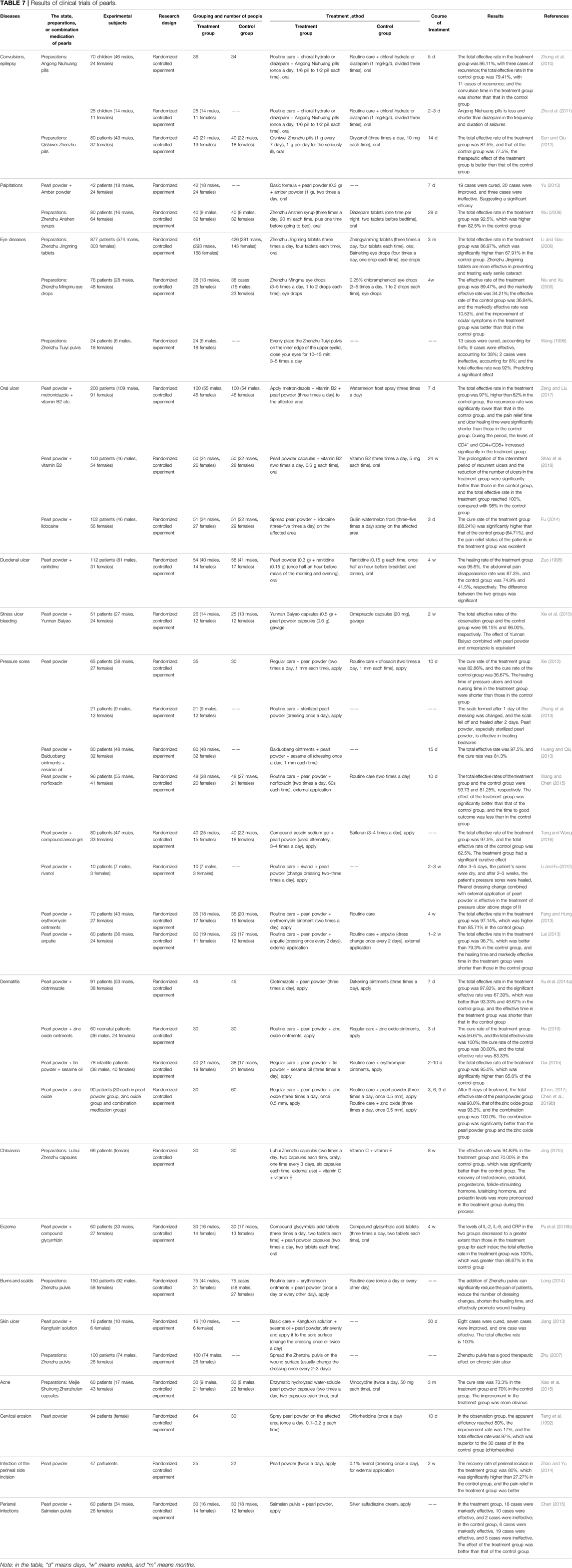
- 1College of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2State Key Laboratory of Southwestern Chinese Medicine Resources, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3College of Ethnomedicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
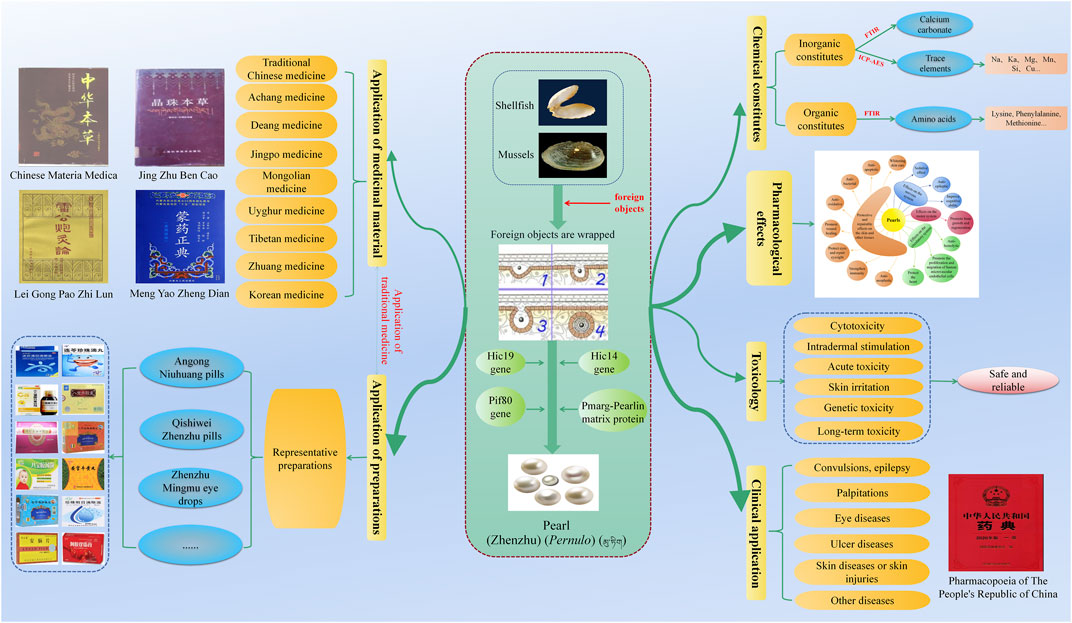
Although pearls are well known by most people, their medicinal value has not been popularized. This article collates the medicinal history of pearls over 2,000 years in China, including the application of pearls in the traditional medicine of China and their various preparations, as well as the progress of their chemical constituents, pharmacology, toxicology, and clinical research. Pearls from three different sources are used as medical materiel by 9 nationalities and 251 prescription preparations in China. In addition, pearls contain various inorganic constituents, such as calcium carbonate, trace elements, and water, and organic constituents, such as amino acids. In terms of pharmacology, pearls have many effects such as calming, improving cognitive ability, being anti-epileptic, promoting bone growth and regeneration, promoting the proliferation and migration of human microvascular endothelial cells, protecting the heart, anti-hemolysis, and anti-oxidation. In terms of toxicology, pearls are safe to take for a long time without exerting obvious adverse reactions. In terms of clinical application, pearls have been used to treat many diseases and conditions, such as convulsions, epilepsy, palpitations, eye diseases, ulcer diseases, skin diseases, or skin lesions. This article provides a reference for the application and research of pearls in the future.
1 Introduction
Pearls are produced by the natural biomineralization process (Bourrat et al., 2012). Biomineralization, the biological process by which an organism produces mineralized tissue (Montagnani et al., 2011), results from a unique biological synergy (Arnaud-Haond et al., 2007). The process of pearl formation, whether by natural means or human intervention, is a response to mantle tissue damage (Cartwright et al., 2013). The formation of pearls under natural conditions rarely occurs (Wang et al., 2009; Liu et al., 2012) and is generally cultivated by artificial means. When pearl shellfish or mussels are stimulated or stressed by foreign objects or external forces, their defensive function is stimulated, and the upper mantle envelops the foreign objects, resulting in pearl formation. The part of the cells of a single layer epithelial tissue is invaginated, constantly secreting nacre on foreign objects and gradually forming a pearl sac. The pearl sac continues to secrete nacre, surrounds it, and finally forms a pearl (Koji, 1999; Ma N. et al., 2011). Pearl formation includes two consecutive stages. One is the irregular CaCO3 deposition on the bare nucleus; the second is that the deposition of CaCO3 becomes more and more regular until a mature nacre is formed on the nucleus, similar to the regeneration of shells (Sato and Komaru, 2019). Pearl formation takes about 2–3 years and is related to the expression and release of a variety of genes and proteins, such as the Hic14 gene (Jin et al., 2019), Hic19 gene (Jin et al., 2019), Pif80 gene (Zhang et al., 2018), Pmarg-Pearlin matrix protein (Bourrat et al., 2012), and pearl shell matrix protein gene (Cartwright et al., 2013). These genes and proteins jointly regulate the formation of pearls, such as their growth, shape, and size. Pearls are mainly composed of the pearl nucleus, amorphous matrix layer, and nacre layer; amongst them, the nacre layer includes the prismatic layer and aragonite layer (Wan et al., 2014; Wan, 2016; Wu, 2017). The pearl nucleus is a small ball after grinding and polishing (Ouyang et al., 2012), which can be formed naturally or artificially implanted. The amorphous matrix layer is an organic matter that adheres to the surface of the pearl nucleus or a mixture of organic matter and inorganic crystal formation (Wan et al., 2014; Wan, 2016; Wu, 2017), and its thickness affects the quality of pearls. The amorphous matrix layer is thinner or even absent in high-quality pearls. The prismatic layer is also called the calcite crystal layer, slightly different from the view that it appears only in inferior pearls and hardly in high-quality pearls. On the contrary, the prismatic layer is universally present in pearls, and its role in pearls can be summarized as regulating spatial orientation by controlling the size and shape of the pearls (Wan et al., 2014). The aragonite layer is the main constituent of pearls as it directly determines their quality (Wan, 2016). Ouyang et al. (2012) described the structure of pearls according to the classification of nucleated and non-nucleated pearls (Ouyang et al., 2012).
Pearls (Latin name: Pernulo, Chinese name and its Pinyin: 珍珠Zhenzhu, Tibetan name:  ) are a mineral medicine used in the traditional medicine of China. It can be obtained from bivalves such as Pteria martensii Dunker, Hyriopsis cumingii Lea, or Cristaria plicata Leach; it can tranquilize and quiet the spirit, improve eyesight and remove nebula, detoxify and promote granulation, and moisturize the skin and remove speckle (Committee, 2020). It has a spherical, oblong, oval, or rod shape; has a characteristic colorful sheen; and is qualitatively hard (Committee, 2020). Pearls have been used for medicinal purposes in China for more than 2,000 years (Zhang X. et al., 2014). A clear record of pearls’ efficacy has been reported as early as the Jin dynasty (AD 266–420) in Bao Pu Zi and Zhou Hou Bei Ji Fang of Ge Hong (Lu et al., 1991), indicating that pearls had been used as medicine before, and its effects were summarized by later ages. Pearls can be divided into many types according to different ways, such as natural and artificial pearls with different genesis (Zhao, 1992). Seawater and freshwater pearls have different ecological environments, and white and black pearls have different colors (Feng et al., 2005). Most of the freshwater pearls have been used in medicine; they are mostly produced amongst Chinese rivers, and China accounts for 80% of the world’s freshwater pearls production. However, most of the common pearls in the market are seawater pearls, which are often used for decoration because of their natural environmental effects, bright color, and large round integuments, but there are also many freshwater pearls in the market. Of course, natural seawater contains more organic substances and nutrients than freshwater, so seawater pearls are better for medicinal purposes (Lin et al., 2007). Pearls as medicine are generally developed in the form of pearl powder by physical grinding and used as a raw material in traditional Chinese medicine and cosmetics (Liu et al., 2018). Pearl powder makes the constituents more bioavailable because of its small particle size and large contact area, which facilitates the release of active components and absorption by the human body (Gao et al., 2008). Some studies have shown (Chen L. et al., 2019) that the particle size of pearl powder will affect its curative effect, from which nano-pearl powder is derived, and the size of pearl powder after nanometerization is smaller, the released protein is increased, and the activity is stronger. Pearls have also been used in various compound preparations due to their good bioactivity and effect, such as Qishiwei Zhenzhu pills, Ershiwuwei Zhenzhu pills, Mengyao Zhenzhu pills, Luhui Zhenzhu capsules, Zhenzhufen capsules, Zhenzhu Shaoshang ointments, Zhenzhu ointments, and Zhenzhu pulvis, which tranquilize and quiet the spirit (Sun and Qiu, 2012), remove decay and promote granulation (Xiang and Zheng, 2010), and exert whitening effect (Xiao et al., 2015).
) are a mineral medicine used in the traditional medicine of China. It can be obtained from bivalves such as Pteria martensii Dunker, Hyriopsis cumingii Lea, or Cristaria plicata Leach; it can tranquilize and quiet the spirit, improve eyesight and remove nebula, detoxify and promote granulation, and moisturize the skin and remove speckle (Committee, 2020). It has a spherical, oblong, oval, or rod shape; has a characteristic colorful sheen; and is qualitatively hard (Committee, 2020). Pearls have been used for medicinal purposes in China for more than 2,000 years (Zhang X. et al., 2014). A clear record of pearls’ efficacy has been reported as early as the Jin dynasty (AD 266–420) in Bao Pu Zi and Zhou Hou Bei Ji Fang of Ge Hong (Lu et al., 1991), indicating that pearls had been used as medicine before, and its effects were summarized by later ages. Pearls can be divided into many types according to different ways, such as natural and artificial pearls with different genesis (Zhao, 1992). Seawater and freshwater pearls have different ecological environments, and white and black pearls have different colors (Feng et al., 2005). Most of the freshwater pearls have been used in medicine; they are mostly produced amongst Chinese rivers, and China accounts for 80% of the world’s freshwater pearls production. However, most of the common pearls in the market are seawater pearls, which are often used for decoration because of their natural environmental effects, bright color, and large round integuments, but there are also many freshwater pearls in the market. Of course, natural seawater contains more organic substances and nutrients than freshwater, so seawater pearls are better for medicinal purposes (Lin et al., 2007). Pearls as medicine are generally developed in the form of pearl powder by physical grinding and used as a raw material in traditional Chinese medicine and cosmetics (Liu et al., 2018). Pearl powder makes the constituents more bioavailable because of its small particle size and large contact area, which facilitates the release of active components and absorption by the human body (Gao et al., 2008). Some studies have shown (Chen L. et al., 2019) that the particle size of pearl powder will affect its curative effect, from which nano-pearl powder is derived, and the size of pearl powder after nanometerization is smaller, the released protein is increased, and the activity is stronger. Pearls have also been used in various compound preparations due to their good bioactivity and effect, such as Qishiwei Zhenzhu pills, Ershiwuwei Zhenzhu pills, Mengyao Zhenzhu pills, Luhui Zhenzhu capsules, Zhenzhufen capsules, Zhenzhu Shaoshang ointments, Zhenzhu ointments, and Zhenzhu pulvis, which tranquilize and quiet the spirit (Sun and Qiu, 2012), remove decay and promote granulation (Xiang and Zheng, 2010), and exert whitening effect (Xiao et al., 2015).
Pearls are not only traditional natural medicine but also a kind of marine shellfish. Marine shellfish have long been used to treat inflammation, burns, scalds, wounds, cuts, and pain healing, among others (Yang, 2020). In addition, the effects of pearls on calming (Pan et al., 1999; Zhang et al., 2016), improving eyesight (Lu et al., 1990), and whitening (Yang et al., 2016a) are also significant. It has been used as a medicine in China for thousands of years and has been used by many ethnic groups in China at the same time (Zhang X. et al., 2014). In modern research, pearls or pearls combined with other medicine have also been applied to epilepsy (Zhong et al., 2010), eye diseases (Niu and Xu, 2005), and skin diseases (Xie, 2013), among others.
With the progress of medical technology and the expansion of people’s perception of traditional Chinese medicine, pearls play an increasingly important role in medical treatment. Thus, this study will further promote the use of pearls and provide a reference for future research by expounding on pearls’ application in the traditional medicine of China and their chemical constituents, pharmacology, toxicology, and clinical research.
