- 1Department of Cardiovascular Medicine, The Second Xiangya Hospital of Central South University, Changsha, China
- 2St. Vincent’s Hospital, Sydney, NSW, Australia
A Corrigendum on
The Transition From Ambrisentan to Macitentan in Patients With Pulmonary Arterial Hypertension: A Real-Word Prospective Study
by Chen, Y., Luo, J., Chen, J., Kotlyar, E., Li, Z., Chen, W. and Li, J. (2022). Front Pharmacol. 12:811700. doi:10.3389/fphar.2021.811700
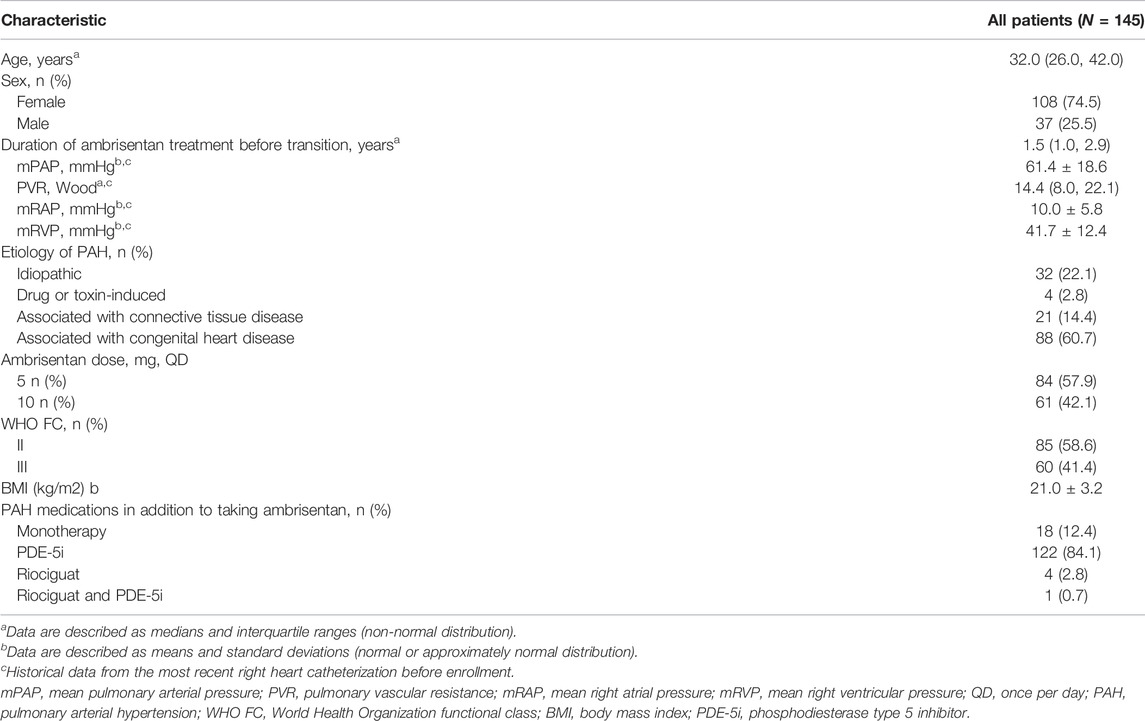
In the original article, there was a mistake in Table 1 and in the Section Results, Patients’ Clinical Characteristics as published. “There were errors in the values for the ‘Ambrisentan dose’ as it was reported that one hundred and twenty-eight patients (88.3%) were receiving 10 mg once per day, whereas seventeen patients (11.7%) were receiving 5 mg once daily. This was corrected to sixty-one patients (42.1%) were receiving 10 mg once per day, and eighty-four patients (57.9%) were receiving 5 mg once daily.” The corrected Table 1 appears below and the text in the Section Results, Patients’ Clinical Characteristics has been updated.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: pulmonary arterial hypertension, macitentan, ambrisentan, endothelin receptor antagonist, real-word study
Citation: Chen Y, Luo J, Chen J, Kotlyar E, Li Z, Chen W and Li J (2022) Corrigendum: The Transition From Ambrisentan to Macitentan in Patients With Pulmonary Arterial Hypertension: A Real-Word Prospective Study. Front. Pharmacol. 13:891907. doi: 10.3389/fphar.2022.891907
Received: 08 March 2022; Accepted: 15 March 2022;
Published: 11 April 2022.
Edited and reviewed by:
Kevin Lu, University of South Carolina, United StatesCopyright © 2022 Chen, Luo, Chen, Kotlyar, Li, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiang Li, bGlqaWFuZ2NzQGNzdS5lZHUuY24=
 Yusi Chen1
Yusi Chen1 Jingyuan Chen
Jingyuan Chen Jiang Li
Jiang Li