- Center of Integrative Medicine, Beijing Ditan Hospital, Capital Medical University, Beijing, China
Objectives: To assess the efficacy of LiangXue JieDu (LXJD) therapy in combination with Western medicine (WM) for acute-on-chronic liver failure (ACLF).
Methods: Articles on randomized controlled trials of LXJD therapy for ACLF were obtained from PubMed, Embase, Cochrane Library, Web of Science, Chinese National Knowledge Infrastructure, VIP, Wanfang, and China Biology Medicine databases, with the search range from database inception to March 2022. We evaluated the quality of data from these articles using the Cochrane risk-of-bias tool. Evaluation indicators were total effective rate, mortality rate, complications, liver and coagulation function, and Traditional Chinese medicine (TCM) syndrome score. We then calculated the risk ratio (RR) for dichotomous variables and mean difference (MD) for continuous variables with a 95% confidence interval (CI).
Results: The meta-analysis included 18 studies with moderate quality and totaling 1,609 patients. Compared with WM alone, LXJD therapy plus WM improved total effective rate [RR = 1.34, 95% CI: (1.24, 1.45)], while reducing mortality rate [RR = 0.54, 95% CI: (0.42, 0.70)] and complications [RR = 0.43, 95% CI: (0.26, 0.71)]. The combined treatment also improved prothrombin activity [MD = 1.30, 95% CI: (1.02, 1.59)], prothrombin time [MD = −0.90, 95% CI: (−1.40, −0.39)], international normalized ratio [MD = −0.59, 95% CI: (−0.93, −0.25)], alanine aminotransferase [MD = −0.92, 95% CI: (−1.30, −0.55)], aspartate aminotransferase [MD = −0.57, 95% CI: (−0.93, −0.21)], total bilirubin [MD = −1.07, 95% CI: (−1.38, −0.76)], and TCM syndrome score [MD = −1.70; 95% CI: (−2.03, −1.37)].
Conclusions: This study suggests that LXJD therapy plus WM can significantly improves ACLF clinical symptoms and short-term outcomes. However, more high-quality trials are required to confirm the efficacy of LXJD therapy.
1 Introduction
Acute-on-chronic liver failure (ACLF) refers to the acute decompensation of chronic liver disease, with jaundice, coagulopathy, ascites, and hepatic encephalopathy as the main clinical manifestations (Wu et al., 2018). In China, the main etiology of ACLF is associated with hepatitis B virus infection (Bernal et al., 2015). A major health problem, ACLF results in high mortality rate and severe multi-organ damage (Li et al., 2021). Currently, effective drugs for ACLF are lacking and treatment mainly involves artificial livers, liver transplantation, and other symptomatic treatments (Sarin and Choudhury., 2016). However, a shortage in donor livers limits widespread implementation of transplants, and overall clinical efficacy has been unsatisfactory (Sarin and Choudhury, 2016). Therefore, novel treatment methods for ACLF are urgently needed.
Traditional Chinese medicine (TCM) has been used for centuries to treat liver disease. TCM is clinically effective for promoting lowering jaundice, endotoxins, and inflammation, while enhancing liver regeneration (Cai et al., 2020; Xu et al., 2020). TCM categorizes ACLF as “jaundice,” and its basic pathogenesis is concentrated in “poison, heat, dampness, and stasis” (Shi et al., 2021). Syndromes associated with heat, poison, and stasis such as ACLF are commonly treated with the cool blood detoxification method (“LiangXue JieDu” in Chinese, abbreviated to LXJD) (Liu et al., 2014; Zhang, 2021). TCM theory postulates that heat, toxins, and blood stasis are pathological products and pathogenic factors. Both stasis and heat negatively affect liver function and cause complications. In principle, LXJD lowers heat, detoxifies, cools blood, and activates blood circulation, resulting in unobstructed Qi and blood flow; as a result, liver damage and disease progression are prevented (China Association of Chinese Medicine, 2019). Several clinical studies have recently evaluated the combined effects of LXJD therapy and Western medicine (WM) on ACLF. For example, a previous study found that detoxification and stasis-resolving granules are beneficial for improving jaundice and alleviating various other symptoms (Wang et al., 2014). Our previous study also reported that Jiedu Liangxue Jianpi prescriptions had a positive effect on ACLF treatment (Shi et al., 2021). However, the efficacy of LXJD therapy has not yet been systematically evaluated.
Accordingly, this study performed a meta-analysis of randomized controlled trials (RCTs) to assess the efficacy of LXJD combined with WM in treating ACLF.
2 Materials and Methods
2.1 Search Strategy
The study was performed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (Cumpston et al., 2019). Two researchers (KS and JH) separately searched eight databases (PubMed, Embase, Cochrane Library, Web of Science, Chinese National Knowledge Infrastructure, Wanfang, VIP, and China Biology Medicine) from their inception until March 2022. Search languages were Chinese and English. We conducted manual retrieval and secondary searches to ensure comprehensive literature retrieval. Table 1 shows the PubMed search strategy as an example.
2.2 Inclusion and Exclusion Criteria
Studies were included if: 1) patients were diagnosed with ACLF according to consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) (Sarin et al., 2019); 2) they were RCTs; 3) treatment involved LXJD therapy plus WM; 4) patients were classified as having heat-toxin-stasis syndrome; 5) at least one of the following indicators were reported: total effective rate, mortality rate, complication, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), albumin (ALB), prothrombin activity (PTA), prothrombin time (PT), international normalized ratio (INR), and TCM syndrome score. Primary outcomes were total effective rate and mortality rate, whereas secondary outcomes were complication rate, liver function, coagulation function, and TCM syndrome score.
Exclusion criteria were as follows: 1) duplicated or redundant study; 2) reviews, animal experiments, and non-RCTs; 3) non-LXJD therapy or LXJD combined with other TCM therapies; 4) acute, subacute, or chronic liver failure; 5) incomplete data.
2.3 Data Extraction and Quality Assessment
Two researchers (KS and JH) independently performed the literature search and extracted data based on inclusion and exclusion criteria. Data extraction included title, author name, publication date, sample size, gender, age, intervention measures, course of treatment, and observed outcome indicators. If the two researchers disagreed, a third party was consulted to reach a resolution (QZ and YFB).
