
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 29 June 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.909914
This article is part of the Research TopicRising Stars in Ethnopharmacology: 2021View all 8 articles
Bilberry (Vaccinium myrtillus L.) fruits are an important part of local diets in many countries and are used as a medicinal herb to treat various disorders. Extracts from fruits are often a part of eye health-promoting supplements, whereas extracts from leaves are advertised for type 2 diabetes mellitus and glycemic control. This review provides an overview of the current knowledge of the phytochemical contents of bilberry fruits and leaves and their bioactivities, critically summarizes origins of the health claims and the outcome of clinical trials, with special attention towards those published in the past 10 years. Overall, the three most referenced indications, which are type 2 diabetes mellitus, vision disorders and circulatory diseases, all include contradictory results with no clear conclusion as to the benefits and recommended dosages. Moreover, the indications for vision disorders and diabetes originate from unproven or false claims that have been repeated in research since the 20th century without consistent fact-checking. Beneficial clinical results have been attested for the treatment of dyslipidemia and chronic inflammatory disorders when applied as dietary supplementation of fresh bilberries or as anthocyanin-rich bilberry fruit extracts. However, there is a general lack of double-blinded controlled research with larger sample sizes.
Bilberry (Vaccinium myrtillus L.) fruits are an important part of local diets in many countries. They are valued for their pleasant taste and are often processed into jams, preserves, pies, juices and alcoholic beverages. Their high market value is caused by their relatively difficult availability. Bilberry bushes only grow in wild, montane areas. It is not possible to cultivate them due to very specific soil demands. Picking the berries is a tedious and tiring work either using hands or small harvesting combs (Zoratti et al., 2016; Vaneková et al., 2020).
Bilberries have also been used as a medicinal herb to treat various disorders. The extracts from the fruits and leaves have a long-standing tradition of use for vision-related ailments, elevated blood sugar levels and several different cardiovascular disorders. The popularity of bilberries is increasing along with the marketing claims of being “functional food” or “superfood” and various health claims that are not always substantiated. To promote an herbal supplement either not backed by research at all or with only superficial proof might offer no benefits to the consumer or, at worst, cause serious harm to the health. Therefore, it is important to keep track of the recent findings in the field of ethnopharmacology.
Moreover, herbal supplements containing bilberry extracts are not subject to detailed quality control due to the generally lax legislation. Although the quality specifications for bilberries are regulated by three monographs of the European Pharmacopoeia (Bilberry fruit, fresh; Bilberry fruit, dried; Fresh bilberry fruit dry extract, refined and standardized) (European Directorate for the Quality of Medicines & HealthCare 2019) herbal supplements do not have to comply to the pharmacopoeial requirements. Indeed, as recently analyzed by Gaspar et al. (2021), supplements found in pharmacies and health food stores are often adulterated with anthocyanins from other sources, with other cheaper berry extracts, or they do not contain any beneficial extracts at all.
This review summarizes the current knowledge of the chemistry and bioactivities of the constituents of bilberry fruits and leaves. It aims to critically assess the origins and the level of scientific evidence behind the above-mentioned health claims with a special focus on clinical studies performed using bilberry fruits and leaves or extracts thereof.
(Syn. Myrtillus niger Gilib., Myrtillus sylvaticus Drejer, Vaccinium oreophilum Rydb., Vitis-idaea myrtillus (L.) Moench) (The Plant List, 2022).
Common bilberry is a small bush, 30–50 cm high, densely branched with erect green triangular twigs. Leaves are deciduous, bright green, 1–3 cm long with short petiole, ovate, with serrulate margin. Flowers singular, growing from axillary buds, with short pedicels, blooming in April–June. Sepals and petals five each, connate and forming an urceolate corolla, white with greenish or reddish hues. Stamens 8–10, anthers with two horn-like hollow appendages. The fruit is a round berry with persisting style, 5–10 mm wide, dark blue and glaucous. The pulp is as dark as the peel, the taste is sweet and astringent (Bertová, 1982). A rare albino form has greenish white fruits; the light color is caused by suppression of genes that code the anthocyanin synthesis (Zorenc et al., 2016).
Bilberry is a chamaephytic plant (plant that bears hibernating buds on persistent shoots near the ground), grows on acidic, wet, humous, rocky or bog soils. It spreads through seed dispersal as well as dense system of rhizomes (Bertová, 1982; Kliment and Valachovič 2007). In arctic and subarctic regions it characteristically grows in moist boreal forests dominated by Norway spruce, abundantly from the west coast of Northern Europe to Caucasus toward the northern Asia Pacific coast. In lower latitudes of Europe it can be found in dryish upland forests, heaths, and mountains. A few disjunct populations have been reported in western North America and central Japan (Zoratti et al., 2016).
‘Blueberry’ is an umbrella term which often causes confusion in nomenclature and can include several species of wild and cultivated Vaccinium plants, mainly of North American origin. It most commonly refers to V. corymbosum L. (northern highbush blueberry), V. angustifolium Aiton (common lowbush blueberry), V. ashei Reade. (rabbiteye blueberry), their cultivated varieties and various hybrids. The exact taxonomic differentiation is extremely difficult, as almost every single hybrid and variety has been pronounced a standalone species at some point. Out of the above-mentioned, V. corymbosum is the one with the highest commercial yields (Kole, 2011).
Northern highbush blueberry is a tall, erect, deciduous bush 0,5–2 m tall, twigs are yellow-green and lenticellar. Leaves alternate, ovate, 3–8 cm long, margin entire to serrulate, color is dark green and changes to red in autumn. Flowers are white or sometimes reddish white, narrowly urceolate, 1 cm long, in terminal racemes. Bloom is in spring, and fruits are round berries, with dark blue and glaucous peel and greenish white pulp, 1–2 cm in diameter and sweet.
This species originates from the eastern United States and Canada where it grows on acidic soils of heathlands, margins of lakes and rivers, swamps, bogs, meadows, and forests. It is cultivated all over the world as a commercial fruit bush, in many different cultivars and hybrids (Horáček 2007; Flora of North America, 2022).
The second most common species of the Vaccinium genus in Europe is the common lingonberry (Vaccinium vitis-idaea L.). Lingonberry fruit is a round berry, the peel is red and covered in shiny wax layer; the pulp is white and has a sour and astringent taste (Bertová, 1982; Horáček 2007). Lingonberry is a chamaephytic, often creeping bush, growing on acidic and humous soils in boreal, subalpine and alpine zones (Bertová, 1982; Kliment and Valachovič 2007), used similarly to cranberry (Vaccinium oxycoccos or V. macrocarpon) because of its similar taste and aroma, albeit more sour and astringent.
Vaccinium × intermedium Ruthe is a rare natural hybrid of bilberry and lingonberry. It was experimentally proven that it is an offspring of lingonberry pollen and a flowering bilberry plant. The biggest barrier to hybridization lies in different flowering times–bilberry usually flowers several weeks earlier than lingonberry when they grow in the same location.
Bilberry and lingonberry display some substantial differences in their growth strategies and morphology, which are visible as intermediate characteristics in the hybrid. In the hybrid some leaves drop in the fall and others overwinter. The fruits of the hybrid grow single in branches, similarly to bilberries, whereas lingonberries grow in clusters. The hybrid species produces notably less flowers and smaller fruits than the parent plants and the colour is dark but has more reddish hue (Lätti et al., 2011).
The species most commonly mistaken for bilberry in its natural habitat is bog bilberry (Vaccinium uliginosum L.). It is a bog species frequent in low heathlands and montane moorlands of Europe, Asia and North America. The fruit is a dark blue round berry with whitish pulp (Bertová, 1982; Horáček 2007). They are universally considered edible and nutritious, however local sources in Europe mention the folklore names “Rauschbeere” (DE: “intoxicating berry”), “šialenica” (SK: “mad berry”), “blinkavka” (CZ: “vomit berry”) or „opilki” (PL: “drunken berry”) and accompanying effects after consumption: headaches, nausea, vomiting and hallucinations. This is unlikely to be caused by the berry itself, rather possibly by a species of parasitic fungus Monilinia megalospora (Woronin) Whetzel., which forms mycelia in ripe and fallen berries, turns them into sclerotia and forms the apothecium the following spring (Woronin 1888; Kotlaba and Pilát 1952; Rätsch 2005). This is, however, merely a hypothesis, as it has not been proven by research. There are currently no studies regarding the potential fungal metabolites or their effects in the scientific literature.
The fruits of each above listed species have a characteristic anthocyanin profile which can be used for fingerprint analysis:
• V. myrtillus contains mainly delphinidin and cyanidin (Figure 1) in a 1 : 1 ratio, followed by petunidin, peonidin and malvidin
• V. vitis-idaea contains mainly cyanidin (>90%) and small amounts of peonidin, other anthocyanins are absent
• V. × intermedium typically contains more cyanidin compared to bilberry
• V. corymbosum contains mainly delphinidin, peonidin is absent
• V. uliginosum contains delphinidin and malvidin in 1 : 1 ratio (Määttä-Riihinen et al., 2004)
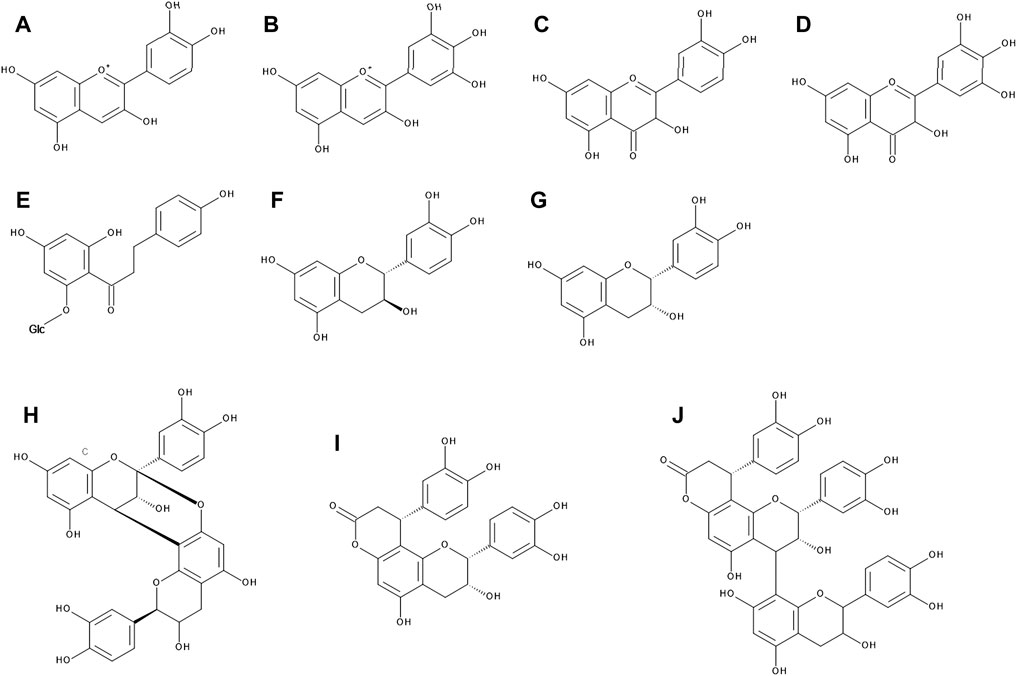
FIGURE 1. The chemical structures of cyanidin (A), delphinidin (B), quercetin (C), myricetin (D), phlorizin (E), catechin (F), epicatechin (G), A-type procyanidin dimer (H), cinchonain Ia (I), and cinchonain IIa (J).
