- 1West China School of Pharmacy, Sichuan University, Chengdu, China
- 2Department of Pharmacy, West China Second University Hospital, Sichuan University, Chengdu, China
- 3Evidence-Based Pharmacy Center, West China Second University Hospital, Sichuan University, Chengdu, China
- 4Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, Chengdu, China
- 5Department of Obstetrics, West China Second University Hospital, Sichuan University, Chengdu, China
- 6West China School of Medicine, Sichuan University, Chengdu, China
- 7National Drug Clinical Trial Institute, West China Second University Hospital, Sichuan University, Chengdu, China
Background: Prelabor rupture of membranes (PROM) is associated with maternal and neonatal infections. Although guidelines suggest prophylactic antibiotics for pregnant women with PROM, the optimal antibiotic regimen remains controversial. Synthesizing the data from different studies is challenging due to variations in reported outcomes.
Objective: This study aimed to form the initial list of outcomes for the core outcome set (COS) that evaluates antibiotic use in PROM by identifying all existing outcomes and patients’ views.
Methods: Relevant studies were identified by searching PubMed, EMBASE, Cochrane Library, Chinese National Knowledge Infrastructure, Wanfang, and VIP databases. We also screened the references of the included studies as a supplementary search. We extracted basic information from the articles and the outcomes. Two reviewers independently selected the studies, extracted the data, extracted the outcomes, and grouped them into domains. Then, semi-structured interviews based on the potential factors collected by the systematic review were conducted at West China Second Hospital of Sichuan University. Pregnant women who met the diagnostic criteria for PROM were enrolled. Participants reported their concerns about the outcomes. Two researchers identified the pregnant women’s concerns.
Results: A total of 90 studies were enrolled in this systematic review. The median outcomes in the included studies was 7 (1–31), and 109 different unique outcomes were identified. Pre-term PROM (PPROM) had 97 outcomes, and term PROM (TPROM) had 70 outcomes. The classification and order of the core outcome domains of PPROM and TPROM were consistent. The physiological domain was the most common for PPROM and TPROM outcomes. Furthermore, 35.1 and 57.1% outcomes were only reported once in PPROM and TPROM studies, respectively. Thirty pregnant women participated in the semi-structured interviews; 10 outcomes were extracted after normalized, and the outcomes were reported in the systematic review. However, studies rarely reported pregnant women’s concerns.
Conclusion: There was considerable inconsistency in outcomes selection and reporting in studies about antibiotics in PROM. An initial core outcomes set for antibiotics in PROM was formed.
1 Introduction
Prelabor rupture of membranes (PROM) is a rupture of membranes before the onset of labor, which consists of “pre-term prelabor rupture of membranes (PPROM)” and “term prelabor rupture of membranes (TPROM)” (Siegler et al., 2020). It affects 2.3%–18.7% of pregnancies and increases the risk of intrauterine infection, neonatal sepsis, neonatal pneumonia, etc. (Kenyon et al., 2001a; Martin et al., 2005; Mercer, 2005; Smith et al., 2005; Clark and Varner, 2011; Reuter et al., 2014; Middleton et al., 2017; Zhuang et al., 2020). Although guidelines suggest that the use of prophylactic antibiotics could reduce infection morbidity and improve the outcomes for mothers and newborns, the optimal antibiotic regimen is still controversial (Yudin et al., 2009; Kenyon et al., 2013; Thomson and Royal College of Obstetricians and Gynaecologists, 2019; Chatzakis et al., 2020; Siegler et al., 2020). Despite many studies about the antibiotics regimens for PROM conducted, it is difficult to synthesize their data due to outcome variations. As a recent systematic review shows, only 70.0% (17/20) of the included studies reported the primary outcome. The risk of bias was 35.0% (7/20) and 90.0% (18/20) of the included studies, including risk in “Measurement of outcome” and “Selection of reported result,” respectively (Chatzakis et al., 2020).
A core outcome set (COS), defined as an agreed standardized set of outcomes that should be measured and reported as a minimum, could improve consistency in outcome measurement and reduce outcome reporting bias. A COS would eliminate unnecessary waste in producing and reporting research findings (Williamson et al., 2012). The COS is drawing increasing attention across all health research areas and is referred to as a starting point for outcome selection in the work of some trialists, systematic reviewers, and guideline developers (COS users) (Gorst et al., 2016).
However, there is no COS for antibiotics in PROM or COS for treating or preventing infection in pregnant women. This systematic review and semi-structured interview would form the initial list of outcomes for the COS of antibiotics in PROM by identifying all existing outcomes and patients’ views.
2 Methods
This COS project is registered on the core outcome measures in effectiveness trials (COMET) database, and further details are available at https://www.comet-initiative.org/Studies/Details/1986.
2.1 Systematic Review
The part of the systematic review was performed and reported per the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines for systematic reviews (Preferred Reporting Items for Systematic Reviews and Meta-Analyses, 2009).
2.1.1 Search Strategy
We conducted an electronic search of PubMed, EMBASE, Cochrane Library, Chinese National Knowledge Infrastructure, Wanfang, and VIP Database from inception to September 2021. The search strategy was adjusted specifically for each database. It combined medical subject headings and free text terms for (“Fetal Membranes, Premature Rupture” “antibiotics” or “Prelabor rupture of membranes”) and (“Anti-Infective Agents” or “antibiotics” or “Penicillins” or” Cephalosporins” or” azithromycin” or” erythromycin” or” Clindamycin” ). Supplementary Table S1 lists the search terms. Citation lists of the included studies were reviewed to identify any intervention reports missed by the search strategy.
2.1.2 Inclusion Criteria
The following studies were included: 1) Participants: pregnant women (no restriction for gestational age) met the diagnostic criteria for PROM according to the guidelines of the Chinese Medical Association, American College of Obstetricians and Gynecologists, Society of Obstetricians & Gynaecologists (SOGC), Royal College of Obstetricians and Gynaecologists (ROGC), etc. 2) Intervention: antibiotics. 3) Type of study: systematic reviews, randomized controlled trials, non-randomized controlled trials, or cohort studies. The following studies were excluded: 1) non-Chinese and non-English literature, 2) unobtainable full-texts.
2.1.3 Data Extraction
Titles and abstracts were independently screened by two reviewers to determine potential eligible studies, and full texts of potentially relevant articles were independently screened by two reviewers to assess for eligibility. Disagreements were resolved by consensus or consulted a third reviewer. Two reviewers independently extracted data from the included studies and cross-checked it. The extracted data included: 1) the basic information of the articles (the first author, published year, study design, country, etc.); 2) the characteristics of participants and interventions; 3) the outcomes reported (names, definitions, and measurements of each outcome).
2.1.4 Assessment of Risk of Bias
There was no assessment of the risk of bias since the purpose of this study was to identify all outcomes reported irrespective of the study quality.
2.1.5 Data Synthesis
All outcomes were extracted verbatim from studies. Variations in the same outcome reporting were revised for consistency, and the composite outcomes were split into unique outcomes by a researcher with clinical experience in obstetrics. Outcome terminologies were assigned to one of the core outcome domains according to the COMET Handbook (Williamson et al., 2017). We calculated the number of unique outcomes for each study and outcome domain, the number of reported studies for each outcome, and the median number of the reported studies for each outcome domain.
2.2 Semi-Structured Interview
According to recommendations of COS-STAndards for Development and COMET handbook (version 1.0) (Kirkham et al., 2016; Williamson et al., 2017), a list of outcomes from published clinical trials may be supplemented with semi-structured interviews with patients. Therefore, we conducted the semi-structured interview to obtain the opinions of patients on PROM treatment.
The semi-structured interview study was conducted at West China Second Hospital of Sichuan University from January to February 2022. The West China Second University Hospital, Sichuan University, provided ethical approval. The participants gave verbal consent before their interviews. The participants’ socioeconomic information of participants came from the hospital information system.
2.2.1 Participants
Pregnant women in West China Second Hospital of Sichuan University, January to February 2022, who met the diagnostic criteria for PROM were enrolled. The exclusion criteria included: 1) pregnant women with serious illnesses who were not suitable to participate in the study; 2) pregnant women with communication difficulties; 3) pregnant women who refused to participate. The sample size was 30 since 30 subjects could achieve data saturation reported in other studies (Keyvanara et al., 2013; Alkadhimi et al., 2020). However, if new information is generated in the final interview, the sample size of the interview will increase.
2.2.2 Procedure
The research team designed a semi-structured interview guide involving open-ended questions (Supplementary). The face-to-face semi-structured interviews took place at the patient’s bedside at mutually convenient times. The researchers would explain the content and purpose of the study to the patients and interview them after obtaining their informed consent. Interviews were digitally audio-recorded using a mobile phone.
2.2.3 Analysis
All the interviews were transcribed literally by a researcher. Our systematic review developed a consensus codebook using a deduction coding process and evaluating the first 10 transcripts to identify emerging codes through an inductive coding process. Each transcript was independently coded by two researchers, and coding inconsistencies were resolved by discussion. Disagreements were resolved by consensus or a discussion in the research group. Data analysis was processed by identifying the codes to judge whether these were new outcomes and whether they should be added to the list of candidate outcomes. We would identify whether these outcomes are new and judge whether they should be added to the list of candidate outcomes.
