
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 19 July 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.915609
This article is part of the Research Topic Organelle Targeting: Focus on Drug Discovery and Theranostics View all 15 articles
Modern cellular biology faces several major obstacles, such as the determination of the concentration of active sites corresponding to chemical substances. In recent years, the popular small-molecule fluorescent probes have completely changed the understanding of cellular biology through their high sensitivity toward specific substances in various organisms. Mitochondria and lysosomes are significant organelles in various organisms, and their interaction is closely related to the development of various diseases. The investigation of their structure and function has gathered tremendous attention from biologists. The advanced nanoscopic technologies have replaced the diffraction-limited conventional imaging techniques and have been developed to explore the unknown aspects of mitochondria and lysosomes with a sub-diffraction resolution. Recent progress in this field has yielded several excellent mitochondria- and lysosome-targeted fluorescent probes, some of which have demonstrated significant biological applications. Herein, we review studies that have been carried out to date and suggest future research directions that will harness the considerable potential of mitochondria- and lysosome-targeted fluorescent probes.
Mitochondria, popularly termed the cellular “powerhouses,” are one of the most significant constituents of eukaryotic cells. The mitochondria not only plays an important role in adenosine triphosphate (ATP) production but also performs numerous essential functions within the cells, such as transmission of information, induction of cell differentiation, growth, and apoptosis (Vakifahmetoglu-Norberg et al., 2017). Mitochondria have a distinctive double-membrane structure, playing important roles in their unique and complicated functions. Lysosomes are “digestion workshops” in cells, participating in the apoptotic process and other types of cell death (Luzio et al., 2007). They can function alone to participate in normal biological processes and can also interact with each other to accelerate the transmission of materials and communication with the external environment, which allows cells to improve their biological functions.
Apart from playing their own distinctive function, mitochondria could interact with lysosomes. At present, the interactions between mitochondria and lysosomes have been extensively explored, such as the fusion of the lysosomes with mitochondria during the process of autophagy (Chen et al., 2019a) and the mitochondrial and lysosomal contact (MLC) (Wong et al., 2019). Their contact promotes transmission of materials and information. When their contacting function is disrupted, the occurrence of human-related diseases, such as Parkinson’s and lysosomal storage–related diseases, is noted (Wong et al., 2019). Therefore, the timely monitoring of their dynamic changes and the estimation of the reactive small-molecule (RSM) levels are very important for identifying their physiological function and the pathogenesis of the related diseases. Several RSMs have been found in the mitochondria and various enzymes exist in the lysosome, whose dysfunction can participate in the progression of human-related diseases, such as metabolic diseases, heart failure, neurodegenerative diseases (Marc et al., 2009), Alzheimer’s disease (Nixon et al., 2000), and Parkinson disease (Daniel et al., 2008).
Fluorescence microscopy (FM) is a powerful tool for studying cellular dynamics, which has been used widely to study the interaction mechanism between mitochondria and lysosomes (Chen et al., 2019b; Chen et al., 2020). However, among imaging organelle interaction, the systematic introduction of mitochondrial and lysosomal imaging strategy is rare. Therefore, the present review focuses on some new imaging strategies of fluorescence probes targeting mitochondria and lysosomes.
Small-molecule fluorescent probes targeting mitochondria can enable the imaging of these organelles to detect the dynamic location and morphological changes of the mitochondria and observe their physiological process (Wei et al., 2022). The fluorescent probes used for imaging mitochondria are mainly divided into the following two types: bearing unique positive charge and targeting the contents of mitochondria and lysosomes. Hence, membrane permeable cationic compounds could be enriched into mitochondria because of their electrophoresis effect. The commercial probe targeting mitochondria, MitoTracker Green (MTG) with positive charge, has been widely used to target mitochondria selectively. In addition, triphenylphosphonium (TPP) with large hydrophobic radius has also been used as a targeting group for mitochondria owing to its high membrane permeability (Figure 1) (Tian et al., 2022).
Mitochondrial membrane potential is the main component of proton motion dynamics, which is formed by protons pumped from the mitochondrial matrix to membrane. The mitochondria keep the negative transmembrane potential up to −180 mV if the potential in the cytoplasm is 0 mV (Tian et al., 2022). Based on these characterizations, positive charge has been widely applied to target mitochondria.
Recently, Gu et al. have designed a novel photoactivatable bio-probe, o-TPE-ON+ (Gu et al., 2016), which can indicate the characterization of spontaneous scintillation without any imaging buffer or additives. Interestingly, this probe can target the mitochondria specifically, which is probably owing to the accumulation of negative charge of mitochondrial outer membrane. In addition, o-TPE-ON+ has been thought as a better choice for fluorescence imaging on the basis of its better cell permeability and outstanding biocompatibility. And the probe o-TPE-ON+ has tracked the high-resolution nanoscopic imaging and dynamic changes of the mitochondria (Gu et al., 2016). Huimin et al. (2022) utilized Cy5 to link with dextran to target and visualize the mitochondrial changes under dextran, which realizes the drug-visualized study at the organelle scale. Saipeng et al. (2015) also designed a mitochondrial fluorescence probe, NPA-TPP, with a fluorescent group of 1,8-naphthalimide group and the targeting group of TPP (Figure 2).
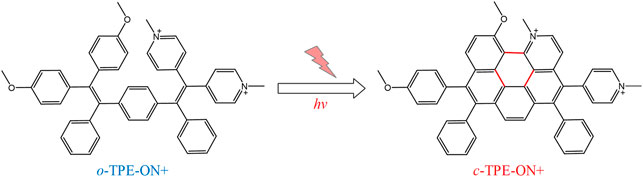
FIGURE 2. The photocyclodehydrogenation process of o-TPE-ON+ (Gu et al., 2016)
It has been demonstrated that there are several RSMs in the mitochondria. The RSMs mainly include reactive oxygen species (ROS), active nitrogen, metal cations, protons, and anions. Therefore, to visualize the distribution and action mechanism of RSMs in the mitochondria, various researchers have designed and synthesized several small-molecule fluorescent probes that target specific mitochondrial contents to further study the action targets and mechanism of drugs, which provides a powerful tool for the integration of diagnosis and treatment of diseases (Liu et al., 2021; Huimin et al., 2022).
The survival of cells depends largely on mitochondrial function, which was recognized as an important target for potential drug development (Bras et al., 2005). In recent years, the mitochondria has been recognized as an important target of many drugs. It has been reported that one of the main ways of causing mitochondrial damage is the abnormal level of intracellular ROS. The ROS includes peroxides, super oxides, hydroxyl radicals, singlet oxygen, etc.
Among them, peroxynitrite (ONOO−) is a major one because of its function of signal transduction and antibacterial activities in the biosystems (Radi, 2013). To detect ONOO− selectively, Liu et al. (2021) designed a probe L-1, a “landmine,” to monitor the ONOO− levels in living cells with higher selectivity. In addition, they proposed a novel strategy “landmine warfare strategy.” “Landmine” L-1 without fluorescence was distributed evenly in the cell matrix and could release fluorophore when “engineer” ONOO− was generated in the mitochondria (Figure 3). Under SIM, it could be found that the “engineer” ONOO− acted alone at the mitochondrial cristae and emitted fluorescence. The “landmine warfare strategy” provided a novel designed method for innovative drugs development of diseases which resulted owing to the abnormal ONOO− concentration and provided a novel research direction for other ROS materials in the mitochondria. H2O2 is one of the major forms of ROS, which participates in the process of a cell’s growth metabolism and energy production (Chen et al., 2014; Kangnan et al., 2020a). Kangnan et al. (2020b) designed a TP probe, Pyp-B, to detect the concentration of H2O2 in the mitochondria. Xiaoyue et al. (2018) developed the near-infrared fluorescent probe Mito-Cy-Tfs to detect the level of superoxide anion (O2•−) and the relationship between O2•− concentration and apoptosis during ischemia/reperfusion.
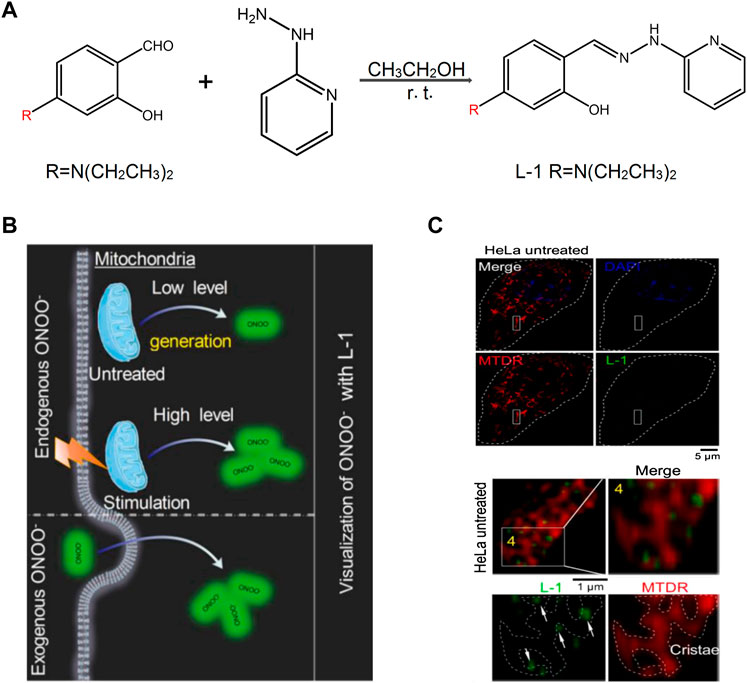
FIGURE 3. The design of L-1 and its response with ONOO− (Liu et al., 2021). (A) Synthetic route of probe L-1. (B) Proposed ONOO− visualization mechanisms for probe L-1 in living cells. (C) Super-resolution visualization of ONOO− using L-1 in living cells.
