
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 20 June 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.906764
Background: Integrative herbal medicine has been reported to have beneficial effects in the treatment of coronavirus disease 2019 (COVID-19).
Aim: To compile up-to-date evidence of the benefits and risks of herbal medicine for the treatment of COVID-19 symptoms.
Methods: Eleven databases, including PubMed, Cochrane Register of Controlled Trials (CENTRAL), Embase, Allied and Complementary Medicine Database (AMED), Chinese National Knowledge Infrastructure Database (CNKI), Wanfang Database, and Chinese Science and Technique Journals Database (VIP), Research Information Service System (RISS), Korean Medical database (KMBase), Korean Association of Medical Journal database (KoreaMed), and OASIS database, were searched from 15 June, 2020, until 28 March 2022. Randomized controlled trials (RCTs), published in any language, reporting the efficacy and safety outcomes of herbal medicine in patients of all ages with a PCR-confirmed diagnosis of COVID-19 were included in this analysis. Data extraction and quality assessments were performed independently.
Results: Random-effects meta-analyses showed evidence of favorable effects of treatment with herbal medicine when added to standard treatment, versus standard treatment alone, on the total effective rate (p = 0.0001), time to remission from fever (p < 0.00001), rate of remission from coughing (p < 0.0001), fatigue (p = 0.02), sputum production (p = 0.004), improvement of manifestations observed on chest computed tomography scans (p < 0.00001), incidence of progression to severe COVID-19 (p = 0.003), all-cause mortality (p = 0.003), time to a negative COVID-19 coronavirus test (p < 0.0001), and duration of hospital stay (p = 0.0003). There was no evidence of a difference between herbal medicine added to standard treatment, versus standard treatment alone, on the rate of remission from symptoms such as a fever, sore throat, nasal congestion and discharge, diarrhea, dry throat, chills, and the rate of conversion to a negative COVID-19 coronavirus test. Meta-analysis showed no evidence of a significant difference in adverse events between the two groups. There was an unclear risk of bias across the RCTs included in this analysis, indicating that most studies had methodological limitations.
Conclusion: Current evidence suggests that herbal medicine added to standard treatment has potential benefits in the treatment of COVID-19 symptoms but the certainty of evidence was low.
Coronavirus disease 2019 (COVID-19), also known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused a significant impact on global health. Although the number of new cases is ebbing in several countries that were hit hard early on, the number of new cases worldwide is growing rapidly each day. As of 25 March 2022, COVID-19 has afflicted more than 476 million people globally with approximately 6.1 million fatalities (World Health Organization (WHO), 2022b). This pandemic has a broad clinical spectrum ranging from self-limiting respiratory tract illness to fatal pneumonia, with therapeutic measures remaining limited (Cao et al., 2020).
Being the first country heavily affected by the COVID-19 outbreak, the Chinese government has attributed its swift turnaround in managing COVID-19 to the integration of herbal medicine with conventional medicine (National Health Commission of the People's Republic of China, 2020; Weeks, 2020). Clinical practice guidelines or memorandums of herbal medicine usage for COVID-19 have also been issued in several countries such as South Korea, Japan, Malaysia, and India.
Compelling evidence supporting integrative herbal medicine have been reported in several review articles amongst numerous studies published following the outbreak of COVID-19. A recent systematic review reported that the integration of herbal medicine and conventional medicine can alleviate the symptoms of COVID-19 without increasing adverse drug reactions (Liu M. et al., 2020). However, such beneficial effects of herbal medicine are inconclusive due to the poor quality of the methodology used in the clinical trials. Another review article critically analyzed the therapeutic evidence of herbal medicine integration in the management of COVID-19 symptoms and concluded that, by combining with conventional medicine, herbal medicine showed positive effects and great potential in complementing the conventional treatment (Chan et al., 2020). Yet, the evidence is not sufficient enough and more clinical studies are required to validate this conclusion. Overall, the evidence has shown a level of uncertainty in the effectiveness of herbal medicine intervention for the treatment of COVID-19, and rigorous clinical studies are highly warranted.
To address the uncertainty in the evidence for herbal medicine intervention for COVID-19 treatment, there is a need for the continuous surveillance of new evidence. This can be performed using a living systematic review (LSR) approach, where a systematic review is continually updated to incorporate newly available evidence (Elliott et al., 2017). According to the International Clinical Trials Registry Platform (ICTRP) of the WHO, >100 randomized controlled trials (RCTs) related to herbal medicine intervention have been registered worldwide, and there is the probability of more trials in the near future [World Health Organization (WHO), 2022a]. Hence, there is likely to be emerging evidence that will allow informed decisions to be made on the effectiveness of treatment using herbal medicine for the treatment of COVID-19 symptoms.
In this LSR, evidence regarding the potential effects and risks of using herbal medicine for the treatment or prevention of COVID-19 was evaluated, focusing on the following questions: 1) Is herbal medicine effective in treating patients with COVID-19? 2) Is herbal medicine effective in preventing COVID-19 infections? 3) Are there any potential adverse events or risks associated with the use of herbal medicine for the treatment of COVID-19? New evidence will be kept under surveillance and summarized quarterly. A major update will be performed if the emerging evidence impacts upon the conclusion of this review. This LSR will continuously inform the best practice for clinical treatment and clinical research of this disease.
This LSR was registered in the PROSPERO international prospective register of systematic reviews database (ID: CRD42020191711) and was reported in accordance with the reporting guideline provided in the Preferred Reporting Items Systematic Reviews and Meta-Analysis (PRISMA) statement (Moher et al., 2009). Standard methods and guidance for conducting living reviews was also followed (Akl et al., 2017; Elliott et al., 2017).
All sources were searched from the date of inception through to 28 March 2022. Two review authors developed the search strategy (see Supplementary Table S1) and conducted the search monthly for eligible articles. The sources included, but was not limited to the following 11 databases: PubMed, Cochrane Register of Controlled Trials (CENTRAL), Embase, Allied and Complementary Medicine Database (AMED), Chinese National Knowledge Infrastructure Database (CNKI), Wanfang Database, and Chinese Science and Technique Journals Database (VIP), Research Information Service System (RISS), Korean Medical database (KMBase), Korean Association of Medical Journal database (KoreaMed), and OASIS database.
Additional sources such as the COVID-19 Study Registry (https://covid-19.cochrane.org/), WHO’s COVID-19 Database (https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/), and The Institute of Medical Information (IMI) and Library, Chinese Academy of Medical Sciences and Peking Union Medical College (CAMS and PUMC) (http://2019ncov.imicams.ac.cn/index.html) were also searched. To identify ongoing trials, the National Institute of Health and Clinical Trials Database (http://www.clinicaltrials.gov/), WHO’s International Clinical Trials Registry Platform (https://www.who.int/ictrp/en/), and Chinese Clinical Trial Registry (http://www.chictr.org.cn/) were searched.