3 Results
3.1 Systematic Review
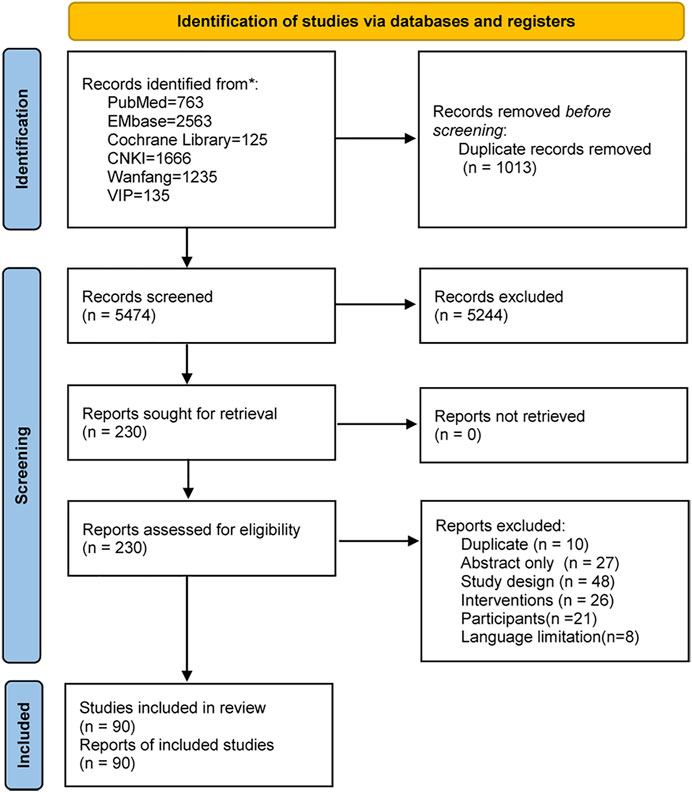
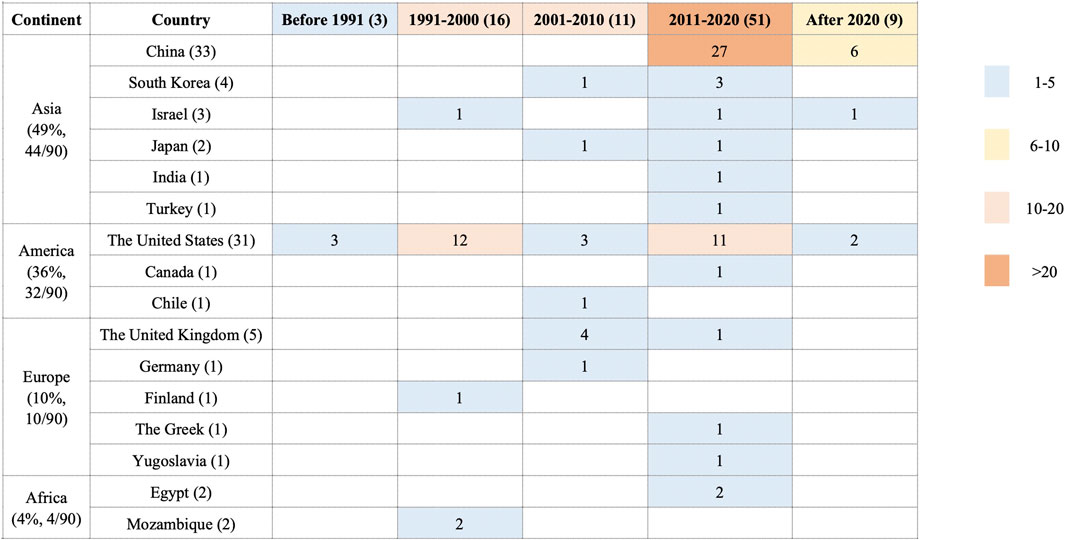
3.1.1 Study Characteristics
The search retrieved 6,487 studies. After removing duplicates and irrelevant records by screening the titles and abstracts, 230 studies were assessed for eligibility by full-text screening. Eventually, 90 studies (Chatzakis et al., 2020) were included in this systematic review (Figure 1). These studies were conducted in 17 countries on five continents from 1966 to 2021 (Figure 2). The study designs were comprised of systematic review (7/90, 7.8%) (Mercer and Arheart, 1995; Maymon et al., 1998; Kenyon et al., 2004; Cousens et al., 2010; Wojcieszek et al., 2014; Saccone and Berghella, 2015; Chatzakis et al., 2020), RCTs (32/90, 35.6%) (Brelje and Kaltreider, 1966; Amon et al., 1988; Johnston et al., 1990; McGregor et al., 1991; Kurki et al., 1992; McCaul et al., 1992; Mercer et al., 1992; Lockwood et al., 1993; Ernest and Givner, 1994; Lewis et al., 1995; Almeida et al., 1996; Grable et al., 1996; Lovett et al., 1997; Kenyon et al., 2001b; Ovalle et al., 2002; Lewis et al., 2003; Segel et al., 2003; August Fuhr et al., 2006; Kwak et al., 2013; Nabhan et al., 2014; Zhang, 2014; Kahramanoglu et al., 2016; Mai and He, 2016; Liang, 2018; Zheng, 2018; Pasquier et al., 2019; Siegel et al., 2019; Deng, 2020; Wolf et al., 2020; Chen, 2021; Cong, 2021; Zheng, 2021) and cohort studies (51/90, 56.6%) (A, 2021; Ali, 2020; Bar et al., 2020; Barišić et al., 2017; Bergström, 1991; Chang et al., 2017; Chen et al., 2020; Dotters-Katz et al., 2017; Du, 2016; Du et al., 2019; Du and Zhang, 2020; Ehsanipoor et al., 2008; Feng, 2020; Finneran et al., 2019; Finneran et al., 2017; Fitzgibbon et al., 2021; Siegel et al., 2019; Ke, 2013; Kenyon et al., 2008; Knupp et al., 2022; Kole-White et al., 2021; Lee et al., 2016; Li, 2017; Li, 2020; Lin et al., 2012; Martingano et al., 2020; Navathe et al., 2019; Pan et al., 2018; Pawar and Reddy, 2020; Pierson et al., 2014; Edwards et al., 2020; Ryo et al., 2005; Smith et al., 2015; Song and Han, 2005; Sung et al., 2017; Tai, 2011; Tanaka et al., 2019; Kramer et al., 1996; Wu, 2018; Yeung et al., 2014; Zeng and Lin, 2020; Zhang, 2017; Zhang, 2019;Zhao, 2019a; Zhao,2019b; Zheng et al., 2016; Zhou et al., 2015; Zhou, 2020; Zou, 2021; Zheng et al., 2020). Out of the 90 studies, 78 (86.7%) studies (Ali, 2020; Almeida et al., 1996; Amon et al., 1988; Bar et al., 2000; Bergström, 1991; Chang et al., 2017; Chatzakis et al., 2020; Chen et al., 2020; Chen, 2021; Cong, 2021; Cousens et al., 2010; Lewis et al., 1995; Deng, 2020; Dotters-Katz et al., 2017; Du, 2016; Du et al., 2019; Du and Zhang, 2020; Ehsanipoor et al., 2008; Ernest and Givner, 1994; Feng, 2020; Finneran et al., 2019; Finneran et al., 2017; Fitzgibbon et al., 2021; August et al., 2006; Grable et al., 1996; Siegel et al., 2019; Johnston et al., 1990; Kahramanoglu et al., 2016; Ke, 2013; Kenyon et al., 2001; Kenyon et al., 2004; Knupp et al., 2022; Kole-White et al., 2021; Kurki et al., 1992; Kwak et al., 2013; Lee et al., 2016; Li, 2017; Li, 2020; Li ,2021; Liang, 2018; Lin et al., 2012; Lockwood et al., 1993; Lovett et al., 1997; Siegel et al., 2019; Mai et al., 2016; Martingano et al., 2020; Maymon et al., 1998; McCaul et al., 1992; McGregor et al., 1991; Mercer et al., 1992; Mercer and Arheart, 1995; Lewis et al., 2003; Nabhan et al., 2014; Navathe et al., 2019; Ovalle et al., 2002; Pan et al., 2018; Pasquier et al., 2019; Pawar and Reddy, 2020; Pierson et al., 2014; Edwards et al., 2020; Ryo et al., 2005; Saccone and Berghella, 2015; Segel et al., 2003; Smith et al., 2015; Song et al. 2005; Sung et al., 2017; Tai, 2011; Tanaka et al., 2019; Kramer et al., 1996; Wojcieszek et al., 2014; Wolf et al., 2020; Wu, 2018; Yeung et al., 2014; Zeng and Lin, 2020; Zhang, 2014; Zhang, 2017; Zhang, 2019; Zhao, 2019a; Zhao, 2019b; Zheng et al., 2016; Zheng, 2021; Zhou et al., 2015; Zhou, 2020; Zou, 2021) included PPROM women, 6 (6.7%) studies (Zheng, 2018; A, 2021; Barišić et al., 2017; Tai, 2011; Zhao, 2019a; Zheng et al., 2020) included term PROM women, 4 (4.4%) studies (Kwak et al., 2013; Nabhan et al., 2014; Wojcieszek et al., 2014; Saccone and Berghella, 2015) included both PPROM and term PROM women and 2 (2.2%) studies (Brelje and Kaltreider, 1966; Kenyon et al., 2008) did not report whether the participants were term. The study interventions/comparisons included: 1) using antibiotics vs placebo/blank control (31/90, 34.4%) (Brelje and Kaltreider, 1966; Amon et al., 1988; Johnston et al., 1990; Bergström, 1991; Kurki et al., 1992; McCaul et al., 1992; Mercer et al., 1992; Lockwood et al., 1993; Ernest and Givner, 1994; Mercer and Arheart, 1995; Almeida et al., 1996; Grable et al., 1996; Kramer et al., 1996; Maymon et al., 1998; Bar et al., 2020; Ovalle et al., 2002; Kenyon et al., 2004; Song and Han, 2005; August Fuhr et al., 2006; Cousens et al., 2010; Lin et al., 2012; Ke, 2013; Nabhan