- 1Department of Clinical Medicine and Surgery, University of Naples “Federico II”, Naples, Italy
- 2Department of Translational Medical Science, University of Naples “Federico II”, Naples, Italy
- 3Department of Public Health, University of Naples Federico II, Naples, Italy
Background: In more than 90% of chronic viral hepatitis C (HCV) patients treated with direct-acting antiviral agents (DAAs), a sustained viral response (SVR) was observed. Unfortunately, there are subgroups of subjects who display enduring liver fibrosis and are at high risk of developing hepatocellular carcinoma (HCC). Thus, liver fibrosis evaluation during the follow-up of these patients plays a pivotal role. The gold standard to evaluate hepatic fibrosis is liver biopsy, which is an invasive procedure. Imaging techniques and serum biomarkers have been proposed as safer and cheaper procedures.
Objectives: In this study, we evaluated the concordance of transient elastography (TE) with ELF score ( enhanced liver fibrosis) in a cohort of patients with HCV before and after direct-acting antiviral (DAAs) treatment. ELF score has been validated in other chronic liver diseases; the evidence is not available in HCV patients treated with DAAs.
Study design: We prospectively recruited all consecutive HCV patient candidates for DAAs therapy at the University of Naples “Federico II” between April 2015 and July 2016. TE and ELF scores were assessed at baseline, at SVR24, and at SVR48.
Results: One-hundred-nineteen patients were treated with DAAs, and 94.1% of them reached SVR. A total of 55.5% of patients were males with a mean age of 64.7 ± 9.6 years. TE results revealed that 12 patients (10%) had F1-2 mild/moderate fibrosis, and 107 (90%) had F3-4 advanced fibrosis. At baseline, SVR24, and SVR48, the concordance between ELF test and TE was poor: 0.11 (p = 0.086), 0.15 (p = 0.124), and 0.034 (p = 0.002), respectively. However, at SVR24 and SVR48, both methods showed a significant amelioration of liver fibrosis compared to baseline (p < 0.001). In addition, both ELF index and TE were significantly associated with portal hypertension at baseline, but not with varices and ascites.
Conclusions: Our findings suggested that ELF test could predict changes in liver fibrosis, independently of TE. In case of TE unavailability, ELF score could represent an appropriate tool. Notably, in the context of the COVID-19 pandemic, ELF testing should be encouraged to reduce unnecessary access to the hospital and prolonged physical contact.
Introduction
Direct-acting antiviral agents (DAAs) represent a milestone in the clinical management of chronic hepatitis C virus (HCV) patients. More than 90% of these subjects reach sustained virologic response (SVR) and effectively recover (Kowdley et al., 2014; Lawitz et al., 2014; Muir et al., 2015). However, in some patients, liver inflammation and fibrosis persist after DAA treatment (Putra et al., 2018), and SVR might not correlate with a reduced risk of hepatocellular carcinoma (HCC) (Sapena et al., 2022). Therefore, assessment of liver fibrosis in chronic hepatitis C virus (HCV) infection is crucial to monitor the response to treatment, the progression of liver damage, and to program an adequate follow-up (Carmona et al., 2016).
The current gold standard to evaluate hepatic fibrosis is liver biopsy, an invasive procedure, poorly tolerated by patients and carrying a small but significant risk of complications. Moreover, the specimen of liver biopsy can be limited by sampling error, and with a significant intra- and inter-observer variability in the assessment of fibrosis stages (Regev et al., 2002; Rockey et al., 2009). In a study, up to 10% variability in the staging of the same specimen after repeated assessments by a single observer was reported (Vuppalanchi et al., 2009). Not surprisingly, noninvasive tests (NTIs) have been identified and validated to indirectly estimate liver fibrosis (Krag et al., 2022). NTIs to identify fibrosis stage allow performing serial follow-up of patients or, in the case of viral hepatitis, to assess therapy response (Stasi and Milani, 2016; Wong, 2018). NTIs comprise imaging techniques and circulating biomarkers (Sharma et al., 2014a). Among imaging approaches, transient elastography (FibroScan®) is one of the most commonly used, showing a high correlation to liver biopsy for staging hepatic fibrosis (Arena et al., 2008) and providing accurate diagnostic and prognostic information (Castera, 2011; Fernandez et al., 2015). Unfortunately, its availability is scarce and requires trained personnel (Fraquelli et al., 2007; Castera et al., 2010). This technique has several limitations, including the cost of the equipment and the lack of standardized cut-offs for the diagnosis of fibrosis stages. Despite these limitations, TE is currently the second-best option for staging hepatic fibrosis.
Serum biomarkers and biomarker scores have also been proposed for the assessment of fibrosis. Biomarker scores are highly reproducible, cost-effective, and do not require trained personnel or expensive instruments (Wong, 2018).
The enhanced liver fibrosis (ELF) score is one of the most widely studied (Patel et al., 2020; Abdel-Hameed et al., 2021). The algorithm of ELF score combines three serum markers of fibrosis: hyaluronic acid (HA); amino-terminal propeptide of type-III-procollagen (PIIINP); and tissue inhibitor of metalloproteinase type-1 (TIMP-1).
Elevated HA levels reflect increased production of HA within a fibrotic liver or a reduced clearance. Elevated TIMP-1 levels were observed in alcoholic patients with fibrosis and cirrhosis. PIIINP (amino-terminal of serum procollagen Ⅲ peptide) is a marker of collagen turnover. Increased levels occur in tissue repair and fibrosis (Sharma et al., 2014b).
Overall, these biomarkers are involved directly in the synthesis and degradation of hepatic extracellular matrix (Patel et al., 2020); thus, it can be assumed that ELF score more directly mirror the extracellular matrix (ECM) turnover, the central event of hepatic fibrosis.