There were no restrictions concerning the language or publication status. The use of indexing terms such as medical subject headings (MeSH) terms, and other equivalent terms, were applied for wider coverage. All of the searches will be performed again on a monthly basis to retrieve any further eligible studies.
All RCTs that included herbal medicine as a treatment for COVID-19 were eligible for inclusion. Other clinical and experimental studies such as case-control studies, cohort studies, cross-sectional studies, case reports, animal studies, and laboratory experiments were excluded. Preprint articles were also excluded because these studies can introduce bias and could undergo major changes before peer-review and publication.
Patients of all ages with a PCR-confirmed diagnosis of COVID-19, regardless of sex, or ethnicity were included. Patients who used herbal medicine for other comorbidities or any other purpose were excluded to ensure the accuracy of evidence. Participants who were suspected of having COVID-19 or asymptomatic PCR-confirmed COVID-19 cases were also excluded. Trials which reported separate data on symptomatic and asymptomatic COVID-19 participants were included in this review.
All types of herbal medicine intervention by oral administration, such as herbal decoctions or a patient’s medicine were considered in this review, irrespective of the composition of herbal medicine, dose, and duration of administration. Co-intervention of standard medical treatment was also eligible so long as the co-intervention was given similarly in both intervention and comparison groups. Herbal medicine interventions in the form of single/multiple herbal extractions, herbal injections, herbal fumigation, or combinations of two or more different types of herbal medicine interventions were excluded.
Comparison groups that received only standard medical treatment, placebo, or no treatment were included. Comparator groups that involved any type of herbal medicine were excluded.
The primary outcomes comprised of:
− Total effective rate (defined by the number of patients who were cured or where treatment was markedly effective)
− Symptom resolution
The secondary outcomes included:
− Chest radiological findings
− Progression to severe or critical COVID-19
− All-cause mortality
− Negative viral assay
− Duration of hospital stay
− Adverse events
Both primary and secondary outcomes were chosen by referring to the core outcome sets (COS) that have been registered on the Core Outcome Measures in Effectiveness Trials (COMET) database (Qiu R. et al., 2020; Jin et al., 2020; Marshall et al., 2020; Tong et al., 2020), with the consideration of applicability to patients with mild-to-critical COVID-19.
Two review authors (LA and ES) performed the literature searches and assessed the eligibility of the studies. To prevent discrepancies between daily database updates, all searches were conducted and results exported on the same day. The full text of the potentially eligible RCTs was then retrieved and screened according to the pre-defined criteria. Any discrepancies regarding the suitability of a study for inclusion in this review were discussed with a third review author (ML) until a consensus was reached.
Trial design, sample size, risk of bias domains (as defined above), length of trial and follow-up, the number of participants (recruited, randomized, withdraw, completed, analyzed, and lost to follow-up), age range, sex ratio, details of the interventions and comparators (regimens), all outcome measures, study results, adverse events, and funding source data were extracted by two review authors (LA and ES) independently using a standard data extraction form. Each trial was named after the first author and year of primary publication, and any relevant secondary publications were classified under that name. The authors of the included studies were contacted for unreported data or missing data.
Subsequently, two review authors (LA and ES) individually assessed the risk of bias of the included studies using the Cochrane Collaboration’s Risk of Bias Assessment tool Version 2 (ROB 2.0) (Sterne et al., 2019). The following five domains were assessed: 1) randomization process, 2) deviations from intended interventions, 3) missing outcome data, 4) measurement of outcome, and 5) selection of the reported results. The risk of bias of each item was categorized into “low risk of bias,” “some concerns,” or “high risk of bias.” The overall risk of bias of the included studies was also assessed. Additionally, the quality of evidence was summarized using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE, https://gradepro.org/). Any disagreements over the data extraction, risk of bias, and quality of evidence in a particular study were resolved through the involvement of a third party.
All data were analyzed using Review Manager (RevMan) Version 5.3 software. The risk ratios (RRs), odds ratio (OR), or risk difference (RD) with 95% confidence intervals (CIs) were calculated for dichotomous data while the mean differences (MDs) with 95% CIs were calculated for continuous data. A narrative synthesis of the key findings from the included studies was presented according to the review questions with summary tables for study characteristics, participants, and outcome details. Quantitative synthesis was also performed to demonstrate overall effect estimates. The random-effects model was used as it incorporates heterogeneity both within and between studies. The heterogeneity levels of the eligible RCTs were assessed using I2 statistics.
A monthly surveillance for new evidence related to the potential benefits and risks of the treatment was planned. The data selection, data extraction, and quality assessment methods described in this review will be performed. The relevant meta-analyses will continuously be updated, and if significant evidence is available, the results will be published quarterly.
Following screening of 14,955 titles and abstracts, and removing duplicates from the literature searches, 51 full-text records were retrieved. A total of 25 randomized clinical trials (2,288 participants) were identified as of 28 March 2022 (Figure 1) and included in this review.(Ai et al., 2020; Ding et al., 2020; Duan et al., 2020; Fu et al., 2020a; Fu et al., 2020b; Hu et al., 2020; Li and Zhang, 2020; Liao, 2020b; a; Lin et al., 2020; Liu Z. et al., 2020; Qiu M. et al., 2020; Sun H.-m. et al., 2020; Wang JB. et al., 2020; Wang Y. et al., 2020; Xiong WZ. et al., 2020; G-CHAMPS Collaborative Group, 2020; Yu et al., 2020; Zhang C. et al., 2020; Zhang Y. et al., 2020; Zhao et al., 2020; Zheng et al., 2020; Liu J. et al., 2021; Zeng et al., 2021; Zhou et al., 2021) Reasons for exclusion of 26 full-text articles are provided in Supplementary Table S2. Most trials included in this review were not registered (18/25; 72%), published in Chinese (16/25; 64%), and evaluated treatment in symptomatic COVID-19 patients (2,272/2,288; 99%). All studies included in this analysis were conducted in China.
The diagnosis of COVID-19 was confirmed by polymerase chain reaction (PCR) for all cases in all studies and was accompanied by radiological findings of pneumonia caused by COVID-19 in some studies. The majority of standard medical treatment received by participants involved the use of lopinavir-ritonavir, arbidol, alpha-interferon nebulization, or a combination of these treatments. Five different forms of herbal medicines were evaluated. The most common form of herbal medicines was herbal decoction (12/25; 48%), followed by herbal granules (9/25; 36%), herbal oral liquid (2/25; 8%), herbal capsules (1/25; 4%), and herbal powder (1/25; 4%). All trials compared herbal medicine added to standard treatment versus standard treatment alone. Due to insufficient data, meta-analysis for several trials and outcomes could not be conducted. We wrote to the authors of those studies requesting the missing data and have yet to receive responses. The detailed study characteristics of the included trials can be found in Tables 1, 2.
