- 1Department of Plant and Soil Sciences, University of Pretoria, Pretoria, South Africa
- 2Metabolon Inc., Morrisville, NC, United States
- 3Department of Agriculture and Animal Health, University of South Africa (UNISA), Pretoria, South Africa
- 4Molecular Virology, Department of Biomedicine, University of Basel, Basel, Switzerland
Several species of the Helichrysum genus have been used ethnobotanically to treat conditions that we today know have been caused by viral infections. Since HIV is a modern disease with no ethnobotanical history, we commenced with a study on the anti-HIV activity of several Helichrysum species. Drug discovery of small molecules from natural resources that is based on the integration of chemical and biological activity by means of metabolomical analyses, enables faster and a more cost-effective path to identify active compounds without the need for a long process of bioassay-guided fractionation. This study used metabolomics to identify anti-HIV compounds as biomarkers from 57 Helichrysum species in a combined study of the chemical and biological data of two previous studies. In the OPLS-DA and hierarchical cluster analyses, anti-HIV activity data was included as a secondary observation, which assisted in the correlation of the phytochemical composition and biological activity of the samples. Clear grouping revealed similarity in chemical composition and bioactivity of the samples. Based on the biological activity of polar extracts, there was a distinct phytochemical difference between active and non-active groups of extracts. This NMR-based metabolomic investigation showed that the chlorogenic acids, compounds with cinnamoyl functional groups, and quinic acid were the most prominent compounds in the Helichrysum species with anti-HIV activity. This study further revealed that the chlorogenic acid type compounds and quinic acid are biomarkers for anti-HIV activity.
Introduction
Medicinal plants treated several diseases throughout the history of mankind and it led to many investigations to identify the metabolites responsible for their curative effects. These bioactive compounds have been the source of many ‘modern’ pharmaceutical drugs (Satheeshkumar et al., 2012; Wang et al., 2017).
The selection of specific biomarkers for bioactivity from thousands of metabolites in medicinal plants has been a difficult challenge (Wang et al., 2017). Due to the variable sources and chemical complexity of medicinal plants, the use of only chromatographic techniques to find bioactive compounds and to standardize botanical extracts, has limitations. DNA-based molecular markers have usually precedent in fields such as taxonomy, physiology, genetics, medicine, etc. to identify biomarkers (Joshi et al., 2004; Pourmohammad, 2013). In contrast, identifying the secondary metabolite biomarkers using NMR-based metabolomic analysis has emerged as a new technique (Markley et al., 2017; Jahangir, et al., 2018).
Some secondary metabolites play important roles in immune function enhancement and exhibit antiviral potential, including against the Human Immunodeficiency Virus (HIV) (Salehi et al., 2018). HIV has infected around 75 million people worldwide. According to the World Health Organization (WHO), approximately 38 million are living with the infection with most of the infected population in sub-Saharan Africa (Deeks et al., 2015; World Health Organization, 2020).
Metabolomic studies of plants are of great importance when one wants to associate bioactivity with the chemical composition of the extract (Castro et al., 2020) and also to identify biomarkers employing metabolic fingerprinting and profiling (Madsen et al., 2010; Takayama et al., 2015). The techniques, 1H NMR spectroscopy and multivariate data analysis, are complimentary for studying the biochemical composition and metabolic pathways for discovering biomarkers from natural resources such as medicinal plants (Satheeshkumar et al., 2012). Principal component analysis (PCA) and orthogonal projections to latent structures discriminant analysis (OPLS-DA) can classify chemical groups with particular bioactivities and also identify the components responsible for the groupings which could well be used as biomarkers (Castro et al., 2020).
The Helichrysum genus of the Asteraceae family, is widely recognised for its many traditional medicinal plants used for the treatment of several medical conditions like nervousness and hysteria, and also to treat wounds, bacterial and viral infections and respiratory conditions (Meyer et al., 1996; Lourens et al., 2004; Lourens et al., 2008; Van Vuuren, 2008). It consists of approximately 500–600 species of which 245 are indigenous to southern Africa including Namibia (Lourens et al., 2004; Lourens et al., 2008). This genus has been the source of many interesting and bioactive compounds (Lourens et al., 2004; Appendino et al., 2007; Bauer et al., 2011; Kutluk et al., 2018). The extracts and the essential oils from this species have exhibited promising biological activities in various in vitro assays, which include anti-oxidant, antimicrobial, antifungal, anti-inflammatory and antiviral activity (Van Vuuren, 2008; Akaberi et al., 2019; Akinyede et al., 2021). In case of antiviral activity of Helichrysum genus there are several reports. According to Viegas et al. (2014), flavonoids and phloroglucinols isolated from H. italicum has inhibition activity against herpes simplex virus 1 (HSV1) and HIV, respectively. Most interesting though are the findings by Appendino et al. (2007) that arzanol (a phloroglucinol α–pyrone) inhibits HIV-1 replication in T-cells and inhibited NF-ҡB (IC50 = 5 μg/ml) indicating that this group of compounds may exhibit both antiviral and anti-inflammatory properties. The ethanol extract of H. cymocum exhibited the virucidal activity against the HSV1, the measles virus strain Edmonston A (MV-EA) as well as the Semliki forest virus A7 (Sindambiwe et al., 1999). Aqueous extracts of H. aureonitens exhibited antiviral activity against the HSV1 in vitro at a concentration of 1.35 mg/ml (Meyer et al., 1996).
Results of two previous studies conducted by Heyman et al. (2015) and Yazdi et al. (2019) on the metabolomic analysis of anti-HIV activity of Helichrysum species have been integrated in this study. This study therefore aimed to identify biomarkers for anti-HIV activity in the aqueous methanolic extracts of the aerial parts of 57 Helichrysum species by using NMR spectroscopy and multivariate modeling (PCA and OPLS-DA).