Metal ions are required for mitochondrial physiology in many aspects. Copper, iron, manganese, and zinc play an important role in organ metalloenzymes and metalloproteins (Pierrel et al., 2007). In biological systems, the zinc ion (Zn2+) mainly participates in certain life processes, such as DNA synthesis, enzyme catalysis, and gene transcription. In recent years, a large number of evidence have proved that Zn2+ is essential to the autophagy process, and autophagy can promote large changes of Zn2+. Therefore, the detection of the levels of Zn2+ has become one of the research hotspots of mitochondrial fluorescent probes. In recent years, researchers have designed and synthesized various fluorescent probes for the intracellular detection of Zn2+ in the cells; however, certain probes cannot target the mitochondria (Hung et al., 2013; Liuzzi and Yoo, 2013; Ding and Zhong, 2017). To determine the importance of Zn2+ in autophagy and signal transformation, Fang’s group developed a series of probe targeting Zn2+ in many organelles simultaneously and proposed a novel concept, Zn-STIMO, of tracking Zn2+ in multiple organelles (Figure 4) (Fang et al., 2021). They found that mitochondrial autophagy inducer CCCP-induced mitophagy in HeLa cells is associated with unstable Zn2+ enhancement. The results showed that SIM technology would become a reliable tool detecting unstable Zn2+, which also demonstrated that the organelle identification related with super resolution morphological study would have an amazing potential in tracking the biological species and events of specific organelles in organoids. Ning et al. (2016) developed a two-photon ratio probe (Mito-MPVQ) targeting the mitochondria to detect Zn2+ levels. Triphenylphosphine was used as the targeting group of the probe. Following the attachment of a fluorescent group, this probe was localized in the mitochondria and improved the two-photon signal detection for Zn2+.
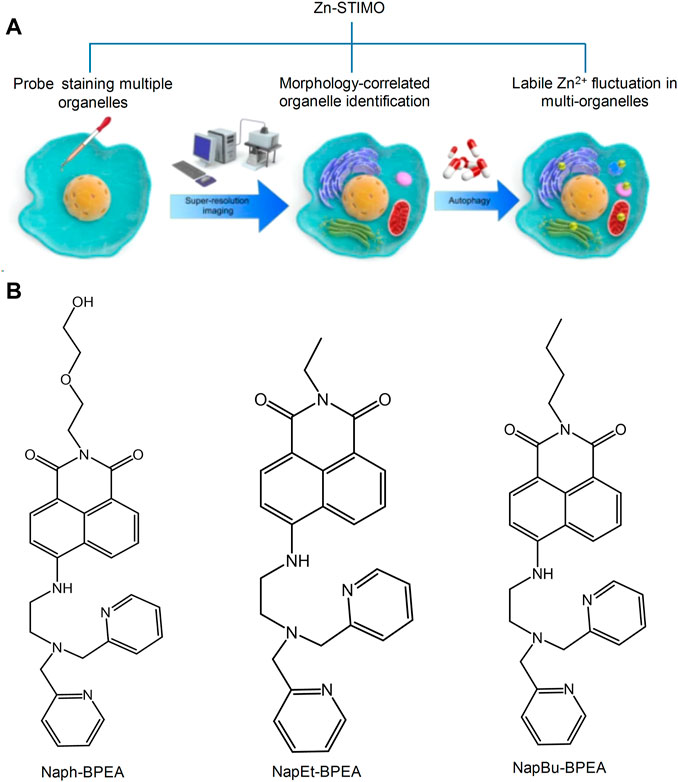
FIGURE 4. Schematic illustration of Zn-STIMO, and the design of probe candidate for Zn-STIMO (Fang et al., 2021). (A) The scheme of Zn-STIMO; (B) the structure of fluorescent probes targeting Zn2+, Naph-BPEA, NapEt-BPEA, and NapBu-BPEA.
In addition, during these years, previous researchers have designed and synthesized several fluorescent probes to detect other metal cations in the mitochondria. For example, Wang et al. (2018) designed the mitochondria-targeting fluorescent probe (PyCM-2) and (PyCM-3) to detect Au3+ levels.
Although the detection of cations in the mitochondria has been extensively examined, the role of anions is also very important. Fluorine ions can cause metabolic diseases following their accumulation in the mitochondria. In 2014, Shiling et al. designed and synthesized the mitochondrial fluorescent probe FP, which was used to detect fluorine (Shiling et al., 2014). This probe was localized in the mitochondria. The fluoride ions could knock out the silyl protecting group, and subsequently the phenoxide reacted with the −CN group, which induced nonfluorescent FP to highly fluorescent YG and emitted strong green fluorescence (Figure 5). Therefore, FP was used to detect and image fluorine in the mitochondria and has been thoroughly examined in the fields of cell biology and medical science. Xu’ group developed a mitochondrial-targeting fluorescent probe for the detection of fluorine in viable cells, which was denoted as Mito-FP (Xu et al., 2019). The probe could be successfully localized in the mitochondria and has been used for the imaging of fluorine in HeLa cells.
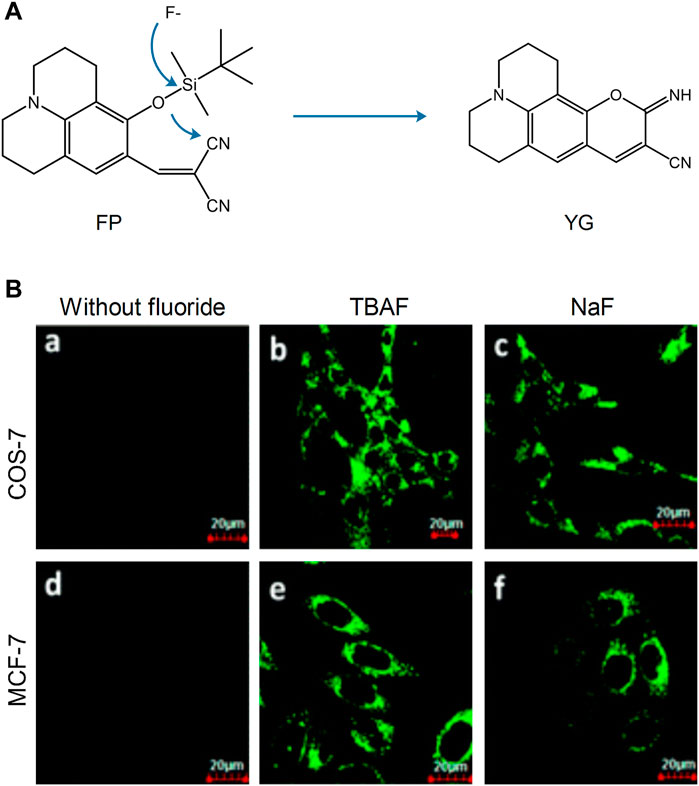
FIGURE 5. The reaction mechanism and fluorescence imaging of probe FP (Shiling et al., 2014). (A) Proposed reaction mechanism of FP. (B) Fluorescence imaging of COS-7 and MCF-7 cells incubated with probe FP (2.5 μM) before (a and d) and after (b, c, e, and f) being treated with TBAF, NaF (100 μM).
In addition to the mitochondrial morphology, several microenvironmental factors are regarded as the significant factors of the mitochondrial status, such as the mitochondrial pH value, polarity, and temperature. Normal polarization is necessary for cellular energy metabolism (Nemoto et al., 2000), and the appropriate pH value could also keep the normal membrane potential, which forces ATP generation and Ca2+ homeostasis regulation (Crompton and Heid, 1978).
Gaoqing et al. (2020) designed the fluorescent probe HBTMP to detect the mitochondrial pH value. This probe emitted red fluorescence in acidic and neutral environments and blue fluorescence in alkaline environments (Figure 6). Furthermore, HBTMP exhibited improved photostability and lower cytotoxicity. The fluorescent image of the viable cells demonstrated that HBTMP could easily spread in the mitochondria and detect changes in the pH with high sensitivity. We concluded that HBTMP could be used to study the pH changes of the mitochondria in viable cells in a more efficient way. Li et al. (2019) reported a near-infrared fluorescence probe of hydroxy-L-lysine in 2019, denoted as HXPI-P. This probe was used to detect mitochondrial polarity changes through drug induction and starvation, which contributed to distinguish the differences in polarity between normal and cancer cells via ratio fluorescence imaging.
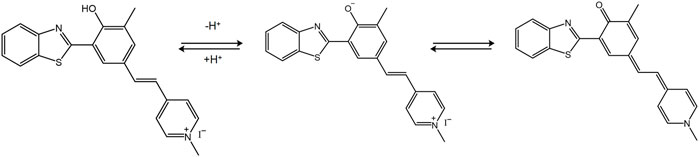
FIGURE 6. The pH sensing mechanism for the probe HBTMP (Gaoqing et al., 2020).