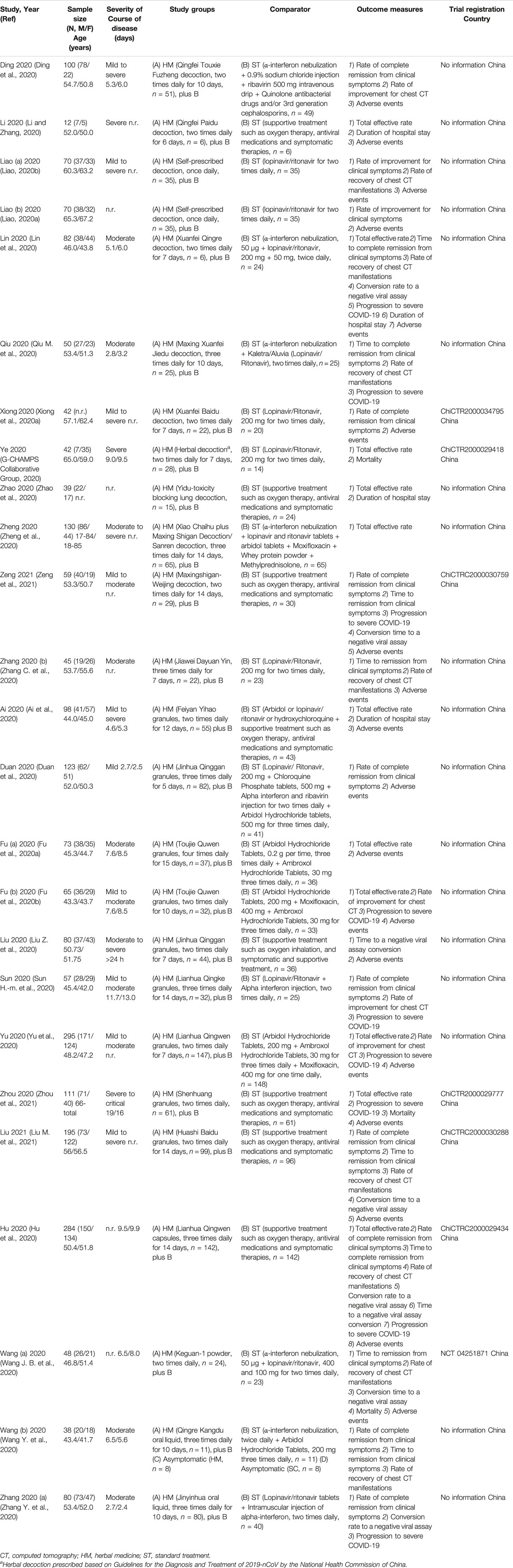
TABLE 1. Study characteristics of trials conducted with herbal medicine for the treatment of COVID-19.
Most of the studies assessed had a concerning level of risk of bias. Of the RCTs included in this review, 16 trials performed adequate randomization using a simple randomization method (Ai et al., 2020; Qiu M. et al., 2020; Wang JB. et al., 2020; Xiong WZ. et al., 2020; Fu et al., 2020b; Sun H.-m. et al., 2020; Wang Y. et al., 2020; Ding et al., 2020; Duan et al., 2020; Hu et al., 2020; Lin et al., 2020; G-CHAMPS Collaborative Group, 2020; Yu et al., 2020; Liu J. et al., 2021; Zeng et al., 2021; Zhou et al., 2021). The specific information on the generation of randomization was absent in nine trials. Most of the trials did not report on allocation sequence concealment. Six trials were open-labeled (Sun H.-m. et al., 2020; Wang Y. et al., 2020; Hu et al., 2020; G-CHAMPS Collaborative Group, 2020; Liu J. et al., 2021; Zeng et al., 2021), one was double-blinded (Wang JB. et al., 2020), and the remaining trials did not provide information on masking. Of the seven trials with publicly accessible protocols or registrations, two trials did not report results for one or more outcomes that were pre-specified in their protocols (Hu et al., 2020; Zhou et al., 2021). The reporting bias in two trials was assessed as concerning due to selective reporting and missing outcome data (Zhao et al., 2020; Zeng et al., 2021). One trial was initially posted as a preprint and subsequently peer-reviewed and published, and no substantial discrepancies in reporting were determined between the preprint and journal publication (G-CHAMPS Collaborative Group, 2020). The full risk of bias assessments for each domain for each study and the summary of findings are available in Supplementary Tables S3, S4, and Figure 1.
Ten RCTs including 1,192 participants, reported total effective rates. Meta-analysis showed that herbal medicine added to standard treatment was more favorable compared with standard treatment alone for total effective rate (RR 1.21, 95% CI 1.10 to1.33, p = 0.0001, I2 = 63; low certainty, Figure 2).
Six RCTs including 365 participants, reported the rate of remission from fever. Meta-analysis showed no evidence of a difference between herbal medicine added to standard treatment versus standard treatment for the reduction of fever (RR 1.21, 95% CI 0.94 to 1.55, p = 0.15, I2 = 91%; low certainty, Figure 3A). In two RCTs including 72 participants, time to remission from fever, using meta-analysis, showed that herbal medicine added to standard treatment reduced time of remission from fever faster compared with standard treatment alone (MD −1.72, 95% CI −2.39 to −1.04, p < 0.00001, I2 = 0%; low certainty, Figure 3B).
