- 1Chinese Evidence-based Medicine Center, West China Hospital, Sichuan University, Chengdu, China
- 2State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, China,
Objective: Motherwort injection (MI) is a modern patented injection extracted from motherwort (Leonurus japonicus Hoult). Empirical studies and systematic reviews have shown the benefits of motherwort injection for preventing postpartum hemorrhage after vaginal delivery and cesarean section. This study was conducted to explore the efficacy and safety of motherwort injection for women with the prevention of post-abortion uterine hemorrhage.
Methods: A comprehensive literature search was conducted to identify RCTs regarding the effect of the use of motherwort injection in women after abortion. Data from trials were pooled by meta-analysis and a random-effects model was used to calculate the summarized relative risks (RRs) and their 95% confidence intervals (CIs). The grading of recommendations assessment, development, and evaluation (GRADE) methodology was used to access the quality of the evidence.
Results: Nine trials with a total of 1,675 participants were identified. Overall, motherwort injection combined with oxytocin compared to oxytocin had a significantly lower blood loss within 2 hours (MD = −50.00, 95% CI −62.92 to −37.08, very low quality); lower blood loss within 24 h (MD = −50.00, 95% CI −62.92 to −37.08, very low quality); however, there was no significant difference between motherwort injection and oxytocin (24 h: MD: 0.72, 95% CI −7.76 to 9.20; 48 h: MD: −0.01, 95% CI −11.35 to 11.33; 72 h: MD: −1.12, 95% CI −14.39 to 12.15, very low quality). Compared with oxytocin or no intervention, both motherwort injection and motherwort injection combined with oxytocin had a significantly decreased duration of blood loss (MI vs. O: MD −2.59, 95% CI −4.59 to −0.60, very low quality; MI + O vs. O: MD −2.62, 95% CI -3.02 to −2.22, very low quality; MI + O vs. No intervention: MD: −1.80, 95% CI −2.28 to −1.33, low quality). Seven of nine included trials reported adverse event outcomes. Three cases were found in the motherwort injection group, and five induced abortion syndromes were found in the motherwort injection plus oxytocin group. 29 adverse events were reported in the oxytocin group instead. The recovery time of normal menstruation after abortion was significantly earlier in the group using motherwort injection compared with oxytocin (MDs −3.77, 95% CI −6.29 to −1.25, very low quality), and the endometrial thickness in the motherwort injection group was significantly different from that in the oxytocin group (MD: 2.24, 95% CI 1.58 to 2.90, very low quality).
Conclusion: The results of this meta-analysis indicate prophylactic use of motherwort injection may reduce the risk of uterine hemorrhage in women after abortion, and more high-quality research is needed to confirm the efficacy and safety of motherwort injection in preventing uterine hemorrhage after abortion.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=274153, identifier CRD42021274153
1 Introduction
An estimated 205 million pregnancies occur each year worldwide, with 20% being terminated by induced abortion. Medication and surgery are both highly effective methods for induced abortion and are determined by the gestational age of the embryo or tutus. Abortion is one of the safest procedures when properly done, but unsafe abortion is associated with significant morbidity (Organization., 2021). Hemorrhage is the common consequence of misled management of abortion care and risks including retained tissues, uterine injury, uterine atony, and vaginal laceration are related to uterine bleeding after an abortion (Kerns and Steinauer, 2013; Perriera et al., 2017). The administration of uterotonic agents, such as oxytocin, misoprostol, and methylergonovine, is recommended to prevent hemorrhage after induced abortion if the uterus is atonic (Evensen et al., 2017; Bienstock et al., 2021; Steinauer and Patil, 2021). However, excessive use of oxytocin and misoprostol may cause high fever, shaking, chills, vomiting, hypertension, and other complications of toxicity (Widmer et al., 2010; Cleland et al., 2013).
Motherwort injection (MI) is a modern patented injection made from aqueous extracts of motherwort (Leonurus japonicus Hoult), which is a traditional Chinese herb used by thousands for gynecological conditions in China (Lulin et al., 2019). Pharmacological studies have shown that the active ingredients of motherwort injection (i.e. alkaloid, leonurine) could significantly facilitate hemostatic outcomes by promoting uterine contraction and blocking the uterine spiral vessels (Jian et al., 2005; Ojewole, 2005; Xiaoju, 2009; Huizhen et al., 2021). Moreover, motherwort injection works on the lower uterus without the receptor saturation effect, which reduces the risk of adverse events caused by the excessive use of uterotonic agents (Wenjing, 2022). Therefore, the prophylactic use of motherwort injection with or without oxytocin has been widely applied in Chinese tertiary hospitals to prevent postpartum and postabortion hemorrhage since 2005.
Empirical studies (Jianhua et al., 2009; Wei et al., 2016) and systematic reviews (Wenwen et al., 2018; Jiajie et al., 2019) have illustrated the benefits of motherwort injection for preventing PPH (postpartum hemorrhage) after vaginal delivery and cesarean section. Meanwhile, some trials have been published to explore the effect of motherwort injection on women after induced abortion and the findings were inconsistent. Therefore, we conducted a systematic review of randomized trials to determine the efficacy and safety of motherwort injection compared to oxytocin in women with induced abortion.
2 Materials and Method
This systematic review has been registered in the PROSPERO database (CRD42021274153) and reported according to The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines (Page et al., 2021) (Supplementary Material S1).