Lysosomes are important acidic organelles in eukaryotic cells. They involve more than 60 hydrolases and proteases and are considered to be the “digestive organs” of the cells. In addition, they can also participate in the regulation of the secretory function of cells. Lysosomes also contain various RSMs that participate in the corresponding biological reactions, such as ROS and metal cations. The visualization of RSMs in the lysosome plays an important role in understanding their mechanism of action and their therapeutic application in the treatment of various related diseases (Chen et al., 2021; Wang and Diao, 2022).
A single functional fluorescent probe targeting lysosomes can enable imaging the lysosomes to detect their dynamic location and morphological changes and track their physiological processes (Qiu et al., 2020). However, the majority of the available commercial probes targeting lysosomes are amine-based compounds and exhibit certain limitations, such as lower specificity of their localization and reducing suitability for long time detection. To address these problems, researchers have carried out research on lysosomal probes (Figure 7).
In cancer cells, the lysosomal content is high, and oncogene-driven transformation will alter the lysosomal membrane in cancer cells, which makes them more sensitive to lysosomal membrane permeability (LMP) and promotes tumor progression (Boya and Kroemer, 2008; Kallunki et al., 2013). Among the various intracellular stimuli (e.g., LMP), the ROS are the most closely related to lysosomal death (Vila et al., 2011; Melina-Theoni and Athanasios, 2014). In order to assess the ROS-related lysosomal cell death in cancer cells, Zhang et al. (2017) designed and synthesized a near-infrared fluorescent probe (PSiR) targeting the lysosomes, which could timely detect the generation of lysosomal ROS in cancer cells (Figure 8). The experimental results indicated that the probe exhibited strong resistance to photooxidation, fast reaction, and high selectivity and sensitivity. The anticancer drug β-lapachone (β-lap) could stimulate the generation of ROS in lysosomes, which was accompanied by a dose-dependent fluorescence enhancement. Due to its sensitivity in detecting ROS in cancer cells, the probe could distinguish normal cells from cancer cells according to specific images; it could also distinguish the presence of cancer cells in healthy tissues (Figure 8C).
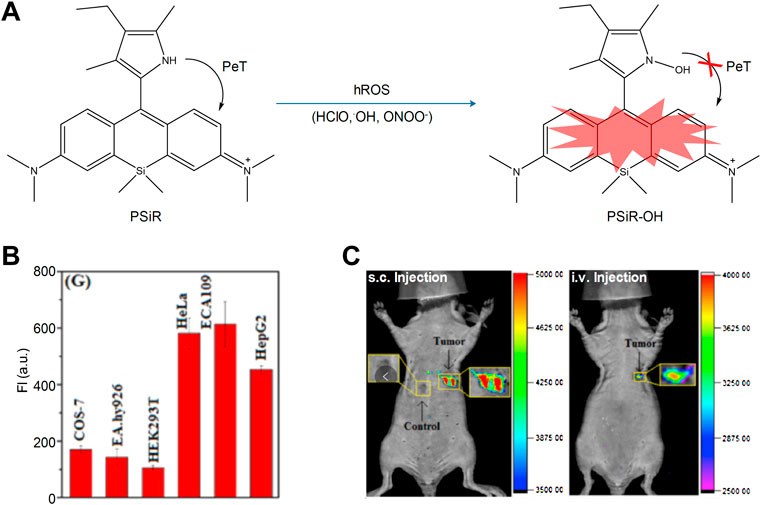
FIGURE 8. The sensing mechanisms of PSiR for hROS and imaging characterization (Zhang et al., 2017). (A) The sensing mechanisms of PSiR for hROS. (B) Average fluorescence intensity from images in various cells. (C) Imaging tumor using PSiR in tumor-bearing mouse with HeLa cells.
The hydroxyl radical (•OH) is one of the most active free radicals noted in ROS, which can destroy various biomacromolecules. In order to detect the generation and function of lysosomal •OH in viable cells, Benitez-Martin et al. (2018) developed an active probe targeting lysosomes, denoted as 1-Red (“off” state). HOCl is a type of ROS and lysosome is one of the main sources of HOCl. Therefore, the detection of lysosomal HOCl plays an important role in studying its biological process. In 2017, Yawei et al. (2017) designed and synthesized a pH-mediated lysosomal targeted fluorescent probe (Lyso-HOCl). The probe contained pH-sensitive phenol, which was used as its targeting group. A similar structure of rhodamine demonstrated a unique chlorination effect in an acidic environment, which could be used to specifically detect HOCl. Meng et al. (2019) developed the fluorescent probe CR-Ly using coumarin as a donor, rhodamine as the receptor, and morpholine as the lysosomal targeting group.
Nitric oxide (NO) plays an important role in the process of cell catabolism, whose quantity can influence lysosomal function. Abnormal NO can induce the development of cardiovascular and nervous system diseases. Therefore, the function of NO in lysosomes remains to be studied, which requires the design and development of ideal lysosomal targeting fluorescent probes detecting the change in the concentration of NO. Feng et al. (2016) synthesized the fluorescent probe LysoNO-Naph to detect NO in lysosomes on the basis of 1,8-naphthalimide (Figure 9). The probe was synthesized by using 4-(2-aminoethyl)-morpholine as a targeting group and o-phenylene-diamine as the reacting site of NO. And it could be used for lysosomal imaging. In addition, hemolysin-Naph exhibited higher selectivity and sensitivity for NO, indicating that this probe could be used to detect lysosomal NO successfully. In the same year, Yinhui et al. (2016) designed the pH-activated fluorescent probe Rhod-H-NO for detecting lysosomal NO levels. When the diameter of the nanoparticles was less than 200 nm, MSNs could enter into the lysosome, resulting in its successful tracking and imaging. Therefore, embedding Rhode-H-NO into the nanopore with MSNs can prevent it from being degraded, which leads to the accumulation of the probe in the lysosomes and the detection of NO. Fengyang et al. (2018) designed and synthesized the novel fluorescent probe MBTD in 2018. MBTD could specifically be used to image NO in lysosomes due to its large stroke shift and stable fluorescence; its D-A-D-structure probe exhibited improved photostability and higher NO selectivity.
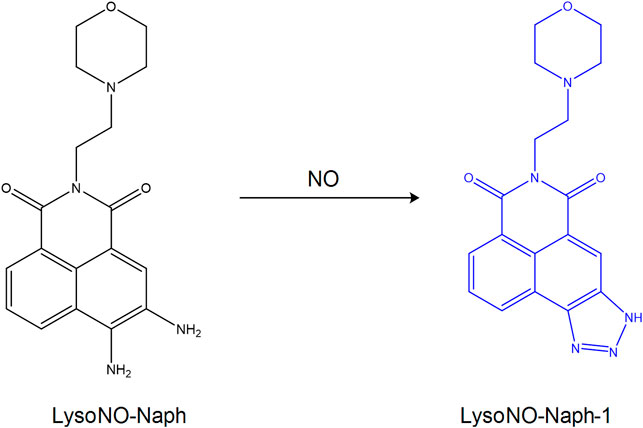
FIGURE 9. The reaction process of probe LysoNO-Naph with NO (Feng et al., 2016).
Hydrogen sulfide (H2S) is very important in various physiological processes. It can regulate cardiovascular and neuronal functions. H2S can also cause instability of the lysosomal membrane, which leads to autophagy and cell death. Low molecular weight mercaptans, such as cysteine (Cys) and glutathione (GSH), play an important role in the pathogenesis of related human diseases (Baocun et al., 2010). Therefore, the detection of the levels of H2S and mercaptans in the cells is very important for the identification of biological processes and the diagnosis and treatment of related diseases.
The hydrolysis of various proteins in the lysosome is closely related to mercaptan (T et al., 2000). In the lysosomes of the liver cells, Cys is a main stimulant for the degradation of albumin (Arunachalam et al., 2000). To investigate the function of Cys in lysosomes, Long et al. synthesized the TP probe MNPO (Figure 10) (Long et al., 2019). The probe was designed and synthesized with a naphthalene derivative as the fluorescent group, morpholine as the lysosomal targeting group, and α,β-unsaturated ketone as the action site of Cys. The introduction of the pyridine group into the molecule could improve water solubility and selectivity of Cys. It was found using specific experiments that the increase of Cys concentration increased the fluorescence intensity of the probe at 524 nm. Moreover, when the concentration range of Cys was 0–10 μM, the fluorescence intensity indicated a linear relationship with Cys. Therefore, the probe could be a useful tool to detect the dynamic changes of Cys in lysosomes. In addition, Tamima et al. (2020) developed a novel lysosome-targeted fluorescent probe ABXO1 for the detection of the levels of Cys. Interestingly, the probe and cysteine adduct exhibited optimal two-photon absorption properties, which could achieve the two-photon imaging of lysosomal cysteine under excitation at near-infrared wavelengths.
