
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 05 August 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.913174
Introduction: Psoriasis is a chronic inflammatory skin disorder characterized by keratinocyte hyperproliferation and differentiation with increased immune cell infiltration. The anti-psoriatic effect of lavender oil has been reported. However, its phytoconstituents, linalool (L) and linalyl acetate (LA), showed a distinctive affinity with psoriasis targets.
Objectives: This investigation was aimed to determine the combined effect of L and LA in ameliorating psoriasis-like skin inflammation and its safety in long-term topical uses.
Methods: The combined effect of L and LA was compared with their individual effects. The anti-psoriatic activity was performed using imiquimod (IMQ)-induced psoriasis in BALB/c mice and evaluated to reduce PASI and CosCam scores and Th-1 and Th-17 cell-specific cytokine levels. The acute and repeated dose dermal toxicities were investigated as per the OECD guidelines.
Results: L and LA combination (LLA) in the 1:1 w/w ratio at 2% concentration showed a synergistic effect. The combination showed 76.31% and 71.29% recovery in PASI and CosCam Scores; however, L2% and LA2% showed 64.28% and 47.61% recovery in PASI and 64.75 and 56.76% recovery in CosCam scores, respectively. It showed >90% and >100% recovery in Th-17 and Th-1 cell-specific cytokines, respectively, and restored epidermal hyperplasia and parakeratosis toward normal compared with psoriatic mice. A marked reduction in NF-κB, cck6, and the IL-17 expression was also observed in the LLA-treated group. This combination was safe in a therapeutically effective dose for 28 days as no significant changes were observed in organ and body weights, liver and kidney parameters, and differential leukocyte counts.
Conclusion: This study proves the synergy between L and LA in a 1:1 w/w ratio at 2% in the treatment of psoriasis-like skin inflammation and provides strong scientific evidence for its safe topical use.
Psoriasis is an immune-mediated disorder of the skin that lasts for several years. It has been characterized by hyper keratinocyte proliferation, leukocyte infiltration, and hyper cytokine expression (Di Meglio et al., 2014; Sharma et al., 2020). As it is an auto-immune disease, it is considered difficult to cure (Roy et al., 2019). However, getting rid of the psoriasis-associated symptoms is a first line strategy as far as the treatment of psoriasis is concerned (Who, 2016).
Lavender oil (LO) is an aromatic oil, i.e., extracted from Lavandula angustifolia Mill (Lamiaceae). It has immense utility as an aromatherapy massage oil, due to its anti-inflammatory and wound healing properties and providing relief in other skin conditions such as psoriasis, dermatitis, and eczema. Linalool and linalyl acetate are the major volatile components of the essential oils of several aromatic species including lavender. These compounds have been reported to possess various pharmacological properties. The anti-inflammatory effect of linalool is reported against ovalbumin-induced pulmonary inflammation (Kim et al., 2019), LPS-induced inflammation in BV2 microglia cells by activating Nrf2 (Li et al., 2015), cigarette smoke-induced lung inflammation by inhibiting NF-κB activation (Ma et al., 2015), and lipopolysaccharide-induced lung inflammation (Huo et al., 2012). Recently, linalyl acetate has been reported to recover the cell damage and cardiovascular changes caused by an acute nicotine-induced cardiovascular disruption in adolescent rats (Kim et al., 2017). However, a report published in 2002 by Peana et al. (2002) exhibits the anti-inflammatory effect of linalool and linalyl acetate against carrageenan-induced paw edema in mice. Additionally, linalool has shown a good psychopharmacological effect in mice, revealing its marked dose-dependent sedative effects on the central nervous system as well (Buchbauer et al., 1991; Jirovetz et al., 1991), including protection against pentylenetetrazol, picrotoxin and transcorneal electroshock-induced convulsions, hypnotic and hypothermic properties (Elisabetsky et al., 1995; Elisabetsky et al., 1999). Another study exhibited that it modulates the expression of glutamate activation in vitro (competitive antagonism of L-[3H] glutamate binding) and in vivo (delayed subcutaneous N-methyl D-aspartate-induced convulsions and blockade of intracerebroventricular quinolinic acid-induced convulsions) (Brum et al., 2001; Silva Brum et al., 2001). Anesthetic activity related to its effects on the nicotinic receptor-ion channel (Ghelardini et al., 2000), spasmolytic effect (Lis-Balchin and Hart, 1999), and antimicrobial activity against several bacteria and fungi (Carson and Riley, 1995; Pattnaik et al., 1997) are the most vital outcomes related to linalool’s pharmacological effects. Moreover, linalool, as well as other terpenes and terpenoids, could enhance the permeability of a number of drugs through skin or mucus membranes (Kunta et al., 1997; Ceschel et al., 1998; Kommuru et al., 1998). For the first time, we reported the anti-psoriatic potential of lavender oil (LO) and its major phytoconstituents, i.e., linalool (L) and linalyl acetate (LA) against imiquimod-induced psoriasis. It was interesting to observe that the linalool and linalyl acetate exhibited anti-psoriatic action by different mode of actions. Linalool showed more than 50% recovery in PASI scores as well as in the levels of Th-17 cell cytokines (IL-17 and IL-22); however, linalyl acetate showed good recovery (more than 90%) in the levels of Th-1 cytokines (TNF-α and IL-1β), specifically at 2% topical dose (Rai et al., 2020). These findings suggested us to investigate the combined effect of linalool and linalyl acetate. Therefore, in the present investigation, different combinations of L and LA were compared for their anti-psoriatic effect in different ratios. During the chemical analysis (GC and GC-MS) of lavender oil, we observed that linalool and linalyl acetate are present in 3:4 ratio approximately (14.2% and 20.0% respectively). Therefore, the anti-psoriatic activity of linalool and linalyl acetate combination was tested in the ratio of 1:4, 2:3, 1:1, 3:2, and 4:1.
As psoriasis expresses both (Th-17 and Th-1) types of cytokines, the combined effect of L and LA, whether additive or synergistic, could be better utilized for the comprehensive treatment of psoriasis. Therefore, we investigated the combined effect of L and LA on the comprehensive treatment of psoriasis and their toxicity studies to ensure its clinical pertinence for human use and to provide a safe and effective alternative to the current therapy.
Materials used in this study were purchased in India. Cyclophosphamide, linalool, linalyl acetate, and albumin 5% solution were from Sigma Aldrich; Imiquimod (IMQ) cream (5%) was the product of Glenmark Pharmaceuticals Ltd.; Polyethylene glycol 200 (PEG200), alcohol, hematoxylin, eosin, DPX mountant, and xylene were from Thomas Baker; Paraffin wax (60–62°C) was from Merck; ELISA Kits (TNF-α, IL-1β, and IL-6) were from Invitrogen Bio-services; IL-17 A and IL-22 were from Koma Biotech; NF-κB, IL-17, and cck6 specific primary and secondary antibodies were from Santa Cruz, and hydrogen peroxide (H2O2; 30%) and DAB chromogen were from TCI Chemicals. The solvents and chemicals used in the study were of analytical grade. We used Milli-Q water throughout the study.
The product specification and description of the linalool (Catalog number W263516) and linalyl acetate (Catalog number W263613) are given in Supplementary Material S1, Supplementary Figure S1; Buchbauer et al. (1991). The combination of the phyto-molecules used in this investigation is endotoxin-free.
Either sex of BALB/c mice (25–30 g) and Wistar rats (220–240 g) were obtained from the animal house facility “Jeevanika” of the institute. Animals were housed and acclimatized under controlled laboratory conditions (25°C ± 3°C room temperature and 60% humidity along with 12 h light and 12 h dark cycle) with free access to food and water ad libitum before starting the actual experiment. Experimental protocols for anti-inflammatory activity (AH-2012-05), acute dermal toxicity study (AH-2012-01), and repeated dose dermal toxicity study (AH-2012-01) were duly approved by the Institutional Animal Ethics Committee (400/01/AB/CPCSEA), Government of India. Animal experiments were conducted following the principles for laboratory animal use and care found in European Community guidelines (EEC Directive of 1986; 86/609/EEC). The safety assessments were commenced in a single trial based on the Organization for Economic Cooperation and Development (OECD) guidelines 404 and 410.
Psoriasis-like skin lesions in BALB/c female mice were induced by topical administration of IMQ. Induction of the disease and dosing was carried out as per the procedure described in our previous study (Rai et al., 2020). Test control was taken as a 2% w/v solution of linalool and linalyl acetate combination in different ratios (4:1, 3:2, 1:1, 2:3, and 1:4 w/w) in PEG200. About 100 µL of the test samples were applied daily to the mice’s skin. Psoriasis area and severity index (PASI) scores and CosCam scores were measured on the 0th, 2nd, 4th, 6th, and 8th days of the study period (Liu et al., 2019). Dorsal skin tissues were harvested for biochemical, immunohistochemical, and histological investigations on the final day.
The collected skin tissues were homogenized in phosphate buffer (10% homogenate). Homogenates were centrifuged (Sigma Laborzentrifugen 3K30) at 10,000 RPM/4°C for 15 min. Supernatants were taken and subjected to antigen-antibody reactions. Quantification of total protein (pg/mL), i.e., pro-inflammatory cytokines (IL-1β and TNF-α) and Th-17 cell cytokines (IL-17 A and IL-22), was performed as per the user’s manual of mouse Enzyme-Linked Immune Sorbent Assay (ELISA) kits (Mishra et al., 2016b).