V. myrtillus has the most intensely colored berries out of all above mentioned species. This is due to the fact that both peel and pulp contain large amount of anthocyanidins (up to 2% of the fresh weight in peels), whereas in other species the pulp is white or light pink at most (Riihinen et al., 2008). In bilberries, anthocyanins comprise about 90% of the total phenolic compounds of the fruit (Kähkönen et al., 2003; Heinonen 2007). In nature, anthocyanins occur mainly in glycosylated form. The most common types in fruits of Vaccinium genus are glucosides, galactosides and arabinosides (Määttä-Riihinen et al., 2004; Lätti et al., 2011; Colak et al., 2016). Fruits of V. myrtillus contain unique cyanidin- and delphinidin-3-O-sambubiosides where the saccharide moiety is glucose (2→1) xylose (Du et al., 2004). It is also possible to isolate 3-O-methyl anthocyanidins in large amounts from bilberry fruits (Ichiyanagi et al., 2020).
Quercetin is the main flavonol of bilberry fruits, accounting for more than 50% of total flavonoid content (Riihinen et al., 2008; Cardeñosa et al., 2016; Zorenc et al., 2016). The second most abundant one is myricetin (Figure 1); other flavonols, such as syringetin, laricitrin and isorhamnetin, have only been detected in low levels (Mikulic-Petkovsek et al., 2014; Zorenc et al., 2016). Kaempferol, although abundant in bilberry leaves (Riihinen et al., 2008; Hokkanen et al., 2009; Cardeñosa et al., 2016), is present in fruits only in trace amounts (Zorenc et al., 2016). Flavonols in V. myrtillus occur mainly in glycosylated form. Studies determined various hexosides, pentosides and glucuronides. The most abundant glycosides appear to be rhamnosides and glucuronides (Zorenc et al., 2016). Phlorizin, a glucoside of a dihydrochalcone phloretin, was found in bilberry fruits, however the aglycone itself was not detected (Ancillotti et al., 2016).
Plants of the entire Vaccinium genus are one of the richest sources of tannins, of both hydrolyzable and condensed type, as well as their various derivatives. Catechin and epicatechin have been found in both leaves and fruits in abundance (Hokkanen et al., 2009; Može et al., 2011; Ancillotti et al., 2016; Zorenc et al., 2016), a B-type dimer of catechin was found in fruits (Määttä-Riihinen et al., 2005). Gallocatechin and epigallocatechin can be found predominantly in leaves (Hokkanen et al., 2009; Zorenc et al., 2016). Both leaves and fruits of V. myrtillus are especially rich in procyanidin, its various dimers and trimers. The universal B-type procyanidins have a single bond between structural units of catechins, whereas the rare A-type procyanidins are double bonded. The A-type linkages (Figure 1) dominate in bilberries over the B-type, which is uncommon in other plant species (Määttä-Riihinen et al., 2005; Riihinen et al., 2008; Hokkanen et al., 2009; Ancillotti et al., 2016).
Various isomers of the flavonolignans cinchonains I and II (Figure 1) were detected in leaves and stems of V. myrtillus (Hokkanen et al., 2009; Bujor et al., 2016). However, in the fruits only cinchonain I isomers were found (Bujor et al., 2016).
The existence of the coumarins, namely umbelliferone (Tumbas Šaponjac et al., 2015) and esculetin (Ancillotti et al., 2016), have been reported from bilberry fruits.
The presence of arbutin (Figure 2) in the leaves V. myrtillus L. has always been widely debated. Ramstad explored the claims in several printed sources from the first half of the 20th century that the drug contains arbutin and concluded that these claims do not seem to be based upon chemical investigation of the plant but rather inferred by virtue of a close botanical relationship of V. myrtillus to other arbutin-bearing species such as Arctostaphylos uva-ursi and V. vitis-idaea (Ramstad 1954). Also Von Friedrich and Schönert did neither prove the presence of arbutin nor hydroquinone in V. myrtillus (Von Friedrich and Schönert 1973). Sticher et al. used HPLC to prove the absence of arbutin, hydroquinone and their derivatives in V. myrtillus (Sticher et al., 1979). Rychlinska and Nowak optimized the HPLC method for proving arbutin and hydroquinone simultaneously and identified them in the leaves of Arctostaphylos uva-ursi and V. vitis-idaea, but not in leaves of V. myrtillus and V. uliginosum (Rychlinska and Nowak 2012). Two separate research teams came to the same conclusion also using HPLC (Ieri et al., 2013; Ștefănescu et al., 2020). Hokkanen et al. identified arbutin in leaves of lingonberry and its hybrid Vaccinium × intermedium, but not in bilberry (Hokkanen et al., 2009).
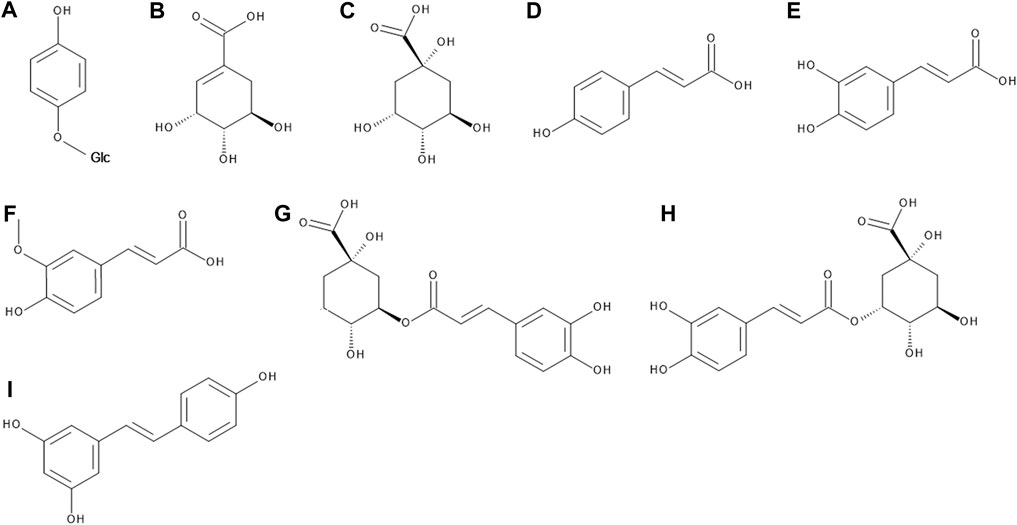
FIGURE 2. The chemical structures of arbutin (A), shikimic acid (B), quinic acid (C), p-coumaric acid (D), caffeic acid (E), ferulic acid (F), chlorogenic acid (G), neochlorogenic acid (H), and resveratrol (I).
Contrarily, two studies describe the determination of arbutin in bilberry leaves using spectrophotometry (Rosłon et al., 2011) or HPLC (Stefkov et al., 2014) but those might suffer from mistakes in their methodology. Overall, studies agree that V. myrtillus does not contain any arbutin unless hybridized with lingonberry.
Bilberry leaves and fruits are an abundant source of organic acids and their derivatives. Their content and exact structures have been researched in great detail in the last 15 years. The studies generally agree that they comprise the majority of the total phenolic compounds in the leaves, whereas in the fruits they are overshadowed by anthocyanins (Riihinen et al., 2008; Bujor et al., 2016). The simple organic acids found in bilberry fruits include shikimic, quinic, citric and malic acid (Mikulic-Petkovsek et al., 2014). As for phenolic and hydroxycinnamic acids, their composition is dominated by p-coumaric, caffeic and ferulic acid (Hokkanen et al., 2009; Tumbas Šaponjac et al., 2015; Ancillotti et al., 2016; Zorenc et al., 2016), followed by syringic acid (Tumbas Šaponjac et al., 2015), gallic and ellagic acid (Može et al., 2011; Ancillotti et al., 2016). In a minority, vanillic acid (Juadjur et al., 2015), dihydroxybenzoic acid (Borowiec et al., 2014) and salicylic acid (Ancillotti et al., 2016) can be found in bilberry fruits.
Above listed organic acids can be detected in these plants either free, esterified, or etherified. They form esters either with each other or with other phenolic compounds. Etherification usually occurs with various saccharide moieties (Ieri et al., 2013; Bujor et al., 2016). The most notable out of these derivatives are chlorogenic and neochlorogenic acid (Figure 2), which often make up the majority of the total phenolic acid content of bilberry fruits and leaves (Riihinen et al., 2008; Ancillotti et al., 2016; Bujor et al., 2016).
Resveratrol (Figure 2) was found in the bilberry fruits by two different research groups (Rimando et al., 2004; Može et al., 2011) and in the leaves (Tadić et al., 2021). It is worth noting that while cultivated blueberries contain both resveratrol and piceatannol, the latter is missing in bilberries completely (Rimando et al., 2004). The presence of pterostilbene is still being debated; some report a complete lack of it (Rimando et al., 2004), others report its presence in bilberry fruit extracts (Habanova et al., 2016; Waszczuk et al., 2020).
The whitish wax cuticle covering the bilberry fruits consists mainly of triterpenoids such as α- and ß-amyrin, oleanolic and ursolic acid (Figure 3), as well as fatty cerotic acid, sitosterol and 2-heneicosanone. The wax layer of bilberries is similar to that of blueberries and much thinner than that of lingonberries and crowberries (Dashbaldan et al., 2019; Trivedi et al., 2019). Oleanolic and ursolic acid, α- and ß-amyrin, lupeol, lupenyl acetate and betulin were identified in the extract of bilberry leaves (Vrancheva et al., 2021).
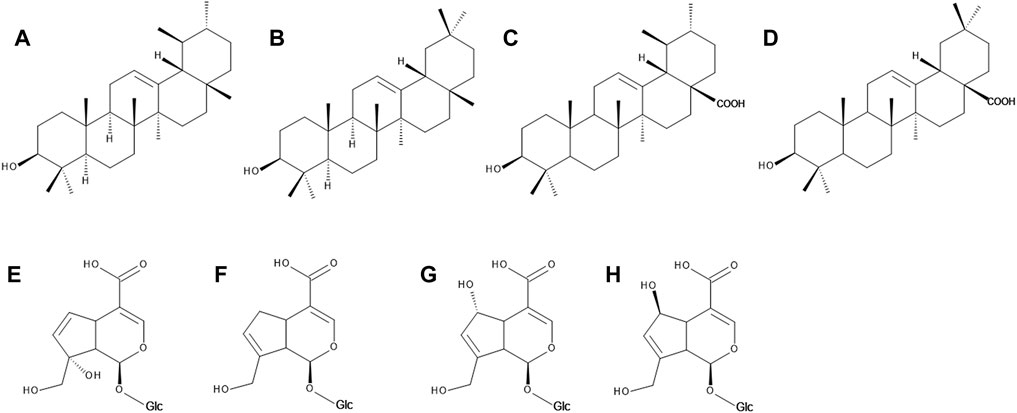
FIGURE 3. The chemical structures of α-amyrin (A), ß-amyrin (B), usolic acid (C), oleanolic acid (D), monotropein (E), geniposidic acid (F), deacetylasperulosidic acid (G) and scandoside (H).
ß-Carotene, as well as the xanthophylls lutein, zeaxanthin and ß-cryptoxanthin were found in bilberry fruits (Mahdavi et al., 1998; Bunea et al., 2012). The bilberry seeds, as well as the oil exctracted from them, is rich in vitamin E, notably γ-tocotrienol (Gustinelli et al., 2018).
In V. myrtillus monotropein, dihydromonotropein and deacetylasperulosidic acid (Figure 3) were identified either as standalone compounds (Jensen et al., 2002; Heffels et al., 2017), or as derivatives, such as p-coumaroyl monotropein, p-coumaroyl dihydromonotropein (Bujor et al., 2016), p-coumaroyl-deacetylasperulosidic acid (Heffels et al., 2017). 10-p-Trans-coumaroyl-1S-monotropein is an iridoid glycoside found in bilberry fruits which subsequently got a name vaccinoside (Aaby et al., 2013). Scandoside, its p-coumaroyl derivative (Heffels et al., 2017) and geniposidic acid (Borowiec et al., 2014) were reported in the bilberry fruits. Several studies also report on a significant number of various iridoid derivatives which, however, could not be further identified (Hokkanen et al., 2009; Ieri et al., 2013; Juadjur et al., 2015; Zorenc et al., 2016; Heffels et al., 2017).
