- 1School of Pharmacy, Lanzhou University, Lanzhou, China
- 2Department of Pathology, Yale University School of Medicine, New Haven, CT, United States
- 3School of Pharmacy, University of Connecticut, Mansfield, CT, United States
- 4School of Basic Medical Science, Lanzhou University, Lanzhou, China
Gastrodiae Rhizoma and its active constituents are known to exhibit neuroprotective effects in Alzheimer’s disease (AD). However, the effect of Rhizoma Gastrodiae water extract (WERG) on AD and the detailed mechanism of action remain unclear. In this study, the mechanism of action of WERG was investigated by the microbiome–gut–brain axis using a D-galactose (D-gal)/AlCl3-induced AD mouse model. WERG improved the cognitive impairment of D-gal/AlCl3-induced mice. The expression level of p-Tauthr231 in the WERG-H treatment group was decreased, and p-Tauthr231 was found negative in hippocampal DG, CA1, and CA3 regions. Here, the diversity and composition of the gut microbiota were analyzed by 16sRNA sequencing. WERG-H treatment had a positive correlation with Firmicutes, Bacilli, Lactobacillus johnsonii, Lactobacillus murinus, and Lactobacillus reuteri. Interestingly, the Rikenellaceae-RC9 gut group in the gut increased in D-gal/AlCl3-induced mice, but the increased L. johnsonii, L. murinus, and L. reuteri reversed this process. This may be a potential mechanistic link between gut microbiota dysbiosis and P-TauThr231 levels in AD progression. In conclusion, this study demonstrated that WERG improved the cognitive impairment of the AD mouse model by enriching gut probiotics and reducing P-TauThr231 levels.
Introduction
Along with the rapid increase in the elderly population (≥ 65 years) worldwide, Alzheimer’s disease (AD) is now considered a major global public health threat and causes a huge economic burden (Prince et al., 2015; Li et al., 2021). AD, a primary degenerative brain disease, is caused by synaptic lesions and neuronal loss (An et al., 2017). The disease is clinically characterized by amyloid plaques and neurofibrillary tangles (NFT), as well as progressive cognitive impairment and memory loss (Prakash and Kumar, 2014). Intraneuronal accumulation of hyperphosphorylated tau is a hallmark pathology shown in over 20 neurodegenerative disorders, which is collectively termed tauopathies, including AD (Zheng et al., 2021). Numerous studies have shown that abnormal hyperphosphorylated tau protein plays an important role in the occurrence and development of neurodegeneration and learning, and memory impairment in AD (Sahara et al., 2010). Therefore, selectively removing or reducing hyperphosphorylated tau is promising for therapies of AD and other tauopathies. However, due to the complex etiology and pathogenesis, there is currently no strategy for specifically targeting tau phosphorylation.
To date, the traditional Chinese herbal medicine has several thousand years’ history as a drug for AD in oriental countries (Chang et al., 2015). Rhizoma Gastrodiae is a perennial parasitic herbal with neuroprotective activities (Zhan et al., 2016) and has shown that it has positive effects on the central nervous system, cardiovascular system, and immune system (Lee et al., 2012; Shu et al., 2013). Some components from Rhizoma Gastrodiae, such as gastrodin, have been reported to suppress inflammation and attenuate liver injury by modulating gut microbiota (Liu et al., 2021a; Ma et al., 2021). Homogeneous polysaccharide GEP-1 can promote the growth of Akkermansia muciniphila (A. muciniphila) and Lacticaseibacillus paracasei (L. paracasei) strains (Huo et al., 2021). Rhizoma Gastrodiae water extract (WERG) modulates neurotransmitters and alters the gut microbiota in a depression mouse model (Huang et al., 2021). Multiple probiotics grew after taking fresh Rhizoma Gastrodiae extract, including Ruminiclostridium, Butyricicoccus, and Parvibacter (Hua et al., 2019). It produced a positive regulation on the mouse gut microbiota (Hua et al., 2019). However, the anti-Alzheimer’s effects of WERG in AD mouse models were little studied. This study aimed to explore the health-promoting effects of long-term WERG intervention on the AD mouse model.
Gut microbiota, also known as “the second brain,” can regulate brain function (Hsiao et al., 2013). Growing evidence suggested that there is an association between the gut–brain axis and AD progression. The gut microbiota affects the brain and behavior through pathways such as the vagus nerve, microbial metabolites, immune stimulation, enteroendocrine cells, the enteric nervous system, and neurotransmitters (Nagpal and Cryan, 2021). Gut microbiota can promote AD pathology, cognitive impairment, and microglial activation in AD mice (Chen et al., 2022). Meanwhile, gut microbiota affects various complex behaviors, including emotional, social, and anxiety-like behaviors (Hsiao et al., 2013). Previous studies reported that the traditional Chinese medicine prescription “Huanglian Jiedu Decoction” could reverse the cognitive impairment of Tg mice, reshape the gut microbiota structure of Tg mice, and enrich the population of short-chain fatty acid-producing gut microbiota (Gu et al., 2021). GV-971 is a new drug for Alzheimer's disease originally developed in China and the first in the world targeting the brain–gut axis. Studies have shown that it can significantly improve the memory dysfunction of animals caused by tau phosphorylation, Aβ deposition, and neuroinflammation (Rao, 2020). Mannan oligosaccharides alleviated cognitive and behavioral impairments in 5xFAD Alzheimer’s mice by modulating the gut–microbiota–brain axis (Liu et al., 2021b). However, the role of gut microbiota in AD pathogenesis remains unclear. D-Galactose/aluminum chloride (D-gal/AlCl3) can induce AD-like symptoms (Zhang et al., 2016a; Wei et al., 2017). Animals exposed to long-term D-gal show aging-related changes such as elevated oxidant levels and cognitive impairment (Lei et al., 2011; Yang et al., 2013). Furthermore, intraperitoneal injection (i.p) of D-gal resulted in increased levels of acetylcholinesterase in the brains of rats (Gao et al., 2016). Aluminum has been linked to the pathogenesis of AD (Kaizer et al., 2008). Accumulating evidence showed that co-administration of D-gal/AlCl3 to rats impaired their cognitive functions, increased AChE activities, altered oxidative balance, and induced neurodegeneration (Zhang et al., 2016b; Li et al., 2016; Chiroma et al., 2018). As a consequence, this study evaluated the health-promoting effects of WERG on D-gal/AlCl3-induced mice via the microbiome–gut–brain axis, and provides a theoretical basis and a new perspective for the development and utilization of WERG.
Materials and Methods
Chemicals and Materials
Rhizoma Gastrodiae comes from Yangba, Kang County, Gansu Province, China. Yangba Town is located 84 km southeast of Kang County. Accurately 10.0 g of Gastrodia elata was weighed, the appropriate amount of distilled water soaked for 1 h was added and boiled three times (each time for 30 min), frying was stopped and the mixture was cooled to room temperature, gauze was filtered, the filtrate was combined, and the filtrate was concentrated to 1.0 ml of crude drug per ml of distilled water decoction. Oxiracetam (99%) was purchased from McLean Biochemical Technology Co., Ltd., Shanghai, China.
Animals
All mice were housed 10 per cage and maintained under 12 h light–dark cycle, temperature (23 ± 1°C), humidity (60% ± 10%), and SPF conditions with free access to food and water. The protocol was approved by the guidelines of the Lanzhou Institute of Animal Science.
Experimental Design and Drug Treatment in D-gal and AlCl3-Induced Mice
SPF-grade two-month-old mice were divided into six groups with eight mice in each group, the first two months: 1) control group (distilled water + physiological saline solution); (2–6) treated groups (120 mg/kg D-galactose + 10 mg/kg AlCl3 daily); three-month: 1) control group (distilled water + physiological saline solution daily); 2) D-gal + AlCl3 group (120 mg/kg D-galactose + 10 mg/kg AlCl3 daily); 3) D-gal + Oxira group (120 mg/kg D-galactose + 10 mg/kg AlCl3 + 289.0 mg/kg oxiracetam daily); 4) D-gal + WERG-L group (120 mg/kg D-galactose + 10 mg/kg AlCl3 + 100 mg/kg WERG daily); 5) D-gal + WERG-M group (120 mg/kg D-galactose + 10 mg/kg AlCl3 + 200 mg/kg WERG daily); 6) D-gal + WERG-H group (120 mg/kg D-galactose + 10 mg/kg AlCl3 + 300 mg/kg WERG daily). Oxiracetam was chosen as a positive control. Figure 1A showed the experimental design and drug treatment schedule.