Immunohistochemistry (IHC) was performed to observe the expression of NF-κB, IL-17, and cck6 in the skin tissue sections as per the procedure reported elsewhere (Somagoni et al., 2015). Briefly, collected skin tissues were fixed in formalin and embedded in paraffin wax. About 5 µm thick sections were cut using a microtome; slides were prepared and hydrated using xylene, followed by different strengths of alcohol. Furthermore, tissues were treated with 3% H2O2 and 5% albumin and incubated at 4°C for 24 h with the primary antibodies against the corresponding markers. Horseradish peroxidase-conjugated secondary antibody was used to find the expression of the primary antibody. Tissues were treated with DAB chromogen and counterstained using hematoxylin. This process was carried out to produce brown staining, indicating activated markers’ presence. Finally, the specimens were observed under Olympus BX40 light microscope, i.e., equipped with a computer-controlled digital camera (DP71, Olympus Center Valley, PA). The intensity of the brown color produced was checked by the ImageJ.exe app. The statistical differences were calculated based on the relative intensity scores of each image.
Skin tissue samples for histopathological analysis were kept in 10% formalin buffer at room temperature. Formalin buffered skin tissues were embedded in paraffin wax, cut into 7 µm sections using a microtome, stained with hematoxylin and eosin (H and E) dye, and observed at 10X magnification for histological changes using Leica DM 750 microscope. Histopathological changes, i.e., epidermal and dermal hyperplasia, para-keratosis, hyper-keratosis, dermal edema, vesicular formation, and granulosis, were evaluated and compared between the groups (Shah et al., 2012; Raza et al., 2013).
The anti-psoriatic effect of LLA was investigated by percentage reduction in the parameters (Yadav et al., 2008) like PASI scores, CosCam scores, and the levels of Th-1 and Th-17 cell expressing cytokines compared to DC group using Eq. (1).
*Sample that showed >50% reduction in the parameters selected previously were considered active (Carlin et al., 2004).
This investigation was carried out using OECD Test guideline no. 404 (adopted on 9 October 2017) (Oecd, 2015) as per the procedure reported by Wang et al. (2017) with modification. Healthy young adult BALB/c mice (25–30 g; either sex) and Wistar Rats (220–240 g; either sex) were used. Non-pregnant females were used in these experiments. Issued animals were acclimatized to the experimental environment for 7 days before the commencement of the investigation. The temperature and humidity in the experimental animal room were maintained at 22°C ± 3°C and 55% ± 5% RH, respectively. The artificial lighting of 12 h light and 12 h dark was maintained. Animals were provided ad libitum access to a commercial diet and drinking water. Animals (n = 6) were randomly selected and marked to provide individual identification. One day before the administration of the test sample, fur was removed from the dorsal/flank area of the animals (i.e., at least 10% of the total body surface area) by closely clipping with a curved scissor. Anesthesia was used to aid in handling animals and minimize animal stress if and when required. Utmost care was taken to avoid abrasion of the skin to avoid the alteration of the skin permeability. The clipped skin area was divided into two test sites of one square inch each. Normal saline was chosen as vehicle control. Test samples used for the investigation were LLA (1:1 w/w) at 2%, 10%, and 20% solutions in PEG 200. About 50 µL of these samples were applied once on one test site of the animal against the vehicle (50 µL). The sites were macroscopically examined on the 1st, 7th, and 14th days for skin irritation in terms of erythema and edema. Animals were observed for any casualty also. Skin reactions were graded separately, each time on a 0–4 grading scale, and the test materials were categorized based on primary irritation index values as reported elsewhere (Yadav et al., 2014; Mishra et al., 2016a). High-definition pictures of the animals were taken on the 1st, 7th, and 14th day for macroscopic examination of the skin surfaces. The body weight of the animals was taken on the last day of the study period. Organ weight (the heart, lung, liver, kidney, and spleen) was measured only in the case of mice. If and when a decrease in organ weight was observed in mice, rats could have also been sacrificed for organ weight measurements.
A repeated dose dermal toxicity study was carried out as per the OECD guideline 410 for testing of chemicals (adopted on 12 May 1981) (Oecd, 2018) as per the reported procedure by Djerrou et al. (2013) with modification. Healthy young adult Wistar rats (220–240 g; either sex) were employed in this study. Non-pregnant females were used in these experiments. Issued animals were acclimatized to the experimental environment for 7 days before the commencement of the investigation. Animals (n = 6) were randomly selected and marked to provide individual identification. On day before the administration of the test sample, the fur of the animals was removed from the dorsal/flank area (i.e., at least 10% of the total body surface area) by clipping with a curved scissor. Clipping was repeated, if needed, every week. Utmost care was taken to avoid the abrasion of the skin that led to the altered skin permeability. The clipped skin area was divided into two test sites of one square inch each. Normal saline was chosen as vehicle control. Test samples used for the investigation were LLA (1:1 w/w) at 0.2%, 2%, and 4% solutions in PEG 200. About 50 µL of the test samples and vehicle were applied once daily on one of the test sites of the animals against the vehicle for 28 days. The pictures of the animals were taken on the 1st, 7th, 14th, 21st, and 28th days for macroscopic examination of the skin surfaces. The body weight of the animals was taken on the 1st, 7th, 14th, 21st, and 28th days of the study period to monitor any significant variation in the weight. The organ (Heart, Lung, Liver, Kidney, and Spleen) weight of the animals was measured on the last day of the investigation, and the blood sample was collected from the retro-orbital route for different biochemical and hematological evaluations (Obara et al., 1997).
Varian CP-3800 Gas Chromatography analyzed linalool and linalyl acetate in a 1:1 w/w ratio. DB-5 capillary column of 30 m length, 0.25 mm internal diameter, and 0.25 µm film thickness was used for the analysis. The column oven temperature was programed with a rate of 3°C/min from 60°C to 240°C for 2 min hold time at 240°C. Hydrogen was used as a carrier gas with a constant flow rate of 1 mL/min in the split ratio of 1:40, and injector and detector (Flame Ionization Detector) temperatures were maintained at 280°C.
The intactness of linalool and linalyl acetate in combination was also confirmed by 13C and 1H 1D NMR by taking the spectra of linalool and linalyl acetate in a 1:1 w/w ratio. This investigation was performed to assess the possible interaction between linalool and linalyl acetate (López-Martínez et al., 2015). Spectra were produced using Bruker Advance spectrometer (Billerica, United States ) in CDCl3 at 301 K.
Data are shown as means ± SEM. Data for treatment groups were compared using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test using GraphPad Prism (R), Version 5.01 (GraphPad software. Inc. United States). #p < 0.05, ##p < 0.01, and ###p < 0.001 (NC vs. DC), *p < 0.05, **p < 0.01, and ***p < 0.001 (DC vs. treatments).
PASI and CosCam parameters were scored to portray the severity of psoriasis-like inflammation. The visual manifestations like erythema, scaling, and thickness increased significantly in the DC group on the 8th day of the study (Data from the 2nd, 4th, and 6th days was not provided as no significant change in the level of erythema, scaling, and thickness was observed on these days). LLA showed significant alleviation in all the PASI and CosCam Parameters (Figure 1). LLA 2% (3:2 w/w) and LLA 2% (1:1 w/w) attained PASI 70 and PASI 75, respectively (Table 1).
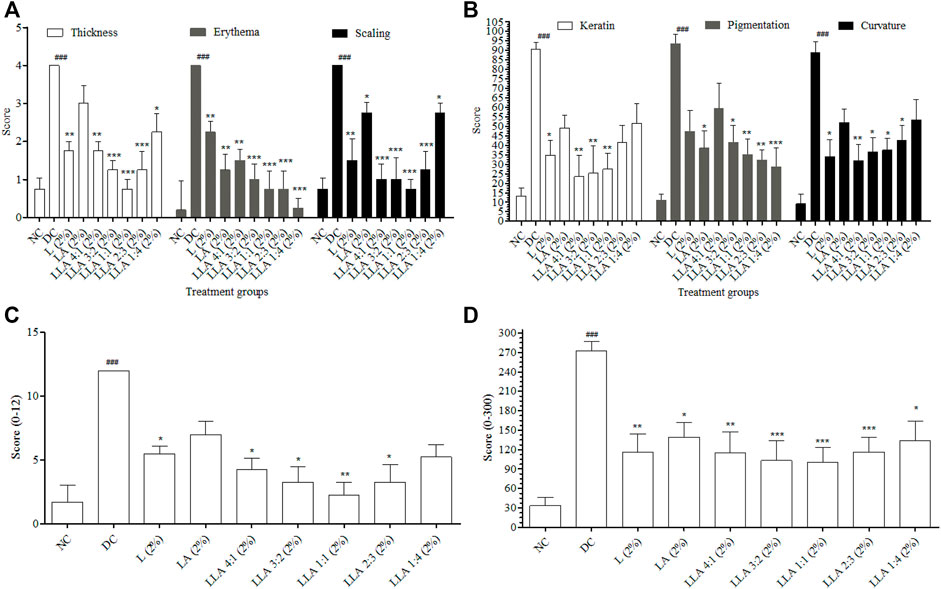
FIGURE 1. Results of PASI and CosCam scores of the mice treated with different ratios of LLA combination. Graph (A) depicts thickness, erythema, and scaling of PASI, (B) depicts keratin, pigmentation, and curvature of CosCam scores, (C) depicts cumulative PASI scores, and (D) depicts cumulative CosCam scores of the treatment groups. NC; normal control, DC; disease control, LLA (2%); linalool + linalyl acetate in 4:1, 3:2, 1:1, 2:3, and 1:4 ratios at 2% concentration. The data represent mean ± SEM, n = 6. ###p < 0.001 (NC vs. DC), *p < 0.05, **p < 0.01, and ***p < 0.001 (DC vs. treatments).