et al., 2014; Wojcieszek et al., 2014; Zhang, 2014; Saccone and Berghella, 2015; Du, 2016; Chang et al., 2017; Dotters-Katz et al., 2017; Pasquier et al., 2019; Feng, 2020); 2) different antibiotics (29/90, 32.2%) (McGregor et al., 1991; Lewis et al., 1995; Lovett et al., 1997; Edwards et al., 2020; Kenyon et al., 2001b; Ryo et al., 2005; Ehsanipoor et al., 2008; Kenyon et al., 2008; Kwak et al., 2013; Pierson et al., 2014; Yeung et al., 2014; Kahramanoglu et al., 2016; Lee et al., 2016; Zheng et al., 2016; Finneran et al., 2017; Sung et al., 2017; Wu, 2018; Zhao, 2019b; Finneran et al., 2019; Navathe et al., 2019; Siegel et al., 2019; Tanaka et al., 2019; Ali, 2020; Chatzakis et al., 2020; Martingano et al., 2020; Pawar and Reddy, 2020; Wolf et al., 2020; Fitzgibbon et al., 2021); 3) different timing of antibiotics administration (17/90, 18.9%) (Deng, 2020; Liang, 2018; Zheng, 2018; A, 2021; Barišić et al., 2017; Tai, 2011; Zhao, 2019a; Zheng et al., 2020; Chen et al., 2020; Du et al., 2019; Du and Zhang, 2020; Knupp et al., 2022; Li, 2017; Pan et al., 2018; Zeng and Lin, 2020; Zhang, 2019; Zhou et al., 2015); 4) antibiotics chosen depending on experience vs culture results (8/90, 8.9%) (Mai and He, 2016; Zhang, 2017; Zhou, 2020; Chen, 2021; Cong, 2021; Zheng, 2021; Zou, 2021); 5) different courses of antibiotics administration (4/90, 4.4%) (Lewis et al., 2003; Segel et al., 2003; Smith et al., 2015; Li, 2020); 6) different administration route (1/90, 1.1%) (Kole-White et al., 2021). The median number of the outcomes in the included studies was 7, with the range 1–31. Only 38.9% (35/90) studies (Chatzakis et al., 2020; Saccone and Berghella, 2015; Amon et al., 1988; Brelje and Kaltreider, 1966; Lewis et al., 1995; Ernest and Givner, 1994; Grable et al., 1996; Siegel et al., 2019; Kahramanoglu et al., 2016; Kenyon et al., 2001b; Kurki et al., 1992; Kwak et al., 2013; Lockwood et al., 1993; Mercer et al., 1992; Nabhan et al., 2014; Pasquier et al., 2019; Segel et al., 2003; A, 2021; Zhao, 2019a; Zheng et al., 2020; Kenyon et al., 2008; Bar et al., 2020; Chang et al., 2017; Dotters-Katz et al., 2017; Kramer et al., 1996; Ehsanipoor et al., 2008; Fitzgibbon et al., 2021; Martingano et al., 2020; Pierson et al., 2014; Sung et al., 2017; Zheng et al., 2016; Knupp et al., 2022; Smith et al., 2015; Kole-White et al., 2021) defined study outcomes and 3.3% (3/90) studies (Kenyon et al., 2008; Kwak et al., 2013; Chang et al., 2017) explained how to measure the outcomes. 16.7% (15/90) of studies used composite outcomes (Lockwood et al., 1993; Kenyon et al., 2001b; Segel et al., 2003; Kenyon et al., 2008; Kwak et al., 2013; Wojcieszek et al., 2014; Smith et al., 2015; Kahramanoglu et al., 2016; Zheng et al., 2016; Chang et al., 2017; Zhao, 2019a; Pasquier et al., 2019; Siegel et al., 2019; Zheng et al., 2020; Knupp et al., 2022). Supplementary Table S2 shows the study characteristics.
3.1.2 Outcomes Reported in the Studies
Extraction of each verbatim outcome domain from each study, a total of 784 verbatim outcomes were identified. After merging outcomes with similar definitions and removing duplicates, we had 109 unique outcomes. Of those, 76.1% (83/109) of outcomes were not clearly defined and often had different definitions for the same term. For example, the definition of “latency period” was provided in 11 studies (Lockwood et al., 1993; Ernest and Givner, 1994; Grable et al., 1996; Pierson et al., 2014; Smith et al., 2015; Chang et al., 2017; Dotters-Katz et al., 2017; Sung et al., 2017; Siegel et al., 2019; Fitzgibbon et al., 2021; Kole-White et al., 2021); however, some studies meant “time from the first dose of antibiotics to delivery” (Pierson et al., 2014; Sung et al., 2017; Kole-White et al., 2021) and other studies meant “from the day of rupture of membranes to the date of delivery” (Lockwood et al., 1993; Ernest and Givner, 1994; Grable et al., 1996; Smith et al., 2015; Chang et al., 2017; Dotters-Katz et al., 2017; Siegel et al., 2019; Fitzgibbon et al., 2021).
Since the antibiotics strategy dramatically differs between PPROM and TPROM, we analyzed these subsets of pregnancy complications separately. Outcomes were categorized according to the populations in the studies reporting these outcomes, with PPROM having more outcomes than TPROM, 97 and 70, respectively.
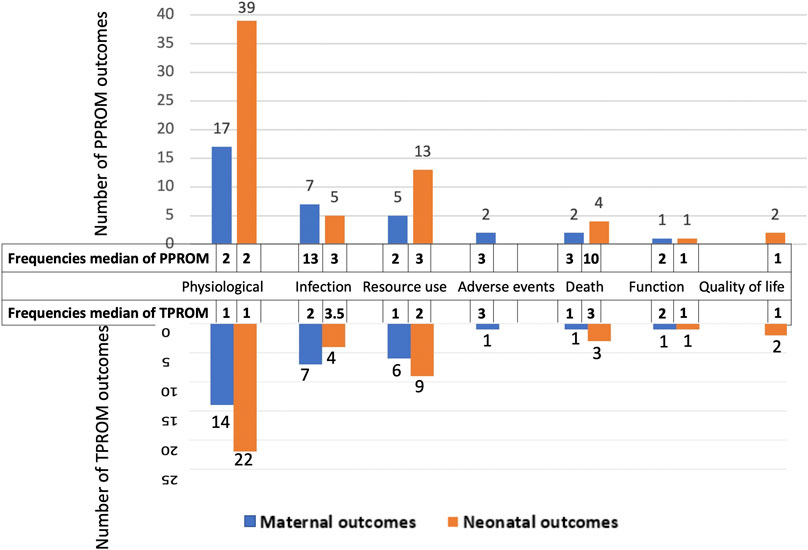
The 97 outcomes for PPROM were grouped into maternal outcomes and neonatal outcomes. Maternal outcomes involved 33 outcomes categorized into six core domains (physiological, infection, resource use, death, adverse events, and function, from most to least). Neonatal outcomes involved 64 outcomes categorized into six core domains (physiological, resource use, infection, death, quality of life, and function, from most to least) (Figure 3). The physiological domain was the most common for maternal and neonatal outcomes, with the 51.5% (17/33) and 60.9% (39/64) outcomes falling into it, respectively.
