- 1Maternal and Child Health, Translational Health Science and Technology Institute, Faridabad, India
- 2School of Agriculture and Food Science, The University of Queensland, Brisbane, QLD, Australia
Stringent balance of the immune system is a key regulatory factor in defining successful implantation, fetal development, and timely parturition. Interference in these primary regulatory mechanisms, either at adolescence or prenatal state led to adverse pregnancy outcomes. Fertility restoration with the help of injectable gonadotrophins/progesterone, ovulation-inducing drugs, immunomodulatory drugs (corticosteroids), and reproductive surgeries provides inadequate responses, which manifest its own side effects. The development of a potential diagnostic biomarker and an effectual treatment for adverse pregnancy outcomes is a prerequisite to maternal and child health. Parent cell originated bi-layered-intraluminal nano-vesicles (30–150 nm) also known as exosomes are detected in all types of bodily fluids like blood, saliva, breast milk, urine, etc. Exosomes being the most biological residual structures with the least cytotoxicity are loaded with cargo in the form of RNAs (miRNAs), proteins (cytokines), hormones (estrogen, progesterone, etc.), cDNAs, and metabolites making them chief molecules of cell-cell communication. Their keen involvement in the regulation of biological processes has portrayed them as the power shots of cues to understand the disease’s pathophysiology and progression. Recent studies have demonstrated the role of immunexosomes (immunomodulating exosomes) in maintaining unwavering immune homeostasis between the mother and developing fetus for a healthy pregnancy. Moreover, the concentration and size of the exosomes are extensively studied in adverse pregnancies like preeclampsia, gestational diabetes mellitus (GDM), and preterm premature rupture of membrane (pPROMs) as an early diagnostic marker, thus giving in-depth information about their pathophysiology. Exosomes have also been engineered physically as well as genetically to enhance their encapsulation efficiency and specificity in therapy for cancer and adverse pregnancies. Successful bench to bedside discoveries and interventions in cancer has motivated developmental biologists to investigate the role of immunexosomes and their active components. Our review summarizes the pre-clinical studies for the use of these power-shots as therapeutic agents. We envisage that these studies will pave the path for the use of immunexosomes in clinical settings for reproductive problems that arise due to immune perturbance in homeostasis either at adolescence or prenatal state.
Introduction
The semi-allogenic fetus develops and resides within the mother’s womb, causing a series of physiological, structural, organismal changes in her body. These profound changes take place proximally in the endometrium and the uterine cavity to protect the fetus from rejection via modulation of the maternal immune system and structural remodeling to provide better nutrition for the growing fetus. Distally the informed changes in maternal physiology are an adaptation process in order to prepare the mother for the rest of the gestational journey. The endocrine signals (progesterone, estrogen, human chorionic gonadotrophin (hCG), genomic (miRNAs), and metabolomic entities (lipids, amino acids, etc.) work in conjunction to progenerate the maternal immune system towards accepting the fetal antigens, which is a kind of stress test for the mother (Bukovsky et al., 2003; Li et al., 2004; Mulac-Jericevic and Conneely, 2004; Rolle et al., 2013; Jabrane-Ferrat, 2019). The fetoplacental communication resembles a webbed structure with every node impersonating an immune cell, required to maintain equilibrium among all cells in the unit. The maternal immune system is renovated, providing a suppressive immune niche for fetal survival, thus establishing a crucial feto-maternal immune crosstalk. Interestingly in cancer, a similar mechanism of reconditioning the immune system for favorable changes is very well studied (Costanzo et al., 2018). Cancer progression is thus a phenomenon of forced changes and has similarities with regulated fetal growth during pregnancy. The host and maternal immune system engage in a contest of strength towards producing a response against developing cancer and the fetus. Ultimately this response modulates the host and maternal immune system resulting in the establishment of cancer and sustenance of the fetus, respectively. This immunomodulation is effectively aided by the signals emanating within the bilayered-intraluminal nanovesicles, which work distally in maintaining the immune crosstalk for their stabilization (Salomon et al., 2014a). Discovered almost 40 years ago in 1989 (Trams et al., 1981; Pan and Johnstone, 1983), the extracellular vesicles named exosomes were characterized later as lipid-bilayered-intraluminal microvesicles (ILVs) (30–150 nm), yielded by invagination of multivesicular bodies (MVBs) derived from endosomes during stress response or for cell-cell communication (Harding et al., 1984). Exosomes are decisive in an aspect because they encapsulate regulatory signals of cellular behavior. Demonstrated in the database, over 9,690 kinds of proteins, more than 3,300 varieties of mRNAs, and 1,010 different types of lipids can exist in an exosome depending upon its origin (Keerthikumar et al., 2016; Kurian and Modi, 2019). Studies have represented that the exosomes are extensively involved in feto-maternal communication facilitating embryo implantation, trophoblast invasion, trophoblast proliferation, angiogenesis, glucose metabolism, and immunological signaling (Salomon et al., 2014b). The mission to these exosomes is assigned by the placenta. Evidential studies have praised the similarities between the placenta and cancer on the behalf of their mechanism for evasion of immune response utilizing exosomes, thus generating a fetal or tumor-sustaining environment (Holtan et al., 2009). Such similarities have puzzled the brilliant scientific mind for ages, hence it is fascinating to connect and observe the underlined mechanisms. This review emphasizes how these factors (immune-exosomes) interact with the immune cells to modify their functions and affect their metabolic rates so as to yield a balanced pro- and anti-inflammatory milieu for successful fetal development and timely parturition. A well-sustained fetal development and timely parturition are based on a well-regulated immune clock implicating a pro-inflammatory milieu in the first and third trimester along with a skewed but required anti-inflammatory milieu in the second trimester (Dekel et al., 2010). Alterations of this stringent immune clock result in pregnancy complications like pre-eclampsia (PE), gestational diabetes (GDM), and preterm birth (PTB) (Erlebacher et al., 2007; Schonkeren et al., 2011; Han et al., 2015). The mass manipulation of the immune system by cancer cells via exosomes, for their survival, can be instigated for the ideas in mending the immune perturbations resulting in pregnancy complications. Therefore, we attempt to explore the role of the immune-exosomes in cancer and pregnancy focusing on taking lessons from the trail followed by cancer-derived immune-exosomes, which can help in the development of future therapeutics for pregnancy complications.
Further, we envisage that bringing about modification at the immune level with the use of exosomes as immunomodulatory effectors may prove as therapeutic tools, as have been studied in building up a strong tolerogenic niche for cancer survival.
Conjunction of Bio-Molecules in a Healthy Pregnancy
Hormones, miRNAs and metabolites impact various immune cells and alter their lineages resulting in modification of their effector functions. This causes disbalance of pro- and anti-inflammatory milieu leading to adverse pregnancy outcomes.
Hormones: The Catalysts of Pregnancy
Progesterone, in most mammals, is essential for successful implantation and maintenance of gestation. Progesterone acts through its two nuclear progesterone receptor (PR) isoforms, PRA and PRB (Li et al., 2004; Mulac-Jericevic and Conneely, 2004). The A isoform is responsible for fertility in mice and B is involved in the development of the mammary gland (Mulac-Jericevic et al., 2000; Mulac-Jericevic et al., 2003; Conneely et al., 2003). It also lays a tolerant immunological environment in the endometrium, to shield the fetus expressing paternal antigens from the maternal immune attack responses. In peripheral blood, both PR isoforms are expressed on NK cells (Arruvito et al., 2008). During a healthy pregnancy, a significantly upregulated expression (approx. 97%) of PRs on γδ-TCR positive T-cells has been reported. However, in non-pregnant individuals the expression of PRs on γδ-TCR positive T-cells was reported to be as low as 14% (Polgar et al., 1999). Interestingly, the increased progesterone levels during a healthy pregnancy have been reported to induce progesterone-induced blocking factor (PIBF), which suppresses NK cytotoxic activity in the decidua thus, aiding successful pregnancy (Kandzija et al., 2019). Progesterone is crucial as a mediator to induce the naïve T cells to differentiate into Th2-type cells and inhibit activities of T effector cells, especially Th1 (Piccinni et al., 1995) (Figure 1A). Lower levels of PR on peripheral blood lymphocytes and serum PIBF have been associated with women having recurrent miscarriages (RM) (Liang et al., 2021). Lymphocyte immunotherapy has shown an improvement in outcomes for RM and is reported to induce increased PR expression on maternal lymphocytes (Hudic et al., 2020). In preeclamptic rat models, administering PIBF displayed normalized Th1/Th2 ratio, it suppressed inflammation, adjusted blood pressure to normal, and prevented fetal growth restriction. PIBF is detectable in the serum after 14 days of embryo transfer in vitro fertilization (IVF) patients PIBF concentration in serum increase with gestational age in normal pregnancy. However, a lower-than-normal threshold can help predict spontaneous pregnancy termination (Lim et al., 2020). Dydrogesterone treatment on peripheral blood mononuclear cells (PBMCs) isolated from women with a history of unexplained RSM induces Th2 responses by elevating IL-4 and IL-6 while suppressing Th1/Th-17 cytokines such as IFN-γ (Interferon-Gamma), TNF-α, and IL-17. Dydrogesterone treatment to women at risk of preterm delivery also resulted in increased PIBF production, IL-10 concentrations, and lower concentrations of IFN-γ (Lim et al., 2020).
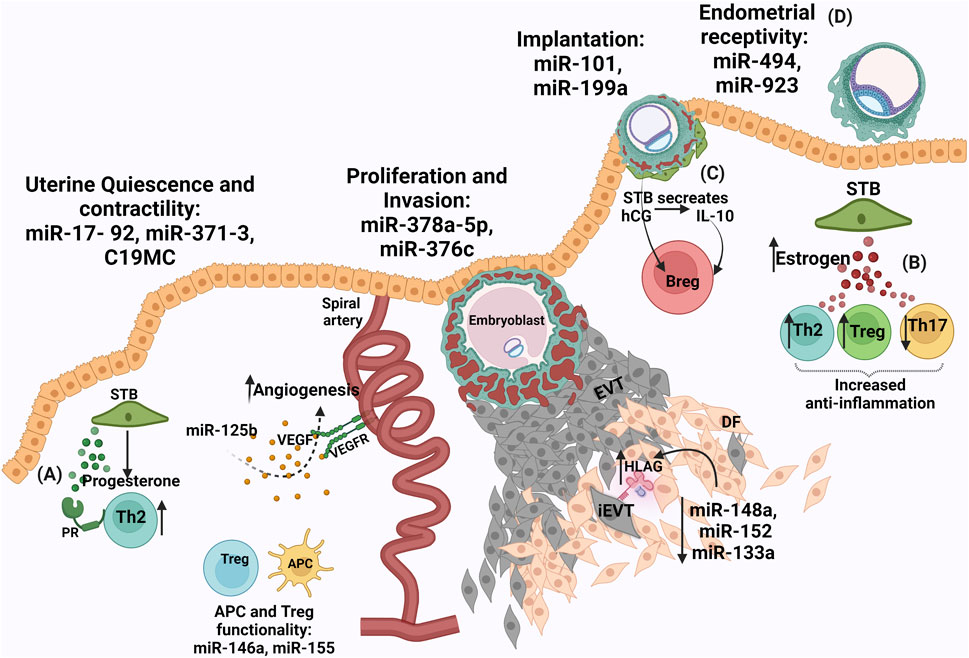
FIGURE 1. Conjunction of biomolecules in healthy pregnancy (A) Progesterone from syncytiotrophoblasts (STB) causes Treg expansion to form a tolerogenic zone (B) Increased estrogen levels from STB aids an anti-inflammatory response (C) Human chorionic gonadotrophin hormone (hCG) released from STB induces interleukin-10 (IL-10) which causes expansion of regulatory B cells and an assured immune environment (D) miRNAs are involved during placentation for endometrial receptivity: miR-30 family, miR-494, miR-923, implantation: miR-101 and miR-199a, proliferation and invasion: miR-378a-5p and miR-376c, uterine quiescence and contractility: miR-17- 92, miR-371–3, C19MC, APC and Treg functionality. miR0146a and miR-155 are involved in both pro- and anti-angiogenic functions. Reduced expression of miR-148a, miR-152, miR-133a in the extravillous trophoblasts (EVTs) ensures high levels of HLA-G to aid in a conducive environment by regulating NK cell cytotoxicity.
Estrogens are extensively produced by the fetoplacental unit and required in maintaining pregnancy as well as for fetal maturation. The receptors for estrogens, similar to progesterone receptors are of two types, estrogen receptors (ER) -alpha (α) and -beta (β) (Bukovsky et al., 2003). These receptors are differentially expressed on subsets of immune cells such as lymphocytes, macrophages (MØ), and dendritic cells (DCs) (Kadel and Kovats, 2018). Increased expression of estrogen is associated with healthy pregnancies (Levitz and Young, 1977). Estrogen expression by the placenta raises the level of the hormone in circulation during gestation. Elevated expression levels of ER-alpha are found on T cells whereas, ER-beta elevated expression is reported on B-cells (Phiel et al., 2005). Estrogen exposed immune cells executes paired responses such that it can enhance NK cell cytotoxicity and interferon-gamma (IFN-γ) production but can also suppress granzyme B and FasL to increase and reduce inflammation, respectively (Hao et al., 2007). A dose-dependent effect of estrogen is observed on monocytes, where lower levels of estrogen result in an increase of pro-inflammatory interleukins (IL) IL-1, IL-6, and TNF- α and the higher level of estrogen reduces these pro-inflammatory cytokines (Bouman et al., 2005). In adaptive immunity, a higher concentration of estrogen promotes Th2 responses, expands regulatory T (Tregs) cells, and causes suppression of Th-17 in mice (Figure 1B) (Arruvito et al., 2007; Mao et al., 2010). Estrogen also aids angiogenesis by upregulating VEGF and VEGFR1, during normal pregnancy (Storment et al., 2000). Lower levels of estrogen in this aspect result in dysfunctional angiogenesis contributing to PE (Cantonwine et al., 2019). Short intramuscular-administration of estrogen in pre-eclamptic women reduces mean arterial blood pressure (Babic et al., 2018). Genistein, a phytoestrogen that works by binding G-protein ER (GPER) is used to treat PE. Lower levels of estrogen resulted in insulin resistance and thus are also associated with GDM pregnancies (Fernandez et al., 2016).
hCG, driven by the endocrine and immune system, induces maternal immune cells via lectin-glycan interactions to promote the attachment of the embryo to aid its invasion. Signals from embryo to endometrial immune environment lay a healthy embryo–endometrial relationship, producing pregnancy-induced immune tolerance in favor of the fetus. This stability deciphers the acceptance of the embryo for successful implantation (Schumacher et al., 2009; Schumacher et al., 2013; Schumacher and Zenclussen, 2019). hCG, via hCG receptors, stimulates IL-10 which is shown to increase CD19 + CD24 (high+) CD27 + regulatory B-cells population (Figure 1C). These regulatory B-cells enhance the positive effects of the immune environment in pregnancy (Rolle et al., 2013). In baboon endometrial stromal cells and glycodelin in the glandular epithelium, hCG was found to be directly involved in the induction of α-smooth muscle actin (SMA) expression. This suggests that the primate blastocyst, prior to implantation mediate changes in the uterine environment. Concomitant signals between the embryo and maternal endometrium form a cross-talk, which directs the event of successful embryo implantation (Fazleabas et al., 1999). A study demonstrated that hCG hormone is encapsulated in placental-derived exosomes and amnion-derived exosomes forming a no-contact bridge between maternal and embryo thus, providing distal effects. hCG from chorionic trophoblast cells is found to be involved in DC differentiation, maturation, and function regulation at the maternal-fetal interface (Fitzgerald et al., 2018).
Pregnancy Involved miRNAs
Shown to be multiplayer, miRNAs are involved in inhibition of mRNA and promotion of translation (Taganov et al., 2007; Vasudevan et al., 2007; Olsen and Ambros, 1999). In humans, endometrial receptivity associated miRNAs are miR-30 family, miR-494, and miR-923, whereas, miR-101 and miR-199a aid the embryo implantation (Altmäe et al., 2013; Chakrabarty et al., 2007). miRNAs regulating placental functions like uterine quiescence and contractility are miR-17- 92, miR-371-3, chromosome 19 miRNA cluster (C19MC), miR-200 whereas, miR-378a-5p and miR-376c are involved in proliferation and invasion of trophoblast (Figure 1D) (Renthal et al., 2010). The myeloid cell differentiation has been reported to be regulated by miR-20a, miR-17-5p, and miR-106a. Clusters namely, C19MC, miR-371-3 (both located on chromosome 19), and C14MC (located on chromosome 14) are reported, out of which the C19MC is the most extensively researched (Flor et al., 2012). C19MC is expressed in trophoblast and placenta-derived stromal cells. miRNAs from this cluster are expressed in human embryonic stem cells and function in cell proliferation, invasion, and differentiation processes. C19MC expression is recorded in extravillous trophoblasts (EVTs) and several malignancies. Several miRNAs are involved in both pro- and anti-angiogenic functions (Donker et al., 2012). Members of the miRNA-17-92 cluster (miR-17, miR -18a, miR -19a, miR -19b, miR-20a, and miR -92a) have been shown to have anti-angiogenic effects on the endothelial cell in vitro, and inhibition of these leads towards pro-angiogenesis (Doebele et al., 2010). This regulation potential towards angiogenesis by miRNAs is exploited by cancer cells (Alpini et al., 2011). miRNAs are also involved in generating tolerance, such that HLA-G expressed mainly by the EVTs of the placenta could be downregulated by miRNA (miR-148a, and miR-152) binding to its 39-untranslated region thus, masking trophoblast antigenicity and shielding it from the attack of NK cells (Manaster et al., 2012). Favorably, the expression of these miRNAs have been reported to be expressed at low levels in the placenta, thus aiding the higher expression of HLA-G to create a tolerogenic realm. Modulating the immune cells, miR-155 is required for DC differentiation and DC endocytic capacity. 109 miRNAs are reported to influence macrophage (MØ) differentiation and exhibit both pro-inflammatory and anti-inflammatory phenotypes (Ferretti and La Cava, 2014). miR-146a, miR-155, and miR-223 miRNAs are involved in Treg cell differentiation. miR-17–92, a polycistronic miRNA mediates the regulation and differentiation of antigen-specific IL10-producing natural Tregs (Tregs) (de Kouchkovsky et al., 2013; Herberth et al., 2014). The maternal blood isolated at the 34th week of gestation and umbilical cord blood isolated at the time of birth had a higher miR-223, expression which was correlated with the lower number of Treg cells implying the increase in inflammation required for parturition. miR-146a enhances the suppressive capacity of Treg cells and in turn, limits Th1 responses (Zhou et al., 2015). miR-146a regulates TLR signaling and produces cytokines by decreasing the inflammatory response. However, decreased expression of miR-146a-5p was present in decidual tissue from patients with recurrent spontaneous abortions (Zhao et al., 2018a). In-vitro culturing of bovine embryos, revealed an increase expression of miR-25, miR-302c, miR-196a2, and miR-181a in embryos that demonstrated ceased development from morula to blastocyst stage, as compared to the embryos that successfully attained blastocyst stage. Thus, indicating a correlation between miRNA expression pattern and embryo development (Kropp et al., 2014). A study concluded that miR-34 is involved in cervical remodelling in normal labor whereas (Hassan et al., 2010), mir-223-3p is associated with preterm labor regulating the immune system. In preterm labor, mir-223-3p regulates inflammasome activity and MØ activation via NLRP3 and Pknox1 thus, regulating IL-1beta production (Bauernfeind et al., 2012; Haneklaus et al., 2012). miRNAs are unstable species thus, are encapsulated in exosomes to increase their stability and provide a targeted delivery. For embryo implantation miR-17, miR-106a and miR-200c are the most abundant miRNAs in placental exosomes (Yang et al., 2011; Ng et al., 2013). Exosomal C19MC family provides anti-viral responses by executing autophagy and thus may protect developing fetus from infections (Dumont et al., 2017).
Immune Metabolome
A healthy pregnancy requires degradation of stored energy to facilitate fetal development and achieve timely parturition, thus causing a shift of an anabolic state in the first and second trimester to a catabolic state in the third trimester. These shifts primarily regulate the physiological immune responses in normal pregnancy whereas, alteration in these can lead to pregnancy complications.
NK Cells
mTOR signaling-dependent regulation of glycolysis and mitochondrial functions are enhanced and most importantly studied in NK cell activation. In response to IL-2/IL-12/IL-5, the NK cells are activated, which leads to upregulation of nutrient receptors like CD71 and CD98 causing increased expression of GLUT-1 in an mTOR-dependent manner. This energy is required by NK cells to interact with villous trophoblasts and produce required responses for trophoblast invasion, proliferation, and tolerance (Jabrane-Ferrat, 2019). This provided the nutrition and energy which are essential at the initial stage of pregnancy (Donnelly et al., 2014; Slattery et al., 2021).
Macrophages
Differentiated phenotypes of MØ have varied glycometabolism pathways. Pro-inflammatory type-1 macrophage (M1) provide spontaneous responses against invading pathogens inside the body receiving their power by anaerobic glycolysis. However, anti-inflammatory M2 responses are long-lasting and generated by mitochondrial oxidative phosphorylation (Van den Bossche et al., 2016). In response to lipopolysaccharide (LPS) and IFN-γ exposures, the MØ mitochondrial oxidative phosphorylation is downregulated, which triggers a shift towards type-1 macrophage (M1) polarization by anaerobic glycolysis and pentose phosphate pathway (PPP). Adding, hexokinases and GLUT1 are positively regulated by accumulated TCA cycle metabolites and increased HIF-1α (Tannahill et al., 2013). M1 are responsible for regulating the trophoblast invasion and proliferation by providing optimal inflammation during the early phase of pregnancy. However, prolonged dysfunction of mitochondrial oxidative phosphorylation is responsible for the generation of pro-inflammatory conditions like PE, gestational diabetes mellitus (GDM), and preterm birth (PTB). Thus, researchers have targeted the metabolic programming for the reversal of M1 to M2 polarization for therapeutic purposes. For instance, a study showed reconstruction of dysregulated mitochondrial oxidative phosphorylation by inhibiting iNOS thereby, reverting polarized M1 into M2 ultimately reducing the inflammation (Van den Bossche et al., 2016).
Dendritic Cells
The activation of DCs and stimulation of DCs via LPS leads to inactivation of mitochondrial oxidative phosphorylation and thus a prompt response is generated to increase glycolysis rate for increasing the ATP production (Brombacher and Everts, 2020). The inhibition of mitochondrial oxidative phosphorylation occurs due to endogenous synthesis of NO by iNOS enzyme and stabilized HIF-1α via mTOR signalling. Amino acids like leucine, glutamine, (required for mTORC1 activity), and arginine (fuel for NO production) also affect mTOR signalling (Everts et al., 2012; Lawless et al., 2017). When DCs interact with T cells for antigen transfer, uptake of glucose and amino acids increases, yielding nutrient competitive surrounding and this competition cause prolonged T cell responses. However, these prolonged T cell responses are regulated on the type of T cell subset demand during the course of pregnancy. Extended inflammatory Th cell responses have been associated with pregnancy complications like GDM (Lawless et al., 2017).
T Cells
Stimulatory responses by T cells are produced via switching between glucose and lipid metabolism, whereas the quiescent state of T cells is provided via oxidative phosphorylation (Warburg, 1956). T cell proliferation consumes a high concentration of ATP which is produced via conversion of pyruvate into lactate during glycolysis. Thus, producing essential bio-macromolecules for executing physiological processes of a cell such as growth and division (Pearce et al., 2013). Moreover, T cell stimulation requires increased absorption, this happens by the interaction between its co-stimulatory molecule (CD28) and TCR on APC. This interaction increases GLUT1 expressions via mTOR signalling resulting in increased glucose uptake by respective cells resulting in the execution of their effector responses (Macintyre et al., 2014). In the T cell subset, Th1, Th2, and Th17 closely rely on mitochondrial metabolism with Th17 being the fastest and longest consumer of glucose in a HIF-1α dependent manner (Dang et al., 2011). In addition, Treg cells have multiple metabolic pathways such as glycolysis, lipid oxidation, and oxidative phosphorylation regulating their responses. A regulated balance between these metabolic pathways for pro and anti-inflammatory cells exists (Michalek et al., 2011) however, mitochondrial metabolism could be targeted to decrease inflammatory T-cell responses. Similarly, to receive Treg prominent responses, its respective metabolic pathways could be targeted in creating therapeutics for chronic inflammation-associated pregnancies. The transport of these metabolic signals to the target cell could be via simple diffusion or carrier-mediated (Hardy et al., 2009; Weiler et al., 2017). During pregnancy, the role of exosomes in carrying immuno-metabolic signals to the target cell is still unclear and requires more attention. Although, the communication in the immune cells during pregnancy is crucial for fetal protection.