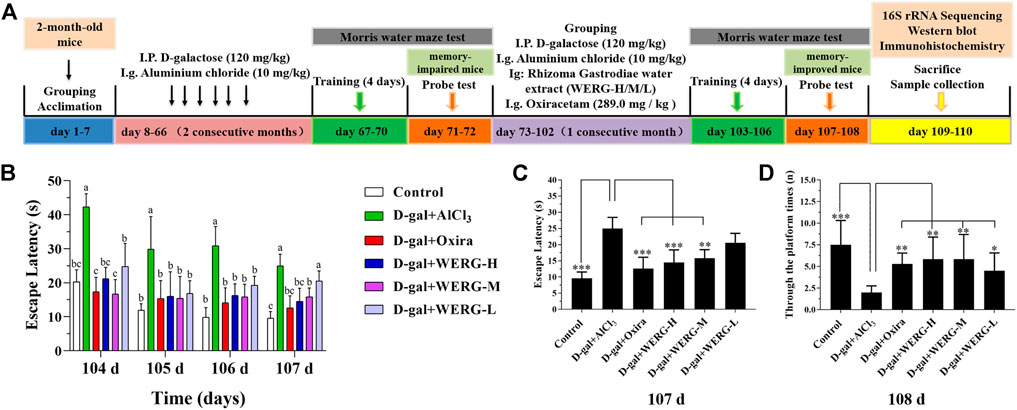
FIGURE 1. WERG-H improved cognitive impairment in AD mouse model. Control, D-gal 120 + AlCl3 10 mg/kg. bwt (D-gal + AlCl3), D-gal 120 + AlCl3 10 + Oxiracetam 298 mg/kg. bwt (D-gal + Oxira), D-gal 120 + AlCl3 10 + Rhizoma Gastrodiae water extract 300 mg/kg. bwt (D-gal + WERG-H), D-gal 120 + AlCl3 10 + Rhizoma Gastrodiae water extract 200 mg/kg. bwt (D-gal + WERG-M), D-gal 120 + AlCl3 10 + Rhizoma Gastrodiae water extract 100 mg/kg. bwt (D-gal + WERG-L). Experimental protocols and prevention strategies (A); the escape latency during training (104–107 days) (B); escape latency (107 days) (C); after removal of the platform, through the platform times (90 s) (D). Data were expressed as mean ± SD (n = 8). “*” presented significant difference at p < 0.05 levels, “**” presented significant difference at p < 0.01 level, “***” presented significant difference at p < 0.001 level.
Morris Water Maze Test
MWMT conditions are as follows: diameter 1.5 m, water depth 21 cm, platform diameter 8 cm, height 20 cm, water temperature 25°C, and milky white water. Navigation test: Mice were randomly placed into the pool and let to swim for 60 s to find the hidden platform. The time required for the mouse to climb on the platform was used as escape latency and stayed for 15 s. If the platform could not be found within 60 s, the escape latency was recorded as 60 s, and it was placed on the platform for 15 s. According to this method, each animal was trained twice a day for four consecutive days. Probe test: After the platform was removed, mice were randomly placed into the water for 90 s, and the number of original platform crossings was recorded.
Western Blot
Western blots were carried out as the previously described method (Cui et al., 2019) with some modifications. Total proteins were extracted from hippocampus tissues using RIPA lysis buffer (MCE, Shanghai, China). The primary antibody was purchased from Bioss (Bioss, Beijing, China, 1:500). Goat-anti-rabbit IgG secondary antibody was used as the secondary antibody (Bioss, Beijing, China, 1:3000). The grayscale analysis of Western blot results was evaluated by ImageJ software.
Hematoxylin and Eosin Staining and Immunohistochemical Staining
Immunohistochemistry was carried out as the previously described method (Zhang et al., 2020) with some modifications. For immunohistochemical staining, the sections were incubated with P-tau (Thr231) antibody (Bioss, Beijing, China, 1:300) and GFAP antibody (Bioss, Beijing, China, 1:200) overnight at 4°C. After washing, the sections were incubated with the appropriate secondary antibody (Bioss, Beijing, China) for 60 min at 25°C. Finally, these sections were observed by using a fluorescence digital photo microscope (OLYMPUS, Japan).
16 S rRNA Sequencing
The genomic DNA of feces was extracted using the CTAB/SDS method, and then the purity and concentration of DNA were detected by agarose gel electrophoresis. An appropriate amount of the sample was taken in a centrifuge tube, and the sample was diluted to 1 ng/μl with sterile water. PCR products were detected by electrophoresis on a 2% agarose gel. Equal amounts of samples were mixed according to the concentration of PCR products, 2% agarose gel electrophoresis was used to detect PCR products after mixing thoroughly, and the target bands were recovered. The TruSeq® DNA PCR-Free Sample Preparation Kit was used for library construction. The constructed library was quantified by Qubit and Q-PCR. After the library was qualified, Illumina HiSeq2500 PE250 was used for on-machine sequencing.
Statistical Analysis
Data are presented as mean ± SEM. The experimental data were analyzed using SPSS version 22.0. A p-value < 0.05 was considered to be statistically significant, and Duncan’s statistical procedure was performed.
Results
WERG Treatment Ameliorated D-gal/AlCl3-Induced Cognitive Impairment and Changes in p-TauThr231 Protein Expression
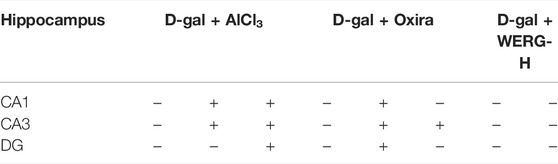
To investigate the improving effects of WERG on the D-gal/AlCl3-induced mice, two cognition-related indicators were examined in mice. The escape latency of each group was improved in a time-dependent manner during the training process, and the D-gal/AlCl3 group was significantly different from the control group, indicating that the AD mouse model induced by D-gal/AlCl3 was effective (Figure 1B). After training, the escape latency at 107 days was significantly different between the treated and D-gal/AlCl3 groups, especially in D-gal + WERG-H and D-gal + Oxira groups (p < 0.001) (Figure 1C). The D-gal + WERG-H and D-gal + Oxira groups also had an increased number of through the platform times compared with the D-gal/AlCl3 group (p < 0.01) (Figure 1D). As shown in Figure 2A, the results of HE staining showed that a large number of swollen neurons with loosen structure, karyopyknosis, and other morphological changes could be observed in hippocampus neurons of CA3 and DG regions in the D-gal/AlCl3 group. When compared with the D-gal/AlCl3 group, the pathological changes of hippocampus neurons were ameliorated in the D-gal + WERG-H group, and the tissue cells in the DG and CA3 regions of the hippocampus were generally lighter in staining, with clear cell boundaries and neat arrangement. In addition, CA1 region hippocampus neurons had no obvious pathological changes in the two groups. Western blot analysis indicated that the levels of p-TauThr231 in the D-gal + WERG-H group were decreased compared with those of the D-gal/AlCl3 group (p < 0.01); WERG down-regulated the levels of p-TauThr231 in a dose-dependent manner (Figure 2B). We further investigated the expression levels of p-TauThr231 by immunohistochemistry, and a semi-quantitative analysis was performed (Figure 3). The distribution pattern of p-TauThr231 in the CA1, CA3, and DG regions of four treatment groups was examined (Table 1). The number of p-TauThr231-positive cells in the D-gal + AlCl3 group was significantly increased compared with that in the control group, and the arrangement was scattered, the overall staining was darker, and the cytoplasm of the cells was brown. The plaques were significantly increased and darker than those in the WERG-H group. Compared with the D-gal + AlCl3 group, the number of positive cells in the WERG-H group decreased, the brown plaques became lighter, and the cells were compactly arranged.
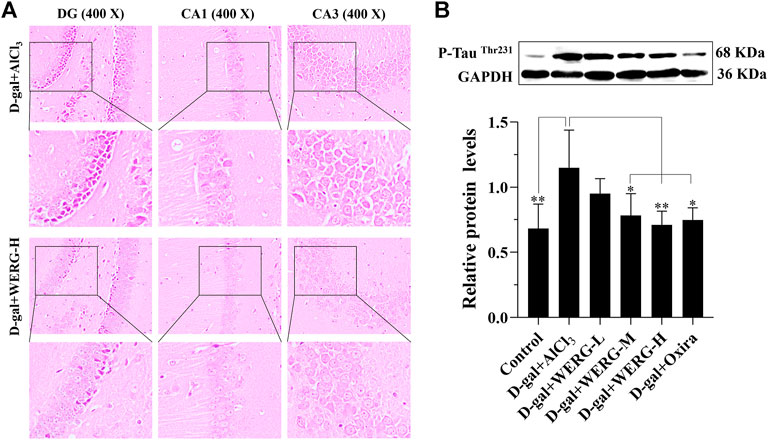
FIGURE 2. WERG decreased hippocampus neuron damage in an AD mouse model. Pathological changes in the hippocampus CA1, CA3, and DG regions were detected by HE staining (400 ×) (A); the expression of p-TauThr231 was quantitatively analyzed by Western blotting (B). Data were presented as mean ± SD repeated three times. “*” presented significant difference at p < 0.05 levels, “**” presented significant difference at p < 0.01 level.
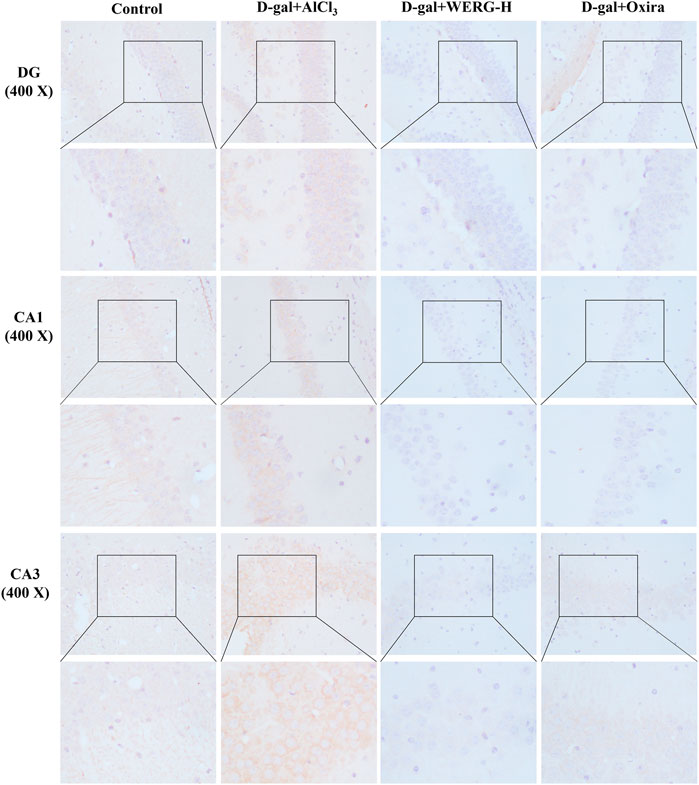
FIGURE 3. Th1e expressions of p-TauThr231 were measured by immunohistochemistry. The sections of the DG, CA1, and CA3 regions were acquired using a fluorescence digital photo microscope (OLYMPUS, Japan) at × 400 magnification (scale bar, 100 μm).