Xyloglucans are heteropolysaccharides with mechanical function. In plants they form bridges between cellulose microfibrils in the cell wall. In bilberry fruits they consist of xylose, glucose, galactose and fucose (Hilz et al., 2007).
A study regarding volatile compounds of V. myrtillus, V. corymbosum and others determined that out of a vast number of volatile compounds present in these fruits, the ones with most prominent blueberry-like aroma were ethyl 3-hydroxy-3-methylbutanoate, a few other esters with similar structure, and hydroxycitronellol (Figure 4). Other compounds responsible for the aroma were identified as trans-2-hexenal, trans-2-hexenoic acid, phenylacetaldehyde, methyl salicylate and 2-phenylethyl formate (Hirvi and Honkanen 1983).
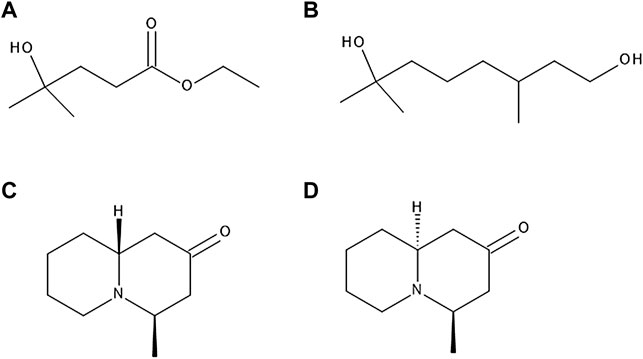
FIGURE 4. The chemical structures of ethyl 3-hydroxy-3-methylbutanoate (A), hydroxycitronellol (B), myrtine (C) and epimyrtine (D).
Alkaloids myrtine and epimyrtine (Figure 4) were detected in above-ground parts of bilberry plants (Slosse and Hootelé 1981). Another, more recent study confirmed these findings (Nardin et al., 2017).
Wild berries are a valuable part of the European nature and tradition. Especially in the northern and eastern parts of Europe, wild berries grow abundantly, and berry picking is an important form of recreation for many people. In these areas, about half of the wild edible berries are picked for personal consumption and the other half for commercial use. They are picked either by hand or by traditional wooden or metal combs. The average bilberry yield in Nordic countries has been estimated to account for more than 500 million kg per year, of which only 5–8% is currently exploited. The wild berry industry in Europe is typically fairly small and fragmented. The distinctions in the forest policies, access rights, and the berry picking traditions also differ to some extent between European countries.
To secure the availability of bilberries for commercial exploitation, methods for field cultivation and semi-cultivation of natural stands have been considered. Semi-cultivation of wild stands of Vaccinium spp. has been a great success in North America for decades. The North American lowbush blueberry, Vaccinium angustifolium Aiton, is semi-cultivated using modern agricultural practices such as fertilization, pruning, and mechanical harvesting. Attempts to cultivate and semi-cultivate the bilberry have been initiated in Denmark, Norway, and Finland, with modest results, because of the plant’s specific environmental demands (Nestby et al., 2011; Zoratti et al., 2016).
Most bilberries picked commercially in Northern Europe are exported frozen and unprocessed to East Asia or to the local food industry. China and Japan have been the major customers, increasingly focusing on health products. In their home countries and abroad alike, bilberries are processed into juices, jams, preserves and other highly demanded products (Kresánek and Kresánek 2008; Zoratti et al., 2016). Other industrial uses include the extraction of anthocyanins as food colorants, utilizing the press cake after juicing and extraction of oil from bilberry seeds (Pires et al., 2020).
The bilberry fruits and fruit extracts are currently on the market as herbal medicinal products with traditional use, according to two herbal monographs by European Medicines Agency (EMA): Bilberry fruit, fresh, which also includes its standardized dry extract (European Medicines Agency, 2015b), and Bilberry fruit, dried (European Medicines Agency 2015c); whereas the former is a herbal drug to relieve symptoms of discomfort and heaviness of legs related to minor venous circulatory disturbances and to relieve symptoms of cutaneous capillary fragility, the latter is applied for symptomatic treatment of mild diarrhoea and minor inflammations of the oral mucosa. The analytic quality of these herbal drugs is regulated by their respective monographs in the European Pharmacopoeia, with an additional monograph for Fresh bilberry fruit dry extract, refined and standardized (European Directorate for the Quality of Medicines & HealthCare 2019).
Natural Standard Research collaboration assessed the clinical evidence on bilberry and its extracts for several indications including diabetes mellitus, glaucoma, retinopathy, cataracts and night vision in 2009. All indications have been assessed with grade C—„Unclear or conflicting scientific evidence”, except for night vision, which was assessed one grade lower, D—„Fair negative scientific evidence“ (Ulbricht et al., 2009).
As there has been an abundance of new clinical studies in the recent years (Supplementary Table S1), these indications will be critically reviewed below.
Berries of all Vaccinium species contain unusually high levels of antioxidant compounds, which effectively makes them valuable food for dietary supplementation of antioxidant compounds. V. myrtillus is mainly characterised by delphinidin and cyanidin glycosides together with quercetin and chlorogenic acid (Ancillotti et al., 2016). When studying the antioxidant activities of the whole extract, a strong synergistic effect was apparent, as most of the measured effects were associated not with the anthocyanin fractions, but with the unfractioned extract (Juadjur et al., 2015).
Recent studies have suggested that anthocyanins and other polyphenols can also have indirect effects by stimulating antioxidative defence mechanisms via induction of enzymes such as GST or GSH-Px (Juadjur et al., 2015) or DAF-16 and HSF-1 (González-Paramás et al., 2020). These mechanisms are usually dose dependent. However, a study on isolated rat hearts showed that high concentrations of bilberry anthocyanins (5–50 mg/L) can have diminished cardioprotection and show cardiotoxic activity despite having their radical scavenging and intracellular antioxidant capabilities increased in a concentration-dependent manner (Žiberna et al., 2010).
Antioxidant activity is prerequisite to many beneficial effects in human body: bilberry extracts inhibit oxidative modification of human LDL in vitro, inhibit lipid peroxidation in rat liver microsomes and liver lipid peroxidation in vivo in mice (Tumbas Šaponjac et al., 2015), attenuate liver damage induced by CCl4 or stress in rats (Bao et al., 2010; Domitrović and Jakovac 2011; Popović et al., 2016), ameliorate oxidative stress after ischaemia-reperfusion injury in isolated rat heart (Žiberna et al., 2010), during metabolic syndrome (Shi et al., 2017) or in cancer cells (Tumbas Šaponjac et al., 2015).
Drawing from its powerful content of antioxidants, bilberry fruits and their extracts have been investigated for their anti-inflammatory activities as well. Bilberry extract Mirtoselect, standardized to contain 40% of anthocyanins, has been found to induce a complex anti-inflammatory response in lipopolysaccharide-activated macrophages. The response consists of attenuation of pro-inflammatory cytokines (including TNF- α, IL-1β, IL-6, and COX-2), attenuation of multiple lipopolysaccharide-induced chemokines and IL receptors almost to control levels (Chen et al., 2008). In human THP-1 monocytic cells, the response of the cells treated with anthocyanin-rich bilberry extract to inflammatory stimulation was more varied—bilberry extract attenuated most of the IFN-γ-induced signal protein activation, pro-inflammatory gene expression, and cytokine secretion, whereas it enhanced TNF-α-induced responses (Roth et al., 2014). This suggests a distinct role of anthocyanins in the inflammatory modulation that might vary from target to target.
These in vitro results are reflected in several clinical studies for inflammatory diseases. For example, after only 7 days of consuming 500 g bilberries per day, the patients suffering from gingivitis showed reduced inflammatory cytokine levels and less bleeding on probing, which is a routine dental clinical parameter of inflammation (Widén et al., 2015). In a study on patients with increased cardiovascular risk, after 4 weeks of consuming 330 ml of bilberry juice daily, some of the participants‘ inflammatory blood markers significantly decreased compared to placebo group (C-reactive protein, IL-6, IL-15, and monokine induced by INF-γ), while other remained unchanged. Unexpectedly, an increase of TNF-α was also observed (Karlsen et al., 2010).
In test subjects with metabolic syndrome, supplementing 400 g fresh bilberries daily for 8 weeks into their diet caused reductions in several inflammatory parameters (C-reactive protein, IL-6, IL-12, and LPS) and generally lower inflammation scores (Kolehmainen et al., 2012). Similar anti-inflammatory results were achieved in metabolic syndrome patients by a supplement containing purified bilberry and blackcurrant anthocyanins, therefore it can be hypothesized that they are the main active principle behind the favorable effects of bilberries on cardiometabolic risk factors (Aboonabi et al., 2020).
Inflammatory bowel diseases are not always satisfactorily treated with standard therapy regimens and the current evidence suggests that a dysregulated immune response to intestinal microbiota induces the relapsing inflammation. In vitro experiments, bilberry fruit extract and the isolated anthocyanins were able to attenuate the inflammatory response of human colon epithelial cell cultures stimulated by IFN-γ/IL-1β/TNF-α (Triebel et al., 2012). In patients with mild to moderate ulcerative colitis, an open, non-blinded and non-controlled trial showed that 90,9% subjects showed a positive response to a preparation made of dried bilberry powder and concentrated bilberry juice, with 63,4% patients achieving remission (Biedermann et al., 2013). This makes bilberries a promising therapeutic for inflammatory intestinal disorders and further studies on mechanisms, as well as randomized blinded trials are warranted.
However, the anti-inflammatory effect of bilberries might not be universally applicable. It was observed that the consumption of bilberry juice before, during and 2 days after a half marathon race paradoxically caused a mild increase of exercise-induced muscle soreness and of C-reactive protein levels in blood (Lynn et al., 2018).
Type 2 diabetes mellitus (T2DM) is a complex disease characterised by hyperglycaemia with an antecedent phase of insulin resistance, often associated with dyslipidemia, increase in pro-inflammatory cytokines and oxidative stress.
According to current studies, the possible pathways of bilberries’ in vitro antidiabetic activity are most likely complex and working in synergy. It has been suggested that tannins may have therapeutic potential in the treatment of T2DM, mainly through two ways: they may lower glucose levels by delaying intestinal glucose absorption through inhibition of intestinal α-glucosidase, and they may delay the onset of T2DM by regulating the antioxidant environment of pancreatic β-cells (Shi et al., 2017). Myricetin is a flavonoid abundant in berries and it was reported that the anti-diabetic effectiveness of myricetin is due to its anti-inflammatory activity (Wu et al., 2016). Anthocyanins have been demonstrated to significantly reduce glucose production by 24–74% in H4IIE hepatocytes (Shi et al., 2017). Abscisic acid is another promising compound with antidiabetic properties. It is a plant signalling molecule that plays an important role in fruit ripening and seed development. It has been shown to up-regulate the peroxisome proliferator-activated receptor, PPAR γ, both in vitro and in vivo. Abscisic acid has been shown to ameliorate the symptoms of T2DM after oral administration, targeting PPAR γ in a similar manner than the thiazolidinediones class of anti-diabetic drugs. It is present in most plant tissues (Bassaganya-Riera et al., 2010). Bilberry fruits contain the highest level of abscisic acid just before the fully ripe stage (38 μg/g dry weight) but fully ripe fruits still contain a considerable amount of this metabolite (13 μg/g dry weight) which can add to the overall antidiabetic effect (Karppinen et al., 2013). Cinchonain Ib has been shown to increase plasma insulin levels in a way similar to glibenclamide in vitro and in vivo after oral administration (Qa'Dan et al., 2009). It has been theorized that the various cinchonains found in leaves of V. myrtillus could be partially responsible for the blood glucose lowering effects (Hokkanen et al., 2009).