Immune System in Pregnancy: Simply Complex
During the first trimester of pregnancy MØ, DCs and NK cells infiltrate the decidual tissue surrounding the invading trophoblast cells (Ashkar et al., 2000; Shimada et al., 2006). The events of implantation and placentation along the first and early second trimester of pregnancy display a close resemblance to “an open wound” which requires strong inflammatory responses (Dekel et al., 2010). In the first trimester, the human decidua has been reported to demonstrate a high number of immune cells, such as NK cells (70%), MØ (20–25%), DCs (1.7%), T lymphocytes (3–10%) with relatively lower expression of B cells in the decidua (Bulmer et al., 1988; King et al., 1997; Aluvihare et al., 2004; Zenclussen, 2005; Wicherek et al., 2009; Benner et al., 2020).
Innate Immune Cell Cross-Talk During Pregnancy
DC-mediated NK cells activation induces innate immune response whereas, NK-mediated DC editing and maturation activate adaptive immune response (Ferlazzo and Morandi, 2014). DCs and NK cells have been successful in establishing a reciprocal cross-talk in the decidual tissue across the pregnancy, in a direct or in an indirect manner by either cell-cell contact or by cytokine secretions, respectively (Figure 2A).

FIGURE 2. Immununological changes in pregnancy across gestation and in complications. (A) Healthy pregnancy: Innate immune responses: HLA disparity on extravillous trophoblasts (EVTs) causes NK suppression (first Trimester), increased M2Ø expression, production of DCs recruiting chemokine CCL5, accumulation of immature immunosuppressive DCs in decidua (second Trimester) for progression of pregnancy, differentiation of immunosuppressive DCs into stimulatory DCs contributing to parturition (third Trimester). Adaptive immune responses: 10–20% CD3+ Tcells exists in first Trimester, increased exhausted and senescent T cell population, increased Th2, decreased Th1 and Th17, pro-inflammatory cytokines (IFN-γ, TNF-α, IL-2) stimulate MØ to regulate trophoblasts invasion by phagocytosis (second Trimester), Increased expression of Th1, TFh, and decreased expression of Th2 promotes parturition as the gestation ends (third Trimester) (B) Immune dysregulation in pre-eclampsia (PE)- Increased expression of decidual MØ, T helper 17 cells, CD8+ cytotoxic T cells, increased suppression of regulatory T cells (Tregs) all these events lead to inflammation. Hyper-activation of Tregs causes decreased cytotoxic and angiogenic responses from NK cells leading to perturbed placentation causing PE (C) Gestational Diabetes mellitus (GDM) Increased levels of non-metabolized glucose induces inflammasome activation causing cytokine storm (IL-1β, IL-18) and increased neutrophilia leading to hyper-inflammation (MØ) activation causes cleavage of CD163 surface marker and its release into circulation, MØ releases MØ migration inhibitory factor (MIF) that further stimulates Th1 and Th17 promoting inflammation in GDM pathogenesis (D) Preterm birth (PTB) Increased CD8+ cytotoxic T cells infiltrate the decidua, increased Th1, Th17, and decreased Treg expression promote inflammation. Decreased expression of exhausted and senescent T cells are involved in PTB pathogenesis.
It was shown that over 60% immature DCs (imDCs) in the decidua were in close vicinity of NK cells (Kämmerer et al., 2003). Displaying a pregnancy-specific interaction, this clustering phenomenon between DCs and NK cells have been observed at the maternal-fetal interface (Tirado-González et al., 2012). The progression towards the second trimester occurs when IL-1β and TNF-α induce MØ and produce DC-recruiting chemokines through the MAPK and NFκB pathways (Li et al., 2011). CCL2 is the main chemoattractant for MØ and CCL5 is the main chemoattractant for immature DCs (imDCs). This results in the accumulation of MØ and DCs in decidual tissue (Figure 2A). Overexpression of anti-inflammatory genes, such as TGF-β is also reported (Dekel et al., 2014). In the second trimester, decidual MØ differentiates into immunosuppressive DC-like cells. There is an interesting shift of such immunosuppressive DC-like cells to immunostimulatory DC-like cells in the third trimester of pregnancy. This demonstrated a conclusive shift of maternal-fetal immunotolerance to maternal-fetal immune-rejection (Figure 2A) (Wang et al., 2016). Decidual MØ is believed to initiate childbirth through increased expression of inflammatory mediators to promote uterine contraction, parturition, and placental detachment (Bollapragada et al., 2009). In humans and rats, the MØ population was found to be increased in the decidua and also recruited to the cervix during ripening prior to the parturition (Päzolt and Henkert, 1990; Sakamoto et al., 2005). MØ subtypes work altogether to execute an optimal trophoblast invasion and spiral artery remodelling during healthy pregnancy. This occurs to meet the nutritional and respiration demands of the growing fetus. During the invasion of EVTs into the uterine stroma, a combinational profile of M1/M2 is established (Jaiswal et al., 2012). For the sustenance of the uterus and hence to avoid its rejection, a shift towards a predominantly M2 phenotype is observed (Figure 2A) (Mor et al., 2011).
On the basis of CD-11c expression, MØ are classified into two distinct groups in the decidual tissue during the first trimester (Houser et al., 2011). CD11chigh and CD11clow secrete pro-and anti-inflammatory cytokines thereby helping in maintaining immune homeostasis during the first trimester while retaining defense against invading pathogens at the maternal-fetal interface (Houser et al., 2011). Conversely, gene expression profiling and surface marker phenotyping demonstrate that the term MØ resembles M2 skewed cells (Gustafsson et al., 2008; Repnik et al., 2008; Xu et al., 2016). Term MØ in decidua exhibit an immunomodulatory property with low expression of CD80/CD86 and produce major volumes of the immunosuppressive cytokine IL-10 (Heikkinen et al., 2003). Along with IL-10, trophoblast-derived macrophage colony-stimulating factor (M-CSF) in maternal monocytes have been proven to induce this M2 regulatory phenotype (Svensson et al., 2011). Soluble HLAG5 has been found to induce MØ by polarizing them to bear immunomodulatory phenotype exhibiting increased numbers of activated MØ (CD163 high) but decreased CD86 expression (Lee et al., 2015). Interestingly, in placental MØ pro-M2 genes like CCL2, CCL13, CCL14, and CD209 are hypomethylated to induce an M2-like phenotype and M1 phenotype is repressed by the hypermethylation of genes such as TLR-9, IL1B, IL-12 receptor β-2, and CD48 (Kim et al., 2012). To regulate angiogenesis in the feto-placental vasculature, a hallmark of organogenesis, placental MØ secretes VEGF and fibroblast growth factors (FGFs) like FGF2 (Demir et al., 2004; Loegl et al., 2016). Phenotypically characterized as M2-like, placental MØ can induce a pro-inflammatory response when activated via TLRs (Young et al., 2015; Thomas et al., 2021) and function to impart host defense within the placenta thus, triggering the local inflammation required for the initial development of the placenta (Young et al., 2015).
Adaptive Immune Responses in Pregnancy
T cells constitute 45–60% of the total leukocytes in the endometrium in the early proliferative phase but the percentage decreases at the time of pre-conception creating a conducive environment for implantation (Gomez-Lopez et al., 2010; Bulmer et al., 1991). CD3+ T lymphocytes are present around 10–20% (Figure 2A) of the endometrial stromal leukocytes in the first trimester. Among the entire T cell population, CD4+ T cells (30–45%) and CD8+ T cells (45–75%) along with Th2 and Th17 cells accounting for 5 and 2% of CD4+ T cells, respectively (Bulmer et al., 1991; Nancy and Erlebacher, 2014). Nearly 5–30% of CD4+ T cells are found to be Th1 (CCR4-CXCR3+CCR6−) cells and nearly 5% CD25hi FOXP3+ Treg cells are CD4+ T cells (Nancy and Erlebacher, 2014).
In the early phase of pregnancy, the inflammatory priming of PBMCs occurs at the implantation site (Germain et al., 2007). Circulating syncytiotrophoblast’s microparticle (STBM) stimulates the production of various inflammatory cytokines, like IL-12, TNF-α along with mild-level of IL-18, from monocytes leading to the establishment of mild systemic inflammation (Sargent et al., 2006). On the surface of CD4+ T cells, chemokine receptor expressions (especially CCR molecules) determine their trafficking patterns which include the recognition of target tissue, timing, and signals to receive (Knieke et al., 2012). To keep track of the number and movement of trophoblast and prevent excessive trophoblast invasion, Th1 cells secrete cytokines IL- 2, TNF- α, and IFN-g (Figure 2A) (Torchinsky et al., 2003). TNF-α has been reported to act as a protector of the fetoplacental unit and regulates trophoblast invasion, by altering trophoblast cell adhesion to laminin and inhibiting the mobility of trophoblast cells studied through in vitro approaches (Todt et al., 1996). TNF-α hikes the trophoblast-derived plasminogen activator inhibitor-1 (PAI-1) levels and neutralizes the invasive capacity of trophoblasts (Bauer et al., 2004; Renaud et al., 2005). It has been stated that IFN-γ is involved in vascular remodelling during the peri-implantation phase and IFN-γ mRNA expression has been visualized at the implantation sites of healthy pregnant women and the murine model (Delassus et al., 1994; Jokhi et al., 1994). IFN-γ has a critical role of regulating EVT invasion, by increasing apoptosis of EVT and/or decreasing protease activity. Contrary to the physiological roles of IFN-γ, it impels pro-inflammatory functions as it increases expression of HLA class I and II antigen and TLR in innate immune cells (Podaný et al., 1975) which in turn promotes various functions like isotype commutation, chemokine secretion, (MØ) activation, and increased phagocytosis (Raphael et al., 2015). Pregnant women in the third trimester when compared to the non-pregnant counterparts have a higher percentage of peripheral blood follicular T helper cells (Tfh), despite co-expressing markers, including programmed death (PD)-1, ICOS, or CXCR3. Pregnant women also reveal a notably higher percentage of CXCR3C Tfh cells than non-pregnant women, which may produce IL-6, IL-10, and IL-21, and particularly, PD-1/CXCR3 (Monteiro et al., 2017). Th9 cells, a subpopulation of Th2 cells differ by altered phenotypical and functional aspects, which subjects to PPARγ involved in fatty acid storage and glucose metabolism (Micossé et al., 2019). In the presence of TGF-β, Th-17 cells produce IL-9 which have an inflammation-inducing function. In mouse, IL-9 was reported to be present in the non-pregnant uterus. However, during pregnancy, high level of IL-9 remained in both the placenta and uterus pointing again to its role in local inflammatory immune responses which might pose a threat to the developing offspring (Habbeddine et al., 2014). IL-22 secreted by Th22 cells has been found to be relevant for physiologic immune regulation and pathologic allograft rejection, therefore could potentially harm the pregnancy (Jia and Wu, 2014). At the maternal-fetal junction, IL-22 promotes proliferation, reduces apoptosis of trophoblast cells, and positively affects their viability (Wang et al., 2013). IL-22 plays an important role in protecting trophoblast cells from pathogens and producing inflammatory immune responses following intrauterine infection (Graham et al., 2011; Dambaeva et al., 2018). IL-22 receptors (IL-22R) are located on placental villi, a subunit of IL-22R, IL-22R1, allows binding of IL-22 from dNK cells and decidual stromal cells (Dambaeva et al., 2018). The downstream IL-22/IL-22R1 pathway is said to be involved in the trophoblasts survival and maintenance of pregnancy. In a successful pregnancy, IL-22, Th17/Th2 and Th17/Th0 subsets were highly prevalent, and the mRNA expression of GATA-3, ROR-C, AHR, IL-4, IL-17A, and IL-22 were recorded at the site of implantation. However, mRNA expression of T-bet and IFN-γ was detected away from the site of implantation. Hence, for a successful pregnancy, the pertinent association of IL-22 and IL-4 production at the implantation site is proved (Logiodice et al., 2019).
Immune Tolerance in Pregnancy
In healthy pregnancy, the earlier defined Th1/Th2 paradigm shifted to Th1/Th2/Th17/Treg paradigm when the advancement in the understanding of feto-maternal immune cross-talk for building a fetal alloantigen tolerogenic environment became clearer. The shift of pro-inflammatory milieu to anti-inflammatory milieu majorly occurs during the second trimester of pregnancy where fetal tolerance is at its maximum while at the end of the third trimester of pregnancy shows the generation of fetal rejecting environment to induce parturition (Chaouat and Voisin, 1979; Saito et al., 2010). In the early pregnancy development of fetal tolerant surroundings takes place when the maternal immune system encounters paternal antigens on the fetus, which causes phenotypic suppression of maternal immune cells. This suppression of immune cells is contributed from both fetal and maternal side. It has been reported that even fetal immune cells in response to maternal antigens cause inactivation of inflammation producing fetal immune cells and expansion of anti-inflammation producing fetal immune cells. In addition, the construction of fetal trophoblasts is in such a way that they escape maternal immune cell attack. The cytotrophoblasts, and STB along with STBM do not express any variety of HLA or NOD-like receptor family CARD domain containing 5 (NLRC5) (Tilburgs et al., 2017). Thus, during healthy pregnancy, the alloreactivity of CD3+CD4+ T helper cell is suppressed in the absence of HLA class I and II antigens on villous trophoblasts. In contrast to villous trophoblast, EVTs expressed HLA C, a classical MHC class I molecule, and a non-classical MHC class I molecules HLA E, F, and G and MHC transcriptional activators such as NLRP2 (Tilburgs et al., 2017; Tilburgs et al., 2010). At the maternal-fetal junction, HLA-C histo-incompatibility has been recorded to induce a tolerogenic microenvironment (Tilburgs et al., 2009). Prior to implantation, paternal antigen-specific Treg cells accumulate and increase in number in the uterus after implantation. Intriguing results from (Mohr et al., 2019) showed how seminal plasma initiates the expansion of Treg cells specific to paternal antigens imparting tolerance to paternal alloantigen (Shima et al., 2015; Robertson et al., 2009). As the pregnancy progresses, the cellular responses of innate and adaptive immunity work in collaboration to strengthen and extend fetal tolerance. DCs drives differentiation of naïve T cells into Th2 and Tregs in response to fetal antigen exposure. Increased Th2 response causes secretion of anti-inflammatory cytokines like IL-4, IL-5, IL-6, IL-10, IL-13, and TGF-β thereby decreasing the local inflammation. IL-4 and IL-13 work in a paracrine manner and represses Th1 and Th17 immunities, respectively, and brings forth allograft tolerance (Figure 2A) (Mitchell et al., 2017). Another subset of T cells like CD8+ Tc cells upon indirect recognition of fetal antigens, undertake the fate of clonal deletion (Erlebacher et al., 2007) whereas, CD4+CD25 + Fox3+ Treg expansion has been found to establish and maintain an allogeneic pregnancy in both mice and humans(Zenclussen et al., 2005). Treg cells play a crucial role in the production of paternal antigen-specific tolerance (Rowe et al., 2012). Another physiological phenomenon of inducing tolerance during pregnancy is T cell exhaustion and senescence which are known to occur because of excessive stimulation of T cells. This causes T cells to lose their proliferative and cytokine secreting properties however, the exact mechanism leading to this is still unknown. T cell exhaustion and senescence is characterized by increased surface expression of inhibitory receptors like PD-1, TIM-3, CTLA4, LAG-3 and CD57, KLRG-1, respectively (Figure 2A) (Sugita et al., 2013). PD-1/PD-L1 (CD274) axis engages in the suppression of autoreactive immune effectors and to achieve T cell homeostasis. Through negative costimulatory interactions, the PD1/PD-L1 pathway can also suppress Th22 and Th9 cells (Wang et al., 2020a). Primarily, identified as a Th1-specific receptor, Tim-3 is present on the surface of the cell. These domains engage galectin-9 (Gal-9) to transduce an apoptotic signal which ultimately results in inhibition of Th1 responses (Zhu et al., 2005; Miyanishi et al., 2007). The interaction of Tim-3 and its ligand Gal-9, causes intracellular calcium influx which commence the supersession of Th1 and Th17 cells (Seki et al., 2008; Oomizu et al., 2012). Conversely, Tim-3 enhances Th2 immunity at the maternal-fetal junction thereby safeguarding the decidual stromal cells from inflammatory damages and apoptosis mediated by TLR (Wang et al., 2015a; Wu et al., 2015). Therefore, Tim-3 signalling during pregnancy may operate as a self-control mechanism in TLR-triggered inflammation (Wang et al., 2015a). CD-57 expression is indicative of shortened telomere inside the cell implying that the cell has lost the ability to proliferate conferring a suppressed state of immune cell which is required for preventing fetal rejection (Slutsky et al., 2019). Later in pregnancy, paternal antigen-specific tolerance disappears post-delivery which is earlier present during pregnancy (Rowe et al., 2012). In a study, cytokine analysis of serum from pregnant women revealed the increased levels of IL-1b, IL-6, IL-8, IL-12p70, L13, IL-15, IP-10, and FLT3-ligand in relation to gestational weeks while, serum IFN alpha-2, IL-1RA, IL-3, IL-9, IL-12p40, and soluble CD40L levels were increased with the advancement of the trimester (Holtan et al., 2015). As interpreted, the optimal increase in pro-inflammation in the third trimester of pregnancy is associated with the preparation for the healthy delivery.
Immune Dysregulation Causing Pregnancy Complications
Immune tolerance built by various diverse cellular interactions is the cornerstone for successful gestation and healthy outcomes. The breakdown of this mechanism is proved to be one of the causes for the pathophysiology observed in adverse pregnancy outcomes. Various studies have been performed to understand the immune dysregulation in the context of pregnancy complications like PE, GDM, and PTB.
Pre-Eclampsia
PE is indicated as a state of hypertension and proteinuria any time after 20 weeks of gestation and is categorized as early-onset PE (EOP) that presents before 34 weeks and late-onset PE (LOP) that initiates after 34 weeks of gestation. A hallmark of PE is a deficiency of EVT infiltration and spiral artery remodelling, which results in a placental microenvironment that is ischemic towards increasing oxidative stress (Cartwright et al., 2017). Hyper-activation of pro-inflammatory cells (M1, Th1, Th17, cytotoxic dNK cells) or hyper-activation of anti-inflammatory cells (M2, Th2, Treg, suppressive dNK cells) causes alterations in the process of placental formation leading to pre-eclampsia. M1 have been reported to have elevated levels than M2 in the decidua of patients with PE, with a total increase in the MØ numbers in PE patients when compared to healthy controls (Schonkeren et al., 2011). Uterine M1 by the action of TNF-α has been reported to inhibit trophoblast invasion and disrupt spiral artery remodelling (Renaud et al., 2005). Similarly, the cytotoxic capacity of CD8+ T cells has been involved in controlling trophoblast invasion. In a human study, CD3+ and CD8+ T cells were significantly increased in the maternal decidua of PE patients compared to normotensive controls, indicating that an inflammatory environment aids in the progression of the disease (Milosevic-Stevanovic et al., 2019). Higher Th17/Treg ratios in umbilical cord blood, peripheral blood, and decidua have been reported to be associated with preeclamptic women when compared to healthy pregnant and non-pregnant controls (Figure 2B) (Milosevic-Stevanovic et al., 2019). In addition, animal studies have shown that depletion of Tregs in early gestation results in the generation of an uncontrolled pro-inflammatory milieu that causes preeclampsia-like phenotype (Care et al., 2018). This is suggestive of an exacerbated pro-inflammatory response that disturbs the trophoblastic properties of migration, invasion, and proliferation thus causing PE. However, contradicting studies have also been reported to be involved in PE pathogenesis. Increased expression of cytotoxic CD8+ T cells in PE patients’ decidua basalis, has also been reported by few studies and is suggestive of their role in the pathophysiology of PE (Milosevic-Stevanovic et al., 2019). Moreover, the increased number of dNK cells, decidual Treg cells, and TGFβ-1 in pre-eclamptic women is connected with a profound notion that excess anti-inflammation or increased suppression of cytotoxic and angiogenic properties of dNK cells can also result in insufficient trophoblasts proliferation, migration, and invasion. Thus, indicating the need for a balanced spatio-temporal relationship between inflammation and anti-inflammation for adequate spiral artery remodeling (Figure 2B) (Zhang et al., 2019). Another important aspect of PE pathogenesis is increased obstructions in maternal blood flow during pre-eclampsia, due to which dNK cells cannot interact with trophoblast cells and with other decidual cells, thus are restrained in promoting an adequate trophoblast invasion, causing dysfunction in spiral artery remodeling in PE (Fraser et al., 2012). However, inconsistent results are found over the varied role of dNK cells in PE giving the explanation of geographical indications, that even the environmental factors have an impact in modulating the immune system (Valenzuela et al., 2012; Shashar et al., 2020; Steinthorsdottir et al., 2020).
Gestational Diabetes Mellitus
Affecting 15% of pregnant mothers in developing countries GDM is a metabolic disorder which if left untreated may result in PTB due to hyperglycemia (Salomon et al., 2016). Hyperglycemia in GDM is associated with increased inflammation which occurs due to activation of inflammasomes in trophoblasts. The potent reason behind this activation of the inflammasome is excessive glucose which induces NLRP3 resulting in the generation of pro-inflammatory cytokine storms mainly IL-1β and IL-18 (Figure 2C) (Han et al., 2015; Corrêa-Silva et al., 2018). Excessive neutrophilia, high glycaemic levels, and increased homeostatic model assessment of insulin resistance are associated with GDM diagnosis as early as in the first trimester (Figure 2C) (Sun et al., 2020). The increased numbers of neutrophils are intended to be more reliable than leukocyte numbers i.e., the neutrophil to leukocyte ratio is used as an inflammatory marker for diagnosis of GDM in the second trimester. In addition, during the third trimester of pregnancy for GDM prediction a serum delta neutrophil index representing increased neutrophil numbers and inflammation is adopted (Sahin Uysal et al., 2020). The innate immune system contributes to increased inflammation in GDM via inflammatory signals secreting monocytes (Chandra et al., 2012). Monocyte/MØ activation has been proposed to be an early predictor of GDM in as early as 14–16 weeks of gestation. A hemoglobin-haptoglobin scavenger receptor CD163 (sCD163) is scraped out of MØ as an activation marker of these cells and this shedding is significantly increased in GDM women thus, the increased circulatory levels of CD163 from the placenta as well as from adipose tissue are reflective of GDM (Figure 2C) (Dige et al., 2014). Another study reveals elevated levels of CD163 + cells, IL-6, TNF-α, and TLR2 are associated with a pro-inflammatory milieu in GDM patients when compared to healthy pregnancies (Ueland et al., 2019; Bari et al., 2014). Another MØ secretory signal, a pro-inflammatory cytokine known as MØ migration inhibitory factor (MIF) which stimulates TH1 cells, induces IL-17 release, and increases TLR-4 expression on MØ is used for GDM prediction (Figure 2C) (Yilmaz et al., 2012). Moreover, GDM susceptibility has also been determined by specific genotypes associated with MIF (Aslani et al., 2011; Zhan et al., 2015). Decreased Treg numbers are associated with GDM prognosis, as shown in multiple studies where subsets of suppressive Tregs, CD4+CD127LOW+/CD25 + Tregs and CD45RA Tregs were evaluated during GDM pregnancies and represented a decline of anti-inflammatory function of Tregs as early as in the first trimester of GDM pregnancy (Schober et al., 2014). In addition, CD4+ CD25 and CD4+CD25 + FOXP3 cells numbers were decreased whereas, TNF-α, a pro-inflammatory cytokine expression by Tregs (CD4+CD25 + FOXP3+CD127-) were found to be significantly upregulated in women with GDM pregnancies compared to women with healthy pregnancies (Schober et al., 2014). Aggravated circulatory CD4+ and CD8+ T cells responses in GDM pregnancy contribute to GDM pro-inflammatory milieu with significantly higher expression of CD69 (T cell activation marker) in insulin-untreated cases and higher expression of HLA-DR in insulin-treated cases (Lobo et al., 2018). Thus, the above-mentioned studies project towards an extensive pro-inflammatory build-up in GDM patients. In addition, increased levels of circulating Th-17 cells, a higher Th17: Treg cells ratio, and Th1: Treg ratios have been associated with GDM pregnancies compared to uncomplicated pregnancies (Sheu et al., 2018; Zhao et al., 2020). Thus, in order to predict a pregnancy complication only studying Th1/Th2 imbalance is insufficient however, a more comprehensive understanding can be attained by taking the Th1/Th2/Th17/Treg paradigm into consideration.