So far, ELF score has been validated in patients with chronic liver diseases, non-alcoholic steatohepatitis (NASH), hepatitis B, and hepatitis C (Guha et al., 2008; Parkes et al., 2010; Parkes et al., 2011; Trembling et al., 2014; Fernandes et al., 2015; Thiele et al., 2018).
No evidence is available on ELF score in subjects with HCV treated with DAAs. In this study, we evaluated ELF score to assess the modification of liver fibrosis in patients with HCV chronic hepatitis before and after DAAs treatment. TE was used as a reference assay to assess liver fibrosis, as most patients were not compliant with liver biopsy.
Patients and methods
Study design and patient population
From April 2015 to July 2016, 119 patients with a diagnosis of chronic hepatitis HCV treated with IFN-free DAA (direct-acting antiviral) regimens were prospectively and consecutively enrolled at the Liver Unit of the University of Naples “Federico II.” The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the local ethics board of the promoting center (Federico II University of Naples, n 245/2013). All patients and controls involved in the study provided written informed consent to participate. Exclusion criteria included patients with current or past HCC and history of other malignancies, HBV or HIV co-infection, liver transplant recipients, and pregnancy or breastfeeding.
Demographic and laboratory data, comorbidities, and information regarding liver disease were collected. All patients were treated with DAA regimens available according to AISF (Italian Association for the Study of Liver) and EASL (European Association for the Study of the Liver) guidelines (European, 20182018). DAA regimens employed for the treatment of HCV infection are shown in Supplementary Table S1.
Liver fibrosis was evaluated with both TE and ELF tests at baseline, 24 weeks after DAA treatment (SVR24), and 48 weeks after DAA treatment (SVR48). Enrolled patients submitted to TE by Fibroscan® and to a fasting blood sample on the same days. Patients were divided into two groups, according to TE: the F1-F2 group with mild/moderate fibrosis and the F3-F4 group with advanced fibrosis.
Portal hypertension was assessed considering the direct presence of gastroesophageal varices, portal hypertensive gastropathy, and/or indirect (liver stiffness >25 kPa, splenomegaly, and thrombocytopenia) signs of clinically significant portal hypertension (CSPH).
The study protocol was approved by the Ethics Committee of the University of Naples “Federico II.” All the study’s procedures were conducted according to the provisions of the Declaration of Helsinki and Good Clinical Practice Guidelines.
Transient elastography measurements
Liver stiffness measurements (LSM) were performed by a single well-trained operator using a TE-FibroScan instrument (502Touch, EchosenseTM, Paris, France). The results were expressed in kiloPascals (kPa) with a range from 2.5 to 75 kPa. IQR was defined as an index of the intrinsic variability of LSM. Only those measurements with more than ten successful acquisitions, with a success rate of at least 60% and an interquartile range lower than 30%, were classified as valid and taken into consideration for statistical evaluation (Nitta et al., 2009).
In HCV patients, LSM correlates strongly with METAVIR fibrosis stages (de Ledinghen and Vergniol, 2008). In this study, the F1-F2 group was defined by LSM <10 kPa, while the F3-F4 group was identified by LSM >10 kPa.
Enhanced liver fibrosis test
Fasting blood samples were obtained. All sera were frozen and stored at −20°C until measurement. Samples were assayed in an automated analyzer that performs magnetic separation enzyme immunoassay tests (ADVIA Centaur; Siemens Healthcare Diagnostics, Tarrytown, NY). Results were entered into the manufacturer’s published algorithm to derive an ELF score [ELF = 2.278 + 0.851 ln (HA) + 0.751 ln (PIIINP) +0.394 ln (TIMP-1)].
The cutoff points suggested by the manufacturer were <7.7, none to mild fibrosis; 7.7 to <9.8, moderate fibrosis; and >9.8, severe fibrosis (Day et al., 2019). In our study, the F1-F2 group was defined by ELF score values <9.8 and the F3-F4 group by values >9.8.
Statistical analysis
All statistical analyses were performed using R Language for Statistical Computing (version 4.0.3). Continuous variables were expressed as mean ± standard deviation (SD) with range or, in the case of skewed variables, as median (25th and 75th percentile) with range; qualitative variables were reported as absolute frequency and percentage. Accordingly, between-group comparisons were based either on the t-test for independent samples and the Mann–Whitney U-test or the chi-square test and the Fisher exact test (when appropriate). The concordance between TE and ELF scores was assessed both by Spearman correlation coefficient and by Cohen's kappa with the corresponding 95% confidence interval (95% CI). Assessment of time trends in the severity of fibrosis was based on the McNemar test for paired samples. All tests were two-sided with p-value < 0.05 denoting statistical significance.
Results
General characteristics of the study population
One-hundred-nineteen patients were enrolled and treated with DAAs from April 2015 to July 2016 at the Liver Unit of University Hospital of Naples Federico II. Most of them (94.1%) showed sustained virological response (SVR). Baseline demographic, clinical, and laboratory characteristics of the patients, stratified in the F1-F2 and F3-F4 groups according to TE values, are summarized in Table 1. According to liver fibrosis, at baseline, 12/119 (10.1%) patients were in the F1-F2 group and 107/119 (89.9%) in the F3-F4 group. The different distribution was due to HCV treatment criteria effective at the time of the enrollment. A total of 55.5% of patients were males with a mean age of 64.7 ± 9.6 (range: 31.1–81.9) years at the start of therapy. The mean BMI was 26.5 ± 3.6 kg/m2. The age and BMI were significantly higher in the F3-F4 group (p = 0.031 and p = 0.032, respectively) than those in the F1-F2 group. Seventy (58.8%) patients were interferon experienced without a significant difference between the two groups. As expected, at baseline, in the F3-F4 group, alfa-fetoprotein (AFP) levels were higher than in the F1-F2 group (p = 0.003), while albumin levels were significantly lower (p = 0.036).