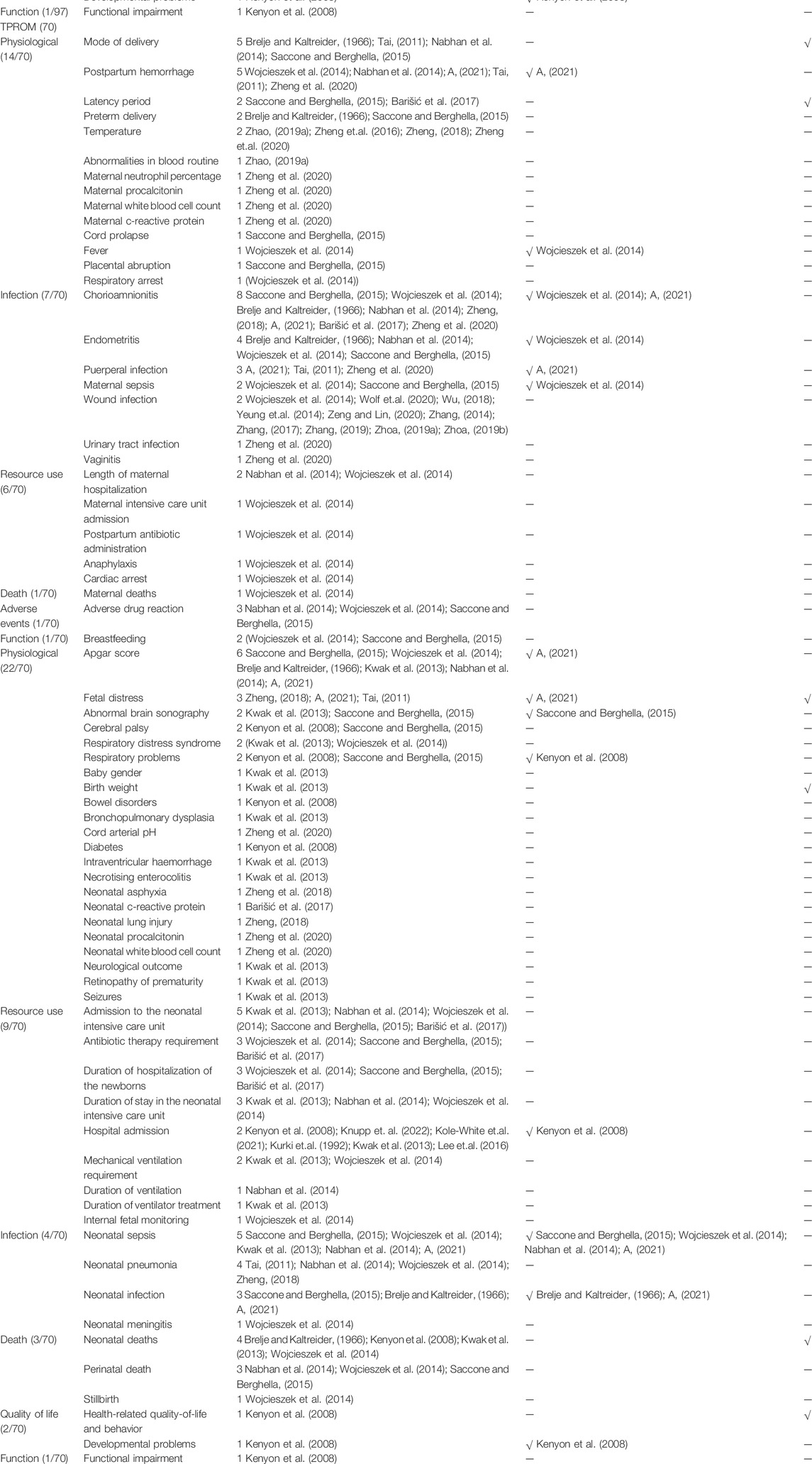
Table 1 presents outcomes for PPROM with the number of reported studies (reported frequencies). Figure 3 ranks the outcome domains by median reported frequencies from high to low. The rank for maternal outcome domains were infection, death, adverse events, physiological, function and resource use, and for neonatal domains were death, infection, resource use, physiological, quality of life, and function. Across all maternal outcomes, the top three most frequently reported outcomes were chorioamnionitis, pregnancy latency period, and mode of delivery, reported in 47.8% (43/90), 45.6% (41/90), and 30.0% (27/90), respectively of the including studies. The top three most frequently reported outcomes for newborns were neonatal sepsis, neonatal deaths, and birth weight, reported in 38.9% (35/90), 37.8% (34/90), and 36.7% (33/90) of the included studies. Nevertheless, 35.1% of outcomes (34/97, eight maternal and 26 neonatal outcomes) were reported only once in the related studies.
The 70 outcomes for TPROM were divided into maternal outcomes and neonatal outcomes. Maternal outcomes included 29 outcomes and were classified into six core domains, while neonatal outcomes included 41 outcomes classified into six core domains. Besides, the order of domains is the same as for PPROM (Figure 3). The physiological domain was the most common for both maternal and neonatal outcomes, with the 48.3% (14/29) and 53.7% (22/41) outcomes belonging to it, respectively.
Table 1presents the outcomes for TPROM and the number of reported studies. The rank for maternal outcome domains by reported frequencies were adverse events, infection, function, death, physiological, and resource use, and for neonatal domains were infection, death, resource use, physiological, and quality of life (Figure 3). The top three most frequently reported maternal outcomes were chorioamnionitis, postpartum hemorrhage, and mode of delivery, reported in 8.9% (8/90), 5.6% (5/90), and 5.6% (5/90), respectively of the included studies. And the top three most frequently reported neonatal outcomes were Apgar score, neonatal sepsis, and admission to the neonatal intensive care unit, reported in 6.7% (6/90), 5.6% (5/90), and 5.6% (5/90) of the including studies. Nevertheless, 57.1% of outcomes (40/70, 16 maternal and 24 neonatal outcomes) were reported only once in the related studies.
3.2 Semi-structured Interview
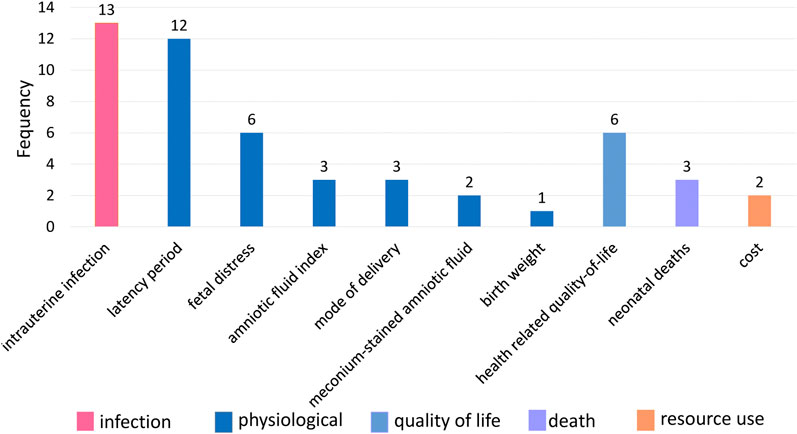
From January 2022 to February 2022, 30 pregnant women took part in the interviews. Their socioeconomic information is in Supplementary Table S3. Two researchers extracted 10 outcomes after normalization, and no new outcomes were obtained (Figure 4). The most frequently reported outcomes by PROM pregnant women were intrauterine infection (43.3%, 13/30), followed by latency period (40.0%, 12/30), fetal distress (20.0%, 6/30), and health-related quality of life and behavior (20.0%, 6/30).
Discussion
To our knowledge, this is the first study to investigate study outcomes and the concerns of pregnant women on antibiotics in PROM. Our study showed a growing number of studies about antibiotics used in PROM; however, a significant inconsistency appeared in outcomes reported in antibiotics used in pregnant women with PROM. Firstly, the current studies reported many different outcomes, some of which were only reported once. Moreover, many outcomes were not clearly defined, and different definitions were frequently found for the same term. Therefore, it might not be possible to compare, contrast or combine the results of the individual studies in a systematic review to provide higher-level evidence for clinical practice (Clarke and Williamson, 2016), which contributes to waste in research (Glasziou et al., 2014). The development of the COS for antibiotics in PROM could improve the research quality of PROM and provide a reference for research about the infection in pregnant women.
Although the classification and order of the core outcome domains of PPROM and TPROM were consistent, there were some differences between the specific outcomes of PPROM and TPROM studies due to the different clinical stages of PPROM and TPROM. For example, neonatal death was one of the most concerned outcomes of PPROM researchers. However, this outcome was seldom reported in TPROM studies because pre-term birth complications are the leading cause of death among children (World Health Organization, 2018).
The outcomes identified in the including studies could cover the outcomes concerned by pregnant women. The physiological domain contained the most outcomes. Despite this, many outcomes were reported only once in studies or by pregnant women. Both the PPROM studies’ researchers and the pregnant women interviewed were very concerned about the latency period. During the latency period of PROM, the fetus would be exposed to the risk of maternofetal infection, abruptio placentae, cord prolapse, and intrauterine death (Mercer, 2003). However, a large cohort study suggested that prolonged latency duration did not worsen neonatal prognosis. Moreover, survival and survival without severe morbidity improved with increased gestational age at birth (Lorthe et al., 2017). Therefore, prolonging latency if there is no contraindication was recommended in pregnant women at 24 0/7–33 6/7 weeks of gestation (Siegler et al., 2020). Nevertheless, some pregnant women’s concerns, such as health-related quality of life and behavior, were rarely reported in the studies. This kind of outcome is used to assess the effect of chronic disease management on an individual’s health status and is drawing the attention of researchers and policymakers (Guyatt et al., 1993). Although PROM is not a chronic disease, the sequelae of premature infants, according to PROM, require constant attention as many pre-term children develop important behavioral and educational difficulties (Bhutta et al., 2002). Future studies could pay attention to these outcomes.
