- 1Jiangsu Key Laboratory of New Drug Research and Clinical Pharmacy and School of Pharmacy, Xuzhou Medical University, Xuzhou, China
- 2Department of Pharmacy, Xuzhou Oriental Hospital Affiliated to Xuzhou Medical University, Xuzhou, China
- 3Department of Endocrinology, Huaian Hospital of Huaian City, Huaian, China
- 4Department of Pharmacy, Suzhou Science and Technology Town Hospital, Suzhou, China
- 5School of Nursing, Xuzhou Medical University, Xuzhou, China
The aim of the present study is to investigate the quantitative effects of sodium–glucose cotransporter-2 (SGLT-2) inhibitors on the quality of life in heart failure (HF) patients. A total of 14,674 HF patients from two dapagliflozin and three empagliflozin studies is included for analysis via the nonlinear mixed-effect modeling (NONMEM) software, among which the change rate of the Kansas City Cardiomyopathy Questionnaire (KCCQ) score is used as the evaluation index. There is no significant difference in the pharmacodynamics influencing the quality of life in HF patients between the SGLT-2 inhibitors: 10 mg/day dapagliflozin and 10 mg/day empagliflozin. For the clinical summary score (CSS), total symptom score (TSS), and overall summary score (OSS), the Emax of the SGLT-2 inhibitors on the quality of life in HF patients is 3.74%, 4.43%, and 4.84%, respectively, and ET50 is 2.23, 4.37, and 7.15 weeks, respectively. In addition, the time duration of achieving 25%, 50%, 75%, and 80% Emax is 0.75, 2.23, 6.69, and 8.92 weeks for the CSS; 1.46, 4.37, 13.11, and 17.48 weeks for the TSS; and 2.39, 7.15, 21.45, and 28.6 weeks for the OSS, respectively. Therefore, to reach the plateau period (80% of Emax) of SGLT-2 inhibitors on the CSS, TSS, and OSS, 10 mg/day dapagliflozin (or 10 mg/day empagliflozin) is required to be taken for 8.92 weeks, 17.48 weeks, and 28.6 weeks, respectively. This is the first time that the quantitative effects of SGLT-2 inhibitors on the quality of life in HF patients are being explored.
Introduction
Heart failure (HF) is a functionally abnormal heart on account of either structural and/or functional deficits inducing an elevation in intracardiac pressures and/or reduced cardiac output, which has become a growing medical problem (Badoer, 2022). Recent data indicate that the incidence of HF is increasing year by year (Roth et al., 2015; Conrad et al., 2018; Cleland et al., 2019). At the same time, it has brought a huge economic burden on society (Bozkurt et al., 2021). Based on world standards, China and India are the two countries with the fastest rising rates of HF (Bragazzi et al., 2021). Combined with the large populations of these two countries, the HF problem in future will be even more serious (Badoer, 2022). Therefore, more efforts are required to be put into the prevention and treatment of HF patients.
Sodium–glucose cotransporter-2 (SGLT-2) inhibitors are a group of antidiabetic drugs, which are located in the S1 segment of the renal proximal tubule and account for the absorption of nearly 90% of the glucose by the kidneys (Turner and Moran, 1982; Kashiwagi and Maegawa, 2017). In addition to lowering blood glucose, SGLT-2 inhibitors also have the power to reduce the risk of cardiovascular outcomes and mortality (Mascolo et al., 2021), especially when HF patients can obtain benefits from therapy treatment by dapagliflozin and empagliflozin (Packer et al., 2020; Butt et al., 2021). However, the quantitative effects of SGLT-2 inhibitors dapagliflozin and empagliflozin on the quality of life in HF patients are still unknown. The present study aims to explore the quantitative effects of SGLT-2 inhibitors on the quality of life in HF patients.
Methods
Enrolled patients
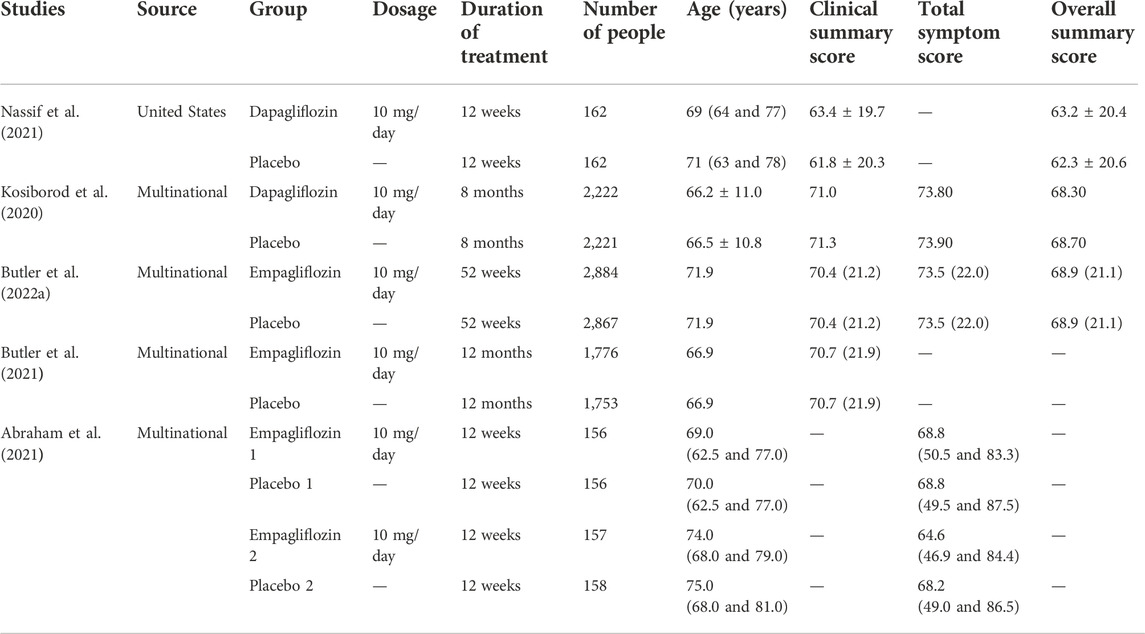
A total of 14,674 HF patients treated by SGLT-2 inhibitors, namely, dapagliflozin and empagliflozin, from published literature have been included for analysis (Kosiborod et al., 2020; Abraham et al., 2021; Butler et al., 2021; Nassif et al., 2021; Butler et al., 2022a). The strategy used for literature search is given in the Supplementary Material. The source, group, dosage, duration of treatment, number of people, age, clinical summary score (CSS), total symptom score (TSS), and overall summary score (OSS) have been collected from the published literature included for analysis.