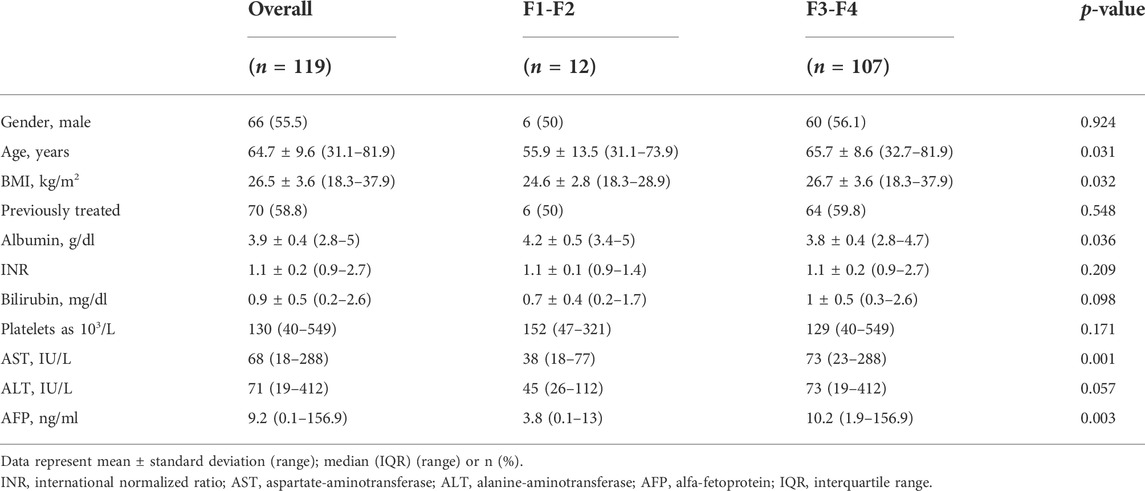
TABLE 1. Baseline demographic, clinical, and laboratory characteristics of 119 patients; overall and stratified to liver fibrosis according to TE values.
Enhanced liver fibrosis and transient elastography concordance
One-hundred-four patients were considered for the analysis, and 15 patients were excluded as both tests were not available.
When considering ELF and TE measures in their original numerical scale, a weak, although significant, correlation was observed in all time points (baseline: r = 0.335, p < 0.001; SVR24: r = 0.347, p < 0.001; SVR48: r = 0.332, p = 0.002).
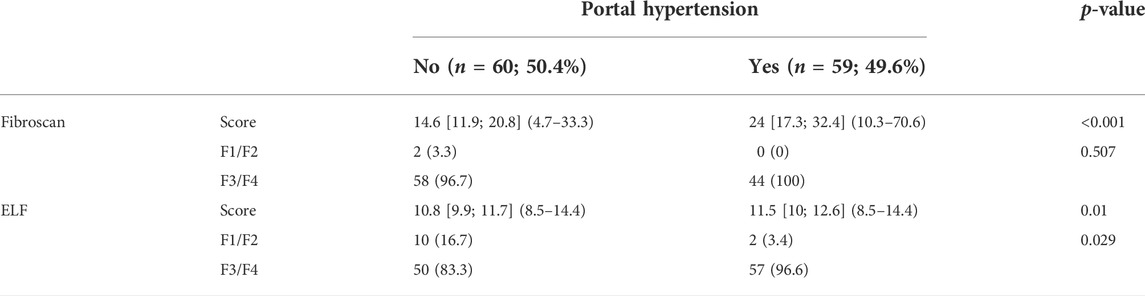
At baseline, ELF score and TE showed a poor concordance, with Cohen’s kappa coefficient of 0.11 (95% confidence interval −0.11 to 0.35; p-value = 0.086) (Table 2). In particular, 102 (98.1%) patients showed, at baseline, advanced liver fibrosis according to TE, whereas 92 (88.5%) subjects had severe liver fibrosis, according to ELF test.
At SVR24, the concordance between the two methods remained poor, similar to that at baseline, with Cohen’s kappa coefficient of 0.15 (95% confidence interval −0.05 to 0.34; p-value = 0.124) (Table 2). Notably, 68/104 (65.4%) patients were classified as having severe liver fibrosis at TE, and 76/104 (73.1%) showed the same grade of liver fibrosis on the ELF test. Regarding F1-F2 fibrosis, 36 (34.6%) and 28 (26.9%) patients showed mild/moderate fibrosis at TE and ELF, respectively.
At SVR48, the concordance between ELF and TE increased to 0.34 (95% CI: 0.12 to 0.50; p = 0.003) (Table 2), with 22 (25.3%) patients showing a mild/moderate fibrosis and 35 (40.2%) a severe fibrosis according to both ELF and TE.
Time trends of enhanced liver fibrosis and transient elastography from baseline to SVR24 and SVR48
To evaluate time trends of the fibrosis, the analysis was conducted on 96 subjects who performed TE at baseline and at SVR24 and in 119 patients with ELF determination for both times.
Both TE values and ELF test results showed a significant reduction at SVR24 compared to baseline, according to the amelioration of liver fibrosis after DAA therapy (Figure 1A).