Limitation and Future Research
Firstly, our study only included articles in Chinese and English, which could have a language limitation. Besides, the semi-structured interview was conducted at a single center, which could have limitations to sample representativeness. Therefore, in the next stage of this COS research, we would conduct a Delphi survey with stakeholder groups, which were based on multicenter, to add important outcomes not identified by our current study and prioritize outcomes for the COS.
Conclusion
An initial list of core outcomes set for antibiotics in pregnant women with prelabor rupture of membranes is formed. We identified 109 outcomes from 90 studies and a semi-structured interview. There was considerable inconsistency in outcomes selection and reporting in current studies for antibiotics in PROM. These results provide a robust foundation for the development of a COS.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
LZh and QY contributed to the conception and design of the study. DL, LW, JL, SL, and YL conducted the systematic review including screening of abstracts and full-text and extracting the data. DL, LW, CZ, and LZe conducted the semi-structured interview including data collection and data analysis. DL, LW, and LZe performed the analyses and wrote the manuscript. All authors revised it critically for important intellectual content and gave their approval of the final version.
Funding
This work was supported by the Science and Technology Plan Project of Sichuan Province(2020YFS0035).
Acknowledgments
We are grateful to the evidence-based pharmacy committee of the Chinese Pharmaceutical Association.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.915698/full#supplementary-material
References
A, W. Q. Z. (2021). The Application Opportunity and Clinical Effect of Antibiotics for Term Premature Rupture of Membranes. Fertil. Health (2), 8–9.
Ali, A. I. (2020). Preterm Premature Rupture of Membranes Management with Erythromycin versus Azithromycin. Ijpr 12, 2117–2122. doi:10.31838/ijpr/2020.12.01.331
Alkadhimi, A., Dawood, O. T., and Hassali, M. A. (2020). Dispensing of Antibiotics in Community Pharmacy in Iraq: a Qualitative Study. Pharm. Pract. (Granada) 18 (4), 2095. doi:10.18549/PharmPract.2020.4.2095
Almeida, L., Schmauch, A., and Bergström, S. (1996). A Randomised Study on the Impact of Peroral Amoxicillin in Women with Prelabour Rupture of Membranes Preterm. Gynecol. Obstet. Invest. 41 (2), 82–84. doi:10.1159/000292046
Amon, E., Lewis, S. V., Sibai, B. M., Villar, M. A., and Arheart, K. L. (1988). Ampicillin Prophylaxis in Preterm Premature Rupture of the Membranes: a Prospective Randomized Study. Am. J. Obstet. Gynecol. 159 (3), 539–543. doi:10.1016/s0002-9378(88)80002-4
August Fuhr, N., Becker, C., van Baalen, A., Bauer, K., and Hopp, H. (2006). Antibiotic Therapy for Preterm Premature Rupture of Membranes - Results of a Multicenter Study. J. Perinat. Med. 34 (3), 203–206. doi:10.1515/JPM.2006.035
Bar, J., Maayan-Metsger, A., Hod, M., Ben Rafael, Z., Orvieto, R., Shalev, Y., et al. (2020). Effect of Antibiotic Therapy in Preterm Premature Rupture of the Membranes on Neonatal Mortality and Morbidity. Am. J. Perinatol. 17 (5), 237–241. doi:10.1055/s-2000-10004
Barišić, T., Mandić, V., Tomić, V., Zovko, A., and Novaković, G. (2017). Antibiotic Prophylaxis for Premature Rupture of Membranes and Perinatal Outcome. J. Maternal-Fetal Neonatal Med. 30 (5), 580–584. doi:10.1080/14767058.2016.1178228
Bergström, S. (1991). A Prospective Study on the Perinatal Outcome in Mozambican Pregnant Women with Pre-term Rupture of Membranes Using Two Different Methods of Clinical Management. Gynecol. Obstet. Invest. 32 (4), 217–219. doi:10.1159/000293035
Bhutta, A. T., Cleves, M. A., Casey, P. H., Cradock, M. M., and Anand, K. J. (2002). Cognitive and Behavioral Outcomes of School-Aged Children Who Were Born Preterm: a Meta-Analysis. JAMA 288 (6), 728–737. doi:10.1001/jama.288.6.728
Brelje, M. C., and Kaltreider, D. F. (1966). The Use of Vaginal Antibiotics in Premature Rupture of the Membranes. Am. J. Obstet. Gynecol. 94 (7), 889–897. doi:10.1016/0002-9378(66)90021-4
Chang, K. H., Kim, H. J., Yu, H. J., Lee, J., Kim, J. S., Choi, S. J., et al. (2017). Comparison of Antibiotic Regimens in Pre-term Premature Rupture of Membranes: Neonatal Morbidity and 2-year Follow-Up of Neurologic Outcome. J. Matern. Fetal Neonatal Med. 30 (18), 2212–2218. doi:10.1080/14767058.2016.1243097
Chatzakis, C., Papatheodorou, S., Sarafidis, K., Dinas, K., Makrydimas, G., and Sotiriadis, A. (2020). Effect on Perinatal Outcome of Prophylactic Antibiotics in Pre-term Pre-labor Rupture of Membranes: Network Meta-Analysis of Randomized Controlled Trials. Ultrasound Obstet. Gynecol. 55, 20–31. doi:10.1002/uog.21884
Chen, H., Lu, C., and Pang, L. (2020). Effects of Timing of Antibiotic Administration on Maternal and Infant Outcomes of Premature Rupture of Membranes at Different Gestational Weeks. Diet. Health 7 (13), 64–65.
Chen, Q. (2021). Therapeutic Effect of Antibiotics on Premature Rupture of Membranes Complicated with Reproductive Tract Infection. Self Care (7), 43–44.
Clark, E. A., and Varner, M. (2011). Impact of Pre-term PROM and its Complications on Long-Term Infant Outcomes. Clin. Obstet. Gynecol. 54, 358–369. doi:10.1097/GRF.0b013e318217ee18
Clarke, M., and Williamson, P. R. (2016). Core Outcome Sets and Systematic Reviews. Syst. Rev. 5, 11. doi:10.1186/s13643-016-0188-6
Cong, J. (2021). To Observe the Effect of Antibiotic Treatment on Premature Rupture of Membranes Complicated with Reproductive Tract Infection. China Health Care & Nutr. 31 (4), 225.
Cousens, S., Blencowe, H., Gravett, M., and Lawn, J. E. Antibiotics for Pre-term Pre-labour Rupture of Membranes: Prevention of Neonatal Deaths Due to Complications of Pre-term Birth and Infection. Int. J. Epidemiol. (2010) 39 Suppl. 1(Suppl 1):i134-43. doi: doi:10.1093/ije/dyq030
Deng, D. (2020). To Analyze the Effect and Safety of Antibiotics in Different Time of Premature Rupture of Membranes. Chin. Baby (17), 55.
Dotters-Katz, S. K., Myrick, O., Smid, M., Manuck, T. A., Boggess, K. A., and Goodnight, W. (2017). Use of Prophylactic Antibiotics in Women with Previable Pre-labor Rupture of Membranes. J. Neonatal Perinat. Med. 10 (4), 431–437. doi:10.3233/NPM-16165
Du, K., and Zhang, D. (2020). Effect of Antibiotics on Delivery Outcome of Preterm Premature Rupture of Membranes at Different Times. China Health Vis. (11), 215.
Du, Y., Zhang, J., and Wang, H. (2019). To Analyze the Effect of Antibiotics Application Time on Maternal and Infant Outcomes in the Treatment of Premature Rupture of Membranes. World Latest Med. Inf. 19 (15), 102–103. doi:10.19613/j.cnki.1671-3141.2019.15.067
Du, J. (2016). Effects of Antibiotics on Maternal and Infant Outcomes of Pre-term Premature Rupture of Membranes. Yiayao Qianyan 6 (16), 121–122.