Figure 2 depicts different treatment groups with the skin condition of each group of mice, keratin analysis chart, pigmentation analysis chart, curvature analysis chart, radar graph, and bar graphs. As depicted by their respective analysis charts, a marked increase in keratin, pigmentation, and curvature was observed in psoriatic mice. Test control groups showed alleviation in these parameters. The highest reduction can be observed in the case of LLA 2% in 3:2, 1:1, and 2:3 ratios. As the size of the triangle of the radar graph directly reflects the severity of the skin inflammation, a smaller size reflects less intense inflammation. The smallest size of the triangle and bar was observed in the case of LLA 2% in 3:2, and 1:1 ratios. This shows the comprehensive utility of these combinations against psoriasis.
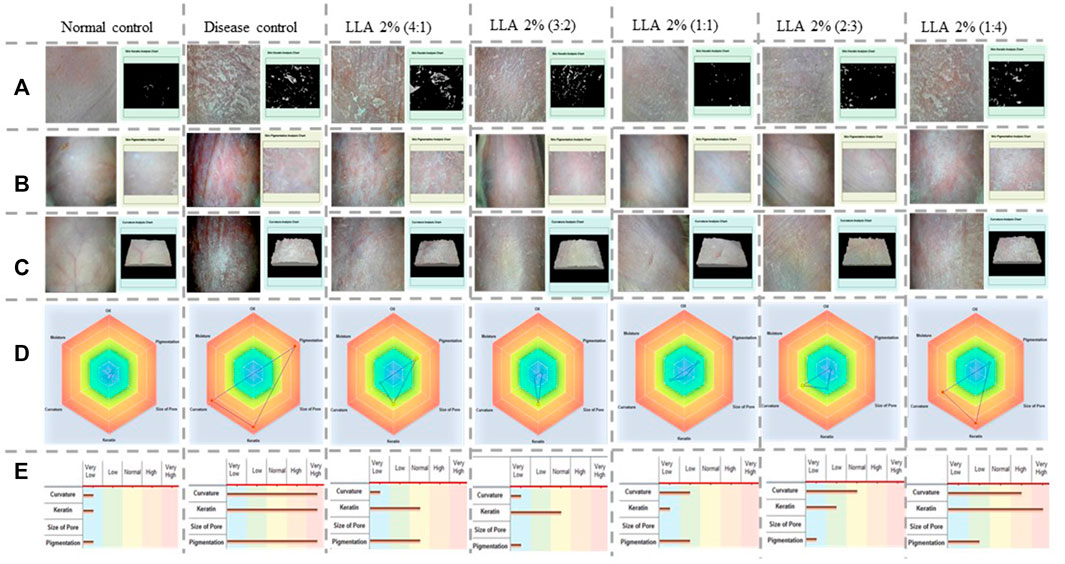
FIGURE 2. Pictorial representation of keratin, pigmentation, curvature analysis charts, radar, and bar graphs showing the severity of skin inflammation in different tested groups, Vertical columns present the different treatment groups, and horizontal rows denote (A) the keratin analysis chart, (B) the pigmentation analysis chart, (C) the curvature analysis chart, (D) the radar graph, and (E) bar graphs of the psoriatic and animals treated with linalool and linalyl acetate.
A significant increase in TNF-α (p < 0.05) and IL-1β (p < 0.05) levels was observed in the DC group. The levels of IL-17 (p < 0.001) and IL-22 (p < 0.01) also increased significantly in psoriatic mice. LLA (2%) ratios 4:1, 3:2, and 1:1 showed significant alleviation in the level of IL-17. However, ratios 4:1, 3:2, 1:1, and 2:3 showed considerable alleviation in the level of IL-22. LLA (2%) ratios 3:2, 1:1, 2:3, and 1:4 showed significant alleviation in the levels of TNF-α, and ratios 4:1, 3:2, 1:1, 2:3, and 1:4 showed significant alleviation in the levels of IL-1β. LLA 2% (1:1 w/w) showed a consistent and better recovery in all these parameters among all combinations (Figure 3).
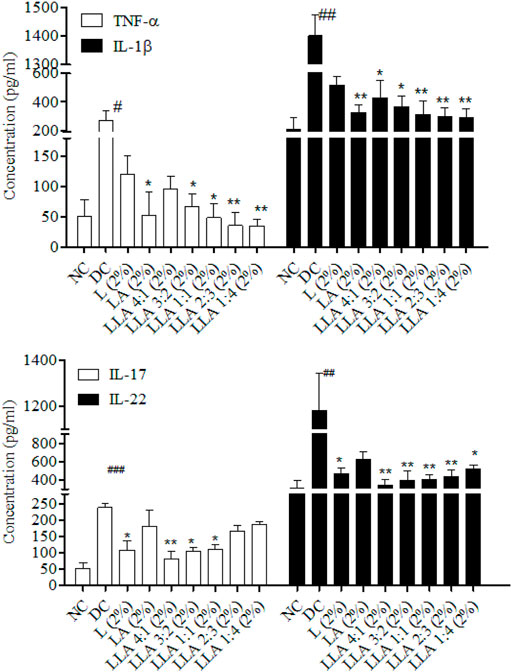
FIGURE 3. Levels of Th-17 and Th-1 cell-specific cytokines in skin tissues of linalool and linalyl acetate combination-treated mice. NC; normal control, DC; disease control, LLA (2%); linalool + linalyl acetate in 4:1, 3:2, 1:1, 2:3, and 1:4 ratios at 2% concentration. The data represent mean ± SEM, n = 6. #p < 0.05, ##p < 0.01 and ###p < 0.001 (NC vs. DC), *p < 0.05 and **p < 0.01 (DC vs. treatments).
Histological investigation depicts the significant presence of psoriasis-like histological changes in the psoriatic mice. These changes got normalized after the topical application of the test samples. The histological changes are summarized in Table 2; Figure 4. LLA 2.0% (1:1 w/w) showed an apparent reduction in keratinocyte proliferation, epidermal hyperplasia, and vacuole formation; hence, there were no erythema, flakiness, and skin thickness observed (very near to normal skin). An increase in the expressions of NF-κB, IL-17, and cck6 was observed in the DC group. LLA 2.0% (3:2 and 1:1 w/w) showed a marked reduction in the expression of NF-κB, IL-17, and cck6 (Figure 4). Quantification of IHC staining differentiated the individual group. As depicted in Figure 4B, the DC group shows significantly higher expression of the particular gene (p < 0.05). Except for LLA 2% (2:3), all the tested groups showed significant recovery in the expressions of cck6, and IL-17. LLA 2% (3:2) and LLA 2% (1:1) showed the best intensity recovery compared to the DC group.
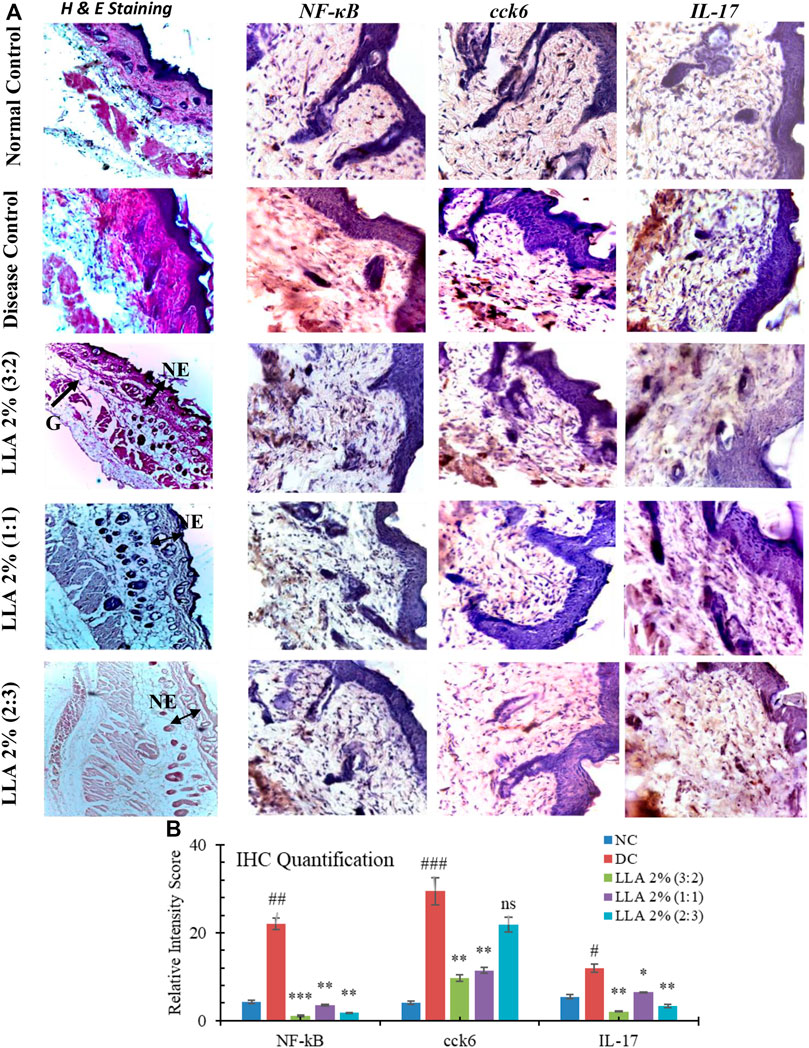
FIGURE 4. Histopathological features of the skin treated with linalool and linalyl acetate in combination. Immunohistochemical images of the mice skin treated with linalool + linalyl acetate combinations. (A) Histopathological features and immunohistochemical images of the mouse skin treated with linalool + linalyl acetate combinations. Sections were stained with H and E, and pictures were visualized at 10X magnification. (B) depicts the individual protein’s relative intensity scores of the IHC expressions. LLA (2%); linalool + linalyl acetate in 3:2, 1:1, and 2:3 ratios at 2% concentration. NE; normal epidermis, G; granulosis. The brown color represents NF-κB, IL-17, and cck6 expression.