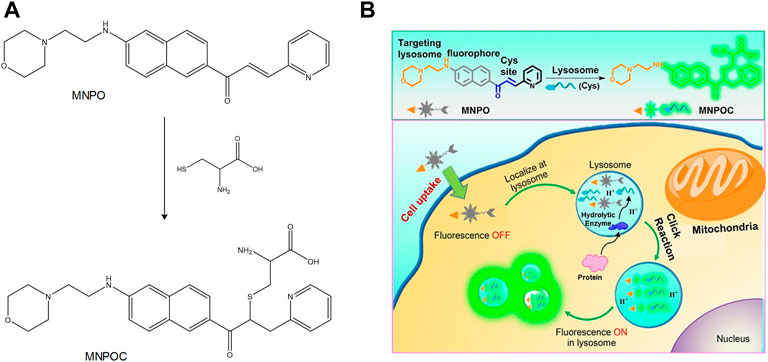
FIGURE 10. The action mechanism of MNPO with Cys in lysosomes (Long et al., 2019). (A) Proposed response mechanism of MNPO to Cys. (B) Schematic showing the general design of lysosome-targeting fluorogenic probe MNPO for Cys.
H2S is an antioxidant that participates in various physiological reactions in the liver, spleen, and kidneys. Traditional lysosomal-targeting H2S fluorescent probes cannot provide adequate imaging of the lysosome with an open fluorescent signal; therefore, the design of fluorescent probes targeting lysosomal and mitochondrial H2S has high application value. Yong et al. (2016) designed and synthesized the TP probe TP-PMVC, which could be used to image lysosomal and mitochondrial H2S by using a dual channel. The probe used carbazole MVC as a TP platform. Since the pKa of pyridine was 5.0, it was used as the site of H+ and as the targeting unit of the lysosome. In addition, indole exhibited potent electrophilicity required for the generation of H2S. In an acidic environment, pyridine was protonated to produce red fluorescence for lysosomal imaging. By using specific experiments, it was shown that the fluorescent intensity was significantly improved under acidic conditions, while lysosomes and lysosomal H2S could be detected at 960 nm and 810 nm. In addition, Cai et al. (2018) synthesized the ratio fluorescent probe SN-N3 for the detection of lysosomal H2S.
In recent years, a novel function of the lysosomes has been discovered which is the regulation of the steady state of the transition metals, such as copper and zinc (Blaby-Haas and Merchant, 2014). Various enzymes in the body can catalyze biochemical reactions by using specific transition metals. Therefore, lysosomes can maintain the metal steady state in cells by regulating the metal content and controlling its downstream signaling (Kurz et al., 2011; E. and S., 2014).
Copper is related to the activity of various essential enzymes in the body. When copper is absent, the activity of the enzymes dependent to copper will decrease leading to the occurrence of related diseases, such as Menkes syndrome (Bie et al., 2007; Tümer and Møller, 2010). In addition, excessive copper can also lead to cellular toxicity, affect lipid metabolism, and other biological processes, and subsequently lead to certain related diseases, such as Wilson’s disease (Seth et al., 2004). Therefore, in recent years, fluorescent probes detecting copper ions (Cu2+) in lysosomes have become a major focus of research investigation. In 2015, Mingguang et al. developed a lysosomal targeted Cu2+ fluorescent probe (Lys-Cu) with dual channel emission, which used rhodamine as a dye (Mingguang et al., 2015). The carbonyl oxygen atoms in 1,8-naphthalimide can combine with metal ions and can be used as a binding unit and a fluorescent group. During the process of photoinduced electron transfer, PET is inhibited following the combination of Cu2+ with 1,8-naphthalimide, leading to a significant enhancement of the fluorescent intensity at 440 nm and 580 nm. In addition, the probe exhibited lower cytotoxicity and higher affinity and could be used to image Cu2+ in lysosomes more efficiently. The hydrazone-containing pyrrole exhibited high affinity for Cu2+ and, in 2018, synthesized a hydrazone probe from sunitinib (Wu et al., 2018). The results indicated that when Cu2+ was combined with the probe, rhodamine was used to induce a ring opening reaction; pyrrole could then react with rhodamine resulting in the imaging of Cu2+ in lysosomes.
Zinc ions (Zn2+) are important metal ions involved in several biological reactions. They are closely related to lysosomal dysfunction and autophagy. The abnormal Zn2+ concentration levels can result in the development of human diseases, such as coronary heart disease (Heyu et al., 2021) and allergic inflammation (Masanobu et al., 2020). Currently, various fluorescent probes have been developed to detect Zn2+; however, only a few can be used to detect Zn2+ in lysosomes. In 2015, Hyo-Jun et al. introduced an N,N-di-(2-pyridyl)ethylenediamine (DPEN) group into the naphthalimide dye (Hyo-Jun et al., 2016). The oxygen atom in the carbonyl group of the imide was combined with Zn2+ and with the nitrogen atom in DPEN, subsequently a two-photon fluorescence probe was synthesized for the detection of Zn2+ in the lysosomes (Figure 11). It was found that the probe exhibited an optimal linear response to Zn2+; the fluorescent intensity of the probe was low following combination with Zn2+ when pH = 7.4. When pH = 4.5–5.5, the fluorescent intensity was significantly enhanced. The high sensitivity and affinity of the probe for Zn2+ was optimal for the detection of the dynamic changes of lysosomal Zn2+. In addition, Duan et al. designed the fluorescent probe DR, which was synthesized using N,N-bis(2-pyridylmethyl)ethylenediamine (BPEN) and morpholine as a ligand (Duan et al., 2019). When BPEN was connected with the fluorescent group by benzene on the imide, the probe could achieve high sensitivity to Zn2+. When the probe was combined with Zn2+, the fluorescent intensity increased at a pH range of 7.0–10.0 and was significantly increased when the pH range was 4.0–7.5, which indicated that the probe could be used to detect Zn2+ in lysosomes.
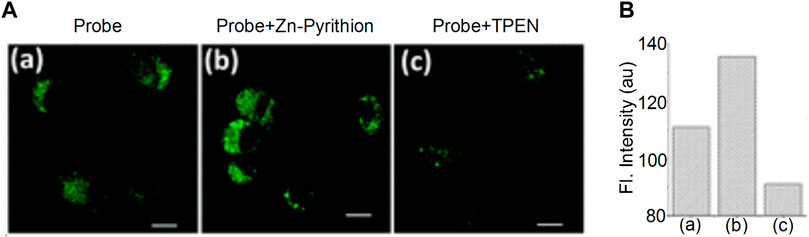
FIGURE 11. The fluorescence characteristics of probe (Hyo-Jun et al., 2016). (A) TPM imaging of Zn(ii) ions in live NIH 3T3 cells. (B) The substantial increase in fluorescence intensity after being uncubated with Zn-Pyrithion in the cells.
The maintenance of lysosomal pH is achieved by the synergy of the proton pump and the chloride channel. Moreover, the chloride (Cl−) ion plays an important role in the development of brain diseases, such as Alzheimer’s disease (Hui et al., 2017). Therefore, the development of fluorescent probes that can specifically detect Cl− in lysosomes has become one of the major research hotspots in recent years. Sang-Hyun et al. (2019) developed the fluorescent probe MQAE-MP in 2019 to specifically detect lysosomal Cl−. It was found by using specific experiments that the concentration of Cl− in lysosomes was decreased following treatment with the substances that could destroy their normal function. The concentration of Cl− depended on whether MQAE-MP was used or not. Therefore, due to the targeting effect of the morpholine group, MQAE-MP was mainly accumulated in lysosomes and could be used to fully detect the levels of Cl− in these organelles (Figure 12).
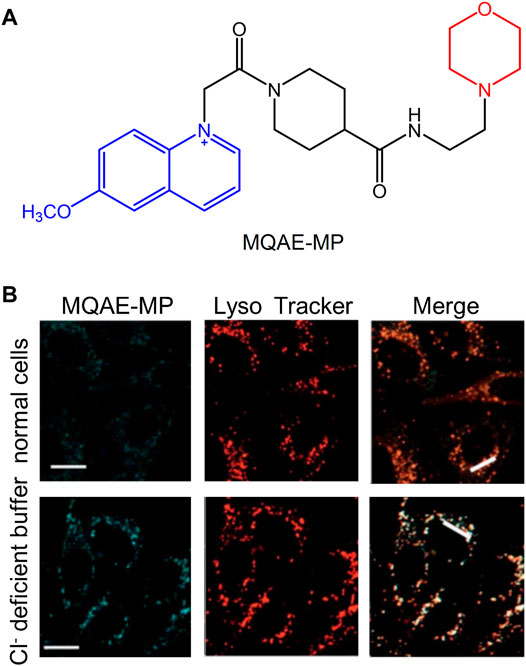
FIGURE 12. The structure and fluorescence characteristic of MQAE-MP (Sang-Hyun et al., 2019). (A) The chemical structure of MQAE-MP. (B) The detection of Cl− ions in cells with MQAE-MP.
The normal pH of the lysosome is 4.5–5.5, which demonstrates weak acidity. An abnormal pH value can lead to corresponding changes in the cell function, and subsequently cause human-related diseases, such as cardiovascular and neurodegenerative diseases (Onyenwoke and Brenman, 2015). Therefore, the detection of the changes in lysosomal pH is important for understanding their biological functions in the related diseases. In the recent 10 years, various small-molecule fluorescent probes have been developed to detect the changes in the lysosomal pH (Wen et al., 2014; Jun et al., 2015; Yongkang et al., 2016; Ji-Ting et al., 2017). Despite these efforts, considerable work is required to improve their detection sensitivity. Peng et al. (2019) developed a two-photon fluorescence probe, which was sensitive to pH changes, and was denoted as Lyso-MPCB (Figure 13). The probe was equipped with the lysosome-located group morpholine, and it could monitor the pH value of the lysosome in real time. Moreover, it could specifically detect autophagy. Lyso-MPCB exhibited blue fluorescent emission at basic conditions and could emit green fluorescence at acidic conditions. The results indicated that the pKa value of the probe was 4.86, which was suitable for detecting the normal pH changes of the lysosomes (4.5–5.5). In addition, the ratio signal of the lysosome was linear with the pH when the range was 4.2–5.6. Therefore, the probe was a powerful tool for monitoring pH changes in lysosomes.