Edwards, R. K., Locksmith, G. J., and Duff, P. (2020). Expanded-spectrum Antibiotics with Pre-term Premature Rupture of Membranes. Obstet. Gynecol. 96 (1), 60–64. doi:10.1016/s0029-7844(00)00843-7
Ehsanipoor, R. M., Chung, J. H., Clock, C. A., McNulty, J. A., and Wing, D. A. (2008). A Retrospective Review of Ampicillin-Sulbactam and Amoxicillin + Clavulanate vs Cefazolin/cephalexin and Erythromycin in the Setting of Pre-term Premature Rupture of Membranes: Maternal and Neonatal Outcomes. Am. J. Obstet. Gynecol. 198 (5), e54–6. doi:10.1016/j.ajog.2007.12.022
Ernest, J. M., and Givner, L. B. (1994). A Prospective, Randomized, Placebo-Controlled Trial of Penicillin in Preterm Premature Rupture of Membranes. Am. J. Obstet. Gynecol. 170 (2), 516–521. doi:10.1016/s0002-9378(94)70220-9
Feng, Y. (2020). Intervention Effect of Anti-infection Treatment on Pregnancy Outcomes and Neonatal Status in GBS Infected Pregnant Women with Premature Rupture of Membranes. J. Rare Uncommon Dis. 27 (2), 54–56,66. doi:10.3969/j.issn.1009-3257.2020.02.020
Finneran, M. M., Appiagyei, A., Templin, M., and Mertz, H. (2017). Comparison of Azithromycin versus Erythromycin for Prolongation of Latency in Pregnancies Complicated by Pre-term Premature Rupture of Membranes. Am. J. Perinatol. 34 (11), 1102–1107. doi:10.1055/s-0037-1603915
Finneran, M. M., Smith, D. D., and Buhimschi, C. S. (2019). Cost Analysis of Azithromycin versus Erythromycin in Pregnancies Complicated by Pre-term Premature Rupture of Membranes. Am. J. Perinatol. 36 (1), 105–110. doi:10.1055/s-0038-1667369
Fitzgibbon, A., Clooney, L., Broderick, D., Eogan, M., McCallion, N., and Drew, R. J. (2021). Erythromycin Compared to Amoxicillin and Azithromycin for Antimicrobial Prophylaxis for Preterm Premature Rupture of the Membranes: a Retrospective Study. J. Obstet. Gynaecol. 41 (4), 569–572. doi:10.1080/01443615.2020.1786806
Glasziou, P., Altman, D. G., Bossuyt, P., Boutron, I., Clarke, M., Julious, S., et al. (2014). Reducing Waste from Incomplete or Unusable Reports of Biomedical Research. Lancet 383, 267–276. doi:10.1016/S0140-6736(13)62228-X
Gorst, S. L., Gargon, E., Clarke, M., Blazeby, J. M., Altman, D. G., and Williamson, P. R. (2016). Choosing Important Health Outcomes for Comparative Effectiveness Research: An Updated Review and User Survey. PLoS ONE 11, e0146444. doi:10.1371/journal.pone.0146444
Grable, I. A., Garcia, P. M., Perry, D., and Socol, M. L. (1996). Group B Streptococcus and Preterm Premature Rupture of Membranes: a Randomized, Double-Blind Clinical Trial of Antepartum Ampicillin. Am. J. Obstet. Gynecol. 175 (4 Pt 1), 1036–1042. doi:10.1016/s0002-9378(96)80049-4
Guyatt, G. H., Feeny, D. H., and Patrick, D. L. (1993). Measuring Health-Related Quality of Life. Ann. Intern Med. 118 (8), 622–629. doi:10.7326/0003-4819-118-8-199304150-00009
Johnston, M. M., Sanchez-Ramos, L., Vaughn, A. J., Todd, M. W., and Benrubi, G. I. (1990). Antibiotic Therapy in Pre-term Premature Rupture of Membranes: a Randomized, Prospective, Double-Blind Trial. Am. J. Obstet. Gynecol. 163 (3), 743–747. doi:10.1016/0002-9378(90)91060-p
Kahramanoglu, I., Baktiroglu, M., Senol, T., Kahramanoglu, O., Ozkaya, E., Ilhan, O., et al. (2016). Comparison of Two Different Antibiotic Regimens for the Prophylaxisis of Cases with Pre-term Premature Rupture of Membranes: a Randomized Clinical Trial. Ginekol. Pol. 87 (10), 701–705. doi:10.5603/GP.2016.0071
Ke, W. (2013). Treatment and Neonatal Outcome of Preterm Membrane Rupture in the Third Trimester of Pregnancy. J. Community Med. 13, 57–58.
Kenyon, S., Boulvain, M., and Neilson, J. (2001a). Antibiotics for Pre-term Premature Rupture of Membranes. Cochrane Database Syst. Rev. (4), CD001058. doi:10.1002/14651858.CD001058
Kenyon, S. L., Taylor, D. J., and Tarnow-Mordi, W.ORACLE Collaborative Group (2001b). Broad-spectrum Antibiotics for Pre-term, Prelabour Rupture of Fetal Membranes: the ORACLE I Randomised Trial. ORACLE Collaborative Group. Lancet 357 (9261), 979–988. doi:10.1016/s0140-6736(00)04233-1
Kenyon, S., Boulvain, M., and Neilson, J. (2004). Antibiotics for Pre-term Rupture of the Membranes: a Systematic Review. Obstet. Gynecol. 104 (5 Pt 1), 1051–1057. doi:10.1097/01.AOG.0000143268.36682.21
Kenyon, S., Pike, K., Jones, D. R., Brocklehurst, P., Marlow, N., Salt, A., et al. (2008). Childhood Outcomes after Prescription of Antibiotics to Pregnant Women with Pre-term Rupture of the Membranes: 7-year Follow-Up of the ORACLE I Trial. Lancet 372 (9646), 1310–1318. doi:10.1016/S0140-6736(08)61202-7
Kenyon, S., Boulvain, M., and Neilson, J. P. (2013). Antibiotics for Preterm Rupture of Membranes. Cochrane Database Syst. Rev. (12), CD001058. doi:10.1002/14651858.CD001058.pub310.1002/14651858.CD001058.pub2
Keyvanara, M., Ojaghi, R., Sohrabi, M. C., and Papi, A. (2013). Experiences of Experts about the Instances of Plagiarism. J. Educ. Health Promot 2, 32. doi:10.4103/2277-9531.115817
Kirkham, J. J., Gorst, S., Altman, D. G., Blazeby, J. M., Clarke, M., Devane, D., et al. (2016). Core Outcome Set-STAndards for Reporting: the COS-STAR Statement. PLoS Med. 13 (10), e1002148. doi:10.1371/journal.pmed.1002148
Knupp, R. J., Pederson, S., Blanchard, C., Szychowski, J., Etikala, D., Sinkey, R., et al. (2022). Antibiotic Timing in Previable Pre-labor Rupture of Membranes Less Than 24 Weeks of Gestation. Am. J. Perinatol. 39 (6), 671–676. doi:10.1055/s-0040-1718876
Kole-White, M. B., Nelson, L. A., Lord, M., Has, P., Werner, E. F., Rouse, D. J., et al. (2021). Pregnancy Latency after Pre-term Premature Rupture of Membranes: Oral versus Intravenous Antibiotics. Am. J. Obstet. Gynecol. MFM 3 (3), 100333. doi:10.1016/j.ajogmf.2021.100333
Kramer, W. B., Saade, G. R., Belfort, M., Samora-Mata, J., Wen, T., and Moise, K. J. (1996). Antibiotic Prophylaxis for Presumptive Group B Streptococcal Infection in Preterm Premature Rupture of the Membranes: Effect on Neonatal and Maternal Infectious Morbidity. Infect. Dis. Obstet. Gynecol. 4 (6), 313–318. doi:10.1155/S1064744996000634
Kurki, T., Hallman, M., Zilliacus, R., Teramo, K., and Ylikorkala, O. (1992). Premature Rupture of the Membranes: Effect of Penicillin Prophylaxis and Long-Term Outcome of the Children. Am. J. Perinatol. 9 (1), 11–16. doi:10.1055/s-2007-994661
Kwak, H. M., Shin, M. Y., Cha, H. H., Choi, S. J., Lee, J. H., Kim, J. S., et al. (2013). The Efficacy of Cefazolin Plus Macrolide (Erythromycin or Clarithromycin) versus Cefazolin Alone in Neonatal Morbidity and Placental Inflammation for Women with Pre-term Premature Rupture of Membranes. Placenta 34 (4), 346–352. doi:10.1016/j.placenta.2013.01.016
Lee, J., Romero, R., Kim, S. M., Chaemsaithong, P., Park, C. W., Park, J. S., et al. (2016). A New Anti-microbial Combination Prolongs the Latency Period, Reduces Acute Histologic Chorioamnionitis as Well as Funisitis, and Improves Neonatal Outcomes in Pre-term PROM. J. Matern. Fetal Neonatal Med. 29 (5), 707–720. doi:10.3109/14767058.2015.1020293
Lewis, D. F., Fontenot, M. T., Brooks, G. G., Wise, R., Perkins, M. B., and Heymann, A. R. (1995). Latency Period after Pre-term Premature Rupture of Membranes: a Comparison of Ampicillin with and without Sulbactam. Obstet. Gynecol. 86 (3), 392–395. doi:10.1016/0029-7844(95)00181-P
Lewis, D. F., Adair, C. D., Robichaux, A. G., Jaekle, R. K., Moore, J. A., Evans, A. T., et al. (2003). Antibiotic Therapy in Pre-term Premature Rupture of Membranes: Are Seven Days Necessary? A Preliminary, Randomized Clinical Trial. Am. J. Obstet. Gynecol. 188 (6), 1413–1417. discussion 1416-7. doi:10.1067/mob.2003.382
Li, S. (2017). Effects of Antibiotics at Different Times on Maternal and Infant Outcomes in the Treatment of Preterm Premature Rupture of Membranes. Health All (14), 71.
Li, J. (2020). Analysis of the Effects of Antibiotic Treatment on Preterm Premature Rupture of Membranes at Term. Spec. Health (5), 111–112.
