- Changzhou Hospital of Traditional Chinese Medicine, Changzhou, China
Curcumin is extracted from the rhizomes of Curcuma longa L. It is now widely used in food processing, cosmetics, dyes, etc. Current researching indicates that curcumin has high medical value, including anti-inflammatory, antioxidant, anti-tumor, anti-apoptotic, anti-fibrosis, immune regulation and other effects, and can be used to treat a variety of diseases. Inflammatory bowel disease (IBD) is a nonspecific inflammatory disease of the intestine including Crohn’s disease (CD) and ulcerative colitis (UC). The drug treatment effect is often limited and accompanied by side effects. A large number of basic and clinical studies have shown that curcumin has the effect of treating IBD and also can maintain the remission of IBD. In this review, the research of curcumin on IBD in recent years is summarized in order to provide reference for further research and application of curcumin.
1 Introduction
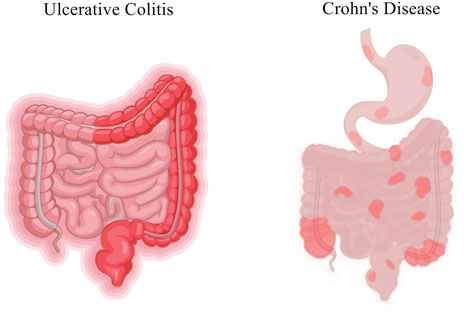
Inflammatory bowel disease (IBD) is a nonspecific inflammatory disease of the intestine with unknown etiology, including Crohn’s disease (CD) and ulcerative colitis (UC). The lesions of CD mainly involve the terminal ileum and proximal colon, and the lesions of UC are mostly accumulated in the rectum and part of the colon (Baumgart and Sandborn, 2012; Ungaro et al., 2017) (Figure 1). In recent years, the prevalence of IBD showed an upward trend year by year. In the United States alone, there are about 70,000 new confirmed cases each year (Colombel and Mahadevan, 2017). Although the prevalence in Asia and Africa is lower than that in western developed countries, it also shows an overall upward trend. Moreover, the prevalence and incidence of IBD in children are gradually increasing worldwide. According to statistics from the Chinese Center for Disease Control and Prevention, the number of IBD patients in China might increase to 1.5 million by 2025. IBD is a chronic recurrent disease, and patients often require lifelong medication to control clinical symptoms, alleviate pathological damage such as mucosal barrier destruction, and reduce recurrence. IBD is often accompanied by intestinal pathological changes, characterized by intestinal inflammation and damage to the intestinal mucosal barrier (van der Veen et al., 2009). The most common symptoms of IBD include abdominal pain, diarrhea, bowel obstruction and weight loss (Table 1). IBD is considered to be the result of the continuous inflammatory process against endogenous microbes in genetically susceptible individuals. The existing studies indicated that IBD outcome is a result of a complex interplay between genetic, immunologic, and modifiable environmental factors in a genetically susceptible host against the subset of gut commensal microbiota (Castro et al., 2015). The rising incidence of IBD indicates the necessity of studying the environmental factors. In addition, clearing environmental factors can help prevent and treat the disease.
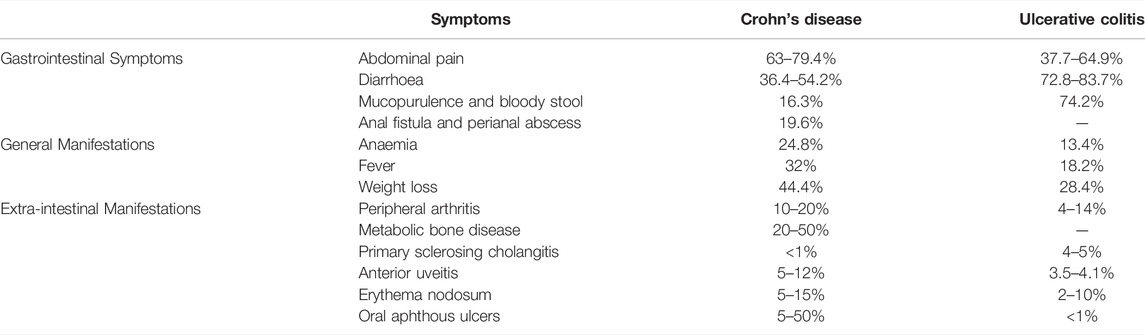
TABLE 1. The proportion of the clinical symptoms of IBD (Li et al., 2017; Annese, 2019; Rogler et al., 2021).
The effect of medicinal treatment is often affected by the compliance and economic status of patients (Khan et al., 2012). At present, the drug effect is limited, the price is high, and often accompanied by side effects (i.e., diarrhea and lymphopenia). IBD is difficult to delay to heal and easy relapse. The economic burden of patients is heavy, and the quality of life is often seriously affected. Researchers have been trying to find plant-derived products or dietary supplements that could treat IBD.
Curcumin is an orange-yellow polyphenolic chemical substance extracted from the rhizomes of Curcuma longa L. It is the main active ingredient of Chinese medicine Curcuma Longa and is now widely used in food processing, cosmetics, dyes, etc. Curcumin has been used as a traditional herbal medicine in India and Southeast Asia for thousands of years. It is often used to treat biliary tract diseases, anorexia, rhinitis, cough, rheumatism, and various chronic inflammatory diseases. Due to its extensive biological activities, it has received widespread attention from researchers in recent years. Current researching indicates that curcumin has high medical value, including anti-inflammatory, antioxidant, anti-tumor, anti-apoptotic, anti-fibrosis, immune regulation and other effects, and can be used to treat a variety of diseases (Salehi et al., 2019). It is involved in many significant genetic and biochemical pathways (Karthikeyan et al., 2020; Moniruzzaman and Min, 2020; Beyene et al., 2021). Curcumin is associated with many cellular targets (i.e., NF-κB, JAKs/STATs, MAPKs, TNF-γ, IL-6, PPARγ, and TRPV1) that effectively reduce the progression of IBD. The research of curcumin and related formulations for IBD treatment has surged over the decade (Kahkhaie et al., 2019; Sharma et al., 2019; Patel et al., 2020). So far, a large number of basic and clinical studies have shown that curcumin has the effect of treating IBD and also can maintain the remission of IBD (Yang H. et al., 2017). In this review, the researches of IBD genetics and pathogenesis and curcumin molecular targets in IBD in recent years are summarized in order to provide reference for further research and application of curcumin.
2 Curcumin
2.1 Ingredient and Structure
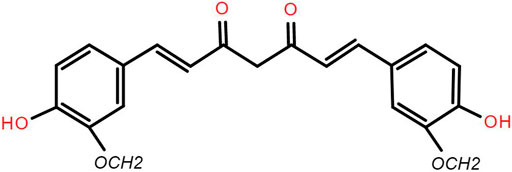
The main components of curcumin are curcumin, demethoxycurcumin and bisdemethoxycurcumin, which are the main components of Curcuma longa to play the pharmacologic effects. Curcuminoid account for about 70% of the total content of curcumin. The backbone of the molecular formula of curcumin is unsaturated fatty hydrocarbons and aromatic group. It is easily soluble in methanol, ethanol, acetone, ethyl acetate and slightly soluble in benzene and ether. Curcumin has low solubility in water and is a strong photosensitive substance. The molecular formula of curcumin is C21H20O6 (Epstein et al., 2010a) (Figure 2).
