- 1Beijing Key Laboratory for HIV/AIDS Research, Center for Infectious Diseases, Beijing Youan Hospital, Capital Medical University, Beijing, China
- 2Travel Clinic, Center for Infectious Diseases, Beijing Youan Hospital, Capital Medical University, Beijing, China
Objectives: To compare the benefits and risks between Rapid ART and standard/delayed treatment for HIV.
Methods: Databases of PubMed, Cochrane Library, Embase and Web of science were searched from the inception to 28 October 2021. Two investigators independently screened studies related to Rapid ART, extracted data, and evaluated the literature quality. The risk of bias was assessed by Cochrane Collaboration Risk of Bias Tool and the statistical software Stata15.0 was used for meta-analysis.
Results: Ten eligible studies were included in this meta-analysis, the results showed Rapid ART was superior to standard/delayed treatment in continuing care for at least 8 months (RR = 1.13, 95%CI: 1.03∼1.25, Z = 2.44, p = 0.015), and severe bacterial infection (RR = 0.42, 95%CI: 0.25∼0.70, Z = 3.33, p = 0.001). At 12 months following treatment, there was no statistically significant difference in viral load <100 copies/mL (RR = 1.05, 95%CI: 0.80∼1.39, Z = 0.35, p = 0.726), mortality (RR = 0.77, 95%CI: 0.47∼1.24, Z = 1.09, p = 0.277), or the incidence of adverse events (RR = 0.52, 95%CI: 0.16∼1.76, Z = 1.05, p = 0.294) compared with standard/delayed treatment.
Conclusion: In comparison to standard/delayed treatment, rapid ART can reduce the incidence of TB and severe bacterial infections in HIV patients. Our findings suggest that rapid ART should be utilized when clinical conditions and the patient’s physical state allow.
Systematic Review Registration: [https://inplasy.com/?s=202210004], identifier [INPLASY202210004].
Introduction
The World Health Organization (WHO) estimates that 37.7 million people globally will be diagnosed with HIV by 2020. Antiretroviral therapy (ART) has been shown to significantly decrease the mortality and transmission of HIV. By the end of 2020, 73% (56–88%) of HIV-infected people were receiving antiretroviral therapy, up from 25% (18–30%) in 2010. However, there were still 1.5 million HIV infections and approximately 68,000 deaths worldwide in 2020 (WHO, 2021).
In 2015, based on the START and TEMPRANO trial data, WHO recommended rapid initiation of ART regardless of CD4+ T cell count, that is, starting treatment within 7 days after diagnosis and encouraging the initiation on the same day (Wilkinson et al., 2015). Currently, there is no uniform and clear definition of the time of Rapid ART. Immediate and same-day ART should refer to within 24 h after diagnosis, but due to practical reasons, such as the complexity of providing health care services or formulating diagnostic examination protocols, the actual initiation time may be 5, 7, 14, 28 days or longer after diagnosis. For example, the START protocol stipulates that the rapid initiation group should start treatment within 60 days (Lundgren et al., 2015).
The CD4+ T lymphocytes of normal adults are 500∼1,600/µL, while in HIV-infected patients, there is a progressive or irregular decline in CD4+ T lymphocytes. In most international guidelines, the threshold of CD4+ T lymphocytes for initiating ART was defined as 350/mm3 for the years 2006–2009 and increased to 500/mm3 in 2009–2013. An important step was taken between 2012 and 2015 to recommend treatment regardless of the CD4+ T lymphocyte count (Eholié et al., 2016). As the result, several international trials defined immediate treatment as Rapid ART, standard treatment as CD4+ T lymphocyte threshold of 500/mm3, and delayed treatment as CD4+ T lymphocyte threshold of 350/mm (Lundgren et al., 2015; Borges et al., 2016; Eholié et al., 2016; Boatman et al., 2019; Amstutz et al., 2020; Maskew et al., 2020).
Continuing care in AIDS treatment is an ongoing challenge to global public health (Rosen and Fox, 2011), and is the key to ensuring the success of ART. Currently, there is still controversy over the care retention rate of Rapid ART. A systematic review based on randomized controlled trials showed that Rapid ART improved care retention for 12 months (Mateo-Urdiales et al., 2019), while an observational study in southern Africa found a lower care retention rate (Joseph Davey et al., 2020), which may be related to the living standards.
HIV viral load measures the amount of virus in the blood, used to monitor the level of virus replication and the effectiveness of ART. The goal of treatment is to reduce the viral load in the blood to an undetectable level (less than 50 copies/mL) (Mateo-Urdiales et al., 2019). However, the thresholds used to define virus inhibition in different settings and periods are inconsistent. The CASCADE HIV trial defined viral inhibition as viral load <100 copies/mL (Labhardt et al., 2018; Amstutz et al., 2020), while in several HIV trials in the United States, viral inhibition was defined as viral load <200 copies/mL (Pilcher et al., 2017; Bacon et al., 2020; McNulty et al., 2020).
HIV weakens a person’s immunity to opportunistic infections, such as tuberculosis and fungal infections, severe bacterial infections, and certain cancers. The leading causes of death among adults with advanced HIV globally include tuberculosis (TB), severe bacterial infection, cryptococcal meningitis, toxoplasmosis, and pneumocystis carinii pneumonia (PCP) (Ford et al., 2015; Low et al., 2016). Several systematic reviews and meta-analyses utilized mortality and continuing care as outcome measures, but the conclusions are controversial (Ford et al., 2018; Mateo-Urdiales et al., 2019; Long et al., 2020). On this basis, mortality, continuing care, adverse events, viral load <100 copies/mL at 12 months following treatment, tuberculosis, and severe bacterial infections were included as outcome measures in this meta-analysis.
Rapid ART has been recommended for the treatment of HIV, but its efficacy and safety remain controversial. Therefore, we conducted this systematic review and meta-analysis to assess the advantages and disadvantages of Rapid ART against standard/delayed treatment and to offer a reliable reference for active treatment decisions.
Methods
Meta Registration
This meta-analysis was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. The protocol for the study has been submitted to INPLASY PROTOCOL (registration number INPLASY202210004).
Research Objective
The English databases were searched for publicly published studies comparing Rapid ART and standard/delayed treatment in HIV patients, with grey literature excluded.
Literature Inclusion and Exclusion Criteria
Literature inclusion criteria: 1) Rapid ART was administered to the intervention group (immediate/same-day diagnosis and treatment), while the control group was treated with standard/delayed treatment; 2) Articles in English only; 3) No restrictions on gender, age and region.