For eliminating the potential baseline effect, the change rate of the KCCQ score is used as the evaluation index, which includes the CSS, TSS, and OSS. Eq. 1 describes the calculation method:
where k represents the KCCQ score which includes the CSS, TSS, and OSS; Sk represents the change rate of the KCCQ score; Sk,time represents the value of the KCCQ score at a time; Sk,base represents the value of the KCCQ score at baseline.
Model building
The quantitative effects of the SGLT-2 inhibitors on the KCCQ in HF patients are evaluated by the Emax model. For obtaining the actual effects of the SGLT-2 inhibitors on the KCCQ in HF patients, the placebo control group effects are subtracted from the sum effects, which are shown in Eqs 2, 3:
where Ss,k,i,j represents the sum effects of the SGLT-2 inhibitors on the KCCQ in HF patients; Sp,k,i,j represents the placebo control group effects on the KCCQ in HF patients; Sa,k,i,j represents the actual effects of the SGLT-2 inhibitors on the KCCQ in HF patients; k represents the KCCQ score, which includes CSS, TSS, and OSS; i represents different studies; and j represents the time point. Emax,k represents the maximal effects on the KCCQ; ET50,k represents the duration of treatment to reach half of the maximal effects; Ɛk,i,j represents the residual error of study i with j time under different KCCQ scores, which include the CSS, TSS, and OSS; Nk,i,j represents the sample size in study i with time point j under different KCCQ scores, which include the CSS, TSS, and OSS; Ɛk,i,j is weighted by the sample size, assumed to be normally distributed, with a mean of 0 and variance of σ2/(Nk,i,j/1,000).
The variability of the inter-study is described by the additive or exponential error model, given by Eqs 4–7:
ηk,1,i and ηk,2,i represent the inter-study variabilities, which are assumed to be normally distributed, with a mean of 0 and variance of ωk,1,i2 and ωk,2,i2, when available, they would be added into Emax, k or ET50, k, respectively; k represents the KCCQ score, which includes the CSS, TSS, and OSS.
Furthermore, Eqs 8–10 describe continuous or categorical covariates:
where Si represents the parameter for a patient with a covariate value of COV; ST represents the typical value of the parameter; COV represents the covariate; COVm represents the median value of the covariable in the population. θc represents a correction coefficient of the covariate to the model parameter. Dapagliflozin and empagliflozin are also selected as potential covariables to evaluate whether there is a significant difference in the pharmacodynamics between the two drugs.
The study is conducted via the nonlinear mixed-effect modeling (NONMEM, edition 7, ICON Development Solutions, Ellicott City, MD, United States) software. After the basic model is set up, the potential covariates are considered for adding into Emax,k or ET50,k. The objective function value (OFV) change is selected as the covariate inclusion criterion, where if the OFV decreases more than 3.84 (χ2, α = 0.05, d.f. = 1), it is considered sufficient for inclusion; however, if the OFV increases more than 6.63 (χ2, α = 0.01, d.f. = 1), it is considered sufficient for significance in the final model (Wang et al., 2021).
Model evaluation
The final models are evaluated by the observations vs. individual predictions and conditional weighted residuals (WRES) vs. individual predictions vs. individual plots. The predictive performance of the final models is assessed by visual predictive check (VPC) plots.
Prediction
The curves of the final models from the CSS, TSS, and OSS are simulated using the Monte Carlo method, which includes the time required to achieve 25%, 50%, 75%, and 80% Emax of the SGLT-2 inhibitors on the KCCQ in HF patients.
Results
Included patients
HF patients from two dapagliflozin and three empagliflozin studies are included for analysis (Kosiborod et al., 2020; Abraham et al., 2021; Butler et al., 2021; Nassif et al., 2021; Butler et al., 2022a). The dosages of dapagliflozin and empagliflozin are 10 mg/day. The KCCQ score of HF patients include the CSS, TSS, and OSS. The detailed information is given in Table 1.