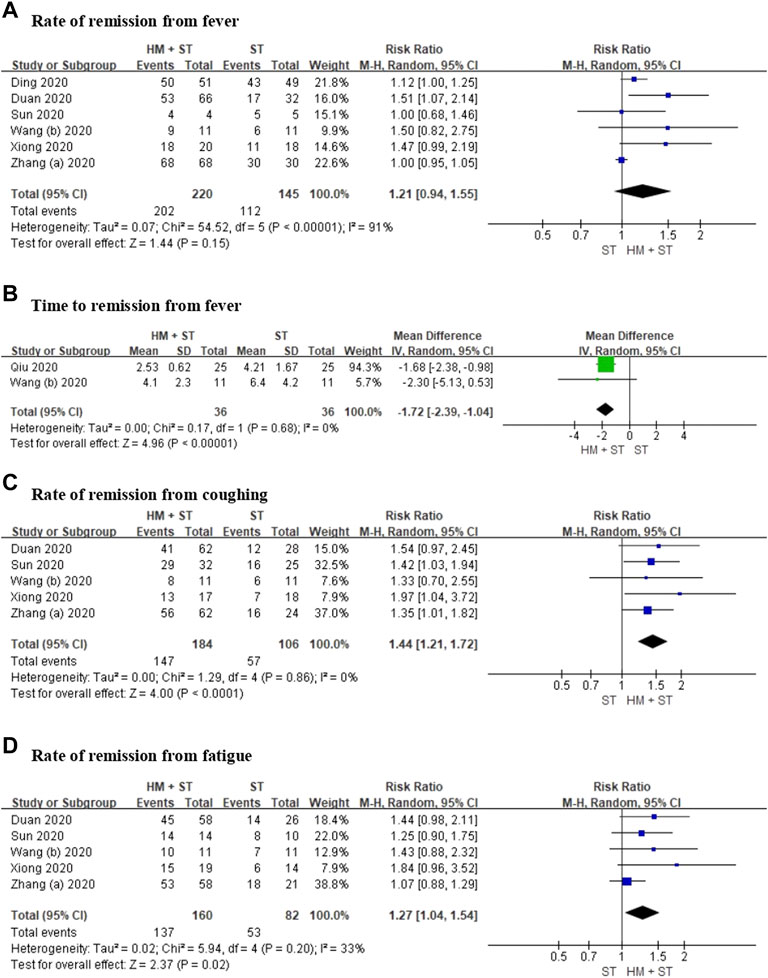
FIGURE 3. Forest plot for (A) rate of remission from fever, (B) time to remission from fever, (C) rate of remission from coughing, (D) rate of remission from fatigue.
Five RCTs including 290 participants, reported the rate of remission from coughing. Meta-analysis showed that herbal medicine added to standard treatment has a significantly greater effect in alleviating coughing compared with standard treatment (RR 1.44, 95% CI 1.21 to 1.72, p < 0.0001, I2 = 0%; low certainty, Figure 3C).
Five RCTs including 242 participants, reported rates of remission from fatigue. Meta-analysis showed that herbal medicine added to standard treatment has a significantly greater effect in reducing fatigue compared with standard treatment (RR 1.27, 95% CI 1.04 to 1.54, p = 0.02, I2 = 33%; low certainty, Figure 3D).
Meta-analysis of two RCTs including 80 participants, also showed that herbal medicine added to standard treatment has a positive effect in reducing sputum production compared with standard treatment (RR 1.73, 95% CI 1.19 to 2.50, p = 0.004, I2 = 0%; low certainty). In contrast, meta-analyses showed no evidence of a significant difference between herbal medicine added to standard treatment versus standard treatment at reducing symptoms such as a sore throat (three trials, n = 31, RR 1.06, 95% CI 0.73 to 1.54, p = 0.76, I2 = 0%; low certainty), nasal congestion and discharge (two trials, n = 14, 95% CI 0.62 to 2.18, p = 0.67, I2 = 0%; low certainty), diarrhea (two trials, n = 28, RR 0.32, 95% CI 0.01 to 8.68, p = 0.0.50, I2 = 82%; very low certainty), a dry throat (two trials, n = 25, RR 0.98, 95% CI 0.65 to 1.48, p = 0.93, I2 = 0%; very low certainty), and chills (two trials, n = 60, RR 0.99, 95% CI 0.75 to 1.32, p = 0.97, I2 = 0%; low certainty). Forest plots figures are provided in Supplementary Figures 2A–F.
Eleven RCTs including 1,117 participants, reported the rate of improvement of chest computed tomography (CT) scans. Meta-analysis showed that herbal medicine added to standard treatment was more favorable compared with standard treatment on improving pulmonary lesions (RR 1.23, 95% CI 1.14 to 1.32, p < 0.00001, I2 = 0%; low certainty, Supplementary Figure S3).
Seven RCTs including 953 participants, reported the incidence of progression to severe COVID-19 following treatment. Meta-analysis showed that herbal medicine added to standard treatment probably reduced deterioration compared with standard treatment alone (RR 0.52, 95% CI 0.34 to 0.80, p = 0.003, I2 = 0%; low certainty, Supplementary Figure S4).
Four RCTs including 495 participants, reported mortality. Meta-analysis showed that herbal medicine added to standard treatment probably reduced deaths compared with standard treatment (OR 0.26, 95% CI 0.12 to 0.54, p = 0.003, I2 = 0%; very low certainty, Supplementary Figure S5).
Two RCTs including 162 participants, reported conversion to a negative COVID-19 test, and meta-analyses showed that herbal medicine added to standard treatment probably reduced the conversion time to a negative COVID-19 test compared with standard treatment (MD −3.14, 95% CI −4.68 to −1.60, p < 0.0001, I2 = 0%; moderate certainty, Supplementary Figure S6A). Meta-analysis of two RCTs including 404 participants, did not show a significant difference for the conversion rate to a negative COVID-19 test between herbal medicine added to standard treatment, versus standard treatment (RR 1.04, 95% CI 0.95 to 1.13, p = 0.39, I2 = 0%; moderate certainty, Supplementary Figure S6B).
Three RCTs including 161 participants, reported the duration of hospital stay. Meta-analysis showed that herbal medicine added to standard treatment reduced the length of stay compared with standard treatment (RR −3.78, 95% CI −5.85 to −1.71, p = 0.0003, I2 = 0%; low certainty, Supplementary Figure S7).
Seventeen RCTs including 1,716 participants, reported adverse effects relating to herbal medicine and standard treatment intervention. Meta-analysis showed no evidence of a significant difference between herbal medicine added to standard treatment versus standard treatment in the adverse events recorded (RD 0.00, 95% CI -0.04 to 0.03, p = 0.88, I2 = 75%; low certainty, Supplementary Figure S8). Of the 12 RCTs assessed for adverse events, no severe adverse events were determined in either group. The extracted data for all studies evaluating adverse events are available in Supplementary Table S5.
There were no studies that directly addressed the prophylactic potential of herbal medicine intervention in COVID-19.
The ongoing RCTs evaluating herbal medicine for the treatment and prevention of COVID-19 are provided in Supplementary Table S6. As of 28 March 2022, 48 RCTs for the treatment of COVID-19 were identified and one RCT investigating prophylaxis. These RCTs are being conducted or scheduled to start in several countries including China, United States, Singapore, and Pakistan, with expected trial completion dates by December 2022.
