
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 13 September 2022
Sec. Pharmacoepidemiology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.892526
This article is part of the Research TopicRising Stars in Pharmacoepidemiology: 2021View all 4 articles
Objective: To assess the cost-effectiveness of nebulized budesonide and intravenous methylprednisolone in the treatment of acute exacerbation of chronic obstructive pulmonary disease (AECOPD) in a real-world setting.
Materials and methods: Data from 291 patients with AECOPD were collected from the information system of a tertiary hospital in China. Patients were categorized into two groups: those treated with nebulized budesonide (n = 148) and those treated with intravenous methylprednisolone (n = 143). Clinical efficacy and the rate of no readmission within 1 year after discharge were used as effect indicators, and a cost-effectiveness analysis was conducted from the perspective of the Chinese healthcare system. Logistic regression, generalized linear regression, and bootstrap methods were used for sensitivity analyses.
Results: There was no statistical difference between the budesonide and methylprednisolone groups in clinical efficacy rates (94.6% vs. 93.7%). The cost-minimization analysis shows that budesonide is not cost-effective owing to higher total cost. In terms of readmission rates, budesonide was again not cost-effective, with an incremental cost-effectiveness ratio (ICER) of 22276.62 CNY, which is higher than the willingness to pay (WTP) of 20206.20 CNY, the mean per admission expenditure in China. The sensitivity analyses confirm that these results are robust.
Conclusion: Compared with intravenous methylprednisolone, nebulized budesonide is not a cost-effective strategy for AECOPD patients in China.
Chronic obstructive pulmonary disease (COPD) is a leading cause of mortality worldwide and entails a significant economic and social burden (Lozano et al., 2012; Vos et al., 2012). In China, COPD is the fifth most common cause of death, accounting for 31.1% of the world’s total deaths from COPD (Yin et al., 2016; GBD 2017 Causes of Death Collaborators, 2018). Acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is an episode of worsening symptoms that negatively impacts health status (Vogelmeier et al., 2017). AECOPD is a frequent cause of hospital admission and accounts for 50%–75% of the health care costs incurred by patients with COPD (Celli et al., 2004).
COPD patients usually experience about 0.5–3.5 exacerbations per year (Hurst et al., 2010). However, greater frequency of exacerbations is associated with accelerated lung function decline (Donaldson et al., 2002), quality of life impairment (Seemungal et al., 1998) and increased mortality (Soler-Cataluña et al., 2005). Systemic corticosteroids (SCs) in COPD exacerbations shorten recovery time and improve lung function (Global, 2021), and a dose of 40 mg prednisone per day for 5 days is recommended for management of exacerbations of COPD (Leuppi et al., 2013). However, because of adverse effects such as osteoporosis and bone fractures, glucose intolerance, and myopathy (Kwong et al., 1987; Decramer et al., 1994; Henzen et al., 2000), nebulized budesonide alone may be a suitable alternative to SCs and provides similar benefits to intravenous methylprednisolone (Ding et al., 2016). Because the cost of budesonide is higher than that of methylprednisolone, the Global Initiative for Chronic Obstructive Lung Disease guideline (GOLD) states that the choice between these options may depend on local cost issues (Global, 2021).
The present real-world study assesses the efficacy, safety, and cost-effectiveness of nebulized budesonide versus intravenous methylprednisolone for AECOPD patients from the perspective of the Chinese healthcare system.
For this single-center retrospective study of patients with AECOPD, data collection was carried out from 1 January 2020 to 31 December 2020 at Lianyungang First People’s Hospital, a tertiary general hospital. Data were collected from patients’ electronic medical records and retrieved for analysis in a real-world setting. Eligible patients were aged between 45 and 80 years old, with a confirmed diagnosis of COPD and currently in acute exacerbation. Patients were ineligible if they received SCs in the past month, or had a history of pneumothorax, pulmonary embolism, or other respiratory diseases. Patients were categorized into two groups: those treated with budesonide suspension (2 mg, tid, nebulized inhalation) and those treated with methylprednisolone (40 mg, qd, intravenous infusion). The duration was 5–7 days in both groups.
In this study, clinical effectiveness was evaluated by professional clinicians according to the patient’s respiratory symptoms (cough, sputum, and dyspnea), pulmonary function, and blood gas analysis. In addition, to assess the long-term effect, the two groups’ rates of no readmission within 1 year after discharge were compared. Adverse events that occurred during the treatment were also extracted from medical records and investigated.
