
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pharmacol. , 15 July 2022
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.913124
This article is part of the Research Topic Emerging Talents in Frontiers in Pharmacology: Gastrointestinal and Hepatic Pharmacology 2022 View all 10 articles
 Zhijun Guo1,2†
Zhijun Guo1,2† Jingjing Duan2†
Jingjing Duan2† Yitian Chen1,2
Yitian Chen1,2 Weijia Cai1,2
Weijia Cai1,2 Chenghua Yang2
Chenghua Yang2 Zhen Yang2
Zhen Yang2 Xiufeng Liu2
Xiufeng Liu2 Feng Xu1,2*
Feng Xu1,2*Background: Chemotherapy-induced nausea and vomiting severely impairs the treatment and prognosis of cancer patients. Depressive mood disorder might aggravate nausea and vomiting in cancer patients; however, the role of neurotransmitters and receptors involved in the mediation of emesis and nausea is still not well elaborated.
Methods: The study was carried out based on the chronic unpredictable mild stress–induced depression-like phenotype rat model and cisplatin-induced pica rat model establishment. Forty male Sprague–Dawley rats were randomized into the non-treated control group and the chronic stress group, which were exposed to 8 weeks of stress. Each group was then sub-divided into vehicle subgroups (n = 10) and cisplatin subgroups (n = 10) which were given cisplatin to induce pica behavior. Kaolin and food intake were recorded after administration. The medulla oblongata and ileum tissues were obtained. Neurotransmitters involved in the mediation of emesis and nausea (5-HT, DA, SP, and AEA) were detected using an ELISA kit. Vomit-related receptors (5-HT3R, DA2R, NK1R, and CB1R) in tissues were assayed for mRNA and protein expression by RT-qPCR and Western blotting.
Results: Behavioral test and sucrose preference validated that depression-like phenotype rat models were established successfully. The kaolin consumption test confirmed that chronic stress pretreatment aggravated anorexia and pica behavior. Vomiting-related molecules’ data showed that chronic stress exposure increased 5-HT and SP levels in the medulla oblongata. Vomiting-related receptor expression data showed that chronic stress pretreatment upregulated 5-HT3R, DA2R, and NK1R expressions and downregulated the CB1R expression in the medulla oblongata. However, chronic stress pretreatment downregulated 5-HT3R, DA2R, and NK1R expressions and upregulated the CB1R expression in the ileum.
Conclusion: Chronic stress pretreatment aggravates anorexia and vomiting progress, which might be via altering neurotransmitters and receptors involved in the mediation of emesis and the nausea level and expression in the central nervous system.
Chemotherapy-induced nausea and vomiting (CINV) is a critically adverse drug reaction in cancer patients (Aapro, 2018), which severely disturbs treatment decision and impairs overall quality of life and therapeutic outcome (Hawkins and Grunberg, 2009; Bray et al., 2018; Natale, 2018).
Serotonin (5-HT) and substance P (SP) are key factors involved in the vomiting process (Darmani and Ray, 2009). When 5-HT or/and SP combines with the corresponding receptors, the vomiting center in the medulla oblongata is triggered and thus results in vomiting. Although new therapeutics have been developed for nausea and vomiting control, CINV is still very challenging for cancer chemotherapy patients (Jordan et al., 2015; Warr, 2018). Up to now, CINV has remained a thorny issue in cancer chemotherapy (Dranitsaris et al., 2017). Approximately 9–30% of cancer patients still suffer from serious vomiting despite current anti-emetic medications (Mersiades et al., 2018). A further complication is that many risk factors are identified to aggravate CINV, including history of nausea/vomiting, expectancy of CINV, alcohol intake, and anxiety/depression (Mosa et al., 2020).
In our previous work, we found that the frequency and extent of subjectively experienced adverse drug reactions (anorexia, nausea, and fatigue) in cancer chemotherapy patients seemed to be well in line with the severity of their depression (Zhou et al., 2010). Chronic stress pretreatment might aggravate the vomiting process; however, the underlying mechanism is still not clear. In addition, many documents indicated that the gastrointestinal function was affected by a specific psychological mood disorder status such as depression and anxiety (Peng and Liang, 1999; Liu et al., 2015), further indicating that chronic stress pretreatment is related to changes in the gastrointestinal function.
In this study, we hypothesized that chronic stress pretreatment might aggravate nausea and vomiting. Due to the special physiological and anatomical structure, rats are lacking a vomiting response. Non-nutritive substance consumption, such as kaolin consumption (pica behavior), can indirectly reflect the degree of vomiting in rats (Borner et al., 2020). We examined the kaolin consumption in this study to explore the vomiting response in rats. As the vomiting process involves many molecules and receptors (Li and Xu, 2020), this study focused on neurotransmitters involved in the mediation of emesis and nausea 5-HT, DA, SP and AEA level, 5-HT3R, DA2R, NK1R, and CB1R expressions in the central nervous system and aimed to explore the relevant molecule profile of chronic stress aggravating CINV based on chronic unpredictable mild stress (CUMS) and/or the pica rat model.