Literature quality was evaluated using the Cochrane Collaboration’s tool, accounting for seven sources of bias: random-sequence generation, allocation concealment, blinding of investigators and participants, blinding of outcome evaluation, incomplete outcome data, selective reporting, and other. Reports in line with quality evaluation criteria were categorized as low risk; otherwise, they were considered high risk. Studies without sufficient information for assessment were labeled as unclear risk.
2.4 Statistical Analysis
All data analyses were performed in Stata 16.0 (Stata Corp, College Station, TX) and R (version 4.0.5, The R Foundation, Vienna, Austria). Risk ratio (RR) and 95% confidence interval (CI) were used for analyzing dichotomous variables. Continuous variables were analyzed using mean difference (MD) and 95% CI. Effect models were selected based on I2 and p-values. A random-effects model was used when I2 > 50% or p < 0.1; otherwise, a fixed-effects model was applied. Sensitivity analysis was conducted to assess the stability of results after removing individual studies. A funnel plot was generated to evaluate potential publication bias.
3 Results
3.1 Literature Search and Patients’ Characteristics
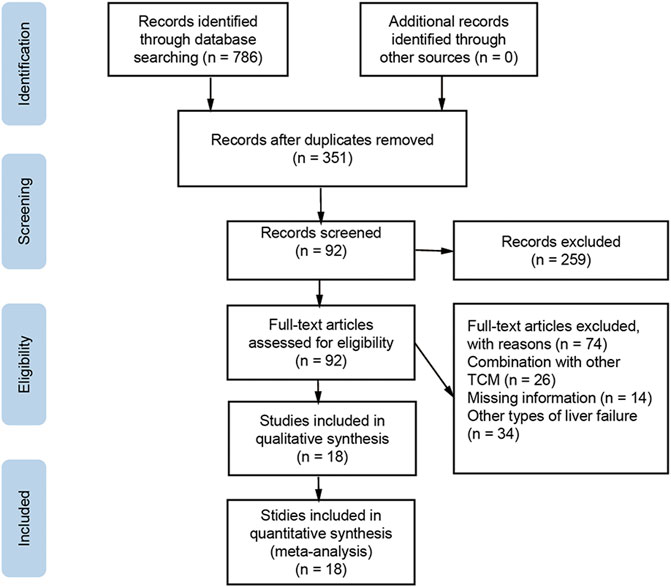
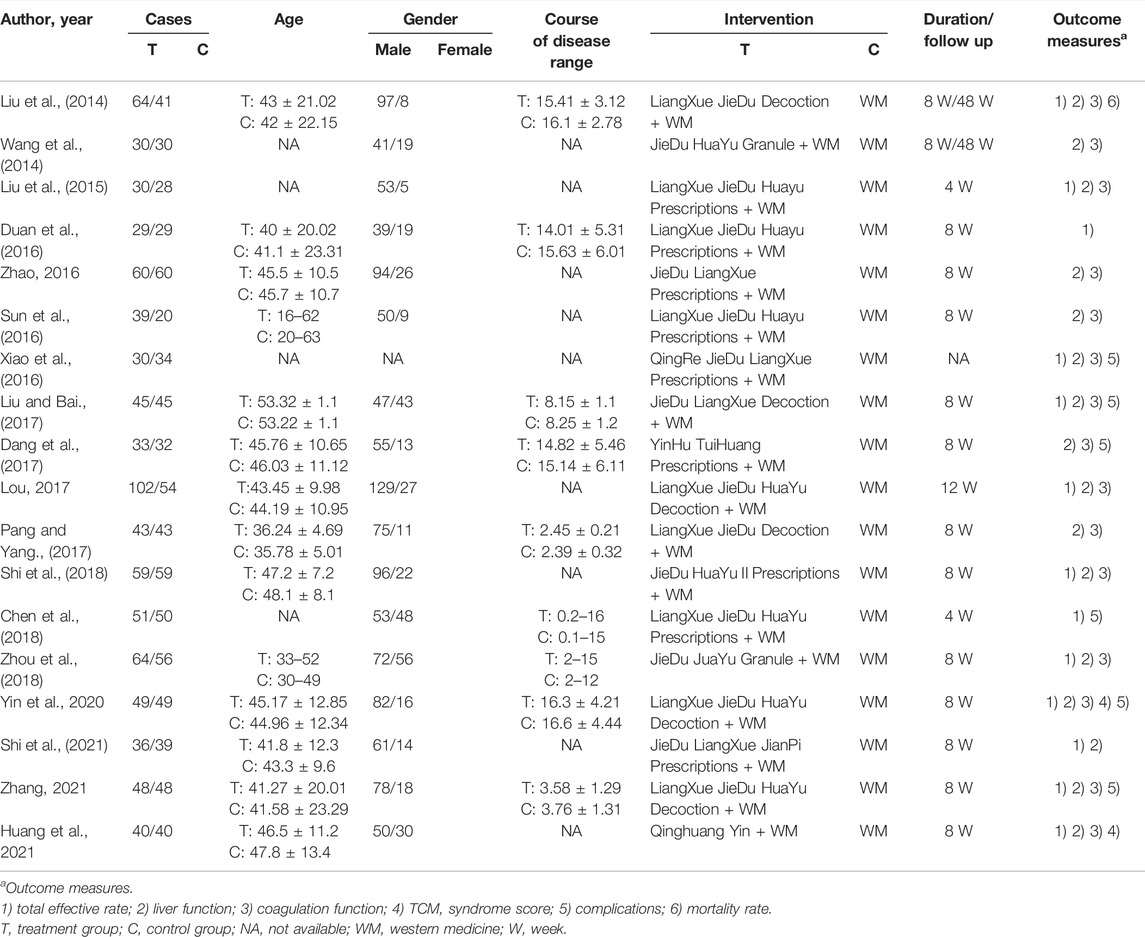
The literature search initially yielded 786 articles, and 435 duplicate studies were excluded. Another 259 articles were excluded because they were reviews, animal experiments, or non-RCTs. The remaining 92 articles were downloaded for full text review. Of these, 74 studies were further excluded because they lacked primary data, combined WM with other TCM, or did not investigate ACLF. The final meta-analysis included 18 studies and 1,609 patients (Liu et al., 2014; Wang et al., 2014; Liu et al., 2015; Duan et al., 2016; Sun et al., 2016; Xiao et al., 2016; Zhao, 2016; Dang et al., 2017; Liu and Bai, 2017; Lou, 2017; Pang and Yang, 2017; Chen et al., 2018; Shi et al., 2018; Yin and Wen, 2018; Zhou et al., 2018; Shi et al., 2021; Zhang, 2021; Huang et al., 2022). Inclusion and exclusion procedures are shown in Figure 1. Features of included studies are presented in Table 2.