In a murine model, the anthocyanin-rich bilberry fruit extract significantly reduced the blood glucose concentration and enhanced insulin sensitivity. AMPK was activated in white adipose tissue, skeletal muscle, and the liver of diabetic mice. This activation was accompanied by upregulation of glucose transporter 4 in white adipose tissue and skeletal muscle and suppression of glucose production and lipid content in the liver. At the same time, acetyl-CoA carboxylase was inactivated and PPARα, acyl-CoA oxidase, and carnitine palmitoyltransferase-1A were upregulated in the liver (Takikawa et al., 2010). Similarly, three different bilberry fruit extracts reduced the trehalose level in the hemolymph of sugar-fed Drosophila melanogaster to 50% compared to the control group (Neamtu et al., 2020).
Generally speaking, a high dietary intake of anthocyanins and flavonoids has been associated with a decreased insulin resistance in women of all ages by a large cohort study (Jennings et al., 2013). In line with this result, the dietary anthocyanin consumption has been associated with a 15% reduction of T2DM risk in a large meta-analysis (Guo et al., 2016).
In clinical trials using V. myrtillus specifically, oral administration of bilberry fruits and extracts thereof yielded mixed results. Saccharide-free extracts tended to show better antidiabetic effect than whole fruits or fruit juices, most likely due to the higher content of biologically active substances instead of sugars, which are present in berries in considerable quantities (Shi et al., 2017). One study showed that patients with T2DM who ingested a single oral dose of bilberry fruit extract (standardized 36% anthocyanins) had lower postprandial insulin and glucose blood levels than the placebo group (Hoggard er al. 2013). Another study, where patients added 400 g fresh bilberries to their diet for 8 weeks, showed an inverse correlation between dietary intake of bilberries and fasting plasma glucose level, but insulin sensitivity remained unchanged (De Mello et al., 2011).
However, other clinical studies have indicated that there were no significant differences in fasting plasma glucose between the treatment and the control groups after dietary supplementation with anthocyanins for 12 (Qin et al., 2009) or 24 weeks (Zhu et al., 2013), or after ingesting 400 g fresh bilberries daily for 2 months (Kolehmainen et al., 2012). Even the newest clinical study on Chinese patients with T2DM using 1400 mg of bilberry extract per day did not report any significant differences compared to placebo in any of the measured biomarkers (Chan et al., 2021).
The first record of bilberry fruits for eye issues goes back to World War II when a bilberry jam was allegedly used by British pilots to increase night vision. There exist multiple (mostly unsourced) claims that this was a hoax purposefully created by British to cover up the technological advancement of airplane radar systems (Canter 2004) or purely a product of R.A.F. pilots’ famous superstitions (Bilberries and Night Vision: Reality Check, 2022). However, in the search for a proof, the claims have been since studied in various models.
The fruits were shown to contain lutein, which is commonly used as a supplement to improve eye health (Bunea et al., 2012). However, the anthocyanins are often commonly referred to as the compound class which improves night vision. The outcomes of clinical trials are inconsistent, as summarized by a systematic review of the available clinical studies (Canter and Ernst 2004). The ones using relatively low doses of standardized anthocyanins (12–150 mg daily) did not prove any beneficial effects (e.g., Mayser and Wilhelm 2001). Studies with higher doses (up to 720 mg/day) showed beneficial effects in some measured aspects of night vision, but never enough to conclude the study as successful. One study in particular used 40 military pilots as test subjects with 400 mg bilberry anthocyanins taken before flight. Subjects reported reduced and shorter-lived post-dazzling after-images and reduced subjective visual fatigue but there was no difference in objective ophthalmological values (Belleoud et al., 1966). Overall, the systematic review of the available clinical studies published in 2004 shows that the extract does not improve night vision in healthy eyes, but there is a serious lack of rigorous clinical trials in patients with impaired night vision or diagnosed eye disease. There are plausible mechanisms by which constituents of V. myrtillus could modify vision and the health of the eye, including accelerated re-synthesis of rhodopsin, antioxidant activity, modulation of retinal enzyme activity, stabilization of collagen, anti-inflammatory action, and improved microcirculation. Whether the extract has any unique properties over and above those of plant-derived antioxidants remains to be elucidated. There is a need of more studies with subjects suffering impaired night vision due to pathological eye conditions, particularly macular degeneration (Canter and Ernst 2004).
The first study on this topic was an open, placebo-controlled study on patients with diabetic retinopathy; the treatment with 200 mg of unspecified bilberry fruit extract combined with 10 mg beta-carotene taken 3 times daily reduced vascular permeability and improved the state of retinal blood vessels (Scharrer and Ober 1981). It was followed by another study on patients with diabetic and hypertensive retinopathy who took 160 mg of a bilberry extract containing 25% anthocyanidins twice daily. The authors reported on 77–90% improvement (compared to placebo) in ophthalmoscopic and fluoro-angiographic anomalies, but the study, similarly to the previous one, suffers from serious methodological issues (Perossini et al., 1987).
Another study tested the effect of an unspecified bilberry fruit extract given in a dose of 510 mg daily over 1 year on patients with diabetic retinopathy. It reported a gradual improvement in contrast sensitivity, but other measured parameters (corrected visual acuity, hard exudates, microaneurysms, leaking points) remained unchanged for the entire duration of the study (Kim et al., 2008).
A combination of two phenolic extracts from bilberry (standardized to 36% anthocyanins) and French maritime pine bark (standardized to 70% procyanidins) lowered elevated intraocular pressure in patients almost as effectively as latanoprost, however, it took much longer (24 vs. 4 weeks). The combination treatment with both extracts and latanoprost was even more effective for lowering intraocular pressure and the combination yielded better retinal blood flow. No serious side effects occurred during the study, apart from common side effects in some of the latanoprost patients (temporarily blurred vision and eyelid redness). However, limitations of this study included a lack of a placebo group, double-blinding and randomization. The use of a multi-ingredient product also makes it difficult to discern the effects of bilberry alone (Steigerwalt et al., 2010).
Twenty-two patients with dry eye symptoms were treated with 160 mg of (unspecified) bilberry nutraceutical. After 30 days, the Ocular Surface Disease Index was statistically improved in the bilberry group compared to placebo (Anderson et al., 2011).
In a double-blind, randomized, placebo-controlled trial, a supplementation of 240 mg standardized bilberry extract daily for 12 weeks significantly improved the tonic accommodation of the ciliary muscle during near-vision tasks on display terminals, and therefore alleviated the ocular fatigue symptoms (Kosehira et al., 2020).
Thirty healthy individuals with myopic eyes have undergone treatment with either 400 mg of yeast-fermented bilberry fruit extract or placebo. After 4 weeks of treatment, there was an improvement in accommodation and mesopic contrast sensitivity, but not in other measured parameters (Kamiya et al., 2013).
It is well-documented that flavonoids, flavonoid chalcones and other related phenolic compounds have beneficial effects on circulatory disorders (Ulloa 2019). The use of bilberry fruits and anthocyanin-rich extracts for microcirculation issues and peripheral venous insufficiency is supported by the review of European Medicines Agency (European Medicines Agency 2015a). Older studies reviewed in the EMA assessment report show positive results, such as reduction of the number of petechiae on the skin of 27 patients with capillary fragility who took 80–120 mg of bilberry anthocyanosides (Coget and Merlen 1968) or reduction of venous insufficiency symptoms such as oedema, cramp-like pain and paresthesia by a regime of 480 mg bilberry fruit extract daily (Gatta 1988), but are generally lacking in study design and statistical analysis. There is also a lack of newer clinical research on this topic.
Several studies have been conducted addressing the influence on plasma lipoproteins. In humans, a study performed on patients after myocardial infarct showed that bilberry powder (40 g per day) significantly potentiated the effect of statins on plasma total cholesterol and LDL cholesterol. The bilberry group patients were also more successful in a 6 min walking test (Arevström et al., 2019) The same group of patients was also evaluated for the presence of circulating microvesicles which are a marker of cardiovascular disease. It was found that bilberry extract improved the blood profile and reduced endothelial vesiculation (Bryl-Górecka et al., 2020). Another clinical study showed that consumption of 150 g of frozen bilberries 3 times a week for 6 weeks led to a significant decrease in total cholesterol, LDL and triglycerides, and a favorable increase in HDL (Habanova et al., 2016). This is in agreement with an older studies which showed significant decrease in LDL and increase in HDL after supplementing the dyslipidemic patients with 320 mg of purified anthocyanins daily for 12 weeks (Qin et al., 2009) or 24 weeks (Zhu et al., 2013).
Drawing from the numerous studies on antioxidant activity of bilberry extracts, there have been several studies on cancer cell lines. Fractions containing catechins, flavonoids and organic acids were able to inhibit the growth of cervix epitheloid carcinoma, breast adenocarcinoma and colon adenocarcinoma cell lines, with IC50 ranging from 125.80 to 300.48 mg/ml (Tumbas Šaponjac et al., 2015). Whole bilberry extract inhibited the growth of three colon cancer cell lines (Caco-2, HT-29, and HCT 116) (Aaby et al., 2013), whole bilberry extract and the standardized mixture of its main phenolic compounds inhibited the growth of prostate cancer cell lines (Del Bubba et al., 2020). The bilberry fruit extract also inhibited early preneoplastic liver cell lesions in rats (Hara et al., 2014). Whole bilberry powder inhibited the viability, proliferation, migration and invasion of oral squamous carcinoma cell lines in zebrafish model (Mauramo et al., 2021).
Only one clinical pilot human study has been published to date. Three different doses of a standardized anthocyanin-rich bilberry fruit extract (1,400, ,2800 or 5,600 mg daily) were given to 25 colorectal cancer patients scheduled for resection for 7 days before the surgery. In all patients, the tumor cell proliferation was decreased by 7% compared to the baseline; the apoptotic index increased from 3.6 to 5.3%, regardless of the dosage. The study however lacks a control group (Thomasset et al., 2009).
Cholinesterases are key enzymes participating in the pathogenesis of Alzheimer’s disease and screening for cholinesterase inhibitors in selected fruits and vegetables is a potential way of finding new treatment options. In bilberry fruit extract, the derivatives of chlorogenic and benzoic acid showed the highest cholinesterase inhibition (Borowiec et al., 2014). Bilberry-supplemented rats achieved better results at maze tests and their hippocampal parvalbumin-immunoreactive neurons were significantly reduced (Borowiec et al., 2019). Likewise, supplementing bilberry anthocyanins improved learning and memory abilities of Alzheimer’s disease model mice (Li et al., 2020). Unfortunately, the relevant clinical study, in which the elderly volunteers showed fewer cognitive symptoms and improved memory discrimination, used commercially available North American blueberries, not European bilberries (McNamara et al., 2018).
A water-soluble V. myrtillus fruit extract (total polyphenols 339.3 mg/100 g FW; total anthocyanins 297.4 mg/100 g FW) was able to reduce UVA- and UVB-induced damage in a human keratinocyte cell line. The extract was able to reduce the UVB-induced cytotoxicity and genotoxicity and UVB-induced lipid peroxidation. With UVA-induced damage, V. myrtillus reduced genotoxicity as well as the imbalance of redox intracellular status. Moreover, the extract reduced the UVA-induced apoptosis, but had no effect against the UVB one (Calò and Marabini 2014). A cream which incorporated both the oil from bilberry seeds and the extract from bilberry leaves improved skin hydration parameter slightly more than placebo cream in a month-long clinical trial on 25 volunteers (Tadić et al., 2021). However, in another very recent study, the bilberry fruit extract was found to reduce the sun protection factor upon incorporation into the tested sunscreens. This is a very unexpected and paradoxical result which requires further scientific attention (Ruscinc et al., 2022).