Materials and Methods
Plant Material
The aerial parts of 59 extracts of selected Helichrysum species (57 species, one variety and one subspecies, Table 1) were collected from different geographical regions in South Africa during spring and summer (Permit no: OP 4928/2010). Herbarium specimens were identified by taxonomists from the South African National Biodiversity Institute (SANBI) together with the personnel at the H.G.W.J. Schweickerdt Herbarium (University of Pretoria).

TABLE 1. Analysed Helichrysum species and their herbarium voucher numbers and anti-HIV screening results (% inhibition). No activity against HIV-1 was observed for the non-polar extracts at 2.5 μg/ml (Heyman et al., 2015; Yazdi et al., 2019).
Plant Extraction
All plants were dried in the dark at room temperature. Dried material (5 g) was ground into small pieces but not to a fine powder. Different solvent systems with increasing polarity [hexane, dichloromethane (DCM), acetone (Ace), and methanol (MeOH): water (50:50)] were used for extractions. Extraction of the collected plant material was done on a SpeedExtractor E-914/E-916 (Buchi, Switzerland) in 40 ml steel pressure vessels. Thereafter, the filtrate was concentrated under vacuum to dryness using a Genevac (EZ-2 Plus, GeneVac, United Kingdom) (Heyman et al., 2015; Yazdi et al., 2019).
1H NMR Analysis
A Varian 600 MHz spectrometer (Council for Scientific and Industrial Research, CSIR) was used for the 1H NMR analysis of the samples. The 12 mg of polar fractions were re-dissolved in 800 ml (15 mg/ml) in a buffered mixture of CD3OD and KH2PO4-D2O solution, with the pH adjusted to pH 6.0 with NaOD (1M). The internal standard trimethylsillyl propionic acid-D4 sodium salt (0.1% TSP- 0.00 ppm) was used for spectral referencing of the 50% methanolic samples. For each spectrum, 64 scans were recorded with a spectral width of 14 ppm. The temperature was kept at 25°C constantly. The total transients of the standard 1D spectra were set to 46 with a three-second relaxation delay, and the acquisition time for each transient scan set to 3 seconds. The magnet shimming was done automatically for optimal and consistent spectral resolution.
All 1H NMR spectra were referenced (based on residual CH3OH; δ3.310 ppm), baseline-corrected (Whittaker smoother), automatic phase corrected, and normalised by scaling the spectral intensities to 0.1% trimethylsilylpropanoic acid (TSP) using MestReNova 14.2 (Mestrelab Research S.L.). The region of 0.00-10.00 ppm was reduced to bins of 0.04 ppm in width. The regions ranging from 3.28 to 3.36 ppm (residual MeOH) and 4.60–5.00 ppm (residual water) were removed before statistical analysis. The ASCII files generated were then processed in Microsoft Excel and imported into SIMCA-P 14 (Umetrics, Umeå, Sweden). The data was Pareto scaled before being subjected to PCA and OPLS analyses.
Anti-HIV Screening Activity
The procedures of anti-HIV screening and the colorimetric HIV-1 reverse transcriptase assay were done as previously described by Heyman et al. (2015) and Yazdi et al. (2019).
Results
The results of the anti-HIV screening of the 59 extracts of selected Helichrysum species, are shown in Table 1, consistent with the results from previous studies (Heyman et al., 2015; Yazdi et al., 2019).
The proton NMR spectra of the Helichrysum species extracts with the highest activity against HIV (Table 1) showed significant similarities with the presence of aromatic compounds (6.50‒7.50 ppm) and carbohydrate moieties (1.80‒2.50 and 3.00‒4.10 ppm), characteristic signals of phenylpropanoids or chlorogenic acids (Figure 1). These compounds are a diverse family of organic compounds that are synthesized by plants from the amino acids, phenylalanine and tyrosine. Various bio-activities have been reported for these phytochemicals such as antiviral, anti-cancer and other biological effects (Miyamae et al., 2011).
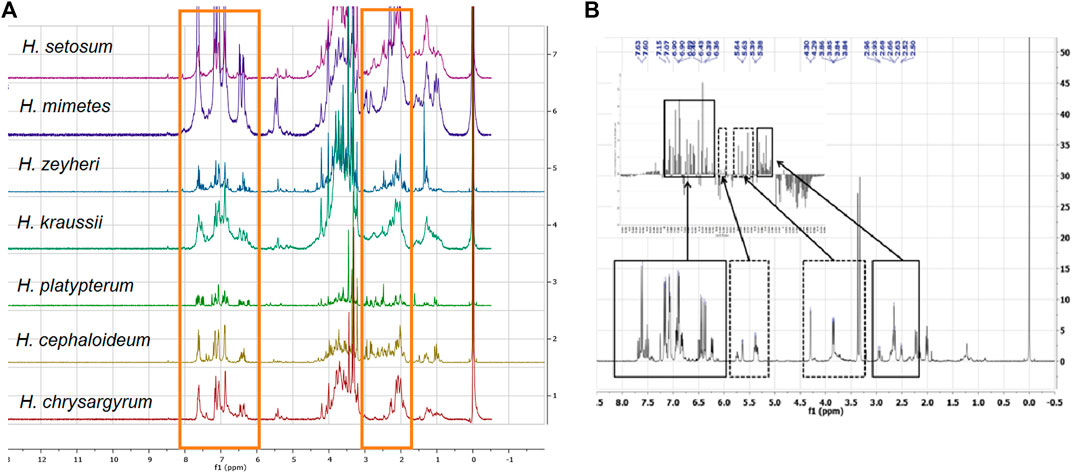
FIGURE 1. Comparison of the stacked 1H NMR spectra of the most active polar Helichrysum species extracts studied by Yazdi et al. (2019). (A). with the NMR spectrum of a fraction isolated from H. populifolium with the best active profile from the study conducted by Heyman et al. (2015). (B). The chemical shifts linked to the caffeoylquinic acid type compounds were present in the Helichrysum polar extracts (red boxes). (A). and areas with the most contribution are highlighted with solid line boxes. (B).