2.1 Eligibility Criteria
Randomized control trials were eligible if they met the following criteria: 1) Participants: pregnant women anticipating an induced abortion; 2) Intervention: motherwort injection given by any route of administration and dose used alone or in combination with oxytocin; 3) Control: no intervention or oxytocin alone; 4) Outcomes measures: duration of uterine hemorrhage, the volume of blood loss, adverse events, the recovery time of normal menstruation, and endometrial thickness. We excluded trials reporting blood loss as a categorical variable due to a lack of classification criteria.
2.2 Data Source and Search Strategy
Relevant studies were identified from PubMed, EMbase, Cochrane Central Register of Controlled Trials (CENTRAL), Chinese National Knowledge Infrastructure Database (CNKI) and WanFang database inception to December 2021, updated to May 2022. An information expert was consulted to optimize our search strategies (Supplementary Material S2). ClinicalTrial.gov and Chinese Clinical Trial Registry were searched to identify unpublished studies, and the reference lists of included trials were searched for additional eligible studies. The search strategy was based on Mesh terms and their variants. No restriction in language was applied.
We also contacted a content expert and industry representatives and searched conference abstracts for additional information.
2.3 Data Selection and Data Extraction
Two reviewers (Xue XY and Tang XT) used predefined, pilot-tested forms to screen studies for eligibility, independently screened titles/abstracts, and full text of potentially eligible articles. They independently assessed the risk of bias, quality of evidence and extracted data. If necessary, discrepancies were resolved through discussion. We collected information regarding study characteristics (sample size, publication year, author name, affiliation, and multicenter study), participants’ characteristics (age, gestational week, and risk factors), interventions (dosage, timing, injection site, and duration of treatment), and outcomes (duration of hemorrhage, blood loss, adverse events, recovery time of normal menstruation, and endometrial thickness).
2.4 Risk of Bias Assessment
We assessed the risk of bias using the revised Cochrane Risk of Bias tool (Higgins et al., 2011; Akl et al., 2012) in our published study. The items included randomization sequence generation, allocation concealment, blinding of patients and personnel, or outcome assessors, infrequent missing outcome data, selective outcome reporting, and funding resources. The risk of bias for each item will be classified into low risk, high risk, and unclear. The risk of bias for each item will be classified into low risk, high risk, and unclear. The options for an overall risk of bias are the same as for individual items and are based on the following criteria: 1) trials were judged to be at low risk of bias if all items were assessed as low risk; 2) to be at high risk in at least one item assessed as high risk, or multiple items were assessed as an unclear risk; 3) to be an unclear risk in at least one item assessed as unclear but not to be at high risk for any item (Higgins et al., 2022).
2.5 Data Analysis and Rating Quality of Evidence
The data were pooled using a random-effects model for potential heterogeneity among studies when two or more studies assessed the same outcome. Heterogeneity among studies was assessed by Cochran’s Q test and the I2 statistic. We expressed dichotomous data as risk ratio (RR) with 95% confidence intervals (CIs) and continuous data as mean differences (MDs) with 95% CIs. If the trial was comparing three groups, we separately analyzed the data in terms of their interventions. The intervention arm was included twice in the analysis; however, this was related to only one trial, and this double inclusion would not influence the outcomes. Subgroup analyses were performed based on the type of administration (immediate administration versus consecutive administration) and risk for hemorrhage after abortion (high risk vs. moderate risk vs. low risk) when applicable. We summarized the adverse event data from all included studies and qualitatively described the data for rare data. Publication bias was assessed using Egger test plots when ten or more studies were available (Higgins JPT et al., 2022). RevMan 5.4 software was used for meta-analysis. We used the grading of recommendations assessment, development, and evaluation (GRADE) methodology to assess the quality of the evidence (Guyatt et al., 2008).
3 Results
3.1 Search Results
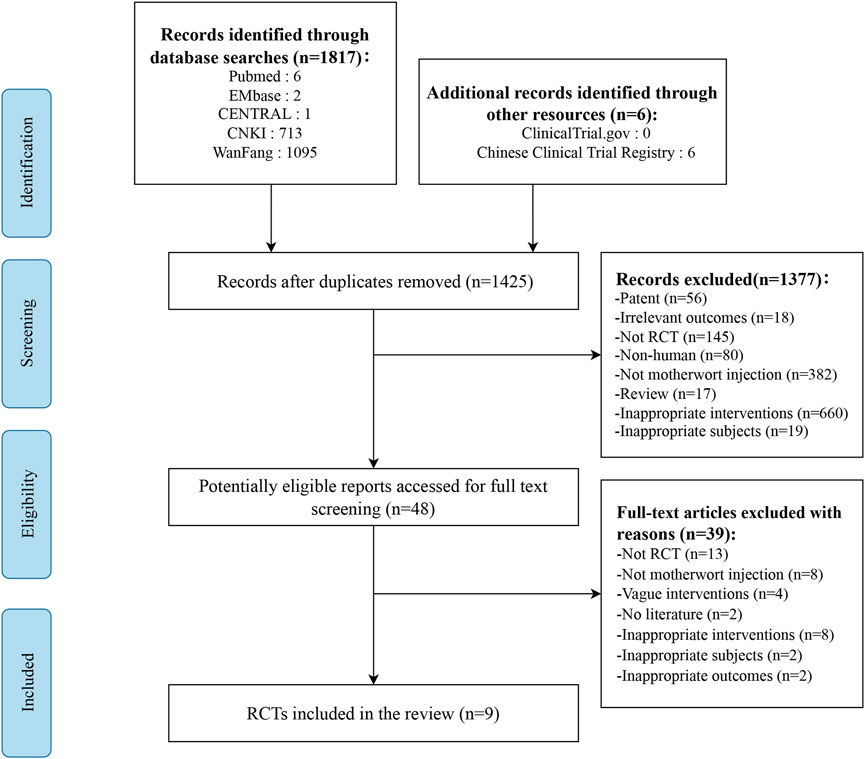
Seven databases were screened yielding a total of 1823 studies. After removing duplicates and title and abstract screening, 48 studies were selected for a full-text review. Of 48 potentially relevant studies, 37 were excluded (e.g., studies were not properly randomized, or did not report relevant outcomes, etc.,) and two were abstracts without outcome measures. Finally, nine studies involving 1,675 women were included in the systematic review. The selection process is listed in Figure 1.
