
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 14 June 2022
Sec. Drug Metabolism and Transport
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.914733
This article is part of the Research Topic Methods and Application in Drug Metabolism and Transport: 2021 View all 14 articles
Voxtalisib, is a specific, effective, and reversible dual inhibitor, which inhibits both pan-class I phosphoinositide 3-kinase (PI3K) and mechanistic target of rapamycin (mTOR). To date, voxtalisib has been studied in trials for melanoma, lymphoma, glioblastoma, breast cancer, and other cancers. In this study, a highly sensitive and rapid ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) technology was applied to the quantitative methodology and pharmacokinetic analysis of voxtalisib in rat plasma. After protein precipitation of the analyte by acetonitrile, the chromatographic separation was performed by gradient elution on an Acquity BEH C18 column (2.1 mm × 50 mm, 1.7 μm) with acetonitrile (solvent A) and 0.1% formic acid (solvent B) as the mobile phase. In the positive ion mode, the mass transfer detection of the analyte and IS was m/z 270.91 > 242.98 and m/z 572.30 > 246.10, respectively. In the concentration range of 1–2000 ng/ml, a good linear relationship of voxtalisib was successfully established by the UPLC-MS/MS technology, and the lower limit of quantification (LLOQ) of the analyte was identified as 1 ng/ml. Intra-day and inter-day precisions for voxtalisib were 7.5–18.7% and 13.0–16.6%, respectively, and the accuracies were in the ranges of −14.0–2.0% and −7.2–3.1%, respectively. The matrix effect, extraction recovery, carryover and stability of the analyte were all in compliance with the acceptance criteria of bioassays recommended by FDA. Finally, the pharmacokinetic profile of the analyte had been availably studied by the UPLC-MS/MS bio-analytical method after rats were treated by intragastric administration with voxtalisib (5 mg/kg). The results indicated that the UPLC-MS/MS technology can effectively and quickly quantify the analyte, and this method can also be used for the pharmacokinetic study of voxtalisib, which can provide reference for the optimization of clinical drug management in the later period.
Voxtalisib (Figure 1A), also called as SAR245409 or XL765, is a dual inhibitor that inhibits both pan-class I phosphoinositide 3-kinase (PI3K) and mechanistic target of rapamycin (mTOR) by competitively binding specific adenosine triphosphate (ATP) to the catalytic domain of PI3K and mTOR (Janne et al., 2014; He et al., 2018). Voxtalisib plays an anti-tumor role mainly by inhibiting the formation of tumor blood vessels and inducing apoptosis of cancer cells, and its anti-tumor activity has been proved (Markman et al., 2010; Prasad et al., 2011; Zhao et al., 2019). Voxtalisib achieved its therapeutic effect by apoptotic caspase-dependent primary chronic lymphocytic leukemia cells with a half-maximum inhibitory concentration (IC50) of 0.86 µM and a maximum duration of action of 48 h. In addition, voxtalisib blocked the adhesion, proliferation, and in vitro survival of chronic lymphocytic leukemia cells and effectively inhibits T-cell-mediated cytokines that support the production of chronic lymphocytic leukemia (Thijssen et al., 2016; Brown et al., 2018; Zhang et al., 2018). As pharmacokinetic information is essential for the optimization of clinical administration, it is necessary to quantify and monitor the plasma concentration of voxtalisib.
Therefore, in this experiment, we intended to measure the concentration of voxtalisib in rat plasma by using a high-efficiency UPLC-MS/MS technology. Then, we demonstrated the robustness of our method by measuring the linearity, carryover, precision and accuracy, matrix effect and extraction recovery, and stability of the analyte. Subsequently, the pharmacokinetic studies of voxtalisib in rat plasma confirmed the specificity and repeatability of this method.