One of the major types of compounds present in Helichrysum species are chlorogenic acids (Albayrak et al., 2010). The 1H NMR spectra of all fractions isolated in two previous studies conducted by Heyman et al. (2015) and Yazdi et al. (2019) showed that the anti-HIV compound(s) could probably be caffeoylquinic acids. Comparison of the stacked NMR spectra showed that all the chemical shifts linked to the caffeoylquinic acid type compounds existed in all Helichrysum species extracted (Figure 1).
All polar extracts were subjected to metabolomic analysis. To fast-track the selection of the possible biomarkers, metabolomic tools were used to investigate similarities and differences in the chemical profiles of the extracts of the 57 Helichrysum species and one subspecies and one variant using 1H NMR spectroscopy (Supplementary Material 1). Since all samples belonged to the Helichrysum genus, it was predicted that not many different groups would be obtained in the PCA. The datasets used for the PCA score plots did not show distinct grouping correlating with the activity of the extracts. The PCA model with R2X = 0.766 and Q2 = 0.571 values for component 2 indicated good predictability and reliability of the model (Figure 2).
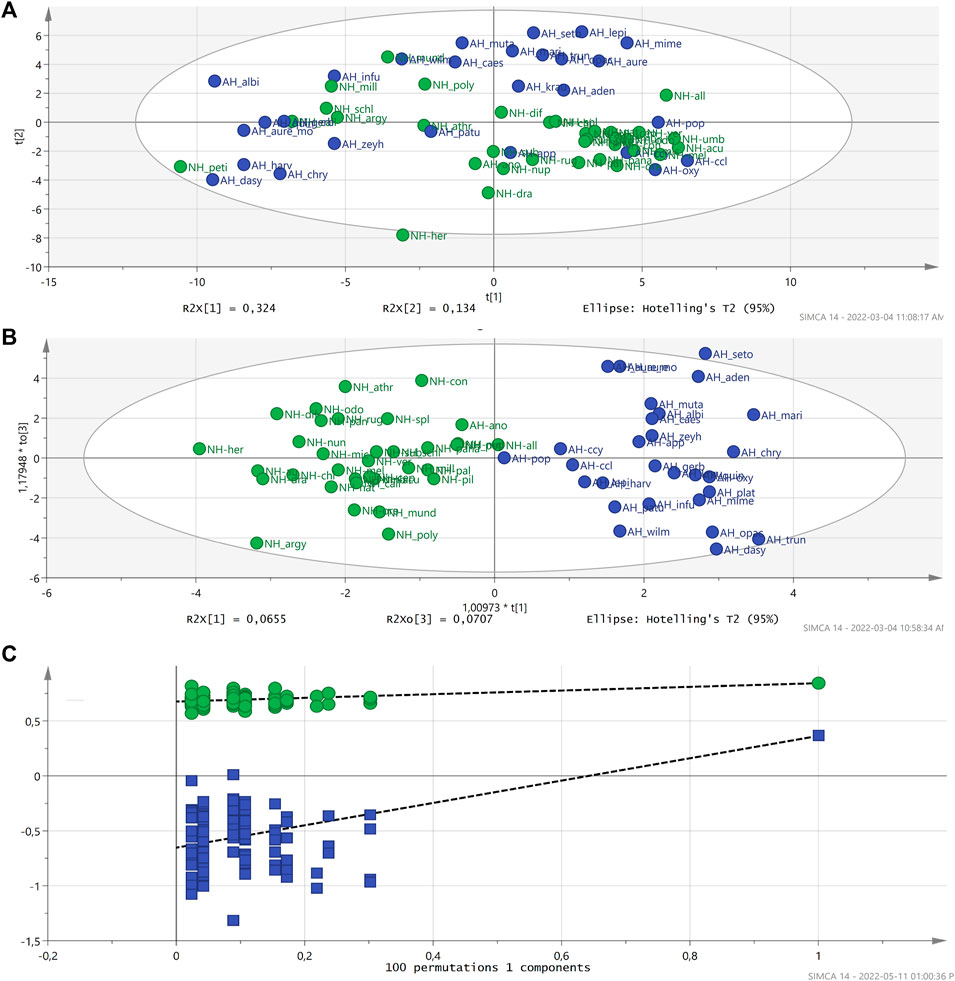
FIGURE 2. PCA score plot did not exhibit a significant correlation between active and non-active Helichrysum polar extracts. R2X: 0.766 and Q2 (cum): 0.571.






In the OPLS-DA analysis (Figure 2), anti-HIV activity data was included as a secondary observation, which assisted in the correlation of the phytochemical composition and biological activity of the samples. The extent of grouping on similarity in chemical composition and bioactivity was satisfactory in this analysis. It indicates that, based on the biological activity of polar extracts, there is a distinct phytochemical difference between these two groups of extracts (active and non-active). The variation in X explains much of the variation with the R2X (cumulative) being 75%. The predictive component (P1) only explained 4.6% of the variation in X related to the separation of the samples based on the activity. Although, the description of the variation in the samples was acceptable the predictability of the model was slightly lower with R2X = 0.683, R2Y = 0.843 and Q2 (cum) = 0.227. Based on the Q2 of approximately 0.25, the predictability of the model was not significant but acceptable (Eriksson et al., 2013; Wu and Wang, 2015). The OPLS-DA plots were generated with a Hotelling’s T2 test of a 95% significance, validated by Permutation (100 permutations on the first five components) (Figure 2) and subjected to cross validated (CV)-ANOVA significance testing (p-value < 0.05).
Hierarchical cluster analysis (HCA), as a complementary data reduction and pattern recognition method, was used for finding the underlying structure of objects through a repetitive process that associates or dissociates object by object until all are equally and completely processed. The HCA showed separated groups in the OPLS-DA analysis in the active and also in non-active extracts (Figures 3A,B).