This LSR and meta-analyses presents a comprehensive overview of herbal medicine trials relating to the treatment of COVID-19 up to 28 March 2022. This is the first version of this systematic review where there were 25 published RCTs, and 50 ongoing trials using many different herbal medicine interventions for the treatment and prevention of COVID-19. No studies investigating the effects of herbal medicine in preventing COVID-19 were identified. Of the 25 randomized trials included in this analysis, from a total of 2,288 participants, 1,185 participants were randomized to receive herbal medicine in addition to their standard treatment. The risk of bias of the included RCTs was overall unclear.
Eighteen meta-analyses were performed in this study. Random effects meta-analyses showed evidence of favorable effects of herbal medicine added to standard treatment, versus standard treatment alone based on the total effective rate (p = 0.0001), time to remission from fever (p < 0.00001), rate of remission from coughing (p < 0.0001), fatigue (p = 0.02), sputum production (p = 0.004), improvement in chest CT scans (p < 0.00001), incidence of progression to severe COVID-19 (p = 0.003), all-cause mortality (p = 0.003), time to a negative COVID-19 test (p < 0.0001), and duration of hospital stay (p = 0.0003). It was also determined that there was no evidence of a difference between herbal medicine added to standard treatment versus standard treatment alone on the rate of remission from symptoms such as fever, sore throat, nasal congestion and discharge, diarrhea, dry throat, and chills, as well as rate of conversion to a negative COVID-19 test. No severe adverse events were determined relating to herbal medicine interventions, and meta-analysis showed no evidence of a significant difference in the risk of adverse events between the two groups. The findings of this review showed that herbal medicine added to standard treatment may be beneficial for COVID-19 patients, but the certainty of evidence was mostly low to very low.
Most RCTs included in this study involved participants who were mildly to severely ill with COVID-19. However, different scales of disease severity and progression were used across the studies despite all included studies having originated from China. The severity of COVID-19 of the study participants varied between studies, and the available data was insufficient to allow an evaluation of whether the benefits of herbal medicine would differ with the severity of disease. The outcome measures of the included trials were also complex and varied widely. We selected and analyzed eight outcomes of interest based on “patient-important outcomes” and the general applicability of these outcomes to participants with mild-to-moderate or severe illness. Due to the lack of relevant data in the majority of studies, we could only include limited data for the analysis of safety outcomes. Several RCTs reporting on the outcomes selected for this current review were also excluded in the meta-analyses due to improper data presentation. Additionally, the comparison of differential treatment effects could not be conducted for the types and forms of herbal medicine due to the small number of included studies for each type of herbal medicine.
The evidence for outcome measures was of moderate to very low certainty, with a vast majority being low to very low certainty. We downgraded the certainty of evidence for most outcomes to low or very low certainty due to the serious risk of bias for inadequate blinding, due to wide CI, or serious inconsistency in statistical heterogeneity (Supplementary Table S4). In addition, the sample size of several studies was small which may have led to higher variability and skewing of findings. Hence, the overall robustness as determined by these review findings was considered to be low.
The primary limitation of this review was the unclear risk of bias across the included RCTs, indicating that most studies had methodological limitations. Only one study was judged to have a low risk of bias. All trials were described as “randomized controlled trials” but the reporting of most trials did not comply with the Consolidated Standards of Reporting Trials (CONSORT). Most trials did not provide the relevant information regarding allocation concealment and blinding and, several trials were open-labeled which may introduce bias to the trial results. The risk of bias across the included studies was concerning and resulted in the downgrading of the certainty of evidence. Another limitation was the reliability of the included studies which was possibly reduced due to the lack of trial registration and publicly available study protocol. Where data were missing, we tried to contact all study authors for the detailed reporting of incomplete data but we have yet to receive responses. In this review a comprehensive search was conducted to identify all completed, and ongoing trials, therefore we are confident that most of the relevant studies have been identified in this review, and further monitoring of the list of identified ongoing studies will be performed. We are also aware that excluding preprints from this review may somehow affect the outcome effect size. The evidence in preprints, however, is unstable and often undergoes major changes during peer-reviewed publication. Nevertheless, we will continue to monitor the updates in the evidence reported in preprints and their peer-reviewed publication in order to update the review accordingly.
To date, 21 systematic reviews have been identified that have similar findings to this current review (Liu M. et al., 2020; Ang et al., 2020; Sun CY. et al., 2020; Xiong X. et al., 2020; Fan et al., 2020; Pang et al., 2020; Zeng et al., 2020; Zhou et al., 2020; Wang H. et al., 2021; Liu M. et al., 2021; Wang Q. et al., 2021; Du et al., 2021; Fan et al., 2021; Jiang et al., 2021; Li et al., 2021; Liang et al., 2021; Luo et al., 2021; Wu et al., 2021; Yan et al., 2021; Yin et al., 2021; Zhuang et al., 2021). The treatment of COVID-19 benefits from the rapid dissemination of evidence, providing data for the synthesizing of evidence disseminated was constantly complied by systematic reviewers, resulting in a large number of systematic reviews with overlapping evidence, each article updated with the inclusion of only one or two additional studies at the time of publication. The multiplicity of systematic reviews of similar topics can confuse and mislead research end-users. Therefore, the intention in performing a LSR is to provide guidance in the form of a systematic up-to-date review. A comprehensive search (e.g., extensive database search) was performed in this review with analytical methods best suited for the evidence compiled (e.g., random-effects model instead of fixed effect model). This LSR and meta-analyses will be periodically updated with changes from each version highlighted for readers.
Evidence that herbal medicine added to standard treatment of patients with COVID-19 results in important benefits, but the harms to relevant patient-important outcomes are highly uncertain, and not definitive. The evidence regarding the adverse effects of using herbal medicine interventions is also very uncertain due to inconsistent reporting of safety outcomes. Herbal medicine interventions could be recommended after their efficacy and safety concerns are adequately addressed. Amongst the included studies, there was also a lack of consistency in the outcome measurements, where the main outcomes reported varied across the studies and included many insubstantial outcomes. Despite the various challenges faced by COVID-19 clinical research, optimal trial designs of substantial sample size, randomization or blinding methods, and outcome measurements with proper reporting is necessary to increase the quality of evidence. Hence, future well-designed RCTs relating to herbal medicine for the treatment of COVID-19 remains highly anticipated.
Current evidence suggests that herbal medicine added to standard treatment has potential benefits for treating COVID-19 regardless of the uncertainties. Many research gaps in the clinical studies of herbal medicine still exist and trials with more rigorous study designs are highly warranted.