3.2 Study Characteristics
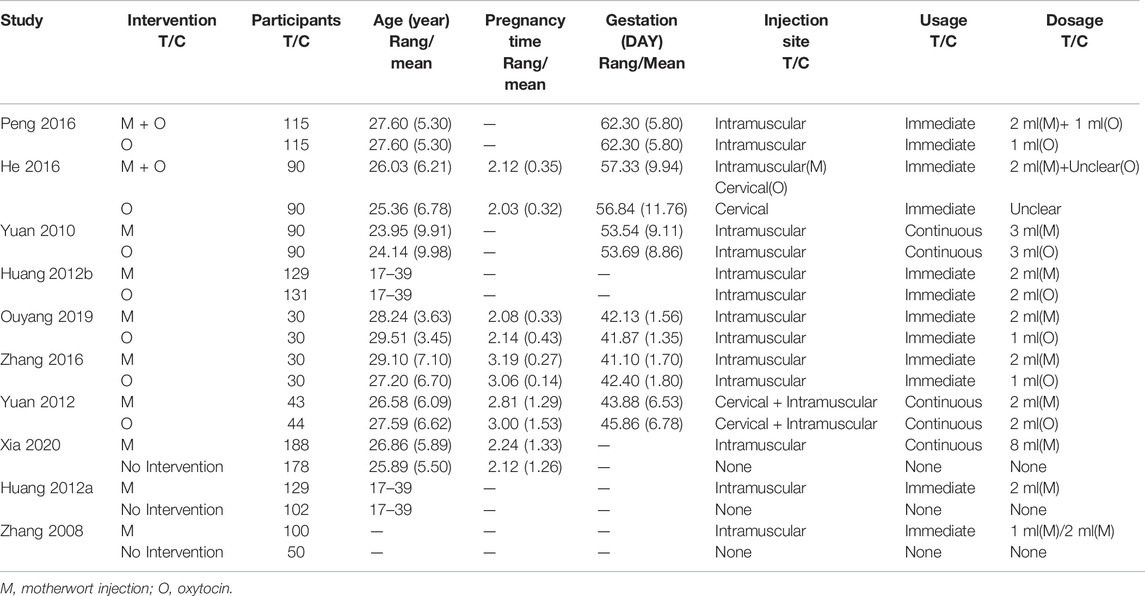
One multicenter RCT was identified and the remaining RCTs were single centers. All studies were reported in Chinese except one (Wanting et al., 2020) in English. These studies were all conducted in China between 2006 and 2019, from 60 to 366, and the characteristics of included studies are shown in Table 1. The mean age of pregnant women was 26.43 (SD:6.72), the mean pregnancy time was 2.31 (SD:1.10), and the mean gestation was 54.18 (SD:10.62) days. Two studies (Juan, 2016; Yun and Dengyu, 2016) evaluated motherwort injection combined with oxytocin versus oxytocin alone, four studies (Jiaogui, 2010; Li and Lixia, 2012; Yingjie and Junling, 2016; Yunyan, 2019) compared motherwort injection with oxytocin, and two studies (Yimei et al., 2008; Wanting et al., 2020) compared motherwort injection to no intervention. There was one trial (Yanxia, 2012) with multiple arms (MI vs. O vs. None).