3.2 Quality Assessment
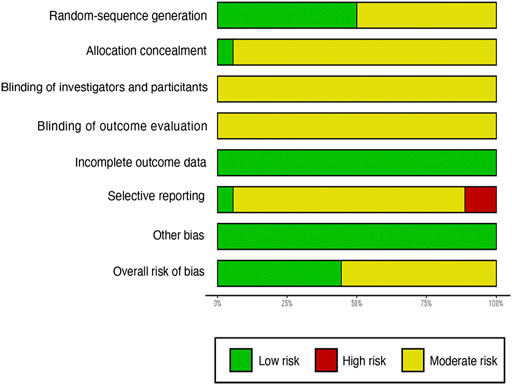
We evaluated article quality using the bias assessment tool recommended by the Cochrane Collaboration (Figures 2, 3). Nine studies used a random number table, while another nine mentioned the word “random,” but did not describe any randomization methods. With insufficient information to determine the risks of blinding investigators or participants, we classified these studies as having unclear risks. No studies were labeled as having incomplete data or other biases, so they were considered low risk. Four studies reported cases of detachment and provided a reasonable explanation. Overall, the studies included in our meta-analysis were of moderate quality.
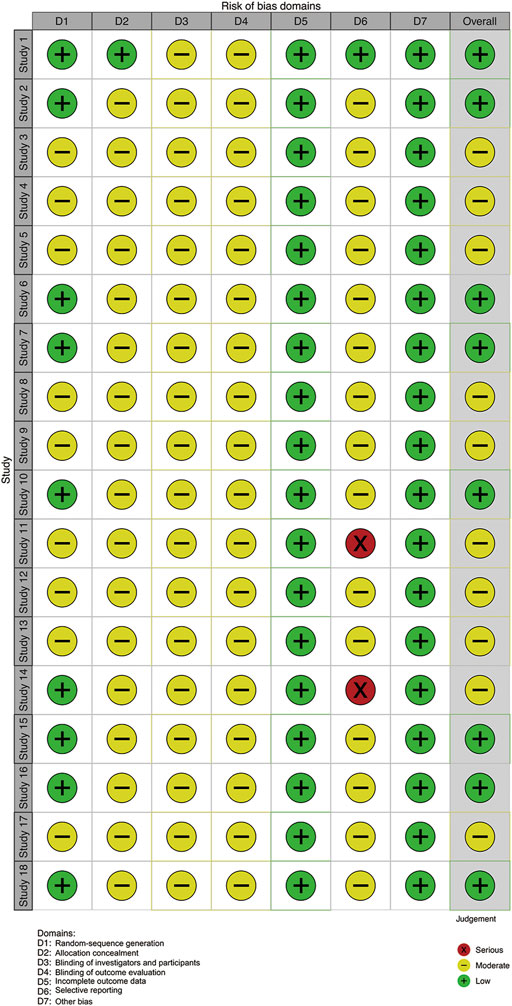
FIGURE 3. Risk of bias summary. Note: Study 1–18 (Liu et al., 2014; Wang et al., 2014; Liu et al., 2015; Duan et al., 2016; Zhao, 2016; Sun et al., 2016; Xiao et al., 2016; Liu and Bai, 2017; Dang et al., 2017; Lou, 2017; Pang and Yang, 2017; Shi et al., 2018; Chen et al., 2018; Zhou et al., 2018; Yin and Wen, 2018; Shi et al., 2021; Zhang, 2021; Huang et al., 2022).
3.3 Outcome Measures
3.3.1 Total Effective Rate and Mortality Rate
We found 14 and 5 studies that compared the effects of LXJD therapy plus WM on total effective rate and mortality rate, respectively. We adopted a fixed-effects model based on the p-value and I2 value. The RRs (95% CI) were 1.34 (1.24, 1.45) and 0.54 (0.42, 0.70). Compared with WM alone, combination therapy improved effectiveness and reduced mortality (Figures 4A,B).