Modeling
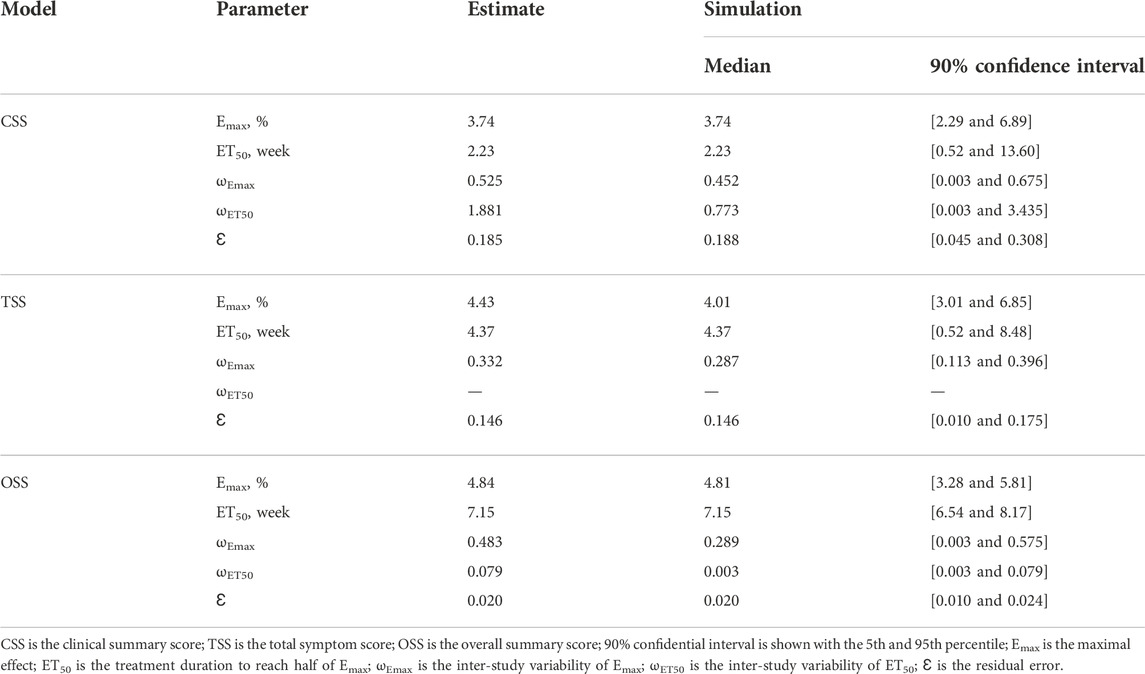
The parameter estimates of the final model are shown in Table 2. For the CSS, TSS, and OSS, the Emax of the SGLT-2 inhibitors on the quality of life in HF patients is 3.74%, 4.43%, and 4.84%, respectively, and the ET50 is 2.23 weeks, 4.37 weeks, and 7.15 weeks, respectively. In addition, the different SGLT-2 inhibitors, namely, dapagliflozin or empagliflozin, are not the covariates included in the final model, showing that there is no significant difference in the pharmacodynamics influencing the quality of life in HF patients between the SGLT-2 inhibitors: 10 mg/day dapagliflozin and 10 mg/day empagliflozin from the studies so far included in the analysis.
Finally, the relationships between the SGLT-2 inhibitors (dapagliflozin and empagliflozin) and the quality of life in HF patients are shown in Eqs. 11–13:
where S represents the change rate of the KCCQ score, and Eqs. 11–13 are the CSS, TSS, and OSS, respectively. Time is the duration of treatment for the SGLT-2 inhibitors in HF patients.
Evaluation
Figure 1 shows the goodness-of-fit plots of the model, which include observations vs. individual predictions and conditional weighted residuals (WRES) vs. individual plots. Figures 1A,B are from the CSS model, Figures 1C,D are from the TSS model, and Figures 1E,F are from the OSS model. They show good linear relationships between individual predictions and observations, indicating the better fitting of the final models. Figure 2 shows individual plots, Figures 2A–C are from the CSS, TSS, and OSS, respectively. They demonstrate acceptable predictability from the perspective of the clinical sparse data. Figure 3 shows the VPC plots, and Figures 3A–C are from the CSS, TSS, and OSS, respectively. They indicate the most observed data that are included in the 95% prediction intervals produced with the simulation data, meaning the predictive power of the final models.