3.3 Risk of Bias Within Studies
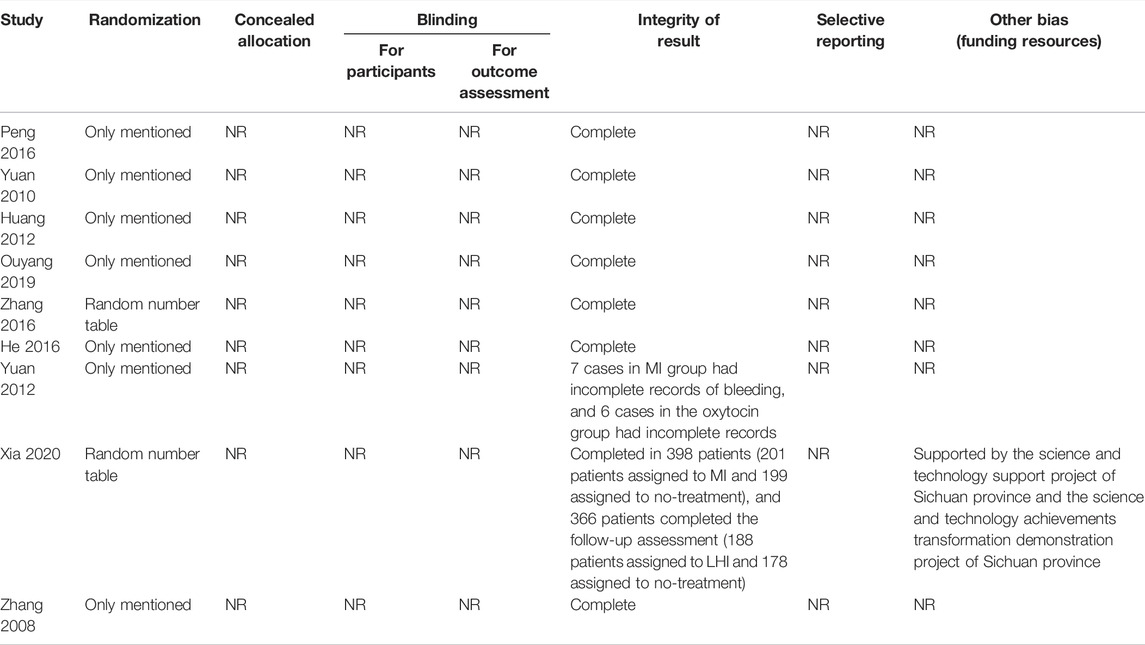
Among these nine trials, two (Yingjie and Junling, 2016; Wanting et al., 2020) adequately generated random sequences by random number table or computer; none of them clearly stated how to conceal the random sequence and blind the participants, health care providers, or outcome assessors; none of them reported selective outcomes, and one trial reported the funding resource. Table 2 contains detailed results of the assessment. Overall, each of the included studies assessed the risk of bias to be unclear.
3.4 Outcome measures
3.4.1 Blood Loss
3.4.1.1Motherwort Injection vs. Oxytocin
Only one RCT (Li and Lixia, 2012) involving 87 participants reported blood loss within 24 h, 48 h, and 72 h. The data from this trial showed no significant difference between motherwort injection and oxytocin in all three assessments (24 h: MD: 0.72, 95% CI −7.76 to 9.20; 48 h: MD: −0.01, 95% CI −11.35 to 11.33; 72 h: MD: −1.12, 95% CI −14.39 to 12.15, very low quality).
3.4.1.2 Motherwort Injection Plus Oxytocin vs. Oxytocin
One trial (n = 230) (Juan, 2016) compared motherwort injection to oxytocin reporting blood loss within 2 hours and 24 h. There was a significant decrease in blood loss in the combined group compared to oxytocin alone (2 h: MD: −50.00, 95% CI −62.92 to −37.08; 24 h: MD: −50.00, 95% CI −62.92 to -37.08, very low quality).
3.5 Duration of Blood Loss (days)
3.4.1 Motherwort Injection vs. Oxytocin
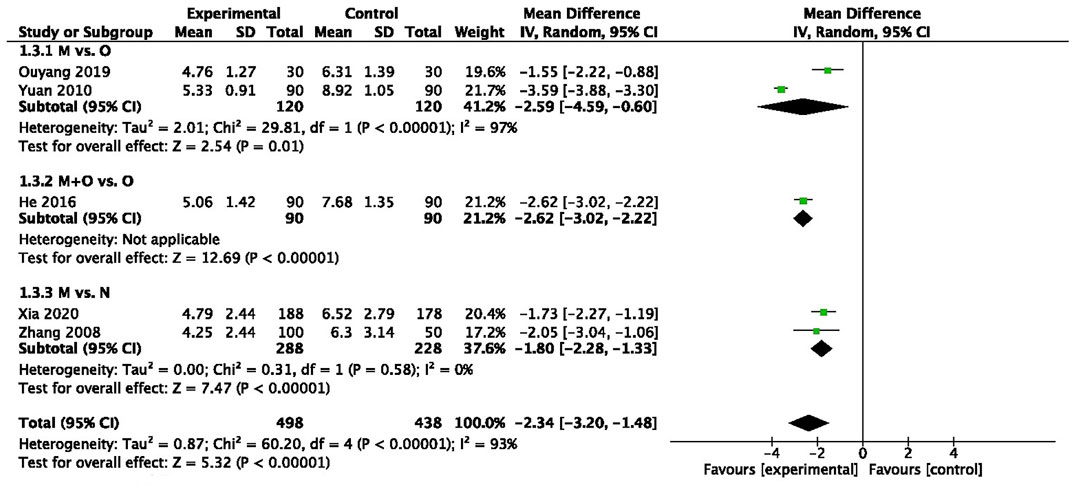
There was statistically significant heterogeneity among two trials (n = 240, I2 = 97%) (Jiaogui, 2010; Yunyan, 2019), and pooled data demonstrated that motherwort injection significantly decreased the duration of blood loss compared to oxytocin (MD −2.59, 95% CI −4.59 to −0.60, very low quality) (Figure 2).
3.5.2 Motherwort Injection Plus Oxytocin vs. Oxytocin
We also observed a significantly decreased duration of blood loss in the combination administration group in one trial (MD -2.62, 95% CI −3.02 to −2.22, very low quality) (Yun and Dengyu, 2016) (Figure 2).