Literature exclusion criteria: 1) Non-English literature; 2) Duplicate, irrelevant literature and unavailable original literature; 3) Interventions to prevent mother-to-child transmission and improve the initiation of antiretroviral therapy in pregnant women; 4) Individuals receiving antiretroviral therapy for reasons other than the treatment of HIV infection (e.g., ART for post-exposure prophylaxis and pre-exposure prophylaxis), and studies including patients with co-infection who were not recommended to receive rapid ART treatment for clinical reasons (e.g., cryptococcal meningitis); 5) The minimum sample size was defined as at least 20 patients.
Literature Retrieval
The literature was collected from English databases such as PubMed, Cochrane Library, Embase, and Web of Science, with the search period set to 28 October 2021. The retrieval strategy used was subject terms + free words, and the subject keywords in PubMed were HIV: “HIV” [Mesh], and anti-retrovirus: “Anti-Retroviral Agents” (Mesh), and the free words without subject terms were “immediate” (Title/Abstract) and “same-day” (Title/Abstract).
Literature Screening and Data Extraction
In our investigation, the entire process of literature screening was conducted by two independent researchers. The first stage of screening was conducted according to the title and abstract to eliminate non-randomized controlled trials, and studies with inconsistent experimental interventions, lack of controls, or failing to match the theme. Then, the included articles were screened according to the full text, and finally the included literature of this study was selected.
Using standardized forms, two researchers independently retrieved data from the included articles. The retrieved data primarily consisted of: basic study information (first author, publication year, country, etc.), patient information (number of patients, gender ratio, mean age), interventions, and outcome measures. The two researchers cross-checked after completing the information extraction, and any disagreement was adjudicated by a third researcher Supplementary Data Sheet 1.
Outcome Measures
Viral load <100 copies/mL at 12 months following treatment, continuing care, mortality, adverse events, infected tuberculosis, and severe bacterial infections were all the outcome measures of this meta-analysis.
Quality Evaluation
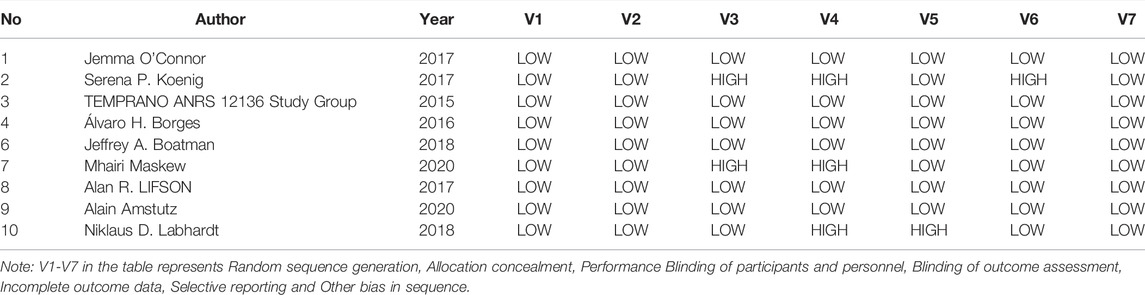
The two researchers independently used the Cochrane Collaboration Risk of Bias Tool (CCRBT) (Cumpston et al., 2019) to assess the risk of bias in the included articles. Following the completion of the evaluation, the two researchers cross-checked their findings, and if there was any controversy, a third researcher participated in the decision-making process. The Cochrane Collaboration Risk of Bias Tool examined the risk of bias specifically using seven items designed from the six aspects listed below: 1) Selection bias (Random sequence generation, Allocation concealment), 2) Performance bias (Blinding of participants and personnel), 3) Detection bias (Blinding of outcome assessment), 4) Attrition bias (Incomplete outcome data), 5) Reporting bias (Selective reporting), 6) Other bias. The risk of bias assessment resulted in “high risk,” “low risk,” and “unclear” for each item.
Statistical Methods
Meta-analysis was performed on the data with Stata15.0 software. In this Meta-analysis, the combined effect sizes of viral load <100 copies/mL, continuing care, mortality, adverse events, infected tuberculosis, severe bacterial infections were expressed by the relative risk rate (RR) and its 95% confidence interval, namely RR (95% CI).
Q test and I2 were used to quantify the heterogeneity among different studies. When I2 < 50% or p > 0.05, a fixed-effect model was used to combine the outcome measures; and when I2 ≥ 50% or p < 0.05, a random-effect model was adopted to combine the outcome measures.
Results
Literature Retrieval Results
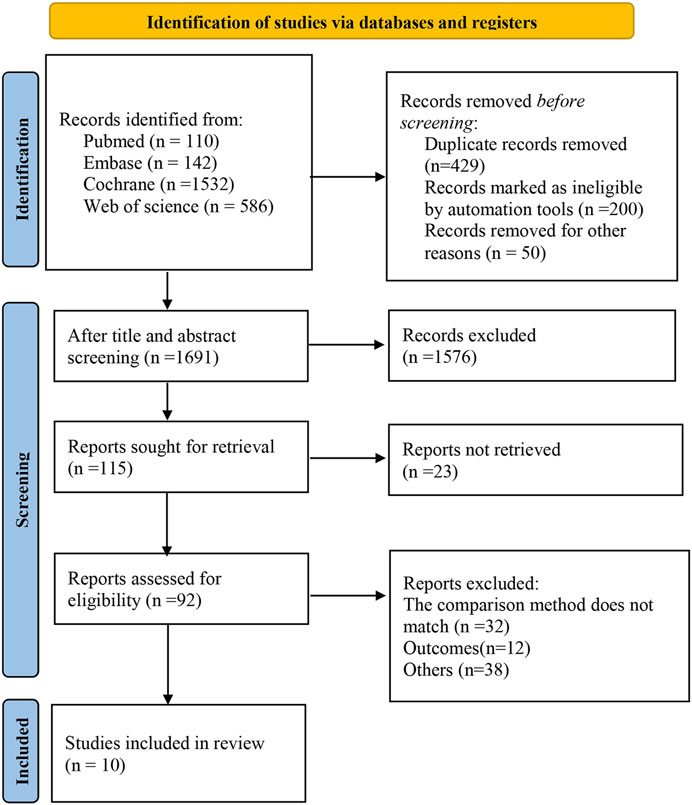
The databases yielded a total of 2,370 relevant studies, and 10 studies (Boatman et al., 2019; Amstutz et al., 2020; Maskew et al., 2020; Borges et al., 2016; Labhardt et al., 2018; The TEMPRANO ANRS 12136 Study Group, 2015; Koenig et al., 2017; Hecht et al., 2006; Lifson et al., 2017; O’Connor et al., 2017) were finally included for this meta-analysis after the gradual screening. Figure 1 depicts the literature screening process.