Male Sprague–Dawley rats weighing 120–150 g were purchased from Shanghai Jiesijie Laboratory Animal Technology Co., Ltd. (Animal Quality Certificate: 20180004058940). Rats were housed in a specific pathogen-free (SPF) laboratory with a regular 12-h light (06:00–18:00)-dark (18:00–06:00) cycle, at the Laboratory Animal Center, East China Normal University, Shanghai. All rats were allowed to adapt to the new environment for 1 week before CUMS model establishment. The body weight of rats was measured biweekly. Animal procedures complied with the ARRIVE guidelines. This study was reviewed and approved by the Animal Research Ethics Committee in Fengxian Hospital, Southern Medical University (No. 201920143).
The OFT was used to evaluate the locomotion activity and exploratory behavior of rats. Each rat was individually placed at the center of a box (100 cm × 100 cm × 40 cm) divided into 25 cm × 25 cm2 squares and observed for 5 min. The crossing numbers of squares (locomotion activity) and the rearing times (exploratory behavior) were monitored using a ZSZFT Video Analysis System (ZSZRDC Science and Technology Co., Ltd., China). The OFT was only conducted before and after 8 weeks of modeling to maintain a relatively unfamiliar environment for rats.
The SPT was used to detect the anhedonia status in depression-like phenotype rats (Liu et al., 2018). Rats were individually housed in cages for isolation adaption. Sucrose training for 48 h helped the rats adapt to the sucrose solution. At the first 24 h, two bottles of 1% sucrose solution (Sigma, St. Louis, MO, United States) were given to the rats. At the second 24 h, a bottle of water and a bottle of 1% sucrose solution were given to the rats. After 23 h of deprivation of water and food, a bottle of water and a bottle of 1% sucrose solution were given to the rats, and the intake of water and sucrose solution for 1 h was recorded. The positions of the two bottles were exchanged every 6 h to eliminate unilateral preference during the whole test.
After OFT and SPT primary screening, rats with normal behavior were divided into the non-treated control group (n = 20) and the chronic stress group (n = 20) with a random number table. Rats in the chronic stress group were individually housed in cages and exposed to the following stressors in a random order once a day for 8 weeks: restraint (activity restriction in a 30 cm × 6 cm plastic bottle, 1 h), hot water swimming (45°C, 5 min), cold water swimming (4°C, 5 min), clip tail (clip 1 cm from the end of the tail, 1 min), cage tilting (45°, 24 h), horizontal shaking (30 min), damp padding (24 h), noise interference (industrial noise from a media player, 2 h), and day/night inversion (24 h) (Antoniuk et al., 2019; Li et al., 2019; Zhao et al., 2020). The use of the same stress for two consecutive days was avoided for the purpose of unpredictability. Rats in the non-treated control group were grouped in cages and fed normally without any stress during this period.
Here, 2% Arabic gum solution was prepared by stirring Arabic gum powder (Shanghai Macklin Biochemical Co., Ltd.) in distilled water at room temperature until it was completely dissolved. Then, 500 g kaolin (Shanghai Yuanye Biological Technology Co., Ltd.) and 200 ml of 2% Arabic gum solution were mixed to make a thick paste. The paste was dried at room temperature and molded into chow food in the shape of pellets. After 8 weeks of chronic stress, rats in both the non-treated control group and the chronic stress group were further sub-divided into the vehicle subgroup (normal saline) and the cisplatin subgroup (cisplatin administration), respectively, as follows: non-treated/saline group (n = 10), non-treated/cisplatin group (n = 10), CUMS/cisplatin group (n = 10), and CUMS group (n = 10). Rats in the non-treated/saline group, non-treated/cisplatin group, and CUMS/cisplatin group were individually housed in cages and given kaolin pellets for 1 week of adaptation. Food was provided simultaneously during the cisplatin-induced pica model. Non-treated/cisplatin group and CUMS/cisplatin group rats were then given 6 mg/kg cisplatin (Qilu Pharmaceutical Co., Ltd.) intraperitoneally; meanwhile, rats in the non-treated/saline group were given normal saline intraperitoneally. The intake of kaolin and food was recorded at 24 h after administration. For the cisplatin-induced pica behavior model, rats were not given any stress to avoid affecting kaolin and food intake.
To further investigate the effect of chronic stress pretreatment on vomit-related molecules, we examined the vomit-related neurotransmitters’ levels and vomit-related receptors’ expression in the non-treated/saline group and CUMS group. Then, 24 h after cisplatin administration, the medulla oblongata and ileum tissues of rats in the non-treated/saline group and CUMS group were collected. Portions of tissues were homogenized at 4°C. The levels of 5-HT, DA, SP, and AEA in the tissue homogenate were detected using the ELISA kit (Jiangsu Jianglai Biotechnology Co., Ltd.), according to the manufacturer’s protocol. Portions of tissues were preserved in liquid nitrogen for receptor mRNA and protein expression measurement.