Voxitalisib, and umbralisib (Figure 1B) as the internal standard (IS) were purchased from Beijing sunflower Technology Development Co., Ltd. (Beijing, China). The LC grade methanol and acetonitrile were supplied by Merck (Darmstadt, Germany) and LC grade pure formic acid was produced by Anaqua Chemicals Supply (ACS, American). At the same time, the ultra-pure water from the laboratory was pretreated by the Milli-Q Water Purification System (Millipore, Bedford, Unnited States).
Six male SD rats (300 ± 20 g) were collected from the Experimental Animal Research Center of The First Affiliated Hospital of Wenzhou Medical University (Wenzhou, China), and were given fresh water and plenty of food every day. In the formal pharmacokinetic study, each rat was given 5 mg/kg voxtalisib in the 0.5% carboxymethylcellulose sodium (CMC-Na) by single intragastric administration after fasting for 12 h. Then, blood samples (approximately 0.3 ml) were acquired at 0.333, 0.667, 1, 1.5, 2, 3, 4, 6, 8, 12, 24 and 48 h, respectively, and placed into polyethylene tubes containing heparin. The samples were immediately centrifuged at 13,000 × g for 10 min at 4°C for separation. The centrifuged supernatants were then transferred into new 0.5 ml polythene tubes and then stored at −80°C for further analysis. In this experiment, the plasma concentration of voxtalisib in rats was measured by UPLC-MS/MS technology, and the main pharmacokinetic parameters of voxtalisib in non-compartmental model were calculated using Drugs and Statistics (DAS) 3.0 software (Mathematical Pharmacology Professional Committee of China, Shanghai, China).
Chromatographic separation of voxtalisib and IS were performed using a Waters Acquity ultra-performance liquid chromatography (UPLC) system (Milford, MA, Unnited States) equipped with an Acquity BEH C18 column (2.1 mm × 50 mm, 1.7 μm; Milford, MA, Unnited States), and it was operated under the condition of 40°C column temperature. The mobile phases of gradient elution were acetonitrile (solvent A) and 0.1% formic acid (solvent B), respectively. At the flow rate of 0.30 ml/min, the elution process was 90% B from 0 to 0.5 min; 90–10% B from 0.5 to 1.0 min; 10% B from 1.0 to 1.4 min; 10–90% B from 1.4 to 1.5 min, and finally at 1.5–2.0 min, 90% B was maintained for equilibration. The whole process of analysis was 2.0 min, the temperature of the auto-sampler was set at 10°C, and the injection volume was 1.0 μL for each running process.
Mass spectrometry information of voxtalisib and IS were obtained by a Waters Xevo TQS triple-quadrupole tandem mass spectrometer (Milford, MA, Unnited States) with an electrospray ionization (ESI) source and a positive ion mode detection by MS/MS. Using selective response monitoring (SRM), the ion transitions of voxtalisib and IS were m/z 270.91 > 242.98 and m/z 572.30 > 246.10, respectively (Figure 2). Optimized cone voltages and collision energies were 20 V and 20 eV for voxtalisib, 30 V and 35 eV for IS, respectively. The flow rate of collision gas, cone gas and desolvation gas filled with high purity of nitrogen was 0.15 ml/min, 200 L/h and 1000 L/h, respectively. The optimized desolvation temperature reached 600°C.
Voxtalisib and IS were dissolved in methanol as the stock solutions, and the concentration of each stock solution was 1 mg/ml. Then, the stock solution was gradient diluted with methanol to obtain the calibration and quality control (QC) samples. The concentrations of the working solutions for calibration curve were 10, 20, 50, 100, 500, 1000, 2000, 5000, 10000, 20000 ng/ml, and the IS concentration was 100 ng/ml. Fresh calibration standards with final concentrations of 1, 2, 5, 10, 50, 100, 200, 500, 1000, 2000 ng/ml were prepared by adding the corresponding concentration of voxtalisib working solutions (10 μL) to the blank rat plasma (90 μL). Then, the same method was used to prepare QC samples with different concentrations (2, 800, 1600 ng/ml) and the lower limit of quantification (LLOQ, 1 ng/ml). Finally, all the stock and working solutions were stored at −80°C until further analysis.