The Diversity and Richness of Gut Microbiota Was Changed by WERG
Given gut microbiota configurations relate to AD progression; the effect of WERG on the alterations of the intestinal bacterial structure was addressed in WERG-treated mice. Alpha diversity was used to analyze microbial community diversity, which can reflect the richness and diversity of microbial communities within a fecal sample. To assess the effect of WERG on the gut microbiota of D-gal/AlCl3-induced AD-like model mice, gut contents were analyzed by 16 S rRNAV3-4 gene sequencing. After WERG treatment for a consecutive month, the observed species number of WERG-H (p = 0.046) and D-gal + Oxira (p = 0.035) groups were reduced significantly compared with the D-gal + AlCl3 group (Figure 4A). The Shannon index of control (p = 0.044) and D-gal + WERG-H (p = 0.016) groups were lower than those of the D-gal/AlCl3 group (Figure 4B). The Chao1 index of the WERG-H group also was significantly lower than that of the D-gal + AlCl3 group (p = 0.046) (Figure 4C). The Simpson index of the control group (p = 0.019), D-gal + Oxira (p = 0.009), D-gal + WERG-M (p = 0.04), and D-gal + WERG-H (p = 0.045) groups were significantly decreased compared with that of the D-gal + AlCl3 group (Figure 4D). The species accumulation curve (Figure 4F) showed that as the number of samples increases, there will be a greater possibility of discovering a large number of new species; it seems that WERG-H treatment tended to correct the gut microbial disorder tendency. These results indicated that WERG-H treatment could reduce the alpha diversity and abundance of microbes in the D-gal/AlCl3-induced AD mouse model and improve the disturbance of gut microbiota.
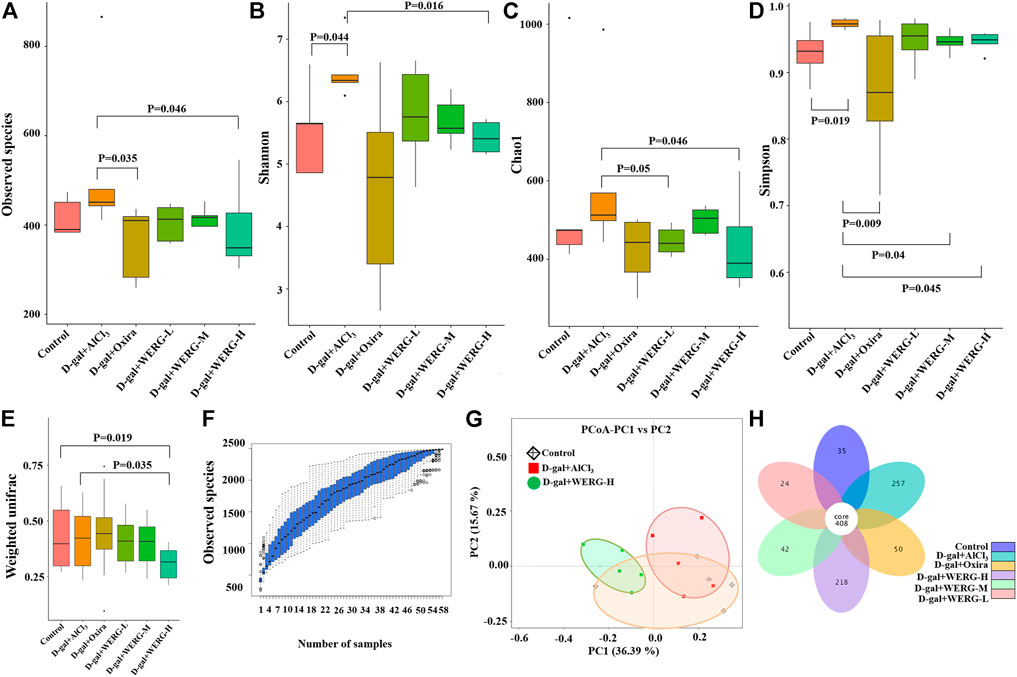
FIGURE 4. Diversity and richness analysis of WERG-H on gut microbiota in D-gal/AlCl3-induced AD mice. The observed species number (A); Shannon diversity index (B); Chao1 diversity index (C); Simpson diversity index (D); weighted UniFrac analysis (E); the species accumulation curve (F); PCoA based on weighted UniFrac distances (G); flower diagrams (H). n = 5 mice per group.
Next, the samples were assessed for beta diversity by using PCoA to investigate differences in microbiota composition in the control, D-gal + AlCl3, and D-gal + WERG-H groups (Figure 4G), which showed that gut microbial community among the three groups formed distinct clusters. The D-gal + AlCl3 and D-gal + WERG-H groups were well separated with 36.39% and 15.67% variation by the principal components PC1 and PC2, respectively. Weighted UniFrac analysis revealed that D-gal + AlCl3 treatment drove a marked difference in gut microbiota composition, whereas WERG-H treatment (p = 0.035) significantly reduced the alterations (Figure 4E). As shown in Figure 4H, the number of OTUs shared by the six groups is 408, and the number of unique OTUs in the D-gal + WERG-H group was 218. As expected, the petal plot showed that the WERG-H treatment group had more specific OTUs than the other treatment groups.
WERG Restores Gut Microbiome Imbalances in the AD Mouse Model
The relative abundances at phylum and genus levels were analyzed. The Phylum level analysis revealed that the relative abundance of Bacteroidetes was significantly lower in the D-gal + AlCl3 group than that in the control group, while the relative abundances of Saccharibacteria, Actinobacteria, Cyanobacteria, Acidobacteria, and Deferribacteres were significantly increased (Figure 5A). In addition, the relative abundances of Bacteroidetes, Proteobacteria, and Saccharibacteria in the D-gal + Oxira and D-gal + WERG-H groups were significantly decreased compared to those in the D-gal + AlCl3 group, while the relative abundances of Firmicutes (p = 0.012) and Bacilli (p = 0.011) were significantly increased. The Rikenellaceae-RC9 gut group (p < 0.001) was enriched in the D-gal + AlCl3 group compared with the treatment group (Figure 5C). The relative abundances at the genus levels were further analyzed (Figure 5B), which showed that the relative abundances of Lactobacillus, Turicibacter, Helicobacter, and Alloprevotella were significantly decreased in the D-gal + AlCl3 group compared to the control group, while the relative abundances of Bacteroides, Ruminococcaceae_UCG-014, Candidatus_Saccharimonas, Staphylococcus, unidentified_Erysipelotrichaceae, Lachnospiraceae_NK4A136_group, Sporosarcina, Parabacteroides, Streptococcus, Desulfovibrio, Sphingomonas, Serratia, Jeotgalicoccus, Anaerotruncus, Roseburia, Rikenella, and Enteractinococcus were increased. However, the relative abundances of Bacteroides, Candidatus_Saccharimonas, Lachnospiraceae_NK4A136_group, Sporosarcina, Parabacteroides, and Desulfovibrio have decreased in the D-gal + Oxira and D-gal + WERG-H groups compared to the D-gal + AlCl3 group, while the relative abundances of Lactobacillus, Turicibacter, and Staphylococcus were markedly increased. As shown in Figure 5D, the effect of WERG treatment on the relative abundance of gut microbial taxa in an AD mouse model was analyzed according to MetaStat. The relative abundances of Lactobacillus-mucosae, Lactobacillus-johnsonii, and Lactobacillus-reuteri in the D-gal + WERG-H group were increased compared with the other treatment group. Then, a linear discriminatory analysis (LDA) effect size (LEfSe) analysis was performed to further determine whether specific individual bacterial taxa were differentially enriched in the D-gal + WERG-H group. As shown in Figure 5E, this analysis identified nine genera, which were differentially abundant between the D-gal + WERG-H and D-gal + AlCl3 groups. The results showed that s-Lactobacillus-murinus, g-Ruminococcus-torques-group, s-Lactobacillus-intestinalis, o-Bacillales, f-Staphylococcaceae, g-Staphylococcus, and s-Staphylococcus-lentus were enriched in the WERG-H treatment group.