Bilberry leaf teas and extracts are a popular folk remedy in the treatment of diabetes. In Russia, for example, it is said to be the most commonly used herbal drug against diabetes (Shikov et al., 2021).
A thorough review of the early history of the bilberry leaf for diabetes was written by Helmstädter and Schuster in 2010. To briefly summarize, there was no mention of antidiabetic activity in the period sources before the end of 19th century. From that point onward, several case studies and anecdotal evidence had begun to show up, followed by the first larger-scale experiments. During the first animal experiments (usually on dogs with surgically removed pancreas to induce partial or complete diabetes), case studies and clinical experiments in the 19th and early 20th century researchers observed various degrees of urinary and blood sugar decrease after pre-prandial administration of bilberry leaf teas or extracts, but some observed no effects at all. Subsequently, Frederick Madison Allen from the United States prepared an unspecified extract of bilberry leaves named Myrtillin. This extract has never been analyzed for the content of specific compounds. The resulting antidiabetic activity was assigned to a hypothetical “glucokinine” entity. Allen’s clinical studies brought very inconsistent results, his search for the active principle was unsuccessful and he was not able to continue funding his research. After World War II the research faded out, despite a couple of further attempts by different research teams which also yielded conflicting results. “Myrtillin” and “glucokinines”, however, kept being incorrectly referenced in later literature and can be still found in current articles and books (Helmstädter and Schuster 2010).
Over the past years the allegedly miraculous antidiabetic effect postulated by bilberry leaves has been coming up again, but the results continue to be contradictory at best. When administered to streptozocin-induced diabetic rats, a bilberry leaf extract reduced the plasma glucose levels by 26% (Cignarella et al., 1996). In another study on diabetic GK rats the bilberry leaf decoction lowered the occasional glycaemia (Ferreira et al., 2010) and, in a similar study, blood glucose level and glycated hemoglobin returned to normal in 50% of the diabetic rats (Sidorova et al., 2017). A study on prediabetic and diabetic mice showed that the extract from bilberry leaves inhibits α-glucosidase and α-amylase enzyme activity and prevents postprandial hyperglycaemia by slowing down the rate of saccharide digestion (Takács et al., 2020). The α-glucosidase inhibitory activity of the applied bilberry leaf extract was found to be comparable to acarbose in an in vitro experiment (Bljajić et al., 2017). However, in an oral glucose tolerance test in healthy rats, an alcoholic extract of V. myrtillus leaves unexpectedly caused an increase of the serum glucose levels compared to controls (Neef et al., 1995). To the best of our knowledge, no clinical study has been performed on this topic in the last 20 years.
Many herbal remedies have had their mechanism of action elucidated by modern scientific approaches and continue to be beneficial for the treatment of various disorders in synergy with standard medication regimes. The current standing of bilberry fruits and extracts, in accordance with European Union herbal monographs, is still on the level of traditional use and more clinical research is necessary towards well-established use.
A complete lack of new clinical research can be observed for the use of bilberry fruits on venous insufficiency and microcirculation since the assessment report by European Medicines Agency from 2015 (European Medicines Agency 2015a). Older studies, while reporting generally positive results, are usually lacking in methodology and statistical analysis (as noted by the EMA assessment report itself), therefore the recommendations towards the use of bilberries for this indication should be treated with caution.
The same can be said for the use of bilberry leaf extracts for glycemic control and T2DM. In this case, dated and contradictory results are amplified by the striking dissonance: bilberry leaf extract is generally labeled as a “traditional remedy for diabetes”, yet there is no mention of it in the period sources, herbals and encyclopedias up until the end of the 19th century. The latest animal models suggest the possible benefit of bilberry leaf teas and extracts as an α-amylase and α-glucosidase inhibitor, but these are yet to be verified by clinical studies. Contradictory clinical results for this application exist for the use of bilberry fruits as well; the improvement in some measured clinical parameters might be attributed to the general antioxidant and anti-inflammatory effects of the bilberry phenolics (Kolehmainen et al., 2012) but in the majority of the performed clinical trials there seems to be no specific benefit against T2DM, despite the fact that in vitro antidiabetic activities have been described for several isolated bilberry phenolic compounds.
When it comes to the use of bilberry fruit extracts for night vision and other eye-related disorders, it needs to be stated that the origin of this indication might have been a purposefully created hoax or a superstition. The systematic review of clinical studies showed no or only marginal benefit of supplementing bilberry fruit extracts to increase night vision (Canter and Ernst 2004). Similarly, only small improvements can be seen for diabetic retinopathy, ocular fatigue and dry eye symptoms.
Bilberry fruits and fruit extracts achieve the most positive clinical results in dyslipidemias and chronic inflammatory diseases such as oral mucosa inflammation, ulcerative colitis, metabolic syndrome and increased cardiovascular risk associated with elevated inflammatory serum values. So far, the consensus on the theorized mechanisms of action for the anti-inflammatory effect includes attenuation of pro-inflammatory cytokines IL-1β, IL-6, IL-12, IL-15 and C-reactive protein. The effects on TNF-α might pose a future challenge because both attenuation and induction was observed in cell cultures and clinical trials alike (Karlsen et al., 2010; Kolehmainen et al., 2012; Roth et al., 2014). The lipid-regulating effect is theorized to stem from the attenuation of the activity of plasma cholesteryl ester transfer protein (CETP) in plasma, upregulation of fatty acid metabolism in mitochondria and reduced oxidation of LDL. The reduction of CRP is also positively correlated with the reduction of plasma LDL (Qin et al., 2009; Zhu et al., 2013; Arevström et al., 2019) (Figure 5).
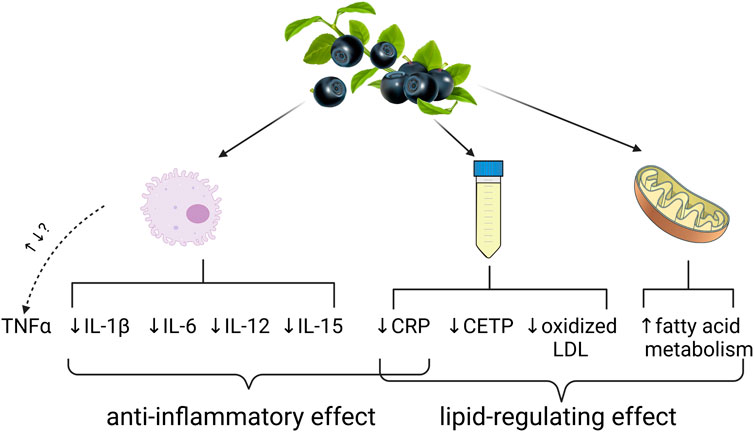
FIGURE 5. A summary of the theorized mechanisms of action of bilberry fruits and extracts in the treatment of inflammatory diseases and dyslipidemias.
This makes bilberries a promising therapeutic for inflammatory disorders and dyslipidemia. However, further studies on the involved mechanisms, as well as larger randomized blinded trials are urgently needed.
JMR conceived the study. ZV extracted the literature data and drafted the manuscript. Both authors discussed the results and contributed to the final manuscript.
This work was supported by the Austrian Science Fund (FWF P34028 of JMR and ESP 149-B of ZV).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.909914/full#supplementary-material
Aaby, K., Grimmer, S., and Holtung, L. (2013). Extraction of Phenolic Compounds from Bilberry (Vaccinium Myrtillus L.) Press Residue: Effects on Phenolic Composition and Cell Proliferation. LWT - Food Sci. Technol. 54, 257–264. doi:10.1016/j.lwt.2013.05.031
Aboonabi, A., Meyer, R. R., Gaiz, A., and Singh, I. (2020). Anthocyanins in Berries Exhibited Anti-atherogenicity and Antiplatelet Activities in a Metabolic Syndrome Population. Nutr. Res. 76, 82–93. doi:10.1016/j.nutres.2020.02.011
Ancillotti, C., Ciofi, L., Pucci, D., Sagona, E., Giordani, E., Biricolti, S., et al. (2016). Polyphenolic Profiles and Antioxidant and Antiradical Activity of Italian Berries from Vaccinium Myrtillus L. And Vaccinium Uliginosum L. Subsp. Gaultherioides (Bigelow) S.B. Young. Food Chem. 204, 176–184. doi:10.1016/j.foodchem.2016.02.106
Anderson, K. G., Anderson, A., and Connor, C. G. (2011). Potential Use of Bilberry for Dry Eye Relief. Optometry - J. Am. Optometric Assoc. 82, 380. doi:10.1016/j.optm.2011.04.081
Arevström, L., Bergh, C., Landberg, R., Wu, H., Rodriguez-Mateos, A., Waldenborg, M., et al. (2019). Freeze-dried Bilberry (Vaccinium Myrtillus) Dietary Supplement Improves Walking Distance and Lipids after Myocardial Infarction: An Open-Label Randomized Clinical Trial. Nutr. Res. 62, 13–22. doi:10.1016/j.nutres.2018.11.008
Bao, L., Abe, K., Tsang, P., Xu, J.-K., Yao, X.-S., Liu, H.-W., et al. (2010). Bilberry Extract Protect Restraint Stress-Induced Liver Damage through Attenuating Mitochondrial Dysfunction. Fitoterapia 81, 1094–1101. doi:10.1016/j.fitote.2010.07.004
Bassaganya-Riera, J., Skoneczka, J., Kingston, D., Krishnan, A., Misyak, S., Guri, A., et al. (2010). Mechanisms of Action and Medicinal Applications of Abscisic Acid. Curr. Med. Chem. 17, 467–478. doi:10.2174/092986710790226110
Belleoud, L., Leluan, D., and Boyer, Y. (1966). Etudes des effets des glucosides d’anthocyan sur las vision nocturne des controleurs d’approche d’aerodrome. Rev. Med. Aeronaut. Spat. 18, 45–50.
Biedermann, L., Mwinyi, J., Scharl, M., Frei, P., Zeitz, J., Kullak-Ublick, G. A., et al. (2013). Bilberry Ingestion Improves Disease Activity in Mild to Moderate Ulcerative Colitis — an Open Pilot Study. J. Crohn's Colitis 7, 271–279. doi:10.1016/j.crohns.2012.07.010
Bilberries and Night Vision: Reality Check (2022). Bilberries and Night Vision: Reality Check. Available at: http://www.paghat.com/bilberry-vision.html (accessed March 30, 2022).