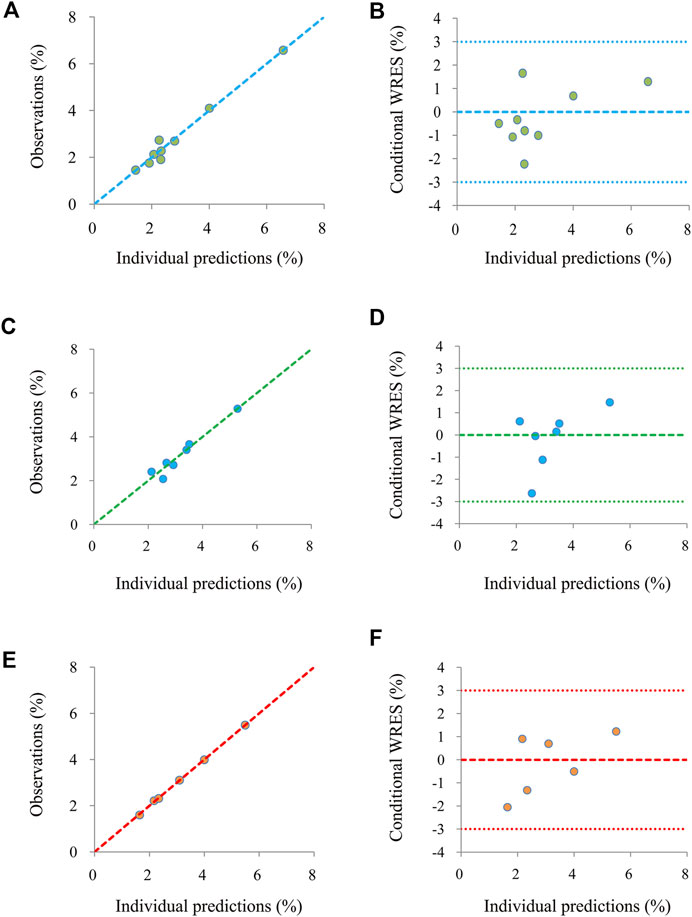
FIGURE 1. Model evaluation. (A,B) were from the CSS, (C,D) were from the TSS, and (E,F) were from the OSS. CSS: clinical summary score; TSS: total symptom score; OSS: overall summary score.
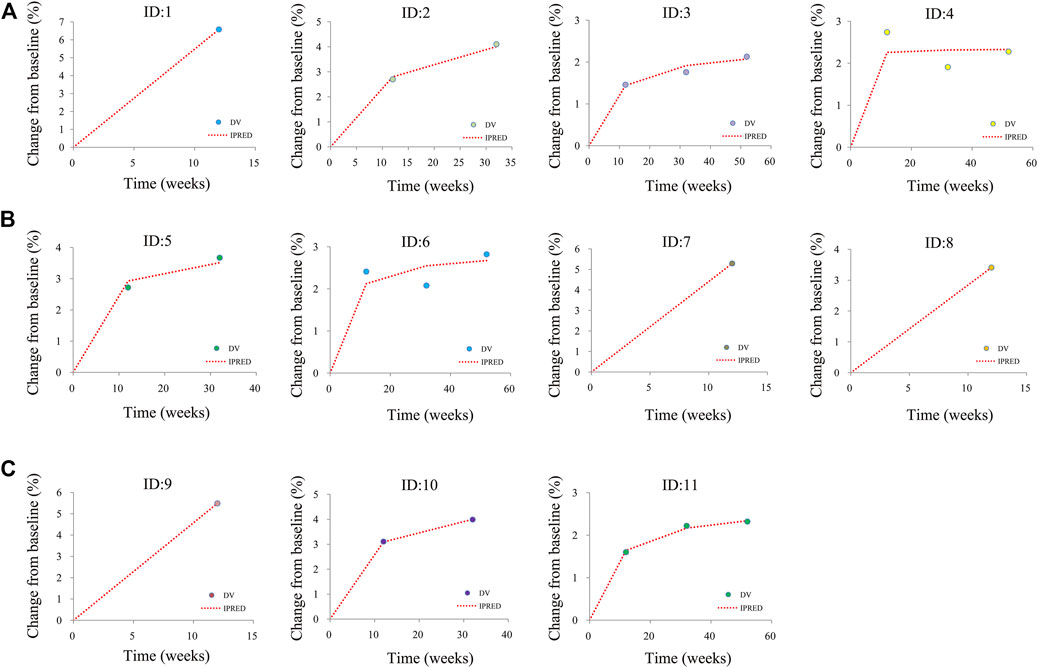
FIGURE 2. Individual plots. (A) was from CSS, (B) was from TSS, and (C) was from OSS; ID: 1–11 were from the studies (Kosiborod et al., 2020; Abraham et al., 2021; Butler et al., 2021; Nassif et al., 2021; Butler et al., 2022a). CSS: clinical summary score; TSS: total symptom score; OSS: overall summary score.
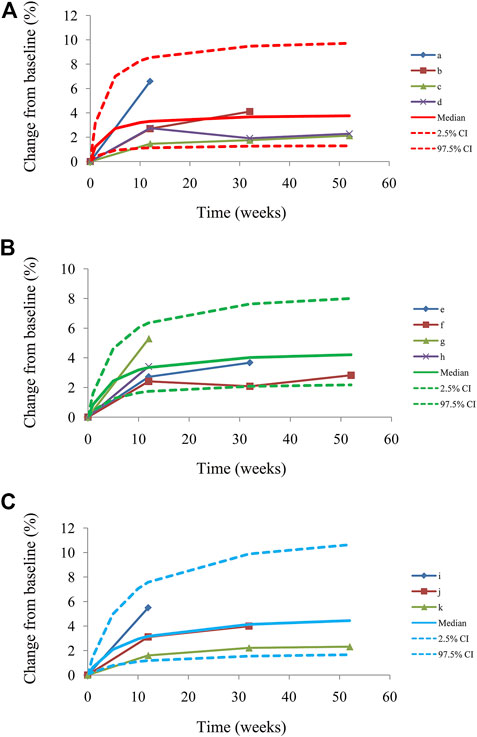
FIGURE 3. Visual predictive check plots from CSS (A), from TSS (B), from OSS (C), and a–k from the studies (Kosiborod et al., 2020; Abraham et al., 2021; Butler et al., 2021; Nassif et al., 2021; Butler et al., 2022a). Median, 2.5% CI and 97.5% CI were simulated by the Monte Carlo method (n = 1,000); CI, confidence interval. CSS: clinical summary score; TSS: total symptom score; OSS: overall summary score.