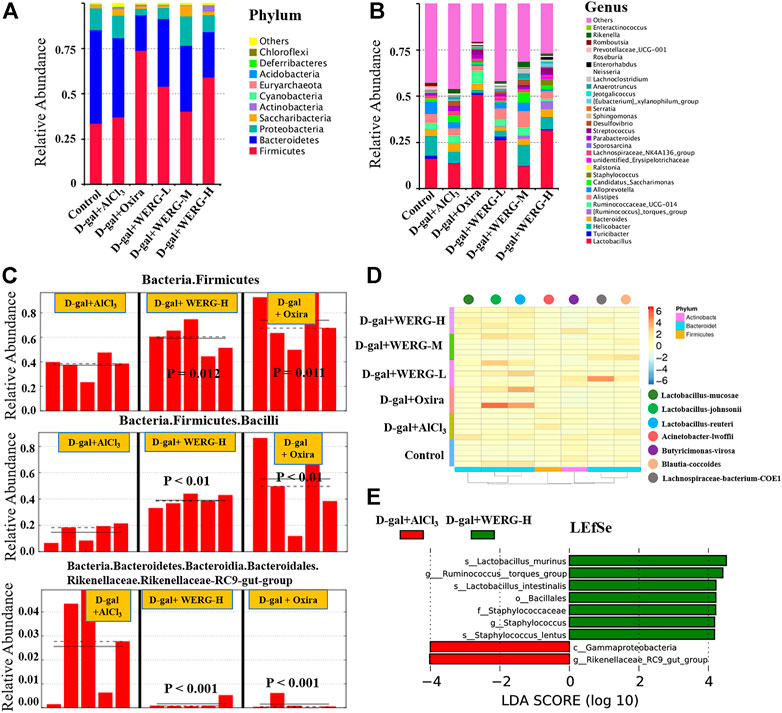
FIGURE 5. WERG-H alleviated gut microbiota dysbiosis in AlCl3/D-gal-induced AD mice. The relative abundance at the phyla level (A); the relative abundance at the genus level (B); biomarker raw images in the sample (C); heatmap analysis of microbial composition (D); LEfSe analysis (E). n = 5 mice per group.
WERG-H Modulated Specific Phylotypes of Gut Microbiome and Increased the Probiotic Species in the AD Mouse Model
LEfSe analysis was further performed to identify statistically significant biomarkers of gut microbiota in different groups. The Linear discriminatory analysis (LDA) score distribution histogram (based on LDA score > 4) and Cladograms analysis were conducted, and a series of biomarkers were identified as shown by the cladogram (Figures 6A,B). A total of 11 OUTs were notably different among all groups. In the D-gal + Oxira group, there were two OUTs and the p-Firmicutes and s-Lactobacillus-murinus were the obvious difference. The three OUTs of f-Rikenellaceae, g-Rikenlla, and g-Anaerotruncu showed remarkable differences in the D-gal + WERG-M group. The most prominent different features in the D-gal + WERG-H group were five OUTs, namely, g-Ruminococcus-torques-group, o-Bacillales, f-Staphylococcaceae, g-Staphylococcus, and s-Staphylococcus-lentus. There was a g-Rikenellaceae-RC9 gut group that exhibited a conspicuous difference in the D-gal + AlCl3 group. It can be seen from Figures 7A–D that there are significant differences in colony distribution between the control group and the D-gal + AlCl3 group (p = 0.048), the D-gal + Oxira group and the D-gal + AlCl3 group (p = 0.017), the D-gal + WERG-M group and the D-gal + AlCl3 group (p = 0.046), and the D-gal + WERG-H group and the D-gal + AlCl3 group (p = 0.046). Ternary plot analysis was used to display common flora or OTUs in three groups. The distribution of species in the D-gal + Oxira and D-gal + WERG-H groups was further analyzed using ternary plots. The results showed that the main enriched species include Lactobacillus-johnsonii, Lactobacillus-murinus, Lactobacillus-reuteri, Staphylococcus-lentus, Firmicutes-bacterium-M10-2, Lactobacillus-intestinalis, Bacteroides-vulgatus, Bacteroides-acidifaciens, Helicobacter-sp-MIT-01–6451, and Streptococcus-hyointestinalis. In general, the three probiotics of Lactobacillus-johnsonii, Lactobacillus-murinus, and Lactobacillus-reuteri were significantly enriched in the D-gal + Oxira and the D-gal + WERG-H groups, which were located in the upper part of the ternary graph (Figures 7E,F). Multiple comparisons were further corrected to show significant differences between D-gal + AlCl3 and D-gal + WERG-H groups at the species levels (Figure 7G). When compared with the D-gal + AlCl3 group, the Lactobacillus-johnsonii (p = 0.022) and Lactobacillus-murinus (p = 0.027) were significantly enriched in the D-gal + WERG-H group.
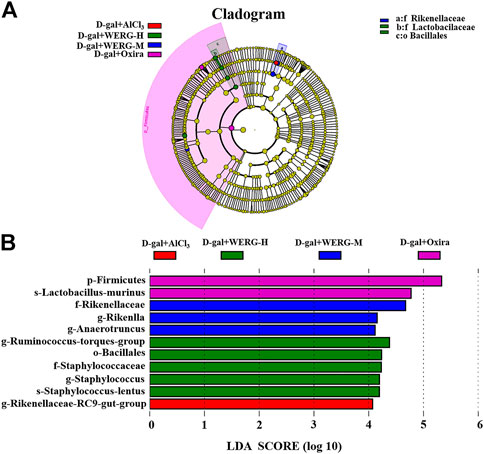
FIGURE 6. Gut microbiota differences. Cladograms reveal microbial phylogenetic branches associated with treatment groups status in the AD mouse model (A); linear discriminant analysis (LDA) (B). Statistical significance reflects both p < 0.05 for Student’s t test and LDA score threshold > 4 was listed, n = 5 mice per group.
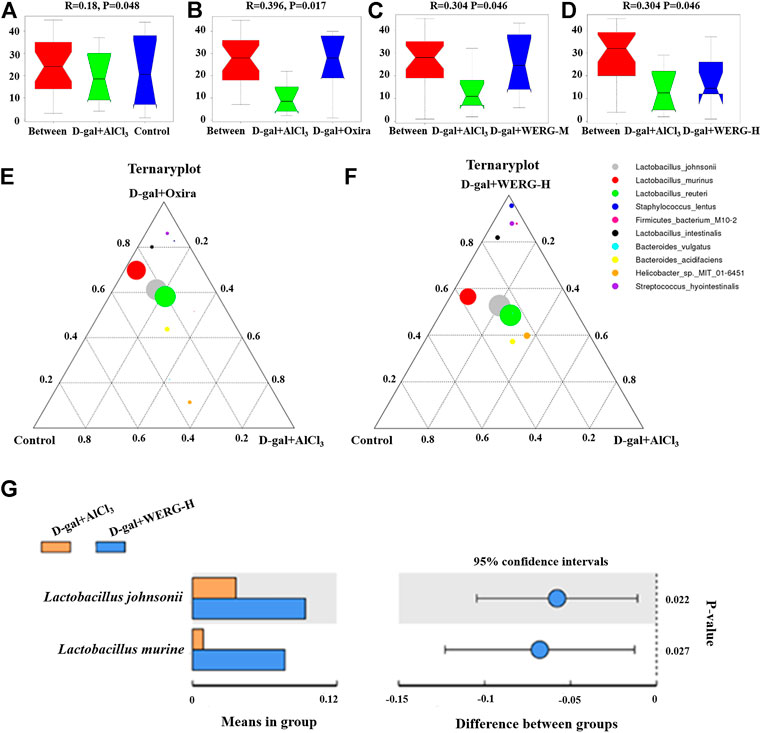
FIGURE 7. WERG-H treatment increased the probiotic species. Anosim analysis results (A–D); ternary plot (E,F); t-test analysis (G). Significant statistical difference by Student’s t-test (p < 0.05). n = 5 mice per group.
Discussion
Currently, amyloid plaques (Aβ) and neurofibrillary tangles (p-Tau) are two typical pathological features in AD pathogenesis (West and Bhugra, 2015). However, the pathogenesis of AD remains unclear. In this study, our main findings are the associations between gut microbiota composition and p-TauThr231 status. To our knowledge, we are the first to report an association between this microbe and AD biomarkers. Tau hyperphosphorylation causes most tau lesions including AD (Mazanetz and Fischer, 2007). Hyperphosphorylated tau was accumulated in the intracellular region and caused neurofibrillary tangles, dysregulated neuronal excitability (Hatch et al., 2017), impaired synaptic plasticity, and neurotransmittance, thus inducing learning and memory impairments. Due to the limited efficiency of new drugs for clearing β-amyloid in AD, tau protein has received more attention as a promising therapeutic target (Panza et al., 2019).
Gut microbiota composition was associated with amyloid and p-tau status. For instance, the abundance of SCFA-producing microorganisms is inversely proportional to the positive rate of amyloid and p-tau status (Verhaar et al., 2021). Animal studies have reported significantly reduced SCFA-producing microbes in AD mice when compared to wild-type mice (Zhang et al., 2017; Sun et al., 2019). Transplantation of fecal microbiota from wild-type mice to APP/PS1 and ADLPAPT mice resulted in a reduction in amyloid, suggesting a causal link between gut microbes and AD (Sun et al., 2019; Kim et al., 2020). In addition, an SCFA, sodium butyrate intervention can reduce AD pathology (Fernando et al., 2020). In this study, we developed a novel tau-based therapeutic strategy, which may provide early treatment of AD and related tau lesions before abnormal tau accumulation.