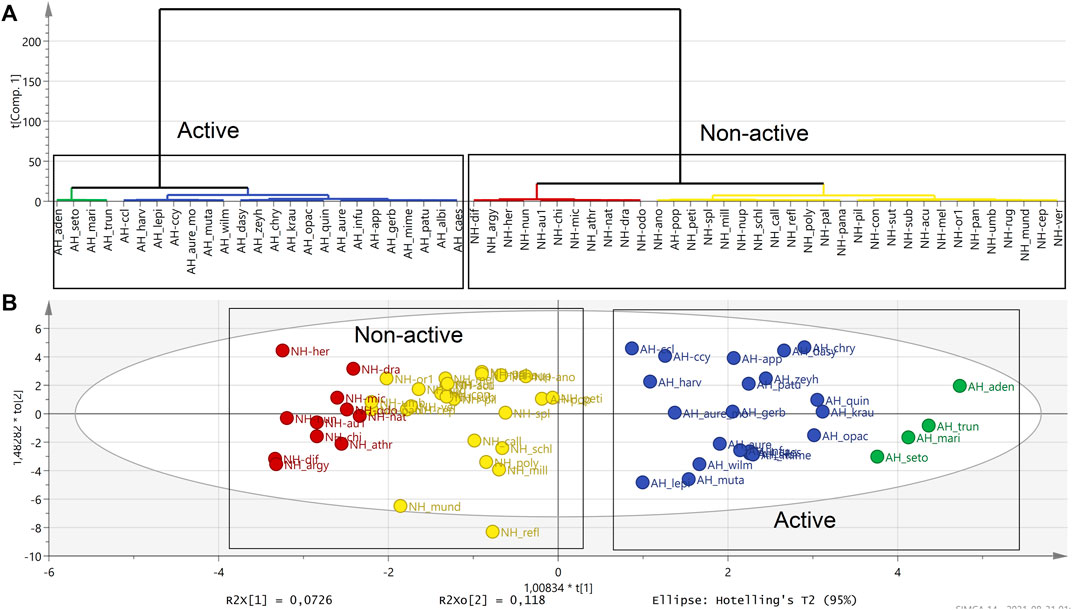
FIGURE 3. HCA dendrogram showing the attribute distances between each group of sequentially merged classes in active (blue and green) and non-active polar extracts (red and yellow). (A). Clustering observed in the OPLS-DA score scatter plot supports the HCA dendrogram analysis by separating active and non-active Helichrysum polar extracts. (B). (AH: active Helichrysum species, NH: non-active Helichrysum).
An S-plot was generated based on the OPLS-DA score plot of the most active Helichrysum species (H. mimetes, H. populifolium, H. platypterum, and H. symosum) and selected the most non-active extracts. The S-plot clearly indicated the typical signals of cinnamoyl units 6.2 to 6.5 and 7.5–7.7 ppm in the active samples (Figure 4). It showed that these signals are the main correlate with for the anti-HIV activity of active Helichrysum species.
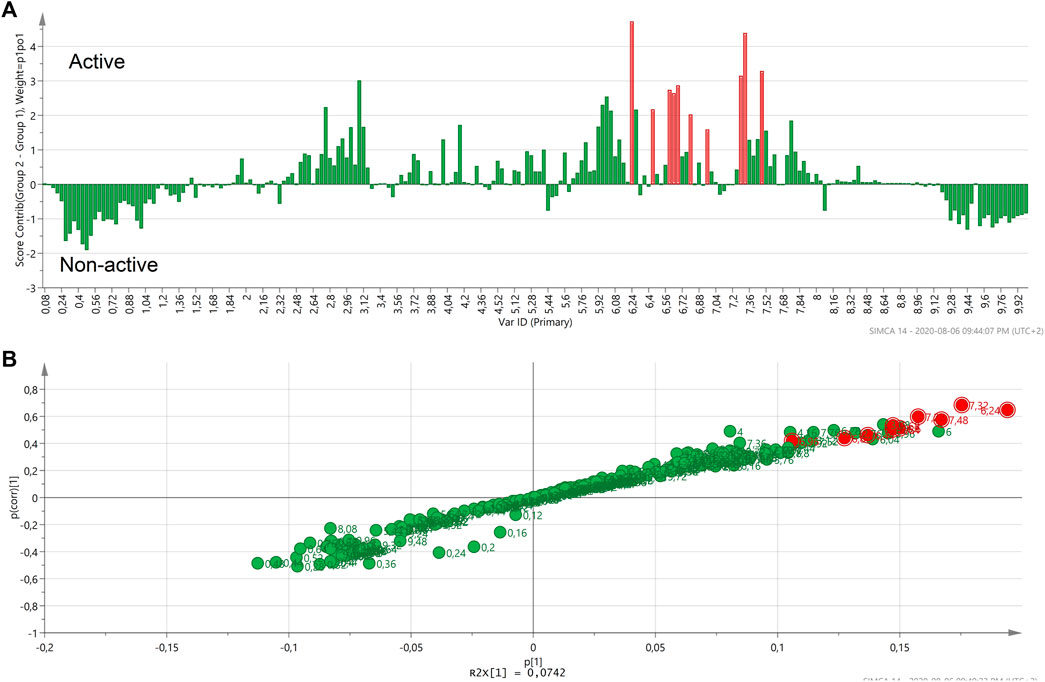
FIGURE 4. Contribution plot (A) and S-plot (B) generated two groups of the most active and non-active Helichrysum extracts and showing the typical signals of cinnamoyl units. In the contribution plot, upward bars indicate the NMR regions associated with the active samples, and downward bars indicate the NMR regions associated with the non-active samples.




A second contribution plot and S-plot (Figures 5B,C) were generated based on the OPLS score plot of selected active and selected non-active groups (Figure 5A). The generated S-plot indicated that the typical signals of the NMR chemical shifts associated with caffeoylquinic acids (CQA): mainly between 2.10–3.10 ppm and 6.20‒8.00 ppm (Figures 5B,C). It revealed that these signals correlate with the anti-HIV-1 activity of Helichrysum species.