As the study context is the Chinese healthcare system, only data relating to direct medical costs were considered. The costs of medication, examinations, laboratory tests, ward beds, nursing, and other costs were collected from the hospital information system, and the total direct medical costs for each patient were calculated as the sum of all cost categories. Mean cost per patient over the entire period was calculated by summing the totals and then dividing the sum by the sample size in each arm. Discounting was not considered in this study because of the short time horizon. All resource costs were represented in Chinese yuan (CNY).
Continuous variables were presented as mean and standard deviation in each group and compared using a two-tailed Student’s t-test if the variables conformed to a normal distribution. In other cases, the minimum, maximum, median, and interquartile ranges were calculated for each group and compared using the Wilcoxon signed-rank test. Categorical variables were presented as count (n) and percentage (%) and compared using Fisher’s exact test or the chi-square test. If a difference in patient characteristics between the two groups was statistically significant, propensity score matching (PSM) was to be used to balance the bias. All analyses were processed using SPSS 23.0 software (IBM), and the significance level was defined as two-sided α = 0.05.
Sensitivity analyses were performed to examine the stability and robustness of the results. First, a logistic regression model was used to control confounding factors, and generalized linear regression was used to identify the influence factors on direct medical costs. Second, the bootstrap method was used for repeated sampling (1,000 times) with replacement, and a cost-effectiveness acceptable curve (CEAC) was drawn according to the sampling results.
Patient characteristics are summarized in Table 1. A total of 291 patients were identified, 148 in the budesonide group and 143 in the methylprednisolone group. As all characteristics were balanced between the two groups, PSM was not used.
Blood gas analysis was carried out for 114 patients, 58 in the budesonide group and 56 in the methylprednisolone group. The results show that PaCO2 and PaO2 levels improved significantly in both groups. However, after treatment, no significant differences in pH, PaO2, or PaCO2 levels were found between the groups (Table 2).
Pulmonary function tests were carried out for 70 patients. The results show that the FEV1 and FEV1/FVC levels improved significantly in both groups. However, after treatment, there were no significant differences in the FEV1 and FEV1/FVC levels between the groups (Table 2).
The clinical efficacy rates of the budesonide and methylprednisolone groups were 94.6% and 93.7%, respectively, and there was no statistical difference between the groups (p = 0.747) (Table 2).
The rate of no readmission within 1 year after discharge in the budesonide group was significantly higher than in the methylprednisolone group (74.3% vs. 60.1%, χ2 = 6.655, p = 0.010) (Table 2).
During hospitalization, adverse events that occurred in the two groups were hyperglycemia, oropharynx fungal infection, sleep disorder, and stomach discomfort. Although the rate of adverse events was significantly higher in the methylprednisolone group than in the budesonide group, all the events were relatively mild, and no special treatment was given (Table 3).
After discharge, there were no adverse events occurred in the two groups.
As there was no statistical difference between the groups in clinical efficacy rates, cost-minimization analysis (CMA) was used. Total costs and a breakdown of the categories are given in Table 4. For most of the costs, including medication, examinations, laboratory tests, treatment, diagnosis, nursing, rehabilitation, and medical consumables, there were no significant differences between the groups. The total costs were significant higher for the budesonide group than for the methylprednisolone group (20460.56 CNY vs. 17297.28 CNY, p = 0.037). Therefore, the CMA results suggest that intravenous methylprednisolone is more economical than nebulized budesonide in the treatment of AECOPD.