3.5.3 Motherwort Injection vs. no Intervention
Two trials (n = 516) (Yimei et al., 2008; Wanting et al., 2020) collected data for the duration of blood loss, and there was a significant reduction in days after motherwort injection (MD: −1.80, 95% CI −2.28 to −1.33, low quality) (Figure 2).
3.6 Adverse Events
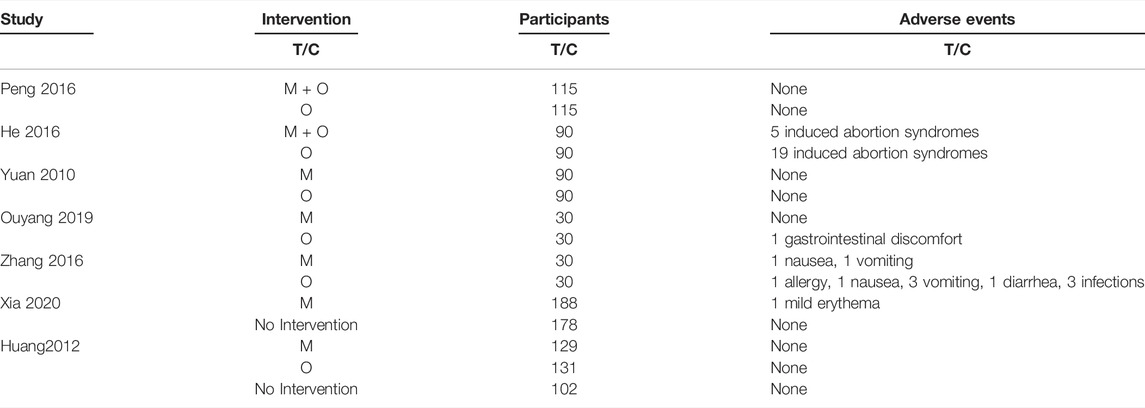
Seven of nine included trials commented on adverse event outcomes, and three of them (Jiaogui, 2010; Yanxia, 2012; Juan, 2016) stated none had occurred. The remaining four trials (Yingjie and Junling, 2016; Yun and Dengyu, 2016; Yunyan, 2019; Wanting et al., 2020) reported adverse events, three cases (mild erythema, nausea and vomiting) (1.2%, 3/248) were found in the motherwort injection group, and five induced abortion syndromes were found in the motherwort injection plus oxytocin group (5.5%, 5/90). 29 adverse events (e.g., induced abortion syndromes, nausea, vomiting, infection, etc.) were reported in the oxytocin group instead (19.3%, 29/150) (Table 3).
3.7 Recovery Time of Normal Menstruation
3.7.1 Motherwort Injection vs. Oxytocin (days)
Only one trial (n = 60) (Yunyan, 2019) comparing motherwort injection with oxytocin discussed the recovery time of normal menstruation. The data showed significantly earlier recovery of normal menstruation in the motherwort injection group than those in the oxytocin group (MDs −3.77, 95% CI −6.29 to −1.25, very low quality).
3.8 Endometrial Thickness
3.8.1 Motherwort Injection vs. Oxytocin (mm)
Only one RCT (Yunyan, 2019) involving 60 participants reported endometrial thickness after abortion. The data from this trial showed a significant difference in endometrial thickness after abortion between the two groups (MD: 2.24, 95% CI 1.58 to 2.90, very low quality).
3.9 Subgroup Analysis and Publication Bias
We failed to conduct the subgroup analysis and assess publication bias for the small number of studies included in each outcome measure.
4 Discussion
This review brings all randomized evidence together to assess the effect of motherwort injection for postabortion hemorrhage. Compared with oxytocin, a significant benefit of prophylactic use of motherwort injection with or without oxytocin was reported for four outcomes: duration of blood loss, adverse events, and the recovery time of normal menstruation and endometrial thickness. In contrast, only two trials reported blood loss as a continuous variable and no significant difference was found between motherwort injection and oxytocin. Considering the low and very low methodological quality, small sample size and the observed difference between groups, this evidence supporting the use of prophylactic use of motherwort injection for women with postabortion hemorrhage must be generalized with caution.
Atony of the uterine body or fundus is a common cause of postabortion hemorrhage (Gill et al., 2022), and the risk of bleeding is associated with prior cesarean section, history of obstetrical hemorrhage, increasing maternal age, gestational age and obesity (Upadhyay et al., 2015; Kerns et al., 2019). Uterotonic agents are a priority protocol for the prevention and treatment of postabortion hemorrhage in women with uterine atony, including methylergonovine, misoprostol and oxytocin. However, little evidence exists to recommend starting with a particular agent. Motherwort injection, approved for marketing in 1971 by the Chinese FDA, has been used for stopping bleeding and regulating menstruation for decades (Yulin et al., 2018). Modern pharmacological studies have demonstrated that the active ingredients of motherwort injection, such as leonurine and stachydrine (Yulin et al., 2018), could exhibit angiogenic activity and have an excitatory effect on the uterus, without adverse effects such as elevated blood pressure (Dan et al., 2013; Xiaofei et al., 2014; Rebonato et al., 2016; Juan et al., 2018; Liefang, 2021; Qiang et al., 2021). These active ingredients can also dilate blood vessels and protect the cardiovascular system (Chengping et al., 2019), and will not affect women’s temperature and respiration (Yanfang et al., 2017). In addition, oxytocin is a polypeptide hormone with uterine contraction and has a rapid onset of action (Aiqun et al., 2007), while motherwort injection causes contractions for a longer period than oxytocin after injection into the uterine wall (half-life is 6 h) with a relatively slow onset of action. Therefore, the additional use of motherwort injection on oxytocin has been widely applied in routine clinical practice.