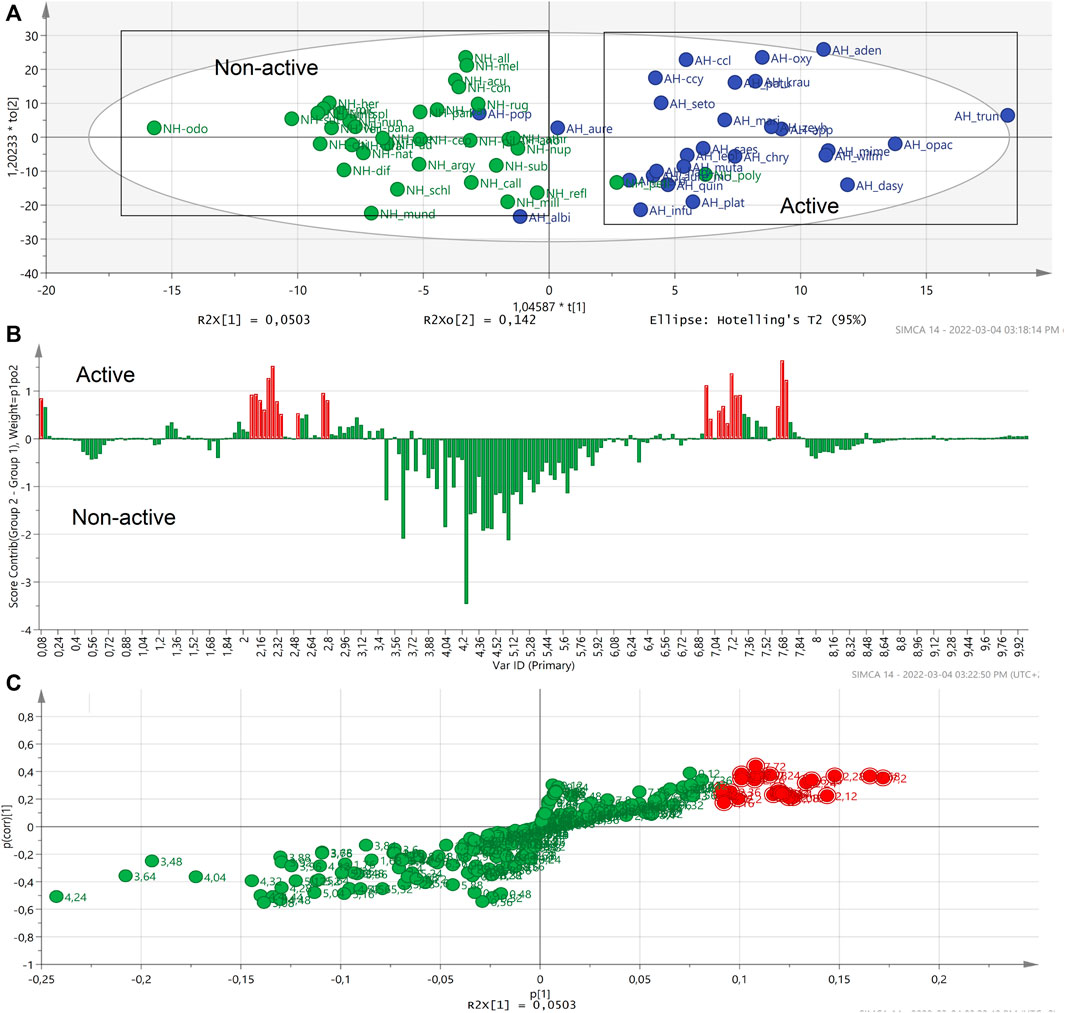
FIGURE 5. The OPLS-DA score plot of the selected active and selected non-active Helichrysum polar extracts. (A). The contribution plot generated by comparing two groups of active and non-active Helichrysum extracts and showing the typical signals of CQA units. In the contribution plot, upward bars indicate the NMR regions associated with the active samples, and downward bars indicate the NMR regions associated with the non-active samples (B), the loading S-plot indicating the buckets that are most responsible with activity of the active Helichrysum extracts. (C).




Contribution and S-plots (Figures 6B,C) were generated based on the OPLS-DA score plot (Figure 6A) of active and a non-active samples identified by the HCA dendrogram. H. odoratissimum, H. sutherlandii, H. oreophilum from the non-active region and H. adenocarpum and H. truncatum from the active region were excluded as outliers to generate this OPLS-DA. Although the presence of CQA peaks is dominant in both plots, quinic acid peaks can also be seen. The generated profile could analyse the specific regions that can be related to the activity of the extract(s). The two plots indicated that the typical signals of quinic acid units: 1.85 to 2.20 and 3.35–4.20 ppm (Figure 6) are contributing to some of the anti-HIV activity. It was also previously reported that these signals are responsible for the activity of the anti-HIV RT of H. mimetes (Yazdi et al., 2019). It seemed that other types of compounds like sugars or amino acids did not play a major role in the activity of the Helichrysum species against HIV RT showing negative contribution for these regions on the contribution plot (Figure 6B). However, it must be considered that the differences in the chemical shifts of the bars above the line and bars below the line could be related to the concentration of the compounds in the two groups of extracts and not a presence or absence of compounds.