2.2 Concentration and Absorption
Curcumin is almost insoluble in aqueous solution because of its lipophilic properties. It is not easy to be absorbed after oral administration. After absorption, curcumin is rapidly discharged from the body through metabolism. Its bioavailability in the body is low, thus affecting its use as a drug directly. Current research results showed that dosage form modification or drug combination can effectively increase the water solubility of curcumin and improve its bioavailability.
Ravindranath et al. administered 400 mg of curcumin orally to rats. After 24 h, the curcumin concentration in the lower part of the intestine (cecum, large intestine) was 38% of the administered dosage. Within to 24 h after oral administration of curcumin, the drug residues in liver and kidney tissue were less than 20 μg per gram of tissue (Ravindranath and Chandrasekhara, 1980). Pan et al. found that 0.1 g/kg body weight of curcumin was injected intraperitoneally into mice, the plasma curcumin content was about 2.25 mg/L after 15 min. The curcumin content in the intestine, spleen, liver and kidney was 177.04 μg/g, 26.06 μg/g, 26.90 μg/g and 7.51 μg/g respectively at 1 h. Only trace amounts (0.41 μg/g) were present in the brain (Pan et al., 1999). The results confirmed that the distribution of curcumin in the gastrointestinal tract and kidney is much larger than that in the liver and plasma. It may be because after oral administration, curcumin is mainly absorbed through the gastrointestinal tract, and is widely distributed in the liver and other organs after entering the blood.
2.3 Metabolism and Toxicity
Sharma et al. studied the pharmacokinetics of curcumin through oral administration of it in 15 patients with advanced colon cancer. Patients received curcumin at 180 mg orally and their plasma and urine were collected. HPLC method was used to determine the content of curcumin. While curcumin and its metabolites were almost not detected, but it was found in the feces of patients. The results showed that curcumin was safe and well tolerated at 180 mg per day orally (Sharma et al., 2001). Lao et al. used dose-escalation to study the maximum tolerated dose and safety of curcumin. Twenty-four healthy subjects took curcumin extract powder orally at doses ranging from 500 mg to 12,000 mg, and only seven of them had minor non-dose-related toxicity. Therefore, curcumin is well tolerated at a single oral dose up to 8000 mg (Lao et al., 2006). Cheng et al. conducted a prospective clinical study on 25 patients with five different malignant diseases to evaluate the safety of curcumin. The initial dose was 500 mg/d, and if there were no consecutive grade 2 or more toxicity, the dose was increased to 1000, 2000, 4000, and 8000 mg/d. The results indicated that there was no curcumin-related toxicity after oral administration of 8000 mg/d for 3 months (Cheng et al., 2001).
2.4 Therapeutic Effects
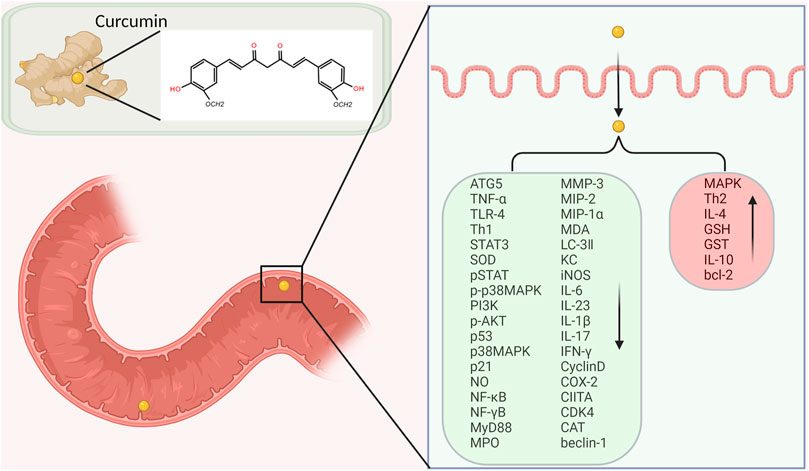
In recent years, studies have shown that curcumin has great pharmacological effects on anti-inflammatory, anti-fibrosis, anti-oxidation, hypolipidemic, anti-atherosclerosis, anti-depression, anti-HIV virus, etc. At the same time, curcumin has very low toxicity, so it has great potential for clinical application. Curcumin can also inhibit the growth of various tumor cells and prevent the occurrence of gastric cancer, duodenal cancer, colon cancer, breast cancer, chemical and radiation-induced skin cancer in rats. Its anti-tumor mechanism mainly includes inducing tumor cell apoptosis, blocking tumor cell signal transduction pathway, anti-oxidation and inhibiting tumor angiogenesis. For inflammatory bowel disease, curcumin can exert immunomodulatory and anti-inflammatory effects by inhibiting inflammatory mediators and cytokines, scavenging oxygen free radicals and other processes. This article will discuss the different mechanisms of curcumin in the treatment of inflammatory bowel disease.
3 Curcumin and Inflammatory Bowel Disease
3.1 In vitro and in vivo Experiment
3.1.1 Effects on Inflammatory Factors
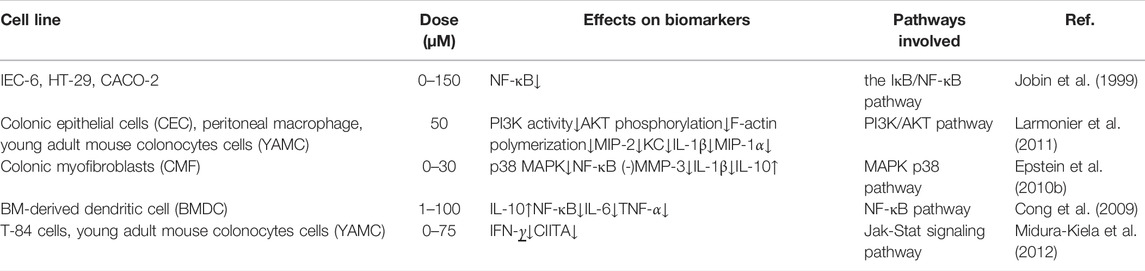
IBD is an autoimmune disease involving both autoantibodies and autoreactive CD4-positive T-lymphocytes. Dysimmunity will cause CD4-positive T-lymphocytes (Th1, Th2, and Th17) to produce a large number of pro-inflammatory factors, pushing the immune response beyond the range of T regulatory cells (Tregs), then triggering IBD. Jobin et al. demonstrated that curcumin inhibits il-β-mediated expression of pro-inflammatory cytokines such as IC-1 and IL-8 in IEC-6, HT-29 and Caco-2 cells, thereby exerting its anti-inflammatory effects (Jobin et al., 1999).
IL-1 mainly activates T cells, macrophages, etc. and its expression level increases significantly in the active period of IBD. Curcumin can inhibit the expression and secretion of inflammatory protein (MIP-2), IL-1β, and cytokine (KC) by macrophages stimulated by lipopolysaccharide (LPS). It blocks the accumulation of neutrophils to the site of intestinal inflammation, thereby alleviating the inflammatory response of IBD (Larmonier et al., 2011). IL-10 is an immunomodulatory cytokine synthesized by Th2, which can fight against pro-inflammatory factors such as IL-2, TNF-α and IFN-γ, and balance the body’s inflammatory response. The researchers found that the mouse model of colitis could be established using IL-10 gene knockout mice, demonstrating that IL-10 expression deficiency is associated with the pathogenesis of IBD (McFadden et al., 2015). Epstein et al. extracted colon tissue cells from IBD patients and used curcumin for intervention. The results showed that curcumin can inhibit the activation of p38MAPK, reduce the expression of IL-1β and matrix metalloproteinase-3 (MMP-3) and increase the expression of IL-10 (Epstein et al., 2010b). Curcumin inhibits LPS-induced expression of IL-12/23p40 in dendritic cells by synergistic action with IL-10 (Larmonier et al., 2008). Curcumin can regulate the expression of ALDH1a and IL-10 in bone marrow-derived dendritic cells (DCs). Curcumin-treated DCs can induce the differentiation of CD4+ T cells into Treg, which in turn inhibits the activation of antigen specific T cells. Thus, it promotes the restoration of immune balance. The results confirmed that curcumin has an effective therapeutic effect on experimental IBD (Cong et al., 2009).