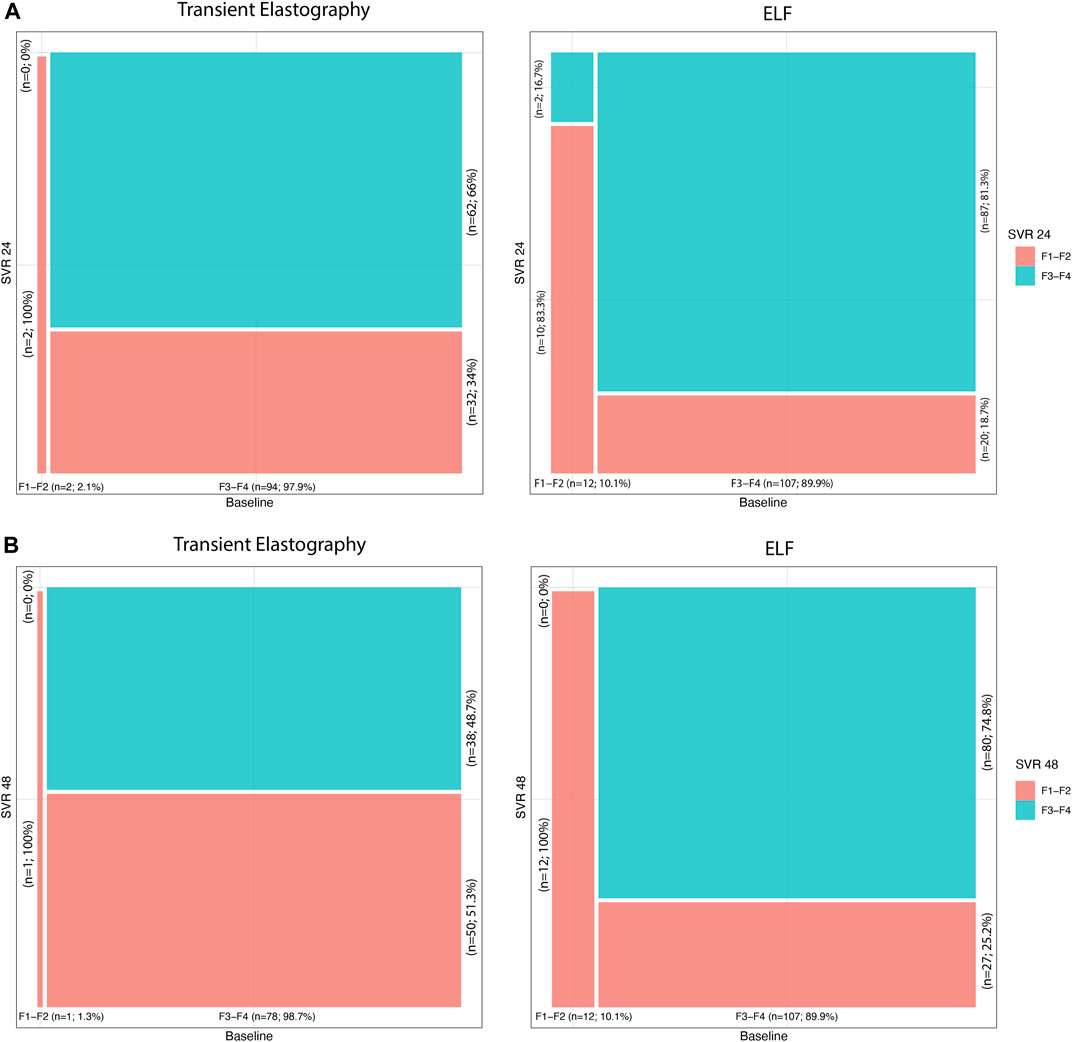
FIGURE 1. (A) Transient elastography and ELF time trends from baseline to SVR24; (B) transient elastography and ELF time trends from baseline to SVR48.
At baseline, two (2.1%) patients showed mild/moderate fibrosis and 94 (97.9%) subjects showed advanced fibrosis, according to TE. Instead, at SVR24, 34 (35.4%) patients had F1-F2 values, and 62 (64.6%), F3-F4 fibrosis, with statistically significant variation from baseline (p-value <0.001).
Similar amelioration of liver fibrosis was detected also considering the ELF test. Indeed, at baseline, 12 (10.1%) patients showed F1-F2, and 107 (89.9%), F3-F4 fibrosis, while at SVR24, 30 (25.2%) and 89 (74.8%) patients showed mild/moderate and advanced fibrosis, respectively. The decline of ELF was significant (p-value <0.001) compared to baseline.
In addition, at SVR48 119 patients were evaluated with ELF scores, and 79 patients, with TE. At baseline, 1 (1.3%) of those patients showed mild/moderate fibrosis, and 78 (98.7%) subjects, advanced fibrosis, according to TE. After 48 weeks of DAA treatment (Figure 1B), 41 (51.9%) patients had F1-F2 values, and 38 (48.1%), F3-F4 fibrosis, with statistically significant variation from baseline (p-value <0.001). As for SVR24, the ELF score showed an improvement in the patient’s liver fibrosis. At SVR48, 39 (32.8%) and 80 (67.2%) patients showed mild/moderate and advanced fibrosis, respectively. The decrease in ELF score value was significant (p-value <0.001) compared to baseline.
Enhanced liver fibrosis score and transient elastography in patients with portal hypertension, varices, and ascites
As shown in Table 3, both TE and ELF scores were significantly elevated in HCV patients with PH in comparison to subjects without PH at basal p < 0.001 and p = 0.010, respectively. Severe fibrosis (F3/F4) was significantly more prevalent in HCV patients with PH based on ELF score (p = 0.029), but not on TE (p = 0.507). Conversely, there was no significant difference in TE and ELF scores between HCV patients with varices and ascites (data not shown).