Basic Characteristics of Included Literature
A total of 21,543 patients were enrolled in the included studies from 2006 to 2020, including 10,684 in the experimental group and 10,859 in the control group. Five studies (Amstutz et al., 2020; Maskew et al., 2020; Labhardt et al., 2018; The TEMPRANO ANRS 12136 Study Group, 2015; Koenig et al., 2017) involved patients from resource-limited African regions (Haiti, South Africa, Lesotho, Ivory Coast), and five multi-center studies (Hecht et al., 2006; Borges et al., 2016; Lifson et al., 2017; O’Connor et al., 2017; Boatman et al., 2019; Amstutz et al., 2020; Maskew et al., 2020) involved patients from multiple regions. Nine randomized controlled trials (Boatman et al., 2019; Amstutz et al., 2020; Maskew et al., 2020; Borges et al., 2016; Labhardt et al., 2018; The TEMPRANO ANRS 12136 Study Group, 2015; Koenig et al., 2017; Lifson et al., 2017; O’Connor et al., 2017) were included, of which three (Labhardt et al., 2018; The TEMPRANO ANRS 12136 Study Group, 2015; Koenig et al., 2017) were non-blind, with rapid initiation of antiretroviral therapy in the intervention group and standard/delayed treatment in the control group. All of the treatments lasted longer than 6 months (see Table 1).
Quality Evaluation of Included Literature
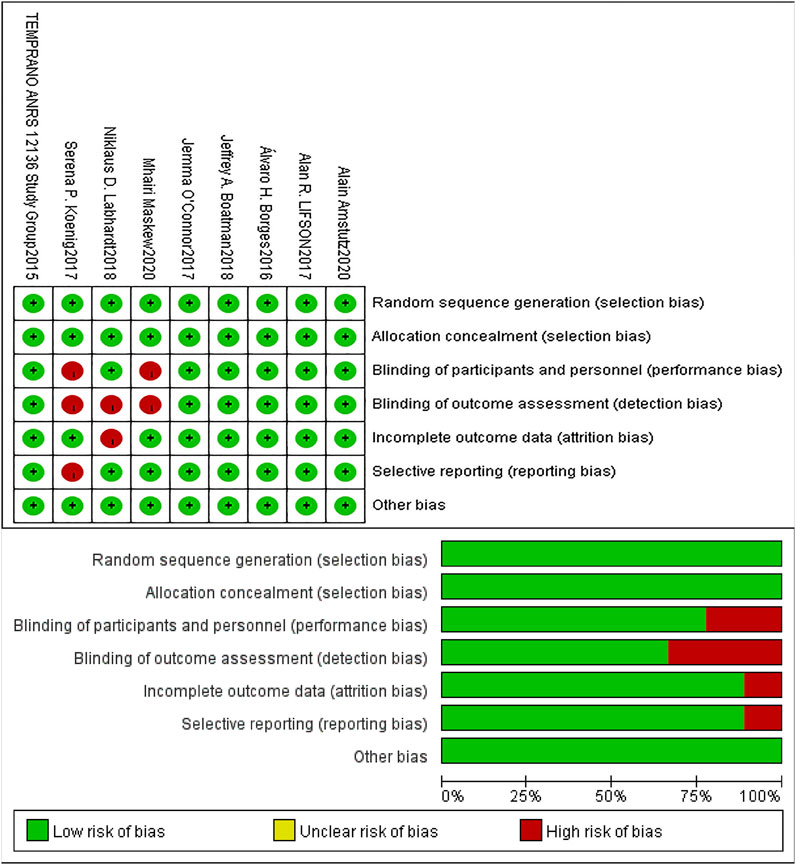
The risk of bias in the included studies was assessed using the Cochrane Collaboration Risk of Bias Tool (Higgins et al., 2019). Table 2 shows the risk ratings of Selection bias, Performance bias, Detection bias, Attrition bias, Reporting bias and Other bias in each study, and the graphics were displayed by Revman 5.4. (See Table 2; Figure 2).
Viral Load <100 Copies/mL at 12 Months After Treatment
Four studies (Koenig et al., 2017; Labhardt et al., 2018; Amstutz et al., 2020; Maskew et al., 2020) reported viral load <100 copies/mL at 12 months following treatment, and a random-effect model was adopted for the combined effect size (I2 = 85.9%, p < 0.001). There was no significant difference between the two groups based on the combined results (RR = 1.05, 95% CI: 0.80–1.39, Z = 0.35, p = 0.726), suggesting that the risk of Rapid ART with viral load <100 copies/mL was close to that of standard/delayed treatment, as shown in Figure 3A and Figure 3B.