2 Application of Pearls in Traditional Medicine of China
2.1 Records of Pearls in Traditional Medicinal Works of China
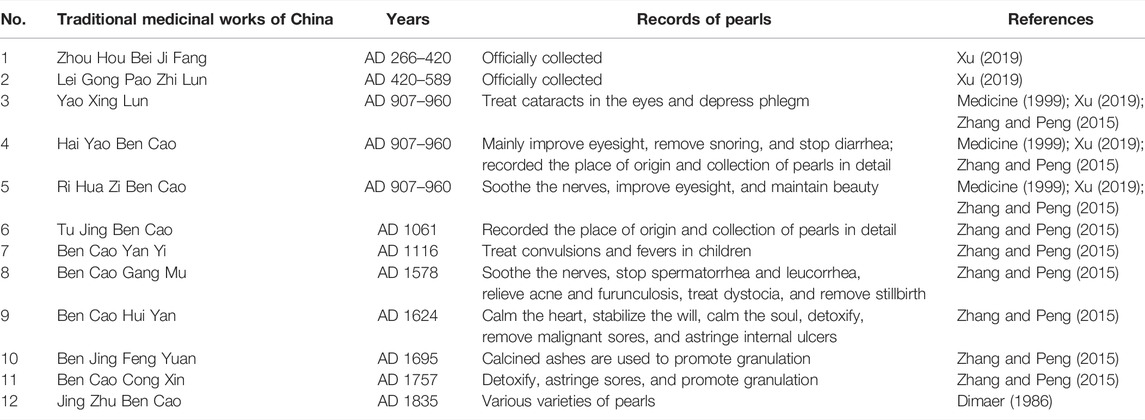
The application of pearls in China has a long history and is collected in numerous medicinal works (see Table 1 for details). The different names of pearls are real bead, mussel bead, Zhenzhuzi, medicinal bead, bead, and Lianzhu (Medicine, 1999). The application of pearls is officially recorded in Ge Hong’s Zhou Hou Bei Ji Fang in the Jin dynasty (AD 266–420) and Lei Xiao’s Lei Gong Pao Zhi Lun in the Southern and Northern dynasty (AD 420–589) (Xu, 2019). Tibetan medical works such as Jing Zhu Ben Cao (AD 1835) also recorded varieties of pearls (Dimaer, 1986). In terms of medicinal use, Yao Xing Lun (AD 907–960) said, “It can treat cataracts in the eyes and also down bear [sic] phlegm.” Hai Yao Ben Cao (AD 907–960) said that pearls “mainly improve eyesight, remove snoring, and stop diarrhea,” and a brief explanation was also given on the compatibility medicine of pearls. Ri Hua Zi Ben Cao (AD 907–960) said that they “soothe the nerves, improve eyesight, and maintain beauty.” The soothing effect of pearls was mentioned for the first time, indicating that our ancestors had a more comprehensive understanding of the main functions of pearls at this time, such as calming the mind, soothing the nerves, and improving eyesight (Medicine, 1999; Zhang and Peng, 2015; Xu, 2019). Ancient books such as Hai Yao Ben Cao (AD 907–960) and Tu Jing Ben Cao (AD 1061) also recorded the origin place and collection of pearls in detail. Ben Cao Yan Yi (AD 1116) said that pearls are also used to treat “convulsions and fevers in children.” During the Ming (AD 1368–1,644) and Qing dynasties (AD 1636–1912), pearls have new progress in medicinal use, especially in the aspects of astringing sores and promoting granulation due to the outstanding progress of the navigation industry. For example, Ben Cao Gang Mu (AD 1578) said that they “soothe the nerves, stop spermatorrhea and leucorrhea, relieve acne and furunculosis, treat dystocia, and remove stillbirth.” Ben Cao Hui Yan (AD 1624) said that they “calm the heart, stabilize the will, calm the soul, detoxify, remove malignant sores, and astringe internal ulcers.” Ben Jing Feng Yuan (AD 1695) recorded that “calcined ashes are used to promote granulation,” which is used to treat “burns.” Ben Cao Cong Xin (AD 1757) believed that pearls had the effects of “detoxifying, astringing sores, and promoting granulation.” At present, Materia Medica’s records on pearls’ functions have gradually improved (Zhang and Peng, 2015).
2.2 Application of Pearls as Medicinal Material
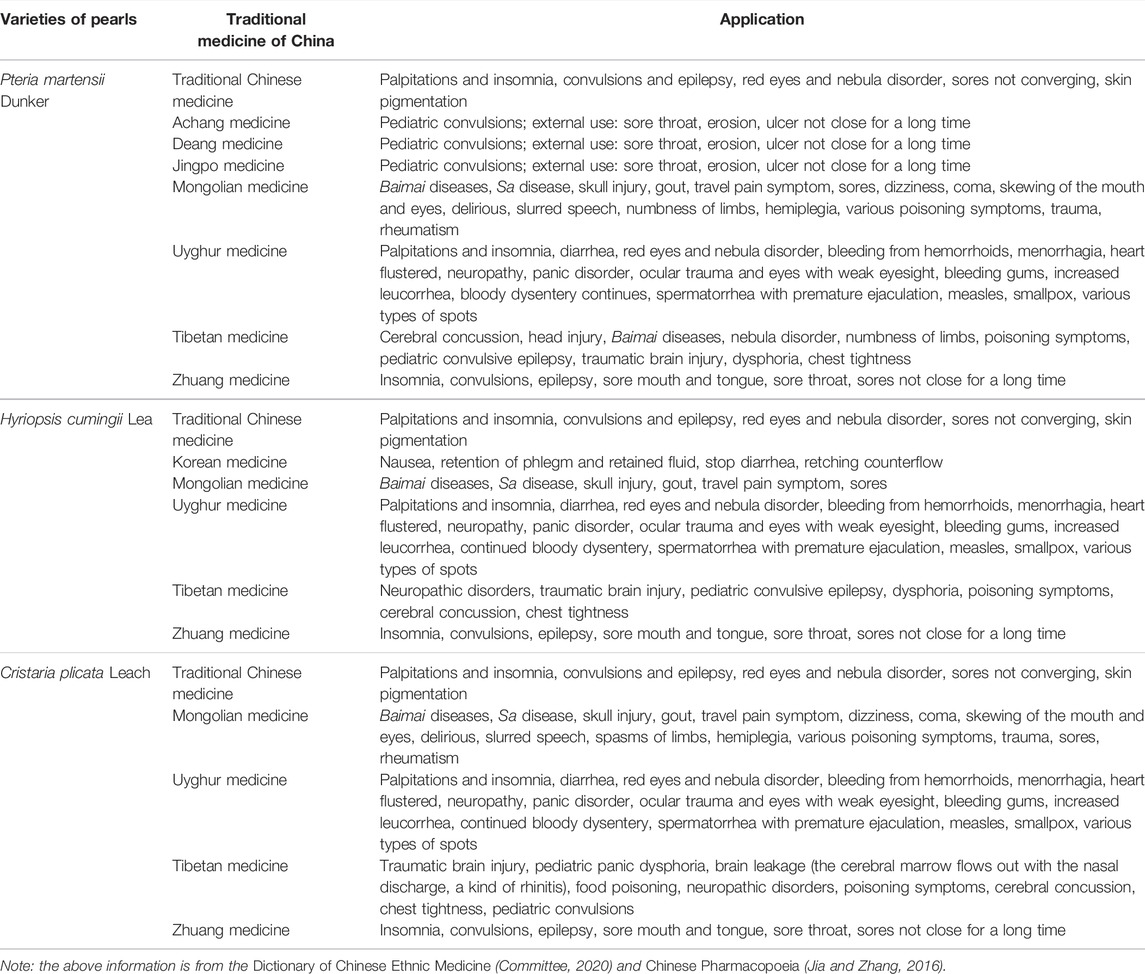
Pearls are derived from bivalves such as Pteria martensii Dunker, Hyriopsis cumingii Lea, or Cristaria plicata Leach, which are used in traditional Chinese medicine and eight ethnic groups (Achang, Deang, Jingpo, Mongolian, Uyghur, Tibetan, Zhuang, and Korean) in China (Table 2) (Jia and Zhang, 2016; Committee, 2020). In traditional Chinese medicine and ethnomedicine, pearls are mainly used to treat nervous system diseases, sores, poisoning, red eyes and nebula disorder, and skin pigmentation with good results. Amongst them, traditional Chinese medicine is mostly used to treat palpitations, eye diseases, ulcers, skin diseases, and other conditions; Achang, Deang, and Jingpo medicines are mostly used to treat convulsive seizures and ulcers; Mongolian and Tibetan medicines are mostly used to treat stroke (brain) and hemiplegia; Uyghur and Zhuang medicines are mainly used to treat insomnia with palpitations and convulsion epilepsy; and Korean medicine has special application, for nausea, diarrhea, phlegm, and other diseases.
2.3 Application and Statistical Analysis of Pearls in Traditional Medicinal Preparations of China
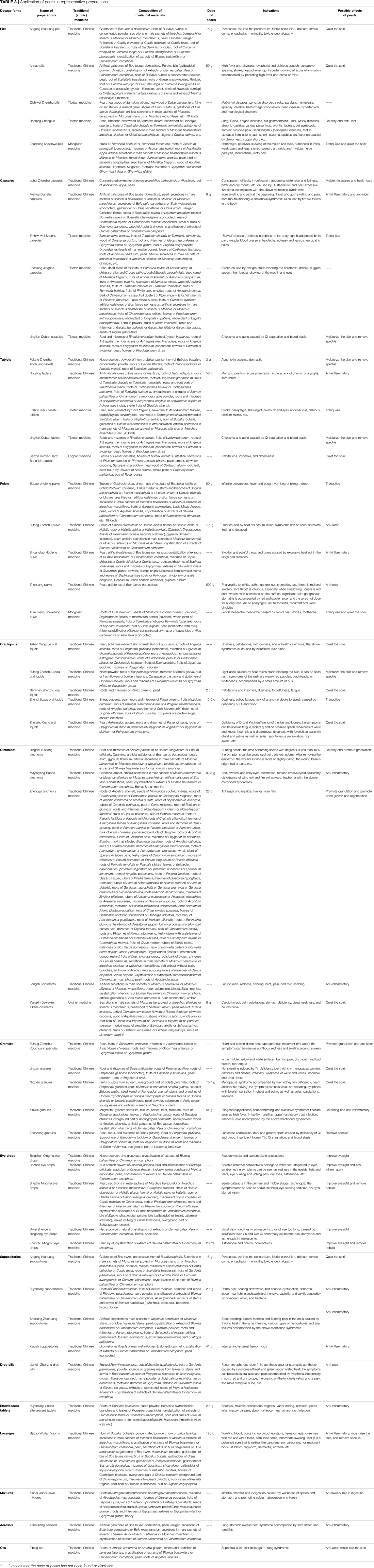
According to statistics, pearls are used in 251 preparations, including 63 pills, 51 capsules, 40 tablets, 50 pulvis, 9 oral liquids, 16 ointments, 6 granules, 6 eye drops, 4 suppositories, and 1 each of other dosage forms (e.g., effervescent tablets, lozenges, mixtures, aerosols, and oils). A total of 227, 18, 5, and 2 preparations containing pearls are used in Chinese, Tibetan, Mongolian, and Uyghur medicines, respectively. Table 3 only displays five representative preparations in each dosage form (including Chinese, Tibetan, Mongolian, and Uyghur medicine), and all are displayed when the number of preparations is less than five. The table contains the dosage form, the name of the preparation, the traditional (ethnic) medicine it belongs to, the composition of medicinal materials, pearls dosage, and indications.
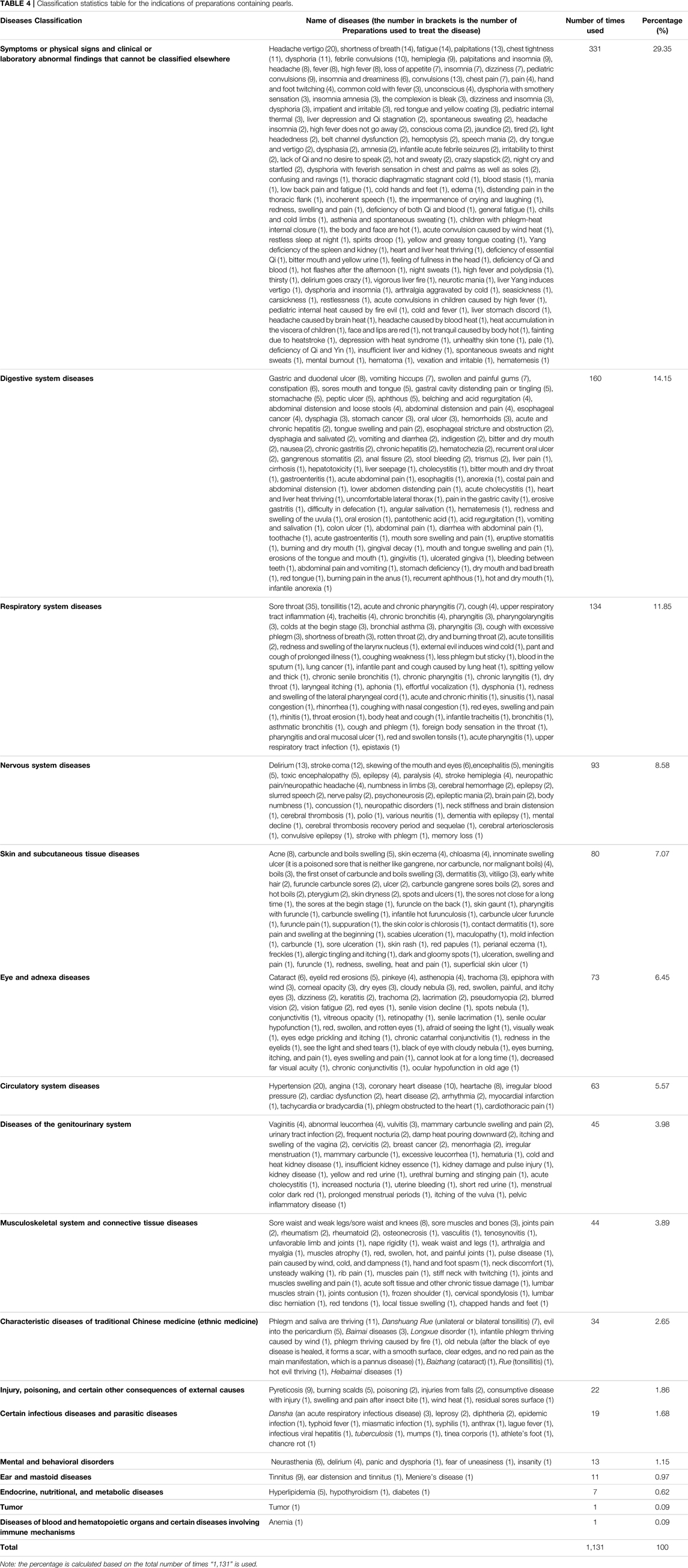
According to our statistics and analysis, the indications of preparations containing pearls involve diseases of 17 systems (see Table 4 for details). The common disease types and frequencies in order are symptoms or physical signs and clinical or abnormal laboratory findings that cannot be classified elsewhere (29.27%), digestive system diseases (14.15%), respiratory system diseases (11.85%), nervous system diseases (8.58%), skin and subcutaneous tissue diseases (7.07%), eye and adnexa diseases (6.45%), circulatory system diseases (5.57%), diseases of the genitourinary system (3.98%), musculoskeletal system and connective tissue diseases (3.89%), and characteristic diseases of traditional Chinese medicine (ethnic medicine) (2.65%), among others. Because of the remarkable effect of pearls, these preparations have great advantages in the treatment of certain diseases, such as skin (Xiao et al., 2015) and eye (Niu and Xu, 2005) diseases.
3 Chemical Constituents of Pearls
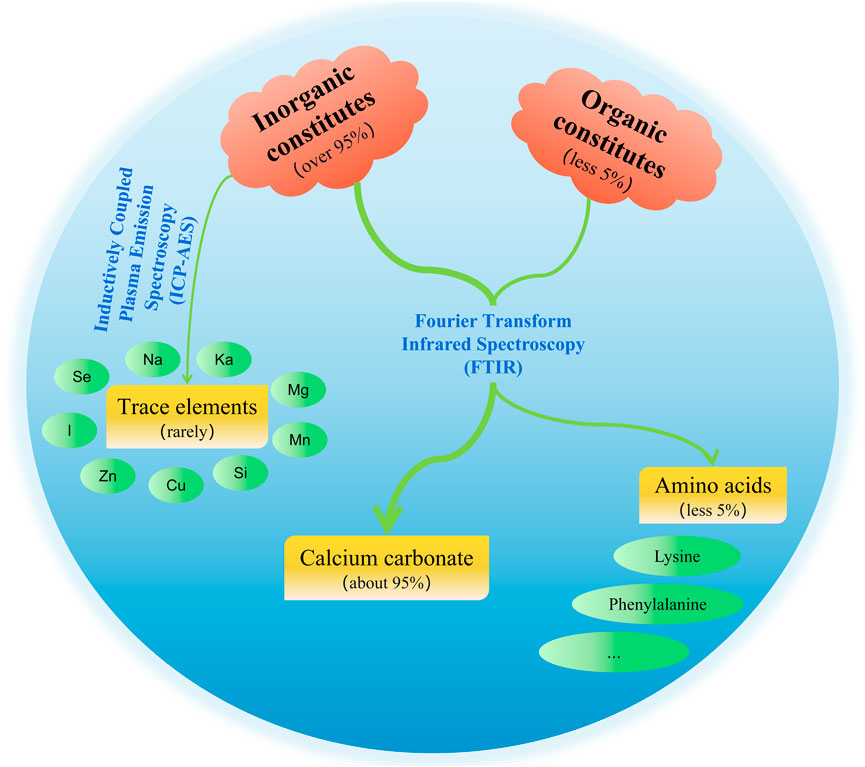
The chemical constituents of pearls include inorganic constituents, organic constituents, and water (see Figure 1 for details) (Ouyang et al., 2012). Inorganic constituents, mainly calcium carbonate, account for more than 95% of pearls content, and there are a variety of trace elements; the content of organic constituents is low, mainly composed of proteins and polysaccharide-like substances; and the water content is less than 2% (Wu, 2017).