The plasma samples were treated with a simple and rapid protein precipitation method. Firstly, adding 10 μL IS working solution to 100 μL plasma sample; Secondly, precipitate the plasma protein by adding 300 μL acetonitrile; Thirdly, the plasma samples in each tube were fully swirled for 2.0 min and centrifuged for 10 min at 13000 × g under 4°C. Finally, we took 100 μL centrifuged supernatant into the special detection bottle of UPLC-MS/MS system, and then used UPLC-MS/MS technology for analysis. In order to ensure the stability of the sample, it is recommended to test immediately. If immediate detection is not available, it can be stored at 4°C in the short-term, and should be stored at −80°C in the long-term.
According to the principle of bio-assay validation of FDA, a novel bio-assay method based on UPLC-MS/MS technology was established, including the specificity, carryover, calibration curve, LLOQ, precision, accuracy, matrix effect, recovery, and stability, as similar with those validated in our previous papers (Qiu et al., 2019; Xu et al., 2019; Tang et al., 2020).
The specificity of UPLC-MS/MS analytical method was determined by analyzing plasma samples from three different batches: blank plasma without analyte and IS, blank plasma spiked with the analyte at LLOQ and IS, as well as rat plasma samples. Then, the retention times of the analyte and IS in the SRM chromatogram were observed to determine whether there were endogenous interference. Carryover was assessed by injecting plasma samples at upper limit of quantification (ULOQ) followed by blank injections.
The linear regression analysis of voxtalisib/IS peak area ratio (Y) and voxtalisib concentration (X) was performed by the least square regression analysis with a weighted factor (1/x2) to obtain the calibration curve. The sensitivity of the UPLC-MS/MS bio-analytical method was evaluated by analyzing six replicates of spiked LLOQ samples.
Six replicate QC samples (2, 800 and 1600 ng/ml) were determined on the same day and on three different days. The intra and inter-day precisions of the method were calculated, which is usually shown as relative standard deviation (RSD%), and the accuracy is commonly represented by relative error (RE%).
At low, medium and high concentrations (2, 800 and 1600 ng/ml, n = 6), the peak areas of voxtalisib added to the post-extracted blank plasma were compared with those of QC sample with corresponding concentration obtained by diluting standard solution with methanol to calculate matrix effect.
Voxtalisib was added into the blank plasma before and after extraction, respectively, and the extraction recovery was evaluated by comparing the peak area of the two, also at three different levels (2, 800 and 1600 ng/ml, n = 6).
We evaluated the stability of this bioassay by measuring rat plasma concentrations of QC samples of voxtalisib at three different concentrations (2, 800 and 1600 ng/ml, n = 5) under four different placement conditions, including short-term stability (3 h at room temperature), long-term stability (3 weeks at −80°C), post-preparation stability (4 h at 10°C in an auto-sampler), and stability through complete three freeze-thaw cycles.
In the pre-experiment, different organic buffers (such as methanol and acetonitrile) and water buffers (such as formic acid and ammonium acetate) on different types of analytical columns (Waters Acquity UPLC BEH C18 column, HSS C18 column, and CSH C18 column) were evaluated. After comparing different types of analytical columns in the experiment, the Acquity BEH C18 column (2.1 mm × 50 mm, 1.7 μm) provided good peak shapes, short chromatographic retention times and high chromatographic responses for both the analyte and IS, and the separation effect was better. In addition, acetonitrile and 0.1% formic acid aqueous solution was the best mobile phase, and the analyte and IS obtained better signals and responses.
In pharmacokinetic study, we chose simple, time-saving and economical organic protein precipitation method to prepare plasma samples. We precipitated plasma proteins with acetonitrile. The results showed that acetonitrile had higher efficiency of protein precipitation.