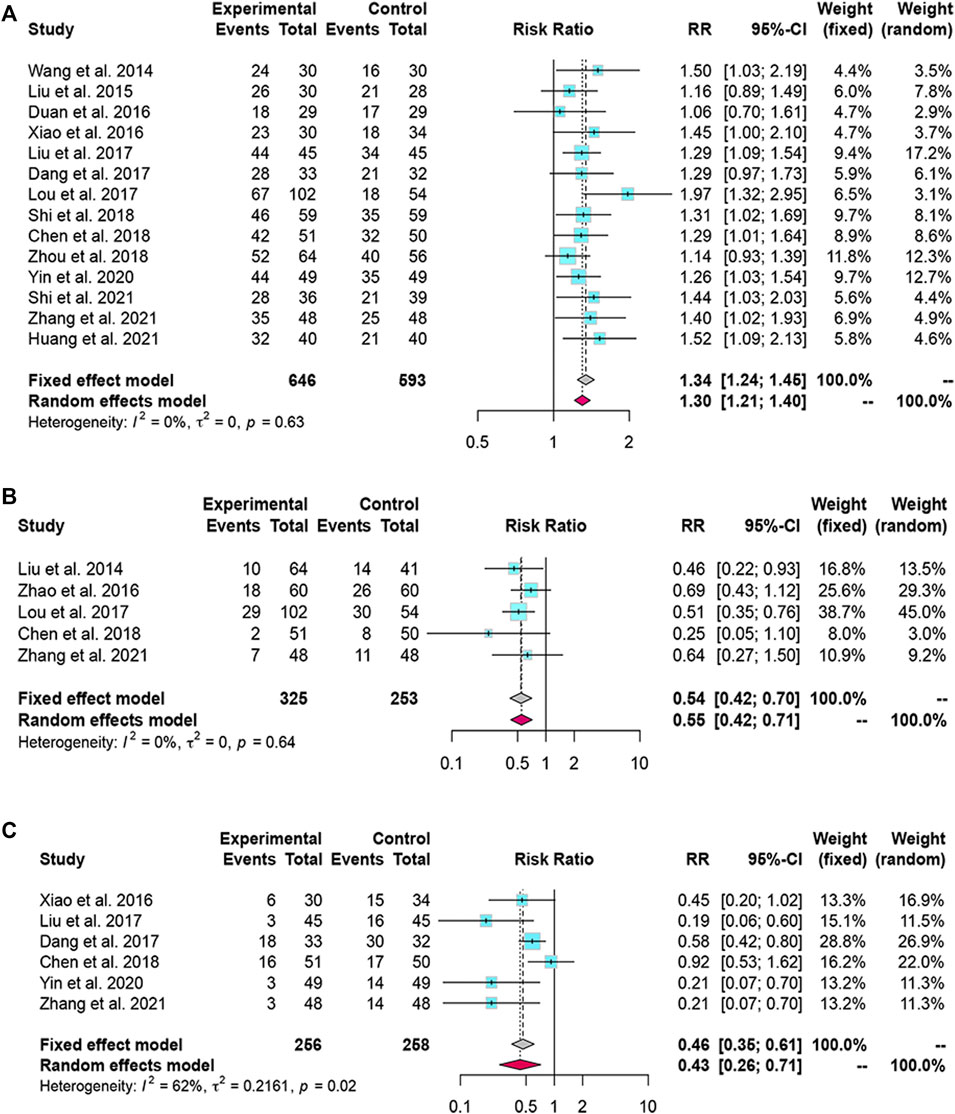
FIGURE 4. Forest plot for the meta-analysis of total effective rate, mortality rate and complications. (A) Forest plot of total effective rate; (B) Forest plot of mortality rate; (C) Forest plot of complications.
3.4 Complication Rate
Six studies reported complication rates. We selected a random-effects model because the complication rate was heterogeneous (I2 = 62%, p = 0.02). The results suggested that LXJD therapy plus WM had a significantly lower complication rate than WM alone (RR = 0.43; 95% CI: [0.26, 0.71]) (Figure 4C).
3.5 Coagulation Function
Fifteen studies reported PTA and seven studies reported PT data. Based on the p-value and I2 value, we adopted a random-effects model. The MDs (95% CI) of PTA and PT were 1.30 (1.02, 1.59) and -0.90 (−1.40, −0.39), respectively, indicating that PTA levels were significantly higher and PT significantly lower in the combined-treatment group than in the control group (Figures 5A,B).
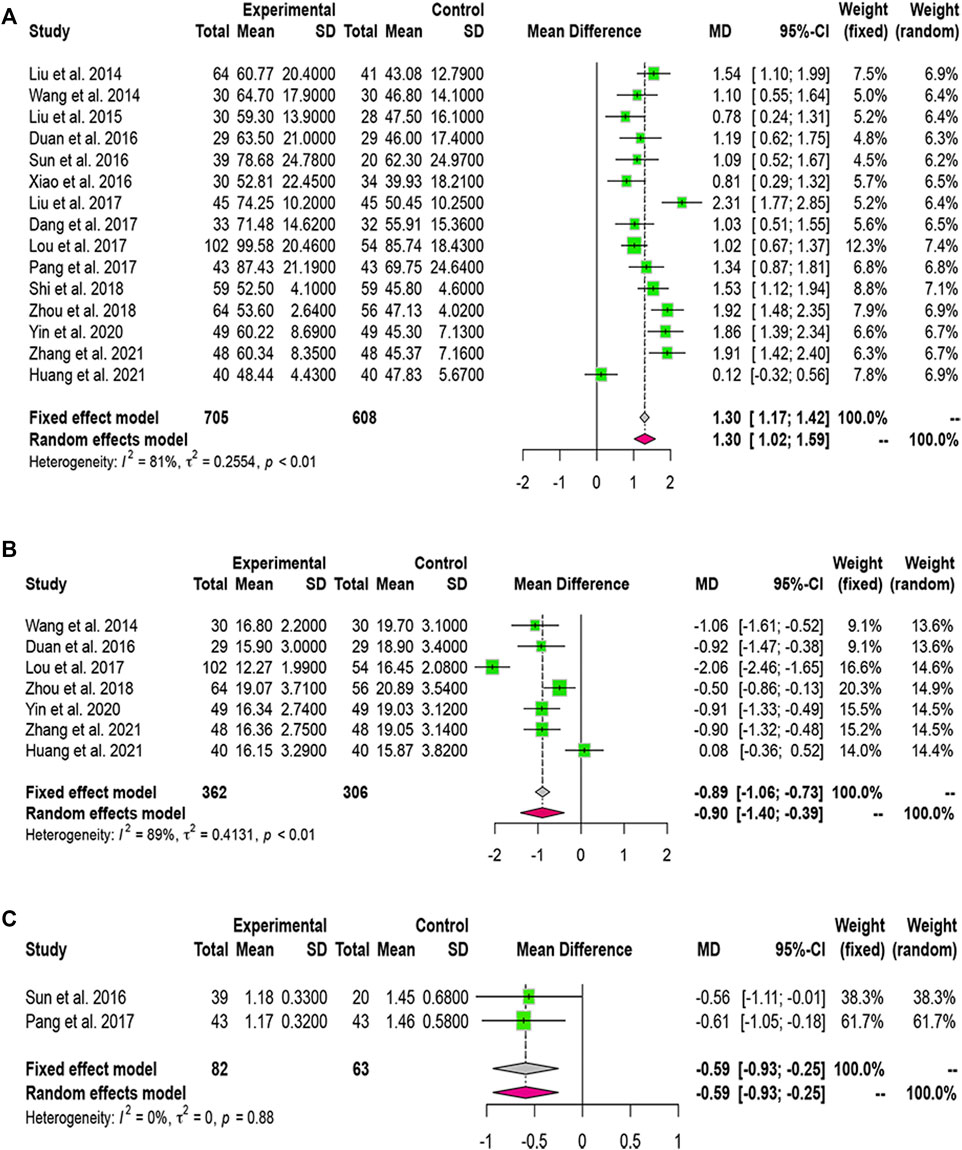
FIGURE 5. Forest plot for the meta-analysis of coagulation function. (A) Forest plot of prothrombin activity; (B) Forest plot of prothrombin time; (C) Forest plot of international normalized ratio.