Primary irritation indices of all the tested groups were found to be in the category of irritation barely perceptible. No significant change in the body weight of any tested animals, whether mice or rats, was observed. Animals in the gross pathological study showed no differences in the organs studied, including their absolute and relative weight (Tables 3, 4; Supplementary Figures S2, S3).
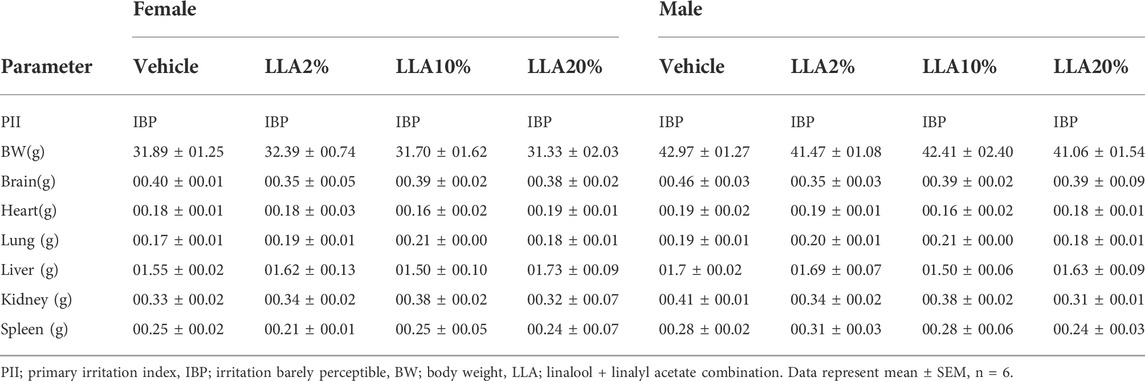
TABLE 3. PII, body, and organ weight of the mice topically treated with linalool + linalyl acetate combinations.
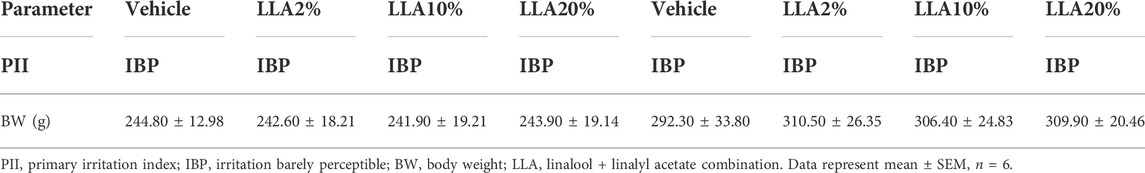
TABLE 4. PII and body weight of the rats topically treated with linalool + linalyl acetate combinations.
Animals in the gross pathological study showed no changes in any of the organs studied, including their absolute and relative weights (Table 5). No observational changes in skin, morbidity, and mortality were observed throughout the experimental period in all the groups of the animals, even on the highest dose level of LLA (4%; 1:1 w/w ratio).
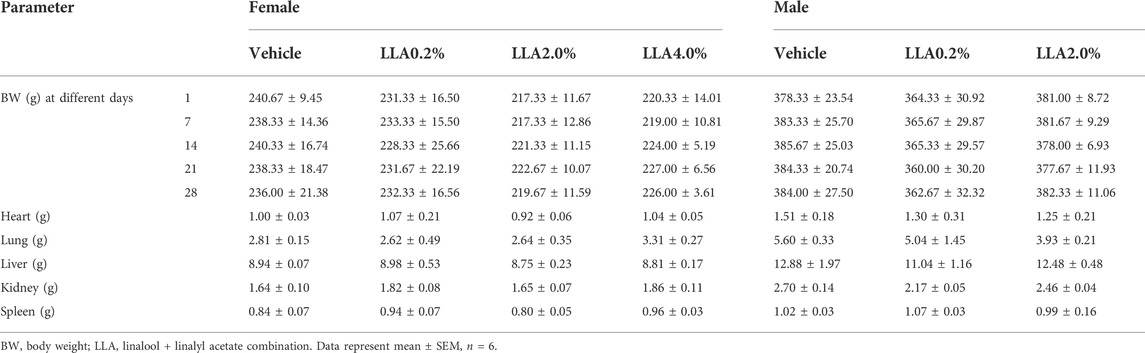
TABLE 5. Body and organ weight of the rats repeatedly treated with linalool + linalyl acetate combinations for 28 days, following topical administration.
The blood and serum analysis showed non-significant changes in all the studied parameters like hemoglobin level, RBC count, WBC count, differential leukocyte count, SGPT, SGOT, serum protein, triglycerides, cholesterol, and albumin, except serum creatinine and ALP (Table 6; Supplementary Figure S4).
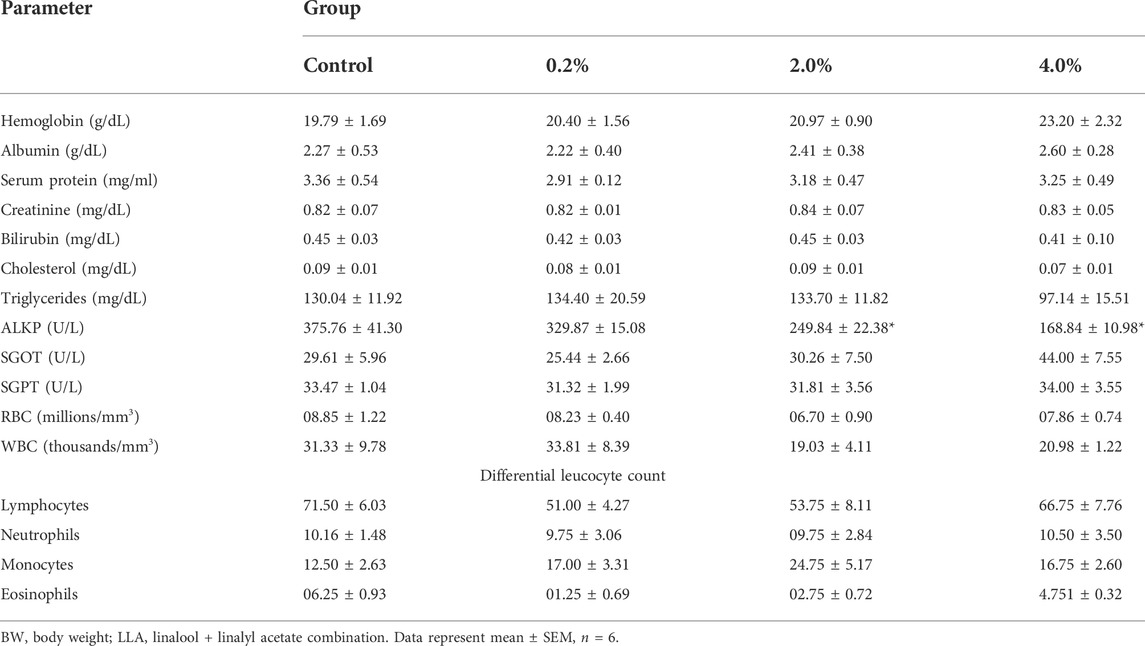
TABLE 6. Effect of LLA (1:1 w/w ratio) at 0.2%, 2%, and 4% PEG200 solutions on different biochemical and hematological parameters when applied topically in Wistar rats once-a-day for 28 days..
Linalool and linalyl acetate in 1:1 w/w ratio were analyzed by gas chromatography to confirm their intactness in combination. The purity of linalool and linalyl acetate was found to be 49.12% and 49.56%, respectively (Figure 5). Linalool and linalyl acetate peaks in 1D 1H NMR spectra are ascribed to the protons at R2CH2, C = C-CH3, R3OH, R2C = CHR, and RCH = CH2. The observed peaks in the 1D13C NMR spectra were found to be in the four central regions that were ascribed to the presence of different types of carbon, such as aliphatic, allylic, hydroxy, and vinylic groups (Figure 5).