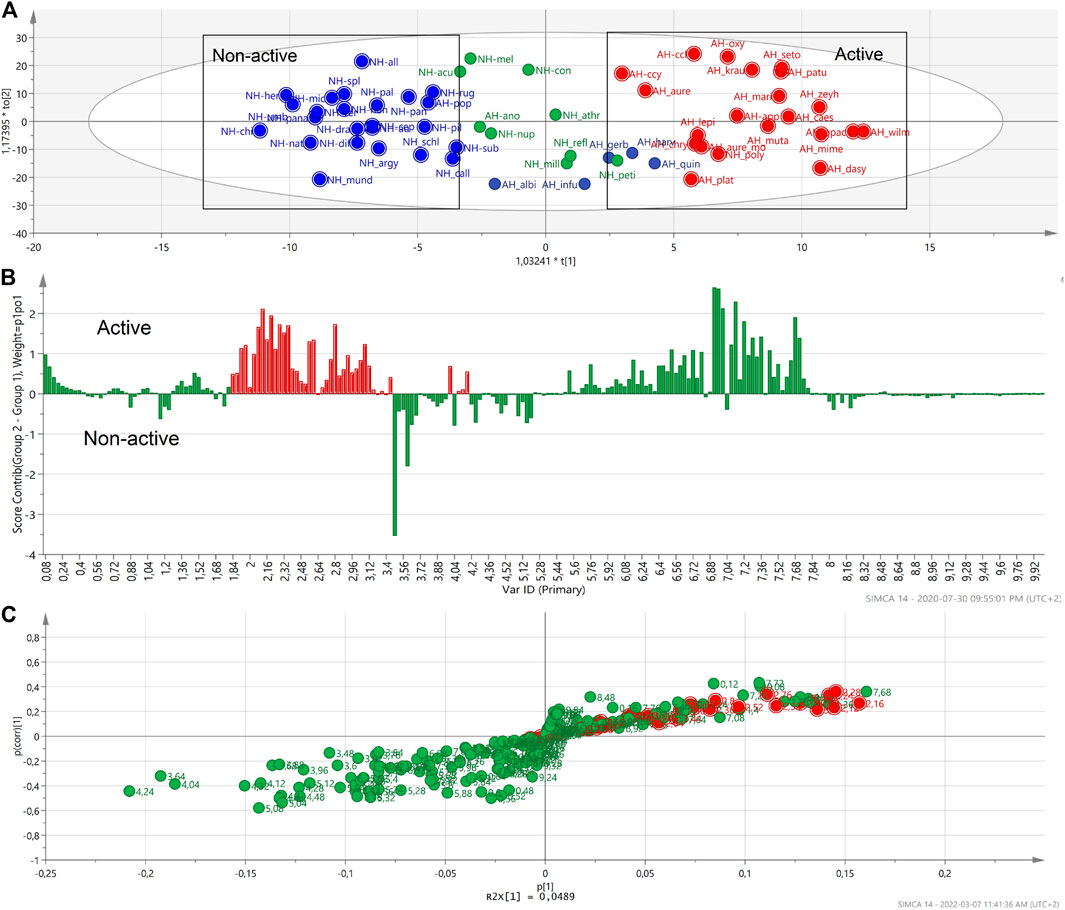
FIGURE 6. The OPLS-DA score plot of the active and non-active Helichrysum polar extracts. (A). The red marked upward bars of the contribution plot and red spots on the S-plot generated by comparing active and non-active groups of Helichrysum extracts show the typical signals of quinic acid units in the active group. (B,C).




All data were analysed statistically of both bioactivity and metabolomic analyses for a better understanding of the anti-HIV activity of Helichrysum species and the p-value of the investigated models was significant (<0.05).
Discussion
Heyman et al. (2015) identified five major compounds observed in the most active fraction six isolated from H. populifolium. The fractions were identified as being chlorogenic acid derivatives using LC-IT-TOF. The fraction patterns showed that three of the major compounds are dicaffeoylquinic acid (DCQA) that is 3,4-DCQA (516 Mr), 3,5-DCQA (516 Mr) and 4,5-DCQA (516 Mr). Two other types of chlorogenic type of compounds were identified by Heyman et al. (2015), tricaffeoylquinic acid (TCQA), 1,3,5-TCQA (678 Mr) and 5-malonyl-1,3,4-TCQA (764 Mr) (Supplementary Material 2A).
In the Yazdi et al. (2019) study, the phytochemical fingerprint of sub-fraction 15 isolated from H. mimetes and standard quinic acid (1,3,4,5-tetrahydroxycyclohexane carboxylic acid), were compared using UPLC-MS to confirm the identity of the compound as quinic acid. The mass spectrum in negative ionization mode is shown in Supplementary Material 2B. A reverse phase C18 column was used with MeOH:H2O as mobile phase. An elemental composition report of the MS analysis confirmed the presence of quinic acid in sub-fraction with the mass of 191.0564 and molecular formula of C7H11O6 (Supplementary Material 2B). The mass spectrum of the contaminant was confirmed as a sodium salt of formic acid.
This study on the data integration of two previous related studies (Heyman et al., 2015; Yazdi et al., 2019) on the chemistry and anti-HIV-1 activity of Helichrysum species showed that the chlorogenic acid type compounds (e.g. caffeoylquinic acids, cinnamoyl groups) and quinic acid, a building block of chlorogenic acids, can serve as biomarkers for anti-HIV activity. There are previous reports that showed the activity of CQA types of compounds against HIV integrase (HIV IN) (McDougall et al., 1998; Tamura et al., 2006). The study of Heyman (Heyman, 2013), also reported anti-HIV IN activity of 3,4-DCQA,3,5-DCQA and 4,5-DCQA at 0.71, 0.66 and 0.30 µM (Heyman, 2013). The obtained results are supported by McDugall et al. (1998) and Tamura et al. (2006) that indicated significant activity of several DCQA against HIV IN, ranging between 2 and 12 µM. There are no previous reports on anti-HIV RT activity of quinic acid apart from that of Yazdi et al. (2019) who isolated it from H. mimetes and showed promising anti-RT activity (IC50 = 53.82 μg/ml) comparable to the positive drug control, doxorubicin (IC50 = 40.31 μg/ml). Also, the molecular docking study on isolated quinic acid from H. mimetes, indicated that the quinic acid–RT complex showed good stability and good H-bonding (Yazdi et al., 2019). Thus, activity-based metabolomic studies on the chemistry and bioactivity of plants represent a unique approach to identify lead compounds without the need for extensive bioassay-guided fractionation. This may also lead to lower input cost and time to discover new therapeutics for various diseases.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
JM, GP, and HH supervised this work. JM, SEY, and HH designed the experiment. HH and SEY contributed to collection and sample handling, preparation voucher specimen no, performed the experiment and analysed the data. HH and SEY performed metabolomic analyses and performed statistical analyses. TK conducted the HIV test. SEY conducted the anti-RT test. SEY performed the data interpretation and wrote the manuscript. JM, GP, TK, and HH revised the manuscript. All authors have read and approved the manuscript.