Bljajić, K., Petlevski, R., Vujić, L., Čačić, A., Šoštarić, N., Jablan, J., et al. (2017). Chemical Composition, Antioxidant and α-glucosidase-inhibiting Activities of the Aqueous and Hydroethanolic Extracts of Vaccinium Myrtillus Leaves. Molecules 22, 703. doi:10.3390/molecules22050703
Borowiec, K., Matysek, M., Szwajgier, D., Biała, G., Kruk-Słomka, M., Szalak, R., et al. (2019). The Influence of Bilberry Fruit on Memory and the Expression of Parvalbumin in the Rat hippocampus. Pol. J. Vet. Sci. 22 (3), 481–487. doi:10.24425/pjvs.2019.129973
Borowiec, K., Szwajgier, D., Targoński, Z., Demchuk, O. M., Cybulska, J., Czernecki, T., et al. (2014). Cholinesterase Inhibitors Isolated from Bilberry Fruit. J. Funct. Foods 11, 313–321. doi:10.1016/j.jff.2014.10.008
Bryl‐Górecka, P., Sathanoori, R., Arevström, L., Landberg, R., Bergh, C., Evander, M., et al. (2020). Bilberry Supplementation after Myocardial Infarction Decreases Microvesicles in Blood and Affects Endothelial Vesiculation. Mol. Nutr. Food Res. 64, 2000108. doi:10.1002/mnfr.202000108
Bujor, O.-C., Le Bourvellec, C., Volf, I., Popa, V. I., and Dufour, C. (2016). Seasonal Variations of the Phenolic Constituents in Bilberry (Vaccinium Myrtillus L.) Leaves, Stems and Fruits, and Their Antioxidant Activity. Food Chem. 213, 58–68. doi:10.1016/j.foodchem.2016.06.042
Bunea, A., Rugină, D., Pintea, A., Andrei, S., Bunea, C., Pop, R., et al. (2012). Carotenoid and Fatty Acid Profiles of Bilberries and Cultivated Blueberries from Romania. Chem. Pap. 66. doi:10.2478/s11696-012-0162-2
Calò, R., and Marabini, L. (2014). Protective Effect of Vaccinium Myrtillus Extract against UVA- and UVB-Induced Damage in a Human Keratinocyte Cell Line (HaCaT Cells). J. Photochem. Photobiol. B Biol. 132, 27–35. doi:10.1016/j.jphotobiol.2014.01.013
Canter, P. H. (2004). Author's Response to J. H. Kramer. Surv. Ophthalmol. 49 (6), 618. doi:10.1016/s0039-6257(04)00142-0
Canter, P. H., and Ernst, E. (2004). Anthocyanosides of Vaccinium Myrtillus (Bilberry) for Night Vision—A Systematic Review of Placebo-Controlled Trials. Surv. Ophthalmol. 49, 38–50. doi:10.1016/j.survophthal.2003.10.006
Cardeñosa, V., Girones-Vilaplana, A., Muriel, J. L., Moreno, D. A., and Moreno-Rojas, J. M. (2016). Influence of Genotype, Cultivation System and Irrigation Regime on Antioxidant Capacity and Selected Phenolics of Blueberries (Vaccinium Corymbosum L.) Food Chem. 202, 276–283. doi:10.1016/j.foodchem.2016.01.118
Chan, S. W., Chu, T. T., Choi, S. W., Benzie, I. F., and Tomlinson, B. (2021). Impact of Short‐term Bilberry Supplementation on Glycemic Control, Cardiovascular Disease Risk Factors, and Antioxidant Status in Chinese Patients with Type 2 Diabetes. Phytotherapy Res. 35, 3236–3245. doi:10.1002/ptr.7038
Chen, J., Uto, T., Tanigawa, S., Kumamoto, T., Fujii, M., and Hou, D.-X. (2008). Expression Profiling of Genes Targeted by Bilberry (Vaccinium Myrtillus) in Macrophages through DNA Microarray. Nutr. Cancer 60, 43–50. doi:10.1080/01635580802381279
Cignarella, A., Nastasi, M., Cavalli, E., and Puglisi, L. (1996). Novel Lipid-Lowering Properties of Vaccinium Myrtillus L. Leaves, a Traditional Antidiabetic Treatment, in Several Models of Rat Dyslipidaemia: A Comparison with Ciprofibrate. Thrombosis Res. 84, 311–322. doi:10.1016/s0049-3848(96)00195-8
Coget, J., and Merlen, J. F. (1968). Clinical Study of a New Chemical Agent for Vascular Protection, Difrarel 20, Composed of Anthocyanosides Extracted from Vaccinum Myrtillus. Phlebologie 21 (2), 221–228.
Colak, N., Torun, H., Gruz, J., Strnad, M., Hermosín-Gutiérrez, I., Hayirlioglu-Ayaz, S., et al. (2016). Bog Bilberry Phenolics, Antioxidant Capacity and Nutrient Profile. Food Chem. 201, 339–349. doi:10.1016/j.foodchem.2016.01.062
Dashbaldan, S., Becker, R., Pączkowski, C., and Szakiel, A. (2019). Various Patterns of Composition and Accumulation of Steroids and Triterpenoids in Cuticular Waxes from Screened Ericaceae and Caprifoliaceae Berries during Fruit Development. Molecules 24, 3826. doi:10.3390/molecules24213826
de Mello, V. D., Schwab, U., Kolehmainen, M., Koenig, W., Siloaho, M., Poutanen, K., et al. (2011). A Diet High in Fatty Fish, Bilberries and Wholegrain Products Improves Markers of Endothelial Function and Inflammation in Individuals with Impaired Glucose Metabolism in a Randomised Controlled Trial: the Sysdimet Study. Diabetologia 54, 2755–2767. doi:10.1007/s00125-011-2285-3
Del Bubba, M., Di Serio, C., Renai, L., Scordo, C. V., Checchini, L., Ungar, A., et al. (2020). Vaccinium Myrtillus L. Extract and its Native Polyphenol‐recombined Mixture Have Anti‐proliferative and Pro‐apoptotic Effects on Human Prostate Cancer Cell Lines. Phytotherapy Res. 35, 1089–1098. doi:10.1002/ptr.6879
Domitrović, R., and Jakovac, H. (2011). Effects of Standardized Bilberry Fruit Extract (Mirtoselect®) on Resolution of CCL4-Induced Liver Fibrosis in Mice. Food Chem. Toxicol. 49, 848–854. doi:10.1016/j.fct.2010.12.006
Du, Q., Jerz, G., and Winterhalter, P. (2004). Isolation of Two Anthocyanin Sambubiosides from Bilberry (Vaccinium Myrtillus) by High-Speed Counter-current Chromatography. J. Chromatogr. A 1045, 59–63. doi:10.1016/j.chroma.2004.06.017
European Directorate for the Quality of Medicines & HealthCare (Edqm), (2019). European Pharmacopoeia. 10th ed. Strasbourg, France: EDQM.
European Medicines Agency (2015b). Herbal Medicinal Products Committee HMPC. European Union herbal monograph on Vaccinium myrtillus L., fructus recens. EMA/HMPC/375808/2014Available at: https://www.ema.europa.eu/en/documents/herbal-monograph/final-european-union-herbal-monograph-vaccinium-myrtillus-l-fructus-recens_en.pdf (Accessed March 30, 2022).
European Medicines Agency (2015c). Herbal Medicinal Products Committee HMPC. European Union herbal monograph on Vaccinium myrtillus L., fructus siccus. EMA/HMPC/678995/2013Available at: https://www.ema.europa.eu/en/documents/herbal-monograph/draft-european-union-herbal-monograph-vaccinium-myrtillus-l-fructus-siccus_en.pdf (Accessed March 30, 2022).
European Medicines Agency (2015a). Assessment Report on Vaccinium Myrtillus L., Fructus Recens and Vaccinium Myrtillus L., Fructus Siccus. EMA/HMPC/555161/2013Available at: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-vaccinium-myrtillus-l-fructus-recens-vaccinium-myrtillus-l-fructus-siccus_en.pdf (Accessed March 30, 2022).Herbal Medicinal Products Committee HMPC
Ferreira, F., Peixoto, F., Nunes, E., Sena, C., Seiça, R., and Santos, M. (2010). Vaccinium Myrtillus Improves Liver Mitochondrial Oxidative Phosphorylation of Diabetic Goto-Kakizaki Rats. J. Med. Plants Res. 4 (8), 692–696.
Flora of North America (2022). Flora of North America Vol. 8: Vaccinium Corymbosum. Available at: http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=242417401 (Accessed March 30, 2022).
Gaspar, D. P., Lechtenberg, M., and Hensel, A. (2021). Quality Assessment of Bilberry Fruits (Vaccinium Myrtillus) and Bilberry-Containing Dietary Supplements. J. Agric. Food Chem. 69, 2213–2225. doi:10.1021/acs.jafc.0c07784
Gatta, L. (1988). Experimental Single-Blind Study: 60 Pts with Venous Insufficiency Received Bilberry Extract Equivalent to 173 Mg Anthocyanins Daily or Placebo for 30 Days. Fitoterapia 59 (1), 19.
González-Paramás, A. M., Brighenti, V., Bertoni, L., Marcelloni, L., Ayuda-Durán, B., González-Manzano, S., et al. (2020). Assessment of the In Vivo Antioxidant Activity of an Anthocyanin-Rich Bilberry Extract Using the Caenorhabditis elegans Model. Antioxidants 9, 509. doi:10.3390/antiox9060509
Guo, X., Yang, B., Tan, J., Jiang, J., and Li, D. (2016). Associations of Dietary Intakes of Anthocyanins and Berry Fruits with Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Eur. J. Clin. Nutr. 70, 1360–1367. doi:10.1038/ejcn.2016.142
Gustinelli, G., Eliasson, L., Svelander, C., Alminger, M., and Ahrné, L. (2018). Supercritical CO2 Extraction of Bilberry (Vaccinium Myrtillus L.) Seed Oil: Fatty Acid Composition and Antioxidant Activity. J. Supercrit. Fluids 135, 91–97. doi:10.1016/j.supflu.2018.01.002
Habanova, M., Saraiva, J. A., Haban, M., Schwarzova, M., Chlebo, P., Predna, L., et al. (2016). Intake of Bilberries (Vaccinium Myrtillus L.) Reduced Risk Factors for Cardiovascular Disease by Inducing Favorable Changes in Lipoprotein Profiles. Nutr. Res. 36, 1415–1422. doi:10.1016/j.nutres.2016.11.010
Hara, S., Morita, R., Ogawa, T., Segawa, R., Takimoto, N., Suzuki, K., et al. (2014). Tumor Suppression Effects of Bilberry Extracts and Enzymatically Modified Isoquercitrin in Early Preneoplastic Liver Cell Lesions Induced by Piperonyl Butoxide Promotion in a Two-Stage Rat Hepatocarcinogenesis Model. Exp. Toxicol. Pathology 66, 225–234. doi:10.1016/j.etp.2014.02.002
Heffels, P., Müller, L., Schieber, A., and Weber, F. (2017). Profiling of Iridoid Glycosides in Vaccinium Species by UHPLC-MS. Food Res. Int. 100, 462–468. doi:10.1016/j.foodres.2016.11.018
Heinonen, M. (2007). Antioxidant Activity and Antimicrobial Effect of Berry Phenolics – a Finnish Perspective. Mol. Nutr. Food Res. 51, 684–691. doi:10.1002/mnfr.200700006
Helmstädter, A., and Schuster, N. (2010). Vaccinium Myrtillus as an Antidiabetic Medicinal Plant – Research through the Ages. Pharmazie 65, 315–321. doi:10.1691/ph.2010.9402
Hilz, H., de Jong, L. E., Kabel, M. A., Verhoef, R., Schols, H. A., and Voragen, A. G. J. (2007). Bilberry Xyloglucan—Novel Building Blocks Containing β-xylose within a Complex Structure. Carbohydr. Res. 342, 170–181. doi:10.1016/j.carres.2006.12.005