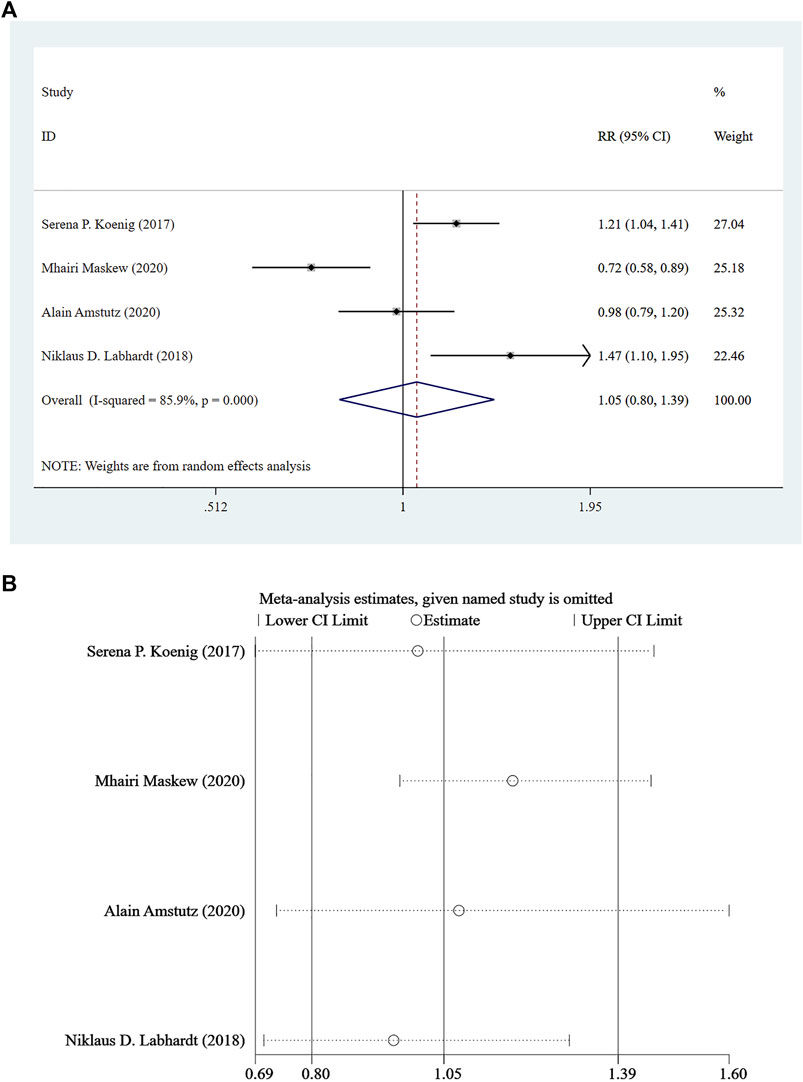
FIGURE 3. (A) Forest plot for Meta-analysis of statistical chart of viral load <100 copies/mL at 12 months after treatment. (B) Sensitivity analysis of statistical chart of viral load <100 copies/mL at 12 months after treatment (After removing the literatures one by one, the circles of each study were within the two edges, indicating that the conclusions of this meta-analysis were stable and reliable).
Continuing Care
Five studies (Amstutz et al., 2020; Maskew et al., 2020; Labhardt et al., 2018; The TEMPRANO ANRS 12136 Study Group, 2015; Koenig et al., 2017) reported continuing care for at least 8 months, and a random-effect model was used for the combined effect size (I2 = 75.5%, p = 0.003). The pooled results showed that the difference between the two groups was statistically significant (RR = 1.13, 95% CI: 1.03–1.25, Z = 2.44, p = 0.015), indicating that Rapid ART could be considered superior to standard/delayed treatment in the risk of continuing care. See Figure 4A and Figure 4B.
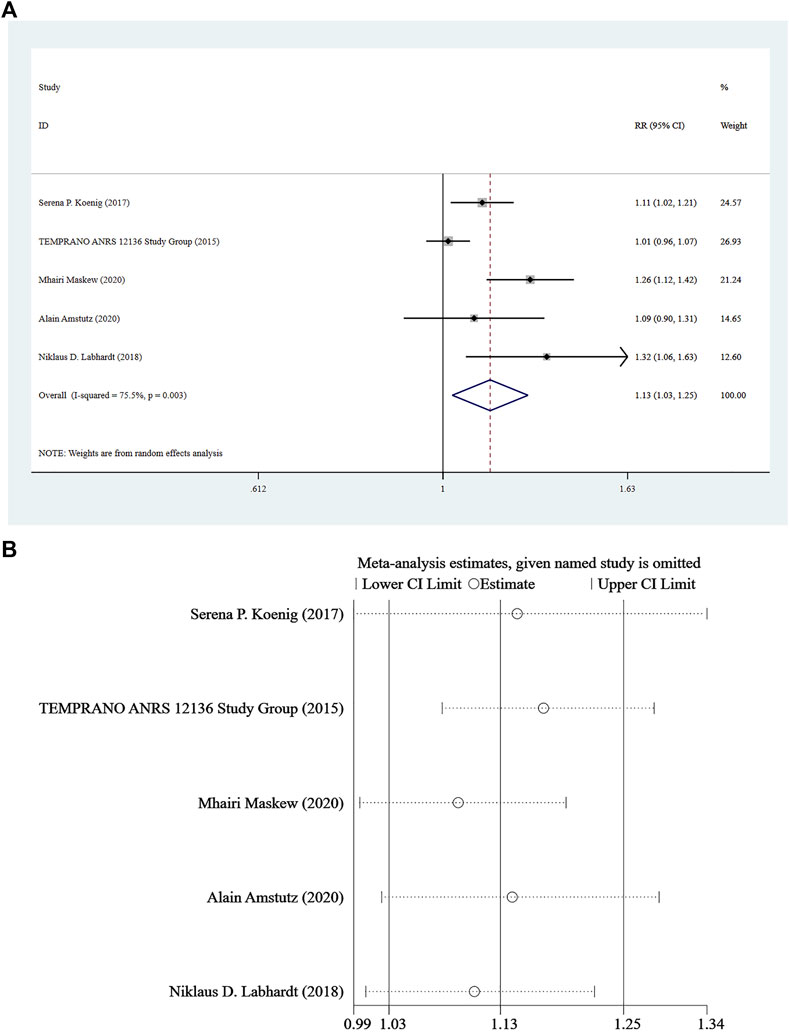
FIGURE 4. (A) Forest plot for Meta-analysis of continuing care statistics chart. (B) Sensitivity analysis of continuing care statistics chart (After removing the literatures one by one, the circles of each study were within the two edges, indicating that the conclusions of this meta-analysis were stable and reliable).
Mortality
Mortality was reported in four studies (Amstutz et al., 2020; Labhardt et al., 2018; The TEMPRANO ANRS 12136 Study Group, 2015; Koenig et al., 2017), and a fixed-effect model was adopted for the combined effect size (I2 = 25.4%, p = 0.259). There was no significant difference between the two groups based on the combined results (RR = 0.77, 95%CI: 0.47–1.24, Z = 1.09, p = 0.277), suggesting that the risk of death associated with Rapid ART was similar to that of standard/delayed treatment. See Figure 5A and Figure 5B.