3.1 Inorganic Constituents of Pearls
3.1.1 Calcium Carbonate
Calcium-like substances are abundant in pearls, and calcium carbonate, in turn, is the main component in calcium type of pearls, accounting for about 95% of the whole calcium-like substances (Yang et al., 2004). The crystalline phase of calcium carbonate is mainly aragonite, with a small amount of calcite and vaterite. Aragonite and calcite are common in nature, whereas vaterite is the most unstable state of calcium carbonate and rare in nature. The nacre is mainly composed of an aragonite layer but contains a small amount of calcite, namely, the prismatic layer (Ouyang et al., 2012). Calcium-like substances based on calcium carbonate can prevent and treat various diseases caused by calcium deficiency, such as rickets, osteoporosis, and dementia. They also have good osteoconductivity and osteogenic effects, which can be used as an alternative material to biological bone and are a good health care product (Zhan, 2010; Li et al., 2015).
3.1.2 Trace Elements
The contents of trace elements in the human body are extremely small, but they have powerful biological effects. They are involved in the metabolic process of enzymes, hormones, vitamins, and nucleic acids (Yang, 2008). Pearls contain more than 10 kinds of trace elements such as Na, K, Mg, Mn, Si, Cu, Fe, Zn, Ba, Ge, Cr, Ni, Co, Ti, Sc, Se, Br, I, and Pb (Li, 2009; Xue and Xu, 2013), of which a large part are essential trace elements for the human body. Studies have found that the different types and content of trace elements can directly affect the treatment efficacy, the color, and the quality of pearls (Yang et al., 2004). However, the types and content of trace elements in pearls largely depend on their growth environment; for example, Na, K, Mg, and Sr are the main enriched elements in seawater pearls, whereas Mn and Ba are the main enriched elements in freshwater pearls (Li, 2009). Moreover, there is a certain regularity for the enriched elements in seawater and freshwater pearls; that is, the contents of Na, Mg, S, and Sr in seawater pearls are high, and the content of Mn is low, whereas freshwater pearls show the opposite trend (Zhang, 2019). This indicates the differences in trace elements in pearls under different environments. The colors of pearls are also related to their element types and content. For example, pearls with Mg and Mn2+ elements exhibit purple color, those with Fe3+ exhibit orange color, and those with organic constituents exhibit black color (Li, 2009). Freshwater pearls contain a higher content of Mn, indicating that the Mn element is ubiquitous in freshwater pearls. Studies have found no significant difference in the content of metallic elements with the same types in white and purple pearls, so they might not be the main factor contributing to the color of pearls. Paradoxically, this experiment also similarly shows that Fe and Mn are most abundant in purple pearls, whereas Fe is not detected in white pearls, leading to the conclusion that the darker the color, the higher the contents of Fe and Mn (Jiang et al., 2019). It shows that the relationship between trace elements and pearls’ color is controversial and needs to be deeply studied and explored.
The trace elements of pearls exert their effects on the human body mainly as follows: Se can enhance human immunity and has an anti-cancer effect; Zn can activate human superoxide dismutase (SOD), thereby clearing peroxidized lipids that predispose to human aging; Mn can protect against cardiovascular diseases and regulate the nervous system, promoting the absorption of Ca in the human body; Ge has an anti-tumor effect; Fe can be used to improve anemia symptoms (Dong et al., 2011; Li et al., 2015). Although trace elements play an indispensable role in the human body because most of them are metals or even heavy metal elements, their excessive levels can also harm the body. The 2020 edition of The Pharmacopoeia of the People’s Republic of China stipulates that the heavy metals and harmful elements in pearls shall not exceed 5 mg/kg for Pb, 0.3 mg/kg for Cd, 2 mg/kg for As, 0.2 mg/kg for Hg, and no more than 20 mg/kg for Cu (Committee, 2020). Although the contents of other metal elements in pearls are not prescribed, we should focus on their intake when they are used. Excessive intake can still cause harm to the human body. For example, excessive Fe intake can cause vomiting, diarrhea, melena, gastroenteritis, and even comatose. When the body ingests too much Fe, it will form goiter and induce hyperthyroidism and thyroid cancer; high intake of Zn can lead to diseases such as hyperglycemia and hypercholesterolemia; and excessive Se can cause alopecia, onycholysis, or skin disorders (Yang et al., 2002). In conclusion, excessive metal intake has adverse effects.
3.2 Organic Constituents of Pearls
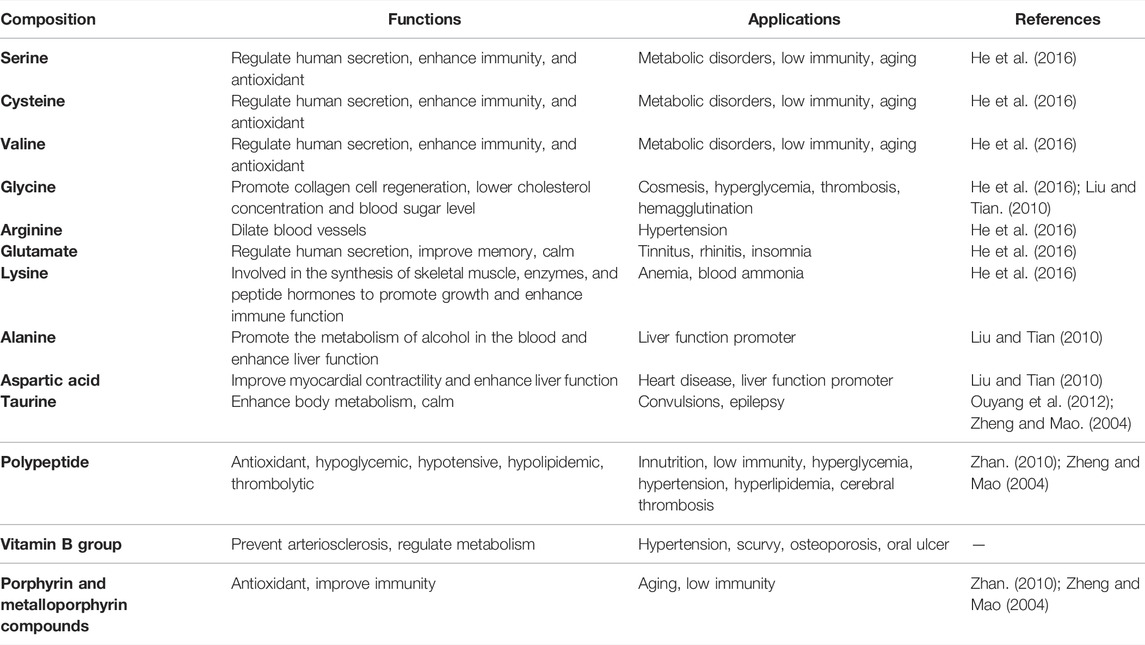
The organic constituents with less than 5% content in pearls (Xia et al., 2010) can be divided into a soluble organic matrix and an insoluble organic matrix (Bédouet et al., 2006; Bédouet et al., 2007; Ma Y. et al., 2011; Ma et al., 2012), which are mainly composed of protein, polypeptide, vitamin B group, porphyrin, and metalloporphyrin compounds. Amongst them, a variety of amino acids can be obtained by protein hydrolysis (pearl hydrolysis), including seven kinds of human essential amino acids (Zhan, 2010; He et al., 2016) (lysine, phenylalanine, methionine, threonine, isoleucine, leucine, and valine) and 10 kinds of non-essential amino acids (aspartate, serine, glutamic acid, proline, glycine, cysteine, alanine, tyrosine, histidine, and arginine) (Bédouet et al., 2007). Studies have also shown that 18 kinds of amino acids can be obtained by hydrolysis from freshwater pearls, including 17 kinds of protein amino acids (including seven kinds of essential amino acids) and a non-protein amino acid (taurine) (Ouyang et al., 2012). Amino acids play an important physiological role in the human body and maintain the body’s normal metabolism. For example, serine, cysteine, and valine can regulate human secretion, enhance immunity, and have an anti-aging effect (He et al., 2016). Glycine can promote the regeneration of skin collagen cells to achieve a cosmetic effect. It can also reduce cholesterol concentration and blood glucose and prevent blood clotting and thrombus. Arginine dilates blood vessels and treats hypertension (Liu and Tian, 2010; He et al., 2016). Aspartate is used medicinally as a cardiological drug and liver function promoter (Liu and Tian, 2010). Glutamate can treat tinnitus, rhinitis, and insomnia (He et al., 2016). The non-protein amino acid taurine can enhance the human body’s metabolism and tranquilize and quiet the spirit (Ouyang et al., 2012). In addition, polypeptides are intermediate products of proteins and have good health care effects (Zheng and Mao, 2004). Vitamin B group also plays an important role in pearls (Zheng and Mao, 2004). Porphyrin and metalloporphyrin compounds, a class of organic substances that can produce various pharmacological effects such as antioxidant activity and improve immunity, play important physiological functions together with proteins in pearls (Zheng and Mao, 2004; Zhan, 2010). The specific functions of various constituents of the organic constituents in pearls are shown in Table 5.
However, the contents of organic constituents in pearls under different environments are different. Compared with freshwater pearls, the nacre of seawater pearls has a higher content of amino acids; in particular, glycine has the highest content, followed by alanine, aspartate, leucine, and arginine (Zhang et al., 2007a; Zhang et al., 2007b). Zhang et al. (2007b) observed that the total amino acid contents in seawater pearls are not only high but also stable, and the relative content and change trend of each amino acid in a certain range for seawater pearls are basically consistent. However, those in freshwater pearls have more obvious changes, indicating that the medicinal value of seawater pearls is more reliable and stable (Zhang et al., 2007b). However, some studies (Weng, 1989) found that the types and content of freshwater and seawater pearls are basically the same, so freshwater pearls can be used instead of seawater pearls to treat diseases. All in all, seawater and freshwater pearls have their own advantages, and the corresponding pearls or blends should be selected according to the specific conditions of clinical diseases to achieve better curative effects.
Organic constituents are also involved in the formation and color of pearls. In terms of formation, the soluble organic matrix is considered to be an important part of the nucleation and growth of pearl crystals (Bédouet et al., 2006; Ma et al., 2012), including crystal form, nucleation location, crystal size, and morphology (Ma et al., 2012). Additionally, the organic matrix plays a regulatory role in the biomineralization process of pearls, especially controlling the formation of CaCO3 crystals (Ouyang, 2019), mainly manifested in organic matrix combination with specific crystal planes, leading to a reduced growth rate in this direction, and these slowly growing planes eventually dominate the morphology of crystals (Ma et al., 2012). A comparative study based on proteomics (Bédouet et al., 2007) determined that certain soluble and insoluble proteins coexist in the nacre, indicating that water-soluble proteins may be the precursors of insoluble protein scaffold in the nacre. In terms of color formation, the main color-causing factors in organic substances are porphyrins and carotenoids, and Raman peaks have been previously detected in natural pearls caused by carotenoids (Ouyang et al., 2012).
4 Pharmacological Effects and Partial Mechanism of Action of Pearls
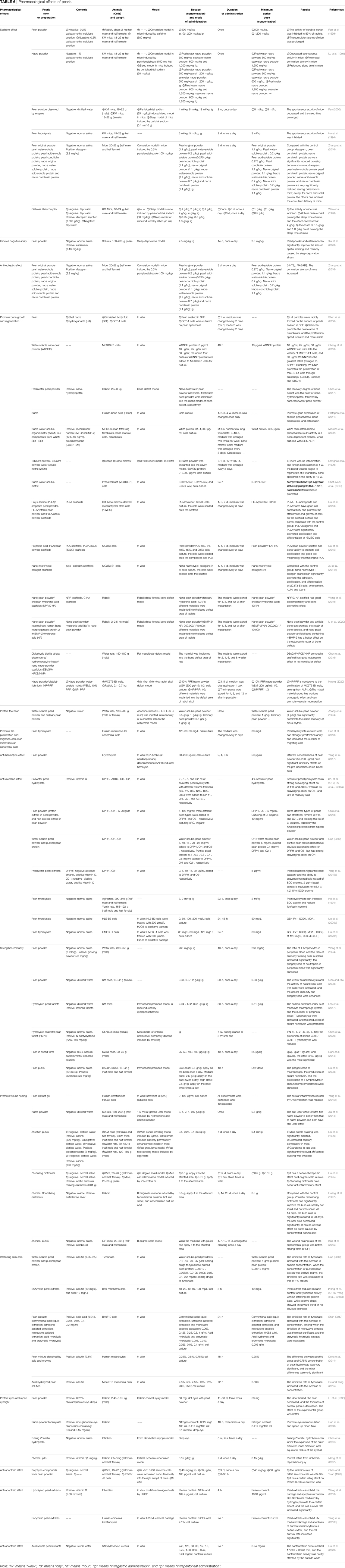
By reviewing the literature, we found that pearl powder, nacre powder, pearl extracts, and various preparations of pearls have various pharmacological effects (see Table 6 for details), and this study mainly expounds on their effects on the nervous system, motor system, circulatory system, skin, and other tissues. The schematic diagram of the pharmacological effects of pearls and part of the mechanism of action is shown in Figure 2.
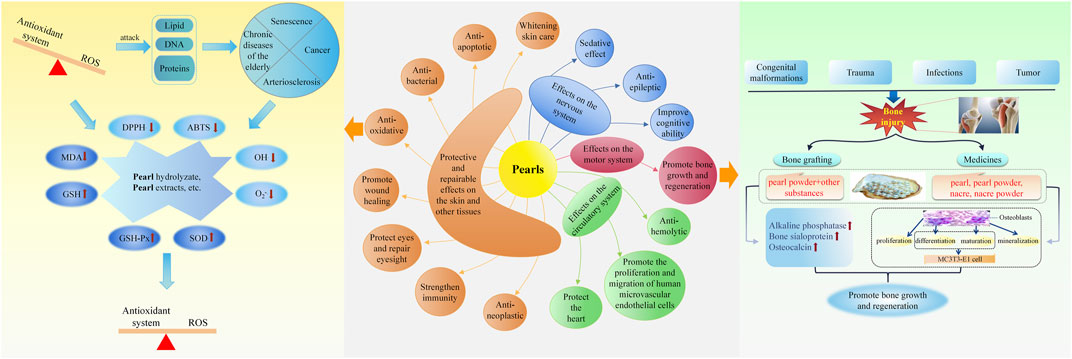
FIGURE 2. Schematic diagram of the pharmacological effects of pearls and part of the mechanism of action.
4.1 Effects on the Nervous System
4.1.1 Sedative Effect
The theory of traditional Chinese medicine believes that the properties of pearls are heavy, sinking, and descending. The property of heavy can be used to suppress timidity and restlessness, so pearls have the effect of calming the nerves and convulsions, treating the disquieted mind, heart palpitations, and insomnia (Zhao, 2015).
In modern scientific research, pearl powder (Pan et al., 1999; Zhang et al., 2016), nacre powder (Lu et al., 1991), pearl solution dissolved by enzyme and acid-base (Hu et al., 1994; Fan, 2000), pearl protein extracts (Zhang et al., 2016) and pearls’ compound preparation Qishiwei Zhenzhu pills (Wen et al., 1998) have a good sedative effect. They achieve a sedative effect by reducing the activity of the cerebral cortex and inhibiting the action of the central nervous system, which are mainly manifested in the inhibition of the spontaneous activity of animals, the prolongation of convulsion latency, the improvement of sleep, and the synergy of pentobarbital sodium or ether to prolong sleep time. In the comparative study of pearl powder and pearl protein extracts, original pearl powder and pearl conchiolin protein most significantly inhibited the activity of mice (Zhang et al., 2016), suggesting that pearl conchiolin protein may be the key constituent of the sedative effect. In addition, the sedative effect of the compound preparation of Qishiwei Zhenzhu pills may be related to the efficacy of pearls, but due to the large number of medicinal materials contained in the preparation, the role of pearls in this preparation still needs to be explored.
4.1.2 Improve Cognitive Ability
Sleep deprivation can mimic insomnia by altering the expression of hippocampal proteins, which in turn can cause cognitive decline. The Morris water maze test confirmed that pearl powder could improve the spatial learning and memory decline induced by sleep deprivation in rats, and the low expression of three hippocampal proteins (RIMS3, Ppp1r14a, and MGR3) was reversed by pearl powder during the process. This indicates that pearl powder can significantly ameliorate hippocampal injury in rats’ brains and improve cognitive ability (Xia et al., 2020).