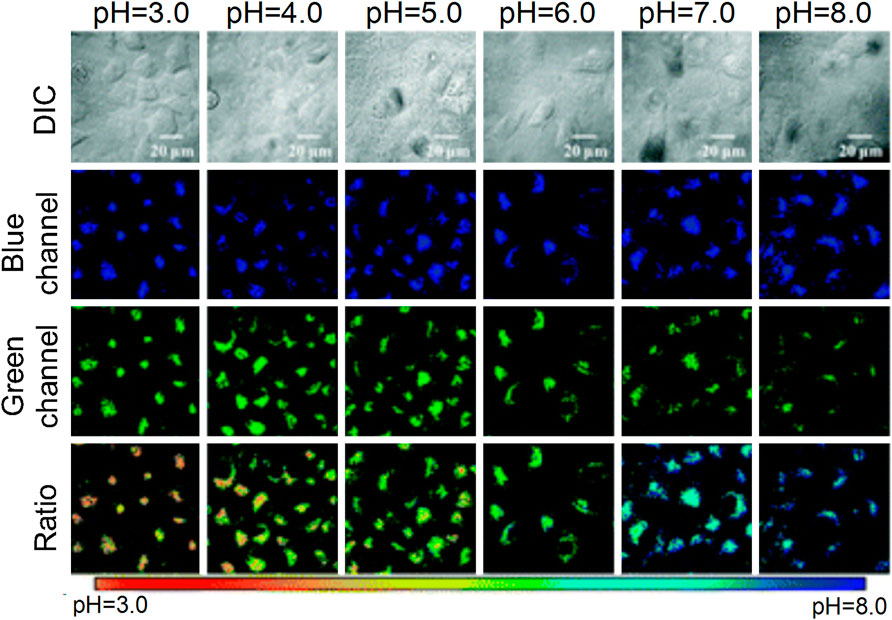
FIGURE 13. The action mechanism of Lyso-MPCB to lysosome pH value (Peng et al., 2019)
In addition, Lee et al. (2018) designed and synthesized a rhodamine pH fluorescent probe, which exhibited improved water solubility, higher quantum yield, and sensitivity and selectivity. The probe’s pKa value was 4.10, and therefore it was used to detect the acidic environment. Gong et al. (2019) designed a fluorescent probe detecting the pH changes in lysosomes, denoted as Ly-HN2AM, which used N-aminomorpholine as a closed loop switch and could react strongly under the range: pH = 4.79–6.07. The probe exhibited lower cytotoxicity and improved photostability and could be used to visualize the pH changes in lysosomes under physiological and pathological conditions. Lingling et al. (2020) synthesized a series of fluorescent probes in 2020 based on imidazole-benzothiadiazole compounds to detect the changes in pH of the lysosomes. The probe MIBTAA could react strongly in an acidic environment and exhibited higher sensitivity and selectivity for detecting pH changes.
The lysosome is an acidic organelle that can decompose proteins, and mitochondria is considered the energy source of the cells. The dynamic changes and biological functions of lysosomes and mitochondria in viable cells can be detected simultaneously with fluorescence microscopy.
The interaction between the mitochondria and lysosomes is an important biological process in eukaryotic cells. When their interaction is dysfunctional, certain neurodegenerative diseases develop (Nixon, 2013), such as cancer (Beth, 2007) and Parkinson’s disease (Burbulla et al., 2017). Therefore, an increased number of studies have visually assessed the interaction between mitochondria and lysosomes. Qixin et al. (2020) developed the hemicyanine fluorescent probe Coupa in 2020, which included a specific organelle-targeting ability (Figure 14). By using the technology of super-resolution illustrated microscopy (SIM), the Coupa dye could label mitochondria and lysosomes simultaneously and reveal the interaction between them by functional fluorescence conversion and co-localization. Staining with Coupa indicated that the local viscosity was increased, which was consistent with the biological characteristics of MLC. This may be the result of protein aggregation in the process of MLC. Therefore, the probe could be used efficiently to locate and track the interaction of the lysosomes with mitochondria in viable cells.
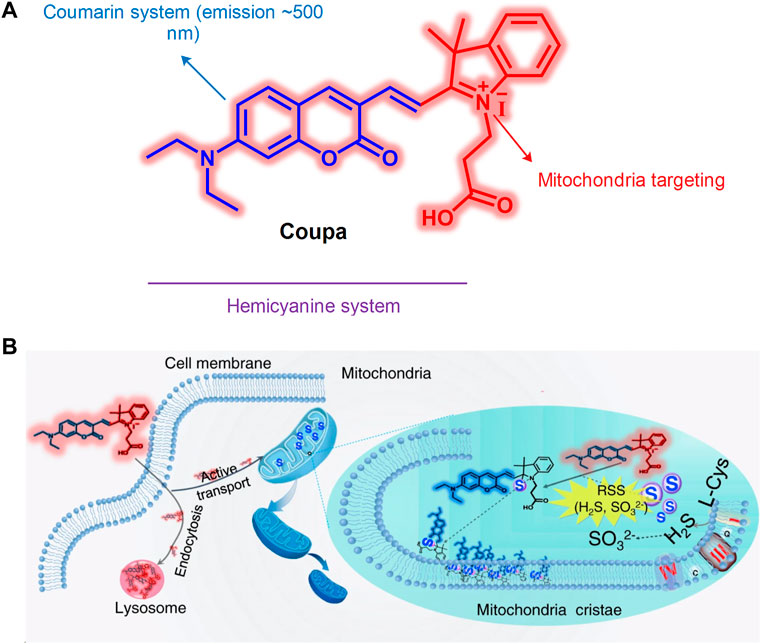
FIGURE 14. The structure and action mechanism of probe Coupa (Qixin et al., 2020). (A) The chemical structure of Coupa; (B) The mitochondria- and lysosome-staining mechanisms of Coupa.
The abnormal viscosity of the mitochondria and lysosomes can lead to their dysfunction and eventually to the development of related diseases (Lu et al., 2013; Bochao et al., 2019). Wei et al. (2021) had developed a cyanine compound in 2020 for the detection of medium viscosity. The probe had near-infrared emission (650 nm) and improved sensitivity and selectivity. It could simultaneously target the lysosomes and mitochondria. In addition, this compound could distinguish normal cells and cancer cells by identifying the changes in viscosity; it could also detect and image the changes in the mitochondrial and lysosomal viscosity in HeLa cells.
SO2 is an important gaseous messenger, which plays a significant role in the induction of apoptosis in lysosomes and mitochondria. When SO2 exhibits abnormal levels, the N-acetylneuraminic acid present in lysosomes and mitochondria will redistribute, which exhibits adverse effects on the induction of apoptosis (Merkur’eva et al., 1981). Therefore, it is necessary to develop a fluorescent probe that can detect SO2 and simultaneously target the lysosomes and mitochondria. Kong et al. (2019) synthesized the novel fluorescent probe DML-P in 2019 by using a FRET-based method, which could simultaneously detect SO2 levels in lysosomes and mitochondria. The probe was the first double-targeted fluorescent probe that could simultaneously track SO2 in mitochondria and lysosomes. DML-P exhibited higher selectivity and stability. In addition, DML-P could detect cellular endogenous SO2 both in the single-photon and two-photon modes, indicating that it could become a powerful tool to study the action mechanism and relationship of the lysosomes and mitochondria.
Nitroreductase (NTR) plays an important role in human health, and the mitochondria and lysosomes are its main sources (Yang et al., 2018; Klockow et al., 2020). Therefore, the development of fluorescent probes for the detection of NTR in mitochondria and lysosomes has become one of the main research hotspots. Sha et al. (2020) developed a dye with aromatic azonia and benzo(e)indol anion skeleton in 2020. On the basis of this dye, the authors of the study synthesized a probe containing 2-ethyl-5-nitrofuran or 4-nitrobenzoyl moiety to detect NTR. The probe emitted in the near infrared. It could target lysosomes and mitochondria simultaneously and exhibited higher sensitivity to NTR. This probe could detect and image lysosomal and mitochondrial NTR in HeLa cells. Therefore, it became the first fluorescent compound which could simultaneously detect lysosomal and mitochondrial NTR.
Small-molecule fluorescent probes located in the mitochondria and lysosomes have been used to detect and image RSMs in recent years. This area has become a research hotspot and involves the identification of fluorescent probes targeting the mitochondria and lysosomes. These probes exhibit specific targeting capabilities and have become a popular research tool in the field of biology, pharmacy, and clinical medicine. Among them, two-photo fluorescent probes have been widely included in the study of lysosomal and mitochondrial targeted fluorescent probes due to the advantages of high resolution and long-time observation. At present, various fluorescent probes have been designed and synthesized to simultaneously target the mitochondria and lysosomes, which provides important tools for the study of RSMs and their mechanisms of action.