A pre-registered protocol of this LSR is available at the International prospective register of systematic reviews database (PROSPERO CRD42020191711). Considering the inconsistency and complexity of outcome measurements reported amongst the included studies, the planned outcomes list was reviewed and the main outcomes were restricted to patient-important outcomes only. We selected the patient-important outcomes based on the COS that have been registered on the Core Outcome Measures in Effectiveness Trials (COMET) database (Qiu R. et al., 2020; Jin et al., 2020; Marshall et al., 2020; Tong et al., 2020). Subsequent to the originally planned risk of bias assessment, we performed an additional assessment on the quality of evidence using the GRADEpro guideline development tool. These changes were implemented before the data analysis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
This work is conceptualized by YC and ML. The study methodology was designed by LA and ES, and validated by HL, ML, and X-YH. Formal analysis and data curation were performed by LA, ES, and ML. The original draft was written by LA, reviewed and edited by X-YH, YC, and ML. This project was supervised and administered by ML. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
This work was supported by Korea Institute of Oriental Medicine (KSN2022210). This funding source did not participate in the design of this study or play any role during its execution, analyses, interpretation of the data, manuscript drafting, or decision to submit results.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.906764/full#supplementary-material
Ai, X., Luo, C., Lin, L., Xie, M., Fan, H., and Tan, X. (2020). Therapeutic Effect of Integrated Traditional Chinese and Western Medicine on COVID-19 in Guangzhou. Chin. Trop. Med. 20 (8), 746–750. doi:10.13604/j.cnki.46-1064/r.2020.08.12
Akl, E. A., Meerpohl, J. J., Elliott, J., Kahale, L. A., and Schünemann, H. J. (2017). Living Systematic Reviews: 4. Living Guideline Recommendations. J. Clin. Epidemiol. 91, 47–53. doi:10.1016/j.jclinepi.2017.08.009
Ang, L., Song, E., Lee, H. W., and Lee, M. S. (2020). Herbal Medicine for the Treatment of Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 9 (5), 1583. doi:10.3390/jcm9051583
Cao, B., Wang, Y., Wen, D., Liu, W., Wang, J., Fan, G., et al. (2020). A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 382 (19), 1787–1799. doi:10.1056/NEJMoa2001282
Chan, K. W., Wong, V. T., and Tang, S. C. W. (2020). COVID-19: An Update on the Epidemiological, Clinical, Preventive and Therapeutic Evidence and Guidelines of Integrative Chinese-Western Medicine for the Management of 2019 Novel Coronavirus Disease. Am. J. Chin. Med. 48 (03), 737–762. doi:10.1142/s0192415x20500378
Ding, X., Zhang, Y., He, D., Zhang, M., Tan, Y., Yu, A., et al. (2020). Clinical Effect and Mechanism of Qingfei Touxie Fuzheng Recipe in the Treatment of COVID-19. Her. Med. 35 (5), 640–644. doi:10.3870/j.issn.1004-0781.2020.05.012
Du, X., Shi, L., Cao, W., Zuo, B., and Zhou, A. (2021). Add-on Effect of Chinese Herbal Medicine in the Treatment of Mild to Moderate COVID-19: A Systematic Review and Meta-Analysis. PLOS ONE 16 (8), e0256429. doi:10.1371/journal.pone.0256429
Duan, C., Xia, W. G., Zheng, C. J., Sun, G. B., Li, Z. L., Li, Q. L., et al. (2020). Clinical Observation of Jinhua Qinggan Granule Combined with Conventional Western Medicine Therapy in Treating Mild Cases of Coronavirus Disease 2019. J. Tradit. Chin. Med. 61 (17), 1473–1477. doi:10.13288/j.11-2166/r.2020.17.001
Elliott, J. H., Synnot, A., Turner, T., Simmonds, M., Akl, E. A., McDonald, S., et al. (2017). Living Systematic Review: 1. Introduction-The Why, what, when, and How. J. Clin. Epidemiol. 91, 23–30. doi:10.1016/j.jclinepi.2017.08.010
Fan, A. Y., Gu, S., and Alemi, S. F. (2020). Chinese Herbal Medicine for COVID-19: Current Evidence with Systematic Review and Meta-Analysis. J. Integr. Med. 18 (5), 385–394. doi:10.1016/j.joim.2020.07.008
Fan, Z., Guo, G., Che, X., Yang, Y., Liu, Y., Li, L., et al. (2021). Efficacy and Safety of Lianhuaqingwen for Mild or Moderate Coronavirus Disease 2019: A Meta-Analysis of Randomized Controlled Trials. Medicine 100 (21), e26059. doi:10.1097/md.0000000000026059
Fu, X., Lin, L., and Tan, X. (2020a). Clinical Study on 37 Case of COVID-19 Treated with Integrated Traditional Chinese and Western Medicine. Tradit. Chin. Drug Res. Clin. Pharmacol. 31 (05), 600–604. doi:10.19378/j.issn.1003-9783.2020.05.016
Fu, X., Lin, L., and Tan, X. (2020b). Clinical Observation on Effect of Toujie Quwen Granules in Treatment of COVID-19. Zhongguo Shi Yan Fang. Ji Xue Za Zhi 26 (12), 44-48. doi:10.13422/j.cnki.syfjx.20201314
Hu, K., Guan, W. J., Bi, Y., Zhang, W., Li, L., Zhang, B., et al. (2020). Efficacy and Safety of Lianhuaqingwen Capsules, a Repurposed Chinese Herb, in Patients with Coronavirus Disease 2019: A Multicenter, Prospective, Randomized Controlled Trial. Phytomedicine 85, 153242. doi:10.1016/j.phymed.2020.153242
Jiang, F., Xu, N., Zhou, Y., Song, J., Liu, J., Zhu, H., et al. (2021). Contribution of Traditional Chinese Medicine Combined with Conventional Western Medicine Treatment for the Novel Coronavirus Disease ( COVID ‐19), Current Evidence with Systematic Review and Meta‐analysis. Phytotherapy Res. 35, 5992–6009. doi:10.1002/ptr.7209
Jin, X., Pang, B., Zhang, J., Liu, Q., Yang, Z., Feng, J., et al. (2020). Core Outcome Set for Clinical Trials on Coronavirus Disease 2019 (COS-COVID). Engineering 6, 1147–1152. doi:10.1016/j.eng.2020.03.002
Li, F., Jiang, Y., Yue, B., and Luan, L. (2021). Use of Traditional Chinese Medicine as an Adjunctive Treatment for COVID-19: A Systematic Review and Meta-Analysis. Medicine 100 (30), e26641. doi:10.1097/md.0000000000026641
Li, Y., and Zhang, W. (2020). Evaluation of the Clinical Effect of Traditional Chinese Medicine and Western Medicine Regimens on COVID-19. Guangming J. Chin. Med. 35 (9), 1273–1275. doi:10.3969/j.issn.1003-8914.2020.09.001
Liang, S. B., Fang, M., Liang, C. H., Lan, H. D., Shen, C., Yan, L. J., et al. (2021). Therapeutic Effects and Safety of Oral Chinese Patent Medicine for COVID-19: A Rapid Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 60, 102744. doi:10.1016/j.ctim.2021.102744
Liao, G. (2020a). Study on the Effect and Safety of Self-Prescribe Traditional Chinese Medicine Decoction in Patients with New Coronavirus Pneumonia. Int. Infect. Dis. 9 (2), 353.