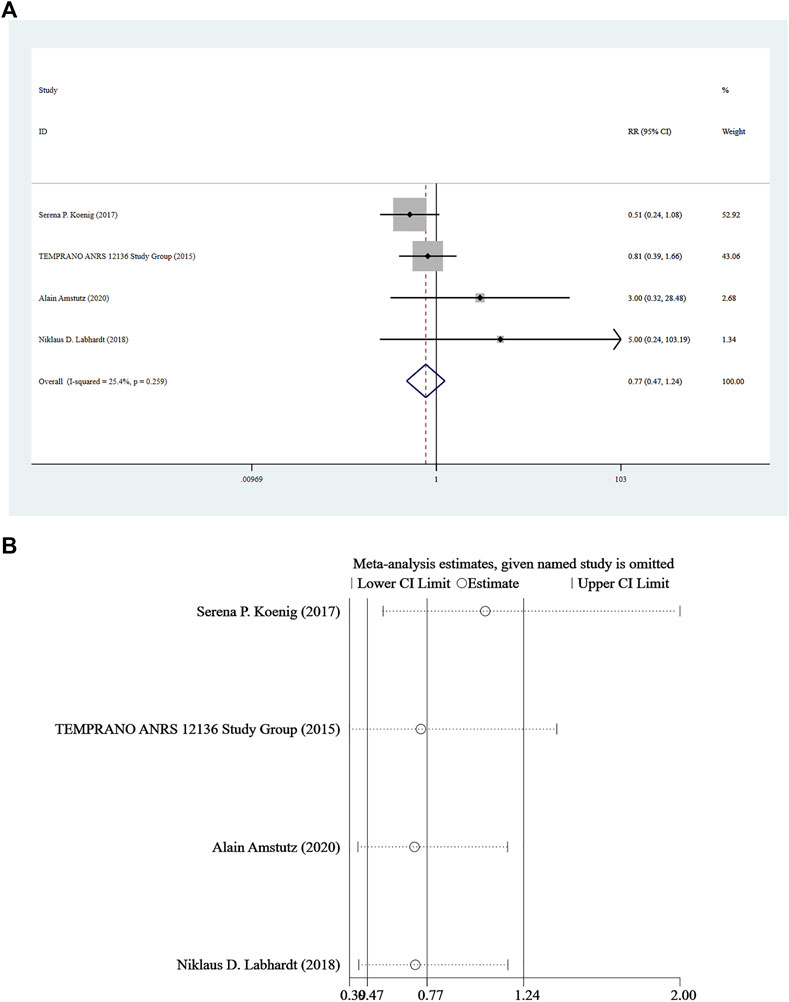
FIGURE 5. (A) Forest plot for Meta-analysis of mortality chart. (B) Sensitivity analysis of mortality chart (After removing the literatures one by one, the circles of each study were within the two edges, indicating that the conclusions of this meta-analysis were stable and reliable).
Adverse Events
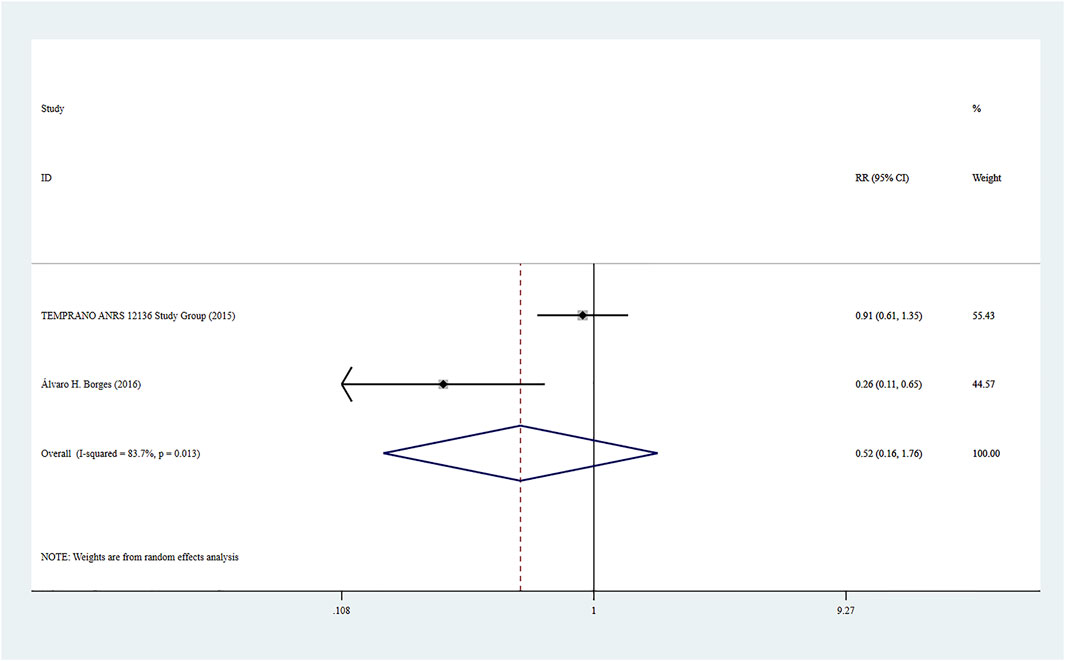
Adverse events were reported in two studies (8, 21), and the combined effect size was based on a random-effect model (I2 = 83.7%, p = 0.013). There was no significant difference between the two groups based on the combined results (RR = 0.52, 95% CI: 0.16–1.76, Z = 1.05, p = 0.294), suggesting that the risk of adverse events caused by Rapid ART was close to that of standard/delayed treatment. See Figure 6.