Two studies included INR data. Heterogeneity analysis led us to use a fixed-effects model. We found that LXJD therapy plus WM significantly reduced the level of INR than WM alone (MD = -0.59; 95% CI: [− 0.93, −0.25]) (Figure 5C).
3.6 Liver Function
Thirteen studies reported serum ALT levels, 12 documented AST and ALB levels, while 16 reported TBIL levels. Based on the p-value and I2 value, we adopted a random-effects model. The MDs (95% CI) of ALT, AST, and TBIL were −0.92 (−1.30, −0.55), −0.57 (−0.93, −0.21), and −1.07 (−1.38, −0.76), respectively (Figures 6A–C). Compared to WM alone, LXJD therapy combined with WM was significantly more effective at decreasing ALT, AST, and TBIL levels. However, the MD (95% CI) of ALB was 0.98 (0.57, 1.39), suggesting that the combined-treatment group did not result in better ALB levels than WM alone (Figure 6D).
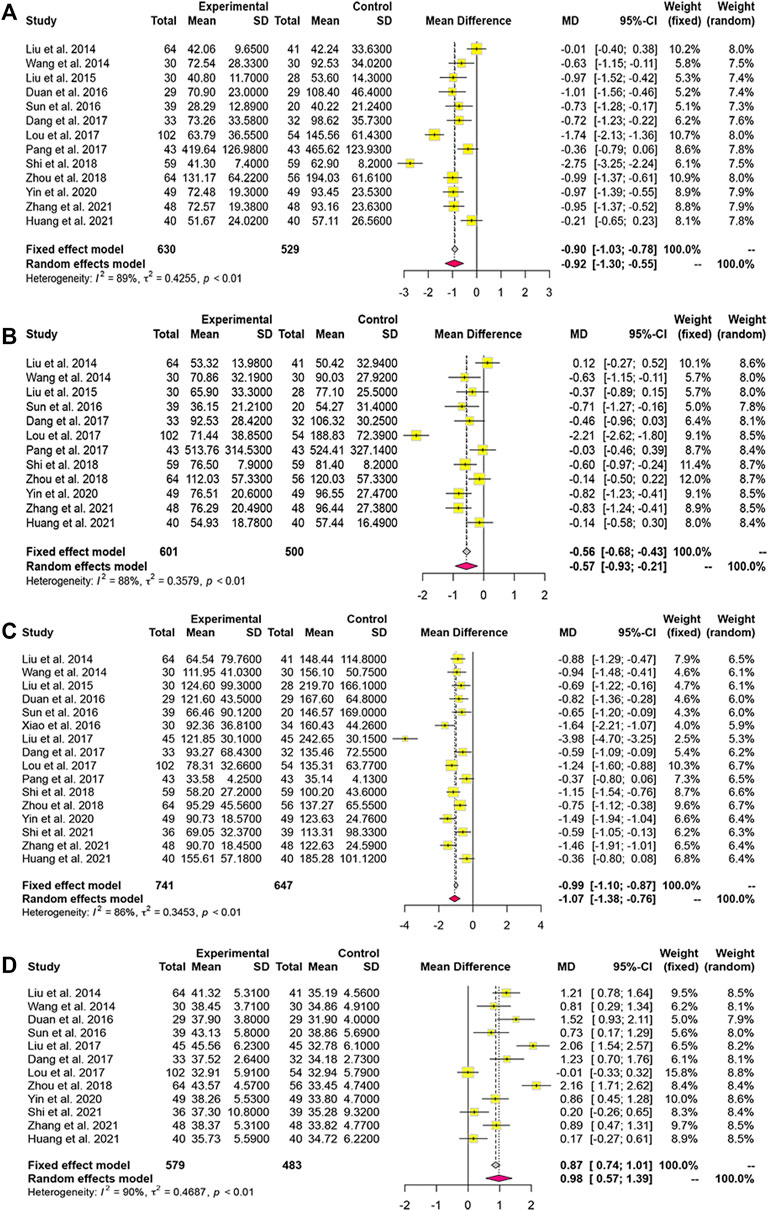
FIGURE 6. Forest plot for the meta-analysis of liver function. (A) Forest plot of alanine aminotransferase; (B) Forest plot of aspartate aminotransferase; (C) Forest plot of total bilirubin; (D) Forest plot of albumin.
3.7 Traditional Chinese Medicine Syndrome Score
Two studies reported TCM syndrome scores, and the heterogeneity analysis revealed homogeneity. The fixed-effects model indicated that TCM syndrome score improved more with combined treatment than with WM alone (MD = −1.70; 95% CI: [−2.03, −1.37]) (Figure 7).
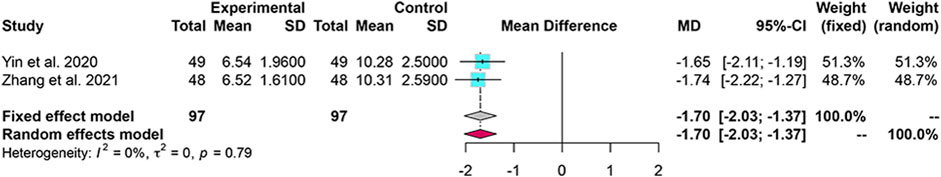
FIGURE 7. Forest plot for the meta-analysis of TCM syndrome score. TCM: Traditional Chinese medicine.
3.8 Commonly Prescribed Chinese Medicines
We analyzed prescription composition in 18 studies and listed the 10 most frequently used Chinese medicines in LXJD therapy. These were Artemisia capillaris Thunb. (Asteraceae), Paeonia lactiflora Pall. (Ranunculaceae), Hedyotis diffusa Willd. (Rubiaceae), Salvia miltiorrhiza Bunge (Lamiaceae), and Gardenia jasminoides Ellis (Rubiaceae) (Table 3).
3.9 Adverse Events
Adverse outcomes were mentioned in two studies. One (Liu et al., 2014) reported five patients developing nausea after taking TCM. The second (Dang et al., 2017) reported no adverse reactions. These conditions can be significantly relieved through symptomatic treatment. None of the included studies described severe adverse events.