Liao, G. (2020b). Study on the Prevention Effect and Mortality of Self-Prescribe Traditional Chinese Medicine Decoction in Patients with New Coronavirus Pneumonia. Int. Infect. Dis. 9 (2), 349.
Lin, F., Huang, J., Yang, J., Zhang, S., Xie, S., and Zhou, F. (2020). Clinical Study on the Adjuvant Treatment of Common COVID-19 with Xuanfeiqingre (Ventilating Lung and Clearing Heat) Decoction. Zhejiang J Integr Trad Chin West Med 30 (12), 977–981. doi:10.3969/j.issn.1005-4561.2020.12.007
Liu, J., Yang, W., Liu, Y., Lu, C., Ruan, L., Zhao, C., et al. (2021). Combination of Hua Shi Bai Du Granule (Q-14) and Standard Care in the Treatment of Patients with Coronavirus Disease 2019 (COVID-19): A Single-Center, Open-Label, Randomized Controlled Trial. Phytomedicine 91, 153671. doi:10.1016/j.phymed.2021.153671
Liu, M., Gao, Y., Yuan, Y., Yang, K., Shi, S., Tian, J., et al. (2021b). Efficacy and Safety of Herbal Medicine (Lianhuaqingwen) for Treating COVID-19: A Systematic Review and Meta-Analysis. Integr. Med. Res. 10 (1), 100644. doi:10.1016/j.imr.2020.100644
Liu, M., Gao, Y., Yuan, Y., Yang, K., Shi, S., Zhang, J., et al. (2020). Efficacy and Safety of Integrated Traditional Chinese and Western Medicine for Corona Virus Disease 2019 (COVID-19): a Systematic Review and Meta-Analysis. Pharmacol. Res. 158, 104896. doi:10.1016/j.phrs.2020.104896
Liu, Z., Li, X., Gou, C., Li, L., Luo, X., Zhang, C., et al. (2020). Effect of Jinhua Qinggan Granules on Novel Coronavirus Pneumonia in Patients. J. Tradit. Chin. Med. 40 (3), 467–472. doi:10.19852/j.cnki.jtcm.2020.03.016
Luo, X., Ni, X., Lin, J., Zhang, Y., Wu, L., Huang, D., et al. (2021). The Add-On Effect of Chinese Herbal Medicine on COVID-19: A Systematic Review and Meta-Analysis. Phytomedicine 85, 153282. doi:10.1016/j.phymed.2020.153282
Marshall, J. C., Murthy, S., Diaz, J., Adhikari, N. K., Angus, D. C., Arabi, Y. M., et al. (2020). A Minimal Common Outcome Measure Set for COVID-19 Clinical Research. Lancet Infect. Dis. 20 (8), e192–e197. doi:10.1016/S1473-3099(20)30483-7
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. BMJ 339 (4), b2535–269. w264. doi:10.7326/0003-4819-151-4-200908180-0013510.1136/bmj.b2535
National Health Commission of the People's Republic of China (2020). Transcript of press conference, Beijing, China, February 17, 2020.
Pang, W., Liu, Z., Li, N., Li, Y., Yang, F., Pang, B., et al. (2020). Chinese Medical Drugs for Coronavirus Disease 2019: a Systematic Review and Meta-Analysis. Integr. Med. Res. 9 (3), 100477. doi:10.1016/j.imr.2020.100477
Qiu, M., Li, Q., Zhu, D., Wang, C., Sun, Q., Qian, C., et al. (2020). Efficacy Observation of Maxing Xuanfei Jiedu Decoction on Moderate COVID-19 Patients. J. Emerg. Tradit. Chin. Med. 29(7), 1129-1132. doi:10.3969/j.issn.1004-745X.2020.07.001
Qiu, R., Zhao, C., Liang, T., Hao, X., Huang, Y., Zhang, X., et al. (2020). Core Outcome Set for Clinical Trials of COVID-19 Based on Traditional Chinese and Western Medicine. Front. Pharmacol. 11 (781), 781. doi:10.3389/fphar.2020.00781
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Sun, C. Y., Sun, Y. L., and Li, X. M. (2020). The Role of Chinese Medicine in COVID-19 Pneumonia: A Systematic Review and Meta-Analysis. Am. J. Emerg. Med. 38 (10), 2153–2159. doi:10.1016/j.ajem.2020.06.069
Sun, H.-m., Xu, F., Zhang, L., Wei, C., Chen, J.-y., Wang, Q.-x., et al. (2020). Study on Clinical Efficacy of Lianhua Qingke Granule in Treatment of Mild and Ordinary COVID-19. Zhongguo Shi Yan Fang. Ji Xue Za Zhi 26 (04), 29–34. doi:10.13422/j.cnki.syfjx.20201438
Tong, A., Elliott, J. H., Azevedo, L. C., Baumgart, A., Bersten, A., Cervantes, L., et al. (2020). Core Outcomes Set for Trials in People with Coronavirus Disease 2019. Crit. Care Med. 48 (11), 1622–1635. doi:10.1097/ccm.0000000000004585
Wang, H., Xu, B., Zhang, Y., Duan, Y., Gao, R., He, H., et al. (2021). Efficacy and Safety of Traditional Chinese Medicine in Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis. Front. Pharmacol. 12. doi:10.3389/fphar.2021.609213
Wang, J. B., Wang, Z. X., Jing, J., Zhao, P., Dong, J. H., Zhou, Y. F., et al. (2020). Exploring an Integrative Therapy for Treating COVID-19: A Randomized Controlled Trial. Chin. J. Integr. Med. 26 (9), 648–655. doi:10.1007/s11655-020-3426-7
Wang, Q., Zhu, H., Li, M., Liu, Y., Lai, H., Yang, Q., et al. (2021). Efficacy and Safety of Qingfei Paidu Decoction for Treating COVID-19: A Systematic Review and Meta-Analysis. Front. Pharmacol. 12. doi:10.3389/fphar.2021.688857
Wang, Y., Xue, J., Dai, E., Xu, Z., Feng, C., Liu, H., et al. (2020). Clinical Study on the Treatment of Patients with Novel Coronavirus Pneumonia and Asymptomatic Infection with Integrated Traditional Chinese and Western Medicine. Hebei J. Trad. Chin. Med. 42 (5), 645–649. doi:10.3969/j.issn.1002-2619.2020.05.001
Weeks, J. (2020). Call to Action: Announcing the Traditional, Complementary and Integrative Health and Medicine COVID-19 Support Registry. J. Altern. Complement. Med. 26 (4), 256–258. doi:10.1089/acm.2020.29083.jjw
World Health Organization (WHO) (2022a). International Clinical Trials Registry Platform (ICTRP). [Online]. Available: https://www.who.int/ictrp/en/ (Accessed June 12, 2020).