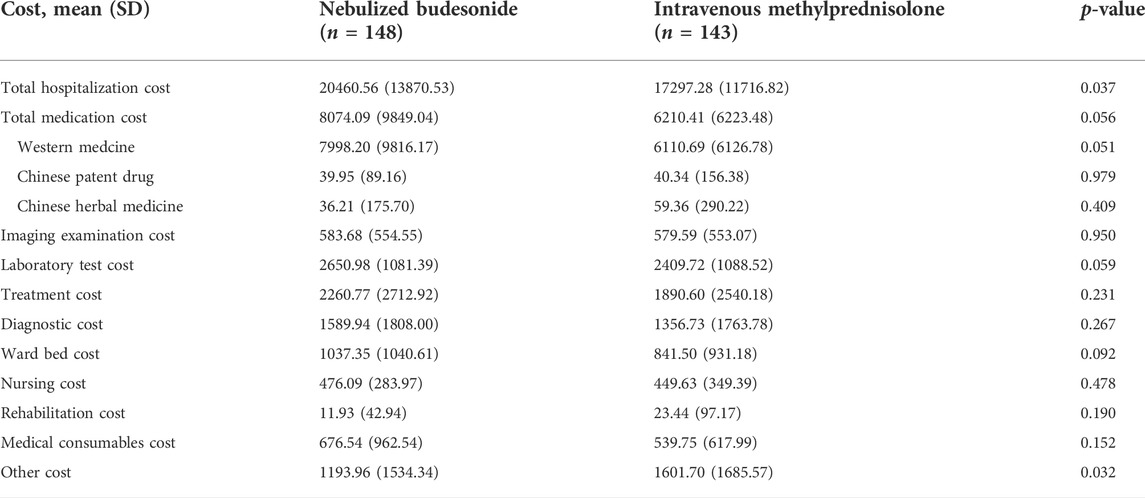
TABLE 4. Comparison of hospitalization costs (CNY) for nebulized budesonide and intravenous methylprednisolone groups.
The rate of no readmission within 1 year after discharge was then adopted as the effect indicator, and a cost-effectiveness analysis (CEA) was used. The results are shown in Table 5. The ICER value of the budesonide group was 22276.62 CNY; thus, compared with intravenous methylprednisolone, nebulized budesonide cost 22276.62 CNY extra and saved one readmission within 1 year after discharge.
For the logistic regression analysis, clinical efficacy and readmission within 1 year after discharge were used as dependent variables, and all possible influencing factors were included as independent variables. Consistent with the results of the basic analysis, the choice of budesonide or methylprednisolone as the main drug had no significant effect on clinical efficacy but had a significant effect on readmission within 1 year after discharge (Table 6).
Because the costs did not follow a normal distribution, generalized linear model analyses were used to examine the associated factors for direct medical and medication costs. Total hospitalization and total medication costs were taken as dependent variables respectively, and all possible influencing factors were included as independent variables. The gamma distribution was used to perform generalized linear regression with identity as the connection function. The results indicate that patients treated with budesonide had greater total hospitalization and total medication costs (Table 7).
Because this study is retrospective, bootstrapping was used to reduce sampling error, and a CEAC was drawn. When the clinical efficacy rate was used as the effect indicator, the results show that the probability of methylprednisolone being cost-effective at a willingness to pay (WTP) of 40,000 CNY was more than 90% (Figure 1). However, when the rate of no readmission within 1 year was used as the effect indicator, and the WTP was more than 23,000 CNY, nebulized budesonide became cost-effective (Figure 2). These findings are consistent with the results of the basic analysis, which indicates that the analysis is robust.
To our knowledge, this is the first study to use real-world patient-level data to investigate the differences in clinical effectiveness and pharmacoeconomics between nebulized budesonide and intravenous methylprednisolone in the treatment of AECOPD. There are three main findings.
First, for hospitalized patients with AECOPD, nebulized budesonide and intravenous methylprednisolone both improved lung function and blood gas analysis and had similar clinical efficacy. These results are in agreement with the findings of Ding et al. (2016) and the recommendations of expert consensus in China (Cai et al., 2014).
Second, in addition to clinical efficacy, this study also explored the effect of the two interventions in reducing the risk of readmission, which is an important indicator for evaluating the long-term effect on COPD patients. Readmission rates within 1 year after discharge were relatively high for both groups, at 25.7% in the budesonide group and 39.9% in the methylprednisolone group. A history of exacerbations is the most reliable predictor of exacerbations in COPD patients (Donaldson and Wedzicha, 2006; Hurst et al., 2010) and the patients in this study were therefore likely to be readmitted for acute exacerbations. This result may therefore be related to the frequent-exacerbation phenotype of hospitalized patients, especially in China, where most patients are already in group D according to the GOLD guidelines when they first visit a doctor.