IFN-γ is mainly produced by Th1 cells. Researchers found that IFN-γ levels in plasma and intestinal mucosa were significantly higher in IBD patients in active stage than in remission stage (Onal et al., 2016). Kiela et al. found that curcumin inhibited IFN-γ signaling in human and mouse colon epithelial cells. It plays a dual regulatory mechanism in colon epithelial cells, thereby improving and alleviating IBD (Midura-Kiela et al., 2012).
TNF-α is a major cytokine involved in inflammatory cascades in IBD. It has independent apoptotic activity and plays a key role in disruption of the intestinal barrier (Vedantam and Viswanathan, 2011). Curcumin also inhibits or kills B-lymphocytes, thereby blocking the release of macrophage-mediated inflammatory cytokines TNF-α and IL-6 (Maradana et al., 2013). Curcumin can down-regulate the production of Indoleamine 2,3-dioxygenase (IDO) through coX-2/PGE2 pathway. It inhibits the expression of surface molecules (CD80, CD86 and MHC class I) and proinflammatory cytokines (IL-12 P70 and TNF-α), thereby playing an immunomodulatory role (Jung et al., 2010). Yeter et al. found that curcumin can effectively inhibited TNF-α release and prevent TNF-α -driven oxidative stress response, thereby significantly reducing inflammatory response in mice with colitis (Mouzaoui et al., 2012).
3.1.2 Effects on Transcription Factors
Regulation of Toll-like receptor 4 (TLR-4) is an intracellular pattern recognition receptor, which plays an important role in response to intestinal epithelial injury and in limiting intestinal bacterial migration in mice with colitis. Nuclear factor-κB (NF-κB) also plays an important role in the pathogenesis of IBD. A high level of NF-κB expression increased the capability to secrete cytokines (IL-1, IL-6, TNF-α, et al). It was associated with mucosal damage in IBD (Atreya et al., 2008). Inhibition of NF-κB activity has been researched as one of the main treatment methods for IBD. Through the TLR-4/MyD88/MAPK/NF-κB pathway, NF-κB can be activated and transferred to the nucleus, thereby inducing the expression of multiple inflammatory mediators, resulting in intestinal inflammatory injury. Curcumin inhibits NF-κB activation by inhibiting TLR-4 receptor activation and reduces cytoplasmic IκB protein degradation. At the same time, the expression level of MMP-9 was significantly reduced, and then the inflammatory cascade was stopped, the intestinal mucosal damage was relieved (Lubbad et al., 2009).
Peroxisomeproliferator-activated receptor γ (PPAR-γ), the member of the nuclear receptor superfamily, is expressed at high level in colon epithelial cells and plays an important role in the gut. Researchers think that PPAR-γ suppresses inflammation by regulating the aggregation of macrophages to inflammatory sites in the colon. Zhang et al. observed that curcumin inhibited trinitrobenzene sulfonic acid (TNBS) induced colitis by activating PPAR-γ, improved the long-term survival rate and reduced the macroscopic score of colitis in rats with IBD. They also observed that curcumin combined with dexamethasone could improve the expression of PPAR-γ and inhibit the expression of COX-2 and PGJ2 (Zhang et al., 2006a).
Curcumin significantly reduced the expression of CD4+T cell subsets and B and T Lymphocyte Attenuator (BTLA) in DSS induced UC mice. The mechanism of its action may be that it inhibits Th1/Tc1 cell differentiation by regulating the balance between CD4+/CD8+T lymphocyte subsets. These processes promote upregulation of BTLA expression on the surface of CD4+T lymphocytes and decrease of immune response, thus exerting anti-inflammatory and therapeutic effects on UC (Zhang et al., 2015).
3.1.3 Curcumin’s Molecular Targets in IBD
The treatment of IBD focuses on controlling inflammation, thereby improving symptoms. At present, the clinical effect of anti-inflammatory or immunosuppressive drugs is not ideal. Curcumin is considered as a potential treatment for IBD due to its significant anti-inflammatory effect and safety. Curcumin may mediate anti-inflammatory effects through the following targeting molecular pathways.
Curcumin controls inflammation by downregulating the genes associated with oxidative stress and fibrogenesis pathways. The activity of PI3K and phosphorylation of AKT will result in decreasing cell death. Meanwhile, curcumin blocks neutrophils and downregulates the phosphorylation of PI3K and AKT (Ghoneim et al., 2002).
Signal transduction pathways play a crucial role in a cascade of inflammatory and are considered as potential molecular targets for the treatment of IBD. P38 mitogen-activated protein kinase (MAPK), a key regulatory enzyme of multiple target genes, regulates intestinal inflammation by regulating monocyte infiltration and intestinal water and electrolyte balance. The activation of MAPK system can lead to the large expression of inflammatory mediators, resulting in intestinal inflammatory damage. Studies have shown that curcumin can significantly inhibit p38MAPK activation and histone acetylation in intestinal mucosal cells cultured in vitro from children and adults with IBD, thereby reducing inflammatory responses (Epstein et al., 2010b). Similarly, curcumin can reduce the release of TNF-α and other pro-inflammatory factors by inhibiting p38MAPK signaling pathway, thus alleviating intestinal mucosal injury and symptoms in DSS induced IBD mice (Yang et al., 2013). Li et al. induced IBD model mice with dextran sodium sulfate (DSS) to explore the mechanism of curcumin in the treatment of IBD. The results showed that the expression of p38MAPK protein and mRNA was significantly decreased in curcumin treatment group, and the production of TNF-α was also decreased. In another experiment, curcumin reduced apoptosis and alleviated acetic acid induced colitis injury in rats by modulating the p38MAPK and JNK pathways (Topcu-Tarladacalisir et al., 2013).
Curcumin can inhibit the antigen presentation and maturation of dendritic cells by down-regulating the ability of spleen dendritic cells to express costimulatory signals, thus reducing the secretion level of pro-inflammatory factors. The infiltration of inflammatory cells was then inhibited, and the inflammatory damage of intestinal cells was relieved (Yang M. et al., 2017).
At present, it is generally believed that intestinal mucosal immune imbalance is one of the main pathogenesis of IBD. Many proinflammatory cytokines, such as IL-1, IL-6 and INF-γ, promote inflammatory responses through the JAK/STAT pathway. Studies have found that STAT3 phosphorylation state is the highest in the STAT family in UC and CD patients and DSS induced IBD model mice, suggesting that STAT3 may play an important role in the pathogenesis of IBD (Suzuki et al., 2001). Curcumin exerts anti-inflammatory effects in DSS-induced IBD mice by inhibiting the STAT3 pathway (Liu et al., 2013). Yang et al. proved that curcumin could inhibit p53 expression by inhibiting STAT3 signaling. P53 is an upstream regulator of the CDK4-cylind1 complex, and therefore the levels of cell cycle regulators CDK4 and Cylind1 are decreased accordingly. Curcumin thus had an anti-inflammatory effect, and reduced the severity of DSS induced colitis in mice (Yang et al., 2013). Yue et al. ‘s experiments confirmed that curcumin can prevent DSS-induced colitis by inhibiting excessive autophagy and regulating subsequent cytokine related pathways (Karthikeyan et al., 2021) (Figure 3).