Preterm Birth
PTB is defined globally as any live birth that occurs before 37 weeks of gestation or less than 259 days. According to the world health organization (WHO), an estimated 15 million infants are born prematurely every year. One-fifth of those 15 million prematurely born infants across the world are, born in India PTB is stratified as spontaneous PTB with an intact membrane (sPTB-IM), induced PTB, preterm premature rupture of membrane (pPROM), and caregiver induced PTB. Among the PTB populations, the prevalence of sPTB is 40–45%, induced is 30–35% and pPROM is 25–30% (Goldenberg et al., 2008). The immunological status of an idiopathic PTB is more complicated than that of PE or GDM because of the absence of pathological cues. Whereas, the infection-induced PTB and labor are more frequently studied. Neutrophils are the phagocytic cells that reach predominantly at the infection site or site of injury thereafter recruiting other effector immune cells. Several rodent studies have reported that depletion of neutrophils prior to LPS administration could not delay the preterm labor however, it did help in reducing the IL-1 beta levels at the feto-maternal interface (Arenas-Hernandez et al., 2019; Gomez-Lopez et al., 2016) implicating an indirect role of neutrophils in creating an inflammatory milieu underlying PTB or pPROM. Histological evidence of PTB placentae has shown a more prominent invasion of CD8+ Tc cells indicating chorioamnionitis as similarly observed in cases of pPROM and fetal death (Figure 2D) (Galaz et al., 2020). Flow cytometric analysis of these cases revealed an influx of effector memory T cells, secreting high levels of perforins and granzymes at the feto-maternal interface in preterm labor (Arenas-Hernandez et al., 2019). The chorioamnionitis membranes in preterm placentae are infiltrated by the increased number of Th17 subtypes that release IL-17 at the maternal-fetal interface and also in amniotic fluid indicating a chronic inflammatory status (Figure 2D) (Ostojic et al., 2003; Wu et al., 2014; Lombardelli et al., 2016; Pinget et al., 2016). At the feto-maternal interface, the elevated expression of Th1 and Th17 related genes with declined FOXP3 expressions were associated with unexplained recurrent pregnancy loss and spontaneous abortion patients (Lee et al., 2011; Wu et al., 2016; Zhu et al., 2017). Invariant NK cells (iNKTs) are the bridges between innate and adaptive immunity, where they provide an intense immune activation by upregulating the signalling pathways responsible for Th1 and Th2 cytokine release (Miller et al., 2018). Studies have reported increased expression of iNKT in the first and third trimester of pregnancy thus, implying their roles during term labor (Wang et al., 2002; Boyson et al., 2002). Preterm murine studies have revealed an inverse relation of iNKT and Tregs at the feto-maternal interface (Gomez-Lopez et al., 2017). The expansion of iNKT cells was accompanied by increased Th17 and decreased Treg expression. Thus, inhibiting iNKT cells activation reduced the immune responses at feto-maternal interface, thus delaying preterm labour in mice (St Louis et al., 2016). Moreover, in humans increased expression of iNKT cells at the decidua were revealed in a transcriptomic analysis and immunophenotyping of lymphocytes in placentae of preterm cases when compared to control terms (St Louis et al., 2016). Given that iNKT cells are present at the murine maternal–fetal interface throughout pregnancy, other than the innate immune cells contributing to infection induced PTB, the adaptive immune cells also have important roles in PTB (Gomez-Lopez et al., 2017; St Louis et al., 2016). Exhausted and senescent T-cells are present at the maternal-fetal interface and help in regulating inflammation throughout gestation in a normal pregnancy. Chronic/repetitive antigen exposure on T cells can result in their functional loss which is identified by the expression of exhaustion markers such as TIM-3, PD-1, CTLA-4, and LAG-3. Whereas, T cell senescence is characterized by vanished proliferative ability along with the absence of these inhibitory markers and presence of senescent markers (increased CD57, KLRG-1 and decreased CD27 and CD28 (Wherry and Kurachi, 2015). In humans, CD4+T cells exhibiting effector memory phenotype showed upregulated expression of inhibitory marker PD-1 at the second trimester during normal pregnancy (Meggyes et al., 2020). During infectious preterm pregnancy, a decline in senescent CD4+/CD8+ T cell numbers and exhausted CD4+ T cell numbers have been reported at the feto-maternal interface (Slutsky et al., 2019). The existence of T cell subsets in the above-mentioned effector memory phenotypes concludes a pro-inflammatory milieu responsible for preterm labour leading to PTB (Figure 2D). Moreover, blocking the inhibitory markers using antibodies to PD-1, TIM-3 has been associated with increased rates of fetal loss and thus emphasizing the fact that balanced cellular exhaustion and senescence are required for the execution of a healthy pregnancy (Wang et al., 2015b). This was further supported by the observation that CD8+PD-1+TIM-3+ T cells were impaired in decidual tissues from women with miscarriage (Wang et al., 2015b; Slutsky et al., 2019). Another aspect contributing to the pregnancy complications as explained in PE and GDM also exists in PTB i.e., decrease in Tregs numbers. Immunophenotyping performed on the lymphocytes isolated from women undergoing preterm labor revealed that chorioamnionitis accompanied preterm labouring women at the time of delivery had significantly lower numbers of Tregs as compared to term labouring women (Xiong et al., 2010). Studies have revealed the existence of reduced Tregs at the feto-maternal interface in women with idiopathic preterm birth. In a mice model of endotoxin (LPS) induced PTB the depletion of Tregs in the third week of mice pregnancy resulted in PTB. The endotoxin-induced PTB was reversible by adoptive transfer of depleted Tregs from allogeneic mice, implying the importance of Tregs in delivering a full-term pregnancy (Gomez-Lopez et al., 2020). Moreover, human cellular studies are accompanied by cytokine studies, which represented a decrease in the levels of IL-10 an anti-inflammatory cytokine with each approaching trimester in PTB. Serum levels of IL-10 and IL-10 receptors in endometrial biopsy of women with preterm labor were also found to be lower when compared to women with normal labor (Pereira et al., 2016). However, the trigger behind the perturbed immune responses in idiopathic PTB still remains unclear and requires thorough investigations.
Pla-Xosomes: Connecting Link Between Immune Clock and Pregnancy Complications
Ongoing research for identification of the one triggering factor responsible for bringing about perturbations of the immune system that lead to such pregnancy complications and adverse pregnancy outcomes is still unknown. However, of the multiple studies underway that are being investigated for identification of this trigger, one such investigation involves the study of extracellular vesicles also known as exosomes (EVs). Discovered almost 40 years ago in 1989 (Trams et al., 1981; Pan and Johnstone, 1983; Harding et al., 2013), the extracellular vesicles named exosomes were characterized later as lipid-bilayered-intraluminal microvesicles (ILVs) (30–150 nm) yielded by invagination of multivesicular bodies (MVBs) derived from endosomes during stress response or for cell-to-cell communication (Harding et al., 1984). Exosomes being the most biological residual structures with the least cytotoxicity are loaded with cargo in the form of RNAs (miRNAs) (Menon et al., 2019), proteins (cytokines) (Pillay et al., 2020), hormones (estrogen, progesterone (Fitzgerald et al., 2018), cDNAs, and metabolites making them chief molecules of cell-cell communication (Kurian and Modi, 2019). Since exosomes act as power shots of clues/factors for regulating the proximal and distal cellular responses, they are being studied to unravel the trail leading to the trigger of immune dysregulation in pregnancy complications. The involvement of exosomes in facilitating feto-maternal cross-talk during a successful pregnancy through reported literature on the cargo investigated at regular stages of gestation has led to a deeper understanding of these power shots as physiological modifiers through their action on the immune system of the pregnant mother. Exosomes act as messengers between the fetal and maternal tissues during pregnancy, delivering their payload to target cells towards making an incremental functional impact. They also have crucial roles e.g., in embryo implantation (Kurian and Modi, 2019), accelerating the glucose metabolism (James-Allan et al., 2020), and acting as a mediator for executing immune responses bring about either activation, suppression, or tolerance (Mincheva-Nilsson and Baranov, 2014a). In early pregnancy, exosomes produced by the placental cells (pla-xosomes) induce endothelial cells and vascular smooth muscle cells to promote angiogenesis (Salomon et al., 2014b). Apart from maintaining the conducive environment for the healthy growth of the developing fetus, the inflammatory signals required to initiate parturition at the last trimester of pregnancy are also provided by exosomes (Sheller-Miller et al., 2018).
Exosomes Facilitate a Fetal Sustaining Environment During a Healthy Pregnancy
Exosomes from trophoblast cell lines have been reported to trigger the recruitment and differentiation of immune cells specifically monocytes. Placenta-derived exosomes (Pla-xosomes) concentration increases with each progressive gestation of a healthy pregnancy (Salomon et al., 2014a). Pla-xosomes can cause phenotypic changes in monocytes i.e., phagocytic classical monocytes (CD14++ CD16+) are transformed into intermediate monocytes (CD14 + CD16+) with enhanced migratory capabilities, and pro-inflammatory factors like IL-1beta, IL-6, serpin1, GM-CSF, M-CSF, and TNF-α are secreted (Al-ofi et al., 2012; Tagliani et al., 2011). These responses are essential to function in an optimal manner so as to provide regulated angiogenesis and invasion of trophoblast cells. Along with pregnancy, M1 polarization to M2 occurs to contribute to an anti-inflammatory phase for fetal survival (Figure 5B). This transition is caused by the presence of an immune checkpoint inhibitory molecule known as PDL-1 on the pla-xosomes (Petroff et al., 2003; Enninga et al., 2018). Effector responses of T cells have to be reduced in order to aid the successful growth of the fetus. Multiple mechanisms such as inhibition of T cell proliferation, T cells apoptosis, T regulatory expansion, and reduction of Tc cells occur so as to shield effector T cell responses (Figure 5B). The immune cells have been reported to express the FAS and TRAIL receptors. Interestingly pla-xosomes isolated from the placenta or that from blood biopsies express apoptotic molecules like FAS ligand and TRAIL, thus inducing apoptosis in Jurkat cells and activating PBMCs via their receptors in a dose-dependent manner (Stenqvist et al., 2013). In addition, pla-xosomes from maternal blood downregulate the expression of CD3 and JAK3 inhibiting T cell activation (CD4+ and CD8+) (Sabapatha et al., 2006). MHC class I chain-related (MIC) and UL-16 binding protein (ULBP) expression on pla-xosomes downregulates expression of NKG2D receptor on CD8+ T cells thus inhibiting their cytotoxic responses (Figure 5B) (Hedlund et al., 2009). Syncytin-2 an endogenous retroviral protein is expressed on pla-xosomes and has been reported to reduce Th1 cytokine secretion using PBMCs invitro culture causing immunosuppression (Figure 5B) (Lokossou et al., 2020). Although, pla-xosomes inhibit lymphocyte proliferation and induce regulatory/memory T cells differentiation in a similar manner the tumor-derived exosomes manipulate the immune cells by inhibiting immune cell attacks (Mikami et al., 2020; Yu et al., 2020). The induction of Tregs is crucial for the sustenance of the fetus during the second trimester of the pregnancy. EVs from BeWo cells showed expression of a 10 KDa heat shock protein which initiated the helper T-cell differentiation to Treg cells (Kovács et al., 2019). As described above the exosomes are potential mediators of cell-cell communication during a healthy pregnancy. The immune perturbations in pregnancy complications alter the cargo of exosomes and their numbers, which have been associated with perturbed pregnancies like pre-eclampsia, GDB, and PTB.
Pla-Xosomes in Adverse Pregnancy Outcomes
Preeclampsia
Compared to a healthy pregnancy, the placental EVs from PE patients remain in circulation for longer. Pla-xosomes levels in pre-eclamptic pregnancies in the third trimester have been reported to be elevated in comparison to healthy control (Pillay et al., 2016). Exosomal cargo has been described as biomarkers for pre-eclampsia. In the C19MC miRNAs, a set of placental unique miRNAs (miR- 517-5p, miR-520a-5p, and miR-525-5p) measured in the first trimester were reported as a biomarker panel (AUC: region underneath the curve 0.719) for predicting the PE prognosis (Figure 3D) (Hromadnikova et al., 2019). Proteomic studies on pre-eclamptic maternal plasma-derived exosomes have revealed higher expression of peptidase inhibitor (PAI)-1, S100 calcium-binding protein (S100b), TGF-β, VEGFR1, and natriuretic peptide B(BNP) (Tan et al., 2017; Tan et al., 2014) compared to their healthy counterparts. Increase in sFLT-1 (soluble fms-like tyrosine kinase-1) and sENG (soluble endoglobin), the causative agents of PE are found to have upregulated expression in PE exosomes compared to controls (Figure 3D) (Chang et al., 2018). Providing the indications of PE pathology, a reduction of immune-suppressive markers like PD-L1 and syncytin 1 or 2 (regulates M1 polarization, T reg cell differentiation, and inhibits T cell activation respectively) on exosomal membranes have been reported in preeclamptic patients (Levine et al., 2020). RNA sequencing has revealed elevated enrichment of mir-210 in preeclamptic patients that downregulates potassium channel modulatory factor 1 and thus inhibits trophoblast invasion (Luo et al., 2014). In pregnant mice, exosomes derived from the plasma of PE patients can induce PE-like phenotypes in the mother as well as the fetus (Sheller-Miller et al., 2019). PE STBs derived-EVs induces the production of superoxide by neutrophils which have been thought to surge the neutrophil extracellular traps (NETs) formation and showed more interaction with monocytes, MØ, thus increasing the pathological inflammation (Gupta et al., 2006). Pla-xosomes carry the destined cargo to prepare the mother by modulating the physiological, structural, and immunological status towards the healthy development of the fetus.
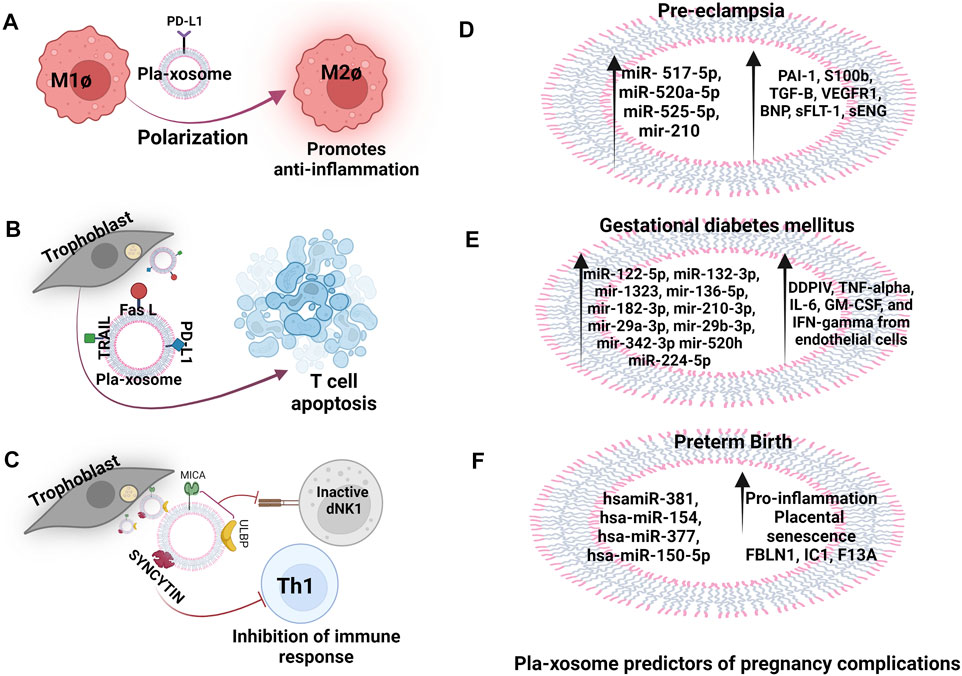
FIGURE 3. Pla-xosomes in heathy, complicated pregnancies and adverse outcomes. (A) Pla-xosome (PD-L1+) causes M1Ø to M2Ø polarization to increase anti-inflammation (B) Pla-xosomes (Fas L + TRAIL + PD-L1+) cause T cell apoptosis for regulated immunosuppression (C) Pla-xosomes (ULBP + MICA + Syncytin+) cause inhibition of innate and adaptive immune response to support restructuring of materno-fetal interface (D) Increased expression of pla-xosomal miR- 517-5p, miR-520a-5p, miR-525-5p, and miR- 210 and the proteins PAI-1, S100b, TGF-B, VEGFR1, BNP, sFLT-1, sENG as predictors (E) miR-122-5p, miR-132-3p, mir-1323, mir-136-5p, mir-182-3p, mir-210-3p, mir-29a-3p, mir-29b-3p, mir-342-3p and mir-520h along with proteins like DDPIV, GLP-1, tumor-necrosis factor-alpha (TNF-α), IL-6, GM-CSF, and IFN-gamma in GDM reported to be predictors of the disease (F) hsamiR-381, hsa-miR-154, hsa-miR-377, and hsa-miR-150-5p and proteins FBLN1, IC1, F13A are associated with preterm labour that causes increased placental senescence and inflammation.
Gestational Diabetes Mellitus
In humans, the PLAP content per exosome (PLAP ratio) is used to define the existence of placental exosomes in total exosomes. In GDM, this ratio has been found to be lower in comparison to normal pregnancy irrespective of the higher number of total and placental exosomes implying that there are alterations in the number of exosomes released by the placentae, increased non-placental exosomes secretion, or convergence of both (Salomon et al., 2016). Exosomes from the plasma of GDM patients also cause glucose intolerance, decreased glucose-induced insulin secretion, and poor insulin responsiveness (James-Allan et al., 2020). Exosomal miRNAs are extensively studied for the prediction of GDM in humans eg. miR-125a-3p, miR-99b-5p, miR-197-3p, miR-22-3p, and miR-224-5p are consistently detected in higher concentrations in the placenta, skeletal muscles, placental and total exosomes representing their metabolic involvement (Nair et al., 2018). In addition, miR-122-5p, miR-132-3p, mir-1323, mir-136-5p, mir-182-3p, mir-210-3p, mir-29a-3p, mir-29b-3p, mir-342-3p, and mir-520h have significantly higher expression in GDM cases than in controls and have been reported to be involved in trophoblast proliferation, differentiation and insulin regulation and glucose transport in pregnant women (Figure 3E) (Gillet et al., 2019). A urine exosomal study in GDM patients in the third trimester of pregnancy revealed that miR-516-5p, miR-517-3p, miR-518-5p, miR-222-3p, and miR-16-5p are present in lower levels compared to a healthy pregnancy (Herrera-Van Oostdam et al., 2020). Increased level of exosomal dipeptidyl peptidase IV (DDPIV) is associated with GDM pathogenesis and a mice study showed that inhibitors of DDPIV inhibit glucose homeostasis by cleaving glucagon-like peptide 1. This could be used to treat type 2 diabetes (Figure 3E) (Kandzija et al., 2019). Thus, not only exosomes can serve as predictors for pathological pregnancy like GDM but can also be used as target molecules for the assessment of given therapeutics. Hyperglycaemic condition induces exosomes release in GDM pregnancy and interestingly these exosomes promote the release of pro-inflammatory cytokines like TNF-α, IL-6, GM-CSF, and IFN-γ from endothelial cells, thus contributing to the pathological inflammation in GDM (Salomon et al., 2016).
Preterm Birth
Studies on placental-derived exosomes in PTB are less and limited. Exosomes have been reported to carry miRNAs involved in the regulation of trophoblast invasion, proliferation and angiogenesis as potential biomarkers for predicting PTB such as hsamiR-381, hsa-miR-154, hsa-miR-377, and hsa-miR-150-5p (Figure 3F) (Menon et al., 2019; Cook et al., 2019). A set of proteins (FBLN1, IC1, F13A etc.) from plasma exosomes collected at 10–12 weeks of gestation are reported to be associated with the diagnosis of moderate PTB with the area under the receiver operating characteristic curve of 0.74 (Figure 3F) (McElrath et al., 2019). A comprehensive analysis of miRNA profiles of maternal plasma-derived exosomes differs at term and preterm and the miRNA’s target genes are associated with TGF-β signaling, p53, and glucocorticoid receptor signalling (Menon et al., 2019). A comprehensive proteomic profiling of PTB plasma-derived placental exosomal cargo has further verified that the alterations in protein compositions are also associated with inflammatory and metabolic signals. Interestingly, the placental senescence that occurs due to the encounter of oxidative and mitochondrial stress is reported to be influenced by these inflammatory signals (Figure 3F) (Cook et al., 2019). Studies performed on amniotic fluid-derived exosomes from preterm patients have confirmed these results (Dixon et al., 2018). A study in mice and cows demonstrated that in-vitro btamiR-499 found in pla-xosomes isolated from early pregnancy collected plasma, inhibited the activation of NF-κB via Lin28B/let-7 axis (lin 28B is an RNA Binding Protein and let7 is its targeted a miRNA) in bovine endometrial epithelial cells, suggesting that placental exosomes have a vital role in regulating uterine inflammatory balance determining a threshold for the onset of labor (Zhao et al., 2018b). In-vivo studies on mice have revealed labor-triggering properties of exosomes isolated from plasma of CD-1 mice from late gestation (E18) (Sheller-Miller et al., 2019). It emphasizes the importance of exosomal signals in the early termination of pregnancy.
Similarities in the Development of the Placenta and Cancer
As pregnancy disorders involve the failure of feto-maternal cells to function normally, cancer begins with the failure of cells to reproduce and differentiate in a regulated manner. The development of the placenta and fetal-placental communication during pregnancy mimics a regulated form of cancer. Cancer manipulates the immune system for its survival in a similar manner as the placenta does for fetal survival. The cross-talk between cancer cells and immune cells is mediated via tumor exosomes (TEVs) (He et al., 2021). Interestingly, the cargo of TEVs also resembles similar to pla-xosomes indicating initiation of some similar pathways e.g., angiogenesis, T cell suppression, and expansion of anti-inflammatory responses during the growth spurt, later we will be exploring these aspects in detail. Expression of factors such as angiopoietins and members of the VEGF family occurs in placental and cancer development to aid in angiogenesis (Shore et al., 1997; Charnock-Jones et al., 2004). Therefore, a similarity can be drawn between the cellular invasion of EVT and cancer cells as early events in both the cases. Both of these cell types use the epithelial-to-mesenchymal transition to promote movement across the endometrium (during placental development) or normal (cancerous growth) tissue (Yang and Weinberg, 2008).