Our published SRs (Wenwen et al., 2018; Jiajie et al., 2019) have suggested the preferable outcomes of motherwort injection for preventing PPH (postpartum hemorrhage) after vaginal delivery and cesarean section. However, even with wide application in clinical settings, limited studies have been conducted to discuss the effect of motherwort injection on a postabortion hemorrhage. To the best of our knowledge, this is the first systematic review and meta-analysis to address the effect of motherwort injection on a postabortion hemorrhage.
4.1 Limitations
We conducted a comprehensive systematic review including all published RCTs with rigorous methods to evaluate the effect of motherwort injection for women with induced abortion. However, our study also has a few significant limitations. First, the trials included suffered from a high risk of bias, and only two trials clearly stated the method of random sequence generation. Second, we were unable to conduct a subgroup analysis to explore the source of heterogeneity for a limited number of studies. Most of the trials included in our analyses had small sample sizes and resulted in an imprecise estimation of effects with very low quality. Third, the trials we included were all conducted in China mainland and included women who were in the first termination; and no trials on the use of motherwort injection in women with intermediate or late abortions. Fourth, the most appropriate dosing schedule is still unknown for the limited evidence we found.
5 Conclusion
In conclusion, due to the small number of events and sample sizes and severe limitations, the current body of evidence is inadequate to establish the positive effects—including blood loss, duration of blood time, adverse events, the recovery time of normal menstruation and endometrial thickness—of motherwort injection preventing hemorrhage after abortion. Given the insufficiently high quality of these trials, future adequately powered, well-designed, and conducted trials are warranted to test the effects of the different treatments preventing hemorrhage fairly. Observational studies that carefully collect and analyze the data may also provide important insights regarding the effects of motherwort injection.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
YJ and LY conceived and designed this study; XX and TX searched the literature and extracted data, XX synthesized data, and developed the first draft of the manuscript; YJ provided critical methodological guidance; WF provided clinical guidance. All authors critically revised the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.916665/full#supplementary-material
References
Aiqun, X., Weiyue, Z., Dairong, W., Yong, Y., Xinghui, L., Aiyun, X., et al. (2007). Primary Effects of Motherwort Injection on Uterine Contraction and Hemostasis. Chin. J. Obstetrics Gynecol. Pediatr. 3 (2), 88–90. doi:10.3969/j.issn.1673-5250.2007.02.009
Akl, E. A., Sun, X., Busse, J. W., Johnston, B. C., Briel, M., Mulla, S., et al. (2012). Specific Instructions for Estimating Unclearly Reported Blinding Status in Randomized Trials Were Reliable and Valid. J. Clin. Epidemiol. 65 (3), 262–267. doi:10.1016/j.jclinepi.2011.04.015
Bienstock, J. L., Eke, A. C., and Hueppchen, N. A. (2021). Postpartum Hemorrhage. N. Engl. J. Med. 384 (17), 1635–1645. doi:10.1056/NEJMra1513247
Chen, W., Yu, J., Tao, H., Cai, Y., Li, Y., and Sun, X. (2018). Motherwort Injection for Preventing Postpartum Hemorrhage in Pregnant Women with Cesarean Section: A Systematic Review and Meta-Analysis. J. Evid. Based Med. 11 (4), 252–260. doi:10.1111/jebm.12300
Chengping, L., Cuiping, X., Jianlong, Y., Jinjin, S., Li, D., Wenying, W., et al. (2019). Protective Effects of the Active Components of Leonurus Japonicus Houtt. In Myocardial Ischemic Reperfusion Injury. Chin. J. Hosp. Pharm. | Chin J Hosp Pharm 39 (07), 708–712. doi:10.13286/j.cnki.chinhosppharmacyj.2019.07.11
Cleland, K., Creinin, M. D., Nucatola, D., Nshom, M., and Trussell, J. (2013). Significant Adverse Events and Outcomes after Medical Abortion. Obstet. Gynecol. 121 (1), 166–171. doi:10.1097/aog.0b013e3182755763
Dan, L., Xiaofang, X., Cheng, P., Liang, X., Xiaomei, Z., and Juan, L. (2013). “Effects of the Extractum of Leonurus Japonicus Injection and its Alkaloid and Non-alkaloid Fraction on Uterus Induced by Drug-Abortion in Rats,” in Proceedings of the 4th International Conference on Modernization of Traditional Chinese Medicine, Chengdu, Septemper 26, 2013.
Evensen, A., Anderson, J. M., and Fontaine, P. (2017). Postpartum Hemorrhage: Prevention and Treatment. Am. Fam. Physician 95 (7), 442–449.
Gill, P., Patel, A., and Van Hook, J. W. (2022). “Uterine Atony,” in StatPearls (Florida: Treasure Island).