3.1.4 Effects on Immune Cells
IBD is an autoimmune disease involving both autoantibodies and autoreactive CD4+T lymphocytes. Both UC and CD are characterized by immune response to intestinal antigens, but their immune response patterns are different. The pathogenesis of CD is mainly related to the inflammatory response dominated by interleukin (IL-1), IL-6, IL-8, tumor necrosis factor (TNF -α) and interferon γ (IFN-γ), which are secreted by Th1 and Th17 cells. UC is associated with inflammatory responses dominated by cytokines such as IL-4, IL-5, IL-9 and IL-13, which are secreted by TH-2 cells (Shale et al., 2013; Vecchi Brumatti et al., 2014; Sreedhar et al., 2016). The results of Zhang et al. showed that curcumin decreased the expression of Th1 cytokines in colonic mucosa, and at the same time up-regulated the expression of Th2 cytokines. Curcumin also upregulated the proportion of IFN-γ/IL-4 in the circulation. By regulating the balance of Th1/Th2, the effect of IBD treatment can be achieved (Zhang et al., 2006b).
3.1.5 The Antioxidant Stress Effect
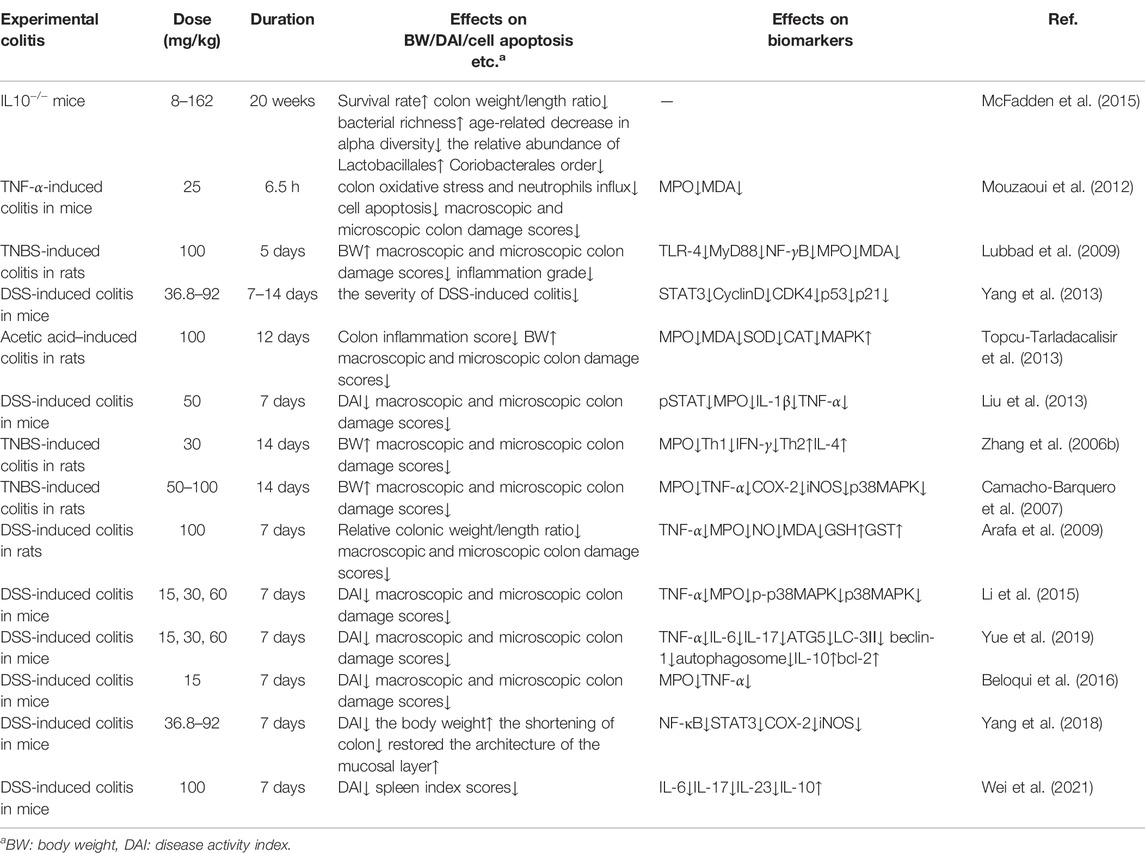
The activity of Myeloperoxidase (MPO) in mice with colitis was significantly reduced after curcumin administration. Therefore, oxidative stress, cytokine cascade and colitis response can be partially reduced (Camacho-Barquero et al., 2007). Shukla et al. demonstrated that after taking curcumin, the level of superoxide dismutase (SOD) in serum of rats increased, indicating that curcumin has good antioxidant and anti-inflammatory effects. Curcumin can eliminate oxygen free radicals in cells, inhibit lipid peroxidation in tissues, and protect SOD activity in tissues, thus playing an anti-inflammatory role in the treatment of ulcerative colitis (Shukla et al., 2003). Curcumin also plays a protective role in ulcerative colitis by regulating the antioxidant/antioxidant balance (Arafa et al., 2009) (Tables 2, 3).
3.2 Clinical Trial
3.2.1 Remission Induction
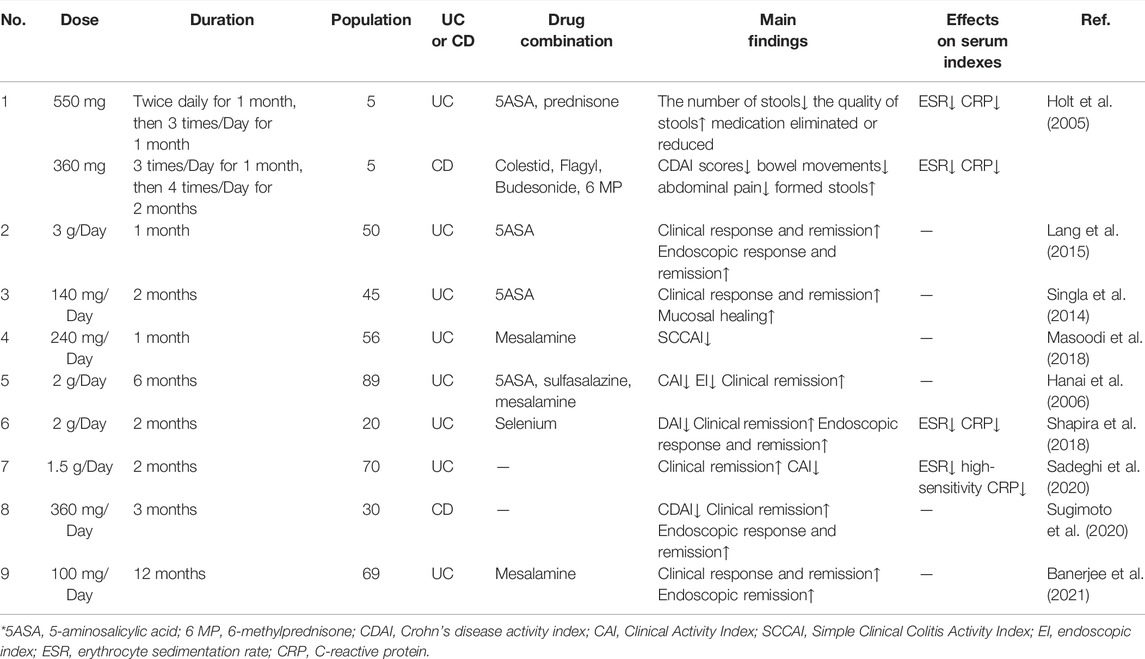
In Holt’s study, five subjects with ulcerative proctitis had received 5-aminosalicylic acid (5-ASA) before entering the study. In treatment group, researchers took curcumin 550 mg twice a day for 1 month. Then it was changed to 550 mg three times a day for 1 month. Biochemical, inflammatory factors and other indicators of subjects were examined after taking the medicine. The results showed that the clinical symptoms of all five patients improved, including two patients who had stopped taking 5-aminosalicylic acid and two patients who had reduced the dosage. Five Crohn’s disease subjects were given curcumin 360 mg 3 times a day for 1 month. Then it was changed to 360 mg 4 time a day for 1 month. The results showed an average decrease of 55% in disease activity index, 10 mm/h in erythrocyte sedimentation rate, and 0.1 mg/dl in C-reactive protein. There was no significant effect on liver and kidney function in all subjects (Holt et al., 2005).