Prediction
The trends of efficacy of the SGLT-2 inhibitors on the quality of life in HF patients are shown in Figure 4. The time duration to achieve 25%, 50%, 75%, and 80% Emax is 0.75, 2.23, 6.69, and 8.92 weeks for the CSS; 1.46, 4.37, 13.11, and 17.48 weeks for the TSS; and 2.39, 7.15, 21.45, and 28.6 weeks for the OSS, respectively. Therefore, to reach the plateau period (80% of Emax) of the SGLT-2 inhibitors on the CSS, TSS, and OSS, 10 mg/day dapagliflozin (or 10 mg/day empagliflozin) is required to be taken for 8.92, 17.48, and 28.6 weeks, respectively.
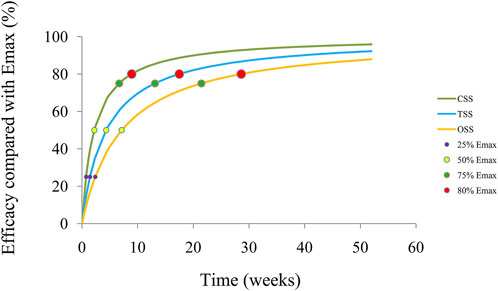
FIGURE 4. Model prediction. CSS: clinical summary score; TSS: total symptom score; OSS: overall summary score.
Discussion
HF is a syndrome of complex diseases, which could lead to death in severe cases and bring some challenges to treatment and nursing (Fiuzat et al., 2020; Wang A. et al., 2022). For patients with HF, the treatment objectives are to alleviate clinical symptoms, delay disease progression, improve long-term prognosis, reduce mortality and hospitalization rates, and improve the quality of life in patients as much as possible. In general, patients with HF need to take comprehensive treatment, which mainly includes removing the inducement, etiology, and symptomatic support treatments such as surgery and drug therapy, among which drug therapy is one of the most common means (Wang Y. et al., 2022).
SGLT-2 inhibitors are a newer group of antidiabetic drugs, with novel and unexpected pleiotropic effect. However, the number of relevant studies is increasing and the evidence of benefit on HF, and patients' quality of life, is mounting. Research in recent years has found that SGLT-2 inhibitors have the power to reduce the risk of cardiovascular outcomes and mortality (Mascolo et al., 2021), especially on HF patients who could gain benefits from therapy treatment with dapagliflozin and empagliflozin (Packer et al., 2020; Butt et al., 2021). The mechanism of the benefits of SGLT-2 inhibitors in HF is not clear, but includes improvement in myocardial energetics (Garcia-Ropero et al., 2019) due to enhanced cardiac consumption of fatty acids and ketone bodies (Santos-Gallego et al., 2019), reverse cardiac remodeling (Santos-Gallego et al., 2021b), improvement in diastolic function (Santos-Gallego et al., 2021a), natriuretic and diuretic effects that reduce congestion (Mordi et al., 2020), inhibition in sodium–hydrogen exchanger (Baartscheer et al., 2017), improvement in aortic stiffness (Requena-Ibanez et al., 2021), and systemic vascular resistance (Chilton et al., 2015).
In addition, the EMPA-TROPISM trial also corroborates the improvement in the quality of life in HF patients with SGLT-2 inhibitors (Requena-Ibanez et al., 2022). The cardiopulmonary exercise test (CPET) is a more sensitive technique to evaluate exercise capacity and quality of life. Of utmost importance, the SGLT-2 inhibitors also improve peak VO2 (the most sensitive parameter for exercise capacity and quality of life) in HF patients, both empagliflozin (Santos-Gallego et al., 2021b) and dapagliflozin (Palau et al., 2022). However, the quantitative effects of SGLT-2 inhibitors dapagliflozin and empagliflozin on the quality of life in HF patients remain unknown. The present study aims to explore the quantitative effects of SGLT-2 inhibitors on the quality of life in HF patients.
The KCCQ, assessing the influence on HF from the perspective of patients in their health status, is a disease-specific measure item (Green et al., 2000), which has been proved to be sensitive, valid, and reliable to clinical changes and at the same time associated with hospitalization, costs, and death (Spertus and Jones, 2015; Pokharel et al., 2017), where the CSS quantifies the physical function and symptoms domains, TSS includes symptom frequency and severity, OSS is sourced from the TSS, physical function, quality of life, and social function, and these are scored from a range of 0–100, with higher scores reflecting a better health status (Butler et al., 2021). At present, more and more studies have taken the KCCQ as a key evaluation index in the treatment of patients with HF. For example, Wohlfahrt et al. (2022) report HF-related quality-of-life impairment after myocardial infarction. DeVore et al. (2022) report the association of improvement in the left ventricular ejection fraction with outcomes in patients with HF with a reduced ejection fraction: data from CHAMP-HF. Butler et al. (2022b) report health status improvements with ferric carboxymaltose in HF with reduced ejection fraction and iron deficiency. In the abovementioned studies, the KCCQ is introduced as an important clinical evaluation indicator. Therefore, in this study, we choose the KCCQ, which includes the CSS, OSS, and TSS, as the evaluation index to explore the quantitative effects of the SGLT-2 inhibitors on the quality of life in HF patients.