4.1.3 Anti-Epileptic Effect
Different types of seizures are associated with the intracerebral excitatory neurotransmitter 5-hydroxytryptamine (5-HT) and inhibitory neurotransmitter γ-aminobutyric acid (GABA). After epileptic seizures were induced by pentylenetetrazole, the expression of 5-HT3 was upregulated, and the level of GABAB was downregulated. After treatment with the original pearl powder, pearl water-soluble protein, pearl acid-soluble protein, and pearl conchiolin protein, the expression of 5-HT3 and the level of GABAB were recovered to some extent, and the protein extracts exhibited the most significant effect (Zhang et al., 2016). This indicates that proteins may be the active constituents exerting the anti-epileptic effect.
4.2 Effects on the Motor System
The motor system contains bone, joints, and skeletal muscle. The role of the bone in the motor system is indispensable. Bone defects are one of the common phenomena in clinics, and their formation is related to many factors, such as congenital malformations, trauma, infection, tumor, and pathological factors, which are difficult to treat. Human intervention is required to promote bone tissue regeneration when the loss is too great, or the self-repair ability declines. Bone grafting is the main way to solve this problem, and the choice of bone grafting materials becomes the key to good or bad bone repair (Mao and Xu, 2016; Li et al., 2018; Wang et al., 2019). Therefore, high biological activity, bio-compatibility, osteoconduction, and biodegradability have become necessary conditions for bone grafting materials (Liu et al., 2013; Cheng et al., 2018). General bone injuries can be treated with drugs, such as pro-bone forming and bone resorption inhibiting drugs (Chen et al., 2004; Wang et al., 2014).
Pearls used in traditional medicine have played an important role in bone injury as a bone-promoting medicine and a good bone grafting material. In terms of promoting bone formation, some studies (Shen et al., 2006) used the shell nacre and hyaluronic acid as control materials, soaked pearls in simulated body fluids, and conducted cell culture to evaluate the osteogenic activity of pearls. The results showed that pearls could stimulate osteoblast proliferation, which is faster and more stable than shell nacre and hyaluronic acid. However, most pearls are ground into powder clinically for bone injury treatment because the physical arrangement of crystals in pearl powder greatly enhances the ability of osteoinduction (Cheng et al., 2018), and the chemical constituents of general and nano-pearl powder are more easily utilized by the human body. Compared with general pearl powder, nano-pearl powder shows better curative efficacy. An in vivo experiment to repair bone defects of the distal femur of rabbits found that nano-pearl powder is superior to micron-size pearl powder in the percentage of the area of newly forming bone tissue, degradation speed, and repair ability, indicating that nano-pearl powder has a stronger ability to restore bone tissue (Chen et al., 2017). Of course, the nacre also has the same osteogenic effect. Pattapon et al. (2011) studied the role of the nacre in inducing bone regeneration through the gene expression of bone markers (alkaline phosphatase, bone sialoprotein, and osteocalcin) and the production of bone sialoprotein (Pattapon et al., 2011). They found that the nacre can promote the expression and production of these bone markers. The good cell biocompatibility of the nacre contributes to its excellent bone repair effect (Liu et al., 2014). Nacre powder has a more significant effect because of its small particle size (Atlan et al., 1997). In addition, pearl water-soluble organic matrix or nacre water-soluble organic matrix has a good osteogenic effect, which is specifically manifested as they can push the differentiation and maturation of fibroblasts, bone marrow stromal cells, and osteoblasts, prolonging the life span of bone cells. (Lamghari et al., 1999; Mouriès et al., 2002; Oliveira et al., 2012; Chaturvedi et al., 2013). The mechanisms of the above osteogenesis can be summarized as follows: firstly, the differentiation of MC3T3-E1 cells is promoted by enhancing autophagy; secondly, autophagy in MC3T3-E1 cells is simulated through the MEK/ERK signaling pathway (Wang et al., 2014; Cheng et al., 2018). In conclusion, MC3T3-E1 cells are indispensable during osteogenesis.
In terms of bone grafting materials, pearls in the form of powder are usually combined with other substances to form composite materials as bone substitutes and promote osteogenesis. For example, polylactic acid (PLA)/pearl or nacre powder scaffolds have two times higher compressive strength than PLA scaffolds alone and have a significantly stronger promoting effect on the proliferation and alkaline phosphatase activity of bone marrow mesenchymal stem cells than PLA (Liu et al., 2013). Dai et al. (2015) also showed that adding pearls to PLA helps the deposition of hydroxyapatite and accelerates the proliferation of MC3T3-E1 cells, which is a better bone repair material (Dai et al., 2015). Another example is the nano-nacre/type I collagen composite scaffolds, which can also promote the growth of MC3T3-E1 cells and increase the related bone marker alkaline phosphatase activity and collagen expression level (Xu J. H. et al., 2014). In addition, nano-pearl powder/chitosan hyaluronic acid scaffolds (Wang et al., 2019) and nano-pearl powder/rhBMP-2/hyaluronic acid composite materials (Li et al., 2020) can better repair bone defects in rabbit distal femurs. Dialdehyde bletilla striata glucomanna/hydroxypropyl chitosan/nano-nacre powder scaffolds can also promote bone formation in rat mandibular defects (Chen et al., 2016). Similarly, the composite composed of nacre powder/platelet-rich fibrin has better osteogenic activity than nacre powder and platelet-rich fibrin alone (Huang, 2020). The above bone repair composite materials have the characteristics of good biocompatibility, biological activity, and good three-dimensional structure. The existence of pearls not only lays a more solid foundation for the properties of composites but also provides better conditions for long-term cell proliferation in the osteogenic activity of some materials (Dai et al., 2015).
4.3 Effects on the Circulatory System
4.3.1 Protect the Heart
Water-soluble pearl powder exhibits cardioprotective effects, mainly in terms of its ability to improve cardiac contractility and accelerate the recovery of sinus rhythm, and it can exhibit certain antiarrhythmic (aconitine triggered) effects after multiple administrations of larger doses of water-soluble pearl powder (1 g/kg). However, ordinary pearl powder has no obvious effect, which may be related to its solubility in water, because the solubility of ordinary pearl powder is not good, and the active constituents are not easily volatilized (Zhang et al., 1994). Therefore, the extraction of the active constituents of pearls in the later stage is also the key point of research.
4.3.2 Promote the Proliferation and Migration of Human Microvascular Endothelial Cells
Vascular endothelial cell dysfunction plays an important role in the occurrence and development of hypertension, and improving vascular endothelial cell function has become an important measure for the treatment of hypertension. A study found that the use of pearl hydrolysate to culture human microvascular endothelial cells can significantly promote their division and proliferation, and the higher the concentration, the stronger the promoting effect. The number of migrating cells also increased significantly. This indicates that pearl hydrolysate may have a positive effect on protecting the function of vascular endothelial cells (Cen et al., 2018).
4.3.3 Anti-Haemolytic Effect
2,2′-Azobis (2-amidinopropane) dihydrochloride (AAPH) is a water-soluble free radical generator that induces hemolysis in cells. In human erythrocyte culture in vitro, pretreatment of erythrocytes with pearl powder can resist AAPH-induced oxidative hemolysis, manifesting as a significant reduction in AAPH-induced hemolysis (Yang et al., 2017). This suggests that pearls can be used as a new therapeutic medicine for hemolytic diseases.
4.4 Protective and Repair Effects on the Skin and Other Tissues
4.4.1 Anti-Oxidative Effect
During the oxidation process, the presence of (1,1-diphenyl-2-picrylhydrazyl) radical (DPPH·), 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) radical (ABTS·), hydroxyl radical (OH·), and superoxide anion radical (O2−·) would aggravate the oxidation reaction; meanwhile, SOD and glutathione peroxidase (GSH-Px) would inhibit the oxidative process (Hu et al., 1994; Yang A. Q. et al., 2015; Pu et al., 2017; Pu W. et al., 2018; Liu et al., 2020; Liu et al., 2020).
Pearls have been proven to be an excellent antioxidant. In terms of scavenging free radicals, seawater pearl hydrolysate and freshwater pearl hydrolysate showed strong scavenging ability to DPPH and ABTS·, whereas their scavenging effect on OH· and O2−· was relatively weak. However, the scavenging rate of all free radicals increased with the increase in volume fraction of pearl hydrolysate, and the concentration of pearl hydrolysate used was much lower than that of ascorbic acid at an equivalent scavenging rate (Pu et al., 2017; Pu W. et al., 2018). In addition, pearl powder, protein extracts in pearl powder, and non-protein extracts in pearl powder showed a scavenging effect on DPPH· and O2−·, and the scavenging ability of protein extracts was stronger (Chiu et al., 2018), indicating that the antioxidant capacity of pearls may depend on its protein composition. However, a study showed that the preliminary purification protein sample of pearls (macroporous resin) did not exhibit obvious scavenging ability for DPPH· and O2−· but had strong scavenging ability for OH·(Liao, 2019). The reason for the different results above may be related to the extraction method of pearls. Moreover, the preliminary purified protein sample may be only a part of the total protein, and the effective protein has not been presented.
In terms of the effect on oxidative enzymes, pearl extracts have a SOD-like effect, which can replace SOD to scavenge free radicals (Yang YL. et al., 2015). Pearl hydrolysate can improve SOD activity and reduce lipid peroxide generation in medium-aged rats (Hu et al., 1994). In vitro experiments also showed that pearl hydrolysate can enhance GSH-Px activity in human lens epithelial cells and microvascular endothelial cells, reduce glutathione, decrease malondialdehyde, scavenge active free radicals, and protect cells from H2O2-induced oxidative damage (Zheng and Mao, 2004; Liu et al., 2020a; Liu et al., 2020b). Antioxidation also has a role in prolonging the life span. Huang and Pan (2000) and Ma and Xiao (2007) showed that timely administration of pearl powder at a young age of Drosophila could improve its vitality. It can be seen from the above that the action of enzymes can affect the scavenging of free radicals, indicating that the oxidation process is a chain reaction. Thus, further research is needed to better reveal the antioxidant mechanism of pearl powder.
4.4.2 Strengthen Immunity
The functions of T and B lymphocytes and mononuclear phagocytes can reflect the immune function in vivo. After taking pearl powder, the proportion of T lymphocytes in peripheral blood and the ratio of spleen antibody formation improved, and the phagocytosis of neutrophils in peripheral blood was enhanced (Wang et al., 1994). In addition, pearl powder can also improve the level of serum hemolysin and the activity of natural killer cells in mice (Qian and Zhu, 2003). Similarly, the better-absorbed pearl hydrolysate can also enhance the cellular immunity and humoral immunity of immunocompromised mice. The hydrolyzed Nanzhu tablet can improve the carbon clearance index K of the monocyte macrophage system and the number of T lymphocytes in peripheral blood and promote the production of serum hemolysin (Lan et al., 2017). Hydrolysis of the seawater pearl tablet can reduce the proportion of spleen CD3+/CD4+ T lymphocytes and plays an immunomodulatory role (Chen et al., 2020). To sum up, pearl powder and pearl hydrolysate have good immunity-enhancing functions, but their respective advantages are unclear. Thus, comparative research needs to be carried out.
A new form of pearls has also been developed as a regulator of Th1 and Th2 immune cells. Cell and molecular level studies confirmed that pearl in ash form increased Toll-like receptor-2 (TLR-2) and specific lymphocytes on murine peritoneal macrophages and improved total immunoglobulin G, immunoglobulin G1, immunoglobulin G2a, and immunoglobulin G2b levels (which were still higher than the control group at 60 days after immunization). In particular, the dose of 50 μg/kg exerted the most significant effect, indicating that pearl in ash form can not only enhance the immune response of the body, but also have a long-term effect. This effect may be mediated by the activation of the TLR-2 signaling pathway to induce the interferon-beta- (TRIF-) dependent pathway, leading to the activation of T cells, which in turn promotes an effective immune response (Elahi et al., 2014). The pearl in ash form is an aggregate of mineral compounds, suggesting that inorganic elements may be its effective constituents.
In addition, pearl pulvis, the compound preparation of pearls, has the function of enhancing the body’s resistance. This effect is achieved by promoting the production of serum hemolysin and significantly improving the phagocytic function of macrophages and the proliferation function of spleen T lymphocytes in immunocompromised mice (Liu et al., 2003).
4.4.3 Promote Wound Healing
The chemical constituents in pearls have a significant role in promoting wound healing. For example, as the main component, calcium can alleviate the permeability of capillaries and reduce exudates, which is conducive to the growth of fresh granulation; potassium and sodium are anti-inflammatory and antiseptic; and zinc can accelerate tissue repair (Mo et al., 2015).
Studies have shown that pearl hydrolysate (Zheng and Mao, 2004), compound preparation Zhushen pulvis (Lin et al., 1996), Zhuhuang ointments (Liu et al., 1995), Zhenzhu Shaoshang ointments (Huang et al., 1997), and Zhenzhu pulvis (Kan et al., 2015) have excellent anti-inflammatory and anti-scalding effects, mainly manifested by the inhibition of various swelling, attenuation of capillary permeability, and promotion of wound healing in second-/third-degree burns. During this process, the level of basic fibroblast growth factor was significantly increased, and the value-added rate of skin cells increased. In vitro experiments also showed that pearl extracts and poly(γ-glutamic acid) combined to form a hydrogel could treat inflammation in human keratinocyte cells (HaCaT cells) caused by ultraviolet radiation B irradiation (Yang YL. et al., 2015). In addition, pearls have a good repair effect on skin or mucosal ulcers. In a rat model of gastric mucosal injury, the ulcer index was significantly reduced after treatment with nacre powder; however, the efficacy of ultrafine nacre powder was better (Xia et al., 2014). In short, the role of pearls in promoting wound healing is closely related to their anti-inflammatory, anti-scalding, and anti-ulcer effects.
4.4.4 Whitening Skin Care
Pearls have been used as a whitening skin care product as early as 4,000 years ago; for example, ancient Egyptian women used pearls with milk to wipe their bodies, and China also used pearls as an important medicine for cosmetology in the traditional medical book Ming Yi Bie Lu during the San Guo (AD 220–280) (Zhang et al., 2002).
Many scholars have studied the skin-whitening efficacy of pearls by using modern pharmacological research means. After the extraction of pearls with different solvents, they all had a certain moisture absorption rate and moisturizing rate, but some were more hygroscopic and adept at moisturizing, suggesting that their combination may lead to better skin care outcomes (Yang et al., 2016b). Whilst the occurrence of the whitening effect is inseparable from the production of melanin and the activity of tyrosinase, studies have found that pearl extracts (Yang et al., 2016b; Shen, 2017; Yang et al., 2018a; Liao, 2019) and hydrolyzed pearl (Pu and Tong, 2015; Deng et al., 2017) can reduce the activity of tyrosinase in B16 melanoma cells, thereby inhibiting melanin production. In this process, the mRNA expression of the tyrosinase gene, tyrosinase-related protein 1, and microphthalmia-associated transcription factor was suppressed. Moreover, a dipeptide compound with the molecular formula C11H12N2O2 was identified as one of the pearls’ active constituents (Liao, 2019).
4.4.5 Protect Eyes and Repair Eyesight
Pearls also have eye protection and vision restoration effects. The pearls were ground into powder and applied to injured rabbit corneas. The pathological conditions of rabbit corneas were significantly improved and almost indistinguishable from those of normal rabbits, and their corneal pannus changes were observed at 3 months after discontinuation of the drug. The results showed that the corneal pannus thickness was significantly reduced to within 2 mm, similar to the effect of clinically used Zhenzhu Mingmu eye drops, which reduce or remove scars (Lu et al., 1990). The effect of nacre powder is also not weaker than that of pearl powder. After using nacre powder hydrolysate to treat rabbits with eyeball microcirculation disorder model, it was found to increase the number of capillary crossings of the ocular conjunctiva and effectively improve the microcirculation of the eyeball (Gao et al., 2000).
Fufang Zhenzhu hydrolysate and Zhenzhu pills also have the effect of protecting the eyes and restoring vision. After treating the deprivation myopia model in chickens with Fufang Zhenzhu hydrolysate, the expansion of the outer diameter, inner diameter, and equatorial radius of the eyeballs was inhibited, indicating that the medicine has a repairing effect on vision (Chen et al., 2001). The eye protection effect of Zhenzhu pills is mainly reflected in their ability to treat retinal ischemia-reperfusion injury in rabbits (Meng et al., 2007).