Inflammation and oxidative damage, two potential triggers for AD symptoms, can cause brain damage and induce impairments in synaptic function and memory (Zheng et al., 2019). D-gal/AlCl3 can cause oxidative stress damage, and further develop many other dysfunctions of the central nervous system by generating ROS and inducing neurodegeneration (Rehman et al., 2017; Wang et al., 2019). Previous studies have shown that D-gal/AlCl3 can cause decreased memory and learning abilities, Aβ deposition, and enhanced p-tau expression, and provide an effective non-transgenic AD-like injury model (Zhang et al., 2016a; An et al., 2017; Chiroma et al., 2018). This study mainly investigated the neuroprotective effect of WERG in D-gal/AlCl3-induced AD mice. Substantially, WERG-H significantly alleviated D-gal/AlCl3-stimulated cognitive impairment, p-TauThr231 protein formation, and pathological changes. Consistent with the present study, gastrodin significantly inhibited lead-induced p-Tau accumulation in the mouse brain (Liu et al., 2020). Similarly, another study confirmed that gastrodin suppressed the deposition of p-Tau in the brain of the unilateral intracerebroventricular injection of the Aβ1-42 mouse model (Luo et al., 2022). Moreover, WERG treatment reduced corticosterone, adrenocorticotrophic hormone, hypothalamic corticotropin-releasing factor, and glucocorticoid receptor levels, and decreased plasma interleukin-1β, interleukin-6, and tumor necrosis factor-α concentrations (Wang et al., 2020a). Rhizoma Gastrodiae powder can significantly improve the learning and cognitive ability of mice in the radiation water maze, and the learning and memory impairment in aluminum chloride-induced rats (Shuchang et al., 2008; Mishra et al., 2011). Rhizoma Gastrodiae water extract (WERG) can improve the learning and memory impairment caused by forced swimming in rats and shorten the dark avoidance latency and platform-seeking time of rats in the MWMT (Chen et al., 2011). Accumulating research suggested that WERG can improve memory and learning cognitive dysfunction (Hu et al., 2014; Park et al., 2015; Liu et al., 2018).
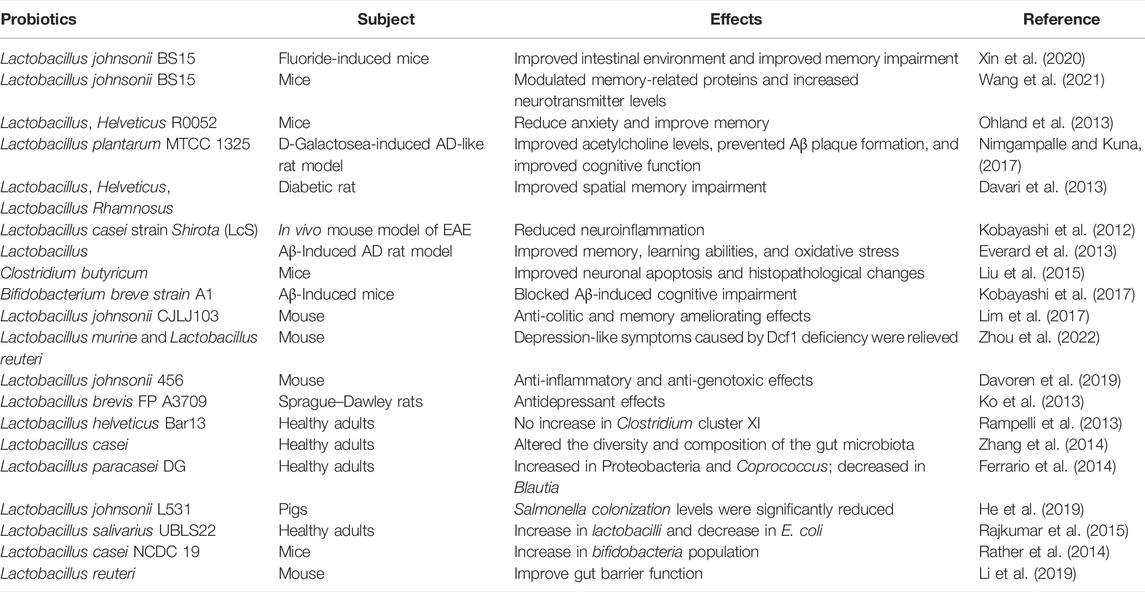
Gut microbiota exerted an important influence on the progression of AD. Gut barrier permeability may be altered by exogenous or endogenous factors as a consequence of the inflammatory process in AD (Wang et al., 2020b). In short, a decreased number of beneficial bacteria and an increased number of pathogenic bacteria led to a disturbance in the composition of the gut microbiota in AD mice. LEfSe can be used to find biomarkers of differences between groups in high-dimensional data. In this study, the D-gal/AlCl3 treatment group had differential biomarkers in the g-Rikenellaceae-RC9 gut group (p < 0.001) and c-Gammaproteobacteria (p < 0.05) at the genus level. A previous study suggested that several specific differential biomarkers were found to be significantly associated with improvements in host parameters, and linolenic acid ameliorated HFD-induced multi-tissue metabolic disorders and gut microbiota disorders. Among them, the Rikenellaceae-RC9 gut group was positively correlated with HFD-induced harmful indicators and negatively correlated with a beneficial indicator (Gao et al., 2020). At the genus level, the Rikenellaceae-RC9 gut group was more abundant in fecal samples from PD patients (Yan et al., 2021). In addition, another study showed that c-Gammaproteobacteria gradually increased from healthy control patients to amnestic mild cognitive impairment patients and then AD patients (Liu et al., 2019). These results provide a preliminary basis for the mining of biomarkers in AD. It has been reported that the abundance of pathogenic bacteria is increased, while the abundance of beneficial bacteria is decreased in Aβ42-induced AD mice (Xu et al., 2020). In the present study, our results showed that the WERG-H treated group had differential biomarkers in L. johnsonii (p = 0.022) and Lactobacillus murine (p = 0.027), and enriched in Lactobacillus-reuteri. The above results showed a stimulatory effect of water extracts from Gastrodiae Rhizoma on probiotic growth at optimal dosage. The D-gal + Oxira group is also mainly enriched in Lactobacillus-johnsonii, Lactobacillus-murinus, and Lactobacillus-reuteri. Studies have reported that the traditional Chinese medicine prescription “Huanglian Jiedu Decoction” can reverse the cognitive impairment of Tg mice and increase Bacteroides S24-7 and Lactobacillus in Tg mice (Gu et al., 2021). As shown in Table 2, many studies have reported the beneficial effects of probiotics on neurological disorders and gut microbiota. A previous study showed that L. johnsonii BS15 intake can improve intestinal inflammation, neuroinflammation, and fluorine-induced and restraint stress-induced memory dysfunction by improving inflammation and permeability (Xin et al., 2020; Wang et al., 2021). In addition, L. johnsonii CJLJ103 was able to alleviate colitis and memory impairment by inhibiting NF-jB activation and intestinal lipopolysaccharide production (Lim et al., 2017). L. johnsonii 456 is associated with reduced inflammation and genotoxicity in vertebrate models (Davoren et al., 2019). Furthermore, probiotic L. johnsonii L531 can promote SCFA production to control Salmonella infection (He et al., 2019). L. murine and L. reuteri intestinal transplantation improved depression-like symptoms caused by Dcf1 deficiency (Zhou et al., 2022). These results suggest that WERG-H treatment can ameliorate intestinal metabolic disturbances by increasing the abundance of probiotics, thereby exerting anti-AD effects by remodeling the gut microbiota and reducing p-tau levels (Figure 8). Regrettably, this study has not yet explored the active ingredients that play a major role in WERG, and will focus on the research on the active ingredients and their mechanism of action on AD later.
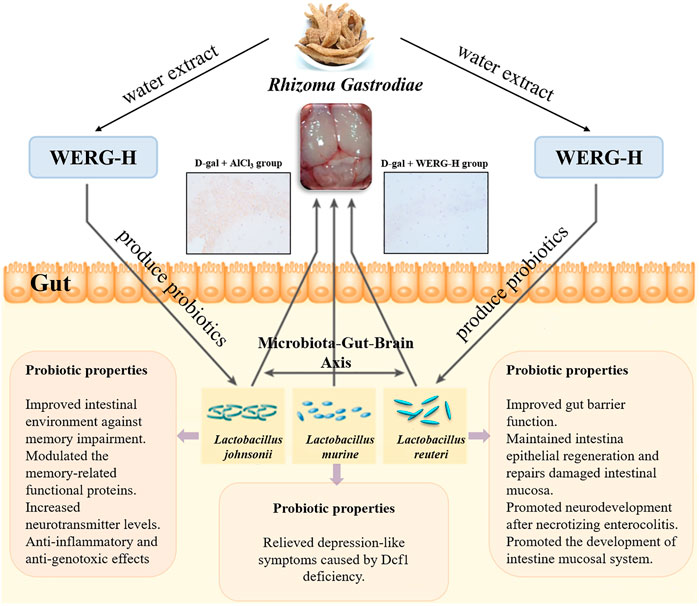
FIGURE 8. Beneficial effects of WERG-H against AD model mouse may be due to inhibition of p-TauThr231 protein expression, amelioration of p-TauThr231-induced toxicity, and alleviation of gut microbiota dysbiosis by enriching probiotics. Finally, through the microbiota–gut–brain axis to improve D-gal/AlCl3-induced cognitive impairment.
Conclusion
Gut microbiota composition was associated with p-tau status. Our study showed observed associations between L. johnsonii, L. murine, and Lactobacillus-reuteri levels and AD biomarkers by showing that higher abundances of probiotic microbes were associated with lower odds of positive p-tau status.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA541119, PRJNA541132, PRJNA541161, PRJNA541165.
Ethics Statement
The animal study was reviewed and approved by the Lanzhou Institute of Animal Science.
Author Contributions
WZ: experimental analysis, Data integration, writing—original draft, and writing—review and editing. JW: methodology. ML: methodology. CW: investigation and methodology. YL and WM: investigation. ZZ: methodology. SH: methodology. YL: methodology. PC: methodology, experimental guidance, funding acquisition, supervision, review, and editing.