In the present study, a total of 14,674 HF patients from two dapagliflozin and three empagliflozin studies have been included for analysis via NONMEM, among which the change rate of the KCCQ, which includes the CSS, OSS, and TSS, are used as the evaluation index. In the final model, there is no significant difference in the pharmacodynamics influencing the quality of life in HF patients between the SGLT-2 inhibitors: 10 mg/day dapagliflozin and 10 mg/day empagliflozin. For the CSS, TSS, and OSS, the Emax of the SGLT-2 inhibitors on the quality of life in HF patients is 3.74%, 4.43%, and 4.84%, respectively, and the ET50 is 2.23, 4.37, and 7.15 weeks, respectively. In addition, the time duration of achieving 25%, 50%, 75%, and 80% Emax is 0.75, 2.23, 6.69, and 8.92 weeks for the CSS; 1.46, 4.37, 13.11, and 17.48 weeks for the TSS; and 2.39, 7.15, 21.45, and 28.6 weeks for the OSS, respectively. Therefore, to reach the plateau period (80% of Emax) of the SGLT-2 inhibitors on the CSS, TSS, and OSS, 10 mg/day dapagliflozin (or 10 mg/day empagliflozin) is required to be taken for 8.92, 17.48, and 28.6 weeks, respectively.
In addition, to eliminate the potential baseline effect, the change rate of the KCCQ score is used as the evaluation index. To obtain the actual effects of the SGLT-2 inhibitors on the KCCQ in HF patients, the placebo control group effects are subtracted from the sum effects. Of course, there is no difference in the ejection fraction between the experimental group and control group from the same research source. By this data calculation, we can exclude the effect of different ejection fractions on the pharmacodynamics of the SGLT-2 inhibitors. In this way, the actual effects that we obtain of the SGLT-2 inhibitors on the KCCQ in HF patients are simply the effects of the SGLT-2 inhibitors without the effects from the ejection fractions.
However, the present study also has objective limitations. The SGLT-2 inhibitors analyzed in this study mainly include dapagliflozin and empagliflozin because other class drugs have not been reported or the number of their studies is too small to be analyzed. Therefore, more drugs from the SGLT-2 inhibitors and more studies are required to be further analyzed in the future.
Conclusion
This is the first time that the quantitative effects of the SGLT-2 inhibitors on the quality of life in HF patients have been explored. To reach the plateau period (80% of Emax) of the SGLT-2 inhibitors on the CSS, TSS, and OSS, 10 mg/day dapagliflozin (or 10 mg/day empagliflozin) is required to be taken for 8.92, 17.48, and 28.6 weeks, respectively.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
Conception and design: D-DW, S-MH, and XC; collection of data: D-DW, CZ, and PZ; data analysis and interpretation: D-DW; manuscript writing: D-DW; final approval of the manuscript: all authors.
Funding
This work was supported by the Initializing Fund of Xuzhou Medical University (No. D2021012), the Fusion Innovation Project of Xuzhou Medical University (No. XYRHCX2021011), and the Xuzhou Special Fund for Promoting Scientific and Technological Innovation (No. KC21257).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, editors, and reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.910858/full#supplementary-material
References
Abraham, W. T., Lindenfeld, J., Ponikowski, P., Agostoni, P., Butler, J., Desai, A. S., et al. (2021). Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur. Heart J. 42 (6), 700–710. doi:10.1093/eurheartj/ehaa943
Baartscheer, A., Schumacher, C. A., Wust, R. C., Fiolet, J. W., Stienen, G. J., Coronel, R., et al. (2017). Empagliflozin decreases myocardial cytoplasmic Na(+) through inhibition of the cardiac Na(+)/H(+) exchanger in rats and rabbits. Diabetologia 60 (3), 568–573. doi:10.1007/s00125-016-4134-x
Badoer, E. (2022). New insights into the role of inflammation in the brain in heart failure. Front. Physiol. 13, 837723. doi:10.3389/fphys.2022.837723
Bozkurt, B., Hershberger, R. E., Butler, J., Grady, K. L., Heidenreich, P. A., Isler, M. L., et al. (2021). 2021 ACC/AHA key data elements and definitions for heart failure: A report of the American college of cardiology/American heart association task force on clinical data standards (writing committee to develop clinical data standards for heart failure). Circ. Cardiovasc. Qual. Outcomes 14 (4), e000102. doi:10.1161/HCQ.0000000000000102