Lang et al. enrolled patients with mild to moderate activity ulcerative colitis who did not respond significantly to the maximum dose of oral 5-ASA. They were randomly divided into treatment and control group. Both groups of patients continued to take 5-ASA orally. Patients in treatment group were given curcumin capsule (3 g/d) and patients in control group were given the same dose of placebo. The experiment lasted for 1 month. The results showed that 53.8% of patients in treatment group had symptomatic relief, while none in control group had symptomatic relief (OR = 42, 95%CI: 2.3–760) (Lang et al., 2015). Singla et al. enrolled patients with mild to moderate UC activity in the distal colon who had taken mesalazine for at least 8 weeks prior to enrollment. They were divided into treatment group and control group. The treatment group was given curcumin enema while mesalazine was taken orally, and the control group was given placebo enema while mesalazine was taken orally. The symptoms of patients in the two groups were observed. The results showed that treatment group had a higher remission rate and clinical response rate (remission rate: 43.4 vs. 22.7%, response rate: 56.5 vs. 36.4%) (Singla et al., 2014). Masoodi et al. also enrolled patients with mild to moderate UC activity. The patients were randomly divided into treatment group and control group. The treatment subjects received curcumin (80 mg, 3 times/d) and mesalazine (3 g/d), and the control group received the same dose of placebo and mesalazine. The disease severity was assessed at the end of the 4th week. The results showed that compared with the control group, the mean score of the simple clinical colitis activity index (SCCAI) in treatment group was significantly decreased (1.71 ± 1.84 vs. 2.68 ± 2.09, p = 0.05) (Masoodi et al., 2018).
3.2.2 Remission Maintain
Hanai et al. studied the ability of curcumin to prevent UC recurrence. A randomized, double-blind, multicenter trial was conducted in 89 patients with UC at resting stage. In addition to salazopyridine or mesalazine, 43 patients received curcumin 1.0 g after breakfast and dinner, and the remaining 46 patients received placebo. The trial lasted for 6 months. Subjects’ clinical activity index and endoscopic index were assessed at the beginning of the trial, every 2 months, and at the end of the trial. The recurrence rate was 4.65% in treatment group and 20.51% in control group. The result indicated that curcumin can significantly improve colitis activity index (CAI) and endoscopic index (EI) in patients (Hanai et al., 2006) (Table 4).
4 Conclusion
IBD is difficult to delay to heal and easy relapse. The economic burden of patients is heavy, and the quality of life is often seriously affected. The effect of medicinal treatment is often affected by the compliance and economic status of patients. At present, the drug effect is limited and often accompanied by side effects (i.e., diarrhea and lymphopenia). Curcumin, as a natural product with low price, has received increasing attention in recent years. Many studies have indicated that it has multiple biological activities. However, some researchers have questioned the effectiveness of curcumin. But the comment co-authored by Dr Heger and other scientists argued that the double-blind, placebo-controlled trial (DBPC) is the best way to get evidence of the medicinal value. At present, many researches have proved that curcumin is safe and effective through DBPC test (Heger, 2017). Curcumin may not fit medical chemists’ definition of the perfect drug, but many in vitro, in vivo and clinical trials have irrefutably confirmed its medicinal potential (Li et al., 2015; Beloqui et al., 2016; Yang et al., 2018; Yue et al., 2019; Wei et al., 2021). We searched ClinicalTrials.gov to identify current clinical trials evaluating curcumin treatment for IBD. A total of 10 trials were included. Three of them have been completed and five are in phase 3. We need to pay close attention to those latest findings.
Curcumin has less adverse reactions and high safety during use. As a potential chemotherapeutic drug, it shows a good application prospect in both basic experimental researches and clinical trials of IBD over the decades (Shapira et al., 2018; Sadeghi et al., 2020; Sugimoto et al., 2020; Banerjee et al., 2021). However, its mechanism of action is complex and related to multiple signaling pathways. Its mechanism of targeting has not been clearly described. More efforts are needed to identify the molecular targets and regulatory mechanisms of curcumin. Curcumin is unstable under physiological conditions and the bioavailability after oral administration is low. In order to improve its characteristics, researchers have tried various ways such as preparation of compounds, using liposomes, synthesis of nanoparticles or curcumin analogues. Some of them have yielded gratifying results (Tsuda, 2018). However, more multi-center large-scale clinical trials are needed to confirm its efficacy and safety. It is hoped that further studies on curcumin will provide new drug research directions for the treatment and remission of IBD in the near future (Li et al., 2017; Annese, 2019; Fança-Berthon et al., 2021; Rogler et al., 2021).
.
Author Contributions
YL, HL, LB, CC, HQ and XY designed research, performed research, analyzed data, and wrote the paper.
Funding
This work was financed by Grant-in-aid for scientific research from the National Natural Science Foundation of China (No. 81970461).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
I would like to express my gratitude to all those who helped me during the writing of this thesis. I gratefully acknowledge my husband. I do appreciate his encouragement, patience, and professional instructions during my thesis writing.
Abbreviations
5-ASA, 5-aminosalicylic acid; BTLA, B and T lymphocyte attenuator; BW, body weight; CAI, clinical activity index; CD, Crohn’s disease; DAI, disease activity index; DCs, dendritic cells; EI, endoscopic index; DSS, dextran sodium sulfate; IBD, inflammatory bowel disease; KC, cytokine; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MMP-3, matrix metalloproteinase-3; MPO, myeloperoxidase; NF-κB, nuclear factor-κB; PPAR-γ, peroxisomeproliferator-activated receptor γ; SCCAI, simple clinical colitis activity index; SOD, superoxide dismutase; Tregs, T regulatory cells; TLR-4, toll-like receptor 4; TNBS, trinitrobenzene sulfonic acid; UC, ulcerative colitis.