According to the present review, the probes detecting lysosomal and mitochondrial RSMs fit the following characteristics: 1) higher selectivity toward certain RSMs compared with other RSMs noted in the organelles; 2) higher quantum yield and longer emission wavelength; 3) higher photostability of product with probe and RSMs; 4) improved biocompatibility. Currently, the emission wavelength of the fluorescent group in certain probes remains short and subject to interference; therefore, the research and development of fluorescent probes require further studies to be completed.
At present, various mitochondrial and lysosomal targeted fluorescent probes have enabled the dynamic monitoring and imaging of RSMs, and certain probes have been used in the diagnosis and treatment of human-related diseases, which will become one of the important research directions in the future.
HC and ZY investigated the literature. HC wrote the manuscript. ZY and SR revised the figures. YQ guided and revised the overall structure and content of the review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arunachalam, B., Phan, U. T., Geuze, H. J., and Cresswell, P. (2000). Enzymatic Reduction of Disulfide Bonds in Lysosomes: Characterization of a Gamma-Interferon-Inducible Lysosomal Thiol Reductase (GILT). Proc. Natl. Acad. Sci. U. S. A. 97, 745–750. doi:10.1073/pnas.97.2.745
Baocun, Z., Xiaoling, Z., Yamin, L., Pengfei, W., Hongyan, Z., and Xiaoqing, Z. (2010). A colorimetric and ratiometric fluorescent probe for thiols and its bioimaging applications. Chemical communications (Camb) 46, 5710-2.doi:10.1039/c0cc00477d
Benitez-Martin, C., Guadix, J. A., Pearson, J. R., Najera, F., Perez-Pomares, J. M., and Perez-Inestrosa, E. (2018). A Turn-On Two-Photon Fluorescent Probe for Detecting Lysosomal Hydroxyl Radicals in Living Cells. Sensors Actuators B. Chem. 284, 744–750. doi:10.1016/j.snb.2018.12.163
de Bie, P., Muller, P., Wijmenga, C., and Klomp, L. W. (2007). Molecular Pathogenesis of Wilson and Menkes Disease: Correlation of Mutations with Molecular Defects and Disease Phenotypes. J. Med. Genet. 44, 673–688. doi:10.1136/jmg.2007.052746
Blaby-Haas, C. E., and Merchant, S. S. (2014). Lysosome-related Organelles as Mediators of Metal Homeostasis. J. Biol. Chem. 289, 28129–28136. doi:10.1074/jbc.R114.592618
Bochao, C., Chengde, L., Jie, Z., Jianfei, K., Tiantian, J., Jin, Z., et al. (2019). Sensing and Imaging of Mitochondrial Viscosity in Living Cells Using a Red Fluorescent Probe with a Long Lifetime. Chem. Commun. (Camb) 55, 7410–7413. doi:10.1039/c9cc03977e
Boya, P., and Kroemer, G. (2008). Lysosomal Membrane Permeabilization in Cell Death. Oncogene 27, 6434–6451. doi:10.1038/onc.2008.310
Bras, M., Queenan, B., and Susin, S. A. (2005). Programmed Cell Death via Mitochondria: Different Modes of Dying. Biochem. (Mosc) 70, 231–239. doi:10.1007/s10541-005-0105-4
Burbulla, L. F., Song, P., Mazzulli, J. R., Zampese, E., Wong, Y. C., Jeon, S., et al. (2017). Dopamine Oxidation Mediates Mitochondrial and Lysosomal Dysfunction in Parkinson's Disease. Science 357, 1255–1261. doi:10.1126/science.aam9080
Cai, G., Xianjun, L., Wen, C., Fenglin, W., and Jian-Hui, J. (2018). A Naphthalene-Based Fluorescent Probe for Ratiometric Imaging of Lysosomal Hydrogen Sulfide in Living Cells. Methods Appl. Fluoresc. 7, 014002. doi:10.1088/2050-6120/aae9c4
Chen, J., Sun, S., Zha, D., Wu, J., Mao, L., Deng, H., et al. (2014). Soyasaponins Prevent H2O2-Induced Inhibition of Gap Junctional Intercellular Communication by Scavenging Reactive Oxygen Species in Rat Liver Cells. Nutr. Cancer 66, 1342–1351. doi:10.1080/01635581.2014.956245
Chen, Q., Shao, X., Tian, Z., Chen, Y., Mondal, P., Liu, F., et al. (2019a). Nanoscale Monitoring of Mitochondria and Lysosome Interactions for Drug Screening and Discovery. Nano Res. 12, 1–222. doi:10.1007/s12274-019-2331-x
Chen, Q., Shao, X., Tian, Z., Chen, Y., Mondal, P., Liu, F., et al. (2019b). Nanoscale Monitoring of Mitochondria and Lysosome Interactions for Drug Screening and Discovery. Nano Res. 12, 1009–1015. doi:10.1007/s12274-019-2331-x
Chen, Q., Fang, H., Shao, X., Tian, Z., Geng, S., Zhang, Y., et al. (2020). A Dual-Labeling Probe to Track Functional Mitochondria-Lysosome Interactions in Live Cells. Nat. Commun. 11, 6290. doi:10.1038/s41467-020-20067-6
Chen, Q., Hao, M., Wang, L., Li, L., Chen, Y., Shao, X., et al. (2021). Prefused Lysosomes Cluster on Autophagosomes Regulated by VAMP8. Cell. Death Dis. 12, 939. doi:10.1038/s41419-021-04243-0
Crompton, M., and Heid, I. (1978). The Cycling of Calcium, Sodium, and Protons across the Inner Membrane of Cardiac Mitochondria. Eur. J. Biochem. 91, 599–608. doi:10.1111/j.1432-1033.1978.tb12713.x
Daniel, S., Peizhou, J., and C, Y.S.-H. (2008). Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry 47, 9678-87.doi:10.1021/bi800699v
Ding, B., and Zhong, Q. (2017). Zinc Deficiency: An Unexpected Trigger for Autophagy. J. Biol. Chem. 292, 8531–8532. doi:10.1074/jbc.H116.762948
Duan, H., Ding, Y., Huang, C., Zhu, W., Wang, R., and Xu, Y. (2019). A Lysosomal Targeting Fluorescent Probe and its Zinc Imaging in SH-Sy5y Human Neuroblastoma Cells. Chin. Chem. Lett. 30, 55–57. doi:10.1016/j.cclet.2018.03.016
Fang, H., Geng, S., Hao, M., Chen, Q., Liu, M., Liu, C., et al. (2021). Simultaneous Zn2+ Tracking in Multiple Organelles Using Super-resolution Morphology-Correlated Organelle Identification in Living Cells. Nat. Commun. 12, 109. doi:10.1038/s41467-020-20309-7
Feng, W., Qiao, Q.-L., Leng, S., Miao, L., Yin, W.-T., Wang, L.-Q., et al. (2016). A 1,8-Naphthalimide-Derived Turn-On Fluorescent Probe for Imaging Lysosomal Nitric Oxide in Living Cells. Chin. Chem. Lett. 27, 1554–1558. doi:10.1016/j.cclet.2016.06.016
Fengyang, W., Shujuan, Y., Zhiai, X., Lingling, L., Yijing, D., Xiaowei, X., et al. (2018). Acid-Promoted D-A-D Type Far-Red Fluorescent Probe with High Photostability for Lysosomal Nitric Oxide Imaging. Anal. Chem. 90, 7953–7962. doi:10.1021/acs.analchem.8b00612
Gaoqing, F., Guoxing, Y., Tingting, N., Wei, W., Hui, H., Haimin, C., et al. (2020). A Novel Ratiometric Fluorescent Probe for the Detection of Mitochondrial pH Dynamics during Cell Damage. Analyst 146, 620–627. doi:10.1039/d0an01240h