Hirvi, T., and Honkanen, E. (1983). The Aroma of Blueberries. J. Sci. Food Agric. 34, 992–996. doi:10.1002/jsfa.2740340916
Hoggard, N., Cruickshank, M., Moar, K.-M., Bestwick, C., Holst, J. J., Russell, W., et al. (2013). A Single Supplement of a Standardised Bilberry (Vaccinium Myrtillus L.) Extract (36 % Wet Weight Anthocyanins) Modifies Glycaemic Response in Individuals with Type 2 Diabetes Controlled by Diet and Lifestyle. J. Nutr. Sci. 2. doi:10.1017/jns.2013.16
Hokkanen, J., Mattila, S., Jaakola, L., Anna Maria, Pirttilä, and Tolonen, A. (2009). Identification of Phenolic Compounds from Lingonberry (Vaccinium vitis-idaeal.), Bilberry (Vaccinium myrtillusL.) and Hybrid Bilberry (Vaccinium X Intermediumruthe L.) Leaves. J. Agric. Food Chem. 57, 9437–9447. doi:10.1021/jf9022542
Ichiyanagi, T., Kashiwada, Y., and Nashimoto, M. (2020). Large-scale Isolation of Three O-Methyl Anthocyanins from Bilberry (Vaccinium Myrtillus L.) Extract. Chem. Pharm. Bull. 68, 1113–1116. doi:10.1248/cpb.c20-00593
Ieri, F., Martini, S., Innocenti, M., and Mulinacci, N. (2013). Phenolic Distribution in Liquid Preparations of Vaccinium Myrtillus L. And Vaccinium Vitis Idaea L. Phytochem. Anal. 24, 467–475. doi:10.1002/pca.2462
Jennings, A., Welch, A. A., Spector, T., Macgregor, A., and Cassidy, A. (2013). Intakes of Anthocyanins and Flavones Are Associated with Biomarkers of Insulin Resistance and Inflammation in Women. J. Nutr. 144, 202–208. doi:10.3945/jn.113.184358
Jensen, H. D., Krogfelt, K. A., Cornett, C., Hansen, S. H., and Christensen, S. B. (2002). Hydrophilic Carboxylic Acids and Iridoid Glycosides in the Juice of American and European Cranberries (Vaccinium Macrocarpon and V. Oxycoccos), Lingonberries (V. Vitis-Idaea), and Blueberries (V. Myrtillus). J. Agric. Food Chem. 50, 6871–6874. doi:10.1021/jf0205110
Juadjur, A., Mohn, C., Schantz, M., Baum, M., Winterhalter, P., and Richling, E. (2015). Fractionation of an Anthocyanin-Rich Bilberry Extract and In Vitro Antioxidative Activity Testing. Food Chem. 167, 418–424. doi:10.1016/j.foodchem.2014.07.004
Kähkönen, M. P., Heinämäki, J., Ollilainen, V., and Heinonen, M. (2003). Berry Anthocyanins: Isolation, Identification and Antioxidant Activities. J. Sci. Food Agric. 83, 1403–1411. doi:10.1002/jsfa.1511
Kamiya, K., Kobashi, H., Fujiwara, K., Ando, W., and Shimizu, K. (2013). Effect of Fermented Bilberry Extracts on Visual Outcomes in Eyes with Myopia: A Prospective, Randomized, Placebo-Controlled Study. J. Ocular Pharmacol. Ther. 29, 356–359. doi:10.1089/jop.2012.0098
Karlsen, A., Paur, I., Bøhn, S. K., Sakhi, A. K., Borge, G. I., Serafini, M., et al. (2010). Bilberry Juice Modulates Plasma Concentration of NF-Κb Related Inflammatory Markers in Subjects at Increased Risk of CVD. Eur. J. Nutr. 49, 345–355. doi:10.1007/s00394-010-0092-0
Karppinen, K., Hirvelä, E., Nevala, T., Sipari, N., Suokas, M., and Jaakola, L. (2013). Changes in the Abscisic Acid Levels and Related Gene Expression during Fruit Development and Ripening in Bilberry (Vaccinium Myrtillus L.) Phytochemistry 95, 127–134. doi:10.1016/j.phytochem.2013.06.023
Kim, E. S., Yu, S. Y., Kwon, S. J., Kwon, O. W., Kim, S. Y., Kim, T. W., et al. (2008). Clinical Evaluation of Patients with Nonproliferative Diabetic Retinopathy Following Medication of Anthocyanoside: Multicenter Study. J. Korean Ophthalmol. Soc. 49, 1629. doi:10.3341/jkos.2008.49.10.1629
Kliment, J., and Valachovič, M. (2007). Rastlinné Spoločenstvá Slovenska. 4. Vysokohorská Vegetácia. 1st ed. Bratislava: Veda.
Kole, Ch. (2011). Wild Crop Relatives: Genomic and Breeding Resources: Temperate Fruits. 1st. Springer Science & Business Media.
Kolehmainen, M., Mykkänen, O., Kirjavainen, P. V., Leppänen, T., Moilanen, E., Adriaens, M., et al. (2012). Bilberries Reduce Low-Grade Inflammation in Individuals with Features of Metabolic Syndrome. Mol. Nutr. Food Res. 56, 1501–1510. doi:10.1002/mnfr.201200195
Kosehira, M., Machida, N., and Kitaichi, N. (2020). A 12-Week-Long Intake of Bilberry Extract (Vaccinium Myrtillus L.) Improved Objective Findings of Ciliary Muscle Contraction of the Eye: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Comparison Trial. Nutrients 12, 600. doi:10.3390/nu12030600
Kotlaba, F., and Pilát, A. (1952). Hlízenka Klikvová, Sclerotina Oxycocci Voron. V ČSR. . Česká Mykol. 6 (3), 41–44.
Kresánek, J., and Kresánek, J. (2008). Atlas Liečivých Rastlín a Lesných Plodov. 1st. Martin: Osveta.
Lätti, A. K., Riihinen, K. R., and Jaakola, L. (2011). Phenolic Compounds in Berries and Flowers of a Natural Hybrid between Bilberry and Lingonberry (Vaccinium×Intermedium Ruthe). Phytochemistry 72, 810–815. doi:10.1016/j.phytochem.2011.02.015
Li, J., Zhao, R., Jiang, Y., Xu, Y., Zhao, H., Lyu, X., et al. (2020). Bilberry Anthocyanins Improve Neuroinflammation and Cognitive Dysfunction in app/PSEN1 Mice via the CD33/trem2/Tyrobp Signaling Pathway in Microglia. Food & Funct. 11, 1572–1584. doi:10.1039/c9fo02103e
Lynn, A., Garner, S., Nelson, N., Simper, T. N., Hall, A. C., and Ranchordas, M. K. (2018). Effect of Bilberry Juice on Indices of Muscle Damage and Inflammation in Runners Completing a half-Marathon: A Randomised, Placebo-Controlled Trial. J. Int. Soc. Sports Nutr. 15. doi:10.1186/s12970-018-0227-x
Määttä-Riihinen, K. R., Kähkönen, M. P., Törrönen, A. R., and Heinonen, I. M. (2005). Catechins and Procyanidins in Berries of Vaccinium Species and Their Antioxidant Activity. J. Agric. Food Chem. 53, 8485–8491. doi:10.1021/jf050408l
Määttä-Riihinen, K. R., Kamal-Eldin, A., Mattila, P. H., González-Paramás, A. M., and Törrönen, A. R. (2004). Distribution and Contents of Phenolic Compounds in Eighteen Scandinavian Berry Species. J. Agric. Food Chem. 52, 4477–4486. doi:10.1021/jf049595y
Mauramo, M., Onali, T., Wahbi, W., Vasara, J., Lampinen, A., Mauramo, E., et al. (2021). Bilberry (Vaccinium Myrtillus L.) Powder Has Anticarcinogenic Effects on Oral Carcinoma In Vitro and In Vivo. Antioxidants 10, 1319. doi:10.3390/antiox10081319
Mayser, H. M., and Wilhelm, H. (2001). Effects of Anthocyanosides on Contrast Vision. Invest Ophthalmol. Vis. Sci. 42 (Suppl. l), 63.
McNamara, R. K., Kalt, W., Shidler, M. D., McDonald, J., Summer, S. S., Stein, A. L., et al. (2018). Cognitive Response to Fish Oil, Blueberry, and Combined Supplementation in Older Adults with Subjective Cognitive Impairment. Neurobiol. Aging 64, 147–156. doi:10.1016/j.neurobiolaging.2017.12.003
Mikulic-Petkovsek, M., Schmitzer, V., Slatnar, A., Stampar, F., and Veberic, R. (2014). A Comparison of Fruit Quality Parameters of Wild Bilberry (Vaccinium myrtillusL.) Growing at Different Locations. J. Sci. Food Agric. 95, 776–785. doi:10.1002/jsfa.6897
Može, Š., Polak, T., Gašperlin, L., Koron, D., Vanzo, A., Poklar Ulrih, N., et al. (2011). Phenolics in Slovenian Bilberries (Vaccinium Myrtillus L.) and Blueberries (Vaccinium Corymbosum L.) J. Agric. Food Chem. 59, 6998–7004. doi:10.1021/jf200765n
Nardin, T., Piasentier, E., Barnaba, C., and Larcher, R. (2017). Alkaloid Profiling of Herbal Drugs Using High Resolution Mass Spectrometry. Drug Test. Analysis 10, 423–448. doi:10.1002/dta.2252
Neamtu, A.-A., Szoke-Kovacs, R., Mihok, E., Georgescu, C., Turcus, V., Olah, N. K., et al. (2020). Bilberry (Vaccinium Myrtillus L.) Extracts Comparative Analysis Regarding Their Phytonutrient Profiles, Antioxidant Capacity along with the In Vivo Rescue Effects Tested on a drosophila Melanogaster High-Sugar Diet Model. Antioxidants 9, 1067. doi:10.3390/antiox9111067
Neef, H., Declercq, P., and Laekeman, G. (1995). Hypoglycaemic Activity of Selected European Plants. Phytother. Res. 9, 45–48. doi:10.1002/ptr.2650090111
Nestby, R., Percival, D., Martinussen, I., Opstad, N., and Rohloff, J. (2011). The European Blueberry (Vaccinium Myrtillus L.) and the Potential for Cultivation. A Review. Eur. J. Plant Sci. Biotechnol. 5 (1), 5–16.
Perossini, M., Guidi, G., Chiellini, S., and Siravo, D. (1987). Diabetic and Hypertensive Retinopathy Therapy with Vaccinium Myrtillus Anthocyanosides (Tegens): Double Blind Placebo Controlled Clinical Trial. Ann. Ottalmaologia Clinica Ocul. 113, 1173–1190.
Pires, T. C., Caleja, C., Santos-Buelga, C., Barros, L., and Ferreira, I. C. F. R. (2020). Vaccinium Myrtillus L. Fruits as a Novel Source of Phenolic Compounds with Health Benefits and Industrial Applications - A Review. Curr. Pharm. Des. 26, 1917–1928. doi:10.2174/1381612826666200317132507
Popović, D., Đukić, D., Katić, V., Jović, Z., Jović, M., Lalić, J., et al. (2016). Antioxidant and Proapoptotic Effects of Anthocyanins from Bilberry Extract in Rats Exposed to Hepatotoxic Effects of Carbon Tetrachloride. Life Sci. 157, 168–177. doi:10.1016/j.lfs.2016.06.007
Qa’dan, F., Verspohl, E. J., Nahrstedt, A., Petereit, F., and Matalka, K. Z. (2009). Cinchonain Ib Isolated from Eriobotrya Japonica Induces Insulin Secretion In Vitro and In Vivo. J. Ethnopharmacol. 124, 224–227. doi:10.1016/j.jep.2009.04.023
Qin, Y., Xia, M., Ma, J., Hao, Y. T., Liu, J., Mou, H. Y., et al. (2009). Anthocyanin Supplementation Improves Serum LDL- and HDL-Cholesterol Concentrations Associated with the Inhibition of Cholesteryl Ester Transfer Protein in Dyslipidemic Subjects. Am. J. Clin. Nutr. 90, 485–492. doi:10.3945/ajcn.2009.27814
Ramstad, E. (1954). Chemical Investigation of Vaccinium Myrtillus L. J. Am. Pharm. Assoc. Sci. 43 (4), 236–240. doi:10.1002/jps.3030430413
Rätsch, Ch. (2005). The Encyclopedia of Psychoactive Plants: Ethnopharmacology and its Applications. 1st ed. New York: Simon & Schuster.