References
Annese, V. (2019). A Review of Extraintestinal Manifestations and Complications of Inflammatory Bowel Disease. Saudi J. Med. Med. Sci. 7, 66–73. doi:10.4103/sjmms.sjmms_81_18
Arafa, H. M., Hemeida, R. A., El-Bahrawy, A. I., and Hamada, F. M. (2009). Prophylactic Role of Curcumin in Dextran Sulfate Sodium (DSS)-induced Ulcerative Colitis Murine Model. Food Chem. Toxicol. 47, 1311–1317. doi:10.1016/j.fct.2009.03.003
Atreya, I., Atreya, R., and Neurath, M. F. (2008). NF-kappaB in Inflammatory Bowel Disease. J. Intern Med. 263, 591–596. doi:10.1111/j.1365-2796.2008.01953.x
Banerjee, R., Pal, P., Penmetsa, A., Kathi, P., Girish, G., Goren, I., et al. (2021). Novel Bioenhanced Curcumin with Mesalamine for Induction of Clinical and Endoscopic Remission in Mild-To-Moderate Ulcerative Colitis: A Randomized Double-Blind Placebo-Controlled Pilot Study. J. Clin. Gastroenterol. 55, 702–708. doi:10.1097/MCG.0000000000001416
Baumgart, D. C., and Sandborn, W. J. (2012). Crohn's Disease. Lancet 380, 1590–1605. doi:10.1016/S0140-6736(12)60026-9
Beloqui, A., Memvanga, P. B., Coco, R., Reimondez-Troitiño, S., Alhouayek, M., Muccioli, G. G., et al. (2016). A Comparative Study of Curcumin-Loaded Lipid-Based Nanocarriers in the Treatment of Inflammatory Bowel Disease. Colloids Surf. B Biointerfaces 143, 327–335. doi:10.1016/j.colsurfb.2016.03.038
Beyene, A. M., Moniruzzaman, M., and Karthikeyan, A. (2021). Curcumin Nanoformulations with Metal Oxide Nanomaterials for Biomedical Applications. Review 11, 460. doi:10.3390/nano11020460
Camacho-Barquero, L., Villegas, I., Sánchez-Calvo, J. M., Talero, E., Sánchez-Fidalgo, S., Motilva, V., et al. (2007). Curcumin, a Curcuma Longa Constituent, Acts on MAPK P38 Pathway Modulating COX-2 and iNOS Expression in Chronic Experimental Colitis. Int. Immunopharmacol. 7, 333–342. doi:10.1016/j.intimp.2006.11.006
Castro, J., Ocampo, Y., and Franco, L. (2015). Cape Gooseberry [Physalis Peruviana L.] Calyces Ameliorate TNBS Acid-Induced Colitis in Rats. J. Crohns Colitis 9, 1004–1015. doi:10.1093/ecco-jcc/jjv132
Cheng, A. L., Hsu, C. H., Lin, J. K., Hsu, M. M., Ho, Y. F., Shen, T. S., et al. (2001). Phase I Clinical Trial of Curcumin, a Chemopreventive Agent, in Patients with High-Risk or Pre-malignant Lesions. Anticancer Res. 21, 2895–2900.
Colombel, J. F., and Mahadevan, U. (2017). Inflammatory Bowel Disease 2017: Innovations and Changing Paradigms. Gastroenterology 152, 309–312. doi:10.1053/j.gastro.2016.12.004
Cong, Y., Wang, L., Konrad, A., Schoeb, T., and Elson, C. O. (2009). Curcumin Induces the Tolerogenic Dendritic Cell that Promotes Differentiation of Intestine-Protective Regulatory T Cells. Eur. J. Immunol. 39, 3134–3146. doi:10.1002/eji.200939052
Epstein, J., Sanderson, I. R., and Macdonald, T. T. (2010). Curcumin as a Therapeutic Agent: the Evidence from In Vitro, Animal and Human Studies. Br. J. Nutr. 103, 1545–1557. doi:10.1017/S0007114509993667
Epstein, J., Docena, G., MacDonald, T. T., and Sanderson, I. R. (2010). Curcumin Suppresses P38 Mitogen-Activated Protein Kinase Activation, Reduces IL-1beta and Matrix Metalloproteinase-3 and Enhances IL-10 in the Mucosa of Children and Adults with Inflammatory Bowel Disease. Br. J. Nutr. 103, 824–832. doi:10.1017/S0007114509992510
Fança-Berthon, P., Tenon, M., Bouter-Banon, S. L., Manfré, A., Maudet, C., Dion, A., et al. (2021). Pharmacokinetics of a Single Dose of Turmeric Curcuminoids Depends on Formulation: Results of a Human Crossover Study. J. Nutr. 151, 1802–1816. doi:10.1093/jn/nxab087
Ghoneim, A. I., Abdel-Naim, A. B., Khalifa, A. E., and El-Denshary, E. S. (2002). Protective Effects of Curcumin against Ischaemia/reperfusion Insult in Rat Forebrain. Pharmacol. Res. 46, 273–279. doi:10.1016/s1043-6618(02)00123-8
Hanai, H., Iida, T., Takeuchi, K., Watanabe, F., Maruyama, Y., Andoh, A., et al. (2006). Curcumin Maintenance Therapy for Ulcerative Colitis: Randomized, Multicenter, Double-Blind, Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 4, 1502–1506. doi:10.1016/j.cgh.2006.08.008
Heger, M. (2017). Drug Screening: Don't Discount All Curcumin Trial Data. Nature 543, 40. doi:10.1038/543040c
Holt, P. R., Katz, S., and Kirshoff, R. (2005). Curcumin Therapy in Inflammatory Bowel Disease: a Pilot Study. Dig. Dis. Sci. 50, 2191–2193. doi:10.1007/s10620-005-3032-8
Jobin, C., Bradham, C. A., Russo, M. P., Juma, B., Narula, A. S., Brenner, D. A., et al. (1999). Curcumin Blocks Cytokine-Mediated NF-Kappa B Activation and Proinflammatory Gene Expression by Inhibiting Inhibitory Factor I-Kappa B Kinase Activity. J. Immunol. 163, 3474–3483.