For RT-qPCR measurement, briefly, total RNA of the medulla oblongata and ileum tissues were extracted with TRIzol (Invitrogen™, Carlsbad, United States), according to the protocol, and the RNA concentration was measured using a NanoDrop ND-100 spectrophotometer (Thermo Scientific, Wilmington, DE, United States). Tli RNaseH Plus was used according to the manufacturer’s protocol. The 2−ΔΔCT method was used to normalize the fold change in the gene expression. The primer sequences are as follows:
GADPH forward primer, 5′-CAAGAAGGTGGTGAAGCAG-3′ and reverse primer, 5′-CAAAGGTGGAAGAATGGG-3’; 5-HT3R forward primer, 5′-CTGTCCTCCATCCGCCACTCC-3′ and reverse primer, 5′- CAGCAGCCTGTCCAGCACATATC-3’; DA2R forward primer, 5′- CACACGCTACAGCTCCAAG-3′ and reverse primer, 5′- GAAGGACAGGACCCAGACA-3’; NK1R forward primer, 5′- CCGCTACCATGAGCAAGT-3′ and reverse primer, 5′- AGGGCAGGAGGAAGAAGA-3’; and CB1R forward primer, 5′- GGACCCAGAAGAGCATCA-3′ and reverse primer, 5′- ATCAACACCACCAGGATCA-3’. The gene expression was normalized with the following: experimental group: target gene/housekeeping gene; control group: target gene/housekeeping gene).
For Western blot, briefly, the medulla oblongata and ileum tissues were individually ground and sonicated in RIPA buffer (Beyotime, China) for 30 min and centrifuged at 14,000 g for 20 min at 4°C. The supernatant was quantified using the BCA protein assay kit (Beyotime, China), analyzed by 10% SDS-PAGE (Beyotime, China), and transferred onto a nitrocellulose filter membrane (Pall, America). After 1 h of blocking, the membranes were incubated with the primary antibody (5-HT3R antibody, CB1R antibody, and β-actin antibody were purchased from Proteintech, NK1R antibody was purchased from Immunoway, and DA2R antibody was purchased from Bioss; dilution rate: 5-HT3R, CB1R, NK1R, and DA2R were 1:1000, and β-actin was 1:5000) overnight at 4°C, followed by incubation with secondary antibodies (Goat anti-rabbit antibody and goat anti-mouse antibody were purchased from Proteintech; dilution rate: goat anti-rabbit and goat anti-mouse were 1:5000), and detected with an ECL buffer (NCM Biotech, China). The intensity of each band was determined by ImageJ software. The protein expression was normalized with the following: experimental group: target protein/housekeeping protein; control group: target protein/housekeeping protein).
All analysis was performed using SPSS 19.0. Data were expressed as mean ± SD. Statistical comparisons between the two groups were performed using the two independent sample t-tests, and the same group was performed with paired samples’ t-test. The inter-groups were performed using one-way analysis of variance (ANOVA). Repeated measurement data were performed using two-way repeated measures ANOVA. LSD and Dunnett’s T3 were used for post hoc multiple comparisons. p < 0.05 was considered to be statistically significant.
After 8 weeks of CUMS model establishment, the locomotion activity scores, exploratory behavior scores, and sucrose preference were decreased in chronic stress group rats compared to baseline (p < 0.01). However, there was little change in the non-treated control rats over time (Figures 1A–C). The results showed that depression-like behavior was successfully induced in rats in the chronic stress group compared with rats in the non-treated control group. Compared to the non-treated control group, a moderate reduction in the body weight in chronic stress group rats was observed from the second week of CUMS model establishment (Figure 1D). Behavioral test and sucrose preference test results indicated that the chronic stress–induced depression-like phenotype rat model was successfully established.
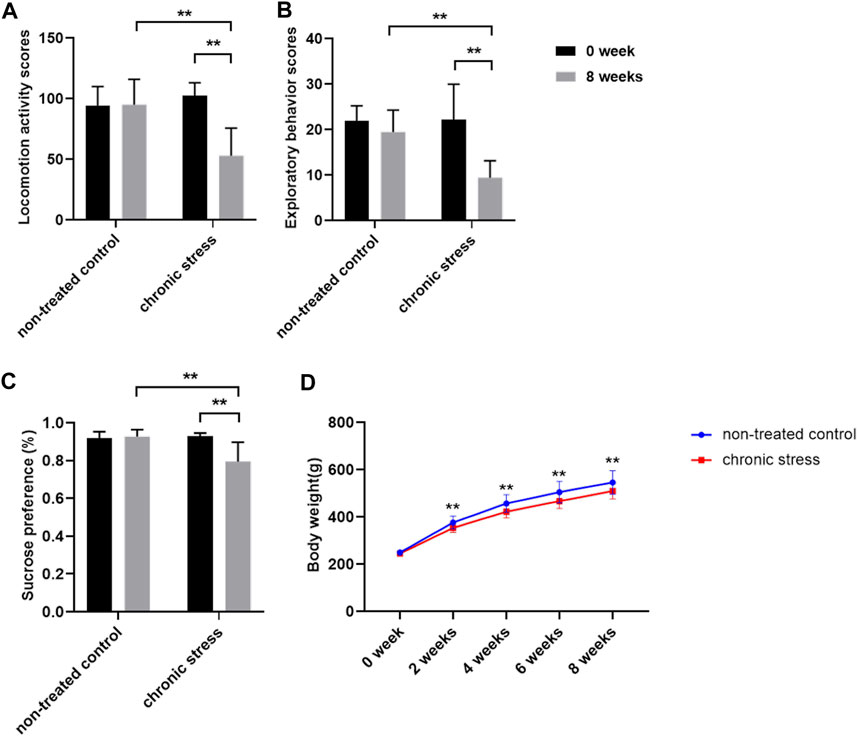
FIGURE 1. Chronic stress model was established successfully (n = 20/group). (A) Locomotion activity scores. (B) Exploratory behavior scores. (C) Sucrose preference. (D) Body weight. Data were expressed as mean ± SD. **p < 0.01 (statistical comparisons between the two groups were performed using the two independent sample t-test, and the same group was performed with the paired sample t-test. Repeated measurement data was performed using two-way repeated measures ANOVA.).