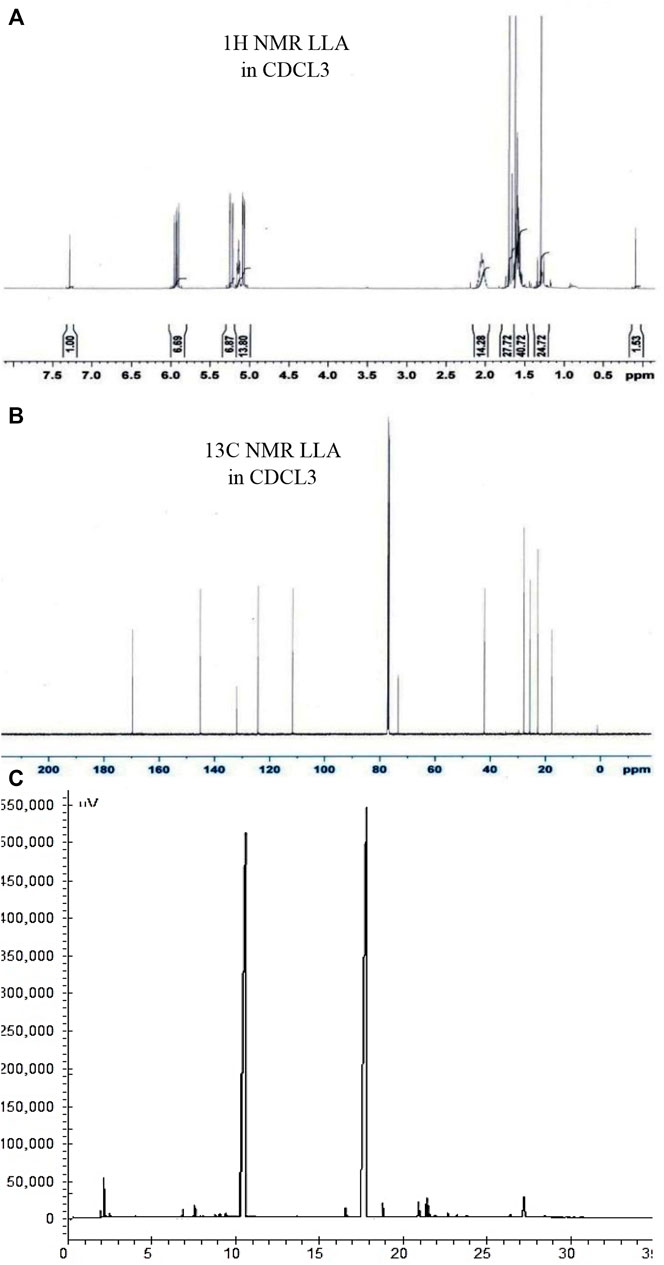
FIGURE 5. Characterizing the phyto-combination of linalool and linalyl acetate by NMR and GC analysis. Pictures (A), (B), and (C) depict 1H NMR, 13C NMR spectrum, and GC chromatogram of linalool + linalyl acetate combination (1:1 w/w), respectively.
In our previous study, we reported the effectiveness of lavender oil (LO) against psoriasis. Linalool, linalyl acetate, and lavandulol were observed as the main components of LO. The most active components against psoriasis were linalool and linalyl acetate. Linalool showed more than 50% recovery in PASI scores and primarily alleviated the level of Th-17 cell cytokines. Linalyl acetate showed the efficient recovery of Th-1 cytokines (94%). Unlike linalyl acetate, linalool showed better improvement in skin thickness as observed from the histological changes in the psoriatic skin (Rai et al., 2020). As these molecules worked independently on different targets to mitigate psoriasis-like conditions, the synergy between L and LA in treating IMQ-induced psoriasis was explored. Therefore, the synergistic or additive effect of L and LA against psoriasis and their safety in the long-term topical application were investigated thoroughly.
PASI and CosCam scores portray the development of the psoriasis-like symptoms. This investigation was performed to have an insight into the induction of the disease and the pattern of cure (Van Der Fits et al., 2009). PASI and CosCam scorings were done based on the phenotypic assessment criteria of the skin (Žurauskas et al., 2020). Reported literature suggests that more than 50% recovery in PASI scores confirms a considerable improvement in psoriasis (Carlin et al., 2004), and more than 75% recovery in PASI is a significant improvement in psoriatic conditions with immense utility (Abrouk et al., 2017). As per these criteria, only LLA 2% (1:1 w/w) could attain PASI 75, and the same was supported by CosCam analysis also. PASI 50 was achieved by all the ratios selected for the investigation. CosCam pictorial and radar graphs show the relevance of different proportions of LLA 2% in psoriasis (Figure 2).
Different types of cytokines (i.e., Th-17 specific and Th-1 specific) are released in IMQ-induced psoriasis-like conditions (Van Der Fits et al., 2009). Attainment of PASI 75 and a significant reduction in cytokine levels are always appreciated. A high percent recovery in the hyper-expressed cytokines was observed. As per our previous report, linalool reduces the level of Th-17 cells, and linalyl acetate reduces Th-1 cells specific cytokines efficiently (Li et al., 2018). However, in combination, these molecules have shown excellent recovery in both types of cytokines. As shown in Table 1, the combination of L and LA in a 1:1 ratio shows a higher reduction (denoted in terms of % recovery towards normalization) in the PASI score, CosCam score, levels of TNF-α, IL-1β, IL-17, and IL-22 when compared with the individual effect of L and LA. In such conditions, where the total dose of drugs in combination is not more than a dose of any particular drug, while the combined pharmacological effect is better than any individual drug used in the treatment, the result is said to be synergistic (Yadav et al., 2008; Chambers (2001)). As per the Huo et al. (2013) report, L and LA inhibit the NF-κB and MAPK activation and reduced the level of IL-6 and TNF-α (Huo et al., 2013). Results of this investigation were found to concur with the reduction in the level of these psoriasis-specific cytokines treated with terpenes and terpenoids (Li et al., 2018).
Thickened skin in psoriatic conditions occurred due to the keratinocyte’s hyper-proliferation and redness/erythema due to the induction of angiogenesis in response to the antigen. These changes led to the exacerbation of psoriasis, and when the condition worsens, the disease gets more severe (Goedkoop et al., 2004). The imbalanced innate and adaptive immune responses initiate psoriatic conditions where IL-1β, IL-6, IL-8, IL-12, IFN-γ, and TNF-α get expressed (Asadullah et al., 1999). In IMQ-induced psoriasis, TLR-7/8 gets activated and initiates Th-17 cells specific cytokines in response to the IL-23 overproduction by plasmacytoid dendritic cells. IL-23 binds with the CD4+ cells available in lymph nodes locally. Matured CD4+ cells are then converted to pathogenic Th-17 cells and produce IL-17 and IL-22 (Van Der Fits et al., 2009; Sharma et al., 2022). IL-17 and IL-22 expressions initiate keratinocyte hyperproliferation and down-regulation of genes associated with keratinocyte differentiation. According to Furue et al., 2020, keratinocytes produce an array of antimicrobial peptides and cytochemokines in response to the produced IL-17 A that further exacerbates the psoriasis like inflammatory condition. The release of IL-17 is directly linked to the worsening of the keratinocyte proliferation, that could be alleviated by reducing IL-17 level (Furue et al., 2020). Our results were in reasonable agreement with this finding, where alleviation in the proliferation of the keratinocytes was observed with the reduction in the level of Th-17 cells specific cytokines.
Literature reports epidermal and dermal hyperplasia, parakeratosis, hyperkeratosis, dermal edema, vesicular formation, and granulosis in the psoriatic skin (Morsy et al., 2010). The significant histological changes were the epidermal thickening due to hyperkeratosis, parakeratosis, dermal hyperplasia, granulosis, para-keratosis, intercellular edema, and vesicular formation in psoriatic mice (Tirumalae, 2013; Huang et al., 2019). Considerable reduction in these histological changes was observed in the case of LLA-treated groups, and the best result was observed in the case of a 1:1 w/w ratio of LLA 2%. Histological improvement confirms the anti-hyperproliferative effect of the tested combination. There is a possibility that linalool is impeding the process of proliferation as some of the investigations cite its anti-proliferative effect as well (Popadic et al., 2008).