Jung, I. D., Jeong, Y. I., Lee, C. M., Noh, K. T., Jeong, S. K., Chun, S. H., et al. (2010). COX-2 and PGE2 Signaling Is Essential for the Regulation of Ido Expression by Curcumin in Murine Bone Marrow-Derived Dendritic Cells. Int. Immunopharmacol. 10, 760–768. doi:10.1016/j.intimp.2010.04.006
Kahkhaie, K. R., Mirhosseini, A., Aliabadi, A., Mohammadi, A., Mousavi, M. J., Haftcheshmeh, S. M., et al. (2019). Curcumin: a Modulator of Inflammatory Signaling Pathways in the Immune System. Inflammopharmacology 27, 885–900. doi:10.1007/s10787-019-00607-3
Karthikeyan, A., Senthil, N., and Min, T. (2020). Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 11, 487. doi:10.3389/fphar.2020.00487
Karthikeyan, A., Young, K. N., and Moniruzzaman, M. (2021). Curcumin and its Modified Formulations on Inflammatory Bowel Disease (IBD): The Story So Far and Future Outlook. Review 13, 484. doi:10.3390/pharmaceutics13040484
Khan, N., Abbas, A. M., Bazzano, L. A., Koleva, Y. N., and Krousel-Wood, M. (2012). Long-term Oral Mesalazine Adherence and the Risk of Disease Flare in Ulcerative Colitis: Nationwide 10-year Retrospective Cohort from the Veterans Affairs Healthcare System. Aliment. Pharmacol. Ther. 36, 755–764. doi:10.1111/apt.12013
Lang, A., Salomon, N., Wu, J. C., Kopylov, U., Lahat, A., Har-Noy, O., et al. (2015). Curcumin in Combination with Mesalamine Induces Remission in Patients with Mild-To-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 13, 1444–1449.e1. doi:10.1016/j.cgh.2015.02.019
Lao, C. D., Ruffin, M. T., Normolle, D., Heath, D. D., Murray, S. I., Bailey, J. M., et al. (2006). Dose Escalation of a Curcuminoid Formulation. BMC Complement. Altern. Med. 6, 10. doi:10.1186/1472-6882-6-10
Larmonier, C. B., Uno, J. K., Lee, K. M., Karrasch, T., Laubitz, D., Thurston, R., et al. (2008). Limited Effects of Dietary Curcumin on Th-1 Driven Colitis in IL-10 Deficient Mice Suggest an IL-10-dependent Mechanism of Protection. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G1079–G1091. doi:10.1152/ajpgi.90365.2008
Larmonier, C. B., Midura-Kiela, M. T., Ramalingam, R., Laubitz, D., Janikashvili, N., Larmonier, N., et al. (2011). Modulation of Neutrophil Motility by Curcumin: Implications for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 17, 503–515. doi:10.1002/ibd.21391
Li, C. P., Li, J. H., He, S. Y., Chen, O., and Shi, L. (2015). Effect of Curcumin on p38MAPK Expression in DSS-Induced Murine Ulcerative Colitis. Genet. Mol. Res. 14, 3450–3458. doi:10.4238/2015.April.15.8
Li, X., Song, P., Li, J., Tao, Y., Li, G., Li, X., et al. (2017). The Disease Burden and Clinical Characteristics of Inflammatory Bowel Disease in the Chinese Population: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 14, 238. doi:10.3390/ijerph14030238
Liu, L., Liu, Y. L., Liu, G. X., Chen, X., Yang, K., Yang, Y. X., et al. (2013). Curcumin Ameliorates Dextran Sulfate Sodium-Induced Experimental Colitis by Blocking STAT3 Signaling Pathway. Int. Immunopharmacol. 17, 314–320. doi:10.1016/j.intimp.2013.06.020
Lubbad, A., Oriowo, M. A., and Khan, I. (2009). Curcumin Attenuates Inflammation through Inhibition of TLR-4 Receptor in Experimental Colitis. Mol. Cell Biochem. 322, 127–135. doi:10.1007/s11010-008-9949-4
Maradana, M. R., Thomas, R., and O'Sullivan, B. J. (2013). Targeted Delivery of Curcumin for Treating Type 2 Diabetes. Mol. Nutr. Food Res. 57, 1550–1556. doi:10.1002/mnfr.201200791
Masoodi, M., Mahdiabadi, M. A., Mokhtare, M., Agah, S., Kashani, A. H. F., Rezadoost, A. M., et al. (2018). The Efficacy of Curcuminoids in Improvement of Ulcerative Colitis Symptoms and Patients' Self-Reported Well-Being: A Randomized Double-Blind Controlled Trial. J. Cell Biochem. 119, 9552–9559. doi:10.1002/jcb.27273
McFadden, R. M., Larmonier, C. B., Shehab, K. W., Midura-Kiela, M., Ramalingam, R., Harrison, C. A., et al. (2015). The Role of Curcumin in Modulating Colonic Microbiota during Colitis and Colon Cancer Prevention. Inflamm. Bowel Dis. 21, 2483–2494. doi:10.1097/MIB.0000000000000522
Midura-Kiela, M. T., Radhakrishnan, V. M., Larmonier, C. B., Laubitz, D., Ghishan, F. K., and Kiela, P. R. (2012). Curcumin Inhibits Interferon-γ Signaling in Colonic Epithelial Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G85–G96. doi:10.1152/ajpgi.00275.2011
Moniruzzaman, M., and Min, T. (2020). Curcumin, Curcumin Nanoparticles and Curcumin Nanospheres: A Review on Their Pharmacodynamics Based on Monogastric Farm Animal, Poultry and Fish Nutrition. Pharmaceutics 12, 12. doi:10.3390/pharmaceutics12050447
Mouzaoui, S., Rahim, I., and Djerdjouri, B. (2012). Aminoguanidine and Curcumin Attenuated Tumor Necrosis Factor (TNF)-α-induced Oxidative Stress, Colitis and Hepatotoxicity in Mice. Int. Immunopharmacol. 12, 302–311. doi:10.1016/j.intimp.2011.10.010
Onal, I. K., Alizadeh, N., and Sargin, Z. G. (2016). Performance of Interferon-Gamma Release Assay for Tuberculosis Screening in Inflammatory Bowel Disease Patients: Disease Activity as an Influencing Factor. Scand. J. Gastroenterol. 51, 381. doi:10.3109/00365521.2015.1092578
Pan, M. H., Huang, T. M., and Lin, J. K. (1999). Biotransformation of Curcumin through Reduction and Glucuronidation in Mice. Drug Metab. Dispos. 27, 486–494.
Patel, S. S., Acharya, A., Ray, R. S., Agrawal, R., Raghuwanshi, R., and Jain, P. (2020). Cellular and Molecular Mechanisms of Curcumin in Prevention and Treatment of Disease. Crit. Rev. Food Sci. Nutr. 60, 887–939. doi:10.1080/10408398.2018.1552244
Ravindranath, V., and Chandrasekhara, N. (1980). Absorption and Tissue Distribution of Curcumin in Rats. Toxicology 16, 259–265. doi:10.1016/0300-483x(80)90122-5
Rogler, G., Singh, A., Kavanaugh, A., and Rubin, D. T. (2021). Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 161, 1118–1132. doi:10.1053/j.gastro.2021.07.042
Sadeghi, N., Mansoori, A., Shayesteh, A., and Hashemi, S. J. (2020). The Effect of Curcumin Supplementation on Clinical Outcomes and Inflammatory Markers in Patients with Ulcerative Colitis. Phytother. Res. 34, 1123–1133. doi:10.1002/ptr.6581
Salehi, B., Stojanović-Radić, Z., Matejić, J., Sharifi-Rad, M., Anil Kumar, N. V., Martins, N., et al. (2019). The Therapeutic Potential of Curcumin: A Review of Clinical Trials. Eur. J. Med. Chem. 163, 527–545. doi:10.1016/j.ejmech.2018.12.016
Shale, M., Schiering, C., and Powrie, F. (2013). CD4(+) T-Cell Subsets in Intestinal Inflammation. Immunol. Rev. 252, 164–182. doi:10.1111/imr.12039
Shapira, S., Leshno, A., Katz, D., Maharshak, N., Hevroni, G., Jean-David, M., et al. (2018). Of Mice and Men: a Novel Dietary Supplement for the Treatment of Ulcerative Colitis. Ther. Adv. Gastroenterol. 11, 1756283x17741864. doi:10.1177/1756283X17741864
Sharma, R. A., McLelland, H. R., Hill, K. A., Ireson, C. R., Euden, S. A., Manson, M. M., et al. (2001). Pharmacodynamic and Pharmacokinetic Study of Oral Curcuma Extract in Patients with Colorectal Cancer. Clin. Cancer Res. 7, 1894–1900.