Liang, M. (2018). Effect of Timing of Combined Anti-infective Therapy on Pregnancy Outcome in Pregnant Women with Premature Rupture of Membranes. China J. Pharm. Econ. 13 (5), 46–48. doi:10.12010/j.issn.1673-5846.2018.05.012
Lin, H., Chen, F., and Zeng, C. (2012). Clinical Significance of Rational Use of Antibiotics in Premature Rupture of Membranes Complicated with Mycotic Vaginitis. GUIDE CHINA Med. 10 (17), 512–513. doi:10.3969/j.issn.1671-8194.2012.17.393
Lockwood, C. J., Costigan, K., Ghidini, A., Wein, R., Chien, D., Brown, B. L., et al. (1993). Double-blind; Placebo-Controlled Trial of Piperacillin Prophylaxis in Preterm Membrane Rupture. Am. J. Obstet. Gynecol. 169 (4), 970–976. doi:10.1016/0002-9378(93)90037-j
Lorthe, E., Ancel, P. Y., Torchin, H., Kaminski, M., Langer, B., Subtil, D., et al. (2017). Impact of Latency Duration on the Prognosis of Pre-term Infants after Preterm Premature Rupture of Membranes at 24 to 32 Weeks’ Gestation: A National Population-Based Cohort Study. J. Pediatr. 182, 47–e2. doi:10.1016/j.jpeds.2016.11.074
Lovett, S. M., Weiss, J. D., Diogo, M. J., Williams, P. T., and Garite, T. J. (1997). A Prospective, Double-Blind, Randomized, Controlled Clinical Trial of Ampicillin-Sulbactam for Preterm Premature Rupture of Membranes in Women Receiving Antenatal Corticosteroid Therapy. Am. J. Obstet. Gynecol. 176 (5), 1030–1038. doi:10.1016/s0002-9378(97)70398-3
Mai, L., and He, J. (2016). Significance of PCT Combined with CRP in Guiding the Treatment of Discontinuation of Antibiotics in Pregnant Women with Subclinical Infection and Premature Rupture of Membranes. J. Liaoning Med. Coll. 37 (4), 36–38.
Martin, J. A., Hamilton, B. E., Sutton, P. D., Ventura, S. J., Menacker, F., and Munson, M. L. (2005). Births: Final Data for 2002. Natl. Vital Stat. Rep. 52, 1–113.
Martingano, D., Singh, S., and Mitrofanova, A. (2020). Azithromycin in the Treatment of Preterm Pre-labor Rupture of Membranes Demonstrates a Lower Risk of Chorioamnionitis and Postpartum Endometritis with an Equivalent Latency Period Compared with Erythromycin Antibiotic Regimens. Infect. Dis. Obstet. Gynecol. 2020, 2093530. doi:10.1155/2020/2093530
Maymon, E., Chaim, W., Sheiner, E., and Mazor, M. (1998). A Review of Randomized Clinical Trials of Antibiotic Therapy in Preterm Premature Rupture of the Membranes. Arch. Gynecol. Obstet. 261 (4), 173–181. doi:10.1007/s004040050218
McCaul, J. F., Perry, K. G., Moore, J. L., Martin, R. W., Bucovaz, E. T., and Morrison, J. C. (1992). Adjunctive Antibiotic Treatment of Women with Preterm Rupture of Membranes or Pre-term Labor. Int. J. Gynaecol. Obstet. 38 (1), 19–24. doi:10.1016/0020-7292(92)90724-w
McGregor, J. A., French, J. I., and Seo, K. (1991). Antimicrobial Therapy in Pre-term Premature Rupture of Membranes: Results of a Prospective, Double-Blind, Placebo-Controlled Trial of Erythromycin. Am. J. Obstet. Gynecol. 165 (3), 632–640. doi:10.1016/0002-9378(91)90299-7
Mercer, B. M., and Arheart, K. L. (1995). Antimicrobial Therapy in Expectant Management of Preterm Premature Rupture of the Membranes. Lancet 346 (8985), 1271–1279. doi:10.1016/s0140-6736(95)91868-x
Mercer, B. M., Moretti, M. L., Prevost, R. R., and Sibai, B. M. (1992). Erythromycin Therapy in Preterm Premature Rupture of the Membranes: a Prospective, Randomized Trial of 220 Patients. Am. J. Obstet. Gynecol. 166 (3), 794–802. doi:10.1016/0002-9378(92)91336-9
Mercer, B. M. (2003). Preterm Premature Rupture of the Membranes. Obstet. Gynecol. 101, 178–193. doi:10.1016/s0029-7844(02)02366-9
Mercer, B. M. (2005). Preterm Premature Rupture of the Membranes: Current Approaches to Evaluation and Management. Obstet. Gynecol. Clin. North Am. 32, 411–428. doi:10.1016/j.ogc.2005.03.003
Middleton, P., Shepherd, E., Flenady, V., McBain, R. D., and Crowther, C. A. (2017). Planned Early Birth versus Expectant Management (Waiting) for Prelabour Rupture of Membranes at Term (37 Weeks or More). Cochrane Database Syst. Rev. 1, CD005302. doi:10.1002/14651858.CD005302.pub3
Nabhan, A. F., Elhelaly, A., and Elkadi, M. (2014). Antibiotic Prophylaxis in Pre-labor Spontaneous Rupture of Fetal Membranes at or beyond 36 Weeks of Pregnancy. Int. J. Gynaecol. Obstet. 124 (1), 59–62. doi:10.1016/j.ijgo.2013.07.018
Navathe, R., Schoen, C. N., Heidari, P., Bachilova, S., Ward, A., Tepper, J., et al. (2019). Azithromycin vs Erythromycin for the Management of Preterm Premature Rupture of Membranes. Am. J. Obstet. Gynecol. 221 (2), 144–e8. e8. doi:10.1016/j.ajog.2019.03.009
Ovalle, A., Martínez, M. A., Kakarieka, E., Gómez, R., Rubio, R., Valderrama, O., et al. (2002). Antibiotic Administration in Patients with Preterm Premature Rupture of Membranes Reduces the Rate of Histological Chorioamnionitis: a Prospective, Randomized, Controlled Study. J. Matern. Fetal Neonatal Med. 12 (1), 35–41. doi:10.1080/jmf.12.1.35.41
Pan, L., Zhang, G., and Wang, N. (2018). Timing of Antibiotic Use on Maternal and Neonatal Outcomes in Pregnant Women with Prematural Rupture of Membranes. Zhejiang Med. J. 40 (13), 1461–1464,1468. doi:10.12056/j.issn.1006-2785.2018.40.13.2017-1443
Pasquier, J. C., Claris, O., Rabilloud, M., Ecochard, R., Picaud, J. C., Moret, S., et al. (2019). Intentional Early Delivery versus Expectant Management for Preterm Premature Rupture of Membranes at 28-32 Weeks’ Gestation: A Multicentre Randomized Controlled Trial (MICADO STUDY). Eur. J. Obstet. Gynecol. Reprod. Biol. 233, 30–37. doi:10.1016/j.ejogrb.2018.11.024
Pawar, L., and Reddy, N. (2020). Comparative Efficacy of Two Prophylactic Antibiotic Regimens on the Maternal and Neonatal Outcomes in Pregnancy with Preterm Premature Rupture of Membrane. Natl. J. Physiol. Pharm. Pharmacol. 10 (6), 1–459. doi:10.5455/NJPPP.2020.10.03066202024032020
Pierson, R. C., Gordon, S. S., and Haas, D. M. (2014). A Retrospective Comparison of Antibiotic Regimens for Preterm Premature Rupture of Membranes. Obstet. Gynecol. 124 (3), 515–519. doi:10.1097/AOG.0000000000000426
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (2009). PRISMA Checklist. Avaialable at: http://www.prisma-statement.org/PRISMAStatement/Checklist.aspx (Accessed January 28, 2022).
Reuter, S., Moser, C., and Baack, M. (2014). Respiratory Distress in the Newborn. Pediatr. Rev. 35, 417–429. doi:10.1542/pir.35-10-417
Ryo, E., Ikeya, M., and Sugimoto, M. (2005). Clinical Study of the Effectiveness of Imipenem/cilastatin Sodium as the Antibiotics of First Choice in the Expectant Management of Patients with Pre-term Premature Rupture of Membranes. J. Infect. Chemother. 11 (1), 32–36. doi:10.1007/s10156-004-0362-y
Saccone, G., and Berghella, V. (2015). Antibiotic Prophylaxis for Term or Near-Term Premature Rupture of Membranes: Meta-analysis of Randomized Trials. Am. J. Obstet. Gynecol. 212 (5), 627–629. doi:10.1016/j.ajog.2014.12.034
Segel, S. Y., Miles, A. M., Clothier, B., Parry, S., and Macones, G. A. (2003). Duration of Antibiotic Therapy after Preterm Premature Rupture of Fetal Membranes. Am. J. Obstet. Gynecol. 189 (3), 799–802. doi:10.1067/s0002-9378(03)00765-8
Siegel, A. M., Heine, R. P., and Dotters-Katz, S. K. (2019). The Effect of Non-penicillin Antibiotic Regimens on Neonatal Outcomes in Pre-term Premature Rupture of Membranes. AJP Rep. 9 (1), e67–e71. doi:10.1055/s-0039-1683378
Siegler, Y., Weiner, Z., and Solt, I. (2020). ACOG Practice Bulletin No. 217: Pre-labor Rupture of Membranes. Obstet. Gynecol. 136, 1061. doi:10.1097/AOG.0000000000004142
Smith, G., Rafuse, C., Anand, N., Brennan, B., Connors, G., Crane, J., et al. (2005). Prevalence, Management, and Outcomes of Preterm Prelabour Rupture of the Membranes of Women in Canada. J. Obstet. Gynaecol. Can. 27, 547–553. doi:10.1016/s1701-2163(16)30711-3
Smith, A., Allen, V. M., Walsh, J., Jangaard, K., and O’Connell, C. M. (2015). Is Preterm Premature Rupture of Membranes Latency Influenced by Single versus Multiple Agent Antibiotic Prophylaxis in Group B Streptococcus Positive Women Delivering Preterm? J. Obstet. Gynaecol. Can. 37 (9), 777–783. doi:10.1016/S1701-2163(15)30147-X
Song, G. A., and Han, M. S. (2005). Effect of Antenatal Corticosteroid and Antibiotics in Pregnancies Complicated by Premature Rupture of Membranes between 24 and 28 Weeks of Gestation. J. Korean Med. Sci. 20 (1), 88–92. doi:10.3346/jkms.2005.20.1.88
Sung, J., Lal, A., Goodman, J. R., and Rugino, A. (2017). Comparison of Latency Antibiotic Regimen for Preterm Premature Rupture of Membranes. Reprod. Sci. 24, 170A. doi:10.1177/1933719117699773
Tai, W. (2011). Effect of Water Duration and Antibiotic Treatment on Delivery Outcome of Term Premature Rupture of Membranes. Chin. J. MISDIAGNOSTICS 11 (13), 3132–3133.