Monocytes, macrophages, and plasmacytoid dendritic cells usually work as Antigen-Presenting Cells (APCs) and begin to express 7th or 8th type TLRs in response to IMQ or other stimuli. However, these APCs accept responses only from specific stimuli. APCs maturation initiates type 1 IFN activity owing to which the rapid influx of various immune cells takes place at the prone site. Van der Fits et al. report that, after pDC activation, further infiltration and influx of pDC takes place that facilitates many inflammatory responses. The initiation of IL23/Th-17 cascade after pDC infiltration and type I IFN activity is the most important hallmark of psoriasis-like conditions (Sharma et al., 2022). NF-κB is triggered in response to the TLR8 activation by IMQ. This activation leads to IL-23 and IL-6 releases. The binding of IL-23 with the receptors present on γδ T cells (cck6) triggers the differentiation of cck6 and hence, initiates the release of IL-17 and IL-22 (Sun et al., 2013). Based on this pathway, NF-κB, IL-17, and cck6 were checked for hyper-expression in skin tissue of different treatment groups (Kim et al., 2020). LLA 2% showed a marked alleviation in the expression of the selected markers in a 1:1 ratio. This may be due to the competitive binding of LLA with the TLR8 resulting in decreased phosphorylation of Ik-β in the cytosol (Somagoni et al., 2015; Li et al., 2018). L and LA are chemically terpenes and isolated from aromatic oils. Interestingly hispidulin, which is an aromatic oil component, significantly alleviates the imiquimod-induced psoriasis-like skin inflammation by inhibiting splenic Th1/Th17 cell population and keratinocyte activation. As per our results, the best possible mechanism that L and LA are following in combination could be the inhibition of Th1/Th17 cell population. Marked reduction in epidermal thickness, inflammation, and the expression of selected proteins (NF-κB, IL-17, and cck6) proves LLA’s immense utility in managing psoriasis. It supports the findings of Huo et al. (2013) that state the blocking of NF-kB activation is the linalool’s major mechanism of action.
As linalool is primarily alleviating Th-17 cell cytokines and reducing skin thickness, it is expected to interfere with the process of activation of NF-kB and binding of IL-17 subset cytokines such as IL-17 A and IL-17 F with their respective receptors present on keratinocytes (the major reason behind the severe keratinocyte proliferation). There are two possibilities that, linalool is modifying both immune responses as well as the process of keratinocyte proliferation. At the same time, linalyl acetate seems to interfere with the IL-12-driven induction of the inflammatory responses owing to which it is working efficiently on Th-1 cells specific cytokines majorly (Figure 6). Mitigating Th-1 and Th-17 cells cytokines specific pathways has been identified as the best approach against IMQ-induced psoriasis (Tokura et al., 2010; Benham et al., 2013; Varma et al., 2017; Moos et al., 2019; Li et al., 2020). Therefore, exploring a combination of L and LA for their synergy in treating IMQ-induced psoriasis was attempted. Here, finding a ratio that shows the best result in the inhibition of the Th1/Th17 cell population is of prime importance.
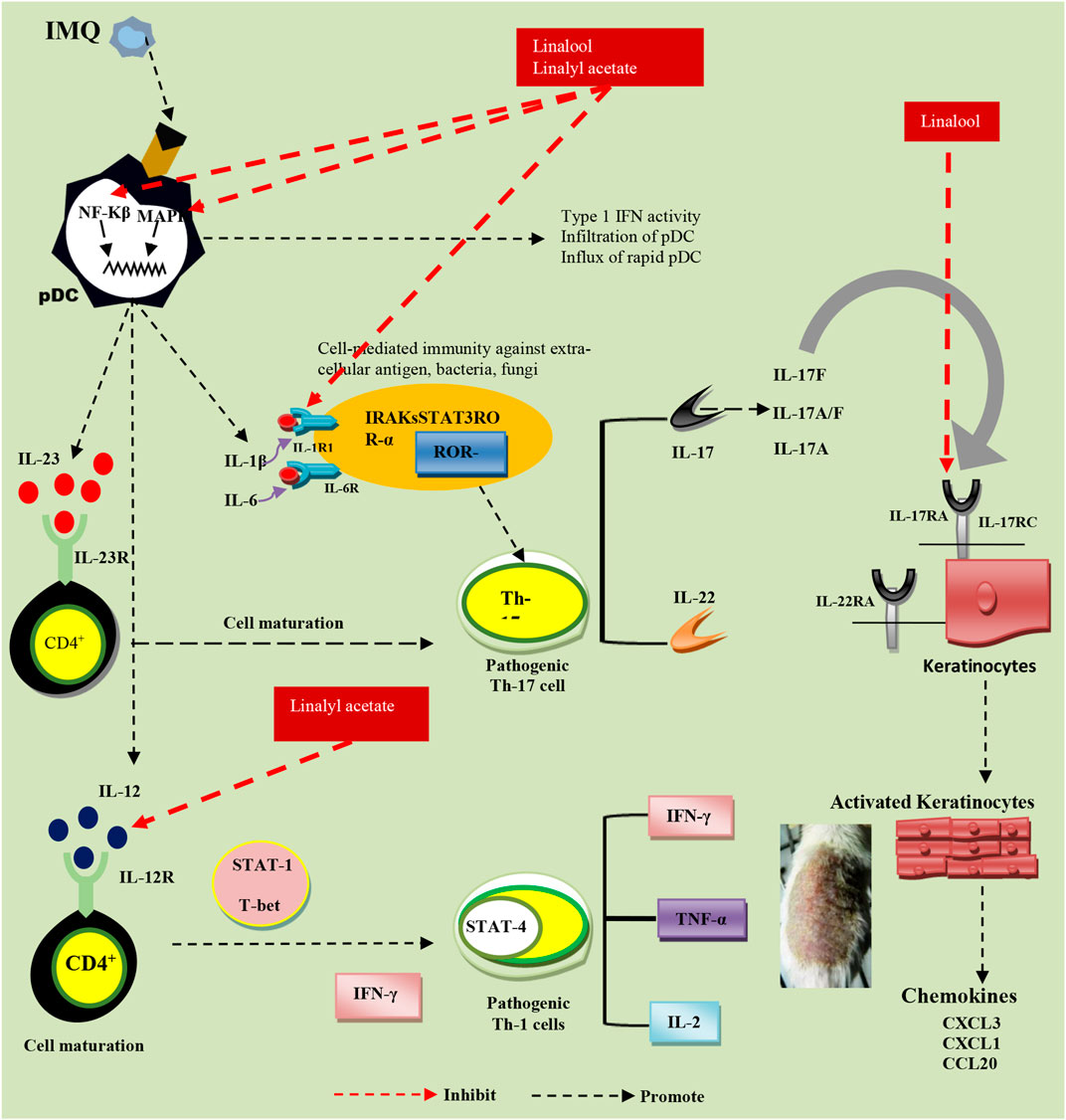
FIGURE 6. Probable pathways of linalool and linalyl acetate effectiveness against IMQ-induced psoriasis.
Skin irritation is barely perceptible at the therapeutic dose, ensuring its high safety. No change in the mice’s body and organ weight and the rat’s body weight ensure the tested combination’s safety if it is available in systemic circulation to any extent (Han et al., 2012).
The period of psoriasis treatment is usually long and requires repeated topical administration of the therapeutic dose for a long duration. In long-term use, the administered dose could have adverse effects. However, LLA 2% (1:1 ratio) did not show any sign of toxicity. No change in the absolute and relative weights of the animal’s body and organ weights and nominal observational changes in skin ensure a good safety profile of the tested combination. It has shown no morbidity and mortality in all the experimental animals, even at the highest dose level of LLA (4%; 1:1 w/w ratio). It ensures its safety in long-term use whether this drug combination is available in systemic circulation or not following topical administration. The systemic availability of this combination could also lead to a change in the blood and hematological parameters (Surekha et al., 2012). However, we did not observe any change in the blood parameters even on LLA 4% dose compared to the vehicle-treated group.
The different combinations of linalool and linalyl acetate tested in this investigation were also checked for their compatibility in a mixture. GC chromatographic analysis confirms the molecules’ intactness when used in a 1:1 w/w ratio. NMR investigation showed the presence of all types of characteristic hydrogens and carbons peaks of linalool and linalyl acetate. This helped us to conclude that these molecules are mutually stable in combination.
The study investigated the combined effect of L and LA for the comprehensive management of psoriasis-like inflammation and its safety in long-term topical use. LLA 2% 1:1 w/w ratio was able to attain PASI 75 compared to the other tested ratios (achieved PASI 50) of L and LA. The combination shows synergistic activity because L and LA in combination (1:1 ratio) consistently exhibit higher anti-psoriatic activity (Table 1, denoted by percent recovery) when compared with either L or LA. This combination showed marked restoration in the histological and immune-histological changes towards normal compared to the psoriatic group. The mixture was found to be safe in single dosing and repeated dosing schedules. It proves the safety profile of the tested combination even in long-term use. Our previous work (Rai et al., 2020) was the scientific validation of the traditional knowledge that lavender oil and its constituents can be used to mitigate the psoriasis-like skin inflammation but the present work provides the novel fact that the linalool and linalyl acetate in combination exhibit the synergistic effect against psoriasis. This fact helped us to file the Indian Patent, which is under evaluation with the Indian Patent Office. Finally, we can conclude that the present study demonstrates the synergistic effect of LLA 2% 1:1 w/w ratio in attaining PASI 75 and provides strong scientific safety evidence favoring its topical use in humans.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the Institutional Animal Ethics Committee, CSIR-CIMAP, Lucknow.
Conceptualization and conception of the idea, analysis of the collected data, and manuscript writing of the original draft were performed by NY. Conceiving the idea, experimentation, compilation of data, and manuscript writing were performed by VR. Experimentation and analysis of data on the toxicity studies were performed by DC, and instrumental analysis and data interpretation were carried out by CC. The final draft of the manuscript was checked and approved by all the authors before submission.
All sources of funding received for the research being submitted.