Kaolin pellets were given to rats in the non-treated/saline group, non-treated/cisplatin group, and CUMS/cisplatin group for 1 week of adaptation. 24 h after administration, kaolin consumption was significantly increased both in the non-treated/cisplatin group and CUMS/cisplatin group compared to that in the non-treated/saline group (Figure 2A). Furthermore, the kaolin consumption in the CUMS/cisplatin group increased more significantly than that in the non-treated/cisplatin group (p < 0.01), suggesting that chronic stress aggravated vomit-like behavior (increased kaolin consumption). In addition, food intake was decreased in the non-treated/cisplatin group and CUMS/cisplatin group (Figure 2B). Chronic stress pretreatment enhanced the reduction of food in the CUMS/cisplatin group. However, there was no significant change in water consumption among the three groups (Figure 2C). Data revealed that cisplatin administration increased the kaolin consumption, and chronic stress pretreatment enhanced this effect in rats.
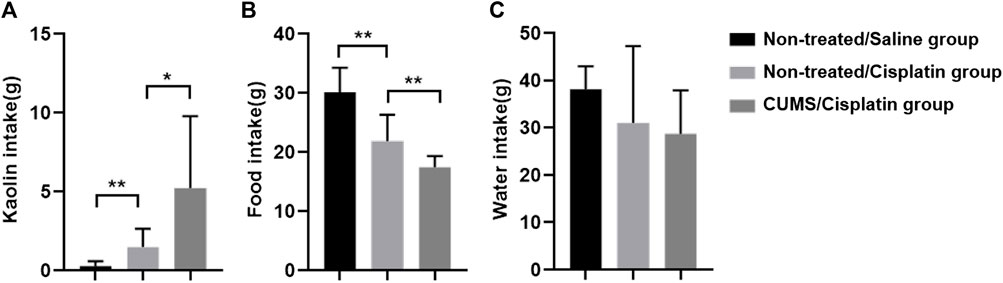
FIGURE 2. Chronic stress pretreatment enhanced cisplatin (6 mg/kg)-induced anorexia and pica behavior in rats (n = 10/group). (A) Kaolin intake. (B) Food intake. (C) Water intake. *p < 0.05 and **p < 0.01(statistical comparisons in the inter-groups were performed using one-way analysis of variance (ANOVA).).
We further investigated the neurotransmitters and receptors involved in the mediation of emesis and the nausea level and receptor expression in the central nervous system (CNS) of the non-treated/saline group and CUMS group. Compared with the non-treated/saline group, chronic stress significantly increased 5-HT and SP levels in the CUMS group (p < 0.01). However, little change occurred in DA and AEA levels between the non-treated/saline group and CUMS group (Figures 3A–D). The immunoblotting and RT-qPCR assay results showed that chronic stress obviously upregulated 5-HT3R, DA2R, and NK1R expressions but downregulated the CB1R expression in the medulla oblongata in the CUMS group (p < 0.01) compared to those in the non-treated/saline group (Figures 3E–G). Results showed that chronic stress increased the levels of vomit-related neurotransmitters 5-HT and SP and altered vomit-related receptors’ expression in the medulla oblongata, which means that more vomit-related neurotransmitters could combine with the corresponding vomit-related receptors and then affect the vomiting process.