Just like tumor cells are found in the systemic circulation, intact trophoblasts are also known to circulate in maternal peripheral blood during the early first trimester of pregnancy. Irrespective of HLA disparity these fetal-derived cells can embed in the maternal system establishing long-term microchimerism that persists for decades after parturition as a change accepted by the maternal immune system (Evans et al., 1999). Apart from the similar mechanism of development, the process for evading host immune response in cancer and trophoblast is also similar. Total or selective loss of HLA class I molecules is a frequently reported mechanism in various human tumors to escape recognition and destruction by cytotoxic T lymphocytes cells (Garcia-Lora et al., 2003). Trogocytosis (i.e., rapid cell-to-cell contacts that are dependent upon membrane transfer) is the primary mechanism by which HLAG + suppressive NK cells are generated within a tumor microenvironment (Caumartin et al., 2007). This mechanism is similar to HLA variants protection of trophoblasts in pregnancy where the trophoblast escape NK cell attack by inducing killer inhibitory receptors on NK cells reference from above (Figure 4A). Cancer cells also present the HLA class II antigen in the absence of the CD80/CD86 universe-stimulating molecules, this frequent representation of cancer cell antigens drives T-cell anergy thus, imparting cancer tolerance (Byrne and Halliday, 2003). Immune tolerance against cancer cells may also be the result of the knockout of lymphocyte lines that respond against autoantigens called tumour-associated antigens (TAA). These TAAs are abnormally expressed or overexpressed on malignant cells and is present in dissolved form in the circulation (Ko et al., 2003). Whereas, in the fetus, a combination of maternal and paternal antigens could contribute in chronic stimulation of T-cells thereby disrupting their effector functions. To ensure clearance from the immune system tumours are able to destroy immunocompetent T cells through a FasR/FasL-dependent mechanism causing T-cell apoptosis (Byrne and Halliday, 2003). A similar mechanism is executed by trophoblast cells for inducing T cell apoptosis. The tumor itself is resistant to Fas-mediated lysis by activated lymphocytes presumably because tumor cells overexpress BCL2 in the cytoplasm (Mese et al., 2000). Expressions of BCL2 have also been shown along the gestations in trophoblast cells however, contradicting studies revealed that expression of BCL2 is higher in the first and second trimester whereas, it has lower expressions in the third trimester of pregnancy emphasizing on the notion of pregnancy mirroring a regulated form of cancer which is a spatio-temporal need of the mother and the developing fetus (Soni et al., 2010). Just like fetal signals drive naïve T-cell differentiation into T regs, the tumor-specific antigens cause expansion of Treg cells in cancer implicating an impaired antitumor immunity, suppressed T cell proliferation, and increased tumor blood vessel density. This dampens the antitumor immune responses to promote angiogenesis (Beyer and Schultze, 2006). Immuno-regulatory mechanisms protect the fetus from the NK cell attack in the decidua. It was shown, Prostaglandin E2 (PGE2) (Figure 4B) which is derived from and localized in decidua aids in protecting the fetus by hindering the production of IL2 and the IL2 receptors on NK and T cells (Munn et al., 1998). This mechanism of host immune protection is hijacked by cancerous cells (Park et al., 2018). During pregnancy, membrane-bound and soluble molecules like LAG-3, Tim-3, PD-1, CTLA-4, and TIGIT are found which influence the Treg cell functions by decreasing the effectiveness of pro-inflammatory T cells (Zhang and Sun, 2020). Signals from cancer cells induce the expression of inhibitory receptor PD-1 on effector T cells setting them in a resting stage also known as T cell exhaustion. During the last decade PD-1, PD-L1 and CTLA-4 inhibitors have been used and were successful in aborting the solid tumours by setting the immune cells in their attacking state (Homet Moreno and Ribas, 2015; Robert, 2020). CD200 (OX-2) (Figure 4C) and carcinoembryonic antigen-related cell adhesion molecules (CEACAM-1), the cell surface tolerance signals exist commonly between trophoblasts and cancer cells (Clark et al., 2003; Gray-Owen and Blumberg, 2006). In-vitro, trophoblasts expressing CD200 can inhibit the generation of CD8+ T cells called cytotoxic lymphocytes (CTLs) and shift the balance of cytokines towards TH2 (Clark et al., 2003). CD200 in TME of melanomas, ovarian cancers, and renal cancers suppresses Th1 cytokines in-vitro (Moreaux et al., 2006). Inhibition of NK-mediated cytolysis also occurs by CEACAM-1 (CD66a), expressed on trophoblasts, whereas, CEACAM-1 in tumor cells diminishes expression of NKG2D receptors on NK cells, thus suppressing NK cytolysis implying another common link between cancer and pregnancy (Gray-Owen and Blumberg, 2006). A chemokine produced by trophoblasts known as RANTES is known to induce apoptosis of fetal-reactive CD3+ cells and the same chemokine is shown to be secreted by tumor-infiltrating lymphocytes following their apoptosis creating a mechanism for immune response evasion (Fraccaroli et al., 2009). Importantly, Indoleamine 2,3-dioxygenase (IDO) (Figure 4D) a tryptophan degrading enzyme is required for maintaining the tolerogenic state at the feto-maternal interface as well as in tumor microenvironment (TME) (Munn and Mellor, 2016). This enzyme converts tryptophan to kyneuirine, an effector T cell toxic compound inhibiting their proliferation and causing T cell apoptosis (Hwu et al., 2000). In a study performed on mouse models the action of enzyme IDO, when expressed at the interface of fetus and mother by MØ and trophoblast cells, was shown to be required for the protection of the semi-allogenic fetus. Moreover, the inhibition of IDO turned out cynical and lead to the death of the semi-allogeneic fetus (Munn et al., 1998). Whereas, IDO in TME, positively regulates the activity of Treg cells and this property has been used for the advantage of immunotherapy with IDO inhibitors (Yentz and Smith, 2018). In women with normal pregnancies, soluble CD30, a member of the tumor necrosis superfamily of receptors and a marker of TH2 polarization, is increased, while it is reduced in women with PE and intrauterine growth retardation (Figure 4D) (Kusanovic et al., 2007). Microarray analysis of placentae from pre-eclamptic pregnancies revealed changes in gene expression pathways including angiogenesis, immune defense responses as well as apoptosis, and cell survival which is also associated with cancer (Louwen et al., 2012).
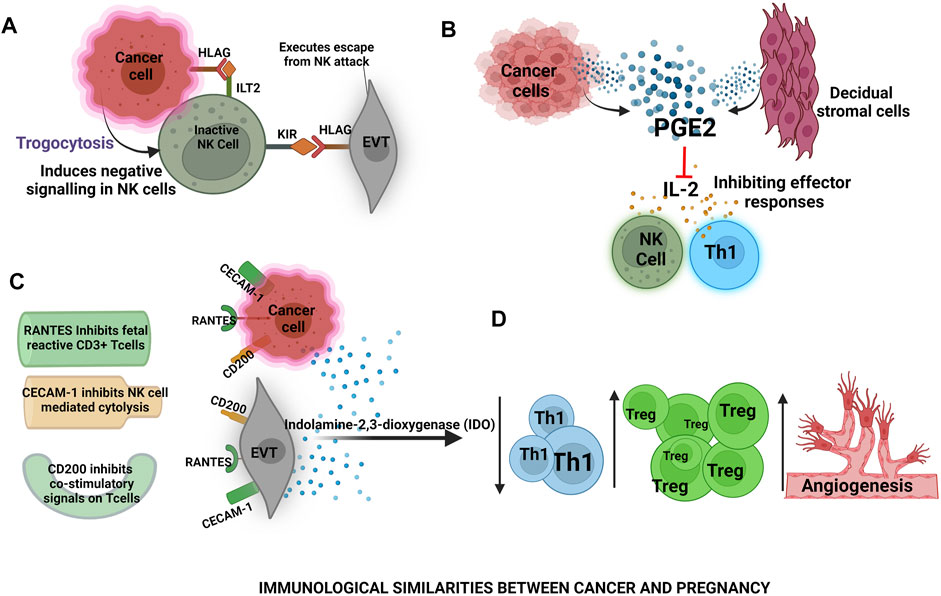
FIGURE 4. Immunological similarities between cancer and pregnancy- (A) In cancer, HLA-G causes suppression of NK cells activity and in placental development trophoblasts expressing the human leukocyte antigen G-5 (HLAG) induce killer inhibitory receptors on NK cells (B) Cancer cells and decidual stromal cells release prostaglandins that inhibit IL-2 production thus, masking pro-inflammatory responses by NK cells and T helper 1 cells (C) RANTES, CECAM-1, CD200 are the common surface molecules among cancer cells and extravillous trophoblasts (EVTs) that inhibit pro-inflammatory cellular responses (D) Cancer cells and EVTs secrete indolamine-2,3-dioxygenase (IDO) which is toxic to Th1 cells thereby ameliorating the Th1 expression and increasing regulatory T (Treg) cell responses to promote angiogenesis for the fetus and cancer survival.
Cancer Escaping the Immune System: Unraveling the Trail of Cancer-Derived Exosomes (CEVS)
Pregnancy and cancer connect with each other at another aspect that is immunomodulation via exosomes. Studies have demonstrated the presence of similar signalling molecules (RNAs and/or proteins) encapsulated inside cancer-derived and placental-derived exosomes. Rigorous studies carried out in the field of cancer provide the initial understanding of the mechanistic pitfalls that may lead to pregnancy complications and adverse outcomes. The manipulation of host immune cells by cancer derived-exosomes to strengthen a tolerogenic milieu for the progression of cancer has been very well studied. This well-trodden path in the field of cancer biology can be tested using appropriate animal models and subsequent clinical trials to restore the lost tolerance and recreation of the anti-inflammatory milieu for the betterment of pregnancy complications. Therefore, it would be interesting to track the trail of cross-talk of cancer- and host immune cells via exosomes.
CEVs Modulate Innate Immune Cells
CEVs deviate the conventional pathway of the expansion of the myeloid and bone marrow precursor cells that are committed towards stimulatory DC into their suppressor phenotypes thus, altering the cancer antigen presentation via DCs and augmenting the tolerogenic niche (Ning et al., 2018; Tung et al., 2018). The miRNA-212 in pancreatic CEVs upon its internalization in DCs, downregulates the expression of transcription factor RFXAP (Regulatory factor X associated protein) which simultaneously demeanours the expression of MHC-II on DCs affecting the antigen presentation via these DCs (Ding et al., 2015). Moreover, CEVs interfere with the expression of co-stimulatory molecules like MHC‐II, CD80, CD86 on DCs and increase the expression of co-inhibitory receptors on DCs like PD-1. Thus, affecting the maturation and migration process of DCs and converting the existing DCs into suppressive phenotypes (Ludwig et al., 2018). Another in vivo study on pancreatic cancer reported that in DCs, CEVs affect their proliferation and expansion by down-regulating TLR4, downstream TNF-α, and IL-12 cytokines via miR-203 (Figure 5A) (Zhou et al., 2014). CEVs also modulate MØ, since mutation acquired abilities of cancer cells enable them to hijack M1 and re-engineer them into M2. The existence of M2 polarized state in malignant cancer forte, expressing functional Arg1, VEGF, and CD163, CD23, CD204, along with cytokines like IL-10, TGF-β, TGF- α, and chemokines including CCL16, CCL17, and CCL22, confirms a congenial M2 state (Cheng et al., 2019). Increased cancer growth creates a hypoxic environment, which results in the release of CEVs that polarizes M1 into M2 in a HIF-1α and HIF-1β dependent manner (Figure 5A) (Hood et al., 2011). Thus, CEVs manipulate M1 to exhibit M2 anti-inflammatory phenotype to help aid angiogenesis for fulfilling the oxygen demand of growing cancer. Interestingly, ovarian CEVs carrying miRNAs like mir-222-3p, have been shown to disrupt Treg/Th17 immune balance. They have been implicated in inducing M2 polarization via STAT-3 signal-dependent pathway thereby, increasing Treg and M2 expansion. Besides a decrease in the Th-17 cell population has been observed contributing to the anti-inflammatory cancer microenvironment (Ying et al., 2016). CEVs also have been reported to inhibit caspases involved in apoptosis and transfer a functional receptor tyrosine kinase initiating the monocyte MAPK pathway (Song et al., 2016). Thus, these altered MØ can then encourage angiogenesis and metastasis required for cancer progression. Another important innate immune subset, NK cells contain switches in the form of activating as well as inhibitory receptors. Apoptosis of cancer cells in prostate cancer and acute leukemia is prevented by CEVs internalization in NK cells, which inhibits the expression of NK activating receptors like NKG2C, NKP30, NKP44, NPK46, and NKG2D (Figure 5A) (Garcia-Iglesias et al., 2009). CEVs have also been shown to target the TGF-β pathway, TGF-β which exists as TGF-latency associated peptide (LAP) in CEVs when bound to integrin a6βV is activated and induces Smad phosphorylation subsequently reducing NKG2D expression thus preventing NK cell cytotoxicity (Szczepanski et al., 2011). In a mice model, CEVs treatment affected the generation of NK cells and also impaired their responses. CEVs encapsulate the stress-inducible NKG2D ligands, MHC-class I related protein chain A/B (MICA/B) and Ul-16 binding protein-1 (ULBP-1) and -2 that acts as a decoy, by down-regulating the NKG2D-mediated cytotoxicity of NK cells in T- and B-cell leukemia/lymphoma (Clayton and Tabi, 2005; Mincheva-Nilsson and Baranov, 2014b). In addition, CEVs suppressed the cyclinD3 expression and inactivate the JAK3 pathway by inhibiting IL-2 stimulation via NK cells thereby, breaking one connective link in innate and adaptive immunity by preventing T cell interaction with NK cells. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function (Liu et al., 2006). However, as disconnecting a single link cannot produce desirable results, thus CEVs interact with adaptive immune cells too.
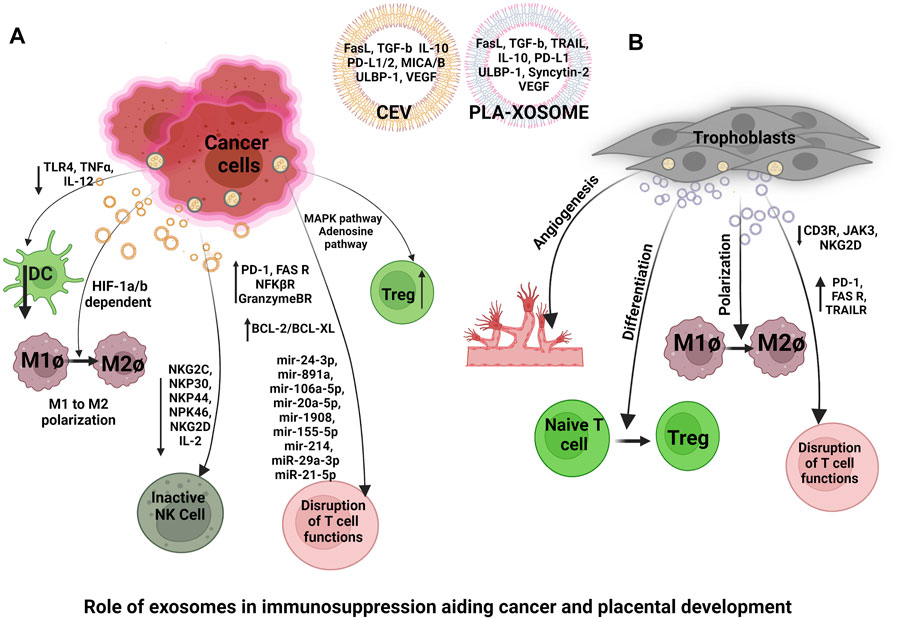
FIGURE 5. Targets of cancer-derived exosomes (CEVs) and placenta derived exosomes (Pla-xosomes)- (A) CEVs contains mir-203 which downregulates expression of toll-like receptor 4 (TLR4), downstream tumor necrosis factor-alpha (TNF-α) and interleukin-12 (IL-12) cytokines responsible for DCs proliferation and expansion, CEVs causes M1Ø to M2Ø polarization in a HIF-1a and HIF-1b dependent manner to promote immune suppression, CEVs internalization in NK cells, inhibits the expression of NK activating receptors like NKG2C, NKP30, NKP44, NPK46, and NKG2D to escape NK cell cytotoxicity, CEVs increases FasL/FasR signalling, PD-L1/PD-1 signalling and BCL2 (anti-apoptotic protein) expressions to evade apoptosis of cancer cells. CEVs carrying miR-29a-3p and miR-21-5p, miRNA 155-5p, miRNA-214, miR-24-3p, miR-891a, miR-106a-5p, miR-20a-5p, and miR-1908 inhibits T-cell activity, CEVs also cause T reg expansion thus aiding cancer development (B) Pla-xosomes promote angiogenesis via VEGF, help in Treg expansion, M2Ø polarization, causes upregulated expression of PD-L1, Fas, TRAIL and downregulates the expression of CD3 receptor, JAKR and NKG2D. All of these are essential to promote an effective immune microenvironment in the mother.
CEVs Modulate Adaptive Immune Cells
CEVs express CD39 (NTP-Dase) and CD73, which work together to convert extracellular ATP to immunosuppressive adenosine and 5 AMP phosphate (Clayton et al., 2011; Muller et al., 2016). Extracellular adenosine production is high, which adversely affects T cells around cancerous tissues, allowing it to evade immune responses. In addition, the presence of CEVs carrying miR-24-3p, miR-891a, miR-106a-5p, miR-20a-5p, and miR-1908 inhibits T-cell activity in nasopharyngeal cancer (Figure 5A) (Bell and Taylor, 2017; Ye et al., 2014). Interestingly, co-culturing CEVs with T cells resulted in elevated expression of BAX (proapoptotic marker) and decline in expression of BCL-2/BCL-XL (anti-apoptotic markers) indicating cancer mediated T cell suppression (Figure 5A). FasL in CEVs causes the apoptosis of FasR + T cells by initiating FasL/FasR signalling (Alzahrani et al., 2018). According to an analysis of EVs recovered from the serum of patients with head and neck cancer and melanoma, cell death ligands such as Fas on CD8+ cytotoxic T lymphocytes (CTLs), were particularly sensitive to CEVs. They affected signal transduction and proliferation of CD8+ CTLs thus, affecting cytotoxic responses on cancer cells (Maybruck et al., 2017). Peritoneal tissue from patients with metastatic ovarian cancer had higher Treg levels than Th17 cells suggesting a requirement of more suppressed TME for metastasis (Zhou et al., 2018). It was found that exosomes play a unique role in this imbalance. Favoring T reg functions, exosomes originating from TAMs transfer miR-29a-3p and miR-21-5p to helper T cells and inhibit intracellular STAT3 signalling which decreases pro-inflammatory cytokine secretion from CD4+ T cells (Figure 5A). This disturbs the Tregs/Th17 balance creating an immunosuppressive environment for ovarian cancer progression (Zhou et al., 2018). In addition, there have been recent reports of CEVs containing PD-L1, which inhibit the immune system by targeting multiple pathways, thus aiding cancer growth (Figure 5A) (Mrizak et al., 2015). The transfer of PD-L1 via CEV from PD-L1high cancer cells to PD-L1low cancer cells elevated the PD-L1 release which further inhibited the T cell response by initiating PD-L1/PD-1 signalling. The membrane-bound PD-L1 carried by exosomes suppresses anti-cancer immune responses both locally in the TME and systemically. PD-L1+ exosomes produced by a breast cancer cell line inhibited co-stimulatory molecule (CD3/CD28) -induced ERK phosphorylation and NFκB activation of T-cells in vitro. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth (Yang et al., 2018). The experiment carried out in-vivo revealed suppression of granzyme B activity of T cells found in the TME, thus reducing cytotoxic T-cell activity (Vignard et al., 2020). In another study, exosomes isolated from head and HNSCC patients’ plasma inhibited the activatory receptor CD69 expression on human activated CD8+ T cells, and the PD-L1 levels on exosomes correlated with their T-cell inhibitory activity. Murine CEVs carrying PD-L1 were immunosuppressive, and blocking of PD-L1 activity with neutralizing mAbs restored the immune competence of T cells and inhibited tumor growth (Theodoraki et al., 2018). CEVs caused the expansion of Tregs. Tregs are one of the most important subsets of T-cells required for sustaining the development and growth of biological entities. Secretion of anti-inflammatory cytokines like IL‐10, TGFβ-1, and CTLA4 promotes the suppressive phenotype of Treg which is immensely exploited by cancer cells. Researchers have confirmed the transformation and proliferation of CD4+CD25+ T-cells into CD4+CD25 + Foxp3+ Tregs in-vivo upon administration of CEVs via MAPK pathway and adenosine pathway (Figure 5A) (Mrizak et al., 2015) miRNA-155-5p and miRNA-214 in CEV inhibited the precursor T-cell differentiation into Th1/Th17 phenotypes and reduces the PTEN-tumor suppressor homolog in T cells respectively, therefore increasing anti-inflammation and decreasing pro-inflammation parallelly (Figure 5A) (Yao et al., 2012; Sharma et al., 2015; Sun et al., 2019). In-vitro, CEV’s surface markers CD39 + CD73+ (NTPDases) bind to the T cell surface adenosine receptor 2 (A2AR) and send out a signal via cAMP. This upregulates the T cells to generate adenosine and prime Tregs thereby inducing their effector responses (Clayton et al., 2011). The elevated content of CD39/CD73 in CEVs reflected the presence of advanced-stage disease in HNSCC patients. These studies give strong evidence of impaired host immune response directed via CEVs (Allard et al., 2017). Interestingly, analyzing T cell-derived exosomes from cancer patient’s plasma for clues of the immune status in CEVs-reprogrammed T cells has recently become possible. Chimeric antigen receptor (CAR+) exosomes derived from CAR-T cells administered in cancer patients are enriched in immunosuppressive proteins and consistently inhibit functions of other T cells, thus their internalization causes intracellular changes in T cells (Fu et al., 2019).
Therapeutic Potential of Exosomes in Pregnancy Complications
The role of exosomes in cancer diagnosis and immune therapy has been extensively studied. As mentioned previously, cancer cells release PD-L1+ exosomes that interact with T cell’s surface PD-1 initiating intracellular suppressive signalling. In the advanced stages of cancer expression levels of soluble PD-L1 are increased that can be detected in circulation thus, cancer-derived exosomal PD-L1 can serve as cancer predicting biomarker (Figure 6A) (Shimada et al., 2021). Even for cancer therapy, the immune checkpoints are known targets for inhibitory antibodies. Moreover, the use of human umbilical cord blood mesenchymal stem cells-derived exosomal mir-503-3p has been reported to abort endometrial cancer and target biological functions of endometrial cancer cells by downregulating mesoderm-specific transcript (Pan et al., 2022). However, the use of exosomes for providing therapies in pregnancy complications is a big challenge because of the need for a balanced treatment at a particular time, simultaneously protecting the fetus from any harm. Irrespective of the challenges, multiple trials for creating therapeutics in restoring the balance of healthy pregnancy processes in pregnancy complications have been attempted. For e.g., in a mouse model study, exosomes from human umbilical cord mesenchymal stem cell-derived (HUMSC) exosomes have been reported to improve endometrial injury by stimulating endometrial regeneration via PTEN/AKT signalling pathway. This further increases the expression of BCL-2 (anti-apoptotic protein) via AKT activation and decreases the expression of activated caspase-3 facilitating cell proliferation thus promoting endometrial regeneration (Wang et al., 2020b). Another study demonstrated that the administration of HUCMSC exosomes results in upregulation of mir-18b-3p, which targets leptin to reduce pro-inflammatory factors and prevent cellular apoptosis in the PE rat placenta (Huang et al., 2021). Interestingly in the mouse model of PE, the therapeutic effects of HUCMSCs derived EVs have been reported where administration of HUCMSC-exos during pregnancy prevented soluble Fms-like Tyrosine kinase (sFLT-1) induce preeclamptic complications. sFLT is a negative regulator of VEGF thus aiding angiogenesis, HUMSCs-exos input resulted in decreased sFLT levels thereby, ultimately improving the fetal and placental weight. The exosomes have engineered to encapsulate IκBa that inhibit pro-inflammatory cytokine transcription factor NFkB in feto-maternal uterine tissues thus, delaying LPS-induced PTB (Sheller-Miller et al., 2021). Administration of mesenchymal stromal cell-derived extracellular vesicles alters inflammatory mediators’ expression in the preeclamptic intrauterine compartment, thus normalizing the formation of fetal lung branches and their morphogenetic gene expressions (Taglauer et al., 2021).
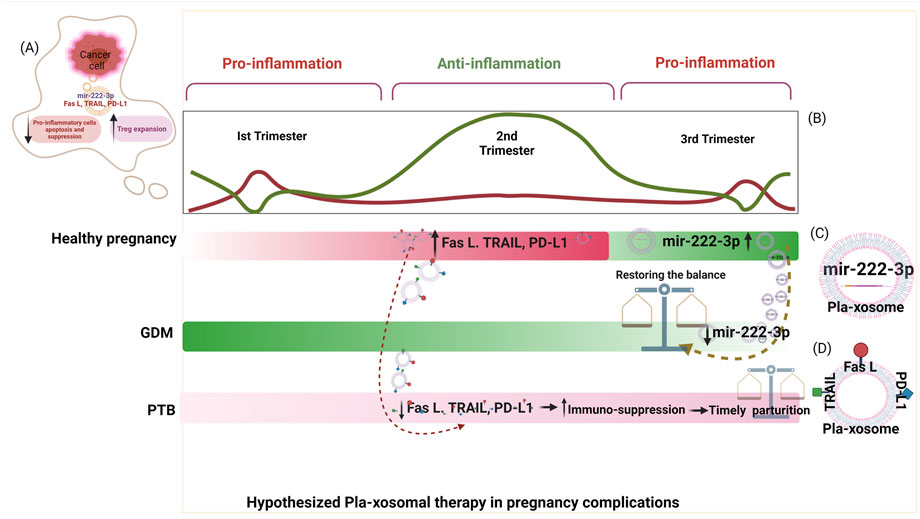
FIGURE 6. Hypothesized pla-xosomal therapy for pregnancy complications: Taking lessons from cancer (A) Cancer cells derived exosomes contain mir-222-3p, PD-L1, Fas L, and TRAIL that cause suppression of pro-inflammatory cells and expansion of anti-inflammatory cells (Tregs) (B) Balanced pro-and anti-inflammatory milieu in a healthy pregnancy with increased expression of PD-L1, Fas L, TRAIL in second Trimester and mir-222-3p in third Trimester (C) Pla-xosome containing mir-223: postulated therapy for GDM (D) Pla-xosome containing PD-L1, Fas, TRAIL: postulated therapy for PTB. Dotted lines represent the donor and recipient gestational windows of plax-osomes intervention.
