Pharmacokinetics, Immunogenicity and Safety Study for SHR-1309 Injection and Perjeta® in Healthy Chinese Male Volunteers
- 1Phase I Clinical Trial Laboratory, Affiliated Hospital of Changchun University of Chinese Medicine, Jilin, China
- 2Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, China
- 3Shanghai Hengrui Pharmaceutical Co., Ltd., Shanghai, China
- 4School of Pharmacy, Jilin University, Jilin, China
- 5Clinical Medical College, Changchun University of Chinese Medicine, Jilin, China
- 6Jilin Province Honesty Medical Technology Consulting Co., Ltd., Jilin, China
A Corrigendum on
Pharmacokinetics, Immunogenicity and Safety Study for SHR-1309 Injection and Perjeta® in Healthy Chinese Male Volunteers
by Cui, Y., Cui, D., Ren, X., Chen, X., Liu, G., Liu, Z., Wang, Y., Qu, X., Zhao, Y. and Yang, H. (2021). Front. Pharmacol. 12:660541. doi: 10.3389/fphar.2021.660541
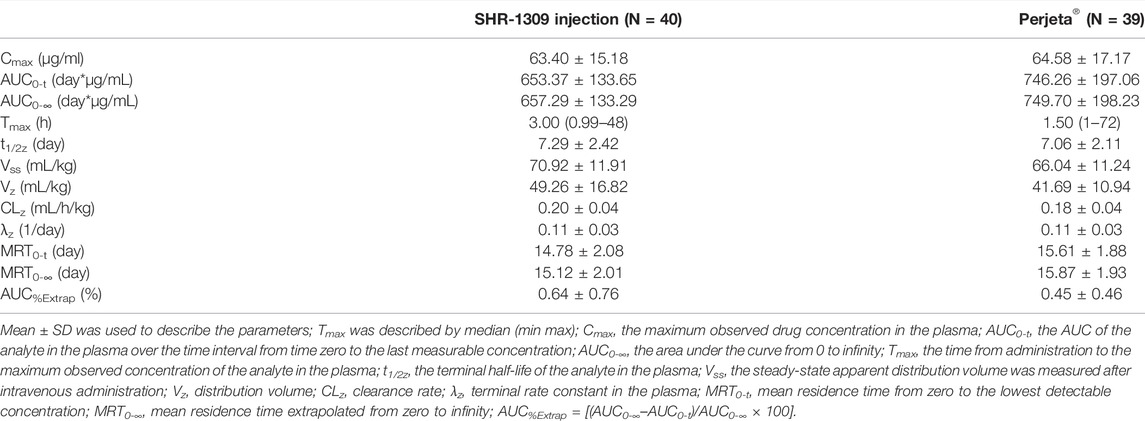
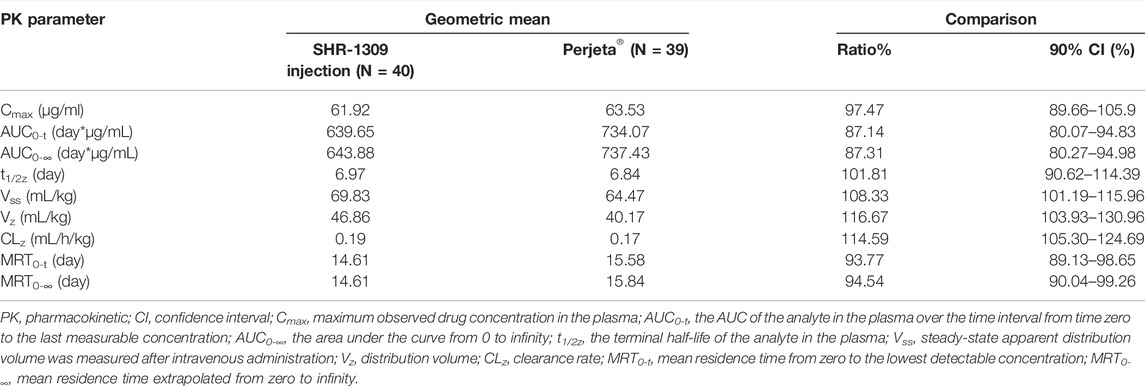
In the original article, there was a mistake in “Tables 2, 3” as published. “In Tables 2, 3, the AUC0-t and AUC0-∞ units were set to h*ng/ml by mistake.” The corrected “Tables 2, 3” appears below.
In the original article, there was an error. “The AUC0-t and AUC0-∞ units were set to h*ng/ml by mistake.”
A correction has been made to “Results,” “Pharmacokinetics,”:
“To evaluate the bioequivalence of SHR-1309 and Perjeta®, we performed PK analysis on two groups of subjects. The subjects were sampled at 21 time points before and after drug administration. Plasma drug concentration was detected by ELISA, and the data were fitted to form the average plasma drug concentration curve of SHR-1309 and Perjeta® (Figure 2A). The logarithmic transformation of the curve is shown in Figure 2B. At the same time, the plasma drug concentration of each subject in the two groups was fitted (Figures 2C,D). There was no significant difference in blood concentration between the two groups after administration. The primary evaluation parameters, secondary evaluation parameters and other pharmacokinetic parameters were obtained through calculation of plasma drug concentration (Table 2; Supplementary Table S1). The mean and standard deviation (SD) values of Cmax were 63.40 ± 15.18 μg/ml and 64.58 ± 17.17 μg/ml for SHR-1309 and Perjeta®, respectively, and the ratio of the geometric mean was 98.30%. The mean and SD values of AUC0-t were 653.37 ± 133.65 and 746.26 ± 197.06 day*μg/mL, respectively, and the ratio of the geometric mean was 88.41%. The mean and SD values of AUC0-∞ were 657.29 ± 133.29 and 749.70 ± 198.23 day*μg/mL, respectively, and the ratio of the geometric mean was 88.58%. Tmax was 1.50 and 3.00 h, respectively. The geometric mean values and ratios of all parameters are shown in Table 3. The primary pharmacokinetic parameters of SHR-1309 and Perjeta® were all up to the standard. Except for Vz, the 90% CI for all values fell within the 80%–125% range (Figure 3A). The PK parameter values of the two drugs were similar, and according to the PK evaluation standard of bioequivalence, SHR-1309 and Perjeta® are bioequivalent.”
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: perjeta®, pharmacokinetics, bioequivalence, immunogenicity, safety, SHR-1309 injection
Citation: Cui Y, Cui D, Ren X, Chen X, Liu G, Liu Z, Wang Y, Qu X, Zhao Y and Yang H (2022) Corrigendum: Pharmacokinetics, Immunogenicity and Safety Study for SHR-1309 Injection and Perjeta® in Healthy Chinese Male Volunteers. Front. Pharmacol. 13:907413. doi: 10.3389/fphar.2022.907413
Received: 29 March 2022; Accepted: 07 April 2022;
Published: 04 May 2022.
Edited and reviewed by:
Wang Lingzhi, National University of Singapore, SingaporeCopyright © 2022 Cui, Cui, Ren, Chen, Liu, Liu, Wang, Qu, Zhao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haimiao Yang, haimiaoyang@outlook.com
†These authors have contributed equally to this work
 Yingzi Cui1†
Yingzi Cui1† Yicheng Zhao
Yicheng Zhao Haimiao Yang
Haimiao Yang