Discussion
Liver health is a major concern in HCV-infected patients (Monga et al., 2001). Thus, an accurate assessment of liver fibrosis degree is required for clinical decision-making. Liver biopsy is the gold standard (Rockey et al., 2009); however, this is an invasive procedure, with significant variability and a substantial lack of standardization. TE and serological tests represent promising alternative strategies to classify liver fibrosis degree (Day et al., 2019; Ueda et al., 2020). However, TE availability is scarce and requires expensive equipment and trained personnel (17,18). On the other hand, serum biomarkers are inexpensive, safe, and highly reproducible. Studies are required to better evaluate their use in different clinical settings.
In this study, we evaluated the ability of the noninvasive ELF score against the TE to reflect liver fibrosis degree in a cohort of HCV chronic hepatitis before and after treatment with DAAs. The ELF score demonstrated a significant association with stages of liver fibrosis. The score can reliably classify F1-F2 as mild/moderate fibrosis and F3-F4 as advanced fibrosis both at baseline and after therapy.
ELF score directly measures the ECM turnover, and we observed that the ELF score changed in a linear manner with fibrosis degree, indicating its value as a suitable prognostic biomarker to monitor the progression or regression of fibrosis. Accordingly, previous studies showed that ELF test can identify advanced liver fibrosis with good accuracy (Wahl et al., 2012; Lichtinghagen et al., 2013; Agbim and Asrani, 2019). Lichtinghagen et al. (2013) showed that there was a considerable overlap of ELF values, especially in F1-F2, because this test has the highest sensitivity to rule out cirrhosis and not identify the intermediate degree of fibrosis. They sustained that this result suggests that extracellular matrix turnover had a higher influence on fibrosis in moderate stages of liver disease. Therefore, ELF, evaluating markers involved in the synthesis and degradation of extracellular matrix, does not clearly evaluate the intermediate degree of liver fibrosis. Wahl et al. (2012) also showed that both TE and ELF had the highest diagnostic accuracy in predicting advanced fibrosis. Thus, to better discriminate intermediate stages of fibrosis, Agbim and Asrani (2019) suggested the combination of noninvasive serum tests and imaging approaches. Notably, Sherman et al. (2019) showed that patients treated with a blockade agent reduced their ELF scores over time with respect to controls (Sherman et al., 2019).
Several authors reported that ELF score was a predictor of liver disease outcome in subjects with chronic pathologies (Parkes et al., 2010; Day et al., 2019) and of mortality in HIV/HCV-coinfected women (Peters et al., 2016).
The identification of severe hepatic fibrosis is fundamental in HCV-treated patients since cirrhotic patients need to be managed in a manner different from that of non-cirrhotic subjects. Indeed, cirrhotic patients must perform semi-annual ultrasound follow-up for HCC screening, endoscopic surveillance for portal hypertension, and outpatient visits to detect early signs of hepatic decompensation (European, 2018).
It is well known that HCV patients treated with DAAs showed a significant regression of liver fibrosis as a consequence of reduced inflammation linked to the elimination of viral replication (Chekuri et al., 2016; Facciorusso et al., 2018; Rout et al., 2019). In our cohort, both ELF score and TE point out the improvement of liver fibrosis at SVR24 compared to baseline, with a significant time variation at both SVR24 and 48. The percentage of patients who improved ELF scores during follow-up was higher than the percentage of patients who improved liver stiffness. This result suggests that ELF score could detect the DAA-related improvement of liver function before liver stiffness. Our data indicate that ELF score could be useful for the follow-up of these patients. A further larger study population need to be carried out in order to clearly assess the sequential use of ELF and TE.
DAAs are highly effective and well-tolerated and require shorter treatment duration and simpler administration leading to a simplification of HCV treatment, which includes reduced testing for HCV RNA load. In the context of the “simplification era” of DAAs, ELF could play a key role to estimate liver fibrosis stage, avoiding the use of more expansive and time-consuming liver fibrosis monitoring techniques. It is worth noting that ELF score is calculated on instruments used for routine tests, available in several laboratories in many countries. Therefore, it is not necessary to purchase expensive instruments and staff training, and it is sufficient for the availability of ELF score kits to perform the assay.
A likely scenario could be the use of ELF as a screening test in the primary care setting. In the case of ELF negative results, the risk of high-degree fibrosis is low. Conversely, if the ELF test is positive for advanced liver fibrosis, the patient should be taken to the hospital and to undergo further expensive and invasive procedures. ELF score could be useful for the follow-up in patients with high BMI, ascites, severe hepatic inflammation, and hepatic congestion, where TE showed failure rates ranging from 6% to 23% (Horowitz et al., 2017; Agbim and Asrani, 2019).
Finally, ELF score helps avoid unnecessary contacts between the operator and the patients. This scenario fits well with the current SARS-CoV-2 pandemic. In the last 2 years, the diagnosis and treatment of HCV infections have been frequently missed with a high impact in the next years on deaths due to HCV liver diseases and with lengthening of times required to eliminate HCV, as recommended by the WHO (Kondili et al., 2021). Thus, it is essential to start again with the path of eradication of HCV and at the same time, implement all the necessary security measures and avoid unnecessary contacts. The use of this “biological” approach instead of a “physical approach” for the measurement of liver fibrosis in HCV patients can reduce the physical contact between the patients and the medical staff while obtaining an adequate stratification of the fibrosis stage to program a personalized follow-up. Furthermore, the ELF test is a cost-effective, readily available method in low-income countries as well, where HCV infection is more prevalent. Indeed, the requirement of a blood sample and no need for expensive investments in exclusive equipment give a chance to patients living in rural and remote areas in limited-resource countries with better healthcare management in the diagnosis and follow-up of liver fibrosis (Omran et al., 2018).