3.10 Sensitivity Analysis
Removing each of the included studies did not significantly alter results, indicating that our conclusions had low sensitivity and high stability.
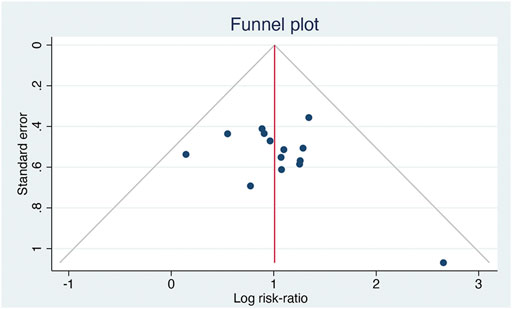
3.11 Publication Bias
Fourteen studies reported the total effective rate. Analysis using inverted funnel plots showed that the distribution was asymmetric, indicating potential publication bias in these studies (Figure 8).
4 Discussion
ACLF is a common and severe liver disease with high short-term mortality. Owing to its complex pathogenesis and lack of effective treatments, patients with ACLF tend to seek complementary and alternative therapies. As an auxiliary treatment, TCM has attracted increasing attention and research. Multiple studies have demonstrated that TCM plus WM has various advantages in improving both the prognosis and clinical symptoms of ACLF (Chen et al., 2018; Huang et al., 2022). Contemporary Chinese medicine generally agrees that the core pathogenesis of ACLF is associated with dampness, heat, and pathogens. Therefore, LXJD is an important principle in TCM-based treatment of ACLF (Zhang, 2021).
Here, our meta-analysis confirmed the advantages of LXJD in combination with WM as a treatment for ACLF. The addition of LXJD therapy to WM resulted in higher total effective rate, notably decreasing both mortality and complications, than WM alone. Coagulation and liver function are widely used as therapeutic indicators in clinical practice. Combined treatment significantly lowered PT, INR, ALT, AST, and TBIL levels while increasing PTA levels. These results indicate that LXJD therapy effectively accelerated jaundice elimination and recovery of liver synthesis in patients with ACLF, both key elements to reducing mortality rate. The TCM syndrome score is a common index for evaluating patient recovery. Two studies in our meta-analysis reported this index and demonstrated that adding LXJD therapy significantly reduced TCM syndrome scores compared with WM alone. However, because this analysis contained a small number of studies, further evaluation of TCM syndrome scores is necessary. We also did not find any beneficial effect of LXJD therapy on ALB levels.
The pathobiology of ACLF is characterized by hepatocyte damage, systemic inflammation, and death (Vanlangenakker et al., 2008). Previous clinical, in vitro, and in vivo studies have reported that TCM has underlying hepatoprotective and pharmacological effects, including anti-inflammatory, antioxidant, anti-apoptotic, and anti-cholestatic effects (Zhuang et al., 2020; Yang et al., 2015; Cai et al., 2020). Specifically, LXJD therapy has multicomponent and multitarget pharmacological effects on the complex pathogenesis of ACLF. For example, paeoniflorin is a main component isolated from Paeonia lactiflora Pall. that alleviates inflammatory response, regulates oxidative stress, and protects liver function (Zhang et al., 2015; Chen et al., 2021). Additionally, cryptotanshinone is a major active ingredient of Salvia miltiorrhiza Bunge (Lamiaceae) that downregulates inflammatory factors, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α (Liu et al., 2021). The Qingchangligan formula significantly enhanced liver failure therapy through regulating hepatitis, promoting autophagy, and limiting hepatocyte apoptosis (Zhang et al., 2017). Moreover, the Jidu Liangxue prescription exerted a protective effect against liver failure in mice, via a mechanism potentially related to inhibiting the mitochondrial apoptosis signaling pathway (Liu et al., 2022). Network pharmacology and basic research have demonstrated that the Jieduan-Niwan formula downregulates the expression of inflammatory factors, protects against oxidative stress, and inhibits the E2F1-mediated apoptosis signaling pathway to treat ACLF (Liang et al., 2020; Hou et al., 2021).
Our meta-analysis suggested that LXJD therapy causes relatively few adverse events. However, because most included trials did not mention adverse events, we should be cautious in our conclusions regarding the safety of LXJD therapy. None of the studies statistically analyzed differences in adverse events between combined-treatment and control groups. Notably, two studies described adverse side-effects, but they were mild and could be alleviated through symptomatic treatment. No serious adverse events were reported.
This meta-analysis had several limitations. First, although random assignment was mentioned in all of the included studies, only half described a specific randomization method, such as a random number table. Therefore, the findings should be further assessed using high-quality RCTs because study quality may have influenced the chosen indicators (Kjaergard et al., 2001). Second, although LXJD therapy was always used in the combined-treatment group, heterogeneity may nevertheless have been present because herbs and administration courses differed between studies. Third, sample size was relatively small among included studies, with only six involving over 100 patients. These RCTs had different experimental periods ranging from 4 to 12 weeks. Few studies focused on long-term follow-up, and only two followed up at 48 weeks. Fourth, since adverse events were not described in most studies, further evaluation is required to verify the safety of LXJD therapy for ACLF. Finally, all included studies were conducted in China, potentially limiting application to populations in foreign countries.
5 Conclusions
Our findings suggest that LXJD therapy combined with WM was more effective than WM alone in treating ACLF, based on improvements to total effective rate, mortality rate, complications, coagulation and liver function, as well as TCM syndrome score. This study provides reliable evidence for clinical practice, showing that LXJD therapy is a promising complementary or alternative treatment for ACLF. However, multicenter, large-sample RCTs with long follow-up periods are needed to better assess the effectiveness and safety of LXJD therapy for ACLF.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
XW and KS designed the study. KS, QZ, and JH contributed to the data collection and data analysis. Results were interpreted by YZ, YB, and KS drafted the original manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Beijing Municipal Science and Technology Commission, NO. Z191100006619033.