Funding
The National Research Foundation of South Africa, grant 119098.
Conflict of Interest
Author HH was employed by Metabolon Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to the Buffelskloof Nature Reserve research manager, Mr. John Burrows, and Mr. Alexander Heunis of the Voortrekker Nature Reserve in Pretoria for giving permission to collect plants and providing us with the plant material. The SWISS-SA collaboration (SA-JRP14) and the financial contributions made by the University of Basel and the CSIR and to the University of Pretoria are acknowledged for their continued support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.904231/full#supplementary-material
References
Akaberi, M., Sahebkar, A., Azizi, N., and Emami, S. A. (2019). Everlasting Flowers: Phytochemistry and Pharmacology of the Genus Helichrysum. Industrial Crops Prod. 138, 111471. doi:10.1016/j.indcrop.2019.111471
Akinyede, K. A., Cupido, C. N., Hughes, G. D., Oguntibeju, O. O., and Ekpo, O. E. (2021). Medicinal Properties and In Vitro Biological Activities of Selected Helichrysum Species from South Africa: a Review. Plants 10 (8), 1566. doi:10.3390/plants10081566
Albayrak, S., Aksoy, A., Sagdic, O., and Hamzaoglu, E. (2010). Compositions, Antioxidant and Antimicrobial Activities of Helichrysum (Asteraceae) Species Collected from Turkey. Food Chem. 119 (1), 114–122. doi:10.1016/j.foodchem.2009.06.003
Appendino, G., Ottino, M., Marquez, N., Bianchi, F., Giana, A., Ballero, M., et al. (2007). Arzanol, an Anti-inflammatory and Anti-HIV-1 Phloroglucinol Alpha-Pyrone from Helichrysum Italicum Ssp. Microphyllum. J. Nat. Prod. 70 (4), 608–612. doi:10.1021/np060581r
Bauer, J., Koeberle, A., Dehm, F., Pollastro, F., Appendino, G., Northoff, H., et al. (2011). Arzanol, a Prenylated Heterodimeric Phloroglucinyl Pyrone, Inhibits Eicosanoid Biosynthesis and Exhibits Anti-inflammatory Efficacy In Vivo. Biochem. Pharmacol. 81 (2), 259–268. doi:10.1016/j.bcp.2010.09.025
Castro, C. B., Luz, L. R., Guedes, J. A., Porto, D. D., Silva, M. F. S., Silva, G. S., et al. (2020). Metabolomics-based Discovery of Biomarkers with Cytotoxic Potential in Extracts of Myracrodruon Urundeuva. J. Braz. Chem. Soc. 31, 775–787. doi:10.21577/0103-5053.20190242
Deeks, S. G., Overbaugh, J., Phillips, A., and Buchbinder, S. (2015). HIV Infection. Nat. Rev. Dis. Prim. 1 (1), 15035. doi:10.1038/nrdp.2015.35
Eriksson, L., Byrne, T., Johansson, E., Trygg, J., and Vikstr€om, C. (2013). “Multi- and Megavariate Data Analysis,” in Technometrics. Malmö, Sweden: MKS Umetrics AB, 323–355. doi:10.1198/tech.2003.s162
Heyman, H. M., Senejoux, F., Seibert, I., Klimkait, T., Maharaj, V. J., and Meyer, J. J. (2015). Identification of Anti-HIV Active Dicaffeoylquinic- and Tricaffeoylquinic Acids in Helichrysum Populifolium by NMR-Based Metabolomic Guided Fractionation. Fitoterapia 103, 155–164. doi:10.1016/j.fitote.2015.03.024
Heyman, H. M. (2013). Identification of Anti-HIV Compounds in Helichrysum Species (Asteraceae) by Means of NMR-Based Metabolomic Guided Fractionation (Pretoria: University of Pretoria). [dissertation’s thesis] http://hdl.handle.net/2263/40273.
Jahangir, M., Nuringtyas, T. R., Ali, K., Wilson, E. G., Choi, Y. H., and Verpoorte, R. (2018). “NMR-based Metabolomics: Understanding Plant Chemistry and Identification of Biologically Active Compounds,” in NMR-based Metabolomics (London: Royal Society of Chemistry), 246–263. doi:10.1039/9781782627937-00246
Joshi, K., Chavan, P., Warude, D., and Patwardhan, B. (2004). Molecular Markers in Herbal Drug Technology. Curr. Sci. 87, 159–165. https://www.jstor.org/stable/24108860.