Our study has some limitations such as the small study population and the low number of patients with low fibrosis stage, but the main one is the lack of the result of the gold standard of histological staging. Nevertheless, others have previously studied ELF score diagnostic and prognostic performance compared to imaging or other serological tests but not to histological findings (French et al., 2016; Peters et al., 2016; Swanson et al., 2016).
Conclusion
In conclusion, our findings support the use of ELF tests in routine clinical practice for the detection of advanced liver fibrosis in HCV patients before and after DAAs. As a noninvasive test, ELF can avoid unnecessary access to hospitals, allowing the identification of high-risk patients. Notably, this strategy could be used to estimate liver fibrosis before and after DAAs therapies in the context of the COVID-19 pandemic.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and the protocols were approved by the local ethics board of the promoting center (Federico II University of Naples, n 245/2013). All patients and controls involved in the study provided written informed consent to participate. The patients/participants provided their written informed consent to participate in this study.
Author contributions
VC and ELC made substantial contributions to the conception and the design; AF, GP (7th author), MG, MC, RS, LV, AL, EM, and SB acquired data; DB analyzed data; VC and DT wrote the article; and FM and GP (13th author) revised the article critically for important intellectual contribution. All authors critically revised the manuscript, approved the final version to be published, and agreed to be accountable for all aspects of the work.
Acknowledgments
We thank Michele Cennamo and Antonietta Liotti for useful discussions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.891398/full#supplementary-material
References
Abdel-Hameed, E. A., Rouster, S. D., Kottilil, S., and Sherman, K. E. (2021). The enhanced liver fibrosis index predicts hepatic fibrosis superior to FIB4 and APRI in HIV/HCV infected patients. Clin. Infect. Dis. 73 (3), 450–459. doi:10.1093/cid/ciaa646
Agbim, U., and Asrani, S. K. (2019). Non-invasive assessment of liver fibrosis and prognosis: An update on serum and elastography markers. Expert Rev. Gastroenterol. Hepatol. 13 (4), 361–374. doi:10.1080/17474124.2019.1579641
Arena, U., Vizzutti, F., Abraldes, J. G., Corti, G., Stasi, C., Moscarella, S., et al. (2008). Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut 57 (9), 1288–1293. doi:10.1136/gut.2008.149708
Carmona, I., Cordero, P., Ampuero, J., Rojas, A., and Romero-Gomez, M. (2016). Role of assessing liver fibrosis in management of chronic hepatitis C virus infection. Clin. Microbiol. Infect. 22 (10), 839–845. doi:10.1016/j.cmi.2016.09.017
Castera, L., Foucher, J., Bernard, P. H., Carvalho, F., Allaix, D., Merrouche, W., et al. (2010). Pitfalls of liver stiffness measurement: A 5-year prospective study of 13, 369 examinations. Hepatology 51 (3), 828–835. doi:10.1002/hep.23425
Castera, L. (2011). Non-invasive assessment of liver fibrosis in chronic hepatitis C. Hepatol. Int. 5 (2), 625–634. doi:10.1007/s12072-010-9240-0
Chekuri, S., Nickerson, J., Bichoupan, K., Sefcik, R., Doobay, K., Chang, S., et al. (2016). Liver stiffness decreases rapidly in response to successful hepatitis C treatment and then plateaus. PLoS One 11 (7), e0159413. doi:10.1371/journal.pone.0159413
Day, J., Patel, P., Parkes, J., and Rosenberg, W. (2019). Derivation and performance of standardized enhanced liver fibrosis (ELF) test thresholds for the detection and prognosis of liver fibrosis. J. Appl. Lab. Med. 3 (5), 815–826. doi:10.1373/jalm.2018.027359
de Ledinghen, V., and Vergniol, J. (2008). Transient elastography (FibroScan). Gastroenterol. Clin. Biol. 32 (6 Suppl. 1), 58–67. doi:10.1016/S0399-8320(08)73994-0
European, e. e. e. (2018). Association for the study of the liver. Electronic address and L. European association for the study of the: EASL recommendations on treatment of hepatitis C. J. Hepatol. 69 (2), 461–511. doi:10.1016/j.jhep.2018.03.026
Facciorusso, A., Del Prete, V., Turco, A., Buccino, R. V., Nacchiero, M. C., Muscatiello, N., et al. (2018). Long-term liver stiffness assessment in hepatitis C virus patients undergoing antiviral therapy: Results from a 5-year cohort study. J. Gastroenterol. Hepatol. 33 (4), 942–949. doi:10.1111/jgh.14008
Fernandes, F. F., Ferraz, M. L., Andrade, L. E., Dellavance, A., Terra, C., Pereira, G., et al. (2015). Enhanced liver fibrosis panel as a predictor of liver fibrosis in chronic hepatitis C patients. J. Clin. Gastroenterol. 49 (3), 235–241. doi:10.1097/MCG.0000000000000128
Fernandez, M., Trepo, E., Degre, D., Gustot, T., Verset, L., Demetter, P., et al. (2015). Transient elastography using Fibroscan is the most reliable noninvasive method for the diagnosis of advanced fibrosis and cirrhosis in alcoholic liver disease. Eur. J. Gastroenterol. Hepatol. 27 (9), 1074–1079. doi:10.1097/MEG.0000000000000392