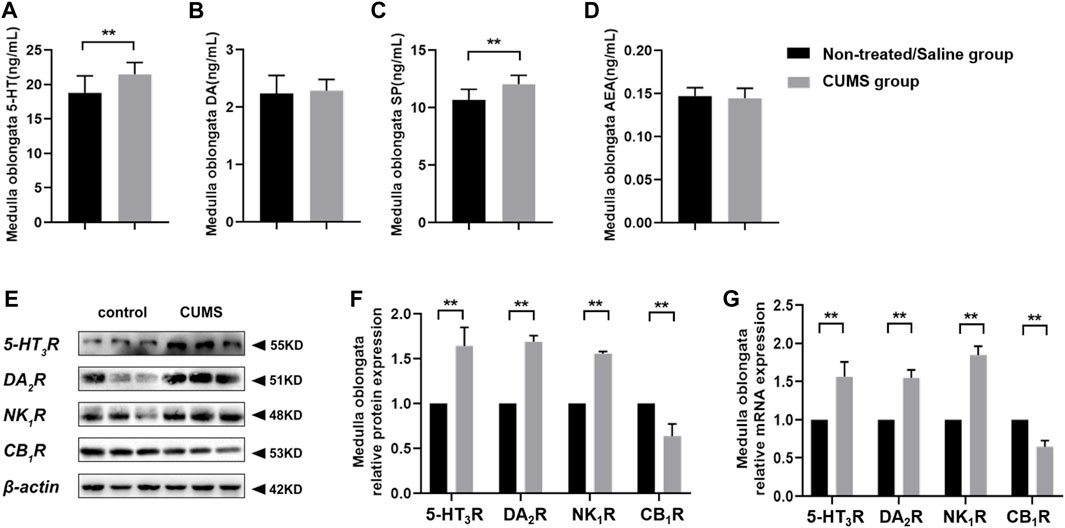
FIGURE 3. Chronic stress increased 5-HT and SP levels, upregulated 5-HT3R, DA2R, and NK1R expressions, and downregulated the CB1R expression in the CNS (n = 10/group). (A–D) Vomit-related neurotransmitter levels in the medulla oblongata. (E) Representative Western blotting bands of vomit-related receptors. (F) Relative protein expression of vomit-related receptors in the medulla oblongata (normalization: experimental group (target protein/housekeeping protein)/control group (target protein/housekeeping protein)). (G) Relative mRNA expression of vomit-related receptors in the medulla oblongata (normalization: experimental group (target gene/housekeeping gene)/control group (target gene/housekeeping gene)). Data were expressed as mean ± SD. **p < 0.01 (statistical comparisons between the two groups were performed using the two independent sample t-test).
To figure out the effect of chronic stress on neurotransmitters involved in the mediation of emesis and nausea in ileum tissue, we measured the 5-HT, DA, SP, and AEA levels and 5-HT3R, DA2R, NK1R, and CB1R expressions. ELISA assay revealed that there was no significant change for relevant molecule levels between the non-treated/saline group and CUMS group (Figures 4A–D). In addition, the Western blot assay and RT-qPCR results displayed that chronic stress downregulated 5-HT3R, DA2R, and NK1R expressions and upregulated the CB1R expression in the ileum in the CUMS group (p < 0.01) compared to the non-treated/saline group (Figures 4E–G). We noticed that chronic stress did not significantly change the vomit-related neurotransmitters’ level in the ileum, and the alteration of vomit-related receptors in the ileum was inconsistent with that in the CNS.
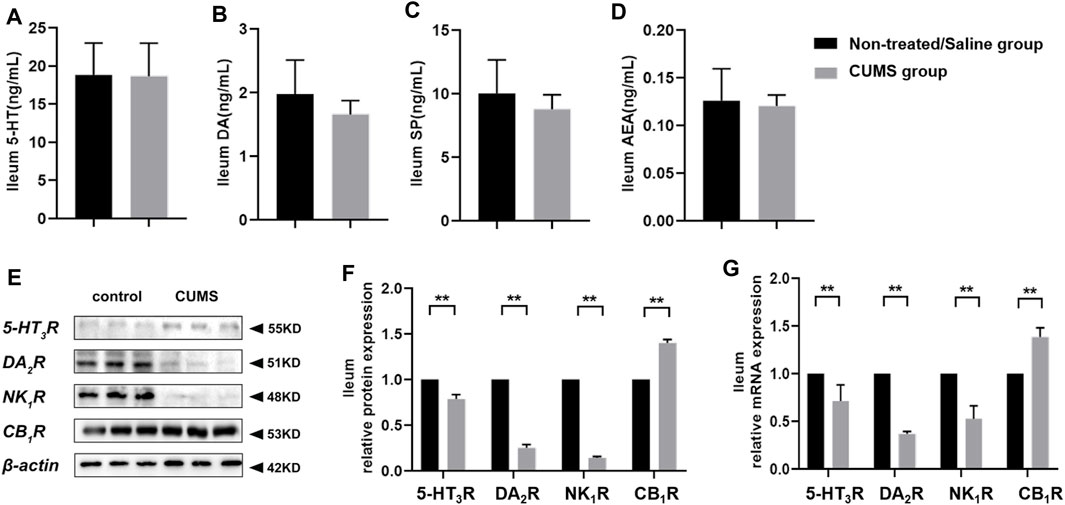
FIGURE 4. Chronic stress did not alter 5-HT, DA, SP, and AEA levels, downregulated 5-HT3R, DA2R, and NK1R expressions, and upregulated the CB1R expression in the peripheral system (n = 10/group). (A–D) Vomit-related neurotransmitter levels in the ileum. (E,F) Representative Western blotting bands and relative protein expressions of vomit-related receptors in the ileum [normalization: experimental group (target protein/housekeeping protein)/control group (target protein/housekeeping protein)]. (G) Relative mRNA expression of vomit-related receptors in the ileum [normalization: experimental group (target gene/housekeeping gene)/control group (target gene/housekeeping gene)]. Data were expressed as mean ± SD. **p < 0.01 (statistical comparisons between the two groups were performed using the two independent sample t-test).