Riihinen, K., Jaakola, L., Kärenlampi, S., and Hohtola, A. (2008). Organ-specific Distribution of Phenolic Compounds in Bilberry (Vaccinium Myrtillus) and ‘northblue’ Blueberry (Vaccinium Corymbosum X V. Angustifolium). Food Chem. 110, 156–160. doi:10.1016/j.foodchem.2008.01.057
Rimando, A. M., Kalt, W., Magee, J. B., Dewey, J., and Ballington, J. R. (2004). Resveratrol, Pterostilbene, and Piceatannol in Vaccinium Berries. J. Agric. Food Chem. 52, 4713–4719. doi:10.1021/jf040095e
Rosłon, W., Osińska, E., Pióro-Jabrucka, E., and Grabowska, A. (2011). Morphological and Chemical Variability of Wild Populations of Bilberry (Vaccinium Myrtillus L.) Pol. J. Environ. Stud. 20 (1), 237–243.
Roth, S., Spalinger, M. R., Müller, I., Lang, S., Rogler, G., and Scharl, M. (2014). Bilberry-derived Anthocyanins Prevent Ifn-γ-Induced Pro-inflammatory Signalling and Cytokine Secretion in Human THP-1 Monocytic Cells. Digestion 90, 179–189. doi:10.1159/000366055
Ruscinc, N., Morocho‐Jácome, A. L., Martinez, R. M., Magalhães, W. V., Escudeiro, C. C., Giarolla, J., et al. (2022). Vaccinium Myrtillus L. Extract Associated with Octocrylene, Bisoctrizole, and Titanium Dioxide: In Vitro and In Vivo Tests to Evaluate Safety and Efficacy. J. Cosmet. Dermatology 2022. doi:10.1111/jocd.14779
Rychlinska, I., and Nowak, S. (2012). Quantitative Determination of Arbutin and Hydroquinone in Different Plant Materials by HPLC. Not. Bot. Horti Agrobot. Cluj-Napoca 40, 109. doi:10.15835/nbha4027987
Scharrer, A., and Ober, M. (1981). Anthocyanoside in der Behandlung von Retinopathien. Klin. Monatsblätter für Augenheilkd. 178, 386–389. doi:10.1055/s-2008-1057228
Shi, M., Loftus, H., McAinch, A. J., and Su, X. Q. (2017). Blueberry as a Source of Bioactive Compounds for the Treatment of Obesity, Type 2 Diabetes and Chronic Inflammation. J. Funct. Foods 30, 16–29. doi:10.1016/j.jff.2016.12.036
Shikov, A. N., Narkevich, I. A., Akamova, A. V., Nemyatykh, O. D., Flisyuk, E. V., Luzhanin, V. G., et al. (2021). Medical Species Used in Russia for the Management of Diabetes and Related Disorders. Front. Pharmacol. 12. doi:10.3389/fphar.2021.697411
Sidorova, Y., Shipelin, V., Mazo, V., Zorin, S., Petrov, N., and Kochetkova, A. (2017). Hypoglycemic and Hypolipidemic Effect of Vaccinium Myrtillus L. Leaf and Phaseolus vulgaris L. Seed Coat Extracts in Diabetic Rats. Nutrition 41, 107–112. doi:10.1016/j.nut.2017.04.010
Slosse, P., and Hootelé, C. (1981). Myrtine and Epimyrtine, Quinolizidine Alkaloids from Vaccinium Myrtillus. Tetrahedron 37 (24), 4287–4294. doi:10.1016/0040-4020(81)85024-7
Ștefănescu, B.-E., Călinoiu, L. F., Ranga, F., Fetea, F., Mocan, A., Vodnar, D. C., et al. (2020). Chemical Composition and Biological Activities of the Nord-West Romanian Wild Bilberry (Vaccinium Myrtillus L.) and Lingonberry (Vaccinium Vitis-Idaea L.) Leaves. Antioxidants 9, 495. doi:10.3390/antiox9060495
Stefkov, G., Hristovski, S., Petreska Stanoeva, J., Stefova, M., Melovski, L., and Kulevanova, S. (2014). Resource Assessment and Economic Potential of Bilberries (Vaccinium Myrtillus and Vaccinium Uliginosum) on Osogovo Mtn., R. Macedonia. Industrial Crops Prod. 61, 145–150. doi:10.1016/j.indcrop.2014.06.053
Steigerwalt, R. D., Belcaro, G., Morazzoni, P., Bombardelli, E., Burki, C., and Schonlau, F. (2010). Mirtogenol® Potentiates Latanoprost in Lowering Intraocular Pressure and Improves Ocular Blood Flow in Asymptomatic Subjects. Clin. Ophthalmol. 4, 471–476. doi:10.2147/OPTH.S9899
Sticher, O., Soldati, F., and Lehman, D. (1979). Hochleistungsflüssigchromatographische Trennung und quantitative Bestimmung von Arbutin. Methylarbutin, Hydrochinon und Hydrochinonmonomethylaether in Arctostaphylos-, Bergenia-, Calluna- und Vaccinium Arten. Planta Med. 35, 235–261. doi:10.1055/s-0028-1097213
Tadić, V. M., Nešić, I., Martinović, M., Rój, E., Brašanac-Vukanović, S., Maksimović, S., et al. (2021). Old Plant, New Possibilities: Wild Bilberry (Vaccinium Myrtillus L., Ericaceae) in Topical Skin Preparation. Antioxidants 10, 465. doi:10.3390/antiox10030465
Takács, I., Szekeres, A., Takács, Á., Rakk, D., Mézes, M., Polyák, Á., et al. (2020). Wild Strawberry, Blackberry, and Blueberry Leaf Extracts Alleviate Starch-Induced Hyperglycemia in Prediabetic and Diabetic Mice. Planta Medica 86, 790–799. doi:10.1055/a-1164-8152
Takikawa, M., Inoue, S., Horio, F., and Tsuda, T. (2010). Dietary Anthocyanin-Rich Bilberry Extract Ameliorates Hyperglycemia and Insulin Sensitivity via Activation of AMP-Activated Protein Kinase in Diabetic Mice. J. Nutr. 140, 527–533. doi:10.3945/jn.109.118216
The Plant List (2022). The Plant List: Ericaceae. Available at: http://theplantlist.org/1.1/browse/A/Ericaceae/(Accessed March 30, 2022).
Thomasset, S., Berry, D. P., Cai, H., West, K., Marczylo, T. H., Marsden, D., et al. (2009). Pilot Study of Oral Anthocyanins for Colorectal Cancer Chemoprevention. Cancer Prev. Res. 2, 625–633. doi:10.1158/1940-6207.capr-08-0201
Triebel, S., Trieu, H.-L., and Richling, E. (2012). Modulation of Inflammatory Gene Expression by a Bilberry (Vaccinium Myrtillus L.) Extract and Single Anthocyanins Considering Their Limited Stability under Cell Culture Conditions. J. Agric. Food Chem. 60, 8902–8910. doi:10.1021/jf3028842
Trivedi, P., Karppinen, K., Klavins, L., Kviesis, J., Sundqvist, P., Nguyen, N., et al. (2019). Compositional and Morphological Analyses of Wax in Northern Wild Berry Species. Food Chem. 295, 441–448. doi:10.1016/j.foodchem.2019.05.134
Tumbas Šaponjac, V., Čanadanović-Brunet, J., Ćetković, G., Djilas, S., and Četojević-Simin, D. (2015). Dried Bilberry (Vaccinium Myrtillus L.) Extract Fractions as Antioxidants and Cancer Cell Growth Inhibitors. LWT - Food Sci. Technol. 61, 615–621. doi:10.1016/j.lwt.2014.04.021
Ulbricht, C., Basch, E., Basch, S., Bent, S., Boon, H., Burke, D., et al. (2009). An Evidence-Based Systematic Review of Bilberry (Vaccinium Myrtillus) by the Natural Standard Research Collaboration. J. Diet. Suppl. 6, 162–200. doi:10.1080/19390210902861858
Ulloa, J. H. (2019). Micronized Purified Flavonoid Fraction (MPFF) for Patients Suffering from Chronic Venous Disease: A Review of New Evidence. Adv. Ther. 36, 20–25. doi:10.1007/s12325-019-0884-4
Vaneková, Z., Vanek, M., Škvarenina, J., and Nagy, M. (2020). The Influence of Local Habitat and Microclimate on the Levels of Secondary Metabolites in Slovak Bilberry (Vaccinium Myrtillus L.) Fruits. Plants 9, 436. doi:10.3390/plants9040436
Von Friedrich, H., and Schönert, J. (1973). Untersuchungen über einige Inhaltsstoffe der Blätter und Früchte von Vaccinium myrtillus. Planta Med. 24, 90–100. doi:10.1055/s-0028-1099474
Vrancheva, R., Ivanov, I., Dincheva, I., Badjakov, I., and Pavlov, A. (2021). Triterpenoids and Other Non-polar Compounds in Leaves of Wild and Cultivated Vaccinium Species. Plants 10, 94. doi:10.3390/plants10010094
Waszczuk, M., Bianchi, S. E., Martiny, S., Pittol, V., Lacerda, D. S., Araújo, A. S., et al. (2020). Development and Validation of a Specific-Stability Indicating Liquid Chromatography Method for Quantitative Analysis of Pterostilbene: Application in Food and Pharmaceutical Products. Anal. Methods 12, 4310–4318. doi:10.1039/d0ay00989j
Widén, C., Coleman, M., Critén, S., Karlgren-Andersson, P., Renvert, S., and Persson, G. (2015). Consumption of Bilberries Controls Gingival Inflammation. Int. J. Mol. Sci. 16, 10665–10673. doi:10.3390/ijms160510665
Woronin, M. (1888). Über die Sclerotienkrankheit der Vaccinieen-Beeren: Entwickelungsgeschichte der diese Krankheit verursachenden Sclerotinien. Mémoires l'Académie impériale Sci. Pétersbg. VIIe Série 36 (6).
Wu, Z., Zheng, X., Gong, M., and Li, Y. (2016). Myricetin, a Potent Natural Agent for Treatment of Diabetic Skin Damage by Modulating TIMP/MMPs Balance and Oxidative Stress. Oncotarget 7, 71754–71760. doi:10.18632/oncotarget.12330
Zhu, Y., Ling, W., Guo, H., Song, F., Ye, Q., Zou, T., et al. (2013). Anti-inflammatory Effect of Purified Dietary Anthocyanin in Adults with Hypercholesterolemia: A Randomized Controlled Trial. Nutr. Metabolism Cardiovasc. Dis. 23, 843–849. doi:10.1016/j.numecd.2012.06.005
Žiberna, L., Lunder, M., Moze, S., Vanzo, A., Tramer, F., Passamonti, S., et al. (2010). Acute Cardioprotective and Cardiotoxic Effects of Bilberry Anthocyanins in Ischemia–Reperfusion Injury: Beyond Concentration-dependent Antioxidant Activity. Cardiovasc. Toxicol. 10, 283–294. doi:10.1007/s12012-010-9091-x
Zoratti, L., Klemettilä, H., and Jaakola, L. (2016). Bilberry (Vaccinium Myrtillus L.) Ecotypes. Nutr. Compos. Fruit Cultiv. 2016, 83–99. doi:10.1016/b978-0-12-408117-8.00004-0
Keywords: Vaccinium myrtillus, bilberry, phenolic compounds, diabetes mellitus, night vision, circulatory disorders
Citation: Vaneková Z and Rollinger JM (2022) Bilberries: Curative and Miraculous – A Review on Bioactive Constituents and Clinical Research. Front. Pharmacol. 13:909914. doi: 10.3389/fphar.2022.909914
Received: 31 March 2022; Accepted: 31 May 2022;
Published: 29 June 2022.
Edited by:
Andrei Mocan, Iuliu Hațieganu University of Medicine and Pharmacy, RomaniaReviewed by:
Adriana Trifan, Grigore T. Popa University of Medicine and Pharmacy, RomaniaCopyright © 2022 Vaneková and Rollinger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zuzana Vaneková, dmFuZWtvdmEyN0B1bmliYS5zaw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.