Funding
This work was supported by the Key Research and Development Program of Gansu Province (Grant No. 21YF5FA112), the Technological Innovation Guidance Program of Gansu Province (Grant No. 21CX6QA127), the Key Program for International S&T Cooperation Projects of China Gansu Province (18YF1WA115), National College Students' Innovation and Entrepreneurship Training Program (Grant No. 202210730011), the College Students' Innovation and Entrepreneurship Program of Lanzhou University, China (Grant No. 20220260010) and Innovation and Entrepreneurship Training Program of Lanzhou University, China (Grant No. cxcy202207).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors acknowledge Nuohe Zhiyuan Biotechnology Co., Ltd. for their technical support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.903659/full#supplementary-material
References
An, S., Lu, W., Zhang, Y., Yuan, Q., and Wang, D. (2017). Pharmacological Basis for Use of Armillaria Mellea Polysaccharides in Alzheimer's Disease: Antiapoptosis and Antioxidation. Oxid. Med. Cell. Longev. 2017, 4184562. doi:10.1155/2017/4184562
Cao, Z., Wang, P., Gao, X., Shao, B., Zhao, S., and Li, Y. (2019). Lycopene Attenuates Aluminum-Induced Hippocampal Lesions by Inhibiting Oxidative Stress-Mediated Inflammation and Apoptosis in the Rat. J. Inorg. Biochem. 193, 143–151. doi:10.1016/j.jinorgbio.2019.01.017
Chang, C. J., Lin, C. S., Lu, C. C., Martel, J., Ko, Y. F., Ojcius, D. M., et al. (2015). Ganoderma Lucidum Reduces Obesity in Mice by Modulating the Composition of the Gut Microbiota. Nat. Commun. 6, 7489. doi:10.1038/ncomms8489
Chen, C., Liao, J., Xia, Y., Liu, X., Jones, R., Haran, J., et al. (2022). Gut Microbiota Regulate Alzheimer’s Disease Pathologies and Cognitive Disorders via PUFA-Associated Neuroinflammation. Gut, 1–20. doi:10.1136/gutjnl-2021-326269
Chen, P. J., Liang, K. C., Lin, H. C., Hsieh, C. L., Su, K. P., Hung, M. C., et al. (2011). Gastrodia Elata Bl. Attenuated Learning Deficits Induced by Forced-Swimming Stress in the Inhibitory Avoidance Task and Morris Water Maze. J. Med. Food 14, 610–617. doi:10.1089/jmf.2010.1209
Chiroma, S. M., Mohd Moklas, M. A., Mat Taib, C. N., Baharuldin, M. T. H., and Amon, Z. (2018). D-Galactose and Aluminium Chloride Induced Rat Model with Cognitive Impairments. Biomed. Pharmacother. 103, 1602–1608. doi:10.1016/j.biopha.2018.04.152
Cui, J. H., Dong, S. M., Chen, C. X., Xiao, W., Cai, Q. C., Zhang, L. D., et al. (2019). Microplitis Bicoloratus Bracovirus Modulates Innate Immune Suppression through the eIF4E-eIF4A axis in the Insect Spodoptera Litura. Dev. Comp. Immunol. 95, 101–107. doi:10.1016/j.dci.2019.02.010
Davari, S., Talaei, S. A., Alaei, H., and Salami, M. (2013). Probiotics Treatment Improves Diabetes-Induced Impairment of Synaptic Activity and Cognitive Function: Behavioral and Electrophysiological Proofs for Microbiome-Gut-Brain axis. Neuroscience 240, 287–296. doi:10.1016/j.neuroscience.2013.02.055
Davoren, M. J., Liu, J., Castellanos, J., Rodríguez-Malavé, N. I., and Schiestl, R. H. (2019). A Novel Probiotic, Lactobacillus Johnsonii 456, Resists Acid and Can Persist in the Human Gut beyond the Initial Ingestion Period. Gut Microbes 10, 458–480. doi:10.1080/19490976.2018.1547612
Everard, A., Belzer, C., Geurts, L., Ouwerkerk, J. P., Druart, C., Bindels, L. B., et al. (2013). Cross-talk between Akkermansia Muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. U. S. A. 110, 9066–9071. doi:10.1073/pnas.1219451110
Fernando, W. M. A. D. B., Martins, I. J., Morici, M., Bharadwaj, P., Rainey-Smith, S. R., Lim, W. L. F., et al. (2020). Sodium Butyrate Reduces Brain Amyloid-β Levels and Improves Cognitive Memory Performance in an Alzheimer's Disease Transgenic Mouse Model at an Early Disease Stage. J. Alzheimers Dis. 74, 91–99. doi:10.3233/JAD-190120
Ferrario, C., Taverniti, V., Milani, C., Fiore, W., Laureati, M., De Noni, I., et al. (2014). Modulation of Fecal Clostridiales Bacteria and Butyrate by Probiotic Intervention with Lactobacillus Paracasei DG Varies Among Healthy Adults. J. Nutr. 144, 1787–1796. doi:10.3945/jn.114.197723
Gao, J., Zhou, R., You, X., Luo, F., He, H., Chang, X., et al. (2016). Salidroside Suppresses Inflammation in a D-Galactose-Induced Rat Model of Alzheimer's Disease via SIRT1/NF-Κb Pathway. Metab. Brain Dis. 31, 771–778. doi:10.1007/s11011-016-9813-2
Gao, X., Chang, S., Liu, S., Peng, L., Xie, J., Dong, W., et al. (2020). Correlations between α-Linolenic Acid-Improved Multitissue Homeostasis and Gut Microbiota in Mice Fed a High-Fat Diet. mSystems 5. doi:10.1128/mSystems.00391-20
Gu, X., Zhou, J., Zhou, Y., Wang, H., Si, N., Ren, W., et al. (2021). Huanglian Jiedu Decoction Remodels the Periphery Microenvironment to Inhibit Alzheimer's Disease Progression Based on the "Brain-Gut" axis through Multiple Integrated Omics. Alzheimers Res. Ther. 13, 44. doi:10.1186/s13195-021-00779-7
Hatch, R. J., Wei, Y., Xia, D., and Götz, J. (2017). Hyperphosphorylated Tau Causes Reduced Hippocampal CA1 Excitability by Relocating the Axon Initial Segment. Acta Neuropathol. 133, 717–730. doi:10.1007/s00401-017-1674-1
He, T., Zhu, Y. H., Yu, J., Xia, B., Liu, X., Yang, G. Y., et al. (2019). Lactobacillus Johnsonii L531 Reduces Pathogen Load and Helps Maintain Short-Chain Fatty Acid Levels in the Intestines of Pigs Challenged with Salmonella enterica Infantis. Vet. Microbiol. 230, 187–194. doi:10.1016/j.vetmic.2019.02.003
Hsiao, E. Y., McBride, S. W., Hsien, S., Sharon, G., Hyde, E. R., McCue, T., et al. (2013). Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell. 155, 1451–1463. doi:10.1016/j.cell.2013.11.024
Hu, Y., Li, C., and Shen, W. (2014). Gastrodin Alleviates Memory Deficits and Reduces Neuropathology in a Mouse Model of Alzheimer's Disease. Neuropathology 34, 370–377. doi:10.1111/neup.12115
Hua, Z. Y., Hong, M., Li, H. M., Sun, J. H., Huo, H. R., Li, X. Q., et al. (2019). Effect of Fresh Gastrodia Elata on Gut Microbiota in Mice. Zhongguo Zhong Yao Za Zhi 44, 1004–1009. doi:10.19540/j.cnki.cjcmm.2019.0018
Huang, Y. J., Choong, L. C., Panyod, S., Lin, Y. E., Huang, H. S., Lu, K. H., et al. (2021). Gastrodia Elata Blume Water Extract Modulates Neurotransmitters and Alters the Gut Microbiota in a Mild Social Defeat Stress-Induced Depression Mouse Model. Phytother. Res. 35, 5133–5142. doi:10.1002/ptr.7091
Huo, J., Lei, M., Li, F., Hou, J., Zhang, Z., Long, H., et al. (2021). Structural Characterization of a Polysaccharide from Gastrodia Elata and its Bioactivity on Gut Microbiota. Molecules 26. doi:10.3390/molecules26154443
Kaizer, R. R., Corrêa, M. C., Gris, L. R., da Rosa, C. S., Bohrer, D., Morsch, V. M., et al. (2008). Effect of Long-Term Exposure to Aluminum on the Acetylcholinesterase Activity in the Central Nervous System and Erythrocytes. Neurochem. Res. 33, 2294–2301. doi:10.1007/s11064-008-9725-6
Kim, M. S., Kim, Y., Choi, H., Kim, W., Park, S., Lee, D., et al. (2020). Transfer of a Healthy Microbiota Reduces Amyloid and Tau Pathology in an Alzheimer's Disease Animal Model. Gut 69, 283–294. doi:10.1136/gutjnl-2018-317431
Ko, C. Y., Lin, H.-T. V., and Tsai, G. J. (2013). Gamma-aminobutyric Acid Production in Black Soybean Milk by Lactobacillus Brevis FPA 3709 and the Antidepressant Effect of the Fermented Product on a Forced Swimming Rat Model. Process Biochem. 48, 559–568. doi:10.1016/j.procbio.2013.02.021
Kobayashi, T., Suzuki, T., Kaji, R., Serata, M., Nagata, T., Ando, M., et al. (2012). Probiotic Upregulation of Peripheral IL-17 Responses Does Not Exacerbate Neurological Symptoms in Experimental Autoimmune Encephalomyelitis Mouse Models. Immunopharmacol. Immunotoxicol. 34, 423–433. doi:10.3109/08923973.2010.617755