In this study, we established a chronic stress–induced depression-like behavior and cisplatin–induced pica rat model. Chronic stress pretreatment decreases food intake and significantly increases kaolin consumption after cisplatin injection, which suggest that chronic stress pretreatment induces anorexia and aggravates cisplatin-induced pica behavior. We explored the relevant molecule profile in this process and found for the first time that chronic stress might aggravate the vomiting progress via altering the level of neurotransmitters and the expression of receptors involved in the mediation of emesis and nausea in the central nervous system of rats.
Clinical evidences indicated that anorexia nervosa often occurs in individuals with stress, anxiety-like, or depression-like behavior (Lian et al., 2017; Lloyd et al., 2019), suggesting the possible association between mood disorder and anorexia. Similar to this finding, our previous work also noted that cancer chemotherapy patients with severe depression seemed more likely to have frequent and severe adverse drug reactions including anorexia, nausea, and fatigue (Zhou et al., 2010). Nausea and vomiting, one of the most terrible adverse reactions experienced by cancer patients, is associated with multiple risk factors including gender, age, anticancer drug dose, history of morning sickness, and alcohol intake. More and more researchers believe that depression and the anxiety mood disorder play a great role in chemotherapy-induced nausea and vomiting (Navari and Aapro, 2016; Kawazoe et al., 2018; Tsuji et al., 2019; Guo and Xu, 2020; Nasu et al., 2020). In addition, expectancy psychology of nausea and vomiting promote vomiting progress for cancer patients (Mosa et al., 2020).
Due to the special physiological structure, rodent animals like rats lack vomiting reflex. Therefore, vomiting in rats is assessed by pica behavior (Takeda et al., 1993). Cisplatin is a classic anticancer drug with high emetogenicity (Bamias et al., 2019), which causes severe nausea and vomiting, especially acute vomiting in cancer patients (Zhang et al., 2018). The cisplatin-induced pica rat model is a widely used animal model for antiemetic screening. Based on this pica model, we further confirmed that chronic stress–induced depression-like phenotype rats are more prone to displaying pica behavior (vomit-like behavior).
As the nausea and vomiting process involves various neurotransmitters and receptors (Di Maio et al., 2018), we studied the 5-HT, DA, SP, and AEA levels and 5-HT3R, DA2R, NK1R, and CB1R expressions in tissue and explored their possible change during the chronic stress process. The results suggested that 5-HT and SP elevation, as 5-HT3R and NK1R expressions increase in the CNS, might play a crucial role in the chronic stress aggravating vomit. As elevated levels of 5-HT and SP are bound with highly expressed 5-HT3R/NK1R, vomiting response is triggered easily, frequently, and severely (Phillips et al., 2016). Why there is no change in 5-HT and SP levels in the ileum is worthy of further in-depth research.
In this study, we mainly focused on the effect of chronic stress on vomit-related molecules’ level while not considering the antiemetic drug efficacy of rats. It would be beneficial to investigate the efficacy for the cisplatin-induced pica behavior in our chronic stress experimental condition in rats in future. In addition, recently, data have shown that different bedding in the cages may influence food intake (Lovasz et al., 2020); bedding change in our CUMS model establishment process might interfere with the results, and it might be an important limitation to the present study.
In this study, chronic stress aggravates cisplatin-induced anorexia and vomiting progress in rats which might be increasing the level of neurotransmitters 5-HT and SP, upregulating the expressions of receptors 5-HT3R, DA2R, and NK1R, and downregulating the CB1R expression in the central nervous system.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by The Animal Research Ethics Committee in Fengxian Hospital, Southern Medical University.
Experimental conception and design: FX. Experimental operation: ZG, JD, YC, WC, CY, ZY, and XL. Writing and revising the manuscript: ZG and FX. All authors reviewed the manuscript.