According to SRM chromatograms under three different conditions in Figure 3, voxtalisib and IS can be significantly distinguished by this chromatographic condition. The three different conditions were as follows: blank rat plasma sample (Figure 3A: no analyte, no IS), LLOQ concentration (1 ng/ml) of the analyte and IS added to blank rat plasma sample (Figure 3B), and a rat sample collected 20 min later in pharmacokinetic study after given intragastric administration of voxtalisib (5 mg/kg) (Figure 3C). Figure 3 showed that the retention times of voxtalisib and IS were 1.32 and 1.77 min, respectively. In addition, no carryover was observed for either analyte or IS in rat plasma, because the peak area of the interference peak was less than 20% for the analyte in the LLOQ samples and less than 5% for the IS following injection of ULOQ samples.

FIGURE 3. Representative chromatograms of voxtalisib and IS in rat plasma: (A) blank plasma; (B) blank plasma spiked with analyte at LLOQ and IS; (C) plasma sample collected from a rat at 20 min after intragastric administration of 5 mg/kg voxtalisib.
In this experiment, the linear regression equation of calibration standard curve in the concentration range of 1–2000 ng/ml was y = 0.0231301x + 0.00967189 (r2 = 0.999). As shown in Table 1, the LLOQ of voxtalisib was 1 ng/ml, where precision and accuracy were acceptable within 20%.
Table 1 indicated that the intra and inter-day precision values (RSD%) of voxtalisib at three QC concentrations were 7.5–10.6% and 13.0–14.3%, respectively, and the intra and inter-day accuracy values (RE%) were −14.0% to −4.0% and −7.2% to −4.4%, respectively. According to the FDA guidance, this bioassay was used to quantify voxtalisib in rat plasma with high accuracy and reproducibility.
Table 2 displayed the results of matrix effect and extraction recovery of QC samples of voxtalisib at three different concentration levels (2, 800, 1600 ng/ml). The matrix effect of voxtalisib in blank rat plasma was 94.1–96.7%. In addition, its extraction recovery ranged from 100.5 to 106.8%. And these results were within the reasonable limits.
Table 3 showed the stability results of QC samples of voxtalisib in rat plasma under four different placement conditions. These indicated that voxtalisib had no significant difference under a variety of placement conditions (3 h at room temperature, 3 weeks at −80°C, 4 h at 10°C in an auto-sampler, and freeze–thaw stability for 3 times).
The pharmacokinetic study of voxtalisib in rat plasma was performed by UPLC-MS/MS technology. Figure 4 depicted the relationship between mean plasma concentration (ng/ml) and time (0–48 h) of voxtalisib in rats. Since voxtalisib was fully eliminated from the body at around 12 h, the relationship between the mean plasma concentration (ng/ml) of voxtalisib in rats and the time of 0–12 h was added to illustrate the relationship more clearly. Table 4 showed the main pharmacokinetic parameters of voxtalisib, which were calculated by non-compartment analysis using DAS software.
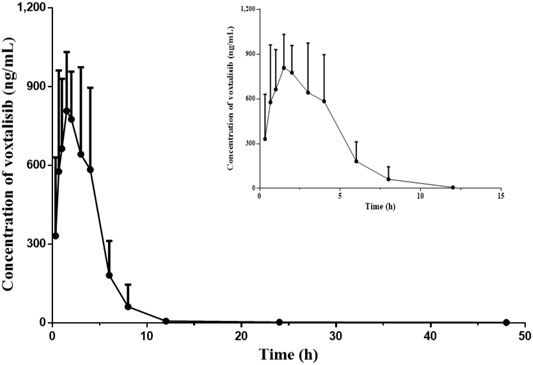
FIGURE 4. Mean plasma concentration-time curves of voxtalisib in rats after intragastric administration of voxtalisib (5 mg/kg). (n = 6).