Conflict of Interest
The handling editor AZ declared a shared affiliation with the authors at the time of review.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to Ting Zhao for her constructive comments in improving the grammar and readability of the paper.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.905215/full#supplementary-material
References
Bernal, W., Jalan, R., Quaglia, A., Simpson, K., Wendon, J., and Burroughs, A. (2015). Acute-on-chronic Liver Failure. Lancet 386, 1576–1587. doi:10.1016/S0140-6736(15)00309-8
Chen, L., Zhao, X., Wei, S., Ma, X., Liu, H., Li, J., et al. (2021). Mechanism of Paeoniflorin on ANIT-Induced Cholestatic Liver Injury Using Integrated Metabolomics and Network Pharmacology. Front. Pharmacol. 12, 737630. doi:10.3389/fphar.2021.737630
Chen, Z. Q., Zhao, Y. W., Lai, Q. Y., and Lv, Z. L. (2018). Curative Effect of Cooling Blood, Resolving Toxin and Removing Stasis Prescription on Patients with Acute-On-Chronic Liver Failure and Syndrome of Damp Heat and Stasis Yellow. J. Baotou. Med. Coll. 34, 100–101. doi:10.16833/j.cnki.jbmc.2018.10.043
China Association of Chinese Medicine (2019). Guidelines for Clinical Diagnosis and Treatment of Acute-On-Chronic Liver Failure in Traditional Chinese Medicine. J. Clin. Hepatol. 35, 494–503. doi:10.3969/j.issn.1001-5256.2019.03.009
Cumpston, M., Li, T., Page, M. J., Chandler, J., Welch, V. A., Higgins, J. P., et al. (2019). Updated Guidance for Trusted Systematic Reviews: a New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane. Database. Syst. Rev. 10, Ed000142. doi:10.1002/14651858.ED000142
Dang, Z. B., Dang, Z. Q., Wang, Y. L., Zhao, C. P., Wang, H. X., and Xi, Y. H. (2017). Clinical Study on Combination of Chinese and Western Medicine Treatment of Hepatitis B Virus-Acute on Chronic Liver Failure. Acta. Chin. Med. 32, 1491–1494. doi:10.16368/j.issn.1674-8999.2017.08.393
Duan, M., Zhu, L., Wu, B., Wang, L., Wang, Y. L., and Li, J. K. (2016). Clinical Observation of Combined Traditional Chinese and Western Medicine in the Treatment of Damp-Heat Stasis Yellow Syndrome of Hepatitis B Related Acute-On-Chronic Liver Failure. Hebei J. Tradit. Chin. Med. 38, 1018–1020. doi:10.3969/j.issn.1002-2619.2016.07.016
Hou, W., Hao, Y., Yang, W., Tian, T., Fang, P., Du, Y., et al. (2021). The Jieduan-Niwan (JDNW) Formula Ameliorates Hepatocyte Apoptosis: a Study of the Inhibition of E2F1-Mediated Apoptosis Signaling Pathways in Acute-On-Chronic Liver Failure (ACLF) Using Rats. Drug. Des. devel. Ther. 15, 3845–3862. doi:10.2147/DDDT.S308713
Huang, R. H., Xie, Q., Yan, X. H., and Chen, X. Y. (2022). Intervention and Prognostic Study of Qinghuang Decoctionon 80 Patients with HBV- Related Acute-On-Chronic Liver Failure. J. Emer. Tradit. Chin. Med. 31, 310–313. doi:10.3969/j.issn.1004-745X.2022.02.033
Kjaergard, L. L., Villumsen, J., and Gluud, C. (2001). Reported Methodologic Quality and Discrepancies between Large and Small Randomized Trials in Meta-Analyses. Ann. Intern Med. 135, 982–989. doi:10.7326/0003-4819-135-11-200112040-00010
Li, J., Liang, X., You, S., Feng, T., Zhou, X., Zhu, B., et al. (2021). Development and Validation of a New Prognostic Score for Hepatitis B Virus-Related Acute-On-Chronic Liver Failure. J. Hepatol. 75, 1104–1115. doi:10.1016/j.jhep.2021.05.026
Liang, J., Wu, M., Bai, C., Ma, C., Fang, P., Hou, W., et al. (2020). Network Pharmacology Approach to Explore the Potential Mechanisms of Jieduan-Niwan Formula Treating Acute-On-Chronic Liver Failure. Evidence-Based Complementary Altern. Med. 2020, 1–16. doi:10.1155/2020/1041307
Liu, H. M., Wang, X. B., Hou, Y. X., Gao, F. Y., Sun, F. X., Jiang, Y. Y., et al. (2014). Efficacy and Safety of Integrative Medical Program Based on Blood Cooling and Detoxification Recipe in Treating Patients with Hepatitis B Virus Related Acute-On Chronic Liver Failure: a Randomized Controlled Clinical Study. Zhongguo Zhong Xi Yi Jie He Za Zhi 34, 412–417. doi:10.7661/CJIM.2014.04.0412
Liu, H. M., Gao, F. Y., Li, Y. X., Wang, X. B., and Jiang, Y. Y. (2022). Therapeutic Effect and Mechanism of Jiedu Liangxue Prescription on Acute Liver Failure Mice. Beijing. J. Tradit. Chin. Med. 41, 248–254. doi:10.16025/j.1674-1307.2022.03.006
Liu, H., Zhan, X., Xu, G., Wang, Z., Li, R., Wang, Y., et al. (2021). Cryptotanshinone Specifically Suppresses NLRP3 Inflammasome Activation and Protects against Inflammasome-Mediated Diseases. Pharmacol. Res. 164, 105384. doi:10.1016/j.phrs.2020.105384
Liu, L., Li, Q., Lin, H., Liu, Z. F., and Huang, W. (2015). Clinical Research on Liangxue Jiedu Huayu Formula in the Treatment of HBV-Related Acute-On-Chronic Liver Failure. Lishizhen Med. Mat. Med. Res. 26, 2936–2937. doi:10.3969/j.issn.1008-0805.2015.12.047
Liu, Y., and Bai, J. (2017). Evaluation of Therapeutic Effect of Detoxification and Cooling Blood Therapy on Hepatitis B and Acute Hepatic Failure and its Mechanism. Chin. Arch. Tradit. Chin. Med. 35, 75–76. doi:10.13193/j.issn.1673-7717.2017.06.07510.3724/sp.j.1123.2016.08006
Lou, H. S. (2017). Short-term Efficacy of Entecavir Combined with Detoxification, Cooling Blood and Removing Stasis in the Treatment of Chronic Hepatitis B Associated Acute on Chronic Liver Failure. Chin. J. Integr. Tradit. West. Med. Liver Dis. 27, 350–352. doi:10.3969/j.issn.1005-0264.2017.06.011
Pang, G. H., and Yang, R. L. (2017). Traditional Chinese Medicine and Western Medicine in the Treatment of Chronic Hepatitis B Patients with Acute Liver Failure. Jilin J. Chin. Med. 37, 363–366. doi:10.13463/j.cnki.jlzyy.2017.04.012
Sarin, S. K., and Choudhury, A. (2016). Acute-on-chronic Liver Failure: Terminology, Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 13, 131–149. doi:10.1038/nrgastro.2015.219
Sarin, S. K., Choudhury, A., Sharma, M. K., Maiwall, R., Al Mahtab, M., Rahman, S., et al. (2019). Acute-on-chronic Liver Failure: Consensus Recommendations of the Asian Pacific Association for the Study of the Liver (APASL): an Update. Hepatol. Int. 13, 353–390. doi:10.1007/s12072-019-09946-3
Shi, K., Hou, Y. X., Hou, J., Li, Y. X., Ran, C. P., and Wang, X. B. (2021). Clinical Effect of Jiedu Liangxue Jianpi Prescription in Treatment of Patients with Hepatitis B Virus-Associated Acute-On-Chronic Liver Failure in Early and Middle Stage. J. Clin. Hepatol. 37, 1065–1069. doi:10.3969/j.issn.1001-5256.2021.05.018
Shi, Q. L., Mao, D. W., Chen, Y. Q., and Lv, J. L. (2018). Effect of Jiedu Huayu Ⅱ Decoction on the Expression of Th17 and Th1 in Peripheral Blood of Patients with HBV Related Acute Liver Failure and its Clinical Efficacy. China. Med. Her. 15, 122–125.