Kobayashi, Y., Sugahara, H., Shimada, K., Mitsuyama, E., Kuhara, T., Yasuoka, A., et al. (2017). Therapeutic Potential of Bifidobacterium Breve Strain A1 for Preventing Cognitive Impairment in Alzheimer's Disease. Sci. Rep. 7, 13510. doi:10.1038/s41598-017-13368-2
Lee, O. H., Kim, K. I., Han, C. K., Kim, Y. C., and Hong, H. D. (2012). Effects of Acidic Polysaccharides from Gastrodia Rhizome on Systolic Blood Pressure and Serum Lipid Concentrations in Spontaneously Hypertensive Rats Fed a High-Fat Diet. Int. J. Mol. Sci. 13, 698–709. doi:10.3390/ijms13010698
Lei, Y., Fu, W., Chen, J., Xiong, C., Wu, G., Wei, H., et al. (2011). Neuroprotective Effects of Abacopterin E from Abacopteris Penangiana against Oxidative Stress-Induced Neurotoxicity. J. Ethnopharmacol. 134, 275–280. doi:10.1016/j.jep.2010.10.062
Li, H., Kang, T., Qi, B., Kong, L., Jiao, Y., Cao, Y., et al. (2016). Neuroprotective Effects of Ginseng Protein on PI3K/Akt Signaling Pathway in the hippocampus of D-galactose/AlCl3 Inducing Rats Model of Alzheimer's Disease. J. Ethnopharmacol. 179, 162–169. doi:10.1016/j.jep.2015.12.020
Li, S., Qi, C., Zhu, H., Yu, R., Xie, C., Peng, Y., et al. (2019). Lactobacillus Reuteri Improves Gut Barrier Function and Affects Diurnal Variation of the Gut Microbiota in Mice Fed a High-Fat Diet. Food Funct. 10, 4705–4715. doi:10.1039/c9fo00417c
Li, Z., Zhang, Z., Ren, Y., Wang, Y., Fang, J., Yue, H., et al. (2021). Aging and Age-Related Diseases: from Mechanisms to Therapeutic Strategies. Biogerontology 22, 165–187. doi:10.1007/s10522-021-09910-5
Lim, S.-M., Jang, H.-M., Jeong, J.-J., Han, M. J., and Kim, D.-H. (2017). Lactobacillus Johnsonii CJLJ103 Attenuates Colitis and Memory Impairment in Mice by Inhibiting Gut Microbiota Lipopolysaccharide Production and NF-Κb Activation. J. Funct. Foods 34, 359–368. doi:10.1016/j.jff.2017.05.016
Liu, C. M., Tian, Z. K., Zhang, Y. J., Ming, Q. L., Ma, J. Q., and Ji, L. P. (2020). Effects of Gastrodin against Lead-Induced Brain Injury in Mice Associated with the Wnt/Nrf2 Pathway. Nutrients 12. doi:10.3390/nu12061805
Liu, F. Y., Wen, J., Hou, J., Zhang, S. Q., Sun, C. B., Zhou, L. C., et al. (2021a). Gastrodia Remodels Intestinal Microflora to Suppress Inflammation in Mice with Early Atherosclerosis. Int. Immunopharmacol. 96, 107758. doi:10.1016/j.intimp.2021.107758
Liu, J., Sun, J., Wang, F., Yu, X., Ling, Z., Li, H., et al. (2015). Neuroprotective Effects of Clostridium Butyricum against Vascular Dementia in Mice via Metabolic Butyrate. Biomed. Res. Int. 2015, 412946. doi:10.1155/2015/412946
Liu, P., Wu, L., Peng, G., Han, Y., Tang, R., Ge, J., et al. (2019). Altered Microbiomes Distinguish Alzheimer's Disease from Amnestic Mild Cognitive Impairment and Health in a Chinese Cohort. Brain Behav. Immun. 80, 633–643. doi:10.1016/j.bbi.2019.05.008
Liu, Q., Xi, Y., Wang, Q., Liu, J., Li, P., Meng, X., et al. (2021b). Mannan Oligosaccharide Attenuates Cognitive and Behavioral Disorders in the 5xFAD Alzheimer's Disease Mouse Model via Regulating the Gut Microbiota-Brain axis. Brain Behav. Immun. 95, 330–343. doi:10.1016/j.bbi.2021.04.005
Liu, Y., Gao, J., Peng, M., Meng, H., Ma, H., Cai, P., et al. (2018). A Review on Central Nervous System Effects of Gastrodin. Front. Pharmacol. 9, 24. doi:10.3389/fphar.2018.00024
Luo, K., Wang, Y., Chen, W. S., Feng, X., Liao, Y., Chen, S., et al. (2022). Treatment Combining Focused Ultrasound with Gastrodin Alleviates Memory Deficit and Neuropathology in an Alzheimer's Disease-like Experimental Mouse Model. Neural Plast. 2022, 5241449. doi:10.1155/2022/5241449
Ma, S., Sun, Y., Zheng, X., and Yang, Y. (2021). Gastrodin Attenuates Perfluorooctanoic Acid-Induced Liver Injury by Regulating Gut Microbiota Composition in Mice. Bioengineered 12, 11546–11556. doi:10.1080/21655979.2021.2009966
Mazanetz, M. P., and Fischer, P. M. (2007). Untangling Tau Hyperphosphorylation in Drug Design for Neurodegenerative Diseases. Nat. Rev. Drug Discov. 6, 464–479. doi:10.1038/nrd2111
Mishra, M., Huang, J., Lee, Y. Y., Chua, D. S., Lin, X., Hu, J. M., et al. (2011). Gastrodia Elata Modulates Amyloid Precursor Protein Cleavage and Cognitive Functions in Mice. Biosci. Trends 5, 129–138. doi:10.5582/bst.2011.v5.3.129
Nagpal, J., and Cryan, J. F. (2021). Microbiota-brain Interactions: Moving toward Mechanisms in Model Organisms. Neuron 109, 3930–3953. doi:10.1016/j.neuron.2021.09.036
Nimgampalle, M., and Kuna, Y. (2017). Anti-Alzheimer Properties of Probiotic, Lactobacillus Plantarum MTCC 1325 in Alzheimer's Disease Induced Albino Rats. J. Clin. Diagn. Res. 11, KC01–KC05. doi:10.7860/JCDR/2017/26106.10428
Ohland, C. L., Kish, L., Bell, H., Thiesen, A., Hotte, N., Pankiv, E., et al. (2013). Effects of Lactobacillus Helveticus on Murine Behavior Are Dependent on Diet and Genotype and Correlate with Alterations in the Gut Microbiome. Psychoneuroendocrinology 38, 1738–1747. doi:10.1016/j.psyneuen.2013.02.008
Panza, F., Lozupone, M., Logroscino, G., and Imbimbo, B. P. (2019). A Critical Appraisal of Amyloid-β-Targeting Therapies for Alzheimer disease. Nat. Rev. Neurol. 15, 73–88. doi:10.1038/s41582-018-0116-6
Park, Y. M., Lee, B. G., Park, S. H., Oh, H. G., Kang, Y. G., Kim, O. J., et al. (2015). Prolonged Oral Administration of Gastrodia Elata Extract Improves Spatial Learning and Memory of Scopolamine-Treated Rats. Lab. Anim. Res. 31, 69–77. doi:10.5625/lar.2015.31.2.69
Prakash, A., and Kumar, A. (2014). Implicating the Role of Lycopene in Restoration of Mitochondrial Enzymes and BDNF Levels in β-amyloid Induced Alzheimer׳s Disease. Eur. J. Pharmacol. 741, 104–111. doi:10.1016/j.ejphar.2014.07.036
Prince, M., Wimo, A., Guerchet, M., Ali, G. C., and Prina, M. (2015). World Alzheimer Report 2015. Glob. Impact Dementia Analysis Preval. Incidence, Cost Trends.
Rajkumar, H., Kumar, M., Das, N., Kumar, S. N., Challa, H. R., and Nagpal, R. (2015). Effect of Probiotic Lactobacillus Salivarius UBL S22 and Prebiotic Fructo-Oligosaccharide on Serum Lipids, Inflammatory Markers, Insulin Sensitivity, and Gut Bacteria in Healthy Young Volunteers: A Randomized Controlled Single-Blind Pilot Study. J. Cardiovasc Pharmacol. Ther. 20, 289–298. doi:10.1177/1074248414555004
Rampelli, S., Candela, M., Severgnini, M., Biagi, E., Turroni, S., Roselli, M., et al. (2013). A Probiotics-Containing Biscuit Modulates the Intestinal Microbiota in the Elderly. J. Nutr. Health Aging 17, 166–172. doi:10.1007/s12603-012-0372-x
Rao, Y. (2020). Omission of Previous Publications by an Author Should Be Corrected. Cell. Res. 30, 819. doi:10.1038/s41422-020-0344-3
Rather, S. A., Pothuraju, R., Sharma, R. K., De, S., Mir, N. A., and Jangra, S. (2014). Anti-obesity Effect of Feeding Probiotic Dahi containingLactobacillus caseiNCDC 19 in High Fat Diet-Induced Obese Mice. Int. J. Dairy Technol. 67, 504–509. doi:10.1111/1471-0307.12154
Rehman, S. U., Shah, S. A., Ali, T., Chung, J. I., and Kim, M. O. (2017). Anthocyanins Reversed D-Galactose-Induced Oxidative Stress and Neuroinflammation Mediated Cognitive Impairment in Adult Rats. Mol. Neurobiol. 54, 255–271. doi:10.1007/s12035-015-9604-5
Sahara, N., Maeda, S., Murayama, M., Suzuki, T., Dohmae, N., Yen, S. H., et al. (2010). Assembly of Two Distinct Dimers and Higher-Order Oligomers from Full-Length Tau. Eur. J. Neurosci. 25, 3020–3029. doi:10.1111/j.1460-9568.2007.05555.x
Shu, G., Yang, T., Wang, C., Su, H., and Xiang, M. (2013). Gastrodin Stimulates Anticancer Immune Response and Represses Transplanted H22 Hepatic Ascitic Tumor Cell Growth: Involvement of NF-Κb Signaling Activation in CD4+ T Cells. Toxicol. Appl. Pharmacol. 269, 270–279. doi:10.1016/j.taap.2013.02.019
Shuchang, H., Qiao, N., Piye, N., Mingwei, H., Xiaoshu, S., Feng, S., et al. (2008). Protective Effects of Gastrodia Elata on Aluminium-Chloride-Induced Learning Impairments and Alterations of Amino Acid Neurotransmitter Release in Adult Rats. Restor. Neurol. Neurosci. 26, 467–473.