4.4.6 Anti-Neoplastic Effect
Chen and Yuan (1990) extracted and isolated porphyrin compounds from pearl powder, and the results showed that porphyrin compounds can inhibit S180 sarcoma and Lewis lung cancer tumors, in which the inhibition rate of S180 sarcoma reached 34.8%, and the inhibition rate against Lewis lung cancer tumors was 13.89%. In addition, porphyrin compounds can prolong the survival time of P388/J lymphocytic leukemia mice and reduce the spleen weight of animals, indicating that it has a certain inhibitory effect on P388/J lymphocytic leukemia, and this inhibitory effect is mainly achieved through killing P388/J3 cells. However, more experiments are needed to confirm the anti-neoplastic effect (Chen and Yuan, 1990).
4.4.7 Anti-Apoptotic Effect
Studies have shown that pearl extracts can inhibit H2O2-mediated apoptosis of human skin fibroblasts and ultraviolet-mediated apoptosis of human keratinocytes to a certain extent (Yang et al., 2018b; Wang et al., 2018). In addition, the nacre water-soluble organic matrix and the preparation of Zhenzhu pills also showed anti-apoptotic effects on osteoblasts and retinal neuron cells, respectively. After using them, the survival rate of osteoblasts increased significantly, and the number of apoptotic retinal neuron cells decreased significantly. In this process, B cell lymphoma-2 (Bcl-2) gene expression was promoted (Mouriès et al., 2002; Meng et al., 2007).
4.4.8 Antibacterial Effect
Pearl extracts (240 mg/ml) have a strong inhibitory effect on Staphylococcus aureus, and its antibacterial circle reached 17.861 ± 0.948 mm. In addition, the antibacterial activity is almost unaffected regardless of the influence of different temperatures, strong acid and alkali, ultraviolet radiation, metal ions, or pancreatic pepsin (Liu et al., 2020). Another compound preparation containing pearls, Zhenzhu Shaoshang ointments, also showed an inhibitory effect on Pseudomonas aeruginosa (Hu et al., 1998).
5 Toxicology of Pearls
As a valuable and common Chinese medicinal material with a specific good curative effect, pearls have been used since ancient times. With the popularization of its application, its toxicity has attracted more and more attention. Ancient Chinese medicinal books have recorded the safety of pearls. For example, Kai Bao Ben Cao (AD 974) said, “Non-toxic.” Shao Xing Ben Cao (AD 1159) also indicated, “Slightly cold, non-toxic (Medicine, 1999).” It can be seen that pearls as medicine have been proven to be safe since ancient times.
In modern pharmacology, much related research on the toxicity of pearls has been conducted. In terms of cytotoxicity, Zhang (2019) and Mao et al. (2018) showed that cells exposed to nano-pearl powder or nano-pearl powder extracts did not exhibit large area abscission or obvious cell morphological changes caused by cell damage. However, when the concentration was high (500 μg/ml or 100% extracts), the cell proliferation rate was relatively reduced, and the extracellular matrix was poorly extended and had certain cytotoxicity (Mao et al., 2018; Zheng et al., 2019). Although the toxicity of high and low concentrations is different, the rating is grade 1, which meets the application standard of biomaterials in China. In terms of acute toxicity, the histopathology of the abdominal cavity and internal organs was observed after the intraperitoneal administration of nano-pearl powder extracts to rats, and there were no abnormalities (Mao et al., 2018). Water-soluble pearl powder, pearl powder, and dyed black pearls were administered to mice at the maximum dose (10, 15, and 10 g/kg, respectively). Similarly, no obvious poisoning symptoms were found, and no animals died within 14 days (Wu, 2009; Shen et al., 2013; Deng et al., 2014). In terms of skin irritation, black pearls were studied and showed no primary irritation to rabbit skin (Deng et al., 2014). In terms of intradermal stimulation, the nano-pearl powder extracts were injected into the back skin of rabbits. After 4 h, the skin mounds disappeared at each injection site, and no skin erythema and edema were observed at 24, 48, and 72 h (Mao et al., 2018). In terms of genotoxicity, neither water-soluble pearl powder nor pearl powder showed mutagenic effects on mice sperm, indicating that they were not genotoxic (Wu and Zhu, 2009; Shen et al., 2013). In terms of long-term toxicity, the general condition, body weight, food utilization rate, hematology, blood biochemistry, visceral body ratio, and histopathology of rats after taking water-soluble pearl powder and pearl powder were detected through a 30-day feeding experiment, and the results were normal (Wu and Zhu, 2009; Shen et al., 2013).
In summary, all forms of pearls are non-toxic and non-irritating in terms of cytotoxicity, acute toxicity, skin irritation, intradermal irritation, genotoxicity, and long-term toxicity, indicating the safety of pearls for internal and external use.
6 Clinical Applications of Pearls
See Table 7 for details.
6.1 Convulsions and Epilepsy
Compound preparations containing pearls are commonly used to treat convulsions and epilepsy. For example, Angong Niuhuang pills are used for opening the orifices and awaking the spirit. Zhong et al. (2010) divided 70 children with febrile convulsion into the treatment and control groups. The treatment group was treated with Angong Niuhuang pills, with a total effective rate of 86.11%, which was better than the control group with conventional treatment, and the time to stop convulsions and recurrence rate were significantly shortened and reduced. In another study, after 25 children with febrile convulsion were treated with Angong Niuhuang pills, the number of seizures and duration of convulsion were reduced and shortened (Zhu et al., 2011).
6.2 Palpitations and Insomnia
In 42 patients with phlegm-stasis block and heart and spleen deficiency accompanied by palpitations, Yu (2013) added pearl powder and amber powder to the basic prescription, which resulted in a satisfactory curative effect: 19 cases were cured, 20 cases improved, and 3 cases were invalid (Yu, 2013). Sun and Qiu (2012) used the compound preparation of pearls, Qishiwei Zhenzhu pills, to treat 40 of 80 patients with insomnia, and the results showed that the total effective rate was 87.5%, which was significantly higher than that of the control group treated with oryzanol, indicating that Qishiwei Zhenzhu pills have a better therapeutic effect on insomnia (Sun and Qiu, 2012). Another compound preparation of pearls, Zhenzhu Anshen Syrup, was also used to treat 40 patients with insomnia and showed a total effective rate of 92.5%, which was higher than that of the diazepam-treated control group (82.5%) (Wu, 2009).
6.3 Eye Diseases
The compound preparation Zhenzhu Jingming tablets are used to prevent and treat the early senile cataract. In a clinical study, 877 patients with early senile cataract were divided into the treatment and control groups. The treatment group was treated with Zhuzhu Jingming tablets, whereas the control group was treated with Zhangyanming tablets and Baineiting eye drops. The results showed that the total effective rate was 86.97% in the treatment group and 67.91% in the control group. Zhenzhu Jingming tablets showed better efficacy (Li and Gao, 2006). In addition, amongst 76 patients with chronic conjunctivitis, the total effective rate of 38 patients treated with Zhuzhu Mingmu eye drops was significantly higher than that of 0.25% chloramphenicol (Niu and Xu, 2005). In another study, Zhenzhu Tuiyi pulvis was used to treat 24 patients with coiled filamentous keratitis, and the total effective rate reached 92% (Wang, 1996).
6.4 Ulcer Diseases
Pearl powder alone or in combination with other drugs can be used to treat various ulcer diseases, such as oral, duodenal, and stress ulcer bleeding.
In oral ulcers, Zeng and Liu (2017) selected 200 patients with recurrent oral ulcers (Zeng and Liu (2017). After 7 days, the total effective rate of the treatment group (metronidazole + vitamin B2 + pearl powder) was 97%, which was significantly higher than that (82%) of the control group (watermelon frost spray). Moreover, the recurrence rate, pain sensation elimination time, and ulcer healing time were significantly lower than those in the control group. During this period, the levels of CD4+ and CD4+/CD8+ of the two groups in the peripheral blood were increased, and the increase was more significant in the treatment group. Shao et al. (2018) also used pearl powder combined with vitamin B2 to treat 50 patients with recurrent oral ulcers, and the total effective rate reached 100%, which was higher than that of vitamin B2 alone. Moreover, the number and the interval of ulcers were significantly reduced and prolonged, indicating that the addition of pearl powder produced faster and better recovery of oral ulcers (Shao et al., 2018). In addition, pearl powder combined with lidocaine was used to treat 51 patients with oral ulcers of grades II–III. The results showed that the cure rate reached 88.24%, which was significantly better than that of the control group treated with watermelon frost, and the pain was also significantly relieved (Fu, 2014).
In duodenal ulcers, the combination of pearl powder and ranitidine has a significant effect. A total of 112 duodenal ulcer patients were taken as the research object. After 4 weeks, the healing rate and abdominal pain disappearance rate of the treatment group (pearl powder + ranitidine) were 95.6% and 100%, respectively, which were significantly higher than those (74.9% and 83%, respectively) of the control group (ranitidine), further proving that the presence of pearl powder made the treatment of duodenal ulcer more prominent (Zuo, 1995).
In stress ulcer bleeding, Yunnan Baiyao combined with pearl powder can effectively treat stress ulcer bleeding caused by senile dementia. A total of 51 patients with senile dementia complicated with stress ulcer bleeding were randomly divided into the treatment group and control group. The treatment group was treated with Yunnan Baiyao combined with pearl powder, whereas the control group was treated with omeprazole. The results showed that the levels of the two groups were equivalent, and both could effectively stop bleeding and repair ulcers (Xie et al., 2015).
6.5 Skin Diseases or Skin Injuries
Common skin diseases or skin injuries include pressure ulcers, dermatitis, chloasma, eczema, burns, skin ulcers, and acne, each of which can be treated with pearl powder as a primary or adjunct treatment.
Pressure ulcers, also known as bedsores, are a major cause of common skin problems. Xie (2013) used pearl powder to treat 35 patients with pressure ulcers, and the results showed that the healing time and local nursing time of pearl powder were significantly lower than those of ofloxacin gel. The cure rate of pearl powder reached 82.86%, which was significantly higher than that of 36.67% of the control group. In another study, pearl powder combined with Baiduobang ointments and sesame oil was used to treat 80 patients with pressure ulcers of stages III–IV. The total effective rate reached 97.5%, and the recovery rate was 81.3% (Huang and Qiu, 2013). The total effective rate also reached 93.75% in 48 pressure ulcer patients treated with the combination of pearl powder and norfloxacin, and the time to improvement was significantly shorter than that in the control group (Wang and Chen, 2015). After treatment with pearl powder and compound aescin gel in 40 patients with pressure ulcers, the average healing time and total effective rate were both shorter and higher than those of saifurun (Tang and Wang, 2016). The combination of pearl powder and rivanol also showed a good effect in the treatment of 10 patients with pressure ulcers (Li and Fu, 2012). In another study, pearl powder and erythromycin ointments were added on the basis of routine nursing to treat 30 patients with decubitus ulcers, and the results showed that the total effective rate was 97.14%, which was higher than that of routine nursing (Fang and Huang, 2013). Similarly, when 30 decubitus ulcer patients were treated with pearl powder combined with anputie, the healing time and marked effect time were significantly shorter than those of the control group, and the total effective rate reached 96.7% and 100% after 1 and 2 weeks, respectively (Lai, 2013). The above examples all show that pearl powder has a significant effect in treating pressure ulcers.
For the treatment of dermatitis, in a clinical study, 91 patients with mycotic dermatitis were randomly divided into two groups: one group was treated with clotrimazole and pearl powder and the other with Dakening ointments. A significant effective rate of 67.39% was found in the group treated with clotrimazole and pearl powder, which was significantly superior to that of 46.67% in the group treated with Dakening ointments. Moreover, the duration of treatment was shorter in the former (Xu X. L. et al., 2014). In another study, 30 neonates with severe diaper dermatitis treated with zinc oxide ointments combined with pearl powder showed a total effective rate of 100%, which was significantly higher than the total effective rate of zinc oxide ointments alone, suggesting that pearl powder played an important role during this period (He, 2019). In another clinical experiment, pearl powder, tin powder, and sesame oil were used to treat 40 infants with neonatal diaper dermatitis. The total effective rate was 95%, and the total effective rate of the buttock cream was only 65.8%, showing a significant difference (Dai, 2015). Pearl powder combined with zinc oxide can also be used to treat incontinence dermatitis, and in 90 patients, the total effective rate of the combined medication was 100%, which was higher than the total effective rate of pearl powder or zinc oxide alone, indicating that combined medication can treat incontinence dermatitis faster and more effectively (Chen, 2017; Chen X. et al., 2019).
Regarding chloasma, Jing (2015) conducted a clinical study on 60 patients with chloasma, and the results showed that the total effective rate of the treatment group (Luhui Zhenzhu capsules + vitamin C + vitamin E) was 87%, which was higher than that of 70% in the control group (vitamin C + vitamin E) (Jing, 2015). During this process, the levels of estrogen such as estradione, progesterone, luteinising hormone, follicle-generating hormone, testosterone, and prolactin were decreased in the two groups, but the decrease was more obvious in the treatment group, in which pearl powder played a great promoting role. In addition, Wei and Feng (2014) and Zhang X. et al. (2014) statistically analyzed the outcomes of 38 and 58 patients with chloasma after treatment with Luhui Zhenzhu capsules, respectively, and found that the total effective rate reached 71.05% and 94.83%, respectively (Zhang X. L. et al., 2014; Wei and Feng, 2014).
In terms of eczema, 60 patients with chronic eczema were randomly divided into the treatment group and control group. The treatment group was given compound glycyrrhizin tablets and pearl powder capsules, whereas the control group was only given compound glycyrrhizin tablets. The results showed that the total effective rate of the treatment group was 100%, whereas that of the control group was only 86.67%. The levels of related indicators, interleukin- (IL-) 2, IL-6, and C-reactive protein, also showed a more obvious decrease in the treatment group, suggesting that pearl powder capsules have the effect of promoting the healing of eczema (Pu Y. H. et al., 2018).
In terms of burns and scalds, 150 patients with burns and scalds were selected as experimental subjects and divided into the treatment group and control group. The treatment group was treated with Zhenzhu pulvis and routine nursing care, whereas the control group only received routine nursing care. The results showed that the pain sensation and healing time in the treatment group were milder and shorter than those in the control group, and the number of dressing changes was lesser (Long, 2014).
Regarding skin ulcers, after treatment of 16 skin ulcer patients with pearl powder and Kangfuxin solution, the total effective rate reached 100% (Jiang, 2013). In addition, Zhenzhu pulvis, a compound preparation of pearls, was used to treat 100 patients with chronic skin ulcers, and all patients were cured (Zhu, 2007).
In acne, Xiao et al. (2015) treated 30 acne patients with enzymatic hydrolyzed water-soluble pearl powder capsules and showed that the cure rate was 73.3% after 3 months, which was slightly higher than that 70% of minocycline (Xiao et al., 2015).
6.6 Other Diseases
Pearls are equally effective in treating cervical erosions, lateral perineal incision infections, and perianal infections.
Clinically, 94 patients with cervical erosions were divided into the treatment and control groups. The treatment group was treated with pearl powder, and the total effective rate reached 97%. However, the total effective rate of the control group treated with chlorhexidine was only 70%, which was far less than that of the treatment group, indicating that pearl powder is better for the treatment of cervical erosions (Tang et al., 1992).
Lateral perineal incision infection is a common incision complication in the postpartum period. Zhao and Yu (2014) divided 47 maternal patients with lateral perineal incision infections into two groups and treated them with pearl powder and 0.1% rivanol gauze, respectively (Zhao and Yu, 2014). The results showed that the pain relief condition was more obvious, and the recovery rate reached 80% in the patients treated with pearl powder, which was much higher than that of patients treated with 0.1% rivanol.
Saimeian pulvis and pearl powder can effectively treat perianal infections during the onset cycle of acute leukemia. Sixty patients were randomly divided into two groups, and the results showed that the number of effective cases in the treatment group (Saimeian pulvis + pearl powder) was greater than that in the control group (silver sulfadiazine cream), indicating that pearl powder combined with Saimeian pulvis has a better effect on perianal infection of acute leukemia (Chen, 2015).
7 Discussion
Pearls have a long history of medicinal use in China as a natural mineral medicine, and modern pharmacology has similarly demonstrated the point of view in ancient books regarding the medicinal effects of pearls (i.e., it can tranquilize and quiet the spirit, improve eyesight and remove nebula, detoxify and promote granulation, moisturize the skin, and remove speckle). However, the exertion of these effects is inseparable from the chemical constituents in pearls (Mo et al., 2015). Amongst these chemical constituents, few active constituents were well defined; however, it is not difficult to speculate on the roles that these constituents play because most of the calcium-like substances, trace elements, and amino acids in pearls are essential constituents of the human body, and many studies have confirmed their value in humans. For example, calcium can promote bone growth and regeneration and may become a bone substitute material (Zhan, 2010; Li et al., 2015). Trace elements such as Se, Zn, Mn, Ge, and Fe can scavenge free radicals in the human body and extend the life of the body; they can treat cardiovascular diseases, regulate the nervous system, resist tumors, and improve anemia symptoms, amongst others (Dong et al., 2011; Li et al., 2015). Amino acids are also essential organic constituents in the human body, maintaining human metabolism and playing other key roles (Liu and Tian, 2010; He et al., 2016). These chemical constituents alone or in conjunction with other constituents achieve the desired physiological function. Therefore, the identification of specific constituents in pearls has also become the cornerstone of subsequent pharmacodynamic studies. Secondly, these specific constituents often have multiple uses for one constituent or one use for multiple constituents, suggesting that we should focus on mining multiple constituents rather than just one constituent in efficacy research.