This work was supported by the Shanghai Municipal Science Commission [grant number 19411971700].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aapro, M. (2018). CINV: Still Troubling Patients after All These Years. Support Care Cancer 26, 5–9. doi:10.1007/s00520-018-4131-3
Antoniuk, S., Bijata, M., Ponimaskin, E., and Wlodarczyk, J. (2019). Chronic Unpredictable Mild Stress for Modeling Depression in Rodents: Meta-Analysis of Model Reliability. Neurosci. Biobehav Rev. 99, 101–116. doi:10.1016/j.neubiorev.2018.12.002
Bamias, A., Tzannis, K., Bamia, C., Harshman, L. C., Crabb, S., Plimack, E. R., et al. (2019). The Impact of Cisplatin- or Non-cisplatin-containing Chemotherapy on Long-Term and Conditional Survival of Patients with Advanced Urinary Tract Cancer. Oncologist 24, 1348–1355. doi:10.1634/theoncologist.2018-0739
Borner, T., Shaulson, E. D., Ghidewon, M. Y., Barnett, A. B., Horn, C. C., Doyle, R. P., et al. (2020). GDF15 Induces Anorexia through Nausea and Emesis. Cell Metab. 31, 351–362. e5. doi:10.1016/j.cmet.2019.12.004
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 68, 1–31. doi:10.3322/caac.21492
Darmani, N. A., and Ray, A. P. (2009). Evidence for a Re-evaluation of the Neurochemical and Anatomical Bases of Chemotherapy-Induced Vomiting. Chem. Rev. 109, 3158–3199. doi:10.1021/cr900117p
Di Maio, M., Baratelli, C., Bironzo, P., Vignani, F., Bria, E., Sperti, E., et al. (2018). Efficacy of Neurokinin-1 Receptor Antagonists in the Prevention of Chemotherapy-Induced Nausea and Vomiting in Patients Receiving Carboplatin-Based Chemotherapy: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 124, 21–28. doi:10.1016/j.critrevonc.2018.02.001
Dranitsaris, G., Molassiotis, A., Clemons, M., Roeland, E., Schwartzberg, L., Dielenseger, P., et al. (2017). The Development of a Prediction Tool to Identify Cancer Patients at High Risk for Chemotherapy-Induced Nausea and Vomiting. Ann. Oncol. 28, 1260–1267. doi:10.1093/annonc/mdx100
Guo, Z., and Xu, F. (2020). Research Progress on Classification and Drug Therapy of Chemotherapy-Induced Nausea and Vomiting. China Pharm. 29, 1–6. doi:10.3969/j.issn.1006-4931.2020.22.001
Hawkins, R., and Grunberg, S. (2009). Chemotherapy-Induced Nausea and Vomiting: Challenges and Opportunities for Improved Patient Outcomes. Clin. J. Oncol. Nurs. 13, 54–64. doi:10.1188/09.CJON.54-64
Jordan, K., Jahn, F., and Aapro, M. (2015). Recent Developments in the Prevention of Chemotherapy-Induced Nausea and Vomiting (CINV): a Comprehensive Review. Ann. Oncol. 26, 1081–1090. doi:10.1093/annonc/mdv138
Kawazoe, H., Murakami, A., Yamashita, M., Nishiyama, K., Kobayashi-Taguchi, K., Komatsu, S., et al. (2018). Patient-related Risk Factors for Nausea and Vomiting with Standard Antiemetics in Patients with Breast Cancer Receiving Anthracycline-Based Chemotherapy: A Retrospective Observational Study. Clin. Ther. 40, 2170–2179. doi:10.1016/j.clinthera.2018.10.004
Li, N., Wang, Q., Wang, Y., Sun, A., Lin, Y., Jin, Y., et al. (2019). Fecal Microbiota Transplantation from Chronic Unpredictable Mild Stress Mice Donors Affects Anxiety-like and Depression-like Behavior in Recipient Mice via the Gut Microbiota-Inflammation-Brain axis. Stress 22, 592–602. doi:10.1080/10253890.2019.1617267
Li, Z., and Xu, F. (2020). Chronic Stress Alters Expression of Vomiting‐related Receptors in CNS in SD Rats. FASEB J. 34, 06802. doi:10.1096/fasebj.2020.34.s1.06802
Lian, Q., Zuo, X., Mao, Y., Luo, S., Zhang, S., Tu, X., et al. (2017). Anorexia Nervosa, Depression and Suicidal Thoughts Among Chinese Adolescents: a National School-Based Cross-Sectional Study. Environ. Health Prev. Med. 22, 30. doi:10.1186/s12199-017-0639-2
Liu, M. Y., Yin, C. Y., Zhu, L. J., Zhu, X. H., Xu, C., Luo, C. X., et al. (2018). Sucrose Preference Test for Measurement of Stress-Induced Anhedonia in Mice. Nat. Protoc. 13, 1686–1698. doi:10.1038/s41596-018-0011-z
Liu, Y., Bai, S., Ma, J., Wu, F., Liu, J., Wu, Z., et al. (2015). Research Progress of Functional Diarrea Treated with Chinese Medicine. J. Liaoning Univ. TCM 17, 78–80. doi:10.13194/j.issn.1673-842x.2015.02.028