Taking Lessons From CEVs
Due to the uncanny resemblance of the underlying biological processes of pregnancy with cancer, the signal carrying exosomal cargo in both are also close to similar. The immunosuppressive entities harbored in the exosomes e.g., PD-L1, VEGF, MICA, ULBP-1, HLA variants, Fas L, TRAIL, IL-10 etc. target similar immune cell subsets like Th1, Tregs, DCs and NK cells thus, promoting the anti-inflammatory niche required for the fetus and cancer development post its implantation and establishment respectively (Figure 6B). Interestingly, ovarian-cancer-derived exosomes contain mir-222-3p that is shown to increase Tregs thus, promoting anti-inflammation required for cancer survival (Stenqvist et al., 2013). Whereas, in GDM patients the expression of placental derived exosomal mir-222-3p significantly decreases by the third trimester and affects the metabolic processes like steroid hormone biosynthesis and tryptophan metabolism triggering insulin resistance and inflammation in GDM (Herrera-Van Oostdam et al., 2020). However, as a healthy pregnancy progresses, elevated levels of mir-222-3p have been observed, implying that the increased expression of this miRNA is a requirement for an uncomplicated pregnancy (Herrera-Van Oostdam et al., 2020). Since the mir-222-3p is enriched within placental exosomes, these exosomes could be used in a spatio-temporal manner to ameliorate pregnancy complications like GDM as a therapeutic agent (Figure 6C). Similarly, the apoptosis-inducing ligands like FasL, TRAIL, and immune exhaustion markers like PD-L1 are enriched on pla-xosomes and their isolation would be more appropriate from the second trimester of a pregnancy where an anti-inflammatory milieu is a necessity for fetal development (Figure 6D) (Stenqvist et al., 2013). These pla-xosomes may be used as therapeutics for treatment in pregnancy complications like PTB where inflammatory responses are high causing early parturition. However, isolation and delivery of these power shots should be carried out in a timely manner i.e., pla-xosmes isolated from the second trimester of a healthy pregnancy need to be administered to a high-risk mother diagnosed for preterm delivery so as to decrease the inflammation and lengthen the gestational age in utero. However, for such a successful execution of this hypothesized therapy, the identification of early predictive markers for adverse pregnancies is an obligation and clinical trials are vital.
Author Contributions
HD contributed to the planning, literature search, writing, and diagrammatic representations. RK contributed in refining some sections of the review. AM was responsible for assisting in the literature search. SB supported the idea and provided inputs. PK contributed to the conceptualization, planning, supervision, implications, and final editing.
Funding
We acknowledge that this research work has been conducted under the Project (BT/PR32851/MED/97/461/2019) funded by the Department of Biotechnology, Ministry of Science, Government of India.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the funding agency and Manju Kumari, Manivannan J, Pragya Tailor and Arpita Singh towards their contribution in preparation the manuscript. I thankfully acknowledge BioRender as the figures are created with BioRender.com.
References
Al-ofi, E., Coffelt, S. B., and Anumba, D. O. (2012). Monocyte Subpopulations from Pre-eclamptic Patients Are Abnormally Skewed and Exhibit Exaggerated Responses to Toll-like Receptor Ligands. PLoS One 7 (7), e42217. doi:10.1371/journal.pone.0042217
Allard, B., Longhi, M. S., Robson, S. C., and Stagg, J. (2017). The Ectonucleotidases CD39 and CD73: Novel Checkpoint Inhibitor Targets. Immunol. Rev. 276 (1), 121–144. doi:10.1111/imr.12528
Alpini, G., Glaser, S. S., Zhang, J. P., Francis, H., Han, Y., Gong, J., et al. (2011). Regulation of Placenta Growth Factor by microRNA-125b in Hepatocellular Cancer. J. Hepatol. 55 (6), 1339–1345. doi:10.1016/j.jhep.2011.04.015
Altmäe, S., Martinez-Conejero, J. A., Esteban, F. J., Ruiz-Alonso, M., Stavreus-Evers, A., Horcajadas, J. A., et al. (2013). MicroRNAs miR-30b, miR-30d, and miR-494 Regulate Human Endometrial Receptivity. Reprod. Sci. 20 (3), 308–317. doi:10.1177/1933719112453507
Aluvihare, V. R., Kallikourdis, M., and Betz, A. G. (2004). Regulatory T Cells Mediate Maternal Tolerance to the Fetus. Nat. Immunol. 5 (3), 266–271. doi:10.1038/ni1037
Alzahrani, F. A., El-Magd, M. A., Abdelfattah-Hassan, A., Saleh, A. A., Saadeldin, I. M., El-Shetry, E. S., et al. (2018). Potential Effect of Exosomes Derived from Cancer Stem Cells and MSCs on Progression of DEN-Induced HCC in Rats. Stem Cell Int 2018, 8058979. doi:10.1155/2018/8058979
Arenas-Hernandez, M., Romero, R., Xu, Y., Panaitescu, B., Garcia-Flores, V., Miller, D., et al. (2019). Effector and Activated T Cells Induce Preterm Labor and Birth that Is Prevented by Treatment with Progesterone. J. Immunol. 202 (9), 2585–2608. doi:10.4049/jimmunol.1801350
Arruvito, L., Giulianelli, S., Flores, A. C., Paladino, N., Barboza, M., Lanari, C., et al. (2008). NK Cells Expressing a Progesterone Receptor Are Susceptible to Progesterone-Induced Apoptosis. J. Immunol. 180 (8), 5746–5753. doi:10.4049/jimmunol.180.8.5746
Arruvito, L., Sanz, M., Banham, A. H., and Fainboim, L. (2007). Expansion of CD4+CD25+and FOXP3+ Regulatory T Cells during the Follicular Phase of the Menstrual Cycle: Implications for Human Reproduction. J. Immunol. 178 (4), 2572–2578. doi:10.4049/jimmunol.178.4.2572
Ashkar, A. A., Di Santo, J. P., and Croy, B. A. (2000). Interferon Gamma Contributes to Initiation of Uterine Vascular Modification, Decidual Integrity, and Uterine Natural Killer Cell Maturation during normal Murine Pregnancy. J. Exp. Med. 192 (2), 259–270. doi:10.1084/jem.192.2.259
Aslani, S., Hossein-nezhad, A., Maghbooli, Z., Mirzaei, K., and Karimi, F. (2011). Genetic Variation in Macrophage Migration Inhibitory Factor Associated with Gestational Diabetes Mellitus and Metabolic Syndrome. Horm. Metab. Res. 43 (8), 557–561. doi:10.1055/s-0031-1275706
Babic, G. M., Markovic, S. D., Varjacic, M., Djordjevic, N. Z., Nikolic, T., Stojic, I., et al. (2018). Estradiol Decreases Blood Pressure in Association with Redox Regulation in Preeclampsia. Clin. Exp. Hypertens. 40 (3), 281–286. doi:10.1080/10641963.2017.1368538
Bari, M. F., Weickert, M. O., Sivakumar, K., James, S. G., Snead, D. R., Tan, B. K., et al. (2014). Elevated Soluble CD163 in Gestational Diabetes Mellitus: Secretion from Human Placenta and Adipose Tissue. PLoS One 9 (7), e101327. doi:10.1371/journal.pone.0101327
Bauer, S., Pollheimer, J., Hartmann, J., Husslein, P., Aplin, J. D., and Knöfler, M. (2004). Tumor Necrosis Factor-Alpha Inhibits Trophoblast Migration through Elevation of Plasminogen Activator Inhibitor-1 in First-Trimester Villous Explant Cultures. J. Clin. Endocrinol. Metab. 89 (2), 812–822. doi:10.1210/jc.2003-031351
Bauernfeind, F., Rieger, A., Schildberg, F. A., Knolle, P. A., Schmid-Burgk, J. L., and Hornung, V. (2012). NLRP3 Inflammasome Activity Is Negatively Controlled by miR-223. J. Immunol. 189 (8), 4175–4181. doi:10.4049/jimmunol.1201516
Bell, E., and Taylor, M. A. (2017). Functional Roles for Exosomal MicroRNAs in the Tumour Microenvironment. Comput. Struct. Biotechnol. J. 15, 8–13. doi:10.1016/j.csbj.2016.10.005
Benner, M., Feyaerts, D., García, C. C., Inci, N., López, S. C., Fasse, E., et al. (2020). Clusters of Tolerogenic B Cells Feature in the Dynamic Immunological Landscape of the Pregnant Uterus. Cell Rep 32 (13), 108204. doi:10.1016/j.celrep.2020.108204
Beyer, M., and Schultze, J. L. (2006). Regulatory T Cells in Cancer. Blood 108 (3), 804–811. doi:10.1182/blood-2006-02-002774
Bollapragada, S., Bollopragada, S., Youssef, R., Jordan, F., Greer, I., Norman, J., et al. (2009). Term Labor Is Associated with a Core Inflammatory Response in Human Fetal Membranes, Myometrium, and Cervix. Am. J. Obstet. Gynecol. 200 (1), 104–111. doi:10.1016/j.ajog.2008.08.032
Bouman, A., Heineman, M. J., and Faas, M. M. (2005). Sex Hormones and the Immune Response in Humans. Hum. Reprod. Update 11 (4), 411–423. doi:10.1093/humupd/dmi008
Boyson, J. E., Rybalov, B., Koopman, L. A., Exley, M., Balk, S. P., Racke, F. K., et al. (2002). CD1d and Invariant NKT Cells at the Human Maternal-Fetal Interface. Proc. Natl. Acad. Sci. U S A. 99 (21), 13741–13746. doi:10.1073/pnas.162491699
Brombacher, E. C., and Everts, B. (2020). Shaping of Dendritic Cell Function by the Metabolic Micro-environment. Front. Endocrinol. (Lausanne) 11, 555. doi:10.3389/fendo.2020.00555
Bukovsky, A., Caudle, M. R., Cekanova, M., Fernando, R. I., Wimalasena, J., Foster, J. S., et al. (2003). Placental Expression of Estrogen Receptor Beta and its Hormone Binding Variant-Ccomparison with Estrogen Receptor Alpha and a Role for Estrogen Receptors in Asymmetric Division and Differentiation of Estrogen-dependent Cells. Reprod. Biol. Endocrinol. 1, 36. doi:10.1186/1477-7827-1-36
Bulmer, J. N., Morrison, L., Longfellow, M., Ritson, A., and Pace, D. (1991). Granulated Lymphocytes in Human Endometrium: Histochemical and Immunohistochemical Studies. Hum. Reprod. 6 (6), 791–798. doi:10.1093/oxfordjournals.humrep.a137430
Bulmer, J. N., Pace, D., and Ritson, A. (1988). Immunoregulatory Cells in Human Decidua: Morphology, Immunohistochemistry and Function. Reprod. Nutr. Dev. 28 (6B), 1599–1613. doi:10.1051/rnd:19881006
Byrne, S. N., and Halliday, G. M. (2003). High Levels of Fas Ligand and MHC Class II in the Absence of CD80 or CD86 Expression and a Decreased CD4+ T Cell Infiltration, Enables Murine Skin Tumours to Progress. Cancer Immunol. Immunother. 52 (6), 396–402. doi:10.1007/s00262-003-0380-0
Cantonwine, D. E., McElrath, T. F., Trabert, B., Xu, X., Sampson, J., Roberts, J. M., et al. (2019). Estrogen Metabolism Pathways in Preeclampsia and normal Pregnancy. Steroids 144, 8–14. doi:10.1016/j.steroids.2019.01.005
Care, A. S., Bourque, S. L., Morton, J. S., Hjartarson, E. P., Robertson, S. A., and Davidge, S. T. (2018). Reduction in Regulatory T Cells in Early Pregnancy Causes Uterine Artery Dysfunction in Mice. Hypertension 72 (1), 177–187. doi:10.1161/HYPERTENSIONAHA.118.10858
Cartwright, J. E., James-Allan, L., Buckley, R. J., and Wallace, A. E. (2017). The Role of Decidual NK Cells in Pregnancies with Impaired Vascular Remodelling. J. Reprod. Immunol. 119, 81–84. doi:10.1016/j.jri.2016.09.002
Caumartin, J., Favier, B., Daouya, M., Guillard, C., Moreau, P., Carosella, E. D., et al. (2007). Trogocytosis-based Generation of Suppressive NK Cells. EMBO J. 26 (5), 1423–1433. doi:10.1038/sj.emboj.7601570
Chakrabarty, A., Tranguch, S., Daikoku, T., Jensen, K., Furneaux, H., and Dey, S. K. (2007). MicroRNA Regulation of Cyclooxygenase-2 during Embryo Implantation. Proc. Natl. Acad. Sci. U S A. 104 (38), 15144–15149. doi:10.1073/pnas.0705917104
Chandra, S., Tripathi, A. K., Mishra, S., Amzarul, M., and Vaish, A. K. (2012). Physiological Changes in Hematological Parameters during Pregnancy. Indian J. Hematol. Blood Transfus. 28 (3), 144–146. doi:10.1007/s12288-012-0175-6
Chang, X., Yao, J., He, Q., Liu, M., Duan, T., and Wang, K. (2018). Exosomes from Women with Preeclampsia Induced Vascular Dysfunction by Delivering sFlt (Soluble Fms-like Tyrosine Kinase)-1 and sEng (Soluble Endoglin) to Endothelial Cells. Hypertension 72 (6), 1381–1390. doi:10.1161/HYPERTENSIONAHA.118.11706
Chaouat, G., and Voisin, G. A. (1979). Regulatory T Cell Subpopulations in Pregnancy. I. Evidence for Suppressive Activity of the Early Phase of MLR. J. Immunol. 122 (4), 1383–1388.
Charnock-Jones, D. S., Kaufmann, P., and Mayhew, T. M. (2004). Aspects of Human Fetoplacental Vasculogenesis and Angiogenesis. I. Molecular Regulation. Placenta 25 (2-3), 103–113. doi:10.1016/j.placenta.2003.10.004
Cheng, H., Wang, Z., Fu, L., and Xu, T. (2019). Macrophage Polarization in the Development and Progression of Ovarian Cancers: An Overview. Front. Oncol. 9, 421. doi:10.3389/fonc.2019.00421
Clark, D. A., Keil, A., Chen, Z., Markert, U., Manuel, J., and Gorczynski, R. M. (2003). Placental Trophoblast from Successful Human Pregnancies Expresses the Tolerance Signaling Molecule, CD200 (OX-2). Am. J. Reprod. Immunol. 50 (3), 187–195. doi:10.1034/j.1600-0897.2003.00086.x
Clayton, A., Al-Taei, S., Webber, J., Mason, M. D., and Tabi, Z. (2011). Cancer Exosomes Express CD39 and CD73, Which Suppress T Cells through Adenosine Production. J. Immunol. 187 (2), 676–683. doi:10.4049/jimmunol.1003884
Clayton, A., and Tabi, Z. (2005). Exosomes and the MICA-NKG2D System in Cancer. Blood Cell Mol Dis 34 (3), 206–213. doi:10.1016/j.bcmd.2005.03.003
Conneely, O. M., Jericevic, B. M., and Lydon, J. P. (2003). Progesterone Receptors in Mammary Gland Development and Tumorigenesis. J. Mammary Gland Biol. Neoplasia 8 (2), 205–214. doi:10.1023/a:1025952924864
Cook, J., Bennett, P. R., Kim, S. H., Teoh, T. G., Sykes, L., Kindinger, L. M., et al. (2019). First Trimester Circulating MicroRNA Biomarkers Predictive of Subsequent Preterm Delivery and Cervical Shortening. Sci. Rep. 9 (1), 5861. doi:10.1038/s41598-019-42166-1
Corrêa-Silva, S., Alencar, A. P., Moreli, J. B., Borbely, A. U., de S Lima, L., Scavone, C., et al. (2018). Hyperglycemia Induces Inflammatory Mediators in the Human Chorionic Villous. Cytokine 111, 41–48. doi:10.1016/j.cyto.2018.07.020
Costanzo, V., Bardelli, A., Siena, S., and Abrignani, S. (2018). Exploring the Links between Cancer and Placenta Development. Open Biol. 8 (6). doi:10.1098/rsob.180081
Dambaeva, S., Schneiderman, S., Jaiswal, M. K., Agrawal, V., Katara, G. K., Gilman-Sachs, A., et al. (2018). Interleukin 22 Prevents Lipopolysaccharide- Induced Preterm Labor in Mice. Biol. Reprod. 98 (3), 299–308. doi:10.1093/biolre/iox182
Dang, E. V., Barbi, J., Yang, H. Y., Jinasena, D., Yu, H., Zheng, Y., et al. (2011). Control of T(H)17/T(reg) Balance by Hypoxia-Inducible Factor 1. Cell 146 (5), 772–784. doi:10.1016/j.cell.2011.07.033
de Kouchkovsky, D., Esensten, J. H., Rosenthal, W. L., Morar, M. M., Bluestone, J. A., and Jeker, L. T. (2013). microRNA-17-92 Regulates IL-10 Production by Regulatory T Cells and Control of Experimental Autoimmune Encephalomyelitis. J. Immunol. 191 (4), 1594–1605. doi:10.4049/jimmunol.1203567
Dekel, N., Gnainsky, Y., Granot, I., and Mor, G. (2010). Inflammation and Implantation. Am. J. Reprod. Immunol. 63 (1), 17–21. doi:10.1111/j.1600-0897.2009.00792.x
Dekel, N., Gnainsky, Y., Granot, I., Racicot, K., and Mor, G. (2014). The Role of Inflammation for a Successful Implantation. Am. J. Reprod. Immunol. 72 (2), 141–147. doi:10.1111/aji.12266
Delassus, S., Coutinho, G. C., Saucier, C., Darche, S., and Kourilsky, P. (1994). Differential Cytokine Expression in Maternal Blood and Placenta during Murine Gestation. J. Immunol. 152 (5), 2411–2420.
Demir, R., Kayisli, U. A., Seval, Y., Celik-Ozenci, C., Korgun, E. T., Demir-Weusten, A. Y., et al. (2004). Sequential Expression of VEGF and its Receptors in Human Placental Villi during Very Early Pregnancy: Differences between Placental Vasculogenesis and Angiogenesis. Placenta 25 (6), 560–572. doi:10.1016/j.placenta.2003.11.011
Dige, A., Støy, S., Thomsen, K. L., Hvas, C. L., Agnholt, J., Dahlerup, J. F., et al. (2014). Soluble CD163, a Specific Macrophage Activation Marker, Is Decreased by Anti-TNF-α Antibody Treatment in Active Inflammatory Bowel Disease. Scand. J. Immunol. 80 (6), 417–423. doi:10.1111/sji.12222
Ding, G., Zhou, L., Qian, Y., Fu, M., Chen, J., Chen, J., et al. (2015). Pancreatic Cancer-Derived Exosomes Transfer miRNAs to Dendritic Cells and Inhibit RFXAP Expression via miR-212-3p. Oncotarget 6 (30), 29877–29888. doi:10.18632/oncotarget.4924
Dixon, C. L., Sheller-Miller, S., Saade, G. R., Fortunato, S. J., Lai, A., Palma, C., et al. (2018). Amniotic Fluid Exosome Proteomic Profile Exhibits Unique Pathways of Term and Preterm Labor. Endocrinology 159 (5), 2229–2240. doi:10.1210/en.2018-00073
Doebele, C., Bonauer, A., Fischer, A., Scholz, A., Reiss, Y., Urbich, C., et al. (2010). Members of the microRNA-17-92 Cluster Exhibit a Cell-Intrinsic Antiangiogenic Function in Endothelial Cells. Blood 115 (23), 4944–4950. doi:10.1182/blood-2010-01-264812
Donker, R. B., Mouillet, J. F., Chu, T., Hubel, C. A., Stolz, D. B., Morelli, A. E., et al. (2012). The Expression Profile of C19MC microRNAs in Primary Human Trophoblast Cells and Exosomes. Mol. Hum. Reprod. 18 (8), 417–424. doi:10.1093/molehr/gas013
Donnelly, R. P., Loftus, R. M., Keating, S. E., Liou, K. T., Biron, C. A., Gardiner, C. M., et al. (2014). mTORC1-dependent Metabolic Reprogramming Is a Prerequisite for NK Cell Effector Function. J. Immunol. 193 (9), 4477–4484. doi:10.4049/jimmunol.1401558
Dumont, T. M. F., Mouillet, J. F., Bayer, A., Gardner, C. L., Klimstra, W. B., Wolf, D. G., et al. (2017). The Expression Level of C19MC miRNAs in Early Pregnancy and in Response to Viral Infection. Placenta 53, 23–29. doi:10.1016/j.placenta.2017.03.011
Enninga, E. A. L., Harrington, S. M., Creedon, D. J., Ruano, R., Markovic, S. N., Dong, H., et al. (2018). Immune Checkpoint Molecules Soluble Program Death Ligand 1 and Galectin-9 Are Increased in Pregnancy. Am. J. Reprod. Immunol. 79 (2). doi:10.1111/aji.12795
Erlebacher, A., Vencato, D., Price, K. A., Zhang, D., and Glimcher, L. H. (2007). Constraints in Antigen Presentation Severely Restrict T Cell Recognition of the Allogeneic Fetus. J. Clin. Invest. 117 (5), 1399–1411. doi:10.1172/JCI28214
Evans, P. C., Lambert, N., Maloney, S., Furst, D. E., Moore, J. M., and Nelson, J. L. (1999). Long-term Fetal Microchimerism in Peripheral Blood Mononuclear Cell Subsets in Healthy Women and Women with Scleroderma. Blood 93 (6), 2033–2037. doi:10.1182/blood.v93.6.2033.406k18_2033_2037
Everts, B., Amiel, E., van der Windt, G. J., Freitas, T. C., Chott, R., Yarasheski, K. E., et al. (2012). Commitment to Glycolysis Sustains Survival of NO-Producing Inflammatory Dendritic Cells. Blood 120 (7), 1422–1431. doi:10.1182/blood-2012-03-419747
Fazleabas, A. T., Donnelly, K. M., Srinivasan, S., Fortman, J. D., and Miller, J. B. (1999). Modulation of the Baboon (Papio anubis) Uterine Endometrium by Chorionic Gonadotrophin during the Period of Uterine Receptivity. Proc. Natl. Acad. Sci. U S A. 96 (5), 2543–2548. doi:10.1073/pnas.96.5.2543
Ferlazzo, G., and Morandi, B. (2014). Cross-Talks between Natural Killer Cells and Distinct Subsets of Dendritic Cells. Front. Immunol. 5, 159. doi:10.3389/fimmu.2014.00159
Fernandez, A. R., Omar, S. Z., and Husain, R. (2016). Role of Genistein in Preeclampsia: A Case-Control Study. J. Reprod. Med. 61 (1-2), 47–51.