Third, in economic terms, when clinical efficacy was used as the short-term efficacy indicator, the CMA results indicated that intravenous methylprednisolone was cost-effective due to lower total costs. However, from a long-term perspective, the CEA results indicated that nebulized budesonide cost 22,276.62 CNY extra compared with intravenous methylprednisolone, while saving one readmission within 1 year after discharge. Therefore, an average cost of one hospitalization for an acute exacerbation can be used as the threshold for WTP. A number of studies have found that AECOPD contributes significantly to the costs of COPD (Rutten-van Mölken et al., 1999; Hilleman et al., 2000; Miravitlles et al., 2002; Toy et al., 2010). In China, a large-scale retrospective study conducted by Liang et al. found that mean expenditure per admission increased from 19,760 CNY in 2009 to 20,118 CNY in 2017 (a growth rate of 0.11%) (Liang et al., 2020). On this trend, by 2021 mean expenditure per admission would have reached 20,206.20 CNY. In terms of willingness to pay, nebulized budesonide was not cost-effective compared with intravenous methylprednisolone because the ICER (22,276.62 CNY) was higher than the WTP (20,206.20 CNY).
A key strength of the present study is that it uses real-world data that are likely to provide reasonably good estimates of absolute event probabilities and costs in actual clinical practice. In addition, for a more comprehensive assessment, its economic evaluation includes different effect indicators, namely, clinical efficacy and no readmission within 1 year after discharge, which correspond to short-term and long-term effects, respectively.
However, the limitations of this study should be noted. First, because it is a single-center retrospective study, the sample size is small and the data identified from the electronic medical record database were incomplete. Second, the analysis focused on direct medical costs and paid no attention to direct nonmedical or indirect costs, which may have influenced the results. Third, the use of mean expenditure per admission, without considering the negative impact of readmission on patients’ lung function and quality of life, may have led to an underestimation of the WTP threshold.
In conclusion, compared with intravenous methylprednisolone, nebulized budesonide is not a cost-effective strategy in terms of either short-term or long-term effect. Large-scale multicenter studies are required to validate the findings of this study.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Concept and design of the study by Y-LG, Z-XS, HG, and YW. Data collection and management of the study by Y-LG, CC, D-LJ, and YS. Statistical analysis by Y-LG, CC, YS, and XG. Drafting of the manuscript by Y-LG and YS. All authors contributed to the article and approved the submitted version.
This work was supported by the Young Talents Fund of the First People’s Hospital of Lianyungang (grant number QN202010).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Cai, B. Q., Cai, S. X., Chen, R. C., Cui, L. Y., Feng, Y. L., Gu, Y. T., et al. (2014). Expert consensus on acute exacerbation of chronic obstructive pulmonary disease in the People's Republic of China. Int. J. Chron. Obstruct. Pulmon. Dis. 9, 381–395. doi:10.2147/copd.S58454
Celli, B. R., and MacNee, W.ATS/ERS Task Force (2004). Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur. Respir. J. 23 (6), 932–946. doi:10.1183/09031936.04.00014304
Decramer, M., Lacquet, L. M., Fagard, R., and Rogiers, P. (1994). Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am. J. Respir. Crit. Care Med. 150 (1), 11–16. doi:10.1164/ajrccm.150.1.8025735
Ding, Z., Li, X., Lu, Y., Rong, G., Yang, R., Zhang, R., et al. (2016). A randomized, controlled multicentric study of inhaled budesonide and intravenous methylprednisolone in the treatment on acute exacerbation of chronic obstructive pulmonary disease. Respir. Med. 121, 39–47. doi:10.1016/j.rmed.2016.10.013
Donaldson, G. C., Seemungal, T. A., Bhowmik, A., and Wedzicha, J. A. (2002). Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 57 (10), 847–852. doi:10.1136/thorax.57.10.847
Donaldson, G. C., and Wedzicha, J. A. (2006). COPD exacerbations .1: Epidemiology. Thorax 61 (2), 164–168. doi:10.1136/thx.2005.041806
GBD 2017 Causes of Death Collaborators (2018). Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the global burden of disease study 2017. Lancet 392 (10159), 1736–1788. doi:10.1016/S0140-6736(18)32203-7
Global (2021) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2022 report. Available from: https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf. [Accessed February 04 2022].