Amongst the numerous pharmacological activities of pearls, there are many studies on the protection of bone tissue and antioxidant effect, indicating that pearls have good application prospects in these two aspects. Amongst them, the protection of bone tissue is not recorded in ancient books previously, which is an innovative discovery; the anti-oxidation effect is another in-depth study of the moisturizing and freckle-removing effect in the ancient books. This not only shows that pearls have great potential development value, but also suggests the importance of ancient books in medical research. In addition, pearls have been applied to a variety of compound preparations, and under a certain efficacy, these compound preparations also show the same effect as when used with pearls alone. However, due to the large number of medicinal materials contained in compound preparations, the role of pearls in compound preparations is unclear, and the possible effect may be weakened or synergistically enhanced. It is also possible to stimulate another effect, so further experiments are needed for exploration and confirmation. In the current pharmacological experiments on pearls, there is a lack of research on their active constituents, which have also become a breakthrough in future experiments. For example, the research on pearl extraction can be strengthened, and the extraction methods and conditions can be explored to obtain the best extracts. Then, the main constituents can be roughly determined by the extraction method; the extraction method can also be combined with related technologies, such as ICP-MS and LC-MS, to analyze the types of amino acids of the protein and trace elements in the pearl extracts, so as to clarify the active constituents. As most of the results of pharmacological studies were obtained by animal models and their effectiveness could not be fully demonstrated, more clinical trials with confirmatory results are needed (Jiang et al., 2016).
So far, pearls have been clinically applied in the treatment of convulsions, epilepsy, palpitations, insomnia, eye disease, ulcer disease, skin disease, skin injury, and some gynecological diseases, all showing a good curative effect. However, some diseases have complex clinical syndromes, and it is difficult to obtain the desired effect with a single medicine. Thus, pearls are often used in combination with other medicines to treat diseases and often receive satisfactory results. At present, the compound preparations of pearls are also used clinically and have a good curative effect, but their mechanism of action is still unclear. Further research is needed. Although most clinical trials have a small sample size and lack multi-center comprehensive comparative research and unified standards, they still have a certain reference value.
The Pharmacopoeia of the People’s Republic of China (Committee, 2020) stipulates that pearls have heavy metals and harmful elements, indicating that their use has certain safety hazards. However, almost all toxicological experimental studies of pearls indicate that they are safe and reliable to use. Although pearls with a slightly higher concentration have certain cytotoxicity, they are within the safety range of the national standard, with a grade of 1, and meet the standards for biomaterial applications in China. However, the number and scope are still insufficient, so more toxicological experiments still need to be carried out to make them more comprehensive and authoritative, laying the foundation for formulating standardized safety standards.
In addition, there is no literature report on the pharmacokinetics of pearls. The absorption, distribution, metabolism, and excretion of pearls in the body after their administration, as well as the changes in blood drug concentrations over time, are unclear. However, some pharmacokinetic studies of pearl preparations, such as Qishiwei Zhenzhu pills (Li and Suo, 2003) and Angong Niuhuang pills (Li et al., 2019), are not enough to replace the pharmacokinetic study of pearls. Therefore, future experiments can also be considered from this aspect to provide a basis for elucidating the pharmacological effects and the design of clinical trials (Wang, 2015). In terms of absorption, the utilization of pearls is not high, so they often enter the human body in the form of powder, which greatly increases the contact area between the medicine and the body, especially nano-pearl powder (Chen L. et al., 2019). On this basis, enzymatic hydrolyzed pearl oral liquid was also derived, and its absorbency became higher due to its solubility (Fan, 2000). Thus, the development of pearls absorption form can be regarded as a research strategy and prospect.
Tibetan medicine is an ethnic medicine that uses the most pearls in addition to traditional Chinese medicine in China, and pearls are used in many Tibetan prescription preparations, such as Qishiwei Zhenzhu pills, Ershiwuwei Zhenzhu pills, and Renqing Changjue. The reasons that Tibetan medicine use pearls can be attributed to two points: one is that pearls are one of the seven treasures of Tibetan Buddhism and have an important position in Tibetan culture (Cao, 2018); the second one is that pearls are a symbol of Tibetan people identity since ancient times, thereby gaining a great deal of attention from the Tibetan people. In addition, from a modern point of view, pearls are mainly produced in Guangxi, Jiangsu, Zhejiang, Anhui, Hunan, Jiangxi, and other places in China, but they are hardly produced in Tibetan areas. Now, convenient transportation has allowed people in Tibetan areas to easily obtain pearls, so the status of pearls in Tibetan areas is not as good as before. However, its medicinal value has been solidified in Tibetan medicine, so pearls are used in a wider range of medicine.
Of course, there will be differences between pearls due to the different production areas. For example, Chinese and Japanese pearls belong to the same variety and are cultivated by Pteria martensii Dunke. The difference between the two is mainly reflected in their size, luster, and color; each type has its own merits. However, there is a saying that west pearls are not as good as east pearls, and east pearls are not as good as south pearls (Nan Zhu). South pearls (Nan Zhu) are Chinese seawater pearls, which shows that Chinese pearls have a high reputation around the world.
More trace elements and amino acids in pearls have been studied, whereas other constituents are less studied. The exploration and development of other constituents should be intensified to improve their quality standards and basic research on pharmacodynamic substances. In addition, the pharmacological effects of pearls are numerous and extensive, but there is a lack of research on their mechanism of action. In the future, we should focus on the mechanism research at the gene and molecular levels so that pearls can be better applied in practice. Toxicology research has also come to the fore. The limited clinical trials are not perfect in quality, but they still have a certain reference value. More scientific and representative clinical trials are needed in the future. At present, pearls have been used in many different fields, such as medicine (Cao et al., 1996), cosmetics (Li, 2002), health care (Zhang et al., 2002), and clothing (Hu and Wu, 2020), and more fields are still under development. The value of pearls in medical treatment is particularly significant and needs more attention and extensive research.
8 Conclusion
This study comprehensively expounds on the medicinal history of pearls in China and their chemical composition, pharmacology, toxicology, and clinical application for the first time. As a natural mineral medicine, the research on the chemical constituents of pearls has certain limitations; the specific effective constituents are unclear. Most of them use extracts, such as water extraction, acidolysis, and enzymatic hydrolysis. Amongst them, the effect of protein extracts is better, but often a single extraction will lead to the loss of many effective constituents. Therefore, it is often necessary to use a variety of extracts together to make the curative effect better. The specific active constituents need to be further studied. As a new research method, proteomics can be used to explore the specific active constituents of pearls containing protein. Most of the pharmacological effects of pearls have been developed, but most of them live in the superficial part, and the mechanism research has a long way to go. Toxicological studies have confirmed its safety. In terms of clinical application, pearls are mostly used combined with other drugs to treat diseases. The cases of pearls alone to treat diseases are limited, which may lead to the inability to substantively prove the effectiveness of pearls, but these clinical trials still have a certain reference significance. In a word, pearls have great potential development value, and its medicinal value should be well known by more people. In the future, we should focus on the development of pearls taking forms and mechanisms: one is to promote the discovery of more active constituents in pearls, and the other is to lay a foundation for its clinical application.
Author Contributions
YS was responsible for collecting data and writing this article, WC was responsible for writing the Latin scientific names of medicinal materials, KF put forward relevant suggestions for the article, and ZW directed the writing of the article and functioned as our corresponding author.
Funding
This study was supported by China Postdoctoral Science Foundation (2012M511916); Project of Sichuan Provincial Administration of Traditional Chinese Medicine (2012-E-040); and Science and Technology Development Fund of Chengdu University of Traditional Chinese Medicine (ZRQN1544).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all authors for their contributions to this article.
References
Arnaud-Haond, S., Goyard, E., Vonau, V., Herbaut, C., Prou, J., and Saulnier, D. (2007). Pearl Formation: Persistence of the Graft during the Entire Process of Biomineralization. Mar. Biotechnol. (NY) 9 (1), 113–116. doi:10.1007/s10126-006-6033-5
Atlan, G., Balmain, N., Berland, S., Vidal, B., and Lopez, E. (1997). Reconstruction of Human Maxillary Defects with Nacre Powder: Histological Evidence for Bone Regeneration. C R. Acad. Sci. III 320 (3), 253–258. doi:10.1016/s0764-4469(97)86933-8
Bédouet, L., Rusconi, F., Rousseau, M., Duplat, D., Marie, A., Dubost, L., et al. (2006). Identification of Low Molecular Weight Molecules as New Components of the Nacre Organic Matrix. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 144 (4), 532–543. doi:10.1016/j.cbpb.2006.05.012
Bédouet, L., Marie, A., Dubost, L., Péduzzi, J., Duplat, D., Berland, S., et al. (2007). Proteomics Analysis of the Nacre Soluble and Insoluble Proteins from the Oyster Pinctada Margaritifera. Mar. Biotechnol. 9 (5), 638–649.
Bourrat, X., Qiao, L., Feng, Q. L., Angellier, M., Dissaux, A., Beny, J.-M., et al. (2012). Origin of Growth Defects in Pearl. Mater. Charact. 72. doi:10.1016/j.matchar.2012.07.010
Cao, C., Xu, Z., Wei, H. Y., Yao, S. H., and Liu, Y. (1996). A Preliminary Study on the New Uses of Pearl and Nacre Powder in Medical and Health Care. China J. Chin. Materia Medica (10), 59–62+65.
Cao, R. J. (2018). The Dzi Beads of the Seven Treasures of Tibetan Buddhism and the Inheritance of Chinese-Tibetan Art. Tomorrow Fashion, 298.08.
Cartwright, J. H., Checa, A. G., and Rousseau, M. (2013). Pearls Are Self-Organized Natural Ratchets. Langmuir 29 (26), 8370–8376. doi:10.1021/la4014202
Cen, Y. H., Lin, Y., Lin, J., Jia, W., and Zhao, J. (2018). Hydrolysis of Nanzhu Fluid Promotes Proliferation and Migration of Human Microvascular Endothelial Cells. Chin. J. Tissue Eng. Res. 22 (16), 2564–2569.
Chaturvedi, R., Singha, P. K., and Dey, S. (2013). Water Soluble Bioactives of Nacre Mediate Antioxidant Activity and Osteoblast Differentiation. PLoS One 8 (12), e84584. doi:10.1371/journal.pone.0084584
Chen, L. (2017). Clinical Application of Pearl Powder Combined with Zinc Oxide in the Treatment of Incontinence-Associated Dermatitis. Guangzhou: Guangzhou University of Chinese Medicine.
Chen, L., Hu, G. Y., Wang, X. J., Wang, H. J., and Zhou, J. (2019a). Therapeutic Effect of Pearl Powder Combined with Zinc Oxide in Treating Incontinence-Associated Dermatitis. J. Guangzhou Univ. Traditional Chin. Med. 36 (01), 53–58.
Chen, L. J., Li, C., and Li, T. (2004). Experimental Study on Anti-osteoporosis of Pearl Active Protein. J. Chin. Med. Mater. (05), 363–365.
Chen, L., Xu, P., Chen, M. W., Li, X. N., Zheng, B., Wang, Q. Z., et al. (2017). In Vivo study on the Repair of Rabbit Distal Femoral Bone Defects with Nano-freshwater Pearl Powder. J. Mod. Stomatology 31 (01), 15–18.
Chen, X., Peng, L. H., Chee, S. S., Shan, Y. H., Liang, W. Q., and Gao, J. Q. (2019b). Nanoscaled Pearl Powder Accelerates Wound Repair and Regeneration In Vitro and In Vivo. Drug Dev. Ind. Pharm. 45 (6), 1009–1016. doi:10.1080/03639045.2019.1593436
Chen, X. M. (2015). Nursing Care of Sanmeian Pulvis and Pearl Powder for Treatment of Perianal Infection in Patients with Acute Leukemia. Fujian Med. J. 37 (04), 163–164.
Chen, Y. H., Liu, X. W., Hou, X. H., and Sun, Q. F. (2016). Study of Mouse Alveolar Bone Defect Reparation by Dialdehyde Bletilla Striata Glucomanna/hydroxypropyl Chitosan/nano-Nacre Powder Composited Scaffolds. J. Shandong Univ. Sci. 54 (06), 7–11+30.
Chen, Y. J., and Yuan, Z. K. (1990). Study on the Anti-tumor Effect of Porphyrins of Pearl. Chin. J. Mod. Appl. Pharm. (06), 48–49.
Chen, Z., Yan, Q., Zhang, Z., Lan, T., Liu, P., Han, S., et al. (2020). Immunomodulatory Effects of Hydrolyzed Seawater Pearl Tablet (HSPT) on Th1/Th2 Functionality in a Mice Model of Chronic Obstructive Pulmonary Disease (COPD) Induced by Cigarette Smoke. Evid. Based Complement. Altern. Med. 2020, 5931652. doi:10.1155/2020/5931652
Chen, Z. J., Han, X. X., Wu, W. Y., Ding, X. Z., Deng, X. G., Tian, X. L., et al. (2001). Experimental Study on Form Deprivation Myopia ( FDM) Model in Chickens. Chin. J. Exp. Ophthalmol. (06), 507–510.
Cheng, Y., Zhang, W., Fan, H., and Xu, P. (2018). Water-soluble Nano-pearl Powder Promotes MC3T3-E1 Cell Differentiation by Enhancing Autophagy via the MEK/ERK Signaling Pathway. Mol. Med. Rep. 18 (1), 993–1000. doi:10.3892/mmr.2018.9052
Chiu, H. F., Hsiao, S. C., Lu, Y. Y., Han, Y. C., Shen, Y. C., Venkatakrishnan, K., et al. (2018). Efficacy of Protein Rich Pearl Powder on Antioxidant Status in a Randomized Placebo-Controlled Trial. J. Food Drug Anal. 26 (1), 309–317. doi:10.1016/j.jfda.2017.05.010
Committee, N. P. (2020). Pharmacopoeia of the People's Republic of China, I. Beijing: China Medical Science and Technology Press.
Dai, J., Bai, J., Jin, J., Yang, S., and Li, G. (2015). Stimulation by Pearl of Mineralization and Biocompatibility of PLA. Adv. Eng. Mat. 17 (11), 1691–1697. doi:10.1002/adem.201500144
Dai, M. F. (2015). The Curative Effect and Nursing Experience of Pearl Powder, Tin Powder and Sesame Oil in the Treatment of Infant Red Buttocks. Strait Pharm. J. 27 (02), 191–192.
Deng, C. M., Yin, G. R., Liu, Y., Yin, L. B., and Lu, D. W. (2014). Safety Evaluation of the Black Pearl. Guangdong Chem. Ind. 41 (05), 19–20.
Deng, Y. Y., Wang, X. Y., Li, C. G., Meng, H., Dong, Y. M., and Li, L. (2017). Skin-whitening Mechanism of Margarita Liquid. Mod. Chin. Med. 19 (07), 992–994+1006.
Dong, N., Liang, J., Liu, Y. Q., Ma, K., Lv, D. Y., Jin, L. M., et al. (2011). Determination of the Trace Element in Pearl Powder. J. Anhui Agric. Sci. 39 (01), 315–316.
Elahi, A., Singh, M. P., Ali, S., and Khan, F. (2014). Antigen Specific Immune Enhancement of Innate and Acquired Immunity by Pearl in Ashed Form. Int. Immunopharmacol. 21 (1), 82–93. doi:10.1016/j.intimp.2014.04.013
Fan, B. L. (2000). The Effect of Enzymolysis Pearl Liquid on Improving Sleep. J. Prev. Med. Inf. (04), 46–47.
Fang, C. P., and Huang, M. S. (2013). Efficacy and Nursing Observation of Pearl Powder and Erythromycin Ointments for Treatment of Bedsores. Strait Pharm. J. 25 (01), 178–179.
Feng, W. L., Wu, Y. T., and Zhao, Y. M. (2005). Pearl’s Classification and True and False Discrimination. Hebei J. Traditional Chin. Med. 05, 361–362.
Fu, Y. M. (2014). Clinical Observation on the Treatment of 51 Cases of Oral Ulcer with Degree Ⅱ∼Ⅲ with Pearl Powder and Lidocaine. Zhejiang J. Traditional Chin. Med. 49 (09), 664.