Infected Tuberculosis
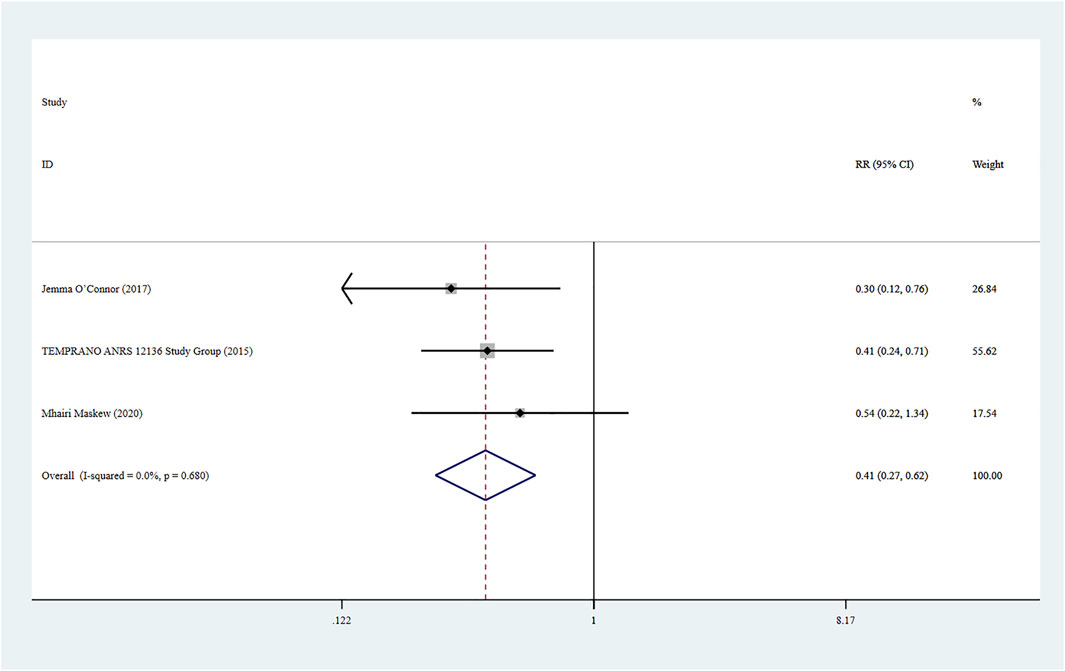
Three studies (Maskew et al., 2020; The TEMPRANO ANRS 12136 Study Group, 2015; O’Connor et al., 2017) reported infected tuberculosis in HIV patients, and a fixed-effect model was adopted for the combined effect size (I2 < 0.01%, p = 0.680). The combined results showed a statistically significant difference between the two groups (RR = 0.41, 95% CI: 0.27–0.62, Z = 4.24, p < 0.001), suggesting that the risk of infected tuberculosis caused by Rapid ART group was lower than that of standard/delayed treatment group, as shown in Figure 7.
Severe Bacterial Infections
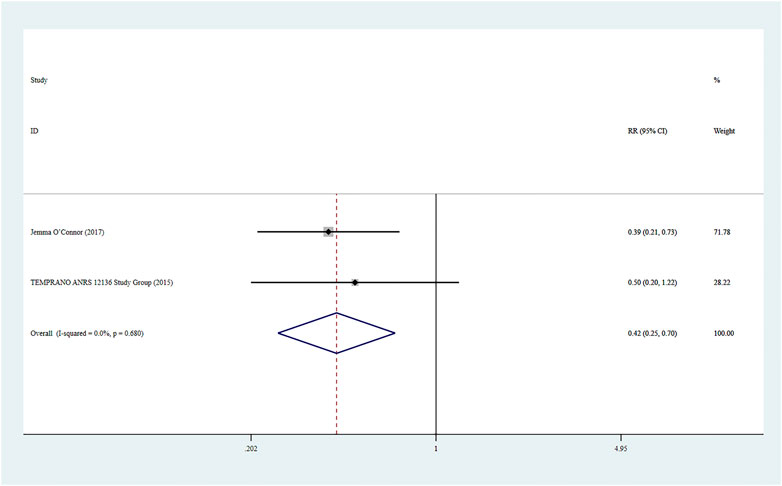
Two studies (O’Connor et al., 2017) reported severe bacterial infections, and a fixed-effect model was used for the combined effect size (I2 < 0.01%, p = 0.680). The combined results indicated a statistically significant difference between the two groups (RR = 0.42, 95% CI: 0.25–0.70, Z = 3.33, p = 0.001), suggesting that Rapid ART had a lower risk of severe bacterial infections than standard/delayed treatment. See Figure 8.
Discussion
Risk of infected tuberculosis caused by Rapid ART standard/delayed treatment. A total of 10 articles were included in this meta-analysis, nine of which were randomized controlled trials, and they were assessed as high-quality literature by the Cochrane Collaboration Risk of Bias Tool. The meta-analysis results showed that compared with standard/delayed treatment, Rapid ART was statistically significant in the risk of continuing care, tuberculosis and severe bacterial infections, and could significantly reduce the risk of tuberculosis and severe bacterial infections (RR = 1.13, 95% CI: 1.03–1.25, Z = 2.44, p = 0.015) (RR = 0.41, 95% CI: 0.27–0.62, Z = 4.24, p < 0.001) (RR = 0.42, 95% CI: 0.25–0.70, Z = 3.33, p = 0.001). There was no statistical difference in adverse events (RR = 0.52, 95% CI: 0.16–1.76, Z = 1.05, p = 0.294), mortality (RR = 0.77, 95%CI: 0.47–1.24, Z = 1.09, p = 0.277), and viral load <100 copies/mL at 12 months after treatment (RR = 1.05, 95% CI: 0.80–1.39, Z = 0.35, p = 0.726). The results indicate that Rapid ART has clinical significance in ensuring the drug safety of patients and reducing the risk of HIV-related diseases in patients.
Currently, the benefits and risks of Rapid ART are still controversial. Mateo-urdiale (Mateo-Urdiales et al., 2019) found that Rapid ART can reduce HIV-related morbidity and mortality and inhibit HIV viral load based on several randomized trials and observational studies. Ford (Ford et al., 2018) found in a randomized trial that Rapid ART shortened the virus inhibition time, but the mortality rate was not significantly reduced. And Rapid ART significantly increased the treatment cost, and loss to follow-up in observational studies showed an increasing trend. According to literature reports, most of the included literature in the current meta-analyses related to Rapid ART were observational studies in Africa. Although the feasibility of Rapid ART has been confirmed, it is difficult to measure the impact on the overall HIV epidemic. This study is a meta-analysis based on multi-region and multi-center RCTs, which confirmed that compared with standard/delayed treatment, Rapid ART was statistically significant in continuing care, and could reduce the risk of tuberculosis and severe bacterial infections in HIV patients.
In addition, our findings revealed that the two treatment choices had a similar risk of adverse events, viral load <100 copies/mL at 12 months following treatment, and mortality, which differed from previous findings. In comparison with standard/delayed treatment, a cohort observational study showed that Rapid ART reduced the possibility of virus inhibition at 6 months (Ford et al., 2018), and a study of adults in South Africa reported a high virus inhibition rate of 94% among HIV-infected people (Wilkinson et al., 2015). This may be due to the different definitions of virus inhibition and Rapid ART in our included literature. Mateo-urdiales (Mateo-Urdiales et al., 2019) included seven studies and found no statistical difference between the two treatments, which is consistent with the results of this study. The possible reason is that the longest follow-up time in the included literature is 3 years, and AIDS has become a chronic disease under the control of ART, so the follow-up time is not enough to draw a conclusion. Adverse events were not analyzed due to the outcome measures of the included literature (Ford et al., 2018; Mateo-Urdiales et al., 2019) as only two trials included in this study used adverse events as an outcome measure, thereby lacking sufficient evidence to draw conclusions on the occurrence of adverse events.