Bragazzi, N. L., Zhong, W., Shu, J., Abu Much, A., Lotan, D., Grupper, A., et al. (2021). Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur. J. Prev. Cardiol. 28 (15), 1682–1690. doi:10.1093/eurjpc/zwaa147
Butler, J., Anker, S. D., Filippatos, G., Khan, M. S., Ferreira, J. P., Pocock, S. J., et al. (2021). Empagliflozin and health-related quality of life outcomes in patients with heart failure with reduced ejection fraction: The EMPEROR-reduced trial. Eur. Heart J. 42 (13), 1203–1212. doi:10.1093/eurheartj/ehaa1007
Butler, J., Filippatos, G., Jamal Siddiqi, T., Brueckmann, M., Bohm, M., Chopra, V. K., et al. (2022a). Empagliflozin, health status, and quality of life in patients with heart failure and preserved ejection fraction: The EMPEROR-preserved trial. Circulation 145 (3), 184–193. doi:10.1161/CIRCULATIONAHA.121.057812
Butler, J., Khan, M. S., Friede, T., Jankowska, E. A., Fabien, V., Goehring, U. M., et al. (2022b). Health status improvement with ferric carboxymaltose in heart failure with reduced ejection fraction and iron deficiency. Eur. J. Heart Fail. 24, 821–832. doi:10.1002/ejhf.2478
Butt, J. H., Nicolau, J. C., Verma, S., Docherty, K. F., Petrie, M. C., Inzucchi, S. E., et al. (2021). Efficacy and safety of dapagliflozin according to aetiology in heart failure with reduced ejection fraction: Insights from the DAPA-HF trial. Eur. J. Heart Fail. 23 (4), 601–613. doi:10.1002/ejhf.2124
Chilton, R., Tikkanen, I., Cannon, C. P., Crowe, S., Woerle, H. J., Broedl, U. C., et al. (2015). Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes. Metab. 17 (12), 1180–1193. doi:10.1111/dom.12572
Cleland, J. G. F., van Veldhuisen, D. J., and Ponikowski, P. (2019). The year in cardiology 2018: Heart failure. Eur. Heart J. 40 (8), 651–661. doi:10.1093/eurheartj/ehz010
Conrad, N., Judge, A., Tran, J., Mohseni, H., Hedgecott, D., Crespillo, A. P., et al. (2018). Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 391 (10120), 572–580. doi:10.1016/S0140-6736(17)32520-5
DeVore, A. D., Hellkamp, A. S., Thomas, L., Albert, N. M., Butler, J., Patterson, J. H., et al. (2022). The association of improvement in left ventricular ejection fraction with outcomes in patients with heart failure with reduced ejection fraction: Data from CHAMP-HF. Eur. J. Heart Fail. 24, 762–770. doi:10.1002/ejhf.2486
Fiuzat, M., Lowy, N., Stockbridge, N., Sbolli, M., Latta, F., Lindenfeld, J., et al. (2020). Endpoints in heart failure drug development: History and future. JACC. Heart Fail. 8 (6), 429–440. doi:10.1016/j.jchf.2019.12.011
Garcia-Ropero, A., Vargas-Delgado, A. P., Santos-Gallego, C. G., and Badimon, J. J. (2019). Inhibition of sodium glucose cotransporters improves cardiac performance. Int. J. Mol. Sci. 20 (13), E3289. doi:10.3390/ijms20133289
Green, C. P., Porter, C. B., Bresnahan, D. R., and Spertus, J. A. (2000). Development and evaluation of the Kansas city Cardiomyopathy Questionnaire: A new health status measure for heart failure. J. Am. Coll. Cardiol. 35 (5), 1245–1255. doi:10.1016/s0735-1097(00)00531-3
Kashiwagi, A., and Maegawa, H. (2017). Metabolic and hemodynamic effects of sodium-dependent glucose cotransporter 2 inhibitors on cardio-renal protection in the treatment of patients with type 2 diabetes mellitus. J. Diabetes Investig. 8 (4), 416–427. doi:10.1111/jdi.12644
Kosiborod, M. N., Jhund, P. S., Docherty, K. F., Diez, M., Petrie, M. C., Verma, S., et al. (2020). Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: Results from the DAPA-HF trial. Circulation 141 (2), 90–99. doi:10.1161/CIRCULATIONAHA.119.044138
Mascolo, A., Scavone, C., Scisciola, L., Chiodini, P., Capuano, A., and Paolisso, G. (2021). SGLT-2 inhibitors reduce the risk of cerebrovascular/cardiovascular outcomes and mortality: A systematic review and meta-analysis of retrospective cohort studies. Pharmacol. Res. 172, 105836. doi:10.1016/j.phrs.2021.105836
Mordi, N. A., Mordi, I. R., Singh, J. S., McCrimmon, R. J., Struthers, A. D., and Lang, C. C. (2020). Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: The RECEDE-CHF trial. Circulation 142 (18), 1713–1724. doi:10.1161/CIRCULATIONAHA.120.048739
Nassif, M. E., Windsor, S. L., Borlaug, B. A., Kitzman, D. W., Shah, S. J., Tang, F., et al. (2021). The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: A multicenter randomized trial. Nat. Med. 27 (11), 1954–1960. doi:10.1038/s41591-021-01536-x
Packer, M., Anker, S. D., Butler, J., Filippatos, G., Pocock, S. J., Carson, P., et al. (2020). Cardiovascular and renal outcomes with empagliflozin in heart failure. N. Engl. J. Med. 383 (15), 1413–1424. doi:10.1056/NEJMoa2022190