Lloyd, E. C., Haase, A. M., Foster, C. E., and Verplanken, B. (2019). A Systematic Review of Studies Probing Longitudinal Associations between Anxiety and Anorexia Nervosa. Psychiatry Res. 276, 175–185. doi:10.1016/j.psychres.2019.05.010
Lovasz, R. M., Marks, D. L., Chan, B. K., and Saunders, K. E. (2020). Effects on Mouse Food Consumption after Exposure to Bedding from Sick Mice or Healthy Mice. J. Am. Assoc. Lab. Anim. Sci. 59, 687–694. doi:10.30802/AALAS-JAALAS-19-000154
Mersiades, A. J., Tognela, A., Haber, P. S., Stockler, M., Lintzeris, N., Simes, J., et al. (2018). Oral Cannabinoid-Rich THC/CBD Cannabis Extract for Secondary Prevention of Chemotherapy-Induced Nausea and Vomiting: a Study Protocol for a Pilot and Definitive Randomised Double-Blind Placebo-Controlled Trial (CannabisCINV). BMJ Open 8, e020745. doi:10.1136/bmjopen-2017-020745
Mosa, A. S. M., Hossain, A. M., Lavoie, B. J., and Yoo, I. (2020). Patient-Related Risk Factors for Chemotherapy-Induced Nausea and Vomiting: A Systematic Review. Front. Pharmacol. 11, 329. doi:10.3389/fphar.2020.00329
Nasu, I., Shimano, R., Kawazoe, H., Nakamura, T., Miura, Y., Takano, T., et al. (2020). Patient-related Risk Factors for Nausea and Vomiting with Standard Antiemetics in Patients with Cancer Receiving Carboplatin: A Retrospective Study. Clin. Ther. 42, 1975–1982. doi:10.1016/j.clinthera.2020.08.007
Natale, J. J. (2018). Overview of the Prevention and Management of CINV. Am. J. Manag. Care 24, S391–S397.
Navari, R. M., and Aapro, M. (2016). Antiemetic Prophylaxis for Chemotherapy- Induced Nausea and Vomiting. New Engl. J. Med. 374, 1356–1357. doi:10.1056/NEJMra1515442
Peng, L., and Liang, H. (1999). Depression and Functional Diseases of the Digestive Tract. Shijie Huaren Xiaohua Zazhi 7 (7), 601–602.
Phillips, R. S., Friend, A. J., Gibson, F., and Pizer, B. (2016). Antiemetic Medication for Prevention and Treatment of Chemotherapy-Induced Nausea and Vomiting in Childhood. Cochrane Database Syst. Rev. 2 (2), CD007786. doi:10.1002/14651858.CD007786.pub3
Takeda, N., Hasegawa, S., Morita, M., and Matsunaga, T. (1993). Pica in Rats Is Analogous to Emesis: an Animal Model in Emesis Research. Pharmacol. Biochem. Be 45, 817. doi:10.1016/0091-3057(93)90126-e
Tsuji, D., Suzuki, K., Kawasaki, Y., Goto, K., Matsui, R., Seki, N., et al. (2019). Risk Factors Associated with Chemotherapy-Induced Nausea and Vomiting in the Triplet Antiemetic Regimen Including Palonosetron or Granisetron for Cisplatin-Based Chemotherapy: Analysis of a Randomized, Double-Blind Controlled Trial. Support Care Cancer 27, 1139–1147. doi:10.1007/s00520-018-4403-y
Warr, D. (2018). Bringing it All Together in the Treatment of CINV: Application of Current Knowledge into Routine Clinical Practice. Support Care Cancer 26, 29–33. doi:10.1007/s00520-018-4117-1
Zhang, L., Lu, S., Feng, J., Dechaphunkul, A., Chang, J., Wang, D., et al. (2018). A Randomized Phase III Study Evaluating the Efficacy of Single-Dose NEPA, a Fixed Antiemetic Combination of Netupitant and Palonosetron, versus an Aprepitant Regimen for Prevention of Chemotherapy-Induced Nausea and Vomiting (CINV) in Patients Receiving Highly Emetogenic Chemotherapy (HEC). Ann. Oncol. 29, 452–458. doi:10.1093/annonc/mdx698
Zhao, Q., Wang, A., Gao, X., Li, L., and Zhao, J. (2020). Research Progress in Depression-like Rat Model Induced by Chronic Unpredictable Mild Stress. Laboratory Animal Comp. Med. 40, 344–353. doi:10.3969/j.issn.1674-5817.2020.04.012
Keywords: chronic unpredictable mild stress, anorexia, pica, cisplatin, chemotherapy-induced nausea and vomiting
Citation: Guo Z, Duan J, Chen Y, Cai W, Yang C, Yang Z, Liu X and Xu F (2022) Cisplatin-Induced Anorexia and Pica Behavior in Rats Enhanced by Chronic Stress Pretreatment. Front. Pharmacol. 13:913124. doi: 10.3389/fphar.2022.913124
Received: 05 April 2022; Accepted: 14 June 2022;
Published: 15 July 2022.
Edited by:
Thomas Brzozowski, Jagiellonian University Medical College, PolandReviewed by:
Feifei Guo, Qingdao University, ChinaCopyright © 2022 Guo, Duan, Chen, Cai, Yang, Yang, Liu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Xu, eHVmQHNtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.