Fraquelli, M., Rigamonti, C., Casazza, G., Conte, D., Donato, M. F., Ronchi, G., et al. (2007). Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut 56 (7), 968–973. doi:10.1136/gut.2006.111302
French, A. L., Hotton, A., Young, M., Nowicki, M., Augenbraun, M., Anastos, K., et al. (2016). Isolated hepatitis B core antibody status is not associated with accelerated liver disease progression in HIV/hepatitis C coinfection. J. Acquir. Immune Defic. Syndr. 72 (3), 274–280. doi:10.1097/QAI.0000000000000969
Guha, I. N., Parkes, J., Roderick, P., Chattopadhyay, D., Cross, R., Harris, S., et al. (2008). Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 47 (2), 455–460. doi:10.1002/hep.21984
Horowitz, J. M., Venkatesh, S. K., Ehman, R. L., Jhaveri, K., Kamath, P., Ohliger, M. A., et al. (2017). Evaluation of hepatic fibrosis: A review from the society of abdominal radiology disease focus panel. Abdom. Radiol. 42 (8), 2037–2053. doi:10.1007/s00261-017-1211-7
Kondili, L. A., Andreoni, M., Alberti, A., Lobello, S., Babudieri, S., Roscini, A. S., et al. (2021). Estimated prevalence of undiagnosed HCV infected individuals in Italy: A mathematical model by route of transmission and fibrosis progression. Epidemics 34, 100442. doi:10.1016/j.epidem.2021.100442
Kowdley, K. V., Lawitz, E., Poordad, F., Cohen, D. E., Nelson, D. R., Zeuzem, S., et al. (2014). Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. N. Engl. J. Med. 370 (3), 222–232. doi:10.1056/NEJMoa1306227
Krag, A., Roskams, T., Pinzani, M., and Mueller, S. (2022). Diagnostic challenges in patients with alcohol-related liver disease. Z. Gastroenterol. 60 (1), 45–57. doi:10.1055/a-1713-4372
Lawitz, E., Poordad, F. F., Pang, P. S., Hyland, R. H., Ding, X., Mo, H., et al. (2014). Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): An open-label, randomised, phase 2 trial. Lancet 383 (9916), 515–523. doi:10.1016/S0140-6736(13)62121-2
Lichtinghagen, R., Pietsch, D., Bantel, H., Manns, M. P., Brand, K., Bahr, M. J., et al. (2013). The enhanced liver fibrosis (ELF) score: Normal values, influence factors and proposed cut-off values. J. Hepatol. 59 (2), 236–242. doi:10.1016/j.jhep.2013.03.016
Monga, H. K., Rodriguez-Barradas, M. C., Breaux, K., Khattak, K., Troisi, C. L., Velez, M., et al. (2001). Hepatitis C virus infection-related morbidity and mortality among patients with human immunodeficiency virus infection. Clin. Infect. Dis. 33 (2), 240–247. doi:10.1086/321819
Muir, A. J., Poordad, F., Lalezari, J., Everson, G., Dore, G. J., Herring, R., et al. (2015). Daclatasvir in combination with asunaprevir and beclabuvir for hepatitis C virus genotype 1 infection with compensated cirrhosis. JAMA 313 (17), 1736–1744. doi:10.1001/jama.2015.3868
Nitta, Y., Kawabe, N., Hashimoto, S., Harata, M., Komura, N., Kobayashi, K., et al. (2009). Liver stiffness measured by transient elastography correlates with fibrosis area in liver biopsy in patients with chronic hepatitis C. Hepatol. Res. 39 (7), 675–684. doi:10.1111/j.1872-034X.2009.00500.x
Omran, D., Yosry, A., Darweesh, S. K., Nabeel, M. M., El-Beshlawey, M., Saif, S., et al. (2018). Enhanced liver fibrosis test using ELISA assay accurately discriminates advanced stage of liver fibrosis as determined by transient elastography fibroscan in treatment naive chronic HCV patients. Clin. Exp. Med. 18 (1), 45–50. doi:10.1007/s10238-017-0463-4
Parkes, J., Guha, I. N., Roderick, P., Harris, S., Cross, R., Manos, M. M., et al. (2011). Enhanced Liver Fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J. Viral Hepat. 18 (1), 23–31. doi:10.1111/j.1365-2893.2009.01263.x
Parkes, J., Roderick, P., Harris, S., Day, C., Mutimer, D., Collier, J., et al. (2010). Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut 59 (9), 1245–1251. doi:10.1136/gut.2009.203166
Patel, P. J., Connoley, D., Rhodes, F., Srivastava, A., and Rosenberg, W. (2020). A review of the clinical utility of the Enhanced Liver Fibrosis test in multiple aetiologies of chronic liver disease. Ann. Clin. Biochem. 57 (1), 36–43. doi:10.1177/0004563219879962
Peters, M. G., Bacchetti, P., Boylan, R., French, A. L., Tien, P. C., Plankey, M. W., et al. (2016). Enhanced liver fibrosis marker as a noninvasive predictor of mortality in HIV/hepatitis C virus-coinfected women from a multicenter study of women with or at risk for HIV. AIDS 30 (5), 723–729. doi:10.1097/QAD.0000000000000975
Putra, J., Schiano, T. D., and Fiel, M. I. (2018). Histological assessment of the liver explant in transplanted hepatitis C virus patients achieving sustained virological response with direct-acting antiviral agents. Histopathology 72 (6), 990–996. doi:10.1111/his.13453
Regev, A., Berho, M., Jeffers, L. J., Milikowski, C., Molina, E. G., Pyrsopoulos, N. T., et al. (2002). Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am. J. Gastroenterol. 97 (10), 2614–2618. doi:10.1111/j.1572-0241.2002.06038.x