TABLE 4. The main pharmacokinetic parameters of voxtalisib in rat plasma after intragastric administration of voxtalisib at a single dose of 5 mg/kg. (n = 6, Mean ± SD).
According to Figure 4 and Table 4, after the rats were given 5 mg/kg voxtalisib in a single dose, voxtalisib was rapidly absorbed into the blood and spread throughout the whole body, reaching a maximum plasma concentration (Cmax) of 977.74 ± 250.40 ng/ml at 2.45 ± 1.42 h (time to Cmax, Tmax). Moreover, the half-life (t1/2) of its elimination in rats was 10.47 ± 5.00 h.
In a phase I dose-escalation study on the safety and pharmacokinetics of voxtalisib tablets in patients with solid tumors, Cmax = 301 ± 101 ng/ml, Tmax = 1.53 h, t1/2 = 3.94 ± 0.79 h on the first day after giving patients 50 mg/kg once daily (Mehnert et al., 2018). The existence of these differences in patient and rat data may be related to ethnic and individual differences, and in addition, our experimental results have only been verified in a few rats (n = 6), compared to 49 patients in the clinical trial. Consequently, the pharmacokinetics of voxtalisib need to be further studied. Furthermore, this pharmacokinetics of voxtalisib in patients with solid tumors did not provide enough data for repeating the approach in other laboratories (specificity, accuracy, precision, etc.), so our method effectively achieved the need of high sample throughput for biological analysis (Mehnert et al., 2018).
In summary, in this experiment, we determined the specificity, carryover, precision, accuracy, extraction recovery, matrix effect, and stability of voxtalisib in rat plasma. This UPLC-MS/MS assay can effectively and quickly quantify the analyte, and this method can also be used for the pharmacokinetic study of voxtalisib in rats, which can provide reference for the optimization of clinical drug management in the later period.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by The First Affiliated Hospital of Wenzhou Medical University.
QL: Investigation; Writing—original draft; Conceptualization; Data curation; Formal analysis; Y‐nL: Investigation; Methodology; Visualization, Writing—original draft; JW: Data curation; editing; YH: Investigation; Methodology; JH: Data curation; Formal analysis; R‐aX: Supervision; Writing—review and editing; Validation; LS: Project administration; Resources; Software; Supervision; Writing—review and editing; Validation; LC: Project administration; Resources; Software; Supervision; Writing—review and editing; Validation.
This work was supported by National Key Research and Development Program of China (2020YFC2008301) and PhD Start-up Funds from The First Affiliated Hospital of Wenzhou Medical University (2022QD23).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Brown, J. R., Hamadani, M., Hayslip, J., Janssens, A., Wagner-Johnston, N., Ottmann, O., et al. (2018). Voxtalisib (XL765) in Patients with Relapsed or Refractory Non-hodgkin Lymphoma or Chronic Lymphocytic Leukaemia: an Open-Label, Phase 2 Trial. Lancet Haematol. 5, e170–e180. doi:10.1016/S2352-3026(18)30030-9
He, J., Fang, P., Zheng, X., Wang, C., Liu, T., Zhang, B., et al. (2018). Inhibitory Effect of Celecoxib on Agomelatine Metabolism In Vitro and In Vivo. Drug Des. Devel Ther. 12, 513–519. doi:10.2147/DDDT.S160316