Gao, H., Chen, H., Chen, W., Tao, F., Zheng, Y., Jiang, Y., et al. (2008). Effect of Nanometer Pearl Powder on Calcium Absorption and Utilization in Rats. Food Chem. 109 (3), 493–498. doi:10.1016/j.foodchem.2007.12.052
Gao, Q. H., Han, X. X., Huang, K. X., and Xu, H. B. (2000). Efects of Margaric Hydrolysate and Zinc on Microcirculation in Bulbar Conjuncitva of Rabbit Eyes. Guangzhou: Journal of Guangdong Pharmaceutical University, 273–276.04.
He, J. F., Deng, Q., Pu, Y. H., Yan, B., and Zeng, M. (2016). Amino Acids Composition Analysis of Pearl Powder and Pearl Layer Powder. Food Industry 37 (04), 270–273.
He, L. (2019). Clinical Observation of Zinc Oxide Ointments Combined with Pearl Powder in the Treatment of Severe Diaper Dermatitis in Newborns. J. Pract. Traditional Chin. Med. 35 (06), 741–742.
Hu, S. H., and Wu, P. (2020). Application of Pearl Fiber Blended Yarn in Weft Knitted Women's Clothing. Knitt. Ind. 05, 26–30.
Hu, S. S., Wang, D. Y., Liu, J. P., Yu, H., Leng, H. W., Tan, L. W., et al. (1994). Experimental Study on the Anti-aging Effect of Pearl Hydrolyzate. Tianjin: Chinese Traditional and Herbal Drugs, 203–204+221.04.
Hu, W. B., Chen, L. M., Liu, S., and Tian, L. (1998). Research Progress of Pharmacological Action and Clinical Application of Pearl. Acta Chin. Med. Pharmacol. 05, 53–56.
Huang, A. L., Liu, J. Q., Zhu, X. Z., Wu, Y. F., Cai, J., Liang, Z. Q., et al. (1997). Research on Animal Experiment and Clinical Application of Zhenzhu Shaoshang Ointments. Guangxi Med. J. 01, 77–79.
Huang, L. H., and Qiu, W. H. (2013). Observation on the Effect of Baiduobang Ointments Combined with Pearl Powder and Sesame Oil in the Treatment of Stage Ⅲ Ⅳ Pressure Ulcer. J. Nurs. Rehabilitation 12 (06), 563–564.
Huang, Q. P., and Pan, H. M. (2000). The Pharmacological Action and Clinical Application of Pearl. Lishizhen Med. Materia Medica Res. 06, 564–565.
Huang, Y. (2020). Study on the Nacre Powder Combine with Platelet-Rich Fibrin in the Cranial Defect of Rabbit. LanzhouLanzhou: Lanzhou UniversityLanzhou University.
Jia, M. R., and Zhang, Y. (2016). Dictionary of Chinese Ethnic Medicine. Beijing: China Medical Science Press, 674–675.
Jiang, J. (2013). Pearl Powder Combined with Kangfu Xin Solution in the Treatment of 16 Cases of Skin Ulcers after Radiotherapy. Zhejiang J. Traditional Chin. Med. 48 (07), 507.
Jiang, N., Zhang, Y. X., and Du, G. H. (2016). Novel Ideas and Methods of Pharmacological and New Drug Researches of Traditional Chinese Medicine. Chin. J. Pharmacol. Toxicol. 30 (09), 893–909.
Jiang, Q., Bai, Z. Y., and Sun, C. H. (2019). Analysis of Metallic Element Types and Contents in Pearls of Different Colors and Their Related Tissues in Hyriopsis Cumingii. J. Shanghai Ocean Univ. 28 (06), 882–889.
Jin, C., Ren, H. Y., Pu, J. W., Liu, X. J., and Li, J. L. (2019). Identification of Nacre Matrix Protein Genes Hic14 and Hic19 and Their Roles in Crystal Growth and Pearl Formation in the Mussel Hyriopsis Cumingii. Biotechnol. Appl. Biochem. 66 (4), 545–554. doi:10.1002/bab.1752
Jing, X. K. (2015). Clinical Observation of Luhui Zhenzhu Capsules in Treating Chloasma and Regulating Hormone Level. Shanxi Med. J. 44 (10), 1156–1158.
Kan, C. G., Xu, S. J., Yang, R. Y., Li, J. Z., Wang, Z., Chen, Z. L., et al. (2015). Experimental Study on the Effect of Zhenzhu Pulvis on Tissue Repair of Acute and Chronic Wounds and Basic Fibroblast Growth Factor. Inf. Traditional Chin. Med. 32 (01), 37–39.
Lai, X. J. (2013). Treatment of 30 Cases of Stage Ⅱ Bedsore after Apoplexy with Pearl Powder and Anputie. China's Naturop. 21 (04), 21–22. doi:10.1111/j.1749-124x.2013.12046.x
Lamghari, M., Almeida, M. J., Berland, S., Huet, H., Laurent, A., Milet, C., et al. (1999). Stimulation of Bone Marrow Cells and Bone Formation by Nacre: In Vivo and In Vitro Studies. Bone 25 (2 Suppl. l), 91S–94S. doi:10.1016/s8756-3282(99)00141-6
Lan, T. J., Lin, J., Mo, M. Y., and Lin, Y. (2017). The Effect of Hydrolyzed Seawater Pearl Tablet (HSPT) on the Immunological Function of Immunocompromised Mice. Genomics Appl. Biol. 36 (05), 1811–1812+18141817.
Li, F. H. (2002). The Application Status and Prospect of Pearls in Cosmetics. China Cosmet. Rev. (09), 66–67.
Li, J., Luo, X. L., Wu, Z. L., Wang, X., and Tang, W. X., (2019). Effect of Angong Niuhuang Pills on Early Cerebral Oxygen Metabolism and Cerebral Protection after Cardiopulmonary Resuscitation. 54(11), 790–791.
Li, L. P. (2009). Composition of Seawater and Fresh Water Cultured Pearls. Earth Sci. 34 (05), 752–758.
Li, N., Xu, P., Chen, Z., Chen, M., He, H., and Zhang, W. (2020). In Vivo study on the Repairment of Distal Femur Defects in Rabbit with Nano-Pearl Powder Bone Substitute. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 45 (06), 684–692. doi:10.11817/j.issn.1672-7347.2020.180676
Li, S. R., Zhang, J. X., Yao, S., Guo, D. A., and Wu, W. Y. (2015). Comparative Study of Trace Elements in Pearls and Nacres. World Chin. Med. 10 (10), 1594–1597.
Li, T. C., and Suo, Y. R. (2003). Stud Y on Metabolism in Mice Means of Mineral Elements after Feeding Them with Tibetan Medicine Seventy Flavor Pearl Pills. Chin. Archives Traditional Chin. Med. 03, 356–359.
Li, Y. J., and Fu, J. H. (2012). Observation of the Effect of Rivanol Combined with Pearl Powder for the Treatment of Ten Cases of Patients with Pressure Sores. Shanghai Med. Pharm. J. 33 (24), 30–31.
Li, Y. Y., and Gao, Q. G. (2006). Treatment of 451 Cases of Early Senile Cataract with Zhenzhu Jingming Tablets. Shandong J. Traditional Chin. Med. 02, 96–97.
Liang, S., Chen, X., Yang, G., Li, Z., and Xu, S. (2018). Application of Dynamic Rod Worth Measurement in a VVER rhBMP-2 and Their Complex in Bone Engineering. Nucl. Eng. Des. 338 (03), 176–180. doi:10.1016/j.nucengdes.2018.08.018
Liao, J. (2019). Separation and Purification of Soluble Components from Pearl Powder and It’s Bioactivities. Hangzhou: Zhejiang University.
Lin, J., Lin, Y., Yang, J. F., and Liu, Q. (2007). Comparison of Seawater Pearls and Freshwater Pearls: Medicinal Value, Identification Method. J. Guangxi Univ. Chin. Med. 04, 80–82.
Lin, Z., Dong, J. X., and Lu, M. (1996). Study on the Anti-inflammatory Effect of Zhenshen Pulvis. J. Changchun Univ. Chin. Med. 01, 61.
Liu, H. Q., Li, J. S., and Ye, J. (1995). Study on Zhuhuang Ointments. Chin. Tradit. Pat. Med. 17 (12), 8–10.
Liu, P., Cen, Y. H., Lin, J., and Lan, T. J. (2020a). Protective Effect of Nanzhu Hydrolysate against H2O2-Induced Oxidative Dam- Age in Human Lens Epithelial Cells. Recent Adv. Ophthalmol. 40 (07), 612–616.
Liu, P., Lin, J., Jia, W., Lan, T. J., and Cen, Y. H. (2020b). Nanzhu Hydrolysate Attenuate Oxidative Stress Damage of Human Microvascular Endothelial Cells by Inhibiting Autophagy. Biotechnology 30 (04), 389–396.
Liu, P., Liu, Y. Q., Lan, T. J., Huang, C. S., Luo, R. J., Lu, A. Y., et al. (2020). Antibacterial Activity and Stability of Hepu Pearl Extracts on the Staphylococcus Aureusin In Vitro. Sci. Technol. Food Industry 41 (14), 108–113.
Liu, S., Wei, W., Bai, Z., Wang, X., Li, X., Wang, C., et al. (2018). Rapid Identification of Pearl Powder from Hyriopsis Cumingii by Tri-step Infrared Spectroscopy Combined with Computer Vision Technology. Spectrochim. Acta A Mol. Biomol. Spectrosc. 189, 265–274. doi:10.1016/j.saa.2017.08.031
Liu, W. D., Liu, Y. E., Wei, X. B., Liu, H. Q., Li, A. G., Chen, X. Y., et al. (2003). Experimental Study of Fufang Zhenzhu Pulvis on Regulating Immune Function. J. Shandong Univ. Traditional Chin. Med. (06), 459–461.
Liu, X., Li, J., Xiang, L., Sun, J., Zheng, G., Zhang, G., et al. (2012). The Role of Matrix Proteins in the Control of Nacreous Layer Deposition during Pearl Formation. Proc. Biol. Sci. 279 (1730), 1000–1007. doi:10.1098/rspb.2011.1661
Liu, X. L., and Tian, J. (2010). Amino Acids Composition and Function Analysis of Nano Pearl Powder. Food Res. Dev. 31 (04), 113–114.
Liu, Y., Huang, Q., and Feng, Q. (2013). 3D Scaffold of PLLA/pearl and PLLA/nacre Powder for Bone Regeneration. Biomed. Mater 8 (6), 065001. doi:10.1088/1748-6041/8/6/065001
Liu, Y. S., Huang, Q. L., and Feng, Q. L. (2014). Research Progress in Nacre as a Bone Repair Material. China Sci. 9 (02), 168–174.
Long, L. E. (2014). Application of Zhenzhu Pulvis in Burns and Scalds Infected Wounds. Hubei J. Traditional Chin. Med. 36 (08), 43.
Lu, H. Q., Hu, S. K., Yang, J. Y., Cao, J. H., Wang, Z. C., and Zhao, F. Z. (1990). The Experimental Research on Pearl Powder in Treating Rabbit’s Corneal. Nat. Prod. Res. Dev. 03, 36–41.
Lu, H. Q., Yang, J. Y., Cong, Y. J., Xiao, Y. X., and Zhang, L. Y. (1991). The Comparison of Medicinal Value of Pearl Powder and Pearl Layer Powder. Nat. Prod. Res. Dev. (03), 35–42.
Ma, L. S., and Xiao, S. X. (2007). The Pharmacology and Clinical Application of Medicinal Pearls. Wuhan: China Pharmacist, 380–381.04.
Ma, N., Liu, Y. X., Wang, T., and Wang, M. Z. (2011a). The Mystery of Pearls. Educ. Chem. (06), 61–63.
Ma, Y., Qiao, L., and Feng, Q. (2012). In-vitro Study on Calcium Carbonate Crystal Growth Mediated by Organic Matrix Extracted from Fresh Water Pearls. Mater Sci. Eng. C Mater Biol. Appl. 32 (7), 1963–1970. doi:10.1016/j.msec.2012.05.030
Ma, Y., Gao, Y., and Feng, Q. (2011b). Characterization of Organic Matrix Extracted from Fresh Water Pearls. Mater. Sci. Eng. C 31 (7), 1338–1342. doi:10.1016/j.msec.2011.04.016
Mao, Q. H., Xu, P., Cheng, Y. N., Liao, J., Chen, L., Xing, L., et al. (2018). Preliminary Study on Preparation and Biological Experimental Assessment of Nano-Scale Freshwater Pearl Powder. J. Mod. Stomatology 32 (05), 257–260.
Mao, Q. H., and Xu, P. (2016). Research Progress of Pearl/nacre in Bone Tissue Repair. J. Oral Sci. Res. 32 (03), 311–313.
Medicine, E. B. (1999). Chinese Materia Medica, 25. Shanghai: Shanghai Scientific & Technical Publishers.
Meng, G. H., Li, H. J., and Wuren, T. Y. (2007). Study of Protecting Rabbits Retinal Ischemic-Reirrigation and Expression of Bcl-2 Gene. J. Med. Pharm. Chin. Minorities (01), 42–46.
Mo, M. Y., Lin, J., and Wei, M. C. (2015). Research Progress on Detoxification and Promoting Granulation of Pearl Powder and its Clinical Application. J. Guangxi Univ. Chin. Med. 18 (03), 77–79.
Montagnani, C., Marie, B., Marin, F., Belliard, C., Riquet, F., Tayalé, A., et al. (2011). Pmarg-pearlin Is a Matrix Protein Involved in Nacre Framework Formation in the Pearl Oyster Pinctada Margaritifera. Chembiochem 12 (13), 2033–2043. doi:10.1002/cbic.201100216
Mouriès, L. P., Almeida, M. J., Milet, C., Berland, S., and Lopez, E. (2002). Bioactivity of Nacre Water-Soluble Organic Matrix from the Bivalve Mollusk Pinctada Maxima in Three Mammalian Cell Types: Fibroblasts, Bone Marrow Stromal Cells and Osteoblasts. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 132 (1), 217–229. doi:10.1016/s1096-4959(01)00524-3
Niu, Y. L., and Xu, W. (2005). Clinical Observation on Pearl Clear-Sighted Eye Drops in Therapy of Chronic Conjunctivitis. J. Tongji Univ. Sci. (02), 62–64.
Oliveira, D. V., Silva, T. S., Cordeiro, O. D., Cavaco, S. I., and Simes, D. C. (20122012). Identification of Proteins with Potential Osteogenic Activity Present in the Water-Soluble Matrix Proteins from Crassostrea gigas Nacre Using a Proteomic Approach. Sci. World J., 765909.
Ouyang, Q. Q., Yang, L., Luo, J. Q., Lao, Z., and Chen, J. (2012). Components and Structure with its Color Generation Mechanism of Pearl. Guangzhou Chem. Ind. 40 (12), 23–26.
Ouyang, Z. Y. (2019). Studies on the Regulatory Mechanism of Synthesis of Seawater Nacre in Vitro. Zhanjiang: Guangdong Ocean University.
Pan, J. X., Gu, Z. L., Qian, Z. N., and Wang, Y. Q. (1999). Research on the Effect of Pearl Powder on the Central Nervous System. Chin. Tradit. Pat. Med. (11), 44–45.
Pattapon, A., Panjit, C., and Theeralaksna, S. (2011). Potential Induction of Bone Regeneration by Nacre: an In Vitro Study. Implant Dent. 20 (1), 32–39.
Pu, W., Yuan, X. P., Zhou, Y., Zhu, Y. M., and Xu, L. L. (2018a). Clinical Study of Pearl Powder Combined with Glycyrrhizin in the Treatment of Chronic Eczema. Chin. J. Ethnomedicine Ethnopharmacy 27 (09), 85–87.
Pu, Y. H., Deng, Q., He, J. F., and Tong, Y. H. (2017). Study on Antioxidant Activities of Pearl Hydrolyzate In Vitro. Chem. Res. Appl. 29 (04), 555–559.
Pu, Y. H., Deng, Q., and Tong, Y. H. (2018b). Study on Antioxidant Activities of Seawater Pearl Hydrolyzate In Vitro. Appl. Chem. Ind. 47 (12), 2635–2637+2642.
Pu, Y. H., and Tong, Y. H. (2015). Research on Skin-Whitening Mechanism of Pearl Hydrolyzate. Flavour Fragr. Cosmet. (03), 43–46.
Qian, R. H., and Zhu, J. P. (2003). Experimental Study on Pearl Powder for Regulating Immune Function. Chin. J. Mod. Appl. Pharm. 20 (S1), 22–23.