This meta-analysis provides the following benefits. First, this meta-analysis provided a complete and systematic evaluation of the advantages and disadvantages of rapid initiation of antiretroviral therapy against standard/delayed therapy, offering a reference for the selection of following clinical treatment options based on high-quality research. Second, compared with trials that only covered the African region, this study included five well-designed multi-center and multi-region RCTs with a total of 21,543 participants, including multiple ethnic groups and non-short-term treatment outcomes.
However, there are some limitations in this study. First, after thoroughly searching major databases, there were just a few publications that could be included in our meta-analysis. Second, the longest follow-up time of the included literature is 3 years, while AIDS has become a chronic disease under the control of ART. More outcome measures need to be confirmed by subsequent studies with longer follow-up times.
Conclusion
Rapid ART can reduce the risk of tuberculosis and severe bacterial infections in HIV patients compared to standard/delayed treatment and appears to have a similar safety profile. These findings provide support for the recommendation of WHO to accelerate the initiation of antiretroviral therapy, and Rapid ART is suggested to be used when medical conditions and the patient’s physical conditions permit. However, this meta-analysis included few studies and failed to effectively evaluate treatment-related adverse events, mortality, and virus inhibition. Therefore, we hope that there will be unified standards for the implementation time and virus inhibition of Rapid ART in the future, and more reasonably designed multi-center RCTs. More attention should be paid to the survival and quality of life of patients, and more reports with continuous follow-up need to be made on the outcomes of Rapid ART such as adverse events, to provide sufficient evidence for the treatment of Rapid ART.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
RB, JD, and SL conceived, designed, and performed the analysis. LD verified the analytical methods. RB wrote the paper and revised the manuscript for important intellectual content. WH revised the manuscript for important intellectual content. All authors discussed the results and contributed to the final manuscript.
Funding
This work was supported by the Beijing Municipal of Science and Technology Major Project (Grant numbers: Z211100002921003 to LD), Beijing Natural Science Foundation (7222092 to LD).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks to all authors for their contributions to this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.898449/full#supplementary-material
References
Amstutz, A., Brown, J. A., Ringera, I., Muhairwe, J., Lejone, T. I., Klimkait, T., et al. (2020). Engagement in Care, Viral Suppression, Drug Resistance, and Reasons for Nonengagement after Home-Based Same-Day Antiretroviral Therapy Initiation in Lesotho: a Two-Year Follow-Up of the CASCADE Trial. Clin. Infect. Dis. 71 (10), 2608–2614. doi:10.1093/cid/ciz1126
Bacon, O., Chin, J., Cohen, S. E., Hessol, N. A., Sachdev, D., Coffey, S., et al. (2020). Decreased Time from Human Immunodeficiency Virus Diagnosis to Care, Antiretroviral Therapy Initiation, and Virologic Suppression during the Citywide RAPID Initiative in San Francisco. Clin. Infect. Dis. 73 (1), e122–e128. doi:10.1093/cid/ciaa620
Boatman, J. A., Baker, J. V., Emery, S., Furrer, H., Mushatt, D. M., Sedláček, D., et al. (2019). Risk Factors for Low CD4+ Count Recovery Despite Viral Suppression Among Participants Initiating Antiretroviral Treatment with CD4+ Counts > 500 Cells/mm3: Findings from the Strategic Timing of AntiRetroviral Therapy (START) Trial. J. Acquir Immune Defic. Syndr. 81 (1), 10–17. doi:10.1097/QAI.0000000000001967
Borges, Á. H., Neuhaus, J., Babiker, A. G., Henry, K., Jain, M. K., Palfreeman, A., et al. (2016). Immediate Antiretroviral Therapy Reduces Risk of Infection-Related Cancer during Early HIV Infection. Clin. Infect. Dis. 63 (12), 1668–1676. doi:10.1093/cid/ciw621
Cumpston, M., Li, T., Page, M. J., Chandler, J., Welch, V. A., Higgins, J. P., et al. (2019). Updated Guidance for Trusted Systematic Reviews: a New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 10, ED000142. doi:10.1002/14651858.ED000142
Eholié, S. P., Badje, A., Kouame, G. M., N'takpe, J. B., Moh, R., Danel, C., et al. (2016). Antiretroviral Treatment Regardless of CD4 Count: the Universal Answer to a Contextual Question. AIDS Res. Ther. 13, 27. doi:10.1186/s12981-016-0111-1
Ford, N., Shubber, Z., Meintjes, G., Grinsztejn, B., Eholie, S., Mills, E. J., et al. (2015). Causes of Hospital Admission Among People Living with HIV Worldwide: a Systematic Review and Meta-Analysis. Lancet HIV. 2 (10), e438–44. doi:10.1016/S2352-3018(15)00137-X
Ford, N., Migone, C., Calmy, A., Kerschberger, B., Kanters, S., Nsanzimana, S., et al. (2018). Benefits and Risks of Rapid Initiation of Antiretroviral Therapy. AIDS 32 (1), 17–23. doi:10.1097/QAD.0000000000001671
Hecht, F. M., Wang, L., Collier, A., Little, S., Markowitz, M., Margolick, J., et al. (2006). A Multicenter Observational Study of the Potential Benefits of Initiating Combination Antiretroviral Therapy during Acute HIV Infection. J. Infect. Dis. 194 (6), 725–733. doi:10.1086/506616
Higgins, J. P., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (2019). Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Hoboken and Chichester: The Cochrane Collaboration and John Wiley & Sons.