The authors are thankful to the Council of Scientific and Industrial Research, New Delhi, India, for awarding the CSIR-JRF GATE (Grant No. 31/GATE/34(01)/2011-EMR-1) to VR and CSIR-Central Institute of Medicinal and Aromatic Plants, Lucknow, India, for providing necessary facilities under the Network Project ChemBio (BSC 203) and AROMA MISSION (HCP 0007).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.913174/full#supplementary-material
cck6, chemokine receptor 6; CD4+, cluster of differentiation 4; DAB, 3,3-diaminobenzidine; DC, disease control; ELISA, enzyme-linked immunosorbent assay; GC, gas chromatography; i.p., intra-peritoneal; IFN-γ, interferon-gamma; IL, interleukin; IMQ, imiquimod; L, linalool; LA, linalyl acetate; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; NC, normal control; NF-κB, nuclear factor kappa-light-chain-enhancer of an activated B cell; OECD, Organization for Economic Co-operation and Development; PASI, psoriasis area and severity index; pDCs, paracitoid dendritic cells; PEG, polyethylene glycol; PII, primary irritation index; Th-1, 1st type helper T cell; Th-17, 17th type helper T cell; Th-22, 22nd type helper T cell; TLR, toll-like receptor; TNF, tumor necrosis factor.
Abrouk, M., Nakamura, M., Zhu, T. H., Farahnik, B., Koo, J., and Bhutani, T. (2017). The impact of PASI 75 and PASI 90 on quality of life in moderate to severe psoriasis patients. J. Dermatol. Treat. 28, 488–491. doi:10.1080/09546634.2016.1278198
Asadullah, K., Döcke, W. D., Volk, H. D., and Sterry, W. (1999). The pathophysiological role of cytokines in psoriasis. Drugs Today (Barc) 35, 913–924.
Benham, H., Norris, P., Goodall, J., Wechalekar, M. D., Fitzgerald, O., Szentpetery, A., et al. (2013). Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res. Ther. 15, R136. doi:10.1186/ar4317
Brum, L. F., Elisabetsky, E., and Souza, D. (2001). Effects of linalool on [(3)H]MK801 and [(3)H] muscimol binding in mouse cortical membranes. Phytother. Res. 15, 422–425. doi:10.1002/ptr.973
Buchbauer, G., Jirovetz, L., Jäger, W., Dietrich, H., and Plank, C. (1991). Aromatherapy: Evidence for sedative effects of the essential oil of lavender after inhalation. Z Naturforsch C J. Biosci. 46, 1067–1072. doi:10.1515/znc-1991-11-1223
Carlin, C. S., Feldman, S. R., Krueger, J. G., Menter, A., and Krueger, G. G. (2004). A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J. Am. Acad. Dermatol 50, 859–866. doi:10.1016/j.jaad.2003.09.014
Carson, C. F., and Riley, T. V. (1995). Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacteriol. 78, 264–269. doi:10.1111/j.1365-2672.1995.tb05025.x
Ceschel, G. C., Maffei, P., Moretti, L., Peana, A., and Demontis, S. (1998). In vitro permeation through porcine buccal mucosa of Salvia sclarea L. essential oil from topical formulations. S.T.P. Pharma Sci. 8, 103–106.
Chambers, H. F. (2001). “Antimicribial agents: General Considerations,” in Goodman & Gilmans The Pharmacological Basis of Therapeutics. Editors J. G. Hardman, and L. E. Limbird. New York: McGraw-Hill, 1162–1163.
Di Meglio, P., Villanova, F., and Nestle, F. O. (2014). Psoriasis. Cold Spring Harb. Perspect. Med. 4, a015354. doi:10.1101/cshperspect.a015354
Djerrou, Z., Djaalab, H., Riachi, F., Serakta, M., Chettoum, A., Maameri, Z., et al. (2013). Irritantcy potential and sub acute dermal toxicity study of Pistacia lentiscus fatty oil as a topical traditional remedy. Afr. J. Tradit. Complement. Altern. Med. 10, 480–489. doi:10.4314/ajtcam.v10i3.15
Elisabetsky, E., Brum, L. F., and Souza, D. O. (1999). Anticonvulsant properties of linalool in glutamate-related seizure models. Phytomedicine 6, 107–113. doi:10.1016/s0944-7113(99)80044-0
Elisabetsky, E., Souza, G. C. D., Santos, M. a. D., Siquieira, I. R., Amador, T. A., and Nunes, D. S. J. F. (1995). Sedative Prop. linalool 66, 407–414.
Furue, M., Furue, K., Tsuji, G., and Nakahara, T. (2020). Interleukin-17A and keratinocytes in psoriasis. Int. J. Mol. Sci. 21, 1275. doi:10.3390/ijms21041275
Ghelardini, C., Galeotti, N., Salvatore, G., and Mazzanti, G. (2000). Local anaesthetic activity of the essential oil of Lavandula angustifolia. Planta Med. 65, 700–703. doi:10.1055/s-1999-14045
Goedkoop, A. Y., Kraan, M. C., Picavet, D. I., De Rie, M. A., Teunissen, M. B., Bos, J. D., et al. (2004). Deactivation of endothelium and reduction in angiogenesis in psoriatic skin and synovium by low dose infliximab therapy in combination with stable methotrexate therapy: A prospective single-centre study. Arthritis Res. Ther. 6, R326–R334. doi:10.1186/ar1182
Han, S. M., Lee, G. G., and Park, K. K. (2012). Acute dermal toxicity study of bee venom (Apis mellifera L.) in rats. Toxicol. Res. 28, 99–102. doi:10.5487/TR.2012.28.2.099
Huang, Z. Z., Xu, Y., Xu, M., Shi, Z. R., Mai, S. Z., Guo, Z. X., et al. (2019). Artesunate alleviates imiquimod-induced psoriasis-like dermatitis in BALB/c mice. Int. Immunopharmacol. 75, 105817. doi:10.1016/j.intimp.2019.105817
Huo, M., Cui, X., Xue, J., Chi, G., Gao, R., Deng, X., et al. (2012). Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J. Surg. Res. 180, e47–54. doi:10.1016/j.jss.2012.10.050
Huo, M., Cui, X., Xue, J., Chi, G., Gao, R., Deng, X., et al. (2013). Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J. Surg. Res. 180, e47–54. doi:10.1016/j.jss.2012.10.050
Jirovetz, L., Jäger, W., Buchbauer, G., Nikiforov, A., and Raverdino, V. (1991). Investigations of animal blood samples after fragrance drug inhalation by gas chromatography/mass spectrometry with chemical ionization and selected ion monitoring. Biol. Mass Spectrom. 20, 801–803. doi:10.1002/bms.1200201210
Kim, J. R., Kang, P., Lee, H. S., Kim, K. Y., and Seol, G. H. (2017). Cardiovascular effects of linalyl acetate in acute nicotine exposure. Environ. Health Prev. Med. 22, 42. doi:10.1186/s12199-017-0651-6
Kim, M. G., Kim, S. M., Min, J. H., Kwon, O. K., Park, M. H., Park, J. W., et al. (2019). Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int. Immunopharmacol. 74, 105706. doi:10.1016/j.intimp.2019.105706
Kim, N., Lee, S., Kang, J., Choi, Y. A., Lee, B., Kwon, T. K., et al. (2020). Hispidulin alleviates imiquimod-induced psoriasis-like skin inflammation by inhibiting splenic Th1/Th17 cell population and keratinocyte activation. Int. Immunopharmacol. 87, 106767. doi:10.1016/j.intimp.2020.106767
Kommuru, T. R., Khan, M. A., and Reddy, I. K. (1998). Racemate and enantiomers of ketoprofen: Phase diagram, thermodynamic studies, skin permeability, and use of chiral permeation enhancers. J. Pharm. Sci. 87, 833–840. doi:10.1021/js9704644
Kunta, J. R., Goskonda, V. R., Brotherton, H. O., Khan, M. A., and Reddy, I. K. (1997). Effect of menthol and related terpenes on the percutaneous absorption of propranolol across excised hairless mouse skin. J. Pharm. Sci. 86, 1369–1373. doi:10.1021/js970161+
Li, B., Huang, L., Lv, P., Li, X., Liu, G., Chen, Y., et al. (2020). The role of Th17 cells in psoriasis. Immunol. Res. 68, 296–309. doi:10.1007/s12026-020-09149-1
Li, Y., Lv, O., Zhou, F., Li, Q., Wu, Z., and Zheng, Y. (2015). Linalool inhibits LPS-induced inflammation in BV2 microglia cells by activating Nrf2. Neurochem. Res. 40, 1520–1525. doi:10.1007/s11064-015-1629-7
Li, Y. L., Du, Z. Y., Li, P. H., Yan, L., Zhou, W., Tang, Y. D., et al. (2018). Aromatic-turmerone ameliorates imiquimod-induced psoriasis-like inflammation of BALB/c mice. Int. Immunopharmacol. 64, 319–325. doi:10.1016/j.intimp.2018.09.015
Lis-Balchin, M., and Hart, S. (1999). Studies on the mode of action of the essential oil of lavender (Lavandula angustifolia P. Miller). Phytother. Res. 13, 540–542. doi:10.1002/(sici)1099-1573(199909)13:6<540::aid-ptr523>3.0.co;2-i
Liu, C., Chen, Y., Lu, C., Chen, H., Deng, J., Yan, Y., et al. (2019). Betulinic acid suppresses Th17 response and ameliorates psoriasis-like murine skin inflammation. Int. Immunopharmacol. 73, 343–352. doi:10.1016/j.intimp.2019.05.030
López-Martínez, L. M., Santacruz-Ortega, H., Navarro, R. E., Sotelo-Mundo, R. R., and González-Aguilar, G. A. (2015). A ¹H NMR investigation of the interaction between phenolic acids found in mango (manguifera indica cv ataulfo) and papaya (carica papaya cv maradol) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals. PloS one 10, e0140242. doi:10.1371/journal.pone.0140242
Ma, J., Xu, H., Wu, J., Qu, C., Sun, F., and Xu, S. (2015). Linalool inhibits cigarette smoke-induced lung inflammation by inhibiting NF-κB activation. Int. Immunopharmacol. 29, 708–713. doi:10.1016/j.intimp.2015.09.005
Mishra, N., Rai, V. K., Yadav, K. S., Sinha, P., Kanaujia, A., Chanda, D., et al. (2016a). Encapsulation of mentha oil in chitosan polymer matrix alleviates skin irritation. AAPS PharmSciTech 17, 482–492. doi:10.1208/s12249-015-0378-x
Mishra, N., Yadav, K. S., Rai, V. K., and Yadav, N. P. (2016b). Polysaccharide encrusted multilayered nano-colloidal system of andrographolide for improved hepatoprotection. AAPS PharmSciTech. 18:381. doi:10.1208/s12249-016-0512-4
Moos, S., Mohebiany, A. N., Waisman, A., and Kurschus, F. C. (2019). Imiquimod-induced psoriasis in mice depends on the IL-17 signaling of keratinocytes. J. Invest. Dermatol 139, 1110–1117. doi:10.1016/j.jid.2019.01.006
Morsy, H., Kamp, S., Thrane, L., Behrendt, N., Saunder, B., Zayan, H., et al. (2010). Optical coherence tomography imaging of psoriasis vulgaris: Correlation with histology and disease severity. Arch. Dermatol Res. 302, 105–111. doi:10.1007/s00403-009-1000-4
Obara, S., Muto, H., Ichikawa, N., Tanaka, O., Otsuka, M., Kawanabe, M., et al. (1997). A repeated-dose dermal toxicity study of hydrophobically modified hydroxypropyl methylcellulose in rats. J. Toxicol. Sci. 22, 255–280. doi:10.2131/jts.22.3_255
Oecd (2018). Test No. 410: Repeated dose dermal toxicity: 21/28-Day study (OECD TG 410). Paris: OECD guidelines for the testing of chemicals, section 4, OECD Publishing.