Sun, J., Xu, J., Ling, Y., Wang, F., Gong, T., Yang, C., et al. (2019). Fecal Microbiota Transplantation Alleviated Alzheimer's Disease-like Pathogenesis in APP/PS1 Transgenic Mice. Transl. Psychiatry 9, 189. doi:10.1038/s41398-019-0525-3
Verhaar, B. J. H., Hendriksen, H. M. A., de Leeuw, F. A., Doorduijn, A. S., van Leeuwenstijn, M., Teunissen, C. E., et al. (2021). Gut Microbiota Composition Is Related to AD Pathology. Front. Immunol. 12, 794519. doi:10.3389/fimmu.2021.794519
Wang, H., He, S., Xin, J., Zhang, T., Sun, N., Li, L., et al. (2021). Psychoactive Effects of Lactobacillus Johnsonii against Restraint Stress-Induced Memory Dysfunction in Mice through Modulating Intestinal Inflammation and Permeability-A Study Based on the Gut-Brain Axis Hypothesis. Front. Pharmacol. 12, 662148. doi:10.3389/fphar.2021.662148
Wang, J., Zhang, T., Liu, X., Fan, H., and Wei, C. (2019). Aqueous Extracts of Se-Enriched Auricularia Auricular Attenuates D-Galactose-Induced Cognitive Deficits, Oxidative Stress and Neuroinflammation via Suppressing RAGE/MAPK/NF-κB Pathway. Neurosci. Lett. 704, 106–111. doi:10.1016/j.neulet.2019.04.002
Wang, M., Dong, W., Wang, R., Xu, X., Wu, Y., Sun, G., et al. (2020a). Gastrodiae Rhizoma Water Extract Ameliorates Hypothalamic-Pituitary-Adrenal Axis Hyperactivity and Inflammation Induced by Chronic Unpredictable Mild Stress in Rats. Biomed. Res. Int. 2020, 8374614. doi:10.1155/2020/8374614
Wang, Y., An, Y., Ma, W., Yu, H., Lu, Y., Zhang, X., et al. (2020b). 27-Hydroxycholesterol Contributes to Cognitive Deficits in APP/PS1 Transgenic Mice through Microbiota Dysbiosis and Intestinal Barrier Dysfunction. J. Neuroinflammation. 17, 199. doi:10.1186/s12974-020-01873-7
Wei, Y., Liu, D., Zheng, Y., Li, H., Hao, C., and Ouyang, W. (2017). Protective Effects of Kinetin against Aluminum Chloride and D-Galactose Induced Cognitive Impairment and Oxidative Damage in Mouse. Brain Res. Bull. 134, 262–272. doi:10.1016/j.brainresbull.2017.08.014
West, S., and Bhugra, P. (2015). Emerging Drug Targets for Aβ and Tau in Alzheimer's Disease: a Systematic Review. Br. J. Clin. Pharmacol. 80, 221–234. doi:10.1111/bcp.12621
Xin, J., Zeng, D., Wang, H., Sun, N., Khalique, A., Zhao, Y., et al. (2020). Lactobacillus Johnsonii BS15 Improves Intestinal Environment against Fluoride-Induced Memory Impairment in Mice-A Study Based on the Gut-Brain axis Hypothesis. PeerJ 8, e10125. doi:10.7717/peerj.10125
Xu, M., Mo, X., Huang, H., Chen, X., Liu, H., Peng, Z., et al. (2020). Yeast β-glucan Alleviates Cognitive Deficit by Regulating Gut Microbiota and Metabolites in Aβ1-42-Induced AD-like Mice. Int. J. Biol. Macromol. 161, 258–270. doi:10.1016/j.ijbiomac.2020.05.180
Yan, Z., Yang, F., Cao, J., Ding, W., Yan, S., Shi, W., et al. (2021). Alterations of Gut Microbiota and Metabolome with Parkinson's Disease. Microb. Pathog. 160, 105187. doi:10.1016/j.micpath.2021.105187
Yang, W. N., Han, H., Hu, X. D., Feng, G. F., and Qian, Y. H. (2013). The Effects of Perindopril on Cognitive Impairment Induced by D-Galactose and Aluminum Trichloride via Inhibition of Acetylcholinesterase Activity and Oxidative Stress. Pharmacol. Biochem. Behav. 114-115, 31–36. doi:10.1016/j.pbb.2013.10.027
Zhan, H. D., Zhou, H. Y., Sui, Y. P., Du, X. L., Wang, W. H., Dai, L., et al. (2016). The Rhizome of Gastrodia Elata Blume - an Ethnopharmacological Review. J. Ethnopharmacol. 189, 361–385. doi:10.1016/j.jep.2016.06.057
Zhang, J., Wang, L., Guo, Z., Sun, Z., Gesudu, Q., Kwok, L., et al. (2014). 454 Pyrosequencing Reveals Changes in the Faecal Microbiota of Adults Consuming Lactobacillus Casei Zhang. FEMS Microbiol. Ecol. 88, 612–622. doi:10.1111/1574-6941.12328
Zhang, L., Wang, Y., Xiayu, X., Shi, C., Chen, W., Song, N., et al. (2017). Altered Gut Microbiota in a Mouse Model of Alzheimer's Disease. J. Alzheimers Dis. 60, 1241–1257. doi:10.3233/JAD-170020
Zhang, W., Hua, H., Guo, Y., Cheng, Y., Pi, F., Yao, W., et al. (2020). Torularhodin from Sporidiobolus Pararoseus Attenuates D-galactose/AlCl3-Induced Cognitive Impairment, Oxidative Stress, and Neuroinflammation via the Nrf2/NF-Κb Pathway. J. Agric. Food Chem. 68, 6604–6614. doi:10.1021/acs.jafc.0c01892
Zhang, Y., Pi, Z., Song, F., and Liu, Z. (2016a). Ginsenosides Attenuate D-Galactose- and AlCl3-Inducedspatial Memory Impairment by Restoring the Dysfunction of the Neurotransmitter Systems in the Rat Model of Alzheimer's Disease. J. Ethnopharmacol. 194, 188–195. doi:10.1016/j.jep.2016.09.007
Zhang, Y., Yang, X., Jin, G., Yang, X., and Zhang, Y. (2016b). Polysaccharides from Pleurotus Ostreatus Alleviate Cognitive Impairment in a Rat Model of Alzheimer's Disease. Int. J. Biol. Macromol. 92, 935–941. doi:10.1016/j.ijbiomac.2016.08.008
Zheng, J., Tian, N., Liu, F., Zhang, Y., Su, J., Gao, Y., et al. (2021). A Novel Dephosphorylation Targeting Chimera Selectively Promoting Tau Removal in Tauopathies. Signal Transduct. Target Ther. 6, 269. doi:10.1038/s41392-021-00669-2
Keywords: Rhizoma Gastrodiae water extract, Alzheimer’s disease, P-Tau protein, gut microbiota, Lactobacillus johnsonii, Lactobacillus murinus, Lactobacillus reuteri
Citation: Zhao W, Wang J, Latta M, Wang C, Liu Y, Ma W, Zhou Z, Hu S, Chen P and Liu Y (2022) Rhizoma Gastrodiae Water Extract Modulates the Gut Microbiota and Pathological Changes of P-TauThr231 to Protect Against Cognitive Impairment in Mice. Front. Pharmacol. 13:903659. doi: 10.3389/fphar.2022.903659
Received: 24 March 2022; Accepted: 09 June 2022;
Published: 15 July 2022.
Edited by:
Andleeb Khan, Jazan University, Saudi ArabiaReviewed by:
Mashoque Ahmad Rather, Annamalai University, IndiaSidharth Mehan, Indo-Soviet Friendship College of Pharmacy, India
Copyright © 2022 Zhao, Wang, Latta, Wang, Liu, Ma, Zhou, Hu, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Chen, Y2hlbnBlbmdAbHp1LmVkdS5jbg==; Yingqian Liu, eXFsaXVAbHp1LmVkdS5jbg==
 Wenbin Zhao
Wenbin Zhao Jianhui Wang
Jianhui Wang Maria Latta3
Maria Latta3 Yuheng Liu
Yuheng Liu Wantong Ma
Wantong Ma Zhongkun Zhou
Zhongkun Zhou Shujian Hu
Shujian Hu Peng Chen
Peng Chen Yingqian Liu
Yingqian Liu