Ferretti, C., and La Cava, A. (2014). miR-126, a New Modulator of Innate Immunity. Cell Mol Immunol 11 (3), 215–217. doi:10.1038/cmi.2014.5
Fitzgerald, W., Gomez-Lopez, N., Erez, O., Romero, R., and Margolis, L. (2018). Extracellular Vesicles Generated by Placental Tissues Ex Vivo: A Transport System for Immune Mediators and Growth Factors. Am. J. Reprod. Immunol. 80 (1), e12860. doi:10.1111/aji.12860
Flor, I., Neumann, A., Freter, C., Helmke, B. M., Langenbuch, M., Rippe, V., et al. (2012). Abundant Expression and Hemimethylation of C19MC in Cell Cultures from Placenta-Derived Stromal Cells. Biochem. Biophys. Res. Commun. 422 (3), 411–416. doi:10.1016/j.bbrc.2012.05.004
Fraccaroli, L., Alfieri, J., Larocca, L., Calafat, M., Mor, G., Leirós, C. P., et al. (2009). A Potential Tolerogenic Immune Mechanism in a Trophoblast Cell Line through the Activation of Chemokine-Induced T Cell Death and Regulatory T Cell Modulation. Hum. Reprod. 24 (1), 166–175. doi:10.1093/humrep/den344
Fraser, R., Whitley, G. S., Johnstone, A. P., Host, A. J., Sebire, N. J., Thilaganathan, B., et al. (2012). Impaired Decidual Natural Killer Cell Regulation of Vascular Remodelling in Early Human Pregnancies with High Uterine Artery Resistance. J. Pathol. 228 (3), 322–332. doi:10.1002/path.4057
Fu, W., Lei, C., Liu, S., Cui, Y., Wang, C., Qian, K., et al. (2019). CAR Exosomes Derived from Effector CAR-T Cells Have Potent Antitumour Effects and Low Toxicity. Nat. Commun. 10 (1), 4355. doi:10.1038/s41467-019-12321-3
Galaz, J., Romero, R., Slutsky, R., Xu, Y., Motomura, K., Para, R., et al. (2020). Cellular Immune Responses in Amniotic Fluid of Women with Preterm Prelabor Rupture of Membranes. J. Perinat Med. 48 (3), 222–233. doi:10.1515/jpm-2019-0395
Garcia-Iglesias, T., Del Toro-Arreola, A., Albarran-Somoza, B., Del Toro-Arreola, S., Sanchez-Hernandez, P. E., Ramirez-Dueñas, M. G., et al. (2009). Low NKp30, NKp46 and NKG2D Expression and Reduced Cytotoxic Activity on NK Cells in Cervical Cancer and Precursor Lesions. BMC Cancer 9, 186. doi:10.1186/1471-2407-9-186
Garcia-Lora, A., Algarra, I., and Garrido, F. (2003). MHC Class I Antigens, Immune Surveillance, and Tumor Immune Escape. J. Cell Physiol 195 (3), 346–355. doi:10.1002/jcp.10290
Germain, S. J., Sacks, G. P., Sooranna, S. R., Soorana, S. R., Sargent, I. L., and Redman, C. W. (2007). Systemic Inflammatory Priming in normal Pregnancy and Preeclampsia: the Role of Circulating Syncytiotrophoblast Microparticles. J. Immunol. 178 (9), 5949–5956. doi:10.4049/jimmunol.178.9.5949
Gillet, V., Ouellet, A., Stepanov, Y., Rodosthenous, R. S., Croft, E. K., Brennan, K., et al. (2019). miRNA Profiles in Extracellular Vesicles from Serum Early in Pregnancies Complicated by Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 104 (11), 5157–5169. doi:10.1210/jc.2018-02693
Goldenberg, R. L., Culhane, J. F., Iams, J. D., and Romero, R. (2008). Epidemiology and Causes of Preterm Birth. Lancet 371 (9606), 75–84. doi:10.1016/S0140-6736(08)60074-4
Gomez-Lopez, N., Arenas-Hernandez, M., Romero, R., Miller, D., Garcia-Flores, V., Leng, Y., et al. (2020). Regulatory T Cells Play a Role in a Subset of Idiopathic Preterm Labor/Birth and Adverse Neonatal Outcomes. Cell Rep 32 (1), 107874. doi:10.1016/j.celrep.2020.107874
Gomez-Lopez, N., Guilbert, L. J., and Olson, D. M. (2010). Invasion of the Leukocytes into the Fetal-Maternal Interface during Pregnancy. J. Leukoc. Biol. 88 (4), 625–633. doi:10.1189/jlb.1209796
Gomez-Lopez, N., Romero, R., Arenas-Hernandez, M., Ahn, H., Panaitescu, B., Vadillo-Ortega, F., et al. (2016). In Vivo T-cell Activation by a Monoclonal αCD3ε Antibody Induces Preterm Labor and Birth. Am. J. Reprod. Immunol. 76 (5), 386–390. doi:10.1111/aji.12562
Gomez-Lopez, N., Romero, R., Arenas-Hernandez, M., Schwenkel, G., St Louis, D., Hassan, S. S., et al. (2017). In Vivo activation of Invariant Natural Killer T Cells Induces Systemic and Local Alterations in T-Cell Subsets Prior to Preterm Birth. Clin. Exp. Immunol. 189 (2), 211–225. doi:10.1111/cei.12968
Graham, A. C., Carr, K. D., Sieve, A. N., Indramohan, M., Break, T. J., and Berg, R. E. (2011). IL-22 Production Is Regulated by IL-23 during Listeria Monocytogenes Infection but Is Not Required for Bacterial Clearance or Tissue protection. PLoS One 6 (2), e17171. doi:10.1371/journal.pone.0017171
Gray-Owen, S. D., and Blumberg, R. S. (2006). CEACAM1: Contact-dependent Control of Immunity. Nat. Rev. Immunol. 6 (6), 433–446. doi:10.1038/nri1864
Gupta, A., Hasler, P., Gebhardt, S., Holzgreve, W., and Hahn, S. (2006). Occurrence of Neutrophil Extracellular DNA Traps (NETs) in Pre-eclampsia: a Link with Elevated Levels of Cell-free DNA? Ann. N. Y Acad. Sci. 1075, 118–122. doi:10.1196/annals.1368.015
Gustafsson, C., Mjösberg, J., Matussek, A., Geffers, R., Matthiesen, L., Berg, G., et al. (2008). Gene Expression Profiling of Human Decidual Macrophages: Evidence for Immunosuppressive Phenotype. PLoS One 3 (4), e2078. doi:10.1371/journal.pone.0002078
Habbeddine, M., Verbeke, P., Karaz, S., Bobé, P., and Kanellopoulos-Langevin, C. (2014). Leukocyte Population Dynamics and Detection of IL-9 as a Major Cytokine at the Mouse Fetal-Maternal Interface. PLoS One 9 (9), e107267. doi:10.1371/journal.pone.0107267
Han, C. S., Herrin, M. A., Pitruzzello, M. C., Mulla, M. J., Werner, E. F., Pettker, C. M., et al. (2015). Glucose and Metformin Modulate Human First Trimester Trophoblast Function: a Model and Potential Therapy for Diabetes-Associated Uteroplacental Insufficiency. Am. J. Reprod. Immunol. 73 (4), 362–371. doi:10.1111/aji.12339
Haneklaus, M., Gerlic, M., Kurowska-Stolarska, M., Rainey, A. A., Pich, D., McInnes, I. B., et al. (2012). Cutting Edge: miR-223 and EBV miR-BART15 Regulate the NLRP3 Inflammasome and IL-1β Production. J. Immunol. 189 (8), 3795–3799. doi:10.4049/jimmunol.1200312
Hao, S., Zhao, J., Zhou, J., Zhao, S., Hu, Y., and Hou, Y. (2007). Modulation of 17beta-Estradiol on the Number and Cytotoxicity of NK Cells In Vivo Related to MCM and Activating Receptors. Int. Immunopharmacol 7 (13), 1765–1775. doi:10.1016/j.intimp.2007.09.017
Harding, C., Heuser, J., and Stahl, P. (1984). Endocytosis and Intracellular Processing of Transferrin and Colloidal Gold-Transferrin in Rat Reticulocytes: Demonstration of a Pathway for Receptor Shedding. Eur. J. Cell Biol 35 (2), 256–263.
Harding, C. V., Heuser, J. E., and Stahl, P. D. (2013). Exosomes: Looking Back Three Decades and into the Future. J. Cell Biol 200 (4), 367–371. doi:10.1083/jcb.201212113
Hardy, K. M., Dillaman, R. M., Locke, B. R., and Kinsey, S. T. (2009). A Skeletal Muscle Model of Extreme Hypertrophic Growth Reveals the Influence of Diffusion on Cellular Design. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296 (6), R1855–R1867. doi:10.1152/ajpregu.00076.2009
Hassan, S. S., Romero, R., Pineles, B., Tarca, A. L., Montenegro, D., Erez, O., et al. (2010). MicroRNA Expression Profiling of the Human Uterine Cervix after Term Labor and Delivery. Am. J. Obstet. Gynecol. 202 (1), 80–88. doi:10.1016/j.ajog.2009.08.016
He, C., Li, L., Wang, L., Meng, W., Hao, Y., and Zhu, G. (2021). Exosome-mediated Cellular Crosstalk within the Tumor Microenvironment upon Irradiation. Cancer Biol. Med. 18 (1), 21–33. doi:10.20892/j.issn.2095-3941.2020.0150
Hedlund, M., Stenqvist, A. C., Nagaeva, O., Kjellberg, L., Wulff, M., Baranov, V., et al. (2009). Human Placenta Expresses and Secretes NKG2D Ligands via Exosomes that Down-Modulate the Cognate Receptor Expression: Evidence for Immunosuppressive Function. J. Immunol. 183 (1), 340–351. doi:10.4049/jimmunol.0803477
Heikkinen, J., Möttönen, M., Komi, J., Alanen, A., and Lassila, O. (2003). Phenotypic Characterization of Human Decidual Macrophages. Clin. Exp. Immunol. 131 (3), 498–505. doi:10.1046/j.1365-2249.2003.02092.x
Herberth, G., Bauer, M., Gasch, M., Hinz, D., Röder, S., Olek, S., et al. (2014). Maternal and Cord Blood miR-223 Expression Associates with Prenatal Tobacco Smoke Exposure and Low Regulatory T-Cell Numbers. J. Allergy Clin. Immunol. 133 (2), 543–550. doi:10.1016/j.jaci.2013.06.036
Herrera-Van Oostdam, A. S., Toro-Ortíz, J. C., López, J. A., Noyola, D. E., García-López, D. A., Durán-Figueroa, N. V., et al. (2020). Placental Exosomes Isolated from Urine of Patients with Gestational Diabetes Exhibit a Differential Profile Expression of microRNAs across Gestation. Int. J. Mol. Med. 46 (2), 546–560. doi:10.3892/ijmm.2020.4626
Holtan, S. G., Chen, Y., Kaimal, R., Creedon, D. J., Enninga, E. A., Nevala, W. K., et al. (2015). Growth Modeling of the Maternal Cytokine Milieu throughout normal Pregnancy: Macrophage-Derived Chemokine Decreases as Inflammation/counterregulation Increases. J. Immunol. Res. 2015, 952571. doi:10.1155/2015/952571
Holtan, S. G., Creedon, D. J., Haluska, P., and Markovic, S. N. (2009). Cancer and Pregnancy: Parallels in Growth, Invasion, and Immune Modulation and Implications for Cancer Therapeutic Agents. Mayo Clin. Proc. 84 (11), 985–1000. doi:10.1016/S0025-6196(11)60669-1
Homet Moreno, B., and Ribas, A. (2015). Anti-programmed Cell Death Protein-1/ligand-1 Therapy in Different Cancers. Br. J. Cancer 112 (9), 1421–1427. doi:10.1038/bjc.2015.124
Hood, J. L., San, R. S., and Wickline, S. A. (2011). Exosomes Released by Melanoma Cells Prepare sentinel Lymph Nodes for Tumor Metastasis. Cancer Res. 71 (11), 3792–3801. doi:10.1158/0008-5472.CAN-10-4455
Houser, B. L., Tilburgs, T., Hill, J., Nicotra, M. L., and Strominger, J. L. (2011). Two Unique Human Decidual Macrophage Populations. J. Immunol. 186 (4), 2633–2642. doi:10.4049/jimmunol.1003153
Hromadnikova, I., Dvorakova, L., Kotlabova, K., and Krofta, L. (2019). The Prediction of Gestational Hypertension, Preeclampsia and Fetal Growth Restriction via the First Trimester Screening of Plasma Exosomal C19MC microRNAs. Int. J. Mol. Sci. 20 (12). doi:10.3390/ijms20122972
Huang, Q., Gong, M., Tan, T., Lin, Y., Bao, Y., and Fan, C. (2021). Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomal MicroRNA-18b-3p Inhibits the Occurrence of Preeclampsia by Targeting LEP. Nanoscale Res. Lett. 16 (1), 27. doi:10.1186/s11671-021-03475-5
Hudic, I., Szekeres-Bartho, J., Vrtacnik, E. B., Virant Klun, I., Brkic, S., Frangez, H. B., et al. (2020). Progesterone Induced Blocking Factor (PIBF) Taken in Early Pregnancy Predicts the Pregnancy Outcome in Women Undergoing In Vitro Fertilization Procedure. J. Reprod. Immunol. 140, 103150. doi:10.1016/j.jri.2020.103150
Hwu, P., Du, M. X., Lapointe, R., Do, M., Taylor, M. W., and Young, H. A. (2000). Indoleamine 2,3-dioxygenase Production by Human Dendritic Cells Results in the Inhibition of T Cell Proliferation. J. Immunol. 164 (7), 3596–3599. doi:10.4049/jimmunol.164.7.3596
Jabrane-Ferrat, N. (2019). Features of Human Decidual NK Cells in Healthy Pregnancy and during Viral Infection. Front. Immunol. 10, 1397. doi:10.3389/fimmu.2019.01397
Jaiswal, M. K., Mallers, T. M., Larsen, B., Kwak-Kim, J., Chaouat, G., Gilman-Sachs, A., et al. (2012). V-ATPase Upregulation during Early Pregnancy: a Possible Link to Establishment of an Inflammatory Response during Preimplantation Period of Pregnancy. Reproduction 143 (5), 713–725. doi:10.1530/REP-12-0036
James-Allan, L. B., Rosario, F. J., Barner, K., Lai, A., Guanzon, D., McIntyre, H. D., et al. (2020). Regulation of Glucose Homeostasis by Small Extracellular Vesicles in normal Pregnancy and in Gestational Diabetes. FASEB J. 34 (4), 5724–5739. doi:10.1096/fj.201902522RR
Jia, L., and Wu, C. (2014). The Biology and Functions of Th22 Cells. Adv. Exp. Med. Biol. 841, 209–230. doi:10.1007/978-94-017-9487-9_8
Jokhi, P. P., King, A., Sharkey, A. M., Smith, S. K., and Loke, Y. W. (1994). Screening for Cytokine Messenger Ribonucleic Acids in Purified Human Decidual Lymphocyte Populations by the Reverse-Transcriptase Polymerase Chain Reaction. J. Immunol. 153 (10), 4427–4435.
Kadel, S., and Kovats, S. (2018). Sex Hormones Regulate Innate Immune Cells and Promote Sex Differences in Respiratory Virus Infection. Front. Immunol. 9, 1653. doi:10.3389/fimmu.2018.01653
Kämmerer, U., Eggert, A. O., Kapp, M., McLellan, A. D., Geijtenbeek, T. B., Dietl, J., et al. (2003). Unique Appearance of Proliferating Antigen-Presenting Cells Expressing DC-SIGN (CD209) in the Decidua of Early Human Pregnancy. Am. J. Pathol. 162 (3), 887–896. doi:10.1016/S0002-9440(10)63884-9
Kandzija, N., Zhang, W., Motta-Mejia, C., Mhlomi, V., McGowan-Downey, J., James, T., et al. (2019). Placental Extracellular Vesicles Express Active Dipeptidyl Peptidase IV; Levels Are Increased in Gestational Diabetes Mellitus. J. Extracell Vesicles 8 (1), 1617000. doi:10.1080/20013078.2019.1617000
Keerthikumar, S., Chisanga, D., Ariyaratne, D., Al Saffar, H., Anand, S., Zhao, K., et al. (2016). ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 428 (4), 688–692. doi:10.1016/j.jmb.2015.09.019
Kim, S. Y., Romero, R., Tarca, A. L., Bhatti, G., Kim, C. J., Lee, J., et al. (2012). Methylome of Fetal and Maternal Monocytes and Macrophages at the Feto-Maternal Interface. Am. J. Reprod. Immunol. 68 (1), 8–27. doi:10.1111/j.1600-0897.2012.01108.x
King, A., Loke, Y. W., and Chaouat, G. (1997). NK Cells and Reproduction. Immunol. Today 18 (2), 64–66. doi:10.1016/s0167-5699(97)01001-3
Knieke, K., Lingel, H., Chamaon, K., and Brunner-Weinzierl, M. C. (2012). Migration of Th1 Lymphocytes Is Regulated by CD152 (CTLA-4)-Mediated Signaling via PI3 Kinase-dependent Akt Activation. PLoS One 7 (3), e31391. doi:10.1371/journal.pone.0031391
Ko, E. C., Wang, X., and Ferrone, S. (2003). Immunotherapy of Malignant Diseases. Challenges and Strategies. Int. Arch. Allergy Immunol. 132 (4), 294–309. doi:10.1159/000074897
Kovács, Á. F., Fekete, N., Turiák, L., Ács, A., Kőhidai, L., Buzás, E. I., et al. (2019). Unravelling the Role of Trophoblastic-Derived Extracellular Vesicles in Regulatory T Cell Differentiation. Int. J. Mol. Sci. 20 (14). doi:10.3390/ijms20143457
Kropp, J., Salih, S. M., and Khatib, H. (2014). Expression of microRNAs in Bovine and Human Pre-implantation Embryo Culture media. Front. Genet. 5, 91. doi:10.3389/fgene.2014.00091
Kurian, N. K., and Modi, D. (2019). Extracellular Vesicle Mediated Embryo-Endometrial Cross Talk during Implantation and in Pregnancy. J. Assist. Reprod. Genet. 36 (2), 189–198. doi:10.1007/s10815-018-1343-x
Kusanovic, J. P., Romero, R., Hassan, S. S., Gotsch, F., Edwin, S., Chaiworapongsa, T., et al. (2007). Maternal Serum Soluble CD30 Is Increased in normal Pregnancy, but Decreased in Preeclampsia and Small for Gestational Age Pregnancies. J. Matern. Fetal Neonatal. Med. 20 (12), 867–878. doi:10.1080/14767050701482993
Lawless, S. J., Kedia-Mehta, N., Walls, J. F., McGarrigle, R., Convery, O., Sinclair, L. V., et al. (2017). Glucose Represses Dendritic Cell-Induced T Cell Responses. Nat. Commun. 8, 15620. doi:10.1038/ncomms15620
Lee, C. L., Guo, Y., So, K. H., Vijayan, M., Guo, Y., Wong, V. H., et al. (2015). Soluble Human Leukocyte Antigen G5 Polarizes Differentiation of Macrophages toward a Decidual Macrophage-like Phenotype. Hum. Reprod. 30 (10), 2263–2274. doi:10.1093/humrep/dev196
Lee, S. K., Kim, J. Y., Hur, S. E., Kim, C. J., Na, B. J., Lee, M., et al. (2011). An Imbalance in Interleukin-17-Producing T and Foxp3⁺ Regulatory T Cells in Women with Idiopathic Recurrent Pregnancy Loss. Hum. Reprod. 26 (11), 2964–2971. doi:10.1093/humrep/der301
Levine, L., Habertheuer, A., Ram, C., Korutla, L., Schwartz, N., Hu, R. W., et al. (2020). Syncytiotrophoblast Extracellular Microvesicle Profiles in Maternal Circulation for Noninvasive Diagnosis of Preeclampsia. Sci. Rep. 10 (1), 6398. doi:10.1038/s41598-020-62193-7
Levitz, M., and Young, B. K. (1977). Estrogens in Pregnancy. Vitam Horm. 35, 109–147. doi:10.1016/s0083-6729(08)60522-1
Li, M., Wu, Z. M., Yang, H., and Huang, S. J. (2011). NFκB and JNK/MAPK Activation Mediates the Production of Major Macrophage- or Dendritic Cell-Recruiting Chemokine in Human First Trimester Decidual Cells in Response to Proinflammatory Stimuli. J. Clin. Endocrinol. Metab. 96 (8), 2502–2511. doi:10.1210/jc.2011-0055
Li, X., Lonard, D. M., and O'Malley, B. W. (2004). A Contemporary Understanding of Progesterone Receptor Function. Mech. Ageing Dev. 125 (10-11), 669–678. doi:10.1016/j.mad.2004.04.007
Liang, Q., Tong, L., Xiang, L., Shen, S., Pan, C., Liu, C., et al. (2021). Correlations of the Expression of Gammadelta T Cells and Their Co-stimulatory Molecules TIGIT, PD-1, ICOS and BTLA with PR and PIBF in the Peripheral Blood and Decidual Tissues of Women with Unexplained Recurrent Spontaneous Abortion. Clin. Exp. Immunol. 203 (1), 55–65.