Palau, P., Amiguet, M., Dominguez, E., Sastre, C., Mollar, A., Seller, J., et al. (2022). Short-term effects of dapagliflozin on maximal functional capacity in heart failure with reduced ejection fraction (DAPA-VO): A randomized clinical trial. Eur. J. Heart Fail. doi:10.1002/ejhf.2560
Pokharel, Y., Khariton, Y., Tang, Y., Nassif, M. E., Chan, P. S., Arnold, S. V., et al. (2017). Association of serial Kansas city Cardiomyopathy Questionnaire assessments with death and hospitalization in patients with heart failure with preserved and reduced ejection fraction: A secondary analysis of 2 randomized clinical trials. JAMA Cardiol. 2 (12), 1315–1321. doi:10.1001/jamacardio.2017.3983
Requena-Ibanez, J. A., Santos-Gallego, C. G., Rodriguez-Cordero, A., Vargas-Delgado, A. P., and Badimon, J. J. (2022). Empagliflozin improves quality of life in nondiabetic HFrEF patients. Sub-analysis of the EMPATROPISM trial. Diabetes Metab. Syndr. 16 (2), 102417. doi:10.1016/j.dsx.2022.102417
Requena-Ibanez, J. A., Santos-Gallego, C. G., Rodriguez-Cordero, A., Vargas-Delgado, A. P., Mancini, D., Sartori, S., et al. (2021). Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: From the EMPA-TROPISM study. JACC. Heart Fail. 9 (8), 578–589. doi:10.1016/j.jchf.2021.04.014
Roth, G. A., Forouzanfar, M. H., Moran, A. E., Barber, R., Nguyen, G., Feigin, V. L., et al. (2015). Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 372 (14), 1333–1341. doi:10.1056/NEJMoa1406656
Santos-Gallego, C. G., Requena-Ibanez, J. A., San Antonio, R., Garcia-Ropero, A., Ishikawa, K., Watanabe, S., et al. (2021a). Empagliflozin ameliorates diastolic dysfunction and left ventricular fibrosis/stiffness in nondiabetic heart failure: A multimodality study. JACC. Cardiovasc. Imaging 14 (2), 393–407. doi:10.1016/j.jcmg.2020.07.042
Santos-Gallego, C. G., Requena-Ibanez, J. A., San Antonio, R., Ishikawa, K., Watanabe, S., Picatoste, B., et al. (2019). Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J. Am. Coll. Cardiol. 73 (15), 1931–1944. doi:10.1016/j.jacc.2019.01.056
Santos-Gallego, C. G., Vargas-Delgado, A. P., Requena-Ibanez, J. A., Garcia-Ropero, A., Mancini, D., Pinney, S., et al. (2021b). Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J. Am. Coll. Cardiol. 77 (3), 243–255. doi:10.1016/j.jacc.2020.11.008
Spertus, J. A., and Jones, P. G. (2015). Development and validation of a short version of the Kansas city Cardiomyopathy Questionnaire. Circ. Cardiovasc. Qual. Outcomes 8 (5), 469–476. doi:10.1161/CIRCOUTCOMES.115.001958
Turner, R. J., and Moran, A. (1982). Heterogeneity of sodium-dependent D-glucose transport sites along the proximal tubule: Evidence from vesicle studies. Am. J. Physiol. 242 (4), F406–F414. doi:10.1152/ajprenal.1982.242.4.F406
Wang, A., Zhao, W., Yan, K., Huang, P., Zhang, H., Zhang, Z., et al. (2022a). Mechanisms and efficacy of traditional Chinese medicine in heart failure. Front. Pharmacol. 13, 810587. doi:10.3389/fphar.2022.810587
Wang, D. D., Mao, Y. Z., He, S. M., and Chen, X. (2021). Analysis of time course and dose effect from metformin on body mass index in children and adolescents. Front. Pharmacol. 12, 611480. doi:10.3389/fphar.2021.611480
Wang, Y., Liu, Y., Liu, Z., Cheng, Y., and Wang, D. (2022b). A systematic review and meta-analysis of the efficacy and safety of xinbao pill in chronic heart failure. Front. Pharmacol. 13, 846867. doi:10.3389/fphar.2022.846867
Keywords: Kansas City Cardiomyopathy Questionnaire, dapagliflozin, empagliflozin, heart failure patients, nonlinear mixed-effect modeling
Citation: Wang D-D, Zhang C, Zhu P, He S-M and Chen X (2022) Quantitative effects of sodium–glucose cotransporter-2 inhibitors dapagliflozin and empagliflozin on quality of life in heart failure patients. Front. Pharmacol. 13:910858. doi: 10.3389/fphar.2022.910858
Received: 01 April 2022; Accepted: 02 November 2022;
Published: 28 November 2022.
Edited by:
Carlos Garcia Santos-Gallego, Mount Sinai Hospital, United StatesReviewed by:
Juan Badimon, Icahn School of Medicine at Mount Sinai, United StatesMaeve Soto-Pérez, Hospital General Universitario de Ciudad Real, Spain
Copyright © 2022 Wang, Zhang, Zhu, He and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Su-Mei He, aGVoZTgyMDRAMTYzLmNvbQ==; Xiao Chen, Y2hlbnhpYW8xMTI3MzNAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Dong-Dong Wang
Dong-Dong Wang Cun Zhang
Cun Zhang Ping Zhu3†
Ping Zhu3† Su-Mei He
Su-Mei He Xiao Chen
Xiao Chen