Henzen, C., Suter, A., Lerch, E., Urbinelli, R., Schorno, X. H., and Briner, V. A. (2000). Suppression and recovery of adrenal response after short-term, high-dose glucocorticoid treatment. Lancet 355 (9203), 542–545. doi:10.1016/s0140-6736(99)06290-x
Hilleman, D. E., Dewan, N., Malesker, M., and Friedman, M. (2000). Pharmacoeconomic evaluation of COPD. Chest 118 (5), 1278–1285. doi:10.1378/chest.118.5.1278
Hurst, J. R., Vestbo, J., Anzueto, A., Locantore, N., Müllerova, H., Tal-Singer, R., et al. (2010). Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 363 (12), 1128–1138. doi:10.1056/NEJMoa0909883
Kwong, F. K., Sue, M. A., and Klaustermeyer, W. B. (1987). Corticosteroid complications in respiratory disease. Ann. Allergy 58 (5), 326–330.
Leuppi, J. D., Schuetz, P., Bingisser, R., Bodmer, M., Briel, M., Drescher, T., et al. (2013). Short-term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: The REDUCE randomized clinical trial. JAMA 309 (21), 2223–2231. doi:10.1001/jama.2013.5023
Liang, L., Shang, Y., Xie, W., Shi, J., Tong, Z., and Jalali, M. S. (2020). Trends in hospitalization expenditures for acute exacerbations of COPD in beijing from 2009 to 2017. Int. J. Chron. Obstruct. Pulmon. Dis. 15, 1165–1175. doi:10.2147/copd.S243595
Lozano, R., Naghavi, M., Foreman, K., Lim, S., Shibuya, K., Aboyans, V., et al. (2012). Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the global burden of disease study 2010. Lancet 380 (9859), 2095–2128. doi:10.1016/s0140-6736(12)61728-0
Miravitlles, M., Murio, C., Guerrero, T., and Gisbert, R.DAFNE Study Group. Decisiones sobre Antibioticoterapia y Farmacoeconomia en la EPOC (2002). Pharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPD. Chest 121 (5), 1449–1455. doi:10.1378/chest.121.5.1449
Rutten-van Mölken, M. P., Postma, M. J., Joore, M. A., Van Genugten, M. L., Leidl, R., and Jager, J. C. (1999). Current and future medical costs of asthma and chronic obstructive pulmonary disease in The Netherlands. Respir. Med. 93 (11), 779–787. doi:10.1016/s0954-6111(99)90262-7
Seemungal, T. A., Donaldson, G. C., Paul, E. A., Bestall, J. C., Jeffries, D. J., and Wedzicha, J. A. (1998). Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 157 (5), 1418–1422. doi:10.1164/ajrccm.157.5.9709032
Soler-Cataluña, J. J., Martínez-García, M. A., Román Sánchez, P., Salcedo, E., Navarro, M., and Ochando, R. (2005). Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 60 (11), 925–931. doi:10.1136/thx.2005.040527
Toy, E. L., Gallagher, K. F., Stanley, E. L., Swensen, A. R., and Duh, M. S. (2010). The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: A review. Copd 7 (3), 214–228. doi:10.3109/15412555.2010.481697
Vogelmeier, C. F., Criner, G. J., Martinez, F. J., Anzueto, A., Barnes, P. J., Bourbeau, J., et al. (2017). Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir. Crit. Care Med. 195 (5), 557–582. doi:10.1164/rccm.201701-0218PP
Vos, T., Flaxman, A. D., Naghavi, M., Lozano, R., Michaud, C., Ezzati, M., et al. (2012). Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the global burden of disease study 2010. Lancet 380 (9859), 2163–2196. doi:10.1016/s0140-6736(12)61729-2
Keywords: budesonide, methylprednisolone, chronic obstructive pulmonary disease, acute exacerbation, minimum cost analysis, cost-effectiveness analysis
Citation: Gu Y-L, Sun Z-X, Sun Y, Wen Y, Guan X, Jiang D-L, Cheng C and Gu H (2022) A real-world cost-effectiveness analysis of nebulized budesonide and intravenous methylprednisolone in acute exacerbation of chronic obstructive pulmonary disease. Front. Pharmacol. 13:892526. doi: 10.3389/fphar.2022.892526
Received: 09 March 2022; Accepted: 04 August 2022;
Published: 13 September 2022.
Edited by:
Chris Gillette, Wake Forest School of Medicine, United StatesReviewed by:
Sergey Avdeev, I.M. Sechenov First Moscow State Medical University, RussiaCopyright © 2022 Gu, Sun, Sun, Wen, Guan, Jiang, Cheng and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huan Gu, NDcwMzQ5MDc0QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.