Sharma, M., Sharma, S., and Wadhwa, J. (2019). Improved Uptake and Therapeutic Intervention of Curcumin via Designing Binary Lipid Nanoparticulate Formulation for Oral Delivery in Inflammatory Bowel Disorder. Artif. Cells Nanomed Biotechnol. 47, 45–55. doi:10.1080/21691401.2018.1543191
Shukla, P. K., Khanna, V. K., Khan, M. Y., and Srimal, R. C. (2003). Protective Effect of Curcumin against Lead Neurotoxicity in Rat. Hum. Exp. Toxicol. 22, 653–658. doi:10.1191/0960327103ht411oa
Singla, V., Pratap Mouli, V., Garg, S. K., Rai, T., Choudhury, B. N., Verma, P., et al. (2014). Induction with NCB-02 (Curcumin) Enema for Mild-To-Moderate Distal Ulcerative Colitis - a Randomized, Placebo-Controlled, Pilot Study. J. Crohns Colitis 8, 208–214. doi:10.1016/j.crohns.2013.08.006
Sreedhar, R., Arumugam, S., Thandavarayan, R. A., Karuppagounder, V., and Watanabe, K. (2016). Curcumin as a Therapeutic Agent in the Chemoprevention of Inflammatory Bowel Disease. Drug Discov. Today 21, 843–849. doi:10.1016/j.drudis.2016.03.007
Sugimoto, K., Ikeya, K., Bamba, S., Andoh, A., Yamasaki, H., Mitsuyama, K., et al. (2020). Highly Bioavailable Curcumin Derivative Ameliorates Crohn's Disease Symptoms: A Randomized, Double-Blind, Multicenter Study. J. Crohns Colitis 14, 1693–1701. doi:10.1093/ecco-jcc/jjaa097
Suzuki, A., Hanada, T., Mitsuyama, K., Yoshida, T., Kamizono, S., Hoshino, T., et al. (2001). CIS3/SOCS3/SSI3 Plays a Negative Regulatory Role in STAT3 Activation and Intestinal Inflammation. J. Exp. Med. 193, 471–481. doi:10.1084/jem.193.4.471
Topcu-Tarladacalisir, Y., Akpolat, M., Uz, Y. H., Kizilay, G., Sapmaz-Metin, M., Cerkezkayabekir, A., et al. (2013). Effects of Curcumin on Apoptosis and Oxidoinflammatory Regulation in a Rat Model of Acetic Acid-Induced Colitis: the Roles of C-Jun N-Terminal Kinase and P38 Mitogen-Activated Protein Kinase. J. Med. Food 16, 296–305. doi:10.1089/jmf.2012.2550
Tsuda, T. (2018). Curcumin as a Functional Food-Derived Factor: Degradation Products, Metabolites, Bioactivity, and Future Perspectives. Food Funct. 9, 705–714. doi:10.1039/c7fo01242j
Ungaro, R., Mehandru, S., Allen, P. B., Peyrin-Biroulet, L., and Colombel, J. F. (2017). Ulcerative Colitis. Lancet 389, 1756–1770. doi:10.1016/S0140-6736(16)32126-2
van der Veen, B. S., de Winther, M. P., and Heeringa, P. (2009). Myeloperoxidase: Molecular Mechanisms of Action and Their Relevance to Human Health and Disease. Antioxid. Redox Signal 11, 2899–2937. doi:10.1089/ars.2009.2538
Vecchi Brumatti, L., Marcuzzi, A., Tricarico, P. M., Zanin, V., Girardelli, M., and Bianco, A. M. (2014). Curcumin and Inflammatory Bowel Disease: Potential and Limits of Innovative Treatments. Molecules 19, 21127–21153. doi:10.3390/molecules191221127
Vedantam, G., and Viswanathan, V. K. (2011). Unlocking the Gates to Inflammatory Bowel Disease: the Role of Enterococcus faecalis Gelatinase. Gastroenterology 141, 795–798. doi:10.1053/j.gastro.2011.07.022
Wei, C., Wang, J. Y., Xiong, F., Wu, B. H., Luo, M. H., Yu, Z. C., et al. (2021). Curcumin Ameliorates DSS-induced Colitis in Mice by Regulating the Treg/Th17 Signaling Pathway. Mol. Med. Rep. 23, 23. doi:10.3892/mmr.2020.11672
Yang, J. Y., Zhong, X., Yum, H. W., Lee, H. J., Kundu, J. K., Na, H. K., et al. (2013). Curcumin Inhibits STAT3 Signaling in the Colon of Dextran Sulfate Sodium-Treated Mice. J. Cancer Prev. 18, 186–191. doi:10.15430/jcp.2013.18.2.186
Yang, J. Y., Zhong, X., Kim, S. J., Kim, D. H., Kim, H. S., Lee, J. S., et al. (2018). Comparative Effects of Curcumin and Tetrahydrocurcumin on Dextran Sulfate Sodium-Induced Colitis and Inflammatory Signaling in Mice. J. Cancer Prev. 23, 18–24. doi:10.15430/JCP.2018.23.1.18
Yang, H., Du, Z., Wang, W., Song, M., Sanidad, K., Sukamtoh, E., et al. (2017). Structure-Activity Relationship of Curcumin: Role of the Methoxy Group in Anti-inflammatory and Anticolitis Effects of Curcumin. J. Agric. Food Chem. 65, 4509–4515. doi:10.1021/acs.jafc.7b01792
Yang, M., Wang, J., Yang, C., Han, H., Rong, W., and Zhang, G. (2017). Oral Administration of Curcumin Attenuates Visceral Hyperalgesia through Inhibiting Phosphorylation of TRPV1 in Rat Model of Ulcerative Colitis. Mol. Pain 13, 1744806917726416. doi:10.1177/1744806917726416
Yue, W., Liu, Y., Li, X., Lv, L., Huang, J., and Liu, J. (2019). Curcumin Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice via Regulation of Autophagy and Intestinal Immunity. Turk J. Gastroenterol. 30, 290–298. doi:10.5152/tjg.2019.18342
Zhang, M., Deng, C., Zheng, J., Xia, J., and Sheng, D. (2006). Curcumin Inhibits Trinitrobenzene Sulphonic Acid-Induced Colitis in Rats by Activation of Peroxisome Proliferator-Activated Receptor Gamma. Int. Immunopharmacol. 6, 1233–1242. doi:10.1016/j.intimp.2006.02.013
Zhang, M., Deng, C. S., Zheng, J. J., and Xia, J. (2006). Curcumin Regulated Shift from Th1 to Th2 in Trinitrobenzene Sulphonic Acid-Induced Chronic Colitis. Acta Pharmacol. Sin. 27, 1071–1077. doi:10.1111/j.1745-7254.2006.00322.x
Keywords: IBD, curcumin, dietary supplements, immune regulation, anti-inflammatory actvity
Citation: Lin Y, Liu H, Bu L, Chen C and Ye X (2022) Review of the Effects and Mechanism of Curcumin in the Treatment of Inflammatory Bowel Disease. Front. Pharmacol. 13:908077. doi: 10.3389/fphar.2022.908077
Received: 01 April 2022; Accepted: 02 June 2022;
Published: 20 June 2022.
Edited by:
Rosa Serio, University of Palermo, ItalyReviewed by:
Karthikeyan Adhimoolam, Jeju National University, South KoreaHala Chaaban, University of Oklahoma Health Sciences Center, United States
Copyright © 2022 Lin, Liu, Bu, Chen and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofeng Ye, drzoelin@163.com
 Yuan Lin
Yuan Lin