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). GRADE: an Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. Bmj 336 (7650), 924–926. doi:10.1136/bmj.39489.470347.AD
He, Y. L., Shi, J. Y., Peng, C., Hu, L. J., Liu, J., Zhou, Q. M., et al. (2018). Angiogenic Effect of Motherwort (Leonurus Japonicus) Alkaloids and Toxicity of Motherwort Essential Oil on Zebrafish Embryos. Fitoterapia 128, 36–42. doi:10.1016/j.fitote.2018.05.002
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. Bmj 343, d5928. doi:10.1136/bmj.d5928
J. P. T. Higgins, J. Thomas, J. Chandler, M. Cumpston, T. Li, M. J. Pageet al. (Editors) (2022). Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3 (Updated February 2022). Cochrane. Available: www.training.cochrane.org/handbook.
Huizhen, Y., Ying, L., and Xizhen, G. (2021). Research Progress on Chemical Components and Pharmacological Effects of Lagopsis Supina, Leonurus Japonicus and Prunella Vulgaris. J. Beijing Union Univ. 35 (02), 85–92. doi:10.16255/j.cnki.ldxbz.2021.02.013
Jianhua, L., Qide, L., Xinghui, L., Jianying, Y., Jing, H., Li, L., et al. (2009). Multi-center Study of Motherwort Injection to Prevent Postpartum Hemorrhage after Caesarian Section. Zhonghua Fu Chan Ke Za Zhi 44 (3), 175–178. doi:10.3969/j.issn.1003-6946.2009.01.017
Jiaogui, Y. (2010). The Clinical Application of Motherwort Injection on Post-surgical Abortion. Strait Pharm. J. 22 (2), 86–88. doi:10.3969/j.issn.1006-3765.2010.02.041
Juan, P. (2016). The Clinical Application of Motherwort Injection in Induced Abortion. J. Pract. Gynecol. Endocrinol. 3 (2), 81–82.
Kerns, J., and Steinauer, J. (2013). Management of Postabortion Hemorrhage: Release Date November 2012 SFP Guideline #20131. Contraception 87 (3), 331–342. doi:10.1016/j.contraception.2012.10.024
Kerns, J. L., Ti, A., Aksel, S., Lederle, L., Sokoloff, A., and Steinauer, J. (2019). Disseminated Intravascular Coagulation and Hemorrhage After Dilation and Evacuation Abortion for Fetal Death. Obstet. Gynecol. 134 (4), 708–713. doi:10.1097/aog.0000000000003460
Li, Y., and Lixia, G. (2012). Motherwort Injection in the Prevention and Treatment of Intraoperative and Postoperative Bleeding in Induced Abortion: Safety and Efficacy. World Health Dig. Med. Period. 9 (26), 276–277. doi:10.3969/j.issn.1672-5085.2012.26.258
Liefang, L. (2021). Curative Effect of Motherwort Injection Combined with Oxytocin in Prevention of Postpartum Hemorrhage After Cesarean Section. J. LiaoNIing Univ. TraditIional Chin. Med. 23 (09), 218–220. doi:10.13194/j.issn.1673-842x.2021.09.046
Liu, J., Peng, C., Zhou, Q.-M., Guo, L., Liu, Z.-H., and Xiong, L. (2018). Alkaloids and Flavonoid Glycosides from the Aerial Parts of Leonurus Japonicus and Their Opposite Effects on Uterine Smooth Muscle. Phytochemistry 145, 128–136. doi:10.1016/j.phytochem.2017.11.003
Liu, W., Ma, S., Pan, W., and Tan, W. (2016). Combination of Motherwort Injection and Oxytocin for the Prevention of Postpartum Hemorrhage after Cesarean Section. J. Maternal-Fetal Neonatal Med. 29 (15), 1–4. doi:10.3109/14767058.2015.1090425
Miao, L.-L., Zhou, Q.-M., Peng, C., Liu, Z.-H., and Xiong, L. (2019). Leonurus Japonicus (Chinese Motherwort), an Excellent Traditional Medicine for Obstetrical and Gynecological Diseases: A Comprehensive Overview. Biomed. Pharmacother. 117, 109060. doi:10.1016/j.biopha.2019.109060
Ojewole, J. A. (2005). Antinociceptive, Antiinflammatory and Antidiabetic Effects of Leonotis Leonurus Leaf Aqueous Extract in Mice and Rats. Methods Find. Exp. Clin. Pharmacol. 27 (4), 257–264. doi:10.1358/mf.2005.27.4.893583
Organization, W. H. (2021). Abortion. Geneva, Switzerland: World Health Organization. [Online]. Available: https://www.who.int/news-room/fact-sheets/detail/abortion ([Accessed].
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 Statement: an Updated Guideline for Reporting Systematic Reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Perriera, L. K., Arslan, A. A., and Masch, R. (2017). Placenta Praevia and the Risk of Adverse Outcomes during Second Trimester Abortion: A Retrospective Cohort Study. Aust. N. Z. J. Obstet. Gynaecol. 57 (1), 99–104. doi:10.1111/ajo.12580
Qiang, H., Mengdie, L., Wei, L., Yalin, Z., Xuemei, G., and Jie, M. (2021). Investigation and Analysis of Postpartum Hemorrhage in Sichuan Provincial People's Hospital from 2013 to 2020. Pract. J. Clin. Med. 18 (06), 194–197. doi:10.3969/j.issn.1672-6170.2021.06.055
Rebonato, A., Mosca, S., Fischer, M., Gerli, S., Orgera, G., Graziosi, L., et al. (2016). Endovascular Management of Massive Post-partum Haemorrhage in Abnormal Placental Implantation Deliveries. Eur. Radiol. 26 (6), 1620–1630. doi:10.1007/s00330-015-4001-z
Shang, X., Pan, H., Wang, X., He, H., and Li, M. (2014). Leonurus Japonicus Houtt.: Ethnopharmacology, Phytochemistry and Pharmacology of an Important Traditional Chinese Medicine. J. Ethnopharmacol. 152 (1), 14–32. doi:10.1016/j.jep.2013.12.052
Steinauer, J., and Patil, M. (2021). Overview of Pregnancy Termination. [Online]. Available: https://www.uptodate.cn/contents/zh-Hans/overview-of-pregnancy-termination?search ([Accessed].