Rockey, D. C., Caldwell, S. H., Goodman, Z. D., Nelson, R. C., Smith, A. D., American Association, D., et al. (2009). For the study of liver: Liver biopsy. Hepatology 49 (3), 1017–1044. doi:10.1002/hep.22742
Rout, G., Nayak, B., Patel, A. H., Gunjan, D., Singh, V., Kedia, S., et al. (2019). Therapy with oral directly acting agents in hepatitis C infection is associated with reduction in fibrosis and increase in hepatic steatosis on transient elastography. J. Clin. Exp. Hepatol. 9 (2), 207–214. doi:10.1016/j.jceh.2018.06.009
Sapena, V., Enea, M., Torres, F., Celsa, C., Rios, J., Rizzo, G. E. M., et al. (2022). Hepatocellular carcinoma recurrence after direct-acting antiviral therapy: An individual patient data meta-analysis. Gut 71 (3), 593–604. doi:10.1136/gutjnl-2020-323663
Sharma, P., Dhawan, S., Bansal, R., Tyagi, P., Bansal, N., Singla, V., et al. (2014). Usefulness of transient elastography by FibroScan for the evaluation of liver fibrosis. Indian J. Gastroenterol. 33 (5), 445–451. doi:10.1007/s12664-014-0491-x
Sharma, S., Khalili, K., and Nguyen, G. C. (2014). Non-invasive diagnosis of advanced fibrosis and cirrhosis. World J. Gastroenterol. 20 (45), 16820–16830. doi:10.3748/wjg.v20.i45.16820
Sherman, K. E., Abdel-Hameed, E., Rouster, S. D., Shata, M. T. M., Blackard, J. T., Safaie, P., et al. (2019). Improvement in hepatic fibrosis biomarkers associated with chemokine receptor inactivation through mutation or therapeutic blockade. Clin. Infect. Dis. 68 (11), 1911–1918. doi:10.1093/cid/ciy807
Stasi, C., and Milani, S. (2016). Non-invasive assessment of liver fibrosis: Between prediction/prevention of outcomes and cost-effectiveness. World J. Gastroenterol. 22 (4), 1711–1720. doi:10.3748/wjg.v22.i4.1711
Swanson, S., Ma, Y., Scherzer, R., Huhn, G., French, A. L., Plankey, M. W., et al. (2016). Association of HIV, hepatitis C virus, and liver fibrosis severity with the enhanced liver fibrosis score. J. Infect. Dis. 213 (7), 1079–1086. doi:10.1093/infdis/jiv567
Thiele, M., Madsen, B. S., Hansen, J. F., Detlefsen, S., Antonsen, S., Krag, A., et al. (2018). Accuracy of the enhanced liver fibrosis test vs FibroTest, elastography, and indirect markers in detection of advanced fibrosis in patients with alcoholic liver disease. Gastroenterology 154 (5), 1369–1379. doi:10.1053/j.gastro.2018.01.005
Trembling, P. M., Lampertico, P., Parkes, J., Tanwar, S., Vigano, M., Facchetti, F., et al. (2014). Performance of Enhanced Liver Fibrosis test and comparison with transient elastography in the identification of liver fibrosis in patients with chronic Hepatitis B infection. J. Viral Hepat. 21 (6), 430–438. doi:10.1111/jvh.12161
Ueda, N., Kawaoka, T., Imamura, M., Aikata, H., Nakahara, T., Murakami, E., et al. (2020). Liver fibrosis assessments using FibroScan, virtual-touch tissue quantification, the FIB-4 index, and mac-2 binding protein glycosylation isomer levels compared with pathological findings of liver resection specimens in patients with hepatitis C infection. BMC Gastroenterol. 20 (1), 314. doi:10.1186/s12876-020-01459-w
Vuppalanchi, R., Unalp, A., Van Natta, M. L., Cummings, O. W., Sandrasegaran, K. E., Hameed, T., et al. (2009). Effects of liver biopsy sample length and number of readings on sampling variability in nonalcoholic Fatty liver disease. Clin. Gastroenterol. Hepatol. 7 (4), 481–486. doi:10.1016/j.cgh.2008.12.015
Wahl, K., Rosenberg, W., Vaske, B., Manns, M. P., Schulze-Osthoff, K., Bahr, M. J., et al. (2012). Biopsy-controlled liver fibrosis staging using the enhanced liver fibrosis (ELF) score compared to transient elastography. PLoS One 7 (12), e51906. doi:10.1371/journal.pone.0051906
Keywords: ELF index, HCV, liver fibrosis, transient elastography (FibroScan), direct-acting agents
Citation: Cossiga V, La Civita E, Bruzzese D, Guarino M, Fiorentino A, Sorrentino R, Pontillo G, Vallefuoco L, Brusa S, Montella E, Terracciano D, Morisco F and Portella G (2022) Enhanced liver fibrosis score as a noninvasive biomarker in hepatitis C virus patients after direct-acting antiviral agents. Front. Pharmacol. 13:891398. doi: 10.3389/fphar.2022.891398
Received: 07 March 2022; Accepted: 04 July 2022;
Published: 17 August 2022.
Edited by:
Stefano Fiorucci, University of Perugia, ItalyReviewed by:
Ciro Celsa, University of Palermo, ItalyGiuseppe Losurdo, University of Bari Medical School, Italy
Copyright © 2022 Cossiga, La Civita, Bruzzese, Guarino, Fiorentino, Sorrentino, Pontillo, Vallefuoco, Brusa, Montella, Terracciano, Morisco and Portella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniela Terracciano, ZGFuaWVsYS50ZXJyYWNjaWFub0B1bmluYS5pdA==; Valentina Cossiga, dmFsZW50aW5hLmNvc3NpZ2FAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Valentina Cossiga
Valentina Cossiga Evelina La Civita
Evelina La Civita Dario Bruzzese
Dario Bruzzese Maria Guarino
Maria Guarino Andrea Fiorentino1
Andrea Fiorentino1 Daniela Terracciano
Daniela Terracciano Filomena Morisco
Filomena Morisco Giuseppe Portella
Giuseppe Portella