Sato, Y., and Komaru, A. (2019). Pearl Formation in the Japanese Pearl Oyster (Pinctada Fucata) by CaCO 3 Polymorphs: Pearl Quality‐specific Biomineralization Processes and Their Similarity to Shell Regeneration. Aquac. Res. 50 (6), 1710–1717. doi:10.1111/are.14057
Shao, Q., Luo, X., Zhang, Q. Q., and Xu, L. L. (2018). Clinical Observation on Treating Recurrent Aphthous Ulcer with Pearl Powder Plus Vitamin B2. Clin. J. Chin. Med. 10 (34), 90–92.
Shen, L. L., Zhong, Y. H., Li, S. F., Hu, Q., Huan, F., and Wang, Y. B. (2013). Toxicological Evaluation for the Edible Safety of Pearl Powder. J. Baotou Med. Coll. 29 (05), 21–24.
Shen, Y., Zhu, J., Zhang, H., and Zhao, F. (2006). In Vitro Osteogenetic Activity of Pearl. Biomaterials 27 (2), 281–287. doi:10.1016/j.biomaterials.2005.05.088
Shen, Z. L. (2017). Optimization of Extraction Process and Efficacy Research for Pearl Powder. Hangzhou: Zhejiang University of Technology.
Sun, M. L., and Qiu, J. (2012). Clinical Observation of Sedative Effects of Rannasampelin. China Mod. Med. 19 (22), 110–111.
Tang, X. Y., and Wang, P. R. (2016). Observation on the Curative Effect of Compound Aescinate Gel Combined with Pearl Powder in the Treatment of Pressure Ulcer. J. Pract. Traditional Chin. Med. 32 (09), 902–903.
Tang, Z. J., Mei, Z. M., Huo, M. Y., and Zou, Y. P. (1992). Treatment of 64 Cases of Cervical Erosion with Pearl Powder. Chin. J. New Drugs Clin. (05), 296–297.
Wan, T. T. (2016). Study on the Microstructure, Elementary Composition and Black-Dyeing Optimized of Inferior Southern Pearls. Zhanjiang: Guangdong Ocean University.
Wan, T. T., Yang, L., Ouyang, Q. Q., Li, S. D., and Chen, J. (2014). The Research Progress of Microstructure of the Sea-Cultured Pearls. J. Chin. Electron Microsc. Soc. 33 (05), 478–486.
Wang, C. L., Ning, Y. Y., Cheng, J. X., Mo, H. L., and Song, Z. J. (1994). The Effects of Pteria Martensii on the Immune System in Rat. J. Guangxi Med. Univ. (03), 280–282.
Wang, J., Yang, A. Q., Chen, Z. X., Zhang, L. H., and Yao, Y. F. (2018). Study on Inhibitory Effects of Pearl Extracton Hydrogen Peroxideinduced Apoptosis of Human Skin Fibroblasts. Pharm. Biotechnol. 25 (05), 402–405.
Wang, K., Wei, B., Tan, J. N., and Ji, H. (2014). Effect of Pearl Powder on the Proliferation Differentiation and Mineralization of MC3T3-E1 Cells and the Expression of Bone Related Genes in the Cells. Chin. J. Osteoporos. 20 (06), 666–672.
Wang, M. L., and Chen, Z. Q. (2015). The Role of Norfloxacin + Pearl Powder in the Care of Pressure Ulcer. J. North Pharm. 12 (03), 191+109.
Wang, N., Kinoshita, S., Riho, C., Maeyama, K., Nagai, K., and Watabe, S. (2009). Quantitative Expression Analysis of Nacreous Shell Matrix Protein Genes in the Process of Pearl Biogenesis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 154 (3), 346–350. doi:10.1016/j.cbpb.2009.07.012
Wang, Q. Z., Xu, P., Li, N., Chen, M. W., and Zhang, W. B. (2019). Repair of Bone Defect of Distal Femur in Rabbits with Nanometer Pearl Powder/chitosan-Hyaluronic Acid Scaffold. Nat. Prod. Res. Dev. 31 (04), 696–703+668.
Wang, W. W. (2015). Talking about the Research Significance and Development Status of the Pharmacokinetics of Traditional Chinese Medicine. Health Guide Med. Res. 17, 179.
Wang, X. S. (1996). Zhenzhu Tuiyi Pulvis for Treatment of Curling Keratitis. Chin. J. Optometry Ophthalmol. Vis. Sci. (04), 227.
Wei, B., and Feng, S. J. (2014). Application of Dermatoscopy to Evaluate the Clinical Efficacy of Luhui Zhenzhu Capsules in Treating Chloasma. Med. Innovation China 11 (08), 114–116.
Wen, Q. J., Ai, C. Q., Hai, P., Zhou, S. X., Shang, H., and Chen, Q. H. (1998). Study on the Sedative Effect of Tibetan Medicine Qisheiwei Zhenzhu Pills. J. Med. Pharm. Chin. Minorities (02), 42–43.
Weng, L. F. (1989). Analysis and Comparison of Chemical Composition of Freshwater Pearls and Seawater Pearls. Chin. Pharm. J. (05), 276–278+316.
Wu, H. (2017). Study on the Composition and Structure of Seawater Cultured Pearls and in Vitro Mineralization Based on Pearl Layer. Zhanjiang: Guangdong Ocean University.
Wu, J. Y., and Zhu, J. P. (2009). Observe the Safety of Water Soluble Pearl Powder: An Expermiental Research. Chin. Archives Traditional Chin. Med. 27 (02), 397–399.
Wu, Y. (2009). Zhenzhu Anshen Syrups in Treating 40 Cases of Insomnia. Shaanxi J. Traditional Chin. Med. 30 (05), 519–520.
Xia, J. F., Qian, G. Y., Chen, L., and Li, C. Y. (2010). Study on the Differentiation of Pearl and Conch Powders by FTIR. Chin. J. Spectrosc. Laboratory 27 (02), 524–528.
Xia, M., Huang, D., Tong, Y., and Lin, J. (2020). Pearl Powder Reduces Sleep Disturbance Stress Response through Regulating Proteomics in a Rat Model of Sleep Deprivation. J. Cell Mol. Med. 24 (9), 4956–4966. doi:10.1111/jcmm.15095
Xia, Q., Lin, C. Q., Mo, Q. Y., Li, R. L., Zhan, R. T., Deng, W., et al. (2014). Preparation and Pharmacodynamics Study of Nacre Powder Ultrafine Powder. Chin. Tradit. Pat. Med. 36 (02), 396–399.
Xiang, S. X., and Zheng, X. X. (2010). Clinical Observation on the Application of Zhenzhu Shaoshang Ointments in Burn Wounds. Chin. Community Dr. 12 (32), 127–128.
Xiao, Y., Liu, G. R., Zhu, X. P., and Yan, J. (2015). Observation on the Curative Effect of Meijie Shuirong Zhenzhufen Capsules in the Treatment of 30 Cases of Acne. Hunan J. Traditional Chin. Med. 31 (07), 75–76.
Xie, C. H., Zhan, J. H., Xie, W. G., Shou, Y. E., Chen, Y. F., and Chen, X. F. (2015). Yunnan Baiyao Combined with Pearl Powder in Treating 51 Cases of Senile Dementia ICU Patients Complicated with Stress Ulcer Hemorrhage Patients. China Mod. Dr. 53 (27), 85–88.
Xie, F. Z. (2013). Observation on the Effect of Pearl Powder in the Nursing of Pressure Sores. Mod. Nurse (01), 135–136.
Xu, J. H., Yu, M. F., Wang, H. M., and Weng, W. J. (2014a). Constitution and Osteogenic Activity of Nano Nacre/I Collagen Scaffold In Vitro. Rare Metal Mater. Eng. 43 (S1), 347–352. doi:10.1016/s1875-5372(14)60137-5
Xu, X. L., Zheng, D. F., Chen, J., and Cai, Y. R. (2014b). Analysis of the Curative Effect of Clotrimazole and Pearl Powder in the Treatment of Mycotic Dermatitis. Chin. J. Sch. Dr. 28 (02), 119+121.
Xue, D. P., and Xu, J. X. (2013). Determination of Rare Earth Elements in Pearls by ICP-XSP. Stud. Trace Elem. Health 30 (04), 43–44.
Yang, A. Q., Chen, Z. X., Zhang, L. H., Yao, Y. F., Mo, J. H., and Wang, J. (2018b). Protective Effect of Pearl Extract on Ultraviolet Radiation Induced Injury in HaCaT Cell. Pharm. Biotechnol. 25 (03), 218–221.
Yang, A. Q., Shen, Y. Q., Zhang, L. H., Mo, J. H., Chen, Z. X., and Wang, J. (2016b). Study on the Skin Care Functions of Active Ingredients in Pearl Extractive. Shanghai: Flavour Fragrance Cosmetics, 58–61.01.
Yang, A. Q., Wang, J., Luo, J. F., Zhang, L. H., Mo, J. H., and Shen, Y. Q. (2015a). Antioxidant Activities of Pearl Extract from Hyriopsis Cumingii. Pharm. Biotechnol. 22 (03), 233–236.
Yang, A. Q., Wang, J., Shen, Y. Q., Zhang, L. H., Mo, J. H., and Chen, Z. X. (2018a). Effects of Pearl Extract on the Tyrosinase Activity and Melanogenesis of Melanoma Cells. Pharm. Biotechnol. 25 (4), 312–315.
Yang, A. Q., Wang, J., Zhang, L. H., Mo, J. H., Chen, Z. X., and Shen, Y. Q. (2016a). Lighting Effect of Pearl Extract on Melanocytes In Vitro. Pharm. Biotechnol. 23 (02), 146–149.
Yang, F. M. (2020). Study on Effect of Enzymatic Hydrolysis from Mantle of Pearl Oyster on Mice Skin Wound Healing. Zhenjiang: Guangdong Ocean University.
Yang, H. L., Korivi, M., Lin, M. K., Chang, H. C., Wu, C. R., Lee, M. S., et al. (2017). Antihemolytic and Antioxidant Properties of Pearl Powder against 2,2'-Azobis(2-Amidinopropane) Dihydrochloride-Induced Hemolysis and Oxidative Damage to Erythrocyte Membrane Lipids and Proteins. J. Food Drug Anal. 25 (4), 898–907. doi:10.1016/j.jfda.2016.10.007
Yang, M. Y., Guo, S. G., Shi, L. Y., and Wang, W. Z. (2004). Study on Compositions and Colouring Mechanism of Freshwater Cultured Pearls. J. Gems Gemmology (02), 10–13.
Yang, X. R., Ke, C. Y., and Deng, H. (2002). Trace Elements and Health. Heilongjiang Environ. J. 26 (277), 63107–63108.
Yang, Y. L., Chang, C. H., Huang, C. C., and Liu, H. W. (2015b). Anti-inflammation and Anti-apoptosis Effects of Pearl Extract Gel on UVB Irradiation HaCaT Cells. Biomed. Mater Eng. 26 (Suppl. 1), S139–S145. doi:10.3233/BME-151299
Yu, H. F. (2013). Experience of Treating Heart Palpitations with Pearl Powder and Amber Powder as a Pair Medicine. Guangming J. Chin. Med. 28 (03), 595.
Zeng, W. Y., and Liu, Z. Y. (2017). Metronidazole, Vitamin B2, Pearl Powder, Such as Compatibility Treatment of Oral Ulcer Clinical Studies. J. North Pharm. 14 (08), 6–8.
Zhan, C. T. (2010). Research Status and Development Prospects of Inferior Pearls. Today Sci. Technol. (02), 44–45.
Zhang, E., Xing, M., and Peng, M. S. (2007b). A Study of Compositional Characteristics of Chinese Cultured Pearls. Acta Petrologica Mineralogica (04), 381–386.
Zhang, E., Xing, M., and Peng, M. S. (2007a). Discussion on the Composition and Structure Characteristics of Pearls. Chin. Soc. Mineralogy Petrology Geochem., 158–159.
Zhang, J. X., Li, S. R., Yao, S., Bi, Q. R., Hou, J. J., Cai, L. Y., et al. (2016). Anticonvulsant and Sedative-Hypnotic Activity Screening of Pearl and Nacre (Mother of Pearl). J. Ethnopharmacol. 181, 229–235. doi:10.1016/j.jep.2016.01.039
Zhang, L. (2019). Comparative Study of Nucleated and Non-nuclear Freshwater Pearls. Beijing: China University of Geosciences.
Zhang, L. H., Ci, H., and Guan, T. (2013). Clinical Application of Pearl Powder in the Treatment of Bedsores. Cap. Food Med. 20 (20), 60–61.
Zhang, L. H., Wang, J. L., Mei, H. P., and Luo, B. (2002). Beauty Effect and Health Care Efficacy of Pearl. Taiyuan: Detergent & Cosmetics, 33–36.04.
Zhang, R., Qin, M., Shi, J., Tan, L., Xu, J., Tian, Z., et al. (2018). Molecular Cloning and Characterization of Pif Gene from Pearl Mussel, Hyriopsis Cumingii, and the Gene Expression Analysis during Pearl Formation. 3 Biotech. 8 (4), 214. doi:10.1007/s13205-018-1233-z
Zhang, T. M., and Peng, C. (2015). Chinese Clinical Pharmacy. Place: Published: People's Medical Publishing House, 1045–1048.
Zhang, X., Hu, C., Yan, Y., Yang, H. F., Li, J. F., Bai, H., et al. (2014a). Identification of Pearl Powder Using Microscopic Infrared Reflectance Spectroscopy. Guang Pu Xue Yu Guang Pu Fen Xi 34 (09), 2424–2428.
Zhang, X. L., Mao, D. J., Ge, B. Y., and Li, H. Y. (2014b). Observation on the Curative Effect of Adding Luhui Zhenzhu Capsule in Treating Chloasma. Guide China Med. 12 (09), 183–184.
Zhang, Y. Y., Gu, W., Wu, Z., and Li, R. (1994). The Pharmacological Effects of Water-Soluble Pearl Powder on the Heart. Chin. Tradit. Pat. Med. (09), 35–37.
Zhao, A. Q. (2015). Pearls: the Top Grade for Beauty and Tranquility. China Drug Store (22), 82. doi:10.1002/cnma.201500036
Zhao, J. Z., and Yu, W. Y. (2014). Observation on Treatment of 47 Cases of Infection and Dehiscence of Perineal Side Incision with Pearl Powder. Zhejiang J. Traditional Chin. Med. 49 (05), 352–353.
Zhao, Q. L. (1992). Lecture 4 Pearl’s Classification and Place of Production. Superhard Mater. Eng. (02), 55–57.
Zheng, B., Xu, P., Li, X. N., Chen, L., Chen, Y. N., Zhang, W. B., et al. (2019). Effect of Nano-Pearl Powder on Cell Viability of Mouse Bone Marrow Mesenchymal Stem Cells. Chin. J. Pract. Stomatology 12 (01), 26–30.
Zheng, Q. Y., and Mao, Y. M. (2004). Comparison of Component, Action and Effects between Freshwater and Seawater Pearl. Shanghai J. Traditional Chin. Med. (03), 54–55.
Zhong, Z. W., Liu, R. C., and Zhou, Z. M. (2010). Angong Niuhuang Pills for the Treatment of 36 Children with Febrile Convulsions. Zhejiang J. Traditional Chin. Med. 45 (09), 663.
Zhu, B., Li, B. Y., and Chen, Y. (2011). Angong Niuhuang Pills to Prevent 25 Children with Febrile Convulsions. China Pharm. 20 (20), 71.
Zhu, H. Y. (2007). Treatment of 100 Cases of Chronic Skin Ulcer with Zhenzhu Pulvis. J. New Chin. Med. (03), 60.
Keywords: pearls, traditional medicine of China, chemical constituents, pharmacology, toxicology, clinical application
Citation: Song Y, Chen W, Fu K and Wang Z (2022) The Application of Pearls in Traditional Medicine of China and Their Chemical Constituents, Pharmacology, Toxicology, and Clinical Research. Front. Pharmacol. 13:893229. doi: 10.3389/fphar.2022.893229
Received: 10 March 2022; Accepted: 21 June 2022;
Published: 23 August 2022.
Edited by:
Ashwell Rungano Ndhlala, University of Limpopo, South AfricaReviewed by:
Kunming Qin, Jiangsu Ocean University, ChinaYi Tao, Zhejiang University of Technology, China
Chan Kam Wa, The University of Hong Kong, Hong Kong SAR, China
Copyright © 2022 Song, Chen, Fu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhang Wang, d2FuZ3poYW5nY3FjZEBjZHV0Y20uZWR1LmNu
 Yinglian Song1,2
Yinglian Song1,2 Zhang Wang
Zhang Wang