Tanaka, S., Tsumura, K., Nakura, Y., Tokuda, T., Nakahashi, H., Yamamoto, T., et al. (2019). New Antibiotic Regimen for Preterm Premature Rupture of Membrane Reduces the Incidence of Bronchopulmonary Dysplasia. J. Obstet. Gynaecol. Res. 45 (5), 967–973. doi:10.1111/jog.13903
Thomson, A. J.Royal College of Obstetricians and Gynaecologists (2019). Care of Women Presenting with Suspected Preterm Prelabour Rupture of Membranes from 24+0 Weeks of Gestation: Green-top Guideline No. 73. BJOG 126, e152–e166. doi:10.1111/1471-0528.15803
Williamson, P. R., Altman, D. G., Blazeby, J. M., Clarke, M., Devane, D., Gargon, E., et al. (2012). Developing Core Outcome Sets for Clinical Trials: Issues to Consider. Trials 13, 132. doi:10.1186/1745-6215-13-132
Williamson, P. R., Altman, D. G., Bagley, H., Barnes, K. L., Blazeby, J. M., Brookes, S. T., et al. (2017). The COMET Handbook: Version 1.0. Trials 18, 280. doi:10.1186/s13063-017-1978-4
Wojcieszek, A. M., Stock, O. M., and Flenady, V. (2014). Antibiotics for Prelabour Rupture of Membranes at or Near Term. Cochrane Database Syst. Rev. (10), CD001807. doi:10.1002/14651858.CD001807.pub2
Wolf, M. F., Sgayer, I., Miron, D., Krencel, A., Sheffer, V. F., Idriss, S. S., et al. (2020). A Novel Extended Prophylactic Antibiotic Regimen in Pre-term Pre-labor Rupture of Membranes: A Randomized Trial. Int. J. Infect. Dis. 96, 254–259. doi:10.1016/j.ijid.2020.05.005
World Health Organization (2018). Pre-term Birth. Avaialable at: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (Accessed May 28, 2022).
Wu, M. (2018). Effects of Different Infection Prevention Programs on the Outcome of Preterm Membrane Rupture Pregnancy. Chin. Baby (2), 35. doi:10.3969/j.issn.1671-2242.2018.02.031
Yeung, S. W., Sahota, D. S., and Leung, T. Y. (2014). Comparison of the Effect of Penicillins versus Erythromycin in Preventing Neonatal Group B streptococcus Infection in Active Carriers Following Preterm Pre-labor Rupture of Membranes. Taiwan J. Obstet. Gynecol. 53 (2), 210–214. doi:10.1016/j.tjog.2014.04.016
Yudin, M. H., van Schalkwyk, J., and Eyk, N. V.Infectious Diseases Committee; Maternal Fetal Medicine Committee (2009). Antibiotic Therapy in Preterm Premature Rupture of the Membranes. J. Obstet. Gynaecol. Can. 31, 863–867. doi:10.1016/S1701-2163(16)34305-5
Zeng, L., and Lin, J. (2020). Effect of Timing of Antibiotic Administration on Maternal and Infant Outcomes of Pre-term Premature Rupture of Membranes. J. Clin. Ration. Drug Use 13 (11), 24–25. doi:10.15887/j.cnki.13-1389/r.2020.11.013
Zhang, Y. (2014). Treatment and Pregnancy Outcome of Patients with Pre-term Membrane Rupture in the Third Trimester of Pregnancy. Jilin Med. J. (16), 3479–3480.
Zhang, W. (2017). Antibiotic Treatment of Premature Rupture of Membranes Complicated with Reproductive Tract Infection and its Effect. World Latest Med. Inf. 33 (16), 39.
Zhang, D. (2019). Standardized Management of Premature Rupture of Membranes. J. Pract. Gynecol. Endocrinol. 6 (26), 167.
Zhao, W. (2019b). Application Time and Clinical Effect of Antibiotics for Term Premature Rupture of Membranes. World Latest Med. Inf. 19 (A3), 193–194. doi:10.19613/j.cnki.1671-3141.2019.103.111
Zhao, L. (2019a). Effect of Erythromycin and Penicillin on Amniotic Cavity Infection and its Effect on Pregnancy and Perinatal Outcomes in Pregnant Women with Premature Rupture of Inner Fetal Membrane. Anti-Infection Pharm. 16 (10), 1806–1808. doi:10.13493/j.issn.1672-7878.2019.10-049
Zheng, C., Cao, J., Luo, S., and Bai, Z. (2016). Clinical Analysis of the Effect of Combination Use of Ceftriaxone, Clarithromycin and Metronidazole on the Neonatal Outcome of Patients with PPROM. Pract. Pharm. Clin. Remedies 19 (8), 988–992. doi:10.14053/j.cnki.ppcr.201608019
Zheng, M., Cao, L., Yi, S., Dong, L., Wang, R., Wang, D., et al. (2020). Influence of Timing of Prophylactic Antibiotics for Premature Rupture of Membranes on Maternal and Infant Outcomes. J. third Mil. Med. Univ. 42 (2), 197–201. doi:10.16016/j.1000-5404.201908053
Zheng, Y. (2018). Analysis and Study on the Influence and Prevention of Premature Rupture of Membranes on Mothers and Children. Contin. Med. Educ. 32 (10), 112–113. doi:10.3969/j.issn.1004-6763.2018.10.058
Zheng, F. (2021). To Observe the Effect of Antibiotic Treatment on Premature Rupture of Membranes Complicated with Reproductive Tract Infection. Jia You Yun Bao 3 (5), 96.
Zhou, Z., Yang, C., Lin, F., Huang, L., and Pan, H. (2015). Influen Ce Exploration of Antibiotics Time PPROM Maternal and Neonatal Outcomes. Mod. Diagnosis Treat. (10), 2243–2245.
Zhou, Y. (2020). Efficacy Evaluation of Antibiotics in the Treatment of Premature Rupture of Membranes Complicated with Reproductive Tract Infection. Women’s Health Res. (9), 85–86.
Zhuang, L., Li, Z. K., Zhu, Y. F., Ju, R., Hua, S. D., Yu, C. Z., et al. (2020). The Correlation between Prelabour Rupture of the Membranes and Neonatal Infectious Diseases, and the Evaluation of Guideline Implementation in China: a Multi-Centre Prospective Cohort Study. Lancet Reg. Health West Pac 3, 100029. doi:10.1016/j.lanwpc.2020.100029
Keywords: core outcome sets, outcome reporting, pregnancy, prelabor rupture of membranes, systematic review, semi-structured interview
Citation: Liu D, Wu L, Luo J, Li S, Liu Y, Zhang C, Zeng L, Yu Q and Zhang L (2022) Developing a Core Outcome Set for the Evaluation of Antibiotic Use in Prelabor Rupture of Membranes: A Systematic Review and Semi-Structured Interview. Front. Pharmacol. 13:915698. doi: 10.3389/fphar.2022.915698
Received: 08 April 2022; Accepted: 02 June 2022;
Published: 01 August 2022.
Edited by:
Nanbert Zhong, Institute for Basic Research in Developmental Disabilities (IBR), United StatesReviewed by:
Ivana Musilova, Charles University, CzechiaMarian Kacerovsky, University Hospital Hradec Kralove, Czechia
Copyright © 2022 Liu, Wu, Luo, Li, Liu, Zhang, Zeng, Yu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingli Zhang, emhhbmdfbGluZ2xpQGFsaXl1bi5jb20=
†This author have contributed equally to this work and share last authorship
 Dan Liu
Dan Liu Lin Wu4,5
Lin Wu4,5 Siyu Li
Siyu Li Yan Liu
Yan Liu