Jänne, P. A., Cohen, R. B., Laird, A. D., Macé, S., Engelman, J. A., Ruiz-Soto, R., et al. (2014). Phase I Safety and Pharmacokinetic Study of the PI3K/mTOR Inhibitor SAR245409 (XL765) in Combination with Erlotinib in Patients with Advanced Solid Tumors. J. Thorac. Oncol. 9, 316–323. doi:10.1097/JTO.0000000000000088
Markman, B., Atzori, F., Pérez-García, J., Tabernero, J., and Baselga, J. (2010). Status of PI3K Inhibition and Biomarker Development in Cancer Therapeutics. Ann. Oncol. 21, 683–691. doi:10.1093/annonc/mdp347
Mehnert, J. M., Edelman, G., Stein, M., Camisa, H., Lager, J., Dedieu, J. F., et al. (2018). A Phase I Dose-Escalation Study of the Safety and Pharmacokinetics of a Tablet Formulation of Voxtalisib, a Phosphoinositide 3-kinase Inhibitor, in Patients with Solid Tumors. Invest. New Drugs 36, 36–44. doi:10.1007/s10637-017-0467-7
Prasad, G., Sottero, T., Yang, X., Mueller, S., James, C. D., Weiss, W. A., et al. (2011). Inhibition of PI3K/mTOR Pathways in Glioblastoma and Implications for Combination Therapy with Temozolomide. Neuro Oncol. 13, 384–392. doi:10.1093/neuonc/noq193
Qiu, X., Xie, S., Ye, L., and Xu, R. A. (2019). UPLC-MS/MS Method for the Quantification of Ertugliflozin and Sitagliptin in Rat Plasma. Anal. Biochem. 567, 112–116. doi:10.1016/j.ab.2018.12.016
Tang, C., Niu, X., Shi, L., Zhu, H., Lin, G., and Xu, R. A. (2020). In Vivo Pharmacokinetic Drug-Drug Interaction Studies between Fedratinib and Antifungal Agents Based on a Newly Developed and Validated UPLC/MS-MS Method. Front. Pharmacol. 11, 626897. doi:10.3389/fphar.2020.626897
Thijssen, R., Ter Burg, J., Van Bochove, G. G., De Rooij, M. F., Kuil, A., Jansen, M. H., et al. (2016). The pan Phosphoinositide 3-kinase/mammalian Target of Rapamycin Inhibitor SAR245409 (voxtalisib/XL765) Blocks Survival, Adhesion and Proliferation of Primary Chronic Lymphocytic Leukemia Cells. Leukemia 30, 337–345. doi:10.1038/leu.2015.241
Xu, R. A., Lin, Q., Qiu, X., Chen, J., Shao, Y., Hu, G., et al. (2019). UPLC-MS/MS Method for the Simultaneous Determination of Imatinib, Voriconazole and Their Metabolites Concentrations in Rat Plasma. J. Pharm. Biomed. Anal. 166, 6–12. doi:10.1016/j.jpba.2018.12.036
Zhang, L., Wang, Z., Khishignyam, T., Chen, T., Zhou, C., Zhang, Z., et al. (2018). In Vitro anti-leukemia Activity of Dual PI3K/mTOR Inhibitor Voxtalisib on HL60 and K562 Cells, as Well as Their Multidrug Resistance Counterparts HL60/ADR and K562/A02 Cells. Biomed. Pharmacother. 103, 1069–1078. doi:10.1016/j.biopha.2018.04.089
Keywords: voxtalisib, quantitative methodology, pharmacokinetic analysis, UPLC-MS/MS technology, rat plasma
Citation: Li Q, Liu Y-n, Wang J, Hu Y, Hu J, Xu R-a, Shao L and Chen L (2022) UPLC-MS/MS Technology for the Quantitative Methodology and Pharmacokinetic Analysis of Voxtalisib in Rat Plasma. Front. Pharmacol. 13:914733. doi: 10.3389/fphar.2022.914733
Received: 07 April 2022; Accepted: 30 May 2022;
Published: 14 June 2022.
Edited by:
Grover Paul Miller, University of Arkansas for Medical Sciences, United StatesReviewed by:
Dustyn Barnette, National Center for Toxicological Research (FDA), United StatesCopyright © 2022 Li, Liu, Wang, Hu, Hu, Xu, Shao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liu Shao, MTA0MTg2MzE5OEBxcS5jb20=; Lianguo Chen, bGlhbmd1b2NoZW5AMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.