Accepted: 01 June 2022;
Published: 18 July 2022.
Edited by:
Ying-Yong Zhao, Northwest University, ChinaReviewed by:
Yue Liu, Xiyuan Hospital, ChinaCopyright © 2022 Wang, Feng, Li, Yang, Shi, Xuan, Yin and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dengke Yin, eWluZGVuZ2tlQGFodGNtLmVkdS5jbg==; Fan Xu, MTM1MDU1MTQ0MDdAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.