Kutluk, I., Aslan, M., Orhan, I. E., and Özçelik, B. (2018). Antibacterial, Antifungal and Antiviral Bioactivities of Selected Helichrysum Species. South Afr. J. Bot. 119, 252–257. doi:10.1016/j.sajb.2018.09.009
Lourens, A. C., Reddy, D., Başer, K. H., Viljoen, A. M., and Van Vuuren, S. F. (2004). In Vitro biological Activity and Essential Oil Composition of Four Indigenous South African Helichrysum Species. J. Ethnopharmacol. 95 (2-3), 253–258. doi:10.1016/j.jep.2004.07.027
Lourens, A. C., Viljoen, A. M., and Van Heerden, F. R. (2008). South African Helichrysum Species: a Review of the Traditional Uses, Biological Activity and Phytochemistry. J. Ethnopharmacol. 119 (3), 630–652. doi:10.1016/j.jep.2008.06.011
Madsen, R., Lundstedt, T., and Trygg, J. (2010). Chemometrics in Metabolomics-Aa Review in Human Disease Diagnosis. Anal. Chim. Acta. 659 (1-2), 23–33. doi:10.1016/j.aca.2009.11.042
Markley, J. L., Brüschweiler, R., Edison, A. S., Eghbalnia, H. R., Powers, R., Raftery, D., et al. (2017). The Future of NMR-Based Metabolomics. Curr. Opin. Biotechnol. 43, 34–40. doi:10.1016/j.copbio.2016.08.001
McDougall, B., King, P. J., Wu, B. W., Hostomsky, Z., Reinecke, M. G., and Robinson, W. E. (1998). Dicaffeoylquinic and Dicaffeoyltartaric Acids Are Selective Inhibitors of Human Immunodeficiency Virus Type 1 Integrase. Antimicrob. Agents Chemother. 42 (1), 140–146. doi:10.1128/AAC.42.1.140
Meyer, J. J., Afolayan, A. J., Taylor, M. B., and Engelbrecht, L. (1996). Inhibition of Herpes Simplex Virus Type 1 by Aqueous Extracts from Shoots of Helichrysum Aureonitens (Asteraceae). J. Ethnopharmacol. 52, 41–43. doi:10.1016/0378-8741(96)01387-6
Miyamae, Y., Kurisu, M., Han, J., Isoda, H., and Shigemori, H. (2011). Structure-activity Relationship of Caffeoylquinic Acids on the Accelerating Activity on ATP Production. Chem. Pharm. Bull. (Tokyo) 59 (4), 502–507. doi:10.1248/cpb.59.502
Pourmohammad, A. (2013). Application of Molecular Markers in Medicinal Plant Studies. Acta Univ. Sapientiae, Acta Univ. Sapientiae, Agric. Environ. 5 (1), 80–90. doi:10.2478/ausae-2014-0006
Salehi, B., Kumar, N., Şener, B., Sharifi-Rad, M., Kılıç, M., Mahady, G., et al. (2018). Medicinal Plants Used in the Treatment of Human Immunodeficiency Virus. Ijms 19 (5), 1459–1460. doi:10.3390/ijms19051459
Satheeshkumar, N., Nisha, N., Sonali, N., Nirmal, J., Jain, G. K., and Spandana, V. (2012). Analytical Profiling of Bioactive Constituents from Herbal Products, Using Metabolomics-Aa Review. Nat. Prod. Commun. 7 (8), 1111–1115. doi:10.1177/1934578X1200700837
Sindambiwe, J. B., Calomme, M., Cos, P., Totté, J., Pieters, L., Vlietinck, A., et al. (1999). Screening of Seven Selected Rwandan Medicinal Plants for Antimicrobial and Antiviral Activities. J. Ethnopharmacol. 65, 71–77. doi:10.1016/s0378-8741(98)00154-8
Takayama, T., Mochizuki, T., Todoroki, K., Min, J. Z., Mizuno, H., Inoue, K., et al. (2015). A Novel Approach for LC-MS/MS-based Chiral Metabolomics Fingerprinting and Chiral Metabolomics Extraction Using a Pair of Enantiomers of Chiral Derivatization Reagents. Anal. Chim. Acta. 898, 73–84. doi:10.1016/j.aca.2015.10.010
Tamura, H., Akioka, T., Ueno, K., Chujyo, T., Okazaki, K., King, P. J., et al. (2006). Anti-human Immunodeficiency Virus Activity of 3,4,5-tricaffeoylquinic Acid in Cultured Cells of Lettuce Leaves. Mol. Nutr. Food Res. 50 (4‐5), 396–400. doi:10.1002/mnfr.200500216
Van Vuuren, S. F. (2008). Antimicrobial Activity of South African Medicinal Plants. J. Ethnopharmacol. 119 (3), 462–472. doi:10.1016/j.jep.2008.05.038
Viegas, D. A., Palmeira-de-Oliveira, A., Salgueiro, L., Martinez-de-Oliveira, J., and Palmeira-de-Oliveira, R. (2014). Helichrysum Italicum: from Traditional Use to Scientific Data. J. Ethnopharmacol. 151, 54–65. doi:10.1016/j.jep.2013.11.005
Wang, H. P., Liu, Y., Chen, C., and Xiao, H. B. (2017). Screening Specific Biomarkers of Herbs Using a Metabolomics Approach: a Case Study of Panax Ginseng. Sci. Rep. 7 (1), 4609–9. doi:10.1038/s41598-017-04712-7
World Health Organization (2020). HIV. Available at: https://www.who.int/docs/default-source/hiv-hq/presentation-international-aidsconference-2020.pdf?sfvrsn=cbd9bbc_4 (Accessed November 3, 2020).
Wu, J.-F., and Wang, Y. (2015). “Multivariate Analysis of Metabolomics Data,” in Plant Metabolomics. Editors X. Qi, X. Chen, and Y. Wang (Dordrecht: Springer), 105–122. doi:10.1007/978-94-017-9291-2_5
Keywords: biomarker, chlorogenic acids, Helichrysum, human immunodeficiency virus, metabolomics, quinic acid
Citation: Emamzadeh Yazdi S, Heyman HM, Prinsloo G, Klimkait T and Meyer JJM (2022) Identification of Anti-HIV Biomarkers of Helichrysum Species by NMR-Based Metabolomic Analysis. Front. Pharmacol. 13:904231. doi: 10.3389/fphar.2022.904231
Received: 25 March 2022; Accepted: 01 June 2022;
Published: 22 July 2022.
Edited by:
Elwira Sieniawska, Medical University of Lublin, PolandReviewed by:
Andrey Stoyanov Marchev, Stephan Angelov Institute of Microbiology, BulgariaŁukasz Pecio, Institute of Soil Science and Plant Cultivation, Poland
Copyright © 2022 Emamzadeh Yazdi, Heyman, Prinsloo, Klimkait and Meyer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simin Emamzadeh Yazdi, simin_ey31@yahoo.com
 Simin Emamzadeh Yazdi
Simin Emamzadeh Yazdi