Oecd (2015). Test No. 404: Acute dermal irritation/corrosion. Paris: OECD guidelines for the testing of chemicals, section 4, OECD Publishing.
Pattnaik, S., Subramanyam, V. R., Bapaji, M., and Kole, C. R. (1997). Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios 89, 39–46.
Peana, A. T., D'aquila, P. S., Panin, F., Serra, G., Pippia, P., and Moretti, M. D. (2002). Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 9, 721–726. doi:10.1078/094471102321621322
Popadic, S., Ramic, Z., Medenica, L., Mostarica Stojkovic, M., Trajković, V., and Popadic, D. (2008). Antiproliferative effect of vitamin A and D analogues on adult human keratinocytes in vitro. Skin. Pharmacol. Physiol. 21, 227–234. doi:10.1159/000135639
Rai, V. K., Sinha, P., Yadav, K. S., Shukla, A., Saxena, A., Bawankule, D. U., et al. (2020). Anti-psoriatic effect of Lavandula angustifolia essential oil and its major components linalool and linalyl acetate. J. Ethnopharmacol. 261, 113127. doi:10.1016/j.jep.2020.113127
Raza, K., Katare, O. P., Setia, A., Bhatia, A., and Singh, B. (2013). Improved therapeutic performance of dithranol against psoriasis employing systematically optimized nanoemulsomes. J. Microencapsul. 30, 225–236. doi:10.3109/02652048.2012.717115
Roy, A. K., Al Basir, F., and Roy, P. K. (2019). A vivid cytokines interaction model on psoriasis with the effect of impulse biologic (TNF-α inhibitor) therapy. J. Theor. Biol. 474, 63–77. doi:10.1016/j.jtbi.2019.04.007
Shah, P. P., Desai, P. R., Patel, A. R., and Singh, M. S. (2012). Skin permeating nanogel for the cutaneous co-delivery of two anti-inflammatory drugs. Biomaterials 33, 1607–1617. doi:10.1016/j.biomaterials.2011.11.011
Sharma, A., Upadhyay, D. K., Gupta, G. D., Narang, R. K., and Rai, V. K. (2022). IL-23/Th17 Axis: A potential therapeutic target of psoriasis. Curr. Drug Res. Rev. 14, 24–36. doi:10.2174/2589977513666210707114520
Sharma, A., Upadhyay, D. K., Sarma, G. S., Kaur, N., Gupta, G. D., Narang, R. K., et al. (2020). Squalene integrated NLC based gel of tamoxifen citrate for efficient treatment of psoriasis: A preclinical investigation. J. Drug Deliv. Sci. Technol. 56, 101568. doi:10.1016/j.jddst.2020.101568
Silva Brum, L. F., Emanuelli, T., Souza, D. O., and Elisabetsky, E. (2001). Effects of linalool on glutamate release and uptake in mouse cortical synaptosomes. Neurochem. Res. 26, 191–194. doi:10.1023/a:1010904214482
Somagoni, J., Boakye, C. H., Godugu, C., Patel, A. R., Mendonca Faria, H. A., Zucolotto, V., et al. (2015). Nanomiemgel--a novel drug delivery system for topical application--in vitro and in vivo evaluation. PLOS ONE 9, e115952. doi:10.1371/journal.pone.0115952
Sun, J., Zhao, Y., and Hu, J. (2013). Curcumin inhibits imiquimod-induced psoriasis-like inflammation by inhibiting IL-1beta and IL-6 production in mice. PLoS One 8, e67078. doi:10.1371/journal.pone.0067078
Surekha, P., Kishore, A. S., Srinivas, A., Selvam, G., Goparaju, A., Reddy, P. N., et al. (2012). Repeated dose dermal toxicity study of nano zinc oxide with Sprague-Dawley rats. Cutan. Ocul. Toxicol. 31, 26–32. doi:10.3109/15569527.2011.595750
Tirumalae, R. (2013). Psoriasiform dermatoses: Microscopic approach. Indian J. Dermatol 58, 290–293. doi:10.4103/0019-5154.113945
Tokura, Y., Mori, T., and Hino, R. (2010). Psoriasis and other Th17-mediated skin diseases. J. uoeh 32, 317–328. doi:10.7888/juoeh.32.317
Van Der Fits, L., Mourits, S., Voerman, J. S., Kant, M., Boon, L., Laman, J. D., et al. (2009). Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182, 5836–5845. doi:10.4049/jimmunol.0802999
Varma, S. R., Sivaprakasam, T. O., Mishra, A., Prabhu, S., Rafiq, M., and Rangesh, P (2017). Imiquimod-induced psoriasis-like inflammation in differentiated Human keratinocytes: Its evaluation using curcumin. Eur. J. Pharmacol. 813, 33–41. doi:10.1016/j.ejphar.2017.07.040
Wang, J., Li, Z., Sun, F., Tang, S., Zhang, S., Lv, P., et al. (2017). Evaluation of dermal irritation and skin sensitization due to vitacoxib. Toxicol. Rep. 4, 287–290. doi:10.1016/j.toxrep.2017.06.003
Yadav, N. P., Pal, A., Shanker, K., Bawankule, D. U., Gupta, A. K., Darokar, M. P., et al. (2008). Synergistic effect of silymarin and standardized extract of Phyllanthus amarus against CCl4-induced hepatotoxicity in Rattus norvegicus. Phytomedicine 15, 1053–1061. doi:10.1016/j.phymed.2008.08.002
Yadav, N. P., Rai, V. K., Mishra, N., Sinha, P., Bawankule, D. U., Pal, A., et al. (2014). A novel approach for development and characterization of effective mosquito repellent cream formulation containing citronella oil. Biomed. Res. Int. 2014, 786084. doi:10.1155/2014/786084
Keywords: psoriasis, imiquimod, topical, PASI, synergistic activity, linalool, linalyl acetate, lavender oil
Citation: Rai VK, Chanda D, Chanotiya CS and Yadav NP (2022) A combination of linalool and linalyl acetate synergistically alleviates imiquimod-induced psoriasis-like skin inflammation in BALB/c mice. Front. Pharmacol. 13:913174. doi: 10.3389/fphar.2022.913174
Received: 05 April 2022; Accepted: 27 June 2022;
Published: 05 August 2022.
Edited by:
Uraiwan Panich, Mahidol University, ThailandReviewed by:
Mingmei Zhou, Shanghai University of Traditional Chinese Medicine, ChinaCopyright © 2022 Rai, Chanda, Chanotiya and Yadav. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Narayan Prasad Yadav, bnAueWFkYXZAY2ltYXAucmVzLmlu, bnB5YWRhdkBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.