Lim, M. K., Ku, C. W., Tan, T. C., Lee, Y. H. J., Allen, J. C., and Tan, N. S. (2020). Characterisation of Serum Progesterone and Progesterone-Induced Blocking Factor (PIBF) Levels across Trimesters in Healthy Pregnant Women. Sci. Rep. 10 (1), 3840. doi:10.1038/s41598-020-59452-y
Liu, C., Yu, S., Zinn, K., Wang, J., Zhang, L., Jia, Y., et al. (2006). Murine Mammary Carcinoma Exosomes Promote Tumor Growth by Suppression of NK Cell Function. J. Immunol. 176 (3), 1375–1385. doi:10.4049/jimmunol.176.3.1375
Lobo, T. F., Borges, C. M., Mattar, R., Gomes, C. P., de Angelo, A. G. S., Pendeloski, K. P. T., et al. (2018). Impaired Treg and NK Cells Profile in Overweight Women with Gestational Diabetes Mellitus. Am. J. Reprod. Immunol. 79 (3). doi:10.1111/aji.12810
Loegl, J., Hiden, U., Nussbaumer, E., Schliefsteiner, C., Cvitic, S., Lang, I., et al. (2016). Hofbauer Cells of M2a, M2b and M2c Polarization May Regulate Feto-Placental Angiogenesis. Reproduction 152 (5), 447–455. doi:10.1530/REP-16-0159
Logiodice, F., Lombardelli, L., Kullolli, O., Haller, H., Maggi, E., Rukavina, D., et al. (2019). Decidual Interleukin-22-Producing CD4+ T Cells (Th17/Th0/IL-22+ and Th17/Th2/IL-22+, Th2/IL-22+, Th0/IL-22+), Which Also Produce IL-4, Are Involved in the Success of Pregnancy. Int. J. Mol. Sci. 20 (2). doi:10.3390/ijms20020428
Lokossou, A. G., Toudic, C., Nguyen, P. T., Elisseeff, X., Vargas, A., Rassart, É., et al. (2020). Endogenous Retrovirus-Encoded Syncytin-2 Contributes to Exosome-Mediated Immunosuppression of T Cells†. Biol. Reprod. 102 (1), 185–198. doi:10.1093/biolre/ioz124
Lombardelli, L., Logiodice, F., Aguerre-Girr, M., Kullolli, O., Haller, H., Casart, Y., et al. (2016). Interleukin-17-producing Decidual CD4+ T Cells Are Not Deleterious for Human Pregnancy when They Also Produce Interleukin-4. Clin. Mol. Allergy 14, 1. doi:10.1186/s12948-016-0039-y
Louwen, F., Muschol-Steinmetz, C., Reinhard, J., Reitter, A., and Yuan, J. (2012). A Lesson for Cancer Research: Placental Microarray Gene Analysis in Preeclampsia. Oncotarget 3 (8), 759–773. doi:10.18632/oncotarget.595
Ludwig, S., Sharma, P., Theodoraki, M. N., Pietrowska, M., Yerneni, S. S., Lang, S., et al. (2018). Molecular and Functional Profiles of Exosomes from HPV(+) and HPV(-) Head and Neck Cancer Cell Lines. Front. Oncol. 8, 445. doi:10.3389/fonc.2018.00445
Luo, R., Shao, X., Xu, P., Liu, Y., Wang, Y., Zhao, Y., et al. (2014). MicroRNA-210 Contributes to Preeclampsia by Downregulating Potassium Channel Modulatory Factor 1. Hypertension 64 (4), 839–845. doi:10.1161/HYPERTENSIONAHA.114.03530
Macintyre, A. N., Gerriets, V. A., Nichols, A. G., Michalek, R. D., Rudolph, M. C., Deoliveira, D., et al. (2014). The Glucose Transporter Glut1 Is Selectively Essential for CD4 T Cell Activation and Effector Function. Cell Metab 20 (1), 61–72. doi:10.1016/j.cmet.2014.05.004
Manaster, I., Goldman-Wohl, D., Greenfield, C., Nachmani, D., Tsukerman, P., Hamani, Y., et al. (2012). MiRNA-mediated Control of HLA-G Expression and Function. PLoS One 7 (3), e33395. doi:10.1371/journal.pone.0033395
Mao, G., Wang, J., Kang, Y., Tai, P., Wen, J., Zou, Q., et al. (2010). Progesterone Increases Systemic and Local Uterine Proportions of CD4+CD25+ Treg Cells during Midterm Pregnancy in Mice. Endocrinology 151 (11), 5477–5488. doi:10.1210/en.2010-0426
Maybruck, B. T., Pfannenstiel, L. W., Diaz-Montero, M., and Gastman, B. R. (2017). Tumor-derived Exosomes Induce CD8+ T Cell Suppressors. J. Immunother. Cancer 5 (1), 65. doi:10.1186/s40425-017-0269-7
McElrath, T. F., Cantonwine, D. E., Jeyabalan, A., Doss, R. C., Page, G., Roberts, J. M., et al. (2019). Circulating Microparticle Proteins Obtained in the Late First Trimester Predict Spontaneous Preterm Birth at Less Than 35 Weeks' Gestation: a Panel Validation with Specific Characterization by Parity. Am. J. Obstet. Gynecol. 220 (5), 488–e11. doi:10.1016/j.ajog.2019.01.220
Meggyes, M., Nagy, D. U., and Szereday, L. (2020). Investigation of the PD-1 and PD-L1 Immune Checkpoint Molecules throughout Healthy Human Pregnancy and in Nonpregnant Women. J. Clin. Med. 9 (8). doi:10.3390/jcm9082536
Menon, R., Debnath, C., Lai, A., Guanzon, D., Bhatnagar, S., Kshetrapal, P. K., et al. (2019). Circulating Exosomal miRNA Profile during Term and Preterm Birth Pregnancies: A Longitudinal Study. Endocrinology 160 (2), 249–275. doi:10.1210/en.2018-00836
Mese, H., Sasaki, A., Alcalde, R. E., Nakayama, S., and Matsumura, T. (2000). Regulation of Apoptosis Reduction in the Cisplatin-Resistant A431 Cell Line by Bcl-2 and CPP32. Chemotherapy 46 (1), 69–76. doi:10.1159/000007258
Michalek, R. D., Gerriets, V. A., Jacobs, S. R., Macintyre, A. N., MacIver, N. J., Mason, E. F., et al. (2011). Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets. J. Immunol. 186 (6), 3299–3303. doi:10.4049/jimmunol.1003613
Micossé, C., von Meyenn, L., Steck, O., Kipfer, E., Adam, C., Simillion, C., et al. (2019). Human "TH9" Cells Are a Subpopulation of PPAR-Γ+ TH2 Cells. Sci. Immunol. 4 (31). doi:10.1126/sciimmunol.aat5943
Mikami, N., Kawakami, R., Chen, K. Y., Sugimoto, A., Ohkura, N., and Sakaguchi, S. (2020). Epigenetic Conversion of Conventional T Cells into Regulatory T Cells by CD28 Signal Deprivation. Proc. Natl. Acad. Sci. U S A. 117 (22), 12258–12268. doi:10.1073/pnas.1922600117
Miller, D., Motomura, K., Garcia-Flores, V., Romero, R., and Gomez-Lopez, N. (2018). Innate Lymphoid Cells in the Maternal and Fetal Compartments. Front. Immunol. 9, 2396. doi:10.3389/fimmu.2018.02396
Milosevic-Stevanovic, J., Krstic, M., Stefanovic, M., Zivadinovic, R., Vukomanovic, P., Trajkovic-Dinic, S. P., et al. (2019). T Lymphocytes in the Third Trimester Decidua in Preeclampsia. Hypertens. Pregnancy 38 (1), 52–57. doi:10.1080/10641955.2019.1575393
Mincheva-Nilsson, L., and Baranov, V. (2014). Cancer Exosomes and NKG2D Receptor-Ligand Interactions: Impairing NKG2D-Mediated Cytotoxicity and Anti-tumour Immune Surveillance. Semin. Cancer Biol. 28, 24–30. doi:10.1016/j.semcancer.2014.02.010
Mincheva-Nilsson, L., and Baranov, V. (2014). Placenta-derived Exosomes and Syncytiotrophoblast Microparticles and Their Role in Human Reproduction: Immune Modulation for Pregnancy success. Am. J. Reprod. Immunol. 72 (5), 440–457. doi:10.1111/aji.12311
Mitchell, R. E., Hassan, M., Burton, B. R., Britton, G., Hill, E. V., Verhagen, J., et al. (2017). IL-4 Enhances IL-10 Production in Th1 Cells: Implications for Th1 and Th2 Regulation. Sci. Rep. 7 (1), 11315. doi:10.1038/s41598-017-11803-y
Miyanishi, N., Nishi, N., Abe, H., Kashio, Y., Shinonaga, R., Nakakita, S., et al. (2007). Carbohydrate-recognition Domains of Galectin-9 Are Involved in Intermolecular Interaction with Galectin-9 Itself and Other Members of the Galectin Family. Glycobiology 17 (4), 423–432. doi:10.1093/glycob/cwm001
Mohr, A., Atif, M., Balderas, R., Gorochov, G., and Miyara, M. (2019). The Role of FOXP3+ Regulatory T Cells in Human Autoimmune and Inflammatory Diseases. Clin. Exp. Immunol. 197 (1), 24–35. doi:10.1111/cei.13288
Monteiro, C., Kasahara, T. M., Castro, J. R., Sacramento, P. M., Hygino, J., Centurião, N., et al. (2017). Pregnancy Favors the Expansion of Circulating Functional Follicular Helper T Cells. J. Reprod. Immunol. 121, 1–10. doi:10.1016/j.jri.2017.04.007
Mor, G., Cardenas, I., Abrahams, V., and Guller, S. (2011). Inflammation and Pregnancy: the Role of the Immune System at the Implantation Site. Ann. N. Y Acad. Sci. 1221, 80–87. doi:10.1111/j.1749-6632.2010.05938.x
Moreaux, J., Hose, D., Reme, T., Jourdan, E., Hundemer, M., Legouffe, E., et al. (2006). CD200 Is a New Prognostic Factor in Multiple Myeloma. Blood 108 (13), 4194–4197. doi:10.1182/blood-2006-06-029355
Mrizak, D., Martin, N., Barjon, C., Jimenez-Pailhes, A. S., Mustapha, R., Niki, T., et al. (2015). Effect of Nasopharyngeal Carcinoma-Derived Exosomes on Human Regulatory T Cells. J. Natl. Cancer Inst. 107 (1), 363. doi:10.1093/jnci/dju363
Mulac-Jericevic, B., and Conneely, O. M. (2004). Reproductive Tissue Selective Actions of Progesterone Receptors. Reproduction 128 (2), 139–146. doi:10.1530/rep.1.00189
Mulac-Jericevic, B., Lydon, J. P., DeMayo, F. J., and Conneely, O. M. (2003). Defective Mammary Gland Morphogenesis in Mice Lacking the Progesterone Receptor B Isoform. Proc. Natl. Acad. Sci. U S A. 100 (17), 9744–9749. doi:10.1073/pnas.1732707100
Mulac-Jericevic, B., Mullinax, R. A., DeMayo, F. J., Lydon, J. P., and Conneely, O. M. (2000). Subgroup of Reproductive Functions of Progesterone Mediated by Progesterone Receptor-B Isoform. Science 289 (5485), 1751–1754. doi:10.1126/science.289.5485.1751
Muller, L., Mitsuhashi, M., Simms, P., Gooding, W. E., and Whiteside, T. L. (2016). Tumor-derived Exosomes Regulate Expression of Immune Function-Related Genes in Human T Cell Subsets. Sci. Rep. 6, 20254. doi:10.1038/srep20254
Munn, D. H., and Mellor, A. L. (2016). IDO in the Tumor Microenvironment: Inflammation, Counter-regulation, and Tolerance. Trends Immunol. 37 (3), 193–207. doi:10.1016/j.it.2016.01.002
Munn, D. H., Zhou, M., Attwood, J. T., Bondarev, I., Conway, S. J., Marshall, B., et al. (1998). Prevention of Allogeneic Fetal Rejection by Tryptophan Catabolism. Science 281 (5380), 1191–1193. doi:10.1126/science.281.5380.1191
Nair, S., Jayabalan, N., Guanzon, D., Palma, C., Scholz-Romero, K., Elfeky, O., et al. (2018). Human Placental Exosomes in Gestational Diabetes Mellitus Carry a Specific Set of miRNAs Associated with Skeletal Muscle Insulin Sensitivity. Clin. Sci. (Lond) 132 (22), 2451–2467. doi:10.1042/CS20180487
Nancy, P., and Erlebacher, A. (2014). T Cell Behavior at the Maternal-Fetal Interface. Int. J. Dev. Biol. 58 (2-4), 189–198. doi:10.1387/ijdb.140054ae
Ng, Y. H., Rome, S., Jalabert, A., Forterre, A., Singh, H., Hincks, C. L., et al. (2013). Endometrial Exosomes/microvesicles in the Uterine Microenvironment: a New Paradigm for Embryo-Endometrial Cross Talk at Implantation. PLoS One 8 (3), e58502. doi:10.1371/journal.pone.0058502
Ning, Y., Shen, K., Wu, Q., Sun, X., Bai, Y., Xie, Y., et al. (2018). Tumor Exosomes Block Dendritic Cells Maturation to Decrease the T Cell Immune Response. Immunol. Lett. 199, 36–43. doi:10.1016/j.imlet.2018.05.002
Olsen, P. H., and Ambros, V. (1999). The Lin-4 Regulatory RNA Controls Developmental Timing in Caenorhabditis elegans by Blocking LIN-14 Protein Synthesis after the Initiation of Translation. Dev. Biol. 216 (2), 671–680. doi:10.1006/dbio.1999.9523
Oomizu, S., Arikawa, T., Niki, T., Kadowaki, T., Ueno, M., Nishi, N., et al. (2012). Cell Surface Galectin-9 Expressing Th Cells Regulate Th17 and Foxp3+ Treg Development by Galectin-9 Secretion. PLoS One 7 (11), e48574. doi:10.1371/journal.pone.0048574
Ostojic, S., Dubanchet, S., Chaouat, G., Abdelkarim, M., Truyens, C., and Capron, F. (2003). Demonstration of the Presence of IL-16, IL-17 and IL-18 at the Murine Fetomaternal Interface during Murine Pregnancy. Am. J. Reprod. Immunol. 49 (2), 101–112. doi:10.1034/j.1600-0897.2003.01150.x
Pan, B. T., and Johnstone, R. M. (1983). Fate of the Transferrin Receptor during Maturation of Sheep Reticulocytes In Vitro: Selective Externalization of the Receptor. Cell 33 (3), 967–978. doi:10.1016/0092-8674(83)90040-5
Pan, Y., Wang, X., Li, Y., Yan, P., and Zhang, H. (2022). Human Umbilical Cord Blood Mesenchymal Stem Cells-Derived Exosomal microRNA-503-3p Inhibits Progression of Human Endometrial Cancer Cells through Downregulating MEST. Cancer Gene Ther. 2022. doi:10.1038/s41417-021-00416-3
Park, A., Lee, Y., Kim, M. S., Kang, Y. J., Park, Y. J., Jung, H., et al. (2018). Prostaglandin E2 Secreted by Thyroid Cancer Cells Contributes to Immune Escape through the Suppression of Natural Killer (NK) Cell Cytotoxicity and NK Cell Differentiation. Front. Immunol. 9, 1859. doi:10.3389/fimmu.2018.01859
Päzolt, H. J., and Henkert, K. (1990). Surgical Treatment of Peripheral Nerve Injuries. Evaluation of a Multicenter Study. Zentralbl Chir 115 (11), 677–684.
Pearce, E. L., Poffenberger, M. C., Chang, C. H., and Jones, R. G. (2013). Fueling Immunity: Insights into Metabolism and Lymphocyte Function. Science 342 (6155), 1242454. doi:10.1126/science.1242454
Pereira, T. B., Thomaz, E. B., Nascimento, F. R., Santos, A. P., Batista, R. L., Bettiol, H., et al. (2016). Regulatory Cytokine Expression and Preterm Birth: Case-Control Study Nested in a Cohort. PLoS One 11 (8), e0158380. doi:10.1371/journal.pone.0158380
Petroff, M. G., Chen, L., Phillips, T. A., Azzola, D., Sedlmayr, P., and Hunt, J. S. (2003). B7 Family Molecules Are Favorably Positioned at the Human Maternal-Fetal Interface. Biol. Reprod. 68 (5), 1496–1504. doi:10.1095/biolreprod.102.010058
Phiel, K. L., Henderson, R. A., Adelman, S. J., and Elloso, M. M. (2005). Differential Estrogen Receptor Gene Expression in Human Peripheral Blood Mononuclear Cell Populations. Immunol. Lett. 97 (1), 107–113. doi:10.1016/j.imlet.2004.10.007
Piccinni, M. P., Giudizi, M. G., Biagiotti, R., Beloni, L., Giannarini, L., Sampognaro, S., et al. (1995). Progesterone Favors the Development of Human T Helper Cells Producing Th2-type Cytokines and Promotes Both IL-4 Production and Membrane CD30 Expression in Established Th1 Cell Clones. J. Immunol. 155 (1), 128–133.
Pillay, P., Maharaj, N., Moodley, J., and Mackraj, I. (2016). Placental Exosomes and Pre-eclampsia: Maternal Circulating Levels in normal Pregnancies and, Early and Late Onset Pre-eclamptic Pregnancies. Placenta 46, 18–25. doi:10.1016/j.placenta.2016.08.078
Pillay, P., Moodley, K., Vatish, M., Moodley, J., Duarte, R., and Mackraj, I. (2020). Exosomal Th1/Th2 Cytokines in Preeclampsia and HIV-Positive Preeclamptic Women on Highly Active Anti-retroviral Therapy. Cytokine 125, 154795. doi:10.1016/j.cyto.2019.154795
Pinget, G. V., Corpuz, T. M., Stolp, J., Lousberg, E. L., Diener, K. R., Robertson, S. A., et al. (2016). The Majority of Murine γδ T Cells at the Maternal-Fetal Interface in Pregnancy Produce IL-17. Immunol. Cel Biol 94 (7), 623–630. doi:10.1038/icb.2016.48
Podaný, V., Vachálková, A., Miertus, S., and Bahna, L. (1975). Electrochemical Properties of Polycyclic Compounds Studied by the Polarographic Method in Anhydrous Systems. II. Polarographic Study of Carcinogenic Nitrogen Compounds in Dimethylformamide and Comparison of Half-Wave Potentials with Quantum-Chemical Calculations of Molecular Orbitals. Neoplasma 22 (5), 469–482.
Polgar, B., Barakonyi, A., Xynos, I., and Szekeres-Bartho, J. (1999). The Role of Gamma/delta T Cell Receptor Positive Cells in Pregnancy. Am. J. Reprod. Immunol. 41 (4), 239–244. doi:10.1111/j.1600-0897.1999.tb00433.x
Raphael, I., Nalawade, S., Eagar, T. N., and Forsthuber, T. G. (2015). T Cell Subsets and Their Signature Cytokines in Autoimmune and Inflammatory Diseases. Cytokine 74 (1), 5–17. doi:10.1016/j.cyto.2014.09.011
Renaud, S. J., Postovit, L. M., Macdonald-Goodfellow, S. K., McDonald, G. T., Caldwell, J. D., and Graham, C. H. (2005). Activated Macrophages Inhibit Human Cytotrophoblast Invasiveness In Vitro. Biol. Reprod. 73 (2), 237–243. doi:10.1095/biolreprod.104.038000
Renthal, N. E., Chen, C. C., Williams, K. C., Gerard, R. D., Prange-Kiel, J., and Mendelson, C. R. (2010). miR-200 Family and Targets, ZEB1 and ZEB2, Modulate Uterine Quiescence and Contractility during Pregnancy and Labor. Proc. Natl. Acad. Sci. U S A. 107 (48), 20828–20833. doi:10.1073/pnas.1008301107
Repnik, U., Tilburgs, T., Roelen, D. L., van der Mast, B. J., Kanhai, H. H., Scherjon, S., et al. (2008). Comparison of Macrophage Phenotype between Decidua Basalis and Decidua Parietalis by Flow Cytometry. Placenta 29 (5), 405–412. doi:10.1016/j.placenta.2008.02.004
Robert, C. (2020). A Decade of Immune-Checkpoint Inhibitors in Cancer Therapy. Nat. Commun. 11 (1), 3801. doi:10.1038/s41467-020-17670-y
Robertson, S. A., Guerin, L. R., Bromfield, J. J., Branson, K. M., Ahlström, A. C., and Care, A. S. (2009). Seminal Fluid Drives Expansion of the CD4+CD25+ T Regulatory Cell Pool and Induces Tolerance to Paternal Alloantigens in Mice. Biol. Reprod. 80 (5), 1036–1045. doi:10.1095/biolreprod.108.074658
Rolle, L., Memarzadeh Tehran, M., Morell-García, A., Raeva, Y., Schumacher, A., Hartig, R., et al. (2013). Cutting Edge: IL-10-producing Regulatory B Cells in Early Human Pregnancy. Am. J. Reprod. Immunol. 70 (6), 448–453. doi:10.1111/aji.12157
Rowe, J. H., Ertelt, J. M., Xin, L., and Way, S. S. (2012). Pregnancy Imprints Regulatory Memory that Sustains Anergy to Fetal Antigen. Nature 490 (7418), 102–106. doi:10.1038/nature11462
Sabapatha, A., Gercel-Taylor, C., and Taylor, D. D. (2006). Specific Isolation of Placenta-Derived Exosomes from the Circulation of Pregnant Women and Their Immunoregulatory Consequences. Am. J. Reprod. Immunol. 56 (5-6), 345–355. doi:10.1111/j.1600-0897.2006.00435.x
Sahin Uysal, N., Eroglu, H., Ozcan, C., Sahin, D., and Yucel, A. (2020). Is the Serum delta Neutrophil index Level Different in Gestational Diabetic Women? J. Matern. Fetal Neonatal. Med. 33 (19), 3349–3354. doi:10.1080/14767058.2020.1760833
Saito, S., Nakashima, A., Shima, T., and Ito, M. (2010). Th1/Th2/Th17 and Regulatory T-Cell Paradigm in Pregnancy. Am. J. Reprod. Immunol. 63 (6), 601–610. doi:10.1111/j.1600-0897.2010.00852.x
Sakamoto, Y., Moran, P., Bulmer, J. N., Searle, R. F., and Robson, S. C. (2005). Macrophages and Not Granulocytes Are Involved in Cervical Ripening. J. Reprod. Immunol. 66 (2), 161–173. doi:10.1016/j.jri.2005.04.005
Salomon, C., Scholz-Romero, K., Sarker, S., Sweeney, E., Kobayashi, M., Correa, P., et al. (2016). Gestational Diabetes Mellitus Is Associated with Changes in the Concentration and Bioactivity of Placenta-Derived Exosomes in Maternal Circulation across Gestation. Diabetes 65 (3), 598–609. doi:10.2337/db15-0966
Salomon, C., Torres, M. J., Kobayashi, M., Scholz-Romero, K., Sobrevia, L., Dobierzewska, A., et al. (2014). A Gestational Profile of Placental Exosomes in Maternal Plasma and Their Effects on Endothelial Cell Migration. PLoS One 9 (6), e98667. doi:10.1371/journal.pone.0098667
Salomon, C., Yee, S., Scholz-Romero, K., Kobayashi, M., Vaswani, K., Kvaskoff, D., et al. (2014). Extravillous Trophoblast Cells-Derived Exosomes Promote Vascular Smooth Muscle Cell Migration. Front. Pharmacol. 5, 175. doi:10.3389/fphar.2014.00175
Sargent, I. L., Borzychowski, A. M., and Redman, C. W. (2006). NK Cells and Human Pregnancy-Aan Inflammatory View. Trends Immunol. 27 (9), 399–404. doi:10.1016/j.it.2006.06.009
Schober, L., Radnai, D., Spratte, J., Kisielewicz, A., Schmitt, E., Mahnke, K., et al. (2014). The Role of Regulatory T Cell (Treg) Subsets in Gestational Diabetes Mellitus. Clin. Exp. Immunol. 177 (1), 76–85. doi:10.1111/cei.12300
Schonkeren, D., van der Hoorn, M. L., Khedoe, P., Swings, G., van Beelen, E., Claas, F., et al. (2011). Differential Distribution and Phenotype of Decidual Macrophages in Preeclamptic versus Control Pregnancies. Am. J. Pathol. 178 (2), 709–717. doi:10.1016/j.ajpath.2010.10.011
Schumacher, A., Brachwitz, N., Sohr, S., Engeland, K., Langwisch, S., Dolaptchieva, M., et al. (2009). Human Chorionic Gonadotropin Attracts Regulatory T Cells into the Fetal-Maternal Interface during Early Human Pregnancy. J. Immunol. 182 (9), 5488–5497. doi:10.4049/jimmunol.0803177
Schumacher, A., Heinze, K., Witte, J., Poloski, E., Linzke, N., Woidacki, K., et al. (2013). Human Chorionic Gonadotropin as a central Regulator of Pregnancy Immune Tolerance. J. Immunol. 190 (6), 2650–2658. doi:10.4049/jimmunol.1202698
Schumacher, A., and Zenclussen, A. C. (2019). Human Chorionic Gonadotropin-Mediated Immune Responses that Facilitate Embryo Implantation and Placentation. Front. Immunol. 10, 2896. doi:10.3389/fimmu.2019.02896
Seki, M., Oomizu, S., Sakata, K. M., Sakata, A., Arikawa, T., Watanabe, K., et al. (2008). Galectin-9 Suppresses the Generation of Th17, Promotes the Induction of Regulatory T Cells, and Regulates Experimental Autoimmune Arthritis. Clin. Immunol. 127 (1), 78–88. doi:10.1016/j.clim.2008.01.006
Sharma, M. D., Shinde, R., McGaha, T. L., Huang, L., Holmgaard, R. B., Wolchok, J. D., et al. (2015). The PTEN Pathway in Tregs Is a Critical Driver of the Suppressive Tumor Microenvironment. Sci. Adv. 1 (10), e1500845. doi:10.1126/sciadv.1500845
Shashar, S., Kloog, I., Erez, O., Shtein, A., Yitshak-Sade, M., Sarov, B., et al. (2020). Temperature and Preeclampsia: Epidemiological Evidence that Perturbation in Maternal Heat Homeostasis Affects Pregnancy Outcome. PLoS One 15 (5), e0232877. doi:10.1371/journal.pone.0232877
Sheller-Miller, S., Radnaa, E., Yoo, J. K., Kim, E., Choi, K., Kim, Y., et al. (2021). Exosomal Delivery of NF-Κb Inhibitor Delays LPS-Induced Preterm Birth and Modulates Fetal Immune Cell Profile in Mouse Models. Sci. Adv. 7 (4). doi:10.1126/sciadv.abd3865