Sun, X. F., Han, Z. Y., Zhang, W., Ma, W. F., Yi, X., and Zhou, X. Z. (2016). Clinical Observation of Chronic Hepatitis B Virus Associated Acute-On-Chronic Liver Failure Treated with Integrated Traditional Chinese and Western Medicine. Beijing J. Tradit. Chin. Med. 35, 195–198. doi:10.16025/j.1674-1307.2016.03.001
Vanlangenakker, N., Vanden Berghe, T., Krysko, D. V., Festjens, N., and Vandenabeele, P. (2008). Molecular Mechanisms and Pathophysiology of Necrotic Cell Death. Curr. Mol. Med. 8, 207–220. doi:10.2174/156652408784221306
Wang, N., Wang, S., Tang, N., and Mao, D. W. (2014). A Detoxification and Stasis-Resolving Granules-Dominated Combination Therapy in Patients with Hepatitis B Acute-On-Chronic Liver Failure. Chin. J. Integr. Tradit. West. Med. Liver Dis. 24, 207–209. doi:10.3969/j.issn.1005-0264.2014.04.005
Wu, T., Li, J., Shao, L., Xin, J., Jiang, L., Zhou, Q., et al. (2018). Development of Diagnostic Criteria and a Prognostic Score for Hepatitis B Virus-Related Acute-On-Chronic Liver Failure. Gut 67, 2181–2191. doi:10.1136/gutjnl-2017-314641
Xiao, Z. H., Wu, L., Lin, L., and Chen, G. L. (2016). Clinical Study of Clearing Heat, Detoxicating and Cooling Blood Therapy for Early Stage of Hepatitis B Virus-Related Acute-On-Chronic Liver Failure. Guangming J. Chin. Med. 31, 1403–1406. doi:10.3969/j.issn.1003-8914.2016.10.025
Yin, G. H., and Wen, T. (2018). Clinical Study on Liangxue Jiedu Huayu Tang for Hepatitis B Virus-Associated Acute-On-Chronic Liver Failure. New. Chin. Med. 52, 81–83. doi:10.13457/j.cnki.jncm.2020.17.024
Zhang, L., Yang, B., and Yu, B. (2015). Paeoniflorin Protects against Nonalcoholic Fatty Liver Disease Induced by a High-Fat Diet in Mice. Biol. Pharm. Bull. 38, 1005–1011. doi:10.1248/bpb.b14-00892
Zhang, X., Ding, J., Gou, C., Wen, T., Li, L., Wang, X., et al. (2017). Qingchangligan Formula Attenuates the Inflammatory Response to Protect the Liver from Acute Failure Induced by D-Galactosamine/lipopolysaccharide in Mice. J. Ethnopharmacol. 201, 108–116. doi:10.1016/j.jep.2016.11.007
Zhang, Z. W. (2021). Clinical Observation of Integrated Traditional Chinese and Western Medicine in the Treatment of Hepatitis B Related Acute-On-Chronic Liver Failure. J. Pract. Tradit. Chin. Med. 37, 1507–1509.
Zhao, X. H. (2016). A Clinical Analysis of Treating Hepatitis B Plus Acute Liver Failure in the Integrative Medicine. Clin. J.Chin. Med. 8, 62–63. doi:10.3969/j.issn.1674-7860.2016.14.029
Keywords: LiangXue JieDu, traditional Chinese medicine, meta-analysis, actue-on-chronic liver failure, randomized controlled trials
Citation: Shi K, Zhang Q, Hou J, Zhang Y, Bi Y and Wang X (2022) Evaluation of LiangXue JieDu Therapy in Combination With Western Medicine for Acute-On-Chronic Liver Failure: A Systematic Review and meta-Analysis. Front. Pharmacol. 13:905215. doi: 10.3389/fphar.2022.905215
Received: 26 March 2022; Accepted: 23 June 2022;
Published: 12 July 2022.
Edited by:
Aimin Zhou, Cleveland State University, United StatesReviewed by:
Xiaoning Wang, Shanghai University of Traditional Chinese Medicine, ChinaJiabo Wang, Capital Medical University, China
Copyright © 2022 Shi, Zhang, Hou, Zhang, Bi and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianbo Wang, d2FuZ3hiQGNjbXUuZWR1LmNu
 Ke Shi
Ke Shi Qun Zhang
Qun Zhang Jie Hou
Jie Hou Xianbo Wang
Xianbo Wang