Gong, Y. J., Kong, Z. Z., Zhang, M.-L., Lv, M. K., and Zhang, G. (2019). A Structure Optimized Fluorescent Probe for Highly Sensitive Monitoring Drug Induced Lysosomal pH Value Changes. Talanta 203, 1–8. doi:10.1016/j.talanta.2019.05.037
Gu, X., Zhao, E., Zhao, T., Kang, M., Gui, C., Lam, J. W., et al. (2016). A Mitochondrion-Specific Photoactivatable Fluorescence Turn-On AIE-Based Bioprobe for Localization Super-Resolution Microscope. Adv. Mater 28, 5064–5071. doi:10.1002/adma.201505906
Heyu, M., Yueying, W., Fengfeng, Z., Jianjun, R., Meiyu, D., Xue, W., et al. (2021). Reduced Serum Zinc Ion Concentration Is Associated with Coronary Heart Disease. Biol. trace Elem. Res. 199, 4109–4118. doi:10.1007/s12011-020-02551-8
Hui, D., Limin, Z., Wei, L., and Yang, T. (2017). Label-Free Electrochemical Biosensor for Monitoring of Chloride Ion in an Animal Model of Alzhemier's Disease. ACS Chem. Neurosci. 8, 339–346. doi:10.1021/acschemneuro.6b00296
Huimin, C., Han, W., Yongchun, W., Maomao, H., Bo, D., Hongbao, F., et al. (2022). Super-resolution Imaging Reveals the Subcellular Distribution of Dextran at the Nanoscale in Living Cells. J. Chin. Chem. Lett. 33, 1865–1869. doi:10.1016/j.cclet.2021.10.025
Hung, H. H., Huang, W. P., and Pan, C. Y. (2013). Dopamine- and Zinc-Induced Autophagosome Formation Facilitates PC12 Cell Survival. Cell. Biol. Toxicol. 29, 415–429. doi:10.1007/s10565-013-9261-2
Hyo-Jun, L., Chang-Woo, C., Hyewon, S., Subhankar, S., Woong, J. Y., Kyung-Ha, L., et al. (2016). A Two-Photon Fluorescent Probe for Lysosomal Zinc Ions. Chem. Commun. (Camb) 52, 124–127. doi:10.1039/C5CC06976A
Ji-Ting, H., Xiu, R. W., Kun, L., Jinho, S., Amit, S., Xiao-Qi, Y., et al. (2017). Fluorescent Bioimaging of pH: from Design to Applications. Chem. Soc. Rev. 46, 2076–2090. doi:10.1039/c6cs00719h
Yin, J., Hu, Y., and Yoon, J. (2015). Fluorescent Probes and Bioimaging: Alkali Metals, Alkaline Earth Metals and pH. Chem. Soc. Rev. 44, 4619–4644. doi:10.1039/c4cs00275j
Kallunki, T., Olsen, O. D., and Jäättelä, M. (2013). Cancer-associated Lysosomal Changes: Friends or Foes? Oncogene 32, 1995–2004. doi:10.1038/onc.2012.292
Kangnan, W., Shuyue, M., Yanyan, M., Yuping, Z., Miaomiao, X., Liyu, Z., et al. (2020a). Aurone Derivative Revealing the Metabolism of Lipid Droplets and Monitoring Oxidative Stress in Living Cells. Anal. Chem. 92, 6631–6636. doi:10.1021/acs.analchem.0c00456
Kangnan, W., Wen, M., Yuchun, X., Xin, L., Gui, C., Meng, Y., et al. (2020b). Design of a Novel Mitochondria Targetable Turn-On Fluorescence Probe for Hydrogen Peroxide and its Two-Photon Bioimaging Applications. Chin. Chem. Lett. 31, 3149–3152. doi:10.1016/j.cclet.2020.08.039
Klockow, J. L., Hettie, K. S., Lagory, E. L., Moon, E. J., Giaccia, A. J., Graves, E. E., et al. (2020). An Activatable NIR Fluorescent Rosol for Selectively Imaging Nitroreductase Activity. Sens. Actuators B Chem. 306, 127446. doi:10.1016/j.snb.2019.127446
Kong, X., Yin, J., Li, M., Zhu, L., Dong, B., Ma, Y., and Lin, W. (2019). Simultaneously imaging of SO 2 in lysosomes and mitochondria based on a dual organelle-targeted fluorescent probe. Sensors & Actuators: B. Chemical 292, 80-87.doi:10.1016/j.snb2019.04.094
Kurz, T., Eaton, J. W., and Brunk, U. T. (2011). The Role of Lysosomes in Iron Metabolism and Recycling. Int. J. Biochem. Cell. Biol. 43, 1686–1697. doi:10.1016/j.biocel.2011.08.016
Lee, D., Swamy, K.M.K., Hong, J., Lee, S., and Yoon, J. (2018). A rhodamine-based fluorescent probe for the detection of lysosomal pH changes in living cells. Sensors and Actuators: B. Chemical 266, 416-421.doi:10.1016/j.snb2018.03.133
Li, X., Li, X., and Ma, H. (2019). A Near-Infrared Fluorescent Probe Reveals Decreased Mitochondrial Polarity during Mitophagy. Chem. Sci. 11, 1617–1622. doi:10.1039/c9sc05505c
Li, L., Li, Y., Dang, Y., Chen, T., Zhang, A., Ding, C., et al. (2020). Imidazole-fused Benzothiadiazole-Based Red-Emissive Fluorescence Probe for Lysosomal pH Imaging in Living Cells. Talanta 217, 121066. doi:10.1016/j.talanta.2020.121066
Liu, Y., Zhang, C., Wei, Y., Chen, H., Kong, L., Chen, Q., et al. (2021). De Novo-Designed Landmine Warfare Strategy Luminophore for Super-resolution Imaging Reveal ONOO- Evolution in Living Cells. Chem. Eng. J. 422, 130151. doi:10.1016/j.cej.2021.130151
Liuzzi, J. P., and Yoo, C. (2013). Role of Zinc in the Regulation of Autophagy during Ethanol Exposure in Human Hepatoma Cells. Biol. Trace Elem. Res. 156, 350–356. doi:10.1007/s12011-013-9816-3
Long, Z., Chen, L., Dang, Y., Chen, D., Lou, X., and Xia, F. (2019). An Ultralow Concentration of Two-Photon Fluorescent Probe for Rapid and Selective Detection of Lysosomal Cysteine in Living Cells. Talanta 204, 762–768. doi:10.1016/j.talanta.2019.06.064
Lu, W., Yi, X., Wenming, T., and Liezheng, D. (2013). Activatable Rotor for Quantifying Lysosomal Viscosity in Living Cells. J. Am. Chem. Soc. 135, 2903–2906. doi:10.1021/ja311688g
Luzio, J. P., Pryor, P. R., and Bright, N. A. (2007). Lysosomes: Fusion and Function. Nat. Rev. Mol. Cell. Biol. 8, 622–632. doi:10.1038/nrm2217
Marc, L., Manuel, P., and Antonio, Z. (2009). Mitochondrial Dynamics in Mammalian Health and Disease. Physiol. Rev. 89, 799–845. doi:10.1152/physrev.00030.2008
Masanobu, S., Takayoshi, S., Masashi, W., Shigetsugu, H., Shogo, K., Akira, N., et al. (2020). Role of Intracellular Zinc in Molecular and Cellular Function in Allergic Inflammatory Diseases. Allergol. Int., 70, 190–200. doi:10.1016/j.alit.2020.09.007
Melina-Theoni, G., and Athanasios, G. P. (2014). Lysosome: the Cell's 'suicidal Bag' as a Promising Cancer Target. Trends Mol. Med. 20.
Meng, H., Huang, X. Q., Lin, Y., Yang, D. Y., Lv, Y. J., Cao, X. Q., et al. (2019). A New Ratiometric Fluorescent Probe for Sensing Lysosomal HOCl Based on Fluorescence Resonance Energy Transfer Strategy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 223, 117355. doi:10.1016/j.saa.2019.117355
Merkur'eva, R. V., Bushinskaia, L. I., Skvortsova, N. I., Dolinskaia, S. I., and Shaternikova, I. S. (1981). Membrane-bound Sialoglycoproteins of the Rat Liver as Affected by the Combined Action of Benz(a)pyrene and Sulfur Dioxide. Vopr. meditsinskoi khimii 27, 791–794.