Joseph Davey, D., Kehoe, K., Serrao, C., Prins, M., Mkhize, N., Hlophe, K., et al. (2020). Same-day Antiretroviral Therapy Is Associated with Increased Loss to Follow-Up in South African Public Health Facilities: a Prospective Cohort Study of Patients Diagnosed with HIV. J. Int. AIDS Soc. 23 (6), e25529. doi:10.1002/jia2.25529
Koenig, S. P., Dorvil, N., Dévieux, J. G., Hedt-Gauthier, B. L., Riviere, C., Faustin, M., et al. (2017). Same-day HIV Testing with Initiation of Antiretroviral Therapy versus Standard Care for Persons Living with HIV: a Randomized Unblinded Trial. PLoS Med. 14 (7), e1002357. doi:10.1371/journal.pmed.1002357
Labhardt, N. D., Ringera, I., Lejone, T. I., Klimkait, T., Muhairwe, J., Amstutz, A., et al. (2018). Effect of Offering Same-Day ART vs Usual Health Facility Referral during Home-Based HIV Testing on Linkage to Care and Viral Suppression Among Adults with HIV in Lesotho: The CASCADE Randomized Clinical Trial. JAMA 319 (11), 1103–1112. doi:10.1001/jama.2018.1818
Lifson, A. R., Grund, B., Gardner, E. M., Kaplan, R., Denning, E., Engen, N., et al. (2017). Improved Quality of Life with Immediate versus Deferred Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. AIDS 31 (7), 953–963. doi:10.1097/QAD.0000000000001417
Long, L., Kuchukhidze, S., Pascoe, S., Nichols, B. E., Fox, M. P., Cele, R., et al. (2020). Retention in Care and Viral Suppression in Differentiated Service Delivery Models for HIV Treatment Delivery in Sub-saharan Africa: a Rapid Systematic Review. J. Int. AIDS Soc. 23 (11), e25640. doi:10.1002/jia2.25640
Low, A., Gavriilidis, G., Larke, N., B-Lajoie, M. R., Drouin, O., Stover, J., et al. (2016). Incidence of Opportunistic Infections and the Impact of Antiretroviral Therapy Among HIV-Infected Adults in Low- and Middle-Income Countries: a Systematic Review and Meta-Analysis. Clin. Infect. Dis. 62 (12), 1595–1603. doi:10.1093/cid/ciw125
Lundgren, J. D., Lundgren, J. D., Babiker, A. G., Gordin, F., Emery, S., Grund, B., et al. (2015). Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N. Engl. J. Med. 373 (9), 795–807. doi:10.1056/NEJMoa1506816
Maskew, M., Brennan, A. T., Fox, M. P., Vezi, L., Venter, W. D. F., Ehrenkranz, P., et al. (2020). A Clinical Algorithm for Same-Day HIV Treatment Initiation in Settings with High TB Symptom Prevalence in South Africa: the SLATE II Individually Randomized Clinical Trial. PLoS Med. 17 (8), e1003226. doi:10.1371/journal.pmed.1003226
Mateo-Urdiales, A., Johnson, S., Smith, R., Nachega, J. B., and Eshun-Wilson, I. (2019). Rapid Initiation of Antiretroviral Therapy for People Living with HIV. Cochrane Database Syst. Rev. 6 (6), CD012962. doi:10.1002/14651858.CD012962.pub2
McNulty, M., Schmitt, J., Friedman, E., Hunt, B., Tobin, A., Maheswaran, A. B., et al. (2020). Implementing Rapid Initiation of Antiretroviral Therapy for Acute HIV Infection within a Routine Testing and Linkage to Care Program in Chicago. J. Int. Assoc. Provid. AIDS Care 19, 2325958220939754. doi:10.1177/2325958220939754
O’Connor, J., Vjecha, M. J., Phillips, A. N., Angus, P. B., Cooper, P. D., Grinsztejn, B., et al. (2017). Effect of Immediate Initiation of Antiretroviral Therapy on Risk of Severe Bacterial Infections in HIV-Positive People with CD4 Cell Counts of More Than 500 Cells Per μL: Secondary Outcome Results from a Randomised Controlled Trial. Lancet HIV 4 (3), e105–12. doi:10.1016/S2352-3018(16)30216-8
Pilcher, C. D., Ospina-Norvell, C., Dasgupta, A., Jones, D., Hartogensis, W., Torres, S., et al. (2017). The Effect of Same-Day Observed Initiation of Antiretroviral Therapy on HIV Viral Load and Treatment Outcomes in a US Public Health Setting. J. Acquir Immune Defic. Syndr. 74 (1), 44–51. doi:10.1097/QAI.0000000000001134
Rosen, S., and Fox, M. P. (2011). Retention in HIV Care between Testing and Treatment in Sub-saharan Africa: a Systematic Review. PLoS Med. 8 (7), e1001056. doi:10.1371/journal.pmed.1001056
The TEMPRANO ANRS 12136 Study Group (2015). A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N. Engl. J. of Med. 373 (9), 808–822. doi:10.1056/NEJMoa1507198
WHO (2021). WHO Number of deaths due to HIV/AIDS Switzerland. Geneva: WHO. Available from: https://www.who.int/data/gho/data/indicators/indicatordetails/GHO/number-of-deaths-due-to-hiv-aids (accessed December 28, 2021).
Wilkinson, L., Duvivier, H., Patten, G., Solomon, S., Mdani, L., Patel, S., et al. (2015). Outcomes from the Implementation of a Counselling Model Supporting Rapid Antiretroviral Treatment Initiation in a Primary Healthcare Clinic in Khayelitsha, South Africa. South Afr. J. HIV Med. 16 (1), 367. doi:10.4102/sajhivmed.v16i1.367
Keywords: HIV, rapid, antiretroviral therapy, systematic review, meta-analysis
Citation: Bai R, Du J, Lv S, Hua W, Dai L and Wu H (2022) Benefits and Risks of Rapid Initiation of Antiretroviral Therapy: A Systematic Review and Meta-Analysis. Front. Pharmacol. 13:898449. doi: 10.3389/fphar.2022.898449
Received: 17 March 2022; Accepted: 09 May 2022;
Published: 03 June 2022.
Edited by:
Raphael Zozimus Sangeda, Muhimbili University of Health and Allied Sciences, TanzaniaReviewed by:
Raveen Parboosing, National Health Laboratory Service (NHLS), South AfricaAshok K. Shakya, Al-Ahliyya Amman University, Jordan
Copyright © 2022 Bai, Du, Lv, Hua, Dai and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lili Dai, bGlseWRhaWVyQGNjbXUuZWR1LmNu; Hao Wu, d2hkb2NAY2NtdS5lZHUuY24=
†These authors have contributed equally to this work
 Ruojing Bai1†
Ruojing Bai1† Wei Hua
Wei Hua Lili Dai
Lili Dai Hao Wu
Hao Wu