Sheller-Miller, S., Richardson, L., Martin, L., Jin, J., and Menon, R. (2018). Systematic Review of P38 Mitogen-Activated Kinase and its Functional Role in Reproductive Tissues. Am. J. Reprod. Immunol. 80 (6), e13047. doi:10.1111/aji.13047
Sheller-Miller, S., Trivedi, J., Yellon, S. M., and Menon, R. (2019). Exosomes Cause Preterm Birth in Mice: Evidence for Paracrine Signaling in Pregnancy. Sci. Rep. 9 (1), 608. doi:10.1038/s41598-018-37002-x
Sheu, A., Chan, Y., Ferguson, A., Bakhtyari, M. B., Hawke, W., White, C., et al. (2018). A Proinflammatory CD4+ T Cell Phenotype in Gestational Diabetes Mellitus. Diabetologia 61 (7), 1633–1643. doi:10.1007/s00125-018-4615-1
Shima, T., Inada, K., Nakashima, A., Ushijima, A., Ito, M., Yoshino, O., et al. (2015). Paternal Antigen-specific Proliferating Regulatory T Cells Are Increased in Uterine-Draining Lymph Nodes Just before Implantation and in Pregnant Uterus Just after Implantation by Seminal Plasma-Priming in Allogeneic Mouse Pregnancy. J. Reprod. Immunol. 108, 72–82. doi:10.1016/j.jri.2015.02.005
Shimada, S., Nishida, R., Takeda, M., Iwabuchi, K., Kishi, R., Onoé, K., et al. (2006). Natural Killer, Natural Killer T, Helper and Cytotoxic T Cells in the Decidua from Sporadic Miscarriage. Am. J. Reprod. Immunol. 56 (3), 193–200. doi:10.1111/j.1600-0897.2006.00417.x
Shimada, Y., Matsubayashi, J., Kudo, Y., Maehara, S., Takeuchi, S., Hagiwara, M., et al. (2021). Serum-derived Exosomal PD-L1 Expression to Predict Anti-PD-1 Response and in Patients with Non-small Cell Lung Cancer. Sci. Rep. 11 (1), 7830. doi:10.1038/s41598-021-87575-3
Shore, V. H., Wang, T. H., Wang, C. L., Torry, R. J., Caudle, M. R., and Torry, D. S. (1997). Vascular Endothelial Growth Factor, Placenta Growth Factor and Their Receptors in Isolated Human Trophoblast. Placenta 18 (8), 657–665. doi:10.1016/s0143-4004(97)90007-2
Slattery, K., Woods, E., Zaiatz-Bittencourt, V., Marks, S., Chew, S., Conroy, M., et al. (2021). TGFβ Drives NK Cell Metabolic Dysfunction in Human Metastatic Breast Cancer. J. Immunother. Cancer 9 (2). doi:10.1136/jitc-2020-002044
Slutsky, R., Romero, R., Xu, Y., Galaz, J., Miller, D., Done, B., et al. (2019). Exhausted and Senescent T Cells at the Maternal-Fetal Interface in Preterm and Term Labor. J. Immunol. Res. 2019, 3128010. doi:10.1155/2019/3128010
Song, X., Ding, Y., Liu, G., Yang, X., Zhao, R., Zhang, Y., et al. (2016). Cancer Cell-Derived Exosomes Induce Mitogen-Activated Protein Kinase-dependent Monocyte Survival by Transport of Functional Receptor Tyrosine Kinases. J. Biol. Chem. 291 (16), 8453–8464. doi:10.1074/jbc.M116.716316
Soni, S., Rath, G., Prasad, C. P., Salhan, S., Saxena, S., and Jain, A. K. (2010). Apoptosis and Bcl-2 Protein Expression in Human Placenta over the Course of normal Pregnancy. Anat. Histol. Embryol. 39 (5), 426–431. doi:10.1111/j.1439-0264.2010.01012.x
St Louis, D., Romero, R., Plazyo, O., Arenas-Hernandez, M., Panaitescu, B., Xu, Y., et al. (2016). Invariant NKT Cell Activation Induces Late Preterm Birth that Is Attenuated by Rosiglitazone. J. Immunol. 196 (3), 1044–1059. doi:10.4049/jimmunol.1501962
Steinthorsdottir, V., McGinnis, R., Williams, N. O., Stefansdottir, L., Thorleifsson, G., Shooter, S., et al. (2020). Genetic Predisposition to Hypertension Is Associated with Preeclampsia in European and Central Asian Women. Nat. Commun. 11 (1), 5976. doi:10.1038/s41467-020-19733-6
Stenqvist, A. C., Nagaeva, O., Baranov, V., and Mincheva-Nilsson, L. (2013). Exosomes Secreted by Human Placenta Carry Functional Fas Ligand and TRAIL Molecules and Convey Apoptosis in Activated Immune Cells, Suggesting Exosome-Mediated Immune Privilege of the Fetus. J. Immunol. 191 (11), 5515–5523. doi:10.4049/jimmunol.1301885
Storment, J. M., Meyer, M., and Osol, G. (2000). Estrogen Augments the Vasodilatory Effects of Vascular Endothelial Growth Factor in the Uterine Circulation of the Rat. Am. J. Obstet. Gynecol. 183 (2), 449–453. doi:10.1067/mob.2000.105910
Sugita, S., Kawazoe, Y., Imai, A., Usui, Y., Takahashi, M., and Mochizuki, M. (2013). Suppression of IL-22-producing T Helper 22 Cells by RPE Cells via PD-L1/PD-1 Interactions. Invest. Ophthalmol. Vis. Sci. 54 (10), 6926–6933. doi:10.1167/iovs.13-12703
Sun, J. F., Zhang, D., Gao, C. J., Zhang, Y. W., and Dai, Q. S. (2019). Exosome-Mediated MiR-155 Transfer Contributes to Hepatocellular Carcinoma Cell Proliferation by Targeting PTEN. Med. Sci. Monit. Basic Res. 25, 218–228. doi:10.12659/MSMBR.918134
Sun, T., Meng, F., Zhao, H., Yang, M., Zhang, R., Yu, Z., et al. (2020). Elevated First-Trimester Neutrophil Count Is Closely Associated with the Development of Maternal Gestational Diabetes Mellitus and Adverse Pregnancy Outcomes. Diabetes 69 (7), 1401–1410. doi:10.2337/db19-0976
Svensson, J., Jenmalm, M. C., Matussek, A., Geffers, R., Berg, G., and Ernerudh, J. (2011). Macrophages at the Fetal-Maternal Interface Express Markers of Alternative Activation and Are Induced by M-CSF and IL-10. J. Immunol. 187 (7), 3671–3682. doi:10.4049/jimmunol.1100130
Szczepanski, M. J., Szajnik, M., Welsh, A., Whiteside, T. L., and Boyiadzis, M. (2011). Blast-derived Microvesicles in Sera from Patients with Acute Myeloid Leukemia Suppress Natural Killer Cell Function via Membrane-Associated Transforming Growth Factor-Beta1. Haematologica 96 (9), 1302–1309. doi:10.3324/haematol.2010.039743
Taganov, K. D., Boldin, M. P., and Baltimore, D. (2007). MicroRNAs and Immunity: Tiny Players in a Big Field. Immunity 26 (2), 133–137. doi:10.1016/j.immuni.2007.02.005
Taglauer, E. S., Fernandez-Gonzalez, A., Willis, G. R., Reis, M., Yeung, V., Liu, X., et al. (2021). Mesenchymal Stromal Cell-Derived Extracellular Vesicle Therapy Prevents Preeclamptic Physiology through Intrauterine Immunomodulation†. Biol. Reprod. 104 (2), 457–467. doi:10.1093/biolre/ioaa198
Tagliani, E., Shi, C., Nancy, P., Tay, C. S., Pamer, E. G., and Erlebacher, A. (2011). Coordinate Regulation of Tissue Macrophage and Dendritic Cell Population Dynamics by CSF-1. J. Exp. Med. 208 (9), 1901–1916. doi:10.1084/jem.20110866
Tan, K. H., Tan, S. S., Ng, M. J., Tey, W. S., Sim, W. K., Allen, J. C., et al. (2017). Extracellular Vesicles Yield Predictive Pre-eclampsia Biomarkers. J. Extracell Vesicles 6 (1), 1408390. doi:10.1080/20013078.2017.1408390
Tan, K. H., Tan, S. S., Sze, S. K., Lee, W. K., Ng, M. J., and Lim, S. K. (2014). Plasma Biomarker Discovery in Preeclampsia Using a Novel Differential Isolation Technology for Circulating Extracellular Vesicles. Am. J. Obstet. Gynecol. 211 (4), 380.e1–80.e13. doi:10.1016/j.ajog.2014.03.038
Tannahill, G. M., Curtis, A. M., Adamik, J., Palsson-McDermott, E. M., McGettrick, A. F., Goel, G., et al. (2013). Succinate Is an Inflammatory Signal that Induces IL-1β through HIF-1α. Nature 496 (7444), 238–242. doi:10.1038/nature11986
Theodoraki, M. N., Yerneni, S. S., Hoffmann, T. K., Gooding, W. E., and Whiteside, T. L. (2018). Clinical Significance of PD-L1+ Exosomes in Plasma of Head and Neck Cancer Patients. Clin. Cancer Res. 24 (4), 896–905. doi:10.1158/1078-0432.CCR-17-2664
Thomas, J. R., Appios, A., Zhao, X., Dutkiewicz, R., Donde, M., Lee, C. Y. C., et al. (2021). Phenotypic and Functional Characterization of First-Trimester Human Placental Macrophages, Hofbauer Cells. J. Exp. Med. 218 (1). doi:10.1084/jem.20200891
Tilburgs, T., Meissner, T. B., Ferreira, L. M. R., Mulder, A., Musunuru, K., Ye, J., et al. (2017). NLRP2 Is a Suppressor of NF-ƙB Signaling and HLA-C Expression in Human Trophoblasts†,‡. Biol. Reprod. 96 (4), 831–842. doi:10.1093/biolre/iox009
Tilburgs, T., Scherjon, S. A., and Claas, F. H. (2010). Major Histocompatibility Complex (MHC)-mediated Immune Regulation of Decidual Leukocytes at the Fetal-Maternal Interface. J. Reprod. Immunol. 85 (1), 58–62. doi:10.1016/j.jri.2010.01.005
Tilburgs, T., Scherjon, S. A., van der Mast, B. J., Haasnoot, G. W., Versteeg-V D Voort-Maarschalk, M., Roelen, D. L., et al. (2009). Fetal-maternal HLA-C Mismatch Is Associated with Decidual T Cell Activation and Induction of Functional T Regulatory Cells. J. Reprod. Immunol. 82 (2), 148–157. doi:10.1016/j.jri.2009.05.003
Tirado-González, I., Muñoz-Fernández, R., Prados, A., Leno-Durán, E., Martin, F., Abadía-Molina, A. C., et al. (2012). Apoptotic DC-SIGN+ Cells in normal Human Decidua. Placenta 33 (4), 257–263. doi:10.1016/j.placenta.2012.01.003
Todt, J. C., Yang, Y., Lei, J., Lauria, M. R., Sorokin, Y., Cotton, D. B., et al. (1996). Effects of Tumor Necrosis Factor-Alpha on Human Trophoblast Cell Adhesion and Motility. Am. J. Reprod. Immunol. 36 (2), 65–71. doi:10.1111/j.1600-0897.1996.tb00141.x
Torchinsky, A., Shepshelovich, J., Orenstein, H., Zaslavsky, Z., Savion, S., Carp, H., et al. (2003). TNF-alpha Protects Embryos Exposed to Developmental Toxicants. Am. J. Reprod. Immunol. 49 (3), 159–168. doi:10.1034/j.1600-0897.2003.01174.x
Trams, E. G., Lauter, C. J., Salem, N., and Heine, U. (1981). Exfoliation of Membrane Ecto-Enzymes in the Form of Micro-vesicles. Biochim. Biophys. Acta 645 (1), 63–70. doi:10.1016/0005-2736(81)90512-5
Tung, S. L., Boardman, D. A., Sen, M., Letizia, M., Peng, Q., Cianci, N., et al. (2018). Regulatory T Cell-Derived Extracellular Vesicles Modify Dendritic Cell Function. Sci. Rep. 8 (1), 6065. doi:10.1038/s41598-018-24531-8
Ueland, T., Michelsen, A. E., Aukrust, P., Henriksen, T., Bollerslev, J., and Lekva, T. (2019). Adipokines and Macrophage Markers during Pregnancy-Possible Role for sCD163 in Prediction and Progression of Gestational Diabetes Mellitus. Diabetes Metab. Res. Rev. 35 (3), e3114. doi:10.1002/dmrr.3114
Valenzuela, F. J., Pérez-Sepúlveda, A., Torres, M. J., Correa, P., Repetto, G. M., and Illanes, S. E. (2012). Pathogenesis of Preeclampsia: the Genetic Component. J. Pregnancy 2012, 632732. doi:10.1155/2012/632732
Van den Bossche, J., Baardman, J., Otto, N. A., van der Velden, S., Neele, A. E., van den Berg, S. M., et al. (2016). Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cel Rep 17 (3), 684–696. doi:10.1016/j.celrep.2016.09.008
Vasudevan, S., Tong, Y., and Steitz, J. A. (2007). Switching from Repression to Activation: microRNAs Can Up-Regulate Translation. Science 318 (5858), 1931–1934. doi:10.1126/science.1149460
Vignard, V., Labbé, M., Marec, N., André-Grégoire, G., Jouand, N., Fonteneau, J. F., et al. (2020). MicroRNAs in Tumor Exosomes Drive Immune Escape in Melanoma. Cancer Immunol. Res. 8 (2), 255–267. doi:10.1158/2326-6066.CIR-19-0522
Wang, C., Lu, Y., Chen, L., Gao, T., Yang, Q., Zhu, C., et al. (2020). Th9 Cells Are Subjected to PD-1/PD-L1-Mediated Inhibition and Are Capable of Promoting CD8 T Cell Expansion through IL-9R in Colorectal Cancer. Int. Immunopharmacol 78, 106019. doi:10.1016/j.intimp.2019.106019
Wang, H., He, M., Hou, Y., Chen, S., Zhang, X., Zhang, M., et al. (2016). Role of Decidual CD14(+) Macrophages in the Homeostasis of Maternal-Fetal Interface and the Differentiation Capacity of the Cells during Pregnancy and Parturition. Placenta 38, 76–83. doi:10.1016/j.placenta.2015.12.001
Wang, J., Hu, R., Xing, Q., Feng, X., Jiang, X., Xu, Y., et al. (2020). Exosomes Derived from Umbilical Cord Mesenchymal Stem Cells Alleviate Mifepristone-Induced Human Endometrial Stromal Cell Injury. Stem Cell Int 2020, 6091269. doi:10.1155/2020/6091269
Wang, S., Cao, C., Piao, H., Li, Y., Tao, Y., Zhang, X., et al. (2015). Tim-3 Protects Decidual Stromal Cells from Toll-like Receptor-Mediated Apoptosis and Inflammatory Reactions and Promotes Th2 Bias at the Maternal-Fetal Interface. Sci. Rep. 5, 9013. doi:10.1038/srep09013
Wang, S., Li, C., Kawamura, H., Watanabe, H., and Abo, T. (2002). Unique Sensitivity to Alpha-Galactosylceramide of NKT Cells in the Uterus. Cell Immunol 215 (1), 98–105. doi:10.1016/s0008-8749(02)00009-6
Wang, S. C., Li, Y. H., Piao, H. L., Hong, X. W., Zhang, D., Xu, Y. Y., et al. (2015). PD-1 and Tim-3 Pathways Are Associated with Regulatory CD8+ T-Cell Function in Decidua and Maintenance of normal Pregnancy. Cell Death Dis 6, e1738. doi:10.1038/cddis.2015.112
Wang, Y., Xu, B., Li, M. Q., Li, D. J., and Jin, L. P. (2013). IL-22 Secreted by Decidual Stromal Cells and NK Cells Promotes the Survival of Human Trophoblasts. Int. J. Clin. Exp. Pathol. 6 (9), 1781–1790.
Warburg, O. (1956). On Respiratory Impairment in Cancer Cells. Science 124 (3215), 269–270. doi:10.1126/science.124.3215.269
Weiler, A., Volkenhoff, A., Hertenstein, H., and Schirmeier, S. (2017). Metabolite Transport across the Mammalian and Insect Brain Diffusion Barriers. Neurobiol. Dis. 107, 15–31. doi:10.1016/j.nbd.2017.02.008
Wherry, E. J., and Kurachi, M. (2015). Molecular and Cellular Insights into T Cell Exhaustion. Nat. Rev. Immunol. 15 (8), 486–499. doi:10.1038/nri3862
Wicherek, L., Basta, P., Pitynski, K., Marianowski, P., Kijowski, J., Wiatr, J., et al. (2009). The Characterization of the Subpopulation of Suppressive B7H4(+) Macrophages and the Subpopulation of CD25(+) CD4(+) and FOXP3(+) Regulatory T-Cells in Decidua during the Secretory Cycle Phase, Arias Stella Reaction, and Spontaneous Abortion - a Preliminary Report. Am. J. Reprod. Immunol. 61 (4), 303–312. doi:10.1111/j.1600-0897.2009.00696.x
Wu, H. X., Jin, L. P., Xu, B., Liang, S. S., and Li, D. J. (2014). Decidual Stromal Cells Recruit Th17 Cells into Decidua to Promote Proliferation and Invasion of Human Trophoblast Cells by Secreting IL-17. Cell Mol Immunol 11 (3), 253–262. doi:10.1038/cmi.2013.67
Wu, L., Li, J., Xu, H. L., Xu, B., Tong, X. H., Kwak-Kim, J., et al. (2016). IL-7/IL-7R Signaling Pathway Might Play a Role in Recurrent Pregnancy Losses by Increasing Inflammatory Th17 Cells and Decreasing Treg Cells. Am. J. Reprod. Immunol. 76 (6), 454–464. doi:10.1111/aji.12588
Wu, M., Zhu, Y., Zhao, J., Ai, H., Gong, Q., Zhang, J., et al. (2015). Soluble Costimulatory Molecule sTim3 Regulates the Differentiation of Th1 and Th2 in Patients with Unexplained Recurrent Spontaneous Abortion. Int. J. Clin. Exp. Med. 8 (6), 8812–8819.
Xiong, H., Zhou, C., and Qi, G. (2010). Proportional Changes of CD4+CD25+Foxp3+ Regulatory T Cells in Maternal Peripheral Blood during Pregnancy and Labor at Term and Preterm. Clin. Invest. Med. 33 (6), E422. doi:10.25011/cim.v33i6.14594
Xu, Y., Romero, R., Miller, D., Kadam, L., Mial, T. N., Plazyo, O., et al. (2016). An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor that Is Attenuated by Rosiglitazone Treatment. J. Immunol. 196 (6), 2476–2491. doi:10.4049/jimmunol.1502055
Yang, J., and Weinberg, R. A. (2008). Epithelial-mesenchymal Transition: at the Crossroads of Development and Tumor Metastasis. Dev. Cell 14 (6), 818–829. doi:10.1016/j.devcel.2008.05.009
Yang, K., He, Y. S., Wang, X. Q., Lu, L., Chen, Q. J., Liu, J., et al. (2011). MiR-146a Inhibits Oxidized Low-Density Lipoprotein-Induced Lipid Accumulation and Inflammatory Response via Targeting Toll-like Receptor 4. FEBS Lett. 585 (6), 854–860. doi:10.1016/j.febslet.2011.02.009
Yang, Y., Li, C. W., Chan, L. C., Wei, Y., Hsu, J. M., Xia, W., et al. (2018). Exosomal PD-L1 Harbors Active Defense Function to Suppress T Cell Killing of Breast Cancer Cells and Promote Tumor Growth. Cell Res 28 (8), 862–864. doi:10.1038/s41422-018-0060-4
Yao, R., Ma, Y. L., Liang, W., Li, H. H., Ma, Z. J., Yu, X., et al. (2012). MicroRNA-155 Modulates Treg and Th17 Cells Differentiation and Th17 Cell Function by Targeting SOCS1. PLoS One 7 (10), e46082. doi:10.1371/journal.pone.0046082
Ye, S. B., Li, Z. L., Luo, D. H., Huang, B. J., Chen, Y. S., Zhang, X. S., et al. (2014). Tumor-derived Exosomes Promote Tumor Progression and T-Cell Dysfunction through the Regulation of Enriched Exosomal microRNAs in Human Nasopharyngeal Carcinoma. Oncotarget 5 (14), 5439–5452. doi:10.18632/oncotarget.2118
Yentz, S., and Smith, D. (2018). Indoleamine 2,3-Dioxygenase (IDO) Inhibition as a Strategy to Augment Cancer Immunotherapy. BioDrugs 32 (4), 311–317. doi:10.1007/s40259-018-0291-4
Yilmaz, Ö., Küçük, M., Kebapçilar, L., Altindag, T., Yüksel, A., Yuvanç, H. O., et al. (2012). Macrophage Migration-Inhibitory Factor Is Elevated in Pregnant Women with Gestational Diabetes Mellitus. Gynecol. Endocrinol. 28 (1), 76–79. doi:10.3109/09513590.2011.588757
Ying, X., Wu, Q., Wu, X., Zhu, Q., Wang, X., Jiang, L., et al. (2016). Epithelial Ovarian Cancer-Secreted Exosomal miR-222-3p Induces Polarization of Tumor-Associated Macrophages. Oncotarget 7 (28), 43076–43087. doi:10.18632/oncotarget.9246
Young, O. M., Tang, Z., Niven-Fairchild, T., Tadesse, S., Krikun, G., Norwitz, E. R., et al. (2015). Toll-like Receptor-Mediated Responses by Placental Hofbauer Cells (HBCs): a Potential Pro-inflammatory Role for Fetal M2 Macrophages. Am. J. Reprod. Immunol. 73 (1), 22–35. doi:10.1111/aji.12336
Yu, Y. R., Imrichova, H., Wang, H., Chao, T., Xiao, Z., Gao, M., et al. (2020). Disturbed Mitochondrial Dynamics in CD8+ TILs Reinforce T Cell Exhaustion. Nat. Immunol. 21 (12), 1540–1551. doi:10.1038/s41590-020-0793-3
Zenclussen, A. C. (2005). CD4(+)CD25+ T Regulatory Cells in Murine Pregnancy. J. Reprod. Immunol. 65 (2), 101–110. doi:10.1016/j.jri.2005.01.003
Zenclussen, A. C., Gerlof, K., Zenclussen, M. L., Sollwedel, A., Bertoja, A. Z., Ritter, T., et al. (2005). Abnormal T-Cell Reactivity against Paternal Antigens in Spontaneous Abortion: Adoptive Transfer of Pregnancy-Induced CD4+CD25+ T Regulatory Cells Prevents Fetal Rejection in a Murine Abortion Model. Am. J. Pathol. 166 (3), 811–822. doi:10.1016/S0002-9440(10)62302-4
Zhan, Y., Li, C., Chen, J., Yu, S., Gao, Q., Wang, Y. P., et al. (2015). Association between Macrophage Migration Inhibitory Factor Rs1007888 and GDM. Genet. Mol. Res. 14 (1), 797–804. doi:10.4238/2015.February.2.4
Zhang, J., Dunk, C. E., Shynlova, O., Caniggia, I., and Lye, S. J. (2019). TGFb1 Suppresses the Activation of Distinct dNK Subpopulations in Preeclampsia. EBioMedicine 39, 531–539. doi:10.1016/j.ebiom.2018.12.015
Zhang, Y. H., and Sun, H. X. (2020). Immune Checkpoint Molecules in Pregnancy: Focus on Regulatory T Cells. Eur. J. Immunol. 50 (2), 160–169. doi:10.1002/eji.201948382
Zhao, G., Yang, C., Yang, J., Liu, P., Jiang, K., Shaukat, A., et al. (2018). Placental Exosome-Mediated Bta-miR-499-Lin28B/let-7 axis Regulates Inflammatory Bias during Early Pregnancy. Cell Death Dis 9 (6), 704. doi:10.1038/s41419-018-0713-8
Zhao, L., Li, J., and Huang, S. (2018). Patients with Unexplained Recurrent Spontaneous Abortion Show Decreased Levels of Microrna-146a-5p in the Deciduae. Ann. Clin. Lab. Sci. 48 (2), 177–182.
Zhao, Y., Zhang, X., Du, N., Sun, H., Chen, L., Bao, H., et al. (2020). Immune Checkpoint Molecules on T Cell Subsets of Pregnancies with Preeclampsia and Gestational Diabetes Mellitus. J. Reprod. Immunol. 142, 103208. doi:10.1016/j.jri.2020.103208
Zhou, J., Li, X., Wu, X., Zhang, T., Zhu, Q., Wang, X., et al. (2018). Exosomes Released from Tumor-Associated Macrophages Transfer miRNAs that Induce a Treg/Th17 Cell Imbalance in Epithelial Ovarian Cancer. Cancer Immunol. Res. 6 (12), 1578–1592. doi:10.1158/2326-6066.CIR-17-0479
Zhou, M., Chen, J., Zhou, L., Chen, W., Ding, G., and Cao, L. (2014). Pancreatic Cancer Derived Exosomes Regulate the Expression of TLR4 in Dendritic Cells via miR-203. Cell Immunol 292 (1-2), 65–69. doi:10.1016/j.cellimm.2014.09.004
Zhou, S., Dong, X., Zhang, C., Chen, X., Zhu, J., Li, W., et al. (2015). MicroRNAs Are Implicated in the Suppression of CD4+CD25− Conventional T Cell Proliferation by CD4+CD25+ Regulatory T Cells. Mol. Immunol. 63 (2), 464–472. doi:10.1016/j.molimm.2014.10.001
Zhu, C., Anderson, A. C., Schubart, A., Xiong, H., Imitola, J., Khoury, S. J., et al. (2005). The Tim-3 Ligand Galectin-9 Negatively Regulates T Helper Type 1 Immunity. Nat. Immunol. 6 (12), 1245–1252. doi:10.1038/ni1271
Keywords: pregnancy, pla-xosomes, cancer, exosomes, adverse pregnancy outcome, immune exhaustion, immune-therapy
Citation: Devvanshi H, Kachhwaha R, Manhswita A, Bhatnagar S and Kshetrapal P (2022) Immunological Changes in Pregnancy and Prospects of Therapeutic Pla-Xosomes in Adverse Pregnancy Outcomes. Front. Pharmacol. 13:895254. doi: 10.3389/fphar.2022.895254
Received: 13 March 2022; Accepted: 31 March 2022;
Published: 20 April 2022.
Edited by:
Nanbert Zhong, New York State Institute for Basic Research in Developmental Disabilities, United StatesReviewed by:
Anne Schumacher, Helmholtz Association of German Research Centres (HZ), GermanyJessian Munoz, Texas Children’s Hospital, United States
Copyright © 2022 Devvanshi, Kachhwaha, Manhswita, Bhatnagar and Kshetrapal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pallavi Kshetrapal, pallavi.kshetrapal@thsti.res.in
†These authors have contributed equally to this work
‡These authors share first authorship
§These authors share senior authorship
 Himadri Devvanshi
Himadri Devvanshi