Mingguang, R., Beibei, D., Jian-Yong, W., Zhan-Rong, L., and Weiying, L. (2015). A Dual-Emission Fluorescence-Enhanced Probe for Imaging Copper(ii) Ions in Lysosomes. J. Mater. Chem. B 3, 6746–6752. doi:10.1039/c5tb01184a
Nemoto, S., Takeda, K., Yu, Z. X., Ferrans, V. J., and Finkel, T. (2000). Role for Mitochondrial Oxidants as Regulators of Cellular Metabolism. Mol. Cell. Biol. 20, 7311–7318. doi:10.1128/mcb.20.19.7311-7318.2000
Ning, P., Jiang, J., Li, L., Wang, S., Yu, H., Feng, Y., et al. (2016). A Mitochondria-Targeted Ratiometric Two-Photon Fluorescent Probe for Biological Zinc Ions Detection. Biosens. Bioelectron. 77, 921–927. doi:10.1016/j.bios.2015.10.061
Nixon, R. A., Cataldo, A. M., and Mathews, P. M. (2000). The Endosomal-Lysosomal System of Neurons in Alzheimer's Disease Pathogenesis: A Review. Neurochem. Res. 25, 1161–1172. doi:10.1023/a:1007675508413
Nixon, R. A. (2013). The Role of Autophagy in Neurodegenerative Disease. Nat. Med. 19, 983–997. doi:10.1038/nm.3232
Onyenwoke, R. U., and Brenman, J. E. (2015). Lysosomal Storage Diseases-Regulating Neurodegeneration. J. Exp. Neurosci. 9, 81–91. doi:10.4137/JEN.S25475
Peng, N., Liling, H., Yan, F., Guoyong, X., Yuyuan, B., Haizhu, Y., et al. (2019). Real-time Visualization of Autophagy by Monitoring the Fluctuation of Lysosomal pH with a Ratiometric Two-Photon Fluorescent Probe. Chem. Commun. (Camb) 55, 1782–1785. doi:10.1039/c8cc09517e
Phan, U. T., Arunachalam, B., and Cresswell, P. (2000). Gamma-interferon-inducible Lysosomal Thiol Reductase (GILT). Maturation, Activity, and Mechanism of Action. J. Biol. Chem. 275, 25907–25914. doi:10.1074/jbc.M003459200
Pierrel, F., Cobine, P. A., and Winge, D. R. (2007). Metal Ion Availability in Mitochondria. BioMetals 20, 675–682. doi:10.1007/s10534-006-9052-9
Qiu, K., Du, Y., Liu, J., Guan, J. L., Chao, H., and Diao, J. (2020). Super-resolution Observation of Lysosomal Dynamics with Fluorescent Gold Nanoparticles. Theranostics 10, 6072–6081. doi:10.7150/thno.42134
Qixin, C., Hongbao, F., Xintian, S., Zhiqi, T., Shanshan, G., Yuming, Z., et al. (2020). A Dual-Labeling Probe to Track Functional Mitochondria-Lysosome Interactions in Live Cells. Nat. Commun. 11, 6290. doi:10.1038/s41467-020-20067-6
Radi, R. (2013). Peroxynitrite, a Stealthy Biological Oxidant. J. Biol. Chem. 288, 26464–26472. doi:10.1074/jbc.R113.472936
Saipeng, H., Rongcheng, H., Qianfen, Z., Libo, D., Hongying, J., Yangping, L., et al. (2015). New Photostable Naphthalimide-Based Fluorescent Probe for Mitochondrial Imaging and Tracking. Biosens. Bioelectron. 71, 313–321. doi:10.1016/j.bios.2015.04.056
Sang-Hyun, P., Young, H. J., and Injae, S. (2019). A Lysosomal Chloride Ion-Selective Fluorescent Probe for Biological Applications. Chem. Sci. 10, 56–66. doi:10.1039/c8sc04084b
Seth, R., Yang, S., Choi, S., Sabean, M., and Roberts, E. A. (2004). In Vitro assessment of Copper-Induced Toxicity in the Human Hepatoma Line, Hep G2. Toxicol. Vitro 18, 501–509. doi:10.1016/j.tiv.2004.01.006
Sha, X.-L., Yang, X.-Z., Wei, X.-R., Sun, R., Xu, Y.-J., and Ge, J.-F. (2020). A Mitochondria/lysosome-Targeting Fluorescence Probe Based on Azonia-Cyanine Dye and its Application in Nitroreductase Detection. Sensors Actuators B. Chem. 307, 127653. doi:10.1016/j.snb.2019.127653
Shiling, Z., Jiangli, F., Shuangzhe, Z., Jingyun, W., Xianwu, W., Jianjun, D., et al. (2014). Lighting up Fluoride Ions in Cellular Mitochondria Using a Highly Selective and Sensitive Fluorescent Probe. Chem. Commun. (Camb) 50, 14021–14024. doi:10.1039/c4cc05094k
Tamima, U., Song, C. W., Santra, M., Reo, Y. J., Banna, H., Islam, M. R., et al. (2020). A Benzo[ B ]xanthene-Derived Fluorescent Probe Capable of Two-Photon Ratiometric Imaging of Lysosomal Cysteine with High Specificity. Sensors Actuators B. Chem. 322, 128588. doi:10.1016/j.snb.2020.128588
Tian, M., Zhan, J., and Lin, W. (2022). Single Fluorescent Probes Enabling Simultaneous Visualization of Duple Organelles: Design Principles, Mechanisms, and Applications. Coord. Chem. Rev. 451, 214266. doi:10.1016/j.ccr.2021.214266
Tümer, Z., and Møller, L. B. (2010). Menkes Disease. Eur. J. Hum. Genet. 18, 511–518. doi:10.1038/ejhg.2009.187
Vakifahmetoglu-Norberg, H., Ouchida, A. T., and Norberg, E. (2017). The Role of Mitochondria in Metabolism and Cell Death. Biochem. Biophys. Res. Commun. 482, 426–431. doi:10.1016/j.bbrc.2016.11.088
Vila, M., Bové, J., Dehay, B., Rodríguez-Muela, N., and Boya, P. (2011). Lysosomal Membrane Permeabilization in Parkinson Disease. Autophagy 7, 98–100. doi:10.4161/auto.7.1.13933
Wang, L., and Diao, J. (2022). VAMP8 Phosphorylation Regulates Lysosome Dynamics during Autophagy. Autophagy Rep. 1, 79–82. doi:10.1080/27694127.2022.2031378
Wang, W., Zhang, W., Feng, Y., Wang, S., Lei, H., Huang, J., et al. (2018). Strategically Modified Highly Selective Mitochondria-Targeted Two-Photon Fluorescent Probe for Au 3+ Employing Schiff-Base: Inhibited C=N Isomerization vs. Hydrolysis Mechanism. Dyes Pigments 150, 241–251. doi:10.1016/j.dyepig.2017.12.019
Wei, Y.-F., Weng, X.-F., Sha, X.-L., Sun, R., Xu, Y.-J., and Ge, J.-F. (2021). Simultaneous Imaging of Lysosomal and Mitochondrial Viscosity under Different Conditions Using a NIR Probe. Sensors Actuators B. Chem. 326, 128954. doi:10.1016/j.snb.2020.128954
Wei, Y., Kong, L., Chen, H., Liu, Y., Xu, Y., Wang, H., et al. (2022). Super-resolution Image-Based Tracking of Drug Distribution in Mitochondria of a Label-free Naturally Derived Drug Molecules. Chem. Eng. J. 429, 132134. doi:10.1016/j.cej.2021.132134
Wen, S., Xiaohua, L., and Huimin, M. (2014). Fluorescent Probes and Nanoparticles for Intracellular Sensing of pH Values. Methods Appl. Fluoresc. 2, 042001. doi:10.1088/2050-6120/2/4/042001
Wong, Y. C., Kim, S., Peng, W., and Krainc, D. (2019). Regulation and Function of Mitochondria-Lysosome Membrane Contact Sites in Cellular Homeostasis. Trends Cell. Biol. 29, 500–513. doi:10.1016/j.tcb.2019.02.004
Wu, W.-N., Wu, H., Zhong, R.-B., Wang, Y., Xu, Z.-H., Zhao, X.-L., et al. (2018). Ratiometric Fluorescent Probe Based on Pyrrole-Modified Rhodamine 6G Hydrazone for the Imaging of Cu 2+ in Lysosomes. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 212, 121–127. doi:10.1016/j.saa.2018.12.041
Xiaoyue, H., Rui, W., Xinyu, S., Fabiao, Y., Changjun, L., and Lingxin, C. (2018). A Mitochondrial-Targeting Near-Infrared Fluorescent Probe for Bioimaging and Evaluating Endogenous Superoxide Anion Changes during Ischemia/reperfusion Injury. Biomaterials, 156, 134–146. doi:10.1016/j.biomaterials.2017.11.039
Xu, X., Chen, W., Yang, M., Liu, X. J., Wang, F., Yu, R. Q., et al. (2019). Mitochondrial-targeted Near-Infrared Fluorescence Probe for Selective Detection of Fluoride Ions in Living Cells. Talanta 204, 655–662. doi:10.1016/j.talanta.2019.06.028
Yang, L., Niu, J.-Y., Sun, R., Xu, Y.-J., and Ge, J.-F. (2018). The Application of Mitochondrial Targetable Pyronine-Pyridinium Skeleton in the Detection of Nitroreductase. Sensors Actuators B. Chem. 259, 299–306. doi:10.1016/j.snb.2017.12.011
Yawei, W., Liu, W., Caiyun, L., Bingpeng, G., Baocun, Z., Zuokai, W., et al. (2017). A Highly Specific and Ultrasensitive Fluorescent Probe for Basal Lysosomal HOCl Detection Based on Chlorination Induced by Chlorinium Ions (Cl+). J. Mater. Chem. B 5, 3377–3382. doi:10.1039/c6tb03345h
Li, Y., Wu, W., Yang, J., Yuan, L., Liu, C., Zheng, J., et al. (2016). Engineering a Nanolab for the Determination of Lysosomal Nitric Oxide by the Rational Design of a pH-Activatable Fluorescent Probe. Chem. Sci. 7, 1920–1925. doi:10.1039/c5sc04415d
Yong, L., Fangfang, M., Longwei, H., Keyin, L., and Weiying, L. (2016). A Dual-Site Two-Photon Fluorescent Probe for Visualizing Lysosomes and Tracking Lysosomal Hydrogen Sulfide with Two Different Sets of Fluorescence Signals in the Living Cells and Mouse Liver Tissues. Chem. Commun. (Camb) 52, 7016–7019. doi:10.1039/c6cc02368a
Yongkang, Y., Fangjun, H., Songyi, L., Caixia, Y., and Juyoung, Y. (2016). A Review: the Trend of Progress about pH Probes in Cell Application in Recent Years. Analyst 142, 30–41. doi:10.1039/c6an01942k
Keywords: lysosomes, mitochondria, small-molecule fluorescent probe, interaction, imaging
Citation: Chen H, Yu Z, Ren S and Qiu Y (2022) Fluorescent Probes Design Strategies for Imaging Mitochondria and Lysosomes. Front. Pharmacol. 13:915609. doi: 10.3389/fphar.2022.915609
Received: 08 April 2022; Accepted: 30 May 2022;
Published: 19 July 2022.
Edited by:
Peixue Ling, Shandong University, ChinaReviewed by:
Kai Wang, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaCopyright © 2022 Chen, Yu, Ren and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuyu Qiu, cXl5dTE5NzlAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.