Sun, J., Huang, S. H., Zhu, Y. C., Whiteman, M., Wang, M. J., Tan, B. K.-H., et al. (2005). Anti-oxidative Stress Effects of Herba Leonuri on Ischemic Rat Hearts. Life Sci. 76 (26), 3043–3056. doi:10.1016/j.lfs.2004.11.024
Upadhyay, U. D., Desai, S., Zlidar, V., Weitz, T. A., Grossman, D., Anderson, P., et al. (2015). Incidence of Emergency Department Visits and Complications after Abortion. Obstet. Gynecol. 125 (1), 175–183. doi:10.1097/aog.0000000000000603
Wang, W. (2022). Image Analysis Application of Motherwort Total Alkaloid Injection in the Treatment of Postabortion Hemorrhage. J. Healthc. Eng. 2022, 8725030. doi:10.1155/2022/8725030
Widmer, M., Blum, J., Hofmeyr, G. J., Carroli, G., Abdel-Aleem, H., Lumbiganon, P., et al. (2010). Misoprostol as an Adjunct to Standard Uterotonics for Treatment of Post-partum Haemorrhage: a Multicentre, Double-Blind Randomised Trial. Lancet 375 (9728), 1808–1813. doi:10.1016/s0140-6736(10)60348-0
Xia, W.-T., Zhou, H., Wang, Y., Hu, H.-Q., Xu, Z.-H., Li, S.-W., et al. (2020). Motherwort Injection in Preventing Post-abortion Hemorrhage after Induced Abortion: A Multi-Center, Prospective, Randomized Controlled Trial. Explore 16 (2), 110–115. doi:10.1016/j.explore.2019.08.004
Xiaoju, B. (2009). Discussion on Drug Reevaluation by Analyzing Adverse Drug Reaction of Traditional Chinese Medicine Injection. Chin. J. Pharmacovigil. 6 (02), 86–90. doi:10.3969/j.issn.1672-8629.2009.05.005
Yanfang, D., Xueling, Z., Mingyang, Z., and Lichuan, T. (2017). Efficacy and Safety Analysis of Leonurus Injection Combined with Gedan and Xinmu Pei in Hemorrhage of Previa Cesarean Section. World Chin. Med. 12 (03), 602–605. doi:10.3969/j.issn.1673-7202.2017.03.032
Yanxia, H. (2012). Clinical Observation of Motherwort Injection in the Treatment of Vaginal Bleeding after Medical Abortion. Med. Innovation China 9 (26), 25–26. doi:10.3969/j.issn.1674-4985.2012.26.014
Yimei, Z., Gang, F., and Yuming, H. (2008). The Application of Motherwort Injection in Reducing Bleeding after Induced Abortion. Pract. Pharm. Clin. Remedies 11 (3), 161–162. doi:10.3969/j.issn.1673-0070.2008.03.020
Yingjie, Z., and Junling, L. (2016). The Application of Motherwort Injection in Reducing Bleeding after Artificial Abortion. China Med. Pharm. 6 (8), 102–104.
Yu, J., Cai, Y., Su, G., and Li, Y. (2019). Motherwort Injection for Preventing Postpartum Hemorrhage in Women with Vaginal Delivery: A Systematic Review and Meta-Analysis of Randomized Evidence. Evid. Based Complement. Altern. Med. 2019, 1803876. doi:10.1155/2019/1803876
Yun, H., and Dengyu, S. (2016). Effect of Motherwort Injection on Uterine Blood Flow and Uterine Contraction Intensity in Women with Induced Abortion. J. Clin. Med. Pract. 20 (5), 157–158. doi:10.7619/jcmp.201605055
Keywords: induced abortion, motherwort injection, Oxytocin, uterine hemorrhage, meta-analysis, systematic review, randomized controlled trials
Citation: Xinyu X, Xintong T, Youping L, Feng W and Jiajie Y (2022) Motherwort Injection for Preventing Uterine Hemorrhage in Women With Induced Abortion: A Systematic Review and Meta-Analysis of Randomized Evidence. Front. Pharmacol. 13:916665. doi: 10.3389/fphar.2022.916665
Received: 09 April 2022; Accepted: 14 June 2022;
Published: 21 July 2022.
Edited by:
Nanbert Zhong, Institute for Basic Research in Developmental Disabilities (IBR), United StatesReviewed by:
Mojtaba Vaismoradi, Nord University, NorwayCheng-Yang Hu, Anhui Medical University, China
Copyright © 2022 Xinyu, Xintong, Youping, Feng and Jiajie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Jiajie, MjAwM3hpb25nQDE2My5jb20=
 Xue Xinyu
Xue Xinyu Tang Xintong1
Tang Xintong1 Yu Jiajie
Yu Jiajie