World Health Organization (WHO) (2022b). WHO Coronavirus Disease (COVID-19) Dashboard. [Online]. Available: https://covid19.who.int/ (Accessed February 21, 2021).
Wu, X., Li, W., Qin, Z., Xue, L., Huang, G., Luo, Z., et al. (2021). Traditional Chinese Medicine as an Adjunctive Therapy for Mild and Common COVID-19: A Systematic Review and Network Meta-Analysis. Medicine 100 (40), e27372. doi:10.1097/md.0000000000027372
Xiong, W. Z., Wang, G., Du, J., and Ai, W. (2020). Efficacy of Herbal Medicine (Xuanfei Baidu Decoction) Combined with Conventional Drug in Treating COVID-19:A Pilot Randomized Clinical Trial. Integr. Med. Res. 9 (3), 100489. doi:10.1016/j.imr.2020.100489
Xiong, X., Wang, P., Su, K., Cho, W. C., and Xing, Y. (2020). Chinese Herbal Medicine for Coronavirus Disease 2019: A Systematic Review and Meta-Analysis. Pharmacol. Res. 160, 105056. doi:10.1016/j.phrs.2020.105056
Yan, L. Z., Mao, F. W., Cao, Y. H., and Xie, M. (2021). Clinical Effects of the Combination of Traditional Chinese and Western Medicines on Coronavirus Disease 2019: a Systematic Review and Meta-Analysi. J. Tradit. Chin. Med. 41 (4), 499–506. doi:10.19852/j.cnki.jtcm.2021.03.001
The G-CHAMPS Collaborative Group (2020). Guideline-Based Chinese Herbal Medicine Treatment Plus Standard Care for Severe Coronavirus Disease 2019 (G-CHAMPS): Evidence from China. Front. Med. (Lausanne) 7, 256. doi:10.3389/fmed.2020.00256
Yin, B., Bi, Y. M., Sun, L., Huang, J. Z., Zhao, J., Yao, J., et al. (2021). Efficacy of Integrated Traditional Chinese and Western Medicine for Treating COVID-19: A Systematic Review and Meta-Analysis of RCTs. Front. Public Health 9 (892), 622707. doi:10.3389/fpubh.2021.622707
Yu, P., Li, Y., Wan, S., and Wang, Y. (2020). Effects of Lianhua Qingwen Granules Plus Arbidol on Treatment of Mild Corona Virus Disease-19. Chin. Pharm. J. 55(12):1042-1045. doi:10.11669/cpj.2020.12.014
Zeng, C., Yuan, Z., Zhu, J., Wang, Y., Xie, Y., Ye, R., et al. (2021). Therapeutic Effects of Traditional Chinese Medicine (Maxingshigan-Weijing Decoction) on COVID-19: An Open-Label Randomized Controlled Trial. Integr. Med. Res. 10 (Suppl. l), 100782. doi:10.1016/j.imr.2021.100782
Zeng, M., Li, L., and Wu, Z. (2020). Traditional Chinese Medicine Lianhua Qingwen Treating Corona Virus Disease 2019(COVID-19): Meta-Analysis of Randomized Controlled Trials. PLOS ONE 15 (9), e0238828. doi:10.1371/journal.pone.0238828
Zhang, C., Yang, Y., You, F., Huang, Q., Gao, P., Tang, J., et al. (2020a). Clinical Study on COVID-19 from the Perspective of "Yidujiashi" Theory. Pharm Clin Chin Mater Med 36 (2), 43–45. doi:10.13412/j.cnki.zyyl.20200327.002
Zhang, Y., Lei, L., Xu, Y., Wei, D., and Hu, F. (2020b). Clinical Efficacy of Jinyinhua Oral Liquid in the Treatment of 80 Patients with Coronavirus Disease 2019. China Pharm. 29 (9). doi:10.3969/j.issn.1006-4931.2020.09.006
Zhao, J., Yang, X., Wang, C., Song, S., Cao, K., Wei, T., et al. (2020). Yidu-toxicity Blocking Lung Decoction Ameliorates Inflammation in Severe Pneumonia of SARS-COV-2 Patients with Yidu-Toxicity Blocking Lung Syndrome by Eliminating IL-6 and TNF-A. Biomed. Pharmacother. 129, 110436. doi:10.1016/j.biopha.2020.110436
Zheng, Z., Bai, Z., Ji, C., Ge, S., Luo, Y., and He, G. (2020). Effect of the Treatment of COVID-19 According to Syndrome Differentiation. Med J Commun. 34 (2), 117–118. doi:10.19767/j.cnki.32-1412.2020.02.005
Zhou, L. P., Wang, J., Xie, R. H., Pakhale, S., Krewski, D., Cameron, D. W., et al. (2020). The Effects of Traditional Chinese Medicine as an Auxiliary Treatment for COVID-19: A Systematic Review and Meta-Analysis. J. Altern. Complement. Med. 27 (3), 225–237. doi:10.1089/acm.2020.0310
Zhou, S., Feng, J., Xie, Q., Huang, T., Xu, X., Zhou, D., et al. (2021). Traditional Chinese Medicine Shenhuang Granule in Patients with Severe/critical COVID-19: A Randomized Controlled Multicenter Trial. Phytomedicine 89, 153612. doi:10.1016/j.phymed.2021.153612
Keywords: COVID-19 evidence, efficacy, integrative herbal medicine, living review, safety
Citation: Ang L, Song E, Hu X-Y, Lee HW, Chen Y and Lee MS (2022) Herbal Medicine Intervention for the Treatment of COVID-19: A Living Systematic Review and Cumulative Meta-Analysis. Front. Pharmacol. 13:906764. doi: 10.3389/fphar.2022.906764
Received: 30 March 2022; Accepted: 23 May 2022;
Published: 20 June 2022.
Edited by:
Yue Liu, Xiyuan Hospital, ChinaReviewed by:
Jingbo Zhai, Tianjin University of Traditional Chinese Medicine, ChinaCopyright © 2022 Ang, Song, Hu, Lee, Chen and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Myeong Soo Lee, ZHJtc2xlZUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.